Abstract
In order to limit the emergence of human immunodeficiency virus (HIV) drug resistance in a context of limited antiretroviral options, we sought to evaluate the efficacy of third-line (3L) regimens considering HIV genotypic resistance profile at initiation of 3L in Cameroon. A cohort-study was conducted from January-September 2020 among patients initiating a 3L antiretroviral therapy regimen at the Yaoundé Central Hospital. HIV-1 protease-reverse transcriptase was sequenced at the Chantal Biya international reference center for research on HIV/AIDS prevention and management and results were interpreted using Stanford HIVdbv8.3. Good virological response (viral load < 390 copies/mL) was assessed after 12 months using OPP-ERA platform. Statistical analyses were performed using Epi Info v7.2.2.6, with P < .05 considered statistically significant. Of the 38 patients initiating 3L with an available genotyping (42% female; median age, 49 [39–57] years), median cluster of differentiation type 4 count and viral load were 173 [34–374] cells/μL and 169,322 [30,382–551,826] copies/mL, respectively. At enrollment, all patients harbored resistance to reverse transcriptase inhibitors and 66% (25/38) to protease-inhibitors, although 63% (24/38) were still susceptible to darunavir/ritonavir. Preferred 3L regimen was dolutegravir + darunavir/r + tenofovir + lamivudine (51%) and median duration on 3L was 21 [17-32] months. Interestingly, 82% (31/38) of the participants achieved good virological response on 3L, regardless of genotypic profile at recruitment, variations in 3L regimens (P = .9) and baseline cluster of differentiation type 4 count (P = .3). Despite the high burden of reverse transcriptase inhibitor - and protease inhibitor boosted by ritonavir drug resistance, genotyping-guided 3L regimens is accompanied by virological success in most patients. This high efficacy, most likely due to use of high genetic barrier antiretrovirals, requires continuous adherence support alongside close monitoring for long-term effectiveness in similar programmatic settings.
Keywords: antiretroviral therapy, Cameroon, HIV drug resistance, third-line, virological response
1. Introduction
In 2021 worldwide, 85% of all people living with HIV (PLHIV) knew their status; 88% had access to antiretroviral therapy (ART) and 92% achieved viral suppression.[1] This progress, over the last decade, is a result of ART scale-up which has dramatically reduced human immunodeficiency virus (HIV)-related morbidity and mortality even in resource-limited settings (RLS), including sub-Saharan Africa (SSA).[1–6] However, achieving a sustained undetectable viral load (VL) ( < 50copies/mL) remains a major challenge due to emerging HIV drug resistance (HIVDR) strains, especially with the growing number of heavily-treated patients.[7–11] Of note, a systematic review and meta-analysis reported 19% of patients failing second-line after 12 to 18 months in SSA.[12] In Cameroon specifically, late detection of treatment failure has led to a low rate of patients switched to second- and third-line ART[7–9,13]; which remains below the minimum threshold of 5% defined by World Health Organization (WHO) for countries with long-term therapeutic experience.[8] Like several SSA countries, ART guidelines in Cameroon followed the WHO-public health approach, consisting of 2 nucleoside reverse transcriptase inhibitors (NRTIs) and 1 non-nucleoside reverse transcriptase inhibitor (NNRTI) as preferred first-line regimen before 2020.[11,14–16] In case of first-line ART failure, second-line regimen for adults consisted of 2 NRTIs and 1 ritonavir-boosted protease inhibitor (PI/r),[14,15,17] while third-line (3L) ART entailed integrase strand transfer inhibitors (preferably dolutegravir) and/or a second-generation PI/r (darunavir/ritonavir [DRV/r]) in combination with 2 NRTIs whose selection is guided by HIV genotypic resistance profile.[17] As we previously reported, heavily-treated patients in Cameroon, unlike those initiating first-line ART, stand at high-risk of acquiring multi-class drug resistance and have limited therapeutic in future.[9] Of relevance, in settings where access to genotypic drug resistance testing is limited, sequencing of optimal regimens prior to 3L initiation would advise on local strategies for selecting 3L-combinations with the greatest virological outcome.[9,18–20] We herein assess the effectiveness of HIVDR profiling in tailoring for optimal third-line ART among people failing second-line treatment with multi-drug resistance patterns in RLS like Cameroon.
2. Materials and methods
2.1. Study design and settings
This was a cohort-study was conducted among consenting adult living with HIV who were failing second-line ART. All participants were followed at the Yaoundé Central Hospital (YCH) and monitored for HIVDR at the Chantal Biya international reference center for research on HIV/AIDS prevention and management.
