Abstract
Background
Long duration trial data for two-dose COVID-19 vaccines primary series’ are uncommon due to unblinding and additional doses. We report one-year follow-up results from a phase 1/2 trial of AZD1222 (ChAdOx1 nCoV-19) in Japan.
Methods
Adults (n = 256) seronegative for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) were stratified by age, 18–55 (n = 128), 56–69 (n = 86) and ≥70-year-old (n = 42), and randomized 3:1 to AZD1222 or placebo. Safety, immunogenicity, and exploratory efficacy data were collected until study Day 365.
Results
Safety was consistent with previous reports. In AZD1222 vaccinees, humoral responses against SARS-CoV-2 steadily declined over time. By Day 365, anti-SARS-CoV-2 spike-binding (spike) and receptor-binding domain (RBD) mean antibody titers remained above Day 15 levels and pseudovirus neutralizing antibodies were undetectable in many participants.
Conclusions
AZD1222 is immunogenic and well tolerated in Japanese adults. Expected waning in anti-SARS-CoV-2 humoral responses was observed; spike and RBD antibody titers remained elevated. (ClinicalTrials.gov: NCT04568031).
Keywords: COVID-19, AZD1222, ChAdOx1 nCoV-19, Japan, Vaccine, Humoral response
1. Introduction
Assessing the duration of humoral responses to COVID-19 vaccines is important, given the potential for waning immunity to necessitate boosting [1], [2], [3] and the emergence of SARS-CoV-2 variants. However, while there continues to be variance in global booster vaccination policies and availability [4], [5], there is limited clinical trial immunogenicity data available past six months in participants without booster vaccinations [6].
We previously reported in our primary analysis of this trial, which evaluated the safety and immunogenicity of AZD1222 (ChAdOx1 nCoV-19) in Japanese adults up to 28 days post-second dose (Day 57), that AZD1222 was well tolerated and elicited strong humoral responses against SARS-CoV-2 [7]. In this final analysis, we report on extended participant safety and humoral immunogenicity as well as exploratory efficacy through one year post-first dose (Day 365). To our knowledge, this is the first randomized, placebo-controlled study to report the one-year immunogenicity of an adenovirus-vector-based vaccine primary series in an East Asian country.
2. Methods
2.1. Study design, participants, and ethics
Full details of this phase 1/2, randomized, double-blind, parallel-group, placebo-controlled trial (NCT04568031) design and participant information up to Day 57, the primary analysis cut-off, have been reported previously [7]. Methods specific to the final analysis, from Day 57 to Day 365, are detailed herein. Data were censored upon receipt of a non-study COVID-19 vaccine, as described below; otherwise, no changes to the previously reported inclusion or exclusion criteria were applied to the study population prior to this final analysis [7]. Briefly, the study included adults seronegative for SARS-CoV-2 and excluded participants with laboratory confirmed SARS-CoV-2 infection prior to randomization, prior or planned receipt of an investigational or licensed vaccine or product that may impact interpretation of trial data, and those with severe and/or uncontrolled cardiovascular, respiratory, gastrointestinal, hepatic, renal, endocrine, or neurologic comorbidities.
This study was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice. The protocol was approved by an institutional review board prior to initiation and participants provided written informed consent before enrollment.
2.2. Randomization and masking
As previously described, adults seronegative for SARS-CoV-2 were stratified by age and randomized (3:1) to receive either two doses of AZD1222 (5 × 1010 viral particles/dose) or saline (0.9 % weight/volume) placebo, delivered via deltoid intramuscular injection on Day 1 and Day 29 [7]. Investigators and participants were unblinded after the primary analysis data lock, 24 February 2021, to facilitate notification of participants when they became eligible for a licensed non-study COVID-19 vaccine during the follow-up period.
2.3. Study procedures
Study procedures have been detailed previously [7]. For safety, serious adverse events (SAEs) and adverse events of special interest (AESIs), as defined in the primary analysis, were recorded until Day 365 in participants who received at least one dose of study intervention [7]. For immunogenic analyses presented herein, serum samples were collected from participants on Days 183 and 365. Immunology and efficacy were evaluated in participants who received two doses of study intervention and had no protocol deviations that could interfere with assessed responses.
