Abstract
BACKGROUND
Endoscopy has rapidly developed in recent years and has enabled further investigation into the origin and features of intestinal tumors. The small size and concealed position of these tumors make it difficult to distinguish them from nonneoplastic polyps and carcinoma in adenoma (CIA). The invasive depth and metastatic potential determine the operation regimen, which in turn affects the overall survival and distant prognosis. The previous studies have confirmed the malignant features and clinicopathological features of de novo colorectal cancer (CRC).
AIM
To provide assistance for diagnosis and treatment, but the lack of a summary of endoscopic features and assessment of risk factors that differ from the CIA prompted us to conduct this retrospective study.
METHODS
In total, 167 patients with small-sized CRCs diagnosed by endoscopy were reviewed. The patients diagnosed as advanced CRCs and other malignant cancers or chronic diseases that could affect distant outcomes were excluded. After screening, 63 cases were excluded, including 33 de novo and 30 CIA cases. Patient information, including their follow-up information, was obtained from an electronic His-system. The characteristics between two group and risk factors for invasion depth were analyzed with SPSS 25.0 software.
RESULTS
Nearly half of the de novo CRCs were smaller than 1 cm (n = 16, 48.5%) and the majority were located in the distal colon (n = 26, 78.8%). The IIc type was the most common macroscopic type of de novo CRC. In a Pearson analysis, the differential degree, Sano, JNET, and Kudo types, surrounding mucosa, and chicken skin mucosa (CSM) were correlated with the invasion depth (P < 0.001). CSM was a significant risk factor for deep invasion and disturbed judgment of endoscopic ultrasound. A high degree of tumor budding and tumor-infiltrating lymphocytes are accompanied by malignancy. Finally, de novo CRCs have worse outcomes than CIA CRCs.
CONCLUSION
This is the first comprehensive study to analyze the features of de novo CRCs to distinguish them from nonneoplastic polyps. It is also the first study paying attention to CSM invasive depth measurement. This study emphasizes the high metastatic potential of de novo CRCs and highlights the need for more research on this tumor type.
Keywords: De novo colorectal cancer, Carcinoma in adenoma, Endoscopic features, Clinical characteristics, Pathological features
Core Tip: De novo colorectal cancer (CRC) is a specific tumor with a small lesion. Many different features of de novo CRCs exist to distinguish them from non-neoplastic polyps. Moreover, the study highlights that de novo CRCs have special endoscopic and pathological features that distinguish them from traditional adenocarcinomas. Different pit pattern types indicate various tumor types; for example, the III-type pit pattern often occurs in tubular adenomas. For CRCs, invasion depth evaluation is a vital issue. Computed tomography imaging and endoscopic ultrasound are used for judging invasive depth. Besides, chicken skin mucosa may also be a risk factor.
INTRODUCTION
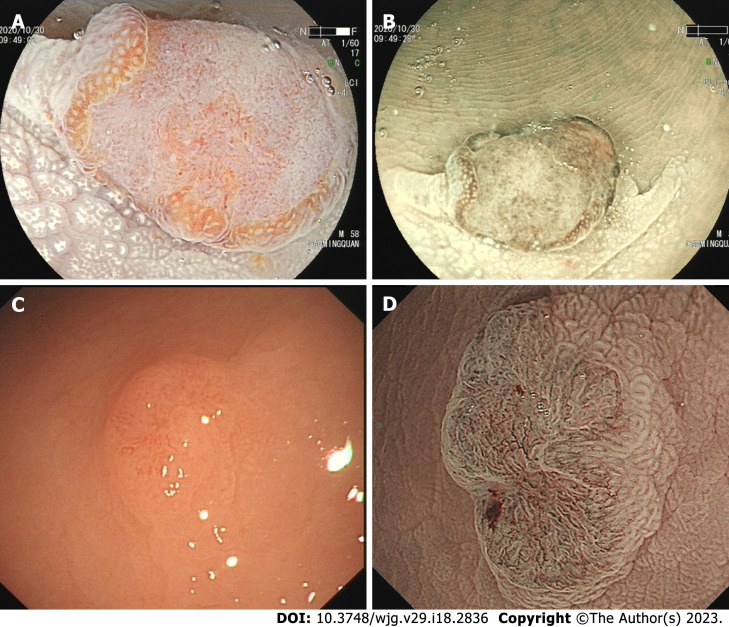
A de novo colorectal cancer (CRC) is a unique CRC that represents a significant public health challenge with high morbidity and mortality globally[1,2]. At present, CRCs have four recognized pathways of development: The carcinoma in adenoma (CIA) pathway (aka chromosomal instability pathway), the de novo carcinoma pathway, the serrated lesion-carcinoma pathway, and the inflammatory-carcinoma pathway. In the 1950s, de novo-originated CRCs emerged increasingly after the definition “de novo” was proposed. In recent decades, the Japanese proposed a de novo carcinoma pathway[3]. Unlike other pathways, a de novo CRC is a tumor derived from cancer cells without progression of adenomatous changes, as shown in Figure 1, and its true pathway remains unknown. Normal I type around tumor and lack of III or IV type pits is a hallmark of de novo CRC under endoscopy[4-6].
Figure 1.
The de novo colorectal cancer under endoscopy. A: A de novo colorectal cancer (CRC) under linked color imaging; B and D: The surface structure under the narrow bind imaging pattern; C: A de novo CRC under white light.
For endoscopists, the endoscopic featrures occupied a key position in diagnosis and treatment. Magnified chromoendoscopy and narrow-band imaging (NBI) technology can be used to observe pit pattern and extimate depth of invasion[7-11]. Apart of pit pattern, there existed several indicators could forecast depth of invasion, such as chicken skin mucosa (CSM), mucosa pulling and converging. The CSM is an area of 0.5 mm of pale-yellow speckles adjacent to colonic neoplasms resulting from fat accumulation in the lamina propria[12]. CSM was more common in neoplastic and advanced polyps[13]. In this study, we concentrate on relationship between CSM and depth of invasion. For the whole CRCs, we should get to know three things: Get to know who they are firstly, estimate depth of invasion secondly, and predict the risk of metastasis at last.
Epithelial-mesenchymal transition (EMT) refers to a cellular reprogramming process in which epithelial cells acquire a mesenchymal phenotype[14]. EMT has an important role in development, wound healing, and malignant progression[15]. Mueller et al[16] have found that E-cadherin express lower in de novo than ex adenoma carcinomas through immunohistochemistry (IHC)[16]. We further want to know the relationship between E-cadherin proportion and distant metastsis.
