Abstract
COVID-19 primarily affects the respiratory system and can cause changes in other systems. Early identification of patients with a higher potential for complications is critical to provide the best possible treatment to reduce the disease's lethality. This study aimed to analyze the behavior of hematologic biomarkers in predicting mortality in patients hospitalized with COVID-19. This retrospective cohort study used data from the medical records of patients hospitalized with COVID-19 between March and August 2020 in two referral hospitals for treatment of the disease in the city of Cuiabá (in the state of Mato Grosso, Brazil). Clinical and laboratory characteristics related to cardiovascular involvement and death during hospitalization were evaluated. Neutrophils, lymphocytes, and monocytes, as well as the neutrophil-to-lymphocyte ratio (NLR) and the monocyte-to-lymphocyte ratio (MRL), were used as potential biomarkers of death. A total of 199 patients were included (male: 113; mean age: 51.4 years). Leukocyte, neutrophil, and lymphocyte counts showed a statistically significant association with death, as did NLR and MRL. Satisfactory accuracy in predicting death was observed for leukocyte, neutrophil, lymphocyte, NLR, and MLR counts. The hematologic biomarkers studied may be useful for prognosticating hospitalized patients for the possibility of death from COVID-19.
Keywords: COVID-19, Biomarkers, Neutrophil, Monocyte, Lymphocyte, Death
1. Introduction
COVID-19 is a viral disease caused by SARS-CoV-2, a coronavirus that primarily affects the respiratory system and can lead to changes in other systems. Since the first cases in the city of Wuhan, China, in December 2019 through July 2022, more than six million deaths have been reported worldwide, according to the World Health Organization [1]. Most patients (81%) with COVID-19 develop mild disease that does not lead to pneumonia. However, critical cases with respiratory failure, septic shock, or multiple organ failure occur in 5% of infected patients. Male patients and patients older than 65 years have a poorer prognosis [2]. Similarly, lethality is twice as high in individuals with comorbidities such as diabetes mellitus, hypertension, and cardiovascular disease compared with individuals without these conditions [3].
The basis of pathophysiological changes in COVID-19 are infiltration of infected tissue by macrophages and the presence of lymphopenia and neutrophilia in hematological examinations. Innate immunity recognizes viral markers, leading to the production of interferon with the intention of reducing viral replication and stimulating the action of lymphocytes. At the same time, endothelial cytokines are released, which increase capillary permeability, resulting in platelet activation, increased coagulation, decreased fibrinolysis, and increased activation of the complement system [4]. All these processes contribute to the emergence of an immune thrombotic phase in the development of the disease. Another important aspect is that patients who are exposed to a higher viral load, elderly, or have comorbidities have fewer T lymphocytes, which serve as biomarkers of infection severity [5].
Changes in the number of one or more blood cells have frequently been reported in patients infected with SARS-CoV-2 [6]. Lymphopenia, which can occur in more than 80% of patients on hospital admission, and an increased neutrophil-to-lymphocyte ratio (NLR) are important complete blood count findings, especially in severe cases of infection [7,8]. Neutrophils, in turn, are the most abundant cells of the immune system and account for approximately 50–70% of leukocytes. Neutrophils are involved in the response to various infections, have homeostatic functions, and play a role in some chronic inflammatory diseases [9]. In COVID-19, the presence of neutrophils is associated with more severe cases and death [10].
Among the various parameters related to leukocytes in the blood, the NLR and monocyte-to-lymphocyte ratio (MLR) stand out as being associated with systemic inflammation [11,12]. This relationship was first studied in 1995 in patients with acute appendicitis [13], and its diagnostic and prognostic significance has since been established [[14], [15], [16]]. Indeed, more than 8000 studies on NLR have been published in the last five years, highlighting the importance of this biomarker in various inflammatory processes. A change in NLR can be used to distinguish viral from bacterial infections or to predict the severe development of neoplasms or infections [17,18]. Yan et al. (2020) conducted a retrospective study in patients with COVID-19 and showed that an elevated NLR on admission can be used as a prognostic marker. An NLR value above 11.75 had a significant association with mortality [19]. It is noteworthy that hematologic biomarkers are easy to obtain and inexpensive, and have satisfactory prognostic accuracy in various pathologies [20], including severe development of COVID-19 [21].
