Background:
Although the cornerstone treatment for deep vein thrombosis (DVT) remains anticoagulation, clinicians perform stenting or angioplasty (SA) in particular patients. To assess the effects of SA in this setting, we performed a systematic review of randomized controlled trials.
Methods:
Based on the Cochrane standards, we searched the Cochrane CENTRAL, MEDLINE, Embase, CINAHL, LILACS and IBECS databases, and trial registries. Our primary outcomes were post-thrombotic syndrome (PTS), venous thromboembolism (VTE) and all-cause mortality.
Results:
We included 7 randomized controlled trial (1485 participants). There was no clinically significant difference between SA and best medical practice (BMP) for the additional treatment of acute DVT regarding PTS (standardized mean difference −7.87, 95% confidence interval [CI] −12.13 to −3.61; very low-certainty) and VTE (risk ratio [RR] 1.19, 95% CI 0.28–5.07, very low-certainty), and no deaths. Compared to BMP, the SA plus BMP and thrombolysis results in little to no difference in PTS (mean difference [MD] −1.07, 95% CI −1.12 to −1.02, moderate-certainty), VTE (RR 1.48, 95% CI 0.95–2.31, low-certainty), and mortality (RR 0.92, 95% CI 0.34–2.52, low-certainty). There was no clinical difference between stenting and BMP for chronic DVT regarding PTS (MD 2.73, 95% CI −2.10 to 7.56, very low certainty) and no VTE and death events.
Conclusions:
SA results in little to no difference in PTS, VTE and mortality in acute DVT compared to BMP. The evidence regarding SA in chronic DVT and whether SA, compared to BMP and thrombolysis, decreases PTS and VTE in acute DVT is uncertain. Open Science Framework (osf.io/f2dm6)
Keywords: angioplasty, deep vein thrombosis, stenting, systematic review, venous thromboembolism
1. Introduction
Venous thromboembolism (VTE) encompasses either the formation of a thrombus in the deep veins, most commonly in the legs (deep vein thrombosis [DVT]), or the subsequent embolization of all or part of the thrombus into the pulmonary circulation (pulmonary embolism [PE]).[1,2] PE is acute, with shortness of breath, pain on inspiration, tachycardia and right heart overload. The global incidence of DVT is 1 to 2/1000 person-years, with 10 million cases per year worldwide, which makes VTE the third leading vascular disease after acute myocardial infarction and stroke.[3,4] Furthermore, the mortality rate associated with VTE is exceptionally high due to PE (18-fold higher than DVT alone).[1,3,4]
Anticoagulants are the cornerstone of treatment for DVT.[1,2,5] They inhibit the coagulation system, reduce the thrombus formation and, with it, reduce the likelihood of PE and death. However, a host of complications of thrombosis can affect the patient’s quality of life (QoL) even with the additional use of compression stockings.[5] The chronic complications of thrombosis can be venous claudication, trophic ulcer, or major edema that adds morbidity to DVT patients. This related group of signs and symptoms is named post-thrombotic syndrome (PTS) and can occur after the DVT event with increasing frequency over time in up to 50% to 75% of DVT cases.[1,3,6–8] Venous injuries, which can occur after a thrombosis, can also increase the rate of new thrombotic occurrences and worsen PTS.
Clinicians can use other non-pharmacological technologies for early thrombus removals, such as pharmaco-mechanical or catheter-directed thrombolysis, with or without associated stenting, aiming to reduce the risk of PTS. Stenting or angioplasty could restore venous patency and reduce the risk of DVT complications, including acute symptoms (pain, edema), ascending DVT, PE, and post-thrombotic morbidity.[9,10] There is current evidence suggesting greater venous patency, even without significant improvement in QoL or late development of PTS (24 months), with current guidelines recommending the use of strategies for thrombus removal in selected people with iliofemoral involvement only.[1,11] However, the evidence supporting these statements is based on low-quality studies or emphasizes the need for more evidence.[1,11]
Aiming to synthesize the best available evidence to answer this utmost question, we assessed the effects of stenting or angioplasty or either alone, in combination with anticoagulation, thrombolysis or thrombectomy, in the management of people with acute or chronic DVT.
2. Methods
This systematic review was done according to the Cochrane Handbook of systematic Reviews of Interventions and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.[12,13] The review protocol was prospectively registered[14] (osf.io/f2dm6) and all differences between the protocol and review are reported in the Item S1, Supplemental Digital Content, http://links.lww.com/MD/J69. All randomized controlled trials (RCTs) comparing stenting or angioplasty alone or plus anticoagulants, thrombolysis or thrombectomy versus any combination of these interventions for DVT were analyzed.
We included participants of both sexes and of any age who were diagnosed with acute or chronic DVT by an expert physician upon clinical and investigative assessment (duplex scanning or angiography (by computed tomography, magnetic resonance or digital subtraction). We considered cases of acute DVT if they were treated within 21 days of developing symptoms.[7,8] Chronic DVT was considered in those patients who were treated after 21 days.
For this review, stenting or angioplasty with or without thrombectomy were pooled into 1 group as the intervention of interest which was compared to the standard-intervention (anticoagulation or thrombolysis). The rationale for this approach is that stenting, angioplasty or thrombectomy are commonly used together aiming to provide better effect compared to the anticoagulation or thrombolysis. Besides, anticoagulation and thrombolysis are already considered as part of the best medical practice (BMP) for VTE.[1,5,8]
We presented the outcomes at 3 different time-points following the start of the intervention: early, at 1 year or less; intermediate, more than 1 to 3 years; long-term, more than 3 years. We considered PTS, VTE and all-cause mortality as primary outcomes. The secondary outcomes were: major bleeding, secondary patency after revascularisation, duration of hospitalization (days), QoL or patient’s subjective perception of improvement (yes or no), adverse events (e.g. hematoma, pain, allergic reactions, vein rupture, contrast-induced nephropathy, etc.).
We searched the following databases for relevant trials on 5 November 2021: the Cochrane Central Register of Controlled Trials (CENTRAL (2021, Issue 11)) via The Cochrane Library,[15] MEDLINE (via PubMed),[16] Embase (via Elsevier),[17] CINAHL,[18] Latin American and Caribbean Health Science Information database (LILACS via Virtual Health Library),[19] Indice Bibliográfico Español de Ciencias de la Salud (IBECS via Virtual Health Library).[19]
In addition, we searched the following trial registries for details of ongoing and unpublished studies: clinicalTrials.gov,[20] World Health Organization International Clinical Trials Registry Platform,[21] ProQuest Dissertations and Theses,[22] Ethos Library,[23] and OpenGrey.eu.[24] We also performed a top-up search of the described databases and trials registries on 2 May 2023 and checked the bibliographies of included trials for further references to relevant trials. We contacted specialists in the field and authors of included trials for any possible unpublished data.
For details of each search strategy, data collection and analysis used, see Items S2–S3, Supplemental Digital Content, http://links.lww.com/MD/J70;http://links.lww.com/MD/J71.
We assessed the certainty of the evidence for each outcome using the GRADE approach.[25] We based the “Summary of findings” tables on Cochrane methods, and justify any departures from the standard methods.[12,25]
We assumed a minimal clinically important difference of 12% absolute improvement (or worsening) for continuous outcomes based on the study conducted by Kahn.[26] We back-translated standardized mean difference (SMD) to a typical scale (selecting a study included in the original meta-analysis that is representative of the population and intervention and at a low risk of bias) in the “Summary of findings” table to facilitate the interpretation, in accordance with Cochrane standards.[27] We also entered the absolute percent difference (calculated by dividing the mean difference by the scale of the measure and expressed as a percentage) in the “Effects of interventions” results section and related table.
