Abstract
Harmful algal blooms produce biotoxins that can injure or kill fish, wildlife, and humans. These blooms occur naturally but have intensified in many locations globally due to recent climatic changes, including ocean warming. Such changes are especially pronounced in northern regions, where the effects of paralytic shellfish toxins (PSTs) on marine wildlife are of growing concern. In Alaska, seabird mortality events have increased in frequency, magnitude, and duration since 2015 alongside anomalously high ocean temperatures. Although starvation has been implicated as the apparent cause of death in many of these die-offs, saxitoxin (STX) and other PSTs have been identified as possible contributing factors. Here, we describe a mortality event at a nesting colony of Arctic Terns (Sterna paradisaea) near Juneau, Alaska in 2019 and report elevated concentrations of PSTs in bird, forage fish, and mussel samples. Concentrations of STX and other PSTs in tern tissues (2.5–51.2 μg 100g−1 STX-equivalents [STX-eq]) were of similar magnitude to those reported from other PST-induced bird die-offs. We documented high PST concentrations in blue mussels (>11,000 μg 100g−1 STX-eq; Mytilus edulis spp.) collected from nearby beaches, as well as in forage fish (up to 494 μg 100g−1 STX-eq) retrieved from Arctic Tern nests, thereby providing direct evidence of PST exposure via the terns’ prey. At maximum concentrations measured in this study, a single 5 g Pacific Sand Lance (Ammodytes personatus) could exceed the median lethal STX dose (LD50) currently estimated for birds, offering strong support for PSTs as a likely source of tern mortality. In addition to describing this localized bird mortality event, we used existing energetics data from adult and nestling Arctic Terns to calculate estimated cumulative daily PST exposure based on ecologically relevant concentrations in forage fish. Our estimates revealed potentially lethal levels of PST exposure even at relatively low (≤30 ug 100g−1 STX-eq) toxin concentrations in prey. These findings suggest that PSTs present a significant hazard to Arctic Terns and other northern seabirds and should be included in future investigations of avian mortality events as well as assessments of population health.
Keywords: Arctic Tern, Food web, Forage fish, Paralytic shellfish toxin, Seabird, Saxitoxin
1. Introduction
Harmful algal blooms (HABs) have been identified as a growing threat to coastal ecosystems worldwide, with deleterious impacts described for a variety of marine wildlife species (Glibert et al., 2014). Although specific drivers of HAB events are often difficult to pinpoint, water temperature influences critical physiological processes of toxin-producing algae (Drent et al., 1992; Gobler et al., 2017; Moore et al., 2008) and the geographic scope, frequency and intensity of blooms are projected to expand in some regions with climate change (Anderson et al., 2021; Glibert et al., 2014). Northern waters are especially vulnerable to ocean warming, which includes periodic marine heatwaves marked by extreme temperature fluctuations (Overland et al., 2018; Walsh et al., 2018). Such perturbations have been linked to increases in the distribution and abundance of harmful algal species (Gobler et al., 2017; Glibert et al. 2014) and, in some cases, have precipitated HAB events unprecedented in scale and duration (McCabe et al., 2016; Trainer et al., 2020). In addition to direct climate-related influences, there is increasing global awareness about impacts of HABs on humans and wildlife, including in regions in which monitoring has not historically been conducted (Hallegraef et al., 2021; Anderson et al., 2022).
In coastal Alaska, like in other parts of the Northeast Pacific Ocean, blooms of toxic dinoflagellates of the genus Alexandrium (Anderson et al., 2012) produce paralytic shellfish toxins (PSTs), a group of potent neurotoxins that cause paralytic shellfish poisoning (PSP; Landsberg et al., 2014). Filter-feeding invertebrates, such as bivalves and zooplankton, directly accumulate PSTs by consuming toxic algae; the toxins are then transferred throughout the food web and into higher trophic-level organisms (Deeds et al., 2008; Landsberg, 2002). Clams, mussels, and other bivalves are common vectors of PSP and a human regulatory limit of 80 ug 100g−1 STX-equivalents (STX-eq) in shellfish been established in the United States and Canada to protect consumer safety (Wekell et al., 2004). The toxicity of individual PST congeners is measured relative to saxitoxin (STX), which is has been well studied in its ability to harm a variety of taxa (Wiese et al., 2010; Landsberg et al., 2014). Paralytic shellfish toxins interfere with functioning of sodium-ion channels and, when ingested, can lead rapidly to severe illness or death via respiratory paralysis (Wiese et al., 2010). Additional to the risks posed to humans, PSTs can negatively impact fishes, birds, and marine mammals, which are exposed to toxins through the marine food web (Landsberg et al., 2014). However, toxicity thresholds among wildlife are poorly understood and no corresponding safety guidelines exist for PST concentrations in prey.
Although PSP has been documented in humans in some regions of Alaska for more than 200 years (Anderson et al., 2021), evidence from the Arctic and Subarctic suggests that local HAB activity may be increasing, thereby presenting new or heightened risks to marine consumers, including many wildlife species (Anderson et al., 2021, 2022; Lewitus et al., 2012; Trainer et al., 2020; Vandersea et al., 2018). Paralytic shellfish toxins have recently been detected in seabirds (Van Hemert et al., 2021; Van Hemert et al., 2020), marine mammals (Hendrix et al., 2021; Lefebvre et al., 2016), and other taxa (Trainer et al., 2014; Van Hemert et al., 2020) throughout coastal Alaska, highlighting the widespread occurrence of these toxins in the local marine environment. As a result, there is growing concern about the potential effects of HAB toxins on northern wildlife, particularly in relation to unexplained wildlife mortality events.
Since 2015, multiple large-scale seabird die-offs have co-occurred with periods of unusually warm ocean water off the coast of Alaska (Jones et al., 2019; Piatt et al., 2020, Van Hemert et al., 2021; Van Hemert et al., 2020). Most of the carcasses in these die-offs were emaciated, with the cause of death presumed to be starvation. However, HABs were also investigated as possible contributing factors and STX was detected in multiple seabird species and locations (Jones et al., 2019; Piatt et al., 2020; Van Hemert et al., 2021; Van Hemert et al., 2020). Seabirds are susceptible to HAB toxin exposure through consumption of forage fish and marine invertebrates, which have been found to contain high levels of PSTs, including in some locations in Alaska (Ben-Gigirey et al., 2021; Van Hemert et al., 2020).
Although many information gaps remain about the cumulative impacts of HABs on seabirds, PST-induced morbidity and mortality has been described in dozens of avian species across a broad geographic range, including in the Northeast Pacific (Ben-Gigirey et al., 2021; Shumway et al., 2003). In 2011 and 2012, up to 21% of Kittlitz’s Murrelet (Brachyramphus brevirostris) nestlings died shortly after consuming Pacific Sand Lance (Ammodytes personatus) at nest sites near Kodiak, Alaska, with mortality attributed to STX ingestion (Shearn-Bochsler et al., 2014). Saxitoxin and other PSTs have subsequently been documented in association with other seabird die-offs in the Chukchi and Bering seas (Van Hemert et al., 2021), Gulf of Alaska (Van Hemert et al., 2020), and along the West coast of the contiguous United States (Gibble et al., 2021). However, establishing causality is challenging from field-collected carcasses (Ben-Gigirey et al., 2021; Shumway et al., 2003) and it remains unclear whether HABs directly or indirectly contributed to recent die-offs in Alaska. An experimental trial with captive Mallards (Anas platyrhynchos) has since provided the first quantitative information on STX toxicity in birds, establishing an estimated lethal dose that presents a useful framework for assessing PST exposure hazards (Dusek et al., 2021).
In mid-late June of 2019, we observed unusual mortality of Arctic Terns (Sterna paradisaea) at two breeding colonies near Juneau, Alaska. Upon routine monitoring of colony sites, biologists discovered multiple nests containing dead nestlings and observed an adult tern convulsing and eventually dying on the beach. Here, we describe the mortality event, report PST levels in tern, forage fish, and blue mussel samples, and investigate the role that HABs may have played in this die-off. We also use data from this investigation and published values on tern energetics to evaluate PST exposure risks in Arctic Terns.
2. Methods
2.1. Field sampling
As part of routine monitoring of two Arctic Tern nesting colonies (Portland Island [58.35° N, −134.75°] and Mendenhall Lake [58.42° N, −134.55°]) near Juneau, Alaska in 2019, U.S. Forest Service biologists observed unusual mortality of nestlings and adults (Fig. 1). Following the onset of the mortality event in mid- to late-June, we opportunistically collected bird carcasses (n = 11) and forage fish (Pacific Sand Lance [n = 6] and Pacific Herring [Clupea pallasii; n = 2]) found near nest sites (Tables 1, 2). Additional sand lance (n = 4) were collected near Juneau at Eagle Beach (58.54°, −134.84°) as part of recreational harvest during low tide events by raking sand to expose buried individuals, and a freshly dead sand lance was picked up off the beach at Tee Harbor (58.41°, −134.76°) as part of a U.S. Geological Survey (USGS) study on HAB toxins in the marine food web (Table 2).
