Abstract
Background:
Left atrial ablation to obtain pulmonary vein isolation (PVI) for the treatment of atrial fibrillation (AF) is a technologically intensive procedure utilizing innovative and continually improving technology. Changes in the technology utilized for PVI can in turn lead to changes in procedure costs. Because of the proximity of the esophagus to the posterior wall of the left atrium, various technologies have been utilized to protect against thermal injury during ablation. The impact on hospital costs during PVI ablation from utilization of different technologies for esophageal protection during ablation has not previously been evaluated.
Objective:
To compare the costs of active esophageal cooling to luminal esophageal temperature (LET) monitoring during left atrial ablation.
Methods:
We performed a time-driven activity-based costing (TDABC) analysis to determine costs for PVI procedures. Published data and literature review were utilized to determine differences in procedure time and same-day discharge rates using different esophageal protection technologies and to determine the cost impacts of same-day discharge versus overnight hospitalization after PVI procedures. The total costs were then compared between cases using active esophageal cooling to those using LET monitoring.
Results:
The effect of implementing active esophageal cooling was associated with up to a 24.7% reduction in mean total procedure time, and an 18% increase in same-day discharge rate. TDABC analysis identified a $681 reduction in procedure costs associated with the use of active esophageal cooling after including the cost of the esophageal cooling device. Factoring in the 18% increase in same-day discharge resulted in an increased cost savings of $2,135 per procedure.
Conclusions:
The use of active esophageal cooling is associated with significant cost-savings when compared to traditional LET monitoring, even after accounting for the additional cost of the cooling device. These savings originate from a per-patient procedural time savings and a per-population improvement in same-day discharge rate.
Keywords: Pulmonary vein isolation, atrial fibrillation, radiofrequency ablation, atrioesophageal fistula, esophageal cooling, luminal esophageal temperature monitoring, time-driven activity-based costing, I19, I1, I, M50, M5, M
Introduction
Left atrial ablation using radiofrequency (RF) energy to obtain pulmonary vein isolation (PVI) for the treatment of atrial fibrillation (AF) is a technologically intensive procedure utilizing innovative and continually improving technology1,2. Because AF is the most common type of heart arrhythmia, with an estimated 2.7 to 6.1 million people in the United States having been diagnosed with AF3, and a prevalence expected to increase to 5.6 million to 12.1 million by 20304, PVI is increasingly being utilized5. As expected, changes in the technology utilized for PVI can in turn lead to changes in procedure costs6–8.
One variable affecting the procedure is the type of esophageal protection strategy chosen to use during the procedure. Because of the proximity of the esophagus to the posterior wall of the left atrium, various technologies have been utilized to protect against thermal injury during ablation9. The traditional standard of care, luminal esophageal temperature (LET) monitoring, alerts electrophysiologists when the temperature in the esophagus has reached dangerous levels, but optimal temperature cutoffs are unknown, and randomized controlled trials have not demonstrated benefits with this approach10,11. Additionally, temperature elevations necessitate pauses in RF energy delivery, which can increase procedure duration and reduce long-term procedural efficacy12,13.
Alternatively, a newer device is available that provides active esophageal cooling to proactively dissipate heat in the esophagus (Figure 1)14. The device is a multi-lumen silicone tube allowing a closed-circuit flow of water as a coolant, which directly cools the esophagus once placed. The largest study of this device randomized 120 patients 1:1 with a control group of LET monitoring with a single-sensor temperature probe. Thermal injury to the mucosa was significantly more common in the control group than in those receiving esophageal cooling (12/60 vs. 2/60; p = .008), with a trend toward reduction in gastroparesis (6/60 vs. 2/60, p = .27)14. This use of this approach avoids the need for pauses during ablation, which in turn has been shown to reduce procedure duration15. For example, a formal study of procedure duration examined procedural data of almost 400 patients undergoing PVI in a healthcare system, comparing the data between patients treated with LET monitoring to those treated with active esophageal cooling. Mean procedure time was 146 ± 51 min in the LET-monitored patients, and 110 ± 39 min in the actively cooled patients (a reduction of 36 min, or 24.7% of total procedure time), while median procedure time was 141 (interquartile range 104–174) min in the LET-monitored patients and 100 (interquartile range 84–122) min in the actively cooled patients (a reduction of 41 min, or 29.1% of total procedure time)15. Additionally, active cooling has been reported to reduce postoperative symptoms such as chest pain, which may facilitate an increased rate of same-day discharge16. Finally, active esophageal cooling has been shown to reduce fluoroscopy requirements, reduce esophageal injury transmurality, and improve long-term freedom from arrhythmias while also reducing operator cognitive load17–20. The impact of choice of esophageal protection method on hospital costs however has not previously been evaluated, but the importance of cost containment continues to increase21. With the growing use of PVI, and the significant costs of these procedures, a valuable opportunity exists to reduce cost to the healthcare system. Therefore, we sought to quantify the costs of PVI procedures and compare any differences in costs between procedures utilizing LET monitoring and those using active esophageal cooling as esophageal protection strategies. Our specific aim is to identify opportunities for cost savings to the overall healthcare system.
Figure 1.
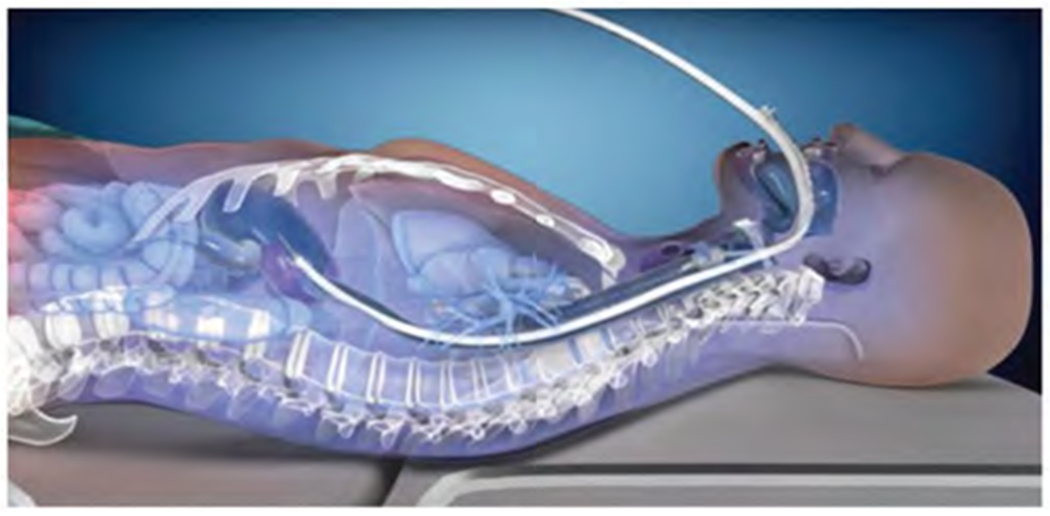
Active esophageal cooling device (with permission, Attune Medical, Chicago, IL).
