Summary
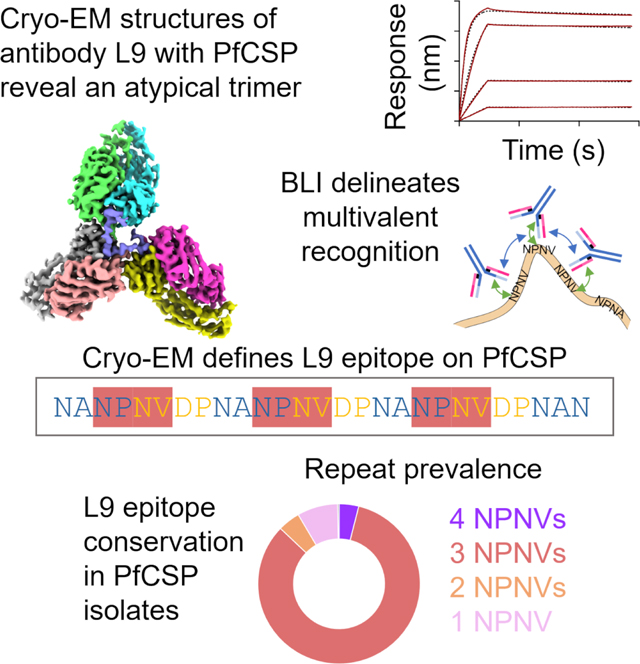
Monoclonal antibody L9 recognizes the Plasmodium falciparum circumsporozoite protein (PfCSP) and is highly protective following controlled human malaria challenge. To gain insight into its function, we determined cryo-EM structures of L9 in complex with full-length PfCSP and assessed how this recognition influenced protection by wild-type and mutant L9s. Cryo-EM reconstructions at 3.6- and 3.7-Å resolution revealed L9 to recognize PfCSP as an atypical trimer. Each of the three L9s in the trimer directly recognized an Asn-Pro-Asn-Val (NPNV) tetrapeptide on PfCSP and interacted homotypically to facilitate L9-trimer assembly. We analyzed peptides containing different repeat tetrapeptides for binding to wild-type and mutant L9s to delineate epitope and homotypic components of L9 recognition; we found both components necessary for potent malaria protection. Last, we found the 27-residue stretch recognized by L9 to be highly conserved in P. falciparum isolates, suggesting the newly revealed complete L9 epitope to be an attractive vaccine target.
Keywords: circumsporozoite protein, CSP, homotypic interactions, malaria, Plasmodium falciparum, R21, RTS, S
eTOC blurb:
Antibody L9 targets the P. falciparum circumsporozoite (PfCSP) protein with high clinical efficacy. Tripathi et al. report cryo-EM structures of L9 in complex with PfCSP and perform structure-function studies. These reveal an atypical trimeric association, implicate homotypic interactions, and delineate a highly conserved 27-residue epitope as a promising vaccine target.
Graphical Abstract
Introduction
Malaria affects hundreds of millions of people each year, killing an estimated 600,000, mainly infants and children in sub-Saharan Africa.1 Malaria is a mosquito-transmitted disease caused by parasites of the Plasmodium family. Plasmodium utilizes multiple mechanisms of immune evasion to enable recurrent infections. One of the most intriguing is embodied by the repeat nature of the circumsporozoite protein (CSP), the dominant antigen covering the surface of sporozoites, the infectious form of Plasmodium. CSP is composed of an N-terminal region, a junctional region containing a functionally critical proteolytic cleavage site (KLKQP), a repeat region comprising several dozen iterations of an NANP tetrapeptide (the CSP major repeat) interspersed with a few NVDP tetrapeptides (the CSP minor repeat), and a C-terminal region. Polyclonal anti-CSP antibodies directed against the major NANP repeats following vaccination with truncated PfCSP require very high titer to mediate protection.2 A few potent antibodies such as mAb-311, −317, −224, and −850 that target the NANP repeat region, however, have been found to mediate potent malaria protection.3–5 Moreover, the discovery of monoclonal antibodies against the junctional region or junctional-proximal minor repeats that can prevent malaria infection in animal models has provided crucial evidence for additional sites of neutralization on PfCSP.6,7 Importantly, two monoclonal antibodies, CIS43 and L9, directed to the junctional region and to the minor repeats, respectively, are highly protective in humans following controlled human malaria infection.7,8
Antibody L9 is ~2–3 fold more potent that CIS43,7 and protection by L9 after low dose subcutaneous administration has been demonstrated in humans making it a lead candidate for further clinical development.9 L9 induces killing of sporozoites through cytolytic mechanism and limits infection of hepatocytes in the liver.7 While structures are known for unbound L9 as well as chimeric versions of L9 with a six-residue fragment (ANPNVD),10 there is limited understanding of how L9 achieves high affinity recognition of the minor repeats in the context of full length PfCSP. Here, we determine cryo-EM structures of L9 with full-length CSP from P. falciparum (PfCSP). We identify residues of L9 involved in direct interaction with CSP as well as residues of L9 involved in homotypic L9-to-L9 interactions. We mutate these residues and assess both the protective capacity as well as the affinity of wild-type and mutant L9s to peptides containing different repeat tetrapeptides. Overall, the results define the molecular mechanism underlying high affinity CSP recognition by L9 and reveal a 27-residue stretch, conserved in most P. falciparum field isolates, as a promising site-of-vulnerability for vaccine development.
Results
Cryo-EM Structures Reveal L9 to Recognize PfCSP as an Atypical Trimer
To provide structural insight into the recognition between antibody L9 and PfCSP, we sought to obtain cryo-electron microscopy (cryo-EM) reconstructions of antibody L9 in complex with full-length PfCSP. Prior binding analysis of L9 recognition suggested that more than a single L9 recognizes PfCSP.7 We therefore incubated the antigen-binding fragment of L9 with an engineered version of full length PfCSP at a 2:1 molar ratio. The engineered construct of PfCSP (PfCSPm) was designed for enhanced expression, and derives from the 3D7 isolate, that encodes the N-terminal region (with four amino acid mutations), the junctional regions, the repeat region (38 NANP major repeats interspersed with 4 NVDP minor repeats), and the C-terminal region.7 We collected and analyzed single-particle cryo-EM data. Two-dimensional (2D)-class averages showed three Fabs to be bound in a trimeric fashion (Figure 1A, left), and we obtained three-dimensional (3D) reconstructions for two classes (Class 1 and Class 2) at 3.6 Å and 3.7 Å resolutions, with 117,479 and 62,735 particles, respectively (Figures 1A and S1–S3).
Figure 1. Cryo-EM structures of L9 with PfCSP at 3.6 Å and 3.7 Å reveal recognition by an L9 trimer.
(A) 2D classes and 3D reconstructions of Class 1 and Class 2 with number of particles listed. (B) Cryo-EM structure of Class 1 at 3.6 Å. Three L9 Fabs bind a 27 residue stretch of PfCSP. The N- and C-termini of PfCSP are labelled. The structure of Class 2 is shown in Figure S1. (C) PfCSP schematic highlighting tetrapeptide motifs and relationship to the NPNV tetrapeptide preferred by L9. Buried surface areas (BSAs) of the PfCSP-L9 complex are shown as a bar plot, with the colors representing heavy and light chains of three Fabs as in panel B. See also Figures S1–S3.