Because of the high lethality of COVID-19, identification of clinical and laboratory markers that have the potential to predict unfavorable outcome of the disease, especially in vulnerable groups, is critical for healthcare professionals. This aspect is particularly important for patients who need to be hospitalized, as they are more likely to progress to a more severe case. Knowledge of this risk allows more efficient allocation of resources available for care, such as the allocation of intensive care beds for patients with a poorer prognosis. The aim of this study was to analyze the prognostic value of hematologic biomarkers determined at admission for predicting death in patients hospitalized for COVID-19 from March to August 2020.
2. Patients and methods
This is a retrospective cohort study of patients hospitalized with COVID-19 at two reference hospitals for the treatment of COVID-19. One is a public hospital (Júlio Müller University Hospital—HUJM) and the other is a private one (Santa Rosa Hospital—HSR) in the city of Cuiabá. The selection of patients was based on the records of patients hospitalized for COVID-19 provided by the Hospital Committee for Infection Control (CCIH) of these hospitals. Patients older than 18 years who were hospitalized from March to August 2020 were eligible for the study. The diagnosis COVID-19 was confirmed by several methods: molecular (RT-PCR), rapid SARS-CoV-2 antigen testing, IgM/IgG antibody testing against SARS-CoV-2, or by combining clinical data and chest CT changes consistent with respiratory changes caused by this viral infection.
Patient demographic, clinical, laboratory, and developmental data were obtained from medical records and recorded on a standard form prepared by the research team. Demographic data included age, sex, and skin color. Clinical data included descriptions of comorbidities in the medical history and the need for ICU transfer or ventilatory support during hospitalization. Medical history of hypertension, diabetes mellitus, cardiac disease, pulmonary disease, cancer, and obesity were considered as risk factors for severe COVID-19 [22] and were therefore described during patient characterization. Particular attention was paid to the evolving complications of each patient and the outcomes of the study, i.e., whether they were discharged from the hospital, or progressed to death.
Although multiple blood tests were performed in all patients during hospitalization, hematologic biomarkers such as total and differential leukocyte counts were collected only for the date of patient admission as baseline data used for analysis of their value in diagnosing severe disease progression. NLR and MLR were calculated by simply dividing the values in mm3 of blood. Hematological assays were performed using an automated blood cell counter (Mindray® BC6800, Shenzhen, China at HUJM; and Sysmex XE 2100, Syxmex® Corporation, Kobe, Japan at HSR).
Descriptive analysis was first performed for all variables of interest to the study. Continuous variables were reported as mean and standard deviation (SD) and categorical variables as proportions. The Shapiro-Wilk test was performed to test the normality of the distribution of hematologic parameters and their ratios. To test the hypothesis of equality of these parameters among patients who died or were discharged, we chose the nonparametric Mann-Whitney test, given the asymmetry of their distributions.
For the hematologic biomarkers that showed a significant association with death from COVID-19, a receiver operating curve (ROC) was constructed to evaluate the sensitivity, specificity, and accuracy of the parameter in predicting the unfavorable evolution of COVID-19 in the study patients. The area under the curve (AUC) was calculated to describe the accuracy of each hematologic biomarker. Higher AUC values indicate a better ability to discriminate between patients who progressed to death. The Youden J index was calculated to define empirical cutoff points corresponding to sensitivity and specificity values that were less likely to have arisen by chance [23]. For all analyses, a p-value <0.05 was considered a statistically significant difference. Statistical analyses were performed using Stata version 12.0 software (Stata Corporation, College Station, TX, USA).
The research project was approved by the Ethics and Research Committee of HUJM on September 1, 2020, under CAAE number 35134820.4.0000.5541 and opinion 4.252.218. The waiver of signing the informed consent form (ICF) was justified with the Ethics Committee because only secondary data from patients were used in a retrospective analysis of medical records.