Methods
For this analysis the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement and guidelines served as our framework to attempt to maximize interpretability and usefulness of the findings22. In particular, we utilized the expanded and updated CHEERS 2022 statement, intended to be used for any form of health economic evaluation22,23.
Health economic analysis plan
Although this analysis utilized a structured time-driven activity-based costing (TDABC) analysis utilizing literature review to determine costs for cardiac radiofrequency ablation procedures, a formal health economic analysis plan was not created.
Study population
The study population includes patients undergoing PVI RF ablation of the left atrium of the heart, using either traditional LET monitoring or active esophageal cooling as the method of esophageal protection. No restrictions on age, the severity of the disease, or the number of comorbidities were placed, and the population is representative of those undergoing PVI in general. For example, a mean age of 67 years, and gender 37% female, with comorbidities of hypertension (67%), diabetes (19%), coronary disease (19%), and congestive heart failure (7%) is reported15,24,25
Setting and location
Patients were treated in electrophysiology departments of either community-based or academic hospitals, all within the United States.
Comparators
We compared procedural data, outcomes, and costs between two primary strategies to esophageal protection during PVI RF ablation. One strategy is LET monitoring, which can utilize either single-sensor, or multi-sensor temperature probes. The other strategy is active esophageal cooling, which utilizes a single device (ensoETM, Attune Medical, Chicago, IL).
Perspective
We performed this analysis from the perspective of the hospital system, representing the institutional system incurring the costs of the procedures, and receiving the reimbursement for procedures from government or third-party payers.
Time horizon
A time horizon focusing on acute procedural costs, and the impacts of procedural outcomes on these costs, was chosen. Although growing data suggest that long-term procedural efficacy may be improved with the use of active esophageal cooling26–29, we did not factor this effect into our analysis.
Discount rate
Because our focus was on the acute procedural costs rather than on long-term economic impacts, we utilized a discount rate of 0%.
Selection, measurement, and valuation of outcomes, resources, and costs
To determine procedural costs and the differences between procedures using LET monitoring and active esophageal cooling, we focused on the outcomes of time to complete each procedure and the effect on same-day discharge rate. These outcomes were obtained from existing data published or presented comparing procedural differences between LET monitoring and active esophageal cooling15,30,31, and published data on cost savings associated with same-day discharge after PVI procedures32. Finally, costs used in the model, such as staff costs, lab space costs, consumables, and capital equipment were obtained from existing online sources and various publications from literature review33–42. Valuation of outcomes was strictly based on hospital costs.
Currency, price date, and conversion
Our analysis utilized U.S. dollar currency, using prices in 2022 U.S. dollars, but including some pricing data from the time frame of 2013–2022. Conversions to 2022 U.S. dollars were performed using the non-seasonally adjusted medical care consumer price indices. No conversions to other currencies were made.
Rationale and description of model
The costing model employed a time-driven activity-based costing (TDABC) analysis, which is a methodology that measures productivity and capacity utilization and identifies performance improvements such as streamlining staffing can be useful to identify costs as patients move through a continuum of care during any given procedure43. TDABC systems have been used across many industries as a management tool to measure costs but have not gained universal appeal in the healthcare industry due to technical and resource constraints involved for process mapping. TDABC is a cost accounting system that can be used to calculate actual healthcare services costs44. TDABC involves process-mapping the clinical tasks involved in left-atrial ablation, and then utilizing personnel costs to calculate cost-per-minute for each staff member45. Figure 2 shows the process map created for this analysis. Since the variation in competing approaches between LET monitoring and active esophageal cooling occurs during the procedure itself, we focused our detailed process map on the intra-procedure time. A key assumption we have made is that there is no difference in process maps between LET and active esophageal cooling in the pre-procedure and post-procedure steps (excepting decreased rate of admission), which we believe is a reasonable assumption. For this analysis, once staff per-minute costs were estimated, published data on ablation procedure duration and consumable costs were utilized to calculate time costs on a per-procedure and daily basis. The cost savings from same day discharge were derived from an economic analysis of the barriers and financial impact of this approach on length of stay management. Specifically, we estimated cost savings of operating an overnight hospital bed from hospital adjusted expenses per inpatient day32.
Figure 2.