Until now, there was no a complete study about de novo CRC, so we make this study to construct a comprehensive system for diagnosis and treatment of de novo CRC.
MATERIALS AND METHODS
We retrospectively reviewed patients diagnosed with CRCs between 2010 to 2020 year. We selected all de novo CRC and CIA-type patients on the His Electric System. All lesions were diagnosed by a senior pathologist and confirmed by two pathologists. The results from two pathologists had more than 90% similarity, and discrepant results were determined by a cheif pathologist. These patients did not have other comorbidities that could significantly impact the distant outcomes. Essential clinical information was collected, including endoscopic features and pathological results (size, endoscopic features, macroscopic type, pit pattern, clinical diagnosis, pathologic diagnosis, infiltration (IFN), immunohistology, invasive depth, tumor budding (TB), grading, lymphocyte IFN, perineural IFN, growth pattern, and lymph node metastasis). Pearson and χ2 tests were performed using SPSS (25.0) software. The difference between each group was statistically significant (P < 0.05). Oral informed consent was obtained from all participants via telephone calls.
The degree of staining was scored according to the staining intensity (0, no staining; 1, weak; 2, moderate; 3, strong) and the proportion of positive cells (0: 0%; 1: < 25%; 2: < 50%; 3: < 75%; 4: ≥ 75%). The score of each slice was determined by the staining index (staining intensity × proportion of positive cells). A staining index > 4 was classified as high-grade expression, while an index ≤ 4 was defined as low-grade expression.
The pathological film used in the study was IHC, with the patient's consent, and the patient's information was kept confidential; therefore, ethical approval was not required.
RESULTS
Clinical features of de novo CRCs
Nearly half (n = 16, 48.5%) of the de novo CRCs were less than 1 cm in size and located in the distal colon (n = 26, 78.8%). In contrast, CIA CRCs were mostly 2 cm (n = 21, 70.0%) and located at the proximal colon (n = 21, 70.0%). There were no significant differences in the other risk factors between the de novo and CIA groups (P > 0.05). In addition, we focused on the choice of treatment. All submucosa de novo CRCs were treated surgically, and seven CIA submucosa CRCs were treated endoscopically. The results are presented in Table 1.
Table 1.
The clinical information of de novo and carcinoma in adenoma colorectal cancers
|
|
|
De novo (n = 33)
|
CIA (n = 30)
|
χ
2
|
P value
|
| Clinical information | |||||
| Age (%) | > 55 | 30 (90.9) | 25 (83.3) | 0.814 | > 0.05 |
| < 55 | 3 (9.1) | 5 (16.7) | |||
| Gender (%) | Male | 16 (48.5) | 16 (53.3) | 0.148 | > 0.05 |
| Female | 17 (51.5) | 14 (46.7) | |||
| Smoking history (%) | Yes | 27 (81.8) | 19 (63.3) | 2.725 | > 0.05 |
| No | 6 (18.2) | 11 (36.7) | |||
| Location (%) | Proximal | 7 (21.2) | 21 (70.0) | 15.149 | < 0.05 |
| Distal | 26 (78.8) | 9 (30.0) | |||
| Depth of invasion | M | 8 (24.2) | 9 (30.0) | 1.504 | > 0.05 |
| SMp | 9 (27.3) | 11 (36.7) | |||
| SMd | 16 (48.5) | 10 (33.3) | |||
| Treatment (%) | Surgery | 21 (63.6) | 16 (53.3) | 0.688 | > 0.05 |
| Endoscopy | 12 (36.4) | 14 (46.7) | |||
| Supplement chemotherapy (%) | Yes | 21 (63.6) | 12 (40.0) | 3.520 | > 0.05 |
| No | 12 (36.4) | 18 (60.0) | |||
| Distal metastasis (%) | Yes | 5 (15.2) | 2 (6.7) | 1.145 | > 0.05 |
| No | 28 (84.8) | 28 (93.3) | |||
| Overall survival (%) | 1 yr | 33 (100.0) | 30 (100.0) | ||
| 3 yr | 30 (90.9) | 28 (93.3) | |||
| 5 yr | 23 (69.7) | 28 (93.3) | |||
| DSS | Yes | 26 (78.8) | 28 (93.3) | 2.715 | > 0.05 |
| No | 7 (21.2) | 2 (6.7) | |||
| Endoscopic gross characteristics | |||||
| Size% | 1 cm < S ≤ 2 cm | 14 (42.4) | 9 (30.0) | 30.514 | < 0.05 |
| ≤ 1 cm | 16 (48.5) | 0 (0.0) | |||
| > 2 cm | 3 (9.1) | 21 (70.0) | |||
| Macroscopic type% | Is | 5 (15.2) | 6 (20.0) | 35.187 | < 0.05 |
| IIa + IIc | 11 (33.3) | 1 (3.3) | |||
| IIc | 17 (51.5) | 4 (13.3) | |||
| Is + IIc | 0 (0.0) | 19 (63.3) | |||
| Surface change | Erosion | 24 (72.7) | 13 (43.3) | 9.457 | < 0.05 |
| Ulceration | 2 (6.1) | 11 (36.7) | |||
| None | 7 (21.2) | 6 (20.0) | |||
| Color change | Reddened mucosa | 26 (78.8) | 2 (6.7) | 33.104 | < 0.05 |
| None | 7 (21.2) | 28 (93.3) | |||
| NICE | Type 1 | 0 (0.0) | 0 (0.0) | > 0.05 | |
| Type 2 | 0 (0.0) | 0 (0.0) | |||
| Type 3 | 33 (100.0) | 30 (100.0) | |||
| Sano | I | 0 (0.0) | 0 (0.0) | 5.292 | < 0.05 |
| II | 0 (0.0) | 0 (0.0) | |||
| IIIA | 17 (51.5) | 7 (23.3) | |||
| IIIB | 16 (48.5) | 23 (76.7) | |||
| JNET | 1 | 0 (0.0) | 0 (0.0) | 3.094 | > 0.05 |
| 2A | 0 (0.0) | 0 (0.0) | |||
| 2B | 12 (36.4) | 5 (16.7) | |||
| 3 | 21 (63.6) | 25 (83.3) | |||
| Chemical staining (crystal violet) | |||||
| Pit pattern | Vi | 1 (3.0) | 6 (20.0) | 15.774 | < 0.05 |
| Vn | 13 (39.4) | 15 (50.0) | |||
| Vi + Vn | 19 (57.6) | 5 (16.7) | |||
| IV + Vi | 0 (0.0) | 4 (13.3) | |||
| Pit pattern at the edge of the tumors | I | 33 (100.0) | 0 (0.0) | 63.000 | < 0.05 |
| III + VI | 0 (0.0) | 30 (100.0) | |||
| Surrounding mucosa | |||||
| Pulling | Yes | 9 (27.3) | 21 (70.0) | 11.501 | < 0.05 |
| No | 24 (72.7) | 9 (30.0) | |||
| Converging folds | Yes | 27 (81.8) | 15 (50.0) | 7.159 | < 0.05 |
| No | 6 (18.2) | 15 (50.0) | |||
| CSM | CSM1 | 11 (33.3) | 4 (13.3) | 3.553 | > 0.05 |
| CSM2 | 17 (51.5) | 21 (70.0) | |||
| CSM3 | 5 (15.2) | 5 (16.7) |
CRC: Colorectal cancer; DSS: Disease specific survival; CSM: Chicken skin mucosa; CIA: Carcinoma in adenoma; NICE: Narrow-band imaging International Colorectal Endoscopic.