Starting with the previously reported structure of unbound L9 Fab (PDB: 7RQP), we were able to build three L9s in each of the classes. Class 1 had well-resolved density for both variable and constant regions for two Fabs and only the variable domain for the other Fab, whereas for Class 2, well-resolved density for both variable and constant regions was observed for one Fab and only the variable domains for the other two Fabs. Electron density for most of PfCSPm could not be resolved (370 out of 397 PfCSPm residues were disordered), though well-ordered density was observed for a 27-amino acid stretch comprising residues 105–131(Figures 1B and S1–S3). Analysis of buried epitope surface areas11 revealed L9 to focus recognition not on the NVDP minor repeat, but on four residues – NPNV – comprising the last two residues of a NANP major repeat and the first two residues of the NVDP minor repeat (Figure 1C). Substantial buried surface was also observed for a fifth residue, Asp (D), the third residue of the NVDP minor repeat. This fifth residue was not involved, however, in sequence-specific direct interactions with L9. The closest interactions were with D98H, with which there were unfavorable charge-charge repulsions, and the closest favorable interactions were with Y32H at ~4 Å (Figure S4). Overall, the structures revealed L9 to recognize PfCSP as an atypical trimer, allowing each of the three L9 Fabs to pack closely and to interact both with their NPNV epitope of PfCSP as well as with an adjacent Fab via antibody-to-antibody homotypic interactions.
PfCSP-L9 Epitope Interactions
To understand the differences between the two major reconstruction classes, Class 1 and Class 2, we analyzed the relative angles of approach between each Fab (labeling each starting from the N-terminus of PfCSP as A, B, or C for those of Class 1 and Aʹ, Bʹ, and Cʹ for those of Class 2). In Class 1, the angles between Fab-A/Fab-B, Fab-B/Fab-C, and Fab-C/Fab-A were 82.0°, 81.3°, and 84.4°, respectively. The angle between the equivalent Fabs (Fab-Aʹ/Fab-Bʹ) in Class 2 was similar at 79.6°; however, Fab-Cʹ was rotated in such a manner as to induce larger differences, with angles between Fab-Bʹ/Fab-Cʹ and Fab-Cʹ/Fab-Aʹ at 89.8° and 71.6°, respectively (Figure 2A). These changes in approach angles did not significantly alter the interactions of the antibody-epitope interface, as evidenced by similarities in epitope contacts and the buried surface area (BSA) (Figures 2B,C). We note that, even considering the angular diversity observed between some of the protomers in the atypical trimer, molecular modeling suggested that for Fab angles observed in the atypical trimer to be maintained, connections to a common constraint region would require hinge linkers to extend ~50 Å, suggesting each of the Fabs in the atypical trimer to likely emanate from separate IgGs.
Figure 2. L9 light chain mediates direct interactions with NPNV motif on PfCSP.
(A) The relative angles of approach between Fabs are shown with red and black cylinders representing the pseudo-2-fold axes for each Fab. The blue arrow shows rotation of Fab-Cʹ along the Z-axis. (B) L9 paratope residues involved in NPNV recognition are shown for each Fab from both the structures. R96L forms a H-bond with third Asn (N) of NPNV represented with yellow dotted lines. (C) Bar plots representing buried surface area for individual antibody-epitope contacts. The residues involved are labelled, colored orange in the sequence, and the somatically mutated residues are shown in the sequence alignment with L9 inferred germline. See also Figures S4, S5, and S8.
Each Fab bound to an NPNV motif with the majority of direct antigen interactions governed by the light chain of L9 (Figure 2B). The first Asn (N) in the NPNV tetrapeptide interacted with the backbone carbonyl of Y91L and T92L via hydrogen bonds (to clarify heavy and light chains, we add a subscript of “H” or “L”). Pro (P) at the second position in the NPNV tetrapeptide was involved in hydrophobic interactions with W52H and Y94L. R96L in the light chain third complementarity determining region (L-CDR3) interacted substantially with the third Asn (N) via several hydrogen bonds. Val (V) at position four had the least BSA to CSP (Figures 1C and S1) and resided in a hydrophobic pocket near W32L in the L-CDR1 (Figures 2B,C). The change from Val to Ala at position four between the preferred (NPNV) and secondary (NPNA) tetrapeptides underscores the promiscuity and lower binding affinity of L9 to the major repeats.7 Lastly, we note that the DPNA tetrapeptide, the motif that followed each NPNV tetrapeptide in the complete L9 epitope, had minimal direct interactions with antibody and served primarily as a spacer to properly orient the next NPNV tetrapeptide for binding to another L9 Fab.
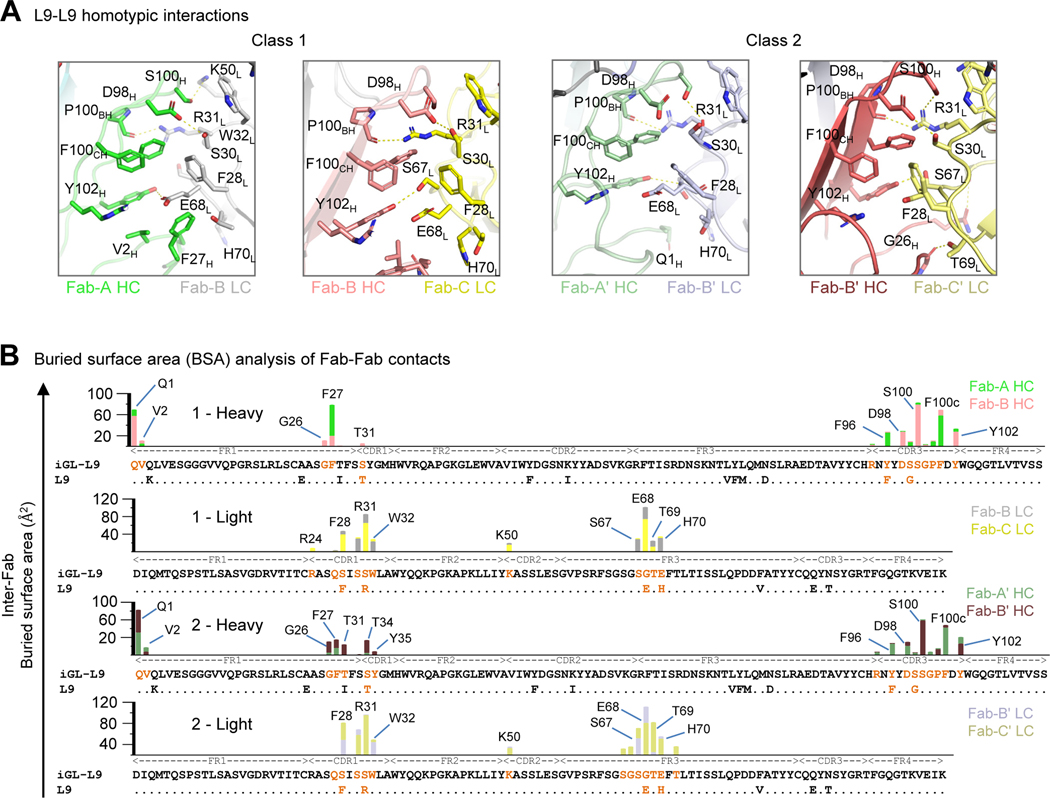
L9-L9 Homotypic Interactions Enable Multivalent Recognition
The repeat nature of the CSP antigen allows multiple antibodies to bind simultaneously.5–7,12 For example, major repeat-targeting (anti-NPNA) antibodies such as mAb-317 also utilize antibody-to-antibody homotypic interactions to enhance affinity to CSP.5,12 Our cryo-EM structures revealed each of the three Fabs in the atypical L9 trimer to be involved in extensive Fab-Fab homotypic interactions: The heavy chain of Fab-A interacted with the light chain of Fab-B, and the heavy chain of Fab-B interacted with the light chain of Fab-C. Similar interactions were observed in the structure of Class 2 as well. Generally, residues involved in the homotypic interface between Fab-A/Fab-B and Fab-B/Fab-C were identical in both classes (Figure 3A). The altered rotation of Fab-Cʹ in the minor structure (Figure 3A, far right panel), however, led to additional homotypic interactions between Fab-Bʹ/Fab-Cʹ (Figure 3B).