For immunogenicity assessment, anti-SARS-CoV-2 spike-binding (spike) and receptor-binding domain (RBD) antibody levels against ancestral SARS-CoV-2 were assessed using the Meso Scale Discovery (MSD) serological assay, a validated multiplexed electrochemiluminescence-based immunoassay (PPD Vaccines, Richmond, VA, USA) [7]. Additionally, pseudovirus neutralizing antibody (nAb) responses to ancestral SARS-CoV-2 were evaluated via a validated HIV-1-based pseudovirus assay (Monogram Biosciences, South San Francisco, CA, USA) [7]. For efficacy, serum samples collected at study visits on Days 57, 183 and 365 were tested for nucleocapsid (NP) seroresponse via MSD assay as previously described [8]. The threshold for NP seroresponse was defined as ≥ 9787 AU/mL, based on a 99th upper percentile serostatus cut point with pre-pandemic samples known to be SARS-CoV-2-negative [8].
Participants who received non-study COVID-19 vaccines were censored from the reported date of non-study COVID-19 vaccination for safety, immunogenicity, and exploratory efficacy outcomes. Participants who continued to attend scheduled site visits after data censoring were evaluated independently for anti-SARS-CoV-2 immunogenicity after non-study COVID-19 vaccination using the methodology described above.
2.4. Statistical methodology
Statistical methodology has been detailed previously [7]. Briefly, a sample size of 128 participants (96 and 32 randomized to AZD1222 and placebo arms, respectively) in each age cohort for the primary analysis (18–55 years and ≥ 56 years) was determined mainly for the evaluation of safety and based on feasibility. Geometric mean titer (GMT) and geometric mean fold rise (GMFR) were calculated along with their 95 % CI based on the Clopper Pearson method.
To evaluate the extent of the waning observed during the follow-up period, a post-hoc mixed model for repeated measures of the log-transformed titer values for anti-SARS-CoV-2 spike and RBD antibodies was performed with Tukey-Kramer multiplicity adjusted post-Day 1 timepoint comparisons in participants who had received two study doses of AZD1222. The model was used to explore differences in GMTs recorded at Days 183 and 365 in participants in the AZD1222 arm relative to respective Day 15 values.
2.5. Outcomes
Safety was evaluated in all participants receiving study intervention until Day 365. AZD1222 immunogenicity outcomes were GMT and GMFR of anti-SARS-CoV-2 spike, RBD and nAb until Day 365. Occurrences of reported positive SARS-CoV-2 reverse transcription polymerase chain reaction (RT-PCR) or antigen tests, or SARS-CoV-2 NP seroresponse in serum samples collected after Day 43 were recorded for exploratory efficacy analyses.
2.6. Immunogenicity after non-study COVID-19 vaccination
Immunogenicity data, including anti-SARS-CoV-2 spike, RBD, and pseudovirus nAb titers, for all participants who had reported a non-study COVID-19 vaccination (mRNA-1273 or BNT162b2) and attended site visits on Days 183 and 365, were plotted according to the time that had elapsed from the date of the first reported non-study COVID-19 vaccination. Peak titers from participants who had been randomized to the placebo arm and who reported receiving two doses of a non-study COVID-19 vaccination were pooled for comparison against respective peak GMTs obtained from AZD1222 vaccinees and corresponding placebo controls at Days 43 or 57.
3. Results
3.1. Participants
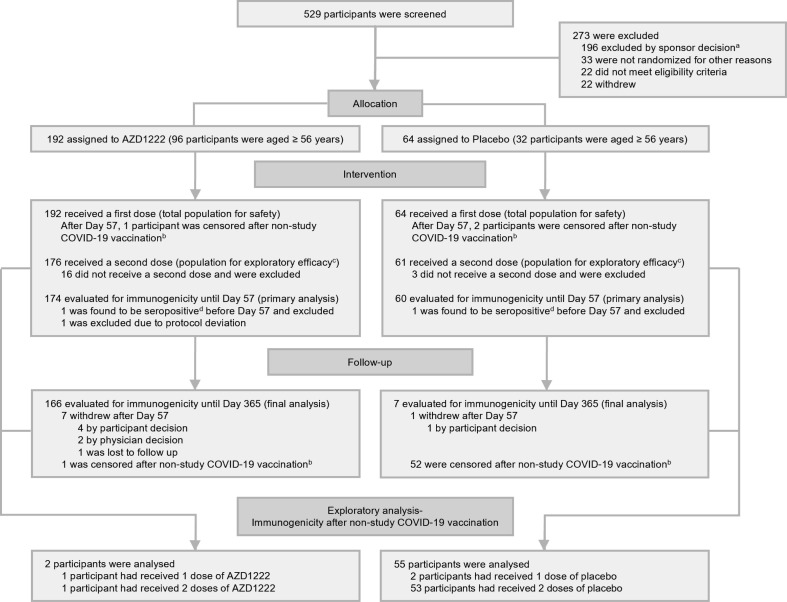
Between August–November 2020, 192 and 64 participants were randomized to receive AZD1222 or placebo, respectively (Fig. 1 ). The baseline demographics of the full study population have been published previously [7]. Among the AZD1222 arm of 65 female and 127 male participants, mean age was 55.3 years; among the placebo arm of 22 female and 42 male participants, mean age was 56.1 years. The last participant study visit occurred in November 2021; clinical data lock was in January 2022. The median follow-up time for safety in participants who received at least one dose of AZD1222 was 355.5 (range: 183, 383) days, compared to 271.5 (range: 140, 368) days in participants who received placebo. As previously reported, at Day 57, 174 and 60 participants remained enrolled in the AZD1222 and placebo arms, respectively, and were evaluated in the primary analysis for immunogenicity (Fig. 1).