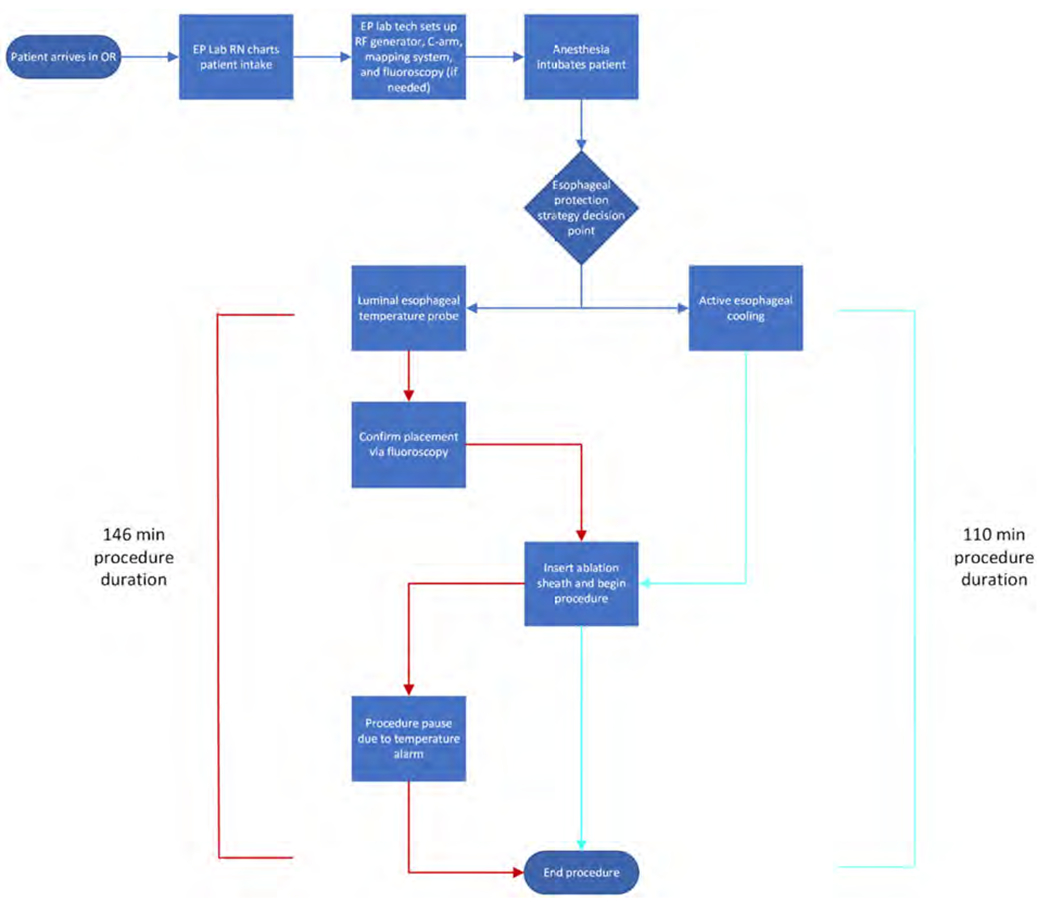
Process map of activities and patient path in the pulmonary vein isolation (PVI) procedure care cycle.
Analytics and assumptions
Our analysis assumes each procedure involves one electrophysiologist, two registered nurses, and two lab technicians per procedure. A total of 70% of procedure time is allocated to the electrophysiologist, 20% of the time to lab technicians, and 10% of the time to the registered nurses. A total of 200 lab days per year and a standard 8-h or 480-min workday were assumptions for this analysis. No statistical transformation or extrapolation methods were required.
Characterizing heterogeneity
We address heterogeneity by performing additional sensitivity analyses as discussed below. Specifically, we performed further analyses by considering significant heterogeneity (up to 50% variation) in the main drivers of outcome, procedure time and same-day discharge rate. We assume homogeneity across the population in the study, since published data incorporate the expected range of heterogeneity in patient baseline characteristics and risks.
Characterizing distributional effects
Distributional concerns were not evaluated in this analysis.
Characterizing uncertainty
We performed additional analyses assuming as much as a 50% worse time savings and same-day discharge improvement to allow for large margins of uncertainty in the outcome. While TDABC is designed for relatively accurate information to estimate the costs of services provided to patients, estimating the cost and time required for hospital activities is a subjective and uncertain task. Healthcare costs often vary according to physician and personnel performance and are often highly varied due to uncertainty involved in staff capacity and performance. This paper aims to present a hybrid model for estimating the costs of healthcare services using literature review and TDABC methodology to overcome the limitations and deficiencies associated with data estimation or uncertainty in TDABC input data such as cost, capacity, and time to determine the exact cost of a PVI ablation procedure.
Approach to engagement with patients and others affected by the study
At this point, engagement with patients and other stakeholders affected by this analysis has not occurred. The authors anticipate that the results will provide data of value to patients and hospitals.
Results
Study parameters
Input values are as shown in the tables below. Specifically, staff costs including fringe benefits, consumables costs, space costs, and capital equipment costs are shown in Tables 1–4.
Table 1.
Staff costs, Including fringe benefits, adjusted to 2022 dollars using the medical component of the consumer price Index (CPI).46
| Staff costs (including fringe benefits) | ||
|---|---|---|
|
| ||
| Daily Staff Cost | Source | |
| Electrophysiologist | $4,123 | 42 |
| EP Lab Staff (RN) | $838 | 37 |
| EP Lab Staff (Lab Tech) | $610 | 37 |
| Total | $5,105 | |
Abbreviations: RN, Registered Nurse; EP, electrophysiologist.
Table 4.
Capital equipment costs, adjusted to 2022 dollars using the medical component of the consumer price index (CPI)34,46.
| Capital equipment costs | |||||
|---|---|---|---|---|---|
|
| |||||
| Equipment cost | Equipment maintenance cost | Life of equipment (working days) | Total cost | Daily cap eq. cost | |
| RF generator | $44,515 | $63,592 | 1000 | $108,107 | $108 |
| CARTOi mapping system | $476,941 | $63,592 | 1000 | $540,533 | $541 |
| C-arm | $508,737 | $63,592 | 1000 | $572,330 | $572 |
| Fluoroscopy machine | $127,184 | $63,592 | 1000 | $190,777 | $191 |
Abbreviation: RF, radiofrequency.
Summary of main results
As described above, median procedural time for ablations using LET monitoring and cooling was reported to be 141 [IQR 104 to 174] min in LET-monitored patients and 100 [IQR 84–122] min in actively cooled patients (Figure 3)15.
Figure 3.
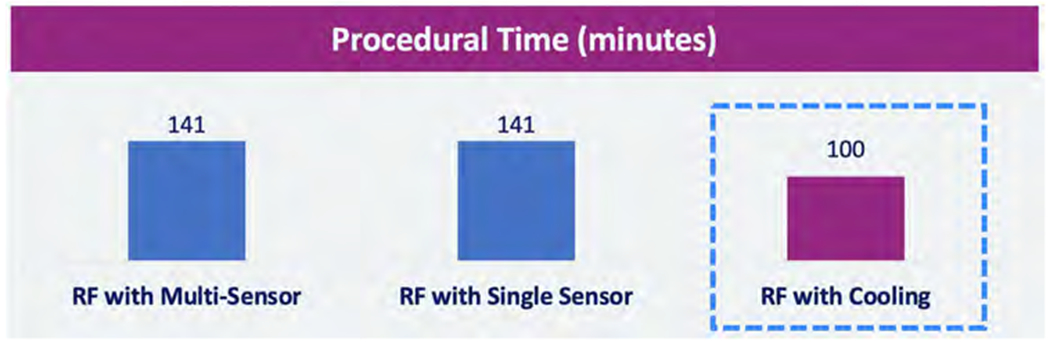
Procedural time differences reported for luminal esophageal temperature (LET) monitoring and active esophageal cooling15. Abbreviation: RF, radiofrequency.