Fig. 1.

Arctic Tern (Sterna paradisaea) nestling found dead at Mendenhall Lake nesting colony on 28 June 2019 near Juneau, Alaska.
Table 1.
Saxitoxin (STX) and total paralytic shellfish toxin (PST) concentrations in Arctic Tern (Sterna paradisaea) tissues collected during a 2019 bird mortality event near Juneau, Alaska. STX analyzed by enzyme-linked immunosorbent assay (ELISA; μg 100g−1 STX) and total PSTs analyzed by high-performance liquid chromatography (HPLC; μg 100g−1 STX-eq) for muscle, liver, and upper and lower gastrointestinal tract (GI) and contents. STX was not detected in any muscle samples. NA = not available. BDL = below detection limit. See Table 3 for PST congener profiles.
| NWHC Accession |
Age | Date | Location | Liver ELISA(μg 100g−1 STX) |
HPLC(μg 100g−1 STX-eq) | Upper GI ELISA(μg 100g−1 STX) |
HPLC(μg 100g−1 STX-eq) | Lower GI ELISA(μg 100g−1 STX) |
HPLC(μg 100g−1 STX-eq) |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 29287–001 |
Adult | 6/27/2019 | Mendenhall Lake | 2.0 | 2.5 | 5.5 | NA | 39.4 | 51.2 |
| 29287–002 | Chick | 6/27/2019 | Mendenhall Lake | 5.9 | 3.9 | 7.1 | 6.6 | NA | NA |
| 29287–003 | Chick | 6/27/2019 | Mendenhall Lake | NA | NA | 2.2* | 2.5* | NA | NA |
| 29287–004 | Chick | 6/27/2019 | Mendenhall Lake | 3.7 | 2.3 | 7.7 | 7.1 | NA | NA |
| 29287–006 | Chick | 7/1/2019 | Portland Island | BDL | NA | BDL | NA | BDL | NA |
*Entire GI
Table 2.
Saxitoxin (STX) measured by enzyme-linked immunosorbent assay (ELISA; μg 100g−1 STX) and total paralytic shellfish toxin (PST; μg 100g−1 STX-eq) measured by high-performance liquid chromatography (HPLC) in whole forage fish (Pacific Sand Lance [Ammodytes personatus] and Pacific Herring [Clupea pallasii]) collected June–July 2019 near Juneau, Alaska. NA = not available. See Table 3 for PST congener profiles.
| Lab ID | Species | Site | Date | ELISA(μg −1 STX) |
HPLC(μg −1 STX-eq) |
|---|---|---|---|---|---|
|
| |||||
| ATS0686 | Pacific Herring |
Portland Island colony |
7/1/ 2019 |
13.1 | 58.1 |
| ATS0687 | Pacific Sand Lance |
Mendenhall Lake colony |
6/28/ 2019 |
13.8 | 91.0 |
| ATS0756 | Pacific Sand Lance |
Tee Harbor | 6/16/ 2019 |
60.8 | 341.1 |
| ATS0761 | Pacific Sand Lance |
Eagle Beach | 6/6/ 2019 |
2.3 | 8.5 |
| ATS0762 | Pacific Sand Lance |
Eagle Beach | 6/6/ 2019 |
4.8 | 6.6 |
| ATS0763 | Pacific Sand Lance |
Eagle Beach | 6/6/ 2019 |
1.5 | 1.9 |
| ATS1042 | Pacific Sand Lance |
Mendenhall Lake colony |
6/28/ 2019 |
56.4 | 125.2 |
| ATS1043 | Pacific Sand Lance |
Mendenhall Lake colony |
6/28/ 2019 |
49.6 | 151.9 |
| ATS1044 | Pacific Sand Lance |
Mendenhall Lake colony |
6/28/ 2019 |
47.5 | 494.1 |
| ATS1045 | Pacific Sand Lance |
Mendenhall Lake colony |
6/28/ 2019 |
25.0 | 139.0 |
| ATS1046 | Pacific Sand Lance |
Mendenhall Lake colony |
6/28/ 2019 |
2.0 | 37.6 |
| ATS1047 | Pacific Herring |
Mendenhall Lake colony |
6/28/ 2019 |
2.6 | NA |
Bird carcasses (Fig. 1) from the two tern colonies were placed in a −20 °C freezer within 4 hours of collection and later shipped frozen to the USGS National Wildlife Health Center for cause-of-death determination, including necropsy and histopathology. Utensils and surfaces were disinfected between birds with Unicide (Brulin, Indianapolis, Indiana). Subsamples of gastrointestinal tract (n = 7), liver (n = 4), and breast muscle (n = 5) were collected at necropsy from carcasses for PST analysis and stored frozen.
To examine the course of the HAB event during the summer of 2019 we examined PST concentration in blue mussels (Mytilus edulis spp.) approximately every two weeks at four beaches in the Juneau area from 3 May to 7 August 2019 (Fig. 2). Two discrete samples were taken from each beach on every sampling occasion. Blue mussels are excellent sentinels for blooms of Alexandrium since they uptake and eliminate PSTs rapidly (Bricelj and Shumway, 1998; Harley et al., 2020a).
Fig. 2.
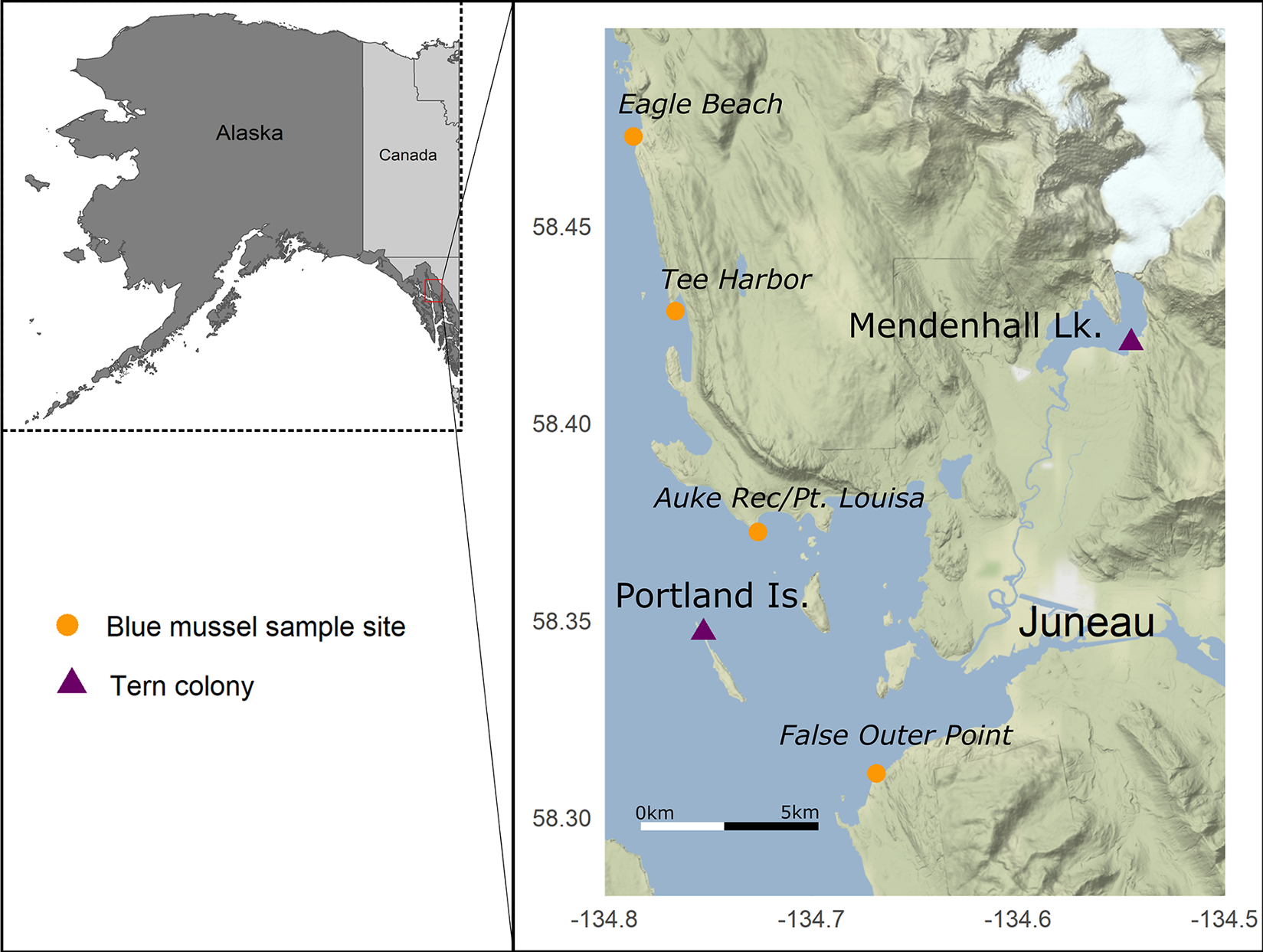
Map of study area near Juneau, Alaska showing Arctic Tern (Sterna paradisaea) colony sites (purple triangles) affected by 2019 bird mortality event and sampling locations for PST monitoring of blue mussels (Mytilus edulis spp.; orange circles).
