Abstract
It is known that the menopause transition (MT) is a complex period during a woman’s life, but there has been ongoing debate on whether the increase in cardiovascular disease (CVD) during midlife is due to chronological aging or ovarian aging. The purpose of this review is to summarize the recent findings on the role of ovarian aging versus chronological aging on cardiovascular disease (CVD) outcomes and its risk factors in women.
Recent data from longitudinal studies have shown that menopause-related factors, such as earlier age at menopause and surgical menopause are associated with higher CVD outcomes. The MT is also associated with detrimental changes in vascular health as well as cardiometabolic risk factors including body composition, visceral fat accumulation, lipids/lipoproteins, blood pressure and the metabolic syndrome.
The robust evidence from recent research indicating increases in CVD risk over the MT beyond aging call for immediate efforts to rise awareness among women and their health care providers of CVD risk acceleration accompanying the MT. Efforts should also be directed toward developing and testing novel preventive approaches that target women during this time period to counteract the expected increase in CVD risk.
Keywords: Menopause, menopause transition, cardiovascular disease, women’s health
INTRODUCTION:
In women, progressing through midlife, a time period which coincides with the menopause transition (MT), is associated with an increase in risk of cardiovascular disease (CVD) 1. Whether the MT plays a role in this increased CVD risk or whether the elevated risk is a mere consequence of chronological aging has been the subject of a major debate for many years. Several studies tried to disentangle the contribution of chronological versus ovarian aging on CVD risk in women using mainly a cross-sectional study design 2-4. However, despite the fact that menopause is defined as a single event marked by the time of the final menstrual period (FMP), the phase of the MT is much more complex. It is characterized by hormonal, physiological, and symptomatic changes that start years before the FMP 5 and last well into the postmenopausal period, with significant variability in these changes among midlife women. As such, understanding the consequences of the MT on health and well-being, and distinguishing its contributions to disease risk independent of chronological aging can be challenging in the context of cross-sectional study designs. Longitudinal studies that follow women over time and characterize menopause-related changes over the MT are essential to comprehend whether the MT increases CVD risk in midlife women.
Over the last 2 decades, the science linking the MT with CVD risk significantly evolved as a result of longitudinal data from cohort studies that have followed women over the MT 6-8. The growing data in the field pushed for the release of the first scientific statement on menopause and CVD in 2020 by the American Heart Association (AHA) 9**. This statement highlighted the MT as a period of increased CVD vulnerability in women, and acknowledged the urgent need to increase awareness in identifying the escalating cardiovascular risk linked to this stage of women’s lives at the levels of women and health care providers. The AHA statement also stressed the need for clinical trials that target women during the MT for better identification of preventive approaches to mitigate the heightened disease risk.
This short review summarizes the latest relevant research from the past 20 years that investigates how the MT might independently increase CVD risk beyond chronological aging. Further, the review identifies the next steps that are essential to move the field of CVD prevention in midlife women forward.
ESTABLISHING A LINK BETWEEN MENOPAUSE AND CVD RISK:
When it comes to establishing a link between menopause and CVD risk, most studies utilized age at which women reach the FMP as a surrogate to understand menopause. Due to the need for a long follow up time, earlier studies collected age at menopause retrospectively. Although, this approach is convenient, recall bias has been a major concern 10. This bias is a problematic if the goal is to characterize changes as related to time relative to the date of the final menstrual period. However, in studies that focused on establishing a link between age at menopause and CVD, meta-analyses provided consistent results 4,11-13. For example, in a recent meta-analyses that included pooled data from 310,329 women from 32 observational studies, women who reached menopause (both naturally or surgically) at an age younger than 45 years (early menopause) had a relative risk (RR) of 1.50 (95% CI: 1.28-1.76) for coronary heart disease (CHD) compared to those who reached menopause after the age of 45 years13. Moreover, women with early menopause had higher risk of CVD mortality [RR: 1.19 (95% CI: 1.08-1.31)] and CHD mortality [RR: 1.11 (95% CI: 1.03-1.20)] compared to women who reached menopause after the age of 45 years 13**. Interestingly, another recent meta-analysis from the InterLACE consortium of 301,438 women suggested that the relation between younger age at natural menopause and increased risk of CVD events may be limited to cardiovascular events occurring before the age of 60 years, but not after age 70 years 14.
