ABSTRACT
Background:
EUS-guided choledoco-duodenostomy using electrocautery-enhanced lumen-apposing metal stents (ECE-LAMS) is becoming the gold standard in case of endoscopic retrograde cholangio-pancreatography failure for distal malignant obstruction. Long-term data in larger samples are lacking.
Methods:
This was a prospective monocentric study including all patients who underwent EUS-guided choledochoduodenostomy (CDS) between September 2016 and December 2021. The primary endpoint was the rate of biliary obstruction during follow-up. Secondary endpoints were technical and clinical success rates, adverse event rates, and identification of risk factors for biliary obstruction.
Results:
One hundred and twenty-three EUS-guided CDS using ECE-LAMS were performed at Limoges University Hospital were performed during the study period and included in the study. The main cause of obstruction was pancreatic adenocarcinoma in 91 (74.5%) cases. The technical and clinical success rates were 97.5% and 91%, respectively. Twenty patients (16.3%) suffered from biliary obstructions during a mean follow-up of 242 days. The clinical success rate for endoscopic desobstruction was 80% (16/20). In uni- and multivariate analyses, only the presence of a duodenal stent (odds ratio [OR]: 3.6, 95% confidence interval [CI] 95%: 1.2–10.2; P = 0.018) and a bile duct thinner than 15 mm (OR: 3.9, CI 95%: 1.3–11.7; P = 0.015) were the significant risk factors for biliary obstruction during the follow-up.
Conclusion:
Obstruction of LAMS occurred in 16.3% of cases during follow-up and endoscopic desobstruction is efficacious in 80% of cases. The presence of duodenal stent and a bile duct thinner than 15 mm are the risk factors of obstruction. Except in these situation, EUS-CDS with ECE-LAMS could be proposed in the first intent in case of distal malignant obstruction.
Key words: EUS-guided biliary drainage, lumen-apposing metal stent, malignant distal biliary obstruction
BACKGROUND
ERCP failure occurs in 10%–30% of distal malignant obstruction cases.[1] EUS-guided biliary drainage is the current gold standard in this situation because it has similar efficiency and lower morbidity than percutaneous biliary drainage (PTBD) in cases of malignant biliary obstruction and failure of classic endoscopic drainage by ERCP.[2,3] EUS-guided hepaticogastrostomy (HGS) and choledochoduodenostomy (CDS) are the two different approaches, but recently, EUS-guided CDS using electrocautery-enhanced lumen-apposing metal stents (ECE-LAMS) has become available, simplifying EUS-guided biliary drainage and allowing endoscopic biliodigestive anastomosis in a single step.
High clinical success rates and safety have been reported, particularly when using free-hand techniques (without device exchange due to previous bile duct puncture with a 19 G needle and a guidewire) and small stents (6 or 8 mm).[4]
The relative simplicity of this procedure has led to a significant increase in its use, sometimes even as a first-line treatment. However, despite growing evidence about the short-term results of EUS-BD using LAMS, most published studies have included few patients[5-11] (<60), and there is a lack of long-term data in larger samples.
In this study, we analyzed the risk of stent obstruction during follow-up and identified preoperative risk factors for obstruction.
METHODS
Study design
We performed a prospective study including all patients who underwent EUS-guided CDS between June 2017 and December 2021 at the Limoges University Hospital, France. All patients provided informed consent prior to enrollment. The Institutional Review Board of Ramsay Generale de santé, Paris, approved this study (COS-RGDS-2017-12-004-Avis IRB-NAPOLEON-B).
As some patients were not followed up at our center for biliary obstructions, one author regularly contacted patients, their general physicians, and oncologists to check for biliary obstruction events (jaundice, cholangitis, and so forth) until patient death or surgery.
Inclusion criteria
18 years old patient who underwent EUS-CDS with ECE-LAMS during the study period.
Exclusion criteria
EUS choledoco-gastric anastomosis with ECE-LAMS
Refusal to participate to the study.
