Abstract
Nucleic acid-based electrochemical (NBE) sensors offer real-time and reagent-free sensing capabilities that overcome limitations of target-specific reactivity via affinity-based molecular detection. By leveraging affinity probes, NBE sensors become modular and versatile, allowing the monitoring of a variety of molecular targets by simply swapping the recognition probe without the need to change their sensor architecture. However, NBE sensors have not been rigorously validated in vivo in terms of analytical performance and clinical agreement relative to benchmark methods. In this article, we highlight reports from the past three years that evaluate NBE sensors performance in vivo. We hope this discussion will inspire future translational efforts with statistically robust experimental design, thus enabling real-world clinical applications and commercial development of NBE sensors.
Keywords: electrochemical sensors, pharmacokinetics, bioassay, preclinical validation, nucleic acids
Graphical Abstract

Introduction
A main goal of electrochemical biosensors is to measure the concentration of specific molecules with clinically relevant selectivity, sensitivity, and accuracy relative to standard-of-care methods [1]. The clinical and commercial success of the continuous glucose monitor (CGM) emphasized the potential value of electrochemical biosensors to enable highly precise and personalized medical treatment [2]. The CGM achieves molecular selectivity through sensor-immobilized glucose oxidase, which converts glucose to gluconolactone while transferring electrons [3]. This biocatalytic approach can provide sensitive on-body measurements with clinically relevant selectivity for glucose [3], lactate [4] and glutamate [5]. However, such a target reactivity-based detection approach can only work with molecules that are metabolized by oxidases or reductases, limiting the scope of molecules that can be sensed.
Overcoming the limitations of target enzymatic reactivity, nucleic acid-based electrochemical (NBE) sensors generate a signal upon target binding via affinity interactions (Figure 1). Because DNA, RNA, and other modified oligonucleotides can interact with a vast array of molecular targets– from ions [6,7] and small molecules [8,9] to proteins [10,11] and even whole cells [12,13] – NBE sensors significantly expand the scope of molecules that can be sensed with a constant sensor architecture. As illustrated in Figure 1, NBE sensors are typically functionalized with redox reporter-modified oligos that undergo target binding-induced changes in electron transfer kinetics [14,15,16]. Such a signal transduction mechanism can support molecular measurements in unprocessed biological fluids [14] and be interrogated via electrochemical methods like square wave voltammetry (illustrated in the inset of Figure 1). Additionally, NBE sensors are easily fabricated via self-assembly mechanisms, and they have modular interfaces, allowing replacement of the affinity probe independently from optimizing other sensor components [17,18]. To date, various NBE sensors have been developed for dozens of molecular targets. However, the developed sensors have largely remained in the proof-of-concept stage. Analytical agreement against benchmark methods (such as clinical laboratory-based measurements) have yet to be demonstrated for most NBE platforms. Therefore, there remains a critical need to not only increase the number of NBE sensors, but to pursue statistically rigorous clinical validations with existing and future sensors.
Figure 1. Anatomy and signaling of nucleic acid-based electrochemical (NBE) sensors.

These sensors are characterized by oligo populations that undergo electron transfer (eT) at different rates. This figure illustrates an NBE sensor made from single stranded nucleic acids, but duplexes and other structures are also used. The inset illustrates the typical target-induced signal change readout of NBE via square wave voltammetry.
The focus of this short opinion is to highlight the efforts undertaken in the past three years to demonstrate NBE sensor performance in preclinical animal models. We carefully note that the examples discussed here are not extensive technology validations, but rather proof-of-concept in vivo demonstrations that need further progression into clinical agreement assays. We feature works that focus on (a) therapeutic drug monitoring for precise drug dosing, and (b) metabolite monitoring for chronic disease management. By illuminating ways in which these studies could be further validated, we hope that this opinion piece will set the framework for future research focused on the pursuit of statistically rigorous clinical validations and technology advancement into the realm of human health.
Pharmacokinetic Monitoring of Therapeutic Drugs
Many drugs for the treatment of cancers, infectious diseases, autoimmune disorders, and cardiovascular problems have a narrow therapeutic window relative to patient-to-patient metabolic variability [19,20]. In these cases, indirect predictors of a patients’ metabolism (such as age, gender, or weight) are often insufficient to ensure effective dosing. For some drugs, concentrations are controlled via therapeutic drug monitoring; however, this process can be prohibitively laborious and time-consuming. The ability to measure drug levels with high precision and clinically relevant time resolution could help achieve a better understanding of individualized pharmacokinetics and more precise therapeutic dosing. In Table 1, we summarize all reports cited in this work for therapeutic drug monitoring, along with the limited animal numbers employed. We note that all published works in Table 1 did not include proper power analyses to justify the animal numbers employed. Most of them only conducted a single measurement under each testing condition. And only a few works included proper statistical analyses of their in vivo results.
Table 1.
