Abstract
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignant disease with a 5-year survival rate of <10%. Aberrant activation or elevated expression of the tyrosine kinase c-SRC (SRC) is frequently observed in PDAC and is associated with a poor prognosis. Preclinical studies have revealed a multifaceted role for SRC activation in PDAC, including promoting chronic inflammation, tumor cell proliferation and survival, cancer cell stemness, desmoplasia, hypoxia, angiogenesis, invasion, metastasis, and drug resistance. Strategies to inhibit SRC signaling include suppressing its catalytic activity, inhibiting protein stability, or by interfering with signaling components of the SRC signaling pathway including suppressing protein interactions of SRC. In this review, we discuss the molecular and immunological mechanisms by which aberrant SRC activity promotes PDAC tumorigenesis. We also provide a comprehensive update of SRC inhibitors in the clinic, and discuss the clinical challenges associated with targeting SRC in pancreatic cancer.
Subject terms: Pancreatic cancer, Cancer microenvironment
Pancreatic ductal adenocarcinoma (PDAC)
PDAC is an aggressive malignant disease that accounts for more than 90% of pancreatic cancer cases [1]. Due to nonspecific clinical symptoms, most patients are diagnosed at advanced stages of the disease, and are not eligible for surgery [2, 3]. Systemic chemotherapy is commonly employed as the first-line treatment in patients with nonresectable tumors; however, durable responses are observed in <30% of cases [4, 5]. Furthermore, immunotherapies have failed to translate into meaningful improvements in a majority of PDAC patients due to the presence of a highly immunosuppressive and desmoplastic tumor microenvironment [6–8]. Given the poor prognosis and limited treatment options for PDAC, a better understanding of key signaling pathways and molecules involved in tumor initiation, development, and metastasis is crucial to guide the use of existing therapies and identify new drug targets.
SRC
Francis Peyton Rous was awarded the Nobel Prize in 1966 for his discovery of the transmissible avian Rous sarcoma virus (RSV), which provided the first evidence of virally-mediated tumorigenesis [9]. Subsequently, it was shown that the genome of RSV encoded a tyrosine kinase referred to as viral SRC (v-Src), which stimulated uncontrolled proliferation in host cells [10, 11]. In 1979, J. Michael Bishop and Harold Varmus discovered that normal chicken cells possessed a cellular homolog of v-Src referred to as cellular SRC (c-Src) that shared striking resemblance to v-Src [10]. Importantly, RSV had integrated a genomic sequence encoding a truncated version of c-Src that lacked the regulatory carboxy-terminal tail. Thus, unlike its cellular counterpart c-SRC, which was referred to as a “proto-oncogene”, v-SRC remained constitutively active [12]. These insights led to a paradigm shift in oncology by demonstrating that mutations in tightly regulated proteins encoded by proto-oncogenes can also promote tumor development, with or without viral involvement.
SRC structure and activation
c-SRC (SRC) belongs to a family of nine non-receptor tyrosine kinases collectively referred to as the SRC family kinase (SFK), which comprises three distinct groups based on the expression pattern of individual members. The first group (SRC, FYN, and YES) are ubiquitously expressed. The second group (HCK, BLK, FGR, LCK, YRK, and LYN) are primarily expressed in hematopoietic cells, whereas the third group (FRK) is predominantly expressed on epithelial cells [13].
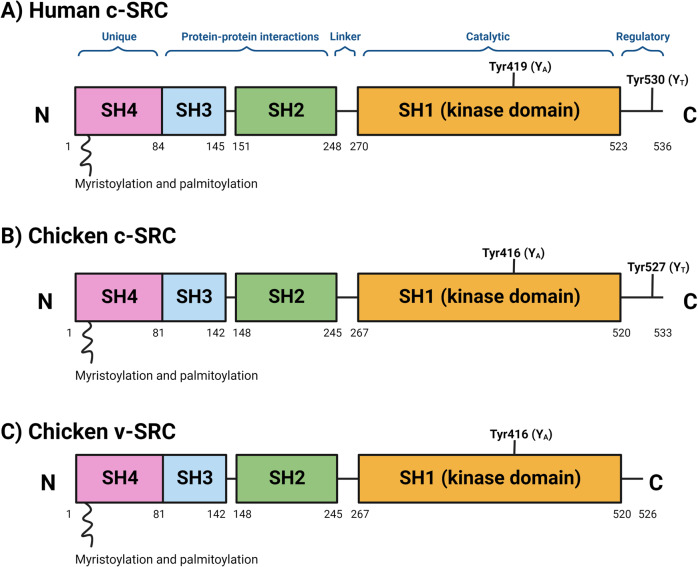
SFKs are comprised of a unique amino-terminal SRC Homology 4 domain (SH4), followed by conserved regulatory SH3 and SH2 domains, a flexible linker, a SH1 protein kinase domain containing a tyrosine residue in the activation loop (YA), and a C-terminal tail containing a negative regulatory tyrosine residue (YT) (Fig. 1) [14].
Fig. 1. Structure of SRC.
