Abstract
Background
Lifetime exposure to the varicella-zoster virus (VZV) has been consistently inversely associated with glioma risk, however, the relationship of VZV with survival in adults with glioma has not been investigated. In this study, we analyzed the survival of adults with glioma in relation to their antibody measurements to 4 common herpes viral infections, including VZV, measured post-diagnosis.
Methods
We analyzed IgG antibody measurements to VZV, cytomegalovirus (CMV), herpes simplex virus 1/2 (HSV), and Epstein-Barr virus (EBV) collected from 1378 adults with glioma diagnosed between 1991 and 2010. Blood was obtained a median of 3 months after surgery. Associations of patient IgG levels with overall survival were estimated using Cox models adjusted for age, sex, self-reported race, surgery type, dexamethasone usage at blood draw, and tumor grade. Models were stratified by recruitment series and meta-analyzed to account for time-dependent treatment effects.
Results
VZV antibody seropositivity was associated with improved survival outcomes in adults with glioma (Hazard ratio, HR = 0.70, 95% Confidence Interval 0.54–0.90, P = .006). Amongst cases who were seropositive for VZV antibodies, survival was significantly improved for those above the 25th percentile of continuous reactivity measurements versus those below (HR = 0.76, 0.66–0.88, P = .0003). Antibody seropositivity to EBV was separately associated with improved survival (HR = 0.71, 0.53–0.96, P = .028). Antibody positivity to 2 other common viruses (CMV, HSV) was not associated with altered survival.
Conclusions
Low levels of VZV or EBV antibodies are associated with poorer survival outcomes for adults with glioma. Differential immune response rather than viral exposure may explain these findings.
Keywords: adult glioma, antibodies to varicella-zoster virus, cytomegalovirus, Epstein-Barr virus, glioblastoma, herpes simplex virus, survival
Key Points.
VZV antibody seropositivity is associated with improved glioma survival outcomes.
Low-reacting VZV seropositive cases have similar survival to seronegative cases.
EBV seropositivity is separately associated with altered survival trajectories.
Importance of the Study.
The potential role of viral infections in the etiology, progression, and ultimately treatment of diffuse glioma tumors in adults is not completely understood. Despite decades of studies, the only consistently implicated virus in altering the lifetime risk of glioma is the varicella-zoster virus (VZV). In this study, we further explore antibody levels to four different herpesviruses and how they may associate with altered glioma prognoses. We primarily demonstrate that low levels of VZV antibodies, as measured post-diagnosis, are associated with poorer survival outcomes in adults with glioma. Our work suggests this association is likely driven by host immunity, as opposed to viral exposure. Research into the role of specific VZV antigens, as well as the effect of chickenpox/shingles vaccines on adults with glioma is warranted. We also believe that prospective clinical investigations into the safety and utility of shingles vaccination post-glioma diagnosis may prove to be a viable approach to the improvement of patient outcomes.
Gliomas make up nearly 80% of malignant central nervous system (CNS) tumors in the United States, with an average of 16 606 deaths per year caused by malignant brain and other CNS tumors.1 Both tumor morphology and specific somatic molecular alterations are used to group gliomas into distinct subtypes, which help to inform specialized treatment strategies. IDH wildtype glioblastoma (IDH WT GBM), the most common and most severe form of diffuse glioma, has a dismal median survival rate of 14–17 months post-diagnosis.2 Infectious challenges have been thought to impact host defense against glioma tumor cell proliferation.3–5 The brain is immunologically a uniquely privileged and protected organ; the blood-brain barrier (BBB) limits the entry of foreign pathogens as well as circulating immune cells, antibodies, and immune mediators.6 However, although herpes and other viruses are known to infect the brain or induce brain-related sequelae after infection, this remains an understudied aspect of viral infections due to difficulties directly studying the healthy human brain.7,8
The interplay between viruses, antibodies, and glioma risk has been studied for decades.9–16 Most studies have focused on herpesviruses and polyomaviruses, both complex DNA viruses that establish lifelong infection through cycles of latent and lytic infection. Varicella-zoster virus (VZV), responsible for chicken pox and shingles, is the only viral infection consistently linked with a reduced risk of adult glioma. First observed in a case-control cohort in the San Francisco Bay Area using self-report data,11 and later replicated in further case-control studies using anti-VZV immunoglobulin G (IgG) antibody assays,12,17,18 previous infection of VZV has been associated with a roughly 20% decrease in the relative lifetime risk of developing a glioma. While the mechanisms underlying anti-VZV IgG measurements and altered glioma risk are generally unknown, one study demonstrated in an ex-vivo model that VZV can infect glioma cells, independent of cellular infection, previous VZV infection may help prime T-cells to recognize cross-reactive epitopes of glioma cells.19 VZV particles have not been detected in human gliomas, lending credence to the plausibility of cross-reactivity. Despite these observations, previous analyses have not found a significant association between VZV antibody seropositivity and glioma prognosis.20 This is likely due to the small sample size of IgG measurements at the time of analysis and the near ubiquity of VZV exposure in adult populations,21,22 resulting in an insufficient number of seronegative glioma cases.
Beyond VZV, the role of other common herpesviruses in the context of glioma remains controversial. The presence of human cytomegalovirus (CMV) in human gliomas is a topic of great debate, with many discordant studies over the last 2 decades.14,23–31 Epstein-Barr virus (EBV), the first implicated human oncovirus,32 although causatively linked to other cancers and CNS disorders,33 has no clear directional association with glioma risk or prognosis despite many suggestive results.34,35 Our recent work demonstrated that elevated levels of genetically predicted IgG seroreactivity to EBV antigens were associated with altered glioma risk and survival,13 particularly in IDH wildtype gliomas, suggesting associations are at least partially mediated by inherited genetic variation in immune function. In the case of VZV, the population-level prevalence of infection has been drastically altered in recent years. In 1996 the Centers for Disease Control and Prevention (CDC) recommended a single VZV vaccine for children aged 12–18 months; this was followed in 2006 by a recommended booster from 4 to 6 years of age.36 The result of the vaccination effort was a 97% reduction in varicella incidence between 1993–1995 and 2013–2014.37 The lack of support for the direct detection of viruses in glioma tumors and new evidence of genetically programmed reactivity to common viruses being associated with glioma leads us to hypothesize that immune reactivity to certain viruses may impact survival.