The reported finding of a greater risk of CVD with younger age at menopause has been attributed to the shorter exposure to endogenous estradiol in women reaching menopause earlier than others. As such, researchers hypothesized that surgical menopause induced by bilateral salpingo-oophorectomy would be associated with a higher CVD risk if it occurred prior to the average age at natural menopause. A recent study of 144,260 women from the UK Biobank who were followed for a median of 7 years, the incidence rate of a composite CVD measure (defined as incident coronary artery disease, heart failure, aortic stenosis, mitral regurgitation, atrial fibrillation, ischemic stroke, peripheral artery disease, or venous thromboembolism) was 11.3/1000 woman-years for women with premature surgical menopause (<40 years), 8.8/1000 woman-years for women with premature natural menopause (<40 years) and 5.7/1000 woman-years for women with natural menopause >40 years 15. In a meta-analysis from the InterLACE consortium, it was reported that in 203,767 postmenopausal women, the positive association of surgical versus natural menopause with the first non-fatal CVD event was attenuated by age at menopause. However, compared to women who achieved natural menopause at 50-54 years, early surgical menopause (<40 years) was significantly associated with a higher hazard for CVD risk [HR for age <35 years: 2.55 (95% CI: 2.22, 2.94); HR for age 35-39 years: 1.91 (95% CI: 1.71, 2.14)]12**.
DISINTANGLING THE CONTRIBUTION OF THE MT VS. CHRONOLOGICAL AGING ON CVD RISK IN MIDLIFE WOMEN:
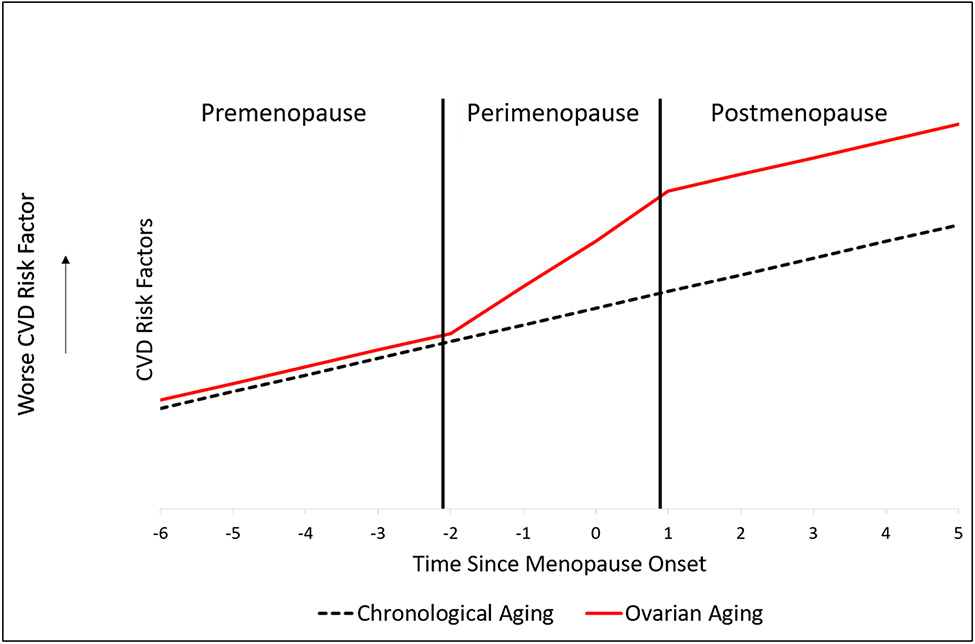
The MT is very complex and rich. As such, it can be characterized and studied in relation to CVD risk factors using multiple dimensions other than age at menopause. These include hormonal changes, changes in cycle length and bleeding patterns, frequency and severity of vasomotor symptom, stages of the MT, and timing relative to the onset of menopause9. Studying CVD risk anchored to time relative to menopause onset has been an attractive approach utilizing data from longitudinal studies followed women over the MT. If the trajectory of a studied CVD risk factor was linearly related to time relative to menopause onset, one can conclude that the association is more driven by chronological aging. However, if trajectory of a studied CVD risk factor was not linear and showed inflection points around menopause, one can conclude that the existing association is more driven by the MT, Figure 1. Another methodological approach involves calculating time elapsed in each stage of the menopause transition by documenting timing of changes in bleeding patterns and then assessing related changes in a studied CVD risk factor over time in each of the reported stages. Increases in risk in a specific stage independent of age would suggest a change that is more likely to be driven by the MT16.