Figure 3. Homotypic interactions between heavy and light chains underlie formation of an L9 trimer.
(A) Antibody-antibody interfaces from both structures are shown. The key residues are labelled, and the H-bonds are shown with a yellow dotted line. Two out of total four light chain residues evolved via SHM, R31L and F28L, strongly stabilize the homotypic interface. (B) Superimposed bar plots depicting individual contributions to inter-Fab BSA from each Fab are shown. The SHM is shown by sequence alignment with L9 inferred-germline. See also Figures S4, S5, and S7.
The homotypic interface comprised residues primarily from H-FR1, H-CDR3, L-CDR1, and L-FR3 was stabilized by both ionic and hydrophobic interactions. Interestingly, the four residues in the light chain of L9 (F28, R31, E68, H70) that contributed most to the BSA had evolved through somatic hypermutation (Figure 3B). F28L and H70L were identified as rare mutations based on the frequencies of amino acid substitutions with respect to the germline V-gene analyzed using the cAb-Rep server13 (Figure S5). R31L formed a strong hydrogen bond with the backbone carbonyl of P100BH in both structures. E68L formed a hydrogen bond with Y102H in the Fab-A/Fab-B interface and may have electrostatic interactions with R94H; however, due to the mobility of the third Fab, this interaction was not conserved. A series of residues, including V2H, F27H, F96H, F100CH, and F28L, lined the interface, creating a hydrophobic pocket.
Binding and Mutational Analyses Delineate Homotypic Contributions
To decipher the contribution of homotypic interactions to the total antibody affinity, we measured the dissociation constants (KD) of L9 Fab, and apparent dissociation constants (KDapp) of L9 IgG and IgM to CSP peptides (Pep-I-IV, Figure 4A), containing different numbers of NPNV and NPNA tetrapeptides, using biolayer interferometry (BLI). Biotinylated Peptides (I-IV) were captured on a streptavidin (SA) biosensor with variable loading, optimized to avoid cross-binding between peptides (Figure S6). The biosensor with captured peptide was then dipped into solutions containing L9 Fab, IgG, or IgM and on- and off-rates determined. Pep-I (1 NPNV; 0 NPNA) had affinities to L9 Fab, IgG, and IgM of 583 nM, 21.5 nM, and 0.042 nM, respectively. L9 Fab and IgG had similar off-rates, but the IgG has a 25-fold higher on-rate than Fab, which led to increased affinity (Figure 4B, first row). The affinity of Fab to Pep-II (1 NPNV; 1 NPNA) did not increase compared with Pep-I (506 nM vs 583 nM), because of the weaker affinity of Fab to NPNA.7 L9 IgG, however, had ~6-fold higher affinity (21.5 nM vs 3.7 nM), and we attributed this increase to homotypic interactions between two IgGs; one tightly bound to NPNV and the other weakly bound to NPNA (Figure 4B, second row). With Pep-III (2 NPNV; 0 NPNA), L9 Fab bound with ~7-fold higher affinity compared to Pep-II, as each Fab now bound an NPNV repeat and interacted via homotypic interactions. Interestingly, even though Pep-II and Pep-III only differ by two residues (NPNAN versus NPNVD; Figure 4A), the ~7-fold higher affinity of L9 Fab to Pep-III highlighted the sequence specificity of L9 to the minor repeat over the major repeats. Similarly, L9 IgG had a ~6-fold higher affinity, which we interpreted as two IgGs, each bound to one NPNV and each with homotypic interactions (Figure 4B, third row). Pep-IV (3 NPNV; 1 NPNA) bound both L9 Fab and L9 IgG with substantially higher affinity, as this peptide could be bound by three Fabs (or IgGs), each recognizing a single NPNV repeat, and the longer peptide could accommodate multiple homotypic interactions (Figure 4B, fourth row).
Figure 4. Binding analysis with CSP peptides defines epitope and homotypic components of L9 recognition.
(A) Correspondence of peptides I-IV and PfCSP sequence. Black italicized font signifies residues not part of the complete L9 epitope. (B) Affinity characterization of L9 Fab/IgG/IgM to CSP peptides is shown by biolayer interferometry (BLI). Y-axis represents the response (nm) and X-axis represents time (s). Units of Kon and Koff are M−1s−1 and s−1, respectively. The potential mode of recognition from Fab/IgG/IgM to different peptides is shown in pictorial representation. The green and blue double headed arrows represent epitope and homotypic interactions, respectively. The cross on the arrows show weak interactions. (C) Free energies, as calculated from KD in panel B, shown after subtraction of the shortest peptide (Pep-I) to demarcate increase in binding energy from additional L9s. See also Figure S6.
To assist in quantifying the contribution of multivalent and homotypic interactions, we calculated the free energy of binding (ΔG) from experimental KDs, subtracting the free energy of binding to Pep-I (which could not accommodate multivalent and homotypic interactions) from peptides Pep-II though Pep-IV (which could accommodate higher-order associations) (Figure 4C). Notably, the increase in affinity was greatest in the IgG context, and lowest in the IgM context. For L9 IgG, adding homotypic and less preferred NPNA with two L9s yielded an increase in affinity of −1.1 kcal/mol; adding homotypic and the preferred NPNV with two L9s yielded an increase in affinity of −2.2 kcal/mol; adding two homotypic and two preferred NPNVs with three L9s yielded an increase in affinity of −3.3 kcal/mol, corresponding to a decrease in KD of over two orders of magnitude.