Forage fish and mussels were frozen upon collection and bird tissues were frozen immediately after necropsy; all samples were shipped on ice in insulated containers to the USGS Alaska Science Center (birds and forage fish) or Sitka Tribe of Alaska Environmental Research Lab (mussels) for PST analysis.
2.2. Necropsy and PST testing
We analyzed bird tissues (n = 16) and forage fish (n = 12) for STX at the USGS Alaska Science Center using commercially available enzyme-linked immunosorbent assay (ELISA) kits (Eurofins Abraxis, Warminster, PA). Following protocols optimized for seabird tissues as described in Van Hemert et al. (2020), we homogenized samples before extraction with 1% acetic acid. We tested available tissues from each bird and whole forage fish except for forage fish <5 g, in which case we pooled multiple individuals from a single site. All samples were run in duplicate, and results averaged prior to analysis. Any duplicate sample results with a coefficient of variation ≥10% were considered invalid and re-run accordingly. Prior validation work described detection limits of 1–2 μg 100g−1 STX in seabird tissues (Van Hemert et al., 2020).
Saxitoxin ELISA kits offer a rapid, high throughput, sensitive screening tool for detection of STX and have previously been used to test seabird and other wildlife samples that may contain low concentrations of toxin (e.g., Lefebvre et al., 2016; Van Hemert et al., 2020). The primary disadvantage of these kits, however, is that they were designed to target STX and have limited cross-reactivity to other PST congeners (per manufacturer’s description; Eurofins Abraxis, Warminster, PA). Therefore, samples that were positive for STX by ELISA were subsequently analyzed using high-performance liquid chromatography (HPLC) to quantify total PSTs and identify specific toxin congeners. Using this approach, we acknowledge that samples containing congeners other than STX may have been missed in the initial ELISA screening; therefore, it is possible that more bird or forage fish samples contained PSTs than what are reported here (also see detailed discussion in Van Hemert et al., 2020). Aliquots of the same extracts were used for both ELISA and HPLC analysis.
For HPLC, we followed methods described in Lawrence et al. (2005) and Van Hemert et al. (2020). Briefly, 1 ml of extract (0.5 g shellfish equivalent) was cleaned using 500 mg, 3 ml solid phase extraction (SPE) cartridges (Supelclean LC-18, Sigma-Aldrich, St Louis, MO) and a Phenomenex HyperSep 10 port glass vacuum manifold (Phenomenex, Torrance, CA) to remove any impurities. Precolumn oxidation was employed under the same conditions as described by Lawrence et al. (2005), with the exception that a Waters Acquity LC system (Waters Corp., Milford, MA) and a Waters Cortecs 2.7 um, 4.6 × 150 mm C18 column was used. Saxitoxin standards used in this analysis were purchased from the Certified Reference Materials Program of the Institute for Marine Biosciences, National Research Council (Halifax, Canada) and included dcGTX2,3; C1,2; dcSTX; GTX2,3; GTX5; STX; GTX1,4; and NEO. Detection limits for HPLC ranged from 1 to 5 μg 100g−1 STX-eq depending on the toxin congener. Typical replicate samples had a relative standard deviation of ≤5%.
Mussel samples (n = 49) were analyzed with the receptor binding assay (RBA) for total PSTs at Sitka Tribe of Alaska Environmental Research Lab, where many non-commercial shellfish samples for Alaska have routinely been processed, as described in Harley et al. (2020b). Each mussel sample consisted of 100 g of pooled individual mussels (generally 15 to 30 mussels) which were homogenized prior to hydrochloric acid extraction (see methods in Harley et al., 2020b; Van Dolah et al., 2012). Samples were analyzed at two different dilutions and both and quality control runs (3 nM [H3] STX) were analyzed in triplicate on an 8-point calibration curve. Provided all samples fell within the calibration curve, replicates were averaged to provide a final concentration. Samples screened by RBA were not available for follow-up HPLC testing, although RBA is designed to quantify the STX-equivalent sum of all PSTs and should therefore provide a reliable indicator of total STX-equivalents in a sample (Turner et al., 2020; Van Dolah et al., 2012). We report mean concentrations (± standard error) of PSTs in mussel samples across 4 beaches. Differences in analytical methods used for PST testing of mussels (RBA) versus birds and forage fish (ELISA followed by HPLC) were due to available laboratory capabilities at the time of analysis as well as existing optimization of specific protocols for target organisms.
2.3. Saxitoxin dose calculations
To assess potential PST exposure to Arctic Terns through their prey, we calculated daily fish intake (g) for adults and nestlings. For adults, we used the mean field metabolic rate (280 kJ d−1) and mass (102 g) reported by Uttley et al. (1994) from an Arctic Tern breeding colony on Coquet Island, Scotland to estimate total daily requirements. For nestlings, we inferred field metabolic rate from Klaassen et al. (1989) by comparing the model developed for total daily energetic demand with the average mass of an n day old bird for the following age classes: 2-d (~20 g, ~70 kJ d−1), 7-d (~50 g, ~160 kJ d−1), and 12-d (~80 g; ~240 kJ d−1). This model was based on doubly labeled water measurements from nestlings at an Arctic Tern colony in Svalbard, Norway (Klaassen et al., 1989), with conclusions supported by subsequent energetics work on tern nestlings from various locations (Drent et al., 1992; Klaassen, 1994). We then calculated average mass of fish consumed per day for each age class, assuming a standard energetic value of 5 kJ g−1 wet weight for Pacific Herring and Pacific Sand Lance (Ammodytes hexapterus; Piatt et al., 2020; Van Pelt et al., 1997).
Next, we derived a range of daily STX doses based on toxin concentrations in forage fish using the following equation:
where
= dose of
= mass of fish consumed
= mass of bird
= concentration of in prey species
For our exposure model we chose a range of reflecting ecologically relevant concentrations observed in forage fish from this and other studies (Nisbet, 1983; Starr et al., 2017). In addition to daily dose calculations, we also estimated maximum STX dose from consumption of a 5 g fish, which is within the size range typical of prey provisioned to Arctic Tern nestlings (Baird et al., 1983; Van Pelt et al., 1997). Because adult terns typically carry one fish at a time for nestling colony deliveries, this estimate reflects a conservative single-dose exposure (Baird et al., 1983; Hatch et al., 2020).
All statistics, figures, and calculations were done using the R Programming Language ver 4.1 (R Core Team) and the tidyverse packages (Harley et al., 2020a).
3. Results
3.1. Field observations
On 17 June, 2019, biologists found a single dead tern nestling at the Mendenhall Lake colony, which consisted of about 40 breeding adults. From this colony, 12 nestlings and two adults were later confirmed dead, and three additional nestling mortalities were suspected based on observations from staff at the nearby U.S. Forest Service Mendenhall Glacier Visitor Center. On a subsequent survey of the Portland Island colony, located approximately 15 km away, biologists counted 60 live adults and 20 dead nestlings; no live nestlings were observed. Prior to and during the mortality event, terns were actively provisioning chicks with Pacific Sand Lance, Pacific Herring, and other forage fish, with regular bill deliveries documented by direct field observation and in remote camera footage at Mendenhall Lake near the Mendenhall Glacier Visitor Center. Video footage from 26 June showed an adult tern convulsing on its back, then later dying on the beach. Biologists returned to the Mendenhall Lake colony on 27 June and retrieved five carcasses, comprising one adult and four nestlings.
3.2. Necropsy results
Eleven Arctic Tern carcasses were examined at the National Wildlife Health Center, including one adult and four nestlings from the Mendenhall Lake colony and six nestlings from the Portland Island colony. The adult from Mendenhall Lake (29287–001) was in fair body condition with mildly diminished fat reserves and had no significant gross or microscopic abnormalities. The nestlings from Mendenhall Lake were in fair to good body condition with mildly diminished to adequate fat reserves, indicating a rapid course of illness; however, poor postmortem condition precluded further diagnostic assessment and tissues were only available for PST testing from three of the four nestlings (29287–002, 003, and 004). One nestling from Portland Island (29287–006) was in poor body condition with pectoral muscle atrophy and depletion of fat reserves and microscopic evidence of renal urate stasis, indicating dehydration. The remaining five nestlings from Portland Island could not be assessed or tested for PST due to advanced decomposition and scavenging.