The YCH is the largest HIV treatment center at country-level, with over 12,000 PLHIV receiving ART on site. This HIV treatment center has first-line, second-line and third-line antiretroviral drug regimens available on site. With over 20 years’ experience on the clinical management of PLHIV and clinical trials, the site has experienced multidisciplinary team of health personnel, including but not limited to HV specialized clinicians, pharmacists, senior nurses, laboratory scientists, health counselors, psychosocial agents, community relay agents, representatives of community-based organizations, and representatives of PLHIV. Most importantly, the site is also a national reference center for the clinical management of patients on 3L regimens, with referral from most health facilities across the country and a cumulative number of 67 patients on third-line ART at the moment of the study. For the routine clinical management of PLHIV, the YCH has approximately 30% of hospital beds occupied by HIV-positive patients.
The Chantal BIYA International Reference Centre for research on HIV/AIDS prevention and management (CIRCB), is a government institution of the Ministry of Public Health dedicated to HIV research and patient monitoring in several aspects, among which: HIV early infant diagnosis in the frame of the national PMTCT program; Diagnosis of co-infections with HIV; Viral load measurement; Cluster of differentiation type 4 (CD4) and CD8 T lymphocytes counts; Biochemical and haematological analyses for monitoring drug safety; HIVDR genotypic resistance testing (GRT) at subsidized costs, and more recently; SARS-CoV-2 real-time PCR and sequencing for COVID-19 variant surveillance (http://www.circb.cm/btc_circb/web/).
2.2. Enrolment of the participants
Following an exhaustive sampling method, all 67 PLHIV receiving third-line ART were contacted by phone-call for possible inclusion in the study after screening for eligibility criteria and provision of a written informed consent. For each participant, we checked medical reports to assess the availability of socio-demographic data (sex, age); clinical data (WHO clinical stage, ART regimens, history of CD4 and viral load and adherence level); and the genotypic resistance profiles at second-line failure.
2.3. Procedure for HIVDR testing prior to 3L initiation
HIV-1 GRT was performed at CIRCB on plasma ARN aliquotes from HIV-infected patients as described elsewhere.[21] Briefly, Viral RNA was extracted from a 1-milliliter aliquot of plasma using a QIAamp Viral RNA Mini Kit (QIAGEN) according to the manufacturer’s instructions and then HIV-1 protease and reverse transcriptase were amplified using an in-house protocol. Drug resistance mutations (DRMs) were interpreted using the Stanford HIVdb v8.3 algorithm, with penalty scores of resistances for drug susceptibility assessment defined as follows: ≥60 (high-resistance); 30 to 59 (intermediate-resistance); <30 (susceptible).[22] Of note, a “susceptible” virus is one with no major DRMs Combinations for third-line ART regimens were provided according to viral susceptibility profile to PI/r and NRTIs. HIV-subtypes were first obtained from Stanford HIVdb algorithm and then assessed using rapid subtyping tools available online: COMET HIV-1 (https://comet.lih.lu/) and REGA HIV-1 Subtyping Tool - v3.0 (http://dbpartners.stanford.edu:8080/RegaSubtyping/stanford-hiv/typingtool/). Subtyping of each individual sequence was confirmed following molecular phylogeny by using MEGA v7 with HIV reference sequences downloaded from LANL and HIVdb.
2.4. Quantification of HIV-1 RNA VL measurement following 3L initiation
After switch on third-line ART, HIV-1 RNA viral load measurement was performed to evaluate the effectiveness of therapeutic outcomes. Following informed consent, 5mL of blood was collected from each participant in an EDTA tube and plasma was obtained after centrifugation at 1800 rpm for 5minutes. Quantification of viral load was done on the OPP-ERA platform as per manufacturer instructions.[23] Of note, the OPP-ERA platform is a point of care with detection thresholds ranging from < 390 to > 10,000,000 copies/mL; therefore, any result with VL < 390 copies/mL was interpreted as good virological response based on this platform that was used at the level of the health facility.
2.5. Statistical analysis
Descriptive statistics were performed for socio-demographic, clinical and biological data when available. Median, and interquartile range were reported for continuous variables. The Fisher exact test and Chi square test were used to compare categorical variables where appropriate. P values ≤ .05 were considered statistically significant. All the analyses were performed using Epi Info version 7.
