Abstract
The Ca2+ signaling genes cpe-1, plc-1, ncs-1, splA2, camk-1, camk-2, camk-3, camk-4, cmd, and cnb-1 are necessary for a normal circadian period length in Neurospora crassa. In addition, the Q10 values ranged between 0.8 and 1.2 for the single mutants lacking cpe-1, splA2, camk-1, camk-2, camk-3, camk-4, and cnb-1, suggesting that the circadian clock exhibits standard temperature compensation. However, the Q10 value for the ∆plc-1 mutant was 1.41 at 25 and 30 °C, 1.53 and 1.40 for the ∆ncs-1 mutant at 20 and 25 °C, and at 20 and 30 °C, respectively, suggesting a partial loss of temperature compensation in these two mutants. Moreover, expression of frq, a regulator of the circadian period, and the blue light receptor wc-1, were increased >2-fold in the Δplc-1, ∆plc-1; ∆cpe-1, and the ∆plc-1; ∆splA2 mutants at 20 °C. The frq mRNA level was increased >2-fold in the Δncs-1 mutant compared to the ras-1bd strain at 20 °C. Therefore, multiple Ca2+ signaling genes regulate the circadian period, by influencing expression of the frq and wc-1 genes that are critical for maintaining the normal circadian period length in N. crassa.
Keywords: calcium signaling, circadian clock, Neurospora crassa, period length, frequency, white collar-1
Multiple calcium signaling genes regulate the circadian period length by modulating expression of the frequency and white collar-1 genes in Neurospora crassa.
Introduction
Circadian rhythms are ubiquitous biological oscillations with an ∼24 h period that impact diverse cell processes, including cell division, homeostasis, immunity, physiology, and sleep-wake cycles in eukaryotes ranging from fungi to mammals (Aronson et al. 1994a). This internal timekeeping mechanism or daily clock phenomenon was termed "circadian" in the 1950s by Halberg, combining the Latin terms ‘circa’ for ‘about’ and ‘dien’ for ‘day’ to explain the biological activity having a frequency of one cycle in every 24 h (Halberg et al. 2003). The circadian clock has a free-running period, which runs at approximately the same rate within a broad range of temperatures, a phenomenon known as temperature compensation, expressed as Q10 that normally ranges between 0.8 and 1.2 (Mattern et al. 1982, Sorek and Levy 2012, Avello et al. 2019).
The calcium ion (Ca2+), a ubiquitous secondary messenger, plays a role in mammalian circadian timekeeping (O'Neill and Reddy 2012). The extracellular signals mediated by Ca2+ regulate amplitude, phase, and period in mammals (O'Neill and Reddy 2012). In mice, a transmembrane Ca2+ flux maintains the molecular rhythmicity by regulating the expression of the clock gene in the hypothalamic suprachiasmatic nucleus (SCN; Lundkvist et al. 2005). The circadian timing in the SCN neurons is entrained by changes in adenylate cyclase and phospholipase C (PLC) activities (An et al. 2011). In addition, inhibition of the inositol 1,4,5-trisphosphate receptor (IP3R) or the endoplasmic-reticulum Ca2+-ATPase (SERCA) increases period length, indicating a role for Ca2+ in modulating the molecular circadian clock in the liver of rats (Báez-Ruiz and Díaz-Muñoz 2011). In both prokaryotes and eukaryotes, Na2+/Ca2+ mediated Ca2+ signaling is conserved in temperature-compensated circadian rhythms (Kon et al. 2021).
The model filamentous fungus Neurospora crassa displays a visible circadian rhythm in the vegetative developmental program (Bell-pedersen et al. 1992, Aronson et al. 1994a). In N. crassa, the circadian period is changed only slightly at different temperatures, thus compensated for the temperature difference, and expressed as the Q10 ratio. The stable interaction of FREQUENCY-casein Kinase 1 (FRQ-CK1) is critical for temperature compensation in N. crassa (Hu et al. 2021). Several molecular components of the clock, such as frequency (frq) and the white collar genes (wc-1 and wc-2) have already been characterized (Aronson et al. 1994a, 1994b, Crosthwaite et al. 1997). The frq transcript oscillates daily and the FRQ protein sets the circadian clock phase (Aronson et al. 1994b). In N. crassa, WC-1 and WC-2 are two proteins in the GATA zinc finger family of nuclear transcription factors that bind to the consensus element within the promoter of light-regulated genes (Ballario et al. 1996, Linden and Macino 1997). The WC proteins play an essential role in maintaining the N. crassa circadian feedback loop (Crosthwaite et al. 1997). The WC-1 and WC-2 proteins interact to form the white collar complex (WCC) via their conserved Per-Arnt-Sim (PAS) domains (Ballario et al. 1998, Cheng et al. 2002, Franchi et al. 2005, Wang et al. 2016) to maintain circadian rhythmicity in constant darkness by regulating rhythmic expression from the frq locus (Crosthwaite et al. 1997, Garceau et al. 1997). The newly synthesized FRQ is progressively phosphorylated by several kinases and regulated by phosphatases (Baker et al. 2012). FRQ inhibits its own transcription through FRQ-dependent phosphorylation of the WCC complex, using a negative feedback loop, because the phosphorylated WCC complex cannot bind to the frq promoter (Aronson et al. 1994b, Baker et al. 2012, Wang et al. 2019). However, when FRQ levels fall below a critical threshold, frq transcription is reactivated (Garceau et al. 1997, Liu and Bell-Pedersen 2006). Therefore, the negative feedback loop causes daily rhythmic accumulation of frq mRNA and FRQ protein, and their oscillations are essential for the normal circadian clock in N. crassa (Garceau et al. 1997, Liu and Bell-Pedersen 2006). Moreover, FRQ acts positively on WC-1 and WC-2 by upregulating the WC-1 protein levels post-transcriptionally and wc-2 mRNA levels transcriptionally, thereby forming an interlocked positive feedback loop (Cheng et al. 2001).
The Ca2+ signaling gene cmd encodes calmodulin (CaM), a Ca2+ sensor required for growth, stress tolerance, circadian clock, and sexual development in N. crassa (Laxmi and Tamuli 2015, 2017). CaM activates several other Ca2+ signaling proteins, including Ca2+/CaM-dependent kinases (Ca2+/CaMKs) and calcineurin. In N. crassa, the camk-1, camk-2, camk-3, and camk-4 genes encode four different Ca2+/CaMKs, including Ca2+/CaMK-1 and Ca2+/CaMK-2, that are both required for full fertility (Tamuli et al. 2011, Kumar and Tamuli 2014). The cna-1 and cnb-1 genes encode the calcineurin catalytic subunit A (CNA-1) and the regulatory subunit B (CNB-1), respectively in N. crassa (Tamuli et al. 2016, Kumar et al. 2019).
