Abstract
Epithelial–mesenchymal transition (EMT) has been implicated in various aspects of tumor development, including tumor invasion and metastasis, cancer stemness, and therapy resistance. Diverse stroma cell types along with biochemical and biophysical factors in the tumor microenvironment impinge on the EMT program to impact tumor progression. Here we provide an in-depth review of various tumor microenvironmental signals that regulate EMT in cancer. We discuss the molecular mechanisms underlying the role of EMT in therapy resistance and highlight new therapeutic approaches targeting the tumor microenvironment to impact EMT and tumor progression.
Keywords: Epithelial-Mesenchymal Transition (EMT), Invasion and metastasis, Extracellular matrix (ECM), Hypoxia, Tumor stroma
1. Introduction
During tumor development, the dynamic interactions between tumor cells and their cellular and extracellular tissue microenvironment foster malignant progression and metastasis. Epithelial–mesenchymal transition (EMT) is a cellular process in which cells lose their epithelial characteristics (E-cadherin) and acquire mesenchymal features (N-cadherin, vimentin). EMT has been shown to promote the tumor initiation ability, linking EMT to cancer stem cells (CSCs) as well as playing integral roles in tumor invasion and metastasis [1–3].
Studies in mouse tumor models and human circulating tumor cells show that EMT is not a binary process during metastasis. In human breast cancer patients, circulating tumor cells (CTCs) present diverse EMT statuses and many CTCs express both epithelial and mesenchymal markers [4]. The intermediate EMT cells, named as hybrid-EMT or partial-EMT, exhibit both mesenchymal and epithelial properties. More recent studies described multiple intermediate EMT states in mouse and human tumor samples by single cell RNA-seq [5–8]. Tumor cells in various partial-EMT states contributes to tumor heterogeneity and cancer stem cell properties [9]. Using a skin tumor model, Tsai et al. show that induction of EMT promotes skin tumor invasion and dissemination; while reversion of EMT is essential for the regrowth of distant metastases [10]. Several in vivo lineage tracing studies have been performed in mouse breast tumor models to determine the role of EMT plasticity in tumor metastasis. Fisher et al. initially performed Fsp1 (fibroblast specific protein 1) and Vimentin promoter-driven Cre-mediated lineage tracing in the MMTV-PyMT breast tumor model and did not observe a requirement of EMT in generating lung metastasis [11]. Later, Li et al. used an elegant dual lineage-tracing system to demonstrate that transient activation of N-cadherin, which marks a partial EMT state, but not activation of vimentin, is required for tumor metastasis in the MMTV-PyMT breast tumor mouse model[12]. EMT marker specificity and lineage tracer sensitivity might contribute to this discrepancy. Bornes et al. reported that Fsp1, the same mesenchymal marker used in Fischer et al. study, was unable to trace most of the mesenchymal cells during tumor metastasis in the PyMT mouse model [13]. More recently, Luond et al. showed that partial EMT, not full EMT, contributes to lung metastasis in the MMTV-PyMT breast cancer mouse model. However, full EMT exhibited higher resistance to chemotherapy and facilitated tumor survival under stress [14]. Activation of partial EMT promoted collective migration and led to higher cell plasticity and metastasis capacity; in contrast, full EMT was difficult to reverse at distant organs, leading to reduced metastasis[14,15]. Therefore, epithelial-mesenchymal plasticity plays a critical role in tumor progression, metastasis, and therapy resistance[9].
The EMT program is orchestrated by a core group of EMT-inducing transcription factors (EMT-TFs), including the SNAIL family SNAI1[16, 17] and SNAI2[18], the ZEB family ZEB1 [19]and ZEB2[20], and the TWIST family TWIST1/2 [21–23]. The SNAIL and ZEB family transcription factors directly repress E-cadherin expression by binding E-boxes at its promoter region, thereby leading to EMT. TWIST1 induces invadopodia-mediated matrix degradation to facilitate tumor invasion and metastasis[21,24]. EMT-TFs recruit epigenetic regulators to regulate their target genes expression. For example, SNAI1 recruits HDAC1 and EZH2 to repress E-cadherin expression. ZEB1 recruits HDAC1 or DNMT1 to repress target gene expression[25]. Of note, the EMT-TFs regulate the transcription of each other and cooperate to orchestrate EMT progression[5,26]. For example, TWIST1 binds to the SNAI2 promoter and induces its transcription [27]. Many EMT-TFs are regulated by miRNAs. miR-200 family members and miR-205 repress ZEB1/2 expression to reverse EMT and suppress migration and invasion in various cancer types[28–30]. Reciprocally, ZEB1 was shown to directly repress the miR-200 family members to promote EMT and stemness acquisition, suggesting a negative feedback loop between miR-200 and ZEB[31,32]. Similarly, the EMT/MET switch could also be toggled by SNAIL/miR-34 circuit regulation[33].
The EMT/MET switches are controlled by both biochemical and biophysical factors in the tumor microenvironment to impact tumor development, progression, and treatment responses. The tumor microenvironment is largely composed of immune cells, stromal cells, blood vessels, and extracellular matrix, though the relative proportion of these components may vary in individual tumors. This review explores these environmental factors regulating EMT to reveal potential therapeutic targets and to predict treatment responses.
2. Regulation of EMT by the tumor microenvironment
2.1. Extracellular matrix in EMT regulation
Extracellular matrix (ECM) is a critical component of the tumor microenvironment that contributes to tumorigenesis and progression. Many ECM proteins, such as collagen I, hyaluronic acid (HA) and fibronectin, have been implicated in promoting EMT. Increasing ECM protein deposition and remodeling drive malignant progression partly via EMT. In addition to direct binding of ECM proteins to tumor cells to activate the downstream biochemical signaling, matrix stiffening during tumor progression exerts increased mechanical forces on tumor cells, which induces EMT and increases the risk of cancer development and progression.
2.1.1. ECM molecules in EMT regulation
The extracellular matrix molecules, including collagens, fibronectin and hyaluronan, all contribute to EMT activation (Fig. 1). Type IV collagen, a major component of the basement membranes, is essential for the maintenance of the epithelial properties of epithelial cells. Disruption of collagen IV deposition could upregulate TGFβ, a major inducer of EMT [34–36]. Collagen I induces EMT in lung cancer cells by activating PI3K/ERK signaling, which in turn stimulates the autocrine secretion of TGF-β3 to induce EMT[37]. Collagen I also binds to the collagen I receptor DDR2 to promote SNAI1 nuclear accumulation and increases SNAI1 protein level by inhibiting SNAI1 ubiquitylation. In turn, SNAI1 further upregulates Membrane type I-matrix metalloproteinase (MT1-MMP) and collagen I to sustain the EMT phenotype and facilitate tumor cell invasion [38]. Fibronectin is a marker of cells that have undergone EMT. During EMT, fibronectin assembly is increased, and fibronectin fibril formation is induced by TGF- β1. Fibronectin fibrils serve as an integration point for mechanical signals and TGF- β1 signaling to induce EMT. High levels of fibronectin have been detected in the stroma of breast tumors [39]. Fibronectin activates FAK and leads to recruitment of Src through binding to integrins. Cooperation between fibronectin and TGFβ is required to activate Src and ERK/MAPK to induce EMT[40]. HA is another important ECM molecule that is overproduced in many types of human tumors. HA is a key ligand of CD44, a cell surface glycoprotein that plays a critical role in cell migration, invasion, and cancer stem cell properties. The binding of HA to CD44 elicits high-affinity interaction between CD44 and the TGFβ receptor I, which increases downstream SMAD2/SMAD3 activation to induce EMT[41]. HA/CD44 interaction was also reported to cause CD44 to translocate into the nucleus and promote de novo transcription of lysyl oxidase (LOX), leading to TWIST1-dependent induction of EMT[42]. Taken together, ECM proteins, when dysregulated in cancer, directly affect EMT.
Fig. 1.
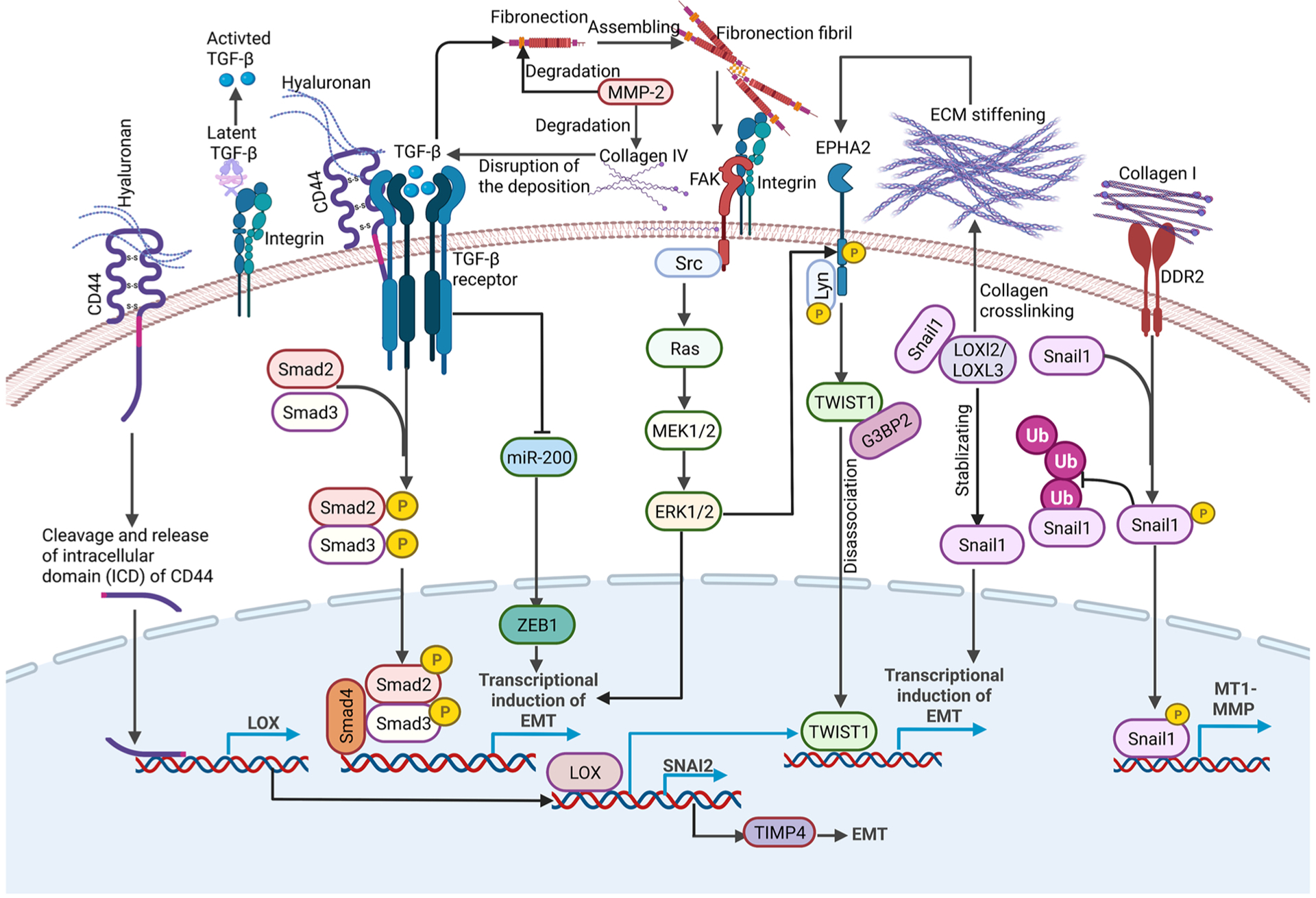
A summary of various extracellular matrix signals implicated in EMT regulation in the tumor microenvironment. Various ECM molecules, ECM remodeling proteins and physical forces exerted from ECM in the tumor microenvironment activate various biochemical and mechanical signaling pathways to regulate the EMT inducers and EMT transcription factors to drive EMT and tumor progression.
2.1.2. ECM remodeling in EMT regulation
Growing evidence suggests that increased ECM remodeling and deposition also drive malignant progression (Fig. 1). Matrix metalloproteinases (MMPs)-mediated ECM protein remodeling and LOX-mediated ECM protein crosslinking both play crucial roles in the EMT process. MMPs in the tumor environments are reported to participate in EMT induction. MMP-2 was found to degrade a wide variety of ECM proteins including fibronectin, collagen IV and collagen V [43–45] . High expression of MMP-3, a stromal enzyme upregulated in many breast tumors, induced expression of an alternatively spliced form of Rac1 and caused an increase in cellular reactive oxygen species. The reactive oxygen species could stimulate SNAI1 expression to facilitate cancer progression[46]. Lysyl oxidase (LOX) is an extracellular copper-dependent enzyme that promotes crosslinking of collagens or elastin to increase ECM tensile strength[47,48]. LOXL2 and LOXL3 are two members of the LOX gene family which can interact with and stabilize SNAI1 to downregulate E-cadherin expression, leading to an induction of EMT. Knockdown of LOXL2 in SNAI1-expressing metastatic carcinoma cells decreased tumor growth and reduced expression of mesenchymal markers and invasion, providing a direct link between LOXL2 and SNAI1 in tumor progression[49]. LOX can also bind and transactivate the SNAI2 promoter, and LOX/SNAI2 axis mediates TIMP4 (TIMP: tissue inhibitors of matrix metalloproteinases that can regulate the proteolytic activity of MMPs) secretion, then facilitating EMT progression[50]. Hypoxia induced the expression of LOX and LOXL2 via HIF1, which then repress E-cadherin to induce EMT [51]. From these studies, it is evident that ECM reorganization directly regulates EMT to impact tumor progression.
2.1.3. ECM-exerted mechanical force in EMT regulation
Tumor cells and stromal cells also respond to ECM stiffening-induced mechanical signals during tumor progression. Matrix rigidities can be sensed and transmitted across the plasma membrane by various mechanosensors, several of which are implicated in EMT regulation. Integrins are the best studied mechanosensors and play a critical role in driving EMT and invasion[52–54]. Blockade of integrin signaling via integrin-blocking antibodies completely abolished stiffness-induced EMT in breast cancer cells [55]. Many αV integrins, especially αVβ6, αVβ3 and αVβ5, are expressed at low levels in healthy epithelial tissues, but are upregulated during EMT [56,57]. Disruption of β1 integrin induced αVβ3 integrin switching and promoted TGFβ activation in E-cadherin-positive triple-negative breast cancer (TNBC) cells via a TGFβ-miR-200-ZEB signaling network to induce EMT and enhance dissemination[58]. DDR2 regulates the activation state of collagen-binding integrins α1β1 and α2β1, thus strengthening cell-ECM interactions and maintaining the mesenchymal phenotype in tumor cells [59,60]. ECM regulates MT1-MMP localization via β1 or αVβ3- integrins [61]. Colocalization and cooperation between β1-integrin and MT1-MMP1 plays an important role in EMT and early cancer dissemination by upregulating the Wnt signaling[62]. Integrin-linked kinase (ILK), as a mechanotransducer, is crucial for TGF-β1-induced EMT via the TWIST1-integrin β1-FAK/ILK pathway[63–65]. ECM stiffening is also reported to activate the mechanosensor Piezo1, which activates TGFβ signaling by recruiting Rab5c, thus promoting EMT and tumor progression[66,67]. TRPV4, a mechano-sensitive ion channel regulating calcium influx, promoted EMT and cell migration in breast cancer cells [68,69].
Several EMT transcription factors are regulated by mechanical forces exerted by ECM in the tumor microenvironment (Fig. 1). Increasing matrix stiffness in the tumor stroma activates TWIST1, a key mechano-responder to drive EMT and invasion at matrix stiffness. Mechanistically, high matrix stiffness releases the TWIST1 protein from binding to its cytoplasmic anchor protein G3BP2. TWIST1 then translocates into the nucleus to drive the transcriptional program of EMT and tumor invasion[55,70,71]. Upstream of TWIST1, high matrix stiffness activates ERK/RSK and leads to ligand independent EPHA2 S897 phosphorylation. EPHA2 then binds to and activates Lyn to phosphorylate TWIST1 and prevents its association with G3BP2, thus leading to TWIST1 nuclear translocation to promote EMT and invasion[55,72]. SNAI1 protein stability is regulated by matrix stiffness via DDR2. DDR2 activation led to activation of ERK, which directly phosphorylates SNAI1 to promote SNAI1 nuclear accumulation and reduces SNAI1 protein ubiquitylation, thus facilitating tumor cells to undergo EMT and invade through collagen I-rich extracellular matrices [38]. In summary, critical ECM molecules and mechanical forces exerted by tumor ECM can impinge on EMT transcription factors to regulate EMT.
2.2. Stromal and immune cells in EMT regulation
In the tumor microenvironment, stromal and immune cells secrete various cytokines and chemokines, which act in a paracrine fashion on nearby carcinoma cells. Some of these paracrine signals, often acting in combination, are potent inducers of EMT in carcinoma cells to promote tumor progression and metastasis.
2.2.1. Cancer-associated fibroblasts
Cancer-associated fibroblasts (CAFs) are a major component of the tumor stroma and play a critical role in facilitating crosstalk between cancer cells and the tumor microenvironment. Numerous studies revealed that CAFs could secret TGFβ[73], HGF[74], FGF[75], SDF-1 [76], IL-6[77], IL-32[78], CCL5[79], CXCL12 [80], MMP-2[81], and MMP-9 [82] to enable the EMT process in various cancer cells[83,84] (Fig. 2). In breast cancer and bladder cancer cells, TGFβ1 secreted by CAFs activated the canonical SMAD-mediated and SMAD-independent pathways, both of which play important roles during EMT induction [73,85,86]. For the canonical SMAD pathway, activated SMAD3/4 upregulated the expression of EMT-TFs, including SNAI1/2, TWIST1/2 and ZEB1/ZEB2 [87–89]. In the SMAD-independent non-canonical pathway, TGFβ activates MAPK/ERK, PI3K/AKT and Rho GTPase to aid EMT initiation[34]. Additionally, IL-6 secreted by CAFs induced EMT via STAT3 in ovarian, bladder, gastric and hepatocellular carcinoma cancer cell lines [77,90–92]. Mesenchymal stem cell (MSC)-derived CAFs or radiation-activated CAFs were shown to secrete enhanced levels of CXCL12, which bound to CXCR4 in tumor cells and stimulated EMT and metastasis in pancreatic and prostate cancer cells respectively [93, 94]. Li et al. suggested the CXCL12/CXCR4 axis activated the p38 kinase pathway to facilitate CAFs-mediated EMT[94]. Moreover, activated CAFs secreted MMP-2 and MMP-9 to remodel ECM, thereby facilitating EMT in prostate cancer cells [82]. In hepatocellular carcinoma cells, CAFs secreted HGF to stimulate the c-MET/FRA1/HEY1 axis and contributed to tumor invasion and metastasis both in vitro and in vivo [74]. More recently, several studies reported the existence of different subtypes of CAFs with distinct functions in the TME [95,96]. In particular, Öhlund et al. demonstrated two subtypes of CAFs, myofibroblastic CAFs (myCAFs) and inflammatory CAFs (iCAFs), in the PDAC microenvironment [95]. myCAFs exhibited high levels of α-SMA and were found in close proximity to tumor cells[95]. In contrast, iCAFs showed lower α-SMA levels but highly expressed IL-6 and were located farther from tumor regions[95]. Co-culture with iCAFs, but not myCAFs, significantly upregulated ZEB1 and Vimentin expression and induced partial EMT in colon tumor organoids[97]. Taken together, CAFs in the tumor microenvironment secret various molecules to impact EMT and metastasis.
Fig. 2.
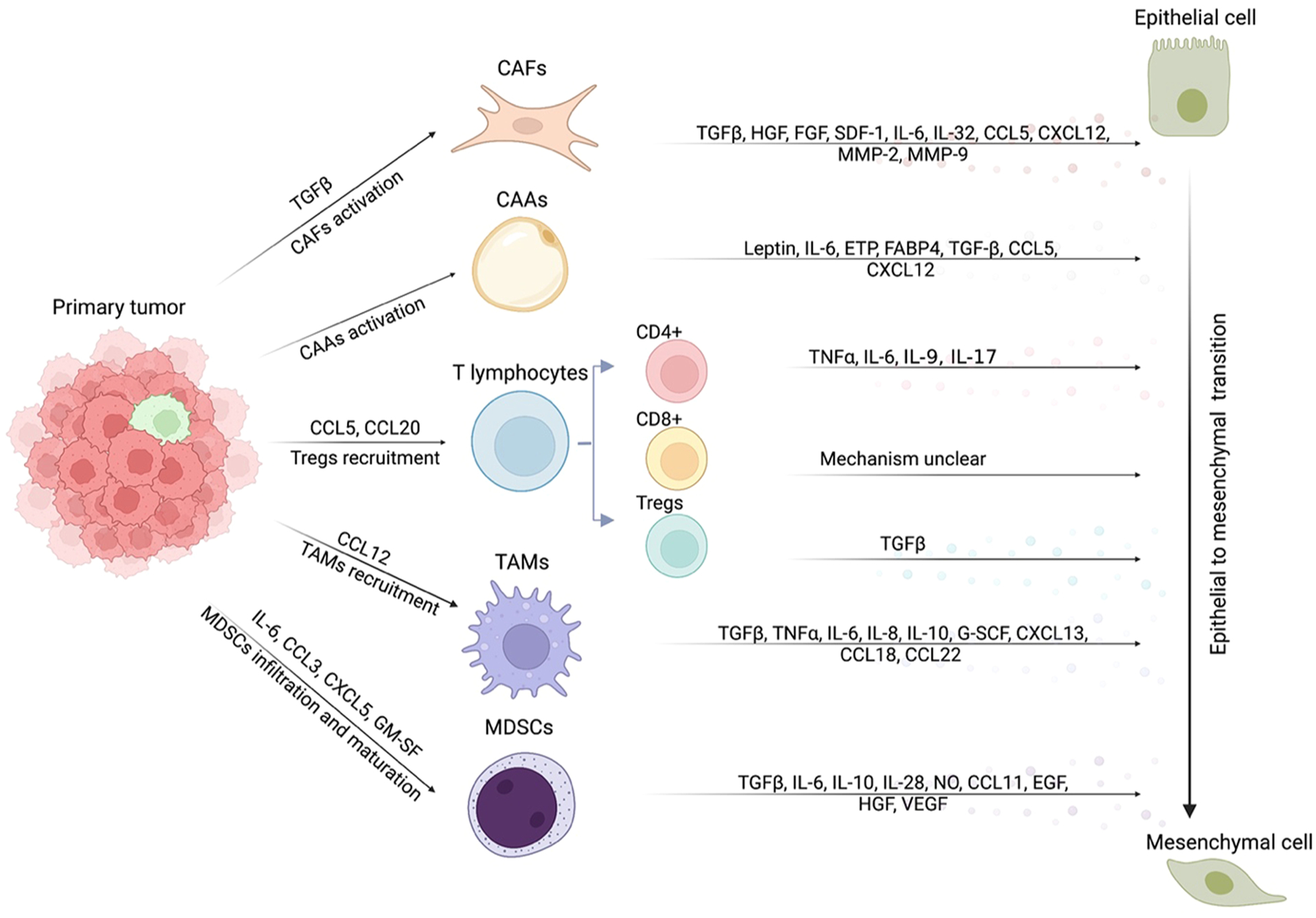
A summary of various secreted factors implicated in EMT induction by stromal cells and immune cells. CAFs, CAAs and immune cells including T lymphocytes, TAMs and MDSCs could promote EMT in cancer cells through the secretion of cytokines, chemokines and growth factors, such as TGFβ, IL-6, CXCL12, CCL18, FGF and HGF. Meanwhile, cancer cells secrete various factors to stimulate CAFs or CAAs formation and recruit more Tregs, TAMs or MDSCs. CAFs: cancer associated fibroblasts; CAAs: cancer associated adipocytes; TAMs: tumor associated macrophages; MDSCs: myeloid-derived suppressor cells.
2.2.2. Cancer-associated adipocytes
Obesity, featuring the expansion of white adipose tissue. has been associated with malignant cancer progression. Adipose tissue consists of adipocytes, adipose mesenchymal stem/stromal cells (ASCs), endothelial cells, and immune cells [98]. Coculturing cancer cells with adipocytes or ASCs were shown to stimulate EMT in cancer cells by secreting IL-6 and activating IL-6/STAT3 pathway [99–101]. IL-6[100], Leptin [102–104], ETP[105], FABP4[106], TGF-β1[107,108], CCL5[109,110] and CXCL12[111] secreted by ASCs or adipocytes were reported to promote EMT in various cancer cell lines(Fig. 2). Leptin binds to leptin receptor (OB-R) and contributes to the EMT process by activating multiple pathways, including PI3K/AKT which phosphorylates GSK3β to increase β-catenin nuclear translocation, STAT3 which recruits G9a to regulate the miR-200c/ZEB1 feedback loop, and ERK which represses E-cadherin and increases vimentin expression[112–115]. FABP4 is shown to activate AKT/G3K3β/SNAI1 pathway, thereby facilitating EMT in cervical squamous cell carcinoma (CSCC) cells[116]. In the PyMT breast tumor mouse model, ETP augments EMT and metastasis by activating the TGF- β signaling pathway[105].
