ABSTRACT
As part of the aging process, fibrotic changes, fatty infiltration, and parenchymal atrophy develop in the pancreas. The pancreatic duct also becomes wider with age. This article provides an overview of the diameter of the pancreatic duct in different age groups and different examination methods. Knowledge of these data is useful to avoid misinterpretations regarding the differential diagnosis of chronic pancreatitis, obstructive tumors, and intraductal papillary mucinous neoplasia (IPMN).
Key words: aging, diameter, ductal adenocarcinoma, EUS, pancreatic duct
INTRODUCTION
Between 1990 and 2019, the proportion of people over the age of 65 has increased from 6 to 11% worldwide, and the United Nations predicts an increase to 16% by 2050. In 17 countries, including Japan, Italy, Germany, and other industrialized nations, people over the age of 65 represent more than one-fifth of the population. However, the greatest increase in the proportion of older people in the total population from 2019 to 2050 is predicted to occur in low- and middle-income countries.[1] Parallel to the increase in life expectancy, medical treatment options for the elderly have also developed, at least in the high-income countries. The importance of the knowledge of aging processes of the human body, their connections with, and their differentiation from disease processes has, therefore, increased considerably over the last decades.
As all other organs, the pancreas undergoes a normal aging process.[2] The morphological changes associated with pancreatic aging have been investigated in autopsy studies and using imaging techniques. Fibrosis and parenchymatous fat degeneration occurs. In addition, parenchymal atrophy may develop.[3-5] Perfusion of the pancreas is diminished.[6] With increasing age, the anteroposterior diameter and parenchymal volume of the pancreas decline, while fat volume increases.[7-11] The pancreatic volume increases until the age of 20, then reaches a plateau and decreases again after the age of 60.[8] Lobulation of the pancreatic contour becomes more pronounced.[10,12] The parenchyma becomes stiffer, which is due to fibrosis.[13-15] Sonographically, the pancreatic parenchyma becomes progressively hyperechoic because of fatty infiltration.[16-20] This is an independent age-related process, although high body mass index (BMI) due to obesity is also an independent factor in pancreatic fatty degeneration [Figure 1].[16,20-23] Interestingly, this affects predominantly the embryologically dorsal parts of the pancreas, whereas the uncinate process is considerably less involved with lipomatosis.[20,23-25] Endosonography shows parenchymal changes mimicking early chronic pancreatitis increasing with age.[26,27] As a result of age-related morphologic changes, exocrine pancreatic insufficiency may occur.[28-32]
Figure 1.
A 75-year-old male. Arterial hypertension, diabetes mellitus type 2 requiring tablets, obesity, BMI 32. Sonography shows a hyperechogenic pancreas with lobulated contour. The diameter of the pancreatic duct is 1.9 mm in the pancreatic corpus. Independent factors for the hyperenhanced pancreatic parenchyma are age and BMI. BMI: Body mass index
The diameter of the main pancreatic duct (MPD) also increases with age.[27,33-35] This may present differential diagnostic difficulties, particularly in symptomatic patients. Differentiation from pancreatic duct dilatation due to chronic pancreatitis, pancreas divisum, benign stenosis of the papilla of Vater, periampullary duodenal diverticulum, solid tumors, or intraductal papillary mucinous neoplasia (IPMN) is important. Knowledge of these age-related changes is useful in the evaluation of imaging findings. We present in this paper data on the diameter of the pancreatic duct in aging.
AUTOPSY STUDIES
Schmitz-Moormann and Hain[36] described a significant correlation between ductal epithelial hyperplasia, intralobular fibrosis, and perilobular fibrosis in autopsy examinations of normal pancreas, and age. All these alterations increase with aging both in frequency and intensity, especially beyond the age of 60.[36] Beyond the age of fifty, the diameter of the pancreatic duct was 10%-13% larger than at younger ages.[37] The inner and outer diameters of the pancreatic duct rise steadily with aging. The relative wall thickness of the pancreatic duct declines. Anatomic measurements revealed an increase in the mean diameter of the pancreatic duct from 1.73 mm at an age of 20 years to 2.36 mm at the age of 80 years. Furthermore, the length of the pancreatic duct was found to increase with aging.[38] In their autopsy study with necropsy retrograde pancreatography, Kreel and Sandin[34] showed that the pancreatic duct diameter was >4 mm in the pancreatic head and 3.5 mm in the pancreatic body from the age of 50. After the 4th decade of life, the diameter of the pancreatic duct increased by 8% in each decade. In people over 80 years of age, the diameter of the pancreatic duct in the pancreatic head, corpus, and tail was 5.3 mm, 4.0 mm, and 2.1 mm, respectively. However, it must be considered that in necropsy retrograde pancreatography, the pancreatic duct system is very strongly filled with contrast medium up to the periphery, which is not a physiological situation and should never be done in ERCP or nonfilling imaging techniques such as EUS or magnetic resonance cholangiopancreatography (MRCP). In the elderly, the dilated pancreatic duct remained evenly tapered and had smooth borders. This is different from chronic pancreatitis or tumor-related prestenotic dilatation. In contrast to the general dilatation of the side branches in duct obstruction, only scattered and intermittent branch dilatations were detectable in “senile” ductal dilatation. In this study, no age-related change in the length of the pancreatic duct was observed,[34] which could be an issue of definition.
In another autopsy study with evaluation of postmortem ductograms, there was a significant age association with ductal epithelial hyperplasia and intralobular fibrosis as well as of ductal changes. Ductograms of the pancreatic duct in elders without preexisting pancreatic disease were misinterpreted by endoscopists experienced in ERCP as chronic pancreatitis in 42%. The changes were primarily in the small lateral ducts, which are not usually visualized during ERCP.[39] Thus, a methodological bias is likely.
In people over 60 years of age, fibrotic foci were found with increasing frequency in the pancreatic parenchyma at autopsy. This can also be reproduced in imaging [Figure 3]. Until the age of 60, these fibrotic foci were very rare. The fibrotic foci occurred in the periphery of the glands and involved one or two lobules. The acinar cells were replaced by connective tissue. In most cases, the pancreas contained more than one fibrotic focus. The fibrosis pattern corresponded to multifocal intralobular fibrosis, which was therefore named “Patchy Lobular Fibrosis in the Elderly” (PLFE).[40] Previous studies have already described an association between fibrosis and ductal papillary hyperplasia.[3] This “ductal papillary hyperplasia” was classified as pancreatic intraepithelial neoplasia type 1B (PanIN-1B). This PLFE was closely associated with PanIN-1B lesions in the excretory ducts, suggesting that narrowing of an excretory duct due to papillary hyperplasia of the epithelium may impede secretion and cause fibrosis of the drained lobule.[40] PanIN-1B lesions were found in only 10.3% of individuals between the age of 29 and 60 years of age, but in 62% of 60-86-year olds. In these individuals, PanIN-1B lesions were more frequently found in or near fibrotic lobules (80.6%) rather than in pancreatic lobules not affected by fibrosis. The association between PanIN-1B and fibrosis was statistically significant.[40]
Figure 3.
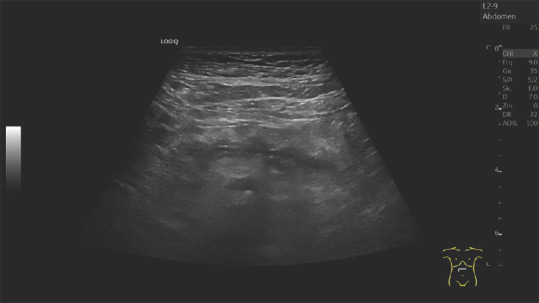
An 80-year-old male. Arterial hypertension, chronic obstructive pulmonary disease. The pancreas is hyperechoic. The pancreatic duct is of normal caliber. The parenchyma around the pancreatic duct is hypoechoic. This is an expression of periductal fibrosis
PANCREATIC DUCT ON IMAGING
Data to determine the diameter of the pancreatic duct are available from ultrasonography, computed tomography (CT), magnetic resonance imaging (MRI), and ERCP.[33,41-48] Ultrasound measurements of the normal diameter of the pancreatic duct have been performed by various authors. Normal values in the pancreatic head/body and tail are 3 mm - 2 mm - 1 mm.[19,44,45,47,49]
In ultrasound screening of more than 130,000 individuals in Japan, regular or irregular pancreatic duct dilatation was found in 0.49% of cases. No specific etiological factor for pancreatic duct dilatation was found in 81.5% of these individuals. It was significantly more common in males, and frequency increased with age in both sexes.[35] Another ultrasound screening study involved 200,000 mostly 40-60-year-old individuals. 0.19% had a pancreatic duct diameter in the pancreatic body >3 mm. In good agreement with the other screening cohort, no underlying disease could be identified in 75.5% of these individuals. A diameter up to 3.5 mm in the pancreatic body was reported as a cut-off for ductal dilation without pancreatic disease [Figure 2 and 4].[50]
Figure 2.