3. Results
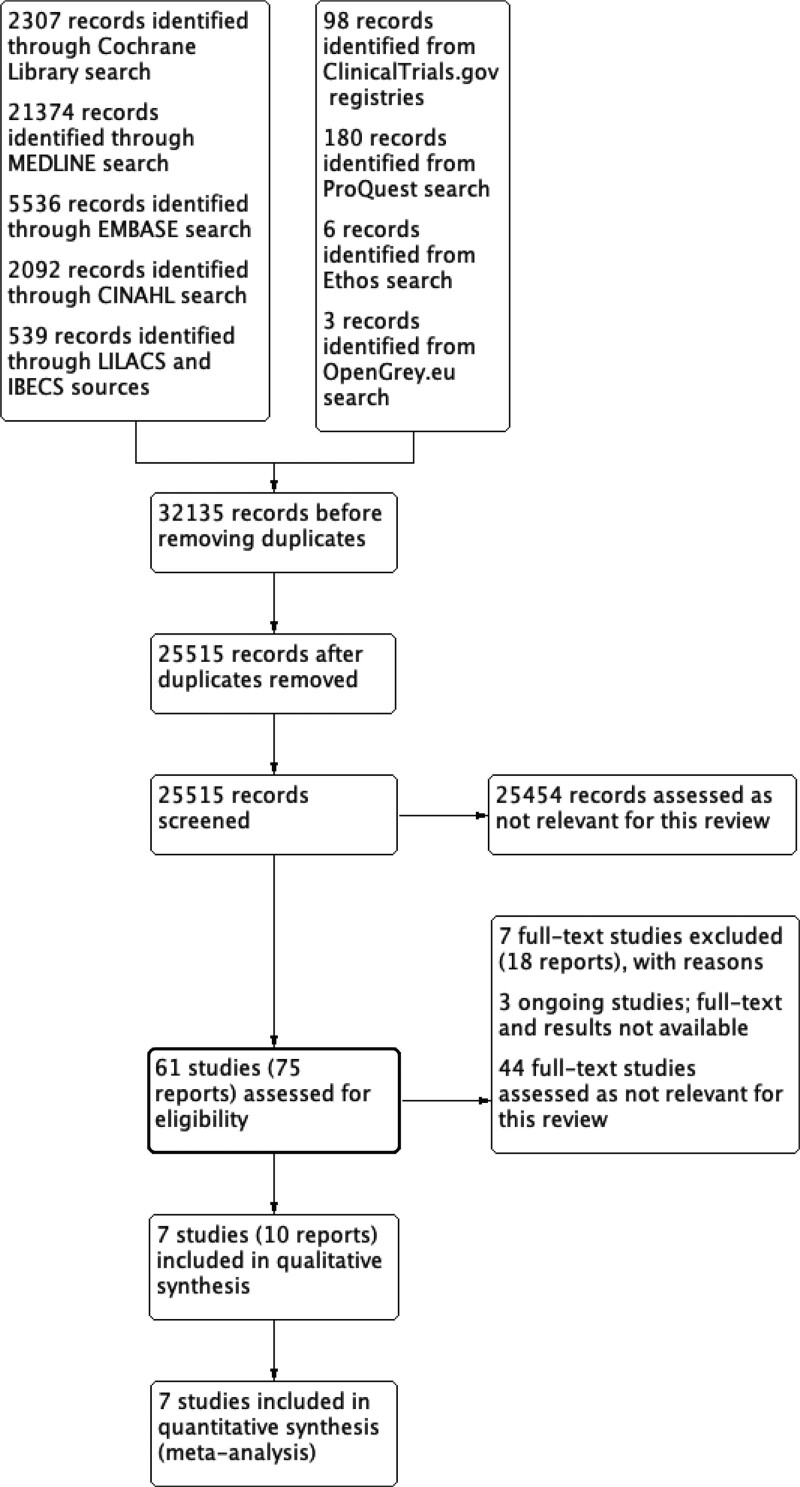
Our searches identified 41,475 records from CENTRAL, MEDLINE, Embase, CINAHL, LILACS and IBECS databases, and from ClinicalTrials.gov, World Health Organization International Clinical Trials Registry Platform, ProQuest, Ethos and OpenGrey.eu. From those initial references, the removal of duplicates resulted in 30,517 records for screening. We assessed 30,439 records as not relevant for this review. We assessed the full text of the other 65 studies for eligibility. From them, we assessed 44 other studies as not relevant, excluded 8 studies with reasons and identified 6 ongoing studies (Fig. 1).[13] See the Items S4–S7, Supplemental Digital Content, http://links.lww.com/MD/J72;http://links.lww.com/MD/J73; http://links.lww.com/MD/J74;http://links.lww.com/MD/J75, for details and a complete list of references from the studies considered for this review.
Figure 1.
Study flow diagram (PRISMA chart).
We included 7 studies (1485 participants), which were conducted between February 2007 and September 2017 (Table 1),[28–34] and excluded other 7 studies.[35–41] Four excluded studies had inadequate study design, two had inadequate intervention and 1 evaluated inadequate participants. We identified 3 ongoing studies that can potentially be included in future versions of this review (Item S5, Supplemental Digital Content, http://links.lww.com/MD/J73).[42–44]
Table 1.
Summary of characteristics of included studies.
| Study (design) | Country | Participant age (mean ± SD) | Setting | Intervention type | Comparator | Post-thrombotic syndrome | Venous thromboembolism† | Follow-up time (mean mo) | Total participants allocated | Intervention group participants |
|---|---|---|---|---|---|---|---|---|---|---|
| ATTRACT[28] (RCT) | Canada and USA | 51 ± 14 (experimental), 51 ± 13 (comparator) | Hospital* | Stenting, angioplasty, thrombolysis and thrombectomy | Anticoagulation, compression stockings and clinical care | MD −0.57 (95% CI −0.60 to −0.54), VCSS | RR 1.48 (95% CI 0.95 to 2.31) | 24 | 692 | Stenting 82/297 (28%); angioplasty: 184/297 (62%); thrombectomy: 183/297 (62%) |
| Cakir[29] (RCT) | Turkey | 53, range 34–79 (experimental), 59, range 30–83 (comparator) | Hospital* | Stenting, angioplasty, and thrombectomy | Anticoagulation, compression stockings and clinical care | NR | RR 0.25 (95% CI 0.03 to 2.05) | 12 | 42 | Stenting 14/21 (66%); angioplasty 19/21 (90%); thrombectomy 21/21 (100%) |
| CAVA[30] (RCT) | Netherlands | 51 ± 22.6 (experimental); 51.7 ± 20.3 (comparator) | Hospital* | Stenting, angioplasty, thrombolysis and thrombectomy | Anticoagulation | MD −0.90 (95% CI −2.09 to 0.29), Villalta score; RR 0.82 (95% CI 0.51 to 1.32), Villalta score dichotomous data‡ | RR 3.65 (95% CI 1.27 to 10.50), recurrent DVT; RR 0.19 (95% CI 0.01 to 3.99), PE | 10 | 184 | Stenting 35/77 (45%); stenting or angioplasty 42/77 (55%); thrombolysis 77/77 (100%) |
| Jiang[31] (RCT) | China | 50.85 ± 1.95 | Hospital* | Stenting, angioplasty and thrombolysis, compression stockings and clinical care | Anticoagulation, thrombolysis, compression stockings and clinical care | MD −2.78 (95% CI −3.48 to −2.09) VCSS, MD −3.95 (95% CI −4.80 to −3.10), CEAP | NR | 24 (experimental), 18 (control) | 66 | Stenting 35/77 27/27 (100%); thrombolysis 27/27 (100%) |
| Meng[32] (RCT) | China | 46 ± 24 | Hospital* | Stenting, thrombolysis, IVCF | Thrombolysis, IVCF | MD −26.37 (95% CI −30.99 to −21.74), VCSS | NR | 24 | 74 | Stenting 45/45 (100%), thrombolysis 45/45 (100%) |
| Rossi[33] RCT | Brazil | 57 (range 26–77) | Hospital* | Stenting, venotonics, compression stockings, and Unna Boot plus elastic bandages for CEAP C6 participants | Venotonics, compression stockings, and Unna Boot plus elastic bandages for CEAP C6 participants | MD 2.73 (95% CI (−2.10 to 7.56), VCSS | NR | 6 | 22 | Stenting 7/7 (100%) |
| Zhang[34] (RCT) | China | Range 21–75 | Hospital* | Stenting and thrombolysis | Thrombolysis | MD −0.05 (95% CI −0.41 to 0.31), Villalta | RR 1.19 (95% CI 0.28 to 5.07), recurrent DVT | 24 | 376 | Stenting 7/7 (100%) |
CEAP = Clinical (C), Etiological (E), Anatomical (A), and Pathophysiological (P), CI = confidence interval, IVCF = inferior vena cava filter, MD = mean difference, NR = not reported, RCT = randomized controlled trial, RR = risk ratio, SD = standard deviation, VCSS = venous clinical severity score.
Hospital: includes operation theater, exclusive endovascular suite or hybrid surgical theater.
Including recurrent DVT and PE, fatal or non-fatal.
Villalta dichotomous data was reported as the proportion of participants with more than 4 points.