To demonstrate that these increases in affinity were dependent on free L9 assembling to form homotypic contacts, we reversed the BLI format, capturing L9 IgG on anti-human Fc (AHC) biosensors and dipping the immobilized L9 IgG into solutions containing varying concentrations of peptide (Figure S6B). Notably, in this reverse format, L9 IgG had comparable affinities to peptides Pep-I through Pep-IV.
To delineate the role of key residues in mediating both direct interactions with CSP as well as homotypic L9-L9 interactions, we produced alanine and germline-reverted mutants of interface residues. These mutants were assessed for their binding to peptides (Pep-I through Pep-IV) using AlphaLISA; a bead-based assay that sensitively quantifies protein-protein interactions. With Pep-I (1 NPNV; 0 NPNA), mutant R96AL, which altered a critical L9-epitope interaction, showed substantially reduced binding (Figure 5A, first column). Other mutants, designed to alter key residues involved in homotypic interactions, showed largely unaltered binding, as anticipated from the inability of the shorter Pep-I (with only a single 1 NPNV site) to interact with multiple IgGs (Figure 5A, first column). Interestingly, D98AH did show enhanced binding, likely explained by a reduction in charge-charge repulsion between D98H and the fifth Asp in NPNVD (Figure S4). With Pep-II (1 NPNV; 1 NPNA), both epitope and homotypic mutants displayed diminished signals compared to L9 wildtype (WT) (Figure 5A second column). With Pep-III (2 NPNV; 0 NPNA), we observed a similar pattern of binding reduction as with Pep-II, though with higher AlphaLISA signals, likely because of the high overall affinity to Pep-III (−12.8 kcal/mol) (Figure 5A, third column). With Pep-IV (3 NPNV; 1 NPNA), against a similar pattern of binding reduction was observed, though even less pronounced then for Pep-III, likely because of the even higher overall affinity (−13.9 kcal/mol). Consistent with this, mutant R96L did not show detectable AlphaLISA signal to peptides (Pep-I-III) but did show weak signal for Pep-IV (Figure 5A, fourth column).
Figure 5. L9 mutant analyses with AlphaLISA and in vivo malaria protection demonstrate both epitope and homotypic interactions are key to function.
(A) L9 epitope and homotypic mutants were analyzed by AlphaLISA assay for their binding to peptides I-IV. X-axis represents AlphaLISA signal (relative to L9 WT) and error bars represent standard deviation (SD). (B) Schema for mouse malaria challenge model. Two hours after passive transfer of antibodies, albino-C57BL/6 mice are infected with rodent malaria parasites (Pb-PfCSP-Luc) engineered to express the P falciparum circumsporozoite protein (PfCSP) and luciferase genes. At 42hrs post infection, luciferin substrate is administered, and bioluminescence is measured by an in vivo imaging system (IVIS) to quantify the liver stage (day 2) burden of infection. (C) Protective efficacy against Pb-PfCSP-Luc sporozoite challenge following passive transfer of 50 μg of WT L9 (black) and L9 mutants (green and blue) is compared with naïve (background, white) and maximum burden (untreated, red) control mice. Bioluminescence or total flux (photon/sec) is shown for each mouse, with horizontal lines denoting the geometric mean; error bars represent SD. N = 10 mice per group. Data shown are a combination of two independent experiments. Mann–Whitney test was used to statistically compare each group to the L9 WT; only significant p values are shown with ** = 0.0016; *** = 0.0005 – 0.0007; ****=<0.0001, respectively. See also Figure S9.
Both Epitope and Homotypic Interactions Are Critical for Potent L9-Protective Efficacy
Our binding analysis with L9 mutants revealed the importance of both epitope and homotypic interactions for high affinity to CSP. To assess the role of these interactions in the in vivo protective capacity of L9, we evaluated L9 mutants for their ability to reduce malaria infection in mice (Figure 5B). Briefly, mice were treated with antibodies (50 ug dose) 2 h prior to intravenous challenge with 2,000 malaria sporozoites, which had been genetically modified to express PfCSP and to encode GFP and luciferase markers.14 Malaria liver stage infection was quantified at 2 d after challenge by injecting mice with D-luciferin and detecting liver luminescence.15
In data combined from two individual experiments, each with five mice per group, naïve mice averaged about 2×105 luminescence, and untreated mice ~107 luminescence (Max burden). For epitope, the R96AL mutant showed about 1×106 luminescence, significantly reduced protection from the L9-treated mice which had about 2×105 luminescence (Figure 5C). For mutants involved in homotypic interactions, residues F28L, R31L, and E68L showed the most significant reductions in protective capacity (Figure 5C), consistent with the key roles each of these residues play in stabilizing the homotypic interface (Figure 3A). Together, these results demonstrated the importance of both epitope and homotypic interactions in maintaining potent L9 protective capacity against malaria.
Conservation of Complete L9 Epitope in P. falciparum Field Isolates
To gain insight into the conservation of the complete L9 epitope of 27 amino acids within the broader context of diverse P. falciparum field isolates, we looked at the residue-by-residue conservation of the complete L9 epitope (Figure 6A) as well as the conservation of NPNV motifs, specifically recognized by L9. The majority (~83%) of P. falciparum isolates contained three NPNVs separated by a tetra-peptide linker (Figure 6B), and phylogenetic analysis indicated conservation of three NPNV to be spread throughout the tree (Figure 6C), with a small fraction (4%) having four NPNV repeats. Interestingly, analysis of the atypical L9 trimer indicated the association enabling trimer formation could not be further extended to accommodate a fourth antibody (Figure 6D). Thus, the molecular mechanism of three L9-antibodies binding as an atypical trimer appears to provide optimal recognition for three NPNV repeats – which is the exact number of NPNV repeats that is most prevalently conserved.
Figure 6. L9 recognizes a 27-residue stretch of CSP, which is conserved in most P. falciparum isolates.
(A, B, C) Sequence logo plot and dendrogram showing sequence conservation of L9 epitope in field isolates of Plasmodium falciparum. Some common strains are highlighted. The scale bar represents number of substitutions per site. (D) Association details of atypical trimeric. Surface representation highlighting PfCSP in purple with ~28 Å distance between the two termini. The fourth Fab (shown in red) was modelled to extend the same interactions as observed in prior protomers, it clashed with Fabs A and C.
Discussion
Antigens like PfCSP are unusual in the repetitive nature of specific peptide segments, and their structures are often highly disordered. This may be a strategy of the parasite to direct antibody responses toward repeat regions and to limit antibodies against other neutralizing sites. Nevertheless, there are now a number of monoclonal antibodies to distinct repeat regions of PfCSP that potently inhibit infection by P. falciparum. For example, antibodies mAb-311, −17, −850 use recognition of the major repeat tetrapeptide, NANP, coupled to homotypic antibody-antibody interactions to induce a continual spiral of antibody-NANP repeats.3,5 Antibody CIS436 recognizes a unique ~15-residue epitope in the junctional region to achieve potent protection. Here, we show that L9 utilizes elements of both homotypic interactions and unique epitope; in specific, homotypic interactions between the heavy chain on one Fab and the light chain of a second Fab induce the formation of an atypical trimer that recognizes a unique 27-residue epitope comprising three NPNV repeats, each followed by a DPNA ‘spacer’.