Procedure: EUS-guided choledochoduodenostomy using electrocautery-enhanced lumen-apposing metal stent
A previously reported free-hand direct technique was used.[4] It consisted of direct fistulotomy of the dilated bile duct using an electrocautery system with a pure cut current delivered through a therapeutic linear echoendoscope with a longroute position (stability of the scope). We recommend using a 6-mm stent to decrease the distance needed for intrabiliary release of the distal flange under EUS guidance. The sheath was gently withdrawn until tubulization of the distal flange (the carrier contacts the distal flange) and then the proximal flange was released inside the working channel before pushing it into the duodenal bulb by pushing the sheath out.
All EUS-CDS procedures were performed using ECE-LAMS under deep propofol sedation or under general anesthesia with endotracheal intubation.
Definitions
Technical success was defined as the ability to correctly deploy the ECE-LAMS stent between the common bile duct (CBD) and the duodenal bulb with the visualization of bile flow
Clinical success was defined as ≥50% decrease in the bilirubin level on day 15 or normalization at 1 month allowing chemotherapy. If death occurred within 15 days despite technical success, it was considered clinical failure based on an intention-to-treat analysis
Early adverse events were defined as those that occurred during hospitalization after the intervention and were classified as mild, moderate, severe, and fatal based on the American Society for Gastrointestinal Endoscopy (ASGE) lexicon[12] and the recently published AGREE classification[13] (classification for adverse events gastrointestinal endoscopy)
Biliary obstruction during the follow-up was defined as the occurrence of cholangitis, cholestasis, or jaundice, even when it did not require hospitalization and/or reintervention. Reintervention for obstruction before achieving clinical success was defined as primary stent dysfunction and an obstruction during follow-up.
Endpoints
The primary endpoint was the rate of biliary obstruction during follow-up. Secondary endpoints were technical and clinical success rates, adverse event rates, and identification of risk factors for biliary obstruction.
Statistical analysis
The data are presented as frequencies and percentages for the categorical variables. Normally, distributed data are expressed as means (standard deviations) and nonnormally distributed data are expressed as medians (interquartile ranges). Linked samples were compared using the two-tailed nonparametric Wilcoxon test because a standardized normal distribution could not be assumed due to the small sample size. The Chi-square and Fisher’s exact tests were used to analyze the qualitative data. P < 0.05 was considered to indicate statistical significance. After the assessment of possible collinearity (using the variance inflation factor), a logistic regression model was formed, using the backward stepwise selection method, with biliary obstruction as the dependent variable. Independent, clinically relevant predictors identified by univariate analyses (P < 0.2) were entered into the model using the “10 events per variable” rule.
Statistical analyses were performed using the opensource software R version 3.0.2 (2013-09-25) (The R foundation, Vienna, Austria) and LaTeX on a i386-w64-mingw32 platform.
RESULTS
Study population
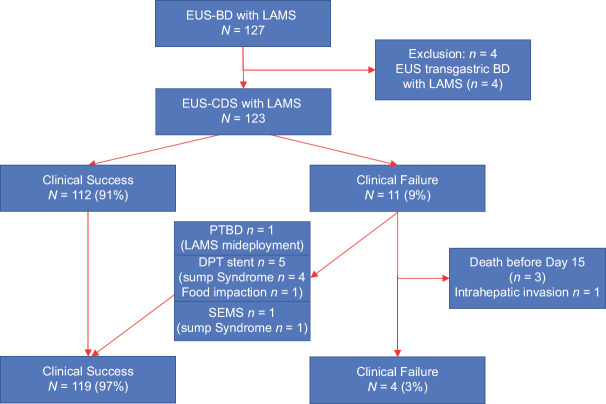
Between June 2017 and December 2021, 127 EUS-guided biliary drainages using ECE-LAMS were performed at Limoges University Hospital. Four patients were excluded because of EUS-guided choledochogastric anastomosis, and the remaining 123 patients were included in the study [Figure 1].