The Ca2+ signaling genes cpe-1, plc-1, and splA2 encode a Ca2+/H+ exchanger (CPE-1), a phospholipase C-1 (PLC-1), and a secretory phospholipase A2 (sPLA2), respectively (Barman and Tamuli 2015). The cpe-1, plc-1, and splA2 genes are necessary for growth, conidiation, carotenoid accumulation, and maintaining Ca2+ homeostasis in N. crassa (Barman and Tamuli 2015, Roy et al. 2020). Moreover, cpe-1 and splA2 exhibit epistatic interactions with plc-1 for normal asexual and sexual development in N. crassa (Barman and Tamuli 2017). In the cell, various Ca2+ sensors respond to high concentrations of Ca2+. The neuronal calcium sensor-1 (NCS-1) protein (Deka et al. 2011) interacts with a Ca2+-permeable channel (MID-1), which has a role in maintaining Ca2+ homeostasis (Lew et al. 2008), and possibly blocks the channel for tolerance to high Ca2+ concentrations (Gohain and Tamuli 2019).
Although the core clock mechanism has been identified in N. crassa, a possible role for Ca2+ signaling genes in regulating the circadian rhythm has remained largely unexplored. In this study, we investigated multiple Ca2+ signaling genes for their roles in regulating N. crassa circadian period length under different temperature conditions. We found that the Ca2+ signaling genes cpe-1, plc-1, ncs-1, splA2, camk-1, camk-2, camk-3, camk-4, cmd, and cnb-1 play roles in maintaining the normal circadian period length and/or temperature compensation in N. crassa. Additionally, expression of the clock regulatory frq and wc-1 genes was altered in the Ca2+ signaling mutants that exhibited abnormal period length.
Materials and methods
Strains, media, and growth conditions
Strains were obtained from the Fungal Genetics Stock Center (FGSC; Kansas State University, Manhattan, KS; McCluskey et al. 2010) or generated in this study (Table S1). For vegetative growth, strains were routinely cultured on Vogel's minimal medium N (VM; Vogel 1964) containing 1.5% D-glucose as a carbon source and 2% Bacto agar (Davis and De Serres 1970). VM was supplemented with calcium-D-pantothenate (CMS168-100GM, Himedia Laboratories, Mumbai, India) at a concentration of 0.5 mg/ml for the growth of the pantothenic acid auxotrophic mutants (pan-2−). For the cnb-1RIP mutants, 50 µM bathocuproinedisulfonic acid (BCS; B1125-500 MG, Sigma–Aldrich, St. Louis, MO, USA) was added to VM in addition to calcium-D-pantothenate. Crosses were performed using the synthetic crossing medium (SCM; Westergaard and Mitchell 1947), containing 1.5% D-glucose and 2% Bacto agar. Ascospores produced from the crosses were germinated by heat shock at 60 °C for 45 min on Petri dishes containing 0.05% fructose, 0.05% glucose, 2% sorbose (FGS), and 2% Bacto agar, and individual progeny were isolated. We crossed the N. crassa Ca2+ signaling knockout mutants to the ras-1bd mutant of opposite mating type and isolated progeny carrying the knockout mutation for the respective Ca2+ signaling genes in the ras-1bd background for visualization of circadian conidiation in race tubes (Table S1 and Supplementary Method).
Determination of the period length, temperature compensation, and real-time studies
The medium containing 1X Vogel's salts, 0.17% L-arginine, 0.1% D-glucose, 50 ng/mL biotin, and 1.5% Bacto agar was used for the circadian conidiation assays in race tubes (Park and Lee 2004). To determine the period length, N. crassa strains were inoculated on one end of race tubes, incubated at 20, 25, or 30 °C for 24 h under constant light, and then shifted to constant darkness. The growth front was marked once per day for 7 days under red safe light. The tubes were then moved to white light, and the position of the conidial bands was marked. Period lengths were calculated by multiplying the distance between conidial bands by the inverse slope of growth front versus time (http://www.fgsc.net/teaching/circad.htm). The Q10 value was calculated using the formula: 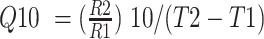 , where R1 and R2 are the frequencies of the period lengths (24/Period) at T1 and T2 temperatures, respectively (Lakin-Thomas 1998, Sorek and Levy 2012).
, where R1 and R2 are the frequencies of the period lengths (24/Period) at T1 and T2 temperatures, respectively (Lakin-Thomas 1998, Sorek and Levy 2012).
For real-time studies of RNA levels, ∼1 × 107 conidia from the 48 h plate cultures were inoculated in flasks containing 25 ml of medium (without agar) used for the circadian conidiation assays (described above) and cultured at 125 rpm on rotary shakers in light at 20 or 25 °C (as indicated) for 2 h and then transferred to dark conditions (Aronson et al. 1994b). The mycelia were harvested after 14 h in the dark, RNA was isolated and quantitative Reverse-Transcriptase PCR (qRT-PCR) was performed (Gohain and Tamuli 2019) to determine the expression of the frq, wc-1, and β-tubulin genes using the primer pairs, RT-FRQ-F and RT-FRQ-R, RT-WC-1-F and RT-WC-1-R, and q-B-tub-FW and q-B-tub-RV, respectively.
Results
Multiple calcium signaling genes play a role in maintaining normal period length in N. crassa
We determined the period lengths in the knockout mutants of N. crassa Ca2+ signaling genes and the ras-1bd control strain (Table 1 and Fig. 1). We determined the period length at three different temperatures (20, 25, and 30 °C) to test if the period length shows temperature compensation over a physiological range of temperatures. The ras-1bd strain, known as the band (bd) mutant, has a T79I point mutation in ras-1 and showed a period length of ∼22.4 h at 25 °C (Belden et al. 2007). The period length of the clock in the ras-1bd control strain was greater at 20 °C compared to 25 and 30 °C (Table 1 and Fig. 1; Gardner and Feldman 1981). We observed significantly longer periods for the Δcamk-1, 2, 3, and 4 mutants, particularly at 25 and 30 °C, relative to the ras-1bd control (Table 1 and Fig. 1). The ∆cpe-1 mutant had a slightly shorter period length than the ras-1bd control at 25 and 30 °C. The ∆ plc-1 mutant showed period lengthening at 20 and 25 °C, and slight shortening at 30 °C relative to the ras-1bd strain. The ∆splA2 mutant did not show significant changes in period length at either of the temperatures tested. Because plc-1 genetically interacts with cpe-1 and splA2 in N. crassa (Barman and Tamuli 2017), we tested the effect of this interaction on the period length. The ∆plc-1; ∆cpe-1 and ∆plc-1; ∆splA2 double mutants showed longer periods at 20 and 25 °C relative to the ras-1bd control; however, the ∆cpe-1; ∆splA2 double mutant did not exhibit any change in period length (Table 1 and Fig. 1). These results suggested that the plc-1 gene is epistatic to both cpe-1 and splA2 for the period length at 20 and 25 °C.