In this study, we evaluate the relationship between the overall survival of adults with glioma and their immunoglobulin G antibody response to antigens for 4 common herpesviruses measured after glioma diagnosis. IgG is the most common type of antibody found in the blood, where measured levels are generally considered stable markers for immunity from the previous infection. High levels of IgG can be a sign of chronic viral infection, and low levels may indicate a lack of prior infection, poor or decreasing immunity, or evidence of immunosuppression. Previous exposure to each of the 4 herpesviruses considered here is common by adulthood for individuals in the United States, which makes this framework a natural experiment of targeted host immune response and glioma outcomes.
Methods
Ethics
Collection of patient samples and associated clinicopathological information were collected with written informed consent and relevant ethical review board approval at the respective study centers per the tenets of the Declaration of Helsinki. Specifically, informed consent and ethical board approval were obtained from the Institutional Review Board of the University of California, San Francisco, Human Research Protection Program in the Office of Ethics and Compliance (USA). The diagnosis of glioma (ICDO-3 codes 9380-9480 or equivalent) was established through histology in all cases by World Health Organization guidelines.
Study Population
Data collection and details of the San Francisco Adult Glioma Study have been previously described.38–40 In brief, any adult 18 years of age and older with a newly diagnosed glioma between 1991 and 1994 (series 1), between 1997 and 1999 (series 2), between 2001 and 2004 (series 3), and between 2006 and 2010 (series 4) who resided in the San Francisco Bay Area (defined by residence within 1 of 6 counties) were eligible to participate. Adults with glioma who were seeking care at the UCSF Neuro-oncology clinic (ages 18 and over) were also recruited to participate, regardless of the place of residence, throughout series 3 and 4 (2001–2010). Non-glioma controls were recruited through random digit dialing within the San Francisco Bay Area and frequency matched to cases by sex, age, and race. The study group of willing participants was interviewed about various factors and provided blood specimens at the time of the interview for research purposes. Blood samples of cases with glioma were obtained with a median of 3 months post-diagnosis (Supplementary Figure S1). Treatment information at the time of the blood draw was recorded through a questionnaire for all cases, including the use of dexamethasone.
Serological Measurements
Details of the measurement of immunoglobulin G (IgG) antibodies to each viral antigen have been previously described.17 Briefly, IgG antibody levels for Varicella-Zoster virus (VZV), Epstein-Barr virus (EBV), cytomegalovirus (CMV), and herpes simplex virus (HSV) were measured from the blood of each participant using commercial enzyme immunoassay kits. EBV, CMV, and HSV IgG measurement assays were performed according to the manufacturer’s instructions. Series 1 assays were run in batches between 1993 and 1995, series 2 and 3 were run in batches between 2001 and 2004, and series 4 samples were run in batches between 2007 and 2011. For series 2, 3, and 4, assays were modified to provide quantitative VZV IgG measurements for each patient sample, with specific details previously provided.17
Statistical Analysis
All statistical analyses were conducted using R v4.0.3. The association between viral IgG-seropositivity and glioma survival was assessed using a Cox proportional hazards regression model with time to event calculated as the date of pathologic diagnosis to either death or censored date of last known contact. For each viral IgG measurement, proportionality assumptions were checked via Schoenfeld residuals, and the effect of influential points/outliers was examined using deviance residuals. Hazard ratios (HR) were estimated using Cox models adjusted for age, sex, self-reported race (binary white/nonwhite), surgery (resection/biopsy only), and current dexamethasone usage at the time of blood draw. Subgroups according to tumor grade (grades II and III, or grade IV) were also independently analyzed. Models testing for association with overall glioma also included stratification by tumor grade. We used a statistical significance threshold of α = 0.05 to determine significant P-values. To test for consistent association across the decades-long range of patient recruitment, survival analyses stratified by AGS recruitment series were combined in a fixed-effects meta-analysis. Heterogeneity in series-specific IgG seropositivity associations was assessed using Cochran’s Q test.
VZV IgG seroreactivity measurements were analyzed separately as continuous and as binned variables. Amongst VZV-seropositive individuals, seroreactivity quartile cut points (25th, 50th, and 75th percentiles) were calculated using the set of seropositive VZV antibody IgG measurements in available AGS non-glioma controls. Cases were then partitioned into 5 groups: seronegative (determined via IgG assay), while IgG-seropositive cases were placed into 1 of 4 seroreactivity bins based on the cut points. Cox analyses, as outlined above, were used to estimate hazard ratios for both continuous association and the differential survival effect of binned high or low reactivity.
Results
The study consisted of 1378 adults with glioma with at least 1 serological (IgG) measure and available survival and covariate information. Adults over the age of 89 (n = 1) and those who had unknown dexamethasone history (n = 16) were excluded from this study. The mean age of included cases was 50.8 years. Basic summary information of study participants overall and by tumor grade along with information on 1124 non-glioma controls is available in Table 1. Supplementary Table S1 provides a breakdown of included glioma cases by somatic IDH mutation subtype. 783 patients recruited from AGS series 1, 2, and 3 were tested for seropositivity to viral antibodies of 4 viral infections of interest: VZV, EBV, CMV, and HSV. Patients recruited from series 4 (n = 595) were solely measured for VZV antibodies. Supplementary Table S2 shows the number of cases from each series with available IgG measurements and the percent who tested seropositive (See Supplementary Table S3 for a breakdown of non-glioma controls). Continuous VZV IgG antibody seroreactivity measurements were also collected for patients in series 2, 3, and 4 (n = 1182). Overall, 585 of the included subjects were diagnosed with an IDH-wildtype GBM, 157 with IDH-mutated astrocytoma (grade II or III), 111 with diffuse astrocytoma (IDH-wildtype), 94 with IDH mutated 1p/19q codeleted oligodendroglioma, 52 with grade-IV astrocytoma, and the 379 remaining with other subtypes (Supplementary Table S4).
Table 1.
