Abstract
Background
COVID-19-related acute respiratory distress syndrome (CARDS) is a severe evolution of the Sars-Cov-2 infection and necessitates intensive care. COVID-19 may subsequently be associated with long COVID, whose symptoms can include persistent respiratory symptoms up to 1 year later. Rehabilitation is currently recommended by most guidelines for people with this condition.
Objectives
To evaluate the effects of exercise training rehabilitation (ETR) on dyspnoea and health-related quality of life measures in people with continuing respiratory discomfort following CARDS.
Methods
In this multicentre, two-arm, parallel, open, assessor-blinded, randomised controlled trial, we enroled adults previously admitted with CARDS to 3 French intensive care units who had been discharged at least 3 months earlier and who presented with an mMRC dyspnoea scale score > 1. Participants received either ETR or standard physiotherapy (SP) for 90 days. The primary outcome was dyspnoea, as measured by the Multidimensional Dyspnoea Profile (MDP), at day 0 (inclusion) and after 90 days of physiotherapy. Secondary outcomes were the mMRC and 12-item Short-Form Survey scores.
Results
Between August 7, 2020, and January 26, 2022, 487 participants with CARDS were screened for inclusion, of whom 60 were randomly assigned to receive either ETR (n = 27) or SP (n = 33). Mean MDP following ETR was 42% lower than after SP (26.15 vs. 44.76); a difference of -18.61 (95% CI -27.78 to -9.44; p<10−4).
Conclusion
People who were still suffering from breathlessness three months after being discharged from hospital with CARDS had significantly improved dyspnoea scores when treated with ETR therapy for 90 days unlike those who only received SP. Study registered 29/09/2020 on Clinicaltrials.gov (NCT04569266).
Keywords: COVID-19, ARDS, Physiotherapy, Rehabilitation, Dyspnoea, HRQOL, Long COVID
Introduction
The COVID-19 pandemic has challenged the worldwide healthcare system with a surge of people admitted to hospitals for acute respiratory failure (ARF). Although most SARS-CoV-2 infections ended naturally, 10% of people developed a hyperinflammatory state leading to severe hypoxaemia that required mechanical ventilation (MV) in intensive care units (ICU). This development of COVID-19-related acute respiratory distress syndrome (CARDS) was associated with, on average, a 90-day mortality rate in about a third of cases [1,2].
While CARDS is broadly similar to acute respiratory distress syndrome (ARDS) [3], several clinical and pathophysiological observations raise questions about the relevance of standard ARDS therapeutic guidelines [4]. “Happy hypoxaemia”, a profound decrease in oxygen saturation, has been repeatedly observed in remarkably non-dyspnoeic people with COVID-19 [5,6]. In addition, while ARDS is associated with a loss of lung compliance, CARDS is often initially associated with a combination of severe hypoxaemia and near-normal lung compliance. Such observations led to the description of 2 contrasting phenotypes of CARDS that only share severe hypoxaemia: Type-H (high elastance) and Type-L (low elastance) [7].
Following ‘regular’ ARDS, approximately 50% of people develop neuromuscular weakness of their limbs and respiratory muscles [8] that alters pulmonary functional capacity at ICU discharge [9]. At present, physiotherapy is offered to people during their ICU stay with early mobilisation, to try and prevent the long-term functional consequences of ARDS. Despite this, functional disability, muscle weakness and dyspnoea can persist for years following discharge, dramatically affecting health-related quality of life (HRQOL) [9,10]. Similar clinical patterns have been observed following long COVID [11], [12], [13], [14]; dyspnoea is the most frequently reported symptom following other respiratory diseases and is a predictive factor for hospitalisation, mortality and is associated with poorer physical and mental HRQOL.
For this reason, international recommendations stress the importance of physiotherapy and rehabilitation for those with long COVID to treat dyspnoea, fatigue and functional disability that persists 4–12 weeks after the initial infection, regardless of its original gravity [15], [16], [17]. Recently several countries, including the French National Authority for Health (Haute Autorité de Santé) and Order of Physiotherapists (Ordre des Masseurs-Kinésithérapeutes) published long COVID rehabilitation guidelines for physiotherapists, based on exercise training rehabilitation (ETR) including both endurance and strength training for pulmonary rehabilitation (PR) in chronic obstructive pulmonary disease (COPD) [17,18]. However, while the benefits of physiotherapy for people in ICU are well-described, little is available about ETR following an ICU stay and methodological quality is poor [19,20]. Finally, in comparison to ARDS, the course of CARDS is significantly different and still not fully understood. People with CARDS may, therefore, experience harsher physical, respiratory and psychological complications [21,22] and specific pathophysiological mechanisms may lead to unexpected responses to rehabilitation [21].
We therefore assessed the effects of ETR compared to standard physiotherapy (SP) on dyspnoea, as measured by the multidimensional dyspnoea profile (MDP) [23] and HRQOL in people with persistent respiratory symptoms following CARDS ≥3 months after being discharged from ICU.
Methods
Study design and participants
This investigator-led, multicentre, randomised–controlled, two-arm, parallel, open-label, assessor-blinded trial took place in physiotherapy practices in Paris, France. People with persistent respiratory symptoms after a CARDS diagnosis were invited to participate if they were ≥18 years, were registered with the French Social Security system, and had: 1) received mechanical ventilation for ≥48 h following a documented SARS-Cov-2 infection; 2) been discharged from any of the 3 participating French Hospital ICU after ≥3 months; and 3) had dyspnoea, defined by the modified Medical Research Council dyspnoea scale [24] (mMRC) score >1 at the time of inclusion.
People were excluded if they: 1) had little/no reported dyspnoea (mMRC dyspnoea scale score ≤1); 2) were unable to participate in rehabilitation sessions due to severe neurological disease or an osteoarticular pathology; 3) were under guardianship; 4) lived >5 km away from the study rehabilitation practice. See Supplementary Material for further details on inclusion criteria.