Figure 1: Hypothetical graph of changes in cardiovascular risk factors related to chronological aging versus ovarian aging over the menopause transition.
With chronological aging, changes in CVD risk factors over the menopause transition are linear. On the other hand, with ovarian aging, inflection points can be identified around the perimenopausal stage, where the worsening of CVD is steepest during this period.
CHANGES IN CARDIOVASCULAR HEALTH AT MIDLIFE THAT ARE MORE DRIVEN BY THE MT:
In the following sections, we focus on the acceleration in CVD risk factors that have been found to be more driven by the MT rather than chronological aging using the time relative to menopause onset approach or the time elapsed in each stage of the MT. These CVD risk factors include body composition, visceral and ectopic fat deposition, lipids and lipoproteins, blood pressure, metabolic syndrome, and vascular health measures.
The MT, Weight Gain, Body Composition and Fat Deposition
The most prominent change that is associated with ovarian aging is the alteration in body composition. Even though studies in midlife women failed to show a relationship between weight gain and the MT beyond the effect of chronological aging 17, changes in body composition has been recently proven to be a MT-related phenomenon 18**. In a longitudinal analysis of 1,246 midlife women, anchoring fat mass and lean mass to time relative to menopause onset showed that rate of fat gain doubled, while lean mass declined until 2 years after menopause18. Interestingly, midlife women are more likely to accumulate visceral fat in the abdomen 19 and in ectopic locations, such as around the heart 20 and in the liver 21. This increase in visceral fat in the abdomen is more driven by the MT rather than chronological aging. In women participating in the Study of Women’s Health Across the Nation (SWAN) Heart ancillary study, abdominal visceral fat increased by 8.2% (95% CI: 4.1%- 12.5%) between 2 years before menopause until natural menopause. This increase continued after menopause but at a slower rate 22**.
The MT and Lipids/Lipoproteins
SWAN provided one of the strongest evidence that changes in lipids/lipoproteins during midlife are linked to the MT independent of aging. Total cholesterol and low-density lipoprotein cholesterol (LDL-C) peaked during the late-peri and early postmenopausal stages 23. This has been consistent with findings from other studies 24,25. The increase in LDL-C and apolipoprotein B (ApoB) is the steepest from one year before until one year after the FMP 26. On the other hand, the association between the MT and high-density lipoproteins (HDL) is complex. Studies have shown contradicting results on the direction of change in HDL-C between the premenopausal and postmenopausal stage 27,28. However, recent work from studies following women over time has shown that HDL-C is higher after menopause 29-32, with this increase associated with higher subclinical atherosclerosis33 suggesting that HDL may become dysfunctional over the MT30**. This was apparent by the decline in the overall size of HDL particles, the increase in small HDL particles and in the triglyceride content of the HDL, as well as the decline in cholesterol efflux capacity per HDL particle around menopause. This HDL profile has been previously associated with worse CVD profile.
The Menopause Transition, Blood Pressure and Metabolic Syndrome
The metabolic syndrome is defined as the clustering of at least 3 of the following five conditions: high blood pressure, high fasting glucose, high triglycerides, large waist circumference and/or low HDL-C. Interestingly, prevalence of the metabolic syndrome significantly rises close to the FMP independent of age 34. Moreover, the progression and increase in severity of the metabolic syndrome are greatest during the late perimenopausal years compared with the postmenopausal period 35**. Although these findings support a strong link between the metabolic syndrome and the MT, studies that evaluated specific components of the metabolic syndrome such as insulin resistance and diabetes showed that changes during midlife are not related to menopause status 36. Until recently, it was believed that the increase in blood pressure in midlife women is attributed to chronological aging 26. However, a recent publication from SWAN showed that women may experience different patterns of changes in their blood pressure during midlife, with one of these patterns displaying an acceleration in blood pressure rise, particularly 1 year after menopause. This pattern was observed in 35% of the SWAN cohort. This supports the theory that blood pressure changes in midlife are related to the MT in some but not all midlife women 37**.