3.3. Toxin results
3.3.1. Birds
All three nestlings and the single adult from the Mendenhall Lake colony had detectable levels of STX. Concentrations were highest for the gastrointestinal tract, with values ranging from 2.2 to 39.4 μg 100g−1 by ELISA. Saxitoxin was also detected in liver tissue of three birds tested (Table 1). We did not detect STX in the single nestling collected at Portland Island or in muscle tissue from any bird. HPLC generally confirmed ELISA results with slightly higher total PSTs (51.2 μg 100g−1 STX-eq) in the lower gastrointestinal sample from the adult tern. Saxitoxin was the dominant congener in most bird samples, although several other PSTs were also present (Table 3; Smith et al., 2022).
Table 3.
Concentrations of paralytic shellfish toxin (PST) congeners (μg 100 g−1 STX-eq) measured by high-performance liquid chromatography in tissues of Arctic Terns (Sterna paradisaea) and whole forage fish (Pacific Sand Lance [Ammodytes personatus] and Pacific Herring [Clupea pallasii]) collected June–July 2019 near Juneau, Alaska. Bird tissues included liver and upper and lower gastrointestinal (GI) tract and contents. NA= not available. BDL = below detection limit.
| Lab ID | NWHC Accession # | Species | Tissue | dcGTX2,3 | C1C2 | dcSTX | GTX2,3 | GTX5 | STX | GTX1,4 | NEO | Total PST |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| ATS0665 | 29287–001 | Arctic Tern | Liver | BDL | 0.1 | BDL | 0.7 | BDL | 0.7 | BDL | 1.0 | 2.5 |
| ATS0667 | 29287–001 | Arctic Tern | Lower GI | BDL | 1.0 | 0.5 | 13.7 | 0.8 | 18.5 | 13.8 | 3.0 | 51.2 |
| ATS0669 | 29287–002 | Arctic Tern | Liver | BDL | BDL | 0.1 | BDL | 0.1 | 3.7 | BDL | BDL | 3.9 |
| ATS0670 | 29287–002 | Arctic Tern | Upper GI | BDL | BDL | 0.3 | BDL | 0.1 | 5.1 | BDL | 1.0 | 6.6 |
| ATS0672 | 29287–003 | Arctic Tern | Entire GI | BDL | 0.5 | BDL | 1.1 | BDL | 0.9 | BDL | BDL | 2.5 |
| ATS0674 | 29287–004 | Arctic Tern | Liver | BDL | BDL | BDL | BDL | BDL | 2.3 | BDL | BDL | 2.3 |
| ATS0675 | 29287–004 | Arctic Tern | Upper GI | BDL | BDL | 1.4 | BDL | BDL | 2.1 | BDL | 3.6 | 7.1 |
| ATS0686 | NA | Pacific Herring | Whole fish | 0.2 | 1.2 | 0.2 | 4.6 | 0.3 | 2.6 | 30.5 | 18.5 | 58.1 |
| ATS0687 | NA | Pacific Sand Lance | Whole fish | 0.4 | 1.2 | 0.3 | 3.7 | 0.4 | 3.3 | 39.6 | 42.0 | 91.0 |
| ATS0756 | NA | Pacific Sand Lance | Whole fish | 1.1 | 7.4 | 1.1 | 20.6 | 2.8 | 20.5 | 159.9 | 127.7 | 341.1 |
| ATS0761 | NA | Pacific Sand Lance | Whole fish | 8.5 | BDL | BDL | BDL | BDL | BDL | BDL | BDL | 8.5 |
| ATS0762 | NA | Pacific Sand Lance | Whole fish | BDL | 0.1 | 0.1 | 0.6 | 0.2 | 2.6 | BDL | 3.0 | 6.6 |
| ATS0763 | NA | Pacific Sand Lance | Whole fish | BDL | BDL | BDL | 0.2 | 0.1 | 0.6 | BDL | 1.0 | 1.9 |
| ATS1042 | NA | Pacific Sand Lance | Whole fish | BDL | 0.8 | 0.8 | 5.6 | 1.8 | 37.1 | 50.1 | 29.0 | 125.2 |
| ATS1043 | NA | Pacific Sand Lance | Whole fish | 48.3 | BDL | BDL | 2.1 | BDL | BDL | 66.5 | 35.0 | 151.9 |
| ATS1044 | NA | Pacific Sand Lance | Whole fish | BDL | 7.2 | 1.9 | 21.3 | 2.5 | 12.8 | 237.4 | 211.0 | 494.1 |
| ATS1045 | NA | Pacific Sand Lance | Whole fish | BDL | 2.0 | 1.9 | 6.2 | 0.9 | 11.1 | 72.9 | 44.0 | 139.0 |
| ATS1046 | NA | Pacific Sand Lance | Whole fish | BDL | 1.1 | BDL | 0.7 | 0.1 | 0.6 | 27.1 | 8.0 | 37.6 |
3.3.2. Forage fish
All Pacific Sand Lance (n = 10) and Pacific Herring (n = 2) samples tested had detectable levels of STX, with values ranging from 1.6 to 60.8 μg 100g−1 by ELISA (Table 2). HPLC analysis revealed much higher total PST concentrations in many of these, with a maximum of 494 μg 100g−1 STX-eq from a sand lance collected at the Mendenhall Lake colony site (Table 2). Unlike bird samples, GTX1,4 and NEO were the dominant congeners in forage fish, at least among samples with total PST concentrations greater than 5 μg 100g−1 (Table 3; Smith et al., 2022).
3.3.3. Mussels
Paralytic shellfish toxins in blue mussel samples exceeded shellfish regulatory limits for human consumption (80 μg 100g−1 STX-eq) at all four routine sampling locations in the Juneau area from 5 June to 10 July 2019. Peak toxin concentrations, with maximum values of up to 11,049 μg 100g−1 STX-eq, were recorded in mid- to late-June, coincident with the observed tern mortality at the Portland Island and Mendenhall Lake colonies (Fig. 3).
Fig. 3.
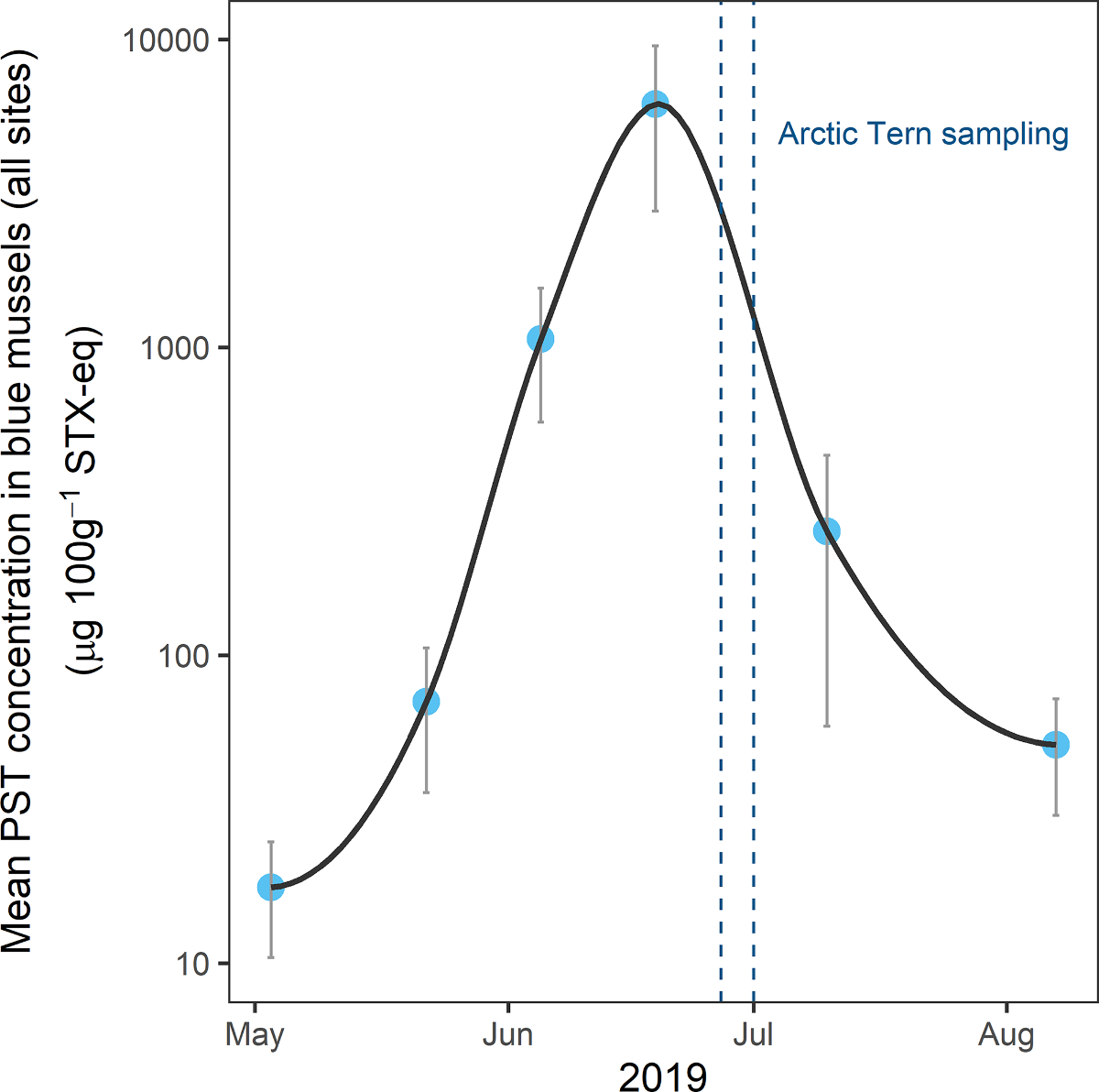
Paralytic shellfish toxin (PST) concentrations (μg 100g−1 STX-eq) measured by receptor binding assay in blue mussels (Mytilus edulis spp.) collected at four locations during May–August 2019 near Juneau, Alaska. Graph displays mean (blue circles) and standard error (gray bars) of mussel samples at each site. The time period of the Arctic Tern (Sterna paradisaea) mortality event and associated sample collection is shown between blue dashed lines. Note that PST concentrations are displayed on a logarithmic scale.
