Abstract
Background
Prognostic models for spinal cord astrocytoma patients are lacking due to the low incidence of the disease. Here, we aim to develop a fully automated deep learning (DL) pipeline for stratified overall survival (OS) prediction based on preoperative MR images.
Methods
A total of 587 patients diagnosed with intramedullary tumors were retrospectively enrolled in our hospital to develop an automated pipeline for tumor segmentation and OS prediction. The automated pipeline included a T2WI-based tumor segmentation model and 3 cascaded binary OS prediction models (1-year, 3-year, and 5-year models). For the tumor segmentation model, 439 cases of intramedullary tumors were used to model training and testing using a transfer learning strategy. A total of 138 patients diagnosed with astrocytomas were included to train and test the OS prediction models via 10 × 10-fold cross-validation using CNNs.
Results
The dice of the tumor segmentation model with the test set was 0.852. The results indicated that the best input of OS prediction models was a combination of T2W and T1C images and the tumor mask. The 1-year, 3-year, and 5-year automated OS prediction models achieved accuracies of 86.0%, 84.0%, and 88.0% and AUCs of 0.881 (95% CI 0.839–0.918), 0.862 (95% CI 0.827–0.901), and 0.905 (95% CI 0.867–0.942), respectively. The automated DL pipeline achieved 4-class OS prediction (<1 year, 1–3 years, 3–5 years, and >5 years) with 75.3% accuracy.
Conclusions
We proposed an automated DL pipeline for segmenting spinal cord astrocytomas and stratifying OS based on preoperative MR images.
Keywords: deep learning , overall survival, prediction, spinal cord astrocytomas
Key Points.
Previous studies on the prognosis of spinal cord astrocytomas remain controversial.
MR images have great potential to predict prognosis in spinal cord astrocytomas.
The cascade model enables automated tumor segmentation and survival prediction.
Importance of the Study.
Primary spinal cord astrocytomas have no well-established prognostic factors. Limited previous studies on prognosis remain controversial. We aim to develop a practical tool to stratify patients’ overall survival (OS). Our study is the first to develop OS prediction models using deep learning in spinal cord astrocytomas. In the study design, the cascade strategy was employed for automated prediction, which avoided overfitting and low accuracy of the direct 4-class model. This strategy may provide new ideas for future prognostic prediction studies. In this study, we analyzed the effect of each clinical factor, pathological result, and image characteristic on models’ performance, and clarified the best input for models. Our results suggested that preoperative MR images had a great ability to stratify OS. Using standard imaging examination, our findings can be readily translated to the clinic and may be used by clinicians to provide prognostic references for this rare tumor.
Primary spinal cord astrocytomas are extremely rare tumors with an incidence of less than 0.1 per 100 000 person-years.1,2 The World Health Organization (WHO) classification of spinal cord astrocytomas mainly describes intracranial midline gliomas.3 However, our and other previous studies have shown that spinal cord astrocytomas differed from their intracranial counterparts, and the prognosis varies greatly even within the same WHO grade.4,5 Existing studies on the prognosis of spinal cord astrocytomas have mainly focused on using the Surveillance, Epidemiology, and End Results (SEER) database to retrospectively analyze the correlation between clinical factors (such as age, sex, surgical section extent, tumor length, and treatment) and prognosis to guide clinical patient management.6,7 In recent years, due to the diagnostic and prognostic value of the H3 K27M mutation in supratentorial midline glioma, some studies have analyzed its correlation with overall survival (OS).4,8,9 However, the limited results are unclear and inconsistent in the stratification of spinal cord astrocytoma outcomes.
Magnetic resonance imaging (MRI) is a standard examination in neuro-oncology for preoperative diagnosis and postoperative review.10 Growing evidence has revealed the feasibility and accuracy of using MR images to predict biomarkers and the prognosis of glioma patients via deep learning (DL), particularly with convolutional neural networks (CNNs).11–13 MR images can provide information for prediction, even independent of clinical factors.14 However, no study has investigated OS prediction in patients with spinal cord astrocytomas due to a lack of adequate training sets in this rare tumor.
Based on our previously published studies, this study further expanded the number of patients with spinal cord astrocytomas.4,15,16 We aim to provide a practical DL-based tool for stratifying OS prediction in patients with spinal cord astrocytomas from preoperative MR images.