2.6. Ethical considerations
Ethical clearance for the study was obtained from the regional ethics committee for research on human health (reference N°1860CRERSHC/2020) and from the institutional research ethics committee of the Faculty of Medicine and Biomedical Sciences of the University of Yaoundé I (reference N° 267UY1/FMSB). Administrative authorization was obtained from the YCH (reference N° 162/20/AR/DHCY) for participant enrollment and VL measurements and from the CIRCB (reference N°0191/019L/CIRCB/DG/SAA/BRH) for analyses of HIV-1 genotypic drug resistance testing.
3. Results
3.1. General clinical features of the study population
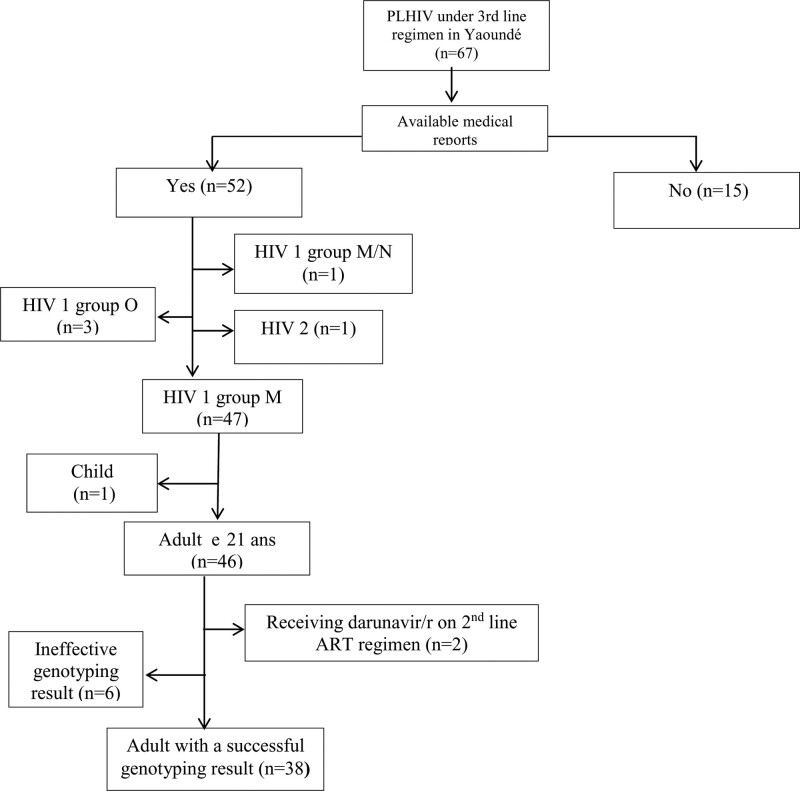
Out of the 67 patients (90.4% with HIV-1 group M, 5.7% with HIV-1 group O and 1.9% with HIV-2), 38 fully met all inclusion criteria (i.e., all data regarding complete clinical history prior 3L initiation were available alongside with the results from a GRT at second-line failure; see Fig. 1). Prior to initiating third-line ART, the median age of study participants was 49 [39–57] years, 57.9% (22/38) were males, 89.5% (34/38) were at WHO clinical stage 1 (asymptomatic), median CD4 count was 173 [34–374] cells/μL, and median viremia was 169,322 [30, 382–551, 826] copies/mL.
Figure 1.
Flow chart enrolment of the study participants. ART = antiretroviral therapy, HIV = human immunodeficiency virus, PLHIV = people living with HIV.