Published NBE sensor validations for therapeutic drug monitoring in live animals
| Target | Year [Ref.] |
Sensor Placement Site | Administration Route | Dose | # Animals | Power Analysis | Statistical Analysis |
|---|---|---|---|---|---|---|---|
| Kanamycin | 2013 [22] |
Ex vivo measurement in collected blood from rat jugular vein | Intravenous bolus | 32, 64, 96 mg/kg | 1 | No | No |
| 2019 [48] |
Rat jugular vein | Intravenous bolus | 40 mg/kg | 1 | No | No | |
| 2020 [25] |
Rat jugular vein, leg muscle and bladder | Intravenous bolus | 30 mg/kg | 2 | No | Yes | |
| 2022 [27] |
Rat bladder | Intravenous bolus | Not reported | 3 | No | No | |
| Doxorubicin | 2013 [22] |
Ex vivo measurement in collected blood from rat jugular vein | Intravenous bolus | 1 mg/m2 | 1 | No | No |
| 2017 [23] |
Ex vivo measurement in collected blood from the ear vein of rabbits and jugular vein of rats | Bolus injection and infusion | 1 mg/m2 injection | 3 (rabbit); 2 (rat) | No | No | |
| 2017 [21] |
Rat jugular vein | Intravenous bolus | 40 mg/kg | 1 | No | No | |
| 2020 [25] |
Tumor tissue in rat | Intravenous bolus | 40 mg/kg | 1 | No | No | |
| 2021 [26] |
Tumor tissue in mouse | Intravenous and intratumoral bolus | 10 mg/kg | 1 | No | No | |
| Tobramycin | 2016 [35] |
Rat jugular vein | Intravenous bolus | 20 mg/kg | 2 | No | Yes |
| 2017 [34] |
Rat jugular vein | Intravenous bolus | 40 mg/kg | 1 | No | No | |
| 2017 [31] |
Rat jugular vein | Intravenous bolus | 30 mg/kg | 1 | No | No | |
| 2018 [33] |
Rat jugular vein | Intravenous bolus | 10 mg/kg | 3 | No | Yes | |
| 2019 [32] |
Rat jugular vein | Intravenous bolus | 20 mg/kg | 6 | No | Yes | |
| 2022 [28] |
Rat skin, abdomen | Intravenous bolus | 10 mg/kg | 1 | No | No | |
| 2022 [42] |
Rat skin, lower back | Intravenous bolus | 10–30 mg/kg | 3 | No | Yes | |
| Irinotecan | 2019 [24] |
Rat jugular vein | Intravenous bolus | 10 & 20 mg/kg | 3 | No | Yes |
| Vancomycin | 2019 [30] |
Rat jugular vein | Intravenous bolus | 30 mg/kg | 3 | No | Yes |
| 2021 [29] |
Rat jugular vein | Intravenous bolus | 40 mg/kg | 1 | No | No | |
| 2022 [36] |
Rat brain, cortex area | intravenous bolus | 75 mg/kg | 8 | No | Yes |
Nevertheless, several groups have demonstrated the feasibility of real-time continuous drug monitoring of antineoplastic agents in research animals [21–26]. Here, we highlight work by Idili and colleagues [24], who developed miniaturized implantable NBE sensors against irinotecan and tested their performance during in vivo pharmacokinetic monitoring (Figure 2A). The reported sensors achieved seconds-resolved measurements of plasma irinotecan in the jugular vein of live rats, allowing the study of intra-subject pharmacokinetic variability. However, the study was done in healthy animals, where the metabolic profile does not match that of a cancerous state. Unfortunately, plasma measurements often do not adequately reflect the abnormal vasculature and physiological heterogeneity of the tumor microenvironment, which can negatively impact drug pharmacokinetic profiles. To address this issue, Li et al. [25] developed NBE sensors suitable for monitoring chemotherapeutics inside tumors. Specifically, their sensors achieved monitoring of doxorubicin penetration into xenograft cervical tumors (Figure 2B). Independently, Seo et al. [26] demonstrated a continuous monitoring platform using doxorubicin NBE sensors to simultaneously measure drug levels across varying tumor depths (Figure 2C). Their results showed a clear trend in which decreasing drug exposure was observed with increasing depth. The measurements also revealed concentration-dependent relationships between pharmacokinetic profiles in tumor tissue versus blood that could be informative of drug disposition into tumors. While both studies deploy NBE sensors in physiologically relevant environments, they remain limited to demonstration in only a few animals (see Table 1). To further advance NBE technology toward the clinic, future studies must include proper power analysis-weighted measurements and should evaluate performance in ex-vivo patient samples.
Figure 2. NBE sensors for continuous monitoring of antineoplastics.

(A) Idili et al. adapted an irinotecan-binding aptamer [24] to create (I) implantable NBE sensors. (II, III) Such sensors supported irinotecan measurements every 10 seconds in a rat model and resolved pharmacokinetic profiles in different animals. (B) Li et al. [25] developed (IV) a two electrode, hydrogel-protected NBE sensor assembly for cervical tumor placement. (V) The sensors achieved measurements of doxorubicin penetration into the tumor following intravenous dosing. (C) Separately, Seo et al. [26] developed (VI) NBE sensors on nano porous gold microelectrode arrays for continuous monitoring of doxorubicin in cancerous B16-F10 tumors. (VII) Their NBE sensors with three sensing channels at different tumor depths achieved spatially resolved doxorubicin measurements following intravenous drug dosing. (VIII) The work also compared the mean doxorubicin concentrations from the three channels versus blood concentrations. Figure panels were reproduced from references [24] and [25] with permission from the Royal Society of Chemistry, and reference [26] with permission from American Association for the Advancement of Science.
Beyond therapeutic monitoring of antineoplastics, NBEs have also been developed for continuous monitoring of antibiotics [27–36]. In this context, we highlight work by Dauphin-Ducharme et al. [30], who reported NBE sensors for high-precision measurements of plasma vancomycin concentrations (Figure 3A). Although their measurements reveal significant subject to subject variability in pharmacokinetic profiles, it is unclear if such a variability arises from differences in sensor performance when implanted in vivo, or from actual metabolic differences between animal subjects. This uncertainty must be addressed in future in vivo implantation work, for example by simultaneously implanting multiple channels to compare sensor to sensor performance during pharmacokinetic measurements. The same report proposes a feedback-controlled drug delivery system based on real-time plasma vancomycin monitoring and drug infusions via a syringe pump. Using this platform, the authors were able to maintain plasma vancomycin concentrations at micromolar levels for over 5 hours, showing potential for the development of closed-loop drug delivery platforms.
Figure 3. Continuous monitoring of antibiotics.