3.1. Risk of bias, subgroup and sensitivity analyses
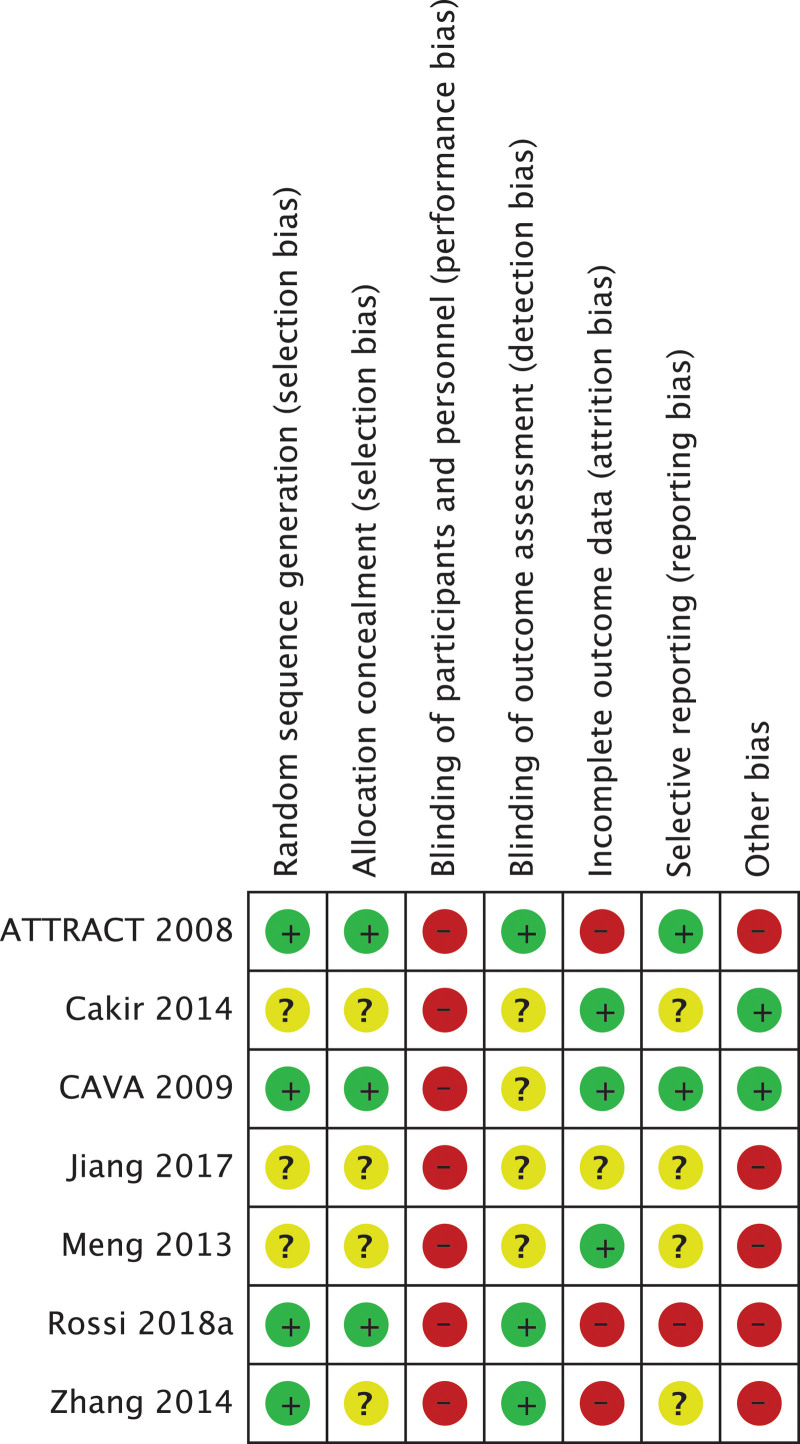
The risk of bias for each study and estimate of the overall risk of bias were assessed with the Cochrane tool version 1.0 (Fig. 2; Item S8, Supplemental Digital Content, http://links.lww.com/MD/J76).[45] We judged three of included studies as having overall high risk of bias[28,33,34] and CAVA[30] as having overall low risk of bias. The other 3 studies were judged as unclear overall risk of bias.[29,31,32] The publication bias assessment by visual inspection of funnel plots was not possible because we included less than 10 studies in each comparison.[14]
Figure 2.
Summary of the review authors’ judgements about each risk of bias item for each included study. Cochrane risk of bias tool version 1.0.
Due to a lack of available data we were unable to perform subgroup analyses for age, gender and intervention material (e.g. self-expanding versus balloon-expanding stent, bare metal stent versus drug-eluting stent) or by venous stenosis (e.g. less than 50% or at least 50% of venous stenosis). Subgroups for different scores and scales used for evaluating continuous variables and for different types of adverse events are presented above.
We performed the sensitivity analyses including only trials at low risk of bias and that had not mixed population data in all possible analyses and reported data also as figures (Item S9, Supplemental Digital Content, http://links.lww.com/MD/J77). We performed the test for subgroup differences when possible: for different scores/scales for PTS and QoL and for different types of adverse events.
3.2. Effects of interventions
We identified 1 comparison for acute DVT with data for the 3 time-points (early, intermediate and long-term),[29,31,32,34] another comparison for acute DVT with data for 2 time-points (early and intermediate)[28,30] and 1 comparison for chronic DVT with data for early time-point.[33] We reported here the longest time-point with primary outcomes data for each comparison and all other available time-points in the Items S9–S10, Supplemental Digital Content, http://links.lww.com/MD/J77; http://links.lww.com/MD/J78.
3.3. Acute DVT – stenting or angioplasty plus BMP and thrombolysis versus BMP and thrombolysis (intermediate time-point)
All of the available outcomes are presented at the 24 month follow-up after the start of intervention (Table 2).[31,32,34]
Table 2.
Stenting or angioplasty plus BMP and thrombolysis compared to BMP and thrombolysis for the treatment of acute deep vein thrombosis (intermediate time point).
| Patient or population: acute deep vein thrombosis (intermediate time point) Setting: In-hospital Intervention: Stenting or angioplasty plus BMP and thrombolysis Comparison: BMP and thrombolysis | ||||||
|---|---|---|---|---|---|---|
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with BMP and thrombolysis | Risk with Stenting or angioplasty plus BMP and thrombolysis | |||||
| Post-thrombotic syndrome assessed with: Villalta score (0–33, lower = better) and VCSS (0–30, lower = better) follow-up: mean 24 mo | The mean post-thrombotic syndrome was −0.69 points | MD 1.82 points lower (2.8 lower to 0.84 lower) |
- | 252 (3 RCTs) |
⨁◯◯◯ Very low†,‡,§,∥ |
Stenting or angioplasty plus BMP and thrombolysis may reduce/have little to no effect on post-thrombotic syndrome but the evidence is very uncertain. Absolute percent difference = 5.5% absolute improvement (95% CI 2.6% improvement to 8.5% improvement). SMD = −7.87 (95% CI −12.13 to −3.61) |
| Post-thrombotic syndrome assessed with: Villalta score (0–33, lower = better) and CEAP-C (0–6, lower = better) follow-up: mean 24 mo | The mean post-thrombotic syndrome was 2.39 points | MD 0.6 points lower (1.2 lower to 0.1 higher) |
- | 252 (3 RCTs) |
⨁◯◯◯ Very low†,§,∥,¶ |
Stenting or angioplasty plus BMP and thrombolysis may reduce/have little to no effect on post-thrombotic syndrome but the evidence is very uncertain. Absolute percent difference = 10% absolute improvement (95% CI 1.7% worsening to 20% improvement). SMD = −2.43 (95% CI −5.17 to 0.31) |
| VTE - recurrent DVT assessed with: Count of events (less = better) follow-up: mean 24 mo | 54 per 1.000 |
64 per 1.000 (15 to 272) |
RR 1.19 (0.28 to 5.07) |
119 (1 RCT) |
⨁◯◯◯ Very low†,# |
Stenting or angioplasty plus BMP and thrombolysis may increase/have little to no effect on incidence of venous thromboembolism - recurrent deep venous thrombosis but the evidence is very uncertain. Absolute percent difference = 1% more events (95% CI 3.9% fewer to 21.8% more) |
| Mortality | No studies measured this outcome | |||||
| Major Bleeding | No studies measured this outcome | |||||
| Secondary patency assessed with: Count of events (less = better) follow-up: mean 24 mo | 500 per 1.000 |
130 per 1.000 (55 to 295) |
RR 0.26 (0.11 to 0.59) |
133 (2 RCTs) |
⨁ ⨁◯◯ Low†,** |
Stenting or angioplasty plus BMP and thrombolysis may result in little to no difference in secondary patency. Absolute percent difference = 37% fewer events (95% CI 44.5% fewer to 20.5% fewer) |
| Duration of hospitalization | No studies measured this outcome | |||||
| Quality of life assessed with: CIVIQ (0–100, higher = better) follow-up: mean 24 mo |
The mean quality of life was 60.66 points | MD 15.05 points higher (1.6 lower to 31.7 higher) |
- | 252 (3 RCTs) |
⨁◯◯◯ Very low†,§,∥,¶ |
Stenting or angioplasty plus BMP and thrombolysis may increase/have little to no effect on quality of life but the evidence is very uncertain. Absolute percent difference = 15.1% absolute improvement (95% CI 1.6% worsening to 31.7% improvement). SMD = 2.26 (95% CI −0.24 to 4.76) |
| Quality of life assessed with: CIVIQ (0–100, higher = better) follow-up: mean 24 mo |
The mean quality of life was 60.66 points | MD 14.92 points higher (2.07 lower to 31.9 higher) |
- | 252 (3 RCTs) |
⨁◯◯◯ Very low†,§,∥,¶ |
Stenting or angioplasty plus BMP and thrombolysis may increase/have little to no effect on quality of life but the evidence is very uncertain. Absolute percent difference = 14.9% absolute improvement (95% CI 2.1% worsening to 31.9% improvement). SMD = 2.24 (95% CI −0.31 to 4.79) |
| Quality of life assessed with: CIVIQ (0–100, higher = better) follow-up: mean 24 mo |
The mean quality of life was 60.66 points | MD 14.92 points higher (2.13 lower to 31.9 higher) |
- | 252 (3 RCTs) |
⨁◯◯◯ Very low†,§,∥,¶ |
Stenting or angioplasty plus BMP and thrombolysis may increase/have little to no effect on quality of life but the evidence is very uncertain. Absolute percent difference = 14.9% absolute improvement (95% CI 2.1% worsening to 31.9% improvement). SMD = 2.24 (95% CI −0.32 to 4.79) |
| Quality of life assessed with: CIVIQ (0–100, higher = better) follow-up: mean 24 mo |
The mean quality of life was 60.66 points | MD 14.92 points higher (2 lower to 31.8 higher) |
- | 252 (3 RCTs) |
⨁◯◯◯ Very low†,§,∥,¶ |
Stenting or angioplasty plus BMP and thrombolysis may increase/have little to no effect on quality of life but the evidence is very uncertain. Absolute percent difference = 14.9% absolute improvement (95% CI 2% worsening to 31.8% improvement). SMD = 2.24 (95% CI −0.3 to 4.78) |
| Adverse events assessed with: Count of events (less = better) follow-up: mean 24 mo |
48 per 1.000 |
61 per 1.000 (18 to 209) |
RR 1.26 (0.37 to 4.34) |
186 (2 RCTs) |
⨁◯◯◯ Very low†,# |
Stenting or angioplasty plus BMP and thrombolysis may increase/have little to no effect on adverse events but the evidence is very uncertain. Absolute percent difference = 1.3% more events (95% CI 3% fewer to 16.1% more) |
CEAP-C = Clinical, Etiologic, Anatomic, and Pathophysiologic classification, CI = confidence interval, CIVIQ = Chronic Venous Insufficiency Questionnaire, DVT = deep venous thrombosis, GRADE = Working Group grades of evidence, MD = mean difference, RCT = randomized controlled trial, RR = risk ratio, VCSS = Venous Clinical Severity Score, VTE = venous thromboembolism
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Downgraded 1 level due to serious study limitations (risk of bias). Unclear or high risk of bias for several domains.