Figure 1.
Flow chart of the study. EUS-BD: EUS-guided biliary drainage; CDS: Choledochoduodenostomy; LAMS: Lumen apposing metal stent; PTBD: Percutaneous biliary drainage; DPT: Double pig tail; SEMS: Self expanding metal stent
During the study period, 21 patients benefited of an hepatico-gastrostomy for malignant bile duct obstruction (6 for a Klastkin tumor and 15 for a modified anatomy). All patients with failed or impossibility of ERCP with access to the CBD underwent and EUS-CDS with ECE-LAMS.
The characteristics of the patients are presented in Table 1. The main cause of obstruction was pancreatic adenocarcinoma in 91 (74.5%) cases. Seventeen (14%) patients had ascites. All but three patients suffered from jaundice with a median serum bilirubin level of 260 µmol/L (±160 DS).
Table 1.
Patient characteristics (n=123)
| n (%) | |
|---|---|
| Age | 73±10 SD |
| Female sex | 52 (43) |
| Etiology of obstruction | |
| Pancreatic adenocarcinoma | 91 (74.5) |
| Cholangiocarcinoma | 9 (7.4) |
| Duodenal cancer | 11 (9) |
| Vater adenocarcinoma | 3 (2.5) |
| Other | 8 (6.6) |
| Jaundice | 120 (97.6) |
| Bilirubinemia (µmol/L) | 260±160 SD |
| Bile duct diameter (mm) | 18±4.5 |
| Fever | 10 (8) |
| Ascites | 17 (14) |
SD: Standard deviation
Overall, 48 patients (39%) had EUS-CDS with ECE-LAMS in first intent without previous ERCP failure: in 27 (56%) cases because of nonpassable duodenal stenosis, in 4 cases (8%) because of inclusion in a multicenter randomized control trial comparing EUS-CDS with ECE-LAMS (NCT03000855) and ERCP for distal malignant biliary obstruction and in 17 (36%) cases because of potentially operable malignant obstruction. Since 2018, after a case of severe post-ERCP pancreatitis in a patient with an operable distal malignant obstruction, we decided in consultation with hepatopancreaticobiliary surgeons and oncologists to perform EUS-CDS in the first intent for patients suffering from operable distal biliary obstructions and those with indications for preoperative biliary drainage.
Procedure
The median diameter of the CBD was 18 mm (±4.5 DS). The EUS-CDS procedures were performed using a 6-mm stent in 117 (95%) patients [Table 2]. A direct free-hand strategy was used in 115 (93%) cases. The proximal flange was released in the digestive tract under EUS-guidance after opening the flange in the working channel in all of the cases. A duodenal stent was simultaneously positioned just after EUS-CDS in 26 (21%) cases.
Table 2.
Characteristics of the procedure (n=123)
| n (%) | |
|---|---|
| Previous ERCP | |
| Number of previous ERCPs | |
| 0 | 48 (39) |
| Duodenal stenosis | 27 (56) |
| RCT | 4 (8) |
| Operable patients | 17 (36) |
| 1 | 63 (51) |
| 2 | 11 (9) |
| 3 | 1 (1) |
| Duodenal stent | |
| Before the procedure | 1 (1) |
| After axios but same session | 26 (21) |
| Pure cut current | |
| EUS-CDS technique | |
| Free hand technique | 115 (93) |
| Preloaded guidewire | 5 (4) |
| 19 G puncture+guidewire before axios | 3 (3) |
| Stent size | |
| 6 | 117 (95) |
| 8 | 4 (3) |
| 10 | 1 (1) |
| 15 | 1 (1) |
| Intraoperative channel release of the proximal flange | 123 (100) |
EUS-CDS: EUS-guided choledochoduodenostomy; RCT: Randomized controlled trials
Endpoints
Technical and clinical success and adverse events
The technical and clinical success rates were 97.5% (120/123) and 91% (112/123), respectively [Table 3]. Three patients (2.5%) suffered from technical failure, all due to intraperitoneal opening of the distal flange of the LAMS. The first one was treated by an OVESCO clip to close the duodenal perforation and a PTBD to treat the jaundice. The second was treated by a salvage cholecysto-duodenostomy with a new ECE-LAMS but need a laparoscopic peritoneal lavage at day two due to bile leakage during the procedure. The last one benefitted from an immediate second EUS-guided CDS with a new 6 mm ECE LAMS that was successful.