(A) Dauphin-Ducharme et al. [30] reported NBE sensors for vancomycin (I) emplaced in the jugular veins of 3 live rats and monitored intravenous doses. (II, III) The work also reported a feedback-controlled drug delivery system able to maintain micromolar plasma vancomycin concentrations for over 5 h. (B) Hydrogel-protected sensors developed by Li et al. [25] were adapted to plasma kanamycin monitoring and tested in various body compartments, including the external (IV) jugular vein, leg (V) muscle, and (VI) bladder. (C) Separately, the same group developed pH-insensitive NBE sensors [27] employing (VII) an exTTF derivative as a pH-independent redox reporter. They tested their NBE sensors in the bladder of a live rat (VIII) by measuring kanamycin pharmacokinetics with micromolar precision and (IX) were not affected by changing pH. (D) Shaver et al. [36] fabricated (X) NBE probes for in brain measurements. The probes were placed within the mouse cerebral cortex (lesion in panel XI) for continuous in vivo measurements. This work demonstrated the first study of drug penetration across the blood-brain-barrier via NBE sensors, achieving highly resolved vancomycin brain measurements (X) following IV dosing. Figure panels were reproduced from references [25] and [27] with permission from the Royal Society of Chemistry, and references [30] and [36] with permission from American Chemical Society.
The hydrogel-protected NBE sensors developed by Li et al. [25] (Figure 2B) also achieved monitoring of kanamycin, an aminoglycoside antibiotic, in the leg muscle and bladder of rats. Relative to the immediate sensor signal increase typical of intravenous dosing, when implanted in the muscle and bladder, the sensors showed delayed responses and slower kinetics (Figure 3B). The work also included parallel blood pharmacokinetic measurements via ex-vivo LC-MS, comparing results relative to gel-protected sensors implanted in the rat the jugular vein. Additionally, in efforts to develop pH-independent NBE sensors, the same group [27] introduced a pH-independent redox-reporter: 9π-extended tetrathiafulvalene (exTTF). The application of this reporter allowed real-time, continuous monitoring of kanamycin in the rat bladder under changing pH conditions (Figure 3C). Again, while these studies deploy sensors in interesting environments, they remain limited to proof-of-concept demonstration, rather than statistically significant validations of the technology.
NBEs have more recently been developed to measure the kinetics of drug transport across the blood−brain barrier. Shaver et al. developed NBE brain probes using methodologies from the field of in vivo fast scan cyclic voltammetry [36]. The brain probes are small enough to be easily placed inside specific regions of the mouse brain and support continuous monitoring of vancomycin brain penetration after intravenous dosing (Figure 2D). In this publication, the NBE sensors were surgically implanted inside the mouse cortex. A 30-min baseline signal was recorded before administering an intravenous bolus of vancomycin through the mouse’s tail vein. The measurements showed a rapid rise in vancomycin brain concentration within a couple of minutes from intravenous dosing, followed by a plateau at ~1 h. Such measurements demonstrate that NBE sensors could be used as a powerful approach to study molecular transport across biological membranes both in vitro and in vivo. The measurements also revealed unexpectedly slow vancomycin excretion kinetics from the brain tissue that had not been reported before. Future studies should increase the number of animals used for rigorous validation of the findings. Additionally, they could expand on the value of these measurements for medical practice, particularly in the context of antibiotic prophylaxis therapy to prevent infections following traumatic brain injury.
Another bodily compartment that has been investigated with NBE sensors is interstitial fluid (ISF), which was originally put in the spotlight by continuous glucose sensing technology. ISF has been recognized as a valuable compartment for biomarker monitoring for years [39], with much of the published work based on microneedle arrays for fluid extraction. However, microneedle supported biosensors have also been extensively demonstrated, and it is now broadly believed that ISF represents the most promising biofluid for wearable continuous molecular monitors [37,38]. To date, benchmark microneedle sensors have been based on biocatalytic or voltametric detection [39–41], limiting sensing to enzymatic- or redox-active molecules. These approaches restrained the possibility to monitor biomarkers and therapeutic drugs that do not undergo redox reaction or enzymatic catalysis. To overcome this obstacle, Wu and colleagues [28] demonstrated the first NBE-supported microneedle sensors and the first in vivo molecular measurements in situ in ISF. They achieved their measurements via 3D-printed, gold-coated microneedle patches that were functionalized with a tobramycin aptamer and placed on the skin of live rats. Their measurements (Figure 4A) highlighted the possibility of minimally invasive continuous molecular monitoring in ISF. However, follow up work by Lin et al. [42] demonstrated a better microneedle NBE sensing platform, based on acupuncture needles, that achieved real-time molecular measurements in ISF with superior operational stability relative to the report by Wu and colleagues. Further, their unprecedented measurements highlighted a strong correlation of drug levels in ISF and blood (Figure 4B), emphasizing the potential value of the approach for minimally invasive health status monitoring.
Figure 4. Therapeutic drug monitoring in interstitial fluid enabled by wearable microneedles.

(A) Wu et al. [28] demonstrated (I) the first continuous tobramycin NBE sensor using microneedle array for drug monitoring in interstitial fluid (ISF). (II, III) The sensors capture discernable signal changes following tobramycin intravenous dosing. (B) Lin et al. [42] further developed (IV) a microneedle patch based on gold nano-particle deposited acupuncture needles. (V) The needle sensors achieved better signal to noise measurements of tobramycin in ISF following different intravenous doses. The corresponding measurements from blood are highlighted in the inserts. Figure panels were reproduced from reference [28] with permission from American Chemical Society, and reference [42] with permission from the American Association for the Advancement of Science.
The above-discussed examples of continuous NBE-based molecular sensing highlight a tremendous potential for clinically meaningful therapeutic monitoring and personalized drug dosing. However, further preclinical validation with adequate statistical power and clinical agreement assays are needed to clarify the extent to which NBE sensor technologies can contribute to improve clinical outcomes.