2.2.3. T Lymphocytes
T lymphocytes, such as CD4+ T cells and CD8+ T cells, play an important role in immune responses to tumors [117]. Numerous studies show that CD4+ T cell or CD8+ T cell infiltration inhibited tumor growth and correlated with a better prognosis [118–122]. However, some reports suggest they also promote tumor metastasis by inducing EMT in certain cancer cell lines. In breast cancer, CD8+ T cells were reported to induce EMT and confer cancer cells with stemness characteristics, although the underlying mechanism is unclear[123]. In pancreatic ductal adenocarcinoma (PDAC) cells, Goebel et al. reported that CD4+ T-effector cells stimulated EMT by secreting TNFα and IL-6. Co-culture with T-effector cells significantly upregulated ZEB1 in tumor cells, suggesting that T-effector cells-induced EMT might depend on ZEB1 [124]. More recently, Salazar et al. showed that CD4+ Th9 and CD4+ Th17 cells secreted IL-9 or IL-17, respectively and enabled lung cancer cells to undergo EMT both in vitro and in vivo[125]. In a xenograft mice model of lung cancer, co-injection of tumor cells with Th9 or Th17 cells significantly increased EMT and metastasis, while Th9 and Th17 blocking antibodies inhibited EMT and tumor progression[125]. Regulatory T cells (Tregs) are a subset of CD4+ T cells mainly responsible for self-tolerance and homeostasis maintenance of T cells. Several studies revealed that Tregs could stimulate EMT in lung epithelial cells, hepatocellular carcinoma and melanoma cells [126–128]. In the Oh et al. study, melanoma cells co-injected with Tregs exhibited increased EMT and metastasis in vivo. TGFβ secreted by Tregs contributed to EMT in hepatocellular carcinoma and melanoma cells[128](Fig. 2). Therefore, in addition to their critical roles in tumor immunity, T lymphocytes could impact EMT in tumor cells via cytokine production.
2.2.4. Macrophages
Macrophages in the tumor microenvironment are known as tumor-associated macrophages (TAMs)[129,130]. TAMs infiltration correlated with higher EMT and poor clinical outcomes in various cancers, including breast cancer, hepatocellular carcinoma, gastric cancer, colorectal cancer and lung cancer [131–138]. Macrophages in tumors are often divided into M1 and M2 macrophages. M1 macrophages secrete numerous pro-inflammatory cytokines and are considered to be anti-tumorigenic. In contrast, M2 macrophages mainly produce anti-inflammatory cytokines and exhibit pro-tumorigenic properties. Yeung et al. reports that M2 macrophages predominantly promoted EMT and metastasis in an orthotopic liver tumor mouse model[135]. Additionally, high M2 macrophages infiltration is positively associated with tumor aggressiveness and poor prognosis in bladder cancer and clear cell renal cell carcinoma(ccRCC) patients [139,140]. TAMs secrete numerous cytokines or chemokines to induce EMT in cancer cells, including TGFβ, TNFα, IL-6, IL-8, IL-10, G-SCF, CXCL13, CCL18, CCL22 and other factors [132–135,140,141–145](Fig. 2). In lung and colon carcinomas, macrophages could secrete TNFα, which synergizes with TGFβ to stimulate EMT [142,146]. Similarly, M2 macrophages also induced EMT by releasing TGFβ, resulting in β-catenin and SMAD2 activation in non-small cell lung cancer(NSCLC) cells and lung epithelial cells[141,147]. Additionally, IL-6 secreted by macrophages induced EMT by various mechanisms. In the Che et al. study, COX2 was upregulated by IL-6, which induced PEG2 and β-catenin nuclear translocation, thereby promoting EMT in lung cancer cells [148].
Reciprocally, tumor cells could secrete cytokines to recruit macrophages and induce M2 polarization of macrophages, reinforcing tumor progression and metastasis. Several studies uncovered the feedback loop between macrophages infiltration and tumor cells. In colon cancer cells, TAMs secreted IL-6 to stimulate EMT. Meanwhile, IL-6 activated the JAK2/STAT3/FoxA1 signaling in colon cancer cells, which led to high levels of CCL12 secretion in cancer cells to recruit more macrophages [149]. Similarly, Su et al. revealed that macrophages secreted CCL18 to activate NF-κB, thus enabling breast cancer cells to undergo EMT [132].
2.2.5. Myeloid-derived suppressor cells (MDSCs)
MDSCs are a population of heterogeneous immature myeloid cells that accumulate in the tumor microenvironment and have been shown to facilitate tumor progression by repressing anti-tumor immune responses and promoting angiogenesis [150,151]. MDSCs are generally divided into two subsets, polymorphonuclear/granulocytic MDSCs (PMN-MDSCs/G-MDSCs), which account for 70%–80% of MDSCs, and monocytic MDSCs (MO-MDSCs) [150,151]. MDSCs are shown to stimulate EMT in various cancer cell types [152–156](Fig. 2). MDSCs stimulated EMT by secreting TGFβ, VEGF, IL-6, IL-10 and IL-28 in melanoma, breast cancer and lung cancer [154,156–160]. Peng et al. reports that IL-6 and Nitric Oxide (NO) produced by MDSCs led to STAT3 and NOTCH activation, respectively, conveying stemness and EMT properties to breast cancer cells[157]. Li et al. reported that co-culture of nasopharyngeal carcinoma cells with MDSCs activated the COX2/β-catenin/TCF4 pathway via TGFβ and NO secretion to provoke EMT[154]. In human NSCLC xenograft models, MDSCs stimulated EMT and metastasis via CCL11/CCR3[161]. Interestingly, primary melanoma cells recruited MDSCs via CXCL5. Consequently, recruited MDSCs promoted EMT and cancer cell dissemination via TGFβ, EGF and HGF[152]. Similarly, breast cancer cells were reported to attract MDSCs via CCL3 production. Reciprocally, infiltrated MDSCs promoted EMT and metastasis by activating the PI3K-Akt-mTOR pathway in cancer cells, suggesting a positive feedback loop between MDSCs recruitment and tumor progression[162]. In summary, tumor cells could secrete GM-SF, IL-6, CXCL5 or CCL3 to promote MDSCs recruitment and maturation. Reciprocally, infiltrated MDSCs stimulate EMT by secreting TGF-β, EGF, HGF, IL-6(target STAT3), IL-10, IL-28(target IFN-λ), NO (target NOTCH), NOS2 or CCL11(target AKT and ERK) (Fig. 2). The crosstalk between cancer cells and their surrounding stromal cells plays a critical role in EMT regulation, thereby impacting tumor progression and metastasis.
2.3. Hypoxia in EMT regulation
Tumor hypoxia is strongly associated with tumor progression, metastasis, and poor clinical outcomes. Hypoxia inducible factors (HIF), including HIF-1α and HIF-2α, are the main mediators of hypoxia responses. HIF target genes are involved in glycolysis, apoptosis, cell proliferation, angiogenesis and metastasis[163,164]. Hypoxia plays a major role in EMT regulation during tumor progression (Fig. 3).
Fig. 3.
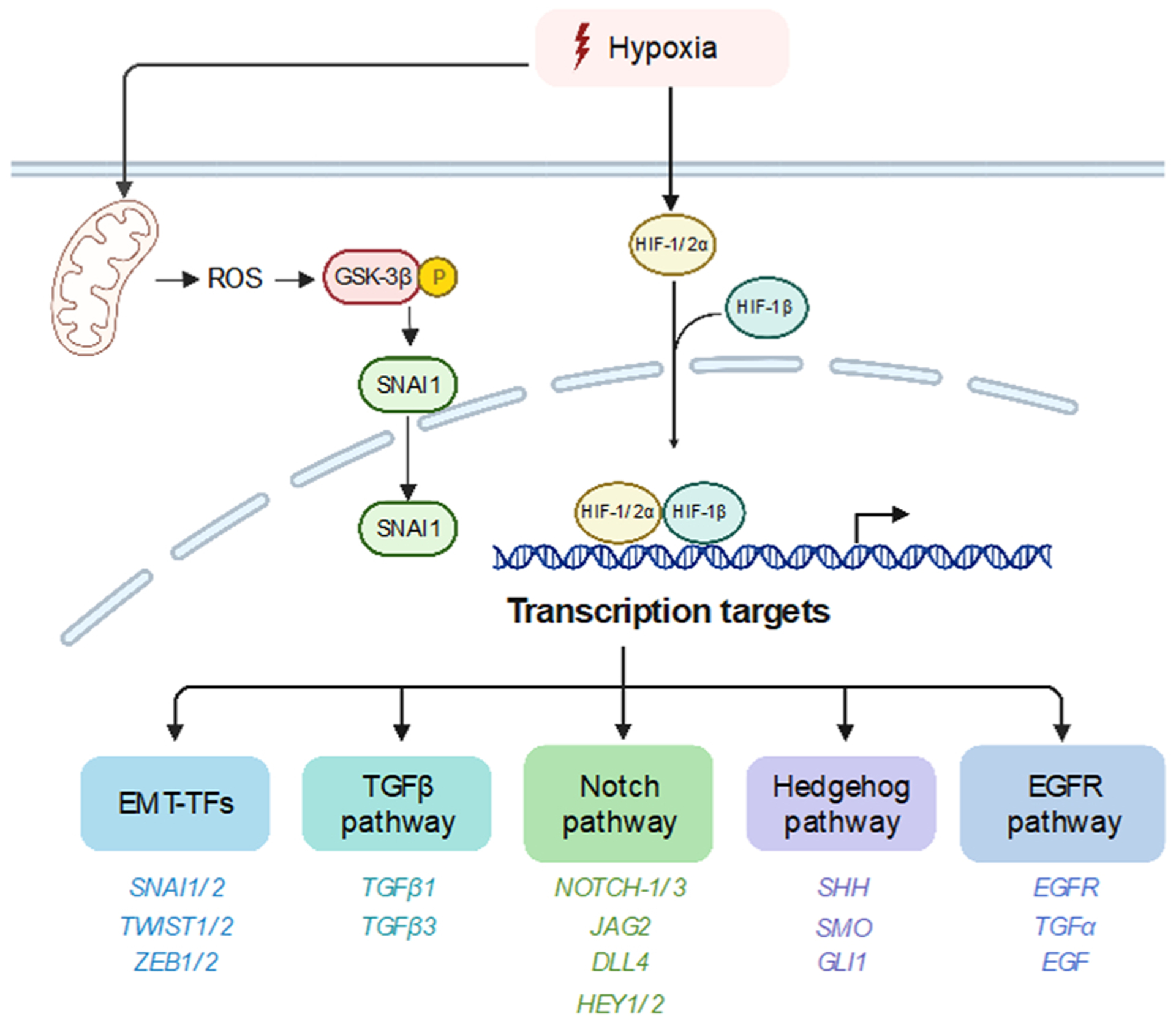
A schematic diagram summarizing mechanisms underlying hypoxia-mediated EMT. Hypoxia activates EMT by directly increasing EMT-TFs expression or stimulating various signaling pathways, including TGFβ, Notch, Hedgehog pathway and EGFR pathway. Direct transcription targets, like SNAI1, TGFβ, NOTCH1–1, SHH and EGFR are listed in diagram. Except for HIF-1/2α, ROS could also stimulate EMT by promoting SNAI1 nuclear translocation under hypoxia condition.
2.3.1. Hypoxia promotes EMT via upregulating EMT transcription factors
Hypoxia is shown to upregulate SNAI1/SNAI2 directly or indirectly in various cell types [165] via multiple mechanisms. Several studies revealed that HIF-1α or HIF-2α could directly regulate SNAI1 and SNAI2 transcriptionally[166–169] [179–182. In human hepatocellular carcinoma and pancreatic cancer cells, HIF-1α binds to SNAI1 at the – 541 hypoxia-response element (HRE) site and promotes SNAI1 transcription to induce EMT[167–169]. Zhang et al. also reported that HIF-1α could directly induce SNAI2 transcription to promote EMT through direct binding to the HRE sequence located in its proximal promoter in HNSCC cells [170]. Hypoxia is also shown to indirectly regulate SNAI1 expression via uPAR or USP47[171,172] to mediate hypoxia-induced EMT in breast cancer cells. Upregulation of uPAR during hypoxia activates the downstream PI3K-AKT signaling, thereby stimulating SNAI1 expression and EMT [171]. Ubiquitin-specific protease 47 (USP47), a deubiquitinating enzyme, promotes SNAI1 protein stabilization through deubiquitylation. Choi et al. reported that HIF-1α indirectly enhances USP47 expression via SOX9 during hypoxia-induced EMT in colorectal cancer cells[172]. Hypoxia is also found to stimulates SNAI1 expression via the Notch pathway. Inhibition of Notch signaling abrogated hypoxia-induced SNAI1 upregulation and EMT in breast cancer cells and ovarian cancer cells [173,174]. ROS produced during hypoxia was shown to promote SNAI1 nuclear translocation via GSK-3β inactivation and stimulated EMT in various cancer cell lines [175]. Regulation of SNAI2 by hypoxia was reported via HIF-1α repressing miR-30c, leading to increased SNAI2 expression and EMT[176] in human renal cell carcinomas.
Hypoxia is also shown to stimulate TWIST1/TWIST2 expression in various cancer cells. HIF-1α directly upregulated TWIST1 expression by binding to the HRE sequence in the proximal TWIST1 promoter in hypopharyngeal cancer, breast cancer and lung cancer cell lines [177]. Similarly, HIF-2α directly induced TWIST1 transcription in cervix carcinoma, prostate cancer and colon cancer cell lines [178]. Prognostic analysis show that co-expression of either two factors among HIF-1α/TWIST1/SNAI1 correlates with poor recurrence-free survival in NSCLC patients[179].
Both ZEB1 and ZEB2 are also frequently upregulated by hypoxia in different cancer cell lines. In colorectal cancer cells, Zhang et al. reported that HIF-1α could directly induce ZEB1 expression by binding to the HRE sequence in the ZEB1 proximal promoter [180]. ZEB1 inhibition could significantly abrogate hypoxia-induced EMT and metastasis [180]. Similarly, hypoxia-induced ZEB1 upregulation led to cell migration and invasion in glioblastoma multiforme (GBM) cells [181, 182]. Indirect regulation of ZEB1 by hypoxia are also observed in multiple studies. In breast cancer cells, hypoxia repressed DICER expression epigenetically to reduce the miR- 16 − 200 level, promoting ZEB1 expression and subsequent EMT [183]. Su et al. reported that increased MEF2D expression in response to hypoxia directly regulated ZEB1 transcription through acetylation of ZEB1 promoter, leading to EMT in colorectal cancer cells[184]. LncRNA was also shown to regulate ZEB1 expression during hypoxia. LncRNA-BX111887 was highly upregulated during hypoxia and recruited YB1 to the ZEB1 promoter, enabling cells to undergo EMT in pancreatic cancer cells[185]. Zhang et al. found that LncRNA-HOTTIP acted as a sponge of miR-101 to stimulate ZEB1 mediated EMT in glioma cells[186]. Interestingly, hypoxia also promoted ZEB2–natural antisense transcript expression to increase ZEB2 translation efficiency[187].
These studies provide strong evidence that hypoxia regulates key EMT transcription factors, such as SNAI1/2, TWIST1/2 and ZEB1/2, to induce EMT in human cancer.
2.3.2. Hypoxia induces EMT via several signaling pathways including TGFβ, EGFR, Notch and Hedgehog pathway
The TGFβ pathway can be activated by hypoxia in various cancer cell lines. In gastric cancer cells, Matsuoka et al. showed that hypoxia upregulated TGFβ1 and TGFβR to stimulate the autocrine TGFβ/TGFβR signaling, resulting in EMT in diffuse-type gastric cancer cells[188]. Similarly, in lung epithelial cells, hypoxia was shown to stimulate EMT via TGF-β1 production, which is dependent on ROS production and HIF-α accumulation[189]. Additionally, HIF-1α directly regulates transcription of TGFβ1 and TGFβ3 through binding to the HREs in their promoters, thereby leading to TGFβ-induced EMT[190,191]. Several papers also suggest indirect regulation of the TGFβ signaling by hypoxia, as the hypoxia-stimulated unfolded-protein response boosted TGFβ expression in gastric cancer cells[192]. Similarly, Nagpal et al. found that miR-191 upregulation by HIF-1α/HIF-2α could increase TGFβ2 levels directly or indirectly through repressing HuR in breast cancer cells [193].
Hypoxia stimulates Notch signaling by increasing both Notch receptor and ligand levels in various cancer cell lines. Upon ligand binding, the active Notch intracellular domain (NICD) could directly upregulate SNAI1/2 transcription to promote EMT [173,194,195]. In ovarian cancer cells, Sahlgren et al. found that hypoxia increased the expression levels of the Notch ligand DLL1 and NICD, thereby leading to SNAI1-mediated EMT[173]. NICD directly activates SNAI1 transcription through binding to the CSL motif in proximal to its promoter[173]. Notch receptor Notch3 and ligand DLL1, JAG1 and JAG2 levels were shown to be upregulated by hypoxia to facilitate EMT in breast cancer cells[174,196,197]. Du et al. found that hypoxia could indirectly increase expression of the Notch receptor Notch1 and the Notch ligand Jagged1 through suppressing miR-34a to promote EMT[198].
The Hedgehog (Hh) signaling also plays important roles in hypoxia-mediated EMT [199–201]. Activated GLI1 was shown to be responsible for upregulation of EMT-TFs, such as SNAI1/2 and TWIST1[202]. In particular, SNAI1 and TWIST1 were shown to be direct targets of GLI1 in hepatocellular carcinoma cells[203,204]. A study in mice shows that hypoxia could activate Hh signaling through HIF-1α mediated Shh upregulation[205]. Similarly, increased transcription of Shh, SMO and GLI1 level or GLI1 nuclear translocation by HIF-1α are also reported to facilitate EMT in various cancer cell lines[200,206]. Interestingly, in cholangiocarcinoma cells, HIF-1α-mediated Shh activation not only provoked EMT, but also increased cancer stemness[206]. Treatment with an Shh inhibitor, cyclopamine, attenuated Shh activation with substantial abrogation of EMT and stemness[206]. HIF-1α also triggered ligand-independent Hh signaling in pancreatic cancer cells, where hypoxia activated GLI1 directly and GLI1 depletion was sufficient to abrogate hypoxia-mediated EMT [201]. Moreover, Liu et al. found that HIF-1α regulated non-canonical Hh through ROS production, which facilitated GLI1-dependent EMT and invasion in hepatocellular carcinomas[207].
Hypoxia also activates EGFR signaling to facilitate EMT. Hypoxia promotes EMT by upregulating EGFR expression in many cancer types, including HNSCC, glioma, gastric cancer, breast cancer and lung cancer [206–210]. Recent studies suggest that HIF-1α directly regulates EGFR transcription through binding to the HRE sequence in EGFR intron 18 in breast cancer cells[211]. Moreover, EGFR translation efficiency was upregulated by hypoxia, whereby HIF-2α boosted EGFR protein synthesis and drove autonomous proliferation in glioma cells[209]. Additionally, EGF is shown to be upregulated by hypoxia to facilitate malignant progression in various cancer types[210,212]. In hepatocellular carcinoma cells, HIF-2α induced TGF-α and promoted EGFR activation under hypoxia [213,214].
In summary, hypoxia in the tumor microenvironment could impinge on a number of EMT-inducing signaling pathways to promote tumor invasion and metastasis.
3. EMT in cancer therapy resistance
3.1. EMT in chemoresistance
Increasing evidence shows that EMT plays a key role in chemoresistance in various human cancer types. Residual breast cancers often displayed a mesenchymal phenotype after chemotherapy in various human cancers[215]. The mesenchymal state induced by EMT confers drug resistance to many types of therapeutic agents. In Cyclophosphamide (CTX)-treated tumor-bearing mice, more than 60% of the surviving tumor cells presented a mesenchymal phenotype, indicating that mesenchymal tumor cells are more resistant to chemotherapy[216]. Doxorubicin could activate the TGF-β signaling and EMT to promote breast cancer stem-like properties and drug resistance[217]. Chemotherapy in combination with TGF-β signaling inhibitors increased therapeutic efficacy and reduced chemoresistance[218]. Inhibition of ZEB1 has been shown to reverse EMT and chemoresistance in docetaxel-resistant human lung adenocarcinoma cell lines[219].
The EMT program confers therapy resistance via several mechanisms. Overexpression of EMT transcription factors is reported to increase the expression of ABC transporters, as expression of ABC transporters in breast cancer cells showed 10-fold more resistance to doxorubicin treatment compared with the control cells[220]. Activation of EMT also strongly induced expression of the AXL receptor tyrosine kinase in breast cancer cells[221]. Expression of the AXL receptor tyrosine kinase in carcinoma cells confers resistance to EGFR inhibitors on EGFR-mutant non-small-cell lung carcinoma (NSCLC) cells[222], thus Axl inhibition restored sensitivity to the EGFR inhibitor Erlotinib [223].
The EMT program has been shown to inhibit apoptosis to promote therapy resistance. EMT activation diminishes E‑cadherin-mediated clustering of the TRAIL receptors DR4 and DR5, thereby making carcinoma cells resistant to TRAIL-induced apoptosis[224]. SNAI1 confers resistance against multiple apoptosis-inducing stimuli, in part by promoting AKT activation, upregulating the expression of the pro-survival protein Bcl-XL and delaying cell-cycle progression[225]. SNAI1 was also shown to confer chemoresistance by reducing the expression of p53 in carcinoma cells through interactions between SNAI1 and p53, thus allowing SNAG domain-associated HDAC1 to deacetylate p53[226]. SNAI2 blocks p53-mediated transcriptional induction of Encoding Bcl-2-binding Component 3 (BBC3) expression by directly repressing the BBC3 promoter region. Multiple lung adenocarcinoma cell lines acquire cisplatin resistance through AKT/NF-κB/Slug-mediated BBC3 reduction [227,228].
Cancer stem cells (CSCs) are a subpopulation of neoplastic cells with stem-cell properties. EMT has been shown to be a critical regulator for the induction and maintenance of CSC properties[229]. CSCs are less sensitive to various chemotherapeutic drugs, including doxorubicin, cisplatin, paclitaxel, temozolomide, and methotrexate[59–64] and contribute to tumor recurrence after drug treatment. Given the critical role of EMT in cancer therapy resistance, understanding the signaling pathways induced by EMT will provide additional drug targets to sensitize cancer cells to chemotherapy.
3.2. EMT in immunotherapy resistance
Numerous studies suggest that EMT is highly associated with an immunosuppressive microenvironment. For example, EMT is correlated with high expression levels of immune checkpoint proteins, including PD-L1, PD-L2, PD-1, TIM-3, B7-H3, BTLA, and CTLA-4, in NSCLC cells [230]. Similarly, the expression levels of CTLA-4/PD-1/PD-L1/TIM-3/LAG-3 were highly associated with MMP-13/TWIST1 in ESCC patients[231]. Additionally, higher Tregs infiltration, M2 macrophage polarization and lower CD8 + T cells were reported to correlate with EMT in ovarian cancer, prostate cancer and NSCLCs[230,232,233]. Interestingly, Hugo et al. found that upregulation of EMT-related gene sets correlated with resistance to anti-PD-1 therapy in melanoma patients[234].
EMT transcription factors contribute to immunotherapy resistance via multiple mechanisms. In melanoma cells, SNAI1-induced EMT was shown to promote Tregs infiltration and impair dendritic cells by secreting TSP1[235]. Akalay et al. found that SNAI1-induced mesenchymal breast cancer cells exhibited resistance to CTL-mediated lysis via autophagy activation[236]. In an aPKCι-induced EMT model, SNAI1 was upregulated by aPKCι/Sp1 to drive EMT, resulting in the induction of immunosuppressive Tregs partially via IL-2 and TGFβ production [237]. Additionally, Taki et al. demonstrated that high SNAI1 expressing tumors secreted high level of CXCL1 and CXCL2 chemokines via NF-κB activation, leading to MDSCs recruitment and CD8+ infiltrating lymphocytes repression[238]. In breast cancer cells, cell surface expression of PD-L1 was shown to be stimulated by SNAI1 via post-translational upregulation of CMTM6 and CMTM7, leading to immune evasion [239]. CD47 is a macrophage immune checkpoint protein that suppresses macrophage phagocytic activity[240]. Overexpression of SNAI1 or ZEB1 increased CD47 expression in breast cancer cells, which significantly protected tumor cells from macrophage attack[241]. SNAI1 and ZEB1 were shown to directly bind to two E-boxes in the CD47 promoter[241]. In the transgenic MMTV-PyMT mouse model of breast adenocarcinoma, SNAI1-high mesenchymal tumor cells exhibited increased Tregs infiltration and M2 macrophage polarization compared to SNAI1-low epithelial tumors. Interestingly, mesenchymal tumor cells that showed high resistance to anti-CTLA4 treatment also conferred epithelial tumor cells resistance to immune attack[242]. Furthermore, SNAI1-high quasi-mesenchymal cells secreted high levels of CD73, CSF1 and SPP1, resulting in M2 polarization of TAMs and T cell suppression. Mechanistically, SNAI1 ChIP-seq data showed that SNAI1 was able to bind to 89 immunomodulatory genes[243].
Similarly, ZEB1 also plays an important role in immunosuppression by regulating PD-L1. In breast cancer cells and NSCLC cells, ZEB1 upregulated PD-L1 expression via repressing miR-200[244,245]. Chen et al. showed that miR-200 directly repressed PD-L1 transcription by binding to miR-200 family seed sequences on its 3’UTR. Therefore, ZEB1/miR-200 axis promotes PD-L1 upregulation to facilitate CD8+ T cell exhaustion [244]. Recently, Guo et al. showed that ZEB1 directly induced PD-L1 and CD47 expression, both of which contain the ZEB1 binding E-box[246]. Thus, EMT in cancer cells leads to an immunosuppressive tumor microenvironment through various mechanisms, including T cell exhaustion and immune cell repression, that decreases the effectiveness of tumor immunotherapies.
4. Targeting EMT-inducing signals in the tumor microenvironment for cancer therapy
4.1. Targeting the extracellular matrix
Most of the currently available therapies targeting EMT are aimed at blocking upstream inducers of EMT. Several studies demonstrated that the extracellular matrix, many regulators of ECM stiffness, various mechanosensors, and mechanotransducers are all targetable. Numerous inhibitors against CD44, DDR, LOX/LOX2, integrins, and FAK have been developed and some have shown anticancer activities in preclinical studies (Table 1).
Table 1.