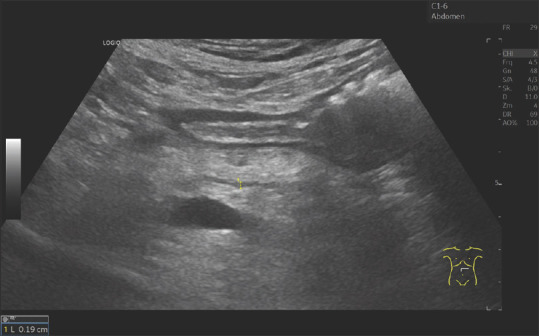
An 80-year-old female. Urinary tract infection, increased renal retention parameters. Narrow pancreatic parenchyma, organ atrophy. Prominent pancreatic duct with a diameter of 2.3 mm on the left pancreatic body
Figure 4.
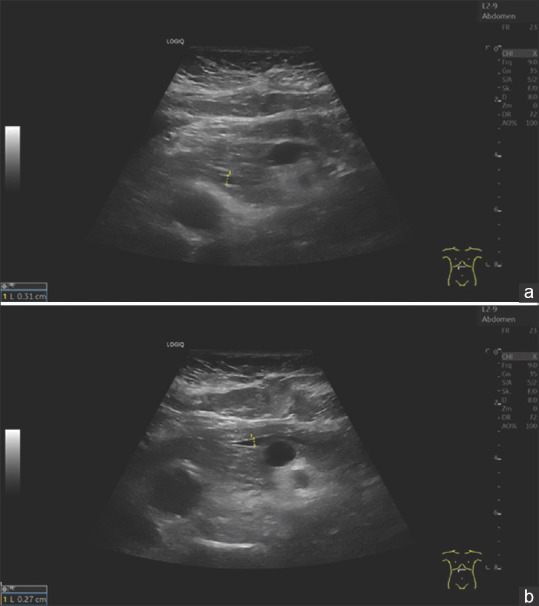
A 79-year-old female. Anemia assessment. The pancreatic parenchyma is narrow. The pancreatic duct is prominent. The duct is delineated at 3.1 mm at the head of the pancreas (a) and 2.7 mm at the body of the pancreas (b). The bile duct was normal, the papilla Vateri inconspicuous. There was no juxtapapillary diverticulum. No mass was found on the head of the pancreas
Tanaka et al. performed an ultrasound screening study including 10,610 hospital patients in 1997. In 10,244 patients without a diagnosis of pancreatic cancer followed until 1999 and with adequate visualization of the pancreatic body, a MPD diameter ≥2 mm or ≥3 mm was observed in 5.03% and 1.21% of cases. The incidence of slight MPD dilatation (≥2 mm/≥3 mm) increased with age: <25 years 0.8%/0.0%; 26-50 years: 2.4%/0.5%; 51-75 years: 5.4%/1.4%; ≥76 years: 10.4%/3.2%.[51] In ultrasound studies by Glaser et al.,[19,49] the diameter of the pancreatic duct in the proximal pancreatic body ranged from 1 to 3 mm (mean 1.9 mm). Individuals in the 20–29-year-old group had a smaller duct diameter, an average of 1.5 mm. A marked increase in duct diameter was observed in the 5th decade of life with an average pancreatic duct diameter of 1.9 mm in the 40–49-year-old group. In the older age groups, a further small increase in the dilatation of the pancreatic duct was observed, which was most pronounced in patients over 80 years of age with an average of 2.3 mm. Age-related dilatation of the duct was significant in subjects 40 years of age and older compared with subjects up to 39 years of age. However, the dilation did not exceed 3 mm, and further significant dilation with older age was not observed [Figure 5a and b].[19,49]
Figure 5.
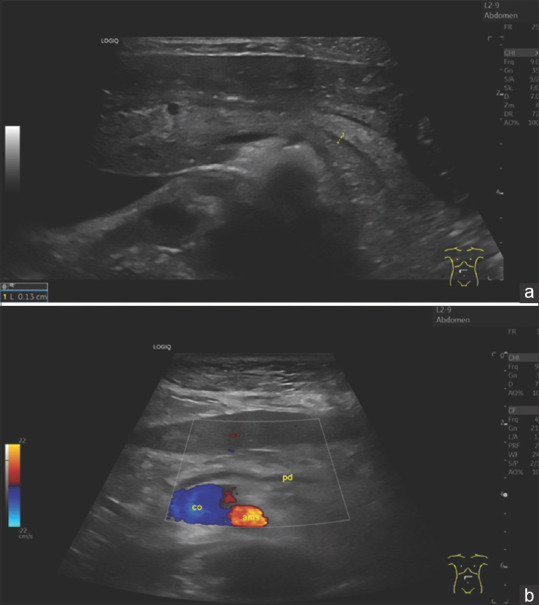
Image of the pancreas of a 29-year-old woman without complaints (as part of an ultrasound course) (a). The pancreas has a smooth border, normal size. The ventral part is slightly hypoechogenic compared to the rest of the parenchyma. The pancreatic duct is very slim and can only be delineated after various maneuvers. Here, visualisation is successful by bulging the abdomen. In contrast, figure (b) shows the age atrophic pancreas of a 96-year-old slim woman, BMI 20. The pancreatic duct is prominently wide. The remaining narrow parenchyma is bright, hyperechoic. Diabetes mellitus was not present. BMI: Body mass index
The age-related dilation of caliber was more pronounced in women than in men.[49] After intravenous injection of the hormone secretin, younger subjects showed a duct dilation of about 110% of the baseline diameter, and older subjects still showed a dilation of about 70%. This may be because older subjects already have a higher basal diameter and, more importantly, periductal fibrosis limits the flexibility of the pancreatic duct which begins in advanced age.[49] This is similar to, but less pronounced in patients with chronic pancreatitis. The latter show no increase in the diameter of the pancreatic duct at all after secretin application.[49] Wachsberg found that the diameter of the pancreatic duct may increase during deep inspiration in some adults without pancreatic disease [Figure 6a and b]. For this reason, different diameters can be determined during a sonographic examination. Whether this process decreases with aging has not been studied.[52]
Figure 6.
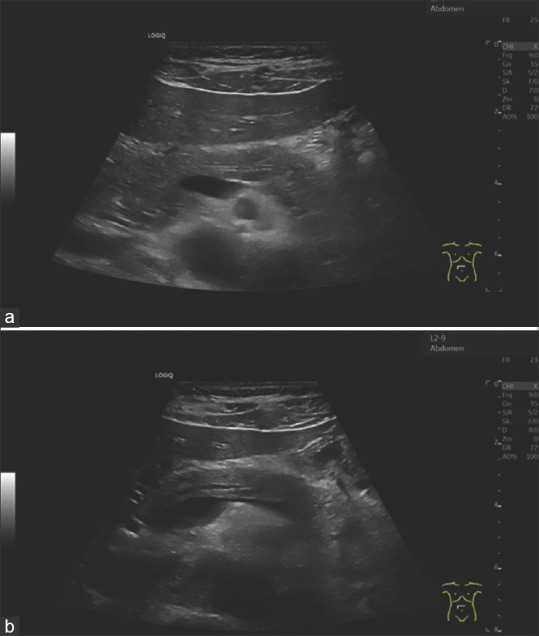
A 40-year-old male. Nonspecific paraumbilical complaints. Deep inspiration is also used in the effort to optimally adjust the pancreas. This shows an increase in the diameter of the pancreatic duct during deep inspiration (b) compared to normal breathing position (a)
In transcutaneous ultrasound due to the physics behind the method the pancreatic duct in the pancreatic body can be displayed in nearly every patient (without gastric surgery or ascites). This is due to the orthogonal orientation of the pancreatic duct in relation to the orientation of the ultrasound wave front. Thus, in transcutaneous ultrasound, a presentation of the pancreatic duct in the pancreatic tail or the head is a hint for dilatation as well.
On ERCP, the diameter of the pancreatic duct remained relatively constant until the 6th decade of life. An increase occurred in the 7th decade of life.[33] When the subjects were subdivided into those <40 years old and 40 years and older, the diameter of the MPD showed a significant increase in the head and body of the pancreas, but not in the tail of the advanced age group. The width of the pancreatic duct at the pancreatic head was 3.78 ± 0.97 mm in those over 40 years of age and 2.97 ± 0.71 mm in those under 40 years of age. At the pancreatic body, it was 2.86 ± 0.9 mm compared to 2.36 ± 0.51 mm. The width of the accessory duct also increased significantly with age.[33]
Beside pressure during injection, in ultrasound techniques measurement differences including or excluding the borders of the pancreatic duct (or even “leading edge” method must be taken into account for biases.