These procedure times affect lab occupancy as shown in Figure 4. Occupancy is defined as the percentage of time in an 8-hour day (480 min) that a single case occupies.
Figure 4.
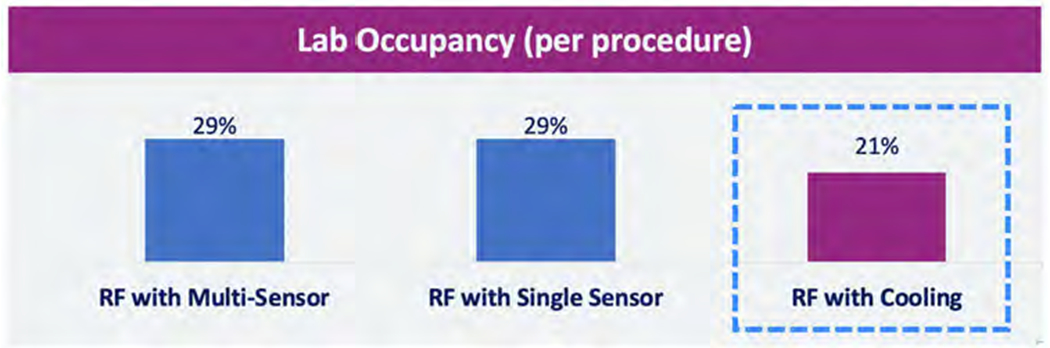
Lab occupancy for luminal esophageal temperature (LET) monitoring compared to active esophageal cooling. Abbreviation: RF, radiofrequency.
Costs for the staff involved in ablation procedures are shown in Figure 5.
Figure 5.
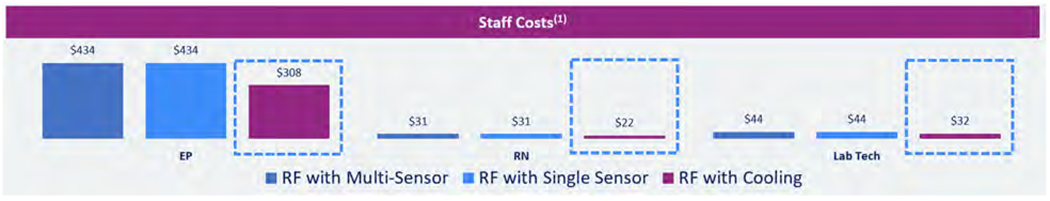
Costs for the staff involved in ablation procedures. Abbreviations: RF, radiofrequency; RN, Registered Nurse; EP, electrophysiologist.
Daily costs for staff, consumables, capital equipment, and laboratory space are as shown in Figure 6.
Figure 6.
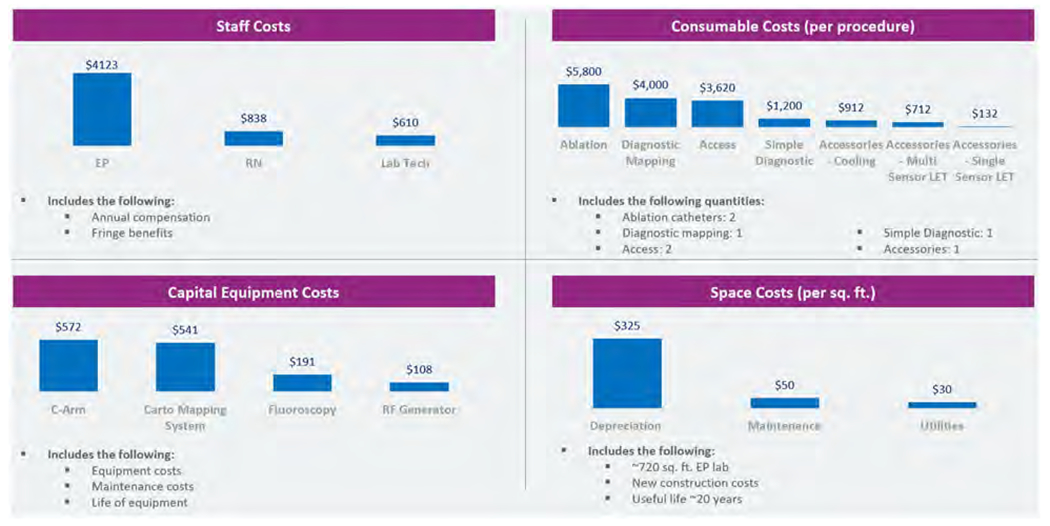
Daily costs for staff, consumables, capital equipment, and laboratory space. Abbreviations: RF, radiofrequency; RN, Registered Nurse; EP, electrophysiologist.
Combining these costs into per-procedure estimates results in the estimates as shown in Figure 7, which includes estimates of profit per procedure based on 2021 average DRG payment36.
Figure 7.
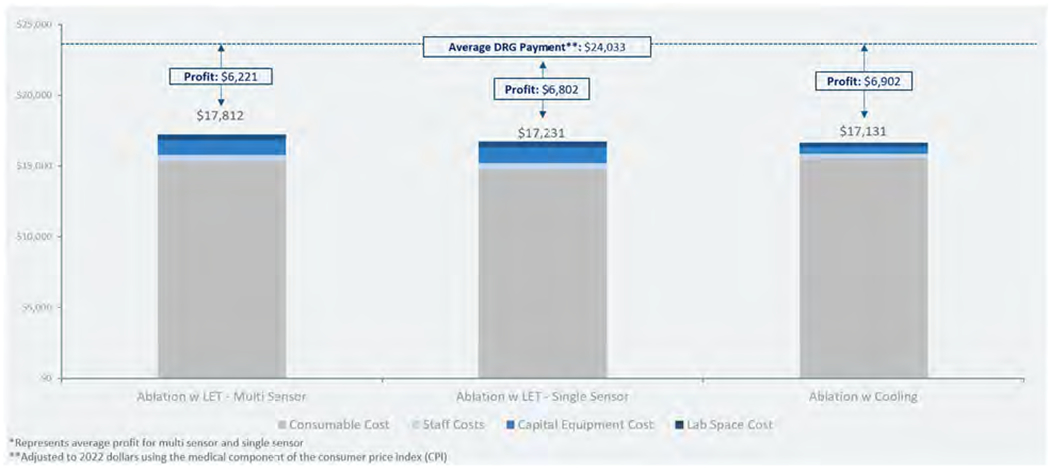
Cost and profit per procedure estimates using LET monitoring (with single and multi-sensor temperature probes) compared to using active esophageal cooling. Abbreviation: LET, luminal esophageal temperature.