Targeting extracellular matrix.
| Drug/inhibitor | Target | Effectiveness | Reference |
|---|---|---|---|
| Bivatuzumab | CD44 | Bivatuzumab can direct against CD44v6, blocking CD44-HA interaction | [247,248] |
| RO5429083 | CD44 | A monoclonal CD44 antibody, block the interaction between CD44 and HA, which have been entered phase 1 clinical trial conducted among patients with CD44-expressing malignant tumors | [249] |
| Verbascoside | CD44 | Verbascoside suppressed growth of glioblastoma cells by inhibiting CD44 dimerization, as well as suppress tumor stem cell formation | [250] |
| 7rh | DDR1 | Inhibition of DDR1 by 7rh reduces collagen-mediated tumorigenicity in pancreatic ductal adenocarcinoma | [251] |
| WRG-28 | DDR2 | By targeting DDR2, WRG-28 can efficiently prevent disrupt DDR2 receptor-collagen ligand interaction and DDR- mediated tumor progression in preclinical models | [252] |
| Imatinib, nilotinib and dasatinib | DDR1 and DDR2 | These 3 compounds are potent inhibitors of both DDR1 and DDR2 by inhibiting collagen- induced discoidin domain receptor 1 and 2 activation | [253–255] |
| Fresolimumab | Collagen | An anti-TGF-β antibody, suppress TGF-β-regulated gene expression, decreases collagen synthesis, it is currently tested in a phase 1 clinical trial | [256,257] |
| Losartan | Collagen | Losartan inhibits collagen I production via TGF-β pathways and improves the distribution and efficacy of nanotherapeuticsin tumors | [258] |
| Simtuzumab | LOXL2 | Simtuzumab (GS-6624) is a selective inhibitor of LOXL2, which suppress LOXL2 enzymatic activity and inhibits collagen crosslinking, it is currently tested in a phase II clinical trial to test the efficacy and safety of GS-6624 combined with gemcitabine as first-line treatment for metastatic pancreatic adenocarcinoma | [259,260] |
| (2-Chloropyridin-4- yl) methanamine hydrochloride | LOXL2 | (2-Chloropyridin-4-yl) methanamine hydrochloride is a small molecule inhibitor of LOXL2, it suppresses transformation abilities by repressing LOX2 induced EMT in cervical cancer | [261] |
| CCT365623 | LOX | CCT365623 is a pharmacological inhibitor of LOX, it disrupts TGFβ1/ HTRA1/ MATN2/EGFR signaling axis and reduces tumor progression. | [262] |
| Cilengitide | αvβ3 and αvβ5 integrin | Cilengitide is an inhibitor of αvβ3 and αvβ5 integrin, demonstrated modest antitumor activity among recurrent glioblastoma multiforme patients in a prior phase I study | [263] |
| Curcumin | αvβ3 integrin | Curcumin is a natural derivative of turmeric, it influences αvβ3 integrin expression and up-regulation of PDK4 in Erlotinib resistant SW480 colon cancer cells | [264] |
| Defactinib(VS-6063) | FAK | Defactinib is a FAK inhibitor which targets FAK catalytic activity, FAK is a key mediator of therapeutic resistance, it is a potential inhibitor to overcome adaptive resistance to chemotherapy, combinations with the other therapy drugs (such as Pembrolizumab; Paclitaxel and carboplatin; VS-6766) have been tested in the clinical trial phase I or II | [265–267] |
| IN10018 | FAK | IN10018 is a highly selective oral inhibitor of FAK, it is currently tested in a phase Ib clinical trial to study of IN10018 in combination with pegylated liposomal doxorubicin in patients with platinum-resistant ovarian cancer | [268] |
| GSK2256098 | FAK | GSK2256098 is an ATPcompetitive inhibitor that binds to the ATP-binding pocket of FAK, it is currently tested in a phase 1 clinical trial to study of GSK2256098 in patients with advanced solid tumors in the United Kingdom | [269,270] |
| BI853520 | FAK | BI853520 is a highly selective ATP-competitive inhibitor of FAK, it is currently tested in a phase I clinical trial to study of BI853520 in patients with advanced or metastatic nonhematologic malignancies | [271] |
Increased collagen crosslinking increases matrix stiffness and promotes EMT. Using recombinant collagenases to remove collagen from tumor ECM has emerged as a potential therapeutic approach, as depleting extracellular collagen could normalize the tumor microenvironment and increase the drug delivery efficiency[272,273]. LOX-mediated collagen crosslinking is a major contributor to ECM stiffening that promotes EMT and breast tumor progression. CCT365623, a LOX inhibitor with great therapeutic promise[262], suppressed breast cancer growth and metastasis in mice[274]. LOXL2 is correlated with ECM formation and induction of EMT. Treatment with a small molecule inhibitor of LOXL2((2-Chloropyridin-4-yl) methanamine hydrochloride) reversed LOXL2-induced EMT and significantly decreased the invasive ability of cervical cancer cells[261].
Several types of collagen molecules bind to and activate the discoidin domain receptors (DDRs). DDR1 and DDR2 are overexpressed in many cancer types. Inhibition of DDR1 with an ATP-competitive small-molecule kinase inhibitor (7rh) inhibited peritoneal metastasis in gastric carcinoma. Inhibition of DDR1 by 7rh also hindered tumor development in pancreatic ductal adenocarcinoma[251,253]. The small molecule allosteric inhibitor of DDR2 WRG-28 is shown to efficiently disrupt DDR2 receptor–collagen ligand interaction and DDR-mediated tumor progression in preclinical tumor models[252]. Dasatinib, another DDR2 inhibitor, shows promising results in preclinical models of DDR2-positive head and neck squamous cell carcinoma[275]. The extracellular domain of CD44 contains binding sites for various ECM molecules such as hyaluronan, collagen, and fibronectin. Activation of CD44 downstream signaling is involved in EMT-induced tumor progression. Several CD44 blocking antibodies and peptides have been developed to target CD44[276,277]. RO5429083, one of the CD44 antibodies, just entered a Phase 1 clinical trial (Clinicaltrials.gov identifier: NCT01358903).
Inhibition of the integrin function has been shown to lead to reduced metastatic burden in various animal tumor models. αVβ3 and αVβ5 expression is significantly upregulated during EMT. Cilengitide is an integrin αvβ3 and αvβ5 inhibitor that is well tolerated and demonstrated modest antitumor activity among recurrent GBM patients in a phase I study [263]. However, several recent clinical trials showed that selective integrin inhibitors did not reach expected efficacy[278]. Emerging studies therefore focused on targeting the integrin downstream signaling, especially focal adhesion kinase (FAK), an important cell signaling hub that is highly activated upon integrin activation. FAK inhibition is identified as a potential strategy to overcome chemotherapy resistance. FAK activation coupled with the WNT-β-catenin signaling sustained tumor growth by promoting cancer stem cell survival and platinum resistance[279]. Combing a small molecule inhibitor of FAK with carboplatin and paclitaxel for the treatment of platinum-resistant high-grade serous ovarian cancer is now entering a clinical trial [280, 281]. Defactinib (VS-6063) is a FAK inhibitor currently tested in patients with advanced solid tumors in multiple clinical trials (Clinicaltrials.gov identifier: NCT04620330; NCT02546531; NCT03287271)[278,282]. Integrin-linked kinase is another mechanotransducer and a critical regulator of intracellular integrin signaling. Preclinical studies show that QLT-0267, an ILK inhibitor, presented anticancer activities in colon cancer [63,283].
4.2. Targeting stromal and immune cells
4.2.1. Targeting cancer-associated fibroblasts
Therapeutically targeting CAF-stimulated EMT has shown promises in cancer treatment. Multiple drugs are shown to impair CAFs-stimulated EMT by interfering with the IL-6/IL-6R signaling, including siltuximab, tocilizumab, retinoic acid (RA), somatostatin analog SOM230 (pasireotide), nab-paclitaxel (nab-PTX), and cucurbitacin I (JSI-124)[284–289]. Siltuximab, an IL-6 neutralizing antibody, is shown to inhibit IL-6-mediated EMT in cholangiocarcinoma cells and exhibited anti-tumor efficacy in a xenograft model with co-injection of CAFs and lung cancer cells [284,290]. Clinically, Siltuximab treatment contributed to stable disease in more than 50% of patients with metastatic renal cell carcinoma[291]. Tocilizumab, an IL-6R inhibitor, blocked paracrine pro-EMT effects of CAFs on breast cancer cells in vitro and in vivo[285]. Interestingly, Billah et al. found that SOM230 specifically impaired IL-6 expression in CAFs through repressing eiF4E-Binding Protein-1 (4E-BP1)-mediated protein synthesis in pancreatic cancer[287]. Bae et al. demonstrates that an AXL inhibitor BGB324 could significantly suppress CAFs-induced EMT in gastric cancer cells, where AXL was activated by CAFs-secreted GAS6[292]. Targeting SDF-1 or the CXCL12/CXCR4 axis is also a promising therapeutic strategy. AMD3100, a CXCR4 antagonist, was shown to prevent CAFs-induced EMT in pancreatic and prostate cancer cells[93,94]. Specifically, AMD3100 treatment blocked the CXCL12/CXCR4 axis and suppressed p38 kinase, leading to reduced EMT, invasion and lung metastasis in pancreatic cancer cells[94]. Another CXCR4 antagonist BL-804, combined with pembrolizumab, showed benefit in metastatic PDAC patients [293]. Inhibitors targeting TGFβ signaling, such as SB431542 or pirfenidone, are also shown to abrogate CAFs-induced EMT in breast cancer cells [294]. PHA-665752, a c-Met kinase inhibitor, attenuated CAFs-stimulated migration, invasion and tumorigenesis in hepatocellular carcinoma [74](Table 2).
Table 2.
Targeting stromal cells and immune cells.
| Inhibitor | Target cell | Target | Effectiveness | Reference |
|---|---|---|---|---|
| AMD3100 | CAFs | CXCR4 specific inhibitor | Inhibit CAFs-induced EMT, migration and invasion | [94] |
| Siltuximab | CAFs | IL-6 blocking antibody | Inhibit tumor growth, showed anti-tumor activity in Phase I/II clinical study | [284,290,291] |
| Tocilizumab | CAFs | IL-6R blocking antibody | Inhibit CAFs-induced EMT, migration and invasion, repress tumor growth | [285] |
| Nab-paclitaxel | CAFs | Decrease IL-6 secretion | Inhibit CAFs-induced EMT and invasion | [288] |
| PHA-665752 | CAFs | c-Met inhibitor | Inhibit CAFs-induced migration and invasion | [74] |
| SOM230 (pasireotide) | CAFs | Protein synthesis inhibitor | Inhibit CAFs-induced EMT, migration and invasion | [287] |
| BGB324 | CAFs | AXL inhibitor | Inhibit CAFs-induced EMT and migration | [292] |
| SB431542,pirfenidone | CAFs | TGF-P1 inhibitor | Inhibit CAFs-induced EMT and migration | [294] |
| ADP335 | CAAs | Adiponectin analog | Inhibit tumor growth | [295,296] |
| PEG-LPrA2 | CAAs | Leptin peptide receptor antagonist | Inhibit tumor growth | [297,298] |
| BMS309403 | CAAs | FABP4 inhibitor | Inhibit Adipocytes-mediated EMT, repress tumor metastasis | [106] |
| Niclosamide | CAAs | Potent STAT3 inhibitor | Inhibit Adipocytes-mediated EMT | [299] |
| D-CAN | CAAs | ASCs depletion | Inhibit obesity-associated EMT, improve chemotherapy outcome | [111,300] |
| α-TGFβ/α-PD-1 | T lymphocytes | PD-1 and TGFβ blocking antibody | α-PD-1 boost T cell response, α-TGFβ block α-PD-1-stimulated EMT, TGFβ/α-PD-1 combination inhibit tumor growth and promote mice survival | [301] |
| SAR439459/α-PD-L1 | T lymphocytes | TGFβ inhibitor /PD-1 blocking antibody | Promote anti-tumor immunity, induce tumor regression | [302] |
| M7824 | T lymphocytes | PD-1 and TGFβ blocking antibody | Inhibit TGFβ-mediated EMT, block PD-L1, booster T cell response, inhibit tumor growth, metastasis, promote mice survival, show promising efficacy in clinical phase I | [303–307] |
| YM101 | T lymphocytes | PD-L1 and TGFβ blocking antibody | Enhance anti-tumor immune response, inhibit EMT, invasion and tumor growth | [308] |
| AZD6244/Anti-PD-L1/ Anti-IL-17 | T lymphocytes | MEK inhibitor, IL-17 and PD-L1 blocking antibody | Overcome Th17-mediated therapy resistance, reduce tumor growth and lung metastasis | [309] |
| RDC018 | TAMs | CCR2 antagonist | Inhibit TAM infiltration, repress M2 polarization, stimulate CD8 +T cell response | [310] |
| BLZ945 | TAMs | CSF-1R inhibitor | Repress M2 polarization, induce established tumor regression | [311] |
| PLX3397 | TAMs | CSF1R inhibitor | Reduce tumor burden, inhibit metastasis, prolong metastasis-free survival | [312] |
| RG7155 | TAMs | CSF-1R mAb | Reduces TAMs, increase T cell infiltration | [313] |
| CP-870,893 | TAMs | CD40 agonist mAb | Reprogram TAMs, FGK45 combined with gemcitabine therapy inhibit PDA tumor in mice, showed anti-tumor activity in Phase I study | [314] |
| BAY11–7082 | TAMs | NF-ĸB inhibitor | Reprogram TAMs, Inhibit TAMs-mediated EMT | [139] |
| IPI-549 | TAMs | PI3Kγ inhibitor | Reprogram TAMs, inhibit tumor metastasis | [315] |
| JWH-015 | TAMs | Cannabinoid receptor-2 agonist | Inhibit TAMs-mediated EMT | [316] |
| Gemcitabine | MDSCs | Thymidylate synthetase inhibitor | Induce MDSCs apoptosis, combined with IFN-β to inhibit tumor growth | [317] |
| 5-fluorouracil (5FU) | MDSCs | Thymidylate synthetase inhibitor | Induce MDSCs apoptosis, inhibit tumor growth | [318] |
| Sunitinib | MDSCs | Receptor tyrosine kinase inhibitor | Induce MDSCs apoptosis | [319] |
| Paclitaxel | MDSCs | Microtubule stabilization | Reduce MDSCs | [320] |
| Peptide-Fc | MDSCs | S100 family protein binding | Reduce MDSCs, restore CD8 +Teff activity, reduce tumor burden and prolong mice survival | [321] |
| PLX647, PLX5622 | MDSCs | CSF-1R inhibitor | Block MDSCs recruitment, combine with anti-PD-L1 and anti CTLA-4 inhibit tumor growth and prolong mice survival | [322] |
| CCR5-Ig | MDSCs | CCR5 ligand neutralizer | Block MDSC and Tregs trafficking, inhibit tumor progression and prolong mice survival | [323] |
| SX682 | MDSCs | CXCR2 inhibitor | Block PMN-MDSC recruitment, combined with anti-PD-1 inhibit tumor growth and prolong mice survival | [324] |
| Sildenafil | MDSCs | PDE5 inhibitor | Repress MDSC function, enhance T cell therapy efficacy | [325] |
| Celecoxib | MDSCs | COX-2 inhibitor | Deplete MDSC, impair MDSC function, combined with DC immunotherapy to improve mice survival | [326] |
| CDDO-Me | MDSCs | Reduce ROS | Neutralize MDSC activity, inhibit tumor growth | [327] |
| TBCA | MDSCs | CK2 inhibitor | Improve myeloid cell differentiation, inhibit tumor growth | [328] |
| ATRA | MDSCs | CK2 inhibitor | Improve dendritic cell differentiation | [329] |
4.2.2. Targeting cancer-associated adipocytes
Several targeting strategies have also been developed to target cancer-associated adipocytes to inhibit EMT. Adiponectin, secreted from normal adipocytes and downregulated in cancer-associated adipocytes (CAAs), was shown to reverse EMT and impair migration and invasion in NSCLC cells[330]. Adiponectin analogue ADP335 was developed to mimic its function on tumor progression. In breast cancer or prostate cancer xenograft models, ADP335 treatment significantly repressed tumor progression by modulating AMPK, Akt, STAT3 and ERK1/2 signaling [295,296]. Targeting leptin is also showing promise in halting tumor progression. PEG-LPrA2, acting as a leptin receptor antagonist, significantly reduced tumor growth in breast cancer xenografts in mice by repressing ERK, AKT or VEGF upregulation [297,298]. Additionally, a FABP4 inhibitor BMS309403 was reported to efficiently inhibit adipocyte-mediated EMT and metastasis in cholangiocarcinoma[106]. Niclosamide treatment repressed adipocyte-induced EMT by inhibiting IL-6/STAT3 axis in breast cancer cells[299]. Depletion of ASCs by D-CAN, also effectively repressed obesity-mediated EMT and prostate tumor progression[111,300](Table 2).
4.2.3. Targeting lymphocytes
Targeting EMT induction in combinations with anti-PD-1/PD-L1 immunotherapies has shown improvement in the response to immuno-therapies in cancer patients. Multiple studies suggest that anti-TGFβ treatment could inhibit EMT and synergize with immunotherapy to boost immune response and attenuate tumor progression[301,302]. In chemically induced squamous cell carcinomas, α-PD-1 therapy not only inhibited tumor growth, but also provoked immunosuppressive Tregs and activated TGFβ/pSMAD3 signaling in tumors, leading to EMT, while addition of anti-TGFβ treatment attenuated EMT and alleviated α-PD-1 resistance[301]. Thus, the anti-TGFβ/anti-PD-1 combination therapy significantly inhibited CCK168 tumors growth and promoted survival in mice[301]. Indeed, bifunctional molecules targeting both TGFβ and PD-L1 exhibited higher anti-tumor efficiency than anti-TGFβ or anti-PD1-L1 monotherapy. M7824, an anti-PD-L1/ TGFβ Trap fusion protein, was shown to revert EMT in vivo and in vitro in lung cancer models and to boost CD8+ T cell and NK cell responses in various cancers, thereby leading to tumor regression and longer survival in mice [303–306]. The M7824 phase I trial showed promising antitumor efficacy in advanced solid tumor patients[307]. More recently, YM101, a new bifunctional antibody against TGFβ and PD-L1 was shown to inhibit EMT and revert immunosuppression in tumor-bearing mice [308]. In KRAS mutant lung cancer models, combination of a MEK inhibitor and anti-PD-L1 therapy inhibited tumor growth, but also led to therapy resistance by increasing CD4+ Th17 infiltration[309]. CD4+ Th17 is implicated in inducing EMT in tumor cells by secreting IL-17. Peng et al. demonstrated that anti-IL-17 in combination with a MEK inhibitor and an anti-PD-L1 antibody significantly reduced tumor metastasis and therapy resistance in tumor-bearing mice[309](Table 2).
4.2.4. Targeting macrophages
TAMs play critical roles in promoting EMT, invasion and metastasis. Numerous macrophages targeting therapies significantly impede tumor progression, including blocking macrophages recruitment, TAMs depletion and reprogramming macrophages polarization[130,331]. Several studies show that blocking the CCL2/CCR2 axis could repress macrophage recruitment. A CCL2 neutralization antibody significantly inhibited macrophage infiltration and tumor metastasis in tumor-bearing mice[131]. RDC018, a CCR2 antagonist, significantly inhibited hepatocellular carcinoma tumor growth and metastasis[310]. Depletion of TAMs could also be achieved through interfering with CSF-1/CSF-1R axis. RG7155, a monoclonal antibody targeting CSF-1 receptor (CSF-1R), could deplete M2 macrophages both in vitro and in vivo, reducing tumor burden in diffuse-type giant cell tumor (Dt-GCT) patients[313]. CSF-1R inhibitors BLZ945 and PLX3397 not only eliminated immunosuppressive M2-like cells, but also boosted T cell response, leading to tumor regression in glioma and pancreatic cancer models[311,312]. Reprogramming TAMs to anti-tumor macrophages has also been tested to induce tumor regression. Hagemann et al. demonstrated that NF- κB inhibition reprogramed M2 macrophages to M1 macrophages and repressed tumor growth by increasing IL-12-dependent NK activity[332]. BAY11–7082, a NF-κB inhibitor, was shown to repress TAMs-mediated EMT and stemness in bladder cancer cells by suppressing M2 polarization[139]. IPI-549, a specific PI3Kγ inhibitor, switched immunosuppressive TAMs to immunostimulatory macrophages by activating NF-κB and inhibiting PI3Kγ/mTOR-S6Kα-C/EBPβ, facilitating spontaneous breast carcinoma regression and reduced lung metastasis[315]. Additionally, the cannabinoid receptor-2 agonist, JWH-015 was shown to repress M2 macrophage-stimulated EMT in NSCLC cells and inhibit tumor growth in mice[316](Table 2).
4.2.5. Targeting MDSCs
Similarly to TAMs, MDSCs were implicated to stimulate EMT and facilitate metastasis. Several approaches were developed to repress MDSC functions, including depleting MDSCs, inhibiting MDSC recruitment, repressing MDSC function and promoting MDSC differentiation [333,334]. CSF-1R, CCR5 and CXCR2 could be targeted to block MDSCs recruitment and revert the immunosuppressive tumor environment [323,322,324]. Blattner et al. demonstrated that the CCR5-Ig fusion protein treatment reduced MDSCs and Tregs infiltration, thereby impeding melanoma tumor progression[323]. Similarly, CSF-1R inhibitors, PLX647 and PLX5622, and CXCR2 inhibitor SX682 were also reported to block MDSCs recruitment, enhancing the response to T cell checkpoint immunotherapy[322,324]. Several compounds including the PDE5 inhibitor, the COX-2 inhibitor and triterpenoid CDDO-Me were reported to impair the immunosuppressive function of MDSCs [325–327]. For example, Sildenafil, a PDE5 inhibitor neutralized MDSC function, thereby facilitating CD8+ T cell activation and inhibiting tumor growth[325]. Song et al. show that Ginsenoside Rg3 blocked MDSC-mediated EMT and stemness acquisition in breast cancer cells by repressing Notch and STAT3 activation, resulting in tumor suppression [335](Table 2). Given these studies, targeting both TAMs and MDSCs in the tumor microenvironment could be a feasible approach to block tumor cell EMT and metastasis.
4.3. Targeting hypoxia
Hypoxia-mediated tumor malignant transformation is mainly orchestrated by HIF-1α/HIF-2α proteins. Numerous inhibitors targeting HIF-1α/ HIF-2α were developed for potential cancer therapies, including 1) PX-478[336,337] that inhibits HIF-1α transcription, translation and de-ubiquitination; 2) digoxin[338,339] and topotecan[340,341] that inhibit HIF-1α/2α protein synthesis; 3) acriflavine[342] that blocks HIF-1α/2α dimerization with HIF-1β; 4) 2-methoxyestradiol (2ME2) that inhibits HIF-1α/2α nuclear accumulation[343]; 5) echinomycin (NSC-13502) [344–346] that inhibits HIF-1α binding to the HRE; 6) chetomin[347], bortezomib[348], YC-1[349], all of which inhibit HIF-1α/2α binding to its transactivator p300 (Table 3). PX-478 treatment efficiently suppressed tumor metastasis in HIF-1α-expressing lung cancer cells[350]. Clinically, PX-478 treatment contributed to improved radiotherapy or chemotherapy efficacy in combination with anti-cancer drugs[351]. Digoxin is shown to prevent hypoxia-mediated EMT by blocking the HIF-1α-ZEB1 axis, further repressing the migration and invasion capacity of GBM cells[182]. Moreover, Carmen et al. demonstrated that either digoxin or acriflavine significantly reduced lung metastasis in breast cancer xenografts in mice [352]. In glioma cells, echinomycin prevented hypoxia-induced EMT and invasion by repressing the HIF-1α/miR-210/TGF-β and HIF-1α/miR-210/NF-κB axis, respectively[353]. Similarly, YC-1 treatment reduced hypoxia-induced migration and invasion, leading to metastasis suppression in hepatocellular carcinoma and breast cancer xenografts in mice[354,355].
Table 3.
Targeting hypoxia.
| Inhibitor | Target | Mechanism | Effectiveness | Reference |
|---|---|---|---|---|
| chetomin | HIF-1α,HIF-2α | Inhibit p300 binding to HIF-1α/2α | Induce tumor necrosis | [347] |
| YC-1 | HIF-1α,HIF-2α | Decrease HIF-1α/2α protein, inhibit p300 binding via FIH | Inhibit hypoxia-mediated migration, invasion and tumor metastasis | [349] |
| Bortezomib | HIF-1α | Inhibit p300 binding via FIH | Induce tumor cell death | [348] |
| Echinomycin (NSC-13502) | HIF-1α | Inhibit HIF-1α/DNA interface | Inhibit hypoxia-mediated EMT, reduce metastasis in mice model, but show toxicity and limited efficacy in clinical phase II | [344–346] |
| PX-478 | HIF-1α | Inhibit HIF-1α transcription, translation and deubiquitination | Inhibit tumor growth and metastasis | [350,351] |
| Acriflavine | HIF-1α,HIF-2α | Inhibit Dimerization with HIF-1β | Inhibit tumor growth and metastasis | [342] |
| Digoxin | HIF-1α,HIF-2α | Inhibit HIF-1α/2α protein synthesis | Inhibit hypoxia-mediated EMT, migration and invasion. Reduce metastasis in mice model, but phase II clinical trial showed no effects | [338,339,356] |
| EZN-2968 | HIF-1α | Reduce HIF-1α mRNA level | Inhibit tumor growth. Clinal trail was premature closed | [357,358] |
| Topotecan | HIF-1α | Inhibit HIF-1α translation | Inhibit tumor growth and angiogenesis, show effective anti-tumor activity in clinical phase I/II/III | [340,341,359] |
| 2-methoxyestradiol (2ME2) | HIF-1α,HIF-2α | Inhibit HIF nuclear accumulation by disrupt tubulin | Inhibit angiogenesis | [343] |
| PT2399 | HIF-2α | blocks the heterodimerization of HIF-2α with HIF-1β | Inhibit tumor growth, induce tumor regression | [360,361] |
| PT2385 | HIF-2α | blocks the heterodimerization of HIF-2α with HIF-1β | Induce tumor regression | [362,363] |
| Belzutifan (MK-6482, PT2977) | HIF-2α | blocks the heterodimerization of HIF-2α with HIF-1β | Induce tumor regression, safe and efficient in Phase I/II clinical trials | [364,365] |
HIF-2α is a key oncogene in ccRCC, especially in VHL-deficient tumors, where stabilized HIF-2α drives tumor invasion and metastasis [366]. Thus, multiple inhibitors specifically targeting HIF-2α were designed to impair its oncogenic activity, such as THS-044[366], PT2385 (MK-3795)[362,363], PT2399[360,361] and belzutifan (MK-6482 or PT2977)[364](Table 3). These inhibitors significantly reduce HIF-2α target gene expression by interrupting its heterodimerization with HIF-1β. PT2385 treatment was shown to inhibit HIF-2α-induced expression of VEGF-A, PAI-1 and cyclin D1, thereby leading to tumor regression of the VHL-deficient ccRCC xenograft model [362]. Furthermore, tumor regression in 34% VHL-deficient ccRCC patients was reported in a phase I clinical study of patients with locally advanced or metastatic ccRCC[363]. Rui et al. developed the second generation HIF-2α inhibitor, belzutifan, which significantly repressed HIF-2α target erythropoietin (EPO) expression and promoted tumor regression in ccRCC bearing mice[364]. Belzutifan showed promising anti-tumor activity in metastatic ccRCC patients[365]. Additionally, belzutifan, in combination with cabozantinib, demonstrated to be an effective treatment for patients with metastatic ccRCC (NCT03634540 ) and has recently been approved by FDA for VHL-related diseases, including ccRCC tumors[367]. Although the action of HIF inhibition on tumor cells is pleiotropic, EMT is likely to be one of the key effects that can be targeted to inhibit metastasis and chemoresistance.