Materials and Methods
Patients
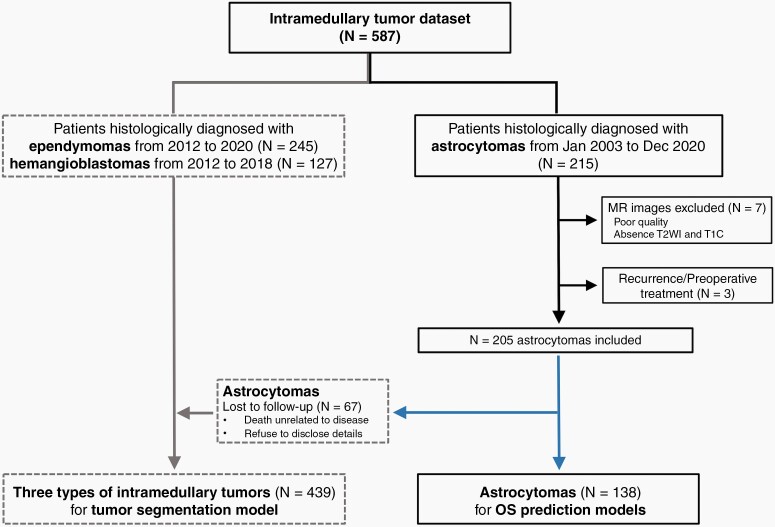
The institutional review board of Beijing Tiantan Hospital, Capital Medical University approved this retrospective study. Patients diagnosed with spinal cord astrocytomas were included from January 2003 to December 2020; the enrollment process is shown in Figure 1. The inclusion criteria were as follows: (1) histopathologically confirmed diagnosis with WHO grade and H3 K27M mutation status (Supplementary eDocument 1 provides pathological diagnostic methods for spinal cord astrocytomas), (2) preoperative T2-weighted (T2W) or contrast-enhanced T1-weighted (T1C) MR images of sufficient quality and no visible motion or artifacts (if the patient had multiple examinations, the 1 closest to the operation time was selected), (3) no therapy prior to MR image acquisition, and (4) available OS information, determined from the time of surgery to tumor-related death. The exclusion criteria were as follows: (1) unknown pathological diagnosis, (2) lack of MR images, (3) therapy before imaging, and (4) loss to follow-up.
Figure 1.
Patient recruitment workflow. All data originated from our intramedullary tumor dataset. This flowchart is divided into 2 parts. For the automated tumor segmentation model, 245 ependymoma patients, 127 hemangioblastoma patients, and 67 spinal cord astrocytoma patients lost to follow-up were included for training and testing. A total of 138 out of 215 patients diagnosed with spinal cord astrocytomas were finally included in developing the OS prediction models based on inclusion and exclusion criteria.
Study Design
The study design is summarized in Figure 2. The research process was divided into 2 steps: First, the determination of the best input for the OS prediction models prior to model development; and second, development of an automated DL pipeline for the stratified prediction of OS, which itself consisted of the following: (1) training of an automated tumor segmentation model using a CNN based on the T2W images of intramedullary tumors; (2) the development of 3 binary models for predicting whether OS exceeded 1 year (1-year model), 3 years (3-year model), or 5 years (5-year model), which were combined through a cascade strategy to achieve 4-class OS prediction (<1 year, 1–3 years, 3–5 years, and >5 years), and (3) implementation of the automated pipeline to connect the tumor segmentation model and the 3 OS prediction models. Details on the data partitioning in these 2 steps are documented in Supplementary eDocument 2 and Supplementary eTable 1.
Figure 2.
Schematic of the fully automated cascade model for OS prediction in patients with spinal cord astrocytomas. Before developing the OS prediction models, the best input for the OS prediction models was first determined. The automated prediction process consists of a tumor segmentation model, OS prediction models, and an automated pipeline. The details of the neural network architecture and training process are described in the following sections.