3. Results
We included 199 patients who were hospitalized for COVID-19 during the study period and met the inclusion criteria. Of these, 133 (66.8%) were admitted to HSR and 66 (33.2%) to HUJM. Of the total number, 113 were male (56.8%) with a mean age (SD) of 51.4 (18.0) years. Elderly patients accounted for 27.7% of cases. COVID-19 was confirmed in 100 (50.3%) patients by RT-PCR, in two (1.0%) by rapid antigen testing, in 45 (22.6%) by rapid IgM/IgG antibody testing, and in 52 patients (26.1%) by combining compatible clinical and imaging data. The mean (SD) duration of hospitalization was 18.8 (15.8) days for patients who died and 20.0 (31.5) days for those who survived (p = 0.030). Likewise, symptom duration was similar between these two groups of patients (p = 0.481). One or more preexisting conditions were identified in 61.3% of the admitted patients. Arterial hypertension (40.2%), diabetes mellitus (22.6%), and obesity (l8.6%) were the most common comorbidities, but they were not associated with death in this cohort of patients. A total of 90.3% of patients required transfer to the intensive care unit and 72.4% to the respiratory care unit Analysis of the outcomes of the patients studied showed that 162 (81.4%) were discharged from the hospital, 34 (17.1%) died, and 3 (1.5%) remained without information due to transfer to another health facility (Table 1).
Table 1.
Demographic, clinical, and evolutionary characteristics of patients admitted for COVID-19 in hospitals in the central region of Brazil from March to August 2020.
| Feature | n | % | |
|---|---|---|---|
| Inpatient Hospital | HSR | 133 | 66.8 |
| HUJM | 66 | 33.2 | |
| Sex | Male | 113 | 56.8 |
| Female | 86 | 43.2 | |
| Skin color (n = 160) | Black | 5 | 3.1 |
| Yellow | 10 | 6.3 | |
| White | 61 | 38.1 | |
| Brown | 84 | 52.5 | |
| Age (years) (n = 195) | 18–49 | 97 | 49.7 |
| 50–64 | 44 | 22.6 | |
| 65–79 | 42 | 21.5 | |
| ≥ 80 | 12 | 6.2 | |
| Confirmation of COVID-19 | RT-PCR | 100 | 50.3 |
| Antigen test | 2 | 1.0 | |
| Rapid IgM/IgG test | 45 | 22.6 | |
| Compatible clinic/imaging | 52 | 26.1 | |
| Comorbidities | Hypertension | 80 | 40.2 |
| Diabetes mellitus | 45 | 22.6 | |
| Obesity | 37 | 18.6 | |
| Cardiopathy | 16 | 8.0 | |
| Pneumopathy | 13 | 6.5 | |
| Hepatopathy | 8 | 4.0 | |
| Chronic kidney disease | 7 | 3.5 | |
| Neoplasia | 5 | 2.5 | |
| HIV in treatment | 2 | 1.0 | |
| Need for intensive care (n = 186) | Yes | 168 | 90.3 |
| No | 18 | 9.7 | |
| Need for ventilatory support (n = 181) | Yes | 131 | 72.4 |
| No | 50 | 27.6 | |
| Outcome | Hospital Discharge | 162 | 81.4 |
| Death | 34 | 17.1 | |
| Transfer | 3 | 1.5 |
Variation in n refers to the lack of information of some patients for the respective variable.
On hospital admission, a high proportion (31.0%) of patients had leukocytosis, with total counts ranging from 1850/mm3 to 42,120/mm3 and a mean (SD) of 9193 (5783)/mm3. The mean (SD) neutrophil, monocyte, and lymphocyte counts were 7318 (5512)/mm3, 582 (432)/mm3, and 1261 (728)/mm3, respectively. Among patients who died, the mean (SD) total leukocyte count was 13,592 (8862)/mm3, significantly higher (p < 0.001) than in discharged patients. The mean (SD) neutrophil count was also higher in the patients who died (p < 0.001). On the other hand, the lymphocyte count (p = 0.013) was lower in patients who died than in those who survived. Although the monocyte count was also higher in the group of patients who died, the difference was not statistically significant compared to the survivor group (p = 0.066). In the group of patients who died during hospitalization, an increase in NLR and MRL was observed compared to those who survived to COVID-19. For NLR, the mean value of the group that died was significantly higher (15.0; SD = 11.9) than that observed in the survivor group (6.44; SD: 6.25; p < 0.001). For TLR, the means (SD) were 0.88 (0.68) and 0.50 (0.50) in patients who died and were discharged, respectively. This difference was also statistically significant (p < 0.001) (Table 2).
Table 2.