Potential participants were screened and informed during a telephone call with the research physiotherapist (CR). After being provided with written information about the protocol, express oral consent was obtained and recorded in the medical record of each participant. Inclusion was confirmed by telephone the following week by the doctor in charge of outcome assessment (PL).
The trial was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. The protocol was approved by the Ethics Committee (Comité de Protection des Personnes Sud-Méditerranée III, final approval 29 June 2020, RCB 2020-A01686-33) and was registered on Clinicaltrials.gov (NCT04569266) on September 29, 2020.
Randomisation
Individuals were screened for eligibility criteria and participants were selected consecutively. Eligible participants were randomly allocated (1:1) to receive 90 days of either ETR or SP (2 sessions per week, 1 week reserved for each baseline and post-intervention outcome assessment measurement). The trial statistician generated a permuted-block randomisation sequence using variably sized blocks of 2 or 4 with stratification according to centre, and sequentially assigned each participant to their intervention using opaque envelopes.
Procedures
Participants in the intervention group were offered 2 × 60 min sessions of ETR per week for 10 weeks, according to outpatient program guidelines [25], [26], [27]. An initial 6-minute walking test was performed during the first assessment by the physiotherapist to determine target heart rate, and again during the final assessment to evaluate progression. Endurance training and exercise tolerance were evaluated using a cycle ergometer, with continuous monitoring of heart rate and oxygen saturation. For the continuous endurance training, participants started at 60–70% of their maximal peak power and the target dyspnoea was 4–6 on the modified Borg scale [28]. Initial effort lasted for 15 min and was gradually increased until an exercise duration of 45–60 min was achieved. Power intensity was adjusted according to each participant's progress until the target heart rate and dyspnoea were reached.
All participants were also offered muscle strength training during every session: strengthening of the lower limbs was prioritised, but additional exercises for the upper limbs and core were also included. Each exercise consisted of 4 sets of 6–12 repetitions. Exercises included leg presses, leg curls, and leg extensions using devices such as steppers, rowing machines, treadmills, and weights. Individual exercise choices were at the discretion of the physiotherapist, but muscle fatigue had to be felt at the end of each set. The ETR protocols are available in the Supplementary Materials (“Exercise Training Modalities”).
Participants in the control group received usual care during the 90 days: SP at the rate of 2 × 30 min sessions per week for 10 weeks. Physiotherapy session exercises were left to the discretion of the physiotherapist according to their mandatory initial assessment. Sessions included activities such as low-to-moderate intensity aerobic training on the exercise bicycle, ergometer or treadmill; strength-training for limbs and trunk muscles using bench weights with guided loads, weights, or elastics; as well as stretching, balance exercises, electrostimulation and respiratory therapy.
Physiotherapists delivering the ETR and SP sessions were all external practitioners who were independent of the 3 recruiting centres; they did not participate in study design, participant selection or result analysis. In order to anticipate participant attrition, both groups received telephone follow-up calls to provide support and ensure they found suitable, available practitioners.
Participants in the ETR group had their exercise training planned in accordance with specialised CARDS guidelines [25,29] and delivered by specialised PR physiotherapists.
Participants in the SP group had the same number of sessions, but they were delivered by general physiotherapist practitioners.
As both ETR and SP are treatments recommended by current COVID-19 guidelines [17,18,29], we designed the protocol to allow the SP group to still benefit from ETR. Thus, after the intervention phase, an ETR prescription was given to those in the SP group to allow them to receive ETR therapy too. Details and rationale of both interventions are given in the Supplementary Materials.
Outcomes
The primary outcome was the measurement of dyspnoea in its 3 dimensions, as assessed by the difference in the MDP score between baseline (at inclusion or day 0) and at 90 days (the end of the intervention). Secondary outcomes were 1) measurement of functional dyspnoea using the Modified Medical Research Council (mMRC) scale and 2) measurement of HRQOL using the 12-item Short-Form Survey (SF-12) at 90 days. The investigator who completed both baseline and 90-day outcome assessments (PL) was blinded to randomisation and group allocations. Participant demographic, medical history and ICU stay data were also collected.
Sample size
At implementation of the protocol, no data were available on the MDP minimal clinically important difference (MCID) for this population; and little-to-no data were available on cardiorespiratory diseases that would result in an 8 -point MCID [30]. We therefore chose a more stringent MDP MCID of 12 points, obtained by anticipating a change of >1 point per item, with a standard deviation of 30 points to account for a pessimistic heterogeneity. Accounting for attrition and using these values, a Student's t-test, a 2-sided alpha of 0.05, and 90% power, we estimated that 100 people needed to be enroled in each group.
Statistical analysis
Presentation of results is made in accordance with the CONSORT guidelines. According to their distribution, continuous variables are presented as either mean (with standard deviation) or median (with interquartile range); categorical data are presented as numbers (with percentages). Before choosing statistical tests, we performed a Shapiro test to assess if data distribution was Gaussian. For 90-day MDP (primary outcome) data, between-groups differences and secondary outcomes were tested using an ANCOVA model adjusted for baseline values. All estimates are presented with their 95% confidence interval.
We performed all analyses with R 4.2.1 software (The R Project for Statistical Computing, https://www.r-project.org), according to the intention-to-treat principle considering a two-sided type I error with an alpha of 0.05. All data were collected using Redcap software (Research Electronic Data Capture).