5. Conclusion
In this review, we summarized various important microenviron-mental cues that impinge on the EMT program to impact tumor development, progression, and therapy response in human tumors, including ECM, hypoxia, stroma cells, and immune cells. Much has been learned from the past two decades of intensive research on EMT and the tumor microenvironment. First, the communication between the tumor microenvironment and the EMT program is not unidirectional. Instead, tumor cells that have undergone EMT can also modulate stromal cells, immune cells, and the ECM to generate a more tumor-prone tumor microenvironment to further facilitate tumor development and progression. Dissecting the bidirectional interactions between tumor cells and their microenvironment and their effects on EMT requires more innovative in vitro culture systems to better mimic the complex three-dimensional biochemical and biophysical tumor microenvironment. Second, the interaction between the tumor microenvironment and EMT is highly dynamic in time and space. As discussed above, the EMT program is a dynamic and transient program during tumor progression. While activation of the transient EMT program promotes tumor cell dissemination into distant organs, tumor cells undergo MET to regain growth at distant organs. Therefore, the same microenvironmental cues in primary tumors and distant organs may impact tumor progression differentially, which should be carefully considered in selecting targeted therapies. Third, extensive clinical and experimental data show a tight association between the mesenchymal state and resistance to various cancer therapeutics. Given many such therapies target cell proliferation, it is conceivable why the mesenchymal state with reduced proliferation is resistant to such therapeutics. However, EMT is also reported to provide resistance to numerous therapeutics, such as immunotherapies, that do not directly target cell proliferation. Elucidating how EMT provides resistance to various cancer therapeutics is critical for developing new approaches to overcome therapy resistance. Lastly, many therapeutic approaches that target tumor microenvironmental cues impact EMT and tumor progression, some of which showed promising clinical benefits in cancer patients. While EMT plays an important role in tumor metastasis, very few cancer therapeutics are designed for metastasis prevention. As our knowledge of EMT and metastasis continues to grow, it becomes evident that metastasis prevention will become an effective approach for high-risk cancer survivors that are prone to developing metastatic recurrence. The EMT research could contribute significantly to the next generation of cancer therapeutics on metastasis prevention.
Acknowledgements
We apologize to many researchers whose work we were unable to cite due to space restrictions. Our research is supported by grants from NCI (1RO1CA262794, 1R01CA174869, 1R01CA206880, and 1R01CA236386), CDMRP DOD Breast Cancer Program BC170283, METAvivor Research Award, California Tobacco-Related Disease Research Program, and AACR-Bayer Innovation and Discovery Grant. The study sponsors are not involved in the writing of the manuscript and the decision to submit the manuscript for publication.
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
Data Availability
No data was used for the research described in the article.
References
- [1].Lambert AW, Weinberg RA, Linking EMT programmes to normal and neoplastic epithelial stem cells, Nat. Rev. Cancer 21 (2021) 325–338, 10.1038/s41568-021-00332-6. [DOI] [PubMed] [Google Scholar]
- [2].Scott LE, Weinberg SH, Lemmon CA, Mechanochemical signaling of the extracellular matrix in epithelial-mesenchymal transition, Front. Cell Dev. Biol 7 (2019) 135, 10.3389/fcell.2019.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Brabletz T, Kalluri R, Nieto MA, Weinberg RA, EMT in cancer, Nat. Rev. Cancer 18 (2018) 128–134, 10.1038/nrc.2017.118. [DOI] [PubMed] [Google Scholar]
- [4].Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, Concannon KF, Donaldson MC, Sequist LV, Brachtel E, Sgroi D, Baselga J, Ramaswamy S, Toner M, Haber DA, Maheswaran S, Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition, Science 339 (2013) 580–584, 10.1126/science.1228522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, Campbell K, Cano A, Casanova J, Christofori G, Dedhar S, Derynck R, Ford HL, Fuxe J, García de Herreros A, Goodall GJ, Hadjantonakis AK, Huang RJY, Kalcheim C, Kalluri R, Kang Y, Khew-Goodall Y, Levine H, Liu J, Longmore GD, Mani SA, Massagúe J, Mayor R, McClay D, Mostov KE, Newgreen DF, Nieto MA, Puisieux A, Runyan R, Savagner P, Stanger B, Stemmler MP, Takahashi Y, Takeichi M, Theveneau E, Thiery JP, Thompson EW, Weinberg RA, Williams ED, Xing J, Zhou BP, Sheng G, Guidelines and definitions for research on epithelial–mesenchymal transition, Nat. Rev. Mol. Cell Biol 21 (2020) 341–352, 10.1038/s41580-020-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Puram SV, Tirosh I, Parikh AS, Patel AP, Yizhak K, Gillespie S, Rodman C, Luo CL, Mroz EA, Emerick KS, Deschler DG, Varvares MA, Mylvaganam R, Rozenblatt-Rosen O, Rocco JW, Faquin WC, Lin DT, Regev A, Bernstein BE, Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer, e24, Cell 171 (2017) 1611–1624, 10.1016/j.cell.2017.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Simeonov KP, Byrns CN, Clark ML, Norgard RJ, Martin B, Stanger BZ, Shendure J, McKenna A, Lengner CJ, Single-cell lineage tracing of metastatic cancer reveals selection of hybrid EMT states, e9, Cancer Cell 39 (2021) 1150–1162, 10.1016/j.ccell.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pastushenko I, Brisebarre A, Sifrim A, Fioramonti M, Revenco T, Boumahdi S, Keymeulen AV, Brown D, Moers V, Lemaire S, Clercq SD, Minguijón E, Balsat C, Sokolow Y, Dubois C, Cock FD, Scozzaro S, Sopena F, Lanas A, Haene ND, Salmon I, Marine J, Voet T, Sotiropoulou PA, Identification of the tumour transition states occurring during EMT, (2018). [DOI] [PubMed] [Google Scholar]
- [9].Pastushenko I, Blanpain C, EMT transition states during tumor progression and metastasis, Trends Cell Biol. 29 (2019) 212–226, 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- [10].Tsai JH, Donaher JL, Murphy DA, Chau S, Yang J, Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis, Cancer Cell 22 (2012) 725–736, 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong STC, Choi H, El Rayes T, Ryu S, Troeger J, Schwabe RF, Vahdat LT, Altorki NK, Mittal V, Gao D, Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance, Nature 527 (2015) 472–476, 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li Y, Lv Z, Zhang S, Wang Z, He L, Tang M, Pu W, Zhao H, Zhang Z, Shi Q, Cai D, Wu M, Hu G, Lui KO, Feng J, Nieto MA, Zhou B, Genetic fate mapping of transient cell fate reveals n-cadherin activity and function in tumor metastasis, e5, Dev. Cell 54 (2020) 593–607, 10.1016/j.devcel.2020.06.021. [DOI] [PubMed] [Google Scholar]
- [13].Bornes L, van Scheppingen RH, Beerling E, Schelfhorst T, Ellenbroek SIJ, Seinstra D, van Rheenen J, Fsp1-mediated lineage tracing fails to detect the majority of disseminating cells undergoing EMT, e3, Cell Rep. 29 (2019) 2565–2569, 10.1016/J.CELREP.2019.10.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lüönd F, Sugiyama N, Bill R, Bornes L, Hager C, Tang F, Santacroce N, Beisel C, Ivanek R, Bürglin T, Tiede S, van Rheenen J, Christofori G, Distinct contributions of partial and full EMT to breast cancer malignancy, e11, Dev. Cell 56 (2021) 3203–3221, 10.1016/j.devcel.2021.11.006. [DOI] [PubMed] [Google Scholar]
- [15].Zhang J, Tian XJ, Zhang H, Teng Y, Li R, Bai F, Elankumaran S, Xing J, TGF-β-induced epithelial-to-mesenchymal transition proceeds through stepwise activation of multiple feedback loops, Sci. Signal 7 (2014) ra91, 10.1126/scisignal.2005304. [DOI] [PubMed] [Google Scholar]
- [16].Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, García De Herreros A, The transcription factor Snail is a repressor of E-cadherin gene expression in epithelial tumour cells, 2000. [DOI] [PubMed] [Google Scholar]
- [17].Cano A, Pérez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Angela Nieto M, The transcription factor Snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression, 2000. [DOI] [PubMed] [Google Scholar]
- [18].Bolós V, Peinado H, Pérez-Moreno MA, Fraga MF, Esteller M, Cano A, The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors, J. Cell Sci 116 (2003) 499–511, 10.1242/jcs.00224. [DOI] [PubMed] [Google Scholar]
- [19].Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, Berx G, Cano A, Beug H, Foisner R, DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells, Oncogene 24 (2005) 2375–2385, 10.1038/sj.onc.1208429. [DOI] [PubMed] [Google Scholar]
- [20].Comijn J, Berx G, Vermassen P, Verschueren K, Van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, Van Roy F, The Two-Handed E Box Binding Zinc Finger Protein SIP1 Downregulates E-Cadherin and Induces Invasion made clear that aberrant E-cadherin expression, re-sulting from somatic inactivating mutations of both E-cadherin alleles, is rare and so far largely confined to diffuse gastric carcinomas and infiltrative lobular breast carcinomas (Becker et al., 2001. [DOI] [PubMed] [Google Scholar]
- [21].Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA, Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis, Cell 117 (2004) 927–939, 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- [22].Ansieau S, Bastid J, Doreau A, Morel AP, Bouchet BP, Thomas C, Fauvet F, Puisieux I, Doglioni C, Piccinin S, Maestro R, Voeltzel T, Selmi A, Valsesia-Wittmann S, Caron de Fromentel C, Puisieux A, Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence, Cancer Cell 14 (2008) 79–89, 10.1016/j.ccr.2008.06.005. [DOI] [PubMed] [Google Scholar]
- [23].Fang X, Cai Y, Liu J, Wang Z, Wu Q, Zhang Z, Yang CJ, Yuan L, Ouyang G, Twist2 contributes to breast cancer progression by promoting an epithelialmesenchymal transition and cancer stem-like cell self-renewal, Oncogene 30 (2011) 4707–4720, 10.1038/onc.2011.181. [DOI] [PubMed] [Google Scholar]
- [24].Eckert MA, Lwin TM, Chang AT, Kim J, Danis E, Ohno-Machado L, Yang J, Twist1-induced invadopodia formation promotes tumor metastasis, Cancer Cell 19 (2011) 372–386, 10.1016/j.ccr.2011.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nowak E, Bednarek I, Aspects of the epigenetic regulation of emt related to cancer metastasis, Cells 10 (2021), 10.3390/cells10123435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Addison JB, Voronkova MA, Fugett JH, Lin CC, Linville NC, Trinh B, Livengood RH, Smolkin MB, Schaller MD, Ruppert JM, Pugacheva EN, Creighton CJ, Ivanov AV, Functional hierarchy and cooperation of emt master transcription factors in breast cancer metastasis, Mol. Cancer Res 19 (2021) 784–798, 10.1158/1541-7786.MCR-20-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Casas E, Kim J, Bendesky A, Ohno-Machado L, Wolfe CJ, Yang J, Snail2 is an essential mediator of twist1-induced epithelial mesenchymal transition and metastasis, Cancer Res. 71 (2011) 245–254, 10.1158/0008-5472.CAN-10-2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Korpal M, Lee ES, Hu G, Kang Y, The miR-200 family inhibits epithelialmesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2, J. Biol. Chem 283 (2008) 14910–14914, 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Park SM, Gaur AB, Lengyel E, Peter ME, The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2, Genes Dev. 22 (2008) 894–907, 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ, The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1, Nat. Cell Biol 10 (2008) 593–601, 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- [31].Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T, A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells, EMBO Rep. 9 (2008) 582–589, 10.1038/embor.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, Hausen AZ, Brunton VG, Morton J, Sansom O, Schüler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T, The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs, Nat. Cell Biol 11 (2009) 1487–1495, 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- [33].Siemens H, Jackstadt R, Hünten S, Kaller M, Menssen A, Götz U, Hermeking H, miR-34 and SNAIL form a double-negative feedback loop to regulate epithelial-mesenchymal transitions, Cell Cycle 10 (2011) 4256–4271, 10.4161/cc.10.24.18552. [DOI] [PubMed] [Google Scholar]
- [34].Xu J, Lamouille S, Derynck R, TGF-В-induced epithelial to mesenchymal transition, Cell Res. 19 (2009) 156–172, 10.1038/cr.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zeisberg M, Bonner G, Maeshima Y, Colorado P, Müller GA, Strutz F, Kalluri R, Renal fibrosis, Am. J. Pathol 159 (2001) 1313–1321, 10.1016/S0002-9440(10)62518-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gong Y, Yang Y, Activation of Nrf2/AREs-mediated antioxidant signalling, and suppression of profibrotic TGF-β1/Smad3 pathway: a promising therapeutic strategy for hepatic fibrosis — a review, Life Sci. 256 (2020), 10.1016/j.lfs.2020.117909. [DOI] [PubMed] [Google Scholar]
- [37].Shintani Y, Maeda M, Chaika N, Johnson KR, Wheelock MJ, Collagen I promotes epithelial-to-mesenchymal transition in lung cancer cells via transforming growth factor-β signaling, Am. J. Respir. Cell Mol. Biol 38 (2008) 95–104, 10.1165/rcmb.2007-0071OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Zhang K, Corsa CA, Ponik SM, Prior JL, Piwnica-Worms D, Eliceiri KW, Keely PJ, Longmore GD, The collagen receptor discoidin domain receptor 2 stabilizes SNAIL1 to facilitate breast cancer metastasis, Nat. Cell Biol 15 (2013) 677–687, 10.1038/ncb2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Griggs LA, Hassan NT, Malik RS, Griffin BP, Martinez BA, Elmore LW, Lemmon CA, Fibronectin fibrils regulate TGF-β1-induced Epithelial-Mesenchymal Transition, Matrix Biol. 60–61 (2017) 157–175, 10.1016/j.matbio.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Park J, Schwarzbauer JE, Mammary epithelial cell interactions with fibronectin stimulate epithelial-mesenchymal transition, Oncogene 33 (2014) 1649–1657, 10.1038/onc.2013.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bourguignon LYW, Singleton PA, Zhu H, Zhou B, Hyaluronan promotes signaling interaction between CD44 and the transforming growth factor β receptor i in metastatic breast tumor cells, J. Biol. Chem 277 (2002) 39703–39712, 10.1074/jbc.M204320200. [DOI] [PubMed] [Google Scholar]
- [42].El-Haibi CP, Bell GW, Zhang J, Collmann AY, Wood D, Scherber CM, Csizmadia E, Mariani O, Zhu C, Campagne A, Toner M, Bhatia SN, Irimia D, Vincent-Salomon A, Karnoub AE, Critical role for lysyl oxidase in mesenchymal stem cell-driven breast cancer malignancy, Proc. Natl. Acad. Sci. U. S. A 109 (2012) 17460–17465, 10.1073/pnas.1206653109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Matrisian LM, The matrix-degrading metalloproteinases, Bioessays 14 (1992) 455–463, 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- [44].Aimes RT, Quigley JP, Matrix Metalloproteinase-2 Is an Interstitial Collagenase, J. Biol. Chem 270 (1995) 5872–5876, 10.1074/jbc.270.11.5872. [DOI] [PubMed] [Google Scholar]
- [45].Song W, Jackson K, McGuire PG, Degradation of Type IV collagen by matrix metalloproteinases is an important step in the epithelial-mesenchymal transformation of the endocardial cushions, Dev. Biol 227 (2000) 606–617, 10.1006/dbio.2000.9919. [DOI] [PubMed] [Google Scholar]
- [46].Radisky DC, Levy DD, Littlepage LE, Liu H, Nelson CM, Fata JE, Leake D, Godden EL, Albertson DG, Angela Nieto M, Werb Z, Bissell MJ, Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability, Nature 436 (2005) 123–127, 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Siegel RC, Fu JCC, Uto N, Horiuchi K, Fujimoto D, Collagen cross-linking: lysyl oxidase dependent synthesis of pyridinoline in vitro: Confirmation that pyridinoline is derived from collagen, Biochem. Biophys. Res. Commun 108 (1982) 1546–1550, 10.1016/S0006-291X(82)80083-1. [DOI] [PubMed] [Google Scholar]
- [48].Csiszar K, Lysyl oxidases: a novel multifunctional amine oxidase family, (2001). [DOI] [PubMed] [Google Scholar]
- [49].Peinado H, del M Carmen Iglesias-de la Cruz, D. Olmeda, K. Csiszar, K.S.K. Fong, S. Vega, M.A. Nieto, A. Cano, F. Portillo, A molecular role for lysyl oxidase-like 2 enzyme in Snail regulation and tumor progression, EMBO J. 24 (2005) 3446–3458, 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Boufraqech M, Zhang L, Nilubol N, Sadowski SM, Kotian S, Quezado M, Kebebew E, Lysyl oxidase (LOX) transcriptionally regulates SNAI2 expression and TIMP4 secretion in human cancers, Clin. Cancer Res 22 (2016) 4491–4504, 10.1158/1078-0432.CCR-15-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Schietke R, Warnecke C, Wacker I, Schödel J, Mole DR, Campean V, Amann K, Goppelt-Struebe M, Behrens J, Eckardt K-U, Wiesener MS, The lysyl oxidases LOX and LOXL2 are necessary and sufficient to repress e-cadherin in hypoxia, J. Biol. Chem 285 (2010) 6658–6669, 10.1074/jbc.M109.042424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kechagia JZ, Ivaska J, Roca-Cusachs P, Integrins as biomechanical sensors of the microenvironment, Nat. Rev. Mol. Cell Biol 20 (2019) 457–473, 10.1038/s41580-019-0134-2. [DOI] [PubMed] [Google Scholar]
- [53].Jang I, Beningo KA, Integrins, CAFs and mechanical forces in the progression of cancer, Cancers 11 (2019), 10.3390/cancers11050721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Hamidi H, Ivaska J, Every step of the way: Integrins in cancer progression and metastasis, Nat. Rev. Cancer 18 (2018) 533–548, 10.1038/s41568-018-0038-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wei SC, Fattet L, Tsai JH, Guo Y, Pai VH, Majeski HE, Chen AC, Sah RL, Taylor SS, Engler AJ, Yang J, Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway, Nat. Cell Biol 17 (2015) 678–688, 10.1038/ncb3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Guo W, Giancotti FG, Integrin signalling during tumour progression, Nat. Rev. Mol. Cell Biol 5 (2004) 816–826, 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- [57].Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, Nishimura SL, Aldape K, Landers DV, Carpenter W, Gillett N, Sheppard D, Matthay MA, Albelda SM, Kramer RH, Pytela R, Expression of the β6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling, (n.d.) 11. [DOI] [PubMed] [Google Scholar]
- [58].Truong HH, Xiong J, Ghotra VPS, Nirmala E, Haazen L, Le Dévédec SE, Balcioğlu HE, He S, Snaar-Jagalska BE, Vreugdenhil E, Meerman JHN, van de Water B, Danen EHJ, β 1 integrin inhibition elicits a prometastatic switch through the TGFβ–miR-200–ZEB network in E-cadherin–positive triple-negative breast cancer, Sci. Signal 7 (2014), 10.1126/scisignal.2004751. [DOI] [PubMed] [Google Scholar]
- [59].Xu H, Bihan D, Chang F, Huang PH, Farndale RW, Leitinger B, Discoidin domain receptors promote α1β1- and α2β1-integrin mediated cell adhesion to collagen by enhancing integrin activation, PLoS One 7 (2012), e52209, 10.1371/journal.pone.0052209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Przybyla L, Muncie JM, Weaver VM, Mechanical control of epithelial-tomesenchymal transitions in development and cancer, Annu. Rev. Cell Dev. Biol 32 (2016) 527–554, 10.1146/annurev-cellbio-111315-125150. [DOI] [PubMed] [Google Scholar]
- [61].Gálvez BG, Matías-Román S, Yáñez-Mó M, Sánchez-Madrid F, Arroyo AG, ECM regulates MT1-MMP localization with β1 or αvβ3 integrins at distinct cell compartments modulating its internalization and activity on human endothelial cells, J. Cell Biol 159 (2002) 509–521, 10.1083/jcb.200205026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Cao J, Chiarelli C, Richman O, Zarrabi K, Kozarekar P, Zucker S, Membrane type 1 matrix metalloproteinase induces epithelial-to-mesenchymal transition in prostate cancer, J. Biol. Chem 283 (2008) 6232–6240, 10.1074/jbc.M705759200. [DOI] [PubMed] [Google Scholar]
- [63].Chadla P, Arbi M, Nikou S, Kalliakoudas T, Papadaki H, Taraviras S, Lygerou Z, Bravou V, Integrin-linked-kinase overexpression is implicated in mechanisms of genomic instability in human colorectal cancer, Dig. Dis. Sci 66 (2021) 1510–1523, 10.1007/s10620-020-06364-6. [DOI] [PubMed] [Google Scholar]
- [64].Górska A, Mazur AJ, Integrin-linked kinase (ILK): the known vs. the unknown and perspectives, Cell. Mol. Life Sci 79 (2022), 10.1007/s00018-021-04104-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Kilinc AN, Han S, Barrett LA, Anandasivam N, Nelson CM, Integrin-linked kinase tunes cell-cell and cell-matrix adhesions to regulate the switch between apoptosis and EMT downstream of TGFβ1, Mol. Biol. Cell 32 (2021) 402–412, 10.1091/mbc.E20-02-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bae C, Sachs F, Gottlieb PA, The mechanosensitive ion channel Piezo1 is inhibited by the peptide GsMTx4, Biochemistry 50 (2011) 6295–6300, 10.1021/bi200770q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Li Y, Xu C, Sun B, Zhong F, Cao M, Yang L, Piezo1 promoted hepatocellular carcinoma progression and EMT through activating TGF ‑ β signaling by recruiting Rab5c, Cancer Cell Int. (2022) 1–19, 10.1186/s12935-022-02574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kärki T, Tojkander S, Trpv protein family—from mechanosensing to cancer invasion, Biomolecules 11 (2021) 1–23, 10.3390/biom11071019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Azimi I, Robitaille M, Armitage K, So CL, Milevskiy MJG, Northwood K, Lim HF, Thompson EW, Roberts-Thomson SJ, Monteith GR, Activation of the ion channel TRPV4 induces epithelial to mesenchymal transition in breast cancer cells, Int. J. Mol. Sci 21 (2020) 1–14, 10.3390/ijms21249417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wei SC, Fattet L, Yang J, The forces behind EMT and tumor metastasis, Cell Cycle 14 (2015) 2387–2388, 10.1080/15384101.2015.1063296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jung HY, Fattet L, Yang J, Molecular pathways: linking tumor microenvironment to Epithelial-mesenchymal transition in metastasis, Clin. Cancer Res 21 (2015) 962–968, 10.1158/1078-0432.CCR-13-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Fattet L, Jung H-Y, Matsumoto MW, Aubol BE, Kumar A, Adams JA, Chen AC, Sah RL, Engler AJ, Pasquale EB, Yang J, Matrix rigidity controls epithelial-mesenchymal plasticity and tumor metastasis via a mechanoresponsive EPHA2/LYN complex, e7, Dev. Cell 54 (2020) 302–316, 10.1016/j.devcel.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Yu Y, Xiao CH, Tan LD, Wang QS, Li XQ, Feng YM, Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling, Br. J. Cancer 110 (2014) 724–732, 10.1038/bjc.2013.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lau EYT, Lo J, Cheng BYL, Ma MKF, Lee JMF, Ng JKY, Chai S, Lin CH, Tsang SY, Ma S, Ng IOL, Lee TKW, Cancer-associated fibroblasts regulate tumor-initiating cell plasticity in hepatocellular carcinoma through c-Met/FRA1/ HEY1 signaling, Cell Rep. 15 (2016) 1175–1189, 10.1016/j.celrep.2016.04.019. [DOI] [PubMed] [Google Scholar]