Fig. 1.
Participant disposition.aAll screen failures categorized as ‘excluded by sponsor decision’ were associated with a temporary interruption in the study, and consequent pause in enrollment following a reported serious adverse event of transverse myelitis in another clinical trial [18]. bData collected from participants who reported receiving a non-study COVID-19 vaccination were censored from safety, AZD1222 immunogenicity, and efficacy outcomes from the date of the first dose of non-study COVID-19 vaccination. cData from all participants who received two doses of study intervention and had not discontinued from the study by Day 43 were included in exploratory efficacy analyses. dHad seroresponse (a ≥ 4-fold rise in titer compared to recorded Day 1 baseline values) to nucleocapsid antibodies as quantified by MSD serology assay up to Day 57. Abbreviation: MSD = Meso Scale Discovery.
After the primary analysis cut-off (Day 57; approximately two months following the first dose of study intervention), 7 participants in the AZD1222 arm (3.6 %, participant withdrawal: n = 4; physician decision: n = 2; lost to follow-up: n = 1) and one in the placebo arm (1.6 %, participant withdrawal) discontinued from the study. The remaining participants were eligible for evaluation until the end of the follow-up period (Day 365) in this final analysis (Fig. 1). Additionally, during the one-year follow-up, one (0.6 %) participant in the AZD1222 arm and 52 (86.7 %) participants in the placebo arm reported a non-study COVID-19 vaccination and were consequently censored from the reported date of vaccination from AZD1222 immunogenicity, efficacy, and safety outcomes (Fig. 1, Supplementary Fig. 1).
3.2. Safety
SAEs were reported in three participants who received AZD1222 (1.6 %, events = 6) and two participants who received placebo (3.1 %, events = 2) during the study. SAEs recorded after Day 57, included 6 events in the AZD1222 and 1 in the placebo arm (Supplementary Table 1, see footnote for event details). None of the reported SAEs were considered related to study intervention, no AESIs, which included thrombotic events, were reported for the duration of follow-up, and no deaths were reported (Supplementary Table 1).