Breakdown of Included Individuals With Available Viral Antibody Assays, San Francisco Bay Area Adult Glioma Study
| Glioma Cases | Grade II/III Glioma | Grade IV Glioma | Non-glioma Controls | |
|---|---|---|---|---|
| Individuals with any IgG data | ||||
| No. | 1378 | 483 | 761 | 1124 |
| Mean age (SE) | 50.8 (2.1) | 44.3 (2.0) | 56.7 (1.7) | 55.6 (2.0) |
| % Male | 58.6 | 55.9 | 62.5 | 50.2 |
| % White | 86.9 | 84.5 | 88.6 | 87.8 |
| % IDH mutated | 30.2 | 71.2 | 8.2 | |
| Median months survival (IQR) | 25.3 (128.6) | 130.0 (251.6) | 16.0 (14.4) | |
| % on Dexamethasone | 34.2 | 20.9 | 45.6 | |
| No. with specific IgG (% seropositive) | ||||
| VZV | 1374 (94.3%) | 480 (95.4%) | 760 (93.8%) | 1121 (94.4%) |
| EBV | 781 (92.6%) | 293 (91.8%) | 415 (93.5%) | 907 (94.6%) |
| HSV | 775 (72.8%) | 290 (69.0%) | 412 (76.7%) | 910 (74.0%) |
| CMV | 781 (54.8%) | 293 (53.2%) | 415 (55.7%) | 911 (54.7%) |
IQR = Interquartile range
IgG Seropositivity-Glioma Survival Associations
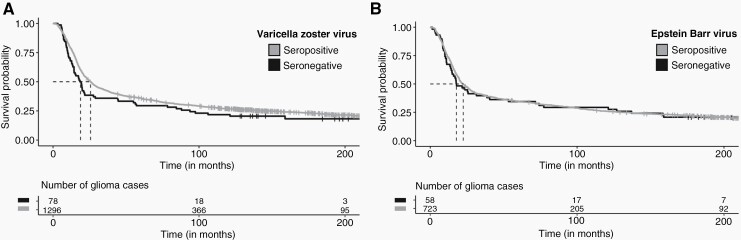
Summarized differences in demographics, tumor type, treatment, and survival outcomes between cases who tested positive or negative to antibodies for the viruses of interest are included in Table 2, along with univariate tests for statistical significance in differences. Kaplan-Meier curves for differential seropositive and seronegative glioma survival visualize the univariate association of each virus on survival trajectory (Figure 1 and Supplementary Figure S1). In multivariate survival analysis, VZV antibody seropositivity at the time of blood draw was associated with improved survival outcomes in individuals with glioma (Hazard ratio, HR = 0.70, 95% Confidence Interval 0.54–0.90, P = .0061), with an association that persists when restricting to only individuals with grade-IV gliomas (HR = 0.65, 0.48–0.87, P = .0046, n = 760). Cases who were positive for VZV antibodies had a median 6.9 months longer survival (4.3 months in grade IV gliomas) than those with negative antibody tests. There was no significant difference in age distribution between the VZV seropositive and seronegative cases (Table 2, Supplementary Figure S2A). EBV antibody seropositivity was also associated with improved survival outcomes in overall glioma (HR = 0.71, 0.53–0.96, P = .028) after adjusting for other covariates, with a median increase in survival of 4.7 months. Of the 777 patients assayed for both VZV and EBV antibodies, only 7 were negative for both, 50 were positive only for VZV antibodies, 51 were positive only for EBV antibodies, and 669 were positive for both. The inclusion of both VZV and EBV seropositivity as covariates in a single cox model only slightly attenuated each association (VZV: HR = 0.69, 0.50–0.94, P = .019/EBV: HR = 0.77, 0.56–1.05, P = .100).
Table 2.
Demographics of IgG Seropositive and Seronegative Adults With Glioma, San Francisco Bay Area Adult Glioma Study
| VZV | EBV | HSV | CMV | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| + | - | P | + | - | P | + | - | P | + | - | P | |
| No. | 1296 | 78 | 723 | 58 | 564 | 211 | 428 | 353 | ||||
| Mean age | 50.8 | 50.9 | .95 | 50.6 | 50.2 | .72 | 52.5 | 45.5 | <.001 | 52.5 | 48.4 | <.001 |
| Median mo. survival | 25.7 | 18.8 | .16 | 22.5 | 17.8 | .79 | 20.0 | 31.4 | .002 | 20.0 | 25.3 | .07 |
| % white | 87.4% | 78.2% | .03 | 87.4% | 89.7% | .77 | 87.2% | 88.2% | .82 | 82.5% | 93.8% | <.001 |
| % on Dexamethasone | 33.9% | 38.5% | .53 | 26.2% | 37.9% | .91 | 36.0% | 37.4% | .61 | 35.7% | 37.1% | .64 |
| % grade IV | 55.2% | 60.3% | .28 | 53.8% | 46.6% | .48 | 56.0% | 46.0% | .03 | 54.0% | 52.4% | .58 |
| % IDH mutated | 30.1% | 29.5% | .99 | 32.0% | 31.9% | .99 | 30.2% | 37.4% | .10 | 29.6% | 35.0% | .16 |
+ = seropositive, − =seronegative, P = P-value for difference between seropositive and seronegative.
Chi-square tests were used by default to test categorical variables. Age differences were assessed using a Wilcoxon rank sum test. Univariate analysis of survival differences was assessed using a log-rank test.
Figure 1.
Kaplan-Meier survival curves for adults with glioma, stratified by seropositivity of antibodies to VZV and EBV, San Francisco Bay Area Adult Glioma Study. Unadjusted percent survival distributions for adults with glioma who were either seronegative or seropositive for anti-viral IgG levels. Dashed lines represent median survival times, measured in months since diagnosis. Censored measurements, where the time of last known contact was not a death event, are demarcated by a “+”. Serostatus was measured from blood drawn a median 3 months after initial glioma diagnosis. Antibodies to two viruses were significant (P < .05) in a multivariate Cox model analysis: A) Varicella-Zoster virus, B) Epstein-Barr virus. All glioma cases were a part of the San Francisco Bay Area Adult Glioma Study.
Similar multivariate analyses for seropositivity to Cytomegalovirus (CMV) and herpes simplex virus (HSV) antibodies resulted in null associations with the survival time of individuals with adult glioma (HRCMV = 1.11, P = .213/HRHSV = 1.13, P = .215). Patients seropositive for HSV or CMV antibodies were significantly older than patients who tested negative (Table 2, Supplementary Figure S2C and S2D). Results of each Cox model analysis provide further details for each test for glioma overall, grades II/III gliomas, and grade IV gliomas (Table 3, Supplementary Figures S3 and S4)
Table 3.