- [75].Sun Y, Fan X, Zhang Q, Shi X, Xu G, Zou C, Cancer-associated fibroblasts secrete FGF-1 to promote ovarian proliferation, migration, and invasion through the activation of FGF-1/FGFR4 signaling, Tumor Biol. 39 (2017) 1–10, 10.1177/1010428317712592. [DOI] [PubMed] [Google Scholar]
- [76].Orimo A, Gupta PB, Sgroi DC, Arenzana-Seisdedos F, Delaunay T, Naeem R, Carey VJ, Richardson AL, Weinberg RA, Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion, Cell 121 (2005) 335–348, 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- [77].Wu X, Tao P, Zhou Q, Li J, Yu Z, Wang X, Li J, Li C, Yan M, Zhu Z, Liu B, Su L, IL-6 secreted by cancer-associated fibroblasts promotes epithelialmesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway, Oncotarget 8 (2017) 20741–20750, 10.18632/oncotarget.15119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wen S, Hou Y, Fu L, Xi L, Yang D, Zhao M, Qin Y, Sun K, Teng Y, Liu M, Cancer-associated fibroblast (CAF)-derived IL32 promotes breast cancer cell invasion and metastasis via integrin β3–p38 MAPK signalling, Cancer Lett. 442 (2019) 320–332, 10.1016/j.canlet.2018.10.015. [DOI] [PubMed] [Google Scholar]
- [79].Mitra AK, Zillhardt M, Hua Y, Tiwari P, Murmann AE, Peter ME, Lengyel E, MicroRNAs reprogram normal fibroblasts into cancer-associated fibroblasts in ovarian cancer, Cancer Discov. 2 (2012) 1100–1108, 10.1158/2159-8290.CD-12-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Soon PSH, Kim E, Pon CK, Gill AJ, Moore K, Spillane AJ, Benn DE, Baxter RC, Breast cancer-associated fibroblasts induce epithelial-to-mesenchymal transition in breast cancer cells, Endocr. -Relat. Cancer 20 (2013) 1–12, 10.1530/ERC-12-0227. [DOI] [PubMed] [Google Scholar]
- [81].Yu B, Chen X, Li J, Qu Y, Su L, Peng Y, Huang J, Yan J, Yu Y, Gu Q, Zhu Z, Liu B, Stromal fibroblasts in the microenvironment of gastric carcinomas promote tumor metastasis via upregulating TAGLN expression, BMC Cell Biol. 14 (2013), 10.1186/1471-2121-14-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Giannoni E, Bianchini F, Masieri L, Serni S, Torre E, Calorini L, Chiarugi P, Reciprocal activation of prostate cancer cells and cancer-associated fibroblasts stimulates epithelial-mesenchymal transition and cancer stemness, Cancer Res. 70 (2010) 6945–6956, 10.1158/0008-5472.CAN-10-0785. [DOI] [PubMed] [Google Scholar]
- [83].Luga V, Zhang L, Viloria-Petit AM, Ogunjimi AA, Inanlou MR, Chiu E, Buchanan M, Hosein AN, Basik M, Wrana JL, Exosomes mediate stromal mobilization of autocrine Wnt-PCP signaling in breast cancer cell migration, Cell 151 (2012) 1542–1556, 10.1016/j.cell.2012.11.024. [DOI] [PubMed] [Google Scholar]
- [84].Donnarumma E, Fiore D, Nappa M, Roscigno G, Adamo A, Iaboni M, Russo V, Affinito A, Puoti I, Quintavalle C, Rienzo A, Piscuoglio S, Thomas R, Condorelli G, Cancer-associated fibroblasts release exosomal microRNAs that dictate an aggressive phenotype in breast cancer, Oncotarget 8 (2017) 19592–19608, 10.18632/oncotarget.14752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Zhang J, Tian XJ, Xing J, Signal transduction pathways of EMT induced by TGF-β, SHH, and WNT and their crosstalks, J. Clin. Med 5 (2016) 1–18, 10.3390/jcm5040041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zhuang J, Lu Q, Shen B, Huang X, Shen L, Zheng X, Huang R, Yan J, Guo H, TGFβ1 secreted by cancer-associated fibroblasts induces epithelial-mesenchymal transition of bladder cancer cells through lncRNA-ZEB2NAT, Sci. Rep 5 (2015) 1–13, 10.1038/srep11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Dongre A, Weinberg RA, New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer, Nat. Rev. Mol. Cell Biol 20 (2019), 10.1038/s41580-018-0080-4. [DOI] [PubMed] [Google Scholar]
- [88].Hua W, ten Dijke P, Kostidis S, Giera M, Hornsveld M, TGFβ-induced metabolic reprogramming during epithelial-to-mesenchymal transition in cancer, Cell. Mol. Life Sci 77 (2020) 2103–2123, 10.1007/s00018-019-03398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Derynck R, Turley SJ, Akhurst RJ, TGFβ biology in cancer progression and immunotherapy, Nat. Rev. Clin. Oncol 18 (2021) 9–34, 10.1038/s41571-020-0403-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Goulet CR, Champagne A, Bernard G, Vandal D, Chabaud S, Pouliot F, Bolduc S, Cancer-associated fibroblasts induce epithelial-mesenchymal transition of bladder cancer cells through paracrine IL-6 signalling, BMC Cancer 19 (2019) 1–13, 10.1186/s12885-019-5353-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Jia C, Wang G, Wang T, Fu B, Zhang Y, Huang L, Deng Y, Chen G, Wu X, Chen J, Pan Y, Tai Y, Liang J, Li X, Hu K, Xie B, Li S, Yang Y, Chen G, Zhang Q, Liu W, Cancer-associated fibroblasts induce epithelial-mesenchymal transition via the transglutaminase 2-dependent il-6/il6r/stat3 axis in hepatocellular carcinoma, Int. J. Biol. Sci 16 (2020) 2542–2558, 10.7150/ijbs.45446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Wang L, Zhang F, Cui JY, Chen L, Chen YT, Liu BW, CAFs enhance paclitaxel resistance by inducing EMT through the IL‑6/JAK2/STAT3 pathway, Oncol. Rep 39 (2018) 2081–2090, 10.3892/or.2018.6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra A, Joseph J, Berry JE, McGee S, Lee E, Sun H, Wang J, Jin T, Zhang H, Dai J, Krebsbach PH, Keller ET, Pienta KJ, Taichman RS, Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis, Nat. Commun 4 (2013) 1–11, 10.1038/ncomms2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Li D, Qu C, Ning Z, Wang H, Zang K, Zhuang L, Chen L, Wang P, Meng Z, Radiation promotes epithelial-to-mesenchymal transition and invasion of pancreatic cancer cell by activating carcinoma-associated fibroblasts, Am. J. Cancer Res 6 (2016) 2192–2206. [PMC free article] [PubMed] [Google Scholar]
- [95].Öhlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ, Chio IIC, Hwang CI, Tiriac H, Baker LA, Engle DD, Feig C, Kultti A, Egeblad M, Fearon DT, Crawford JM, Clevers H, Park Y, Tuveson DA, Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer, J. Exp. Med 214 (2017) 579–596, 10.1084/jem.20162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Costa A, Kieffer Y, Scholer-dahirel A, Soumelis V, Vincent-salomon A, Costa A, Kieffer Y, Scholer-dahirel A, Pelon F, Bourachot B, Cardon M, Fibroblast heterogeneity and immunosuppressive environment in human breast cancer article fibroblast heterogeneity and immunosuppressive environment in human breast cancer, e10, Cancer Cell 33 (2018) 463–479, 10.1016/j.ccell.2018.01.011. [DOI] [PubMed] [Google Scholar]
- [97].Mosa MH, Michels BE, Menche C, Nicolas AM, Darvishi T, Greten FR, Farin HF, A wnt-induced phenotypic switch in cancer-associated fibroblasts inhibits EMT in colorectal cancer, Cancer Res. 80 (2020) 5569–5582, 10.1158/0008-5472.CAN-20-0263. [DOI] [PubMed] [Google Scholar]
- [98].Lengyel E, Makowski L, DiGiovanni J, Kolonin MG, Cancer as a matter of fat: the crosstalk between adipose tissue and tumors, Trends Cancer 4 (2018) 374–384, 10.1016/j.trecan.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Walter M, Liang S, Ghosh S, Hornsby PJ, Li R, Interleukin 6 secreted from adipose stromal cells promotes migration and invasion of breast cancer cells, 2009 28:30. 28, Oncogene (2009) 2745–2755, 10.1038/onc.2009.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, Wang YY, Meulle A, Salles B, Le Gonidec S, Garrido I, Escourrou G, Valet P, Muller C, Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion, Cancer Res. 71 (2011) 2455–2465, 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- [101].Gyamfi J, Lee YH, Eom M, Choi J, Interleukin-6/STAT3 signalling regulates adipocyte induced epithelial-mesenchymal transition in breast cancer cells, 2018 8:1. 8, Sci. Rep (2018) 1–13, 10.1038/s41598-018-27184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Wei L, Li K, Pang X, Guo B, Su M, Huang Y, Wang N, Ji F, Zhong C, Yang J, Zhang Z, Jiang Y, Liu Y, Chen T, Leptin promotes epithelial-mesenchymal transition of breast cancer via the upregulation of pyruvate kinase M2, J. Exp. Clin. Cancer Res. CR 35 (2016), 10.1186/S13046-016-0446-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Al-Khalaf HH, Amir M, Al-Mohanna F, Tulbah A, Al-Sayed A, Aboussekhra A, Obesity and p16 INK4A downregulation activate breast adipocytes and promote their protumorigenicity, Mol. Cell. Biol 37 (2017), 10.1128/mcb.00101-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Haque I, Ghosh A, Acup S, Banerjee S, Dhar K, Ray A, Sarkar S, Kambhampati S, Banerjee SK, Leptin-induced ER-α-positive breast cancer cell viability and migration is mediated by suppressing CCN5-signaling via activating JAK/AKT/STAT-pathway, BMC Cancer 18 (2018) 1–14, 10.1186/S12885-018-3993-6/FIGURES/7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Park J, Scherer PE, Adipocyte-derived endotrophin promotes malignant tumor progression, J. Clin. Investig 122 (2012) 4243–4256, 10.1172/JCI63930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Nie J, Zhang J, Wang L, Lu L, Yuan Q, An F, Zhang S, Jiao Y, Adipocytes promote cholangiocarcinoma metastasis through fatty acid binding protein 4, J. Exp. Clin. Cancer Res 36 (2017) 1–15, 10.1186/S13046-017-0641-Y/FIGURES/9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Xu Q, Wang L, Li H, Han Q, Li J, Qu X, Huang S, Zhao RC, Mesenchymal stem cells play a potential role in regulating the establishment and maintenance of epithelial-mesenchymal transition in MCF7 human breast cancer cells by paracrine and induced autocrine TGF-β, Int. J. Oncol 41 (2012) 959–968, 10.3892/ijo.2012.1541. [DOI] [PubMed] [Google Scholar]
- [108].Wu S, Wang Y, Yuan Z, Wang S, Du H, Liu X, Wang Q, Zhu X, Human adipose-derived mesenchymal stem cells promote breast cancer MCF7 cell epithelial-mesenchymal transition by cross interacting with the TGF-β/Smad and PI3K/AKT signaling pathways, Mol. Med. Rep 19 (2019) 177–186, 10.3892/mmr.2018.9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].D’esposito V, Liguoro D, Ambrosio MR, Collina F, Cantile M, Spinelli R, Raciti GA, Miele C, Valentino R, Campiglia P, De Laurentiis M, Di Bonito M, Botti G, Franco R, Beguinot F, Formisano P, Adipose microenvironment promotes triple negative breast cancer cell invasiveness and dissemination by producing CCL5, n.d. 〈www.impactjournals.com/oncotarget/〉. [DOI] [PMC free article] [PubMed]
- [110].Song X, Zhou X, Qin Y, Yang J, Wang Y, Sun Z, Yu K, Zhang S, Liu S, Emodin inhibits epithelial-mesenchymal transition and metastasis of triple negative breast cancer via antagonism of CC-chemokine ligand 5 secreted from adipocytes, Int. J. Mol. Med 42 (2018) 579–588, 10.3892/ijmm.2018.3638. [DOI] [PubMed] [Google Scholar]
- [111].Su F, Daquinag AC, Ahn S, Saha A, Dai Y, Zhao Z, DiGiovanni J, Kolonin MG, Progression of prostate carcinoma is promoted by adipose stromal cell-secreted CXCL12 signaling in prostate epithelium, npj Precis. Oncol 5 (2021), 10.1038/s41698-021-00160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Yan D, Avtanski D, Saxena NK, Sharma D, Leptin-induced epithelialmesenchymal transition in breast cancer cells requires β-catenin activation via Akt/GSK3- and MTA1/Wnt1 protein-dependent pathways, J. Biol. Chem 287 (2012) 8598–8612, 10.1074/jbc.M111.322800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Chang CC, Wu MJ, Yang JY, Camarillo IG, Chang CJ, Leptin-STAT3-G9a signaling promotes obesity-mediated breast cancer progression, Cancer Res. 75 (2015) 2375–2386, 10.1158/0008-5472.CAN-14-3076. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- [114].Villanueva-Duque A, Daniela Zuniga-Eulogio M, Dena-Beltran J, Castaneda Saucedo E, Calixto-Galvez M, Mendoza-Catalán MA, Ortuno-Pineda C, Navarro-Tito N, Leptin induces partial epithelial-mesenchymal transition in a FAKERK dependent pathway in MCF10A mammary non-tumorigenic cells, 2017.〈www.ijcep.com/〉. [PMC free article] [PubMed]
- [115].Olea-Flores M, Juárez-Cruz JC, Mendoza-Catalán MA, Padilla-Benavides T, Navarro-Tito N, Signaling pathways induced by leptin during epithelial–mesenchymal transition in breast cancer, Int. J. Mol. Sci Vol. 19 (2018) 3493, 10.3390/IJMS19113493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Jin J, Zhang Z, Zhang S, Chen X, Chen Z, Hu P, Wang J, Xie C, Fatty acid binding protein 4 promotes epithelial-mesenchymal transition in cervical squamous cell carcinoma through AKT/GSK3β/Snail signaling pathway, Mol. Cell. Endocrinol 461 (2018) 155–164, 10.1016/j.mce.2017.09.005. [DOI] [PubMed] [Google Scholar]
- [117].Xie Q, Ding J, Chen Y, Role of CD8+ T lymphocyte cells: Interplay with stromal cells in tumor microenvironment, Acta Pharm. Sin. B 11 (2021) 1365–1378, 10.1016/j.apsb.2021.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Hiraoka K, Miyamoto M, Cho Y, Suzuoki M, Oshikiri T, Nakakubo Y, Itoh T, Ohbuchi T, Kondo S, Katoh H, Concurrent infiltration by CD8+ T cells and CD4 + T cells is a favourable prognostic factor in non-small-cell lung carcinoma, Br. J. Cancer 94 (2006) 275–280, 10.1038/sj.bjc.6602934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay NEH, Mosseri V, Laccourreye O, Bruneval P, Fridman WH, Brasnu DF, Tartour E, Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers, Clin. Cancer Res 12 (2006) 465–472, 10.1158/1078-0432.CCR-05-1886. [DOI] [PubMed] [Google Scholar]
- [120].Ali HR, Provenzano E, Dawson SJ, Blows FM, Liu B, Shah M, Earl HM, Poole CJ, Hiller L, Dunn JA, Bowden SJ, Twelves C, Bartlett JMS, Mahmoud SMA, Rakha E, Ellis IO, Liu S, Gao D, Nielsen TO, Pharoah PDP, Caldas C, Association between CD8+ T-cell infiltration and breast cancer survival in 12 439 patients, Ann. Oncol 25 (2014) 1536–1543, 10.1093/annonc/mdu191. [DOI] [PubMed] [Google Scholar]
- [121].Xu X, Tan Y, Qian Y, Xue W, Wang Y, Du J, Jin L, Ding W, Clinicopathologic and prognostic significance of tumor-infiltrating CD8+ T cells in patients with hepatocellular carcinoma: a meta-analysis, Medicine 98 (2019), 10.1097/MD.0000000000013923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Oh DY, Kwek SS, Raju SS, Li T, McCarthy E, Chow E, Aran D, Ilano A, Pai CCS, Rancan C, Allaire K, Burra A, Sun Y, Spitzer MH, Mangul S, Porten S, Meng MV, Friedlander TW, Ye CJ, Fong L, Intratumoral CD4+ T cells mediate anti-tumor cytotoxicity in human bladder cancer, e13, Cell 181 (2020) 1612–1625, 10.1016/j.cell.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Santisteban M, Reiman JM, Asiedu MK, Behrens MD, Nassar A, Kalli KR, Haluska P, Ingle JN, Hartmann LC, Manjili MH, Radisky DC, Ferrone S, Knutson KL, Immune-induced epithelial to mesenchymal transition in vivo generates breast cancer stem cells, Cancer Res. 69 (2009) 2887–2895, 10.1158/0008-5472.CAN-08-3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Goebel L, Grage-Griebenow E, Gorys A, Helm O, Genrich G, Lenk L, Wesch D, Ungefroren H, Freitag-Wolf S, Sipos B, Röcken C, Schäfer H, Sebens S, CD4+ T cells potently induce epithelialmesenchymal- transition in premalignant and malignant pancreatic ductal epithelial cells–novel implications of CD4+ T cells in pancreatic cancer development, OncoImmunology 4 (2015), 10.1080/2162402X.2014.1000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Salazar Y, Zheng X, Brunn D, Raifer H, Picard F, Zhang Y, Winter H, Guenther S, Weigert A, Weigmann B, Dumoutier L, Renauld JC, Waisman A, Schmall A, Tufman A, Fink L, Brüne B, Bopp T, Grimminger F, Seeger W, Pullamsetti SS, Huber M, Savai R, Microenvironmental Th9 and Th17 lymphocytes induce metastatic spreading in lung cancer, J. Clin. Investig 130 (2020) 3560–3575, 10.1172/JCI124037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Xiong S, Pan X, Xu L, Yang Z, Guo R, Gu Y, Li R, Wang Q, Xiao F, Du L, Zhou P, Zhu M, Regulatory T cells promote β-catenin-mediated epithelium-tomesenchyme transition during radiation-induced pulmonary fibrosis, Int. J. Radiat. Oncol. Biol. Phys 93 (2015) 425–435, 10.1016/j.ijrobp.2015.05.043. [DOI] [PubMed] [Google Scholar]
- [127].Shi C, Chen Y, Chen Y, Yang Y, Bing W, Qi J, Cd4 + cd25 + regulatory t cells promote hepatocellular carcinoma invasion via tgf-β1-induced epithelial–mesenchymal transition, OncoTargets Ther 12 (2019) 279–289, 10.2147/OTT.S172417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Oh E, Hong J, Yun C-O, Regulatory T cells induce metastasis by increasing Tgf- β and enhancing the epithelial–mesenchymal transition, Cells 8 (2019) 1387, 10.3390/cells8111387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Yang J, Li Y, Sun Z, Zhan H, Macrophages in pancreatic cancer: An immunometabolic perspective, Cancer Lett. 498 (2021) 188–200, 10.1016/j.canlet.2020.10.029. [DOI] [PubMed] [Google Scholar]
- [130].Tan Y, Wang M, Zhang Y, Ge S, Zhong F, Xia G, Sun C, Tumor-associated macrophages: a potential target for cancer therapy, Front. Oncol 11 (2021) 1–17, 10.3389/fonc.2021.693517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Qian BZ, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, Kaiser EA, Snyder LA, Pollard JW, CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis, Nature 475 (2011) 222–225, 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Su S, Liu Q, Chen J, Chen J, Chen F, He C, Huang D, Wu W, Lin L, Huang W, Zhang J, Cui X, Zheng F, Li H, Yao H, Su F, Song E, A Positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis, Cancer Cell 25 (2014) 605–620, 10.1016/j.ccr.2014.03.021. [DOI] [PubMed] [Google Scholar]
- [133].Fan QM, Jing YY, Yu GF, Kou XR, Ye F, Gao L, Li R, Zhao QD, Yang Y, Lu ZH, Wei LX, Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma, Cancer Lett 352 (2014) 160–168, 10.1016/j.canlet.2014.05.008. [DOI] [PubMed] [Google Scholar]
- [134].Fu XT, Dai Z, Song K, Zhang ZJ, Zhou ZJ, Zhou SL, Zhao YM, Xiao YS, Sun QM, Ding ZB, Fan J, Macrophage-secreted IL-8 induces epithelialmesenchymal transition in hepatocellular carcinoma cells by activating the JAK2/STAT3/Snail pathway, Int. J. Oncol 46 (2015) 587–596, 10.3892/ijo.2014.2761. [DOI] [PubMed] [Google Scholar]
- [135].Yeung OWH, Lo CM, Ling CC, Qi X, Geng W, Li CX, Ng KTP, Forbes SJ, Guan XY, Poon RTP, Fan ST, Man K, Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma, J. Hepatol 62 (2015) 607–616, 10.1016/j.jhep.2014.10.029. [DOI] [PubMed] [Google Scholar]
- [136].Zhang J, Yan Y, Yang Y, Wang L, Li M, Wang J, Liu X, Duan X, Wang J, High infiltration of tumor-associated macrophages influences poor prognosis in human gastric cancer patients, associates with the phenomenon of EMT, Medicine 95 (2016) 1–6, 10.1097/MD.0000000000002636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Gao L, Zhang W, Zhong WQ, Liu ZJ, Li HM, Yu ZL, Zhao YF, Tumor associated macrophages induce epithelial to mesenchymal transition via the EGFR/ERK1/2 pathway in head and neck squamous cell carcinoma, Oncol. Rep 40 (2018) 2558–2572, 10.3892/or.2018.6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Gao S, Hu J, Wu X, Liang Z, PMA treated THP-1-derived-IL-6 promotes EMT of SW48 through STAT3/ERK-dependent activation of Wnt/β-catenin signaling pathway, Biomed. Pharmacother 108 (2018) 618–624, 10.1016/j.biopha.2018.09.067. [DOI] [PubMed] [Google Scholar]
- [139].Zhang Q, Mao Z, Sun J, NF-κB inhibitor, BAY11–7082, suppresses M2 tumor-associated macrophage induced EMT potential via miR-30a/NF-κB/Snail signaling in bladder cancer cells, Gene 710 (2019) 91–97, 10.1016/j.gene.2019.04.039. [DOI] [PubMed] [Google Scholar]
- [140].Xie Y, Chen Z, Zhong Q, Zheng Z, Chen Y, Shangguan W, Zhang Y, Yang J, Zhu D, Xie W, M2 macrophages secrete CXCL13 to promote renal cell carcinoma migration, invasion, and EMT, Cancer Cell Int. 21 (2021) 1–13, 10.1186/s12935-021-02381-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Bonde AK, Tischler V, Kumar S, Soltermann A, Schwendener RA, Intratumoral macrophages contribute to epithelial-mesenchymal transition in solid tumors, BMC Cancer 12 (2012), 10.1186/1471-2407-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Bates RC*, Mercurio AM, Tumor necrosis factor-α stimulates the epithelial-tomesenchymal transition of human colonic organoids, Mol. Biol. Cell 14 (2003) 1790–1800, 10.1091/mbc.E02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Dehai C, Bo P, Qiang T, Lihua S, Fang L, Shi J, Jingyan C, Yan Y, Guangbin W, Zhenjun Y, Enhanced invasion of lung adenocarcinoma cells after co-culture with THP-1-derived macrophages via the induction of EMT by IL-6, Immunol. Lett 160 (2014) 1–10, 10.1016/j.imlet.2014.03.004. [DOI] [PubMed] [Google Scholar]
- [144].Liu CY, Xu JY, Shi XY, Huang W, Ruan TY, Xie P, Ding JL, M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway, Lab. Investig 93 (2013) 844–854, 10.1038/labinvest.2013.69. [DOI] [PubMed] [Google Scholar]
- [145].Ding J, Yang C, Zhang Y, Wang J, Zhang S, Guo D, Yin T, Yang J, M2 macrophage-derived G-CSF promotes trophoblasts EMT, invasion and migration via activating PI3K/Akt/Erk1/2 pathway to mediate normal pregnancy, J. Cell. Mol. Med 25 (2021) 2136–2147, 10.1111/jcmm.16191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Kawata M, Koinuma D, Ogami T, Umezawa K, Iwata C, Watabe T, Miyazono K, TGF-β-induced epithelial-mesenchymal transition of A549 lung adenocarcinoma cells is enhanced by pro-inflammatory cytokines derived from RAW 264.7 macrophage cells, J. Biochem 151 (2012) 205–216, 10.1093/jb/mvr136. [DOI] [PubMed] [Google Scholar]
- [147].Zhu L, Fu X, Chen X, Han X, Dong P, M2 macrophages induce EMT through the TGF-β/Smad2 signaling pathway, Cell Biol. Int 41 (2017) 960–968, 10.1002/cbin.10788. [DOI] [PubMed] [Google Scholar]
- [148].Che D, Zhang S, Jing Z, Shang L, Jin S, Liu F, Shen J, Li Y, Hu J, Meng Q, Yu Y, Macrophages induce EMT to promote invasion of lung cancer cells through the IL-6-mediated COX-2/PGE2/β-catenin signalling pathway, Mol. Immunol 90 (2017) 197–210, 10.1016/j.molimm.2017.06.018. [DOI] [PubMed] [Google Scholar]
- [149].Wei C, Yang C, Wang S, Shi D, Zhang C, Lin X, Liu Q, Dou R, Xiong B, Crosstalk between cancer cells and tumor associated macrophages is required for mesenchymal circulating tumor cell-mediated colorectal cancer metastasis, Mol. Cancer 18 (2019) 1–23, 10.1186/s12943-019-0976-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Safarzadeh E, Orangi M, Mohammadi H, Babaie F, Baradaran B, Myeloid-derived suppressor cells: important contributors to tumor progression and metastasis, J. Cell. Physiol 233 (2018) 3024–3036, 10.1002/jcp.26075. [DOI] [PubMed] [Google Scholar]