The MT and Vascular Health
In a longitudinal analysis of 249 women from SWAN 38, carotid intima media thickness and adventitial diameter both increased dramatically during the late-perimenopausal compared to the pre and early peri- menopausal stages. SWAN also reported an increase in arterial stiffness during the perimenopausal stage beyond aging 39**. Postmenopausal stage may also be associated with lower brachial artery flow-mediated dilation compared to premenopausal and perimenopausal women 40. However, this was a cross-sectional study and findings did not account for differences in age.
SUMMARY AND IMPLICATIONS:
A recent survey by the American Heart Association (AHA) reported that awareness of CVD as the most common cause of death in women has decreased from 65% in 2009 to 44% in 201941. This is disturbing since CVD remains the number one killer in women1. Moreover, since the latest available sex-specific guidelines on CVD prevention were published in 201142, and since abundant research on the relationship between menopause and CVD risk was published at a later time, there is a need for up-to-date recommendations and guidelines regarding the MT as a stage of CVD risk acceleration. It is important to note that premature menopause (menopause before the age of 40 years) has been recognized as an enhancing CVD risk factor in the most recent ACA/AHA guidelines for managing cholesterol43. With the increasing life expectancy in the US population, women are predicted to spend a longer period of their life in the postmenopausal stage. As such, there is an urgent need to incorporate menopause-related factors and the MT in upcoming new CVD prevention guidelines specific to women.
Lifestyle and behavioral modifications are the first line targets for primary prevention against CVD. In 2011, the American Heart Association developed the Life’s Simple 7 score, which evaluates cardiovascular health based on 7 health behaviors and health factors (body mass index, smoking, physical activity, diet, blood pressure, total cholesterol and fasting glucose)1. Despite data from different cohorts showing a reduction in CVD events with healthier lifestyle and higher Life’s Simple 7 score, only 20% of adult American women have an ideal score on >5 cardiovascular health metrics 44. In midlife and postmenopausal women, a very small proportion of women meet the recommendations for ideal physical activity and diet status 45. Research on lifestyle interventions in midlife women who are experiencing ovarian aging is limited, but promising. One randomized clinical trial of 535 premenopausal women (age at baseline 44-50 years) randomized to a control group or a behavioral program of low-calorie diet and increased leisure activity showed that after 5 years, the control group had an increase in LDL-C that was not observed in the intervention group. Intervention group also showed a decrease in triglycerides, blood pressure, glucose and insulin46, and a decline in cIMT progression in perimenopausal and postmenopausal women47. These data strongly call for more efforts to increase women’s awareness of the critical need of adopting a heart healthy lifestyle at midlife if not even earlier. Focusing on a healthy lifestyle for preventing CVD is of particular importance, since the exact relationship between postmenopausal hormone therapy replacement and risk of CVD has not been established yet, and that the timing of initiating hormone therapy may affect its role in altering CVD risk48.
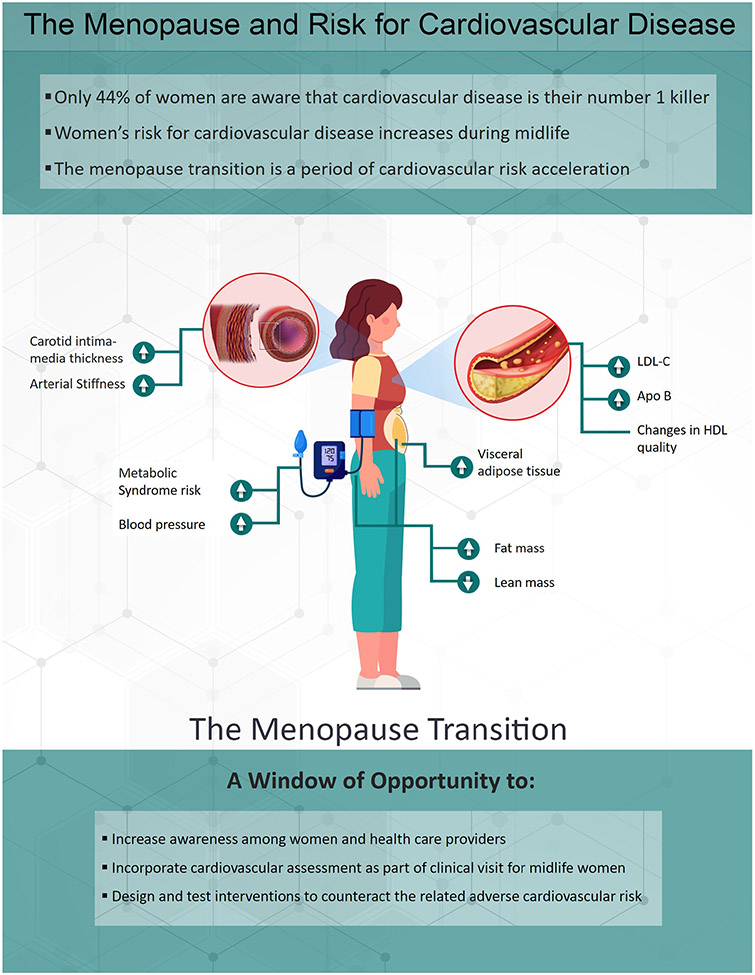
Midlife encompassing the MT is a window of opportunity to increase women awareness of sex-specific CVD risk factors and the implementation of evidence-based prevention strategies to improve the cardiovascular health in women, Figure 2. The accumulated data support incorporating menopause-related factors as important components of CVD risk assessment in clinical practice of midlife women.