Endoscopic characteristics of de novo CRCs
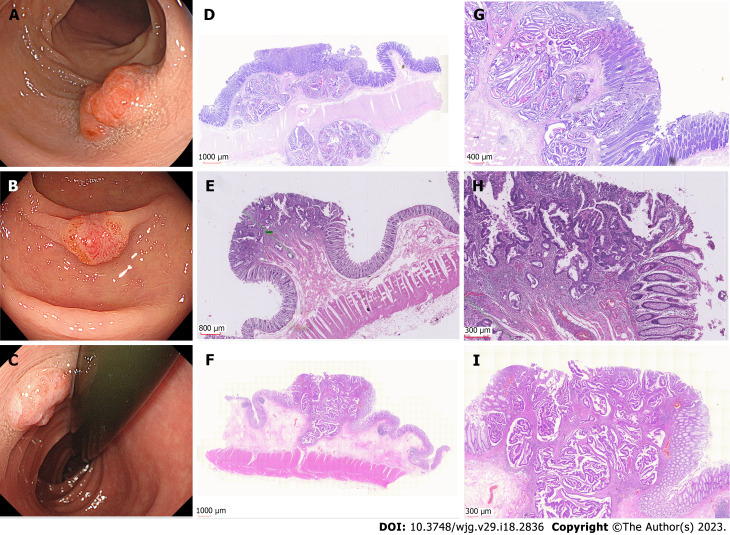
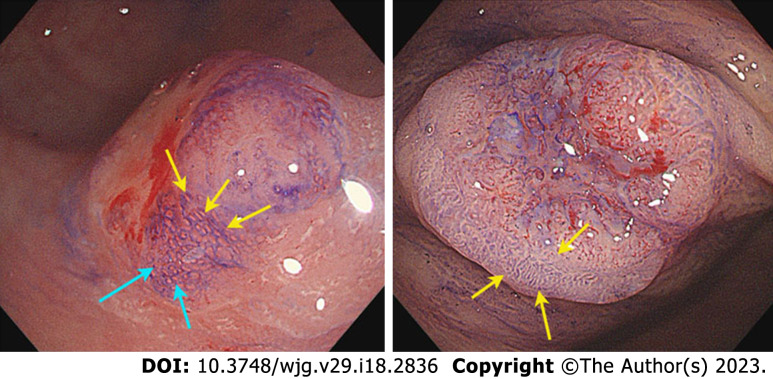
Based on endoscopic appearance, small CRCs were classified as type I (polypoid), type IIa (slightly raised), type IIb (flat), or type IIc (depressed)[12]. Type IIa + IIc is the most common type of de novo CRC. Notably, 15 (45.5%) de novo CRCs were Is type. We showed different macroscopic types of de novo CRCs, as shown in Figure 2. For CIA CRCs, the Is + IIc type (polypoid with depression) was the most common presentation (n = 19, 63.3%). Another significant finding was surface changes: We found that erosion and reddened mucosa are hallmark features of de novo CRCs. In the CIA group, erosion was the same as ulceration. Furthermore, reddened mucosa was seldom observed in the CIA group (n = 28, 93.3%). The endoscopic results are shown in Table 2.
Figure 2.
The examples of I-type de novo colorectal cancer. A-C: Endoscopic images of different types de novo colorectal cancers (CRCs); D-I: Pathological images of de novo CRCs.
Table 2.
The endoscopic characteristics in de novo and carcinoma in adenoma groups
|
|
|
De novo (n = 22)
|
CIA (n = 20)
|
χ2
|
P value
|
| Budding | BD1 | 8 (24.2) | 4 (13.3) | 6.161 | > 0.05 |
| BD2 | 8 (24.2) | 6 (20.0) | |||
| BD3 | 11 (33.3) | 6 (20.0) | |||
| None | 5 (18.2) | 14 (46.7) | |||
| Grading | G1 | 6 (18.2) | 13 (43.3) | 8.878 | > 0.05 |
| G2 | 11 (33.3) | 9 (30.0) | |||
| G1-2 | 10 (30.3) | 8 (26.7) | |||
| G3-2 | 6 (18.2) | 0 (0.0) | |||
| TIL | Yes | 27 (81.8) | 19 (63.3) | 2.725 | > 0.05 |
| No | 6 (18.2) | 11 (36.7) | |||
| Perineural infiltration | Yes | 16 (48.5) | 3 (10.0) | 11.050 | < 0.05 |
| No | 17 (51.5) | 27 (90.0) | |||
| Lymphovascular invasion | Yes | 16 (48.5) | 7 (23.3) | 4.289 | < 0.05 |
| No | 17 (51.5) | 23 (76.7) | |||
| Growth pattern | INFa | 0 (0.0) | 27 (90.0) | 63.000 | < 0.05 |
| INFb | 0 (0.0) | 3 (10.0) | |||
| INFc | 33 (100.0) | 0 (0.0) | |||
| Lymph node metastasis | Yes | 10 (30.3) | 1 (3.3) | 7.931 | > 0.05 |
| No | 23 (69.7) | 29 (96.7) | |||
| Adjacent lesions | Inflammation | 33 (100.0) | 0 (0.0) | 63.000 | < 0.05 |
| Adenoma | 0 (0.0) | 17 (56.7) | |||
| Hyperplasia | 0 (0.0) | 13 (43.3) | |||
| IHC-E-cadherin | No staining | 0 (0.0) | 0 (0.0) | 22.609 | < 0.05 |
| Weak | 17 (51.5) | 2 (6.7) | |||
| Moderate | 15 (48.5) | 19 (63.3) | |||
| Strong | 0 (0.0) | 9 (30.0) | |||
| IHC-Vimentin | No staining | 0 (0.0) | 0 (0.0) | 23.182 | < 0.05 |
| Weak | 13 (39.4) | 18 (60.0) | |||
| Moderate | 1 (3.0) | 10 (33.3) | |||
| Strong | 19 (57.6) | 2 (6.7) |
CIA: Carcinoma in adenoma; TIL: Tumor Infiltrating Lymphocyte; IHC: Immunohistochemistry; BD: Budding grade.