Table 1.
The period length of Ca2+ signaling mutants at different temperatures.
| Strain name | Period length (h)+ | ||
|---|---|---|---|
| 20 ºC | 25 ºC | 30 ºC | |
| ras-1bd (Control) | 23.5 ± 0.1 | 22.0 ± 0.2 | 20.4 ± 0.1 |
| ∆mid-1 (5) | 23.5 ± 0.1 | 21.5 ± 0.7 | 20.0 ± 0.2 (*) |
| ∆cpe-1 (74) | 23.5 ± 0.3 | 21.3 ± 0.2 (**) | 19.6 ± 0.1 (***) |
| ∆plc-1 (60) | 25.2 ± 0.1 (***) | 23.4 ± 0.2 (***) | 19.7 ± 0.2 (**) |
| ∆ncs-1 (11) | 25.0 ± 0.2 (***) | 20.2 ± 0.2 (***) | 18.3 ± 0.1 (***) |
| ∆splA2 (4) | 23.3 ± 0.6 | 21.4 ± 0.2 (**) | 20.0 ± 0.3 |
| Δcamk-1 (12) | 24.2 ± 0.1 (*) | 23.5 ± 0.7 (*) | 22.8 ± 0.3 (**) |
| Δcamk-2 (15) | 24.1 ± 0.1 (*) | 23.2 ± 0.7 (*) | 22.9 ± 0.3 (**) |
| Δcamk-3 (22) | 24.1 ± 0.3 | 23.5 ± 0.5 (*) | 22.8 ± 0.04 (**) |
| Δcamk-4 (31) | 24.1 ± 0.2 | 23.6 ± 0.9 (*) | 22.8 ± 0.1 (***) |
| ∆cna-1; Cna-1 RIP (28–20) | 23.5 ± 0.8 | 21.8 ± 0.5 | 19.7 ± 0.4 (*) |
| ∆cnb-1; cnb-1 RIP (599–1) | 21.8 ± 0.3 (***) | 20.9 ± 0.4 (*) | 19.6 ± 0.6 (***) |
| ∆cnb-1; cnb-1 RIP (600–8) | 21.5 ± 0.2 (***) | 20.5 ± 0.5 (*) | 19.5 ± 0.1 (***) |
| ∆cnb-1; cnb-1 RIP (602–82) | 21.7 ± 0.5 (***) | 20.4 ± 0.2 (**) | 19.3 ± 0.1 (***) |
| ∆plc-1; ∆cpe-1 (3) | 24.5 ± 0.3 (**) | 23.4 ± 0.3 (**) | 20.9 ± 0.1 (**) |
| ∆plc-1; ∆splA2 (37) | 24.3 ± 0.2 (**) | 23.5 ± 0.5 (**) | 20.8 ± 0.9 |
| ∆cpe-1; ∆splA2 (73) | 23.3 ± 0.4 (*) | 22.0 ± 0.3 | 20.1 ± 0.4 |
| cmd RIP (19) | Not determined | Not determined | Not determined |
Results are shown as mean ± SD for three independent experiments (n = 3) with P values < 0.05 (*), < 0.01 (**), and < 0.001 (***) compared with the ras-1bd strain as measured by a one-way ANOVA test.
Figure 1.
Circadian period and period length in the N. crassa Ca2+ signaling mutant strains. (A) Circadian-regulated conidiation in N. crassa. The indicated N. crassa strains (Table S1) were assayed for circadian-regulated conidiation at 20, 25, and 30°C using race tubes. The black lines show the growth front, marked every 24 h. The orange growth indicates the location of conidial bands. (B) Period lengths in N. crassa strains at 20, 25, and 30°C. Strains were inoculated on race tubes, and the cultures were incubated at 25°C in constant light for 24 h and then incubated at 20, 25, and 30°C under constant darkness. Period lengths were calculated by multiplying the distance between conidial bands by the inverse of the slope of the growth rate. Error bars show SDs calculated from the data for three independent experiments (n = 3) with P values < 0.05 (*), < 0.01 (**), and < 0.001 (***) relative to the ras-1bd strain as measured by a one-way ANOVA test.
The ∆ncs-1 mutant displayed a longer period at 20 °C, and a period shortening at 25 and 30 °C (Table 1 and Fig. 1). The cnb-1RIP mutants (Strain #599, 600, and 602; Table S1) exhibited shorter periods at all temperatures (Table 1 and Fig. 1). However, the Cna-1RIP did not exhibit a significant change in the period length (Table 1 and Fig. 1). Because the cmdRIP mutant (Strain #19; Table S1) showed severe growth retardation, period length could not be determined in this mutant.
Loss of certain calcium signaling genes influences temperature compensation in N. crassa
The Q10 value, which reflects the ratio of period lengths relative to a 10 °C rise in temperature, is calculated using the formula 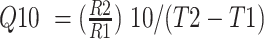 , where R1 and R2 are the frequencies of period lengths at temperatures T1 and T2, respectively (Lakin-Thomas 1998, Sorek and Levy 2012). We used this equation to calculate Q10 values for each strain, using all combinations of period lengths at 20, 25, and 30 °C (Table 2).
, where R1 and R2 are the frequencies of period lengths at temperatures T1 and T2, respectively (Lakin-Thomas 1998, Sorek and Levy 2012). We used this equation to calculate Q10 values for each strain, using all combinations of period lengths at 20, 25, and 30 °C (Table 2).
Table 2.
Q10 values of the Ca2+ signaling mutants.