Association analysis between hematologic biomarkers and progress to death in patients admitted for COVID-19 in hospitals in the central region of Brazil from March to August 2020.
| Hematologic biomarker | Total of patients Mean (SD) | Final outcome |
p∗ | |
|---|---|---|---|---|
| Death Mean (SD) | High Mean (SD) | |||
| Total leukocytes (/mm3) | 9.193 (5.783) | 13.592 (8.862) | 8.372 (4.556) | <0.001 |
| Neutrophils (/mm3) | 7.318 (5.512) | 11.770 (8.277) | 6.476 (4.290) | <0.001 |
| Monocytes (/mm3) | 582 (432) | 744 (621) | 549 (383) | 0.066 |
| Lymphocytes (/mm3) | 1.261 (728) | 1.011 (614) | 1.306 (747) | 0.013 |
| Neutrophil/lymphocyte ratio | 7.81 (8.09) | 15.0 (11.9) | 6.44 (6.25) | <0.001 |
| Monocyte/lymphocyte ratio | 0.56 (0.55) | 0.88 (0.68) | 0.50 (0.50) | <0.001 |
SD: standard deviation.
∗Mann-Whitney non-parametric test.
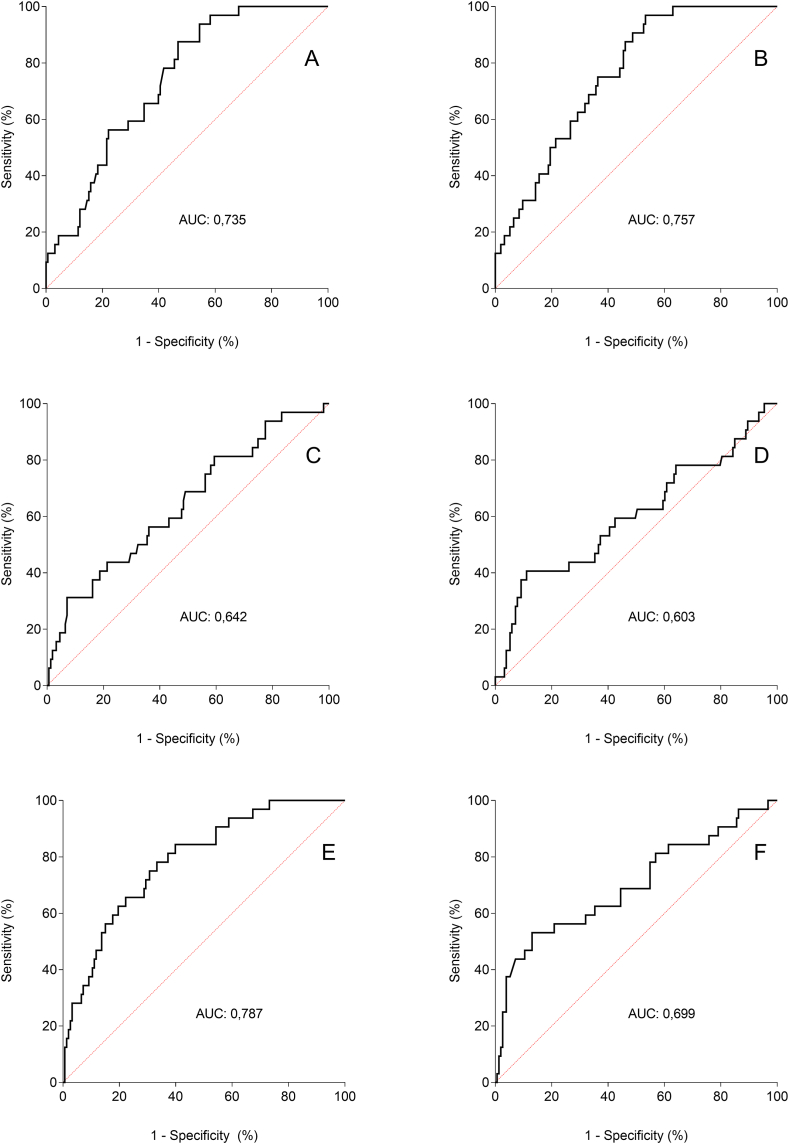
The ROC curve constructed from the hematologic parameters studied here showed a high AUC, i.e., more than 65%, to predict outcome death when the biomarkers were total leukocyte count (0.735), neutrophils (0.757), NLR (0.787), and MRL (0.699) (Fig. 1 - A to F). For lymphocyte and monocyte counts, the observed AUCs were less than 65%. Empirical cutoff points corresponding to the highest sensitivity and specificity values of the analyzed hematologic biomarkers are listed in Table 3. Leukocytosis higher than 7190/mm3, neutrophilia higher than 4833/mm3, and lymphopenia lower than 590/mm3 were the cutoff points that showed higher sensitivity for predicting death with satisfactory accuracy. NLR higher than 6.13 and MRL higher than 0.73 showed sensitivity and specificity of 78%/67% and 53%/87%, respectively.