Monitoring of metabolites, proteins, and other biomarkers
A second promising application for NBE sensors beyond the in vivo tracking of therapeutic drugs involves their use as health monitors for physiologically relevant biomolecular species such as metabolites, proteins, and neurotransmitters [43–50]. The development of NBE sensors for such endogenous molecules requires additional selectivity screenings to prevent false positive readouts given close structural similarities across biomolecular classes (e.g., across monoamine neurotransmitters). Yet, with the advancement of better affinity reagents (e.g., aptamers) and improvements to NBE sensing architectures, we anticipate NBE sensors will play a key role in molecular monitors intended for biomedical research and health status monitoring. In Table 2, we summarized published reports on NBE sensors for the monitoring of neurotransmitters, proteins, and other biomarkers in living animals. Unfortunately, none of these works have discusses appropriate power analyses to justify animal numbers, and most did not perform rigorous statistical analyses of the resulting pharmacokinetic data. Despite these shared missing elements, we highlight recent studies that explore new directions for advancing NBE sensors.
Table 2.
Published NBE sensor validations for monitoring metabolites, proteins, and other biomarkers in live animals
| Target | Year [Ref] |
Sensor site | Method | Dose | # Animals | Power Analysis | Statistical Analysis |
|---|---|---|---|---|---|---|---|
| Adenosine triphosphate | 2015 [50] |
Ex vivo measurements in rat brain dialysate | Microdialysis | N/A | 3 | No | Yes |
| 2018 [49] |
Ex vivo measurements in rat brain dialysate | Microdialysis | N/A | 1 | No | No | |
| 2019 [48] |
Rat jugular vein | Intravenous bolus injection | 30 mg/kg | 1 | No | No | |
| Adenosine | 2019 [47] |
Rat muscle | Intramuscular injection | 0.1 μL, 1 mM | 1 | No | No |
| Dopamine | 2020 [46] |
Rat brain, striatum | Electrical stimulation | 300 μA, 60 Hz, 2 ms per phase | 1 | No | No |
| Cocaine | 2017 [45] |
Rat brain, striatum | Bolus injection and infusion | 2 mg/kg injection, 250 μM infusion | 4 | No | Yes |
| Phenylalanine | 2021 [44] |
Rat jugular vein | Intravenous bolus injection | 100 mg/kg | 4 | No | Yes |
| Troponin I | 2021 [43] |
Mouse mouth | Intravenous bolus injection | 50 μg/mouse | 1 | No | No |
The current benchmark methods for determining disposition kinetics of endogenous metabolites are based on immunoassays or liquid chromatography-mass spectrometry detection [51]. These methods are cumbersome and limited in temporal resolution to tens of minutes if not hours or days, depending on laboratory backlogs. Seeking to provide more convenient strategies to monitor metabolites in biofluids, Idili et al. [44] demonstrated an NBE platform achieving seconds-resolved monitoring of phenylalanine in rodents. Their measurements (Figure 5A) achieved micromolar precision and cover the clinically relevant range for patients with phenylketonuria. Once again, this study discusses the observation of subject-to-subject variability in metabolism kinetics. However, the limited number of animal subjects (Table 2) precludes rigorous statistical analysis to support that point.
Figure 5. Continuous measurements of small molecules and peptide metabolites in vivo.
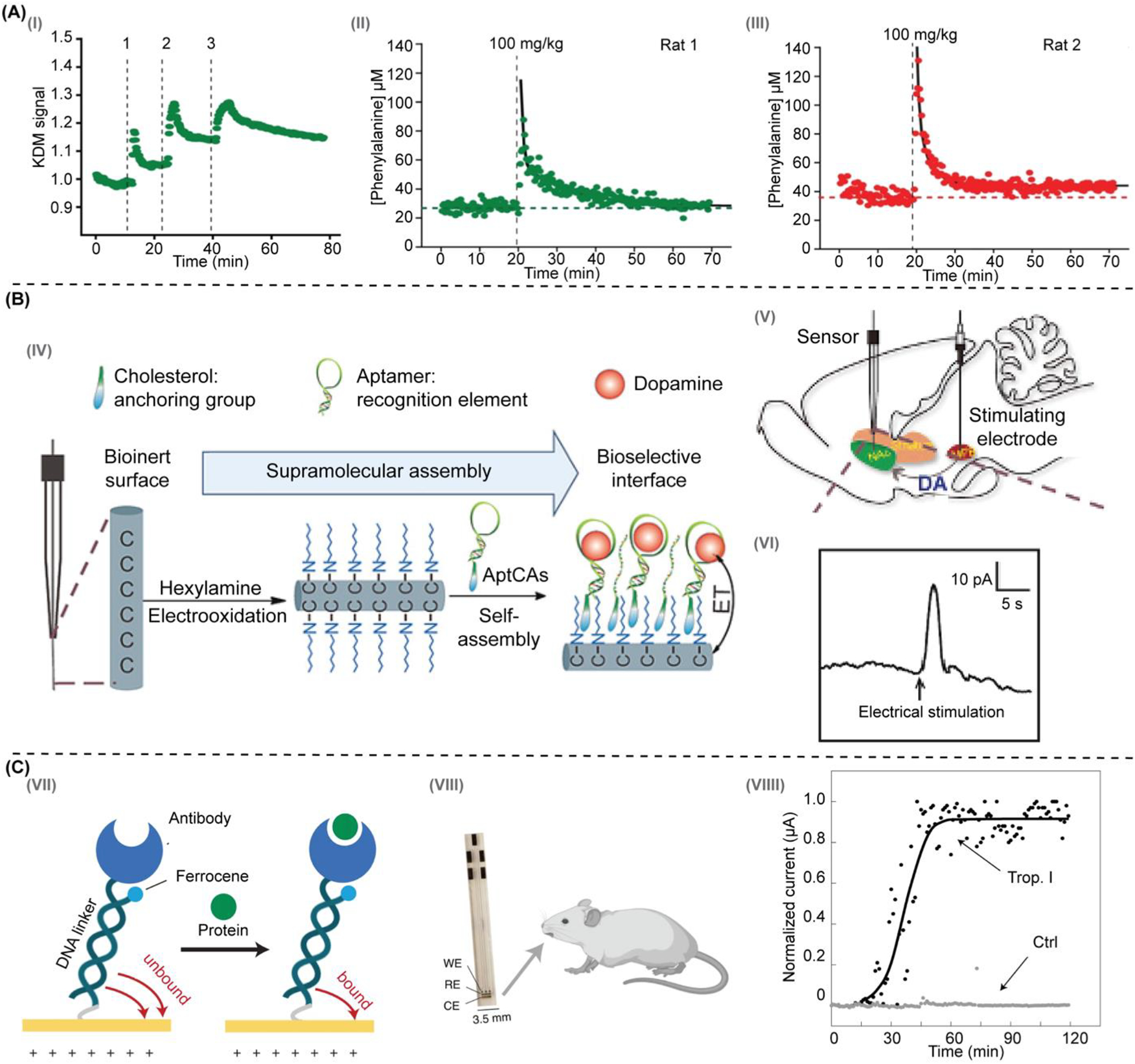
(A) Idili et al. [44] developed NBE sensors for phenylalanine that responsed to (I) serial intravenous doses of increasing concentration and (II, III) presented full pharmacokinetic profiles of plasma phenylalanine levels. (B) Hou et al. [46] proposed (IV) to immobilize nucleic acid aptamers onto carbon fiber microelectrodes (CFE). (V) They placed their sensors in the nucleus accumbens (NAc) of rats to measure (VI) dopamine transients after electrical stimulation of the medial forebrain bundle (MFB). (C) Das et al. [43] developed (VII) an NBE sensing approach based on the motion of a protein-binding molecular pendulum (MP), which was constructed from double-stranded DNA and antibodies. For example, (VIII) using anti-troponin I antibodies in an implantable sensor format, these MP sensors supported continuous monitoring of troponin levels (VIIII) in the mouth of live mice. Figure panels were reproduced from reference [44] with permission from the American Chemical Society, reference [46] with permission from the John Wiley and Sons, and reference [43] with permission from Springer Nature.