3.2. Genotypic resistance profiles prior third-line initiation
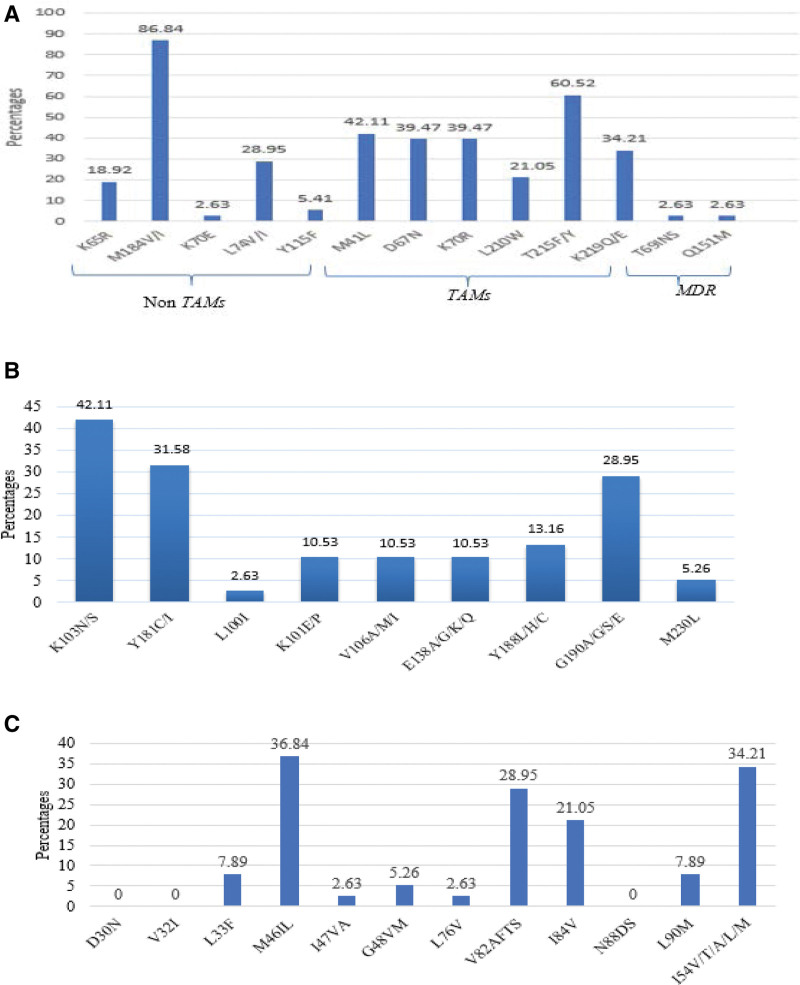
At the moment of initiating 3L ART, all patients harbored DRMs to reverse transcriptase inhibitors, specifically 97.4% (37/38), 86.8% (33/38) to NNRTI and NRTI respectively whereas 65.8% (25/38) harbored DRMs to PI/r. Furthermore, 84.2% (32/38) and 52.6% (20/38) of patients harbored dual (NRTI + NNRTI) and triple class (NRTI + NNRTI + PI/r) resistance respectively. Figure 2 gives a breakdown of all DRMs observed.
Figure 2.
HIVDR was interpreted according to Stanford HIVdb.v8.9-1. HIVDR = HIV drug resistance, MDR = multiple drug resistance mutation, NRTI = nucleoside reverse transcriptase inhibitors, NNRTI = nucleoside reverse transcriptase inhibitors, Non-TAMs = Non- thymidine analogue mutations, PI/r = protease inhibitor boosted by ritonavir, TAMs = thymidine analogue mutations.
3.3. Drug susceptibility prior third-line initiation
According to individual drug class susceptibility profiling, only 5.3% (2/38) of patients appeared still fully susceptible to nevirapine, efavirenz and drotaverine, whereas 26.3% (10/38) were susceptible to etravirine and 21.1% (8/38) to rilpivirine as far as NNRTI were concerned. Regarding NRTI, susceptibility to lamivudine, abacavir and diagnosing was observed in 2.7% (1/38), while susceptibility to zidovudine and tenofovir (TDF) was found in 18.4% (7/38); this underscores the need to select or recycle NRTIs (specifically between zidovudine and TDF) following indications from genotypic resistance profile. Regarding PI/r, 63.2% (24/38) patients harbored viruses fully susceptible to DRV/r, 39.5% (15/38) fully susceptible to ritonavir-boosted lopinavir and 39.5% (15/38) fully susceptible to ritonavir-boosted atazanavir; this further underscores the relevance of genotypic profiling in guiding either a recycling of first generation PI/r (atazanavir/ritonavir or lopinavir/ritonavir) or a switch to DRV/r-containing third-line regimens (with BID for those with partial efficacy).
3.4. HIV-1 genetic diversity at second-line failure, prior to initiating third-line ART
We had 9 (09) different HIV-1 subtypes in our study population. Recombinant forms were the most represented (71.1%; 27/38), with prevailing CRF02_AG (57.9%; 22/38), CRF11_cpx (7.9%; 3/38), CRF02_AG/CRF06_cpx (2.6%; 1/38) and CRF01_AE (2.6%; 1/38). Pure subtypes accounted for 28.9% (11/38) and comprised subtype A1 (13.2%; 5/38), D (5.3%; 2/38), F2 (5.3%; 2/38), G (5.3%; 2/38).