Table 3.
Endpoints (n=123)
| n (%) | |
|---|---|
| Technical success | 120 (97.5) |
| Clinical success | 112 (91) |
| Stent primary dysfunction | 8 (6.5) |
| Biliary obstruction during follow-up (including primary stent dysfunction) | 20 (16) |
Among the 11 (9%) clinical failures, 3 were due to death before day 15 (not related to the procedure, due to malignant disease final evolution). The remaining 8 clinical failures included 5 sump syndromes successfully treated by double pigtail stent placement, one stent misdeployment successfully treated by PTBD, one food impaction treated by double pigtail stent, and one case of intra-hepatic cancer involvement.
Per-procedural adverse events occurred in four cases, all due to distal flange misdeployment in the peritoneal cavity. One was rescued by PTBD, two by immediate new EUS-CDS with another ECE-LAMS, and one by EUS-guided gallbladder drainage.[14] All of these adverse events were classified as mild according to the ASGE classification; 2 were classified Grade I, 1 Grade IIIa and 1 Grade IIIb of the AGREE classification.
Biliary obstruction during follow-up
Twenty patients (16.3%) suffered from biliary obstructions during a mean follow-up of 242 days. A total of 95 (77.2%) patients died during follow-up, while 21 (17%) benefited from Whipple surgery after EUS-CDS with ECE-LAMS. In uni-and multivariate analyses, only the presence of a duodenal stent (odds ratio [OR]: 3.6, 95% confidence interval [CI] 95%: 1.2–10.2; P = 0.018) and a bile duct thinner than 15 mm (OR: 3.9, CI 95%: 1.3–11.7; P = 0.015) were significant risk factors for biliary obstruction during follow-up [Table 4].
Table 4.
Univariate and multivariate analysis of risk factors for stent obstruction during follow-up
| Data | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
|
|||
| OR (CI 95%) | P | CI 95% | P | |
| Etiology of obstruction | ||||
| Pancreatic adenocarcinoma | 1 | - | - | - |
| Cholangiocarcinoma | 3.3 (0.7–15) | 0.12 | - | - |
| Duodenal cancer | 1.48 (0.29–7.9) | 0.64 | - | - |
| Vater adenocarcinoma | 3.3 (0.28–39.6) | 0.34 | - | - |
| Other | 2.2 (0.4–12.3) | 0.36 | - | - |
| Bile duct diameter | 0.88 (0.78–1) | 0.052 | - | - |
| Duodenal stenosis | ||||
| No | 1 | - | - | |
| Yes | 1.8 (0.68–4.8) | 0.231 | - | - |
| Duodenal stent | ||||
| No | 1 | |||
| Yes | 3.9 (1.4–10.7) | 0.009 | 3.6 (1.2–10.2) | 0.018 |
| Bile duct diameter <15 mm | ||||
| No | 1 | - | - | - |
| Yes | 4.2 (1.47–12.2) | 0.007 | 3.9 (1.3–11.7) | 0.015 |
CI: Confidence interval; OR: Odds ratio
The 8 cases of primary dysfunction detailed above were considered early obstructions during follow-up, compared to 12 cases of late (after 1 month) obstructions. All but one reflux-cholangitis were managed endoscopically. The clinical success rate for endoscopic deobstruction was 80% (16/20). The mean and median times of biliary obstruction were 118 days and 38 days, respectively.