3.4. Daily fish intake and STX dose calculations
Given maximum PST concentrations in sand lance collected at colony sites (494 μg kg−1 STX-eq; Table 2), a single 5 g fish could deliver as much as 24.7 μg STX-eq, equaling a dose of 242 μg kg−1 for a 102-g adult bird and 1235, 494, and 309 μg kg−1 for a 20-, 50- and 80 g nestling, respectively. These values far exceed the estimated STX LD50 (lethal dose for 50% of individuals) for Mallards (167 μg/kg; Dusek et al., 2021), the only estimate currently available for an avian species.
Based on estimated field metabolic rates (Klaassen et al. 1989), total daily fish requirements would be 14, 32, 48, and 56 g for 2-d, 7-d, 12-d, and adult terns, respectively (Fig. 4). At these rates of consumption, our projected dose curves indicated that Arctic Terns could rapidly accumulate STX, even at relatively low prey toxin levels (Fig. 4). Over a 24 h period, an average STX concentration of 30 μg 100g−1 in prey would result in a cumulative dose close to or greater than the Mallard LD50 for both nestlings and adults (Fig. 4).
Fig. 4.
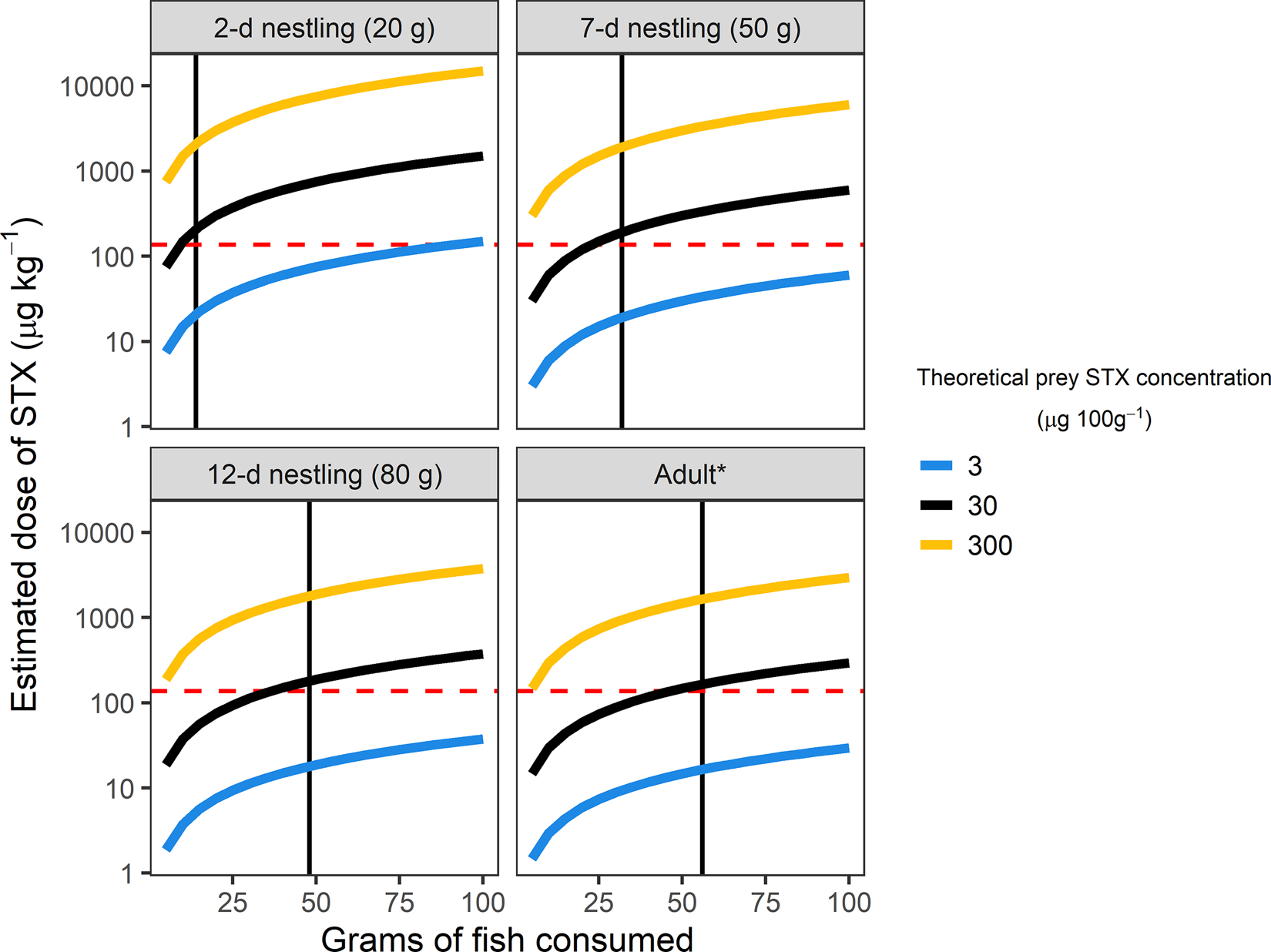
Projected dose curves (μg kg−1) for saxitoxin (STX) exposure in Arctic Terns (Sterna paradisaea) given a range of ecologically relevant mean concentrations in forage fish (3, 30, and 300 μg 100g−1 STX) consumed over a 24 h period. Daily fish requirements (black vertical line) estimated from previous reports of field metabolic rates in adult (Uttley et al., 1994) and nestling (Klaassen et al., 1989) Arctic Terns, assuming a standard energetic value of 5 kJ g−1 wet weight for Pacific Sand Lance (Ammodytes hexapterus) and Pacific Herring (Clupea pallasii; Piatt et al., 2020; Van Pelt et al., 1997). Dashed red line shows LD50 (lethal dose in 50% of individuals) for Mallards (Anas platyrhynchos; Dusek et al., 2021). Note that estimated STX dose curves are displayed on a logarithmic scale. *Mean adult mass of 102 g taken from Uttley et al. (1994).
4. Discussion
In June 2019, unusual mortality of Arctic Tern nestlings and adults occurred at two breeding colonies near Juneau, Alaska, likely impacting at least half of the total active nests. Thirty-two nestlings and two adults were confirmed dead, although more individuals were presumed to have been affected. We detected PSTs in four of the five tern carcasses suitable for algal toxin testing at concentrations associated with mortality in other bird species (Ben-Gigirey et al., 2021). We also detected high levels of PSTs in mussels and forage fish, including Pacific Sand Lance and Pacific Herring collected at colony sites shortly before and coincident with the tern die-off event. Although we were only able to sample a fraction of all birds found dead, high PST concentrations in prey combined with observational data corroborate a likely toxic cause of death.