Results
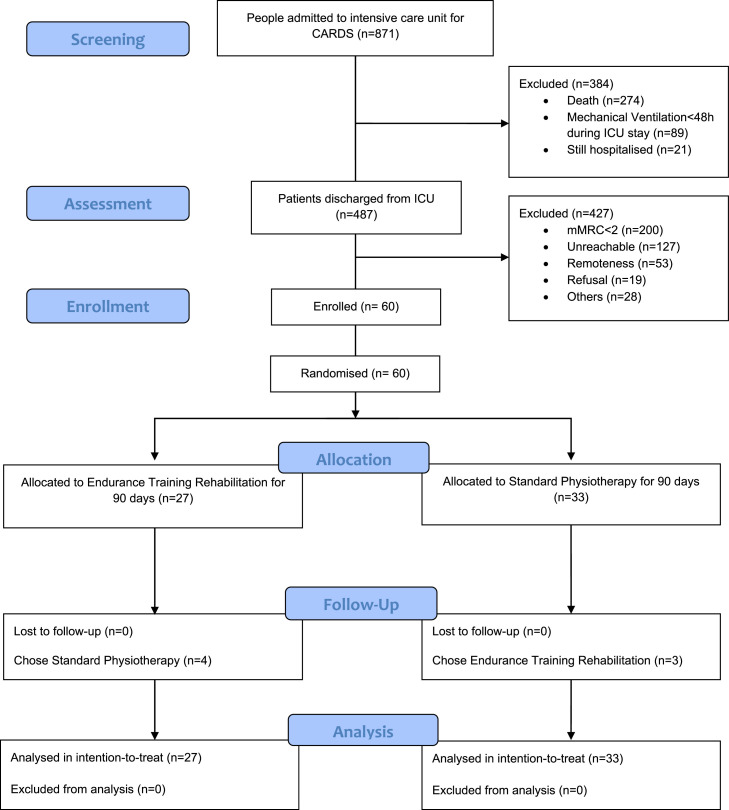
Between July 22, 2020 and January 26, 2022, 487 people were potentially eligible but only 60 could be enroled, 55% (32/60) of whom were recruited from the Groupe Hospitalier Paris Saint-Joseph. Participants were randomly allocated to either the ETR (n = 27) or the SP (n = 33) treatment groups. All participants received SP either in the hospital after their discharge, from ICU, or in post-ICU follow-up and rehabilitation care (FRC) structures as per international guidelines [31] before inclusion in the study. All participants received either ETR or SP physiotherapy care. Nobody withdrew consent or dropped out of their training program. Four participants placed in the ETR group had SP instead as no nearby practices offering ETR had free appointments for several months. Similarly, 3 participants originally assigned to the SP program ultimately received ETR instead as their physiotherapist decided to refer them to a PR practice with a new, more specific, medical prescription. All participants completed the last follow-up assessment. The last follow-up visit took place on March 25, 2022, and the study was closed on March 28, 2022, due to the phasing out of ICU admissions for CARDS. The mean time between ICU discharge and inclusion was 173 days (95% CI 147.36 to 198.64) and 174 days (95% CI 144.42 to 204.92) for the ETR and SP groups, respectively. Details of participant characteristics and follow-up data are shown in Fig. 1 .
Fig. 1.
CONSORT flow diagram illustrating recruitment and analysis in a study comparing the effect of exercise therapy rehabilitation versus standard physiotherapy on dyspnoea in people previously hospitalised with COVID-19-related acute respiratory distress syndrome (CARDS). This was an intention-to-treat trial thus participant data were analysed with their initially allocated treatment group, even though 7 patients ultimately received the other treatment. This 'imperfection' reflected the real-life effect of patient free choice. Dyspnoea was measured by the Modified Medical Research Council dyspnoea scale (mMRC).
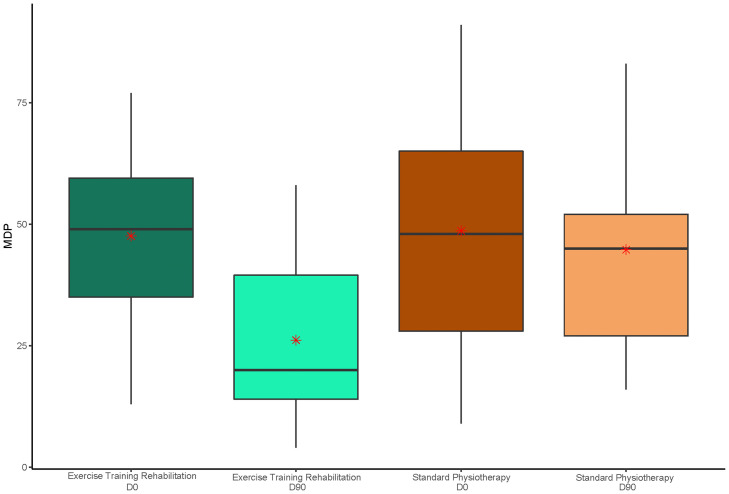
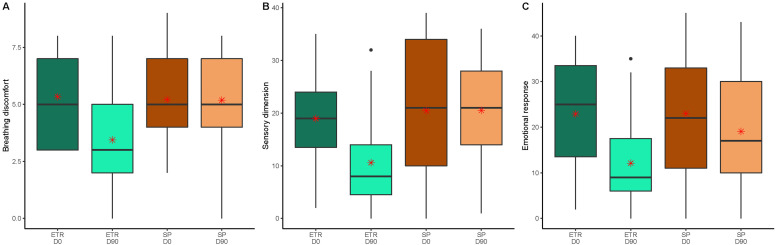
Demographic and baseline characteristics were comparable between groups (see Table 1 ). The mean age of all 60 participants was 58 years (SD 12), 37 were men (62%), 23 were women (38%) and the main comorbidity was diabetes (n = 22, 37%). The mean MDP after ETR was 42% lower than after SP at 26.15 vs. 44.76, a mean difference of −18.61 (95% CI −27.78 to −9.44; p<10−4) (Fig. 2 ). A statistically significant decrease was also observed in all 3 subcategories of the MDP: mean breathing discomfort, sensory dimension and emotional response were 34%, 48% and 36% lower, respectively, at day 90 following ETR compared to after SP. Respective differences were: −1.74 (95% CI −2.81 to −0.67; p<10−3) for breathing discomfort; −9.92 (95% CI −14.67 to −5.18; p<10−4) for sensory dimension scores; and −6.95 (95% CI −12.66 to −1.24; p = 0.014) for emotional response scores (see Fig. 3 ). Full details of primary and key secondary outcomes are shown in Table 2 . The mean mMRC at day 90 was 36% lower in the ETR group than in the SP group. The mean difference was −0.76 (95% CI −1.21 to −0.30; p<10−3).