3.3. AZD1222 immunogenicity
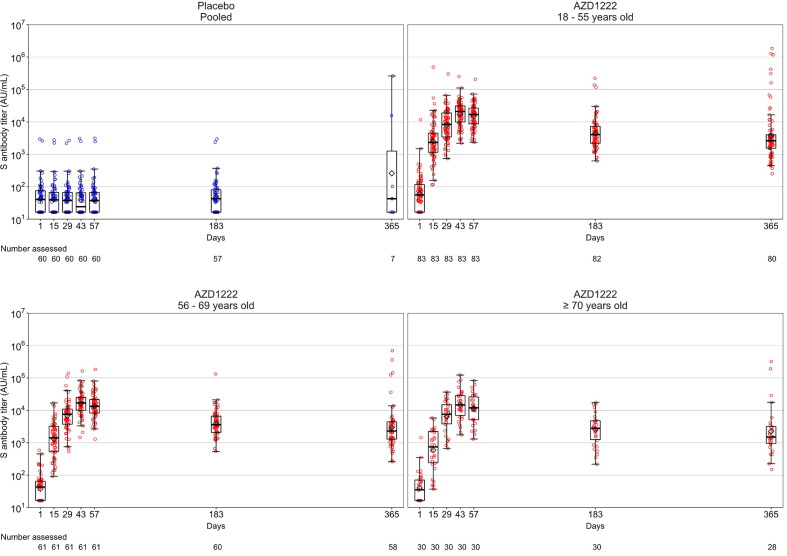
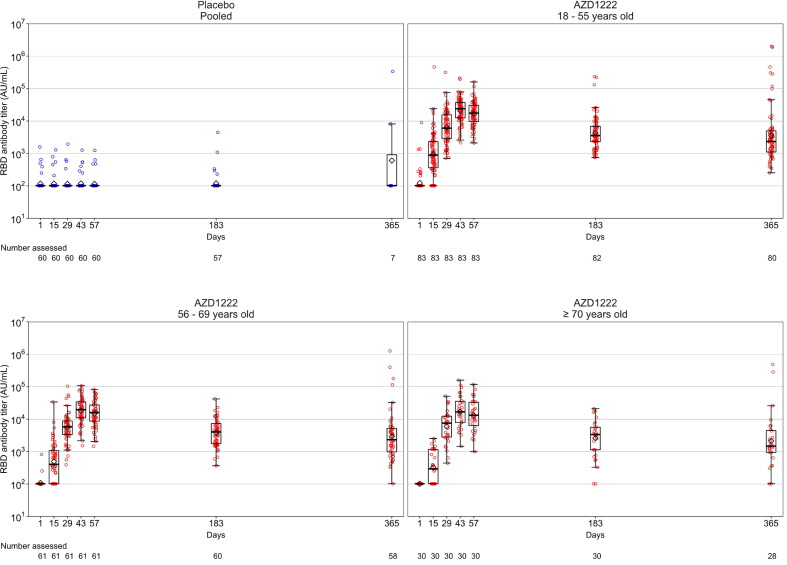
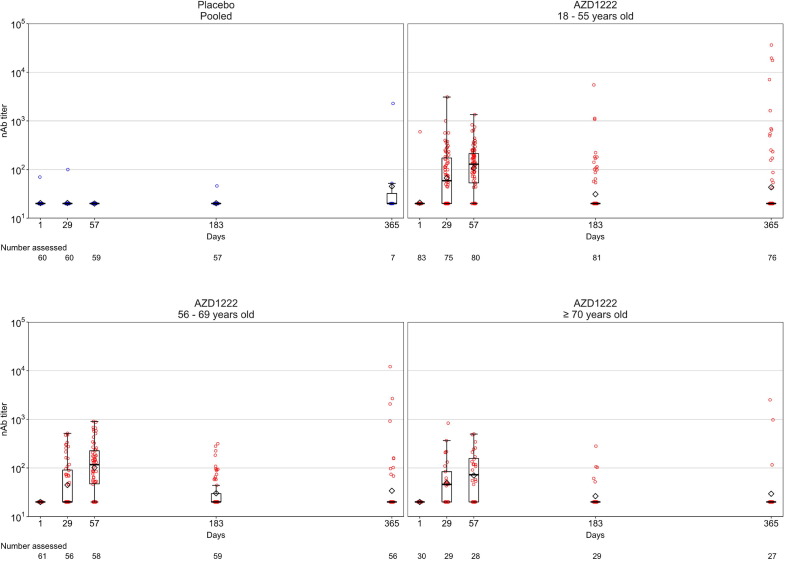
The median follow-up time for immunogenicity was 355.5 (range: 183, 383) days in the AZD1222 arm and 268.0 (range: 140, 365) days in the placebo arm, respectively. As previously reported [7], vaccination with AZD1222 elicited strong humoral responses in participants, which increased substantially after both the first and second doses, peaking at or before Day 57 (Fig. 2, Fig. 3, Fig. 4 ; Supplementary Table 2). After Day 57, anti-SARS-CoV-2 spike and RBD antibody levels in participants who received AZD1222 gradually declined over time (Fig. 2, Fig. 3; Supplementary Table 2). Post-hoc analysis of Day 183 and Day 365 anti-SARS-CoV-2 spike and RBD levels in participants who received AZD1222 indicated that GMTs remained significantly elevated above Day 15 levels for the duration of follow-up (p < 0.0001 for all comparisons). The decline in pseudovirus nAb titers was more pronounced than binding titer, approaching baseline values by Day 183 and falling below the lower limit of quantification in most participants by Day 365 (Fig. 4; Supplementary Table 2). In the placebo arm, humoral responses remained near baseline throughout the study; excepting high anti-SARS-CoV-2 antibody levels recorded in two NP seronegative participants at Day 365, likely caused by unreported non-study COVID-19 vaccination (Fig. 2, Fig. 3, Fig. 4; Supplementary Table 2). Overall, mean humoral responses trended downwards as age increased (Fig. 2, Fig. 3, Fig. 4; Supplementary Table 2).