Figure 2: Summary of changes in CVD risk factors related to ovarian aging in women.
During the menopause transition, women experience changes in CVD risk factors, including increases in LDL-C and Apo-B, deteriorations in quality of HDL, increased accumulation of visceral adipose tissue and fat mass, loss of lean mass, increase in blood pressure and metabolic syndrome, and worsening of subclinical atherosclerosis. This period provides a “window of opportunity” where targeted prevention could counteract the adverse changes in CVD risk.
HIGHLIGHTS:
The menopause transition (MT) is an impactful period for cardiovascular risk
Lipids, visceral fat, metabolic syndrome risk, and vascular health adversely change over the MT
More work is needed to better identify preventive methods to reduce CVD risk during the MT
Funding:
This work was supported by the National Institute on Aging (Grant: AG058690).
Abbreviations:
- ApoB
Apolipoprotein B
- AHA
American Heart Association
- CI
Confidence Interval
- CHD
Coronary Heart Disease
- CVD
Cardiovascular disease
- FMP
Final menstrual period
- HDL
High-density lipoprotein
- HDL-C
High-density lipoprotein cholesterol
- HR
Hazard Ratio
- LDL-C
Large-density lipoprotein cholesterol
- MT
Menopause transition
- RR
Relative Risk
- SWAN
Study of Women’s Health Across the Nation
Footnotes
Declaration of Interest:
None
Declaration of Interest:
Dr. El Khoudary: Supported by funds from NIA and NHLBI
Dr. Nasr: Graduate Student Researcher on The Study Of women’s Health Across the Nation (SWAN) HDL
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141(9):e139–e596 10.1161/CIR.0000000000000757 [DOI] [PubMed] [Google Scholar]
- 2.Vaidya D, Becker DM, Bittner V, Mathias RA, Ouyang P. Ageing, menopause, and ischaemic heart disease mortality in England, Wales, and the United States: modelling study of national mortality data. BMJ. 2011;343:d5170. 10.1136/bmj.d5170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dam V, van der Schouw YT, Onland-Moret NC, Groenwold RHH, Peters SAE, Burgess S, Wood AM, Chirlaque MD, Moons KGM, Oliver-Williams C, et al. Association of menopausal characteristics and risk of coronary heart disease: a pan-European case-cohort analysis. Int J Epidemiol. 2019;48(4):1275–1285 10.1093/ije/dyz016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atsma F, Bartelink ML, Grobbee DE, van der Schouw YT. Postmenopausal status and early menopause as independent risk factors for cardiovascular disease: a meta-analysis. Menopause. 2006;13(2):265–279 10.1097/01.gme.0000218683.97338.ea [DOI] [PubMed] [Google Scholar]
- 5.McKinlay SM, Brambilla DJ, Posner JG. The normal menopause transition. Maturitas. 2008;61(1-2):4–16 10.1016/j.maturitas.2008.09.005 [DOI] [PubMed] [Google Scholar]
- 6.El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell K. Endogenous sex hormones impact the progression of subclinical atherosclerosis in women during the menopausal transition. Atherosclerosis. 2012;225(1):180–186 10.1016/j.atherosclerosis.2012.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szoeke C, Coulson M, Campbell S, Dennerstein L, Investigators W. Cohort profile: Women's Healthy Ageing Project (WHAP) - a longitudinal prospective study of Australian women since 1990. Womens Midlife Health. 2016;2:5. 10.1186/s40695-016-0018-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freeman EW, Sammel MD. Methods in a longitudinal cohort study of late reproductive age women: the Penn Ovarian Aging Study (POAS). Womens Midlife Health. 2016;2:1. 10.1186/s40695-016-0014-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD, Limacher MC, Manson JE, Stefanick ML, Allison MA, et al. Menopause Transition and Cardiovascular Disease Risk: Implications for Timing of Early Prevention: A Scientific Statement From the American Heart Association. Circulation. 2020;142(25):e506–e532 10.1161/CIR.0000000000000912 [DOI] [PubMed] [Google Scholar]