Fig. 1.
ROC curves of the accuracy of different hematologic biomarkers with potential prognostic value of progress to death of patients admitted for COVID-19 in hospitals in the central region of Brazil from March to August 2020. [A] Leukocyte count; [B] Neutrophil count; [C] Lymphocyte count; [D] Monocyte count; [E] Neutrophil/lymphocyte ratio; [F] Monocyte/lymphocyte ratio.
Table 3.
Cutoff points of hematologic biomarkers with potential diagnostic value for the occurrence of death in patients admitted with COVID-19 in hospitals in the central region of Brazil from March to August 2020.
| Biomarker | Cutoff point |
J Index (Youden) |
Sensitivity % |
Specificity % |
Accuracy % |
|---|---|---|---|---|---|
| Progress to death | |||||
| Leukocytes/mm3 | 7.190 | 0.407 | 88.0 | 53.0 | 70.0 |
| Neutrophils/mm3 | 4.833 | 0.436 | 97.0 | 47.0 | 72.0 |
| Monocytes/mm3 | 841 | 0.295 | 41.0 | 89.0 | 65.0 |
| Lymphocytes/mm3 | 590 | 0.242 | 93.0 | 31.0 | 62.0 |
| Neutrophil/lymphocyte ratio | 6.13 | 0.448 | 78.0 | 67.0 | 72.0 |
| Monocyte/lymphocyte ratio | 0.73 | 0.401 | 53.0 | 87.0 | 70.0 |
4. Discussion
The results of this study show that several hematologic biomarkers assessed at hospital admission are associated with death in patients hospitalized for COVID-19 in hospitals in the central region of Brazil, with satisfactory accuracy for predicting death in these patients. The sociodemographic and clinical profile of the patients analyzed here did not differ from that observed in a large Brazilian study that examined the general characteristics of the first 250,000 hospitalizations for COVID-19 in Brazil between February and August 2020. Most patients were over 50 years of age, male, high frequency of co-morbidities, and strongly needed invasive and noninvasive ventilatory support. In the present study, the frequency of progression to death (17.1%) was lower than in this Brazilian review (35.0%), even considering only the central region of the country [24]. A likely explanation for this difference was the high frequency (17.0%) of invasive ventilatory support outside the ICU reported in this review for the central region of Brazil, in contrast to all patients in this study, whose invasive ventilatory support was performed in the ICU in all patients who required it. The overall mortality of patients hospitalized for COVID-19 is about 15%, and increases to 29% if the patient requires intensive care, with a higher risk if the patient is male and over 65 years of age [2]. Lethality rates are also twice as high in people with comorbidities such as diabetes mellitus, systemic arterial hypertension, and cardiovascular disease compared with people without these conditions [3].
Leukocytosis, neutrophilia, and lymphopenia were laboratory changes observed on admission in the patients analyzed here and showed an association with the occurrence of death in patients hospitalized for COVID-19. Changes in monocyte and platelet counts were not relevant in the patients studied. In fact, elevated white blood cell counts occur in patients with COVID-19 and appear to be related to secondary bacterial infection [25]. Zhou et al. (2020) compared laboratory markers from hospitalized patients who did or did not survive COVID-19 and concluded that lymphopenia and leukocytosis were factors associated with death. In individuals who died, laboratory markers such as procalcitonin, C-reactive protein, and interleukin-6 were also elevated, indicating concomitant bacterial infection [26].
Neutrophils play a central role in the innate immune response against bacteria and fungi. However, an inappropriately enhanced neutrophil response can lead to multiple organ failure and death in patients with critical illness, including COVID-19 [27,28]. Urra et al. (2020), in a study of 172 patients, compared neutrophil counts in patients hospitalized for COVID-19 and observed a predominance of neutrophilia in those requiring intensive care, the progress to death of which is generally more common [29].