The homotypic interactions that we observe with L9 differ from those observed with the major repeat recognizers mAb-311, −317, −850, and other IGHV3–33-utilizing antibodies.3–5 mAb-311 and mAb-850 display an open side-on homotypic interface that allows multiple Fabs to bind simultaneously and to induce formation of a continual spiral utilizing homotypic interactions between both heavy and light chains (Figure S7). By contrast L9 has a closed side-on homotypic interface that induces the formation of an atypical trimer through interactions between heavy chain of each Fab and the light chain of each adjacent Fab.
With L9, avidity-enhancing homotypic interactions increased binding energy versus that of a single NPNV repeat by 1–3 kcal/mol, depending on the number of additional repeat sequences. Despite adding only a few kcal/mol of binding energy, homotypic interactions were optimized by affinity maturation, with critical contact residues such as the rare mutation S28FL arising during affinity maturation. By contrast, epitope contacts appeared to be fully present in the inferred germline light chain, suggesting appropriate recombination as the basis for initial affinity, and maturation adding affinity-enhancing recognition of a highly conserved 27-residue stretch containing three NPNV repeats. Indeed, comparison of the L9 structure obtained here with that of the previously determined structure of L9 chimera bound to a six-residue peptide (PDB: 7RQQ)10 showed virtually identical recognition (Figure S8).
The defined molecular mechanism of L9 recognition, with homotypic interactions enhancing binding to a unique extended epitope, provides insight for developing an improved L9 antibody. For epitope-based improvement, the Fab affinity of L9 to the preferred NPNV tetrapeptide was only ~500 nM, far lower in affinity than expected for such a potently protective antibody suggesting considerable opportunities for enhancing this aspect of L9 recognition. We note, however, that such improvement might be tempered by a requirement for reduced affinity to the more prevalent NPNA, as higher NPNA affinity might compete for binding to the less prevalent NPNV-minor repeats. In this context, we note that the highest correlation between protective efficacy and peptide binding occurred with Pep-II and Pep-III (R2 correlations of 0.47 and 0.62, respectively), whereas binding to Pep-I (the shortest) and Pep-IV (the longest) did not correlate with protection (Figure S9). Overall, it will likely be important to balance homotypic affinity and aggregation, as inter-IgG interactions can cause solubility issues if they extend in an unconstrained manner.
In terms of the complete L9 27-residue epitope –we show that three- or four-NPNV repeats are present in ~90% of P. falciparum isolates - although the residue-by-residue conservation is even higher, as the replaced sequence is most often NPNA, which is identical in 3 out of 4 positions with NPNV. Its high conservation and its recognition by a potently neutralizing antibody make the complete L9 epitope an interesting target for vaccine design. To advance, much depends on the ease by which antibodies like L9 can be elicited by class-based vaccine approaches, as is being tried for HIV-1.10,16 It would also be interesting to substitute the newly defined 27-residue epitope alone as an epitope for vaccination or consider adding it to the recently approved RTS,S/AS0117–19 and R2120–22 subunit vaccines, which do not currently display any of the L9 epitope. A closely related alternative would be to create a separate multivalent vaccine incorporating the 27-residue epitope, with and without the neighboring junctional region recognized by CIS43, to see if such an L9-epitope-based vaccine would either protect by itself or synergize with RTS,S and R21 vaccines.
STAR★METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Peter D. Kwong (pdkwong@nih.gov).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
Cryo-EM structure coordinates and electron density maps for L9 and PfCSP complex have been deposited with the Protein Data Bank and Electron Microscopy Data Bank (Class 1: PDB 8EK1, EMD-28192; Class 2: PDB 8EKA, EMD-28196). This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
Female 6- to 8-weeks old B6(Cg)-Tyrc-2J/J albino and Balb/c mice were obtained from The Jackson Laboratory (Bar Harbor, ME). All animals were maintained and cared for in accordance with the American Association for Accreditation of Laboratory Animal Care Standards. All mouse procedures were performed according to protocols approved by the Institutional Animal Care and Use Ethics Committees of the National Institute of Allergy and Infectious Diseases (Animal Study Protocol VRC-20-0855).
Sporozoites
Transgenic P. berghei (strain ANKA 676m1c11, MRA-868) sporozoites expressing full-length P. falciparum CSP and a green fluorescent protein/luciferase fusion protein (Pb-PfCSP-Luc) were harvested from salivary glands of infected mosquitoes, as previously described.7,15 Briefly, Anopheles stephensi (Nijmegan) mosquitoes were obtained from a colony reared at the Laboratory of Malaria and Vector Research (NIAID, NIH). Female mosquitoes were allowed to feed on 6- to 8-week-old female Balb/c mice infected with blood-stage Pb-PfCSP-Luc parasites. After infection, mosquitoes were maintained in an incubator at 19–20°C and supplied with a sterile cotton pad soaked in 10% sucrose every 48 hrs. Eighteen to 21 days following mosquito infections, salivary glands were dissected and ground in 400 μL of L-15 medium (Millipore-Sigma, Burlington, MA), and viable sporozoites were counted in a Neubauer chamber.
Cell Lines
FreeStyle 293-F (cat# R79007) and Expi293F cells (cat# A14528; RRID: CVCL_D615) were purchased from ThermoFisher Scientific Inc. FreeStyle 293-F cells were maintained in FreeStyle 293 Expression Medium, while Expi293F cells were maintained in Expi Expression Medium. The above cell lines were used directly from the commercial sources and cultured according to manufacturer suggestions.
METHOD DETAILS
Antibody Expression
Antibody variable heavy chain and light chain sequences were codon optimized, synthesized and cloned into a VRC8400 (CMV/R expression vector)-based IgG1 vector as previously described.23 The variants were expressed by transient transfection in Expi293 cells (ThermoFisher Scientific, Waltham, MA) using Turbo293 transfection reagent (SPEED BioSystems, Gaithersburg, MD) according to the manufacturer’s recommendation. 50 μg plasmid encoding heavy-chain and 50 μg plasmid encoding light-chain variant genes were mixed with the transfection reagents, added to 100 ml of cells at 2.5 × 106/ml, and incubated in a shaker incubator at 120 rpm, 37°C, 9% CO2. At 5 days post-transfection, cell culture supernatant was harvested and purified with a Protein A (GE Healthcare, Chicago, IL) column. The antibody was eluted using IgG Elution Buffer (ThermoFisher Scientific, Waltham, MA) and were brought to neutral pH with 1 M Tris-HCl, pH 8.0. Eluted antibodies were dialyzed against PBS overnight before use.