Cox Regressions of Survival by Anti-viral IgG Seropositivity, San Francisco Bay Area Adult Glioma Study
| Overall Glioma | Grades II and III Glioma | Grade IV Glioma | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n Cases | n Died | Median Months Survival | P | n Cases | n Died | Median Months Survival | P | n Cases | n Died | Median Months Survival | P | |
| HR (95% CI)a | HR (95% CI)a | HR (95% CI)a | ||||||||||
| IgG for VZV | 1374 | 1072 | .0061 0.70 (0.54–0.90) |
480 | 278 | .226 0.70 (0.40–1.24) |
760 | 739 | .0046 0.65 (0.48–0.87) |
|||
| Negative | 78 | 63 | 18.8 | 22 | 13 | 102.0 | 47 | 47 | 11.8 | |||
| Positive | 1296 | 1009 | 25.7 | 458 | 265 | 133.0 | 713 | 692 | 16.1 | |||
| IgG for CMV | 781 | 640 | .213 1.11 (0.94–1.31) |
293 | 196 | .386 1.14 (0.84–1.55) |
415 | 411 | .758 1.03 (0.84–1.27) |
|||
| Negative | 353 | 284 | 25.3 | 137 | 90 | 135.8 | 184 | 182 | 14.4 | |||
| Positive | 428 | 356 | 20.0 | 156 | 106 | 98.7 | 231 | 229 | 13.4 | |||
| IgG for EBV | 781 | 640 | .028 0.71 (0.53–0.96) |
293 | 196 | .322 0.77 (0.47–1.28) |
415 | 411 | .154 0.75 (0.50–1.12) |
|||
| Negative | 58 | 48 | 17.8 | 24 | 17 | 99.3 | 27 | 27 | 13.4 | |||
| Positive | 723 | 592 | 22.5 | 269 | 179 | 119.4 | 388 | 384 | 14.0 | |||
| IgG for HSV | 775 | 634 | .215 1.13 (0.93–1.36) |
290 | 193 | .163 1.27 (0.91–1.77) |
412 | 408 | .367 1.12 (0.88–1.42) |
|||
| Negative | 211 | 165 | 31.4 | 90 | 57 | 142.0 | 96 | 96 | 15.7 | |||
| Positive | 564 | 469 | 20.0 | 200 | 136 | 111.0 | 316 | 312 | 13.7 |
aHR adjusted for age, sex, race (white/nonwhite), series, surgery (resection/biopsy only), and dexamethasone usage; Overall glioma models include stratification by tumor grade. Hazard ratio for seropositive individuals.
The above results remained consistent when defining survival as the time to the event from the blood draw, instead of from initial glioma diagnosis (Supplementary Table S5), when removing potentially gravely ill cases (removing adults with glioma who died within 30 days of blood draw, n = 25, and subtracting 30 days from remaining survival times, Supplementary Table S6, or within 60 days of blood draw, n = 60, results not shown), and when removing cases whose blood draw was more than 167 days (75th percentile of time to blood collection, Supplementary Figure S1) after initial diagnosis (n = 338 cases removed, Supplementary Table S7). Results also remained consistent when stratifying by World Health Organization (WHO) 2016 glioma subtypes (Supplementary Table S4) in place of tumor grade (comparison of results in Supplementary Table S8). Subtype-specific survival models based on WHO 2016 molecular classification were conducted but were largely underpowered due to a lack of seronegative samples (Supplementary Table S9).
Stability of Survival Associations Across Study Periods
We examined the consistency of the estimated hazard ratios across the 4 independent recruitment series from the AGS to investigate the role of time-dependent survival associations. Glioma survival has modestly improved over time with an evolving standard of care treatment because of improvements in prognosis across the 2 decades of data collection. Supplementary Figure S6 provides forest plots of survival associations by viral antigen, stratified by patient recruitment series. In a fixed-effects meta-analysis, using AGS recruitment series as a proxy for calendar time of diagnosis, the association between anti-VZV IgG seropositivity and improved survival outcomes was consistent across the four independent patient groups (meta-HR = 0.71, 95% CI 0.55–0.93, P = .013), for measurements collected in series 1, 2, 3, and 4, with consistent effect across series (Q = 0.17, Phet = .98) (Supplementary Figure S6A). Similarly, EBV antibody seropositivity was consistently associated with improved survival outcomes across the 3 available recruitment series (meta-HR = 0.66, 0.49–0.90, P = .0093) (Supplementary Figure S6B). The lack of evidence (P > .05) for survival associations was consistent for CMV (meta-HR = 1.21, 0.95–1.33, P = .187) across the series (Supplementary Figure S6C). IgG seropositivity for HSV was inconsistently (Phet = .01) associated with worse survival outcomes across the 3 available series (meta-HR = 1.16, 0.95–1.40, P = .138), with significant associations in series 1 and 2, but null in series 3, whose sample size was larger than 1 and 2 combined (Supplementary Figure S6D).
IgG Seroreactivity-Glioma Survival Associations
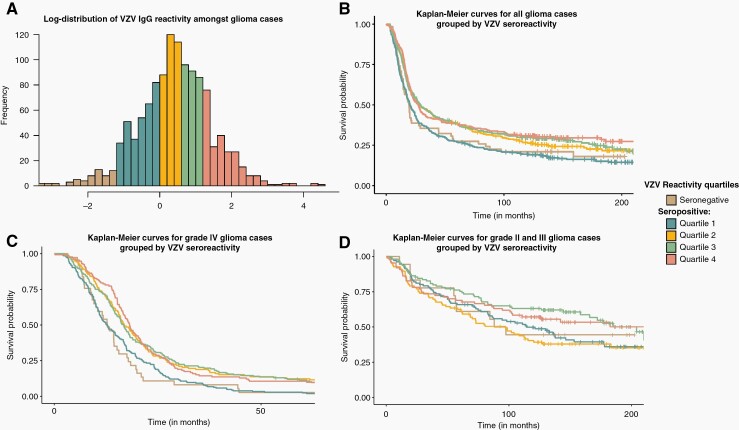
A total of 910 VZV-seropositive non-glioma controls with continuous seroreactivity measurements (Supplementary Table S10) were used to generate quartile bins of VZV seroreactivity. The determined cut points corresponding to the 25th, 50th, and 75th percentiles were 0.987, 1.646, and 3.313 mlU/mL, respectively. VZV-seropositive glioma cases with corresponding continuous IgG seroreactivity measurements were binned into these 4 quartiles of reactivity (Supplementary Table S11). A complete breakdown of multivariate survival analysis between quartiles is provided in Supplementary Table S12. Amongst VZV antibody seropositive cases, individuals with anti-VZV IgG seroreactivity levels in the top 3 quartiles (quartiles 2, 3, or 4, ie, seroreactivity >0.987) of measurements had significantly improved survival outcomes compared to cases in the bottom quartile, Q1, of reactivity (HR = 0.76, 0.66–0.88, P = .0003, n = 1182) (Supplementary Table S12, Supplementary Figure S7A). This effect was localized to patients with grade IV gliomas (HR = 0.75, 0.63–0.89, P = .0013, n = 608). Having IgG levels in the highest quartile was not associated with altered survival (quartile 4 of VZV IgG seroreactivity levels versus quartiles 1, 2, and 3 amongst VZV seropositive cases) amongst glioma cases (HR = 0.94, 0.79–1.11, P = .443) (Supplementary Figure S7B). Cases with seroreactivity in quartile 1 amongst seropositive cases had survival trajectories indistinguishable from VZV-seronegative cases (HR = 0.87, 0.64–1.18, P = .368, n = 416) (Supplementary Figure S7C). Overall, moderate to high reacting cases (VZV quartiles 2, 3, and 4) had significantly improved survival times compared to those with low antibodies (quartile 1 and seronegative cases) (HR = 0.75, 0.65–0.86, P = .00003) (Supplementary Figure S7D). Figure 2B provides a visual of survival trajectories for adults with glioma-based seronegative and seropositive quartiles (quartiles 1, 2, 3, and 4). Figures 2C and 2D present the same stratification restricted to individuals with grade-IV or II-III gliomas, respectively. Anti-VZV IgG seroreactivity modeled as a continuous variable amongst seropositive cases was not associated with glioma survival outcomes (HR = 1.00, 0.93–1.07, P = .91).