Downgraded 2 levels due to very serious inconsistency. High unexplained heterogeneity (I2 = 99%).
Downgraded 1 level due to serious indirectness.
Downgraded 1 level due to serious imprecision. Less than 400 participants were analyzed in the meta-analysis.
Downgraded 2 levels due to very serious inconsistency. High unexplained heterogeneity (I2 = 98%).
Downgraded 2 levels due to very serious imprecision. Less than 300 events were analyzed in the analysis and wide confidence interval of the absolute difference.
Downgraded 1 level due to serious imprecision. Less than 300 events were analyzed in the meta-analysis.
To assess PTS, the trial authors used Villalta score (scale 0–33), venous clinical severity score (VCSS) (scale 0–30) and the clinical, etiological, anatomical, and pathological elements (CEAP) (scale 0–6), where a higher score indicates more severe disease.[31,32,34] We analyzed data by SMD and random-effect models because of the substantial heterogeneity and differences of participants and intervention between the studies. There was neither a significant difference between the experimental and control groups for a combination of Villalta and VCSS data nor for Villalta and CEAP data (Figures S9.1, Supplemental Digital Content, http://links.lww.com/MD/J77, S9.2, Supplemental Digital Content, http://links.lww.com/MD/J77).
Sensitivity analyses including only trials at low risk of bias, and that had not mixed population data did not change the effect estimate substantially when analyzed by VCSS (SMD −14.46, 95% CI −37.57 to 8.65). However, the sensitivity analyses including only trials at low risk of bias, and that had not mixed population data changed the effect estimate when analyzed by CEAP-C (SMD −3.62, 95% CI −4.21 to −3.04). Figures S9.3, Supplemental Digital Content, http://links.lww.com/MD/J77, S9.4, Supplemental Digital Content, http://links.lww.com/MD/J77.
The test for subgroup differences suggests that the tool used to analyze the PTS does not have a modifying effect on PTS when comparing Villalta score and VCSS (P = .22, I2 = 33.0%), but had a modifying effect on PTS when comparing Villalta and CEAP-C (P < .00001, I2 = 99.0%). Figures S9.1, Supplemental Digital Content, http://links.lww.com/MD/J77, S9.2, Supplemental Digital Content, http://links.lww.com/MD/J77.
PE was not an outcome foreseen by the included studies, but the trial authors of 3 reported no fatal PE events in the experimental and control groups.[31,32,34] Therefore, the overall effect could not be estimated for PE. Jiang et al[31] reported 3 DVT events during the total follow-up period without details by each group or each time-point. Zhang et al[34] provided recurrent DVT data in the late period of follow-up (24 months), i.e. the intermediate time-point. There was no significant difference between the experimental and control groups for recurrent DVT at the intermediate time-point (Figure S9.5, Supplemental Digital Content, http://links.lww.com/MD/J77).
Mortality was not an outcome specified for analysis by included trials but all included studies mentioned no deaths during the 24 months of follow-up and, therefore, the overall effect could not be estimated.[31,32,34] Major bleeding was not an outcome specified for analysis by the included trials. Jiang et al[31] mentioned one event but did not provide details (e.g. events by each group, data in intermediate time-point). Zhang et al[34] reported no cases of major bleeding during the 24 months of follow-up. The overall effect could not be estimated.
Jiang et al[31] and Meng et al[32] reported secondary patency with significantly better results for experimental group defined by lower vein occlusion events of the affected segment after treatment (Figure S9.6, Supplemental Digital Content, http://links.lww.com/MD/J77). Although there is no substantial heterogeneity (I2 = 37%), we considered the participants and intervention differences as significant and we analyzed these data with a random effect.
Duration of hospitalization was not measured in the included studies.
Three studies[31,32,34] reported clinically assessed QoL data in a format that could be combined, with a total of 152 participants. Jiang et al[31] and Meng et al[32] reported QoL using the Chronic Venous Insufficiency Questionnaire (score 0–100), while Zhang et al[34] reported QoL using the VEINES-QoL (25 items), VEINES-Sym (10 items), SF-36 PCS (score 0–100) and SF-36 MCS (score 0–100) scales. We subgrouped this outcome according to the type of QoL questionnaire and they were combined in 4 meta-analyses. For all possible meta-analyses, there was no difference between the study arms (stenting or angioplasty versus control) (Figures S9.7, Supplemental Digital Content, http://links.lww.com/MD/J77, S9.8, Supplemental Digital Content, http://links.lww.com/MD/J77, S9.9, Supplemental Digital Content, http://links.lww.com/MD/J77, S9.10, Supplemental Digital Content, http://links.lww.com/MD/J77). Sensitivity analyses including only trials at low risk of bias, and that had not mixed population data changed the effect estimate (Chronic Venous Insufficiency Questionnaire: SMD 3.37, 95% CI 2.83–3.92) (Figure S9.11, Supplemental Digital Content, http://links.lww.com/MD/J77). The test for subgroup differences suggests that tools used to assess QoL have a modifying effect on QoL (P < .00001, I2 = 99.0%) (Figures S9.7, S9.8, Supplemental Digital Content, http://links.lww.com/MD/J77, S9.9, Supplemental Digital Content, http://links.lww.com/MD/J77, S9.10, Supplemental Digital Content, http://links.lww.com/MD/J77).
Jiang et al[31] related adverse events (2 puncture site hemorrhages, 1 retroperitoneal hemorrhage and 3 inferior vena cava filter thrombosis) but did not provide sufficient details for data inclusion in the meta-analysis (e.g. number of events by each group). Neither Meng et al[32] nor Zhang et al[34] pre-specified the adverse events as outcomes to be analyzed, so there are other adverse events that were not reported by the studies. Zhang et al[34] defined major and minor bleeding and reported no adverse events such as nephropathy, major vital organ bleeding and vein rupture. Although not planned, Meng et al[32] reported 1 participant with limb pain in each group and Zhang et al[34] reported popliteal puncture area hematoma in 3/56 participants in the control group and 5/63 participants in the stenting or angioplasty group at the intermediate time-point. There was no significant difference regarding adverse events between the experimental and control groups at an intermediate time-point and the test for subgroup differences suggests that the type of adverse event did not have a modifying effect on adverse events (P = .61, I2 = 0%) (Figure S9.12, Supplemental Digital Content, http://links.lww.com/MD/J77). Sensitivity analyses including only trials at low risk of bias, and that had not mixed population data did not change the effect estimate (Figure S9.13, Supplemental Digital Content, http://links.lww.com/MD/J77).
3.4. Acute DVT – stenting or angioplasty plus BMP and thrombolysis versus BMP (intermediate time-point)
All of the available outcomes are presented at the 24 month follow-up after the start of intervention (Table 3).[28]
Table 3.