There was a significant difference in the Sano and pit pattern types (P < 0.05) but no significant difference in the NBI International Colorectal Endoscopic (NICE) classification and JNET types between the two groups (P > 0.05). About half of the de novo CRCs were IIIA type (n = 17, 51.5%) with a Vi + Vn type pit pattern (n = 19, 57.6%). The most striking difference was in the pit pattern around the edges of the lesions: I type pit patterns (small and round pits) were observed in all de novo CRCs (n = 33, 100%), and III + VI type pit patterns were observed in all CIA CRCs (n = 30, 100%).
We also analyzed the risk factors associated with the invasive depth (Table 3). A third (33.3%) of the de novo group had a CSM1 × vs 13.3% of the CIA group. CSM was associated with the depth of invasion [r (correlation coefficient) = -0.796, P < 0.001]. CSM1 has a high invasive potential for CRC. In addition to CSM, pulling and converging folds also appear in the mucosa surrounding CRCs. Mucosal pulling appeared more frequently in the CIA group (n = 21, 70.0%) than in the de novo group (n = 9, 27.3%). In contrast, the converging fold was more frequent in the de novo group (n = 27, 81.8%) than that in the CIA group (n = 15, 50%). After analyzing the relationship between these parameters and invasive depth, we found that mucosal pulling (r = 0.567, P < 0.001) and converging folds (r = 0.620, P < 0.001) were also significantly associated with invasive depth.
Table 3.
The risk factors are related to the depth of invasion
|
Depth of invasion
|
M
|
SM-S
|
SM-d and deeper
|
r
|
P value
|
| Size, % | -0.002 | > 0.05 | |||
| 1 cm < S ≤ 2 cm | 1 (12.5) | 0 (0.0) | 13 (81.3) | ||
| ≤ 1 cm | 7 (87.5) | 9 (100.0) | 0 (0.0) | ||
| > 2 cm | 0 (0.0) | 0 (0.0) | 3 (18.8) | ||
| Growth pattern | > 0.05 | ||||
| IFNa | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| IFNb | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| IFNc | 8 (100.0) | 9 (100.0) | 16 (100.0) | ||
| Differential degree | 0.904 | < 0.001 | |||
| G1 | 5 (62.5) | 1 (11.1) | 0 (0.0) | ||
| G2 | 3 (37.5) | 8 (88.9) | 0 (0.0) | ||
| G1-2 | 0 (0.0) | 0 (0.0) | 10 (62.5) | ||
| G3-2 | 0 (0.0) | 0 (0.0) | 6 (37.5) | ||
| Macroscopic type | -0.093 | > 0.05 | |||
| Is | 2 (25.0) | 0 (0.0) | 13 (81.3) | ||
| IIa + IIc | 6 (75.0) | 9 (100.0) | 0 (0.0) | ||
| IIc | 0 (0.0) | 0 (0.0) | 3 (18.8) | ||
| Is + IIc | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| NICE | > 0.05 | ||||
| Type 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Type 2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Type 3 | 8 (100.0) | 9 (100.0) | 16 (100.0) | ||
| Sano | 0.938 | < 0.001 | |||
| I | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| II | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| IIIA | 8 (100.0) | 9 (100.0) | 0 (0.0) | ||
| IIIB | 0 (0.0) | 0 (0.0) | 16 (100.0) | ||
| JNET | 0.649 | < 0.001 | |||
| 1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| 2A | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| 2B | 5 (62.5) | 7 (77.8) | 0 (0.0) | ||
| 3 | 3 (37.5) | 2 (22.2) | 16 (100.0) | ||
| Pit pattern | -0.491 | < 0.001 | |||
| Vi | 1 (12.5) | 0 (0.0) | 0 (0.0) | ||
| Vn | 0 (0.0) | 0 (0.0) | 13 (81.3) | ||
| Vi + Vn | 7 (87.5) | 9 (100.0) | 3 (18.8) | ||
| IV + Vi | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Pit pattern at the edge of the tumors | > 0.05 | ||||
| I | 8 (100.0) | 9 (100.0) | 16 (100.0) | ||
| III + VI | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Surrounding mucosa | |||||
| Pulling | -0.567 | < 0.001 | |||
| Yes | 0 (0.0) | 0 (0.0) | 9 (56.3) | ||
| No | 8 (100.0) | 9 (100.0) | 7 (43.8) | ||
| Converging folds | -0.62 | < 0.001 | |||
| Yes | 3 (37.5) | 8 (88.9) | 16 (100.0) | ||
| No | 5 (62.5) | 1 (11.1) | 0 (0.0) | ||
| CSM | -0.796 | < 0.001 | |||
| CSM1 | 0 (0.0) | 0 (0.0) | 11 (68.8) | ||
| CSM2 | 3 (37.5) | 9 (100.0) | 5 (31.3) | ||
| CSM3 | 5 (62.5) | 0 (0.0) | 0 (0.0) |
IFN: Infiltration; CSM: Chicken skin mucosa; NICE: Narrow-band imaging International Colorectal Endoscopic.
Pathological characteristics of de novo CRCs
Pathological results revealed that TB was significantly associated with invasive depth (r = 0.669, P < 0.001) (Table 4). Although there was no significant difference between the two groups, budding grade 3 (BD3) accounted for 33.3% of the de novo group, which is far more than that of the CIA group (n = 6, 20%). Regarding other pathological characteristics, perineural IFN, lymph node metastasis, and tumor-infiltrating lymphocytes (TIL) have also emerged in CRC tissues. There was a significant difference in perineural IFN and lymph node metastasis between the two groups (P < 0.05).
Table 4.