- [151].Kumar V, Patel S, Tcyganov E, Gabrilovich DI, The nature of myeloid-derived suppressor cells in the tumor microenvironment, Trends Immunol. 37 (2016) 208–220, 10.1016/j.it.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, Kato M, PrevostBlondel A, Thiery JP, Abastado JP, Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor, PLoS Biol. 9 (2011), 10.1371/journal.pbio.1001162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Panni RZ, Sanford DE, Belt BA, Mitchem JB, Worley LA, Goetz BD, Mukherjee P, Wang-Gillam A, Link DC, Denardo DG, Goedegebuure SP, Linehan DC, Tumor-induced STAT3 activation in monocytic myeloid-derived suppressor cells enhances stemness and mesenchymal properties in human pancreatic cancer, Cancer Immunol., Immunother 63 (2014) 513–528, 10.1007/s00262-014-1527-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Li ZL, Ye SB, OuYang LY, Zhang H, Chen YS, He J, Chen QY, Qian CN, Zhang XS, Cui J, Zeng YX, Li J, COX-2 promotes metastasis in nasopharyngeal carcinoma by mediating interactions between cancer cells and myeloid-derived suppressor cells, OncoImmunology 4 (2015), 10.1080/2162402X.2015.1044712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Ouzounova M, Lee E, Piranlioglu R, El Andaloussi A, Kolhe R, Demirci MF, Marasco D, Asm I, Chadli A, Hassan KA, Thangaraju M, Zhou G, Arbab AS, Cowell JK, Korkaya H, Monocytic and granulocytic myeloid derived suppressor cells differentially regulate spatiotemporal tumour plasticity during metastatic cascade, Nat. Commun 8 (2017), 10.1038/ncomms14979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Fu X-G, Deng J, Xu W-J, Chen J-Y, Sun J, Deng H, Histidine decarboxylase-expressing PMN-MDSC-derived TGF-β1 promotes the epithelial-mesenchymal transition of metastatic lung adenocarcinoma, Int. J. Clin. Exp. Pathol 13 (2020) 1361–1371. [PMC free article] [PubMed] [Google Scholar]
- [157].Peng D, Tanikawa T, Li W, Zhao L, Vatan L, Szeliga W, Wan S, Wei S, Wang Y, Liu Y, Staroslawska E, Szubstarski F, Rolinski J, Grywalska E, Stanisławek A, Polkowski W, Kurylcio A, Kleer C, Chang AE, Wicha M, Sabel M, Zou W, Kryczek I, Myeloid-derived suppressor cells endow stem-like qualities to breast cancer cells through IL6/STAT3 and NO/NOTCH cross-talk signaling, Cancer Res. 76 (2016) 3156–3165, 10.1158/0008-5472.CAN-15-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Zhu H, Gu Y, Xue Y, Yuan M, Cao X, Liu Q, CXCR2+ MDSCs promote breast cancer progression by inducing EMT and activated T cell exhaustion, Oncotarget 8 (2017) 114554–114567, 10.18632/oncotarget.23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Mucha J, Majchrzak K, Taciak B, Hellmén E, Król M, MDSCs mediate angiogenesis and predispose canine mammary tumor cells for metastasis via IL-28/IL-28RA (IFN-λ) signaling, PLoS ONE 9 (2014) 1–11, 10.1371/journal.pone.0103249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Ma X, Wang M, Yin T, Zhao Y, Wei X, Myeloid-derived suppressor cells promote metastasis in breast cancer after the stress of operative removal of the primary cancer, Front. Oncol 9 (2019) 1–11, 10.3389/fonc.2019.00855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Lin S, Zhang X, Huang G, Cheng L, Lv J, Zheng D, Lin S, Wang S, Wu Q, Long Y, Li B, Wei W, Liu P, Pei D, Li Y, Wen Z, Cui S, Li P, Sun X, Wu Y, Yao Y, Myeloid-derived suppressor cells promote lung cancer metastasis by CCL11 to activate ERK and AKT signaling and induce epithelial-mesenchymal transition in tumor cells, Oncogene 40 (2021) 1476–1489, 10.1038/s41388-020-01605-4. [DOI] [PubMed] [Google Scholar]
- [162].Luo A, Meng M, Wang G, Han R, Zhang Y, Jing X, Zhao L, Gu S, Zhao X, Myeloid-derived suppressor cells recruited by chemokine (C-c motif) ligand 3 promote the progression of breast cancer via phosphoinositide 3-kinase-protein kinase b-mammalian target of rapamycin signaling, J. Breast Cancer 23 (2020) 141–161, 10.4048/jbc.2020.23.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Ikeda H, Kakeya H, Targeting hypoxia-inducible factor 1 (HIF-1) signaling with natural products toward cancer chemotherapy, J. Antibiot 74 (2021) 687–695, 10.1038/s41429-021-00451-0. [DOI] [PubMed] [Google Scholar]
- [164].Hapke RY, Haake SM, Hypoxia-induced epithelial to mesenchymal transition in cancer, Cancer Lett. 487 (2020) 10–20, 10.1016/j.canlet.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Imai T, Horiuchi A, Wang C, Oka K, Ohira S, Nikaido T, Konishi I, Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells, Am. J. Pathol 163 (2003) 1437–1447, 10.1016/S0002-9440(10)63501-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Luo D, Wang J, Li J, Post M, Mouse snail is a target gene for HIF, Mol. Cancer Res 9 (2011) 234–245, 10.1158/1541-7786.MCR-10-0214. [DOI] [PubMed] [Google Scholar]
- [167].Zhang L, Huang G, Li X, Zhang Y, Jiang Y, Shen J, Liu J, Wang Q, Zhu J, Feng X, Dong J, Qian C, Hypoxia induces epithelial-mesenchymal transition via activation of SNAI1 by hypoxia-inducible factor −1α in hepatocellular carcinoma, BMC Cancer 13 (2013) 24–27, 10.1186/1471-2407-13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Zhu GH, Huang C, Feng ZZ, Lv XH, Qiu ZJ, Hypoxia-induced snail expression through transcriptional regulation by HIF-1α in pancreatic cancer cells, Dig. Dis. Sci 58 (2013) 3503–3515, 10.1007/s10620-013-2841-4. [DOI] [PubMed] [Google Scholar]
- [169].Xu X, Tan X, Tampe B, Sanchez E, Zeisberg M, Zeisberg EM, Snail Is a direct target of hypoxia-inducible factor 1α (HIF1α) in hypoxia-induced endothelial to mesenchymal transition of human coronary endothelial cells, J. Biol. Chem 290 (2015) 16553–16664, 10.1074/jbc.M115.636944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Zhang J, Cheng Q, Zhou Y, Wang Y, Chen X, Slug is a key mediator of hypoxia induced cadherin switch in HNSCC: Correlations with poor prognosis, Oral. Oncol 49 (2013) 1043–1050, 10.1016/j.oraloncology.2013.08.003. [DOI] [PubMed] [Google Scholar]
- [171].Lester RD, Jo M, Montel V, Takimoto S, Gonias SL, uPAR induces epithelialmesenchymal transition in hypoxic breast cancer cells, J. Cell Biol 178 (2007) 425–436, 10.1083/jcb.200701092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Choi BJ, Park SA, Lee SY, Cha YN, Surh YJ, Hypoxia induces epithelialmesenchymal transition in colorectal cancer cells through ubiquitin-specific protease 47-mediated stabilization of Snail: a potential role of Sox9, Sci. Rep 7 (2017) 1–15, 10.1038/s41598-017-15139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U, Notch signaling mediates hypoxia-induced tumor cell migration and invasion, Proc. Natl. Acad. Sci. USA 105 (2008) 6392–6397, 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Chen J, Imanaka N, Chen J, Griffin JD, Hypoxia potentiates Notch signaling in breast cancer leading to decreased E-cadherin expression and increased cell migration and invasion, Br. J. Cancer 102 (2010) 351–360, 10.1038/sj.bjc.6605486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Cannito S, Novo E, Compagnone A, di Bonzo LV, Busletta C, Zamara E, Paternostro C, Povero D, Bandino A, Bozzo F, Cravanzola C, Bravoco V, Colombatto S, Parola M, Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal transition in cancer cells, Carcinogenesis 29 (2008) 2267–2278, 10.1093/carcin/bgn216. [DOI] [PubMed] [Google Scholar]
- [176].Huang J, Yao X, Zhang J, Dong B, Chen Q, Xue W, Liu D, Huang Y, Hypoxia-induced downregulation of miR-30c promotes epithelial-mesenchymal transition in human renal cell carcinoma, Cancer Sci. 104 (2013) 1609–1617, 10.1111/cas.12291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, Teng SC, Wu KJ, Direct regulation of TWIST by HIF-1α promotes metastasis, Nat. Cell Biol 10 (2008) 295–305, 10.1038/ncb1691. [DOI] [PubMed] [Google Scholar]
- [178].Gort EH, Van Haaften G, Verlaan I, Groot AJ, Plasterk RHA, Shvarts A, Suijkerbuijk KPM, Van Laar T, Van Der Wall E, Raman V, Van Diest PJ, Tijsterman M, Vooijs M, The TWIST1 oncogene is a direct target of hypoxiainducible factor-2α, Oncogene 27 (2008) 1501–1510, 10.1038/sj.onc.1210795. [DOI] [PubMed] [Google Scholar]
- [179].Hung JJ, Yang MH, Hsu HS, Hsu WH, Liu JS, Wu KJ, Prognostic significance of hypoxia-inducible factor-1α, TWIST1 and Snail expression in resectable non-small cell lung cancer, Thorax 64 (2009) 1082–1089, 10.1136/thx.2009.115691. [DOI] [PubMed] [Google Scholar]
- [180].Zhang W, Shi X, Peng Y, Wu M, Zhang P, Xie R, Wu Y, Yan Q, Liu S, Wang J, HIF-1α promotes epithelial-mesenchymal transition and metastasis through direct regulation of ZEB1 in colorectal cancer, PLoS One 10 (2015) 1–16, 10.1371/journal.pone.0129603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Kahlert UD, Suwala AK, Raabe EH, Siebzehnrubl FA, Suarez MJ, Orr BA, Bar EE, Maciaczyk J, Eberhart CG, ZEB1 promotes invasion in human fetal neural stem cells and hypoxic glioma neurospheres, Brain Pathol 25 (2015) 724–732, 10.1111/bpa.12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Joseph JV, Conroy S, Pavlov K, Sontakke P, Tomar T, Eggens-Meijer E, Balasubramaniyan V, Wagemakers M, den Dunnen WFA, Kruyt FAE, Hypoxia enhances migration and invasion in glioblastoma by promoting a mesenchymal shift mediated by the HIF1α-ZEB1 axis, Cancer Lett. 359 (2015) 107–116, 10.1016/j.canlet.2015.01.010. [DOI] [PubMed] [Google Scholar]
- [183].Van Den Beucken T, Koch E, Chu K, Rupaimoole R, Prickaerts P, Adriaens M, Voncken JW, Harris AL, Buffa FM, Haider S, Starmans MHW, Yao CQ, Ivan M, Ivan C, Pecot CV, Boutros PC, Sood AK, Koritzinsky M, Wouters BG, Hypoxia promotes stem cell phenotypes and poor prognosis through epigenetic regulation of DICER, Nat. Commun 5 (2014), 10.1038/ncomms6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Su L, Luo Y, Yang Z, Yang J, Yao C, Cheng F, Shan J, Chen J, Li F, Liu L, Liu C, Xu Y, Jiang L, Guo D, Prieto J, Avila MA, Shen J, Qian C, MEF2D transduces microenvironment stimuli to ZEB1 to promote epithelial-mesenchymal transition and metastasis in colorectal cancer, Cancer Res. 76 (2016) 5054–5067, 10.1158/0008-5472.CAN-16-0246. [DOI] [PubMed] [Google Scholar]
- [185].Deng SJ, Chen HY, Ye Z, Deng SC, Zhu S, Zeng Z, He C, Liu ML, Huang K, Zhong JX, Xu FY, Li Q, Liu Y, Wang CY, Zhao G, Hypoxia-induced LncRNA-bx111 promotes metastasis and progression of pancreatic cancer through regulating ZEB1 transcription, Oncogene 37 (2018) 5811–5828, 10.1038/s41388-018-0382-1. [DOI] [PubMed] [Google Scholar]
- [186].Zhang S, Wang W, Liu G, Xie S, Li Q, Li Y, Lin Z, Long non-coding RNA HOTTIP promotes hypoxia-induced epithelial-mesenchymal transition of malignant glioma by regulating the miR-101/ZEB1 axis, Biomed. Pharmacother 95 (2017) 711–720, 10.1016/j.biopha.2017.08.133. [DOI] [PubMed] [Google Scholar]
- [187].Nakuluri K, Mukhi D, Nishad R, Saleem MA, Mungamuri SK, Menon RK, Pasupulati AK, Hypoxia induces ZEB2 in podocytes: Implications in the pathogenesis of proteinuria, J. Cell. Physiol 234 (2019) 6503–6518, 10.1002/jcp.27387. [DOI] [PubMed] [Google Scholar]
- [188].Matsuoka J, Yashiro M, Doi Y, Fuyuhiro Y, Kato Y, Shinto O, Noda S, Kashiwagi S, Aomatsu N, Hirakawa T, Hasegawa T, Shimizu K, Shimizu T, Miwa A, Yamada N, Sawada T, Hirakawa K, Hypoxia Stimulates the EMT of Gastric Cancer Cells through Autocrine TGFβ Signaling, PLoS One 8 (2013), 10.1371/journal.pone.0062310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Zhou G, Dada LA, Wu M, Kelly A, Trejo H, Zhou Q, Varga J, Sznajder JI, Hypoxia-induced alveolar epithelial-mesenchymal transition requires mitochondrial ROS and hypoxia-inducible factor 1, Am. J. Physiol. Lung Cell. Mol. Physiol 297 (2009) 1120–1130, 10.1152/ajplung.00007.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Schäffer L, Scheid A, Spielmann P, Breymann C, Zimmermann R, Meuli M, Gassmann M, Marti HH, Wenger RH, Oxygen-regulated expression of TGF-β3, a growth factor involved in trophoblast differentiation, Placenta 24 (2003) 941–950, 10.1016/S0143-4004(03)00166-8. [DOI] [PubMed] [Google Scholar]
- [191].Hung SP, Yang MH, Tseng KF, Lee OK, Hypoxia-induced secretion of TGF-b1 in mesenchymal stem cell promotes breast cancer cell progression, Cell Transplant. 22 (2013) 1869–1882, 10.3727/096368912X657954. [DOI] [PubMed] [Google Scholar]
- [192].Shen X, Xue Y, Si Y, Wang Q, Wang Z, Yuan J, Zhang X, The unfolded protein response potentiates epithelial-to-mesenchymal transition (EMT) of gastric cancer cells under severe hypoxic conditions, Med. Oncol 32 (2015) 1–7, 10.1007/s12032-014-0447-0. [DOI] [PubMed] [Google Scholar]
- [193].Nagpal N, Ahmad HM, Chameettachal S, Sundar D, Ghosh S, Kulshreshtha R, HIF-inducible miR-191 promotes migration in breast cancer through complex regulation of TGFβ-signaling in hypoxic microenvironment, Sci. Rep 5 (2015) 1–14, 10.1038/srep09650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Niessen K, Fu YX, Chang L, Hoodless PA, McFadden D, Karsan A, Slug is a direct Notch target required for initiation of cardiac cushion cellularization, J. Cell Biol 182 (2008) 315–325, 10.1083/jcb.200710067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Cho ES, Kang HE, Kim NH, Yook JI, Therapeutic implications of cancer epithelial-mesenchymal transition EMT, Arch. Pharmacal Res 42 (2019) 14–24, 10.1007/s12272-018-01108-7. [DOI] [PubMed] [Google Scholar]
- [196].Xing F, Okuda H, Watabe M, Kobayashi A, Pai SK, Liu W, Pandey PR, Fukuda K, Hirota S, Sugai T, Wakabayshi G, Koeda K, Kashiwaba M, Suzuki K, Chiba T, Endo M, Mo YY, Watabe K, Hypoxia-induced Jagged2 promotes breast cancer metastasis and self-renewal of cancer stem-like cells, Oncogene 30 (2011) 4075–4086, 10.1038/onc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Ishida T, Hijioka H, Kume K, Miyawaki A, Nakamura N, Notch signaling induces EMT in OSCC cell lines in a hypoxic environment, Oncol. Lett 6 (2013) 1201–1206, 10.3892/ol.2013.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Du R, Sun W, Xia L, Zhao A, Yu Y, Zhao L, Wang H, Huang C, Sun S, Hypoxia-induced down-regulation of microRNA-34a promotes EMT by targeting the Notch signaling pathway in tubular epithelial cells, PLoS One 7 (2012), 10.1371/journal.pone.0030771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Lei J, Fan L, Wei G, Chen X, Duan W, Xu Q, Sheng W, Wang K, Li X, Gli-1 is crucial for hypoxia-induced epithelial-mesenchymal transition and invasion of breast cancer, Tumor Biol 36 (2015) 3119–3126, 10.1007/s13277-014-2948-z. [DOI] [PubMed] [Google Scholar]
- [200].Wang G, Zhang Z, Xu Z, Yin H, Bai L, Ma Z, DeCoster MA, Qian G, Wu G, Activation of the sonic hedgehog signaling controls human pulmonary arterial smooth muscle cell proliferation in response to hypoxia, Biochim. Et. Biophys. Acta Mol. Cell Res 1803 (2010) 1359–1367, 10.1016/j.bbamcr.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Lei J, Ma J, Ma Q, Li X, Liu H, Xu Q, Duan W, Sun Q, Xu J, Wu Z, Wu E, Hedgehog signaling regulates hypoxia induced epithelial to mesenchymal transition and invasion in pancreatic cancer cells via a ligand-independent manner, Mol. Cancer 12 (2013) 1–11, 10.1186/1476-4598-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Tang C, Mei L, Pan L, Xiong W, Zhu H, Ruan H, Zou C, Tang L, Iguchi T, Wu X, Hedgehog signaling through GLI1 and GLI2 is required for epithelialmesenchymal transition in human trophoblasts, Biochim. Et. Biophys. Acta Gen. Subj 1850 (2015) 1438–1448, 10.1016/j.bbagen.2015.04.005. [DOI] [PubMed] [Google Scholar]
- [203].Zheng X, Rumie Vittar NB, Gai X, Fernandez-Barrena MG, Moser CD, Hu C, Almada LL, McCleary-Wheeler AL, Elsawa SF, Vrabel AM, Shire AM, Comba A, Thorgeirsson SS, Kim Y, Liu Q, Fernandez-Zapico ME, Roberts LR, The transcription factor GLI1 mediates TGFβ1 driven EMT in hepatocellular carcinoma via a SNAI1-dependent mechanism, PLoS ONE 7 (2012), 10.1371/journal.pone.0049581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Li J, He Y, Cao Y, Yu Y, Chen X, Gao X, Hu Q, Upregulation of Twist is involved in Gli1 induced migration and invasion of hepatocarcinoma cells, Biol. Chem 399 (2018) 911–919, 10.1515/hsz-2018-0131. [DOI] [PubMed] [Google Scholar]
- [205].Bijlsma MF, Groot AP, Oduro JP, Franken RJ, Schoenmakers SHHF, Peppelenbosch MP, Spek CA, Hypoxia induces a hedgehog response mediated by HIF-1α, J. Cell. Mol. Med 13 (2009) 2053–2060, 10.1111/j.1582-4934.2008.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Bhuria V, Xing J, Scholta T, Bui KC, Nguyen MLT, Malek NP, Bozko P, Plentz RR, Hypoxia induced Sonic Hedgehog signaling regulates cancer stemness, epithelial-to-mesenchymal transition and invasion in cholangiocarcinoma, Exp. Cell Res 385 (2019), 111671, 10.1016/j.yexcr.2019.111671. [DOI] [PubMed] [Google Scholar]
- [207].Liu Z, Tu K, Wang Y, Yao B, Li Q, Wang L, Dou C, Liu Q, Zheng X, Hypoxia accelerates aggressiveness of hepatocellular carcinoma cells involving oxidative stress, epithelial-mesenchymal transition and non-canonical hedgehog signaling, Cell. Physiol. Biochem 44 (2018) 1856–1866, 10.1159/000485821. [DOI] [PubMed] [Google Scholar]
- [208].Wang X, Schneider A, HIF-2α-mediated activation of the epidermal growth factor receptor potentiates head and neck cancer cell migration in response to hypoxia, Carcinogenesis 31 (2010) 1202–1210, 10.1093/carcin/bgq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Franovic A, Gunaratnam L, Smith K, Robert I, Patten D, Lee S, Translational up-regulation of the EGFR by tumor hypoxia provides a nonmutational explanation for its overexpression in human cancer, Proc. Natl. Acad. Sci. USA 104 (2007) 13092–13097, 10.1073/pnas.0702387104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Yang Z, Wang R, Zhang T, Dong X, Hypoxia/lncRNA-AK123072/EGFR pathway induced metastasis and invasion in gastric cancer, Int. J. Clin. Exp. Med 8 (2015) 19954–19968. [PMC free article] [PubMed] [Google Scholar]
- [211].Mamo M, Ye IC, Digiacomo JW, Park JY, Downs B, Daniele M, Hypoxia alters the response to anti-EGFR therapy by regulating EGFR expression and downstream signaling in a DNA methylation-specific and HIF-dependent manner, (2020). 10.1158/0008-5472.CAN-20-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Wang P, Zhao L, Gong S, Xiong S, Wang J, Zou D, Pan J, Deng Y, Yan Q, Wu N, Liao B, HIF1α/HIF2α–Sox2/Klf4 promotes the malignant progression of glioblastoma via the EGFR–PI3K/AKT signalling pathway with positive feedback under hypoxia, Cell Death Dis. 12 (2021), 10.1038/s41419-021-03598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].He C, Sun XP, Qiao H, Jiang X, Wang D, Jin X, Dong X, Wang JJ, Jiang H, Sun X, Downregulating hypoxia-inducible factor-2a improves the efficacy of doxorubicin in the treatment of hepatocellular carcinoma, Cancer Sci. 103 (2012) 528–534, 10.1111/j.1349-7006.2011.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Zhao D, Zhai B, He C, Tan G, Jiang X, Pan S, Dong X, Wei Z, Ma L, Qiao H, Jiang H, Sun X, Upregulation of HIF-2α induced by sorafenib contributes to the resistance by activating the TGF-α/EGFR pathway in hepatocellular carcinoma cells, Cell. Signal 26 (2014) 1030–1039, 10.1016/j.cellsig.2014.01.026. [DOI] [PubMed] [Google Scholar]
- [215].Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, Rimm DL, Wong H, Rodriguez A, Herschkowitz JI, Fan C, Zhang X, He X, Pavlick A, Carolina Gutierrez M, Renshaw L, Larionov AA, Faratian D, Hilsenbeck SG, Perou CM, Lewis MT, Rosen JM, Chang JC, Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features, 2009. 〈www.pnas.org/cgi/content/full/〉. [DOI] [PMC free article] [PubMed]
- [216].Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong STC, Choi H, El Rayes T, Ryu S, Troeger J, Schwabe RF, Vahdat LT, Altorki NK, Mittal V, Gao D, Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance, Nature 527 (2015) 472–476, 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Bandyopadhyay A, Wang L, Agyin J, Tang Y, Lin S, Yeh I-T, De K, Sun L-Z, Doxorubicin in combination with a small TGFβ inhibitor: a potential novel therapy for metastatic breast cancer in mouse models, PLoS One 5 (2010), e10365, 10.1371/journal.pone.0010365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T, Serebryiskaya T, Beroukhim R, Hu M, Halushka MK, Sukumar S, Parker LM, Anderson KS, Harris LN, Garber JE, Richardson AL, Schnitt SJ, Nikolsky Y, Gelman RS, Polyak K, Molecular definition of breast tumor heterogeneity, Cancer Cell 11 (2007) 259–273, 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- [219].Ren J, Chen Y, Song H, Chen L, Wang R, Inhibition of ZEB1 reverses EMT and chemoresistance in docetaxel-resistant human lung adenocarcinoma cell line, J. Cell. Biochem 114 (2013) 1395–1403. [DOI] [PubMed] [Google Scholar]
- [220].Saxena M, Stephens MA, Pathak H, Rangarajan A, Transcription factors that mediate epithelial–mesenchymal transition lead to multidrug resistance by upregulating ABC transporters, e179–e179, Cell Death Dis 2 (2011), 10.1038/cddis.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Gjerdrum C, Tiron C, Høiby T, Stefansson I, Haugen H, Sandal T, Collett K, Li S, McCormack E, Gjertsen BT, Micklem DR, Akslen LA, Glackin C, Lorens JB, Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 1124–1129, 10.1073/pnas.0909333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger S, Cosper AK, Akhavanfard S, Heist RS, Temel J, Christensen JG, Wain JC, Lynch TJ, Vernovsky K, Mark EJ, Lanuti M, Iafrate AJ, Mino-Kenudson M, Engelman JA, Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors, Sci. Transl. Med 3 (2011), 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Byers LA, Diao L, Wang J, Saintigny P, Girard L, Peyton M, Shen L, Fan Y, Giri U, Tumula PK, An epithelial–mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a Therapeutic target for overcoming egfr inhibitor resistanceEMT predicts EGFR and PI3K inhibitor resistance in NSCLC, Clin. Cancer Res 19 (2013) 279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Lu M, Marsters S, Ye X, Luis E, Gonzalez L, Ashkenazi A, E-cadherin couples death receptors to the cytoskeleton to regulate apoptosis, Mol. Cell 54 (2014) 987–998, 10.1016/j.molcel.2014.04.029. [DOI] [PubMed] [Google Scholar]
- [225].Vega S, Morales AV, Ocaña OH, Valdés F, Fabregat I, Nieto MA, Snail blocks the cell cycle and confers resistance to cell death, Genes Dev. 18 (2004) 1131–1143, 10.1101/gad.294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Ni T, Li X-Y, Lu N, An T, Liu Z-P, Fu R, Lv W-C, Zhang Y-W, Xu X-J, Grant Rowe R, Lin Y-S, Scherer A, Feinberg T, Zheng X-Q, Chen B-A, Liu XS, Guo Q-L, Wu Z-Q, Weiss SJ, Snail1-dependent p53 repression regulates expansion and activity of tumour-initiating cells in breast cancer, Nat. Cell Biol 18 (2016) 1221–1232, 10.1038/ncb3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Wu W-S, Heinrichs S, Xu D, Garrison SP, Zambetti GP, Adams JM, Look AT, Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma, Cell 123 (2005) 641–653, 10.1016/j.cell.2005.09.029. [DOI] [PubMed] [Google Scholar]