Stenting or angioplasty plus BMP and thrombolysis compared to BMP for the treatment of acute deep vein thrombosis (intermediate time point).
| Patient or population: acute deep vein thrombosis (intermediate time point) Setting: In-hospital Intervention: Stenting or angioplasty plus BMP and thrombolysis Comparison: BMP | ||||||
|---|---|---|---|---|---|---|
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with BMP | Risk with Stenting or angiop\lasty plus BMP and thrombolysis | |||||
| Post-thrombotic syndrome assessed with: Villalta score (0–33, lower = better) follow-up: mean 24 mo | The mean post-thrombotic syndrome was 4.5 points | MD 1.07 points lower (1.12 lower to 1.02 lower) |
- | 497 (1 RCT) |
⨁⨁⨁◯ Moderate† |
Stenting or angioplasty plus BMP and thrombolysis probably results in little to no difference in post-thrombotic syndrome. Absolute percent difference = 3.2% absolute improvement (95% CI 3.1% improvement to 3.4% improvement) |
| Post-thrombotic syndrome assessed with: Villalta score (dichotomous data - more than 4 points higher, less = better) follow-up: mean 24 mo | 434 per 1.000 |
429 per 1.000 (360 to 508) |
RR 0.99 (0.83 to 1.17) |
691 (1 RCT) |
⨁⨁◯◯ Low†,‡ |
The evidence suggests that Stenting or angioplasty plus BMP and thrombolysis may result in little to no difference in post-thrombotic syndrome. Absolute percent difference = 0.4% fewer events (95% CI 7.4% fewer to 7.4% more) |
| Post-thrombotic syndrome assessed with: VCSS (0–30, lower = better) follow-up: mean 24 mo | The mean post-thrombotic syndrome was 2.42 points | MD 0.55 points lower (0.58 lower to 0.52 lower) |
- | 449 (1 RCT) |
⨁⨁⨁◯ Moderate† |
Stenting or angioplasty plus BMP and thrombolysis probably results in little to no difference in post-thrombotic syndrome. Absolute percent difference = 1.8% absolute improvement (95% CI 1.7% improvement to 1.9% improvement) |
| VTE - recurrent DVT assessed with: Count of events (less = better) follow-up: mean 24 mo | 85 per 1.000 |
125 per 1.000 (80 to 195) |
RR 1.48 (0.95 to 2.31) |
691 (1 RCT) |
⨁⨁◯◯ Low†,‡ |
The evidence suggests that Stenting or angioplasty plus BMP and thrombolysis may result in little to no difference in incidence of venous thromboembolism - recurrent deep vein thrombosis. Absolute percent difference = 4.1% more events (95% CI 0.4% fewer to 11.1% more) |
| Mortality assessed with: Count of events (less = better) follow-up: mean 24 mo | 23 per 1.000 |
21 per 1.000 (8 to 57) |
RR 0.92 (0.34 to 2.52) |
691 (1 RCT) |
⨁⨁◯◯ Low†,‡ |
The evidence suggests that Stenting or angioplasty plus BMP and thrombolysis may result in little to no difference in death. Absolute percent difference = 0.2% fewer events (95% CI 1.5% fewer to 3.4% more) |
| Major bleeding assessed with: Count of events (less = better) follow-up: mean 24 mo | 37 per 1.000 |
56 per 1.000 (28 to 113) |
RR 1.54 (0.77 to 3.08) |
691 (1 RCT) |
⨁⨁◯◯ Low†,‡ |
The evidence suggests Stenting or angioplasty plus BMP and thrombolysis may increase major bleeding slightly. Absolute percent difference = 2% more events (95% CI 0.8% fewer to 7.6% more) |
| Secondary patency | No studies measured this outcome | |||||
| Duration of hospitalization | No studies measured this outcome | |||||
| Quality of life assessed with: SF-36 - Physical Health (0–100, higher = better) follow-up: mean 24 mo | The mean quality of life was 10.06 points | MD 1.12 points higher (0.95 higher to 1.29 higher) |
- | 467 (1 RCT) |
⨁⨁⨁◯ Moderate† |
Stenting or angioplasty plus BMP and thrombolysis probably results in little to no difference in quality of life. Absolute percent difference = 1.1% absolute improvement (95% CI 1% improvement to 1.3% improvement) |
| Quality of life assessed with: SF-36 - Mental Health (0–100, higher = better) follow-up: mean 24 mo |
The mean quality of life was 2.7 points | MD 0 points (0.16 lower to 0.16 higher) |
- | 467 (1 RCT) |
⨁⨁⨁◯ Moderate† |
Stenting or angioplasty plus BMP and thrombolysis probably results in little to no difference in quality of life. Absolute percent difference = 0% absolute improvement (95% CI 0.16% worsening to 0.16% improvement) |
| Quality of life assessed with: VEINES-QoL (0–100, higher = better) follow-up: mean 24 mo | The mean quality of life was 23.47 points | MD 4.2 points higher (3.88 higher to 4.52 higher) |
- | 475 (1 RCT) |
⨁⨁⨁◯ Moderate† |
Stenting or angioplasty plus BMP and thrombolysis probably results in little to no difference in quality of life. Absolute percent difference = 4.2% absolute improvement (95% CI 3.9% improvement to 4.5% improvement) |
| Quality of life assessed with: VEINES-Sym (0–100, higher = better) follow-up: mean 24 mo | The mean quality of life was 17.31 points | MD 3.27 points higher (2.95 higher to 3.59 higher) |
- | 474 (1 RCT) |
⨁⨁⨁◯ Moderate† |
Stenting or angioplasty plus BMP and thrombolysis probably results in little to no difference in quality of life. Absolute percent difference = 3.3% absolute improvement (95% CI 3% improvement to 3.6% improvement) |
| Adverse events - any bleeding assessed with: Count of events (less = better) follow-up: mean 24 mo | 107 per 1.000 |
137 per 1.000 (91 to 204) |
RR 1.28 (0.85 to 1.91) |
691 (1 RCT) |
⨁⨁◯◯ Low†,‡ |
The evidence suggests Stenting or angioplasty plus BMP and thrombolysis may increase adverse events - any bleeding slightly. Absolute percent difference = 3% more events (95% CI 1.6% fewer to 9.7% more) |
CI = confidence interval, CIVIQ = Chronic Venous Insufficiency Questionnaire, DVT = deep venous thrombosis, GRADE = Working Group grades of evidence, MD = mean difference, RCT = randomized controlled trial, RR = risk ratio, SF-36 = Medical Outcomes Study 36-Item Short Form Health Survey, VCSS = Venous Clinical Severity Score, VEINES-QoL = Venous Insufficiency Epidemiological and Economic Study-Quality of Life, VEINES-Sym = Venous Insufficiency Epidemiological and Economic Study-Symptoms, VTE = venous thromboembolism.
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Downgraded 1 level due to serious study limitations (risk of bias). High risk of bias for several domains related to only 1 study.
Downgraded 1 level due to serious imprecision. Less than 300 events were included in the analysis.
One trial[28] evaluated PTS using dichotomous (Villalta score more than 4 = positive) and continuous data. The trial authors used the Villalta score (scale 0–33) and VCSS (scale 0–30), higher score indicates more severe disease. Stenting or angioplasty plus BMP and thrombolysis results in little to no difference in PTS assessed by Villalta dichotomous data (Figure S9.14, Supplemental Digital Content, http://links.lww.com/MD/J77). When assessed by continuous data, stenting or angioplasty plus BMP and thrombolysis probably results in little to no difference in PTS, irrespective of instrument used (Figures S9.15, Supplemental Digital Content, http://links.lww.com/MD/J77, S9.16, Supplemental Digital Content, http://links.lww.com/MD/J77).
Isolated PE was not an outcome foreseen by the included study, but the trial authors reported 1 fatal PE event in experimental groups.[28] The overall effect could not be estimated for PE because we did not have access to all cases of PE in both groups. ATTRACT[28] reported recurrent DVT, and the evidence suggests that stenting or angioplasty plus BMP and thrombolysis results in little to no difference (Figure S9.17, Supplemental Digital Content, http://links.lww.com/MD/J77).
The evidence suggests that stenting or angioplasty plus BMP and thrombolysis results in little to no difference in death (Figure S9.18, Supplemental Digital Content, http://links.lww.com/MD/J77) and increases major bleeding slightly (Figure S9.19, Supplemental Digital Content, http://links.lww.com/MD/J77). Secondary patency and Duration of hospitalization were not measured in the included study.
Adverse events were not a foreseen outcome, but ATTRACT[28] reported data on any bleeding. The evidence suggests that stenting or angioplasty plus BMP and thrombolysis increases adverse events slightly (Figure S9.20, Supplemental Digital Content, http://links.lww.com/MD/J77).
ATTRACT[28] reported QoL with the Medical Outcomes Study 36-Item Short Form Health Survey (SF-36) questionnaire (0–100) using 2 components (physical health and mental health) and with Venous Insufficiency Epidemiological and Economic Study (VEINES) using other 2 components (QoL and symptoms). Stenting or angioplasty plus BMP and thrombolysis probably results in little to no difference in QoL when assessed by all these tools (Figures S9.21, Supplemental Digital Content, http://links.lww.com/MD/J77, S9.22, Supplemental Digital Content, http://links.lww.com/MD/J77, S9.23, Supplemental Digital Content, http://links.lww.com/MD/J77).
3.5. Chronic DVT – stenting or angioplasty plus BMP versus BMP (early time-point)
All of the available outcomes are presented at the 6 month follow-up after the start of the intervention and the number of participants reflect the included limbs because Rossi et al[33] randomized limbs and not individuals (Table 4).
Table 4.