Lymphopenia during COVID-19 is present in more than 80% of patients [8]. It occurs between seven and 14 days after the onset of symptoms and is associated with a worsening of the patient's clinical condition and an increase in inflammatory mediators. The decrease in lymphocytes is due to several mechanisms, such as direct viral invasion of lymphocytes via the angiotensin-converting enzyme 2 receptor (ACE2) located on their surface, interleukin-induced apoptosis of lymphocytes, atrophy of lymphoid organs due to cytokine storm causing decreased lymphocyte turnover and decreased lymphocyte proliferation due to lactic acidosis. An association with death has been described in SARS patients who required treatment in the intensive care unit and in those who died [30]. Lymphocyte levels below 500 cells/mm3 are associated with a 12-fold increase in the risk of progression to death [31,32]. Both B and T lymphocytes are involved in the antiviral adaptive response. Activated B cells produce neutralizing antibodies that can bind to extracellular viral particles to prevent infection of host cells [33]. Virus-infected cells can be recognized and eliminated by CD8+ T lymphocytes. Therefore, lymphopenia not only impairs the antiviral response but may also make the host more susceptible to an enhanced inflammatory response [34,35].
In the present study, the NLR was found to be high in patients hospitalized for COVID-19 and statistically higher in those resulting in death. With a cutoff point of 6.13 at hospital admission, the sensitivity and accuracy of NLR for diagnosing patients at higher risk of death were satisfactory, i.e., 78.0% and 78.7%, respectively. A similar observation was previously made by Erdogan et al. (2021), who associated the increase in NLR with more severe and complicated disease patterns [36]. In a study conducted to evaluate the usefulness of NLR as a prognostic marker at COVID-19, Kulkarni et al. (2021) showed that patients who progressed to death had higher NLR than patients who survived. Using a cutoff point of 6.6 at admission, they confirmed the diagnostic value of NLR in predicting mortality in patients with COVID-19, with a sensitivity of 100% and an accuracy of 79.2% [37]. Similar results consistent with these studies were also reported in 2020 and 2021 b y different authors [[38], [39], [40]]. Considering the studies by Holub et al. (2012) and Naess et al. (2017), who used NLR to distinguish between viral and bacterial infection, it is plausible that the patients who died in the present study must have had an accompanying bacterial infection that exacerbated their clinical evolution [17,18].
Although MLR was associated with death in this study, it was not a measure with satisfactory sensitivity and accuracy to predict patients at higher risk of death. A similar result was reported by Yang et al. (2020), who failed to demonstrate the usefulness of the lymphocyte/monocyte ratio to predict death in patients with COVID-19 [41].
The present study has some limitations. The selection of patients from only two hospital centers and over a short period, compared with the long period of the pandemic, may have introduced selection bias in the study. Because this is an analysis of medical records, the quality of the information collected is always questionable, especially because of the different numbers of patients for the different parameters evaluated. Therefore, analyses with a larger number of patients, in a longitudinal approach, and with subgroup analyses should be performed. Despite the lack of novelty, our results confirm similar observations published by different authors [6,19,21,31,[36], [37], [38], [39], [40], [41]] and suggest that the assessment of these biomarkers at the baseline of hospitalization can predict the severe outcome of patients with COVID-19.
In conclusion, leukocyte, neutrophil, and lymphocyte counts and NLR at hospital admission have shown satisfactory accuracy and sensitivity in predicting patients at higher risk of death by COVID-19. These inexpensive and easily obtainable hematologic biomarkers may be useful in clinical practice for prognostic evaluation of patients hospitalized for COVID-19 in relation to their risk of death.