| Strain name | Q10 values+,^ | ||
|---|---|---|---|
| T1 = 20 ºC, T2 = 25 ºC (P20ºC and P25ºC) |
T1 = 25 ºC, T2 = 30 ºC (P25ºC and P30ºC) |
T1 = 20 ºC, T2 = 30 ºC (P20ºC and P30ºC) |
|
| ras-1bd (Control) | 1.14 (23.5 and 22.0) |
1.16 (22.0 and 20.4) |
1.15 (23.5 and 20.4) |
| ∆mid-1 (5) | 1.19 (23.5 and 21.5) |
1.16 (21.5 and 20.0) |
1.17 (23.5 and 20.0) |
| ∆cpe-1 (74) | 1.22 (23.5 and 21.3) |
1.18 (21.3 and 19.6) |
1.20 (23.5 and 19.6) |
| ∆plc-1 (60) | 1.16 (25.2 and 23.4) |
1.41 (23.4 and 19.7) |
1.28 (25.2 and 19.7) |
| ∆ncs-1 (11) | 1.53 (25.0 and 20.2) |
1.22 (20.2 and 18.3) |
1.40 (25.0 and 18.3) |
| ∆splA2 (4) | 1.18 (23.3 and 21.4) |
1.14 (21.4 and 20.0) |
1.20 (23.3 and 20.0) |
| Δcamk-1 (12) | 1.06 (24.2 and 23.5) |
1.06 (23.5 and 22.8) |
1.06 (24.2 and 22.8) |
| Δcamk-2 (15) | 1.08 (24.1 and 23.2) |
1.02 (23.2 and 22.9) |
1.05 (24.1 and 22.9) |
| Δcamk-3 (22) | 1.05 (24.1 and 23.5) |
1.06 (23.5 and 22.8) |
1.06 (24.1 and 22.8) |
| Δcamk-4 (31) | 1.04 (24.1 and 23.6) |
1.07 (23.6 and 22.8) |
1.06 (24.1 and 22.8) |
| ∆cna-1; Cna-1 RIP (28–20) | 1.16 (23.5 and 21.8) |
1.22 (21.8 and 19.7) |
1.19 (23.5 and 19.7) |
| ∆cnb-1; cnb-1 RIP (599–1) | 1.09 (21.8 and 20.9) |
1.14 (20.9 and 19.6) |
1.11 (21.8 and 19.6) |
| ∆cnb-1; cnb-1 RIP (600–8) | 1.10 (21.5 and 20.5) |
1.10 (20.5 and 19.5) |
1.10 (21.5 and 19.5) |
| ∆cnb-1; cnb-1 RIP (602–82) | 1.13 (21.7 and 20.4) |
1.12 (20.4 and 19.3) |
1.12 (21.7 and 19.3) |
| ∆plc-1; ∆cpe-1 (3) | 1.10 (24.5 and 23.4) |
1.25 (23.4 and 20.9) |
1.17 (24.5 and 20.9) |
| ∆plc-1; ∆splA2 (37) | 1.07 (24.3 and 23.5) |
1.28 (23.5 and 20.8) |
1.17 (24.3 and 20.8) |
| ∆cpe-1; ∆splA2 (73) | 1.12 (23.3 and 22.0) |
1.20 (22.0 and 20.1) |
1.16 (23.3 and 20.1) |
| cmd RIP (19) | Not determined |
Not determined |
Not determined |
The related period lengths for the calculated Q10 values are given in parentheses.
Q10 values marked in bold suggest partial loss of temperature compensation.
The Q10 value ranges from 0.8 to 1.2 for normal circadian rhythms (Mattern et al. 1982, Sorek and Levy 2012). The Q10 value ranged from 0.8 to 1.2 for the strains lacking cpe-1, splA2, camk-1, camk-2, camk-3, camk-4, and cnb-1, as well as for the Cna-1RIP mutant, indicating that the circadian clock was temperature compensated in these mutants (Table 2). However, the Q10 value was 1.41 for the ∆plc-1 mutant between 25 and 30 °C, and the Q 10 value for the ∆ncs-1 mutant was 1.53 and 1.40 when comparing 20 and 25 °C and 20 and 30 °C, suggesting a partial loss of temperature compensation of the circadian clock in these two mutants in these temperature ranges (Table 2).
Transcription of frq and wc-1 was altered in certain calcium signaling mutants that displayed variation in period length
The N. crassa circadian clock is regulated through the interaction of three major genes frq, wc-1, and wc-2 (Aronson et al. 1994a, 1994b, Crosthwaite et al. 1997). Differences in period length are often associated with the transcription of frq (Aronson et al. 1994a). In the nucleus, FRQ regulates the expression of its activators wc-1 and wc-2 (Cheng et al. 2001).
We performed quantitative Real-Time PCR (qRT-PCR) to measure the expression of the frq and wc-1 genes at 20 and 25 °C in the strains showing period length and temperature compensation phenotypes. Transcript levels of frq and wc-1 were significantly increased at 20 °C and marginally at 25 °C in the Δ plc-1 single and ∆plc-1; ∆cpe-1 and ∆plc-1; ∆splA2 double mutants, temperatures at which these strains also had longer periods than the control (Fig. 2A). The difference in frq expression levels was most striking, with more than a 2-fold increase in the Δncs-1 mutant at 20 °C, but slightly reduced expression at 25 °C (Fig. 2B, upper panel). In contrast, wc-1 transcript levels were normal in the Δncs-1 mutant at both temperatures (Fig. 2B, lower panel). Thus, there was a correlation between frq expression and period length in the Δncs-1 mutant; this strain had a longer period at 20 °C but a shorter period at 25 °C, relative to the control. Although the strains lacking the Ca 2+/CaM dependent kinase genes camk-1, camk-2, camk-3, and camk-4 had longer periods, we did not observe a significant difference in the frq and wc-1 transcript levels in these mutants relative to the control (Fig. 2C).
Figure 2.
Expression of frequency (frq) and white collar-1 (wc-1) during circadian-regulated conidiation at 20 and 25°C. RNA was isolated from the indicated groups of strains (A, B, and C) cultured under circadian-regulated conidiation conditions at 20 or 25°C and the expression of the frq (upper panels) and wc-1 (lower panels) genes were determined using qRT-PCR with three biological replicates for each strain. The relative expression of each gene was normalized to the expression of the β-tubulin gene, and expression values were compared with those in the ras-1bd control strain. Error bars indicate SDs calculated from the data for three independent experiments (n = 3) with P values < 0.05 (*), < 0.01 (**), and < 0.001 (***) relative to the ras-1bd strain as measured by a one-way ANOVA test.
Discussion
We investigated the circadian-regulated period length for several N. crassa Ca2+ signaling mutants at three different temperatures (20, 25, and 30 °C). The Δ camk-1, 2, 3, and 4 mutants showed period lengthening (Table 1 and Fig. 1). A longer period phenotype has been previously reported for a Δcamk-1 mutant strain (Yang et al. 2001). The ∆cpe-1 mutant displayed a slightly shorter period length than the control at 25 and 30 °C. The ∆ plc-1 mutant showed a slight period shortening at 30 °C. However, the ∆ plc-1 mutant showed significant period lengthening at 20 and 25 °C. The ∆ plc-1; ∆cpe-1 and ∆plc-1; ∆splA2 double mutants displayed longer periods at 20 and 25 °C; however, period length in the ∆ cpe-1; ∆splA2 double mutant was like the ras-1bd control (Table 1 and Fig. 1). These results suggested that plc-1 genetically interacts with cpe-1 and splA2 to regulate circadian period length in N. crassa. Previously, the genetic interactions of plc-1, cpe-1, and splA2 were also found to regulate asexual and sexual developments in N. crassa (Barman and Tamuli 2017). In addition, the Q10 value was 1.41 for the ∆plc-1 mutant (T1 = 25 ºC, T 2 = 30 ºC), and 1.53 and 1.40 for the ∆ ncs-1 mutant (T1 = 20 ºC, T 2 = 25 ºC; and T 1 = 20 ºC, T 2 = 30 ºC), suggesting a partial loss of temperature compensation of circadian clock in these mutants under the temperature conditions tested (Table 2). In addition to an increased Q10 value, the ∆plc-1 and ∆ncs-1 mutants also appeared to have an increased growth rate, as evident from the daily markings on the race tubes (Fig. 1A), and this could be due to difference in the media composition and the conditions used for the circadian-regulated conidiation assays compared to the routine cultures using VM (described in the "Materials and Methods" section). However, ∆ ncs-1 displays a slow growth phenotype (Deka et al. 2011), and ∆plc-1 grows like the wild type (Barman and Tamuli 2015) when standard VM and growth conditions are used. In N. crassa, frq7, a long-period mutant, also showed an increased period and a larger Q10 value with partial loss of temperature compensation (Gardner and Feldman 1981, Ruoff et al. 2005).