Figure 2.
Distribution of VZV IgG seroreactivity measurements in adults with glioma, and binned reactivity survival curves. Unadjusted percent survival distributions for adults with glioma who were assayed for anti-VZV IgG bodies. A) The log-transformed distribution of continuous anti-VZV seroreactivity measurements in glioma cases. Colors represent assigned seronegativity or seropositive reactivity quartile, quartiles as determined by the distribution of measurement in matched controls. Raw values of seropositive quartile breakpoints, measured in mlU/mL, were 0.987, 1.646, and 3.313. B) Percent survival distributions for adults with glioma grouped by VZV seroreactivity IgG assay. C) Percent survival distributions for adults with grade IV glioma grouped by VZV seroreactivity IgG assay. D) Percent survival distributions for adults with either a grade II or III glioma grouped by VZV seroreactivity IgG assay.
Influence of Dexamethasone on Survival Associations
Under the hypothesis that survival associations we observed may be mediated by dexamethasone (dex) usage, a corticosteroid, and known immunosuppressant, we partitioned cases based on the reported use of dex at the time of blood draw to evaluate the differential impact on measured IgG levels and glioma survival associations (Supplementary Table S13). For cases with reported dex usage at the time of blood draw, VZV seropositivity was associated with improved survival (HR = 0.67, 0.45–0.99, P = .046, n = 469), while EBV seropositivity was not significantly associated (HR = 0.78, 0.49–1.23, P = .285, n = 484). For individuals with no dexamethasone usage at the time of blood draw, the effects of VZV and EBV seropositivity were slightly attenuated (HRVZV = 0.76, 0.54–1.08, P = .126, n = 889/HREBV = 0.67, 0.45–1.01, P = .056, n = 483), and did not reach statistical significance. Overall, the direction of the estimated effect sizes remained consistent for cases with or without reported dex usage (Table 4, grade-specific associations in Supplementary Tables S14 and S15). Associations with CMV and HSV seroreactivity remained null (P > .05) when restricted to either dexamethasone-specific analysis.
Table 4.
Cox Regressions of Survival by Anti-viral IgG Seropositivity, for Patients With or Without Reported Dexamethasone Usage at Time of Blood Draw, San Francisco Bay Area Adult Glioma Study
| Received Dexamethasone | No Dexamethasone Reported | |||||||
|---|---|---|---|---|---|---|---|---|
| n Cases | n Died | Median Months Survival | P, HR | n Cases | n Died | Median Months Survival | P, HR | |
| (95% CI) a | ||||||||
| (95% CI) a | ||||||||
| IgG for VZV | 469 | 434 | .046 0.67 (0.45–0.99) |
889 | 622 | .13 0.76 (0.54–1.08) |
||
| Negative | 30 | 29 | 11.3 | 48 | 34 | 50.5 | ||
| Positive | 439 | 405 | 15.4 | 841 | 588 | 49.6 | ||
| IgG for CMV | 284 | 265 | .17 1.2 (0.92–1.56) |
483 | 363 | .84 1.02 (0.82–1.27) |
||
| Negative | 131 | 118 | 14.9 | 213 | 159 | 44.9 | ||
| Positive | 153 | 147 | 13.2 | 270 | 204 | 44.4 | ||
| IgG for EBV | 284 | 265 | .28 0.78 (0.49–1.23) |
483 | 363 | .056 0.67 (0.45–1.01) |
||
| Negative | 22 | 21 | 13.0 | 35 | 27 | 40.5 | ||
| Positive | 262 | 244 | 14.3 | 448 | 336 | 44.9 | ||
| IgG for HSV | 282 | 263 | .069 1.30 (0.98–1.72) |
380 | 360 | .64 0.94 (0.73–1.21) |
||
| Negative | 79 | 72 | 17.1 | 125 | 88 | 83.0 | ||
| Positive | 203 | 191 | 13.7 | 355 | 272 | 37.3 |
aHR adjusted for age, sex, race (white/nonwhite), series, and surgery (resection/biopsy only). Models include stratification by tumor grade. Hazard ratio for seropositive individuals.
Discussion
Our analysis demonstrates that low or no seroreactivity to VZV and seronegativity to EBV are associated with decreased survival time in adults with glioma. To the best of our knowledge, this is the first observation of these associations and the first time that high reactivity to common viral infections has been shown to positively impact survival in any cancer. We found that the VZV and EBV associations are largely independent and are not driven by the same seronegative cases. We found that adults with glioma who tested positive for VZV antibodies roughly 3 months post-diagnosis were 30% less likely to die at any time point during the study period than those who tested negative. Low seroreactivity was also associated with a poorer prognosis, with indistinguishable survival outcomes from VZV-seronegative patients when restricted solely to VZV-seropositive cases. As the AGS data was collected across multiple independent recruitment series, we were able to verify that the direction and effect size of these seropositivity associations were consistently observed across all 4 AGS recruitment series as an internal validation of consistency. This suggests that individuals with low-to-no detectable circulating antibodies to VZV at the time of diagnosis had significantly poorer glioma prognosis. Similarly, seronegativity to EBV was also a negative marker in overall glioma prognosis, with seropositive patients living a median 4.7 months longer. We found no association between CMV or HSV antibodies and altered glioma survival outcomes.