Incorporating the savings from same-day discharge after atrial fibrillation ablation, which has been estimated at $8082 (adjusted to 2022 dollars using CPI) as a result of the reimbursement available from the bed made available by discharging on the same day as the procedure rather than admitting for overnight observation32, results in the total costs and profit as shown in Figure 8. This incorporates the 18% absolute improvement in same-day discharge reported in published data30. A study of 164 patients who underwent RF ablation between January 2020 and January 2022 was performed to compare same-day discharge rates between 63 patients treated with LET monitoring and 101 patients treated with active esophageal cooling. Of the patients treated with LET monitoring, 13 (20.6%) were discharged within 12 h of admission compared to 39 (38.6%) of the patients treated with active esophageal cooling, for an absolute improvement in same-day discharge rate of 18% (p = .016)30.
Figure 8.
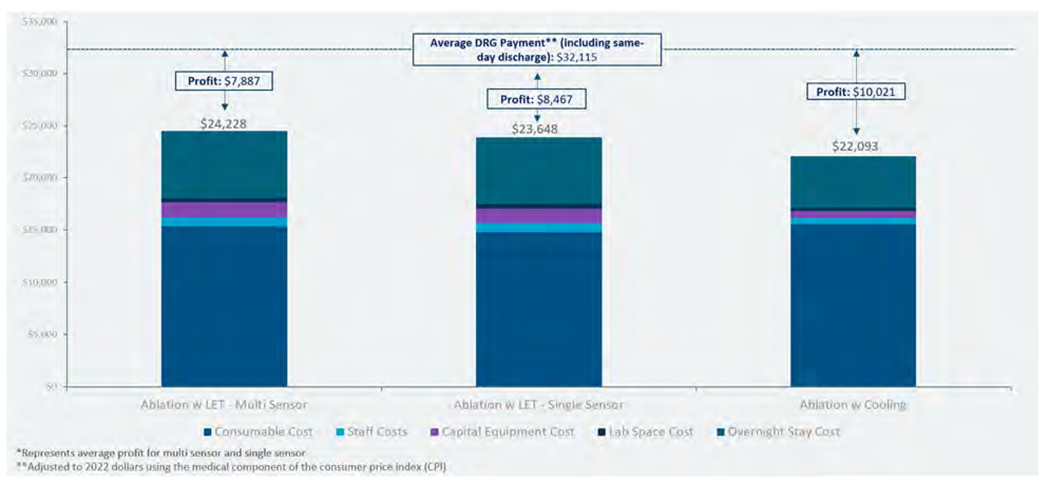
Total costs and net profit per procedure after including savings from same-day discharge. Abbreviation: LET, luminal esophageal temperature.
Effect of uncertainty
To examine the effect of uncertainty or possible heterogeneity around the primary drivers of the cost differences found, we performed additional sensitivity analyses, evaluating the influence of a 50% reduced procedural time savings and same-day discharge rate, on outcomes. Incorporating only a 20-min reduction in procedure duration (rather than the reported 41-min reduction in procedure duration) when utilizing active cooling as compared to luminal esophageal temperature monitoring, the increased cost attributed to increased staff and capital equipment time is $336. The increased cost from a 29.6% same-day discharge rate rather than the reported 38.6% discharge rate is $727. In total, the overall cost of the procedure is $23,157, with a profit of $8958.
Effect of engagement with patients and others affected by the study
Engagement with patients and others affected by this analysis has not yet occurred.
Discussion
In this first reported analysis of the influence of different esophageal protective strategies on costs of left atrial RF ablation using a time-driven activity-based costing methodology, we found notable savings when using active esophageal cooling compared to using more traditional LET monitoring. This savings translates into a per-procedure profit of $10,021 using active esophageal cooling, compared to a per-procedure profit of $8,467 using single-sensor LET monitoring, and $7,887 using multi-sensor LET monitoring. The two drivers of the cost difference between strategies are the shorter procedural time and the improved same-day discharge rate seen with active esophageal cooling.
This analysis identified a cost per minute in the electrophysiology lab of $21.27 per minute. This is in line with other reported values, which range from $11.53 per minute (not including anesthesia costs, which add a further $5.28 per minute), to as high as $33 per minute39–41. Our findings appear to be robust to variations in procedure time and same-day discharge rates. For example, repeating our analysis with an assumption that the comparative benefit in procedure time reduction and same-day discharge rates were reduced by 50%, the per-procedure profit for an ablation performed with active esophageal cooling would be reduced to $8958, still providing an improved savings over the use of single-sensor or multi-sensor LET monitoring.
Our analysis does not take into account the downstream impacts of the choice in esophageal protective strategy. Recent data suggest that long-term efficacy may be improved with the use of active esophageal cooling26–29, and as a result, costs to the healthcare system would be expected to be lower, but without acute change to procedural costs. On the other hand, differences in complication rates would be expected to impact acute costs; however, data to date suggest either no difference, or a reduction in complication rates when using active esophageal cooling14,47. For Accountable Care Organization (ACO) models that rely on per covered life cost reduction, even small improvements in outcomes, reductions in readmission, reductions in repeat procedures, and reductions in catastrophic events (such as atrioesophageal fistula) result in dramatic cost improvements48.
Overall, this analysis underestimates the overall financial impact to the hospital when considering the enhanced throughput from an increased rate of same-day-discharge. Same-day-discharge allows not only a reduction in operating costs but also the ability to care for an incremental patient. This is particularly beneficial for patient care in our current labor and resource constrained environment. The financial impact of these incremental patients can be estimated as the additional reimbursement for a similar patient for chest pain management, $4,21632.