The individualized details of obstructions and deobstruction endoscopic procedures are presented in Table 5.
Table 5.
Details of stent obstruction and endoscopic deobstruction procedures
| Etiology of biliary obstruction | Bile duct diameter | Presence of duodenal stenosis | Cause of LAMS obstruction | Delay of obstruction | Treatment for deobstruction | Success of deobstruction |
|---|---|---|---|---|---|---|
| Pancreatic adenocarcinoma | 9 | Yes | Food impaction | 247 | SEMS | Yes |
| Duodenal adenocarcinoma | 22 | Yes | Food impaction | 370 | Endoscopic deobstruction | Yes |
| Pancreatic adenocarcinoma | 14 | Yes | Food impaction | 44 | Endoscopic deobstruction+double pigtail stent | Yes |
| Cholangiocarcinoma | 13 | Yes | Food impaction | 39 | Endoscopic deobstruction | Yes |
| Pancreatic adenocarcinoma | 16 | No | Food impaction | 16 | Endoscopic deobstruction + double pigtail stent | Yes |
| Cholangiocarcinoma | 23 | No | LAMS migration | 486 | SEMS | Yes |
| Vater adenocarcinoma | 14 | Yes | Tumoral invasion | 37 | Double pigtail stent | Yes |
| Pancreatic adenocarcinoma | 16 | No | Sump syndrome | 66 | Double pigtail stent | Yes |
| Pancreatic adenocarcinoma | 18 | Yes | Food impaction | 6 | Endoscopic deobstruction + double pigtail stent | Yes |
| Pancreatic adenocarcinoma | 19 | Yes | Tumoral invasion | 231 | ERCP | No |
| Lymph node compression | 16 | No | Reflux cholangitis | 205 | Antibiotics | Yes |
| Pancreatic adenocarcinoma | 16 | No | Sump syndrome | 9 | Double pigtail stent | Yes |
| Vater adenocarcinoma | 17 | No | Food impaction | 155 | Endoscopic deobstruction + double pigtail stent | Yes |
| Lymph node compression | 19 | No | Sump syndrome | 378 | Double pigtail stent | Yes |
| Pancreatic adenocarcinoma | 14 | No | Sump syndrome | 9 | Double pigtail stent | Yes |
| Pancreatic adenocarcinoma | 12 | No | Sump syndrome | 7 | Double pigtail stent | Yes |
| Pancreatic adenocarcinoma | 25 | Yes | Sump syndrome | 23 | SEMS+duodenal stent | Yes |
| Cholangiocarcinoma | 13 | No | Liver tumoral involvement | 6 | Double pig tail stent | No |
| Pancreatic adenocarcinoma | 14 | No | Sump syndrome | 12 | Double pig tail stent | Yes |
| Pancreatic adenocarcinoma | 20 | Yes | Food impaction | 37 | Double pig tail stent+duodenal stent | Yes |
LAMS: Lumen-apposing metal stents; SEMS: Self-expanding metal stents
Effect of bile duct size on the results of EUS-guided choledochoduodenostomy
Area under the curve (AUC) calculations allowed us to identify a 14 mm threshold for the risk of obstruction during follow-up (<15 mm). We compared the outcomes of patients based on this threshold. Larger bile ducts had superior technical success (99% [>15 mm] vs. 90.9% [<15 mm]; P = 0.082), superior clinical success (95% [>15 mm] vs. 73% [<15 mm]; P = 0.004) and lower obstruction rates (12% [>15 mm] vs. 36% [<15 mm]; P = 0.009) during follow-up. We also observed a four-fold increase in per-procedural adverse events in cases with bile ducts thinner than 15 mm (9.5% vs. 2%, P = 0.146), but this result did not achieve statistical significance [Table 6].
Table 6.