Cryo-EM sample preparation, grid preparation and data collection
The sample for cryo-EM was prepared by mixing L9 Fab to PfCSPm at 2:1 molar ratio. The complex was incubated overnight at 4 C and flash frozen until ready for use. To prepare grids of the L9-PfCSP complex, 3 μL of protein sample were applied to freshly glow-discharged (easiGLow) C-flat grids (Protochips, CF1.2/1.3–3Au). Blotting was done using a Vitrobot Mark IV (Thermo-Fisher), with 5 s blotting time and 8 pN blotting force at 6 °C in 100 % humidity. Grids were vitrified by plunging into liquid ethane and stored in liquid nitrogen before examination by cryo-EM. Images were recorded on a Glacios TEM (Thermo Fisher) at 200 kV and recorded at 36,000X magnification with a defocus range of −0.3 to −2.2 μm on K3 direct electron detector (Gatan) in super-resolution mode.
Cryo-EM data processing and refinement
Motion correction, contrast transfer function (CTF) estimation, particle picking, extraction, 2D classification, ab initio model generation, 3D refinements and local resolution estimation were carried out in cryoSPARC 3.3.1.24 The 3D reconstructions were performed using C1 symmetry for both the classes. The coordinates of apo-L9 and L9/F10 chimera structures, PDB entries 7RQP and 7RQQ,10 were employed as initial models for fitting the sharpened cryo-EM map of the L9-PfCSP structures (Table 1). Manual and automated model building were iteratively performed using Coot25 and real space refinement in Phenix26 to accurately fit the coordinates to the electron density map. Molprobity27 and EMRinger28 was used to validate geometry and check structure quality. UCSF ChimeraX29 was used for map-fitting cross correlation calculation (Fit-in-Map tool) and for figure preparation.
Table 1.
Cryo-EM single particle data collection and refinement statistics.
| PfCSP in complex with L9 (Class 1) | PfCSP in complex with L9 (Class 2) | |
|---|---|---|
| EMDB ID | 28192 | 28196 |
|
| ||
| PDB ID | 8EK1 | 8EKA |
|
| ||
| Data Collection | ||
| Microscope | FEI Glacios | FEI Glacios |
| Voltage (kV) | 200 | 200 |
| Electron dose (e− /Å2) | 63.7 | 63.7 |
| Detector | Gatan K3 | Gatan K3 |
| Pixel Size (Å) | 0.46 | 0.46 |
| Defocus Range (μm) | −0.3 to −2.2 | −0.3 to −2.2 |
| Magnification | 36000 | 36000 |
| Reconstruction | ||
| Software | cryoSparcV3.3 | cryoSparcV3.3 |
| Particles | 117,479 | 62,735 |
| Symmetry | C1 | C1 |
| Box size (pix) | 320 | 320 |
| Resolution (Å) (FSC0.143) | 3.64 | 3.72 |
| Refinement | ||
| Software | Phenix 1.18 | Phenix 1.18 |
| Protein residues | 1102 | 905 |
| Chimera CC | 0.83 | 0.84 |
| EMRinger Score | 2.83 | 2.26 |
| R.m.s. deviations | ||
| Bond lengths (Å) | 0.004 | 0.003 |
| Bond angles (°) | 0.735 | 0.681 |
| Validation | ||
| Molprobity score | 2.04 | 2.03 |
| Clash score | 9.39 | 7.54 |
| Favored rotamers (%) | 99.47 | 100 |
| Ramachandran | ||
| Favored regions (%) | 90.31 | 87.40 |
| Allowed regions (%) | 9.23 | 12.26 |
| Outlier regions (%) | 0.46 | 0.34 |
Affinity measurements by BLI
Antibody (Fab/IgG/IgM) binding affinity to various ligands were measured using biolayer interferometry on an Octet Red384 instrument (fortéBio) with streptavidin capture biosensors (fortéBio) in solid black tilt-well 96-well plates (Geiger Bio-One). Assays were performed with agitation at 30°C. Biotinylated Peptides (I-IV) were diluted to 0.1 ug/mL in (PBS + 1% BSA) and were immobilized with varying loading times (45s, 30s, 15s, 3s) for each peptide to reduce the density (<0.01 nm) on the biosensor to avoid avidity. This step was followed by a 60s baseline in buffer (PBS + 1% BSA). Association with Fab (serially diluted from 2000 to 2.7 nM), IgG (serially diluted from 1000 to 1.3 nM), and IgM (serially diluted from 100 to 0.1 nM) was done for 240–300s, followed by a dissociation step in buffer for 1200s. For reverse format, IgGs were diluted to 5 ug/mL in (PBS + 1% BSA), immobilized on AHC biosensors to a density of 0.1 nm, and dipped into varying peptide concentrations. In all Octet measurements, parallel correction to subtract systematic baseline drift was carried out by subtracting the measurements recorded for a loaded sensor incubated in PBS. Data analysis was carried out using Octet software, version 9.0. Experimental data were fitted globally with a 1:1 Langmuir model of binding for all the antigens.
AlphaLISA Characterization of L9 Variants
AlphaLISA® (Perkin-Elmer, Waltham, MA) is a bead-based proximity assay in which singlet oxygen molecules, generated by high energy irradiation of Donor beads, transfer to Acceptor beads, which are within a distance of approximately 200 nm. It is a sensitive high throughput screening assay that does not require washing steps. A cascading series of chemical reactions results in a chemiluminescent signal. Purified antibodies were diluted to 10 nM in AlphaLISA® buffer (PBS + 0.05% Tween-20 + 0.5 mg/mL BSA). Subsequently, 5 μL of the IgGs were transferred to an OptiPlate-384 assay plate (white opaque, PerkinElmer, Waltham, MA), mixed with 10μL (10 nM final conc.) of biotinylated peptide probe and 10 uL (10 μg/mL final conc.) of Anti-human IgG (Fc specific; Perkin-Elmer, Waltham, MA) acceptor beads. After an hour of incubation at RT, non-shaking, 25 uL (40 μg/mL final conc.) of streptavidin donor beads (Perkin-Elmer, Waltham, MA) were added. The plate was then incubated for 30 min at RT in the dark before the AlphaLISA signal was detected using a SpectraMax® i3x multi-mode microplate reader (Molecular Devices, San Jose, CA).
Evaluation of Protective Efficacy of Antibodies in a Mouse Malaria Challenge Model
To assess the protective efficacy of L9 variant mAbs in vivo, 50 μg of mAbs were diluted in sterile filtered 1x PBS (pH 7.4; total volume 200 μL/mouse) and administered into the tail veins of female 6- to 8-week old B6(Cg)-Tyrc-2J/J albino mice (The Jackson Laboratory). After 2 hours, mice were intravenously challenged in the tail vein with 2,000 freshly harvested Pb-PfCSP-Luc sporozoites in Leibovitz’s L-15 medium (Thermo Fisher Scientific, Waltham, MA, USA). Forty-two hours post challenge, mice were injected intraperitoneally (i.p.) with 150 μL d-luciferin (30 mg/mL, PerkinElmer, Waltham, MA), anesthetized with isoflurane and imaged with an IVIS® Spectrum in vivo imaging system (PerkinElmer) 10 minutes after luciferin injection. Parasite liver load was quantified by analyzing the corresponding region of interest (ROI) in the upper abdominal region; bioluminescence or the total flux (photons/second; p/s) was measured using the manufacturer’s software (Living Image 4.5, PerkinElmer).