One consequence of shorter procedure times of the magnitude reported to date is the potential for a given electrophysiology lab to perform additional cases in the workday. For example, if a typical case took 140 min using LET monitoring but was reduced to 100 min using active esophageal cooling, the extra 120 min made available during a procedural day in which three cases were completed would allow another case to be added to the schedule. The total annual profit to the hospital system would then be increased by this additional case completion rate. This strategy has already been implemented in a number of healthcare systems, such that a significant increase in procedure volume has been obtained in part due to the adoption of active esophageal cooling with increases of between 10% to 38% in procedural volume being realized. Combined with the improved long-term procedural efficacy reported with active esophageal cooling, this also has the potential to reduce overall healthcare system costs49.
Our findings are in line with other studies evaluating the effects of other new technologies used in complex medical procedures. For example, Mansour et al. found that the adoption of contact force sensing catheters for cardiac ablation decreased costs by $3402 per subject in the first year50. An analysis comparing robotic-assisted to conventional total knee arthroplasty found that robotic-assisted procedures actually cost more than conventional as a result of longer operating room times51.
These findings have the potential to influence the decision-making process of electrophysiology practices. Healthcare affordability remains a growing concern for commercial payers and the federal government52. Recently proposed reductions to work relative value units (RVUs) for electrophysiology ablation services as part of the proposed 2023 Medicare Physician Fee Schedule by the Centers for Medicare and Medicaid Services (CMS) have further highlighted the need for new approaches to maintaining provider revenue levels53. With CMS proposals to further reduce the work RVUs below the revised recommendations from the American Medical Association/Specialty Society RVS Update Committee (RUC) by an average of 12.9%54, one clear route to recover reduced fees is by increasing the procedure volume. Adoption of active esophageal cooling appears to be one way to achieve this goal.
Limitations
Process mapping within the TDABC model is the most accurate methodology to capture the current state of a procedure or process and its associated costs. For each procedure, clinical, administrative staff and other contributing personnel are brought together to complete a process map which involves process steps, decision points, human, materials and equipment resources involved in the activity, time spent doing the activity (stopwatch method) and the variability around each input55. In this study, this methodology was not utilized but rather supplemented the information through literature review. This use of existing data from literature and databases poses a limitation to this study. Key variables affecting costs other than the type of esophageal protection utilized remained the same, however, allowing comparison between the approaches to be the primary focus of the analysis. Operator volume and experience are factors that can influence performance and efficiency, and centers with different levels of volume and experience may see impacts that differ from those found in this analysis. We used published data on procedural time impacts of esophageal cooling that were reported from higher-volume clinical sites with experienced clinicians, rather than from teaching hospitals with electrophysiology fellows in training, since training sites generally have unique facets to procedural workflow that are not reflected in more typical hospital settings. This analysis did not include costs for anesthesia. Variability in approaches to anesthesia in the electrophysiology lab (whether using physician anesthesiologists or certified registered nurse anesthetists, for example) add further complexity to incorporating this cost, but the comparison between esophageal protective modality in a given system is less likely to be affected by this. Likewise, some electrophysiology labs may use different numbers or combinations of nurses and technicians. This study does not include the longitudinal impact and outcomes in the study cohort including the need for repeat procedures and the effects of comorbidities and complications, since these are generally influenced by numerous variables with relatively uncertain associations. As such, the long-term economic impacts are not included in this analysis. For example, increasing data have emerged showing improved long-term efficacy from left atrial ablations performed with active esophageal cooling20,29,56, which has the potential to reduce overall healthcare system costs, but the impacts of this improvement are outside the scope of this analysis. We have not performed a deterministic sensitivity analysis or probabilistic sensitivity analysis varying other input parameters; however, the fact that the same personnel and equipment (other than choice of esophageal protection device) are used for each scenario reduces the potential for significant differences in overall conclusions.
Conclusions
The use of active esophageal cooling is associated with significant cost-savings when compared to traditional LET monitoring, even after accounting for the additional cost of the cooling device. These savings originate from a per-patient procedural time savings and a per-population improvement in same-day discharge rate.
Table 2.
| Consumables costs | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Access cost | Simple Dx cost | Dx mapping cost | Accessory cost | Ablation cost | Daily Consumable Cost | |
| Ablation with multi sensor probe | $3,620 | $1,200 | $ 4,000.00 | $712 | $5,800 | $15,332 |
| Ablation with single sensor probe | $3,620 | $1,200 | $ 4,000.00 | $132 | $5,800 | $14,752 |
| Ablation with esophageal cooling | $3,620 | $1,200 | $ 4,000.00 | $912 | $5,800 | $15,532 |
Abbreviation: Dx, diagnostic.
Table 3.
Space costs in 2022 dollars35.
| Space costs | |||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Square Feet | New construction cost per sq. ft | New construction Total cost | Useful life (yrs) | Depreciation per sq. ft. for new lab construction | Maintenance per sq. ft. | Utilities per sq. ft. | Total cost per sq. ft | Daily space cost | |
| EP Lab | 720 | $6,500 | $4,680,000 | 20 | $325 | $50 | $30 | $405 | $1,458 |
Abbreviation: EP, electrophysiologist.
Acknowledgements
The authors wish to thank the staff of the UT Southwestern Department of Electrophysiology: Cheryl Thomas RN, Roma Alfonso RN, Eileen Dwyer RN, Anish Varghese RN, Josey George RCIS, Pam Harrison RCIS, and Carolyn Carlson RN.
Disclosure statement of funding
Research reported in this publication was supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number R44HL158375 (the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health).
Disclosure statement of financial/other interests
CJ: internship with Attune Medical; JC: funding for data collection from Attune Medical; JZ: consulting for Biosense Webster and Attune Medical; RS: investment in Attune Medical; SM: none; RT: none; EK: employment and equity in Attune Medical; JD: none
Footnotes
CARTO mapping system (Boston Scientific, Marlborough, MA).
Reviewer disclosures
Peer reviewers on this manuscript have received an honorarium from JME for their review work but have no other relevant financial relationships to disclose.