VZV and EBV infection/exposures are nearly ubiquitous in the United States adult population, with the majority of people being exposed in early life.22,41 After the implementation of the VZV vaccine program in 1996, there was a substantial, near complete, reduction in active VZV infections.36,37 However, our patient population was not of age to receive childhood VZV vaccination, the impact which should be addressed in future studies. As there is a low probability that a person who has reached adulthood has had no lifetime exposure to either VZV or EBV, one may ask if the results in this study are driven by elevated rates in the co-occurrence of IgG seronegativity to both VZV and EBV antibodies, which would likely be the result of a lack of durable antibody production, potentially a consequence of the tumor or from medication. However, within our data, we observed that less than 1% of adults with glioma who were measured for both VZV and EBV antibodies were seronegative for both (7 of 777 cases). This observation is in line with the expected rate obtained by sampling the general glioma-free population (4 of 904 non-glioma controls). This simple comparison suggests that there is little-to-no evidence for VZV/EBV seronegativity in adults with glioma being driven by some overall immune suppression and is instead likely driven by natural exposure and differential immune response.
Unlike VZV and EBV, the assumption of early life exposure for both CMV and HSV is more dynamic. Both viruses are common in the United States, but CMV seroprevalence gradually increases with age from ~59% in 6-year-olds to nearly ~91% in 80-year-old adults.42 HSV-1 prevalence is lower, ~60%, and appears to be slowly decreasing over calendar time.43 As the natural variance of HSV/CMV infection is high in the population, it is more challenging to disentangle differences in immune response from lifetime exposure to these 2 viruses. Measurements of IgG seroreactivity to these viruses amongst seropositive adults with glioma would help to address this issue and are recommended for future study.
The IgG measurements used in this study were from serum samples obtained post-diagnosis. Therefore, the measurements likely could have been influenced by tumor or associated treatments, as gliomas have been shown to have various mechanisms of immunosuppression.44–46 The inclusion of the drug, dexamethasone, a known immunosuppressor,47 as a covariate in the models did not influence the reported prognostic associations with IgG seropositivity/reactivity. While attenuated, the direction of effect of seropositivity was consistent across cases with or without reported dexamethasone usage at the time of the blood draw. Interestingly, our study found that significant survival associations came from VZV and EBV IgG measurements. There were no significant associations with CMV or HSV, suggesting a possibility of virus-specific immune mechanisms. One would expect, if the results were due to general immune suppression, associations may have been significant or at least suggestive for all common viruses studied, and that the frequency of individuals who test negative for VZV and EBV together would have been elevated from the background control population, which we saw no evidence of.
In addition to inconsistent timing of blood collection after primary glioma diagnosis (Supplementary Figure S1), there are several other limitations in this study. We only have a single time point per subject and cannot assess any changes in response to viruses across the course of treatment. Continuous seroreactivity measurements were generated only for VZV and not EBV, CMV, or HSV-1/2, limiting our analysis to only seropositivity for these three viruses, which precludes the analysis of continuous reactivity modeling in seropositive individuals. Although the use of dexamethasone was routinely collected, patient history of other potentially explanatory immune suppressive medications and phenotypes, such as Keppra or history of seizures, was not consistently collected across our recruitment series, and should be included in future studies. Our patient population was collected prior to both shingles vaccination recommendations. Zostavax was approved for adults greater than 50 in 2011 and Shingrix was approved in 2017, therefore we cannot analyze the effect of these vaccines on glioma survival in our cohort. Further investigation into the specific VZV antigens driving the survival association will help to elucidate if vaccination confers similar benefits as natural infection for adults with glioma. This is especially important considering the current vaccine in widespread use, Shingrix, targets VZV glycoprotein E, while Zostavax is a broader live attenuated vaccine that is less utilized due to side effects.
This study shows that lower seroreactivity to VZV and seronegativity to EBV are associated with decreased glioma survival time in adults, but the mechanisms remain unclear. Our evidence demonstrating low-reacting VZV seropositive cases having similar survival trajectories to seronegative cases suggests this is not an association directly implicating the lifetime exposure of VZV, but rather the differential immune response by the hosts. Previous research showed that T-cells primed by VZV may recognize glioma cells more efficiently, which suggests a potential mechanism,19 but further research is required to investigate the in vivo effects of VZV in glioma, and investigations into EBV are needed. Further, our previous work demonstrated a significant germline genetic contribution to the EBV seroreactivity-glioma survival association,13 positing that individuals immunogenetically primed to react highly to specific EBV antigens have more favorable glioma survival outcomes. Intriguingly, the results presented here suggest that this association is virus-specific and not the result of treatment effects or systemic immune suppression. This leads to many questions: Is EBV seroreactivity, independent of germline genetics, associated with survival? Does seroreactivity to these viruses change longitudinally across the course of treatment? How do the childhood varicella or adult zoster vaccines alter this observed effect? Are the B or T cell repertoires specific to VZV or EBV increasing glioma survival? Could VZV and EBV be leveraged in immunotherapeutic approaches? Further research into the association of immune responses to VZV and EBV with glioma survival and potential treatment is warranted.
Supplementary Material
Acknowledgments
The authors wish to acknowledge study participants, the clinicians, and research staff at the participating medical centers, the UCSF Cancer Registry, and the UCSF Neurosurgery Tissue Bank. The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP006344; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute, Cancer Registry of Greater California. The ideas and opinions expressed herein are those of the authors and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors. All analyses, interpretations, and conclusions reached in this manuscript from the mortality data are those of the authors and not the State of California Department of Public Health.
Contributor Information
Geno Guerra, Department of Neurological Surgery, University of California San Francisco, San Francisco, California, USA.
Lucie McCoy, Department of Neurological Surgery, University of California San Francisco, San Francisco, California, USA.
Helen M Hansen, Department of Neurological Surgery, University of California San Francisco, San Francisco, California, USA.