- [228].Wu D-W, Lee M-C, Hsu N-Y, Wu T-C, Wu J-Y, Wang Y-C, Cheng Y-W, Chen CY, Lee H, FHIT loss confers cisplatin resistance in lung cancer via the AKT/NF-κB/Slug-mediated PUMA reduction, Oncogene 34 (2015) 2505–2515, 10.1038/onc.2014.184. [DOI] [PubMed] [Google Scholar]
- [229].Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF, Therapeutic implications of cancer stem cells, Curr. Opin. Genet. Dev 14 (2004) 43–47, 10.1016/j.gde.2003.11.007. [DOI] [PubMed] [Google Scholar]
- [230].Lou Y, Diao L, Cuentas ERP, Denning WL, Chen L, Fan YH, Byers LA, Wang J, Papadimitrakopoulou VA, Behrens C, Rodriguez JC, Hwu P, Wistuba II, Heymach JV, Gibbons DL, Epithelial-mesenchymal transition is associated with a distinct tumor microenvironment including elevation of inflammatory signals and multiple immune checkpoints in lung adenocarcinoma, Clin. Cancer Res 22 (2016) 3630–3642, 10.1158/1078-0432.CCR-15-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Mahmoudian RA, Mozhgani S, Abbaszadegan MR, Mokhlessi L, Montazer M, Gholamin M, Correlation between the immune checkpoints and EMT genes proposes potential prognostic and therapeutic targets in ESCC, J. Mol. Histol 52 (2021) 597–609, 10.1007/s10735-021-09971-3. [DOI] [PubMed] [Google Scholar]
- [232].Kolijn K, Verhoef EI, Smid M, Bottcher R, Jenster GW, Debets R, Van Leenders GJLH, Epithelial–mesenchymal transition in human prostate cancer demonstrates enhanced immune evasion marked by IDO1 expression, Cancer Res. 78 (2018) 4671–4679, 10.1158/0008-5472.CAN-17-3752. [DOI] [PubMed] [Google Scholar]
- [233].Hu Z, Cunnea P, Zhong Z, Lu H, Osagie OI, Campo L, Artibani M, Nixon K, Ploski J, Gonzalez LS, Alsaadi A, Wietek N, Damato S, Dhar S, Blagden SP, Yau C, Hester J, Albukhari A, Aboagye EO, Fotopoulou C, Ahmed A, The oxford classic links epithelial-to-mesenchymal transition to immunosuppression in poor prognosis ovarian cancers, Clin. Cancer Res 27 (2021) 1570–1579, 10.1158/1078-0432.CCR-20-2782. [DOI] [PubMed] [Google Scholar]
- [234].Hugo W, Zaretsky JM, Sun L, Song C, Moreno BH, Hu-Lieskovan S, BerentMaoz B, Pang J, Chmielowski B, Cherry G, Seja E, Lomeli S, Kong X, Kelley MC, Sosman JA, Johnson DB, Ribas A, Lo RS, Genomic and transcriptomic features of response to Anti-PD-1 therapy in metastatic melanoma, Cell 165 (2016) 35–44, 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y, Cancer metastasis is accelerated through immunosuppression during snail-induced EMT of cancer cells, Cancer Cell 15 (2009) 195–206, 10.1016/j.ccr.2009.01.023. [DOI] [PubMed] [Google Scholar]
- [236].Akalay I, Janji B, Hasmim M, Noman MZ, Andŕe F, De Cremoux P, Bertheau P, Badoual C, Vielh P, Larsen AK, Sabbah M, Tan TZ, Keira JH, Hung NTY, Thiery JP, Mami-Chouaib F, Chouaib S, Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from t-cell-mediated lysis, Cancer Res. 73 (2013) 2418–2427, 10.1158/0008-5472.CAN-12-2432. [DOI] [PubMed] [Google Scholar]
- [237].Qian Y, Yao W, Yang T, Yang Y, Liu Y, Shen Q, Zhang J, Qi W, Wang J, aPKC-ι/P-Sp1/Snail signaling induces epithelial–mesenchymal transition and immunosuppression in cholangiocarcinoma, Hepatology 66 (2017) 1165–1182, 10.1002/hep.29296. [DOI] [PubMed] [Google Scholar]
- [238].Taki M, Abiko K, Baba T, Hamanishi J, Yamaguchi K, Murakami R, Yamanoi K, Horikawa N, Hosoe Y, Nakamura E, Sugiyama A, Mandai M, Konishi I, Matsumura N, Snail promotes ovarian cancer progression by recruiting myeloid-derived suppressor cells via CXCR2 ligand upregulation, Nat. Commun 9 (2018) 1–12, 10.1038/s41467-018-03966-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Xiao M, Hasmim M, Lequeux A, Van Moer K, Tan TZ, Gilles C, Hollier BG, Thiery JP, Berchem G, Janji B, Noman MZ, Epithelial to mesenchymal transition regulates surface pd-l1 via cmtm6 and cmtm7 induction in breast cancer, Cancers 13 (2021) 1–12, 10.3390/cancers13051165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Takimoto CH, Chao MP, Gibbs C, McCamish MA, Liu J, Chen JY, Majeti R, Weissman IL, The Macrophage “Do not eat me” signal, CD47, is a clinically validated cancer immunotherapy target, Ann. Oncol 30 (2019) 486–489, 10.1093/annonc/mdz006. [DOI] [PubMed] [Google Scholar]
- [241].Noman MZ, Van Moer K, Marani V, Gemmill RM, Tranchevent LC, Azuaje F, Muller A, Chouaib S, Thiery JP, Berchem G, Janji B, CD47 is a direct target of SNAI1 and ZEB1 and its blockade activates the phagocytosis of breast cancer cells undergoing EMT, OncoImmunology 7 (2018), 10.1080/s2162402X.2017.1345415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Dongre A, Rashidian M, Reinhardt F, Bagnato A, Keckesova Z, Ploegh HL, Weinberg RA, Epithelial-to-mesenchymal transition contributes to immunosuppression in breast carcinomas, Cancer Res. 77 (2017) 3982–3989, 10.1158/0008-5472.CAN-16-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Dongre A, Rashidian M, Eaton EN, Reinhardt F, Thiru P, Zagorulya M, Nepal S, Banaz T, Martner A, Spranger S, Weinberg RA, Direct and indirect regulators of epithelial–mesenchymal transition– mediated immunosuppression in breast carcinomas, Cancer Discov. 11 (2021) 1286–1305, 10.1158/2159-8290.CD-20-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, Zhang X, Yi X, Dwyer D, Lin W, Diao L, Wang J, Roybal JD, Patel M, Ungewiss C, Peng D, Antonia S, Mediavilla-Varela M, Robertson G, Jones S, Suraokar M, Welsh JW, Erez B, Wistuba II, Chen L, Peng D, Wang S, Ullrich SE, Heymach JV, Kurie JM, Qin FXF, Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression, Nat. Commun 5 (2014), 10.1038/ncomms6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [245].Noman MZ, Janji B, Abdou A, Hasmim M, Terry S, Tan TZ, Mami-Chouaib F, Thiery JP, Chouaib S, The immune checkpoint ligand PD-l1 is upregulated in EMT-activated human breast cancer cells by a mechanism involving ZEB-1 and miR-200, OncoImmunology 6 (2017) 1–7, 10.1080/2162402X.2016.1263412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Guo Y, Lu X, Chen Y, Rendon B, Mitchell RA, Cuatrecasas M, Cortés M, Postigo A, Liu Y, Dean DC, Zeb1 induces immune checkpoints to form an immunosuppressive envelope around invading cancer cells, Sci. Adv 7 (2021), 10.1126/sciadv.abd7455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Tijink BM, Buter J, De Bree R, Giaccone G, Lang MS, Staab A, Leemans CR, Van Dongen GA, A phase I dose escalation study with anti-CD44v6 bivatuzumab mertansine in patients with incurable squamous cell carcinoma of the head and neck or esophagus, Clin. Cancer Res 12 (2006) 6064–6072. [DOI] [PubMed] [Google Scholar]
- [248].Riechelmann H, Sauter A, Golze W, Hanft G, Schroen C, Hoermann K, Erhardt T, Gronau S, Phase I trial with the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head and neck squamous cell carcinoma, Oral. Oncol 44 (2008) 823–829. [DOI] [PubMed] [Google Scholar]
- [249].Perez A, Neskey DM, Wen J, Goodwin JW, Slingerland J, Pereira L, Weigand S, Franzmann EJ, Targeting CD44 in head and neck squamous cell carcinoma (HNSCC) with a new humanized antibody RO5429083, Cancer Res. 72 (2012), 2521–2521. [Google Scholar]
- [250].Wang C, Wang Z, Chen C, Fu X, Wang J, Fei X, Yan X, Xu R, A low MW inhibitor of CD44 dimerization for the treatment of glioblastoma, Br. J. Pharmacol 177 (2020) 3009–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [251].Aguilera KY, Huang H, Du W, Hagopian MM, Wang Z, Hinz S, Hwang TH, Wang H, Fleming JB, Castrillon DH, Ren X, Ding K, Brekken RA, Inhibition of discoidin domain receptor 1 reduces collagen-mediated tumorigenicity in pancreatic ductal adenocarcinoma, Mol. Cancer Ther 16 (2017) 2473–2485, 10.1158/1535-7163.MCT-16-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [252].Grither WR, Longmore GD, Inhibition of tumor–microenvironment interaction and tumor invasion by small-molecule allosteric inhibitor of DDR2 extracellular domain, Proc. Natl. Acad. Sci. U. S. A 115 (2018), 10.1073/pnas.1805020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [253].Jin H, Ham I-H, Oh HJ, Bae CA, Lee D, Kim Y-B, Son S-Y, Chwae Y-J, Han SU, Brekken RA, Hur H, Inhibition of discoidin domain receptor 1 prevents stroma-induced peritoneal metastasis in gastric carcinoma, Mol. Cancer Res 16 (2018) 1590–1600, 10.1158/1541-7786.MCR-17-0710. [DOI] [PubMed] [Google Scholar]
- [254].Berestjuk I, Lecacheur M, Carminati A, Diazzi S, Rovera C, Prod’homme V, Ohanna M, Popovic A, Mallavialle A, Larbret F, Targeting Discoidin Domain Receptors DDR1 and DDR2 overcomes matrix-mediated tumor cell adaptation and tolerance to BRAF-targeted therapy in melanoma, EMBO Mol. Med 14 (2022), e11814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [255].Kothiwale S, Borza CM, Lowe EW Jr, Pozzi A, Meiler J, Discoidin domain receptor 1 (DDR1) kinase as target for structure-based drug discovery, Drug Discov. Today 20 (2015) 255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [256].Morris JC, Tan AR, Olencki TE, Shapiro GI, Dezube BJ, Reiss M, Hsu FJ, Berzofsky JA, Lawrence DP, Phase I study of GC1008 (fresolimumab): a human anti-transforming growth factor-beta (TGFβ) monoclonal antibody in patients with advanced malignant melanoma or renal cell carcinoma, PloS One 9 (2014), e90353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [257].Rice LM, Padilla CM, McLaughlin SR, Mathes A, Ziemek J, Goummih S, Nakerakanti S, York M, Farina G, Whitfield ML, Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients, J. Clin. Investig 125 (2015) 2795–2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK, Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors, Proc. Natl. Acad. Sci 108 (2011) 2909–2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [259].Vallet SD, Ricard-Blum S, Lysyl oxidases: from enzyme activity to extracellular matrix cross-links, Essays Biochem. 63 (2019) 349–364. [DOI] [PubMed] [Google Scholar]
- [260].Benson III AB, Wainberg ZA, Hecht JR, Vyushkov D, Dong H, Bendell J, Kudrik F, A phase II randomized, double-blind, placebo-controlled study of simtuzumab or placebo in combination with gemcitabine for the first-line treatment of pancreatic adenocarcinoma, Oncologist 22 (2017) 241, e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [261].Peng T, Lin S, Meng Y, Gao P, Wu P, Zhi W, Ding W, Cao C, Wu P, LOXL2 small molecule inhibitor restrains malignant transformation of cervical cancer cells by repressing LOXL2 -induced epithelial-mesenchymal transition (EMT), Cell Cycle (2022) 1–15, 10.1080/15384101.2022.2073047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [262].Tang H, Leung L, Saturno G, Viros A, Smith D, Di Leva G, Morrison E, Niculescu-Duvaz D, Lopes F, Johnson L, Dhomen N, Springer C, Marais R, Lysyl oxidase drives tumour progression by trapping EGF receptors at the cell surface, Nat. Commun 8 (2017) 14909, 10.1038/ncomms14909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Reardon DA, Fink KL, Mikkelsen T, Cloughesy TF, O’Neill A, Plotkin S, Glantz M, Ravin P, Raizer JJ, Rich KM, Schiff D, Shapiro WR, Burdette-Radoux S, Dropcho EJ, Wittemer SM, Nippgen J, Picard M, Nabors LB, Randomized phase II study of cilengitide, an integrin-targeting arginine-glycineaspartic acid peptide, in recurrent glioblastoma multiforme, JCO 26 (2008) 5610–5617, 10.1200/JCO.2008.16.7510. [DOI] [PubMed] [Google Scholar]
- [264].Javadi S, Rostamizadeh K, Hejazi J, Parsa M, Fathi M, Curcumin mediated down-regulation of αVβ3 integrin and up-regulation of pyruvate dehydrogenase kinase 4 (PDK4) in Erlotinib resistant SW480 colon cancer cells, Phytother. Res 32 (2018) 355–364. [DOI] [PubMed] [Google Scholar]
- [265].Capelletto E, Bironzo P, Denis L, Koustenis A, Bungaro M, Novello S, Single agent VS-6766 or VS-6766 plus defactinib in KRAS-mutant non-small-cell lung cancer: the RAMP-202 phase II trial, Future Oncol. 18 (2022) 1907–1915. [DOI] [PubMed] [Google Scholar]
- [266].Wang-Gillam A, Lockhart AC, Tan BR, Suresh R, Lim K-H, Ratner L, DeNardo DG, Phase I study of defactinib combined with pembrolizumab and gemcitabine in patients with advanced cancer., American Society of Clinical Oncology, 2018. [Google Scholar]
- [267].Dawson JC, Serrels B, Byron A, Muir MT, Makda A, García-Muñoz A, von Kriegsheim A, Lietha D, Carragher NO, Frame MC, A synergistic anticancer FAK and HDAC inhibitor combination discovered by a novel chemical–genetic high-content phenotypic screenantitumor effects of FAK and HDAC inhibitors, Mol. Cancer Ther 19 (2020) 637–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [268].Wu L, Wang J, Wang L, Lu W, Wang K, Lin A, Li N, Li L, Su N, Xie S, A phase Ib study of IN10018 in combination with pegylated liposomal doxorubicin (PLD) in patients with platinum-resistant ovarian cancer., American Society of Clinical Oncology, 2022. [Google Scholar]
- [269].Mak G, Soria J-C, Blagden SP, Plummer R, Fleming RA, Nebot N, Zhang J, Mazumdar J, Rogan D, Gazzah A, A phase Ib dose-finding, pharmacokinetic study of the focal adhesion kinase inhibitor GSK2256098 and trametinib in patients with advanced solid tumours, Br. J. Cancer 120 (2019) 975–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [270].Soria J-C, Gan HK, Blagden SP, Plummer R, Arkenau HT, Ranson M, Evans TRJ, Zalcman G, Bahleda R, Hollebecque A, A phase I, pharmacokinetic and pharmacodynamic study of GSK2256098, a focal adhesion kinase inhibitor, in patients with advanced solid tumors, Ann. Oncol 27 (2016) 2268–2274. [DOI] [PubMed] [Google Scholar]
- [271].de Jonge MJ, Steeghs N, Lolkema MP, Hotte SJ, Hirte HW, van der Biessen DA, Abdul Razak AR, De Vos FY, Verheijen RB, Schnell D, Phase I study of BI 853520, an inhibitor of focal adhesion kinase, in patients with advanced or metastatic nonhematologic malignancies, Target. Oncol 14 (2019) 43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [272].Dolor A, Szoka FC, Digesting a path forward: the utility of collagenase tumor treatment for improved drug delivery, Mol. Pharm 15 (2018) 2069–2083, 10.1021/acs.molpharmaceut.8b00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [273].Ng MR, Brugge JS, A stiff blow from the stroma: collagen crosslinking drives tumor progression, Cancer Cell 16 (2009) 455–457, 10.1016/j.ccr.2009.11.013. [DOI] [PubMed] [Google Scholar]
- [274].Leung L, Niculescu-Duvaz D, Smithen D, Lopes F, Callens C, McLeary R, Saturno G, Davies L, Aljarah M, Brown M, Johnson L, Zambon A, Chambers T, Ménard D, Bayliss N, Knight R, Fish L, Lawrence R, Challinor M, Tang H, Marais R, Springer C, Anti-metastatic inhibitors of lysyl oxidase (LOX): design and structure-activity relationships, J. Med. Chem 62 (2019) 5863–5884, 10.1021/acs.jmedchem.9b00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [275].von Mässenhausen A, Sanders C, Brägelmann J, Konantz M, Queisser A, Vogel W, Kristiansen G, Duensing S, Schrock A, Bootz F, Brossart P, Kirfel J, Lengerke C, Perner S, Targeting DDR2 in head and neck squamous cell carcinoma with dasatinib: DDR2 inhibition with dasatinib in HNSCC, Int. J. Cancer 139 (2016) 2359–2369, 10.1002/ijc.30279. [DOI] [PubMed] [Google Scholar]
- [276].Li L, Hao X, Qin J, Tang W, He F, Smith A, Zhang M, Simeone DM, Qiao XT, Chen Z-N, Lawrence TS, Xu L, Antibody against CD44s inhibits pancreatic tumor initiation and postradiation recurrence in mice, e12, Gastroenterology 146 (2014) 1108–1118, 10.1053/j.gastro.2013.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [277].Zheng J, Zhao S, Yu X, Huang S, Liu HY, Simultaneous targeting of CD44 and EpCAM with a bispecific aptamer effectively inhibits intraperitoneal ovarian cancer growth, Theranostics 7 (2017) 1373–1388, 10.7150/thno.17826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [278].Gerstner ER, Ye X, Duda DG, Levine MA, Mikkelsen T, Kaley TJ, Olson JJ, Nabors BL, Ahluwalia MS, Wen PY, Jain RK, Batchelor TT, Grossman S, A phase I study of cediranib in combination with cilengitide in patients with recurrent glioblastoma, Neuro Oncol. 17 (2015) 1386–1392, 10.1093/neuonc/nov085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [279].Diaz Osterman CJ, Ozmadenci D, Kleinschmidt EG, Taylor KN, Barrie AM, Jiang S, Bean LM, Sulzmaier FJ, Jean C, Tancioni I, Anderson K, Uryu S, Cordasco EA, Li J, Chen XL, Fu G, Ojalill M, Rappu P, Heino J, Mark AM, Xu G, Fisch KM, Kolev VN, Weaver DT, Pachter JA, Győrffy B, McHale MT, Connolly DC, Molinolo A, Stupack DG, Schlaepfer DD, FAK activity sustains intrinsic and acquired ovarian cancer resistance to platinum chemotherapy, ELife 8 (2019), e47327, 10.7554/eLife.47327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Falandry C, Rousseau F, Mouret-Reynier M-A, Tinquaut F, Lorusso D, Herrstedt J, Savoye A-M, Stefani L, Bourbouloux E, Sverdlin R, D’Hondt V, Lortholary A, Brachet P-E, Zannetti A, Malaurie E, Venat-Bouvet L, Tŕedan O, Mourey L, Pujade-Lauraine E, Freyer G, Groupe d’investigateurs nationaux pour l’Étude des Cancers de l’Ovaire et du sein (GINECO), efficacy and safety of first-line single-agent carboplatin vs carboplatin plus paclitaxel for vulnerable older adult women with ovarian cancer: a GINECO/GCIG randomized clinical trial, JAMA Oncol. 7 (2021) 853, 10.1001/jamaoncol.2021.0696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Machida H, Tokunaga H, Matsuo K, Matsumura N, Kobayashi Y, Tabata T, Kaneuchi M, Nagase S, Mikami M, Survival outcome and perioperative complication related to neoadjuvant chemotherapy with carboplatin and paclitaxel for advanced ovarian cancer: a systematic review and meta-analysis, Eur. J. Surg. Oncol 46 (2020) 868–875, 10.1016/j.ejso.2019.11.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Shimizu T, Fukuoka K, Takeda M, Iwasa T, Yoshida T, Horobin J, Keegan M, Vaickus L, Chavan A, Padval M, Nakagawa K, A first-in-Asian phase 1 study to evaluate safety, pharmacokinetics and clinical activity of VS-6063, a focal adhesion kinase (FAK) inhibitor in Japanese patients with advanced solid tumors, Cancer Chemother. Pharm 77 (2016) 997–1003, 10.1007/s00280-016-3010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Almasabi S, Ahmed AU, Boyd R, Williams BRG, A potential role for integrin-linked kinase in colorectal cancer growth and progression via regulating senescence and immunity, Front. Genet 12 (2021), 10.3389/fgene.2021.638558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Nguyen MLT, Bui KC, Scholta T, Xing J, Bhuria V, Sipos B, Wilkens L, Nguyen Linh T, Velavan TP, Bozko P, Plentz RR, Targeting interleukin 6 signaling by monoclonal antibody siltuximab on cholangiocarcinoma, J. Gastroenterol. Hepatol. (Aust. ) 36 (2021) 1334–1345, 10.1111/jgh.15307. [DOI] [PubMed] [Google Scholar]
- [285].Al-Jomah N, Al-Mohanna FH, Aboussekhra A, Tocilizumab suppresses the pro-carcinogenic effects of breast cancer-associated fibroblasts through inhibition of the STAT3/AUF1 pathway, Carcinogenesis 42 (2021) 1439–1448, 10.1093/carcin/bgab102. [DOI] [PubMed] [Google Scholar]
- [286].Guan J, Zhang H, Wen Z, Gu Y, Cheng Y, Sun Y, Zhang T, Jia C, Lu Z, Chen J, Retinoic acid inhibits pancreatic cancer cell migration and EMT through the downregulation of IL-6 in cancer associated fibroblast cells, Cancer Lett. 345 (2014) 132–139, 10.1016/j.canlet.2013.12.006. [DOI] [PubMed] [Google Scholar]
- [287].Moatassim-Billah S, Duluc C, Samain R, Jean C, Perraud A, Decaup E, Cassant-Sourdy S, Bakri Y, Selves J, Schmid H, Martineau Y, Mathonnet M, Pyronnet S, Bousquet C, Anti-metastatic potential of somatostatin analog SOM230: Indirect pharmacological targeting of pancreatic cancerassociated fibroblasts, Oncotarget 7 (2016) 41584–41598, 10.18632/oncotarget.9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Feng R, Morine Y, Ikemoto T, Imura S, Iwahashi S, Saito Y, Shimada M, Nabpaclitaxel interrupts cancer-stromal interaction through C-X-C motif chemokine 10-mediated interleukin-6 downregulation in vitro, Cancer Sci. 109 (2018) 2509–2519, 10.1111/cas.13694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Al-Harbi B, Aboussekhra A, Cucurbitacin I (JSI-124)-dependent inhibition of STAT3 permanently suppresses the pro-carcinogenic effects of active breast cancer-associated fibroblasts, Mol. Carcinog 60 (2021) 242–251, 10.1002/mc.23287. [DOI] [PubMed] [Google Scholar]
- [290].Song L, Smith MA, Doshi P, Sasser K, Fulp W, Altiok S, Haura EB, Antitumor efficacy of the anti-interleukin-6 (IL-6) antibody siltuximab in mouse xenograft models of lung cancer, J. Thorac. Oncol 9 (2014) 974–982, 10.1097/JTO.0000000000000193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Rossi JF, Ńegrier S, James ND, Kocak I, Hawkins R, Davis H, Prabhakar U, Qin X, Mulders P, Berns B, A phase I/II study of siltuximab (CNTO 328), an anti-interleukin-6 monoclonal antibody, in metastatic renal cell cancer, Br. J. Cancer 103 (2010) 1154–1162, 10.1038/sj.bjc.6605872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].Bae CA, Ham IH, Oh HJ, Lee D, Woo J, Son SY, Yoon JH, Lorens JB, Brekken RA, Kim TM, Han SU, Park WS, Hur H, Inhibiting the GAS6/AXL axis suppresses tumor progression by blocking the interaction between cancer-associated fibroblasts and cancer cells in gastric carcinoma, Gastric Cancer 23 (2020) 824–836, 10.1007/s10120-020-01066-4. [DOI] [PubMed] [Google Scholar]
- [293].Bockorny B, Semenisty V, Macarulla T, Borazanci E, Wolpin BM, Stemmer SM, Golan T, Geva R, Borad MJ, Pedersen KS, Park JO, Ramirez RA, Abad DG, Feliu J, Muñoz A, Ponz-Sarvise M, Peled A, Lustig TM, Bohana-Kashtan O, Shaw SM, Sorani E, Chaney M, Kadosh S, Vainstein Haras A, Von Hoff DD, Hidalgo M, BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial, Nat. Med 26 (2020) 878–885, 10.1038/s41591-020-0880-x. [DOI] [PubMed] [Google Scholar]
- [294].Ren Y, huan Jia H, qi Xu Y, Zhou X, hui Zhao X, fei Wang Y, Song X, yan Zhu Z, Sun T, Dou Y, ping Tian W, lan Zhao X, sheng Kang C, Mei M, Paracrine and epigenetic control of CAF-induced metastasis: the role of HOTAIR stimulated by TGF-ß1 secretion, Mol. Cancer 17 (2018) 1–14, 10.1186/s12943-018-0758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [295].Otvos L, Haspinger E, La Russa F, Maspero F, Graziano P, Kovalszky I, Lovas S, Nama K, Hoffmann R, Knappe D, Cassone M, Wade J, Surmacz E, Design and development of a peptide-based adiponectin receptor agonist for cancer treatment, BMC Biotechnol. 11 (2011) 90, 10.1186/1472-6750-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Philp LK, Rockstroh A, Lehman M, Sadowski MC, Bartonicek N, Wade JD, Otvos L, Nelson CC, Adiponectin receptor activation inhibits prostate cancer xenograft growth, Endocr. -Relat. Cancer 27 (2020) 711–729, 10.1530/ERC-20-0297. [DOI] [PubMed] [Google Scholar]