Table 1.
Baseline characteristics of participants with COVID-19 related acute respiratory distress syndrome (CARDS) who received either exercise training rehabilitation (ETR) or standard physiotherapy (SP) after discharge from intensive care.
| ETR (n = 27) | SP (n = 33) | |
|---|---|---|
| Demographics | ||
| Age, years | 57 (14.28) | 59 (9.94) |
| Women | 11 (40%) | 12 (36%) |
| Men | 16 (60%) | 21 (67%) |
| Body mass index, kg/m2 | 31.44 (7.49) | 28.94 (5.25) |
| Risk factors | ||
| Smoker | 2 (7%) | 5 (15%) |
| Former smoker | 3 (11%) | 7 (22%) |
| Alcoholism | 0 (0%) | 4 (13%) |
| Chronic obstructive pulmonary disease | 1 (4%) | 4 (12%) |
| Cardiac insufficiency | 1 (4%) | 1 (3%) |
| Ischaemic cardiopathy | 0 (0%) | 3 (9%) |
| Occlusive arteriopathy of the lower limbs | 0 (0%) | 1 (3%) |
| Atrial fibrillation arrhythmia | 1 (4%) | 2 (6%) |
| Cirrhosis | 0 (0%) | 1 (3%) |
| Diabetes | 8 (30%) | 14 (42%) |
| Cancer | 1 (4%) | 2 (6%) |
| Human immunodeficiency virus | 0 (0%) | 1 (3%) |
| Clinical history | ||
| Referred to ICU from… | ||
| Home | 3 (11%) | 4 (12%) |
| Surgical Ward | 7 (26%) | 8 (24%) |
| Hospital Ward | 12 (44%) | 11 (34%) |
| Other Hospital | 5 (19%) | 10 (30%) |
| Length of stay intensive care unit, days | 12.00 (8.50–23.00) | 17.00 (12.00–35.00) |
| Length of stay hospitalisation, days | 26.00 (16.50–37.50) | 26.00 (19.00–38.25) |
| Discharge destination after ICU | ||
| Home | 12 (44%) | 11 (33%) |
| Rehabilitation facility | 15 (56%) | 22 (67%) |
| Clinical parameters at ICU admission | ||
| SAPS II | 37.77 (12.19) | 43.13 (15.39) |
| Mechanical ventilation duration, days | 18.00 (7.00–25.50) | 18.00 (9.25–30.25) |
| PaO2/FiO2 | 121.00 (91.00–153.00) | 97.00 (80.00–165.00) |
Data are shown as mean (SD), n (%) or median (IQR Q1-Q3). ICU, intensive care unit; SAPS II, Simplified Acute Physiology Score N°2. SAPS II scores range from 0 to 163 and provide an estimate of the risk of death without having to specify a primary diagnosis. A higher score indicating a higher risk of death. PaO2/FiO2 ratio is the ratio of arterial oxygen partial pressure to fractional inspired oxygen. A lower score indicates a more profound hypoxaemia.
Fig. 2.
Box and whisker plots comparing multidimensional dyspnoea profile (MDP) data for groups receiving exercise training rehabilitation and standard physiotherapy at baseline and 90 days. The middle horizontal line represents the median. Whisker values are the lower quartile minus 1·5 times the interquartile range, or the upper quartile plus 1·5 times the interquartile range. Means are represented by a red asterisk. X-axis categories: D0, Day 0 or baseline data; D90, data at 90 days, end of the study; MDP scores (shown on y-axis) ranged from 0 to 110 (a higher score indicated greater breathlessness).
Fig. 3.
Comparison of multidimensional dyspnoea profile (MDP) changes for people previously hospitalised with COVID-19-related acute respiratory distress syndrome who received exercise training rehabilitation (ETR) or standard physiotherapy (SP) for 90 days. MDP subcategories (y-axis) were breathing discomfort (Fig. 3A), sensory dimension (Fig. 3B) and emotional response (Fig. 3C). The middle horizontal line represents the median; outliers are displayed as separate black points. Whisker values are the lower quartile minus 1·5 times the interquartile range or the upper quartile plus 1·5 times the interquartile range. Means are represented by a red asterisk. X-axis categories: D0, Day 0 or baseline data; D90, data at 90 days, end of the study; MDP scores ranged from 0 to 10 (breathing discomfort), 0–50 (sensory dimension), and 0–50 (emotional response). For all MDP scores, higher values indicated greater breathlessness.
Table 2.
Primary Outcomes and key secondary outcomes of participants with COVID-19 related acute respiratory distress syndrome (CARDS) who had received 90 days of either exercise training rehabilitation (ETR) or standard physiotherapy (SP) following discharge from intensive care.