3.5. Virological response after switch to third-line ART regimens and its determinants
For treatment switch from second- to third-line ART after GRT, the most prescribed 3L regimen was dolutegravir + DRV/r + TDF + lamivudine (51%) and the median duration on 3L ART was 21 [17–32] months (see Table 1 for overall features of study population). Overall, up to 81.6% (31/38) of patients with < 390 copies/mL, indicating a good virological response to 3L ART. Only 7.9% (3/38) of participants had 390 to 999 copies/mL (low-level viremia), and 10.5% (4/38) had > 1000 copies/mL (unsuppressed viremia). Overall, the rate of viral suppression was 89.5% (34/38) on 3L ART in our study population.
Table 1.
Overall features of included participants (N = 37)*.
| Characteristics | |
|---|---|
| Socio-demographic | |
| Median [IQR] age (years), | 49 [39–57] |
| Male, n (%) | 22 (58) |
| Immuno-virological parameters | |
| Median CD4 count (cell/mm3), | 173 [34–374] |
| Median VL (copies/ml), | 169,322 [30, 382–551, 826] |
| HIV-1 genetic diversity (%) | |
| Pure subtypes | 28.97 |
| Circulating recombinant form | 71.03 |
| Third-line ART regimens (%) | |
| TDF + 3TC + DTG + DRV/r51.3 | |
| TDF + 3TC + DRV/r13.5 | |
| TDF + 3TC + DTG | 8.1 |
| TDF + 3TC + DTG + ATV/r | 5.4 |
| TDF + 3TC + DTG + LPV/r | 5.4 |
| AZT + 3TC + DTG + ATV/r | 8.1 |
| ABC + 3TC + DTG + DRV/ r | 2.7 |
| ABC + 3TC + DRV/r + ATV/r | 2.7 |
| DTG + DRV/r + ETR | 2.7 |
| Median time on third-line therapy (mo) | 21 [17–32] |
% = percentage, 3TC = lamivudine, ABC = abacavir; ART = antiretroviral therapy, ATV/r = ritonavir-boosted atazanavir, AZT = zidovudine, CD = clusters of differentiation, CD4 = cluster of differentiation type 4, DRV/r = ritonavir-boosted darunavir, DTG = dolutegravir, ETR = etravirine, HIV = human immunodeficiency virus, IQR = interquartile range, LPV/r = ritonavir-boosted lopinavir, n = number, TDF = tenofovir, VL = viral load.
one participant did not have a clear treatment history regarding third-line prescription and was not included in this table. However, as previous treatment history as well as genotypic resistance results at 3L initiation were available we did not exclude him from the global analysis (therefore N = 37 here in this table but N = 38 in the study).
Regarding determinants of virological response, no association was found between gender distribution (<49 vs > 49 years) and virological response in our study population (P = .6). Furthermore, with respect to baseline viremia and CD4 count categorical variables (viral load < 1000 vs >1000 copies/mL; CD4 < 200 vs >200 cells/μL) at the moment of failure to second-line ART, no association was found with virological response (P = .6 and P = .3 respectively). As for 3L regimens received, no association was found with virological response (P = .9 respectively). With respect to HIV-1 genetic diversity, no association was found between the virological response and the highly prevalent CRF02_AG vs non-AG clades (P = .9). Finally, no association was found between patients genotypic resistance profiles at second-line failure and virological response on 3L. Thus, among these adherent patients, 3L regimens alone represented the driven factor for the viral suppression (89.5%).
4. Discussion and Conclusion
Management of treatment-experienced HIV-infected patients remains very challenging in RLS where drug options and GRT are limited, poor adherence and lost-to-follow-up are frequent, and programmatic monitoring remains suboptimal.[9,24–26] Our findings reveal that the majority (⁓90%) of participants were experiencing viral suppression (<1.000 copies/mL) under 3L regimens, supporting that a third-line ART guided by genotypic resistance profiling would ensure a good treatment response despite the multi-drug resistance patterns. This would indeed contribute in achieving the elimination of AIDS as an epidemic by 2030 in this difficult-to-treat population living in RLS like Cameroon.[5] Furthermore, the encouraging rate of viral suppression suggests that virological success is mainly related to the use of very potent antiretrovirals with high genetic barrier to resistance, acting on a susceptible viral strain as informed by genotyping.[9,13,27–30] Thus, in RLS like Cameroon with growing number of patients requiring third-line ART, scaling-up access to drug resistance testing, be it in terms of geographical distribution, affordability and staff training, will foster a long-term successful performance of ART programs at global level. This evidence, generated from the Cameroonian context, supports the need for enlarged analyses including other SSA countries, to ensure a wider representativeness of the observed outcomes. Of note, the relatively low sample size, obtained from an exhaustive sampling strategy, reflects the actual number of patients receiving third-line ART locally[12,13]; a number expected to increase overtime with growing access to VL coverage/monitoring. Beside the critical role of genotypic resistance testing, ensuring a regular implementation of enhanced adherence counseling, psychosocial support centered around the patient’s experience and daily challenges with ART intake, limiting drug stock outs and patient lost-to follow-up or defaulters, while promoting good dispensing practices and community engagement in ART response, will trigger a timely achievement of the Joint United Nations Programme on HIV/AIDS goals, following this holistic model.[5,31] In this perspective of an integrated and holistic management of third-line ART patients, RLS may achieve similar ART performance as standard of care currently reported in high-income settings.