Stenting or angioplasty plus BMP compared to BMP for the treatment of chronic deep vein thrombosis (early time point).
| Patient or population: chronic deep vein thrombosis (early time point) Setting: In-hospital Intervention: Stenting or angioplasty plus BMP Comparison: BMP | ||||||
|---|---|---|---|---|---|---|
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with BMP | Risk with Stenting or angioplasty plus BMP | |||||
| Post-thrombotic syndrome assessed with: VCSS (0–30, lower = better) follow-up: mean 6 mo | The mean post-thrombotic syndrome was 15.13 points | MD 2.73 points higher (2.1 lower to 7.56 higher) |
- | 22 (1 RCT) |
⨁◯◯◯ Very low†,‡ |
Stenting or angioplasty plus BMP may increase/have little to no effect on post-thrombotic syndrome but the evidence is very uncertain. Absolute percent difference = 9.1% absolute worsening (95% CI 25.2% worsening to 7% improvement) |
| Venous thromboembolism follow-up: mean 6 mo | One study reported no fatal PE events in experimental and control groups. The overall effect could not be estimated | |||||
| Mortality follow-up: mean 6 mo | One study reported no death in experimental and control groups. The overall effect could not be estimated | |||||
| Major bleeding | No studies measured this outcome | |||||
| Secondary patency follow-up: mean 6 mo | One study reported 100% of secondary patency for the experimental group, but no revascularisation was performed in the control group. The overall effect could not be estimated | |||||
| Duration of hospitalization follow-up: mean 6 mo | One study reported that all participants were discharged within 2 days but did not provide details. The overall effect could not be estimated | |||||
| Quality of life assessed with: SF-36 - Physical functioning (0–100, higher = better) follow-up: mean 6 mo | The mean quality of life was 48.55 points | MD 5.69 points lower (20.97 lower to 9.59 higher) |
- | 22 (1 RCT) |
⨁◯◯◯ Very low†,‡ |
Stenting or angioplasty plus BMP may reduce/have little to no effect on quality of life but the evidence is very uncertain. Absolute percent difference = 5.7% absolute worsening (95% CI 21% worsening to 9.6% improvement) |
| Quality of life assessed with: SF-36 - Physical role functioning (0–100, higher = better) follow-up: mean 6 mo | The mean quality of life was 48.89 points | MD 13.06 points lower (37.55 lower to 11.43 higher) |
- | 22 (1 RCT) |
⨁◯◯◯ Very low†,‡ |
Stenting or angioplasty plus BMP may reduce/have little to no effect on quality of life: SF-36 - Physical role functioning but the evidence is very uncertain. Absolute percent difference = 13.1% absolute worsening (95% CI 37.6% worsening to 11.4% improvement) |
| Quality of life assessed with: SF-36 - Limb pain (0–100, higher = better) follow-up: mean 6 mo | The mean quality of life was 46.65 points | MD 7.16 points lower (22.11 lower to 7.79 higher) |
- | 22 (1 RCT) |
⨁◯◯◯ Very low†,‡ |
Stenting or angioplasty plus BMP may reduce/have little to no effect on quality of life but the evidence is very uncertain. Absolute percent difference = 7.2% absolute worsening (95% CI 22.1% worsening to 7.8% improvement) |
| Quality of life assessed with: SF-36 - General health perceptions (0–100, higher = better) follow-up: mean 6 mo | The mean quality of life was 59.04 points | MD 4.63 points higher (7.89 lower to 17.15 higher) |
- | 22 (1 RCT) |
⨁◯◯◯ Very low†,‡ |
Stenting or angioplasty plus BMP may increase/have little to no effect on quality of life but the evidence is very uncertain. Absolute percent difference = 4.6% absolute improvement (95% CI 7.9% worsening to 17.2% improvement) |
| Quality of life assessed with: SF-36 - Vitality (0–100, higher = better) follow-up: mean 6 mo | The mean quality of life was 57.1 points | MD 1.11 points higher (13.51 lower to 15.73 higher) |
- | 22 (1 RCT) |
⨁◯◯◯ Very low†,‡ |
Stenting or angioplasty plus BMP may increase/have little to no effect on quality of life but the evidence is very uncertain. Absolute percent difference = 1.1% absolute improvement (95% CI 13.5% worsening to 15.7% improvement) |
| Quality of life assessed with: SF-36 - Social role functioning (0–100, higher = better) follow-up: mean 6 mo | The mean quality of life was 16.35 points | MD 2.3 points lower (19.95 lower to 15.35 higher) |
- | 22 (1 RCT) |
⨁◯◯◯ Very low†,‡ |
Stenting or angioplasty plus BMP may reduce/have little to no effect on quality of life but the evidence is very uncertain. Absolute percent difference = 2.3% absolute worsening (95% CI 20% worsening to 15.4% improvement) |
| Quality of life assessed with: SF-36 - Emotional role functioning (0–100, higher = better) follow-up: mean 6 mo | The mean quality of life was 52.78 points | MD 11.51 points higher (17.29 lower to 40.31 higher) |
- | 22 (1 RCT) |
⨁◯◯◯ Very low†,‡ |
Stenting or angioplasty plus BMP may increase/have little to no effect on quality of life but the evidence is very uncertain. Absolute percent difference = 11.5% absolute improvement (95% CI 17.3% worsening to 40.3% improvement) |
| Quality of life assessed with: SF-36 - Mental health (0–100, higher = better) follow-up: mean 6 mo | The mean quality of life was 57.93 points | MD 0.5 points lower (18.23 lower to 17.23 higher) |
- | 22 (1 RCT) |
⨁◯◯◯ Very low†,‡ |
Stenting or angioplasty plus BMP may reduce/have little to no effect on quality of life but the evidence is very uncertain. Absolute percent difference = 0.5% absolute worsening (95% CI 18.2% worsening to 17.2% improvement) |
| Adverse events assessed with: Pain by VAS (0–10, lower = better) follow-up: mean 6 mo | The mean adverse events was 7.4 points | MD 1.74 points higher (0.62 higher to 2.86 higher) |
- | 22 (1 RCT) |
⨁◯◯◯ Very low†,‡ |
Stenting or angioplasty plus BMP may increase/have little to no effect on adverse events but the evidence is very uncertain. Absolute percent difference = 17.4% absolute worsening (95% CI 28.6% worsening to 6.2% worsening) |
CI = confidence interval, DVT = deep venous thrombosis, GRADE = Working Group grades of evidence, MD = mean difference, RCT = randomized controlled trial, RR = risk ratio, SF-36 = Medical Outcomes Study 36-Item Short Form Health Survey, VAS = Visual Analogue Scale, VTE = venous thromboembolism.
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Downgraded 1 level due to serious study limitations (risk of bias). High risk of bias for several domains related to only 1 study.
Downgraded 2 levels due to very serious imprecision. Less than 400 participants were analyzed and the wide confidence interval of the absolute difference.
One trial[33] evaluated PTS and performed it only using continuous data. The trial authors used VCSS, and we analyzed data by MD and fixed-effect model. Stenting or angioplasty plus BMP may increase/have little to no effect on PTS but the evidence is very uncertain (Figure S9.24, Supplemental Digital Content, http://links.lww.com/MD/J77).
PE was not an outcome foreseen by the included study, but the trial authors reported no fatal PE events in experimental and control groups.[33] The overall effect could not be estimated for PE. Regarding recurrent DVT, Rossi et al[33] reported no events during the follow-up period, therefore, the overall effect could not be estimated.
Mortality was not an outcome specified for analysis by included trial but they mentioned no deaths during the 6 months of follow-up.[33] The overall effect could not be estimated for mortality. Major bleeding was not measured in the included study. Rossi et al[33] reported 100% of secondary patency for the experimental (angioplasty and stenting) until the 6 month follow-up, but no revascularisation was performed in the control group (only BMP), therefore, the overall effect could not be estimated.
Rossi et al[33] reported that all participants of both groups (test and control) were discharged within 2 days but the trial authors did not provide additional detail and we could not include these data in the meta-analysis (e.g. standard deviation, CI, P value). The overall effect could not be estimated.
Rossi et al[33] reported QoL with the SF-36 questionnaire (0–100) only by “change from baseline”; however, after requesting information, the mean and SD for continuous variables were kindly provided. The trial authors considered patients to be positive clinical responders to the randomized allocated treatment if, at the 6 month visit, SF-36 > 30 points. Although the SF-36 questionnaire is an individual based questionnaire for assessment of QoL, the trial authors performed an adaptation of SF-36 to evaluate the contribution of each lower limb for the QoL. It was performed because Rossi et al[33] randomized limbs and not individuals. We subgrouped this outcome according to SF-36 domains and there was no difference between the experimental and control groups (22 participants and 1 study for all analysis) in all domains (Figure S9.25, Supplemental Digital Content, http://links.lww.com/MD/J77).
Adverse events were not a foreseen outcome, but Rossi et al[33] reported data on back pain (25% of all trial participants), venous ulcer cicatrization (ulcer healing rate at 6 months was 90% for test and 40% for control group; P = .345), stent fracture (none events until 6 months) and access site hematomas (2 participants; 4% of all trial participants). We did not include these data in the statistical analysis because the trial authors did not provide details on the subgroup of interest for this review (participants with DVT) or did not provide details by each group (test and control).
Rossi et al[33] reported pain as change from baseline on the pain VAS (from 0 to 10) at each visit until the 6-month visit and it was the unique available data about adverse events for statistical analysis in this review. The trial authors considered participants to be positive clinical responders to the randomized allocated treatment if, at the 6 month visit, VAS pain was <3 points. After requesting, the trial authors kindly provided the mean and SD for VAS pain outcome by personal correspondence. We used MD (continuous data) and fixed-effect model. The control group had less VAS pain than the experimental (angioplasty and stenting) (Figure S9.26, Supplemental Digital Content, http://links.lww.com/MD/J77).