Fig. 2.
Anti-SARS-CoV-2 spike responses over time in study participants who received two doses of study intervention. Box plots and individual plots of anti-SARS-CoV-2 spike antibody titers over time in participants, stratified by age, who received AZD1222 or placebo (on Days 1 and 29) and had not been censored at the time of study visits, as determined by Meso Scale Discovery serological assay. The pooled placebo arm includes all participants who received two doses of placebo; participants who received two doses of AZD1222 are stratified by age. The bottom and top edges of the box indicate the first and third quartiles [the difference is the IQR], the line is the median, and the diamond mark is the geometric mean. The whiskers that extend from the box indicate the minimum and maximum within the range of 1.5 × IQR from the box. Box plots are created using log-transformed values. Titer values measured as below the LLoQ (33 AU/mL) are imputed to a value that is half of the LLoQ. Titer values measured as above the ULoQ (2,000,000 AU/mL) are imputed at the ULoQ value. Abbreviations: AU/mL = arbitrary units per milliliter, IQR = interquartile range, LLoQ = lower limit of quantification, S = spike, SARS-CoV-2 = severe acute respiratory syndrome coronavirus 2, ULoQ = upper limit of quantification.
Fig. 3.
Anti-SARS-CoV-2 receptor-binding domain (RBD) responses over time in participants who received two doses of study intervention. Box plots and individual plots of anti-SARS-CoV-2 RBD antibody titers over time in participants who received AZD1222 or placebo (Days 1 and 29) and were not censored at the time of study visits, determined by Meso Scale Discovery serological assay. The pooled placebo arm includes all participants who received two doses of placebo; participants who received two doses of AZD1222 are stratified by age. The bottom and top edges of the box indicate the first and third quartiles [the difference is the IQR], the line is the median, and the diamond mark is the geometric mean. The whiskers that extend from the box indicate the minimum and maximum within the range of 1.5 × IQR from the box. Box plots are created using log-transformed values. Titer values measured as below the LLoQ (204 AU/mL) are imputed to a value that is half of the LLoQ. Titer values measured as above the ULoQ (2,000,000 AU/mL) are imputed at the ULoQ value. Abbreviations: AU/mL = arbitrary units per milliliter, IQR = interquartile range, LLoQ = lower limit of quantification, RBD = receptor-binding domain, SARS-CoV-2 = severe acute respiratory syndrome-coronavirus 2, ULoQ = upper limit of quantification.
Fig. 4.
Pseudovirus neutralizing antibody titers over time in study participants who received two doses of study intervention. Box plots and individual plots of nAb antibody titers over time in participants who received AZD1222 or placebo (on Days 1 and 29) and had not been censored at the time of study visits, as determined by pseudovirus neutralization assay. The pooled placebo arm includes all participants who received two doses of placebo; participants who received two doses of AZD1222 are stratified by age. The bottom and top edges of the box indicate the first and third quartiles [the difference is the IQR], the line is the median, and the diamond mark is the geometric mean. The whiskers that extend from the box indicate the minimum and maximum within the range of 1.5 × IQR from the box. Box plots are created using log-transformed values. Titer values measured as below the LLoQ (40) are imputed to a value that is half of the LLoQ. Titer values measured as above the ULoQ (787,339) are imputed at the ULoQ value. Abbreviations: IQR = interquartile range, LLoQ = lower limit of quantification, nAb = pseudovirus neutralizing antibodies, ULoQ = upper limit of quantification.
3.4. SARS-CoV-2 infection incidence
Occurrence of SARS-CoV-2 infections was recorded for an exploratory assessment of efficacy. Median follow-up time was longer in participants who received AZD1222, at 355.0 (n = 176, range: 183, 383) days, than in participants who received placebo, at 270.0 (n = 61, range: 140, 365) days. Positive tests for SARS-CoV-2 were reported by two (1.0 %) participants aged 18–55 years in the AZD1222 arm – one asymptomatic participant had taken a RT-PCR test as a requirement for travel and one symptomatic participant had taken an antigen test. Additionally, some participants in the AZD1222 arm (Day 57: n = 1: Day 183: n = 2, Day 365: n = 5) exhibited a SARS-CoV-2 NP seroresponse (Supplementary Table 3). By Day 365, the majority of participants (86.7 %, n = 53) in the placebo arm were censored after reporting a non-study COVID-19 vaccine, hindering assessments of efficacy. No SARS-CoV-2 infections were reported in the placebo arm during the follow-up period (Supplementary Table 3).