Acknowledgements
We thank all the participants to this study and especially psychosocial agents and monitors from study site (the Yaoundé Central hospital) for their dynamisms and assistances which has smoothed the work on site. We are also appreciative of the “Chantal BIYA International Reference Centre (CIRCB)” for all the facilitations during data analysis and interpretation.
Author contributions
Conceptualization: Pretty Rosereine Mbouyap, Joseph Fokam, Ezechiel Ngoufack Jagni Semengue, François–Xavier Mbopi Keou.
Data curation: Pretty Rosereine Mbouyap, Joseph Fokam, Ezechiel Ngoufack Jagni Semengue.
Formal analysis: Pretty Rosereine Mbouyap, Désiré Takou, Collins Ambe Chenwi, Alex Durand Nka, Beatrice Dambaya, Georges Teto, Grâce Angong Beloumou, Sandrine Claire Djupsa Ndjeyep, Aude Christelle Ka’e.
Investigation: Pretty Rosereine Mbouyap, Leonella Mossiang, Charles Kouanfack.
Methodology: Pretty Rosereine Mbouyap, Joseph Fokam, Ezechiel Ngoufack Jagni Semengue, Leonella Mossiang.
Project administration: Joseph Fokam, Charles Kouanfack, Alexis Ndjolo, François–Xavier Mbopi Keou.
Resources: Joseph Fokam, Charles Kouanfack, Alexis Ndjolo, François–Xavier Mbopi Keou.
Software: Ezechiel Ngoufack Jagni Semengue.
Supervision: Joseph Fokam, Leonella Mossiang, Charles Kouanfack, Alexis Ndjolo, François–Xavier Mbopi Keou.
Validation: Joseph Fokam, Ezechiel Ngoufack Jagni Semengue, Charles Kouanfack, Alexis Ndjolo, François–Xavier Mbopi Keou.
Visualization: Pretty Rosereine Mbouyap, Joseph Fokam, Ezechiel Ngoufack Jagni Semengue, Charles Kouanfack, Alexis Ndjolo, François–Xavier Mbopi Keou.
Writing – original draft: Pretty Rosereine Mbouyap, Joseph Fokam, Ezechiel Ngoufack Jagni Semengue, Leonella Mossiang, Désiré Takou, Collins Ambe Chenwi, Alex Durand Nka, Beatrice Dambaya, Georges Teto, Grâce Angong Beloumou, Sandrine Claire Djupsa Ndjeyep, Aude Christelle Ka’e.
Writing – review & editing: Pretty Rosereine Mbouyap, Joseph Fokam, Ezechiel Ngoufack Jagni Semengue, Leonella Mossiang, Désiré Takou, Collins Ambe Chenwi, Alex Durand Nka, Beatrice Dambaya, Georges Teto, Grâce Angong Beloumou, Sandrine Claire Djupsa Ndjeyep, Aude Christelle Ka’e, Charles Kouanfack, Alexis Ndjolo, François–Xavier Mbopi Keou.