| Baseline (day 0) |
Day 90 |
|||||
|---|---|---|---|---|---|---|
| ETR (n = 27) | SP (n = 33) | ETR (n = 27) | SP (n = 33) | Difference | p-value | |
| MDP subcategory | ||||||
| Total score | 47.56 (16.10) | 48.64 (24.35) | 26.15 (15.48) | 44.76 (19.25) | −18.61 (−27.78 to −9.44) |
<0.0001 |
| Breathing discomfort | 5.33 (1.94) | 5.21 (1.85) | 3.44 (2.10) | 5.18 (2.02) | −1.74 (−2.81 to −0.67) |
0.0006 |
| Sensory dimension | 18.96 (7.66) | 20.45 (12.64) | 10.59 (8.90) | 20.52 (9.33) | −9.92 (−14.67 to −5.18) |
<0.0001 |
| Emotional response | 22.89 (11.67) | 22.97 (13.17) | 12.11 (9.88) | 19.06 (11.82) | −6.95 (−12.66 to −1.24) |
0.0140 |
| mMRC | 2.37 (0.63) | 2.30 (0.59) | 1.33 (0.62) | 2.09 (1.10) | −0.76 (−1.21 to −0.30) |
0.001 |
| SF-12 | ||||||
| Total score | 74.52 (13.10) | 70.47 (11.21) | 83.36 (14.97) | 75.13 (15.79) | 8.24 (0.22 to 16.25) |
0.14 |
| Physical component | 35.10 (6.55) | 31.45 (5.98) | 39.76 (8.57) | 32.80 (8.15) | 6.95 (2.62 to 11.29) |
0.016 |
| Mental component | 40.57 (8.46) | 39.03 (10.80) | 45.08 (11.05) | 43.02 (10.88) | 2.06 (−3.63 to 7.75) |
0.72 |
Data are mean (SD). Differences are expressed as means (95% CI); MDP, Multidimensional Dyspnoea Profile. Scores range from 0 to 110 with a higher score indicating greater breathlessness: 0–10 for breathing discomfort, 0–50 for the sensory dimension, and 0–50 for the emotional response; mMRC, modified Medical Research Council dyspnoea scale. Scores range from 0 to 4 with a higher score indicating a greater severity of dyspnoea; SF-12, 12-Item Short Form Survey. Both physical and mental components of this scale have a mean score of 50 (SD 10). Lower scores on each component indicate greater disability: <30 for a severe disability, ≥30 - <40 for a moderate disability, ≥40 - <50 for a mild disability and ≥50 no disability.
Mean SF-12 total scores after the rehabilitation intervention were similar for ETR and SP groups. Separate analysis of the SF-12 physical component results, however, revealed a 21% increase of the score following ETR; the difference was 6.95 (95% CI 2.62 to 11.29; p = 0.016). No statistically significant differences were observed between groups for the mental component of the SF-12 score.
Discussion
In this multicentre, randomised trial, a 3-month ETR course decreased dyspnoea for people with long COVID following CARDS more than standard physiotherapy. An improvement of the physical component of the SF-12 HRQOL scale was also observed, without significant changes in either the mental component or the overall HRQOL.
Dyspnoea is a broad symptom that often persists beyond the acute phase of COVID-19 and may worsen in the first year, particularly for women, the elderly and people with multiple comorbidities [32], [33], [34]. During pre-trial screening, 287 of the 487 (59%) people with long COVID were affected by breathlessness during daily activities, confirming dyspnoea as a major reported symptom [11,22,[35], [36], [37], [38]]. Other studies have also reported that shortness of breath, breathlessness and associated sensations of breathing impairment are described by 40–50% of people with long COVID and are more marked following CARDS [39]. Such symptoms are usually noted during classical ARDS evolution.
Interestingly, however, people who had severe hypoxaemia without dyspnoea (‘happy hypoxemia’) during their initial COVID infection, subsequently reported considerable respiratory discomfort during long COVID without severe documented gas exchange abnormalities [5]. Those in this chronic phase of long COVID felt breathless, despite having normalised haematosis at rest. This peculiar reversal of expected symptoms could be explained by alveolocapillary membrane compromise, illustrated by altered diffusing capacity of the lung for carbon monoxide (DLCO), pulmonary function test results and computed-tomography scan abnormalities reported in a growing number of studies [12,38,39]. Moreover, dyspnoea, decreased DLCO and pulmonary fibrosis seem to be closely associated [39]. These functional data are consistent with early histological studies that emphasised the important role of local inflammation and diffuse alveolar damage with observed histological evolution of ARDS.
Whereas physiotherapy has long been used as a key rehabilitation method, few data exist concerning its implementation. Without clear, evidence-based guidelines, considerable variability has developed between physiotherapists when treating patients [35,40]. In contrast, management of people with chronic respiratory insufficiency and dyspnoea with ETR is based on a standardised procedure, allowing accurate evaluation of the treatment's efficacy on breathlessness and HRQOL [25]. Our observation of reduced dyspnoea following ETR is in accordance with previous randomised controlled trials into chronic obstructive pulmonary disease [41,42] and studies of low -to -moderate severity COVID-19 [43,44], including a recent observational cohort study [35] and a pilot study [45]. The improvement of physical abilities observed in those studies agrees with the 45% decrease of MDP scores after a 90-day ETR course that we observed.
This result raises questions about the interdependency between dyspnoea and disability. Whether disability was reduced following improved dyspnoea, or whether the decrease in perceived dyspnoea was merely a consequence of a growth in functional ability has long been debated and remains a matter for future research [46]. While all 3 components of dyspnoea were significantly reduced by ETR, it should be noted that the emotional response was the least decreased. Together with an improvement in physical function, but not the mental dimension of HRQOL, these findings suggest that ETR reduced dyspnoea and breathlessness through physical and functional improvement, rather than through an emotional component. It has previously been suggested by several authors that persistent dyspnoea in long COVID is explained not only by pathophysiological lung abnormalities or physical impairment, but also by intricate mechanisms of hyperventilation believed to be of neurological and emotional origin [47,48].
Assessing dyspnoea is not an easy task. Most studies of PR assess dyspnoea through 1-dimensional assessments such as the Borg or mMRC scales, or disease-specific HRQOL tools like the St. George's Respiratory Questionnaire. Dyspnoeic mechanisms are naturally intricate and multifactorial; they should be assessed using both emotional and sensorial components [23]. Moreover, recent studies into long COVID are shedding light on the direct effect of dyspnoea on anxiety, depression and HRQOL [11,22,[35], [36], [37],43]. We demonstrated a reduction in breathing discomfort and in the sensory dimension of, and emotional response to, dyspnoea. Conversely, the benefit from rehabilitation on HRQOL beyond initial hospitalisation remains poorly studied [20,35,43].