- 10.den Tonkelaar I. Validity and reproducibility of self-reported age at menopause in women participating in the DOM-project. Maturitas. 1997;27(2):117–123 10.1016/s0378-5122(97)01122-5 [DOI] [PubMed] [Google Scholar]
- 11.Roeters van Lennep JE, Heida KY, Bots ML, Hoek A, collaborators of the Dutch Multidisciplinary Guideline Development Group on Cardiovascular Risk Management after Reproductive D. Cardiovascular disease risk in women with premature ovarian insufficiency: A systematic review and meta-analysis. Eur J Prev Cardiol. 2016;23(2):178–186 10.1177/2047487314556004 [DOI] [PubMed] [Google Scholar]
- 12.Zhu D, Chung HF, Dobson AJ, Pandeya N, Brunner EJ, Kuh D, Greenwood DC, Hardy R, Cade JE, Giles GG, et al. Type of menopause, age of menopause and variations in the risk of incident cardiovascular disease: pooled analysis of individual data from 10 international studies. Hum Reprod. 2020;35(8):1933–1943 10.1093/humrep/deaa124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muka T, Oliver-Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, Kavousi M, Franco OH. Association of Age at Onset of Menopause and Time Since Onset of Menopause With Cardiovascular Outcomes, Intermediate Vascular Traits, and All-Cause Mortality: A Systematic Review and Meta-analysis. JAMA Cardiol. 2016;1(7):767–776 10.1001/jamacardio.2016.2415 [DOI] [PubMed] [Google Scholar]
- 14.Zhu D, Chung HF, Dobson AJ, Pandeya N, Giles GG, Bruinsma F, Brunner EJ, Kuh D, Hardy R, Avis NE, et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health. 2019;4(11):e553–e564 10.1016/S2468-2667(19)30155-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honigberg MC, Zekavat SM, Aragam K, Finneran P, Klarin D, Bhatt DL, Januzzi JL Jr., Scott NS, Natarajan P. Association of Premature Natural and Surgical Menopause With Incident Cardiovascular Disease. JAMA. 2019;322(24):2411–2421 10.1001/jama.2019.19191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El Khoudary SR, Qi M, Chen X, Matthews K, Allshouse AA, Crawford SL, Derby CA, Thurston RC, Kazlauskaite R, Barinas-Mitchell E, et al. Patterns of menstrual cycle length over the menopause transition are associated with subclinical atherosclerosis after menopause. Menopause. 2021;29(1):8–15 10.1097/GME.0000000000001876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthews KA, Abrams B, Crawford S, Miles T, Neer R, Powell LH, Wesley D. Body mass index in mid-life women: relative influence of menopause, hormone use, and ethnicity. Int J Obes Relat Metab Disord. 2001;25(6):863–873 10.1038/sj.ijo.0801618 [DOI] [PubMed] [Google Scholar]
- 18.Greendale GA, Sternfeld B, Huang M, Han W, Karvonen-Gutierrez C, Ruppert K, Cauley JA, Finkelstein JS, Jiang SF, Karlamangla AS. Changes in body composition and weight during the menopause transition. JCI Insight. 2019;4(5) 10.1172/jci.insight.124865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond). 2008;32(6):949–958 10.1038/ijo.2008.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El Khoudary SR, Shields KJ, Janssen I, Hanley C, Budoff MJ, Barinas-Mitchell E, Everson-Rose SA, Powell LH, Matthews KA. Cardiovascular Fat, Menopause, and Sex Hormones in Women: The SWAN Cardiovascular Fat Ancillary Study. J Clin Endocrinol Metab. 2015;100(9):3304–3312 10.1210/JC.2015-2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venetsanaki V, Polyzos SA. Menopause and Non-Alcoholic Fatty Liver Disease: A Review Focusing on Therapeutic Perspectives. Curr Vasc Pharmacol. 2019;17(6):546–555 10.2174/1570161116666180711121949 [DOI] [PubMed] [Google Scholar]