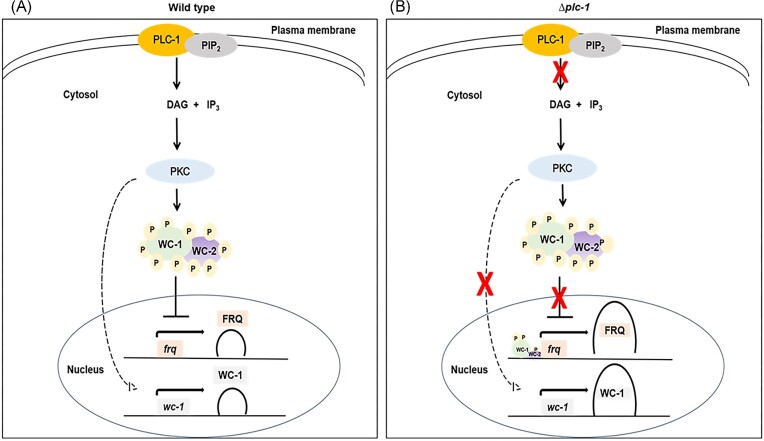
We also determined the expression of the circadian regulators frq and wc-1 under two different temperatures. The transcript levels of the frq and wc-1 genes were increased >2-fold in the Δplc-1 single, and ∆plc-1; ∆cpe-1 and ∆plc-1; ∆splA2 double mutants at 20 °C (Fig. 2A). The membrane-bound phosphoinositide-specific phospholipase C (PLC) hydrolyzes phosphatidylinositol 4, 5-bisphosphate (PIP2) to inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG), inducing the release of intracellular Ca2+ and activation of protein kinase C (PKC), respectively (Rhee and Bae 1997). The N. crassa PKC is a regulator of light-responsive genes (Arpaia et al. 1999), and most of the light responses are regulated by modulating the blue light photoreceptor WC-1 (Franchi et al. 2005). In addition, PKC phosphorylates WC-1 in vitro (Franchi et al. 2005), and hyperphosphorylated WC-1 cannot bind to the frq promoter to drive its transcription (He and Liu 2005). The unphosphorylated WC-1 could efficiently bind to the frq promoter, leading to higher expression of frq mRNA (Fig. 3). Moreover, the transcription of wc-1 is autoregulated by either light-induction or transcript stabilization processes (Ballario et al. 1996). Moreover, activation of PKC significantly decreases both frq and wc-1 at the transcriptional and protein levels (Franchi et al. 2005), and endogenous DAG levels in N. crassa show circadian oscillation (Ramsdale and Lakin-Thomas 2000). These results suggested that loss of PLC-1 might lower DAG levels, causing PKC to remain in an inactive state that cannot phosphorylate WC-1; this could be a possible mechanism of increased expression of frq in the ∆plc-1 mutant compared to the ras-1bd strain (Fig. 2A, upper panel).
Figure 3.
Model showing the role of PLC-1 in circadian-regulated conidiation. (A) PLC-1 regulates FRQ and WC-1 in the wild type strain. PLC-1 hydrolyzes PIP2 to produce two essential second messengers, IP3 and DAG. IP3 causes Ca2+ release from the intracellular stores, and DAG activates PKC (Rhee and Bae 1997). PKC modulates the blue light photoreceptor WC-1 to control the light-responsive genes in N. crassa (Arpaia et al. 1999), and frq expression (Franchi et al. 2005). Down-regulation of PKC abolishes its effect on WC-1, causing increased wc-1 mRNA expression and enhanced stability of the WC-1 protein (Franchi et al. 2005). In addition, the transcription of wc-1 is autoregulated (Ballario et al. 1996). When activated, PKC interacts with the WCC complex and phosphorylates it. The hyperphosphorylated WCC complex cannot bind to the frq promoter to drive its expression (Aronson et al. 1994b, Wang et al. 2019), but a fall of the FRQ protein below a critical level reactivates frq transcription (Garceau et al. 1997, Liu and Bell-Pedersen 2006). FRQ also positively regulates the WC-1 protein level (Cheng et al. 2001). These mechanisms are essential for the normal circadian clock in N. crassa. (B) The effect of the plc-1 deletion mutation on the regulation of FRQ and WC-1. We propose that the deletion of plc-1 may negatively affect DAG levels, causing PKC to remain in an inactive state. Inactive PKC cannot phosphorylate the WCC complex, resulting in increased expression of frq and wc-1 in the Δplc-1 single and double mutants compared to the wild type control strain.
The above model is supported by the longer period length and higher expression of frq and wc-1 mRNA observed in the Δplc-1 single and Δplc-1; Δcpe-1 and Δplc-1; ΔsplA2 double mutants, particularly at 20 °C. The cpe-1 gene encodes for a Ca2+/H+ exchanger, and this family of proteins family plays a role in controlling the resting level of [Ca2+]c, by transporting Ca2+ out of the cells and into intracellular Ca2+ stores in exchange for movement of H+ ions across membranes (Zelter et al. 2004, Tamuli et al. 2013). In addition, the sPLA2 enzyme catalyzes Ca2+-dependent hydrolysis of the sn2 ester linkage of glycerophospholipids to release free fatty acids (FFAs) and 1-acyl-lysophospholipid (1-acyl-LPL), that can both act as potential signaling molecules to regulate various biological functions (Dennis et al. 2011).