Amongst other physiologically important small molecules, neurotransmitters are fundamental to maintaining the balance of brain function [52]. Motivated to understand the temporal dynamics of neuromodulation and neurotransmission, several groups have developed NBEs to monitor neurotransmitter concentrations. However, few successful cases have been reported for detecting neurotransmitters in vivo. This lack of translation to in vivo applications could be due to the rapid fouling of NBEs when deployed in biological fluids. One strategy to mitigate fouling was reported by Hou et al., [46] who proposed replacing the gold electrodes typically used for NBE development with carbon fiber microelectrodes, which are hypothesized to foul less (Figure 5B). The authors assembled aptamer cholesterol amphiphiles (aptCAs) onto alkyl chain-functionalized carbon electrodes (HxA/CFE) to generate their NBE sensors, so called aptCFEs. They provide an example aptCFE for dopamine measurements that was implanted into the nucleus accumbens (NAc) of a rat brain along with a stimulating electrode placed in the medial forebrain bundle (MFB) region. Their experiment showed aptCFE currents associated with dopamine release following electrical stimulation in a rat’s MFB region. The measured signal reached its maximum within a few seconds and quickly decayed back to baseline levels in a trend that aligns with a previous study on dopamine release [53]. Although a novel implementation of NBE sensors, the study generates qualitative results in need of further validation against standard carbon fiber electrodes and the benchmark fast scan cyclic voltammetry.
Finally, we highlight the only work to our knowledge reporting continuous protein biomarker measurements in vivo. Das et al. [43] introduced a novel sensing mechanism for monitoring protein biomarkers of cardiovascular health, allergies, and cancers. Their approach method (Figure 5C) is based on the motion of a protein-binding molecular pendulum (MP), which was constructed from double-stranded DNA and a target-specific antibody. The pendulum is functionalized with a ferrocene redox reporter attached at the distal end of the DNA linker, next to the antibody. By modulating the voltage of the NBE sensor to electrostatically actuate the negatively charged DNA backbone, the MP sensor allows electron transfer from ferrocene at a frequency that is affected by target binding to the antibody. To validate this innovative technology in vivo, the team demonstrated that MP sensors using anti-troponin I antibodies allowed for real-time monitoring of troponin in the mouth of live mice following systemic dosing of troponin. The MP sensors produced signals with outstanding signal-to-noise ratios relative to a serum albumin control. While an exciting new platform for continuous protein monitoring, further technology validations are needed to demonstrate the generalizability of the approach, and to demonstrate that the measurements produced have biological significance and/or clinical value.
Critical Outlook
In this short opinion piece, we highlighted the success and the missing elements of reports from the past three years focused on in vivo demonstrations of continuous molecular monitors based on NBE sensors. We discussed technology applications to therapeutic drug monitoring, feedback-controlled dosing and metabolite and biomarker monitoring. It is encouraging to see some recent research efforts focus on the exploration of new electrode materials and redox reporters. However, we encourage more work towards engineering the bio-interface of NBE sensors to achieve better stability and longevity in the body. Meanwhile we hope that properly powered groups sizes in future technology demonstrations can better address the current lack of serious analytical validations for the technology. We remain enthusiastic about the future of NBE sensors in health monitoring and drug therapy and hope that the community will take this article as motivation to help the pursuit of rigorous analytical and clinical technology validations.