In our work, the mitigation of dyspnoea is associated with an improvement of the physical dimension of HRQOL, as measured by the overall SF-12 score, which did not differ between groups. A possible explanation could be a lack of power. Using a more sensitive evaluation tool, such as the Short Form-36, might have detected a difference as has been previously demonstrated for COPD or COVID-19 [35,40,41]. The SF-36 was our first choice for assessing HRQOL, but for practical reasons during the pandemic we ultimately opted for the more time-efficient SF-12. The relative role of dyspnoea within multiple chronic symptoms is difficult to establish. A wide range of organ dysfunctionality is unaffected by physiotherapy and conditions such as ageusia, headaches, hair loss, and intestinal disorders may play a major part in overall HRQOL, especially in a younger population with few comorbidities [11]. Another explanation, suggested by Grosbois et al., would be that fatigue, anxiety and depression (the mental component of SF-12) seem to have a greater impact on dyspnoea than the physical component [40]. Therefore, an improvement in dyspnoea could have a greater effect on the physical, rather than on the mental part of the HRQOL.
To the best of our knowledge, this is the first randomised controlled work studying the effects of physiotherapist-led, high-intensity exercise training therapy after CARDS, which is a major strength of our study. Furthermore, we chose to collect outcomes using an independent assessor, blinded to the intervention, to minimise bias due to the open nature of this type of trial. The formalisation of intervention, using existing guidelines, and the recruitment of PR physiotherapists allowed us to expect reproducible training, reinforcing the reproducibility of the method in any location [17,25]. Finally, as participants were recruited, assessed and followed-up via phone calls by the practitioners who participated in their initial care in ICU, participant retention and treatment observance were 100%, which is high for such a study. Most participants reported feeling supported by the ICU staff after discharge from hospital at a time when access to care was made difficult by recommendations, mobility restrictions and curfews. They thus showed excellent compliance with all rehabilitation procedures, and everyone completed therapy.
Our study has several limitations. First, the impossibility of recruiting 200 participants as planned forced us to prematurely stop the study. However, our sample size estimation was based on a pessimistic expected MDP inter-treatment group score difference of 12 with a large SD of 30, in order to account for cohort heterogeneity. The actual observed difference was larger with a much smaller SD. Using our results to retrospectively calculate the required sample size gave us a participant cohort size of only 40. Second, vaccination and better overall control of the contagion, while fortunately reducing the pandemic, decreased the possibilities for us to find participants for the study. Third, we faced a major challenge with reference to PR practices. PR practices, usually reserved for people with COPD, are scarce and no official directory is available. Despite the slowing pandemic, specialist PR practices were overwhelmed by a surge in people with COPD and long COVID. The scarcity of such resources, already contributing to difficulties for first-line care provision, also played a role in the lack of post-hospitalisation care availability [49]. Unfortunately, 53 participants could not be included in this study because they lived too far from a rehabilitation practice.
Fourth, due to our study design, no functional data (such as the distance walked during the 6-minute walking test or peak oxygen consumption) were available from the physiotherapists. This significant limitation prevented us from drawing conclusions about the effects of ETR or SP on functional endpoints used in clinical practice.
Lastly, as mentioned earlier, even when delivered by the same physiotherapist ETR and SP can differ substantially because, unlike SP, ETR follows a protocol. Participants in the SP group received aerobic and muscle training (as prescribed) but at a lower intensity (low-to-moderate), for a shorter duration (30-minute sessions) and without standardisation than those in the ETR group. SP is tailored to the patient by the therapist and varies considerably between therapists and sessions. In contrast, consistency of therapy duration and intensity is what defines ETR for PR [25]. While participants in the SP group may well have been subjectively improved by the physiotherapy treatment, SP heterogeneity could explain its lack of a statistically significant effect on dyspnoea. Hence, the results of ETR are more consistent and homogeneous when compared with SP, even though SP currently represents standard care yet failed to improve dyspnoea or HRQOL in this trial.
Conclusion
A 90-day exercise training rehabilitation course, improved dyspnoea in its 3 dimensions amongst participants who had remained dyspnoeic after developing CARDS, compared to standard physiotherapy. An increased physical component of HRQOL was also found in the ETR group. High-intensity exercise training (ie, longer sessions with a higher target exercise intensity) may therefore be the most appropriate rehabilitation treatment for people presenting with dyspnoea after COVID-19. Future studies should investigate the long -term effects of ETR on people still suffering from breathlessness and the intricate mechanisms linking dyspnoea with HRQOL.
Contributors
This manuscript was initially drafted by CR, JW & FPh. GC designed the statistical plan & AF did the statistical analysis. CB, FPh, CR, PL, GP & FPe contributed to participants’ selection. CR & PL included participants. All authors contributed to data interpretation, and critical review and revision of the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Funding
This work was sponsored by the Groupe Hospitalier Paris Saint-Joseph.
Role of funders
The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Declaration of Competing Interest
None.
Acknowledgments
The authors thank the members of the clinical research centre for their constant help throughout the trial. This study was carried out as part of a PhD in clinical medicine and public health at the university of Granada.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.rehab.2023.101765.
Appendix. Supplementary materials
Data availability
Data will be made available on request.