The ERCP study by Hastier et al.[48] evaluated the findings of patients over 70 years of age who had no pancreatic disease. The MPD diameters in head, body and tail in the 70 years and older group were 5.3, 3.7 and 2.6 mm. The increase in diameter in the older group was statistically significant for each region of the MPD compared to the control group of those under 50 years of age. In only 31.4% of the elderly patients was the diameter of the pancreatic duct within the established normal limits. In the majority of patients (63.3%) the dilatation was global, in a minority it was limited to the head and/or body. The increase in the diameter of the pancreatic duct in the pancreatic head was significant in all age groups from 70 to 99 years, while the increase in the duct diameter of the body and tail was significant in the subgroups of 70-79-and 80-89-year olds. The enlargement was most pronounced in the head of the pancreas. For the subgroups of patients in the 8th, 9th or 10th decade, a mean pancreatic duct diameter of 4.8 ± 1.3 mm/5.4 ± 2.1 mm/6.4 ± 1. 6 mm at the pancreatic head, 3.2 ± 1.1 mm/4.1 ± 1.7 mm/ 4.2 ± 1.4 mm at the pancreatic body, and 2.1 ± 0.8 mm/2.9 ± 1.4 mm/3.0 ± 1.2 mm at the pancreatic tail were reported. Dilatation of the side branches was observed in 26.7%.[48]
The maximum diameter of the normal MPD in the head, body, and tail in the Korean ERCP study by Kim et al.[46] was 3.2 mm, 2.5 mm, and 1.6 mm, respectively. These were values measured in people over 40 years of age. The mean MPD diameters in patients over 40 years of age were significantly greater compared with the corresponding measurements in patients <40 years of age. There was no correlation between ductal diameters and gender.[46]
Sato et al. found that there was no statistically significant association between pancreatic duct diameter visualized with MRI and aging. The duct could not be identified in 64%. There was no case of MPD diameter of more than 3 mm.[10] However, the authors observed that the anteroposterior diameter of the pancreas decreased significantly with age and that lobulation and parenchymal fat infiltration became more evident.[10]
The MRI/MRCP study by Wang et al.[53] showed that the increase in duct diameter occurs firstly in the pancreatic head and later in the pancreatic body: this process starts in the 40-49-year age group. In patients in the 6th decade, the duct is wider on the pancreatic body as well. All segments of the pancreatic duct in the age group of 80-89 years were also significantly larger than in the age group 20-29 years. In men, the size of the pancreatic gland first increased and then decreased with age, while the diameter of the pancreatic duct gradually increased with age. Compared with the 20-29 years age group, the head of the gland significantly increased in size at the ages of 40-49 and 50-59 years, and the body of the gland significantly increased in size at the ages of 60-69 years. All segments of the pancreas gland in the 80-89 years age group were significantly smaller than in the 20-29 years age group. In female patients, a similar abrupt decrease in gland size has been noted after the age of 80, with no increase in gland size in the decades prior. A statistically significant increase in pancreatic duct diameter compared with 20-29-year olds occurred in women at 70-79-and 80-89-year olds. In contrast, this statistically significant difference in men occurred only in the 80-89 years group. For all gender–age combinations, the largest change in gland and duct size occurred between the 70-79 and 80-89 age groups. This applies to both genders.[53]
In the MRI study by Elgasim et al.,[42] the mean pancreatic duct diameter for all age groups was determined to be 3.80 ± 0.50 mm. The duct was measured at its widest point, without specifying which area of the pancreas was involved. Already in the 20-30-year olds, the mean diameter was 3.50 ± 0.51 mm. The MPD diameter increased slightly up to the age of 60, and then decreased with increasing age. However, the results were not statistically significant. These data differ from MRI data from Wang et al. However, the group over 70 years of age was not further differentiated. A reasonable upper limit of 4 mm was defined for the pancreatic duct diameter for asymptomatic subjects without specifying to which area of the pancreas this diameter refers.[42]
Endosonography also showed that the diameter of the pancreatic duct at the pancreatic head and pancreatic body increased slightly with age. However, only at the pancreatic head the difference was significant in the three age groups <40 years, 40-60 years, and >60 years. At the pancreatic head, the measurements corresponding to the age groups were 2.0 (1.6-2.2)/2.4 (2.0-3.1)/2.9 (2.2-3.5) mm. In the pancreatic body, they were 1.3 (1.0-1.8)/1.6 (1.3-2.0)/1.8 (1.3-2.1) mm. And, in the pancreas tail, there was no difference 1.0 (0.9-1.2)/1.2 (1.0-1.6)/1.0 (1.0-1.3) mm.[26]
In contrast to the data from the MRI studies,[53] the diameters of the pancreatic head, pancreatic body, and pancreatic tail did not differ among the three age groups.[53] The group of 80-year olds, which showed atrophy by Wang et al.,[53] was not specifically represented here. At least one parenchymal and/or ductal abnormality was found in 28% of patients, with abnormalities increasing with age: 23% under 40 years, 25% between 40 and 60 years and 39% over 60 years. The most common abnormality was a hyperechoic strand, followed by lobular pattern accentuation, irregular ductal contour, echogenic foci, hyperechoic ductal wall, cyst(s), and side branch dilatation. Ductal stenosis, dilatation and stones were not found in any patient.[26] In the EUS study by Petrone et al.,[27] dilatation of the pancreatic duct was the only EUS finding significantly associated with age. However, only 3.3% of a patient collective had pancreatic duct dilatation >3 mm at the pancreatic head, >2 mm at the pancreatic body and >1 mm at the pancreatic tail. Patients with dilated MPD were significantly older (71.8 ± 7.7 years) than those without (63.2 ± 13.7 years).[27]
The diameter of the pancreatic duct in different examination procedures and age groups is shown in parts in Table 1.
Table 1.
Diameter of the pancreatic duct in different examination procedures and age groups
| Autor | Method | n | Age group (years) | Pancreatic duct (mm), pancreatic head | Pancreatic duct (mm), pancreatic body | Pancreatic duct (mm), pancreatic tail | Pancreatic duct (mm), without indication of localization |
|---|---|---|---|---|---|---|---|
| Glaser 1987[49]/2000[19] | US | 101 | All | 1.9 (1-3, SD = 0.5) | |||
| 18-29 | 1.5 ± 0.5 | ||||||
| 30-39 | 1.6 ± 0.5 | ||||||
| 40-49 | 1.9 ± 0.3 | ||||||
| 50-59 | 2.0 ± 0.5 | ||||||
| 60-69 | 2.1 ± 0.4 | ||||||
| 70-81 | 2.0 ± 0.5 | ||||||
| >80 | Mean 2.3 (mm) | ||||||
| Rajan 2005[26] | EUS | 120 | <40 | 2.0 (1.6-2.2) | 1.3 (1.0-1.8) | 1.0 (0.9-1.2) | |
| 40-60 | 2.4 (2.0-3.1) | 1.6 (1.3-2.0) | 1.2 (1.0-1.6) | ||||
| >60 | 2.9 (2.2-3.5) | 1.8 (1.3-2.1) | 1.0 (1.0-1.3) | ||||
| Wang 2019[53] | MRI | 140, males | All | 1.99 ± 0.50 | 1.57 ± 0.35 | 1.27 ± 0.23 | |
| 20-29 | 1.74 ± 0.35 | 1.43 ± 0.16 | 1.19 ± 0.16 | ||||
| 80-89 | 2.59 ± 0.40 | 1.96 ± 0.22 | 1.45 ± 0.27 | ||||
| 140, females | All | 1.99 ± 0.57 | 1.53 ± 0.33 | 1.27 ± 0.24 | |||
| 20-29 | 1.78 (0.29) | 1.35 ± 0.11 | 1.19 ± 0.17 | ||||
| 80-89 | 2.44 (0.44) | 1.78 ± 0.27 | 1.41 ± 0.19 | ||||
| Elgasim 2020[42] | MRI | 80 | 20-30 | 3.50 ± 0.51 | |||
| >70 | 3.68 ± 0.47 | ||||||
| Anand 1989[33] | ERCP | 55 | <40 | 2.97 ± 0.71 | 2.36 ± 0.51 | 1.23 ± 0.38 | |
| >40 | 3.78 ± 0.97 | 2.86 ± 0.9 | 1.15 ± 0.37 | ||||
| Hastier 1998[48] | ERCP | 136 | <50 | 3.3 ± 1.2 | 2.3 ± 0.7 | 1.6 ± 0.4 | |
| 70-79 | 4.8 ± 1.3 | 3.2 ± 1.1 | 2.1 ± 0.8 | ||||
| 80-89 | 5.4 ± 2.1 | 4.1 ± 1.7 | 2.9 ± 1.4 | ||||
| 90-99 | 6.4 ± 1.6 | 4.2 ± 1.4 | 3.0 ± 1.2 | ||||
