Abstract
Background
Several studies report increases in the incidences of primary central nervous system (CNS) tumors. The reasons for this are unclear.
Methods
Data on all 188 340 individuals diagnosed with a primary CNS tumor in England (1993–2017) were obtained from the National Cancer Registration and Analysis Service. Data on all computerized tomography (CT) head and magnetic resonance imaging (MRI) brain scans in England (2013–2017) were obtained from the National Health Service Digital. Age-sex-standardized annual incidence rates per 100 000 population (ASR) were calculated by calendar year, tumor behavior, tumor location, and method of diagnosis. Temporal trends were quantified using average annual percent change (AAPC).
Results
The ASR for all CNS tumors increased from 13.0 in 1993 to 18.6 in 2017 (AAPC: +1.5%, 95% CI: 1.3, 1.7). The ASR for malignant tumors (52% overall) remained stable (AAPC: +0.5%, 95% CI: −0.2, 1.3), while benign tumors (37% overall) increased (AAPC: +2.6%, 95% CI: 1.2, 4.0). Among the 66% of benign tumors that were microscopically confirmed, the ASR increased modestly (AAPC: +1.3%, 95% CI: 0.5, 2.1). However, among the 25% of benign tumors that were radiographically confirmed, the ASR increased substantially (AAPC: 10.2%, 95% CI: 7.9, 12.5), principally driven by large increases in those who are aged 65+ years. The rate of CT head scans in Accident & Emergency (A&E) increased during 2013–2017, with especially large increases in 65–84 and 85+-year-olds (AAPCs: +18.4% and +22.5%).
Conclusions
Increases in CNS tumor incidence in England are largely attributable to the greater detection of benign tumors. This could be the result of the increasing use of neuroimaging, particularly CT head scans in A&E in people who are aged 65+ years.
Keywords: brain tumor, cancer registry, diagnosis, epidemiology, incidence
Key Points.
The incidence rate of malignant central nervous system (CNS) tumors has remained stable in England during 1993–2017.
Over the same period, the incidence rate of benign CNS tumors has increased.
Most of the increase is among radiographically confirmed tumors and in people aged 65+ years.
Importance of the Study.
This is the first study to report temporal trends in the incidence of primary central nervous system (CNS) tumors for the entire population of England according to tumor behavior, method of diagnosis, and age. For all CNS tumors, there was a modest increase in the age-sex-standardized annual incidence rate over the 25-year study period (1993–2017). This was driven chiefly by a rapid increase in tumors that were radiographically rather than microscopically confirmed. Among radiographically confirmed tumors, the rate of increase was much greater for benign tumors than for malignant tumors, but for both groups, the majority of the increase was in people aged at least 65 years. These increases may be due in large part to an increase in the number of cases diagnosed incidentally by CT scans of the head conducted among older people attending accident and emergency departments, rather than to an increase in the underlying disease incidence rates.
Introduction
Primary tumors of the brain and central nervous system (CNS), hereafter collectively referred to as CNS tumors, comprise a heterogeneous group of neoplasms originating in or impinging on the brain, spinal cord, meninges (3-layered membrane that covers the brain and spinal cord), or the endocrine glands located at the base of the brain.1 CNS tumors can be categorized as malignant or nonmalignant (ie, exhibiting benign or uncertain behavior). While malignant tumors are usually associated with poor prognosis, nonmalignant tumors can also result in adverse outcomes through raised intracranial pressure, cerebral edema, and compression of healthy tissue. These can lead to significant neurological deficits and in the most severe cases can result in fatality. Over 90% of primary CNS tumors occur in and around the brain while spinal cord tumors account for the remainder.2
The morbidity and mortality caused by CNS tumors are disproportionate to their incidence.3 Malignant brain tumors were the leading cause of cancer death in people aged under 40 years in England in 2018.4 Many survivors experience considerable morbidity, due to either the tumor itself or the side effects of treatment, causing the reduction in, or loss of independent functioning and long-term neurological deficits.5 Globally, an increase has been reported in the burden of CNS tumors over the past 3 decades, with increasing incidence across all sociodemographic levels and geographic regions except for eastern Europe, where it has remained stable.6
Data on the incidence rates of CNS tumors in England comes mainly from two studies, one that found an increase during 1979–1992 followed by a leveling off during 1993 to 20037 and another that reported an overall increase in brain tumors, during 1995–2017.8 Other studies of temporal trends in incidence in the United Kingdom have been more limited in scope, and restricted to particular CNS tumor subtypes, specific geographic regions, age groups, or shorter time periods.9–15
Diagnosis of a CNS tumor may be achieved through various methods. Microscopic confirmation (MC) of a tumor, occurs when tumor tissue is analyzed by a neuropathologist following surgical resection or biopsy. Radiographic confirmation (RC) occurs via neuroimaging such as computerized tomography (CT) or magnetic resonance imaging (MRI). This is accepted in clinical practice when the radiological findings are compelling and immediate neurosurgical intervention is not required, or when surgery/biopsy carries substantial risks and does not affect clinical management.3 Recent studies in the United States and Wales have shown distinct trends in incidence rates by the method of diagnosis and have suggested that future reports of incidence trends should provide information on the method of diagnosis to avoid misinterpretation of the data.16,17 None of the aforementioned English studies quantified temporal trends by the method of diagnosis.
The aim of this study was to characterize temporal trends in the incidence of primary CNS tumors diagnosed in England during a recent 25-year period (1993–2017) and to investigate factors that might have contributed to the observed trends.
Methods
Study Design and Data Sources
This population-based cohort study used data from the National Cancer Registration and Analysis Service (NCRAS)18 and included all individuals diagnosed with a primary CNS tumor in England between January 1st, 1993 and December 31st, 2017. NCRAS registers all malignant and selected nonmalignant neoplasms in all individuals resident in England. Tumor behavior was determined according to the 5th digit of the International Classification of Diseases for Oncology Third Edition (ICD-O-3) morphology code, which indicates whether a tumor exhibits malignant, benign, or uncertain behavior. Tumor anatomical locations were identified according to the International Classification of Diseases 9th (ICD-9) and 10th Revision (ICD-10) topography codes. Specially trained clinical coders within NCRAS code the data before entering it into the cancer registration system.
Data on CT head and MRI brain scans performed within the National Health Service (NHS), which is the publicly funded healthcare system in England, were available for the period 2013–2017 from the Diagnostic Imaging Dataset via NHS Digital. These data were categorized according to the following patient settings: Accident & emergency, inpatient, general practitioner request, and outpatient.
Method of Diagnosis
Within NCRAS, the method of diagnosis is determined using the 9 categories outlined in ICD-O-3 (Supplementary Table 1).19
In this study, the term microscopically confirmed (MC) indicates that the diagnosis was based on histological examination of tissue from a primary tumor and radiographically confirmed (RC) indicates that the diagnosis was based on imaging. The remaining 7 categories were combined together as “Other.”
Inclusion Criteria
Primary CNS tumors were identified using topography codes for ICD-10 (C70, C71, C72, C75.1–C75.3, D32, D33, D35.2–D35.4, D42, D43, and D44.3–D44.5) and ICD-9 (191, 192, 194.3–194.4, 225, 227.3–227.4, 237.0–237.1, 237.6, and 237.9) (Supplementary Table 2). Secondary (metastatic) CNS tumors were excluded, as were individuals missing key cancer registration variables (age, sex, date of diagnosis, tumor behavior, and tumor location; <0.0001% of all cases).
Statistical Analyses
Age-sex-standardized annual incidence rates per 100 000 person-years (ASR) were calculated by calendar year both overall and stratified by tumor behavior, tumor location, quintiles of the index of multiple deprivation (IMD)20 (a measure of socioeconomic status in England), and method of diagnosis. Annual rates of CT head and MRI brain scans were also calculated per 100 000 person-years. Denominators used annual mid-year population estimates obtained from the Office for National Statistics. Standardization was to the 2013 European Standard Population. Trends for males and females were similar, so we present results for the sexes combined.
Age-specific annual incidence rates, standardized for sex, were calculated for the entire study period using 5-year age groups, stratified by the method of diagnosis, tumor behavior, and tumor location. To examine age-specific temporal trends, due to smaller numbers, 5 broader age groups (pediatric—0 to 14, teenagers and young adults (TYA)—15 to 24, adults—25 to 64, older people—65 to 84, and elderly—85+ years) were used. Incidence rates were age-sex-standardized by 5-year intervals within these broad age groups.
Temporal trends were quantified by estimating the average annual percent change (AAPC) in the incidence rates and their 95% confidence intervals using joinpoint regression models.21 To compute this, the best fitting regression model using joined log-linear segments is selected to identify calendar years during which there was a significant change in the annual percent change for each segment. A single AAPC to describe the average change over the entire 25-year study period is then estimated by taking a weighted average of the slope coefficients of each individual annual percent change segment from the regression model. The AAPC estimate is a useful summary measure that allows for the comparison of trends.
Analyses were performed using Stata 17.0 or Joinpoint 4.9.1.0.
Ethical Approval
This study was approved by the London–London Bridge Research Ethics Committee, NHS Health Research Authority. Informed consent was not required as this study used de-personalized data, collected via routine methods by NCRAS. This study was conducted in accordance with the principles of the Declaration of Helsinki.
Data Availability
The data for this study were obtained from NCRAS via the Office for Data Release and from the Diagnostic Imaging Dataset via NHS Digital. De-personalized study data may be made available on request to accredited researchers who submit an application to the NHS Digital Data Access Request Service. Population estimates and standard populations are publicly available via the Office for National Statistics.