Funding
Y.Y. and N.A.C. are supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R01GM140143. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Netzahualcoyotl Arroyo-Curras reports financial support was provided by National Institute of General Medical Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Reference
Papers of particular interest have been highlighted with an asterisk*
- [1].Dincer C, Bruch R, Costa-Rama E, Fernandez-Abedul MT, Merkoci A, Manz A, Urban GA, Guder F: Disposable sensors in diagnostics, food, and environmental monitoring. Adv Mater 2019, 31, e1806739. [DOI] [PubMed] [Google Scholar]
- [2].Heller A, Feldman B: Electrochemical glucose sensors and their applications in diabetes management. Chem Rev 2008,108:2482–2505. [DOI] [PubMed] [Google Scholar]
- [3].Wang J: Electrochemical glucose biosensors. Chem Rev 2008, 108:814–825. [DOI] [PubMed] [Google Scholar]
- [4].Ohara TJ, Rajagopalan R, Heller A: “Wired” enzyme electrodes or amperometric determination of glucose or lactate in the presence of interfering substances. Anal Chem 1994, 66: 2451–2457. [DOI] [PubMed] [Google Scholar]
- [5].Qin S, van der Zeyden M, Oldenziel WH, Cremers T, Westerink BHC: Microsensors for in vivo measurement of glutamate in brain tissue. Sensors 2008, 8:6860–6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Breaker RR, Joyce GF: A DNA enzyme that cleaves rna. Chem Biol 1994, 1:223–229. [DOI] [PubMed] [Google Scholar]
- [7].Chen Y, Li HH, Gao T, Zhang TT, Xu LJ, Wang B, Wang JN, Pei RJ: Selection of DNA aptamers for the development of light-up biosensor to detect pb(ii). Sensor Actuator B Chem 2018, 254:214–221. [Google Scholar]
- [8].Huizenga DE, Szostak JW: A DNA aptamer that binds adenosine and atp. Biochemistry 1995, 34:656–665. [DOI] [PubMed] [Google Scholar]
- [9].Stojanovic MN, de Prada P, Landry DW: Aptamer-based folding fluorescent sensor for cocaine. J Am Chem Soc 2001, 123: 4928–4931. [DOI] [PubMed] [Google Scholar]
- [10].Bock LC, Griffin LC, Latham JA, Vermaas EH, Toole JJ: Selection of single-stranded DNA molecules that bind and inhibit human thrombin. Nature 1992, 355:564–566. [DOI] [PubMed] [Google Scholar]
- [11].Wang J, Yu J, Yang Q, McDermott J, Scott A, Vukovich M, Lagrois R, Gong Q, Greenleaf W, Eisenstein M, Ferguson BS, Soh HT: Multiparameter particle display (mppd): a quantitative screening method for the discovery of highly specific aptamers. Angew Chem Int Ed Engl 2017, 56: 744–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li X, Zhang W, Liu L, Zhu Z, Ouyang G, An Y, Zhao C, Yang CJ: In vitro selection of DNA aptamers for metastatic breast cancer cell recognition and tissue imaging. Anal Chem 2014, 86:6596–6603. [DOI] [PubMed] [Google Scholar]
- [13].Xiao Z, Shangguan D, Cao Z, Fang X, Tan W: Cell-specific internalization study of an aptamer from whole cell selection. Chem Eur J 2008, 14:1769–1775. [DOI] [PubMed] [Google Scholar]
- [14].Li H, Dauphin-ducharme P, Ortega G, Plaxco KW: Calibration-Free electrochemical biosensors supporting accurate molecular measurements directly in undiluted whole blood. J Am Chem Soc 2017, 139:11207–11213, 10.1021/jacs.7b05412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Soleymani L, Fang Z, Lam B, Bin X, Vasilyeva E, Ross AJ, Sargent EH, Kelley SO: Hierarchical nanotextured microelectrodes overcome the molecular transport barrier to achieve rapid, direct bacterial detection. ACS Nano 2011, 5:3360–3366, 10.1021/nn200586s. [DOI] [PubMed] [Google Scholar]
- [16].Pellitero MA, Shaver A, Arroyo-Currás N: Critical review—approaches for the electrochemical interrogation of DNA based sensors: a critical review. J Electrochem Soc 2020, 167, 037529, 10.1149/2.0292003jes. [DOI] [Google Scholar]
- [17].Chen H, Xie S, Liang H, Wu C, Cui L, Huan SY, Zhang X: Generation of biostable L-aptamers against achiral targets by chiral inversion of existing D-aptamers. Talanta 2017, 164:662–667, 10.1016/j.talanta.2016.11.001. [DOI] [PubMed] [Google Scholar]
- [18].Schoukroun-Barnes LR, Macazo FC, Gutierrez B, Lottermoser J, Liu J, White RJ. Reagentless, Structure-Switching, Electrochemical Aptamer-Based Sensors. Annu Rev Anal Chem (Palo Alto Calif). 2016;9(1):163–181. doi: 10.1146/annurev-anchem-071015-041446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Blix HS, Viktil KK, Moger TA, Reikvam A. Drugs with narrow therapeutic index as indicators in the risk management of hospitalised patients. Pharm Pract (Granada). 2010;8(1):50–55. doi: 10.4321/s1886-36552010000100006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Habet S Narrow Therapeutic Index drugs: clinical pharmacology perspective. J Pharm Pharmacol. 2021;73(10):1285–1291. doi: 10.1093/jpp/rgab102. [DOI] [PubMed] [Google Scholar]
- [21].Li H, Dauphin-Ducharme P, Arroyo-Currás N, et al. A Biomimetic Phosphatidylcholine-Terminated Monolayer Greatly Improves the In Vivo Performance of Electrochemical Aptamer-Based Sensors. Angew Chemie Int Ed. 2017;56(26):7492–7495. doi: 10.1002/anie.201700748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ferguson BS, Hoggarth DA, Maliniak D, et al. Real-Time, Aptamer-Based Tracking of Circulating Therapeutic Agents in Living Animals. Sci Transl Med. 2013;5(213):213ra165–213ra165. doi: 10.1126/scitranslmed.3007095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mage PL, Ferguson BS, Maliniak D, Ploense KL, Kippin TE, Soh HT. Closed-loop control of circulating drug levels in live animals. Nat Biomed Eng. 2017;1(5):1–10. doi: 10.1038/s41551-017-0070. [DOI] [Google Scholar]
- [24].*Idili A, Arroyo-Currás N, Ploense KL, et al. Seconds-resolved pharmacokinetic measurements of the chemotherapeutic irinotecan: In situ in the living body. Chem Sci. 2019;10(35):8164–8170. doi: 10.1039/c9sc01495k. [DOI] [PMC free article] [PubMed] [Google Scholar]; Annotation: The authors developed an aptamer with good signal-noise ratio against irinotecan, a member of the camptothecin family of cancer chemotherapeutics. The 200 mm-diameter, 3 mm-long NBE sensor achieved 20 s-resolved, multi-hour measurements of plasma irinotecan providing high-precision monitoring of chemotherapeutics in living animals.