- 22.Samargandy S, Matthews KA, Brooks MM, Barinas-Mitchell E, Magnani JW, Janssen I, Kazlauskaite R, El Khoudary SR. Abdominal visceral adipose tissue over the menopause transition and carotid atherosclerosis: the SWAN heart study. Menopause. 2021;28(6):626–633 10.1097/GME.0000000000001755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Derby CA, Crawford SL, Pasternak RC, Sowers M, Sternfeld B, Matthews KA. Lipid changes during the menopause transition in relation to age and weight: the Study of Women's Health Across the Nation. Am J Epidemiol. 2009;169(11):1352–1361 10.1093/aje/kwp043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukami K, Koike K, Hirota K, Yoshikawa H, Miyake A. Perimenopausal changes in serum lipids and lipoproteins: a 7-year longitudinal study. Maturitas. 1995;22(3):193–197 10.1016/0378-5122(95)00927-d [DOI] [PubMed] [Google Scholar]
- 25.Bonithon-Kopp C, Scarabin PY, Darne B, Malmejac A, Guize L. Menopause-related changes in lipoproteins and some other cardiovascular risk factors. Int J Epidemiol. 1990;19(1):42–48 10.1093/ije/19.1.42 [DOI] [PubMed] [Google Scholar]
- 26.Matthews KA, Crawford SL, Chae CU, Everson-Rose SA, Sowers MF, Sternfeld B, Sutton-Tyrrell K. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transition? J Am Coll Cardiol. 2009;54(25):2366–2373 10.1016/j.jacc.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Kat AC, Dam V, Onland-Moret NC, Eijkemans MJ, Broekmans FJ, van der Schouw YT. Unraveling the associations of age and menopause with cardiovascular risk factors in a large population-based study. BMC Med. 2017;15(1):2. 10.1186/s12916-016-0762-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matthews KA, Meilahn E, Kuller LH, Kelsey SF, Caggiula AW, Wing RR. Menopause and risk factors for coronary heart disease. N Engl J Med. 1989;321(10):641–646 10.1056/NEJM198909073211004 [DOI] [PubMed] [Google Scholar]
- 29.Matthews KA, Chen X, Barinas-Mitchell E, Brooks MM, Derby CA, Harlow S, Jackson EA, Thurston RC, El Khoudary SR. Age at Menopause in Relationship to Lipid Changes and Subclinical Carotid Disease Across 20 Years: Study of Women's Health Across the Nation. J Am Heart Assoc. 2021:e021362. 10.1161/JAHA.121.021362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.El Khoudary SR, Chen X, Nasr A, Billheimer J, Brooks MM, McConnell D, Orchard T, Crawford S, Matthews KA, Rader DJ. HDL (High-Density Lipoprotein) Subclasses, Lipid Content, and Function Trajectories Across the Menopause Transition: SWAN-HDL Study. Arterioscler Thromb Vasc Biol. 2020:ATVBAHA120315355 10.1161/ATVBAHA.120.315355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Do KA, Green A, Guthrie JR, Dudley EC, Burger HG, Dennerstein L. Longitudinal study of risk factors for coronary heart disease across the menopausal transition. Am J Epidemiol. 2000;151(6):584–593 10.1093/oxfordjournals.aje.a010246 [DOI] [PubMed] [Google Scholar]
- 32.Hashemi Nazari SS, Shakiba M, Khalili D, Hadaegh F, Tohidi M, Azizi F. High-density lipoprotein cholesterol, a protective or a risk factor for developing coronary heart disease? Tehran Lipid and Glucose Study. J Clin Lipidol. 2015;9(4):553–558 10.1016/j.jacl.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 33.El Khoudary SR, Wang L, Brooks MM, Thurston RC, Derby CA, Matthews KA. Increase HDL-C level over the menopausal transition is associated with greater atherosclerotic progression. J Clin Lipidol. 2016;10(4):962–969 10.1016/j.jacl.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: the Study of Women's Health Across the Nation. Arch Intern Med. 2008;168(14):1568–1575 10.1001/archinte.168.14.1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gurka MJ, Vishnu A, Santen RJ, DeBoer MD. Progression of Metabolic Syndrome Severity During the Menopausal Transition. J Am Heart Assoc. 