MR Image Acquisition and Preprocessing
All MR images were obtained on different 1.5 T or 3.0 T scanners. The details of the scanners and image acquisition parameters are listed in Supplementary eTable 2. Briefly, the main scanners were developed by GE, Siemens, or Philips. Fifty-four patients (39.1%) were scanned with 3.0 T devices, and 84 patients (60.9%) were scanned with 1.5 T devices. Sagittal T2W images were acquired with a TR 1800–3200 ms, TE 80–160 ms, in-plane resolution of 0.3–1.0 mm, and 3–4 mm slice thickness. T1C images were acquired with a TR 100–1300 ms, TE 3–15 ms, and identical in-plane resolution and slice thickness as the T2W images.
For tumor segmentation, the z score was used to normalize the 3D array, and all T2W images were resized to 256 × 256. The ground truth was defined as tumor and peritumor areas manually segmented on the T2W images by 2 independent neuroradiologists (J.Z. with 3 years of experience and T.S. with 3 years of experience) and confirmed by a senior neuroradiologist (Y.D. with more than 20 years of experience) using 3D Slicer (https://www.slicer.org/). For the image preprocessing of the OS prediction models, the T1C images were registered to the T2W images, and both sets of registered images were then subjected to N4 bias correction. Similar to the tumor segmentation step, the z score was used to normalize the 3D array, and images were resized to 256 × 256. The details of the image processing methods are described in Supplementary eDocument 3.
Tumor Segmentation
In total, 439 patients diagnosed with intramedullary tumors were used to build the segmentation model with a transfer learning strategy, including 245 ependymomas, 127 hemangioblastomas, and 67 astrocytomas without OS. The data of 70% of the 439 patients (n = 307) were used for model training and validation, and the remaining 30% (n = 132) were used as the test set. The segmentation model firstly was pretrained on ependymomas and hemangioblastomas and fine-tuned on astrocytomas. The classic 2D Unet17 was modified to take T2W images of size 256 × 256 as input and generate the whole tumor area. The detailed training process and CNN architecture for the tumor segmentation step are described in Supplementary eDocument 4. The performance of the tumor segmentation step was measured using the Dice similarity score, which was evaluated by the mean value of the Dice coefficient of all 2D images (slice level), 3D images (patient level), and maximum tumor area on the 2D images (max-slice level) for each patient in the test set.
Determining the Best Input for the OS Prediction Models
This step was divided into 3 substeps: (1) investigation of the effect of the 5 clinical factors (age, sex, duration of chief complaints, tumor length, and extent of resection) and 2 pathological results (WHO grade, H3 K27M mutation status) on OS prediction using a DL multilayer perceptron (MLP) network, (2) investigation of the effect of different input images (T2W images, T1C images, and tumor masks) on OS prediction using a 2D CNN model, and (3) simplification of the final input for the OS prediction models, involving sequential deletion of clinical factors, pathological results, and input MR images according to the above 2 substep results using a multi-input 2D CNN. All network architectures and training processes are summarized in detail in Supplementary eDocument 5.
Developing the OS Prediction Models
For each patient, the best input is determined in the above step. The architecture and training process of the associated CNN were similar to those of the CNN above, with some adjustments in the input layer based on the final input. In the model training and testing phases, the ground truth and predicted masks were used to validate the performance of the pipeline. To perform 4-class OS prediction, the 1-year model, 3-year model, and 5-year model were cascaded with the following logic: The 3 binary models were ranked according to their cross-validation performance. The best-performing model was first used to predict the OS for each patient. If the predictive results fell into 1 of the 4 intervals (<1 year, 1–3 years, 3–5 years, and >5 years), the models stopped the next prediction. Otherwise, the second best-performing model was selected to repeat this process until the OS of the patients was stratified.
Automated OS Prediction Pipeline
An automated pipeline was built to connect the tumor segmentation model and the 3 cascaded binary models. When a new case entered the pipeline, a tumor mask was first generated, then the best input combination was entered into the cascade model, and finally, the predicted OS interval was obtained. For each binary model, the predicted OS result was ensembled from 10 × 10-fold submodels by calculating the mean value of the 100 probabilities and binarized with a 0.5 cutoff value.
Model Explanation
To understand which parts of the image inputs were related to OS prediction, gradient-weighted class activation mapping (Grad-CAM)18 was used to visualize the contributing regions. The output of Grad-CAM was assessed by a neuroradiologist (Q.L. with 3 years of experience) regarding the consistency of the activation areas.