- [25].*Li S, Dai J, Zhu M, et al. Hydrogel-coating improves the in-vivo stability of electrochemical aptamer-based biosensors. bioRxiv. January 2020:2020.11.15.383992. doi: 10.1101/2020.11.15.383992. [DOI] [Google Scholar]; Annotation: The authors demonstrated that the application of an agarose hydrogel coating significantly reduces drift in NBE sensors placed in the veins, the bladder, solid healthy tissue, or solid neoplastic tissues of living animals.
- [26].*Seo J-W, Fu K, Correa S, Eisenstein M, Appel EA, Soh HT. Real-time monitoring of drug pharmacokinetics within tumor tissue in live animals. Sci Adv. 2023;8(1):eabk2901. doi: 10.1126/sciadv.abk2901 [DOI] [PMC free article] [PubMed] [Google Scholar]; Annotation: The authors integrated an array of gold nanoporous microelectrodes into a flexible, polyimide polymer-based probe. The implantable microelectrode array sensor can be implanted directly into tumor tissue in a living mouse. Their results revealed a clear relation between drug exposure and drug depth of penetration into tumors. They also highlighted differences in pharmacokinetic profiles between tumor tissue and blood.
- [27].*Li S, Ferrer-Ruiz A, Dai J, et al. A pH-independent electrochemical aptamer-based biosensor supports quantitative, real-time measurement in vivo. Chem Sci. 2022;13(30):8813–8820. doi: 10.1039/D2SC02021A. [DOI] [PMC free article] [PubMed] [Google Scholar]; Annotation: The authors introduced a pH-independent redox reporter that made it possible to use NBE sensors in pH varying media, such as inside the bladder. They tested the sensor both in vitro using urine samples and in vivo in bladders of living rats.
- [28].*Wu Y, Tehrani F, Teymourian H, et al. Microneedle Aptamer-Based Sensors for Continuous, Real-Time Therapeutic Drug Monitoring. Anal Chem. 2022. doi: 10.1021/acs.analchem.2c00829. [DOI] [PMC free article] [PubMed] [Google Scholar]; Annotation: The authors demonstrated the first platform combining the use of microneedle and electrochemical aptamer-based sensors. This platform achieved continuous molecular measurements of therapeutic drugs in the interstitial fluid of a rodent.
- [29].Downs AM, Gerson J, Hossain MN, et al. Nanoporous Gold for the Miniaturization of In Vivo Electrochemical Aptamer-Based Sensors. ACS Sensors. 2021;6(6):2299–2306. doi: 10.1021/acssensors.1c00354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].*Dauphin-Ducharme P, Yang K, Arroyo-Currás N, et al. Electrochemical Aptamer-Based Sensors for Improved Therapeutic Drug Monitoring and High-Precision, Feedback-Controlled Drug Delivery. ACS sensors. 2019;4(10):2832–2837. doi: 10.1021/acssensors.9b01616. [DOI] [PMC free article] [PubMed] [Google Scholar]; Annotation: The authors demonstrated subject specific vancomycin pharmacokinetic measurements and closed-loop control over plasma concentration levels of the drug in a living animal model.
- [31].Arroyo-Currás N, Dauphin-Ducharme P, Ortega G, Ploense KL, Kippin TE, Plaxco KW. Subsecond-Resolved Molecular Measurements in the Living Body Using Chronoamperometrically Interrogated Aptamer-Based Sensors. ACS Sensors. 2018;3(2):360–366. doi: 10.1021/acssensors.7b00787. [DOI] [PubMed] [Google Scholar]
- [32].Vieira PA, Shin CB, Arroyo-Currás N, et al. Ultra-High-Precision, in-vivo Pharmacokinetic Measurements Highlight the Need for and a Route Toward More Highly Personalized Medicine. Front Mol Biosci. 2019;6(August):1–10. doi: 10.3389/fmolb.2019.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Arroyo-Currás N, Ortega G, Copp DA, et al. High-Precision Control of Plasma Drug Levels Using Feedback-Controlled Dosing. ACS Pharmacol Transl Sci. 2018;1(2):110–118. doi: 10.1021/acsptsci.8b00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Arroyo-Currás N, Scida K, Ploense KL, Kippin TE, Plaxco KW. High Surface Area Electrodes Generated via Electrochemical Roughening Improve the Signaling of Electrochemical Aptamer-Based Biosensors. Anal Chem. 2017;89(22):12185–12191. doi: 10.1021/acs.analchem.7b02830. [DOI] [PubMed] [Google Scholar]
- [35].Arroyo-Currás N, Somerson J, Vieira PA, Ploense KL, Kippin TE, Plaxco KW. Real-time measurement of small molecules directly in awake, ambulatory animals. Proc Natl Acad Sci U S A. 2017;114(4):645–650. doi: 10.1073/pnas.1613458114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].*Shaver A, Mahlum JD, Scida K, et al. Optimization of Vancomycin Aptamer Sequence Length Increases the Sensitivity of Electrochemical, Aptamer-Based Sensors In Vivo. ACS Sensors. 2022;7(12):3895–3905. doi: 10.1021/acssensors.2c01910. [DOI] [PMC free article] [PubMed] [Google Scholar]; Annotation: The authors presented improved sensitivity of a vancomycin NBE sensor in vivo in the brain cortex of living mice. This is the first demonstration of continuous, NBE-based molecular monitoring across biological membranes.