Terri Rice, Department of Neurological Surgery, University of California San Francisco, San Francisco, California, USA.
Annette M Molinaro, Department of Neurological Surgery, University of California San Francisco, San Francisco, California, USA; Department of Epidemiology and Biostatistics, University of California San Francisco, San Francisco, California, USA; Weill Institute for Neurosciences, University of California San Francisco, San Francisco, California, USA.
Joseph L Wiemels, Center for Genetic Epidemiology, Department of Population and Public Health Sciences, University of Southern California, Los Angeles, California, USA.
John K Wiencke, Department of Neurological Surgery, University of California San Francisco, San Francisco, California, USA; Department of Epidemiology and Biostatistics, University of California San Francisco, San Francisco, California, USA; Institute of Human Genetics, University of California San Francisco, San Francisco, California, USA; Weill Institute for Neurosciences, University of California San Francisco, San Francisco, California, USA.
Margaret Wrensch, Department of Neurological Surgery, University of California San Francisco, San Francisco, California, USA; Institute of Human Genetics, University of California San Francisco, San Francisco, California, USA; Weill Institute for Neurosciences, University of California San Francisco, San Francisco, California, USA.
Stephen S Francis, Department of Neurological Surgery, University of California San Francisco, San Francisco, California, USA; Department of Epidemiology and Biostatistics, University of California San Francisco, San Francisco, California, USA; Weill Institute for Neurosciences, University of California San Francisco, San Francisco, California, USA.
Funding
This work was supported by the National Institutes of Health (grant numbers T32CA151022, T32CA112355, R01CA52689, P50CA097257, R01CA126831, R01CA139020, and R01CA266676); the National Brain Tumor Foundation; the Stanley D. Lewis and Virginia S. Lewis Endowed Chair in Brain Tumor Research; the Robert Magnin Newman Endowed Chair in Neuro-oncology; and by donations from families and friends of John Berardi, Helen Glaser, Elvera Olsen, Raymond E. Cooper, and William Martinusen. This publication was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences; National Institutes of Health, through UCSF-CTSI [grant number UL1 RR024131]. Its contents are solely the authors’ responsibility and do not necessarily represent the official views of the NIH.
Conflict of interest statement. The authors declare no competing interests.
Authorship
G.G. and S.S.F. conceived of the study and wrote the main drafts of the manuscript. G.G., L.M., and S.S.F. conducted the statistical analyses. G.G., S.S.F., A.M.M., J.L.W., and M.W. advised on result interpretations. H.M.H., A.M.M., J.L.W., T.R., L.M., J.K.W., and M.W. were involved in primary data collection. All authors contributed to, reviewed, and approved the final manuscript.
References
- 1. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014–2018. Neuro Oncol. 2021;23(Supplement_3):iii1–iii105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Molinaro AM, Taylor JW, Wiencke JK, Wrensch MR. Genetic and molecular epidemiology of adult diffuse glioma. Nat Rev Neurol. 2019;15(7):405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Song GY, DeJong G, Jia W. Cell surface expression of MHC molecules in glioma cells infected with herpes simplex virus type-1. J Neuroimmunol. 1999;93(1–2):1–7. [DOI] [PubMed] [Google Scholar]
- 4. Jiang H, Clise-Dwyer K, Ruisaard KE, et al. Delta-24-RGD oncolytic adenovirus elicits anti-glioma immunity in an immunocompetent mouse model. Castro MG, ed. PLoS One. 2014;9(5):e97407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hellums EK, Markert JM, Parker JN, et al. Increased efficacy of an interleukin-12-secreting herpes simplex virus in a syngeneic intracranial murine glioma model. Neuro Oncol. 2005;7(3):213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muldoon LL, Alvarez JI, Begley DJ, et al. Immunologic privilege in the central nervous system and the blood-brain barrier. J Cereb Blood Flow Metab Off J Int Soc Cereb Blood Flow Metab. 2013;33(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang N, Zuo Y, Jiang L, et al. Epstein-B arr virus and neurological diseases. Front Mol Biosci. 2022;8:1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Skripuletz T, Pars K, Schulte A, et al. Varicella zoster virus infections in neurological patients: a clinical study. BMC Infect Dis. 2018;18(1):238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gerber P, Kirschstein RL. SV40-induced ependymomas in newborn hamsters. Virology. 1962;18(4):582–588. [DOI] [PubMed] [Google Scholar]
- 10. Walker DL, Padgett BL, ZuRhein GM, Albert AE, Marsh RF. Human papovavirus (JC): induction of brain tumors in hamsters. Science. 1973;181(4100):674–676. [DOI] [PubMed] [Google Scholar]
- 11. Wrensch M, Weinberg A, Wiencke J, et al. Does prior infection with varicella-zoster virus influence risk of adult glioma? Am J Epidemiol. 1997;145(7):594–597. [DOI] [PubMed] [Google Scholar]
- 12. Amirian ES, Scheurer ME, Zhou R, et al. History of chickenpox in glioma risk: a report from the glioma international case–control study (GICC). Cancer Med. 2016;5(6):1352–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guerra G, Kachuri L, Wendt G, et al. The immunogenetics of viral antigen response is associated with subtype-specific glioma risk and survival. Am J Hum Genet. 2022;109(6):1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cobbs CS, Harkins L, Samanta M, et al. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 2002;62(12):3347–3350. [PubMed] [Google Scholar]
- 15. Kofman A, Marcinkiewicz L, Dupart E, et al. The roles of viruses in brain tumor initiation and oncomodulation. J Neurooncol. 2011;105(3):451–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Coghill AE, Kim Y, Hodge JM, et al. Prospective investigation of herpesvirus infection and risk of glioma. Int J Cancer. 2022;151(2):222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wrensch M, Weinberg A, Wiencke J, et al. History of chickenpox and shingles and prevalence of antibodies to varicella-zoster virus and three other herpesviruses among adults with glioma and controls. Am J Epidemiol. 2005;161(10):929–938. [DOI] [PubMed] [Google Scholar]