We observed that the frq transcript level in the Δncs-1 mutant was increased >2-fold relative to the ras-1bd at 20 °C. NCS-1 responds to the increased intracellular Ca2+ levels and plays a role in maintaining Ca2+ homeostasis and tolerance to high concentrations of Ca2+ (Deka et al. 2011, Gohain and Tamuli 2019). Environmental stress, including low temperature, can result in increased intracellular Ca2+ levels (Chinnusamy et al. 2007). In addition, the increase of intracellular Ca2+ due to the loss of NCA-2 causes phosphorylation of FRQ and decreased period length in N. crassa (Wang et al. 2021). Because 30 °C is the ambient growth temperature of N. crassa, 20 °C may act as a stress condition that might cause an increase in the intracellular Ca2+ levels. Thus, Ca2+ homeostasis at low temperatures might be disrupted in the Δncs-1 mutant. The calcineurin pathway is activated in response to high concentrations of Ca2+ in N. crassa (Gohain and Tamuli 2019, Kumar et al. 2019, Roy and Tamuli 2022 ). A transient increase in Ca2+ level at 20 °C might activate the calcineurin pathway and cause nuclear localization of WC-1 for the upregulation of the frq transcript in the Δncs-1 mutant, resulting in lengthening of the period length in the mutant (Table 1 and Fig. 2B). In addition, intracellular Ca2+ induces transcription of the circadian-related period 1 and 2 (mPer1 and mPer2) genes via MAP kinase pathways in mouse NIH3T3 cells (Oh-hashi et al. 2002). In mammals, a Ca2+ flux is required for maintaining circadian rhythmicity in the hypothalamic SCN (Lundkvist et al. 2005). In Arabidopsis thaliana and Nicotiana benthamiana, cytosolic free Ca2+ exhibits a rhythmic oscillation that relays signals relating to the circadian clock (Dodd et al. 2005). In addition, Ca2+ also plays a role in the regulation of the circadian rhythm and clock gene expression in Euglena (Goto et al. 1985), mollusks (Khalsa et al. 1993), and insects (Harrisingh et al. 2007).
Calcineurin (CNA-1) is the only serine/threonine protein phosphatase that requires Ca2+/CaM for its activity (Klee et al. 1979). In response to increased [Ca2+]c, Ca2+ binds to CaM and CNB-1, which then activate CNA-1 for dephosphorylation of target transcription factors to induce expression of target genes (Rumi-Masante et al. 2012, Roy and Tamuli 2022). CaM is required to activate protein kinases and might be involved in the signal transduction from light-perceiving components to the N. crassa circadian clock (Sadakane and Nakashima 1996). In N. crassa, CNB-1 binds to the calcineurin-dependent response element (CDRE), possibly to regulate target gene expression (Kumar et al. 2006). It is conceivable that the CNB-1RIP protein also has low affinity for the frq promoter, causing low levels of frq transcript (Fig. 2B). However, no direct genetic interaction could be established between these genes and wc-1, as wc-1 expression was not significantly different from the control under any condition.
The strains lacking the Ca2+/CaM dependent kinase genes camk-1, camk-2, camk-3, and camk-4 had longer periods, but we did not observe any change in the level of frq and wc-1 transcripts in these mutants (Fig. 2C). In a previous study, Ca2+/CaMK-1 was shown to phosphorylate FRQ in vitro, and a knockout mutant of camk-1 was found to affect the phase, period, and phase-shifting of the N. crassa circadian clock (Yang et al. 2001). Moreover, certain frq phosphorylation sites and camk-2 were shown to be epistatic to nca-2 (Wang et al. 2021). Another protein, casein kinase-2 (CK-2), also directly phosphorylates FRQ and plays a role in circadian temperature compensation in N. crassa (Mehra et al. 2009). Our results suggested that Ca2+/CaMKs are not involved in regulating the expression of the frq gene. The increased period length observed in these mutants might result from insufficient phosphorylation and/or a longer time to phosphorylate FRQ before it is ubiquitinated.
In conclusion, similar to the long-period mutant frq7 (Ruoff et al. 2005), a partial loss of temperature compensation was observed in the Δplc-1 and Δncs-1 mutants. The frq and wc-1 transcript levels were increased in the Δplc-1, ∆plc-1; ∆cpe-1, and ∆plc-1; ∆splA2 mutants at 20 °C, suggesting that plc-1 plays a role in the circadian period length by regulating the expression of frq and wc-1. In addition, the frq transcript level was also increased in the Δncs-1 mutant at 20 °C, suggesting that ncs-1 regulates the frq transcription via a mechanism yet to be identified. Further studies will establish the detailed molecular pathways used by these Ca2+ signaling genes to regulate circadian period length in N. crassa.
Supplementary Material
Acknowledgements
We thank Kevin McCluskey and John Leslie at the Fungal Genetics Stock Center for generously waiving charges for strains. DB, CM, DG, AK, and AR were supported by Research Fellowships from the Ministry of Human Resource Development, Government of India. We thank the Department of Biotechnology, Govt. of India, for DBT-NER twinning grant BT/PR24473/NER/95/737/2017. This work was partially supported by NIGMS grants GM068087 and GM086565 and the National Institute of Food and Agriculture Hatch Project #CA-R-PPA-6980-H to KAB.
Contributor Information
Darshana Baruah, Department of Biosciences and Bioengineering, Indian Institute of Technology Guwahati, Guwahati 781039, Assam, India.
Christy Noche K Marak, Department of Biosciences and Bioengineering, Indian Institute of Technology Guwahati, Guwahati 781039, Assam, India.
Avishek Roy, Department of Biosciences and Bioengineering, Indian Institute of Technology Guwahati, Guwahati 781039, Assam, India.
Dibakar Gohain, Department of Biosciences and Bioengineering, Indian Institute of Technology Guwahati, Guwahati 781039, Assam, India.
Ajeet Kumar, Department of Biosciences and Bioengineering, Indian Institute of Technology Guwahati, Guwahati 781039, Assam, India.
Pallavi Das, Department of Biosciences and Bioengineering, Indian Institute of Technology Guwahati, Guwahati 781039, Assam, India.
Katherine A Borkovich, Department of Microbiology and Plant Pathology, Institute for Integrative Genome Biology, College of Natural and Agricultural Sciences, University of California Riverside, Riverside 92521, CA, USA.
Ranjan Tamuli, Department of Biosciences and Bioengineering, Indian Institute of Technology Guwahati, Guwahati 781039, Assam, India.
Author Contribution
D.B., C.M., A.R., D.G., and A.K. performed experiments, and prepared figures and tables. D.B. also wrote the revised drafts and prepared the final figures. P.D. helped in some experiments. K.A.B. helped in generating some of the N. crassa strains used in this study, provided suggestions, and corrected the manuscript. RT designed experiments, wrote, and edited the manuscript.
Ethical Statement
The proper ethical standard was followed in this research.
Conflict of Interest
The authors declare no conflict of interests.
Funding
DBT-NER twinning grant BT/PR24473/NER/95/737/2017 from DBT, Govt. of India, to RT. NIGMS grants GM068087 and GM086565 and the National Institute of Food and Agriculture Hatch Project #CA-R-PPA-6980-H to KAB.
Data Availability
Strains are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present in the article, figures, and tables.