3.5. Immunogenicity after non-study COVID-19 vaccination
Immunogenicity was assessed in all participants following non-study COVID-19 vaccinations, including 2 participants randomized to AZD1222 (1.0 %, mRNA-1273: n = 2) and 55 participants randomized to placebo (89.1 %, mRNA-1273: n = 18; BNT162b2: n = 35; mRNA vaccine not specified by study center or able to be inferred from the dosing interval: n = 2). Individual anti-SARS-CoV-2 spike, RBD, and nAb titers in participants who received non-study COVID-19 vaccines were generally higher than respective peak GMTs recorded in participants who received AZD1222 (Supplementary Fig. 2). As in participants who received AZD1222, peak antibody titers in participants who received non-study COVID-19 vaccinations were observed after second doses were reported – between 30 and 60 days after the reported first dose of non-study COVID-19 vaccination. Waning of humoral responses over time was also noted in participants who received non-study COVID-19 vaccines (Supplementary Fig. 2). Pooled mean titers captured from ten participants who received non-study COVID-19 vaccination (randomized to placebo) within the 30–60 day post-first dose peak time-window were higher than the peak GMTs captured after AZD1222 or placebo (Supplementary Fig. 3).
3.6. Subgroup analysis
No notable patterns or trends were observed in subgroup analyses of immunogenicity by gender (Supplementary Table 2). Though these results were generally consistent with those of the overall population, they should be interpreted with caution given the exploratory nature of these analyses and the small sample sizes.
4. Discussion
Our analysis of the one-year immunogenicity following an AZD1222 primary series in Japanese adult participants in this phase 1/2 trial demonstrates an expected, gradual waning of humoral immunity, consistent with previous reports of both AZD1222 and other COVID-19 vaccines [1], [2], [3], [6]. However, we found anti-SARS-CoV-2 spike and RBD antibody levels remained above post-first-dose (Day 15) levels for the duration of the one-year follow-up, indicating some level of protection may persist. This theory is supported by recent results which suggest that a lower incidence of COVID-19, including severe or critical disease, is maintained for at least 6 months after the first dose despite notable waning of anti-SARS-CoV-2 humoral responses [6], [9]. This long-term efficacy could potentially be mediated by cellular responses not explored herein [10].
These results contain the longest reported primary series immunogenicity data in an East Asian population, including the duration of immunogenicity in high-risk age groups [11]. Thus, this work may inform the potential impact of the ongoing challenges in vaccine availability that persist in many areas of the world [5]. Additionally, our results are consistent with the established AZD1222 safety profile [6], [12], indicating that AZD1222 remains well tolerated.
The small study population size coupled with the frequency, demographics, and timing of non-study COVID-19 vaccination, especially in the placebo arm, prevented meaningful between-group comparisons of efficacy against SARS-CoV-2 infection. Interpretation was further complicated by low rates of SARS-CoV-2 exposure experienced in Japan; under 200 daily COVID-19 cases/million persons were reported for the study duration [13]. Furthermore, an increase in pseudovirus nAb titers observed in several participants at late time points likely reflected unreported non-study COVID-19 vaccination or subclinical infection. Consistent with the primary analysis, our follow-up used standardized assays against ancestral SARS-CoV-2. Other studies of SARS-CoV-2 variant specific neutralizing antibody responses post-AZD1222 indicate responses against the Omicron variant are low following two-dose primary vaccination but increase after additional AZD1222 doses [14], [15].
Consistent with previous reports, early anti-SARs-CoV-2 antibody GMTs were higher in participants after non-study mRNA-1273 or BNT162b2 vaccinations than post-AZD1222 [1], [3], [12]. However, similar effectiveness against severe disease has been observed in global studies of mRNA COVID-19 vaccines and AZD1222 [9], [16] and there is no established threshold for protection against COVID-19. Additionally, a similar pattern of waning of humoral responses and subsequent decline in protection against SARS-CoV-2 infection has been observed after mRNA-based COVID-19 vaccines and AZD1222, an effect amplified by the emergence of variants with increased immune evasion [1], [2], [3], [17].