4. Discussion
In this comprehensive systematic review including all available RCTs (7 studies and 1485 participants) assessing the effects of stenting or angioplasty for the treatment of DVT with or without additional interventions, we found that stenting or angioplasty was associated with little to no difference effects in PTS, VTE, major bleeding, death, secondary patency or QoL with a follow-up 12 to 36 months; and with very low to moderate certainty evidence.
We believe that all relevant studies were identified and included. We identified duplicate reports of studies in the selection process and searched multiple sources, with no language or time restriction. However, the possibility remains that we may have missed some trials, particularly in the “gray” literature. We identified 3 ongoing studies that may bring additional evidence for future versions of this review.[42–44]
Although we followed standard methodological procedures to reduce bias in the review process,[14] there are limitations in this review: a need for PTS and VTE data at long-term follow-up for all comparisons; a need for more data about the duration of hospitalization and adverse events for some time-points; a very low to low certainty evidence for most results; we used trial-level rather than individual-level data to assess outcomes.
We identified other systematic reviews which aimed to evaluate the effects of stenting or angioplasty for the treatment of DVT, but they had significant methodological differences when compared to this work:[46–51] they used limited or repeated databases, they used language or date of publication limits, they considered a heterogeneous source of evidence merging retrospective and prospective studies, case series and non-randomized studies to base their conclusions, they used outdated risk of bias assessment tools (e.g. Newcastle-Ottawa Quality Assessment Scale), and few of them used a GRADE approach to assess the certainty of evidence.
Our data are dissonant with the previous hypotheses that stenting or angioplasty may have a role in the management of proximal DVT in particular people.[11] Although stenting or angioplasty showed up as a safe intervention in selected participants, it may not be a procedure with clinical relevant effect for all patient-relevant outcomes. Since we based our conclusions on 7 RCTs and applied more rigorous methodological procedures, they are more decisive for clinical practice and should be used to better inform the current guidelines.[1,11]
5. Conclusions
Stenting or angioplasty results in little to no difference in PTS and QoL in people with acute DVT compared to BMP. In addition, the best available evidence suggests that stenting or angioplasty also results in little to no difference in VTE and mortality but increases major bleeding and adverse events (any bleeding) in the same comparison.
It is uncertain whether stenting or angioplasty compared to BMP and thrombolysis, decreases PTS, VTE and adverse events or improves secondary patency and QoL in people with acute DVT because the certainty of the evidence is very low for all outcomes. The effects of PE, mortality, major bleeding and duration of hospitalization were not estimable or not available in the included studies. The evidence regarding stenting or angioplasty in a chronic DVT setting is also uncertain for these outcomes due to the very low certainty.
Acknowledgments
We would like to thank Cochrane Brazil and the Division of Vascular and Endovascular Surgery, Universidade Federal de São Paulo, Brazil, for their support. We would like to thank Dr Edina Mariko Koga da Silva, Cochrane Brazil for their assistance and advice in completing this review.
Author contributions
Conceptualization: Ronald Luiz Gomes Flumignan.
Formal analysis: Ronald Luiz Gomes Flumignan.
Funding acquisition: Ronald Luiz Gomes Flumignan.
Methodology: Ronald Luiz Gomes Flumignan, Vinicius Tassoni Civile, Libnah Leal Areias, Carolina Dutra Queiroz Flumignan.
Resources: Ronald Luiz Gomes Flumignan, Vinicius Tassoni Civile, Libnah Leal Areias, Carolina Dutra Queiroz Flumignan.
Software: Ronald Luiz Gomes Flumignan, Vinicius Tassoni Civile, Libnah Leal Areias, Carolina Dutra Queiroz Flumignan.
Supervision: Jorge Eduardo Amorim, Renato Delascio Lopes, Luis Carlos Uta Nakano, Jose Carlos Costa Baptista-Silva.
Validation: Jorge Eduardo Amorim, Renato Delascio Lopes, Luis Carlos Uta Nakano, Jose Carlos Costa Baptista-Silva.
Writing – original draft: Ronald Luiz Gomes Flumignan.
Writing – review & editing: Vinicius Tassoni Civile, Carolina Dutra Queiroz Flumignan, Jorge Eduardo Amorim, Renato Delascio Lopes, Luis Carlos Uta Nakano, Jose Carlos Costa Baptista-Silva.
Supplementary Material
Abbreviations:
- BMP
- best medical practice
- CEAP
- clinical, etiological, anatomical, and pathological elements
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- DSA
- digital subtraction angiography
- DVT
- deep vein thrombosis
- GRADE
- Working Group grades of evidence
- IBECS
- Indice Bibliográfico Español de Ciencias de la Salud
- IVC
- inferior vena cava
- LILACS
- Latin American and Caribbean Health Science Information database
- MD
- mean difference
- PE
- pulmonary embolism
- PTS
- post-thrombotic syndrome
- QoL
- quality of life
- RCTs
- randomized controlled trials
- RR
- risk ratio
- SF-36
- Medical Outcomes Study 36-Item Short Form Health Survey
- SMD
- standardized mean difference
- VCSS
- venous clinical severity score
- VEINES-QoL
- Venous Insufficiency Epidemiological and Economic Study-Quality of Life
- VEINES-Sym
- Venous Insufficiency Epidemiological and Economic Study-Symptoms
- VTE
- venous thromboembolism
The content presented in this paper was produced with personal funds without any external support. This publication was financed in part by Universidade Federal de Sao Paulo and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil, finance code 001.
No individual level data are included in this manuscript. All data are aggregated data from clinical trials. Therefore, ethical approval was waived or not necessary.
Dissemination to participants and related patient and public communities: Results will be disseminated using social media such as LinkedIn, at international conferences, and to relevant stakeholders at nonprofit organizations and within healthcare.
This study is registered in the Open Science Framework (osf.io/f2dm6).
R.D.L. declares grants from Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer, and Sanofi via institution and consulting fees from Bayer, Boehringer Ingleheim, Bristol-Myers Squibb, Daiichi Sankyo, GlaxoSmithKline, Medtronic, Merck, Pfizer, Portola, and Sanofi; all other authors declare no support from any organisation for the submitted work; no other relationships or activities that could appear to have influenced the submitted work.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental Digital Content is available for this article.
How to cite this article: Flumignan RLG, Civile VT, Areias LL, Flumignan CDQ, Amorim JE, Lopes RD, Nakano LCU, Baptista-Silva JCC. Stenting or angioplasty for the treatment of deep vein thrombosis: Systematic review and meta-analysis of randomized controlled trials. Medicine 2023;102:22(e33924).
Contributor Information
Vinicius Tassoni Civile, Email: vinicius_civile@yahoo.com.br.
Libnah Leal Areias, Email: libnahleal@gmail.com.
Carolina Dutra Queiroz Flumignan, Email: carolina.flumignan@gmail.com.
Jorge Eduardo Amorim, Email: jorgeamorim@terra.com.br.
Renato Delascio Lopes, Email: renato.lopes@duke.edu.
Luis C. U. Nakano, Email: luiscnakano@uol.com.br.
Jose Carlos Costa Baptista-Silva, Email: cobapva@gmail.com.
References
- [1].Kakkos SK, Gohel M, Baekgaard N, et al. Clinical practice guidelines on the management of venous thrombosis. Eur J Vasc Endovasc Surg. 2021;61:9–82. [DOI] [PubMed] [Google Scholar]
- [2].Flumignan RL, Civile VT, de Tinôco JDS, et al. Anticoagulants for people hospitalised with COVID-19. Cochrane Database Syst Rev. 2022;3:CD013739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Di Nisio M, van Es N, Büller HR. Deep vein thrombosis and pulmonary embolism. Lancet. 2016;388:3060–73. [DOI] [PubMed] [Google Scholar]
- [4].Amaral FC, Baptista-Silva JC, Nakano LC, et al. Pharmacological interventions for preventing venous thromboembolism in people undergoing bariatric surgery. Cochrane Database Syst Rev. 2022;11:CD013683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:e545–608. [DOI] [PubMed] [Google Scholar]
- [6].de Ávila RB, Marcondes GB, Dias SVM, et al. External validation of Villalta score in high-middle income country patients with deep vein thrombosis. Medicine (Baltimore). 2022;101:e29367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Flumignan CD, Nakano LC, Baptista-Silva JC, et al. Antiplatelet agents for the treatment of deep venous thrombosis. Cochrane Database Syst Rev. 2022;7:CD012369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Broderick C, Watson L, Armon MP. Thrombolytic strategies versus standard anticoagulation for acute deep vein thrombosis of the lower limb. Cochrane Database Syst Rev. 2021;1:CD002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Holper P, Kotelis D, Attigah N, et al. Longterm results after surgical thrombectomy and simultaneous stenting for symptomatic iliofemoral venous thrombosis. Eur J Vasc Endovasc Surg. 2010;39:349–55. [DOI] [PubMed] [Google Scholar]
- [10].Titus JM, Moise MA, Bena J, et al. Iliofemoral stenting for venous occlusive disease. J Vasc Surg. 2011;53:706–12. [DOI] [PubMed] [Google Scholar]
- [11].Farsad K, Kapoor BS, Fidelman N, et al. ACR Appropriateness Criteria® radiologic management of iliofemoral venous thrombosis. J Am Coll Radiol. 2020;17:S255–64. [DOI] [PubMed] [Google Scholar]
- [12].Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.2 (updated February 2021). 2021. Cochrane Available at: www.training.cochrane.org/handbook [Access date June 3, 2021].