4.1. Evidence for PSTs as cause of bird mortalities
Unusually warm weather in Southeast Alaska during summer 2019 preceded an intense HAB event (Southeast Alaska Tribal Ocean Research 2019), which we documented in mussel samples collected at coastal field sites in the Juneau area. Peaking in mid-June, shortly before the first tern mortalities were observed, maximum PSTs in mussel samples exceeded shellfish regulatory limits for human consumption (80 μg 100g−1; Wekell et al., 2004) by more than a factor of 100, with concentrations >11,000 μg 100g−1 (Fig. 3). These are among the highest concentrations ever recorded in Southeast Alaska for any shellfish species (Harley et al., 2020b). Mussels are known to be reliable sentinels of PSTs in the environment due to their role as filter feeders and their rapid acquisition and depuration of toxins, thereby providing near real-time information about bioavailability of STX and other PST congeners (Beyer et al., 2017; Bricelj and Shumway, 1998). Although human health guidelines do not directly apply to wildlife, PST concentrations above shellfish regulatory limits indicate blooms of Alexandrium spp. or other STX-producing algal species that can impact the food web and potentially harm birds and marine mammals. Results from mussel samples collected around Juneau throughout the summer suggested a prolonged bloom period, with elevated concentrations observed at some sites from late May until early August (Fig. 3).
We detected PSTs in four Arctic Tern carcasses found dead at colony sites. Although quantitative data describing the correlation between toxicity and specific tissue concentrations are not yet available for seabirds, results from the lower gastrointestinal tract of the single adult specimen (51.2 μg 100g−1 STX-eq; Table 3) were comparable to those measured in Mallards that died acutely from oral STX administration in experimental trials (Dusek et al., 2021). Concentrations of STX in tern nestlings at the Mendenhall Lake colony (Tables 1, 3) also overlapped those reported in an incident of saxitoxicosis involving Kittlitz’s Murrelet chicks near Kodiak Island AK in 2012, with STX liver concentrations of 5.6 to 10.6 μg 100g−1 and upper gastrointestinal content concentrations ranging from 5.2 to 21.6 μg 100g−1 (Shearn-Bochsler et al., 2014). Furthermore, our results correspond with tissue STX concentrations of up to 134 μg 100g−1 measured in several seabird species from the St. Lawrence Estuary during a well-documented STX-induced mass mortality event (Starr et al., 2017). Remote camera footage of an adult tern convulsing on its back prior to its death near Mendenhall Lake corroborates a possible toxic exposure, as prior reports of saxitoxicosis in birds have described convulsions, as well as regurgitation, discoordination, ataxia, and, ultimately, respiratory failure (Ben-Gigirey et al., 2021; Dusek et al., 2021).
Observations of tern foraging and provisioning behavior combined with toxin results from fish collected at colony nest sites also lend support to the hypothesis that PSTs contributed to bird mortalities. Fish account for the largest proportion of biomass of Arctic Tern diets (Hatch et al., 2020) and forage fish were by far the most commonly observed prey items delivered to nestlings at Juneau-area colonies, with other foods (dragonflies, salmonid fry, marine invertebrates) only occasionally observed. Pacific Sand Lance, known to be vectors of PSTs (Ben-Gigirey et al., 2021), were regularly seen in bill deliveries of adult terns returning to colonies and sand lance samples collected at the Mendenhall Lake colony (n = 6) were all positive for PSTs, with concentrations between 37.6 and 494.1 μg 100g−1 STX-eq (Table 2). The two Pacific Herring samples collected from nest sites also contained PSTs. Notably, the single forage sample collected at Portland Island was a partially consumed herring with 58.1 μg 100g−1 STX-eq (Table 2). Although STX was not detected in the single nestling tern carcass available from this site, the herring sample provided evidence of direct exposure to breeding adults and nestlings through their prey.
Given the apparently rapid depuration rates of PSTs in birds (Dusek et al. 2021), a short detection window exists for assessing prior exposure. As a result, failure to detect PSTs (as was the case with the Portland Island nestling) does not rule out the possibility of toxic exposure. Partially consumed fish found near nest sites may also have reflected regurgitation or vomiting resulting from PST ingestion (Nisbet, 1983). A mortality event attributed to PSTs among breeding Common Terns (Sterna hirundo) in Massachusetts, USA reported some findings similar to ours, including regurgitated forage fish samples containing potentially lethal concentrations of STX (Nisbet, 1983).
It is plausible that parental neglect could have contributed to nestling mortality, particularly if adults experienced PST-related illness, although starvation did not appear to be widespread among nestlings. We noted high rates of provisioning by adults at the Mendenhall Lake colony and, with the exception of the single nestling collected at Portland Island, necropsy data indicated fair to good body condition of most carcasses. Some researchers have suggested that birds and other wildlife species may have the ability to detect and avoid prey with elevated PST concentrations (Kvitek and Bretz, 2005; Kvitek, 1991; Kvitek et al., 1991) but it is unclear whether such a hypothesis is supported among seabirds, especially given the large number of previously reported mortalities (Ben-Gigirey et al., 2021). In this instance, even if terns were able to detect PSTs in prey, it is unknown whether nontoxic alternatives were available due to the long duration and apparently widespread nature of the bloom event. Elevated PST concentrations documented in mussels and other shellfish at various Southeast Alaska locations during June and July 2019 (Southeast Alaska Tribal Ocean Research 2019) suggest that the food web was impacted across a relatively large geographic area.
4.2. Tern energetics and PST exposure
Toxicity thresholds for PSTs have not yet been determined for seabirds, but a recent study of STX in captive Mallards established the first quantitative estimate of the LD50 (167 μg kg−1) in an avian species. Given this estimate, and the maximum PST concentrations measured in sand lance retrieved from the Mendenhall colony (494 μg 100g−1 STX-eq), a single 5 g fish would contain enough toxin to cause acute mortality in an adult tern (Fig. 4). For young chicks, prey STX-eq concentrations of as little as 70 μg 100g−1 could be similarly lethal with a single feeding. More than 60% (5/8) of the forage fish samples we collected at colony sites exceeded this threshold. Even if a single fish did not contain sufficient PSTs to cause immediate death, the high energetic demands of nesting terns and other seabirds (Drent et al., 1992; Hatch et al., 2020; Uttley et al., 1994) imply that repeated exposure could quickly accrue to a potentially lethal dose.
Prior investigations of Arctic Tern energetics provide useful insights about estimated daily STX exposure given a range of ecologically relevant concentrations as measured in prey items from this and other studies. To meet their daily energetic demands, adult terns provisioning nestlings on Coquet Island in Scotland required an average of 280 kJ d−1, which would translate to about 56 g of fish (Uttley et al., 1994). With these high rates of consumption, terns eating fish with average concentrations of 30 μg 100g−1 STX (well below the human shellfish advisory limit of 80 μg 100g−1) could meet or exceed the Mallard LD50 over a 24 hr period (Fig. 4). Nestlings have proportionally greater energetic demand compared to adults (Klaassen, 1994; Klaassen et al., 1989; Uttley et al., 1994) and, as a result of their smaller body size, would require fewer individual fish to reach potentially lethal STX doses. Thus, tern chicks may be especially vulnerable to PST exposure although additional studies are required to determine how sensitivity varies by developmental stage.
Our dose curves estimate cumulative STX ingestion over a 24 h period, but it is important to acknowledge that timing of exposure may be a key factor in actual toxicity calculations. Given the rapid depuration rates of STX, we expect that acute toxicity is more likely to result if a bird consumes a large quantity of STX-containing prey in a short period of time or ingests prey that are highly toxic than if the cumulative exposure occurs over a longer duration. In addition to the risk of acute illness or death, many questions remain about chronic, sublethal PST exposure and potential effects on seabirds, including foraging ability, thermoregulation, and behavior. More research is needed to establish clear toxicity thresholds in wild birds.
4.3. Congener profiles and methodological considerations
As expected, HPLC generally reported greater total concentrations of PSTs than ELISA, particularly among samples with >10 μg 100g−1 STX-eq, which is the lower detection limit for HPLC (Table 3). This difference reflects the fact that the ELISA rapid screening method primarily targets STX and is known to have lower cross-reactivity with other PSTs (Eurofins Abraxis, Warminster, PA). This difference was especially pronounced for forage fish, which on average had the greatest proportions of GTX1,4, NEO, and STX but showed high variability in congener profiles (Table 3). Most bird samples contained detectable concentrations of STX, which corresponds with reports from avian tissues in other studies (Starr et al., 2017; Van Hemert et al., 2021), but other congeners were also present. Although STX is one of the most potent congeners among the various PSTs (Wiese et al., 2010), processes of biotransformation are not well understood for non-model species and to date we have no specific information about metabolism of PSTs in birds. Given small sample sizes, we cannot draw any general conclusions about pharmacokinetics or potential metabolites in Arctic Terns. We report values using STX-eq, which is an accepted method of measuring total toxicity of PSTs, but the potential effects of biotransformation or co-occurrence of congeners on effective toxicity should not be discounted. Our results indicate that seabirds in Alaska are exposed to multiple PST congeners simultaneously and more detailed study on this topic is needed.