Abbreviations:
- 3L
- third-line
- ART
- antiretroviral therapy
- CD4
- cluster of differenciation type 4
- CIRCB
- Centre international de référence Chantal Biya pour la recherche sur la prise en charge et la prevention du VIH/SIDA (Chantal Biya international reference center for research on HIV/AIDS prevention and management)
- DRMs
- drug resistance mutations
- DRV/r
- darunavir/ritonavir
- GRT
- genotypic resistance testing
- HIV
- human immunodeficiency virus
- HIVDR
- HIV drug resistance
- NNRTI
- non-nucleoside reverse transcriptase inhibitor
- NRTI
- nucleoside reverse transcriptase inhibitor
- PI/r
- protease inhibitor boosted by ritonavir
- PLHIV
- people living with HIV
- RLS
- resource-limited settings
- SSA
- sub-Saharan Africa
- TDF
- tenofovir
- VL
- viral load
- WHO
- world health organization,
- YCH
- Yaoundé Central Hospital
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
PRM, JF, and ENJS contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
How to cite this article: Mbouyap PR, Fokam J, Ngoufack Jagni Semengue E, Mossiang L, Takou D, Ambe Chenwi C, Nka AD, Dambaya B, Teto G, Angong Beloumou G, Djupsa Ndjeyep SC, Ka’e AC, Kouanfack C, Ndjolo A, Mbopi Keou F–X. HIV-1 genotypic profiling ensures effective response to third-line antiretroviral therapy in Cameroon. Medicine 2023;102:22(e33897).
Contributor Information
Pretty Rosereine Mbouyap, Email: prmbouyap@yahoo.fr.
Joseph Fokam, Email: josephfokam@gmail.com.
Leonella Mossiang, Email: nellka82@yahoo.fr.
Désiré Takou, Email: dtakou@yahoo.com.
Collins Ambe Chenwi, Email: collinschen@yahoo.co.uk.
Alex Durand Nka, Email: nkaalexdurand@yahoo.com.
Beatrice Dambaya, Email: bdambay@yahoo.fr.
Georges Teto, Email: ggteto@yahoo.fr.
Grâce Angong Beloumou, Email: graceangong12@yahoo.fr.
Sandrine Claire Djupsa Ndjeyep, Email: djupsans@yahoo.fr.
Aude Christelle Ka’e, Email: kae.audechristelle@gmail.com.
Charles Kouanfack, Email: charleskouanfack@yahoo.fr.
Alexis Ndjolo, Email: andjolo@yahoo.com.
References
- [1].ONUSIDA. Fiche d’information – Dernières statistiques sur l’état de l’épidémie de sida | ONUSIDA. 2022. Available at: https://www.unaids.org/fr/resources/fact-sheet. [Access date April 13, 2022].
- [2].Fokam J, Nangmo A, Wandum C, et al. Programme quality indicators of HIV drug resistance among adolescents in urban versus rural settings of the centre region of Cameroon. AIDS Res Ther. 2020;17:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ekollo Mbange A, Malick Diouara AA, Diop-Ndiaye H, et al. High HIV-1 virological failure and drug resistance among adult patients receiving first-line ART for at least 12 months at a decentralized urban HIV clinic setting in Senegal before the test-and-treat. Infect Dis (Auckl). 2021;14:11786337211014504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Billong SC, Fokam J, Aghokeng AF, et al. Population- based monitoring of emerging HIV-1 drug b resistance on antiretroviral therapy and associated factors in a sentinel site in Cameroon: low levels of resistance but poor programmatic performance serge. PLoS One. 2013;8:e72680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. 2016. Available at: https://www.who.int/publications/i/item/9789241549684. [PubMed]
- [6].Bland RM. Management of HIV-infected children in Africa: progress and challenges. Arch Dis Child. 2011;96:911–5. [DOI] [PubMed] [Google Scholar]
- [7].Rusine J, Asiimwe-Kateera B, van de Wijgert J, et al. Low primary and secondary HIV drug-resistance after 12 months of antiretroviral therapy in Human Immune-Deficiency Virus Type 1 (HIV-1)-infected individuals from Kigali, Rwanda. Fox MP, ed. PLoS One. 2013;8:e64345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].WHO. Combating HIV drug resistance, a little known but growing threat. 2017. Available at: https://apps.who.int/iris/bitstream/handle/10665/255896/9789241512831-eng.pdf.
- [9].Takou D, Fokam J, Teto G., et al. HIV-1 drug resistance testing is essential for heavily-treated patients switching from first- to second-line regimens in resource-limited settings: evidence from routine clinical practice in Cameroon. BMC Infect Dis. 2019:19;246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Koay WLA, Kose-Otieno J, Rakhmanina N. HIV drug resistance in children and adolescents: always a challenge? Curr Epidemiol Rep 2021;8:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Moorhouse M, Maartens G, Venter WDF, et al. Third-line antiretroviral therapy program in the South African public sector. JAIDS J Acquir Immune Defic Syndr. 2018;80:73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Edessa D, Sisay M, Asefa F. Second-line HIV treatment failure in sub-Saharan Africa: a systematic review and meta-analysis. Blackard J, ed. PLoS One. 2019;14:e0220159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ndahimana JDA, Riedel DJ, Muhayimpundu R, et al. HIV drug resistance mutations among patients failing second-line antiretroviral therapy in Rwanda. Antivir Ther. 2016;21:253–9. [DOI] [PubMed] [Google Scholar]
- [14].MINSANTE. Guide national de prise en charge des personnes vivant avec le VIH/SIDA − Cameroun. 2012.