Statistical Analysis
The performance of the tumor segmentation model was assessed using the Dice similarity score,19 which was evaluated at the slice level, patient level, and max-slice level in both test sets. The area under the receiver operating characteristic (ROC) curve (AUC) was used to compare the performance of the binary models in the first step. The mean of 100 AUCs was calculated for each group based on 10 × 10-fold cross-validation. The paired T-test was used to calculate the P value for the means of the 100 AUCs of the 2 groups. Delong test was used to test the significance of the difference between 1.5T and 3.0T ROC curves in 1-year model, 3-year model, and 5-year model. P < .05 was considered statistically significant. The statistical analysis was performed in Python (version 3.6.5).
The performance of the 3 binary models was estimated by the sensitivity, specificity, accuracy, and AUC in the test set. The AUC with its 95% confidence interval (CI) was calculated from 1000 bootstrapped replications with the predicted probabilities and labels. The performance of the cascaded model was measured with the overall accuracy and recall for each interval.
Results
Patient Characteristics
A total of 138 patients with spinal cord astrocytomas were finally enrolled, including 59 females (mean age, 30.1 years; range, 4–63 years) and 79 males (mean age, 31.3 years; range, 4–67 years). The average duration of chief complaints was 6 (3–12) months. Among all patients, 57 had mutant-type H3 K27M, 65 had wild-type H3 K27M, and 16 did not have available mutation status information. The average number of vertebral segments involved was 4.3 ± 2.2. All data are summarized in Supplementary eTable 1.
Tumor Segmentation
The tumor segmentation model was trained for 200 epochs. The Dice scores with the test were 0.802, 0.784, and 0.852 at the slice level, patient level, and max-slice level, respectively. These results demonstrate that the performance at the max-slice level was superior to that at the other 2 and reduced the influence of the segmentation model on OS prediction in our automated pipeline. Representative images showing automatic segmentations by the model are shown in Supplementary eFigure 1.
Determining the Best Input for the OS Prediction Models
The accuracy of the 3 binary models when 5 clinical factors and 2 pathological results as input was 84.2%, 83.6%, and 87.6%, respectively. In the 1-year model, WHO grade, age, and duration of chief complaints significantly contributed to OS prediction (P < .05), among which WHO grade played the most critical role (P < .001). The other 4 clinical factors did not significantly affect OS prediction, that is, they did not significantly improve the accuracy of the OS prediction. Similarly, WHO grade contributed most to OS prediction in the 3-year and 5-year models (P < .001). After deleting the WHO grade, the accuracy of the 3 OS prediction models with the other factors decreased by approximately 6, 5, and 4 percentage points, respectively. Supplementary eTable 3 summarizes the contribution of 5 clinical factors and 2 pathological results both together and individually to model prediction.
Supplementary eTables 4 and 5 summarize the effect of different MR inputs on OS prediction with the 1-year model, 3-year model, and 5-year model. The combination of T2W and T1C images predicted OS more accurately than either sequence alone for all 3 binary models (P < .05). Whether using single or both imaging sequences, the performance of the 3 binary models was improved by 4–5 percentage points when the tumor masks were also included, indicating that the latter were important for OS prediction (P < .001). For the 1-year model, the T1C images improved model accuracy by 1.5 percentage points, suggesting that they were more effective than the T2W images in predicting OS (P = .024). However, there was no significant difference between the use of T1C and T2W images for the 3-year model (P = .543) or the 5-year model (P = .582). The best MR image input was the T2W images, T1C images, and tumor mask.
To streamline our model input, we attempted to delete some features without affecting the accuracy of the models. A feature was deleted if doing so did not significantly affect the accuracy of the model. The 5 clinical factors and 2 pathological results were deleted sequentially, and the accuracy of the 1-year model, 3-year model, and 5-year model was ultimately decreased by 0.9, 0.7, and 1.1 percentage points, respectively (P > .05). Thus, the deletion of the 5 clinical factors and 2 pathological results did not significantly affect the prediction effect of the OS models. All results are shown in Supplementary eTable 6. Finally, the T2W images + T1C images + tumor mask combination was considered the best input for the OS models.