| Kim 2002[46] | ERCP | 4097 | 15-39 | ||||
| Maximal ± SD | 2.6 ± 1.0 | 2.0 ± 0.7 | 1.4 ± 0.5 | ||||
| Midportion ± SD | 2.2 ± 0.9 | 1.9 ± 0.7 | 1.2 ± 0.5 | ||||
| All >40 | |||||||
| Maximal ± SD | 3.2 ± 1.2 | 2.5 ± 2.5 | 1.6 ± 0.7 | ||||
| Midportion ± SD | 2.7 ± 1.0 | 2.2 ± 0.9 | 1.4 ± 0.6 | ||||
| >70 | |||||||
| Maximal ± SD | 3.6 ± 1.3 | 2.4 ± 2.3 | 1.8 ± 0.8 | ||||
| Midportion ± SD | 3.1 ± 1.1 | 2.5 ± 1.0 | 1.6 ± 0.7 |
Mean±SD. SD: Standard deviation; MRI: Magnetic resonance imaging; US: Ultrasound
AGING AND PANCREATIC CANCER: A CALL FOR CAUTION
Pancreatic cancer is a disease affecting predominantly older age groups. Approximately 90% of pancreatic cancers are diagnosed in patients >55 years of age. Incidence is increasing with age with highest incidence reported in people older than 70 years. Globally the 5-years-survival is only 9%.[54-56] Detection in early stages is rare, but 5-year-survival of patients with resected small (up to 10 mm), early detected pancreatic cancer was reported as 36% in the large U.S. Surveillance, Epidemiology and End Results (SEER) cohort[57] and 68.7% (Stage Ia) or 59.7% (Stage Ib) in the Japanese Pancreatic Cancer registry.[58,59] Among patients with early-stage pancreatic cancer with a median overall survival of 8.7 months, the median survival decreased with advancing age, from 11.2 months for ages 66 to 69 years to 4.8 months for the age-group >85 years.[60]
Two of the three known precursor lesions of pancreatic cancer are related to age: Pancreatic intraepithelial neoplasia type 3 (PanIN-3) and intraductal papillary mucinous neoplasia (IPMN) of both side-branch-and main-duct-type.[61,62] PanIN-3 lesions (classified also as carcinoma in situ) were observed in an autopsy study in 4% of 173 consecutive cases (mean age 80.5 years), and frequency was associated with diabetes mellitus and/or increasing age. Interestingly, PanIN3 lesions occurred always multifocally, and affected predominantly branch ducts of the pancreatic body and tail and were associated with small (<10 mm) cystic changes in 71% of cases.[33,63-65] Moreover, an association of PanIN lesions with fibrosis and multilobular pancreatic parenchymal atrophy is evident from several studies, and there seems to be a link to pancreatic carcinogenesis.[40,66-68]
SLIGHT DILATATION OF THE MAIN PANCREATIC DUCT AS A PREDICTOR OF EARLY PANCREATIC CANCER
Japanese data have shown that slight dilatation of the MPD is observed in a high percentage of very early pancreatic cancer cases. In 200 cases with Stage 0 (Carcinoma in situ) and stage I pancreatic cancer, MPD dilatation was observed in 74.8%, 79.6%, 82.7% and 88.4% of cases on abdominal ultrasound (US), CT, MRI, and EUS, respectively.[59] Similar results with a somewhat lower rate of MPD dilatation with US (61%), CT or MRI (73% each), and EUS (77%) were reported in a Japanese multicentre study including 69 patients with early pancreatic cancer.[69] Okaniwa et al. report comparable data provided by other Japanese authors.[70]
In the large-scale screening US study described previously including 10,610 individuals, Tanaka et al. also reported retrospectively on a group of 39 patients (51-75 years) operated for pancreatic cancer with an abdominal ultrasound examination performed for reasons not related to suspicion of pancreatic disease ≥1 year pre-operatively. A MPD diameter ≥2 mm or ≥3 mm was reported in 84.6% or 82.1% of these pre-cancer patients 1-12 month before pancreatic cancer surgery (n = 39, mean diameter 4.5 mm), in 82.1% or 51.3% ≥1 year before surgery (n = 39; mean diameter 3.8 mm), in 67.9% or 46.2% ≥2 years before surgery (n = 28; mean diameter 3.5 mm), and in 64.7% or 35.3% ≥4 years before surgery (n = 17; mean diameter 3.0 mm). Compared to the large screening group without concomitant or later diagnosis of pancreatic cancer, MPD dilatation ≥2 mm predicted diagnosis of pancreatic cancer ≥2 years later with an Odds ratio of 37.4.[51] The same group of authors prospectively reported a significant increased risk of pancreatic cancer in subjects with US-detected slight MPD dilatation ≥2.5 mm (Hazard ratio 6.38). A second independent predictor of pancreatic cancer was the presence of pancreatic cysts ≥5 mm (Hazard ratio 6.23). The cumulative 5-year risk of pancreatic cancer was 1.84% in individuals with MPD dilatation ≥2.5 mm, 2.65% in individuals with pancreatic cyst(s), and 5.62% in individuals with a combination of both ultrasound findings.[71] However, MPD dilatation predicts not only pancreatic cancer, but also various other pancreatic diseases. In a cross-sectional ultrasound screening study of a large, presumed healthy Japanese population (n = 281,384), MPD dilatation ≥3 mm was detected in 0.19% of cases. Detailed further examination of these cases revealed the presence of underlying pancreatic pathology in 24.8% of these patients, including pancreatic cysts (15.6%), chronic pancreatitis (4.9%) and pancreatic cancer (1.3%).[50] In 498 individuals with MPD dilatation ≥2.5 mm and/or pancreatic cysts ≥5 mm at baseline ultrasound, long-term ultrasound surveillance (≥3 years; median 5.9 years) was performed and revealed pancreatic cancer in 11 patients (2.3%).[72]
The role of slight MPD dilatation as an important indirect imaging finding preceding pancreatic cancer and facilitating its early diagnosis was also supported by smaller studies using CT.[73-75]
DISCUSSION
With aging, not only fibrosis, parenchymal fatty degeneration and atrophy occur in the pancreas, but the pancreatic duct diameter also increases. Mild focal or segmental ductal ectasia of the main pancreatic main duct and/or its side branches are part of the spectrum of age-related pancreatic changes. This process starts at the head of the pancreas and subsequently involves the pancreatic body. However, no values above 3 mm were measured on ultrasound. Although an increase with age was described using all procedures, the extent of age-related ductal dilatation varied depending on the imaging modality. The largest values were measured in autopsy studies using pancreatography, which seems likely to be related to the fact that here the duct was completely filled with contrast medium up to the side branches. Consequently, the ductal diameters were less wide in ERCP but still larger than in ultrasonography or MRI. ERCP is now a therapeutic procedure and diagnosis of the pancreatic duct pathology uses noninvasive methods. In clinical practice, the question arises as to the tolerance range within which the diameter of the pancreatic duct may be found. In the ultrasound studies by Glaser et al., the diameter of the pancreatic duct was no larger than 3 mm.[19,49] In the MRI study by Wang et al.,[53] statistically relevant changes from 20 to 29 years of age occurred only after 80 years of age in men and from 70 years of age in women. However, this was primarily a tendential increase, not a striking dilatation of the pancreatic duct.[53] Elgasim et al.[42] cited a diameter of 4 mm on MRI without specifying whether at the pancreatic head or other location.[42]
In endosonography, a maximum of 3.5 mm was measured at the pancreatic head in patients over 60 years of age [Figure 7].[26]
Figure 7.
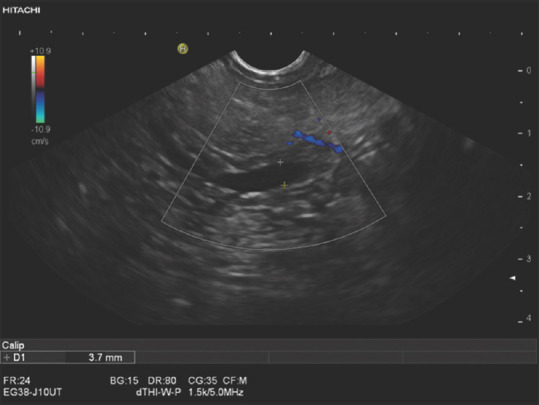
An 80-year-old female. Endosonography was performed to exclude choledocholithiasis. In the hypoechoic ventral part, the pancreatic duct had a maximum diameter of 3.7 mm. There was no outflow obstruction in the periampullary pancreatic parenchyma or in the papillary region. The duct was normal in the pancreatic body and tail
In age-related ductal dilatation, the duct is uniformly tapering and only single side branches are dilated [Figure 8].[34] In contrast, caliber variations are seen in chronic pancreatitis. In obstructing tumors, the upstream duct is usually markedly uniformly dilated, and the upstream side branches are also dilated.
Figure 8.