5. Conclusion
These data suggest that AZD1222 is well tolerated and elicits persistent anti-SARS-CoV-2 spike and RBD antibody responses in Japanese adults, regardless of age, with expected waning over the course of one year.
CRediT authorship contribution statement
Kensuke Ishikawa: Conceptualization, Data curation, Supervision. Maria-Claudia Nascimento: Validation, Methodology, Supervision. Michiko Asano: Conceptualization, Data curation, Supervision. Hajime Hirata: Conceptualization, Data curation. Yohji Itoh: Conceptualization, Data curation, Supervision, Formal analysis. Elizabeth J. Kelly: Conceptualization, Data curation, Supervision. Akiko Matsui: Data curation, Supervison. Urban Olsson: Conceptualization, Data curation, Validation, Methodology, Supervision. Kathryn Shoemaker: Data curation, Formal analysis, Supervision. Justin Green: Conceptualization, Data curation, Supervision.
Data Sharing
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure. Data for studies directly listed on Vivli can be requested through Vivli at www.vivli.org. Data for studies not listed on Vivli could be requested through Vivli at https://vivli.org/members/enquiries-about-studies-not-listed-on-the-vivli-platform/. AstraZeneca Vivli member page is also available outlining further details: https://vivli.org/ourmember/astrazeneca/.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Justin Green reports financial support for the study was provided by AstraZeneca K.K. Justin Green reports a relationship with AstraZeneca that includes: employment and may hold equity or stocks. Kensuke Ishikawa reports a relationship with AstraZeneca that includes: employment and may hold equity or stocks. Maria-Claudia Nascimento reports a relationship with AstraZeneca that includes: employment and may hold equity or stocks. Michiko Asano reports a relationship with AstraZeneca that includes: employment and may hold equity or stocks. Yohji Itoh reports a relationship with AstraZeneca that includes: employment and may hold equity or stocks. Elizabeth J. Kelly reports a relationship with AstraZeneca that includes: employment and may hold equity or stocks. Akiko Matsui reports a relationship with AstraZeneca that includes: employment and may hold equity or stocks. Urban Olsson reports a relationship with AstraZeneca that includes: employment and may hold equity or stocks. Kathryn Shoemaker reports a relationship with AstraZeneca that includes: employment and may hold equity or stocks.
Acknowledgments
Acknowledgements
The authors wish to thank the participants, their families and all investigators (Akiyoshi Uchiyama, Tokyo Asbo Clinic, formerly Shinagawa East One Medical Clinic, Tokyo, Japan; Atsuko Abe, Seikoukai New Medical Research System Clinic, Hachioji City, Tokyo, Japan; Takuma Yonemura, Souseikai Sumida Hospital, Sumida City, Tokyo, Japan; Kenjiro Nakamura, Tenjin Sogo Clinic, Fukuoka City, Fukuoka, Japan; Akira Numata, Ikebukuro Metropolitan Clinic, Toshima City, Tokyo, Japan), Tatsuya Nakamura (study leader, AstraZeneca K.K.), clinical site staff and all relevant persons involved in this study. The authors would like to acknowledge Tonya Villafana and Rebecca A. Bachmann for providing a critical appraisal of the manuscript, Johan Vekemans for his contributions to the study design, and Seth Seegobin and Dongmei Lan for assistance with the post-hoc analysis design and validation. The authors would also like to thank Monogram Biosciences (South San Francisco, USA) and PPD Vaccines (Richmond, VA, USA) for the sample testing performed for this study. Medical writing support for the development of this manuscript, under the direction of the authors, was provided by Rose Follis, PhD, of Ashfield MedComms (Macclesfield, UK), an Inizio company, in accordance with Good Publication Practice (GPP 2022) guidelines (www.ismpp.org/gpp-2022; Ann Intern Med. 2022; 175(9):1298-1304) and funded by AstraZeneca.