Schematic of (A) human c-SRC, (B) chicken c-SRC and (C) chicken v-SRC. Human c-SRC is comprised of a 14-carbon myristic acid moiety attached to the N-terminal SRC Homology 4 domain (SH4), a unique domain, followed by an SH3 and SH2 domain, a linker, a SH1 protein kinase domain containing a tyrosine residue at Tyr419 (YA; Tyr416 in chickens), and a C-terminal negative regulatory tail containing a tyrosine residue at Tyr530 (YT; Tyr527 in chickens). Chicken v-SRC lacks the C-terminal segment containing the YT residue, which renders it constitutively active and capable of inducing cellular transformation. Figure created in Biorender.
The SH4 domain contains myristoylation and palmitoylation sites that anchor SFKs to the inner leaf of the cytoplasmic membrane (Fig. 1). The SH3 domain is a small protein domain (61 amino acid residues in SRC), which is folded into β-barrels of five antiparallel strands. The SH3 domain mediates protein–protein interactions by binding to proline-rich motifs of client proteins [15], and is necessary for substrate recognition and to some extent regulation of kinase activity [16, 17]. Meanwhile, the SH2 domain (97 amino acid residues in SRC) is comprised of a β-sheet flanked by opposing α-helices [18]. The SH2 domain has a conserved arginine-containing recognition pocket that binds to short phosphotyrosine motifs [18]. Lastly, the SH1 catalytic domain contains the active site of the kinase domain nestled between a small N-lobe and a large C-lobe. The N-lobe consists of five-stranded antiparallel β-sheets and a regulatory αC-helix, while the C-lobe contains α-helical segments [19].
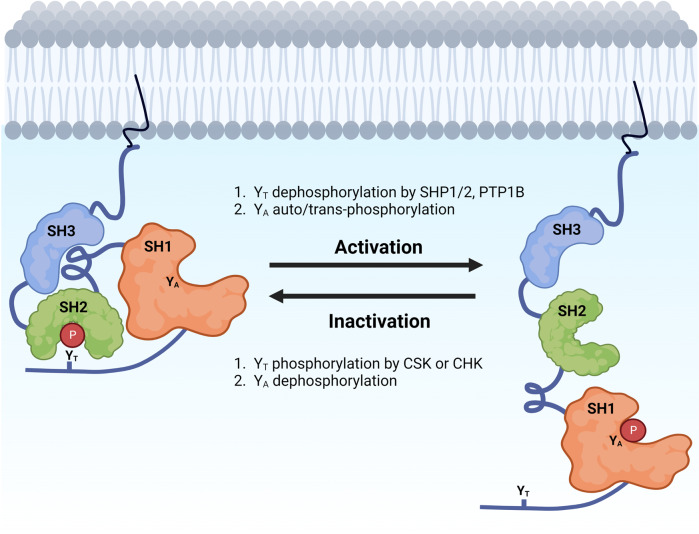
SFKs exist in dynamic equilibria between active and inactive conformations. The inactive ‘closed’ conformation is maintained by two intra-molecular associations (Fig. 2). This closed conformation primarily results from the association of the SH2 domain to the phosphorylated YT residue, and is further strengthened by binding of the SH3 domain to the linker separating the SH2 and SH1 domains [20]. Phosphorylation of YT is tightly regulated by members of the C-terminal SRC kinase (CSK) family, including CSK and CHK [14, 20]. Since v-SRC lacks the C-terminal negative regulatory segment containing the YT residue, it is constitutively active and able to induce cellular transformation [14].
Fig. 2. Regulation of SRC activation.
SRC is maintained in an inactive conformation by the binding of the SH2 linker to the SH3 domain, and by the binding of the phosphorylated YT to the SH2 domain. Activation of SRC occurs following dephosphorylation of YT, as well as auto/trans-phosphorylation of YA. Figure created in Biorender.
Activation of SFKs is mediated by dephosphorylation of YT and auto-phosphorylation of YA [21]. Dephosphorylation of YT by phosphatases such as protein tyrosine phosphatase (PTP)1, and SH2-containing phosphatases (SHP) 1 and SHP2 results in an ‘open’ conformation that facilitates the binding of the SH2 and SH3 domains with their corresponding ligands [14]. Subsequently, auto-phosphorylation of YA results in full SFK activation [22].
Mechanisms underpinning aberrant SRC signaling in cancer
SRC mediates a wide range of signaling pathways that play a pivotal role in normal cellular processes, including proliferation, adhesion, angiogenesis, and migration (Fig. 3). Given the contribution of these features to some of the “hallmarks of cancer,” it is unsurprising that aberrant activation or expression of SRC is observed in many tumor types and correlates with poor patient outcomes. However, while activating mutations in SRC are observed in <2% of pancreatic cancer patients [23–25], elevated expression, and/or activation of SRC in PDAC confers a weakly oncogenic outcome [26–28].