2016;5(8) 10.1161/JAHA.116.003609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soriguer F, Morcillo S, Hernando V, Valdes S, Ruiz de Adana MS, Olveira G, Fuentes EG, Gonzalez I, Tapia MJ, Esteva I, et al. Type 2 diabetes mellitus and other cardiovascular risk factors are no more common during menopause: longitudinal study. Menopause. 2009;16(4):817–821 10.1097/gme.0b013e31819d4113 [DOI] [PubMed] [Google Scholar]
- 37.Samargandy S, Matthews KA, Brooks MM, Barinas-Mitchell E, Magnani JW, Thurston RC, El Khoudary SR. Trajectories of Blood Pressure in Midlife Women: Does Menopause Matter? Circ Res. 2022;130(3):312–322 10.1161/CIRCRESAHA.121.319424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell K. Progression rates of carotid intima-media thickness and adventitial diameter during the menopausal transition. Menopause. 2013;20(1):8–14 10.1097/gme.0b013e3182611787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samargandy S, Matthews KA, Brooks MM, Barinas-Mitchell E, Magnani JW, Janssen I, Hollenberg SM, El Khoudary SR. Arterial Stiffness Accelerates Within 1 Year of the Final Menstrual Period: The SWAN Heart Study. Arterioscler Thromb Vasc Biol. 2020;40(4):1001–1008 10.1161/ATVBAHA.119.313622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moreau KL, Hildreth KL, Meditz AL, Deane KD, Kohrt WM. Endothelial function is impaired across the stages of the menopause transition in healthy women. J Clin Endocrinol Metab. 2012;97(12):4692–4700 10.1210/jc.2012-2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cushman M, Shay CM, Howard VJ, Jimenez MC, Lewey J, McSweeney JC, Newby LK, Poudel R, Reynolds HR, Rexrode KM, et al. Ten-Year Differences in Women's Awareness Related to Coronary Heart Disease: Results of the 2019 American Heart Association National Survey: A Special Report From the American Heart Association. Circulation. 2021;143(7):e239–e248 10.1161/CIR.0000000000000907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Pina IL, Roger VL, Shaw LJ, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the american heart association. Circulation. 2011;123(11):1243–1262 10.1161/CIR.0b013e31820faaf8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139(25):e1082–e1143 10.1161/CIR.0000000000000625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139(10):e56–e528 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 45.Stewart AL, Magnani JW, Barinas-Mitchell E, Matthews KA, El Khoudary SR, Jackson EA, Brooks MM. Social Role Stress, Reward, and the American Heart Association Life's Simple 7 in Midlife Women: The Study of Women's Health Across the Nation. J Am Heart Assoc. 2020;9(24):e017489. 10.1161/JAHA.120.017489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuller LH, Simkin-Silverman LR, Wing RR, Meilahn EN, Ives DG. Women's Healthy Lifestyle Project: A randomized clinical trial: results at 54 months. Circulation. 2001;103(1):32–37 10.1161/01.cir.103.1.32 [DOI] [PubMed] [Google Scholar]
- 47.Wildman RP, Schott LL, Brockwell S, Kuller LH, Sutton-Tyrrell K. A dietary and exercise intervention slows menopause-associated progression of subclinical atherosclerosis as measured by intima-media thickness of the carotid arteries. J Am Coll Cardiol. 2004;44(3):579–585 10.1016/j.jacc.2004.03.078 [DOI] [PubMed] [Google Scholar]
- 48.Hodis HN, Mack WJ. The timing hypothesis and hormone replacement therapy: a paradigm shift in the primary prevention of coronary heart disease in women. Part 2: comparative risks. J Am Geriatr Soc. 2013;61(6):1011–1018 10.1111/jgs.12281 [DOI] [PubMed] [Google Scholar]