Previous presentations
Presented at The 5th Annual Architecture of High Value Health Care National Conference, held October 6–9, 2022, at the Baltimore Convention Center. https://hvpaa.org/2022nc/
References
- [1].Antman EM, Leopold JA, Sauer WH, et al. Atrial fibrillation and catheter ablation. N Engl J Med. 2022;387(14):e31. [DOI] [PubMed] [Google Scholar]
- [2].Mujović N, Marinković M, Lenarczyk R, et al. Catheter ablation of atrial fibrillation: an overview for clinicians. Adv Ther. 2017;34(8):1897–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American college of cardiology/American heart association task force on practice guidelines and the heart rhythm society. Circulation. 2014;130(23):2071–2104. [DOI] [PubMed] [Google Scholar]
- [4].Colilla S, Crow A, Petkun W, et al. Estimates of current and future incidence and prevalence of atrial fibrillation in the U.S. adult population. Am J Cardiol. 2013;112(8):1142–1147. [DOI] [PubMed] [Google Scholar]
- [5].Hosseini SM, Rozen G, Saleh A, et al. Catheter ablation for cardiac arrhythmias: utilization and in-Hospital complications, 2000 to 2013. JACC Clin Electrophysiol. 2017;3(11):1240–1248. [DOI] [PubMed] [Google Scholar]
- [6].Hunter TD, Palli SR, Rizzo JA. Cost comparison of radiofrequency catheter ablation versus cryoablation for atrial fibrillation in hospitals using both technologies. J Med Econ. 2016;19(10):959–964. [DOI] [PubMed] [Google Scholar]
- [7].Barnow A, Goldstein L, Kalsekar I, et al. Use of the THERMOCOOL SMARTTOUCH catheter for ablation of atrial fibrillation: the relationship between hospital procedure volume, re-admissions, and economic outcomes. J Med Econ. 2018;21(5):481–487. [DOI] [PubMed] [Google Scholar]
- [8].Lau D, Sandhu RK, Andrade JG, et al. Cost-Utility of catheter ablation for atrial fibrillation in patients with heart failure: an economic evaluation. J Am Heart Assoc. 2021;10(14):e019599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Leung LWM, Akhtar Z, Sheppard MN, et al. Preventing esophageal complications from atrial fibrillation ablation: a review. Heart Rhythm O2. 2021;2(6Part A):651–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Grosse Meininghaus D, Blembel K, Waniek C, et al. Temperature monitoring and temperature-driven irrigated radiofrequency energy titration do not prevent thermally induced esophageal lesions in pulmonary vein isolation: a randomized study controlled by esophagoscopy before and after catheter ablation. Heart Rhythm. 2021;18(6):926–934 [DOI] [PubMed] [Google Scholar]
- [11].Schoene K, Arya A, Grashoff F, et al. Oesophageal probe evaluation in radiofrequency ablation of atrial fibrillation (OPERA): results from a prospective randomized trial. Europace. 2020;22(10):1487–1494. [DOI] [PubMed] [Google Scholar]
- [12].Kautzner J, Neuzil P, Lambert H, et al. EFFICAS II: optimization of catheter contact force improves outcome of pulmonary vein isolation for paroxysmal atrial fibrillation. Europace. 2015;17(8):1229–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Jankelson L, Dai M, Bernstein S, et al. Quantitative analysis of ablation technique predicts arrhythmia recurrence following atrial fibrillation ablation. Am Heart J. 2020;220:176–183. [DOI] [PubMed] [Google Scholar]
- [14].Leung LWM, Bajpai A, Zuberi Z, et al. Randomized comparison of oesophageal protection with a temperature control device: results of the IMPACT study. Europace. 2021;23(2):205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Joseph C, Sherman J, Ro A, et al. Procedural time reduction associated with active esophageal cooling during pulmonary vein isolation. J Interv Card Electrophysiol. 2022;65(3):617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Joseph C, Daniels J, Kawasaki R, et al. The effect of active esophageal cooling during left atrial ablation on postoperative chest pain – an updated survey of operators. AF Symp. 2022;33(4). [Google Scholar]
- [17].Zagrodzky J, Bailey S, Shah S, et al. Impact of active esophageal cooling on fluoroscopy usage during left atrial ablation. J Innov Card Rhythm Manag. 2021;12(11):4749–4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mercado Montoya M, Gomez Bustamante T, Berjano E, et al. Proactive esophageal cooling protects against thermal insults during high-power short-duration radiofrequency cardiac ablation. Int J Hyperthermia. 2022;39(1):1202–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cooper J, Joseph C, Zagrodzky J, et al. Active esophageal cooling during radiofrequency ablation of the left atrium: data review and update. Expert Rev Med Devices. 2022:1–9. DOI: 10.1080/17434440.2022.2150930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Joseph C, Zagrodzky W, Sherman J, et al. One-year outcomes after active cooling during left atrial radiofrequency ablation. J Am Coll Cardiol. 2022;79(9):114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rehman H, Ali F, Mangi MA. Choosing wisely campaign - innovations in cardiovascular science and the United States healthcare system. Cureus. 2018;10(7):e2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards (CHEERS) 2022 explanation and elaboration: a report of the ISPOR CHEERS II good practices task force. Value Health. 2022;25(1):10–31. [DOI] [PubMed] [Google Scholar]
- [23].Drummond M. Methods for the economic evaluation of health care programmes. 4th ed. Oxford (United Kingdom): Oxford University Press; 2015. [Google Scholar]
- [24].Di Biase L, Monir G, Melby D, et al. Composite index tagging for PVI in paroxysmal AF: a prospective, multicenter postapproval study. JACC Clin Electrophysiol. 2022;8(9):1077–1089. [DOI] [PubMed] [Google Scholar]
- [25].Ayoub T, El Hajjar AH, Singh Sidhu GD, et al. Esophageal temperature during atrial fibrillation ablation poorly predicts esophageal injury: an observational study. Heart Rhythm O2. 2021;2(6):570–577. 11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Joseph C, Nazari J, Zagrodzky J, et al. Esophageal cooling during ablation of persistent atrial fibrillation is associated with improved freedom from arrhythmia at one-year follow up. Eur Heart J. 2022;43(Supplement_2):ehac544.461. [Google Scholar]