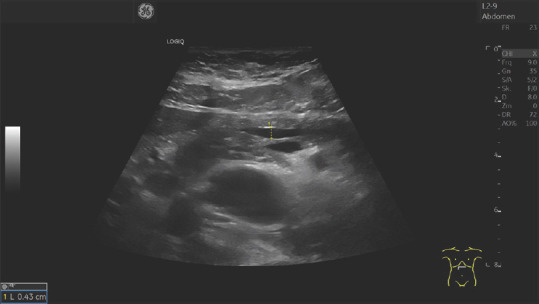
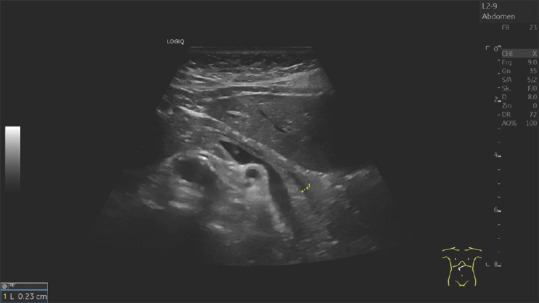
A 79-year-old female. Nonspecific upper abdominal discomfort. The pancreatic duct was up to 4.3 mm wide in the pancreatic body. Sonography and endosonography diagnosed no tumor. However, the conspicuous finding should be a reason for sonographic follow-up
Finally, the question arises as to the cause of the increase in the diameter of the pancreatic duct. It is still unknown whether there is a correlation between pancreas size and ductal diameter. The results of Wang et al.[53] suggest that the two processes responsible for changes in parenchymal size and ductal diameter may be occurring asynchronously. It has been documented that the decrease in pancreatic size is secondary to fatty infiltration and fibrosis of the parenchyma, whereas the dilation of the pancreatic duct is more likely the result of epithelial hyperplasia, periductal fibrosis, and cystic dilation secondary to atrophy of the gland. This finding also suggests that dilatation of the pancreatic duct should not always be expected when pancreatic atrophy is present and vice versa.[53]
Detlefsen et al. assigned a key position to ductal papillary hyperplasia, classified as pancreatic intraepithelial neoplasia type 1B (PanIN-1B), in all aging processes in the pancreas.[40] Parenchymal atrophy is discussed as a cause. However, pancreatic ductal dilatation was first seen at the pancreatic head, whereas parenchymal atrophy was described at the pancreatic tail rather than at the pancreatic head. In tumor-related pancreatic duct obstruction preceded by dilatation, atrophy is a secondary process. Other causes discussed include adenomatous fibrosis of the sphincter Oddi with obstruction of the outflow, reflux from the bile duct into the pancreatic duct, and increased viscosity of pancreatic secretions with age.[48]
CONCLUSIONS
A prominent pancreatic duct in advanced age does not automatically mean a pathological process. On the other hand, slight MPD dilatation is associated with pancreatic pathology in nearly one fourth of individuals[50] and may be the earliest imaging sign of small and potentially curable ductal pancreatic adenocarcinoma [Figures 9-11].[58,59] Therefore, in all patients with a dilated MPD above the age average more than 2 mm for under 50-year-old and more than 2.5 mm for over 50-year-old, a systematic and thorough evaluation of the whole pancreas should be attempted. In particular, the dilated pancreatic duct should be followed to the region of the papilla to detect strictures, abrupt caliber changes, focal parenchymal atrophy, focal fatty changes, hypoechoic regions surrounding MPD strictures, anatomical variations of the duct, cystic pancreatic lesions, criteria of chronic pancreatitis (in particular honeycombing and hyperechoic reflexes with shadowing) and pathology of the papilla of Vater.[76-82] Positional and respiratory variations in the MPD diameter should be considered.[52,83] Japanese authors have suggested a dedicated examination protocol for pancreatic US including a semi-sitting position, liquid-filled stomach and several standard sections.[70,72,84,85] EUS has been shown to be the most sensitive nearly noninvasive imaging modality to clarify the underlying cause of a dilated MPD, to detect and characterize small solid pancreatic lesions using indirect and direct criteria, to evaluate the periampullary region and to diagnose early chronic pancreatitis.[69,79,81,82,86-96] The question whether curvilinear or radial echoendoscopes are better suited to clarify small pancreatic lesions and the cause of ductal caliber changes is answered controversially.[82,97,98] The efficacy of long-term follow-up of patients with slight MPD dilatation warrants further study. Ultimately, all decisions should be made in the overall context of the patients’ clinical symptoms and complaints, as well as the extent of pancreatic parenchymal and ductal changes. Modern ultrasound diagnostics, high-resolution EUS and MRI are available for this purpose.
Figure 9.
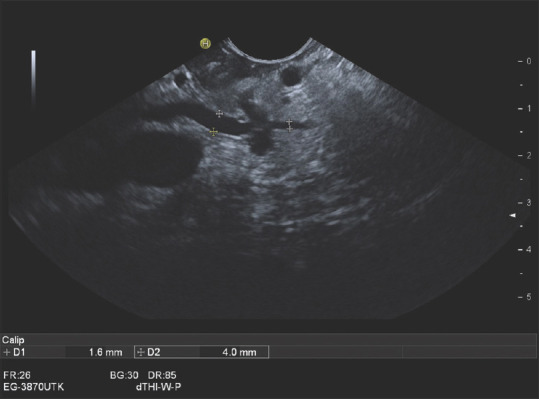
A 72-year-old female. Acute pancreatitis. Imaging showed a prominent pancreatic duct on the left pancreas. Endosonography diagnosed a small infiltrative process on the left-sided pancreas. The upstream pancreatic duct was up to 4 mm wide. As this infiltrative process was not visible on imaging, EUS-FNA was performed, which revealed a ductal adenocarcinoma. Left pancreatic resection was performed. Histologically, the patient had a T1N0M0 stage
Figure 11.
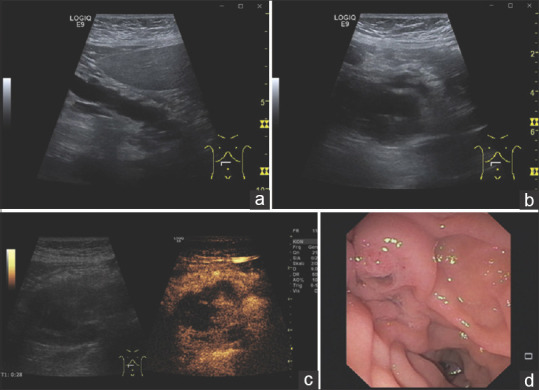
An 82-year-old female. Upper abdominal discomfort and mild lipemia. ultrasonography demonstrates a dilated pancreatic duct (a). At the head of the pancreas, an anechoic lesion appears, which contains internal structures (b). In CEUS, the internal structures are contrast-enhanced and thus correspond to solid tumor tissue (c). Endoscopy with a side-view duodenoscope diagnoses a fish mouth papilla (d). The primary cause of the pancreatic duct dilatation is a main duct intrapapillary mucinous neoplasia (MD IPMN) with solid tumor structures in the pancreatic head. CEUS: Contrast-enhanced ultrasound
Figure 10.
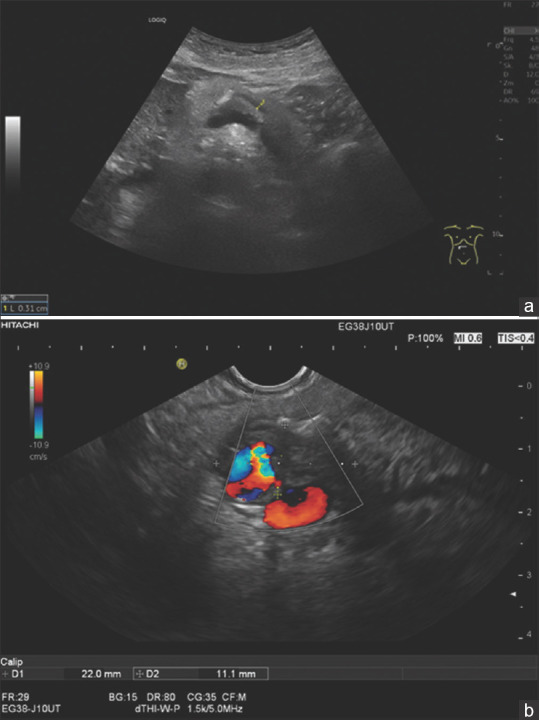
A 78-year-old female. Secondary event of acute pancreatitis. Sonographically, there was a prominent pancreatic (3, 1 mm) duct with a single calcification in the pancreatic body (a). Endosonographically (b) there was a 22 × 11 mm hypoechoic infiltrative process adjacent to the pancreatic duct and calcification which walled off the splenic artery
Recommendation as to what is normal for each age range
In general, normal values for the pancreatic duct in the pancreatic head/pancreatic corpus/and pancreatic tail are given as up to 3 mm/2 mm/1 mm. However, the reference values differ in various studies depending on the examination method and age.[19,26,33,41,42,46,48,49,53]
In ultrasonography in most cases, the measurement is taken in the pancreatic body. The largest difference occurred after the age of 40 years. The diameter of the pancreatic duct in the pancreatic body was 1.6 ± 0.5 mm (1-2 mm) in those under 35, 1.9 ± 0.3 mm in those aged 40-49 and 1.9 ± 0.4 mm (1.5-2.5 mm) in those over 50 years. Therefore, a diameter of 2 mm in the pancreatic body seems plausible as a limit value for those under 40 years of age, and an upper limit of 2.5 mm is acceptable for those over 50 years of age.[19,49]
In endosonography, the maximum measured values at the head of the pancreas were 2.2 mm in under 40-year olds, 3.1 mm in 40-60-year olds, and 3.5 mm in over 60-year olds. In those under 40, the maximum diameter in the pancreatic body was 1.8 mm; in 40-60-year olds, 2.0 mm; and in those over 60, 2.1 mm. The mean values for the tail of the pancreas were 1 mm for those under 40, 1.2 mm for those aged 40-60 and 1.2 mm for those over 60. A maximum value of 1.6 mm was not exceeded.[26]
On MRI, there was a statistically significant increase in diameter at the head of the pancreas after the age of 40 and at the body of the pancreas after the age of 50. The mean increase in width was 0.1 mm. On MRI, the upper values on average for all age groups were 2.6 mm at the head of the pancreas, <2 mm at the body of the pancreas and <1.5 mm at the tail of the pancreas. In the MRI study by Wang et al., the normal values for the pancreatic duct at the pancreatic head/pancreatic body/pancreatic tail were a maximum of 2.6 mm/2 mm/1.5 mm in women younger than 80 years; a maximum of 3 mm/2.1 mm/1.6 mm in women older than 80 years; and correspondingly, a maximum of 2.5 mm/2 mm/1.5 mm in men younger than 80 years and 3 mm/2.1 mm/1.6 mm in men older than 80 years.[53]
What is definitely abnormal
In Tanaka et al.,[51] >80% of patients had a pancreatic duct diameter of more than 2 mm up to 2 years before the diagnosis of pancreatic carcinoma. A pancreatic duct diameter >2,5 mm in those over 50-year-old in the pancreatic body should therefore be a reason for follow-up checks. A control at 6-month intervals seems reasonable. In case of an increase of the diameter in the course, concomitant complaints further diagnostics with endosonography and/or MRI should be performed. The simultaneous presence of an abrupt caliber change should be an indication for intensified diagnostics with endosonography and MRI. Unless a mass is detected on endosonography in the absence of chronic pancreatitis, a tumor can be excluded with a negative predictive value (NPV) of 100%.[99]
Definitely abnormal are segmental dilatation of the pancreatic duct due to pancreatic duct stenosis. A pancreatic duct dilatation with simultaneous parenchymal atrophy, with dilatation of the lateral branches of the pancreatic duct, cystic lesions and also a simultaneous dilatation of the common bile duct (double-duct sign) need to be clarified. Last but not least parenchyma criteria of chronic pancreatitis. Simultaneous evidence of hypoechoic changes in the parenchyma around ductal stenosis is highly tumor suspicious.