Comparison of endpoints between patients with bile duct diameters < and >15 mm
| Bile duct <15 mm (n=101), n (%) | Bile duct >15 mm (n=22), n (%) | P | |
|---|---|---|---|
| Technical failure | 1 (1) | 2 (9.1) | 0.082 |
| Perprocedural complications | 2 (2) | 2 (9.1) | 0.146 |
| Clinical success | 96 (95) | 16 (73) | 0.004 |
| Biliary obstruction during follow-up | 12 (12) | 8 (36) | 0.009 |
DISCUSSION
This is the largest reported study about EUS-CDS using ECE LAMS with long-term follow-up, as 88% of the participants were followed-up until death or Whipple surgery.
Stent obstruction occurred in 16% of the cases, mostly due to alimentary obstruction or sump syndrome. This is consistent with a recent meta-analysis that included 6 studies and 311 patients, and reported a patency rate of 86.2%.[8]
There was a high technical and clinical success rate, as previously reported.[4,10,15] Periprocedural safety was significantly better than several of previous studies. As previously reported, we use only the free-hand technique with a 6 mm stent if the biliary duct is at least 15 mm. Avoiding device changes and biliary tract opacifications significantly decreased the risk for biliary leaks and cholangitis reported with the use of classical EUS-BD strategy with 19G puncture and guidewire positioning before ECE-LAMS deployment.[5]
No studies have identified risk factors for reobstruction. Multivariate analysis (combined with AUC calculation for bile duct size) showed that a bile duct size <15 mm and the presence of a duodenal stent were predictive factors for reobstruction during follow-up.
Duodenal stents are indicated in cases of significant duodenal stenosis linked to tumor invasion. This condition is responsible for food impaction due to alimentary stasis in the duodenal stent.
HGS and/or endoscopic gastrojejunal-anastomosis may prevent biliary reobstruction in cases of duodenal stenosis, as recently reported by an Italian team during the European Congress of Digestive Endoscopy.[16]
In biliary ducts <15 mm, the obstruction rate was 36% during the follow-up, three times higher than for patients with larger CBDs. In addition, as technical, clinical, and safety profiles were significantly poorer for CBDs <15 mm, ECE-LAMS may be less considered in this situation. Hepatico-gastrostomy in cases of dilated intrahepatic ducts, rendezvous strategy, or antegrade stenting could be used as an alternative. However, using a guidewire in this situation seems to be mandatory, and placing a double pig-tail stent over a guidewire at the end of the procedure could avoid sump syndrome. An ongoing Spanish randomized study is comparing systematic double pigtail stents inside LAMS with classic LAMS positioning in this indication.[9,17] No pigtail stent was positioned before obstruction of the LAMS in our study, prophylactic double pigtail stent inside LAMS could perhaps avoid food impaction consequences and sump syndrome that represent 75% (15/20) of LAMS obstruction in our study.
Our study allowed positioning refinements in this technique in cases of distal tumor obstructions. In cases with CBD ≥15 mm without duodenal stenosis, this should be the standard management of ERCP failure because of its efficiency, safety, and superiority compared to radiological drainage.[2,3] In cases with CBD <15 mm, the degree of dilation of the intrahepatic bile ducts and the expertise of the physician must be considered while choosing between transhepatic techniques (HGS, rendezvous, or anterograde drainage) and EUS-CDS with ECE-LAMS, guide wire positioning, and pigtail stenting.
Further investigation is required to determine whether HGS or endoscopic gastroenteroanastomosis is more effective for avoiding biliary obstructions in cases of duodenal stenosis to improve oncological outcomes and quality of life.
A recent unpublished international study, presented at Digestive Disease Week 2021, reported superiority of EUS-CDS with ECE-LAMS in terms of technical success and procedure time, but no differences in terms of biliary obstruction at 1 year compared to ERCP in cases of distal tumoral biliary obstruction in the first intention.[18] Our results challenge this viewpoint. In cases of CBDs <15 mm, ERCP should be systematically attempted in the first intention. Meanwhile, in cases with large CBP (≥15 mm), the use of EUS-CDS with ECE-LAMS in the first intention will probably become the standard of care in the near future.