- [297].Gonzalez RR, Cherfils S, Escobar M, Yoo JH, Carino C, Styer AK, Sullivan BT, Sakamoto H, Olawaiye A, Serikawa T, Lynch MP, Rueda BR, Leptin signaling promotes the growth of mammary tumors and increases the expression of vascular endothelial growth factor (VEGF) and its receptor type two (VEGF-R2), J. Biol. Chem 281 (2006) 26320–26328, 10.1074/jbc.M601991200. [DOI] [PubMed] [Google Scholar]
- [298].Rene Gonzalez R, Watters A, Xu Y, Singh UP, Mann DR, Rueda BR, Penichet ML, Leptin-signaling inhibition results in efficient anti-tumor activity in estrogen receptor positive or negative breast cancer, Breast Cancer Res. 11 (2009) 1–12, 10.1186/BCR2321/COMMENTS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [299].Gyamfi J, Lee YH, Min BS, Choi J, Niclosamide reverses adipocyte induced epithelial-mesenchymal transition in breast cancer cells via suppression of the interleukin-6/STAT3 signalling axis, Sci. Rep 9 (2019), 10.1038/s41598-019-47707-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [300].Su F, Ahn S, Saha A, DiGiovanni J, Kolonin MG, Adipose stromal cell targeting suppresses prostate cancer epithelial-mesenchymal transition and chemoresistance, Oncogene 38 (2019) 1979–1988, 10.1038/s41388-018-0558-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [301].Dodagatta-Marri E, Meyer DS, Reeves MQ, Paniagua R, To MD, Binnewies M, Broz ML, Mori H, Wu D, Adoumie M, Del Rosario R, Li O, Buchmann T, Liang B, Malato J, Arce Vargus F, Sheppard D, Hann BC, Mirza A, Quezada SA, Rosenblum MD, Krummel MF, Balmain A, Akhurst RJ, α-PD-1 therapy elevates Treg/Th balance and increases tumor cell pSmad3 that are both targeted by α-TGFβ antibody to promote durable rejection and immunity in squamous cell carcinomas 11 Medical and Health Sciences 1107 Immunology 11 Medical and Health Sc, J. Immunother. Cancer 7 (2019) 1–15, 10.1186/s40425-018-0493-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [302].Greco R, Qu H, Qu H, Theilhaber J, Shapiro G, Gregory R, Winter C, Malkova N, Sun F, Jaworski J, Best A, Pao L, Hebert A, Levit M, Protopopov A, Pollard J, Bahjat K, Wiederschain D, Sharma S, Pan-TGFβ inhibition by SAR439459 relieves immunosuppression and improves antitumor efficacy of PD-1 blockade, OncoImmunology 9 (2020), 10.1080/2162402X.2020.1811605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [303].David JM, Dominguez C, McCampbell KK, Gulley JL, Schlom J, Palena C, A novel bifunctional anti-PD-L1/TGF-β Trap fusion protein (M7824) efficiently reverts mesenchymalization of human lung cancer cells, OncoImmunology 6 (2017), 10.1080/2162402X.2017.1349589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [304].Knudson KM, Hicks KC, Luo X, Chen JQ, Schlom J, Gameiro SR, M7824, a novel bifunctional anti-PD-L1/TGFβ Trap fusion protein, promotes anti-tumor efficacy as monotherapy and in combination with vaccine, OncoImmunology 7 (2018) 1–14, 10.1080/2162402X.2018.1426519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [305].Lan Y, Zhang D, Xu C, Hance KW, Marelli B, Qi J, Yu H, Qin G, Sircar A, Hernández VM, Jenkins MH, Fontana RE, Deshpande A, Locke G, Sabzevari H, Radvanyi L, Lo KM, Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-, Sci. Transl. Med 10 (2018), 10.1126/scitranslmed.aan5488. [DOI] [PubMed] [Google Scholar]
- [306].Lind H, Gameiro SR, Jochems C, Donahue RN, Strauss J, Gulley JL, Palena C, Schlom J, Dual targeting of TGF-β and PD-L1 via a bifunctional anti-PD-L1/TGF-βRII agent: Status of preclinical and clinical advances, J. Immunother. Cancer 8 (2020) 1–10, 10.1136/jitc-2019-000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [307].Strauss J, Heery CR, Schlom J, Madan RA, Cao L, Kang Z, Lamping E, Marte JL, Donahue RN, Grenga I, Cordes L, Christensen O, Mahnke L, Helwig C, Gulley JL, Phase I trial of M7824 (MSB0011359C), a bifunctional fusion protein targeting PD-L1 and TGFb, in advanced solid tumors, Clin. Cancer Res 24 (2018) 1287–1295, 10.1158/1078-0432.CCR-17-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [308].Yi M, Zhang J, Li A, Niu M, Yan Y, Jiao Y, Luo S, Zhou P, Wu K, The construction, expression, and enhanced anti-tumor activity of YM101: a bispecific antibody simultaneously targeting TGF-β and PD-L1, J. Hematol. Oncol 14 (2021) 1–22, 10.1186/s13045-021-01045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [309].Peng DH, Rodriguez BL, Diao L, Gaudreau PO, Padhye A, Konen JM, Ochieng JK, Class CA, Fradette JJ, Gibson L, Chen L, Wang J, Byers LA, Gibbons DL, Th17 cells contribute to combination MEK inhibitor and anti-PD-L1 therapy resistance in KRAS/p53 mutant lung cancers, Nat. Commun 12 (2021) 1–15, 10.1038/s41467-021-22875-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [310].Li X, Yao W, Yuan Y, Chen P, Li B, Li J, Chu R, Song H, Xie D, Jiang X, Wang H, Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma, Gut 66 (2017) 157–167, 10.1136/gutjnl-2015-310514. [DOI] [PubMed] [Google Scholar]
- [311].Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, Setty M, Leslie CS, Oei Y, Pedraza A, Zhang J, Brennan CW, Sutton JC, Holland EC, Daniel D, Joyce JA, CSF-1R inhibition alters macrophage polarization and blocks glioma progression, Nat. Med 19 (2013) 1264–1272, 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [312].Zhu Y, Knolhoff BL, Meyer MA, Nywening TM, West BL, Luo J, WangGillam A, Goedegebuure SP, Linehan DC, De Nardo DG, CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models, Cancer Res. 74 (2014) 5057–5069, 10.1158/0008-5472.CAN-13-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [313].Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, Rey-Giraud F, Pradel LP, Feuerhake F, Klaman I, Jones T, Jucknischke U, Scheiblich S, Kaluza K, Gorr IH, Walz A, Abiraj K, Cassier PA, Sica A, Gomez-Roca C, deVisser KE, Italiano A, LeTourneau C, Delord JP, Levitsky H, Blay JY, Rüttinger D, Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy, Cancer Cell 25 (2014) 846–859, 10.1016/j.ccr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- [314].Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, Torigian DA, O’Dwyer PJ, Vonderheide RH, CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans, Science 331 (2011) 1612–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [315].Kaneda MM, Messer KS, Ralainirina N, Li H, Leem CJ, Gorjestani S, Woo G, Nguyen AV, Figueiredo CC, Foubert P, Schmid MC, Pink M, Winkler DG, Rausch M, Palombella VJ, Kutok J, McGovern K, Frazer KA, Wu X, Karin M, Sasik R, Cohen EEW, Varner JA, PI3Kγ 3 is a molecular switch that controls immune suppression, Nature 539 (2016) 437–442, 10.1038/nature19834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [316].Ravi J, Elbaz M, Wani NA, Nasser MW, Ganju RK, Cannabinoid receptor-2 agonist inhibits macrophage induced EMT in non-small cell lung cancer by downregulation of EGFR pathway, Mol. Carcinog 55 (2016) 2063–2076, 10.1002/mc.22451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [317].Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM, Gemcitabine selectively eliminates splenic Gr-1+/CD11b + myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity, Clin. Cancer Res 11 (2005) 6713–6721, 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- [318].Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, Martin F, Apetoh L, Ŕebé C, Ghiringhelli F, 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity, Cancer Res. 70 (2010) 3052–3061, 10.1158/0008-5472.CAN-09-3690. [DOI] [PubMed] [Google Scholar]
- [319].Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P, Golshayan A, Rayman PA, Wood L, Garcia J, Dreicer R, Bukowski R, Finke JH, Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients, Clin. Cancer Res 15 (2009) 2148–2157, 10.1158/1078-0432.CCR-08-1332. [DOI] [PubMed] [Google Scholar]
- [320].Sevko A, Michels T, Vrohlings M, Umansky L, Beckhove P, Kato M, Shurin GV, Shurin MR, Umansky V, Antitumor effect of paclitaxel is mediated by inhibition of myeloid-derived suppressor cells and chronic inflammation in the spontaneous melanoma model, J. Immunol 190 (2013) 2464–2471, 10.4049/jimmunol.1202781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [321].Qin H, Lerman B, Sakamaki I, Wei G, Cha SC, Rao SS, Qian J, Hailemichael Y, Nurieva R, Dwyer KC, Roth J, Yi Q, Overwijk WW, Kwak LW, Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice, Nat. Med 20 (2014) 676–681, 10.1038/nm.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [322].Holmgaard RB, Zamarin D, Lesokhin A, Merghoub T, Wolchok JD, Targeting myeloid-derived suppressor cells with colony stimulating factor-1 receptor blockade can reverse immune resistance to immunotherapy in indoleamine 2,3-dioxygenase-expressing tumors, EBioMedicine 6 (2016) 50–58, 10.1016/j.ebiom.2016.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [323].Blattner C, Fleming V, Weber R, Himmelhan B, Altevogt P, Gebhardt C, Schulze TJ, Razon H, Hawila E, Wildbaum G, Utikal J, Karin N, Umansky V, CCR5+ myeloid-derived suppressor cells are enriched and activated in melanoma lesions, Cancer Res. 78 (2018) 157–167, 10.1158/0008-5472.CAN-17-0348. [DOI] [PubMed] [Google Scholar]
- [324].Sun L, Clavijo PE, Robbins Y, Patel P, Friedman J, Greene S, Das R, Silvin C, Van Waes C, Horn LA, Schlom J, Palena C, Maeda D, Zebala J, Allen CT, Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy, JCI Insight 4 (2019) 1–12, 10.1172/jci.insight.126853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [325].Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, Dolcetti L, Bronte V, Borrello I, Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function, J. Exp. Med 203 (2006) 2691–2702, 10.1084/jem.20061104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [326].Veltman JD, Lambers ME, Van Nimwegen M, Hendriks RW, Hoogsteden HC, Aerts JG, Hegmans P, COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid- derived suppressor cells in mesothelioma. Celecoxib influences MDSC function, BMC Cancer 10 (2010) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [327].Nagaraj S, Youn JI, Weber H, Iclozan C, Lu L, Cotter MJ, Meyer C, Becerra CR, Fishman M, Antonia S, Sporn MB, Liby KT, Rawal B, Lee JH, Gabrilovich DI, Anti-inflammatory triterpenoid blocks immune suppressive function of MDSCs and improves immune response in cancer, Clin. Cancer Res 16 (2010) 1812–1823, 10.1158/1078-0432.CCR-09-3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [328].Cheng P, Kumar V, Liu H, Youn JI, Fishman M, Sherman S, Gabrilovich D, Effects of notch signaling on regulation of myeloid cell differentiation in cancer, Cancer Res. 74 (2014) 141–152, 10.1158/0008-5472.CAN-13-1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [329].Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI, All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients, Cancer Res. 66 (2006) 9299–9307, 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [330].Cui E, Guo H, Shen M, Yu H, Gu D, Mao W, Wang X, Adiponectin inhibits migration and invasion by reversing epithelial-mesenchymal transition in non-small cell lung carcinoma, Oncol. Rep 40 (2018) 1330–1338, 10.3892/or.2018.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [331].Cui R, Yue W, Lattime EC, Stein MN, Xu Q, Tan XL, Targeting tumor-associated macrophages to combat pancreatic cancer, Oncotarget 7 (2016) 50735–50754, 10.18632/oncotarget.9383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [332].Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR, “Re-educating” tumor-associated macrophages by targeting NF-κB, J. Exp. Med 205 (2008) 1261–1268, 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [333].Law AMK, Valdes-mora F, Gallego-ortega D, Myeloid-derived suppressor cells as a therapeutic, Cells 27 (2020) 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [334].Tang H, Li H, Sun Z, Targeting myeloid-derived suppressor cells for cancer therapy, Cancer Biol. Med 18 (2021) 992–1009, 10.20892/j.issn.2095-3941.2020.0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [335].Song JH, Eum DY, Park SY, Jin YH, Shim JW, Park SJ, Kim MY, Park SJ, Heo K, Choi YJ, Inhibitory effect of ginsenoside Rg3 on cancer stemness and mesenchymal transition in breast cancer via regulation of myeloidderived suppressor cells, PLoS ONE 15 (2020) 1–15, 10.1371/journal.pone.0240533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [336].Welsh S, Williams R, Kirkpatrick L, Paine-Murrieta G, Powis G, Antitumor activity and pharmacodynamic properties of PX-478, an inhibitor of hypoxiainducible factor-1α, Mol. Cancer Ther 3 (2004) 233–244. [PubMed] [Google Scholar]
- [337].Koh MY, Spivak-Kroizman T, Venturini S, Welsh S, Williams RR, Kirkpatrick DL, Powis G, Molecular mechanisms for the activity of PX-478, an antitumor inhibitor of the hypoxia-inducible factor-1α, Mol. Cancer Ther 7 (2008) 90–100, 10.1158/1535-7163.MCT-07-0463. [DOI] [PubMed] [Google Scholar]
- [338].Zhang H, Wong CCL, Wei H, Gilkes DM, Korangath P, Chaturvedi P, Schito L, Chen J, Krishnamachary B, Winnard PT, Raman V, Zhen L, Mitzner WA, Sukumar S, Semenza GL, HIF-1-dependent expression of angiopoietin-like 4 and L1CAM mediates vascular metastasis of hypoxic breast cancer cells to the lungs, Oncogene 31 (2012) 1757–1770, 10.1038/onc.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [339].Zhang H, Qian DZ, Tan YS, Lee KA, Gao P, Ren YR, Rey S, Hammers H, Chang D, Pili R, Dang CV, Liu JO, Semenza GL, Digoxin and other cardiac glycosides inhibit HIF-1α synthesis and block tumor growth, Proc. Natl. Acad. Sci. USA 105 (2008) 19579–19586, 10.1073/pnas.0809763105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [340].Kummar S, Raffeld M, Juwara L, Horneffer Y, Strassberger A, Allen D, Steinberg SM, Rapisarda A, Spencer SD, Figg WD, Chen X, Turkbey IB, Choyke P, Murgo AJ, Doroshow JH, Melillo G, Multihistology, target-driven pilot trial of oral topotecan as an inhibitor of hypoxia-inducible factor-1α in advanced solid tumors, Clin. Cancer Res 17 (2011) 5123–5131, 10.1158/1078-0432.CCR-11-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [341].Martínez-Sáez O, Gajate Borau P, Alonso-Gordoa T, Molina-Cerrillo J, Grande E, Targeting HIF-2 α in clear cell renal cell carcinoma: a promising therapeutic strategy, Crit. Rev. Oncol. /Hematol 111 (2017) 117–123, 10.1016/j.critrevonc.2017.01.013. [DOI] [PubMed] [Google Scholar]
- [342].Lee KA, Zhang H, Qian DZ, Rey S, Liu JO, Semenza GL, Acriflavine inhibits HIF-1 dimerization, tumor growth, and vascularization, Proc. Natl. Acad. Sci. USA 106 (2009) 17910–17915, 10.1073/pnas.0909353106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [343].Mabjeesh NJ, Escuin D, LaVallee TM, Pribluda VS, Swartz GM, Johnson MS, Willard MT, Zhong H, Simons JW, Giannakakou P, 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF, Cancer Cell 3 (2003) 363–375, 10.1016/S1535-6108(03)00077-1. [DOI] [PubMed] [Google Scholar]
- [344].Kong D, Park EJ, Stephen AG, Calvani M, Cardellina JH, Monks A, Fisher RJ, Shoemaker RH, Melillo G, Echinomycin, a small-molecule inhibitor of hypoxia-inducible factor-1 DNA-binding activity, Cancer Res. 65 (2005) 9047–9055, 10.1158/0008-5472.CAN-05-1235. [DOI] [PubMed] [Google Scholar]
- [345].Nickols NG, Jacobs CS, Farkas ME, Dervan PB, Modulating hypoxiainducible transcription by disrupting the HIF-1–DNA interface, 2 (2007) 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [346].Bailey CM, Liu Y, Peng G, Zhang H, He M, Sun D, Zheng P, Liu Y, Wang Y, Liposomal formulation of HIF-1α inhibitor echinomycin eliminates established metastases of triple-negative breast cancer, Nanomed.: Nanotechnol. Biol. Med 29 (2020), 102278, 10.1016/j.nano.2020.102278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [347].Kung AL, Zabludoff SD, France DS, Freedman SJ, Tanner EA, Vieira A, Cornell-Kennon S, Lee J, Wang B, Wang J, Memmert K, Naegeli HU, Petersen F, Eck MJ, Bair KW, Wood AW, Livingston DM, Small molecule blockade of transcriptional coactivation of the hypoxia-inducible factor pathway, Cancer Cell 6 (2004) 33–43, 10.1016/j.ccr.2004.06.009. [DOI] [PubMed] [Google Scholar]
- [348].Shin DH, Chun YS, Lee DS, Huang LE, Park JW, Bortezomib inhibits tumor adaptation to hypoxia by stimulating the FIH-mediated repression of hypoxiainducible factor-1, Blood 111 (2008) 3131–3136, 10.1182/blood-2007-11-120576. [DOI] [PubMed] [Google Scholar]
- [349].Shan HL, Dong HS, Chun YS, Myung KL, Kim MS, Park JW, A novel mode of action of YC-1 in HIF inhibition: stimulation of FIH-dependent p300 dissociation from HIF-1α, Mol. Cancer Ther 7 (2008) 3729–3738, 10.1158/1535-7163.MCT-08-0074. [DOI] [PubMed] [Google Scholar]
- [350].Jacoby JJ, Erez B, Korshunova MV, Williams RR, Furutani K, Takahashi O, Kirkpatrick L, Lippman SM, Powis G, O’Reilly MS, Herbst RS, Treatment with hif-1α antagonist PX-478 inhibits progression and spread of orthotopic human small cell lung cancer and lung adenocarcinoma in mice, J. Thorac. Oncol 5 (2010) 940–949, 10.1097/JTO.0b013e3181dc211f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [351].Tang W, Zhao G, Small molecules targeting HIF-1α pathway for cancer therapy in recent years, Bioorg. Med. Chem 28 (2020), 115235, 10.1016/j.bmc.2019.115235. [DOI] [PubMed] [Google Scholar]
- [352].Wong CCL, Zhang H, Gilkes DM, Chen J, Wei H, Chaturvedi P, Hubbi ME, Semenza GL, Inhibitors of hypoxia-inducible factor 1 block breast cancer metastatic niche formation and lung metastasis, J. Mol. Med 90 (2012) 803–815, 10.1007/s00109-011-0855-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [353].Liu H, Chen C, Zeng J, Zhao Z, Hu Q, MicroRNA-210–3p is transcriptionally upregulated by hypoxia induction and thus promoting EMT and chemoresistance in glioma cells, PLoS ONE 16 (2021) 1–16, 10.1371/journal.pone.0253522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [354].Shin DH, Kim JH, Jung YJ, Kim KE, Jeong JM, Chun YS, Park JW, Preclinical evaluation of YC-1, a HIF inhibitor, for the prevention of tumor spreading, Cancer Lett. 255 (2007) 107–116, 10.1016/j.canlet.2007.03.026. [DOI] [PubMed] [Google Scholar]
- [355].Hiraga T, Kizaka-Kondoh S, Hirota K, Hiraoka M, Yoneda T, Hypoxia and hypoxia-inducible factor-1 expression enhance osteolytic bone metastases of breast cancer, Cancer Res. 67 (2007) 4157–4163, 10.1158/0008-5472.CAN-06-2355. [DOI] [PubMed] [Google Scholar]
- [356].Lin J, Zhan T, Duffy D, Hoffman-censits J, Kilpatrick D, Trabulsi EJ, Lallas CD, Ph D, Limentani K, Kennedy B, Kessler S, Gomella L, Antonarakis ES, Carducci MA, Kelly WK, A pilot phase II Study of digoxin in patients with recurrent prostate cancer as evident by a rising PSA, Am. J. Cancer Ther. Pharm 2 (2014) 21–32. [PMC free article] [PubMed] [Google Scholar]
- [357].Greenberger LM, Horak ID, Filpula D, Sapra P, Westergaard M, Frydenlund HF, Albæk C, Schrøder H, Ørum H, A RNA antagonist of hypoxiainducible factor-1α, EZN-2968, inhibits tumor cell growth, Mol. Cancer Ther 7 (2008) 3598–3608, 10.1158/1535-7163.MCT-08-0510. [DOI] [PubMed] [Google Scholar]
- [358].Jeong W, Rapisarda A, Park SR, Kinders RJ, Chen A, Melillo G, Turkbey B, Steinberg SM, Choyke P, Doroshow JH, Kummar S, Pilot trial of EZN-2968, an antisense oligonucleotide inhibitor of hypoxia-inducible factor-1 alpha (HIF-1α), in patients with refractory solid tumors, Cancer Chemother. Pharmacol 73 (2014) 343–348, 10.1007/s00280-013-2362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [359].Rapisarda A, Zalek J, Hollingshead M, Braunschweig T, Uranchimeg B, Bonomi CA, Borgel SD, Carter JP, Hewitt SM, Shoemaker RH, Melillo G, Schedule-dependent inhibition of hypoxia-inducible factor-1α protein accumulation, angiogenesis, and tumor growth by topotecan in U251-HRE glioblastoma xenografts, Cancer Res. 64 (2004) 6845–6848, 10.1158/0008-5472.CAN-04-2116. [DOI] [PubMed] [Google Scholar]
- [360].Cho H, Du X, Rizzi JP, Liberzon E, Chakraborty AA, Gao W, Carvo I, Signoretti S, Bruick RK, Josey JA, Wallace EM, Kaelin WG, On-target efficacy of a HIF-2α antagonist in preclinical kidney cancer models, Nature 539 (2016) 107–111, 10.1038/nature19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [361].Chen W, Hill H, Christie A, Kim MS, Holloman E, Pavia-Jimenez A, Homayoun F, Ma Y, Patel N, Yell P, Hao G, Yousuf Q, Joyce A, Pedrosa I, Geiger H, Zhang H, Chang J, Gardner KH, Bruick RK, Reeves C, Hwang TH, Courtney K, Frenkel E, Sun X, Zojwalla N, Wong T, Rizzi JP, Wallace EM, Josey JA, Xie Y, Xie XJ, Kapur P, McKay RM, Brugarolas J, Targeting renal cell carcinoma with a HIF-2 antagonist, Nature 539 (2016) 112–117, 10.1038/nature19796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [362].Wallace EM, Rizzi JP, Han G, Wehn PM, Cao Z, Du X, Cheng T, Czerwinski RM, Dixon DD, Goggin BS, Grina JA, Halfmann MM, Maddie MA, Olive SR, Schlachter ST, Tan H, Wang B, Wang K, Xie S, Xu R, Yang H, Josey JA, A small-molecule antagonist of HIF2α is efficacious in preclinical models of renal cell carcinoma, Cancer Res. 76 (2016) 5491–5500, 10.1158/0008-5472.CAN-16-0473. [DOI] [PubMed] [Google Scholar]
- [363].Courtney KD, Infante JR, Lam ET, Figlin RA, Rini BI, Brugarolas J, Zojwalla NJ, Lowe AM, Wang K, Wallace EM, Josey JA, Choueiri TK, Phase I dose-escalation trial of PT2385, a first-in-class hypoxia-inducible factor-2a antagonist in patients with previously treated advanced clear cell renal cell carcinoma, J. Clin. Oncol 36 (2018) 867–874, 10.1200/JCO.2017.74.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [364].Xu R, Wang K, Rizzi JP, Huang H, Grina JA, Schlachter ST, Wang B, Wehn PM, Yang H, Dixon DD, Czerwinski RM, Du X, Ged EL, Han G, Tan H, Wong T, Xie S, Josey JA, Wallace EM, 3-[(1 S,2 S,3 R)-2,3-Difluoro-1-hydroxy-7-methylsulfonylindan-4-yl]oxy-5-fluorobenzonitrile (PT2977), a hypoxia-inducible Factor 2α (HIF-2α)inhibitor for the treatment of clear cell renal cell carcinoma, J. Med. Chem 62 (2019) 6876–6893, 10.1021/acs.jmedchem.9b00719. [DOI] [PubMed] [Google Scholar]
- [365].Choueiri TK, Bauer TM, Papadopoulos KP, Plimack ER, Merchan JR, McDermott DF, Michaelson MD, Appleman LJ, Thamake S, Perini RF, Zojwalla NJ, Jonasch E, Inhibition of hypoxia-inducible factor-2α in renal cell carcinoma with belzutifan: a phase 1 trial and biomarker analysis, Nat. Med 27 (2021) 802–805, 10.1038/s41591-021-01324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [366].Yu Y, Yu Q, Zhang X, Allosteric inhibition of HIF-2α as a novel therapy for clear cell renal cell carcinoma, Drug Discov. Today 24 (2019) 2332–2340, 10.1016/j.drudis.2019.09.008. [DOI] [PubMed] [Google Scholar]
- [367].Deeks ED, Belzutifan: first approval, Drugs 81 (2021) 1921–1927, 10.1007/s40265-021-01606-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.