What needs further study
It would be interesting to know how frequently the pancreatic duct can be visualized with modern ultrasound equipment and use of linear transducers at the pancreatic head and tail.
Do patients with pancreatic ductal diameters >2 or 3 mm at long-term follow-up of more than 4 years have an increased risk of carcinoma? How long do you need to monitor these patients?
Financial support and sponsorship
Nil.
Conflicts of interest
Siyu Sun is the Editor-in-Chief of the journal; Christoph F. Dietrich is a Co-Editor-in-Chief; Christian Jenssen, Michael Hocke and Julio Iglesias-Garcia are Editorial Board Members. This article was subject to the journal’s standard procedures, with peer review handled independently of the editors and their research groups.
REFERENCES
- 1.United Nations. World Population Ageing 2019:Highlights. New York: United Nations; 2019. [Google Scholar]
- 2.Löhr JM, Panic N, Vujasinovic M, et al. The ageing pancreas:A systematic review of the evidence and analysis of the consequences. J Intern Med. 2018;283:446–60. doi: 10.1111/joim.12745. [DOI] [PubMed] [Google Scholar]
- 3.Stamm BH. Incidence and diagnostic significance of minor pathologic changes in the adult pancreas at autopsy:A systematic study of 112 autopsies in patients without known pancreatic disease. Hum Pathol. 1984;15:677–83. doi: 10.1016/s0046-8177(84)80294-4. [DOI] [PubMed] [Google Scholar]
- 4.Olsen TS. Lipomatosis of the pancreas in autopsy material and its relation to age and overweight. Acta Pathol Microbiol Scand A. 1978;86:367–73. doi: 10.1111/j.1699-0463.1978.tb02058.x. [DOI] [PubMed] [Google Scholar]
- 5.Smith ZL, Nickel KB, Olsen MA, et al. Type of sedation and the need for unplanned interventions during ERCP:Analysis of the clinical outcomes research initiative national endoscopic database (CORI-NED) Frontline Gastroenterol. 2020;11:104–10. doi: 10.1136/flgastro-2019-101175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsushima Y, Kusano S. Age-dependent decline in parenchymal perfusion in the normal human pancreas:Measurement by dynamic computed tomography. Pancreas. 1998;17:148–52. doi: 10.1097/00006676-199808000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Heuck A, Maubach PA, Reiser M, et al. Age-related morphology of the normal pancreas on computed tomography. Gastrointest Radiol. 1987;12:18–22. doi: 10.1007/BF01885094. [DOI] [PubMed] [Google Scholar]
- 8.Saisho Y, Butler AE, Meier JJ, et al. Pancreas volumes in humans from birth to age one hundred taking into account sex, obesity, and presence of type-2 diabetes. Clin Anat. 2007;20:933–42. doi: 10.1002/ca.20543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caglar V, Songur A, Yagmurca M, et al. Age-related volumetric changes in pancreas:A stereological study on computed tomography. Surg Radiol Anat. 2012;34:935–41. doi: 10.1007/s00276-012-0988-x. [DOI] [PubMed] [Google Scholar]
- 10.Sato T, Ito K, Tamada T, et al. Age-related changes in normal adult pancreas:MR imaging evaluation. Eur J Radiol. 2012;81:2093–8. doi: 10.1016/j.ejrad.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Koç U, Taydaş O. Evaluation of pancreatic steatosis prevalence and anthropometric measurements using non-contrast computed tomography. Turk J Gastroenterol. 2020;31:640–8. doi: 10.5152/tjg.2020.19434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sartoris R, Calandra A, Lee KJ, et al. Quantification of Pancreas surface lobularity on CT:A feasibility study in the normal pancreas. Korean J Radiol. 2021;22:1300–9. doi: 10.3348/kjr.2020.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janssen J, Papavassiliou I. Effect of aging and diffuse chronic pancreatitis on pancreas elasticity evaluated using semiquantitative EUS elastography. Ultraschall Med. 2014;35:253–8. doi: 10.1055/s-0033-1355767. [DOI] [PubMed] [Google Scholar]
- 14.Kolipaka A, Schroeder S, Mo X, et al. Magnetic resonance elastography of the pancreas:Measurement reproducibility and relationship with age. Magn Reson Imaging. 2017;42:1–7. doi: 10.1016/j.mri.2017.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chantarojanasiri T, Hirooka Y, Kawashima H, et al. Age-related changes in pancreatic elasticity:When should we be concerned about their effect on strain elastography? Ultrasonics. 2016;69:90–6. doi: 10.1016/j.ultras.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Worthen NJ, Beabeau D. Normal pancreatic echogenicity:Relation to age and body fat. AJR Am J Roentgenol. 1982;139:1095–8. doi: 10.2214/ajr.139.6.1095. [DOI] [PubMed] [Google Scholar]
- 17.Choi CW, Kim GH, Kang DH, et al. Associated factors for a hyperechogenic pancreas on endoscopic ultrasound. World J Gastroenterol. 2010;16:4329–34. doi: 10.3748/wjg.v16.i34.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marks WM, Filly RA, Callen PW. Ultrasonic evaluation of normal pancreatic echogenicity and its relationship to fat deposition. Radiology. 1980;137:475–9. doi: 10.1148/radiology.137.2.7433680. [DOI] [PubMed] [Google Scholar]
- 19.Glaser J, Stienecker K. Pancreas and aging:A study using ultrasonography. Gerontology. 2000;46:93–6. doi: 10.1159/000022141. [DOI] [PubMed] [Google Scholar]
- 20.Coulier B. Hypoechogenic aspects of the ventral embryonic cephalic pancreas:A large prospective clinical study. J Belge Radiol. 1996;79:120–4. [PubMed] [Google Scholar]
- 21.Okada K, Watahiki T, Horie K, et al. The prevalence and clinical implications of pancreatic fat accumulation identified during a medical check-up. Medicine (Baltimore) 2021;100:e27487. doi: 10.1097/MD.0000000000027487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kühn JP, Berthold F, Mayerle J, et al. Pancreatic steatosis demonstrated at MR imaging in the general population:Clinical relevance. Radiology. 2015;276:129–36. doi: 10.1148/radiol.15140446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coulier B. Pancreatic lipomatosis:An extensive pictorial review. J Belg Soc Radiol. 2016;100:39. doi: 10.5334/jbr-btr.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schmitz-Moormann P, Pittner PM, Heinze W. Lipomatosis of the pancreas. A morphometrical investigation. Pathol Res Pract. 1981;173:45–53. doi: 10.1016/S0344-0338(81)80006-4. [DOI] [PubMed] [Google Scholar]
- 25.Atri M, Nazarnia S, Mehio A, et al. Hypoechogenic embryologic ventral aspect of the head and uncinate process of the pancreas: In vitro correlation of US with histopathologic findings. Radiology. 1994;190:441–4. doi: 10.1148/radiology.190.2.8284396. [DOI] [PubMed] [Google Scholar]
- 26.Rajan E, Clain JE, Levy MJ, et al. Age-related changes in the pancreas identified by EUS:A prospective evaluation. Gastrointest Endosc. 2005;61:401–6. doi: 10.1016/s0016-5107(04)02758-0. [DOI] [PubMed] [Google Scholar]
- 27.Petrone MC, Arcidiacono PG, Perri F, et al. Chronic pancreatitis-like changes detected by endoscopic ultrasound in subjects without signs of pancreatic disease:Do these indicate age-related changes, effects of xenobiotics, or early chronic pancreatitis? Pancreatology. 2010;10:597–602. doi: 10.1159/000314599. [DOI] [PubMed] [Google Scholar]
- 28.Rothenbacher D, Löw M, Hardt PD, et al. Prevalence and determinants of exocrine pancreatic insufficiency among older adults:Results of a population-based study. Scand J Gastroenterol. 2005;40:697–704. doi: 10.1080/00365520510023116. [DOI] [PubMed] [Google Scholar]
- 29.Hedström A, Haas SL, Berger B, et al. Frequency of exocrine pancreatic insufficiency in 1105 patients with gastrointestinal symptoms. Pancreatology. 2015;15:S74. [Google Scholar]
- 30.Wang L, Zheng S. Pancreatic senescence and its clinical manifestations. Aging Med (Milton) 2020;3:48–52. doi: 10.1002/agm2.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kromrey ML, Friedrich N, Hoffmann RT, et al. Pancreatic steatosis is associated with impaired exocrine pancreatic function. Invest Radiol. 2019;54:403–8. doi: 10.1097/RLI.0000000000000554. [DOI] [PubMed] [Google Scholar]
- 32.Krill JT, Szafron D, Elhanafi S, et al. Endoscopic ultrasound finding of diffuse echogenicity in the pancreas, is it relevant? Dig Dis Sci. 2022;67:3244–51. doi: 10.1007/s10620-021-07181-1. [DOI] [PubMed] [Google Scholar]