References
- 1.Cummings M.J., Baldwin M.R., Abrams D., Jacobson S.D., Meyer B.J., Balough E.M., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet Lond Engl. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmidt M., Hajage D., Demoule A., Pham T., Combes A., Dres M., et al. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47:60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The ARDS Definition Task Force Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 4.Lascarrou J.-B. COVID-19-related ARDS: one disease, two trajectories, and several unanswered questions. Lancet Respir Med. 2021;9:1345–1347. doi: 10.1016/S2213-2600(21)00381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhont S., Derom E., Van Braeckel E., Depuydt P., Lambrecht B.N. The pathophysiology of “happy” hypoxemia in COVID-19. Respir Res. 2020;21:198. doi: 10.1186/s12931-020-01462-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tobin M.J., Laghi F., Jubran A. Why COVID-19 silent hypoxemia is baffling to physicians. Am J Respir Crit Care Med. 2020;202:356–360. doi: 10.1164/rccm.202006-2157CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gattinoni L., Chiumello D., Caironi P., Busana M., Romitti F., Brazzi L., et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46:1099–1102. doi: 10.1007/s00134-020-06033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dres M., Dubé B.-P., Mayaux J., Delemazure J., Reuter D., Brochard L., et al. Coexistence and impact of limb muscle and diaphragm weakness at time of liberation from mechanical ventilation in medical intensive care unit patients. Am J Respir Crit Care Med. 2017;195:57–66. doi: 10.1164/rccm.201602-0367OC. [DOI] [PubMed] [Google Scholar]
- 9.Wieske L., Dettling-Ihnenfeldt D.S., Verhamme C., Nollet F., van Schaik I.N., Schultz M.J., et al. Impact of ICU-acquired weakness on post-ICU physical functioning: a follow-up study. Crit Care. 2015;19:196. doi: 10.1186/s13054-015-0937-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herridge M.S., Tansey C.M., Matté A., Tomlinson G., Diaz-Granados N., Cooper A., et al. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 11.Montani D., Savale L., Noel N., Meyrignac O., Colle R., Gasnier M., et al. Post-acute COVID-19 syndrome. Eur Respir Rev. 2022;31 doi: 10.1183/16000617.0185-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antoniou K.M., Vasarmidi E., Russell A.-M., Andrejak C., Crestani B., Delcroix M., et al. European Respiratory Society statement on long COVID follow-up. Eur Respir J. 2022;60 doi: 10.1183/13993003.02174-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez-Ramirez D.C., Normand K., Zhaoyun Y., Torres-Castro R. Long term impact of COVID-19: a systematic review of the literature and meta-analysis. Biomedicines. 2021;9:900. doi: 10.3390/biomedicines9080900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vitacca M., Carone M., Clini E.M., Paneroni M., Lazzeri M., Lanza A., et al. Joint statement on the role of respiratory rehabilitation in the COVID-19 crisis: the Italian position paper. Respir Int Rev Thorac Dis. 2020;99:493–499. doi: 10.1159/000508399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas P., Baldwin C., Bissett B., Boden I., Gosselink R., Granger C.L., et al. Physiotherapy management for COVID-19 in the acute hospital setting: clinical practice recommendations. J Physiother. 2020;66:73–82. doi: 10.1016/j.jphys.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spruit M.A., Holland A.E., Singh S.J., Tonia T., Wilson K.C., Troosters T. COVID-19: interim guidance on rehabilitation in the hospital and post-hospital phase from a European Respiratory Society- and American Thoracic Society-coordinated international task force. Eur Respir J. 2020;56 doi: 10.1183/13993003.02197-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kinésithérapie - Réentrainement à l'effort au cours des symptômes prolongés de la COVID-19. Haute Aut Santé. 2021;4 [Google Scholar]
- 19.Tipping C.J., Harrold M., Holland A., Romero L., Nisbet T., Hodgson C.L. The effects of active mobilisation and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med. 2017;43:171–183. doi: 10.1007/s00134-016-4612-0. [DOI] [PubMed] [Google Scholar]
- 20.Connolly B., Salisbury L., O'Neill B., Geneen L., Douiri A., Grocott M.P.W., et al. Exercise rehabilitation following intensive care unit discharge for recovery from critical illness: executive summary of a Cochrane Collaboration systematic review. J Cachexia Sarcopenia Muscle. 2016;7:520–526. doi: 10.1002/jcsm.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alba G.A., Ziehr D.R., Rouvina J.N., Hariri L.P., Knipe R.S., Medoff B.D., et al. Exercise performance in patients with post-acute sequelae of SARS-CoV-2 infection compared to patients with unexplained dyspnea. EClinicalMedicine. 2021;39 doi: 10.1016/j.eclinm.2021.101066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghosn J., Piroth L., Epaulard O., Le Turnier P., Mentré F., Bachelet D., et al. Persistent COVID-19 symptoms are highly prevalent 6 months after hospitalization: results from a large prospective cohort. Clin Microbiol Infect. 2021;27:1041.e1–1041.e4. doi: 10.1016/j.cmi.2021.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banzett R.B., O'Donnell C.R., Guilfoyle T.E., Parshall M.B., Schwartzstein R.M., Meek P.M., et al. Multidimensional Dyspnea Profile: an instrument for clinical and laboratory research. Eur Respir J. 2015;45:1681–1691. doi: 10.1183/09031936.00038914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bestall J.C., Paul E.A., Garrod R., Garnham R., Jones P.W., Wedzicha J.A. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54:581–586. doi: 10.1136/thx.54.7.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spruit M.A., Singh S.J., Garvey C., ZuWallack R., Nici L., Rochester C., et al. An official American Thoracic Society/European Respiratory Society Statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med. 2013;188:e13–e64. doi: 10.1164/rccm.201309-1634ST. [DOI] [PubMed] [Google Scholar]
- 26.Gloeckl R., Marinov B., Pitta F. Practical recommendations for exercise training in patients with COPD. Eur Respir Rev. 2013;22:178–186. doi: 10.1183/09059180.00000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grosbois J.-M., Gicquello A., Langlois C., Le Rouzic O., frederic Bart, Wallaert B., et al. Long-term evaluation of home-based pulmonary rehabilitation in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:2037–2044. doi: 10.2147/COPD.S90534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horowitz M.B., Littenberg B., Mahler D.A. Dyspnea ratings for prescribing exercise intensity in patients with COPD. Chest. 1996;109:1169–1175. doi: 10.1378/chest.109.5.1169. [DOI] [PubMed] [Google Scholar]
- 29.Symptômes prolongés suite à une COVID-19 de l'adulte - diagnostic et prise en charge. Haute Aut Santé n.d. https://www.has-sante.fr/jcms/p_3237041/fr/symptomes-prolonges-suite-a-une-covid-19-de-l-adulte-diagnostic-et-prise-en-charge (accessed September 17, 2022).