OS Model Performance
Among our automated models, the 1-year model, 3-year model, and 5-year model had an outstanding performance, with accuracies of 86.0%, 84.0%, and 88.0% and AUCs of 0.881 (95% CI 0.839–0.918), 0.862 (95% CI 0.827–0.901), and 0.905 (95% CI 0.867–0.942), respectively (Supplementary Table 1). Figure 3 shows the receiver operating characteristic (ROC) curves of the automated binary models. When using the ground-truth images, the accuracies of the models were 86.7%, 84.7%, and 88.0%, with AUCs of 0.888 (95% CI 0.841–0.923), 0.879 (95% CI 0.832–0.936), and 0.901 (95% CI 0.862–0.940), respectively (Supplementary eTable 7). The results indicate that there was no significant difference between the automated models and ground-truth models in OS stratification prediction. To understand the differences between MR field intensity and the models’ performance, ROC curves were plotted for 1.5T and 3.0 T-test sets in the 1-year, 3-year, and 5-year models, respectively. Our results showed that there was no significant difference in the performance of OS prediction models on 1.5T and 3.0T (Supplementary eTable 8 and Supplementary eFigure 3).
Table 1.
Performance of the Automated OS Prediction Models
| SENS (%) | SPEC (%) | ACC (%) | AUC | AUC [95% CI] | |
|---|---|---|---|---|---|
| 1-year model | 87.0 | 84.0 | 86.0 | 88.1 | [0.839, 0.918] |
| 3-year model | 84.3 | 83.8 | 84.0 | 86.2 | [0.827, 0.901] |
| 5-year model | 86.0 | 89.0 | 88.0 | 90.5 | [0.867, 0.942] |
| Cascade model | N/A | N/A | 75.3 | N/A | N/A |
Note: SENS (%), sensitivity in percentage; SPEC (%), specificity in percentage; ACC (%), accuracy in percentage; AUC, area under the curve; AUC [95% CI], 95% confidence interval of the AUC; N/A, not available.
Figure 3.
ROC curves of the 1-year model, 3-year model, and 5-year model in predicting OS.
According to the above results, the logic of the cascade model was finally decided: Figure 4A shows that the 5-year model, 1-year model, and 3-year model were sequentially connected through the pipeline. The cascaded model achieved accuracies of 75.3% and 75.2% with automated masks and ground-truth masks, respectively (Table 1 and Supplementary eTable 7). The confusion matrix further showed the predictive ability of the cascaded model in the test set, with a recall of 80%, 53.3%, 60%, and 86%, respectively (Figure 4B). Representative images of the outputs of the OS predictive models are shown in Figure 5.
Figure 4.
Cascade model for OS prediction. (A) The logic of the cascade model. (B) Confusion matrix with the different survival intervals (<1 year, 1–3 years, 3–5 years, and >5 years).
Figure 5.
Preoperative MR images of representative cases predicted by the OS prediction models. The yellow curves show the manual ground truth and automatically segmented masks in the OS prediction process.
Model Explanation
As shown in Supplementary eFigure 2, regions of color represent areas activated by the CNN; deeper reds indicate stronger correlations with the OS prediction. Our results indicated that the automated pipeline focused on the tumor regions. The consistency of heatmaps and tumor regions was assessed by a neuroradiologist, revealing values of 94.7%, 98.7%, and 85.7% for the 1-year model, 3-year model, and 5-year model, respectively.
Discussion
In this study, we developed a fully automated pipeline for tumor segmentation with a Dice coefficient of 85.2% and stratified OS prediction for spinal cord astrocytomas with accuracy of 75.3% using T2W images, T1C images, and tumor masks. Our fully automated cascaded model incorporated an automated tumor segmentation model, 3 binary OS prediction models, and a fully automated pipeline without requiring any manual operation. In addition, this cascaded model is flexible, and capable of being as 3 independent models (1-year model, 3-year model, and 5-year model) for predicting whether OS can exceed 1, 3, and 5 years, respectively.