- [37].Friedel M, Thompson IAP, Kasting G, et al. Opportunities and challenges in the diagnostic utility of dermal interstitial fluid. Nat Biomed Eng. 2023. doi: 10.1038/s41551-022-00998-9. [DOI] [PubMed] [Google Scholar]
- [38].Tran BQ, Miller PR, Taylor RM, et al. Proteomic Characterization of Dermal Interstitial Fluid Extracted Using a Novel Microneedle-Assisted Technique. J Proteome Res. 2018;17(1):479–485. doi: 10.1021/acs.jproteome.7b00642. [DOI] [PubMed] [Google Scholar]
- [39].Miller PR, Taylor RM, Tran BQ, et al. Extraction and biomolecular analysis of dermal interstitial fluid collected with hollow microneedles. Commun Biol. 2018;1(1):173. doi: 10.1038/s42003-018-0170-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Singh P, Carrier A, Chen Y, et al. Polymeric microneedles for controlled transdermal drug delivery. J Control Release. 2019;315:97–113. doi: 10.1016/j.jconrel.2019.10.022. [DOI] [PubMed] [Google Scholar]
- [41].Van der Maaden K, Jiskoot W, Bouwstra J. Microneedle technologies for (trans)dermal drug and vaccine delivery. J Control Release. 2012;161(2):645–655. doi: 10.1016/j.jconrel.2012.01.042. [DOI] [PubMed] [Google Scholar]
- [42].*Lin S, Cheng X, Zhu J, et al. Wearable microneedle-based electrochemical aptamer biosensing for precision dosing of drugs with narrow therapeutic windows. Sci Adv. 2022;8(38):eabq4539. doi: 10.1126/sciadv.abq4539. [DOI] [PMC free article] [PubMed] [Google Scholar]; Annotation: The authors presented an electrochemical aptamer biosensing microneedle-based patch that enables the reliable in vivo detection of wide range of analytes in interstitial fluid, including antibiotics with narrow therapeutic windows (tobramycin and vancomycin).
- [43].*Das J, Gomis S, Chen JB, et al. Reagentless biomolecular analysis using a molecular pendulum. Nat Chem. 2021;13(5):428–434. doi: 10.1038/s41557-021-00644-y. [DOI] [PubMed] [Google Scholar]; Annotation: The authors presented a new sensing mechanism to expand the target analytes pool. This novel mechanism utilized the motion of a protein-binding molecular pendulum (MP). The MP was constructed from double-stranded DNA and a target-specific antibody. It was functionalized with a ferrocene redox reporter attached at the distal end of the DNA linker, next to the antibody. By modulating the voltage to the negatively charged DNA backbone, electron transfers would undergo from ferrocene at a frequency which would be affected by the antibody binding target. For proof-of-concept, they monitored troponin level changes in live rat’s mouth.
- [44].*Plaxco KW, Idili A, Gerson J, Kippin T. Seconds-resolved, in situ measurements of plasma phenylalanine disposition kinetics in living rats. Anal Chem. 2021;93(8):4023–4032. doi: 10.1021/acs.analchem.0c05024. [DOI] [PMC free article] [PubMed] [Google Scholar]; Annotation: The authors pointed out that there is lack of tools for real time or high time-resolved monitoring of endogenous metabolites. They presented an in vivo sensing platform to realize clinically relevant specificity and time resolution for metabolites like phenylalanine. It showed the capability of NBE sensors to achieve precise disposition kinetics and real-time monitoring of metabolites in living animals.
- [45].Taylor IM, Du Z, Bigelow ET, et al. Aptamer-functionalized neural recording electrodes for the direct measurement of cocaine in vivo. J Mater Chem B. 2017;5(13):2445–2458. doi: 10.1039/C7TB00095B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].*Hou H, Jin Y, Wei H, et al. A Generalizable and Noncovalent Strategy for Interfacing Aptamers with a Microelectrode for the Selective Sensing of Neurotransmitters In Vivo. Angew Chemie Int Ed. 2020;59(43):18996–19000. doi: 10.1002/anie.202008284. [DOI] [PubMed] [Google Scholar]; Annotation: The authors presented an interfacial functionalization strategy to assemble aptamer cholesterol amphiphiles on alkyl chain-functionalized carbon fiber electrodes for dopamine detection. The sensor showed enhanced selectivity in the detection of dopamine in living rat brain for the first time.
- [47].Zhang D, Ma J, Meng X, et al. Electrochemical aptamer-based microsensor for real-time monitoring of adenosine in vivo. Anal Chim Acta. 2019;1076:55–63. doi: 10.1016/j.aca.2019.05.035. [DOI] [PubMed] [Google Scholar]
- [48].Li H, Li S, Dai J, et al. High frequency, calibration-free molecular measurements in situ in the living body. Chem Sci. 2019;10(47):10843–10848. doi: 10.1039/C9SC04434E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Jiang Y, Ma W, Ji W, Wei H, Mao L. Aptamer superstructure-based electrochemical biosensor for sensitive detection of ATP in rat brain with in vivo microdialysis. Analyst. 2019;144(5):1711–1717. doi: 10.1039/C8AN02077A. [DOI] [PubMed] [Google Scholar]
- [50].Yu P, He X, Zhang L, Mao L. Dual recognition unit strategy improves the specificity of the adenosine triphosphate (ATP) aptamer biosensor for cerebral ATP assay. Anal Chem. 2015;87(2):1373–1380. doi: 10.1021/ac504249k. [DOI] [PubMed] [Google Scholar]
- [51].Xiao JF, Zhou B, Ressom HW. Metabolite identification and quantitation in LC-MS/MS-based metabolomics. Trends Analyt Chem. 2012;32:1–14. doi: 10.1016/j.trac.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cooper JR. Neurotransmitters. In: Smelser NJ, Baltes PBBT-IE of the S& BS, eds. Oxford: Pergamon; 2001:10612–10619. doi: 10.1016/B0-08-043076-7/03447-1. [DOI] [Google Scholar]
- [53].Liu X, Xiao T, Wu F, et al. Ultrathin Cell-Membrane-Mimic Phosphorylcholine Polymer Film Coating Enables Large Improvements for In Vivo Electrochemical Detection. Angew Chemie Int Ed. 2017;56(39):11802–11806. doi: 10.1002/anie.201705900. [DOI] [PubMed] [Google Scholar]