Results
Characteristics of the Study Population
During the 25-year study period (1993–2017), 188 340 individuals were diagnosed with a primary CNS tumor in England (Table 1). The annual case count rose from 5571 in 1993 to 9835 in 2017. Malignant tumors accounted for 52% of all CNS tumors, while 37% were benign and 11% were of uncertain behavior (Supplementary Table 3). The majority of CNS tumors were located in the brain (58%) or meninges (22%), while the remainder were in the endocrine glands (11%) or in the spinal cord and other parts of the CNS (9%). Over the study period, the percentage of diagnoses that were microscopically confirmed (MC) each year, varied only between 62% and 70% with no trend. In contrast, the percentage that were radiographically confirmed (RC) each year rose steadily and by a much larger amount, from 10% in 1993 to 35% in 2017. The median age at diagnosis was 61 years (IQR: 46–73). Individuals with RC diagnoses or diagnosed by other methods tended to be older (median ages: 74, 73 IQRs: 61–82, 60–81 years) than those with MC diagnoses (median age: 55; IQR: 41–66 years). Males accounted for 50% of all diagnoses, 52% of MC diagnoses, and 44% of RC diagnoses.
Table 1.
Patient and Tumor Characteristics of 188 340 Individuals Diagnosed With a Primary CNS Tumor According to the Method of Diagnosis—England, 1993–2017 (Row Percentages Presented—See Supplementary Table 3 for Column Percentages)
| Characteristic | MC | RC | Other | Total | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| Sex | ||||||||
| Male | 64 124 | 69 | 18 679 | 20 | 10 444 | 11 | 93 247 | 100 |
| Female | 60 066 | 63 | 23 378 | 25 | 11 649 | 12 | 95 093 | 100 |
| Age group at diagnosis | ||||||||
| Pediatric (0–14) | 7600 | 83 | 1197 | 13 | 404 | 4 | 9201 | 100 |
| Teenagers and young adults (15–24) | 4890 | 83 | 642 | 11 | 393 | 7 | 5925 | 100 |
| Adults (25–64) | 75 596 | 82 | 10 530 | 11 | 6066 | 7 | 92 192 | 100 |
| Older people (65–84) | 35 252 | 50 | 22 843 | 33 | 11 847 | 17 | 69 942 | 100 |
| Elderly (85+) | 852 | 8 | 6845 | 62 | 3383 | 31 | 11 080 | 100 |
| Age at diagnosis | ||||||||
| Median (IQR) | 55 | (41–66) | 74 | (61–82) | 73 | (60–81) | 61 | (46–73) |
| Calendar year of diagnosis | ||||||||
| 1993 | 3569 | 64 | 558 | 10 | 1444 | 26a | 5571 | 100 |
| 1994 | 3630 | 65 | 772 | 14 | 1167 | 21 | 5569 | 100 |
| 1995 | 3949 | 65 | 835 | 14 | 1305 | 21 | 6089 | 100 |
| 1996 | 4208 | 69 | 746 | 12 | 1186 | 19 | 6140 | 100 |
| 1997 | 4369 | 68 | 836 | 13 | 1244 | 19 | 6449 | 100 |
| 1998 | 4381 | 69 | 864 | 14 | 1092 | 17 | 6337 | 100 |
| 1999 | 4283 | 66 | 849 | 13 | 1383 | 21 | 6515 | 100 |
| 2000 | 4475 | 66 | 1132 | 17 | 1181 | 17 | 6788 | 100 |
| 2001 | 4478 | 68 | 1195 | 18 | 952 | 14 | 6625 | 100 |
| 2002 | 4646 | 69 | 1289 | 19 | 769 | 11 | 6704 | 100 |
| 2003 | 4569 | 69 | 1328 | 20 | 752 | 11 | 6649 | 100 |
| 2004 | 4841 | 69 | 1494 | 21 | 647 | 9 | 6982 | 100 |
| 2005 | 5061 | 69 | 1422 | 20 | 808 | 11 | 7291 | 100 |
| 2006 | 5091 | 70 | 1474 | 20 | 739 | 10 | 7304 | 100 |
| 2007 | 5407 | 70 | 1608 | 21 | 659 | 9 | 7674 | 100 |
| 2008 | 5645 | 69 | 1701 | 21 | 864 | 11 | 8210 | 100 |
| 2009 | 5284 | 66 | 1908 | 24 | 865 | 11 | 8057 | 100 |
| 2010 | 5304 | 66 | 1988 | 25 | 805 | 10 | 8097 | 100 |
| 2011 | 5367 | 66 | 2023 | 25 | 756 | 9 | 8146 | 100 |
| 2012 | 5312 | 62 | 2307 | 27 | 921 | 11 | 8540 | 100 |
| 2013 | 6081 | 63 | 2511 | 26 | 1009 | 11 | 9601 | 100 |
| 2014 | 5951 | 62 | 3031 | 32 | 564 | 6 | 9546 | 100 |
| 2015 | 6102 | 62 | 3289 | 33 | 462 | 5 | 9853 | 100 |
| 2016 | 6063 | 62 | 3433 | 35 | 272 | 3 | 9768 | 100 |
| 2017 | 6124 | 62 | 3464 | 35 | 247 | 3b | 9835 | 100 |
| Tumor behaviorc | ||||||||
| Malignant | 66 684 | 68 | 20 280 | 21 | 11 067 | 11 | 98 031 | 100 |
| Benign | 46 033 | 66 | 17 076 | 25 | 6249 | 9 | 69 358 | 100 |
| Uncertain | 11 473 | 55 | 4701 | 22 | 4777 | 23 | 20 951 | 100 |
| Anatomical locationd | ||||||||
| Meninges (C70.0–C70.9, D32.0–D32.9, D42.0–D42.9) | 26 604 | 63 | 11 518 | 27 | 4121 | 10 | 42 243 | 100 |
| Brain (C71.0–C71.9, D33.0–D33.2, D43.0–D43.2) | 70 620 | 65 | 23 447 | 22 | 14 325 | 13 | 108 392 | 100 |
| Spinal cord and other CNS (C72.0–C72.9, D33.3–D33.9, D43.3–D43.9) | 12 318 | 71 | 3466 | 20 | 1545 | 9 | 17 329 | 100 |
| Endocrine CNS (C75.1–C75.3, D35.2–D35.4, D44.3–D44.5) | 14 648 | 72 | 3626 | 18 | 2102 | 10 | 20 376 | 100 |
| Tumor behavior according to anatomical locationc,d | ||||||||
| Malignant | ||||||||
| Meninges | 1129 | 65 | 332 | 19 | 276 | 16 | 1737 | 100 |
| Brain | 62 760 | 68 | 19 224 | 21 | 10 266 | 11 | 92 250 | 100 |
| Spinal cord and other CNS | 1948 | 70 | 552 | 20 | 295 | 11 | 2795 | 100 |
| Endocrine Glands in CNS | 847 | 68 | 172 | 14 | 230 | 18 | 1249 | 100 |
| Benign | ||||||||
| Meninges | 23 194 | 61 | 10 936 | 29 | 3676 | 10 | 37 806 | 100 |
| Brain | 1842 | 67 | 559 | 20 | 335 | 12 | 2736 | 100 |
| Spinal cord and other CNS | 8996 | 71 | 2652 | 21 | 1012 | 8 | 12 660 | 100 |
| Endocrine glands in CNS | 12 001 | 74 | 2929 | 18 | 1226 | 8 | 16 156 | 100 |
| Uncertain | ||||||||
| Meninges | 2281 | 84 | 250 | 9 | 169 | 6 | 2700 | 100 |
| Brain | 6018 | 45 | 3664 | 27 | 3724 | 28 | 13 406 | 100 |
| Spinal cord and other CNS | 1374 | 73 | 262 | 14 | 238 | 13 | 1874 | 100 |
| Endocrine glands in CNS | 1800 | 61 | 525 | 18 | 646 | 22 | 2971 | 100 |
| Total (all CNS tumors) | 124 190 | 66 | 42 057 | 22 | 22 093 | 12 | 188 340 | 100 |
Abbreviation: CNS, central nervous system; MC, microscopically confirmed; RC, radiographically confirmed.
aIn 1993, “Other” comprised: Death Certificate Only-4%; Clinical-14%; Other special tests/Specific tumor marker/Cytology/Histology of metastases- <1%; Unknown-7%
bIn 2017 “Other” comprised: Death Certificate Only-0%; Clinical-1%; Other special tests/Specific tumor marker/Cytology/Histology of metastases- <1%; Unknown-1%
cBehavior based on the 5th digit of the ICD-O-3 histology code.
dAnatomical location based on the ICD-10 topography code.