Robust spinal tumor segmentation is one of the major challenges in spinal cord astrocytomas OS prediction. Compared with those of supratentorial gliomas, the ground-truth images of spinal cord astrocytomas for model training can be difficult to accurately label due to the smaller lesion sizes and unclear margins, and the Dice scores will fluctuate substantially as the label changes. Previously, our study developed an automated model to segment intramedullary tumors, cavities, and edema, achieving a Dice score of 76.7 ± 1.5%.16 In this study, we expanded the dataset from 349 to 439 patients to further improve the performance of the segmentation model. Notably, the Dice score in the test was 85.2% at the max-slice level. Meanwhile, whole lesions including tumors, cavities, and edema were used as input for OS prediction models. The main reason was that the relationship between these imaging characteristics and prognosis remains unclear in spinal cord astrocytomas. However, a few studies of other intramedullary tumors have suggested that these imaging characteristics were associated with disease severity.20,21
The relationship between multiple clinical factors and pathological results and prognosis in spinal cord astrocytomas remains unclear. To simplify OS prediction models and avoid overfitting, it is necessary to analyze in detail whether these factors are necessary as input to models. Our study demonstrated that adding preoperative MR images can further improve the performance of OS prediction models compared to using only clinical factors and pathological results (Supplementary eTables 3 and 6). Considering the practicality and flexibility of prediction models in clinical practice, we tied to delete all clinical factors and pathological results because they were not all available for every patient; for example, WHO grade and H3 K27M can only be obtained postoperatively. Our results showed that using only preoperative MR images to build OS prediction models didn’t significantly reduce the models’ performance (Supplementary eTable 6). We speculated some MR imaging characteristics were intrinsically related to these factors. Therefore, these factors contributed little to improving the performance of models in the presence of these imaging characteristics. For example, the more obvious and extensive tumor enhancement, the higher the WHO grade. These imaging characteristics can indirectly provide useful information for building OS prediction models. The relationship between imaging characteristics and prognosis of spinal cord astrocytomas needs further study in the future.
Accurate prognosis prediction in patients with spinal cord astrocytomas is critical for patient management and postoperative treatment. Few studies have focused on prediction models of spinal cord astrocytomas due to the rarity of this tumor. Compared to previous studies,6,15 our study is the first to develop OS prediction models using DL. The cascade strategy was designed for the prediction models to avoid the overfitting caused by small dataset. This strategy had 3 advantages as follows: First, compared with the 4-class model, the cascade strategy makes full use of the data. For example, if a patient was still alive at the follow-up date, we can only know that the patient OS was more than 1 year, but we cannot determine whether the patient will survive more than 3 years. Such data were not available for the 4-class model, but we could still use it to develop the binary 1-year model. The cascade strategy can also prevent overfitting in the 4-class model due to limited data. Second, this strategy can improve the overall prediction accuracy and minimize error accumulation to the greatest degree possible. During the model development phase, we noticed that the 5-year model and 1-year model misclassified some patients with 1–3 years and 3–5 years OS into the <1 year and >5 years classes, with recalls of 60% and 53.3%, respectively (Figure 4B). This was the reason why the accuracy of the cascade model was lower than that of the 3 binary models. However, in the 4-class model, the overall prediction accuracy only reached 62.5% (results not shown). Finally, the 3 binary models were more flexible than the 4-class model. The 3 binary models can be used independently to predict whether the OS will exceed 1 year, 3 years, or 5 years, and the combination of any 2 models will produce 3 prediction intervals, while the cascade model can produce 4. Thus, the cascade strategy was the best choice for OS prediction under the current data conditions.
It should be noted that the performance of the OS prediction models could be further improved with further studies of spinal cord astrocytomas. Several promising molecular prognostic features have great potential to improve models’ performance. In this study, H3 K27M mutation status had no significant effect on models’ performance (Supplementary eTable 3), but other histone H3 gene families associated with radiotherapy and recurrences, such as H3F3A and HIST1H3B, may contribute to stratifying the prognosis of astrocytomas.22 Although the incidence of BRAFV600E mutations in spinal cord astrocytomas is low, it was associated with a better prognosis in histological grade II/III astrocytaoms.4,23 Additionally, BRAF-KIAA1549 fusion was related to midline brain tumors, which are generally difficult to remove and prone to recurrence.24
This study had 2 limitations. First, it was a single-center study, and the generalizability of the prediction models has not been verified. A further prospective study with a large multicentre dataset is warranted to validate the current findings. Second, although 10-fold cross-validation was used to maximize the reliability of the results, an independent prospective cohort of spinal cord astrocytoma patients needs to be included in future studies.