The risk factors are related to tumor budding
|
|
BD1
|
BD2
|
BD3
|
No budding
|
r
|
P value
|
| Infiltrative depth, % | 0.669 | < 0.001 | ||||
| M | 0 (0.0) | 3 (21.4) | 0 (0.0) | 14 (70.0) | ||
| SMp | 10 (83.3) | 1 (7.1) | 3 (17.6) | |||
| SMd | 2 (16.7) | 10 (71.4) | 14 (82.4) | |||
| Lymphovascular invasion | 0.663 | < 0.001 | ||||
| Yes | 2 (28.6) | 4 (21.1) | 16 (94.1) | 1 (5.0) | ||
| No | 5 (71.4) | 15 (78.9) | 1 (5.9) | 19 (95.0) | ||
| Lymph node metastasis | 0.489 | < -0.001 | ||||
| Yes | 1 (14.3) | 1 (5.3) | 9 (52.9) | 0 (0.0) | ||
| No | 6 (85.7) | 18 (94.7) | 8 (41.7) | 20 (100.0) | ||
| Perineural infiltration | 0.601 | < 0.001 | ||||
| Yes | 2 (28.6) | 4 (21.1) | 13 (76.5) | 0 (0.0) | ||
| No | 5 (71.4) | 15 (78.9) | 4 (23.5) | 20 (100.0) | ||
| Tumor infiltrating lymphocytes | 0.476 | < 0.001 | ||||
| Yes | 7 (100.0) | 19 (100.0) | 14 (82.4) | 6 (30.0) | ||
| No | 0 (0.0) | 0 (0.0) | 3 (17.6) | 14 (70.0) | ||
| Differential degree | 0.706 | < 0.001 | ||||
| G1 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 19 (95.0) | ||
| G1-2 | 2 (28.6) | 9 (47.4) | 7 (41.2) | 0 (0.0) | ||
| G2 | 5 (71.4) | 10 (52.6) | 4 (23.5) | 1 (5.0) | ||
| G3-2 | 0 (0.0) | 0 (0.0) | 6 (35.3) | 0 (0.0) |
BD: Budding grade.
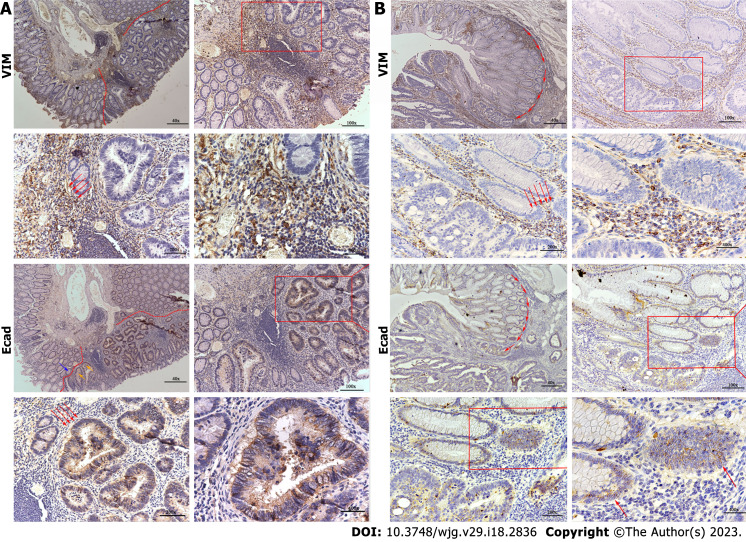
Another critical issue that deserves attention is IHC. In both groups, approximately 70% of the cases were MLH1 (+), MSH2 (+), MSH6 (+), PMS2 (+), CDX2 (+), Ki67 (+), and P53 (+). We extracted several sections that were stained for E-cadherin and vimentin (VIM). In the de novo group, the E-cadherin expression was low in the epithelial regions and VIM was high in the mesenchyma. The CIA group showed opposite results to the de novo group, as shown in Figure 3. We analyzed risk factors for distant metastasis in de novo group, the results are shown in Supplementary Table 1. Differential degree, Sano classification, expression of E-cad and VIM were correlated with distant metastasis. High expression of E-cad was negatively related with distant metastasis and VIM had an opposite result.
Figure 3.
The immunohistory results. A: A de novo colorectal cancer (CRC) pathological section stained with E-cad and vimentin (VIM); B: A carcinoma in adenoma CRC pathological section stained with E-cad and VIM. VIM: Vimentin.
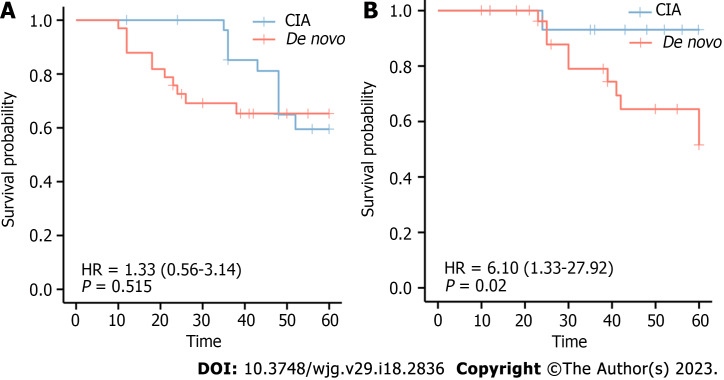
In addition, we explored whether the de novo type was correlated with relapse and survival. In the de novo group, 11 subjects developed neoplastic polyps again within 5 years, and 10 relapsed within 5 years. The 5-year survival rate was 93.3% for CIA and 69.7% for de novo CRCs. Log-rank analysis revealed no significant difference in relapse between the two groups (χ2 = 0.49, P = 0.515); however, there was a significant difference in the survival probability between the two groups (χ2 = 7.08, P = 0.020). Survival curves are shown in Figure 4.
Figure 4.
The association of de novo colorectal cancer and over survival. A: Relationship between relapse rate and colorectal cancer (CRC) types; B: Relationship between rate of death and CRC types. CIA: Carcinoma in adenoma.