Temporal Trends Overall, and Separately by Tumor Behavior, Method of Diagnosis, Age, and Index of Multiple Deprivation
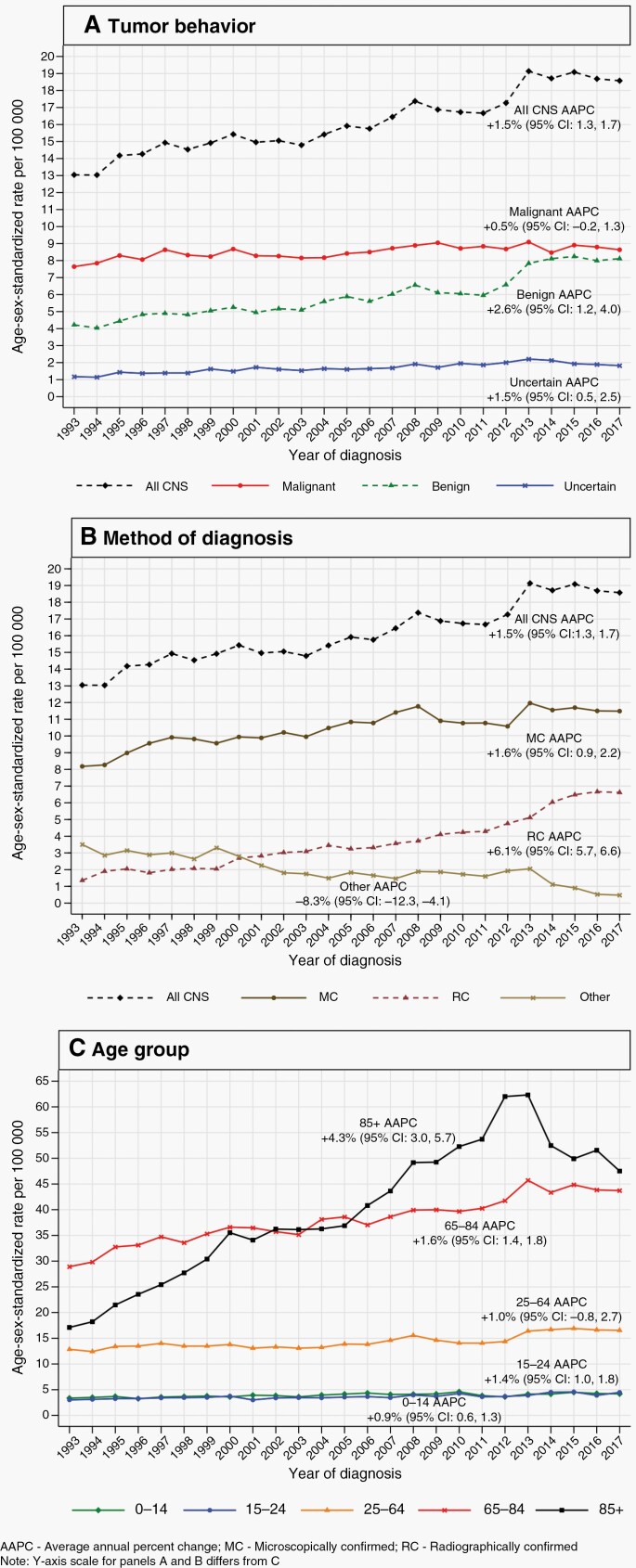
The ASR for all CNS tumors increased from 13.0 in 1993 to 18.6 in 2017 (Figure 1; Table 2). This was chiefly due to an increase in the ASR for benign tumors, which rose steadily from 4.2 in 1993 to 8.1 in 2017 (AAPC: +2.6%, 95% CI: 1.2, 4.0). In contrast, the ASR for malignant tumors changed only from 7.6 in 1993 to 8.6 in 2017 (AAPC: +0.5%, 95% CI: −0.2, 1.3), while the ASR for tumors of uncertain behavior increased modestly from 1.2 in 1993 to 1.8 in 2017 (AAPC: +1.5%, 95% CI: 0.5, 2.5).
Figure 1.
Incidence of primary central nervous system (CNS) tumors by (A) tumor behavior, (B) method of diagnosis, and (C) age group—England, 1993–2017.
Table 2.
Age-Sex-Standardizeda Incidence Rates (ASR) Per 100 000 for Calendar Years 1993 and 2017 Separately and Average Annual Percentage Change (AAPC) for 188 340 Individuals Diagnosed With a Primary CNS Tumor According to Tumor Behaviorb and Method of Diagnosis—England, 1993–2017
| Characteristic | Method of diagnosis | 1993 only | 2017 only | 1993–2017 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | ASR | 95% CI | n | % | ASR | 95% CI | n | % | AAPC | 95% CI | P-Value | ||
| All CNS tumors | ||||||||||||||
| All ages | All | 5571 | 100 | 13.0 | (12.7, 13.4) | 9835 | 100 | 18.6 | (18.2, 18.9) | 188 340 | 100 | 1.5* | (1.3, 1.7) | < .001 |
| MC | 3569 | 64 | 7.4 | (7.9, 8.5) | 6124 | 62 | 11.0 | (11.2, 11.8) | 124 190 | 66 | 1.6* | (0.9, 2.2) | < .001 | |
| RC | 558 | 10 | 1.2 | (1.2, 1.5) | 3464 | 35 | 6.2 | (6.4, 6.8) | 42 057 | 22 | 6.1* | (5.7, 6.6) | < .001 | |
| Other | 1444 | 26 | 3.0 | (3.3, 3.7) | 247 | 3 | 0.4 | (0.4, 0.5) | 22 093 | 12 | −8.3* | (−12.3, −4.1) | < .001 | |
| All CNS tumors | ||||||||||||||
| Pediatric (0–14) | All | 315 | 100 | 3.4 | (3.0, 3.7) | 422 | 100 | 4.2 | (3.8, 4.6) | 9201 | 100 | 0.9* | (0.6, 1.3) | < .001 |
| MC | 252 | 80 | 2.7 | (2.4, 3.0) | 341 | 81 | 3.4 | (3.0, 3.7) | 7600 | 83 | 0.7* | (0.3, 1.1) | .002 | |
| RC | 18 | 6 | 0.2 | (0.1, 0.3) | 69 | 16 | 0.7 | (0.5, 0.8) | 1197 | 13 | 5.8* | (3.4, 8.3) | < .001 | |
| Other | 45 | 14 | 0.5 | (0.3, 0.6) | 12 | 3 | 0.1 | (0.1, 0.2) | 404 | 4 | −5.3* | (−6.8, −3.9) | < .001 | |
| Teenagers and young adults (15–24) | All | 193 | 100 | 3.0 | (2.6, 3.5) | 298 | 100 | 4.5 | (4.0, 5.0) | 5925 | 100 | 1.4* | (1.0, 1.8) | < .001 |
| MC | 140 | 73 | 2.2 | (1.8, 2.6) | 235 | 79 | 3.5 | (3.1, 4.0) | 4890 | 83 | 1.2* | (0.8, 1.7) | < .001 | |
| RC | 4 | 2 | 0.1 | (0.0, 0.2) | 59 | 20 | 0.9 | (0.7, 1.1) | 642 | 11 | 6.4* | (4.8, 8.0) | < .001 | |
| Other | 49 | 25 | 0.8 | (0.6, 1.0) | 4 | 1 | 0.1 | (0.0, 0.2) | 393 | 7 | −4.6* | (−6.8, −2.3) | < .001 | |
| Adults (25–64) | All | 2936 | 100 | 12.9 | (12.4, 13.3) | 4688 | 100 | 16.6 | (16.1, 17.0) | 92 192 | 100 | 1.0 | (−0.8, 2.7) | .283 |
| MC | 2247 | 77 | 9.8 | (9.4, 10.2) | 3598 | 77 | 12.7 | (12.3, 13.1) | 75 596 | 82 | 0.9* | (0.6, 1.2) | < .001 | |
| RC | 167 | 6 | 0.7 | (0.6, 0.8) | 1025 | 22 | 3.6 | (3.4, 3.9) | 10 530 | 11 | 6.6* | (3.3, 10.0) | < .001 | |
| Other | 522 | 18 | 2.3 | (2.1, 2.5) | 65 | 1 | 0.2 | (0.2, 0.3) | 6066 | 7 | −6.5* | (−7.8, −5.3) | < .001 | |
| Older people (65−84) | All | 1983 | 100 | 28.9 | (27.6, 30.2) | 3783 | 100 | 43.7 | (42.3, 45.1) | 69 942 | 100 | 1.6* | (1.4, 1.8) | < .001 |
| MC | 909 | 46 | 13.2 | (12.3, 14.1) | 1911 | 51 | 22.0 | (21.0, 23.0) | 35 252 | 50 | 2.2* | (0.6, 3.9) | .008 | |
| RC | 331 | 17 | 4.8 | (4.3, 5.4) | 1748 | 46 | 20.2 | (19.3, 21.2) | 22 843 | 33 | 5.6* | (3.4, 7.8) | < .001 | |
| Other | 743 | 37 | 10.9 | (10.1, 11.7) | 124 | 3 | 1.4 | (1.2, 1.7) | 11 847 | 17 | −8.7* | (−10.9, −6.5) | < .001 | |
| Elderly (85+) | All | 144 | 100 | 17.1 | (14.3, 20.0) | 644 | 100 | 47.5 | (43.8, 51.2) | 11 080 | 100 | 4.3* | (3.0, 5.7) | < .001 |
| MC | 21 | 15 | 2.6 | (1.5, 3.7) | 39 | 6 | 2.8 | (1.9, 3.7) | 852 | 8 | 0.7 | (−0.3, 1.7) | .184 | |
| RC | 38 | 26 | 4.4 | (3.0, 5.9) | 563 | 87 | 41.5 | (38.1, 45.0) | 6845 | 62 | 9.8* | (8.2, 11.4) | < .001 | |
| Other | 85 | 59 | 10.2 | (8.0, 12.5) | 42 | 7 | 3.2 | (2.2, 4.1) | 3383 | 31 | −5.2* | (−8.1, −2.3) | .001 | |
| Malignant behavior | ||||||||||||||
| Pediatric (0–14) | All | 216 | 100 | 2.3 | (2.0, 2.6) | 223 | 100 | 2.2 | (1.9, 2.5) | 5344 | 100 | 0.0 | (−0.4, 0.5) | .885 |
| MC | 177 | 82 | 1.9 | (1.6, 2.2) | 182 | 82 | 1.8 | (1.5, 2.0) | 4220 | 79 | −0.4 | (−0.8, 0.0) | .062 | |
| RC | 12 | 6 | 0.1 | (0.1, 0.2) | 36 | 16 | 0.4 | (0.2, 0.5) | 880 | 16 | 4.1* | (1.6, 6.7) | .001 | |
| Other | 27 | 13 | 0.3 | (0.2, 0.4) | 5 | 2 | 0.1 | (0.0, 0.3) | 244 | 5 | −3.9* | (−5.9, −1.9) | .001 | |
| Teenagers and young adults (15–24) | All | 124 | 100 | 2.0 | (1.6, 2.3) | 123 | 100 | 1.8 | (1.5, 2.2) | 2855 | 100 | 0.1 | (−0.4, 0.6) | .605 |
| MC | 94 | 76 | 1.5 | (1.2, 1.8) | 108 | 88 | 1.6 | (1.3, 1.9) | 2461 | 86 | 0.3 | (−0.2, 0.8) | .264 | |
| RC | 2 | 2 | 0.0 | (0.0, 0.1) | 13 | 11 | 0.2 | (0.1, 0.3) | 221 | 8 | 1.7 | (−0.3, 3.7) | .094 | |
| Other | 28 | 23 | 0.4 | (0.3, 0.6) | 2 | 2 | 0.0 | (0.0, 0.1) | 173 | 6 | −5.3 | (−11.4, 1.2) | .108 | |
| Adults (25–64) | All | 1719 | 100 | 7.6 | (7.2, 7.9) | 1973 | 100 | 7.0 | (6.7, 7.3) | 46 167 | 100 | −0.3 | (−1.4, 0.8) | .581 |
| MC | 1299 | 76 | 5.7 | (5.3, 6.0) | 1767 | 90 | 6.2 | (5.9, 6.5) | 39 551 | 86 | 0.4 | (−0.8, 1.6) | .511 | |
| RC | 103 | 6 | 0.5 | (0.4, 0.6) | 187 | 9 | 0.7 | (0.6, 0.8) | 3648 | 8 | 1.5* | (0.6, 2.4) | .002 | |
| Other | 317 | 18 | 1.4 | (1.3, 1.6) | 19 | 1 | 0.1 | (0.1, 0.1) | 2968 | 6 | −8.6* | (−9.7, −7.4) | < .001 | |