Epitope Conservation Analysis
PfCSP protein sequences were retrieved from GenBank. Partial or duplicated sequences were removed from the dataset. The unique sequences used to count the consecutive NVDP repeats. Logo plot of 27-residue L9 epitope was prepared by weblogo.30 The neighbor joining tree was visualized by FigTree (http://tree.bio.ed.ac.uk/software/figtree/).
QUANTIFICATION AND STATISTICAL ANALYSIS
For protection studies, statistical analysis was performed in Prism 9.01 (GraphPad) using two-tailed Mann–Whitney test assuming non-normal distribution, as described in the figure legend. The correlation between the normalized liver burden and AlphaLISA signal was calculated using two-tailed Pearson’s correlation method in Prism 9.01 (GraphPad).
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, Peptides, and Recombinant Proteins | ||
| Recombinant PfCSP | Produced in house | N/A |
| L9 IgG | Produced in house | N/A |
| L9 Fab | Produced in house | N/A |
| L9 IgM | Produced in house | N/A |
| Biotinylated junctional peptide I | Genscript | Order ID:# U134AFB120 |
| Biotinylated junctional peptide II | Genscript | Order ID:# U134AFB120 |
| Biotinylated junctional peptide III | Genscript | Order ID:# U134AFB120 |
| Biotinylated junctional peptide IV | Genscript | Order ID:# U134AFB120 |
| Critical Commercial Assays | ||
| Streptavidin Donor Beads, for AlphaLISA | PerkinElmer | CAT#:6760002 |
| Anti-human Fc Acceptor Beads, for AlphaLISA | PerkinElmer | CAT#:AL103M |
| Octet® Streptavidin (SA) Biosensor | Sartorius | CAT#:18-5019 |
| Octet® Anti-Human Fc Capture (AHC) Biosensors | Sartorius | CAT#:18-5060 |
| Deposited Data | ||
| PDB file | This paper | 8EK1 |
| PDB file | This paper | 8EKA |
| EMDB file | This paper | 28192 |
| EMDB file | This paper | 28196 |
| Experimental Models: Cell Lines | ||
| Human: Expi293 cell | Thermo Fisher Scientific | Cat#A14527 |
| Experimental Models: Organisms/Strains | ||
| Mouse: B6(Cg)-Tyrc-2J/J albino | The Jackson Laboratory | JAX:000058 |
| Mouse: Balb/c | The Jackson Laboratory | JAX:000651 |
| Sporozoite: P. berghei sporozoite expressing PfCSP, GFP, and luciferase | Flores-Garcia et al., 2019 | N/A |
| Recombinant DNA | ||
| Plasmodium falciparum circumsporozoite protein (clone 3D7) | PlasmoDB | PF3D7_0304600.1 |
| pVRC8400 huIgG1 | Genscript | N/A |
| pVRC8400 huIgK | Genscript | N/A |
| Software and Algorithms | ||
| Prism 9.0.1 | GraphPad | https://www.graphpad.com/ |
| Microsoft Office | Microsoft | https://www.office.com/ |
| IMGT/V-quest | Brochet et al., 2008 | https://www.imgt.org/IMGTindex/V-QUEST.php |
| Geneious 2020.2 | Biomatters | https://www.geneious.com |
| cryoSPARC v3.3.1 | Punjani et al., 2017 | https://cryosparc.com/ |
| Coot v0.9.4 | Emsley et al., 2004 | https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ |
| ChimeraX v1.2.5 | Goddard et al., 2018 | https://www.cgl.ucsf.edu/chimerax/docs/user/index.html |
| Phenix v1.18.2 | Adams et al., 2010 | https://www.ks.uiuc.edu/Development/Download/download.cgi?PackageName=NAMD |
| EMRinger | Barad et al., 2015 | http://emringer.com/ |
| MolProbity | Williams et al., 2018 | http://molprobity.biochem.duke.edu |
| PISA | Krissinel and Henrick, 2007 | https://www.ebi.ac.uk/pdbe/pisa/ |
| The PyMOL Molecular Graphics System | Schrödinger, LLC | https://pymol.org/2/ |
| Gene-specific substitution profile | Sheng et al., 2017 | https://cab-rep.c2b2.columbia.edu/ |
| Other | ||
| IVIS® Spectrum In Vivo Imaging System | PerkinElmer | N/A |
| SpectraMax® i3x | Molecular Devices | N/A |
Highlights.
Cryo-EM structures of L9 with PfCSP reveal atypical trimer recognition
Each L9 Fab in the atypical trimer mediates direct interactions with an NPNV repeat
Both epitope and homotypic interactions are critical to L9 recognition of PfCSP
The 27 residue L9 epitope is conserved in ~90% of P. falciparum isolates
Acknowledgements
We thank the cryo-EM center at NIDDK and NIAID for assistance with cryo-EM data collection. We also thank J. Netland, D. Rawlings, and M. Pepper for providing L9 IgM, B. Flynn for assistance with peptides, J. Gorman for assistance with cryo-EM data processing, and J. Stuckey for assistance with figures, and members of the Vaccine Research Center, NIAID, NIH, for discussions and comments on the manuscript. Support for this work was provided by the Intramural Research Program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases (NIAID).
Footnotes
Declaration of Interests
L.T.W and R.A.S. have submitted a US Provisional Patent Application 62/842,590, filed 3 May 2019, describing antibody L9. The other authors declare no competing interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. (2021). https://www.who.int/publications/i/item/9789240040496.