It is also worth noting that we collected bird carcasses and forage fish from tern colonies after the onset of the mortality event, and the length of time since death was unknown. Several of the specimens were noted to be in poor postmortem condition and many of them were unsuitable for examination and testing. Prior studies have documented degradation of STX and other PSTs over time, with toxin concentrations declining with heat and bacterial activity (Donovan et al., 2008; Indrasena and Gill, 2000). Therefore, STX concentrations in bird tissues may have originally been higher than the values we report here, although such differences would only strengthen evidence for PSTs as the suspected cause of death.
5. Conclusions
The detection of STX in bird carcasses combined with high PST concentrations measured in forage fish delivered to colony nest sites suggest saxitoxicosis as a likely cause of death among Arctic Terns. Although it is not possible to assign causality without established toxicity thresholds, this study supports a building body of evidence implicating HABs as possible threats to seabird health. The high energetic demands of Arctic Terns are not unique among northern seabirds (Drent et al., 1992; Piatt et al., 2020) and similar concentrations of PST exposure could be expected for other species that consume large quantities of potentially toxic forage fish and marine invertebrates. Given rapid ocean warming and projected increases in HAB activity in the Arctic and Subarctic (e.g., Anderson et al., 2021, 2022; Vandersea et al., 2018), managers and biologists should be aware of potential future impacts on seabirds and, when possible, incorporate HAB toxin screening of bird and forage samples into die-off investigations. During known blooms, monitoring for unusual mortality among seabirds and other marine taxa is also warranted. Information on algal toxins in northern regions is lacking, meaning that such events are important to document even if sample sizes are limited. Additionally, experimental trials to identify physiological and behavioral effects, depuration rates, and cumulative impacts of STX and other PSTs on seabirds are needed to better understand the significance of HABs to wild bird populations.
Acknowledgments
We thank Amber Wendler and Frank Spellman for their assistance monitoring tern nests and collecting samples. Thanks to the Central Council of Tlingit and Haida Indian Tribes of Alaska as well as the Sitka Tribe of Alaska Environmental Research Lab. We acknowledge the collective contributions of the pathologists, epidemiologists, laboratorians, and technicians that worked on the referenced case reports at the USGS National Wildlife Health Center. D. Gerik assisted in the lab and J. Pearce supported development of HAB toxin testing capability at the USGS Alaska Science Center. This work was funded by the USGS Eco-systems Mission Area, U.S. Fish and Wildlife Service, U.S. Forest Service, and NOAA National Ocean Service, National Centers for Coastal Ocean Science program funds. Funding was also provided by the University of Alaska BLaST program: BLaST is supported by the NIH Common Fund, through the Office of Strategic Coordination, Office of the NIH Director with the linked awards: TL4GM118992, RL5GM118990, & UL1GM118991. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health but does represent the official views of USGS. This article has been peer reviewed and approved for publication consistent with the USGS Fundamental Science Practices (https://pubs.usgs.gov/circ/1367/). Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government. University of Alaska is an affirmative action/equal employment opportunity employer and educational institution: www.alaska.edu/nondiscrimination.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anderson DM, Alpermann TJ, Cembella AD, Collos Y, Masseret E, Montresor M, 2012. The globally distributed genus Alexandrium: multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 14, 10–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Fachon E, Pickart RS, Lin P, Fischer AD, Richlen ML, Uva V, Brosnahan ML, McRaven L, Bahr F, 2021. Evidence for massive and recurrent toxic blooms of Alexandrium catenella in the Alaskan Arctic. P. Natl. Acad. Sci. USA 118 (41) e2107387118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Fachon E, Hubbard K, Lefebvre KA, Lin P, Pickart R, Richlen M, Sheffield G, Van Hemert C, 2022. Harmful algal blooms in the Alaskan Arctic: an emerging threat as the ocean warms. Oceanography. 10.5670/oceanog.2022.121. [DOI] [Google Scholar]
- Baird PA, Gould PJ, Lensink C, Sanger G, Hatch S, 1983. The Breeding Biology and Feeding Ecology of Marine Birds in the Gulf of Alaska. U.S. Fish and Wildlife Service. [Google Scholar]
- Ben-Gigirey B, Soliño L, Bravo I, Rodríguez F, Casero MV, 2021. Paralytic and amnesic shellfish toxins impacts on seabirds, analyses and management. Toxins 13 (7), 454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer J, Green NW, Brooks S, Allan IJ, Ruus A, Gomes T, Bråte ILN, Schøyen M, 2017. Blue mussels (Mytilus edulis spp.) as sentinel organisms in coastal pollution monitoring: a review. Mar. Environ. Res. 130, 338–365. [DOI] [PubMed] [Google Scholar]
- Bricelj VM, Shumway SE, 1998. Paralytic shellfish toxins in bivalve molluscs: occurrence, transfer kinetics, and biotransformation. Rev. Fish Sci. 6 (4), 315–383. [Google Scholar]
- Deeds JR, Landsberg JH, Etheridge SM, Pitcher GC, Longan SW, 2008. Non-traditional vectors for paralytic shellfish poisoning. Mar. Drugs 6 (2), 308–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan CJ, Ku JC, Quilliam MA, Gill TA, 2008. Bacterial degradation of paralytic shellfish toxins. Toxicon 52 (1), 91–100. [DOI] [PubMed] [Google Scholar]
- Drent R, Klaassen M, Zwaan B, 1992. Predictive growth budgets in terns and gulls. Ardea 80 (1), 5–17. [Google Scholar]
- Dusek RJ, Smith MM, Van Hemert C, Shearn-Bochsler VI, Hall S, Ridge CD, Hardison DR, Kaler RS, Bodenstein BL, Hofmeister EK, 2021. Acute oral toxicity and tissue residues of saxitoxin in the mallard (Anas platyrhynchos). Harmful Algae 109, 102109. [DOI] [PubMed] [Google Scholar]
- Gibble CM, Kudela RM, Knowles S, Bodenstein B, Lefebvre KA, 2021. Domoic acid and saxitoxin in seabirds in the United States between 2007 and 2018. Harmful Algae 103, 101981. [DOI] [PubMed] [Google Scholar]
- Glibert PM, Icarus Allen J, Artioli Y, Beusen A, Bouwman L, Harle J, Holmes R, Holt J, 2014. Vulnerability of coastal ecosystems to changes in harmful algal bloom distribution in response to climate change: projections based on model analysis. Glob. Change Biol. 20 (12), 3845–3858. [DOI] [PubMed] [Google Scholar]
- Gobler CJ, Doherty OM, Hattenrath-Lehmann TK, Griffith AW, Kang Y, Litaker RW, 2017. Ocean warming since 1982 has expanded the niche of toxic algal blooms in the North Atlantic and North Pacific oceans. P. Natl. Acad. Sci. USA 114 (19), 4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallegraeff GM, Anderson DM, Belin C, Bottein MYD, Bresnan E, Chinain M, Enevoldsen H, Iwataki M, Karlson B, McKenzie CH, Sunesen I, 2021. Perceived global increase in algal blooms is attributable to intensified monitoring and emerging bloom impacts. Comm. Earth Environ. 2 (1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley JR, Lanphier K, Kennedy E, Whitehead C, Bidlack A, 2020a. Random forest classification to determine environmental drivers and forecast paralytic shellfish toxins in Southeast Alaska with high temporal resolution. Harmful Algae 99, 101918. [DOI] [PubMed] [Google Scholar]
- Harley JR, Lanphier K, Kennedy EG, Leighfield TA, Bidlack A, Gribble MO, Whitehead C, 2020b. The Southeast Alaska Tribal Ocean Research (SEATOR) partnership: Addressing data gaps in harmful algal bloom monitoring and shellfish safety in Southeast Alaska. Toxins 12 (6), 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch JJ, Gochfeld M, Burger J, Garcia EFJ, 2020. Arctic Tern (Sterna paradisaea). In: Billerman SM. (Ed.), Birds of the World. Cornell Lab of Ornithology, Ithaca, NY. 10.2173/bow.arcter.01. [DOI] [Google Scholar]