DISCUSSION
In this study, we present our novel findings pertaining to the characteristics of de novo CRC. The key findings included the following: (1) The incidence rate of de novo CRCs is considered lower than CIA CRCs, but our study showed that it is related to the classification of CRC. Although some de novo CRCs are limited to intramucosal cancer, they are not the same as high-grade dysplasia or cancer with high-grade intraepithelial neoplasia, which could be called advancing adenoma. An advancing adenoma is a precancerous lesion, whereas a de novo CRC is a type of early CRC. Some endoscopic physicians misdiagnose advanced adenomas as early CRCs; this will improve the diagnostic rate of CRC but reduce the incidence rate of de novo cancer. Therefore, we believe that the diagnostic bias of endoscopic physicians affects the incidence rate. One of the aims of this study was to bolster the standardized diagnostic requirements of endoscopic physicians and to understand de novo cancer from the molecular, pathological, and endoscopic perspectives. According to the Vienna classification, category 4 includes advanced adenoma/dysplasia and noninvasive carcinoma. The reason for the lower incidence of de novo CRCs in the previous study may be eliminated by comprehensively evaluating the characteristics comprehensively[17-20]; (2) In our study, 48.5% of de novo CRCs were < 1 cm in size. Some investigators excluded tumors > 1 cm because they thought that the adenomatous component might be obliterated by the expanding tumor mass[12]. In our study, 42.4% of the de novo CRC-derived tumors diagnosed by endoscopists and pathologists were ≥ 1 cm and ≤ 2 cm. In addition, there were three lesions larger than 2 cm. Therefore, warning regarding small lesions without adenomatous components should be escalated; and (3) In addition to platform lift, converging mucosa, pulling, and CSM are significant indicators of invasive depth. Fibroplasia causes surface changes during cancer cell invasion. Converging mucosa was more likely to occur in de novo CRCs than that in CIA group, and nearly half of the lesions (n = 16, 48.5%) had deeper invasion. Mucosal pulling may be caused by shrinkage of the mucosa or muscularis mucosa, indicating superficial layer invasion.
The cause of CSM may be fat accumulation in macrophages, which may result from the breakdown of lipids within colonocytes or adjacent tumors. These findings suggest that CSM is a valuable marker for differentiating between neoplastic and advanced polyps using conventional white colonoscopy[21]. Lee et al[13] divided CSM into three types based on their characteristics. Type 1 CSM was confirmed before injection; type 2 CSM was observed after injection; and type 3 CSM was not observed. Types 1 and 2 were considered positive CSM findings. CSM is accompanied by an increased IFN of foam macrophages in the lamina propria, representing a large-scale inflammatory reaction. The appearance of CSM is also related to the increased expression of Ki-67 and COX2, which suggests that the early appearance of CSM symbolizes a high malignancy. However, whether the appearance of CSM and the IFN of macrophages in the lamina propria affect the determination of the depth of IFN by endoscopic ultrasound (EUS) is unknown. After diagnosing white light endoscopy, magnifying endoscopy, and endoscopic ultrasonography, we concluded that the lesion was a de novo cancer with deep invasion, which was no longer suitable for endoscopic treatment. However, after surgical resection, pathology showed that the depth of invasion only reached the M2 layer. After further analysis of the pathology, we found that many inflammatory cells infiltrated the lesion, leading to errors in determining the IFN depth.
Under NBI and magnifying endoscopy patterns, the surface and vascular microstructures can be clearly evaluated. The NICE classification is a method for assessing the characteristics of lesions under the NBI model, which is based on the color, vessel, and general surface pattern[22]. The NICE classification can help reduce the risk of failing to detect diminutive and small lesions, which are easily regarded as polys[23]. Nevertheless, the NICE classification had no differential ability for de novo CRCs and CIA CRCs. The pit pattern of CRC characteristics was categorized according to the Kudo and Tsuruta classification system. Type IIIL and IV patterns are often observed in adenomas and CIA CRCs. Type V is subclassified into Vi and Vn, and occurs in cancers. The surrounding I type pit is a landmark in the diagnosis of de novo CRCs. However, the normal I type often deforms and confounds with an abnormal pit due to the extrusion or invasion of cancer. The IIIs type pit was round and smaller than the I type pit, and IIIL was more irregular than the elongated I type pit. An example is shown in Figure 5.
Figure 5.
The surrounding pit of de novo colorectal cancer and carcinoma in adenoma group. The surrounding pit around de novo colorectal cancer (CRC) is elongated I-type and IIIL-type around carcinoma in adenoma CRC.
De novo CRCs have greater metastatic potential, and all pathological indicators indicate that de novo carcinoma has a higher metastatic potential and worse prognosis. All de novo cancers were classified as microsatellite stability (MSS). In the de novo group, the degree of TB and lymphocyte IFN was higher than that in the CIA group and was positively correlated with the depth of invasion and poor prognosis. The IHC results also showed that the expression of E-cadherin in the de novo group was lower than that in the CIA group. At the same time, the interstitial-related index VIM was higher than that in the CIA group, which means that the metastatic potential of the tumor in the de novo group was higher than that in the CIA group. TB is defined as “a single cancer cell or a cell cluster of 5 cancer cells or less". Larger tumors are called poorly differentiated tumor cell nests. TB is closely correlated with disease recurrence and poor prognosis in SM-invasive CRCs[24-27].
In addition, the TB degree had a relationship with other pathological features. In general, tumor tissues with BD2/3 are considered for further surgery[28]. The lymphocytic reaction is another crucial part of pathological changes in CRCs, which includes Crohn's-like reaction, peritumoral reaction, intratumoral periglandular reaction, and TIL[29]. TIL are defined as lymphocytes on the top of cancer cells, which can be used to predict immunotherapy response and survival outcomes. One study reported that tumors with microsatellite instability-high and sufficient CD8+ TIL had better outcomes than those with MSS/microsatellite instability-low and lower CD8+ TIL[30]. Compared to typical pathological indicators, TIL are a better factor for overall survival[29,31,32]. TIL are related to the expression of exhaustion and senescence markers in the tumor microenvironment[33,34].
This study have several promising implications during clinical practice. Due to the highly invasive and metastatic ability of de novo CRC, endoscopic mucosal resection or endoscopic submucosal dissection could not be performed without exact observation when we find small protruded or depressed lesions. Observing the lesions with magnified endoscopy and chromoendoscopy is necessary to confirm the diagnosis. If we suspect the lesions may be de novo CRCs, it is crucial evaluating invasion depth by magnified endoscopy and chromoendoscopy, or by computed tomography imaging and EUS when necessary. Then, the treatment regimens should be chosen cautiously. In the future, we will further study the molecular biological difference between de novo CRC and CIA CRC to find out the molecular mechanism of invasion and metastasis of de novo CRCs.
However, there exists some limitations in the study. The sample size appear to be small for deficience of acknowledge of de novo CRCs. Moreover, this appears as a single center study, and the pathogenesis/incidence of de novo CRC might be different in other countries.
CONCLUSION
This review summarizes the characteristics of de novo CRCs and confirms the conclusions of our study. A de novo CRC is a small, but malignant tumor that requires more attention during colonoscopy examination. De novo CRCs have special endoscopic and pathological features that distinguish them from the traditional adenocarcinomas. However, more studies are needed to determine the molecular characteristics to explain the genesis mechanism.
ARTICLE HIGHLIGHTS
Research background
The small colorectal small tumors usually be ignored during colonoscopy. However, many depressed or flat lesions have substantial invasion and metastasis. De novo colorectal cancer (CRC) is one type of small tumor related to poor prognosis. And some endoscopists could not distinguish de novo CRC during the examination.