Twenty-one of our patients benefited from Whipple surgery after ECE-LAMS positioning. We chose to perform EUS-CDS with ECE-LAMS in the first intention in resectable cases. The presence of an endoscopic choledochoduodenal anastomosis did not impact the surgical procedure according to our hepatopancreaticobiliary surgeons.[19,20] This strategy avoids the risk for post-ERCP pancreatitis that could prevent the surgery. A French multicenter randomized trial will soon begin to confirm this choice of biliary drainage in patients suffering from distal malignant biliary obstructions suitable for surgery.
A bias of expertise can be reproached. However, three operators had never performed EUS guided biliary drainage before the start of the study (41 (33%) of the total included cases) emphasizing the short learning curve of this procedure for well-trained physicians.
CONCLUSION
In conclusion, this large study confirmed the high technical and clinical success rates and the good safety profile of this procedure. We identified thin bile ducts and presence of duodenal stents as risk factors for obstruction during follow-up. Endoscopists caring for patients with distal malignant biliary obstruction should master both therapeutic EUS and ERCP, which are different tools to treat the same disease. The choice of strategy should take into consideration the size of the bile duct, presence of duodenal stenosis, and the possibility of pancreatic surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ekkelenkamp VE, de Man RA, Ter Borg F, et al. Prospective evaluation of ERCP performance:Results of a nationwide quality registry. Endoscopy. 2015;47:503–7. doi: 10.1055/s-0034-1391231. [DOI] [PubMed] [Google Scholar]
- 2.Ginestet C, Sanglier F, Hummel V, et al. EUS-guided biliary drainage with electrocautery-enhanced lumen-apposing metal stent placement should replace PTBD after ERCP failure in patients with distal tumoral biliary obstruction:A large real-life study. Surg Endosc. 2022;36:3365–73. doi: 10.1007/s00464-021-08653-1. [DOI] [PubMed] [Google Scholar]
- 3.Sawas T, Bailey NJ, Yeung KY, et al. Comparison of EUS-guided choledochoduodenostomy and percutaneous drainage for distal biliary obstruction:A multicenter cohort study. Endosc Ultrasound. 2022;11:223–30. doi: 10.4103/EUS-D-21-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacques J, Privat J, Pinard F, et al. EUS-guided choledochoduodenostomy by use of electrocautery-enhanced lumen-apposing metal stents:A French multicenter study after implementation of the technique (with video) Gastrointest Endosc. 2020;92:134–41. doi: 10.1016/j.gie.2020.01.055. [DOI] [PubMed] [Google Scholar]
- 5.Tsuchiya T, Teoh AY, Itoi T, et al. Long-term outcomes of EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction:A prospective multicenter study. Gastrointest Endosc. 2018;87:1138–46. doi: 10.1016/j.gie.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 6.Teoh AY, Kongkam P, Bapaye A, et al. Use of a novel lumen apposing metallic stent for drainage of the bile duct and gallbladder:Long term outcomes of a prospective international trial. Dig Endosc. 2021;33:1139–45. doi: 10.1111/den.13911. [DOI] [PubMed] [Google Scholar]
- 7.On W, Paranandi B, Smith AM, et al. EUS-guided choledochoduodenostomy with electrocautery-enhanced lumen-apposing metal stents in patients with malignant distal biliary obstruction:Multicenter collaboration from the United Kingdom and Ireland. Gastrointest Endosc. 2022;95:432–42. doi: 10.1016/j.gie.2021.09.040. [DOI] [PubMed] [Google Scholar]