- [15].MINSANTE. Plan national multisectoriel De Lutte Contre Le Vih, Le Sida Et Les Ist (Pnm) Annee 2014-2017. 2013:1–163.
- [16].Dinesha TR, Gomathi S, Boobalan J, et al. Genotypic HIV-1 drug resistance among patients failing tenofovir-based first-line HAART in South India. AIDS Res Hum Retroviruses. 2016;32:1234–6. [DOI] [PubMed] [Google Scholar]
- [17].MINSANTE. Directives nationales de Prevention et de prise en charge du VIH au Cameroun. 2014:193. Available at: https://www.childrenandaids.org/sites/default/files/2017-05/Cameroon_National-Integrated-HIV-Guidelines2014.pdf [access date February 13, 2021]. [Google Scholar]
- [18].Jordan MR, Parkin N, Yang C, et al. HIV-1 drug resistance mutations: potential applications for point-of-care genotypic resistance testing. PLoS One. 2015;10:e0145772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Evans D, Hirasen K, Berhanu R, et al. Predictors of switch to and early outcomes on third-line antiretroviral therapy at a large public-sector clinic in Johannesburg, South Africa. AIDS Res Ther. 2018;15:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Inzaule SC, Hamers RL, Doherty M, et al. Personal view curbing the rise of HIV drug resistance in low-income and middle-income countries: the role of dolutegravir-containing regimens. Lancet Infect Dis. 2019;3099:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fokam J, Salpini R, Santoro MM, et al. Performance evaluation of an in-house human immunodeficiency virus type-1 protease-reverse transcriptase genotyping assay in Cameroon. Arch Virol. 2011;156:1235–43. [DOI] [PubMed] [Google Scholar]
- [22].HIVdb Program: Mutations analysis - Stanford coronavirus antiviral & resistance database (CoVDB). Available at: https://hivdb.stanford.edu/hivdb/by-patterns/. [Access date February 2, 2023].
- [23].Laboratoire • OPP-ERA. Available at: https://toolkit-chargevirale-oppera.solthis.org/laboratoire/. [Access date February 2, 2023].
- [24].Grinsztejn B, Hughes MD, Ritz J, et al. Third-line antiretroviral therapy in low-income and middle-income countries (ACTG A5288): a prospective strategy study. Lancet HIV. 2019;6:e588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Phillips AN, Stover J, Cambiano V, et al. Impact of HIV drug resistance on HIV/AIDS-associated mortality, new infections, and antiretroviral therapy program costs in Sub-Saharan Africa. J Infect Dis. 2017;215:1362–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Fokam J, Takou D, Semengue ENJ, et al. First case of Dolutegravir and Darunavir/r multi drug-resistant HIV-1 in Cameroon following exposure to Raltegravir: lessons and implications in the era of transition to dolutegravir-based regimens. Antimicrob Resist Infect Control. 2020;9:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Siedner MJ, Moorhouse MA, Simmons B, et al. Reduced efficacy of HIV-1 integrase inhibitors in patients with drug resistance mutations in reverse transcriptase. Nat Commun. 2020;11:5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Papot E, Kaplan R, Vitoria M, et al. Optimizing switching strategies to simplify antiretroviral therapy: the future of second-line from a public health perspective. AIDS. 2021;35(Supplement 2):S153–63. [DOI] [PubMed] [Google Scholar]
- [29].Grinsztejn B, Hughes MD, Ritz J, et al. A5288 Team. Third-line antiretroviral therapy in low-income and middle-income countries (ACTG A5288): a prospective strategy study. Lancet HIV. 2019;6:e588–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gill N, Van den Bergh R, Wut Yee Kyaw K, et al. Genotyping and outcomes of presumptive second line ART failure cases switched to third line or maintained on second line ART in Mumbai, India. Yotebieng M, ed. PLoS One. 2019;14:e0225631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Eholie SP, Moh R, Benalycherif A, et al. Implementation of an intensive adherence intervention in patients with second-line antiretroviral therapy failure in four west African countries with little access to genotypic resistance testing: a prospective cohort study. Lancet HIV. 2019;6:e750–9. [DOI] [PubMed] [Google Scholar]