- 2.Cockburn IA, and Seder RA (2018). Malaria prevention: from immunological concepts to effective vaccines and protective antibodies. Nat Immunol 19, 1199–1211. 10.1038/s41590-018-0228-6. [DOI] [PubMed] [Google Scholar]
- 3.Kucharska I, Binter S, Murugan R, Scally SW, Ludwig J, Prieto K, Thai E, Costa G, Li K, Horn GQ, et al. (2022). High-density binding to Plasmodium falciparum circumsporozoite protein repeats by inhibitory antibody elicited in mouse with human immunoglobulin repertoire. PLoS Pathog 18, e1010999. 10.1371/journal.ppat.1010999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pholcharee T, Oyen D, Flores-Garcia Y, Gonzalez-Paez G, Han Z, Williams KL, Volkmuth W, Emerling D, Locke E, Richter King C, et al. (2021). Structural and biophysical correlation of anti-NANP antibodies with in vivo protection against P. falciparum. Nat Commun 12, 1063. 10.1038/s41467-02121221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oyen D, Torres JL, Cottrell CA, Richter King C, Wilson IA, and Ward AB (2018). Cryo-EM structure of P. falciparum circumsporozoite protein with a vaccine-elicited antibody is stabilized by somatically mutated inter-Fab contacts. Sci Adv 4, eaau8529. 10.1126/sciadv.aau8529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kisalu NK, Idris AH, Weidle C, Flores-Garcia Y, Flynn BJ, Sack BK, Murphy S, Schon A, Freire E, Francica JR, et al. (2018). A human monoclonal antibody prevents malaria infection by targeting a new site of vulnerability on the parasite. Nat Med 24, 408–416. 10.1038/nm.4512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang LT, Pereira LS, Flores-Garcia Y, O’Connor J, Flynn BJ, Schon A, Hurlburt NK, Dillon M, Yang ASP., Fabra-Garcia A., et al. (2020). A Potent Anti-Malarial Human Monoclonal Antibody Targets Circumsporozoite Protein Minor Repeats and Neutralizes Sporozoites in the Liver. Immunity 53, 733–744 e738. 10.1016/j.immuni.2020.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaudinski MR, Berkowitz NM, Idris AH, Coates EE, Holman LA, Mendoza F, Gordon IJ, Plummer SH, Trofymenko O, Hu Z, et al. (2021). A Monoclonal Antibody for Malaria Prevention. N Engl J Med 385, 803–814. 10.1056/NEJMoa2034031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu RL, Idris AH, Berkowitz NM, Happe M, Gaudinski MR, Buettner C, Strom L, Awan SF, Holman LA, Mendoza F, et al. (2022). Low-Dose Subcutaneous or Intravenous Monoclonal Antibody to Prevent Malaria. N Engl J Med 387, 397–407. 10.1056/NEJMoa2203067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang LT, Hurlburt NK, Schon A, Flynn BJ, Flores-Garcia Y, Pereira LS, Kiyuka PK, Dillon M, Bonilla B, Zavala F, et al. (2022). The light chain of the L9 antibody is critical for binding circumsporozoite protein minor repeats and preventing malaria. Cell Rep 38, 110367. 10.1016/j.celrep.2022.110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krissinel E, and Henrick K (2007). Inference of macromolecular assemblies from crystalline state. J Mol Biol 372, 774–797. 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Imkeller K, Scally SW, Bosch A, Martí GP, Costa G, Triller G, Murugan R, Renna V, Jumaa H, Kremsner PG, et al. (2018). Antihomotypic affinity maturation improves human B cell responses against a repetitive epitope. Science 360, 1358–1362. 10.1126/science.aar5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo Y, Chen K, Kwong PD, Shapiro L, and Sheng Z (2019). cAb-Rep: A Database of Curated Antibody Repertoires for Exploring Antibody Diversity and Predicting Antibody Prevalence. Front Immunol 10, 2365. 10.3389/fimmu.2019.02365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espinosa DA, Yadava A, Angov E, Maurizio PL, Ockenhouse CF, and Zavala F (2013). Development of a chimeric Plasmodium berghei strain expressing the repeat region of the P. vivax circumsporozoite protein for in vivo evaluation of vaccine efficacy. Infect Immun 81, 2882–2887. 10.1128/IAI.00461-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flores-Garcia Y, Herrera SM, Jhun H, Perez-Ramos DW, King CR, Locke E, Raghunandan R, and Zavala F (2019). Optimization of an in vivo model to study immunity to Plasmodium falciparum pre-erythrocytic stages. Malar J 18, 426. 10.1186/s12936-019-3055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowe JE Jr. (2016). Teaching a Clone to Walk, One Step at a Time. Cell 166, 1360–1361. 10.1016/j.cell.2016.08.039. [DOI] [PubMed] [Google Scholar]
- 17.Rts SCTP, Agnandji ST, Lell B, Soulanoudjingar SS, Fernandes JF, Abossolo BP, Conzelmann C, Methogo BG, Doucka Y, Flamen A, et al. (2011). First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 365, 1863–1875. 10.1056/NEJMoa1102287. [DOI] [PubMed] [Google Scholar]
- 18.Rts SCTP, Agnandji ST, Lell B, Fernandes JF, Abossolo BP, Methogo BG, Kabwende AL, Adegnika AA, Mordmuller B, Issifou S, et al. (2012). A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med 367, 2284–2295. 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olotu A, Fegan G, Wambua J, Nyangweso G, Leach A, Lievens M, Kaslow DC, Njuguna P, Marsh K, and Bejon P (2016). Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children. N Engl J Med 374, 2519–2529. 10.1056/NEJMoa1515257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collins KA, Snaith R, Cottingham MG, Gilbert SC, and Hill AVS (2017). Enhancing protective immunity to malaria with a highly immunogenic virus-like particle vaccine. Sci Rep 7, 46621. 10.1038/srep46621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Datoo MS, Natama MH, Some A, Traore O, Rouamba T, Bellamy D, Yameogo P, Valia D, Tegneri M, Ouedraogo F, et al. (2021). Efficacy of a low-dose candidate malaria vaccine, R21 in adjuvant Matrix-M, with seasonal administration to children in Burkina Faso: a randomised controlled trial. Lancet 397, 1809–1818. 10.1016/S0140-6736(21)00943-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Datoo MS., Natama HM., Some A., Bellamy D., Traore O., Rouamba T., Tahita MC., Ido NFA., Yameogo P., Valia D., et al. (2022). Efficacy and immunogenicity of R21/Matrix-M vaccine against clinical malaria after 2 years’ follow-up in children in Burkina Faso: a phase 1/2b randomised controlled trial. Lancet Infect Dis. 10.1016/S1473-3099(22)00442-X. [DOI] [PubMed]
- 23.Kong R, Duan H, Sheng Z, Xu K, Acharya P, Chen X, Cheng C, Dingens AS, Gorman J, Sastry M, et al. (2019). Antibody Lineages with Vaccine-Induced Antigen-Binding Hotspots Develop Broad HIV Neutralization. Cell 178, 567–584 e519. 10.1016/j.cell.2019.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Punjani A, Rubinstein JL, Fleet DJ, and Brubaker MA (2017). cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat Methods 14, 290–296. 10.1038/nmeth.4169. [DOI] [PubMed] [Google Scholar]
- 25.Emsley P, and Cowtan K (2004). Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60, 2126–2132. 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 26.Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, et al. (2010). PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr 66, 213–221. 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis IW, Murray LW, Richardson JS, and Richardson DC (2004). MOLPROBITY: structure validation and all-atom contact analysis for nucleic acids and their complexes. Nucleic Acids Res 32, W615–619. 10.1093/nar/gkh398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barad BA, Echols N, Wang RY, Cheng Y, DiMaio F, Adams PD, and Fraser JS (2015). EMRinger: side chain-directed model and map validation for 3D cryo-electron microscopy. Nat Methods 12, 943–946. 10.1038/nmeth.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goddard TD, Huang CC, Meng EC, Pettersen EF, Couch GS, Morris JH, and Ferrin TE (2018). UCSF ChimeraX: Meeting modern challenges in visualization and analysis. Protein Sci 27, 14–25. 10.1002/pro.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crooks GE, Hon G, Chandonia JM, and Brenner SE (2004). WebLogo: a sequence logo generator. Genome Res 14, 1188–1190. 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Cryo-EM structure coordinates and electron density maps for L9 and PfCSP complex have been deposited with the Protein Data Bank and Electron Microscopy Data Bank (Class 1: PDB 8EK1, EMD-28192; Class 2: PDB 8EKA, EMD-28196). This paper does not report original code. Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.