- 33.Anand BS, Vij JC, Mac HS, et al. Effect of aging on the pancreatic ducts:A study based on endoscopic retrograde pancreatography. Gastrointest Endosc. 1989;35:210–3. doi: 10.1016/s0016-5107(89)72760-7. [DOI] [PubMed] [Google Scholar]
- 34.Kreel L, Sandin B. Changes in pancreatic morphology associated with aging. Gut. 1973;14:962–70. doi: 10.1136/gut.14.12.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikeda M, Sato T, Morozumi A, et al. Morphologic changes in the pancreas detected by screening ultrasonography in a mass survey, with special reference to main duct dilatation, cyst formation, and calcification. Pancreas. 1994;9:508–12. doi: 10.1097/00006676-199407000-00014. [DOI] [PubMed] [Google Scholar]
- 36.Schmitz-Moormann P, Hein J. Changes of the pancreatic duct system associated with aging:Their relations to parenchyma (author's transl) Virchows Arch A Pathol Anat Histol. 1976;371:145–52. doi: 10.1007/BF00444930. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz-Moormann P, Thoma W, Hein J, et al. Comparative radiological and morphological study of the human pancreas. 2. Morphometrical investigation of the human pancreatic duct system (author's transl) Anat Anz. 1977;141:507–11. [PubMed] [Google Scholar]
- 38.Schmitz-Moormann P, Otte CA, Ihm P, et al. Comparative radiological and morphological study of the human pancreas. III. Morphometric investigation of the major pancreatic duct (author's transl) Z Gastroenterol. 1979;17:256–63. [PubMed] [Google Scholar]
- 39.Schmitz-Moormann P, Himmelmann GW, Brandes JW, et al. Comparative radiological and morphological study of human pancreas. Pancreatitis like changes in postmortem ductograms and their morphological pattern. Possible implication for ERCP. Gut. 1985;26:406–14. doi: 10.1136/gut.26.4.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Detlefsen S, Sipos B, Feyerabend B, et al. Pancreatic fibrosis associated with age and ductal papillary hyperplasia. Virchows Arch. 2005;447:800–5. doi: 10.1007/s00428-005-0032-1. [DOI] [PubMed] [Google Scholar]
- 41.Chantarojanasiri T, Hirooka Y, Ratanachu-Ek T, et al. Evolution of pancreas in aging:Degenerative variation or early changes of disease? J Med Ultrason (2001) 2015;42:177–83. doi: 10.1007/s10396-014-0576-2. [DOI] [PubMed] [Google Scholar]
- 42.Elgasim R, Abukonna A, Abd Elgyoum DA, et al. Measurement of common bile duct and pancreatic duct diameter among healthy adult sudanese subjects using magnetic resonance cholangiopancreatography. International Journal of Biomedicine. 2020;10:392–6. [Google Scholar]
- 43.Chamokova B, Bastati N, Poetter-Lang S, et al. The clinical value of secretin-enhanced MRCP in the functional and morphological assessment of pancreatic diseases. Br J Radiol. 2018;91:20170677. doi: 10.1259/bjr.20170677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Serafino M, Vitale V, Severino R, et al. Pediatric ultrasonography of the pancreas:Normal and abnormal findings. J Ultrasound. 2019;22:261–72. doi: 10.1007/s40477-018-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raut DS, Raje DV, Dandge VP, et al. Percentile reference curves for normal pancreatic dimensions in Indian children. Indian J Radiol Imaging. 2018;28:442–7. doi: 10.4103/ijri.IJRI_189_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim HJ, Kim MH, Lee SK, et al. Normal structure, variations, and anomalies of the pancreaticobiliary ducts of Koreans:A nationwide cooperative prospective study. Gastrointest Endosc. 2002;55:889–96. doi: 10.1067/mge.2002.124635. [DOI] [PubMed] [Google Scholar]
- 47.Zamboni GA, Ambrosetti MC, D'Onofrio M, et al. Ultrasonography of the pancreas. Radiol Clin North Am. 2012;50:395–406. doi: 10.1016/j.rcl.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 48.Hastier P, Buckley MJ, Dumas R, et al. Astudy of the effect of age on pancreatic duct morphology. Gastrointest Endosc. 1998;48:53–7. doi: 10.1016/s0016-5107(98)70129-4. [DOI] [PubMed] [Google Scholar]
- 49.Glaser J, Högemann B, Krummenerl T, et al. Sonographic imaging of the pancreatic duct. New diagnostic possibilities using secretin stimulation. Dig Dis Sci. 1987;32:1075–81. doi: 10.1007/BF01300191. [DOI] [PubMed] [Google Scholar]
- 50.Fujisawa T, Isayama H, Gunji T, et al. Prevalence rate and predictive factors of pancreatic diseases in cases with pancreatic duct dilatation:A cross-sectional study of a large, healthy Japanese population. Intern Med. 2020;59:769–77. doi: 10.2169/internalmedicine.3702-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka S, Nakaizumi A, Ioka T, et al. Main pancreatic duct dilatation:A sign of high risk for pancreatic cancer. Jpn J Clin Oncol. 2002;32:407–11. doi: 10.1093/jjco/hyf093. [DOI] [PubMed] [Google Scholar]
- 52.Wachsberg RH. Respiratory variation of the diameter of the pancreatic duct on sonography. AJR Am J Roentgenol. 2000;175:1459–61. doi: 10.2214/ajr.175.5.1751459. [DOI] [PubMed] [Google Scholar]
- 53.Wang Q, Swensson J, Hu M, et al. Distribution and correlation of pancreatic gland size and duct diameters on MRCP in patients without evidence of pancreatic disease. Abdom Radiol (NY) 2019;44:967–75. doi: 10.1007/s00261-018-1879-3. [DOI] [PubMed] [Google Scholar]
- 54.Gaddam S, Abboud Y, Oh J, et al. Incidence of pancreatic cancer by age and sex in the US, 2000-2018. JAMA. 2021;326:2075–7. doi: 10.1001/jama.2021.18859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gordon-Dseagu VL, Devesa SS, Goggins M, et al. Pancreatic cancer incidence trends:Evidence from the Surveillance, Epidemiology and End Results (SEER) population-based data. Int J Epidemiol. 2018;47:427–39. doi: 10.1093/ije/dyx232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer:Global trends, etiology and risk factors. World J Oncol. 2019;10:10–27. doi: 10.14740/wjon1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hur C, Tramontano AC, Dowling EC, et al. Early pancreatic ductal adenocarcinoma survival is dependent on size:Positive implications for future targeted screening. Pancreas. 2016;45:1062–6. doi: 10.1097/MPA.0000000000000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Egawa S, Toma H, Ohigashi H, et al. Japan Pancreatic Cancer Registry;30th year anniversary: Japan Pancreas Society. Pancreas. 2012;41:985–92. doi: 10.1097/MPA.0b013e318258055c. [DOI] [PubMed] [Google Scholar]
- 59.Kanno A, Masamune A, Hanada K, et al. Multicenter study of early pancreatic cancer in Japan. Pancreatology. 2018;18:61–7. doi: 10.1016/j.pan.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 60.Nipp R, Tramontano AC, Kong CY, et al. Disparities in cancer outcomes across age, sex, and race/ethnicity among patients with pancreatic cancer. Cancer Med. 2018;7:525–35. doi: 10.1002/cam4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–87. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 62.Basturk O, Hong SM, Wood LD, et al. Arevised classification system and recommendations from the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am J Surg Pathol. 2015;39:1730–41. doi: 10.1097/PAS.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Matsuda Y. Age-related morphological changes in the pancreas and their association with pancreatic carcinogenesis. Pathol Int. 2019;69:450–62. doi: 10.1111/pin.12837. [DOI] [PubMed] [Google Scholar]
- 64.Matsuda Y, Kimura W, Matsukawa M, et al. Association between pancreatic cystic lesions and high-grade intraepithelial neoplasia and aging:An autopsy study. Pancreas. 2019;48:1079–85. doi: 10.1097/MPA.0000000000001374. [DOI] [PubMed] [Google Scholar]
- 65.Matsuda Y, Furukawa T, Yachida S, et al. The prevalence and clinicopathological characteristics of high-grade pancreatic intraepithelial neoplasia:Autopsy study evaluating the entire pancreatic parenchyma. Pancreas. 2017;46:658–64. doi: 10.1097/MPA.0000000000000786. [DOI] [PubMed] [Google Scholar]
- 66.Lüttges J, Reinecke-Lüthge A, Möllmann B, et al. Duct changes and K-ras mutations in the disease-free pancreas:Analysis of type, age relation and spatial distribution. Virchows Arch. 1999;435:461–8. doi: 10.1007/s004280050428. [DOI] [PubMed] [Google Scholar]
- 67.LeBlanc JK, Chen JH, Al-Haddad M, et al. Can endoscopic ultrasound predict pancreatic intraepithelial neoplasia lesions in chronic pancreatitis?:A retrospective study of pathologic correlation. Pancreas. 2014;43:849–54. doi: 10.1097/MPA.0000000000000142. [DOI] [PubMed] [Google Scholar]