- 18. Lee ST, Bracci P, Zhou M, et al. Interaction of allergy history and antibodies to specific varicella-zoster virus proteins on glioma risk: reactivity to individual varicella proteins in glioma. Int J Cancer. 2014;134(9):2199–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Canniff J, Donson AM, Foreman NK, Weinberg A. Cytotoxicity of glioblastoma cells mediated ex vivo by varicella-zoster virus-specific T cells. J Neurovirol. 2011;17(5):448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wrensch M, Wiencke JK, Wiemels J, et al. Serum IgE, tumor epidermal growth factor receptor expression, and inherited polymorphisms associated with glioma survival. Cancer Res. 2006;66(8):4531–4541. [DOI] [PubMed] [Google Scholar]
- 21. Reynolds MA, Kruszon-Moran D, Jumaan A, Schmid DS, McQuillan GM. Varicella seroprevalence in the U.S.: data from the National Health and Nutrition Examination Survey, 1999-2004. Public Health Rep Wash DC 1974. 2010;125(6):860–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kilgore PE, Kruszon-Moran D, Seward JF, et al. Varicella in Americans from NHANES III: implications for control through routine immunization. J Med Virol. 2003;70(S1):S111–S118. [DOI] [PubMed] [Google Scholar]
- 23. Rahman M, Dastmalchi F, Karachi A, Mitchell D. The role of CMV in glioblastoma and implications for immunotherapeutic strategies. Oncoimmunology. 2019;8(1):e1514921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scheurer ME, Bondy ML, Aldape KD, Albrecht T, El-Zein R. Detection of human cytomegalovirus in different histological types of gliomas. Acta Neuropathol (Berl). 2008;116(1):79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Slinger E, Maussang D, Schreiber A, et al. HCMV-encoded chemokine receptor US28 mediates proliferative signaling through the IL-6-STAT3 axis. Sci Signal. 2010;3(133):ra58. [DOI] [PubMed] [Google Scholar]
- 26. Priel E, Wohl A, Teperberg M, Nass D, Cohen ZR. Human cytomegalovirus viral load in tumor and peripheral blood samples of patients with malignant gliomas. J Clin Neurosci Off J Neurosurg Soc Australas. 2015;22(2):326–330. [DOI] [PubMed] [Google Scholar]
- 27. Dziurzynski K, Chang SM, Heimberger AB, et al. Consensus on the role of human cytomegalovirus in glioblastoma. Neuro-Oncol. 2012;14(3):246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Strong MJ, Blanchard E, Lin Z, et al. A comprehensive next generation sequencing-based virome assessment in brain tissue suggests no major virus-tumor association. Acta Neuropathol Commun. 2016;4(1):71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin CTM, Leibovitch EC, Almira-Suarez MI, Jacobson S. Human herpesvirus multiplex ddPCR detection in brain tissue from low- and high-grade astrocytoma cases and controls. Infect Agent Cancer. 2016;11(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ursu R, Doridam J, Chaugne E, et al. Predictive factors of human cytomegalovirus reactivation in newly diagnosed glioblastoma patients treated with chemoradiotherapy. J Neurovirol. 2021;27(1):94–100. [DOI] [PubMed] [Google Scholar]
- 31. Cai Z, Yang S, Li X, Chen F, Li W. Viral infection and glioma: a meta-analysis of prognosis. BMC Cancer. 2020;20(1):549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from burkitt’s lymphoma. The Lancet. 1964;283(7335):702–703. [DOI] [PubMed] [Google Scholar]
- 33. Fujimoto H, Asaoka K, Imaizumi T, et al. Epstein-Barr virus infections of the central nervous system. Intern Med Tokyo Jpn. 2003;42(1):33–40. [DOI] [PubMed] [Google Scholar]
- 34. Akhtar S, Vranic S, Cyprian FS, Al Moustafa AE. Epstein–Barr virus in gliomas: cause, association, or artifact? Front Oncol. 2018;8:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Limam S, Missaoui N, Mestiri S, et al. Epstein-Barr virus infection in gliomas. Curr Res Transl Med. 2019;67(4):129–133. [DOI] [PubMed] [Google Scholar]
- 36. Marin M, Güris D, Chaves SS, Schmid S, Seward JF; Advisory Committee on Immunization Practices, Centers for Disease Control and Prevention (CDC). Prevention of varicella: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Rep. 2007;56(RR-4):1–40. [PubMed] [Google Scholar]
- 37. Lopez AS, Zhang J, Marin M. Epidemiology of Varicella during the 2-dose Varicella vaccination program - United States, 2005-2014. MMWR Morb Mortal Wkly Rep. 2016;65(34):902–905. [DOI] [PubMed] [Google Scholar]
- 38. Eckel-Passow JE, Drucker KL, Kollmeyer TM, et al. Adult diffuse glioma GWAS by molecular subtype identifies variants in D2HGDH and FAM20C. Neuro-Oncol. 2020;22(11):1602–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Melin BS, Barnholtz-Sloan JS, Wrensch MR, et al. Genome-wide association study of glioma subtypes identifies specific differences in genetic susceptibility to glioblastoma and non-glioblastoma tumors. Nat Genet. 2017;49(5):789–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wrensch M, Jenkins RB, Chang JS, et al. Variants in the CDKN2B and RTEL1 regions are associated with high-grade glioma susceptibility. Nat Genet. 2009;41(8):905–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Balfour HH, Sifakis F, Sliman JA, et al. Age-specific prevalence of Epstein–Barr virus infection among individuals aged 6–19 years in the United States and factors affecting its acquisition. J Infect Dis. 2013;208(8):1286–1293. [DOI] [PubMed] [Google Scholar]
- 42. Staras SAS, Dollard SC, Radford KW, et al. Seroprevalence of cytomegalovirus infection in the United States, 1988-1994. Clin Infect Dis. 2006;43(9):1143–1151. [DOI] [PubMed] [Google Scholar]
- 43. Xu F, Sternberg MR, Kottiri BJ, et al. Trends in herpes simplex virus Type 1 and Type 2 Seroprevalence in the United States. JAMA. 2006;296(8):964. [DOI] [PubMed] [Google Scholar]
- 44. Wu A, Wei J, Kong LY, et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro-Oncol. 2010;12(11):1113–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gomez GG, Kruse CA. Mechanisms of malignant glioma immune resistance and sources of immunosuppression. Gene Ther Mol Biol. 2006;10(A):133–146. [PMC free article] [PubMed] [Google Scholar]
- 46. Bloch O, Crane CA, Kaur R, et al. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. 2013;19(12):3165–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Giles AJ, Hutchinson MKND, Sonnemann HM, et al. Dexamethasone-induced immunosuppression: mechanisms and implications for immunotherapy. J ImmunoTher Cancer. 2018;6(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.