Research motivation
Some small lesions were cut off directly in the examination without computed tomography imaging and endoscopic ultrasound. This may lead to mistreatment because endoscopists often ignore the judgment of invasion depth. The de novo CRC may have a deep invasion layer.
Research objectives
This study aimed to comprehensively review de novo CRCs and provide a reference atlas for future studies and analyze the features of de novo CRCs to distinguish them from non-neoplastic polyps.
Research methods
This study collected clinical and pathological information on de novo patients and stained E-cadherin and vimentin by immunohistochemistry. Based on this information, we analyzed the characteristic of de novo CRC and the relative correlation between different indicators.
Research results
This study highlights that de novo CRCs have special endoscopic and pathological features that distinguish them from traditional adenocarcinomas. It is also the first study paying attention to chicken skin mucosa invasive depth measurement. More importantly, this study summarized several factors relevant to invasion depth and provide tremendous help in clinical practice to increase diagnostic ability.
Research conclusions
This first study pointed out the relationship between de novo CRC and epithelial-mesenchymal transition related genes. And it is the first study put forward that chicken skin mucosa indicates the depth of invasion.
Research perspectives
We will further study the molecular biological difference between de novo CRC and carcinoma in adenoma CRC to discover the mechanism of invasion and metastasis of de novo CRCs.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Research Ethics Committee of the First Affiliated Hospital of China Medical University Institutional Review Board, No. [2021]2021-68-2.
Informed consent statement: All study participants or their legal guardian provided informed written consent about personal and medical data collection prior to study enrolment.
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
STROBE statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Peer-review started: March 28, 2023
First decision: April 10, 2023
Article in press: April 19, 2023
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Schijven MP, Netherlands; Tie J, Australia S-Editor: Fan JR L-Editor: A P-Editor: Cai YX
Contributor Information
Shi-Yang Li, Department of Endoscopy, The First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China.
Mei-Qi Yang, Department of Endoscopy, The First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China.
Yi-Ming Liu, Department of Endoscopy, The First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China.
Ming-Jun Sun, Department of Endoscopy, The First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China.
Hui-Jing Zhang, Department of Endoscopy, The First Affiliated Hospital of China Medical University, Shenyang 110001, Liaoning Province, China. hjzhang@cmu.edu.cn.
Data sharing statement
Participants gave informed consent for data sharing.
References
- 1.Lieberman DA, Weiss DG, Bond JH, Ahnen DJ, Garewal H, Chejfec G. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med. 2000;343:162–168. doi: 10.1056/NEJM200007203430301. [DOI] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 3.Papagiorgis PC, Zizi AE, Tseleni S, Oikonomakis IN, Nikiteas NI. Clinicopathological differences of colorectal cancers according to tumor origin: Identification of possibly de novo lesions. Biomed Rep. 2013;1:97–104. doi: 10.3892/br.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koga Y, Hirahashi M, Ohishi Y, Oda Y. Clinicopathological features and phenotypic classification of de novo-type colorectal carcinomas differ from those of colorectal carcinomas derived from flat adenomas. Pathol Int. 2019;69:331–340. doi: 10.1111/pin.12803. [DOI] [PubMed] [Google Scholar]
- 5.Trinh A, Lädrach C, Dawson HE, Ten Hoorn S, Kuppen PJK, Reimers MS, Koopman M, Punt CJA, Lugli A, Vermeulen L, Zlobec I. Tumour budding is associated with the mesenchymal colon cancer subtype and RAS/RAF mutations: a study of 1320 colorectal cancers with Consensus Molecular Subgroup (CMS) data. Br J Cancer. 2018;119:1244–1251. doi: 10.1038/s41416-018-0230-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goto H, Oda Y, Murakami Y, Tanaka T, Hasuda K, Goto S, Sasaki Y, Sakisaka S, Hattori M. Proportion of de novo cancers among colorectal cancers in Japan. Gastroenterology. 2006;131:40–46. doi: 10.1053/j.gastro.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 7.Matsuda T, Fujii T, Saito Y, Nakajima T, Uraoka T, Kobayashi N, Ikehara H, Ikematsu H, Fu KI, Emura F, Ono A, Sano Y, Shimoda T, Fujimori T. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol. 2008;103:2700–2706. doi: 10.1111/j.1572-0241.2008.02190.x. [DOI] [PubMed] [Google Scholar]
- 8.Glover B, Patel N, Ashrafian H, Teare J. Diagnostic accuracy of i-scan image enhancement for real-time endoscopic diagnosis of small colorectal polyps: a meta-analysis. Therap Adv Gastroenterol. 2018;11:1756284818814948. doi: 10.1177/1756284818814948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuramoto S, Oohara T. How do colorectal cancers develop? Cancer. 1995;75:1534–1538. doi: 10.1002/1097-0142(19950315)75:6+<1534::aid-cncr2820751525>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 10.Mueller J, Mueller E, Hoepner I, Jütting J, Bethke B, Stolte M, Höfler H. Expression of bcl-2 and p53 in de novo and ex-adenoma colon carcinoma: a comparative immunohistochemical study. J Pathol. 1996;180:259–265. doi: 10.1002/(SICI)1096-9896(199611)180:3<259::AID-PATH654>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Kinzler KW, Vogelstein B. Lessons from hereditary colorectal cancer. Cell. 1996;87:159–170. doi: 10.1016/s0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 12.Shatz BA, Weinstock LB, Thyssen EP, Mujeeb I, DeSchryver K. Colonic chicken skin mucosa: an endoscopic and histological abnormality adjacent to colonic neoplasms. Am J Gastroenterol. 1998;93:623–627. doi: 10.1111/j.1572-0241.1998.177_b.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee YM, Song KH, Koo HS, Lee CS, Ko I, Lee SH, Huh KC. Colonic Chicken Skin Mucosa Surrounding Colon Polyps Is an Endoscopic Predictive Marker for Colonic Neoplastic Polyps. Gut Liver. 2022;16:754–763. doi: 10.5009/gnl210271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–890. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 15.Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–629. doi: 10.1038/nrclinonc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mueller JD, Bethke B, Stolte M. Colorectal de novo carcinoma: a review of its diagnosis, histopathology, molecular biology, and clinical relevance. Virchows Arch. 2002;440:453–460. doi: 10.1007/s00428-002-0623-z. [DOI] [PubMed] [Google Scholar]