| Older People (65–84) | All | 1166 | 100 | 17.0 | (16.0, 18.0) | 1966 | 100 | 22.7 | (21.7, 23.7) | 39 242 | 100 | 1.4* | (0.6, 2.2) | .001 |
| MC | 504 | 43 | 7.3 | (6.7, 7.9) | 1105 | 56 | 12.7 | (12.0, 13.5) | 20 205 | 51 | 2.4* | (1.8, 3.0) | < .001 | |
| RC | 221 | 19 | 3.2 | (2.8, 3.7) | 798 | 41 | 9.3 | (8.6, 9.9) | 12 597 | 32 | 3.9* | (2.9, 4.9) | < .001 | |
| Other | 441 | 38 | 6.5 | (5.9, 7.1) | 63 | 3 | 0.7 | (0.5, 0.9) | 6440 | 16 | −9.7* | (−13.1, −6.2) | < .001 | |
| Elderly (85+) | All | 55 | 100 | 6.3 | (4.6, 8.0) | 283 | 100 | 20.9 | (18.4, 23.3) | 4423 | 100 | 3.7* | (2.6, 4.8) | < .001 |
| MC | 2 | 4 | 0.3 | (−0.1, 0.7) | 14 | 5 | 1.0 | (0.5, 1.6) | 247 | 6 | −0.2 | (−1.8, 1.4) | .791 | |
| RC | 19 | 35 | 2.1 | (1.2, 3.1) | 251 | 89 | 18.5 | (16.2, 20.8) | 2934 | 66 | 8.9* | (6.8, 11.0) | < .001 | |
| Other | 34 | 62 | 3.9 | (2.6, 5.3) | 18 | 6 | 1.3 | (0.7, 2.0) | 1242 | 28 | −5.1* | (−9.0, −0.9) | .016 | |
| Benign behavior | ||||||||||||||
| Pediatric (0–14) | All | 11 | 100 | 0.1 | (0.1, 0.2) | 37 | 100 | 0.4 | (0.3, 0.5) | 763 | 100 | 2.8* | (1.5, 4.1) | < .001 |
| MC | 8 | 73 | 0.1 | (0.0, 0.1) | 25 | 68 | 0.3 | (0.2, 0.4) | 627 | 82 | 1.8* | (0.3, 3.3) | .021 | |
| RC | 0 | 0 | 10 | 27 | 0.1 | (0.1, 0.2) | 90 | 12 | 4.1* | (0.1, 8.2) | .046 | |||
| Other | 3 | 27 | 0.0 | (0.0, 0.1) | 2 | 5 | 0.0 | (0.0, 0.1) | 46 | 6 | −0.7 | (−2.2, 0.9) | .359 | |
| Teenagers and young adults (15–24) | All | 54 | 100 | 0.8 | (0.6, 1.1) | 95 | 100 | 1.4 | (1.1, 1.7) | 1673 | 100 | 1.5* | (0.6, 2.4) | .002 |
| MC | 37 | 69 | 0.6 | (0.4, 0.8) | 56 | 59 | 0.8 | (0.6, 1.1) | 1246 | 74 | 0.3 | (−0.6, 1.3) | .478 | |
| RC | 1 | 2 | 0.0 | (0.0, 0.1) | 38 | 40 | 0.6 | (0.4, 0.8) | 305 | 18 | 6.9* | (4.8, 9.1) | < .001 | |
| Other | 16 | 30 | 0.2 | (0.1, 0.4) | 1 | 1 | 0.0 | (0.0, 0.1) | 122 | 7 | −2.6* | (−4.7, −0.5) | .018 | |
| Adults (25–64) | All | 1057 | 100 | 4.6 | (4.3, 4.9) | 2263 | 100 | 8.0 | (7.7, 8.3) | 38 265 | 100 | 2.3* | (0.1, 4.5) | .038 |
| MC | 875 | 83 | 3.8 | (3.6, 4.1) | 1462 | 65 | 5.2 | (4.9, 5.4) | 30 442 | 80 | 1.1* | (0.6, 1.5) | < .001 | |
| RC | 40 | 4 | 0.2 | (0.1, 0.3) | 767 | 34 | 2.7 | (2.5, 2.9) | 5837 | 15 | 11.0* | (8.5, 13.6) | < .001 | |
| Other | 142 | 13 | 0.6 | (0.5, 0.7) | 34 | 2 | 0.1 | (0.1, 0.2) | 1986 | 5 | −3.2* | (−5.0, −1.3) | .002 | |
| Older people (65–84) | All | 597 | 100 | 8.7 | (8.0, 9.4) | 1559 | 100 | 18.0 | (17.1, 18.9) | 23 773 | 100 | 2.9* | (1.5, 4.3) | < .001 |
| MC | 389 | 65 | 5.7 | (5.1, 6.2) | 668 | 43 | 7.7 | (7.1, 8.3) | 13 188 | 55 | 1.6* | (0.8, 2.5) | < .001 | |
| RC | 59 | 10 | 0.9 | (0.6, 1.1) | 850 | 55 | 9.8 | (9.2, 10.5) | 7779 | 33 | 9.5* | (8.3, 10.8) | < .001 | |
| Other | 149 | 25 | 2.2 | (1.8, 2.5) | 41 | 3 | 0.5 | (0.3, 0.6) | 2806 | 12 | −6.0* | (−11.3, −0.4) | .037 | |
| Elderly (85+) | All | 58 | 100 | 7.1 | (5.3, 9.0) | 312 | 100 | 23.0 | (20.4, 25.5) | 4884 | 100 | 4.9* | (3.8, 6.0) | < .001 |
| MC | 17 | 29 | 2.0 | (1.1, 3.0) | 22 | 7 | 1.6 | (0.9, 2.3) | 530 | 11 | 0.6 | (−0.6, 1.9) | .319 | |
| RC | 8 | 14 | 0.9 | (0.3, 1.6) | 269 | 86 | 19.8 | (17.4, 22.2) | 3065 | 63 | 9.4* | (7.9, 10.9) | < .001 | |
| Other | 33 | 57 | 4.2 | (2.7, 5.6) | 21 | 7 | 1.6 | (0.9, 2.3) | 1289 | 26 | −4.3 | (−11.3, 3.2) | .252 | |
| Uncertain behavior | ||||||||||||||
| Pediatric (0–14) | All | 88 | 100 | 0.9 | (0.7, 1.1) | 162 | 100 | 1.6 | (1.4, 1.9) | 3094 | 100 | 2.0* | (1.4, 2.6) | < .001 |
| MC | 67 | 76 | 0.7 | (0.6, 0.9) | 134 | 83 | 1.3 | (1.1, 1.6) | 2753 | 89 | 2.0* | (1.3, 2.7) | < .001 | |
| RC | 6 | 7 | 0.1 | (0.0, 0.2) | 23 | 14 | 0.2 | (0.1, 0.3) | 227 | 7 | 3.4* | (2.1, 4.7) | < .001 | |
| Other | 15 | 17 | 0.2 | (0.1, 0.2) | 5 | 3 | 0.0 | (0.0, 0.1) | 114 | 4 | −3.3* | (−5.7, −0.9) | .008 | |
| Teenagers and young adults (15–24) | All | 15 | 100 | 0.2 | (0.1, 0.3) | 80 | 100 | 1.2 | (0.9, 1.5) | 1397 | 100 | 3.7* | (2.6, 4.7) | < .001 |
| MC | 9 | 60 | 0.1 | (0.0, 0.2) | 71 | 89 | 1.1 | (0.8, 1.3) | 1183 | 85 | 3.9* | (2.9, 5.0) | < .001 | |
| RC | 1 | 7 | 0.0 | (0.0, 0.1) | 8 | 10 | 0.1 | (0.0, 0.2) | 116 | 8 | 2.1* | (0.2, 3.9) | .028 | |
| Other | 5 | 33 | 0.1 | (0.0, 0.1) | 1 | 1 | 0.0 | (0.0, 0.1) | 98 | 7 | 0.9 | (−3.8, 5.7) | .726 | |
| Adults (25–64) | All | 160 | 100 | 0.7 | (0.6, 0.8) | 452 | 100 | 1.6 | (1.4, 1.7) | 7760 | 100 | 3.5* | (2.9, 4.1) | < .001 |
| MC | 73 | 46 | 0.3 | (0.2, 0.4) | 369 | 82 | 1.3 | (1.2, 1.4) | 5603 | 72 | 5.3* | (4.7, 5.9) | < .001 | |
| RC | 24 | 15 | 0.1 | (0.1, 0.1) | 71 | 16 | 0.2 | (0.2, 0.3) | 1045 | 13 | 2.5 | (−2.5, 7.8) | .328 | |
| Other | 63 | 39 | 0.3 | (0.2, 0.4) | 12 | 3 | 0.1 | (0.0, 0.1) | 1112 | 14 | −3.9* | (−5.3, −2.4) | < .001 | |
| Older people (65–84) | All | 220 | 100 | 3.2 | (2.8, 3.6) | 258 | 100 | 3.0 | (2.6, 3.3) | 6927 | 100 | −0.7 | (−1.9, 0.5) | .240 |
| MC | 16 | 7 | 0.2 | (0.1, 0.3) | 138 | 53 | 1.6 | (1.3, 1.9) | 1859 | 27 | 7.3* | (5.6, 9.0) | < .001 | |
| RC | 51 | 23 | 0.8 | (0.5, 1.0) | 100 | 39 | 1.1 | (0.9, 1.4) | 2467 | 36 | 1.2 | (−0.4, 2.8) | .145 | |
| Other | 153 | 70 | 2.2 | (1.9, 2.6) | 20 | 8 | 0.2 | (0.1, 0.3) | 2601 | 38 | −10.1* | (−13.9, −6.1) | < .001 | |
| Elderly (85+) | All | 31 | 100 | 3.9 | (2.5, 5.3) | 49 | 100 | 3.7 | (2.6, 4.7) | 1773 | 100 | 0.0 | (−2.5, 2.5) | .992 |
| MC | 2 | 6 | 0.3 | (−0.1, 0.7) | 3 | 6 | 0.4 | (0.0, 0.7) | 75 | 4 | 1.3 | (−9.2, 12.9) | .820 | |
| RC | 11 | 35 | 1.2 | (0.5, 2.0) | 43 | 88 | 3.2 | (2.3, 4.2) | 846 | 48 | 5.5* | (3.2, 8.0) | < .001 | |
| Other | 18 | 58 | 2.2 | (1.1, 3.2) | 3 | 6 | 0.2 | (0.0, 0.5) | 852 | 48 | −8.7* | (−13.9, −3.2) | 0.002 |
Abbreviations: MC, microscopically confirmed; RC, radiographically confirmed; CNS, central nervous system.