References
- An S, Irwin RP, Allen CNet al. Vasoactive intestinal polypeptide requires parallel changes in adenylate cyclase and phospholipase C to entrain circadian rhythms to a predictable phase. J Neurophysiol. 2011;105:2289–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson BD, Johnson KA, Dunlap JC. Circadian clock locus frequency: protein encoded by a single open reading frame defines period length and temperature compensation. Proc Natl Acad Sci USA. 1994a;91:7683–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson BD, Johnson KA, Loros JJet al. Negative feedback defining a circadian clock: autoregulation of the clock gene frequency. Sci. 1994b;263:1578–84. [DOI] [PubMed] [Google Scholar]
- Arpaia G, Cerri F, Baima Set al. Involvement of protein kinase C in the response of Neurospora crassa to blue light. Mol Gen Genet. 1999;262:314–22. [DOI] [PubMed] [Google Scholar]
- Avello PA, Davis SJ, Ronald Jet al. Heat the clock: entrainment and compensation in Arabidopsis circadian rhythms. J Circadian Rhythms. 2019;17:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Báez-Ruiz A, Díaz-Muñoz M. Chronic inhibition of endoplasmic reticulum calcium-release channels and calcium-atpase lengthens the period of hepatic clock gene Per1. J Circadian Rhythms. 2011;9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CL, Loros JJ, Dunlap JC. The circadian clock of Neurospora crassa. FEMS Microbiol Rev. 2012;36:95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballario P, Talora C, Galli Det al. Roles in dimerization and blue light photoresponse of the PAS and LOV domains of Neurospora crassa white collar proteins. Mol Microbiol. 1998;29:719–29. [DOI] [PubMed] [Google Scholar]
- Ballario P, Vittorioso P, Magrelli Aet al. White collar-1, a central regulator of blue light responses in Neurospora, is a zinc finger protein. EMBO J. 1996;15:1650–7. [PMC free article] [PubMed] [Google Scholar]
- Barman A, Tamuli R. Multiple cellular roles of Neurospora crassa plc-1, splA 2, and cpe-1 in regulation of cytosolic free calcium, carotenoid accumulation, stress responses, and acquisition of thermotolerance. J Microbiol. 2015;53:226–35. [DOI] [PubMed] [Google Scholar]
- Barman A, Tamuli R. The pleiotropic vegetative and sexual development phenotypes of Neurospora crassa arise from double mutants of the calcium signaling genes plc-1, splA 2, and cpe-1. Curr Genet. 2017;63:861–75. [DOI] [PubMed] [Google Scholar]
- Belden WJ, Larrondo LF, Froehlich ACet al. The band mutation in Neurospora crassa is a dominant allele of ras-1 implicating RAS signaling in circadian output. Genes Dev. 2007;21:1494–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell-pedersen D, Dunlap JC, Loros JJ. The Neurospora circadian clock-controlled gene, ccg-2, is allelic to eas and encodes a fungal hydrophobin required for formation of the conidial rodlet layer. Genes Dev. 1992;6:2382–94. [DOI] [PubMed] [Google Scholar]
- Cheng P, Yang Y, Gardner KHet al. PAS domain-mediated WC-1/WC-2 interaction is essential for maintaining the steady-state level of WC-1 and the function of both proteins in circadian clock and light responses of Neurospora. Mol Cell Biol. 2002;22:517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng P, Yang Y, Liu Y. Interlocked feedback loops contribute to the robustness of the Neurospora circadian clock. Proc Natl Acad Sci USA. 2001;98:7408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnusamy V, Zhu J, Zhu JK. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007;12:444–51. [DOI] [PubMed] [Google Scholar]
- Crosthwaite SK, Dunlap JC, Loros JJ. Neurospora wc-1 and wc-2: transcription, photoresponses, and the origins of circadian rhythmicity. Sci. 1997;276:763–9. [DOI] [PubMed] [Google Scholar]
- Davis RH, De Serres FJ. Genetic and microbiological research techniques for Neurospora crassa. Methods Enzymol. 1970;17:79–143. [Google Scholar]
- Deka R, Kumar R, Tamuli R. Neurospora crassa homologue of Neuronal Calcium Sensor-1 has a role in growth, calcium stress tolerance, and ultraviolet survival. Genetica. 2011;139:885–94. [DOI] [PubMed] [Google Scholar]
- Dennis EA, Cao J, Hsu YHet al. Phospholipase A2 enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev. 2011;111:6130–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd AN, Salathia N, Hall Aet al. Plant circadian clocks increase photosynthesis, growth, survival, and competitive advantage. Science. 2005;309:630–3. [DOI] [PubMed] [Google Scholar]
- Franchi L, Fulci V, Macino G. Protein kinase C modulates light responses in Neurospora by regulating the blue light photoreceptor WC-1. Mol Microbiol. 2005;56:334–45. [DOI] [PubMed] [Google Scholar]
- Garceau NY, Liu Y, Loros JJet al. Alternative initiation of translation and time-specific phosphorylation yield multiple forms of the essential clock protein FREQUENCY. Cell. 1997;89:469–76. [DOI] [PubMed] [Google Scholar]
- Gardner GF, Feldman JF. Temperature compensation of circadian period length in clock mutants of Neurospora crassa. Plant Physiol. 1981;68:1244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohain D, Tamuli R. Calcineurin responsive zinc-finger-1 binds to a unique promoter sequence to upregulate neuronal calcium sensor-1, whose interaction with MID-1 increases tolerance to calcium stress in Neurospora crassa. Mol Microbiol. 2019;111:1510–28. [DOI] [PubMed] [Google Scholar]
- Goto K, Laval-Martin DL, Edmunds LN. Biochemical modeling of an autonomously oscillatory circadian clock in Euglena. Science. 1985;228:1284–8. [DOI] [PubMed] [Google Scholar]
- Halberg F, Cornélissen G, Katinas Get al. Transdisciplinary unifying implications of circadian findings in the 1950 s. J Circadian Rhythms. 2003;1:1–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrisingh MC, Wu Y, Lnenicka GAet al. Intracellular Ca2+ regulates free-running circadian clock oscillation in vivo. J Neurosci. 2007;27:12489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q, Liu Y. Molecular mechanism of light responses in Neurospora: from light-induced transcription to photoadaptation. Genes Dev. 2005;19:2888–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Liu X, Lu Qet al. FRQ-CK1 interaction underlies temperature compensation of the Neurospora circadian clock. MBio. 2021;12:e01425–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalsa SBS, Block GD, Ralph MR. The role of extracellular calcium in generating and in phase-shifting the Bulla ocular circadian rhythm. J Biol Rhythms. 1993;8:125–39. [DOI] [PubMed] [Google Scholar]
- Klee CB, Crouch TH, Krinks MH. Calcineurin: a calcium-and calmodulin-binding protein of the nervous system. Proc Natl Acad Sci. 1979;76:6270–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon N, Wang HT, Kato YSet al. Na+/Ca2+ exchanger mediates cold Ca2+ signaling conserved for temperature-compensated circadian rhythms. Sci Adv. 2021;7:abe8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Roy A, Deshmukh MVet al. Dominant mutants of the calcineurin catalytic subunit (CNA-1) showed developmental defects, increased sensitivity to stress conditions, and CNA-1 interacts with CaM and CRZ-1 in Neurospora crassa. Arch Microbiol. 2019;202:921–34. [DOI] [PubMed] [Google Scholar]