- 8.Krishnamoorthi R, Dasari CS, Thoguluva Chandrasekar V, et al. Effectiveness and safety of EUS-guided choledochoduodenostomy using lumen-apposing metal stents (LAMS):A systematic review and meta-analysis. Surg Endosc. 2020;34:2866–77. doi: 10.1007/s00464-020-07484-w. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Sumalla A, Loras C, Sanchiz V, et al. Multicenter study of lumen-apposing metal stents with or without pigtail in endoscopic ultrasound-guided biliary drainage for malignant obstruction-bampi trial:An open-label, randomized controlled trial protocol. Trials. 2022;23:181. doi: 10.1186/s13063-022-06106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderloni A, Fugazza A, Troncone E, et al. Single-stage EUS-guided choledochoduodenostomy using a lumen-apposing metal stent for malignant distal biliary obstruction. Gastrointest Endosc. 2019;89:69–76. doi: 10.1016/j.gie.2018.08.047. [DOI] [PubMed] [Google Scholar]
- 11.Amato A, Sinagra E, Celsa C, et al. Efficacy of lumen-apposing metal stents or self-expandable metal stents for endoscopic ultrasound-guided choledochoduodenostomy:A systematic review and meta-analysis. Endoscopy. 2021;53:1037–47. doi: 10.1055/a-1324-7919. [DOI] [PubMed] [Google Scholar]
- 12.Cotton PB, Eisen G, Romagnuolo J, et al. Grading the complexity of endoscopic procedures:Results of an ASGE working party. Gastrointest Endosc. 2011;73:868–74. doi: 10.1016/j.gie.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 13.Nass KJ, Zwager LW, van der Vlugt M, et al. Novel classification for adverse events in GI endoscopy:The agree classification. Gastrointest Endosc. 2022;95:1078–85.e8. doi: 10.1016/j.gie.2021.11.038. [DOI] [PubMed] [Google Scholar]
- 14.Issa D, Irani S, Law R, et al. Endoscopic ultrasound-guided gallbladder drainage as a rescue therapy for unresectable malignant biliary obstruction:A multicenter experience. Endoscopy. 2021;53:827–31. doi: 10.1055/a-1259-0349. [DOI] [PubMed] [Google Scholar]
- 15.Jacques J, Privat J, Pinard F, et al. Endoscopic ultrasound-guided choledochoduodenostomy with electrocautery-enhanced lumen-apposing stents:A retrospective analysis. Endoscopy. 2019;51:540–7. doi: 10.1055/a-0735-9137. [DOI] [PubMed] [Google Scholar]
- 16.Vanella G, Bronswijk M, van Wanrooij RL, et al. Outcomes of combined management biliary and gastric outlet obstruction (cabriolet study):A multicentre retrospective analysis. Endoscopy. 2022;54:OP169. doi: 10.1002/deo2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garcia-Sumalla A, Loras C, Guarner-Argente C, et al. Is a coaxial plastic stent within a lumen-apposing metal stent useful for the management of distal malignant biliary obstruction? Surg Endosc. 2021;35:4873–81. doi: 10.1007/s00464-021-08435-9. [DOI] [PubMed] [Google Scholar]
- 18.Teoh AY, Napoleon B, Kunda R, et al. ID:3523404 Eus-Guided choledochoduodenostomy versus ercp with covered metallic stents in patients with unresectable malignant distal biliary obstruction. A multi-centered randomized controlled trial (DRA-MBO trial) Gastrointest Endosc. 2021;93:AB216–7. [Google Scholar]
- 19.Gaujoux S, Jacques J, Bourdariat R, et al. Pancreaticoduodenectomy following endoscopic ultrasound-guided choledochoduodenostomy with electrocautery-enhanced lumen-apposing stents an ACHBT –SFED study. HPB (Oxford) 2021;23:154–60. doi: 10.1016/j.hpb.2020.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Fabbri C, Fugazza A, Binda C, et al. Beyond palliation:Using EUS-guided choledochoduodenostomy with a lumen-apposing metal stent as a bridge to surgery. A case series. J Gastrointestin Liver Dis. 2019;28:125–8. doi: 10.15403/jgld.2014.1121.281.eus. [DOI] [PubMed] [Google Scholar]