- [13].Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Flumignan R. Systematic review and meta-analysis of stenting or angioplasty for the treatment of deep vein thrombosis (Protocol). 2021. Available at: https://osf.io/f2dm6/. [DOI] [PMC free article] [PubMed]
- [15].Cochrane. Cochrane library. 2022. Available at: https://www.cochranelibrary.com/ [Access date December 31, 2022].
- [16].NLM. PubMed. PubMed. Available at: https://pubmed.ncbi.nlm.nih.gov/ [Access date December 31, 2022].
- [17].Elsevier. Embase. Available at: https://www.embase.com/landing?status=grey [Access date December 31, 2022].
- [18].EBSCO. CINAHL Complete. EBSCO Information Services, Inc. | www.ebsco.com/pt. Available at: https://www.ebsco.com/pt/produtos/bases-de-dados/cinahl-complete [Access date December 31, 2022].
- [19].PAHO. VHL regional portal. Available at: https://bvsalud.org/en/ [Access date December 31, 2022].
- [20].NLM. ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ [Access date December 31, 2022].
- [21].WHO. ICTRP search portal. Available at: https://trialsearch.who.int/Default.aspx [Access date December 31, 2022].
- [22].Clarivate. ProQuest dissertations & theses global. Available at: https://about.proquest.com/en/products-services/pqdtglobal/ [Access date December 31, 2022].
- [23].The British Library Board. British Library EThOS. Available at: https://ethos.bl.uk/Home.do;jsessionid=30BF1B9E27156757007AB4498CEA3442 [Access date December 31, 2022].
- [24].Collister S. OPENGREY.EU - Grey Literature Database. Available at: https://opengrey.eu/ [Access date December 31, 2022].
- [25].GRADE working group. GRADE working group. 2017. Available at: http://www.gradeworkinggroup.org/index.htm [Access date February 21, 2016].
- [26].Kahn SR. Measurement properties of the Villalta scale to define and classify the severity of the post-thrombotic syndrome. J Thromb Haemost. 2009;7:884–8. [DOI] [PubMed] [Google Scholar]
- [27].Schünemann HJ, Vist GE, Higgins JP, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022). Cochrane; 2022. Available at: www.training.cochrane.org/handbook. [Google Scholar]
- [28].Vedantham S, Goldhaber SZ, Julian JA, et al.; ATTRACT Trial Investigators. Pharmacomechanical catheter-directed thrombolysis for deep-vein thrombosis. N Engl J Med. 2017;377:2240–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cakir V, Gulcu A, Akay E, et al. Use of percutaneous aspiration thrombectomy vs. anticoagulation therapy to treat acute iliofemoral venous thrombosis: 1-year follow-up results of a randomised, clinical trial. Cardiovasc Intervent Radiol. 2014;37:969–76. [DOI] [PubMed] [Google Scholar]
- [30].Notten P, Arnoldussen CWKP, Brans R, et al. Association of successful ultrasound-accelerated catheter-directed thrombolysis with postthrombotic syndrome: a post hoc analysis of the CAVA trial. Thromb Haemost. 2020;120:1188–99. [DOI] [PubMed] [Google Scholar]
- [31].Jiang K, Li X-Q, Sang H-F, et al. Mid-term outcome of endovascular treatment for acute lower extremity deep venous thrombosis. Phlebology. 2017;32:200–6. [DOI] [PubMed] [Google Scholar]
- [32].Meng Q-Y, Li X-Q, Jiang K, et al. Stenting of iliac vein obstruction following catheter-directed thrombolysis in lower extremity deep vein thrombosis. Chin Med J (Engl). 2013;126:3519–22. [PubMed] [Google Scholar]
- [33].Rossi FH, Kambara AM, Izukawa NM, et al. Randomized double-blinded study comparing medical treatment versus iliac vein stenting in chronic venous disease. J Vasc Surg Venous Lymphat Disord. 2018;6:183–91. [DOI] [PubMed] [Google Scholar]
- [34].Zhang X, Ren Q, Jiang X, et al. A prospective randomized trial of catheter-directed thrombolysis with additional balloon dilatation for iliofemoral deep venous thrombosis: a single-center experience. Cardiovasc Intervent Radiol. 2014;37:958–68. [DOI] [PubMed] [Google Scholar]
- [35].AbuRahma AF, Perkins SE, Wulu JT, et al. Iliofemoral deep vein thrombosis: conventional therapy versus lysis and percutaneous transluminal angioplasty and stenting. Ann Surg. 2001;233:752–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Engelberger RP, Spirk D, Willenberg T, et al. Ultrasound-assisted versus conventional catheter-directed thrombolysis for acute iliofemoral deep vein thrombosis. Circ Cardiovasc Interv. 2015;8:e002027. [DOI] [PubMed] [Google Scholar]
- [37].Bi Y, Yu Z, Chen H, et al. Long-term outcome and quality of life in patients with iliac vein compression syndrome after endovascular treatment. Phlebology. 2019;34:536–42. [DOI] [PubMed] [Google Scholar]
- [38].Enden T, KløW N-E, Sandvik L, et al. Catheter-directed thrombolysis vs. anticoagulant therapy alone in deep vein thrombosis: results of an open randomized, controlled trial reporting on short-term patency. J Thromb Haemost. 2009;7:1268–75. [DOI] [PubMed] [Google Scholar]
- [39].Duan P-F, Ni C-F. Randomized study of different approaches for catheter-directed thrombolysis for lower-extremity acute deep venous thrombosis. J Formos Med Assoc. 2016;115:652–7. [DOI] [PubMed] [Google Scholar]
- [40].Sharifi M, Mehdipour M, Bay C, et al. Endovenous therapy for deep venous thrombosis: the TORPEDO trial. Catheter Cardiovasc Interv. 2010;76:316–25. [DOI] [PubMed] [Google Scholar]
- [41].Wang Q, Li K, He C, et al. Angioplasty with versus without routine stent placement for Budd-Chiari syndrome: a randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4:686–97. [DOI] [PubMed] [Google Scholar]
- [42].NCT03250247. Chronic Venous Thrombosis: Relief With Adjunctive Catheter-Directed Therapy (The C-TRACT Trial) - Tabular View - ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/show/record/NCT03250247 [Access date December 31, 2022].
- [43].IRCT201108035625N3. IRCT | Comparing the effect of conventional therapy (Heparin followed by warfarin) with imterventional therapy (thrombolysis with or without angioplasty and stenting) on venous patency in patients who admitted with acute iliofemoral DVT in Tehran Heart Center emergency department. Available at: https://en.irct.ir/trial/6154?revision=6154 [Access date December 31, 2022].
- [44].van Vuuren TM, van Laanen JHH, de Geus M, et al. A randomised controlled trial comparing venous stenting with conservative treatment in patients with deep venous obstruction: research protocol. BMJ Open. 2017;7:e017233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Higgins J. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS, eds. Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (Updated June 2017), Cochrane. 2017. Available at: www.training.cochrane.org/handbook [Access date October 29, 2018]. [Google Scholar]
- [46].Eckenrode G, Baltich Nelson B, Belarmino A, et al. Meta-analysis and systematic review of interventional therapy versus anticoagulation for isolated femoropopliteal deep venous thrombosis. J Vasc Surg Venous Lymphat Disord. 2019;7:272–6. [DOI] [PubMed] [Google Scholar]
- [47].Qiu P, Zha B, Xu A, et al. Systematic review and meta-analysis of iliofemoral stenting for post-thrombotic syndrome. Eur J Vasc Endovasc Surg. 2019;57:407–16. [DOI] [PubMed] [Google Scholar]
- [48].Razavi MK, Jaff MR, Miller LE. Safety and effectiveness of stent placement for iliofemoral venous outflow obstruction: systematic review and meta-analysis. Circ Cardiovasc Interv. 2015;8:e002772. [DOI] [PubMed] [Google Scholar]
- [49].Taha MA, Busuttil A, Bootun R, et al. A systematic review on the use of deep venous stenting for acute venous thrombosis of the lower limb. Phlebology. 2019;34:115–27. [DOI] [PubMed] [Google Scholar]
- [50].Thomas M, Hollingsworth A, Mofidi R. Endovascular management of acute lower limb deep vein thrombosis: a systematic review & meta-analysis. Ann Vasc Surg. Epub ahead of print February 12, 2019. [DOI] [PubMed] [Google Scholar]
- [51].Wang C-N, Deng H-R. Percutaneous endovenous intervention plus anticoagulation versus anticoagulation alone for treating patients with proximal deep vein thrombosis: a meta-analysis and systematic review. Ann Vasc Surg. 2018;49:39–48. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.