- Kumar KS, Kumar BR, Siddavattam Det al. Characterization of calcineurin-dependent response element binding protein and its involvement in copper-metallothionein gene expression in Neurospora. Biochem Biophys Res Commun. 2006;345:1010–3. [DOI] [PubMed] [Google Scholar]
- Kumar R, Tamuli R. Calcium/calmodulin-dependent kinases are involved in growth, thermotolerance, oxidative stress survival, and fertility in Neurospora crassa. Arch Microbiol. 2014;196:295–305. [DOI] [PubMed] [Google Scholar]
- Lakin-Thomas PL. Choline depletion, frq mutations, and temperature compensation of the circadian rhythm in Neurospora crassa. J Biol Rhythms. 1998;13:268–77. [DOI] [PubMed] [Google Scholar]
- Laxmi V, Tamuli R. The Neurospora crassa cmd, trm-9, and nca-2 genes play a role in growth, development, and survival in stress conditions. Genomics Appl Biol. 2015;6:1–8. [Google Scholar]
- Laxmi V, Tamuli R. The calmodulin gene in Neurospora crassa is required for normal vegetative growth, ultraviolet survival, and sexual development. Arch Microbiol. 2017;199:531–42. [DOI] [PubMed] [Google Scholar]
- Lew RR, Abbas Z, Anderca MIet al. Phenotype of a mechanosensitive channel mutant, mid-1, in a filamentous fungus, Neurospora crassa. Eukaryot Cell. 2008;7:647–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linden H, Macino G. White collar 2, a partner in blue-light signal transduction, controlling expression of light–regulated genes in Neurospora crassa. EMBO J. 1997;16:98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Bell-Pedersen D. Circadian rhythms in Neurospora crassa and other filamentous fungi. Eukaryot Cell. 2006;5:1184–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundkvist GB, Kwak Y, Davis EKet al. A calcium flux is required for circadian rhythm generation in mammalian pacemaker neurons. J Neurosci. 2005;25:7682–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattern DL, Forman LR, Brody S. Circadian rhythms in Neurospora crassa: a mutation affecting temperature compensation. Proc Natl Acad Sci USA. 1982;79:825–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluskey K, Wiest A, Plamann M. The fungal genetics stock center: a repository for 50 years of fungal genetics research. J Biosci. 2010;35:119–26. [DOI] [PubMed] [Google Scholar]
- Mehra A, Shi M, Baker CLet al. A role for Casein Kinase 2 in the mechanism underlying circadian temperature compensation. Cell. 2009;137:749–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh-hashi K, Naruse Y, Tanaka M. Intracellular calcium mobilization induces period genes via MAP kinase pathways in NIH3T3 cells. FEBS Lett. 2002;516:101–5. [DOI] [PubMed] [Google Scholar]
- O'Neill JS, Reddy AB. The essential role of cAMP/Ca2+ signaling in mammalian circadian timekeeping. Portlandpress. 2012:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Lee K. Inverted race tube assay for circadian clock studies of the Neurospora accessions. Fungal Genet Rep. 2004;51:12–4. [Google Scholar]
- Ramsdale M, Lakin-Thomas PL. sn-1, 2-diacylglycerol levels in the fungus Neurospora crassa display circadian rhythmicity. J Biol Chem. 2000;275:27541–50. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Bae YS. Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem. 1997;272:15045–8. [DOI] [PubMed] [Google Scholar]
- Roy A, Kumar A, Baruah Det al. Calcium signaling is involved in diverse cellular processes in fungi. Mycol. 2020;12:10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Tamuli R. Regulation of Hsp80 involved in the acquisition of induced thermotolerance, and NCA-2 involved in calcium stress tolerance by the calcineurin-CRZ-1 signaling pathway in Neurospora crassa. Mycol Prog. 2022;21:84. [Google Scholar]
- Rumi-Masante J, Rusinga FI, Lester TEet al. Structural basis for activation of calcineurin by calmodulin. J Mol Biol. 2012;415:307–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoff P, Loros JJ, Dunlap JC. The relationship between FRQ-protein stability and temperature compensation in the Neurospora circadian clock. Proc Natl Acad Sci USA. 2005;102:17681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadakane Y, Nakashima H. Light-induced phase shifting of the circadian conidiation rhythm is inhibited by calmodulin antagonists in Neurospora crassa. J Biol Rhythms. 1996;11:234–40. [DOI] [PubMed] [Google Scholar]
- Sorek M, Levy O. The effect of temperature compensation on the circadian rhythmicity of photosynthesis in Symbiodinium, coral-symbiotic alga. Sci Rep. 2012;2:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamuli R, Deka R, Borkovich KA. Calcineurin subunits A and B interact to regulate growth and asexual and sexual development in Neurospora crassa. PLoS One. 2016;11:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamuli R, Kumar R, Deka R. Review cellular roles of neuronal calcium sensor-1 and calcium/calmodulin-dependent kinases in fungi. J Basic Microbiol. 2011;51:120–8. [DOI] [PubMed] [Google Scholar]
- Tamuli R, Kumar R, Srivastava DAet al. Calcium signaling. In: Kasbekar DP, McCluskey K (eds.), Neurospora: Gen Mol Biol. Caister Academic Press, Norfolk: 2013:209–25. [Google Scholar]
- Vogel HJ. Distribution of lysine pathways among fungi: evolutionary implications. Am Nat. 1964;98:435–46. [Google Scholar]
- Wang B, Kettenbach AN, Zhou Xet al. The phospho-code determining circadian feedback loop closure and output in Neurospora. Mol Cell. 2019;74:771–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Zhou X, Gerber SAet al. Cellular calcium levels influenced by NCA-2 impact circadian period determination in Neurospora. MBio. 2021;12:e01493–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Zhou X, Loros JJet al. Alternative use of DNA binding domains by the Neurospora white collar complex dictates circadian regulation and light responses. Mol Cell Biol. 2016;36:781–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westergaard M, Mitchell HK. Neurospora V. A synthetic medium favoring sexual reproduction. Amer J Bot. 1947;34:573–7. [Google Scholar]
- Yang Y, Cheng P, Zhi Get al. Identification of a Calcium/Calmodulin-dependent protein kinase that phosphorylates the Neurospora circadian clock protein FREQUENCY. J Biol Chem. 2001;276:41064–72. [DOI] [PubMed] [Google Scholar]
- Zelter A, Bencina M, Bowman BJet al. A comparative genomic analysis of the calcium signaling machinery in Neurospora crassa, Magnaporthe grisea, and Saccharomyces cerevisiae. Fungal Genet Biol. 2004;41:827–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Strains are available upon request. The authors affirm that all data necessary for confirming the conclusions of the article are present in the article, figures, and tables.