- 17.Tsuda S, Veress B, Tóth E, Fork FT. Flat and depressed colorectal tumours in a southern Swedish population: a prospective chromoendoscopic and histopathological study. Gut. 2002;51:550–555. doi: 10.1136/gut.51.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rembacken BJ, Fujii T, Cairns A, Dixon MF, Yoshida S, Chalmers DM, Axon AT. Flat and depressed colonic neoplasms: a prospective study of 1000 colonoscopies in the UK. Lancet. 2000;355:1211–1214. doi: 10.1016/s0140-6736(00)02086-9. [DOI] [PubMed] [Google Scholar]
- 19.Gao Z, Jiang J, Hou L, Zhang B. Dysregulation of MiR-144-5p/RNF187 Axis Contributes To the Progression of Colorectal Cancer. J Transl Int Med. 2022;10:65–75. doi: 10.2478/jtim-2021-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornick JL, Farraye FA, Odze RD. Clinicopathologic and immunohistochemical study of small apparently "de novo" colorectal adenocarcinomas. Am J Surg Pathol. 2007;31:207–215. doi: 10.1097/01.pas.0000213383.17418.a9. [DOI] [PubMed] [Google Scholar]
- 21.Hayashi N, Tanaka S, Hewett DG, Kaltenbach TR, Sano Y, Ponchon T, Saunders BP, Rex DK, Soetikno RM. Endoscopic prediction of deep submucosal invasive carcinoma: validation of the narrow-band imaging international colorectal endoscopic (NICE) classification. Gastrointest Endosc. 2013;78:625–632. doi: 10.1016/j.gie.2013.04.185. [DOI] [PubMed] [Google Scholar]
- 22.Hattori S, Iwatate M, Sano W, Hasuike N, Kosaka H, Ikumoto T, Kotaka M, Ichiyanagi A, Ebisutani C, Hisano Y, Fujimori T, Sano Y. Narrow-band imaging observation of colorectal lesions using NICE classification to avoid discarding significant lesions. World J Gastrointest Endosc. 2014;6:600–605. doi: 10.4253/wjge.v6.i12.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka S, Hayashi N, Oka S, Chayama K. Endoscopic assessment of colorectal cancer with superficial or deep submucosal invasion using magnifying colonoscopy. Clin Endosc. 2013;46:138–146. doi: 10.5946/ce.2013.46.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landau MA, Zhu B, Akwuole FN, Pai RK. Histopathological Predictors of Recurrence in Stage III Colon Cancer: Reappraisal of Tumor Deposits and Tumor Budding Using AJCC8 Criteria. Int J Surg Pathol. 2019;27:147–158. doi: 10.1177/1066896918787275. [DOI] [PubMed] [Google Scholar]
- 25.Liu P, Zhu H, Zhang X, Feng A, Zhu X, Sun Y. Predicting Survival for Hepatic Arterial Infusion Chemotherapy of Unresectable Colorectal Liver Metastases: Radiomics Analysis of Pretreatment Computed Tomography. J Transl Int Med. 2022;10:56–64. doi: 10.2478/jtim-2022-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagata K, Shinto E, Yamadera M, Shiraishi T, Kajiwara Y, Okamoto K, Mochizuki S, Hase K, Kishi Y, Ueno H. Prognostic and predictive values of tumour budding in stage IV colorectal cancer. BJS Open. 2020;4:693–703. doi: 10.1002/bjs5.50300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y, Liu Y, Qiu X, Yan B. Concurrent Comparison of the Prognostic Values of Tumor Budding, Tumor Stroma Ratio, Tumor Infiltrating Pattern and Lymphocyte-to-Monocyte Ratio in Colorectal Cancer Patients. Technol Cancer Res Treat. 2021;20:15330338211045826. doi: 10.1177/15330338211045826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan S, Sun S, Liu B, Hou Y. Pan-cancer Landscape of the RUNX Protein Family Reveals their Potential as Carcinogenic Biomarkers and the Mechanisms Underlying their Action. J Transl Int Med. 2022;10:156–174. doi: 10.2478/jtim-2022-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogino S, Nosho K, Irahara N, Meyerhardt JA, Baba Y, Shima K, Glickman JN, Ferrone CR, Mino-Kenudson M, Tanaka N, Dranoff G, Giovannucci EL, Fuchs CS. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res. 2009;15:6412–6420. doi: 10.1158/1078-0432.CCR-09-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zou Q, Wang X, Ren D, Hu B, Tang G, Zhang Y, Huang M, Pai RK, Buchanan DD, Win AK, Newcomb PA, Grady WM, Yu H, Luo Y. DNA methylation-based signature of CD8+ tumor-infiltrating lymphocytes enables evaluation of immune response and prognosis in colorectal cancer. J Immunother Cancer. 2021;9 doi: 10.1136/jitc-2021-002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phillips SM, Banerjea A, Feakins R, Li SR, Bustin SA, Dorudi S. Tumour-infiltrating lymphocytes in colorectal cancer with microsatellite instability are activated and cytotoxic. Br J Surg. 2004;91:469–475. doi: 10.1002/bjs.4472. [DOI] [PubMed] [Google Scholar]
- 32.Jenkins MA, Hayashi S, O'Shea AM, Burgart LJ, Smyrk TC, Shimizu D, Waring PM, Ruszkiewicz AR, Pollett AF, Redston M, Barker MA, Baron JA, Casey GR, Dowty JG, Giles GG, Limburg P, Newcomb P, Young JP, Walsh MD, Thibodeau SN, Lindor NM, Lemarchand L, Gallinger S, Haile RW, Potter JD, Hopper JL, Jass JR Colon Cancer Family Registry. Pathology features in Bethesda guidelines predict colorectal cancer microsatellite instability: a population-based study. Gastroenterology. 2007;133:48–56. doi: 10.1053/j.gastro.2007.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Newman AM, Steen CB, Liu CL, Gentles AJ, Chaudhuri AA, Scherer F, Khodadoust MS, Esfahani MS, Luca BA, Steiner D, Diehn M, Alizadeh AA. Determining cell type abundance and expression from bulk tissues with digital cytometry. Nat Biotechnol. 2019;37:773–782. doi: 10.1038/s41587-019-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang R, Cheng S, Luo N, Gao R, Yu K, Kang B, Wang L, Zhang Q, Fang Q, Zhang L, Li C, He A, Hu X, Peng J, Ren X, Zhang Z. Distinct epigenetic features of tumor-reactive CD8+ T cells in colorectal cancer patients revealed by genome-wide DNA methylation analysis. Genome Biol. 2019;21:2. doi: 10.1186/s13059-019-1921-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Participants gave informed consent for data sharing.