*Indicates a statistically significant departure (P < .05) from a slope of 0.
aFor ASRs standardized to the United States and World Standard Populations, please refer to Supplementary Table 4 where data for 2017 is presented and Supplementary Figure 1 for comparison purposes.
bBehavior based on the 5th digit of the ICD-O-3 histology code.
Considering the method of diagnosis, the ASR for all MC tumors increased from 7.4 in 1993 to 11.0 in 2017 (AAPC: +1.6%, 95% CI: 0.9, 2.2). The ASR for all RC tumors rose by a similar absolute amount, from 1.2 in 1993 to 6.2 in 2017, but constituted a larger relative change (AAPC: +6.1%, 95% CI: 5.7, 6.6). Meanwhile, the ASR for tumors diagnosed via other methods decreased from 3.0 in 1993 to 0.4 in 2017 (AAPC: −8.3%, 95% CI −12.3, −4.1).
For pediatric tumors and tumors in TYA, the ASRs rose by small absolute amounts (pediatric: 3.4–4.2; TYA: 3.0–4.5) which were, nevertheless, significant (pediatric AAPC: +0.9%, 95% CI: 0.6, 1.3; TYA AAPC: +1.4%, 95% CI: 1.0, 1.8). Meanwhile, for adults aged 25–64 years, the ASR rose from 12.9 in 1993 to 16.6 in 2017, which was not significant (AAPC: +1.0, 95% CI: −0.8, 2.7). In contrast, the ASR for tumors in older people aged 65–84 years increased by a large absolute amount from 28.9 in 1993 to 43.7 in 2017 (AAPC: +1.6%, 95% CI: 1.4, 1.8). In those who are aged 85+ years, the ASR increased by an even larger absolute amount, from 17.1 in 1993 to 47.5 in 2017 (AAPC: +4.3%, 95% CI: 3.0, 5.7).
To see whether the large increases in the ASRs of RC tumors in older age groups were associated with socioeconomic status, we computed ASRs by age at diagnosis, method of diagnosis, and index of multiple deprivation (Supplementary Figure 2). There were some differences in the ASRs according to the index of multiple deprivation quintiles, but no evidence that these differences change substantially with the calendar year.
Temporal Trends by Tumor Behavior
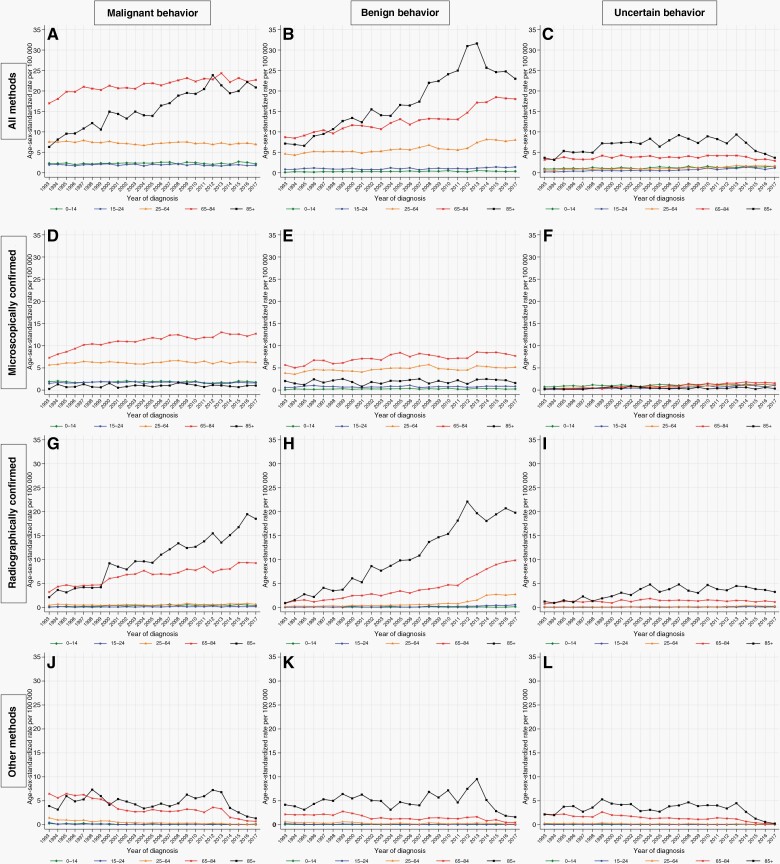
The large absolute increase in the ASR for those who are aged 85+ years was primarily accounted for by large increases in the ASR for all malignant RC tumors, where the ASR increased from 2.1 in 1993 to 18.5 in 2017 (AAPC: +8.9%, 95% CI: 6.8, 11.0), and for all benign RC tumors where the ASR increased from 0.9 in 1993 to 19.8 in 2017 (AAPC: +9.4%, 95% CI: 7.9, 10.9) (Figure 2). The ASR for all tumors of uncertain behavior in this age group (85+ years) did not change significantly, while the ASR for tumors diagnosed by other methods decreased irrespective of tumor behavior.
Figure 2.
Incidence of primary central nervous system (CNS) tumors by tumor behavior, method of diagnosis and age group—England, 1993–2017.
Considerable absolute increases were also seen in the ASRs for those aged 65–84 years for all malignant MC tumors, where the ASR increased from 7.3 in 1993 to 12.7 in 2017 (AAPC: +2.4%, 95% CI 1.8, 3.0), and for all malignant RC tumors where the ASR increased from 3.2 in 1993 to 9.3 in 2017 (AAPC: +3.9%, 95% CI 2.9, 4.9). Similar trends were seen for benign MC tumors which increased from 5.7 in 1993 to 7.7 in 2017 (AAPC: +1.6, 95% CI: 0.8,2.5) and benign RC tumors which increased from 0.9 in 1993 to 9.8 in 2017 (AAPC: +9.5%, 95% CI 8.3, 10.8). The ASRs for MC and RC tumors of uncertain behavior were small and did not contribute materially to overall trends. For tumors diagnosed by other methods, the ASRs decreased during the study period in this age group irrespective of tumor behavior.
For adults aged 25–64 years, for teenagers and young adults aged 15–24, and for pediatric tumors in ages 0–14, there were significant increases for some categories, most notably for benign RC tumors in 25–64-year-olds which increased from 0.2 in 1993 to 2.7 in 2017 (AAPC: +11.0%, 95% CI 8.5, 13.6). However, this and other increases were balanced by several decreases, especially for tumors diagnosed by other methods.