Author contribution statement
Marcia Duarte Sejópoles: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
João Paulo Souza-Silva; Cristiane Silva-Santos; Matheus Moreira Paula-Duarte: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Cor Jesus Fernandes Fontes; Luciano Teixeira Gomes, PhD: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Additional information
Supplementary content related to this article has been published online at [URL].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors wish to thank the teams responsible for the medical files at the Hospital Universitário Júlio Müller and Hospital Santa Rosa, for their valuable help in obtaining the study data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e16964.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.World Health Organization . 2020. WHO Coronavirus (COVID-19)https://covid19.who.int/ Dashboard [Internet] [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Abate S.M., Checkol Y.A., Mantefardo B. Global prevalence and determinants of mortality among patients with COVID-19: a systematic review and meta-analysis. Ann Med Surg. 2021;64 doi: 10.1016/j.amsu.2021.102204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kadkhoda K. COVID-19: an Immunopathological view. mSphere. 2020;29(2) doi: 10.1128/mSphere.00344-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yuki K., Fujiogi M., Koutsogiannaki S. COVID-19 pathophysiology: a review. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gajendra S. Spectrum of hematological changes in COVID-19. Am J Blood Res. 2022;12(1):43–53. [PMC free article] [PubMed] [Google Scholar]
- 7.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020;1(15):762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X., et al. China medical treatment expert group for covid-19. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 Apr 30;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soehnlein O., Steffens S., Hidalgo A., Weber C. Neutrophils as protagonists and targets in chronic inflammation. Nat. Rev. Immunol. 2017;17(4):248–261. doi: 10.1038/nri.2017.10. [DOI] [PubMed] [Google Scholar]
- 10.Wang J., Li Q., Yin Y., Zhang Y., Cao Y., Lin X., et al. Excessive neutrophils and neutrophil extracellular traps in COVID-19. Front. Immunol. 2020;11:1–13. doi: 10.3389/fimmu.2020.02063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papa A., Emdin M., Passino C., Michelassi C., Battaglia D., Cocci F. Predictive value of elevated neutrophil-lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin. Chim. Acta. 2008;395(1–2):27–31. doi: 10.1016/j.cca.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Benites-Zapata V.A., Hernandez A.V., Nagarajan V., Cauthen C.A., Starling R.C., Wilson Tang W.H. Usefulness of neutrophil-to-lymphocyte ratio in risk stratification of patients with advanced heart failure. Am. J. Cardiol. 2015;115(1):57–61. doi: 10.1016/j.amjcard.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodman D., Goodman C., Monk J. Use of the neutrophil:lymphocyte ratio in the diagnosis of appendicitis. Am. Surg. 1995;61(3):257–259. [PubMed] [Google Scholar]
- 14.de Jager C.P.C., Wever P.C., Gemen E.F.A., Kusters R., van Gageldonk-Lafeber A.B., van der Poll T., et al. The Neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia. PLoS One. 2012;7(10) doi: 10.1371/journal.pone.0046561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gomes L.T., Morato-Conceição Y.T., Gambati A.V.M., Maciel-Pereira C.M., Fontes C.J.F. Diagnostic value of neutrophil-to-lymphocyte ratio in patients with leprosy reactions. Heliyon. 2020;6(2) doi: 10.1016/j.heliyon.2020.e03369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klemt C., Tirumala V., Smith E.J., Xiong L., Kwon Y.M. Complete blood platelet and lymphocyte ratios increase diagnostic accuracy of periprosthetic joint infection following total hip arthroplasty. Arch. Orthop. Trauma. Surg. 2022 doi: 10.1007/s00402-021-04309-w. [DOI] [PubMed] [Google Scholar]
- 17.Holub M., Beran O., Kaspříková N., Chalupa P. Neutrophil to lymphocyte count ratio as a biomarker of bacterial infections. Cent. Eur. J. Med. 2012;7(2):258–261. [Google Scholar]
- 18.Naess A., Nilssen S.S., Mo R., Eide G.E., Sjursen H. Role of neutrophil to lymphocyte and monocyte to lymphocyte ratios in the diagnosis of bacterial infection in patients with fever. Infection. 2017;45(3):299–307. doi: 10.1007/s15010-016-0972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yan X., Li F., Wang X., Yan J., Zhu F., Tang S., et al. Neutrophil to lymphocyte ratio as prognostic and predictive factor in patients with coronavirus disease 2019: a retrospective cross-sectional study. J. Med. Virol. 2020;92(11):2573–2581. doi: 10.1002/jmv.26061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haybar H., Pezeshki S.M.S., Saki N. Evaluation of complete blood count parameters in cardiovascular diseases: an early indicator of prognosis? Exp Mol Pathol [Internet] 2019;110 doi: 10.1016/j.yexmp.2019.104267. [DOI] [PubMed] [Google Scholar]