- Hendrix AM, Lefebvre KA, Quakenbush L, Bryan A, Stimmelmayr R, Sheffield G, Wisswaesser G, Willis ML, Bowers EK, Kendrick P, 2021. Ice seals as sentinels for algal toxin presence in the Pacific Arctic and subarctic marine ecosystems. Mar. Mamm. Sci. 37 (4), 1292–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indrasena W, Gill T, 2000. Storage stability of paralytic shellfish poisoning toxins. Food Chem. 71 (1), 71–77. [Google Scholar]
- Jones T, Divine LM, Renner H, Knowles S, Lefebvre KA, Burgess HK, Wright C, Parrish JK, 2019. Unusual mortality of Tufted Puffins (Fratercula cirrhata) in the eastern Bering Sea. PLoS One 14 (5), e0216532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen M, 1994. Growth and energetics of tern chicks from temperate and polar environments. The Auk 111 (3), 525–544. [Google Scholar]
- Klaassen M, Bech C, Masman D, Slagsvold G, 1989. Growth and energetics of Arctic Tern chicks (Sterna paradisaea). The Auk 106 (2), 240–248. [Google Scholar]
- Kvitek R, Bretz C, 2005. Shorebird foraging behavior, diet, and abundance vary with harmful algal bloom toxin concentrations in invertebrate prey. Mar. Ecol. Prog. Ser. 293, 303–309. [Google Scholar]
- Kvitek RG, 1991. Sequestered paralytic shellfish poisoning toxins mediate Glaucous-winged Gull predation on bivalve prey. The Auk 108 (2), 381–392. [Google Scholar]
- Kvitek RG, DeGunge AR, Beitler MK, 1991. Paralytic shellfish poisoning toxins mediate feeding behavior of sea otters. Limnol. Oceanogr. 36 (2), 393–404. [Google Scholar]
- Landsberg JH, 2002. The effects of harmful algal blooms on aquatic organisms. Rev. Fish Sci. 10 (2), 113–390. [Google Scholar]
- Landsberg JH, Lefebvre KA, Flewelling LJ, 2014. Effects of toxic microalgae on marine organisms. In: Rossini GP(Ed.), Toxins and Biologically Active Compounds from Microalgae. CRC Press, Boca Raton, pp. 379–449. [Google Scholar]
- Lawrence JF, Niedzwiadek B, Menard C, 2005. Quantitative determination of paralytic shellfish poisoning toxins in shellfish using prechromatographic oxidation and liquid chromatography with fluorescence detection: collaborative study. J. AOAC Int. 88 (6), 1714–1732. [PubMed] [Google Scholar]
- Lefebvre KA, Quakenbush L, Frame E, Huntington KB, Sheffield G, Stimmelmayr R, Bryan A, Kendrick P, Ziel H, Goldstein T, 2016. Prevalence of algal toxins in Alaskan marine mammals foraging in a changing arctic and subarctic environment. Harmful Algae 55, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewitus AJ, Horner RA, Caron DA, Garcia-Mendoza E, Hickey BM, Hunter M, Huppert DD, Kudela RM, Langlois GW, Largier JL, 2012. Harmful algal blooms along the North American west coast region: History, trends, causes, and impacts. Harmful Algae 19, 133–159. [Google Scholar]
- McCabe RM, Hickey BM, Kudela RM, Lefebvre KA, Adams NG, Bill BD, Gulland F, Thomson RE, Cochlan WP, Trainer VL, 2016. An unprecedented coastwide toxic algal bloom linked to anomalous ocean conditions. Geophys. Res. Lett. 43 (19), 10366–10376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore SK, Trainer VL, Mantua NJ, Parker MS, Laws EA, Backer LC, Fleming LE, 2008. Impacts of climate variability and future climate change on harmful algal blooms and human health. Environ. Health 7 (2), S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbet IC, 1983. Paralytic shellfish poisoning: effects on breeding terns, 85. Condor, pp. 338–345. [Google Scholar]
- Overland JE, Wang M, Ballinger TJ, 2018. Recent increased warming of the Alaskan marine Arctic due to midlatitude linkages. Adv. Atmos. Sci. 35 (1), 75–84. [Google Scholar]
- Piatt JF, Parrish JK, Renner HM, Schoen SK, Jones TT, Arimitsu ML, Kuletz KJ, Bodenstein B, García-Reyes M, Duerr RS, 2020. Extreme mortality and reproductive failure of Common Murres resulting from the northeast Pacific marine heatwave of 2014–2016. PLoS One 15 (1), e0226087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearn-Bochsler V, Lance EW, Corcoran R, Piatt J, Bodenstein B, Frame E, Lawonn J, 2014. Fatal paralytic shellfish poisoning in Kittlitz’s murrelet (Brachyramphus brevirostris) nestlings, Alaska, USA. J. Wildl. Dis. 50 (4), 933–937. [DOI] [PubMed] [Google Scholar]
- Shumway SE, Allen SM, Boersma PD, 2003. Marine birds and harmful algal blooms: sporadic victims or under-reported events? Harmful Algae 2 (1), 1–17. [Google Scholar]
- Smith MM, Van Hemert C, Gerik DE, 2022. Tissue concentrations and congener profiles of harmful algal toxins in seabirds, forage fish, and other organisms. U.S. Geological Survey data release. 10.5066/P9MLNP9H. [DOI] [Google Scholar]
- Starr M, Lair S, Michaud S, Scarratt M, Quilliam M, Lefaivre D, Robert M, Wotherspoon A, Michaud R, Ménard N, 2017. Multispecies mass mortality of marine fauna linked to a toxic dinoflagellate bloom. PLoS One 12 (5), e0176299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southeast Alaska Tribal Ocean Research, 2019. Public service announcement: Elevated levels of paralytic shellfish toxins in Southeast Alaska and Kodiak. Sitka, AK. August 15, 2019. https://www.seator.org/PDF_Documents/SEATOR%20PSA%20Elevated%20Levels%20of%20Paralytic%20Shellfish%20Toxins%20in%20Southeast%20Alaska%20and%20Kodiak%20August%2015%202019.pdf. [Google Scholar]
- Trainer VL, Moore SK, Hallegraeff G, Kudela RM, Clement A, Mardones JI, Cochlan WP, 2020. Pelagic harmful algal blooms and climate change: Lessons from nature’s experiments with extremes. Harmful Algae 91, 101591. [DOI] [PubMed] [Google Scholar]
- Trainer VL, Sullivan K, Le Eberhart B-T, Shuler A, Hignutt E, Kiser J, Eckert GL, Shumway SE, Morton SL, 2014. Enhancing shellfish safety in Alaska through monitoring of harmful algae and their toxins. J. Shellfish Res. 33 (2), 531–539. [Google Scholar]
- Uttley J, Tatner P, Monaghan P, 1994. Measuring the daily energy expenditure of free-living Arctic terns (Sterna paradisaea). The Auk 111 (2), 453–459. [Google Scholar]
- Turner AD, Tarnovius S, Hatfield RG, Teixeira Alves M, Broadwater M, Van Dolah F, Garcia-Mendoza E, Medina D, Salhi M, Goya AB, Barrera F, 2020. Application of six detection methods for analysis of paralytic shellfish toxins in shellfish from four regions within Latin America. Mar. Drugs 18 (12), 616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dolah FM, Fire SE, Leighfield TA, Mikulski CM, Doucette GJ, 2012. Determination of paralytic shellfish toxins in shellfish by receptor binding assay: collaborative study. J. AOAC Int. 95 (3), 795–812. [DOI] [PubMed] [Google Scholar]
- Van Hemert C, Dusek RJ, Smith MM, Kaler R, Sheffield G, Divine LM, Kuletz KJ, Knowles S, Lankton JS, Hardison DR, 2021. Investigation of algal toxins in a multispecies seabird die-off in the Bering and Chukchi seas. J. Wildl. Dis. 57 (2), 399–407. [DOI] [PubMed] [Google Scholar]
- Van Hemert C, Schoen SK, Litaker RW, Smith MM, Arimitsu ML, Piatt JF, Holland WC, Hardison DR, Pearce JM, 2020. Algal toxins in Alaskan seabirds: Evaluating the role of saxitoxin and domoic acid in a large-scale die-off of Common Murres. Harmful Algae 92, 101730. [DOI] [PubMed] [Google Scholar]
- Van Pelt TI, Piatt JF, Lance BK, Roby DD, 1997. Proximate composition and energy density of some North Pacific forage fishes. Comp. Biochem. Physiol. Part A Physiol. 118 (4), 1393–1398. [Google Scholar]
- Vandersea MW, Kibler SR, Tester PA, Holderied K, Hondolero DE, Powell K, Baird S, Doroff A, Dugan D, Litaker RW, 2018. Environmental factors influencing the distribution and abundance of Alexandrium catenella in Kachemak Bay and lower Cook Inlet, Alaska. Harmful Algae 77, 81–92. [DOI] [PubMed] [Google Scholar]
- Walsh JE, Thoman RL, Bhatt US, Bieniek PA, Brettschneider B, Brubaker M, Danielson S, Lader R, Fetterer F, Holderied K, 2018. The high latitude marine heat wave of 2016 and its impacts on Alaska. Bull. Amer. Meteor. Soc. 99 (1), S39–S43. [Google Scholar]
- Wekell JC, Hurst J, Lefebvre KA, 2004. The origin of the regulatory limits for PSP and ASP toxins in shellfish. J. Shellfish Res. 23 (3), 927–930. [Google Scholar]
- Wiese M, D’Agostino PM, Mihali TK, Moffitt MC, Neilan BA, 2010. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 8 (7), 2185–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]