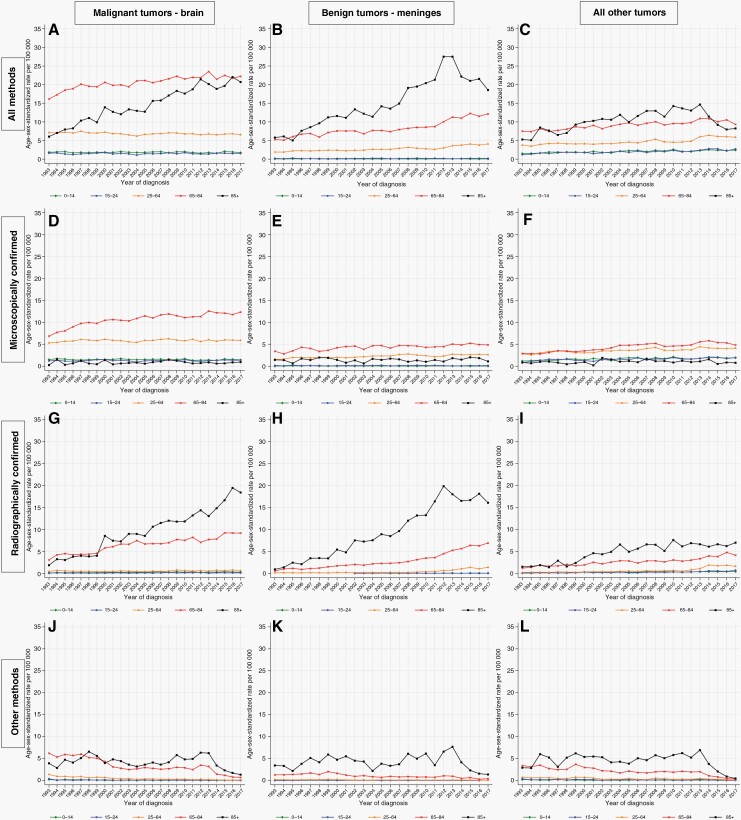
Temporal Trends by Anatomical Location
The commonest anatomical location for malignant tumors was the brain (n = 92 250; 49% of all CNS tumors), and the commonest anatomical location for benign tumors was the meninges (n = 37 806; 20% of all CNS tumors). For both of these categories, there were striking absolute increases in the ASR for individuals aged 85+ years. This was due to the predominant increases in the ASR for RC tumors, from 1.9 in 1993 to 18.4 in 2017 (AAPC: +9.5%, 95% CI: 7.6, 11.5) for the former, and from 0.8 in 1993 to 16.1 in 2017 (AAPC: +9.3% (95% CI: 7.7, 10.8) for the latter (Figure 3; Supplementary Table 5A). For malignant brain tumors, there have also been considerable absolute increases at ages 65–84 years in both MC and RC tumors, no significant changes at ages 0–14, 15–24, and 25–64 years in MC tumors, and significant increases at ages 0–14 and 25–64 years in RC tumors. Over the same period, there have been significant decreases in malignant brain tumors diagnosed by other methods in all age groups. For benign tumors of the meninges, there have been substantial increases in RC tumors at ages 65–84 from 0.4 in 1993 to 6.9 in 2017 (AAPC: +9.0%, 95% CI: 6.5, 11.5), and at ages 25–64 from 0.1 in 1993 to 1.4 in 2017 (AAPC: +9.9%, 7.7, 12.1). There were also significant but numerically smaller increases in benign tumors of the meninges at ages 65–84 and 25–64 years, and a significant decrease in tumors diagnosed by other methods at ages 65–84 years.
Figure 3.
Incidence of primary central nervous system (CNS) tumors by anatomical location, method of diagnosis and age group—England, 1993–2017.
For tumors diagnosed in all other combinations of behavior and anatomical location combined, there were significant increases in MC tumors in all age groups under 65 years, with AAPCs ranging from +1.5 to +2.1%, while RC tumors increased significantly in all age groups, with AAPCs ranging from +4.5 to +8.6%. During the same period, tumors diagnosed via other methods significantly decreased in all age groups, with AAPCs ranging from −3.2 to −9.2% (see Supplementary Table 5a–d and Supplementary Figures 3–5 for data on all combinations of behavior and anatomical location separately).
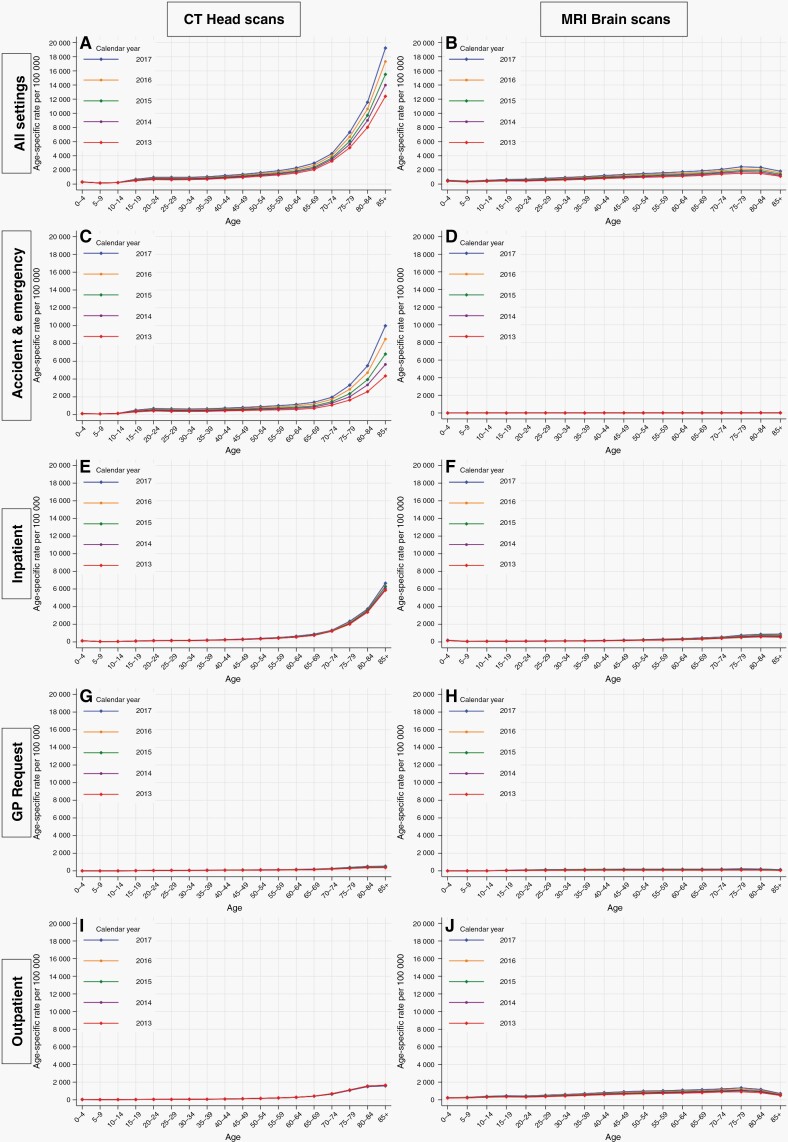
Imaging Trends (CT Head and MRI Brain Scans) in England, 2013–2017
During the 5 years spanning 2013–2017, there were a total of 5 012 335 CT head scans and 2 676 420 MRI brain scans performed within the NHS in England. For CT head scans performed in England across all patient settings combined, rates were higher at older ages (Figure 4). There were also striking increases in the rate of scans in those aged 65 years and above, over each calendar year between 2013 and 2017. The most notable increase was observed in individuals aged 85+ years, for whom the rate rose from 12 409 scans per 100 000 in 2013 to 19 232 scans per 100 000 in 2017.
Figure 4.
Age-specific rates of computerized tomography (CT) head and magnetic resonance imaging (MRI) brain scans by patient setting—England, 2013–2017.
Most of the increase in the calendar year was due to CT head scans performed in Accident & Emergency (A&E) settings, especially in those aged 65–84 and 85+ years, where the rate of scans per 100 000 approximately doubled between 2013 and 2017, from 1312 to 2613 and from 4349 to 9984 respectively. Inpatient CT head scans also increased substantially with advancing age, but these rates have remained largely stable over each calendar year. Meanwhile, there were very low rates of scans performed due to a general practitioner request or those in an outpatient setting, with slight increases after the age of 65 years, but little change over the calendar year.
Considering MRI brain scans, the absolute rates were substantially lower than those for CT head scans and there was little change according to age or calendar year.
Discussion
Summary of Findings
We observed a modest increase in the overall incidence rate of primary CNS tumors in England between 1993 and 2017, using national cancer registry data. Increases were substantially greater for benign tumors compared to tumors of malignant or uncertain behavior, for RC diagnoses compared to MC diagnoses, and for older individuals aged 65–84 and 85+ years compared to younger people. The most notable increase was in the incidence rate of RC benign tumors of the meninges. Meanwhile, incidence rates for malignant CNS tumors have remained stable over the 25-year study period.
For most cancers, MC is regarded as the most accurate method of diagnosis and, together with very low proportions of “death certificate only (DCO)” or “unknown” diagnoses, indicates high data quality within a cancer registry. In the context of CNS tumors, however, MC involves complex neurosurgical intervention such as brain surgery or biopsy. This carries substantially greater risks than similar procedures for other organs, and will only be conducted if the benefits are likely to outweigh the risks. Determining tumor histology via MC can, however, be an important prerequisite for administering appropriate treatment, as histology can predict response to radiotherapy and chemotherapy.
In older age groups, RC was more common than MC, perhaps reflecting shifts in the risk-benefit ratio with increasing age. Surgery may be performed less often in older patients due to poorer health, comorbidities, reduced functional status, and the inherent risks associated with major cranial surgery.22 Furthermore, older patients are more likely to undergo brain scans for falls or other indications, and so a greater proportion of diagnoses could arise incidentally. Support for this theory is provided by our analysis of national imaging data, which revealed considerable increases in the rate of CT head scans performed in England over a recent 5-year period (2013–2017), particularly among individuals aged at least 65 years attending A&E departments. Although such scans are often used to diagnose brain and other CNS tumors, they do have wider clinical uses and we are unable to determine the rationale behind each individual scan. In clinical practice across hospitals in England, there is a low threshold for requesting CT head scans in older patients presenting with common neurological symptoms or deficits. CT scans are a convenient imaging modality that can be used quickly to rule out serious structural brain lesions such as bleeds, strokes, and tumors. The benefit of using CT scans in this emergency setting outweighs any concerns about radiation exposure to patients, hence its widespread use. The substantial volume of CT head scans may result in increased incidental findings of tumors that are small or in inoperable locations.