- 68.Brune K, Abe T, Canto M, et al. Multifocal neoplastic precursor lesions associated with lobular atrophy of the pancreas in patients having a strong family history of pancreatic cancer. Am J Surg Pathol. 2006;30:1067–76. [PMC free article] [PubMed] [Google Scholar]
- 69.Ikemoto J, Serikawa M, Hanada K, et al. Clinical analysis of early-stage pancreatic cancer and proposal for a new diagnostic algorithm:A multicenter observational study. Diagnostics (Basel) 2021;11:287. doi: 10.3390/diagnostics11020287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Okaniwa S. How does ultrasound manage pancreatic diseases?Ultrasound findings and scanning maneuvers. Gut Liver. 2020;14:37–46. doi: 10.5009/gnl18567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tanaka S, Nakao M, Ioka T, et al. Slight dilatation of the main pancreatic duct and presence of pancreatic cysts as predictive signs of pancreatic cancer:A prospective study. Radiology. 2010;254:965–72. doi: 10.1148/radiol.09090992. [DOI] [PubMed] [Google Scholar]
- 72.Fukuda J, Ikezawa K, Nakao M, et al. Predictive factors for pancreatic cancer and its early detection using special pancreatic ultrasonography in high-risk individuals. Cancers (Basel) 2021;13:502. doi: 10.3390/cancers13030502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gonoi W, Hayashi TY, Okuma H, et al. Development of pancreatic cancer is predictable well in advance using contrast-enhanced CT:A case-cohort study. Eur Radiol. 2017;27:4941–50. doi: 10.1007/s00330-017-4895-8. [DOI] [PubMed] [Google Scholar]
- 74.Toshima F, Watanabe R, Inoue D, et al. CT abnormalities of the pancreas associated with the subsequent diagnosis of clinical stage I pancreatic ductal adenocarcinoma more than 1 year later:A case-control study. AJR Am J Roentgenol. 2021;217:1353–64. doi: 10.2214/AJR.21.26014. [DOI] [PubMed] [Google Scholar]
- 75.Kawaji Y, Yoshikawa T, Nakagawa K, et al. Computed tomography findings for predicting the future occurrence of pancreatic cancer:Value of pancreatic volumetry. Int J Clin Oncol. 2021;26:1304–13. doi: 10.1007/s10147-021-01915-x. [DOI] [PubMed] [Google Scholar]
- 76.Sagami R, Yamao K, Nakahodo J, et al. Pre-operative imaging and pathological diagnosis of localized high-grade pancreatic intra-epithelial neoplasia without invasive carcinoma. Cancers (Basel) 2021;13:945. doi: 10.3390/cancers13050945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim SW, Kim SH, Lee DH, et al. Isolated main pancreatic duct dilatation:CT differentiation between benign and malignant causes. AJR Am J Roentgenol. 2017;209:1046–55. doi: 10.2214/AJR.17.17963. [DOI] [PubMed] [Google Scholar]
- 78.Miura S, Takikawa T, Kikuta K, et al. Focal parenchymal atrophy of the pancreas is frequently observed on pre-diagnostic computed tomography in patients with pancreatic cancer:A case-control study. Diagnostics (Basel) 2021;11:1693. doi: 10.3390/diagnostics11091693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nakahodo J, Kikuyama M, Nojiri S, et al. Focal parenchymal atrophy of pancreas:An important sign of underlying high-grade pancreatic intraepithelial neoplasia without invasive carcinoma, i.e. carcinoma in situ. Pancreatology. 2020;20:1689–97. doi: 10.1016/j.pan.2020.09.020. [DOI] [PubMed] [Google Scholar]
- 80.Yamao K, Takenaka M, Ishikawa R, et al. Partial pancreatic parenchymal atrophy is a new specific finding to diagnose small pancreatic cancer (≤10 mm) including carcinoma in situ:Comparison with localized benign main pancreatic duct stenosis patients. Diagnostics (Basel) 2020;10:445. doi: 10.3390/diagnostics10070445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology. 2012;142:796–804. doi: 10.1053/j.gastro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shin EJ, Topazian M, Goggins MG, et al. Linear-array EUS improves detection of pancreatic lesions in high-risk individuals:A randomized tandem study. Gastrointest Endosc. 2015;82:812–8. doi: 10.1016/j.gie.2015.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Molo C, Cui XW, Pirri C, et al. Pancreas mobile. Z Gastroenterol. 2013;51:1165–70. doi: 10.1055/s-0033-1335185. [DOI] [PubMed] [Google Scholar]
- 84.Ashida R, Tanaka S, Yamanaka H, et al. The role of transabdominal ultrasound in the diagnosis of early stage pancreatic cancer:Review and single-center experience. Diagnostics (Basel) 2018;9:2. doi: 10.3390/diagnostics9010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nakao M, Katayama K, Fukuda J, et al. Evaluating the ability to detect pancreatic lesions using a special ultrasonography examination focusing on the pancreas. Eur J Radiol. 2017;91:10–4. doi: 10.1016/j.ejrad.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 86.Kurihara K, Hanada K, Shimizu A. Endoscopic ultrasonography diagnosis of early pancreatic cancer. Diagnostics (Basel) 2020;10:1086. doi: 10.3390/diagnostics10121086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Téllez-Ávila FI, Tepox-Padrón A, Duarte-Medrano G, et al. Patients with unexplained dilated pancreatic duct have high risk of biliopancreatic malignancy detected by EUS. Surg Laparosc Endosc Percutan Tech. 2021;31:304–6. doi: 10.1097/SLE.0000000000000922. [DOI] [PubMed] [Google Scholar]
- 88.Kataoka K, Ishikawa T, Ohno E, et al. Endoscopic ultrasound elastography for small solid pancreatic lesions with or without main pancreatic duct dilatation. Pancreatology. 2021;21:451–8. doi: 10.1016/j.pan.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 89.Dietrich CF, Jenssen C. Modern ultrasound imaging of pancreatic tumors. Ultrasonography. 2020;39:105–13. doi: 10.14366/usg.19039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dietrich CF, Sahai AV, D'Onofrio M, et al. Differential diagnosis of small solid pancreatic lesions. Gastrointest Endosc. 2016;84:933–40. doi: 10.1016/j.gie.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 91.Ignee A, Jenssen C, Arcidiacono PG, et al. Endoscopic ultrasound elastography of small solid pancreatic lesions:A multicenter study. Endoscopy. 2018;50:1071–9. doi: 10.1055/a-0588-4941. [DOI] [PubMed] [Google Scholar]
- 92.Takasaki Y, Ishii S, Fujisawa T, et al. Endoscopic ultrasonography findings of early and suspected early chronic pancreatitis. Diagnostics (Basel) 2020;10:1018. doi: 10.3390/diagnostics10121018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kogekar N, Diaz KE, Weinberg AD, et al. Surveillance of high-risk individuals for pancreatic cancer with EUS and MRI:A meta-analysis. Pancreatology. 2020;20:1739–46. doi: 10.1016/j.pan.2020.10.025. [DOI] [PubMed] [Google Scholar]
- 94.Will U, Bosseckert H, Meyer F. Correlation of endoscopic ultrasonography (EUS) for differential diagnostics between inflammatory and neoplastic lesions of the papilla of Vater and the peripapillary region with results of histologic investigation. Ultraschall Med. 2008;29:275–80. doi: 10.1055/s-2008-1027327. [DOI] [PubMed] [Google Scholar]
- 95.Yamashita Y, Tanioka K, Kawaji Y, et al. Utility of contrast-enhanced harmonic endoscopic ultrasonography for early diagnosis of small pancreatic cancer. Diagnostics (Basel) 2020;10:23. doi: 10.3390/diagnostics10010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoshida T, Yamashita Y, Kitano M. Endoscopic ultrasound for early diagnosis of pancreatic cancer. Diagnostics (Basel) 2019;9:81. doi: 10.3390/diagnostics9030081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ishikawa-Kakiya Y, Maruyama H, Yamamoto K, et al. Comparison of the diagnostic efficiency of radial- and convex-arrayed echoendoscopes for indirect findings of pancreatic cancer:A retrospective comparative study using propensity score method. Cancers (Basel) 2021;13:1217. doi: 10.3390/cancers13061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kanno Y, Ito K, Koshita S, et al. Capability of radial- and convex-arrayed echoendoscopes for visualization of the pancreatobiliary junction. Clin Endosc. 2018;51:274–8. doi: 10.5946/ce.2017.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Deerenberg EB, Poley JW, Hermans JJ, et al. Role of endoscopic ultrasonography in patients suspected of pancreatic cancer with negative helical MDCT scan. Dig Surg. 2011;28:398–403. doi: 10.1159/000334074. [DOI] [PubMed] [Google Scholar]