- [27].Joseph C, Francisco G, Ruppert A, et al. Reduced arrhythmia recurrence at one-year when using active esophageal cooling. AHA scientific sessions, Chicago, IL; 2022. [Google Scholar]
- [28].Joseph C, Francisco G, Ruppert A, et al. Arrhythmia recurrence reduction with an active esophageal cooling device during radiofrequency ablation. EP Europace. 2022;24(Supplement_1):euac053.096. [Google Scholar]
- [29].Leung LWM, Akhtar Z, Elbatran AI, et al. Effect of esophageal cooling on ablation lesion formation in the left atrium: insights from ablation index data in the IMPACT trial and clinical outcomes. Cardiovasc Electrophysiol. 2022;33(12):2546–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cooper J, Joseph C, Turer R, et al. Procedural impacts of active esophageal cooling during radiofrequency ablation in an academic medical center. AHA scientific sessions, Chicago, IL; 2022. [Google Scholar]
- [31].Joseph C, Zagrodzky JD, Zagrodzky W, et al. Reduced procedural duration with proactive esophageal cooling during left atrial ablation: a multicenter Analysis – PO-681-08. Heart Rhythm. 2022;19(5):S364. [Google Scholar]
- [32].Chu E, Zhang C, Musikantow DR, et al. Barriers and financial impact of same-day discharge after atrial fibrillation ablation. Pacing Clin Electrophysiol. 2021;44(4):711–719. [DOI] [PubMed] [Google Scholar]
- [33].Winkle RA, Mead RH, Engel G, et al. Physician-controlled costs: the choice of equipment used for atrial fibrillation ablation. J Interv Card Electrophysiol. 2013;36(2):157–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Yokokawa M, Chugh A, Latchamsetty R, et al. Ablation of paroxysmal atrial fibrillation using a second-generation cryoballoon catheter or contact-force sensing radiofrequency ablation catheter: a comparison of costs and long-term clinical outcomes. J Cardiovasc Electrophysiol. 2018;29(2):284–290. [DOI] [PubMed] [Google Scholar]
- [35].Elrod J. Building a state-of-the-Art EP lab: considerations and technology: interview with Sean Tierney, MD. EP Lab Digest. 2022;6(12). [Google Scholar]
- [36].CRHF reimbursement – coding reference guides. https://www.medtronic.com/us-en/healthcare-professionals/reimbursement/cardiac-rhythm-heart-failure/coding-reference-guides.html.
- [37].Lafontant MP, Smith VE, Sadule Rios N, et al. Implanting loop recorders in a hospital unit versus the electrophysiology laboratory: a retrospective chart review. Nursing & Health Sciences Research Journal. 2019;2(1):30–35. [Google Scholar]
- [38].Physician Employment Benefits See Some Shifts. https://resources.nejmcareercenter.org/article/physician-employment-benefits-see-some-shifts/. [Google Scholar]
- [39].Kawasaki MK, Cleary DJ, Sa D, et al. Understanding the costs associated with cardiac ablations. Circulation. 2019;140(Suppl_1)A9552. [Google Scholar]
- [40].Thording L. Why hospital leaders should look to cardiology for sustained future reprocessing savings. Providence (RI): Innovative Health; 2018. [Google Scholar]
- [41].Electrophysiology market size by product. Selbyville (DE): Global Market Insights; 2021. [Google Scholar]
- [42].Slachta A. Cardiologist salaries trending up for the 5th year in a row. Providence (RI): Cardiovascular Business. Innovate Healthcare; 2019. [Google Scholar]
- [43].Kaplan RS, Anderson SR. Time-driven activity-based costing. Harv Bus Rev. 2004;82(11):131–138, 150. [PubMed] [Google Scholar]
- [44].Repurposing TDABC: case examples from a New York Hospital. https://www.hfma.org/topics/hfm/2018/september/61817.html.
- [45].Shankar PR, Hayatghaibi SE, Anzai Y. Time-driven activity-based costing in radiology: an overview. J Am Coll Radiol. 2020;17(1 Pt B):125–130. [DOI] [PubMed] [Google Scholar]
- [46].BLS Data Viewer. https://beta.bls.gov/dataViewer/view/timeseries/CUUR0000SAM.
- [47].Tschabrunn CM, Attalla S, Salas J, et al. Active esophageal cooling for the prevention of thermal injury during atrial fibrillation ablation: a randomized controlled pilot study. J Interv Card Electrophysiol. 2022;63(1):197–205. [DOI] [PubMed] [Google Scholar]
- [48].McWilliams MEC, Michael J. The case for ACOs: why payment reform remains necessary. Health Affairs. 2022. [Google Scholar]
- [49].Kowalski M, Shah R, Akhrass P, et al. Economics and laboratory efficiency of atrial fibrillation ablation. Curr Opin Cardiol. 2022;37(1):22–29. [DOI] [PubMed] [Google Scholar]
- [50].Mansour M, Reddy VY, Karst E, et al. Economic impact of contact force sensing in catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2019;30(11):2302–2309. [DOI] [PubMed] [Google Scholar]
- [51].Fang CJ, Mazzocco JC, Sun DC, et al. Total knee arthroplasty hospital costs by time-driven activity-based costing: robotic vs conventional. Arthroplast Today. 2022;13:43–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Swagel P. Policy approaches to reduce what commercial insurers pay for hospitals’ and physicians’ services. Washington (DC): Congressional Budget Office; 2022. [Google Scholar]
- [53].Medicare cuts could devastate the field of electrophysiology. https://cardiovascularbusiness.com/node/224666.
- [54].Proposed. 2023. Medicare cuts target ablation services; ACC prepares responses - american college of cardiology. http://www.acc.org/latest-in-cardiology/articles/2022/07/14/14/49/proposed-2023-medicare-cuts-target-ablation-services.
- [55].McLaughlin N, Burke MA, Setlur NP, et al. Time-driven activity-based costing: a driver for provider engagement in costing activities and redesign initiatives. Neurosurg Focus. 2014;37(5):E3. [DOI] [PubMed] [Google Scholar]
- [56].Joseph C, Athill C, Ruppert A, et al. Reduced arrhythmia recurrence at one-year when using active esophageal cooling. Circulation. 2022;146(Suppl_1):A14986. [Google Scholar]