Findings in Context
Globally, the majority of incidence studies include only malignant CNS tumors since the registration of nonmalignant CNS tumors is limited or nonexistent in many regions. Over time, the registration of nonmalignant CNS tumors has improved due to increasing recognition of the serious impact of these tumors on both individuals and healthcare systems. A recent study of 96 population-based registries in 39 countries reported a five-fold difference in the incidence of malignant CNS tumors between the highest incidence registries, mainly in Europe, and the lowest incidence registries, mainly in Asia.23
There is also notable global variation in temporal trends in CNS tumor incidence. Population-based studies from regions in Europe, the United States, and China have reported increasing incidence rates of all CNS tumors,6,8,24–28 consistent with the findings of our study, while others, in Italy and the Nordic countries have reported stable,7,29–32 or in the case of Japan, decreasing incidence rates.33 When restricted to malignant CNS tumors, the majority of studies report stable26,28,34–38 or decreasing incidence rates,2,39–41 while some studies report increasing incidence rates over time.42,43 Among the few studies of nonmalignant CNS tumors, increasing rates have been reported in parts of the United States, Wales, and Spain,16,17,28 which is consistent with our study, but stable rates have been reported in another US study, across Nordic countries, and Australia.2,35,38
CNS Tumor Incidence Trends in England
Two large studies from England have reported inconsistent trends in the incidence of CNS tumors. A study covering the period 1979–2003 reported an increase in the incidence of CNS tumors during 1979–1992, followed by a leveling off during 1993–2003.7 Authors found the early increases were mainly in the young (0–14 years, AAPC: +1.3%) and elderly (65–84 years, AAPC: +2.5%). A more recent study of brain tumors in adults in England during 1995–2017 reported an overall increase, but did not quantify the observed trends.8 Both studies reported variation in the temporal trends by subtype, and hypothesized that increases may be attributed to the emergence and availability of neuroimaging, advances in clinical practice, diagnostic specificity, and improvements in cancer registration practices. Our quantification of temporal trends by the method of diagnosis illustrates greater increases in the incidence rates of RC tumors over time, particularly in older people, and provides evidence consistent with changes in neuroimaging and clinical practice.
Temporal Trends by the Method of Diagnosis and Tumor Behavior
When not accounting for the method of diagnosis, the increase in the incidence rate for all CNS tumors combined found in our study (AAPC: +1.5%, 1993–2017) is largely consistent with studies in Wales17 (AAPC: +1.6%, 1997–2015) and Australia44 (AAPC: +1.2%, 2000–2008), but lower than studies in the US SEER17 (AAPC: +1.9%, 2004–2015), regions within Spain28 (AAPC: +2.1% 1994–2013) and France25 (AAPC: +2.7% 2000–2012), and higher than those who reported from Nordic countries31 (AAPC: +0.6% to +0.9%, 1969–1998). In Japan,33 CNS tumor incidence rates were increasing during 1975–1987 (AAPC: +3.1%), but have been reported to be declining during 1987–2004 (AAPC: −1.8%).
Few studies have investigated incidence rate trends according to both methods of diagnosis and tumor behavior. For CNS tumors of all behaviors, MC incidence rates increased more rapidly in Wales17 (AAPC: +3.6%, 1997–2015) than in the United States17 (AAPC: +0.1%, 2004–2015) or our study in England (AAPC: +1.6%, 1993–2017). Data on RC diagnoses are very limited. We showed substantial increases in rates of RC tumors of all behaviors (AAPC: +6.1%, 1993–2017) which were greater than a recent study during a similar period from Girona, Spain28 (AAPC: +3.9%, 1994–2013). When restricted to malignant tumors, for MC diagnoses, our study (AAPC: +1.0%, 1993–2017) found an opposite trend to a study over a similar period from Finland45 (AAPC: −0.9%, 1990–2016).
In the United States,16 rates of nonmalignant MC tumors have decreased (AAPC: −1.9% to −0.3%, 2004–2017) which differs from the increasing trends observed in our study (AAPC: +1.3%, 1993–2017). Meanwhile, we showed higher increases in rates of RC benign tumors (AAPC: +10.2%, 1993–2017) compared to the US16 (AAPC: +1.7% to +2.3%, 2004–2017). While the central brain tumor registry of the United States (CBTRUS)2 and a US study16 using the SEER database reported high increases in rates of RC nonmalignant tumors during an earlier period (AAPC: +9.9% and +9.5%, 2004–2009), these increases have attenuated more recently (AAPC: +1.8% and +2.3%, 2009–2018 and 2010–2017). An increase in rates of RC diagnoses and nonmalignant CNS tumors in the data may reflect better data collection over time. In 1998, the European Network of Cancer Registries Working Group on Brain and nervous system tumors recommended that all cancer registries include all intracranial and intraspinal neoplasms irrespective of their behavior.
Strengths and Limitations
NCRAS has collected high-quality data on all cancers and on nonmalignant CNS tumors diagnosed each year in England throughout the study period. Data are collected from multiple sources ensuring all avenues are exhausted to capture a complete dataset of diagnosed cases. Full details on the structure and robustness of the NCRAS dataset have been published previously.18 Our study, which covers 25 years, is the longest-spanning study to systematically investigate the influence of the method of diagnosis on temporal trends in CNS tumor incidence rates. While other studies have alluded to the increased availability of neuroimaging as a potential explanation for increasing incidence rates, few have examined trends by the method of diagnosis. By doing so, this study may contribute to our understanding of the role of the method of diagnosis on temporal trends in CNS tumor incidence.
As our study is based on routinely collected data, we are reliant on data being input and coded correctly. With respect to the completeness, only NHS healthcare providers are currently mandated to submit data to NCRAS. While some diagnoses occurring under private healthcare may be missed, it is estimated that the NHS funds 98–99% of hospital activity46 and thus we may assume near complete coverage of CNS tumor diagnoses in England.
Although a lower proportion of MC diagnoses may be seen as a limitation since they are regarded as more reliable due to being based on tumor histology, an increase in RC diagnoses could indicate that case ascertainment has improved over time, thus capturing a wider range of tumors. Interpretation of temporal trends should therefore be considered in context, as an increase in RC diagnoses will include some tumors that were found incidentally upon scanning for another indication, and without which they might never have been identified during the individual’s lifetime. Currently, little is known regarding the implications of these incidental diagnoses in terms of subsequent patient morbidity and mortality as it is not possible to distinguish between incidental and symptomatic diagnoses in the data available to us.
Conclusion
Overall increases in the incidence rates of CNS tumors in England may be attributed to greater detection of benign tumors, particularly those in the meninges and those that are radiographically confirmed. This could be due to the more widespread use of neuroimaging, particularly CT head scans performed in older people aged at least 65 years in A&E departments, in addition to improved registration practices. The incidence of malignant brain tumors, which comprise over 50% of all CNS tumors, has remained relatively stable over the past 25 years.
Due to rates of RC diagnoses increasing rapidly over time, we recommend all future studies of incidence trends report results according to the method of diagnosis, where the source of data allows. This will help provide a better understanding of temporal trends and allow clinically meaningful interpretations and comparisons to be made, leading to a clearer picture of the true burden of this disease.
Supplementary Material
Acknowledgments
This research uses data that have been provided by patients and collected by the NHS as part of their care and support. The data are collated, maintained and quality assured by the National Cancer Registration and Analysis Service, which is part of the National Disease Registration Service (NDRS). We thank Jackie Charman and Sally Vernon of the NDRS for extracting the data for us and providing helpful advice. We also thank Mariko Nakahara for help with proofreading the manuscript.
Contributor Information
Usama M Ali, Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Diana R Withrow, Nuffield Department of Primary Care Health Sciences, University of Oxford, Oxford, UK.
Andrew D Judge, Nuffield Department of Orthopaedics, Rheumatology, and Musculoskeletal Sciences, University of Oxford, Oxford, UK; Bristol NIHR Biomedical Research Centre and University of Bristol, Bristol, UK.
Puneet Plaha, Nuffield Department of Surgical Sciences, University of Oxford, Oxford, UK; Department of Neurosurgery, John Radcliffe Hospital, Oxford University Hospital NHS Foundation Trust, Oxford, Oxford, UK.
Sarah C Darby, Nuffield Department of Population Health, University of Oxford, Oxford, UK.
Funding
UMA was supported by a doctoral scholarship from the Nuffield Department of Population Health, University of Oxford. ADJ was supported by the National Institute for Health and Care Research (NIHR) Biomedical Research Centre at the University Hospitals Bristol and Weston NHS Foundation Trust and the University of Bristol. PP was supported by Oxford University Hospitals and a 5-year grant from the NIHR Efficacy and Mechanism Evaluation (EME) programme (grant no. 127930). SCD was supported by the Nuffield Department of Population Health, University of Oxford and Cancer Research UK (grant no C8225/A21133). None of the funding sources had any involvement in the conduct of this study or the preparation of this manuscript.
Conflict of Interest.
None.
Authorship Statement
Conceptualization and design: UMA, DRW, SCD. Data preparation and statistical analysis: UMA. Interpretation of results: All authors. Drafting of the manuscript: All authors. Revision of manuscript: All authors. All authors read and approved the final manuscript.