- 21.Taj S., Kashif A., Arzinda-Fatima S., Imran S., Lone A., Ahmed Q. Role of hematological parameters in the stratification of COVID-19 disease severity. Ann Med Surg. 2021;62:68–72. doi: 10.1016/j.amsu.2020.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallo-Marin B., Aghagoli G., Lavine K., Yang L., Siff E.J., Chiang S.S., et al. Predictors of COVID-19 severity: a literature review. Rev. Med. Virol. 2021;31(1):1–10. doi: 10.1002/rmv.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bantis L.E., Nakas C.T., Reiser B. Construction of confidence regions in the ROC space after the estimation of the optimal Youden index-based cut-off point. Biometrics. 2014;70(1):212–223. doi: 10.1111/biom.12107. [DOI] [PubMed] [Google Scholar]
- 24.Ranzani O.T., Bastos L.S.L., Gelli J.G.M., Marchesi J.F., Baião F., Hamacher S., et al. Characterisation of the first 250 000 hospital admissions for COVID-19 in Brazil: a retrospective analysis of nationwide data. Lancet Respir. Med. 2021;9(4):407–418. doi: 10.1016/S2213-2600(20)30560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lippi G., Plebani M. The critical role of laboratory medicine during coronavirus disease 2019 (COVID-19) and other viral outbreaks. Clin. Chem. Lab. Med. 2020;58(7):1063–1069. doi: 10.1515/cclm-2020-0240. [DOI] [PubMed] [Google Scholar]
- 26.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., et al. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe. 2016;19(2):181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lefrançais E., Mallavia B., Zhuo H., Calfee C.S., Looney M.R. Maladaptive role of neutrophil extracellular traps in pathogen-induced lung injury. JCI insight. 2018;3(3):1–15. doi: 10.1172/jci.insight.98178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urra J.M., Cabrera C.M., Porras L., Ródenas I. Selective CD8 cell reduction by SARS-CoV-2 is associated with a worse prognosis and systemic inflammation in COVID-19 patients. Clin. Immunol. 2020;217 doi: 10.1016/j.clim.2020.108486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henry B.M., Cheruiyot I., Vikse J., Mutua V., Kipkorir V., Benoit J., et al. Lymphopenia and neutrophilia at admission predicts severity and mortality in patients with COVID-19: a meta-analysis. Acta Biomed. 2020;91(3) doi: 10.23750/abm.v91i3.10217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang I., Pranata R. Lymphopenia in severe coronavirus disease-2019 (COVID-19): systematic review and meta-analysis. J Intensive Care. 2020;8:36. doi: 10.1186/s40560-020-00453-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang S., Hillyer C., Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41:355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J., Lau Y.F., Lamirande E.W., Paddock C.D., Bartlett J.H., Zaki S.R., et al. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4 + T cells are important in control of SARS-CoV infection. J. Virol. 2010;84(3):1289–1301. doi: 10.1128/JVI.01281-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Channappanavar R., Zhao J., Perlman S. T cell-mediated immune response to respiratory coronaviruses. Immunol. Res. 2014;59(1–3):118–128. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erdogan A., Can F.E., Gönüllü H. Evaluation of the prognostic role of NLR, LMR, PLR, and LCR ratio in COVID-19 patients. J. Med. Virol. 2021;93(9):5555–5559. doi: 10.1002/jmv.27097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kulkarni A., Prabhu D., Likitesh A.B., Anil-Kumar T., Rao Vvp P., Murthy S.P. Utility of Neutrophil-lymphocyte ratio (NLR) as an indicator of disease severity and prognostic marker among patients with Covid-19 infection in a tertiary care centre in Bangalore - a retrospective study. J Evid Based Med Healthc. 2021;8(16):2349–2370. [Google Scholar]
- 38.Del Carpio-Orantes L., García-Méndez S., Hernández-Hernández S.N. Neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and systemic immune-inflammation index in patients with COVID-19-associated pneumonia. Gac. Med. Mex. 2020;156(6):527–531. doi: 10.24875/GMM.M21000480. [DOI] [PubMed] [Google Scholar]
- 39.Alagbe A., Pedroso G., Oliveira B., Maia G., Costa E., Albuquerque D., et al. Association of lymphocyte counts, NLR and PLR with mortality in COVID-19 patients. Hematol Transfus Cell Ther. 2021;43:S508–S3509. [Google Scholar]
- 40.Yildiz H., Castanares-Zapatero D., Pierman G., Pothen L., De Greef J., Nana F.A., et al. Validation of neutrophil-to-lymphocyte ratio cut-off value associated with high in-hospital mortality in covid-19 patients. Int. J. Gen. Med. 2021;14:5111–5117. doi: 10.2147/IJGM.S326666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang A.P., Liu J.P., Tao W.Q., Li H.M. The diagnostic and predictive role of NLR, d-NLR and PLR in COVID-19 patients. Int. Immunopharm. 2020;84 doi: 10.1016/j.intimp.2020.106504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.