In conclusion, this study is the first to stratify OS in patients with spinal cord astrocytomas using a fully automated DL process based on preoperative MR images, with the largest number of patients over 17 years. Our prediction models can be translated for clinical use to preoperatively offer prognostic information for patients newly diagnosed with spinal cord astrocytoma.
Supplementary Material
Acknowledgments
We acknowledged all the colleagues who helped with the patient recruitment and MR imaging.
Contributor Information
Ting Sun, Department of Radiology, Beijing Tiantan Hospital, Capital Medical University, Beijing 100070, China.
Yongzhi Wang, Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing 100070, China.
Xing Liu, Department of Pathology, Beijing Tiantan Hospital, Capital Medical University, Beijing 100070, China.
Zhaohui Li, Department of Machine learning, BioMind Inc., Beijing, 100070, China.
Jie Zhang, Department of Radiology, Beijing Tiantan Hospital, Capital Medical University, Beijing 100070, China; Department of Radiology, Beijing Renhe Hospital, Beijing 102600, China.
Jing Lu, Department of Radiology, Beijing Tiantan Hospital, Capital Medical University, Beijing 100070, China; Department of Radiology, Third Medical Center of Chinese PLA General Hospital, Beijing 100089, China.
Liying Qu, Department of Radiology, Beijing Tiantan Hospital, Capital Medical University, Beijing 100070, China.
Sven Haller, Department of Imaging and Medical Informatics, University Hospitals of Geneva and Faculty of Medicine of the University of Geneva, Geneva, Switzerland.
Yunyun Duan, Department of Radiology, Beijing Tiantan Hospital, Capital Medical University, Beijing 100070, China.
Zhizheng Zhuo, Department of Radiology, Beijing Tiantan Hospital, Capital Medical University, Beijing 100070, China.
Dan Cheng, Department of Radiology, Beijing Tiantan Hospital, Capital Medical University, Beijing 100070, China.
Xiaolu Xu, Department of Radiology, Beijing Tiantan Hospital, Capital Medical University, Beijing 100070, China.
Wenqing Jia, Department of Neurosurgery, Beijing Tiantan Hospital, Capital Medical University, Beijing 100070, China.
Yaou Liu, Department of Radiology, Beijing Tiantan Hospital, Capital Medical University, Beijing 100070, China.
Funding
Beijing Municipal Natural Science Foundation for Distinguished Young Scholars (No. JQ20035), Beijing Hospitals Authority Clinical Medicine Development of special funding support (XMLX2021092), the Capital’s funds for Health Improvement and Research Key Projects (CFH2022-1-2042), Beijing Hospital Management Center “DengFeng” talent training program (DFL20220503), The Strategic Priority Research Program of the Chinese Academy of Sciences (XDA16040303).
Conflict of interest statement. None declared.
Author Contributions
Study design, manuscript drawing, tumor segmentation, statistical analysis, and response to reviewers (Ting Sun). Study design, manuscript drawing, and response to reviewers (Yongzhi Wang). Histological information collection, and response to reviewers (Liu Xing). Model development and response to reviewers (Zhaohui Li and Zhizheng Zhuo). Model development (Zhizheng Zhuo). Tumor segmentation, determining activation areas (Jie Zhang, Liying Qu, and Duan YunYun). Critical revision and response to reviewers (Sven Haller). Clinical information collection (Dan Cheng and Xiaolu Xu). Study design, manuscript editing, review, and response to reviewers (Yaou Liu and Wenqing Jia).