- 30.Ekström M., Bornefalk H., Sköld C.M., Janson C., Blomberg A., Sandberg J., et al. Minimal clinically important differences for Dyspnea-12 and MDP scores are similar at 2 weeks and 6 months: follow-up of a longitudinal clinical study. Eur Respir J. 2021;57 doi: 10.1183/13993003.02823-2020. [DOI] [PubMed] [Google Scholar]
- 31.Smondack P., Gravier F.-É., Prieur G., Repel A., Muir J.-F., Cuvelier A., et al. Kinésithérapie et COVID-19 : de la réanimation à la réhabilitation à domicile. Synthèse des recommandations internationales. Rev Mal Respir. 2020;37:811–822. doi: 10.1016/j.rmr.2020.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nasserie T., Hittle M., Goodman S.N. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.11417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie Y., Bowe B., Al-Aly Z. Burdens of post-acute sequelae of COVID-19 by severity of acute infection, demographics and health status. Nat Commun. 2021;12:6571. doi: 10.1038/s41467-021-26513-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang L., Yao Q., Gu X., Wang Q., Ren L., Wang Y., et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet Lond Engl. 2021;398:747–758. doi: 10.1016/S0140-6736(21)01755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nopp S., Moik F., Klok F.A., Gattinger D., Petrovic M., Vonbank K., et al. Outpatient pulmonary rehabilitation in patients with long COVID improves exercise capacity, functional status, dyspnea, fatigue, and quality of life. Respiration. 2022;101:593–601. doi: 10.1159/000522118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lerum T.V., Aaløkken T.M., Brønstad E., Aarli B., Ikdahl E., Lund K.M.A., et al. Dyspnoea, lung function and CT findings 3 months after hospital admission for COVID-19. Eur Respir J. 2021;57 doi: 10.1183/13993003.03448-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heesakkers H., van der Hoeven J.G., Corsten S., Janssen I., Ewalds E., Simons K.S., et al. Clinical outcomes among patients with 1-year survival following intensive care unit treatment for COVID-19. JAMA. 2022;327:559–565. doi: 10.1001/jama.2022.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.González J., Benítez I.D., Carmona P., Santisteve S., Monge A., Moncusí-Moix A., et al. Pulmonary function and radiologic features in survivors of critical COVID-19: a 3-month prospective cohort. Chest. 2021;160:187–198. doi: 10.1016/j.chest.2021.02.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long Q., Li J., Hu X., Bai Y., Zheng Y., Gao Z. Follow-ups on persistent symptoms and pulmonary function among post-acute COVID-19 patients: a systematic review and meta-analysis. Front Med. 2021;8 doi: 10.3389/fmed.2021.702635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grosbois J.-M., Gephine S., Kyheng M., Henguelle J., Rouzic O.L., Saey D., et al. Physical and affective components of dyspnoea are improved by pulmonary rehabilitation in COPD. BMJ Open Respir Res. 2022;9 doi: 10.1136/bmjresp-2021-001160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Higashimoto Y., Ando M., Sano A., Saeki S., Nishikawa Y., Fukuda K., et al. Effect of pulmonary rehabilitation programs including lower limb endurance training on dyspnea in stable COPD: a systematic review and meta-analysis. Respir Investig. 2020;58:355–366. doi: 10.1016/j.resinv.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 42.Liao W., Chen J., Chen X., Lin L., Yan H., Zhou Y., et al. Impact of resistance training in subjects with COPD: a systematic review and meta-analysis. Respir Care. 2015;60:1130–1145. doi: 10.4187/respcare.03598. [DOI] [PubMed] [Google Scholar]
- 43.Liu K., Zhang W., Yang Y., Zhang J., Li Y., Chen Y. Respiratory rehabilitation in elderly patients with COVID-19: a randomized controlled study. Complement Ther Clin Pract. 2020;39 doi: 10.1016/j.ctcp.2020.101166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jimeno-Almazán A., Franco-López F., Buendía-Romero Á., Martínez-Cava A., Sánchez-Agar J.A., Sánchez-Alcaraz Martínez B.J., et al. Rehabilitation for post-COVID-19 condition through a supervised exercise intervention: a randomized controlled trial. Scand J Med Sci Sports. 2022;32:1791–1801. doi: 10.1111/sms.14240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hockele L.F., Sachet Affonso J.V., Rossi D., Eibel B. Pulmonary and functional rehabilitation improves functional capacity, pulmonary function and respiratory muscle strength in post COVID-19 patients: pilot clinical trial. Int J Environ Res Public Health. 2022;19:14899. doi: 10.3390/ijerph192214899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dyspnea. Mechanisms, assessment, and management: a consensus statement. American Thoracic Society. Am J Respir Crit Care Med. 1999;159:321–340. doi: 10.1164/ajrccm.159.1.ats898. [DOI] [PubMed] [Google Scholar]
- 47.Motiejunaite J., Balagny P., Arnoult F., Mangin L., Bancal C., d'Ortho M.-P., et al. Hyperventilation: a possible explanation for long-lasting exercise intolerance in mild COVID-19 survivors? Front Physiol. 2021;11 doi: 10.3389/fphys.2020.614590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bouteleux B., Henrot P., Ernst R., Grassion L., Raherison-Semjen C., Beaufils F., et al. Respiratory rehabilitation for Covid-19 related persistent dyspnoea: a one-year experience. Respir Med. 2021;189 doi: 10.1016/j.rmed.2021.106648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheiber B., Spiegl C., Wiederin C., Schifferegger E., Schiefermeier-Mach N. Post-COVID-19 rehabilitation: perception and experience of Austrian physiotherapists and physiotherapy students. Int J Environ Res Public Health. 2021;18:8730. doi: 10.3390/ijerph18168730. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.