Funding
This study was sponsored by AstraZeneca K.K. The sponsor was involved in the study design, collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.05.015.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data underlying the findings described may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
References
- 1.Levin E.G., Lustig Y., Cohen C., Fluss R., Indenbaum V., Amit S., et al. Waning immune humoral response to BNT162b2 Covid-19 vaccine over 6 months. N Engl J Med. 2021;385(24):e84. doi: 10.1056/NEJMoa2114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews N., Stowe J., Kirsebom F., Toffa S., Rickeard T., Gallagher E., et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doria-Rose N., Suthar M.S., Makowski M., O'Connell S., McDermott A.B., Flach B., et al. Antibody persistence through 6 months after the second dose of mRNA-1273 vaccine for Covid-19. N Engl J Med. 2021;384(23):2259–2261. doi: 10.1056/NEJMc2103916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shekhar R., Garg I., Pal S., Kottewar S., Sheikh A.B. COVID-19 vaccine booster: To boost or not to boost. Infect Dis Rep. 2021;13(4):924–929. doi: 10.3390/idr13040084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watson O.J., Barnsley G., Toor J., Hogan A.B., Winskill P., Ghani A.C. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22(9):1293–1302. doi: 10.1016/S1473-3099(22)00320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sobieszczyk M.E., Maaske J., Falsey A.R., Sproule S., Robb M.L., Frenck R.W., Jr, et al. Durability of protection and immunogenicity of AZD1222 (ChAdOx1 nCoV-19) COVID-19 vaccine over 6 months. J Clin Invest. 2022;132(18):e160565. doi: 10.1172/JCI160565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asano M., Okada H., Itoh Y., Hirata H., Ishikawa K., Yoshida E., et al. Immunogenicity and safety of AZD1222 (ChAdOx1 nCoV-19) against SARS-CoV-2 in Japan: a double-blind, randomized controlled phase 1/2 trial. Int J Infect Dis. 2022:114165–114174. doi: 10.1016/j.ijid.2021.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilkins D., Aksyuk A.A., Ruzin A., Tuffy K.M., Green T., Greway R., et al. Validation and performance of a multiplex serology assay to quantify antibody responses following SARS-CoV-2 infection or vaccination. Clin Transl Immunology. 2022;11(4):e1385. doi: 10.1002/cti2.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasreen S., Chung H., He S., Brown K.A., Gubbay J.B., Buchan S.A., et al. Effectiveness of COVID-19 vaccines against symptomatic SARS-CoV-2 infection and severe outcomes with variants of concern in Ontario. Nat Microbiol. 2022;7(3):379–385. doi: 10.1038/s41564-021-01053-0. [DOI] [PubMed] [Google Scholar]
- 10.Swanson P.A., 2nd, Padilla M., Hoyland W., McGlinchey K., Fields P.A., Bibi S., et al. AZD1222/ChAdOx1 nCoV-19 vaccination induces a polyfunctional spike protein-specific T(H)1 response with a diverse TCR repertoire. Sci Transl Med. 2021;13(620):eabj7211 doi: 10.1126/scitranslmed.abj7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Asai Y., Nomoto H., Hayakawa K., Matsunaga N., Tsuzuki S., Terada M., et al. Comorbidities as risk factors for severe disease in hospitalized elderly COVID-19 patients by different age-groups in Japan. Gerontology. 2022;68(9):1027–1037. doi: 10.1159/000521000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falsey A.R., Sobieszczyk M.E., Hirsch I., Sproule S., Robb M.L., Corey L., et al. Phase 3 safety and efficacy of AZD1222 (ChAdOx1 nCoV-19) Covid-19 vaccine. N Engl J Med. 2021;385(25):2348–2360. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Our World in Data (University of Oxford). Coronavirus (COVID-19) Cases; 2022. Available at: https://ourworldindata.org/covid-cases. Last accessed 14 September 2022.
- 14.Dejnirattisai W., Huo J., Zhou D., Zahradnik J., Supasa P., Liu C., et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185(3):467–84 e15. doi: 10.1016/j.cell.2021.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maaske J., Sproule S., Falsey A.R., Sobieszczyk M.E., Luetkemeyer A.F., Paulsen G.C., et al. Robust humoral and cellular recall responses to AZD1222 attenuate breakthrough SARS-CoV-2 infection compared to unvaccinated. Front Immunol. 2022:131062067. doi: 10.3389/fimmu.2022.1062067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pritchard E., Matthews P.C., Stoesser N., Eyre D.W., Gethings O., Vihta K.D., et al. Impact of vaccination on new SARS-CoV-2 infections in the United Kingdom. Nat Med. 2021;27(8):1370–1378. doi: 10.1038/s41591-021-01410-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas S.J., Moreira E.D., Jr., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N Engl J Med. 2021;385(19):1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data underlying the findings described may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.