References
- 1. Milano MT, Johnson MD, Sul J, et al. Primary spinal cord glioma: a surveillance, epidemiology, and end results database study. J Neurooncol. 2010;98(1):83–92. [DOI] [PubMed] [Google Scholar]
- 2. Schellinger KA, Propp JM, Villano JL, McCarthy BJ. Descriptive epidemiology of primary spinal cord tumors. J Neurooncol. 2008;87(2):173–179. [DOI] [PubMed] [Google Scholar]
- 3. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 4. Chai RC, Zhang YW, Liu YQ, et al. The molecular characteristics of spinal cord gliomas with or without H3 K27M mutation. Acta Neuropathol Commun. 2020;8(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alvi MA, Ida CM, Paolini MA, et al. Spinal cord high-grade infiltrating gliomas in adults: clinico-pathological and molecular evaluation. Mod Pathol. 2019;32(9):1236–1243. [DOI] [PubMed] [Google Scholar]
- 6. Yuan C, Yao Q, Cheng L, et al. Prognostic factors and nomogram prediction of survival probability in primary spinal cord astrocytoma patients. J Neurosurg Spine. 2021; 35( 5): 651– 662. [DOI] [PubMed] [Google Scholar]
- 7. Khalid S, Kelly R, Carlton A, et al. Adult intradural intramedullary astrocytomas: a multicenter analysis. J Spine Surg. 2019;5(1):19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Meyronet D, Esteban-Mader M, Bonnet C, et al. Characteristics of H3 K27M-mutant gliomas in adults. Neuro Oncol. 2017;19(8):1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang L, Li Z, Zhang M, et al. H3 K27M-mutant diffuse midline gliomas in different anatomical locations. Hum Pathol. 2018;78:89–96. [DOI] [PubMed] [Google Scholar]
- 10. Weller M, van den Bent M, Preusser M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi YS, Bae S, Chang JH, et al. Fully automated hybrid approach to predict the IDH mutation status of gliomas via deep learning and radiomics. Neuro Oncol. 2021;23(2):304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Alhasan AS. Clinical applications of artificial intelligence, machine learning, and deep learning in the imaging of gliomas: a systematic review. Cureus. 2021;13(11):e19580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nie D, Lu J, Zhang H, et al. Multi-channel 3D deep feature learning for survival time prediction of brain tumor patients using multi-modal neuroimages. Sci Rep. 2019;9(1):1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Macyszyn L, Akbari H, Pisapia JM, et al. Imaging patterns predict patient survival and molecular subtype in glioblastoma via machine learning techniques. Neuro Oncol. 2016;18(3):417–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pang B, Chai RC, Zhang YW, et al. A comprehensive model including preoperative peripheral blood inflammatory markers for prediction of the prognosis of diffuse spinal cord astrocytoma following surgery. Eur Spine J. 2021;30(10):2857–2866. [DOI] [PubMed] [Google Scholar]
- 16. Lemay A, Gros C, Zhuo Z, et al. Automatic multiclass intramedullary spinal cord tumor segmentation on MRI with deep learning. Neuroimage Clin. 2021;31:102766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ronneberger O, Fischer P, Brox T. U-Net: Convolutional Networks for Biomedical Image Segmentation. Medical Image Computing and Computer-Assisted Intervention – MICCAI 2015. 2015: 234– 241. [Google Scholar]
- 18. Selvaraju RR, Cogswell M, Das A, et al. Grad-CAM: visual explanations from deep networks via gradient-based localization. Int J Comput Vis. 2019;128(2):336–359. [Google Scholar]
- 19. Milletari F, Navab N, Ahmadi S-A. V-Net: Fully Convolutional Neural Networks for Volumetric Medical Image Segmentation. 2016 Fourth International Conference on 3D Vision (3DV), Stanford, CA, USA, 2016, pp. 565– 571.
- 20. Huntoon K, Wu T, Elder JB, et al. Biological and clinical impact of hemangioblastoma-associated peritumoral cysts in von Hippel-Lindau disease. J Neurosurg. 2016;124(4):971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Balériaux DL. Spinal cord tumors. Eur Radiol. 1999;9(7):1252–1258. [DOI] [PubMed] [Google Scholar]
- 22. Castel D, Philippe C, Calmon R, et al. Histone H3F3A and HIST1H3B K27M mutations define two subgroups of diffuse intrinsic pontine gliomas with different prognosis and phenotypes. Acta Neuropathol. 2015;130(6):815–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Schindler G, Capper D, Meyer J, et al. Analysis of BRAF V600E mutation in 1,320 nervous system tumors reveals high mutation frequencies in pleomorphic xanthoastrocytoma, ganglioglioma and extra-cerebellar pilocytic astrocytoma. Acta Neuropathol. 2011;121(3):397–405. [DOI] [PubMed] [Google Scholar]
- 24. Faulkner C, Ellis HP, Shaw A, et al. BRAF fusion analysis in pilocytic astrocytomas: KIAA1549-BRAF 15-9 fusions are more frequent in the midline than within the cerebellum. J Neuropathol Exp Neurol. 2015;74(9):867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.