References
- 1. Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014–2018. Neuro Oncol. 2021;23(Suppl_3):iii1–iii105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hardwidge C, Hettige S. Tumours of the central nervous system. Surgery (Oxford). 2012;30(3):155–161. [Google Scholar]
- 4. Office for National Statistics. Deaths registered in England and Wales: 2018. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/datasets/deathsregisteredinenglandandwalesseriesdrreferencetables. Accessed August 6, 2019.
- 5. Davis FG, McCarthy BJ, Freels S, Kupelian V, Bondy ML. The conditional probability of survival of patients with primary malignant brain tumors: surveillance, epidemiology, and end results (SEER) data. Cancer. 1999;85(2):485–491. [PubMed] [Google Scholar]
- 6. Global, regional, and national burden of brain and other CNS cancer. 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(4):376–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arora RS, Alston RD, Eden TOB, et al. Are reported increases in incidence of primary CNS tumours real? an analysis of longitudinal trends in England, 1979–2003. Eur J Cancer. 2010;46(9):1607–1616. [DOI] [PubMed] [Google Scholar]
- 8. Wanis HA, Møller H, Ashkan K, Davies EA. The incidence of major subtypes of primary brain tumors in adults in England 1995–2017. Neuro Oncol. 2021;23(8):1371–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stiller CA, Bayne AM, Chakrabarty A, Kenny T, Chumas P. Incidence of childhood CNS tumours in Britain and variation in rates by definition of malignant behaviour: population-based study. BMC Cancer. 2019;19(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Maile EJ, Barnes I, Finlayson AE, Sayeed S, Ali R. Nervous system and intracranial tumour incidence by ethnicity in england, 2001–2007: a descriptive epidemiological study. PLoS One. 2016;11(5):e0154347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sehmer EAJ, Hall GJ, Greenberg DC, et al. Incidence of glioma in a northwestern region of England, 2006–2010. Neuro Oncol. 2014;16(7):971–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Feltbower RG, Picton S, Bridges LR, et al. Epidemiology of central nervous system tumors in children and young adults (0–29 years), Yorkshire, United Kingdom. Pediatr Hematol Oncol. 2004;21(7):647–660. [DOI] [PubMed] [Google Scholar]
- 13. McKinney PA, Parslow RC, Lane SA, et al. Epidemiology of childhood brain tumours in Yorkshire, UK, 1974–95: geographical distribution and changing patterns of occurrence. Br J Cancer. 1998;78(7):974–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pobereskin LH, Chadduck JB. Incidence of brain tumours in two English counties: a population based study. J Neurol Neurosurg Psychiatry. 2000;69(4):464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stevens MC, Cameron AH, Muir KR, et al. Descriptive epidemiology of primary central nervous system tumours in children: a population-based study. Clin Oncol (R Coll Radiol). 1991;3(6):323–329. [DOI] [PubMed] [Google Scholar]
- 16. Withrow DR, Devesa SS, Deapen D, et al. Nonmalignant meningioma and vestibular schwannoma incidence trends in the United States, 2004–2017. Cancer. 2021;127(19):3579–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Poon MTC, Brennan PM, Jin K, Sudlow CLM, Figueroa JD. Might changes in diagnostic practice explain increasing incidence of brain and central nervous system tumors? A population-based study in Wales (United Kingdom) and the United States. Neuro Oncol. 2021;23(6):979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Henson KE, Elliss-Brookes L, Coupland VH, et al. Data Resource Profile: National Cancer Registration Dataset in England. International Journal of Epidemiology, 2019;49(1):16–16h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fritz AG, Percy CL. International classification of diseases for oncology. 3rd ed. Geneva: World Health Organization; 2000.
- 20. Noble S, Noble M, McLennan D. . English Indices of Deprivation 2019: Research report: Ministry of Housing, Communities & Local Government; 2019. [Google Scholar]
- 21. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. [DOI] [PubMed] [Google Scholar]
- 22. Tantawy MF, Nazim WM. Brain tumor surgery in the elderly: a single institution experience of short-term outcome—a retrospective case study. Egypt J Neurol Psychiatry Neurosurg. 2021;57(1):1–6. [Google Scholar]
- 23. Miranda-Filho A, Pineros M, Soerjomataram I, Deltour I, Bray F. Cancers of the brain and CNS: global patterns and trends in incidence. Neuro Oncol. 2017;19(2):270–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baldi I, Gruber A, Alioum A, et al. Descriptive epidemiology of CNS tumors in France: results from the gironde registry for the period 2000–2007. Neuro Oncol. 2011;13(12):1370–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pouchieu C, Gruber A, Berteaud E, et al. Increasing incidence of central nervous system (CNS) tumors (2000–2012): findings from a population based registry in Gironde (France). BMC Cancer. 2018;18(1):653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Caldarella A, Crocetti E, Paci E. Is the incidence of brain tumors really increasing? a population-based analysis from a cancer registry. J Neurooncol. 2011;104(2):589–594. [DOI] [PubMed] [Google Scholar]
- 27. Zhu B, Wu X, Piao H, Xu S, Yao B. A comparison of epidemiological characteristics of central nervous system tumours in china and globally from 1990 to 2019. Neuroepidemiology. 2021;55(6):460–472. [DOI] [PubMed] [Google Scholar]
- 28. Fuentes-Raspall R, Solans M, Roca-Barcelo A, et al. Descriptive epidemiology of primary malignant and non-malignant central nervous tumors in Spain: Results from the Girona Cancer Registry (1994–2013). Cancer Epidemiol. 2017;50(Pt A):1–8. [DOI] [PubMed] [Google Scholar]
- 29. Chebil C, Boumediene F, Cicero CE, et al. Epidemiology of primary brain tumors in the province of catania during the 2003–2016 period. Neuroepidemiology. 2021;55(6):473–483. [DOI] [PubMed] [Google Scholar]
- 30. Johannesen TB, Angell-Andersen E, Tretli S, Langmark F, Lote K. Trends in incidence of brain and central nervous system tumors in Norway, 1970–1999. Neuroepidemiology. 2004;23(3):101–109. [DOI] [PubMed] [Google Scholar]
- 31. Lonn S, Klaeboe L, Hall P, et al. Incidence trends of adult primary intracerebral tumors in four nordic countries. Int J Cancer. 2004;108(3):450–455. [DOI] [PubMed] [Google Scholar]
- 32. Cordera S, Bottacchi E, D’Alessandro G, et al. Epidemiology of primary intracranial tumours in NW Italy, a population based study: stable incidence in the last two decades. J Neurol. 2002;249(3):281–284. [DOI] [PubMed] [Google Scholar]
- 33. Nomura E, Ioka A, Tsukuma H. Trends in the incidence of primary intracranial tumors in Osaka, Japan. Jpn J Clin Oncol. 2011;41(2):291–294. [DOI] [PubMed] [Google Scholar]
- 34. Ho VKY, Reijneveld JC, Enting RH, et al. Changing incidence and improved survival of gliomas. Eur J Cancer. 2014;50(13):2309–2318. [DOI] [PubMed] [Google Scholar]
- 35. Deltour I, Johansen C, Auvinen A, et al. Time trends in brain tumor incidence rates in Denmark, Finland, Norway, and Sweden, 1974–2003. J Natl Cancer Inst. 2009;101(24):1721–1724. [DOI] [PubMed] [Google Scholar]
- 36. Chien LN, Gittleman H, Ostrom QT, et al. Comparative brain and central nervous system tumor incidence and survival between the united states and taiwan based on population-based registry. Front Public Health. 2016;4:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lin YJ, Chiu HY, Chiou MJ, et al. Trends in the incidence of primary malignant brain tumors in Taiwan and correlation with comorbidities: A population-based study. Clin Neurol Neurosurg. 2017;159:72–82. [DOI] [PubMed] [Google Scholar]
- 38. Australian Institute of Health and Welfare. Brain and other central nervous system cancers. Cat. no, CAN 106. Canberra: AIHW; 2017. [Google Scholar]
- 39. Deorah S, Lynch CF, Sibenaller ZA, Ryken TC. Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurg Focus. 2006;20(4):E1. [DOI] [PubMed] [Google Scholar]
- 40. Kim SJ, Ioannides SJ, Elwood JM. Trends in incidence of primary brain cancer in New Zealand, 1995 to 2010. Aust N Z J Public Health. 2015;39(2):148–152. [DOI] [PubMed] [Google Scholar]
- 41. Miller KD, Ostrom QT, Kruchko C, et al. Brain and other central nervous system tumor statistics, 2021. CA Cancer J Clin. 2021;71(5):381–406. [DOI] [PubMed] [Google Scholar]
- 42. Voisin MR, Sasikumar S, Mansouri A, Zadeh G. Incidence and prevalence of primary malignant brain tumours in Canada from 1992 to 2017: an epidemiologic study. CMAJ Open. 2021;9(4):E973–E979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Etxeberria J, Román ES, Burgui R, et al. Brain and central nervous system cancer incidence in navarre (Spain), 1973–2008 and projections for 2014. J Cancer. 2015;6(2):177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Dobes M, Shadbolt B, Khurana VG, et al. A multicenter study of primary brain tumor incidence in Australia (2000–2008). Neuro Oncol. 2011;13(7):783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Natukka T, Raitanen J, Haapasalo H, Auvinen A. Incidence trends of adult malignant brain tumors in Finland, 1990–2016. Acta Oncol. 2019;58(7):990–996. [DOI] [PubMed] [Google Scholar]
- 46. Herbert A, Wijlaars L, Zylbersztejn A, Cromwell D, Hardelid P. Data resource profile: hospital episode statistics admitted patient care (HES APC). Int J Epidemiol. 2017;46(4):1093–1093i. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data for this study were obtained from NCRAS via the Office for Data Release and from the Diagnostic Imaging Dataset via NHS Digital. De-personalized study data may be made available on request to accredited researchers who submit an application to the NHS Digital Data Access Request Service. Population estimates and standard populations are publicly available via the Office for National Statistics.