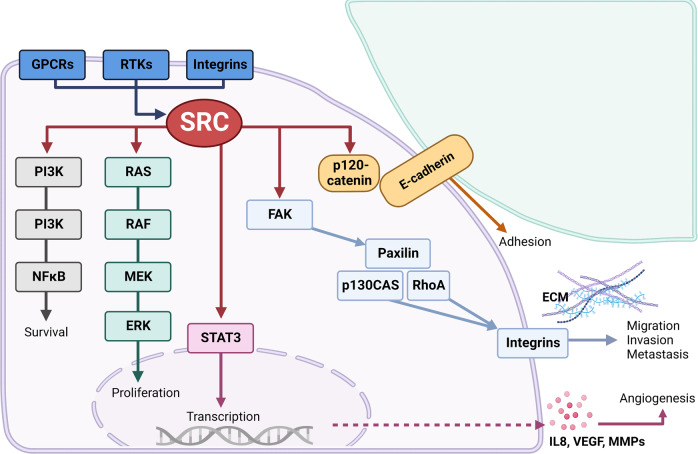
Fig. 3. Examples of SRC signal transduction pathways.
SRC can interact with G-protein coupled receptors (GPCRs), receptor tyrosine kinases (RTKs; e.g., MET, PDGFR, EGFR), and integrins to activate downstream signaling pathways (e.g., PI3K, RAS/ERK, STAT3) that promote cell proliferation, survival and angiogenesis. In addition, SRC can phosphorylate p120-catenin to disrupt adherens junctions stabilized by E-cadherin to enhance cell adhesion. Conversely, SRC and FAK signaling mediates the activation of downstream targets (e.g., p130CAS, Paxillin, RhoA) and results in the formation of complexes with integrin molecules that modulate the extracellular matrix (ECM) to stimulate cell migration, invasion, and metastasis. Figure created in Biorender.
Aberrant activation of SRC can result from several mechanisms, including amplification of upstream signaling pathways, loss of negative regulation, and impaired degradation. Indeed, SRC directly interacts with a variety of cellular factors, including signal transducers and activators of transcription (STAT) proteins, cyclins, and tyrosine kinases (e.g., colony-stimulating factor 1 receptor (CSF1R), platelet derived growth factor receptor (PDGFR), epidermal growth factor receptor (EGFR), human epidermal growth factor receptor (HER), and focal adhesion kinase (FAK)) [29]. FAK signaling enables the fibrotic and immune suppressive tumor microenvironment of PDAC, and its elevated expression is associated with poor overall survival [30, 31]. Following integrin engagement or ligand stimulation, FAK forms a complex with SRC and triggers the activation of downstream pathways involved in tumor cell migration, invasion, survival, and immune suppression [27, 30, 32, 33]. Moreover, amplification of genes associated with SRC effector networks such as PI3K/AKT/mTor and FAK are reported in 17% and 6% of pancreatic tumors, respectively [23–25], while aberrant expression of integrin signaling components that activate SRC are observed in 67% of pancreatic tumors [34]. Collectively, these results highlight a mechanism by which cooperation of SRC with its binding partners may promote feed-forward loops to reinforce its kinase activity.
Aberrant SRC activity may also arise due to disruption to proteins that regulate its function. Gain-of-function mutations in SHP2 leads to increased SRC activation and tumor development in preclinical models [35–37], while reduced expression of CSK correlates with enhanced SRC activity in cancer cells [38]. Another possible mode of increased SRC activation includes altered protein stability, which may occur due to deregulation of the ubiquitin ligase c-CBL that mediates proteasomal degradation of SRC [39, 40].
Effects of tumor cell-intrinsic SRC activity in pancreatic cancer
Tumor cell-intrinsic mechanisms by which SRC signaling facilitates PDAC development and progression include enhancing cancer cell growth and survival, promoting stemness, induction of metabolic reprogramming, facilitating tumor invasion and metastasis, and mediating chemoresistance (Fig. 3).
Cancer cell growth and survival
RAS/RAF/MEK/ERK signaling
Activating mutations in KRAS are a hallmark of pancreatic cancer, and are observed in approximately 95% of patients [41, 42]. Under steady-state conditions, SRC phosphorylates RAS, and triggers downstream activation of the MAPK signaling cascade to stimulate cellular proliferation, differentiation, and survival [43]. Notably, pancreas-specific oncogenic KrasG12D expression and deletion of the SRC negative regulator CSK accelerates PDAC development in mice compared to littermates that express KrasG12D and wild-type levels of SRC [44].
PI3K signaling
SRC regulates PI3K signaling by directly phosphorylating the p85 subunit of PI3K [45], and by inhibiting the PI3K negative regulatory phosphatase and tensin homolog (PTEN) [46]. This results in downstream phosphorylation of AKT, which enhances the growth and survival of pancreatic cancer cells [47].
STAT3 signaling
Reciprocal activation of SRC and STAT3 is implicated in PDAC progression [48–50]. v-SRC directly associates with STAT3 to promote its phosphorylation, DNA binding, and transcriptional activity [51, 52]. Reciprocally, STAT3 activation is required for v-SRC to promote cellular transformation [53–55]. SRC and STAT3 signaling enhances the transcription of angiogenic genes (e.g., IL8, VEGF) in human pancreatic cancer cells [56–58], while inhibition of SRC activity decreases STAT3 phosphorylation and tumorigenicity [56].
YAP signaling
YES1-associated protein (YAP) is a major effector of the Hippo signaling pathway, and enables an immune suppressive PDAC microenvironment [59, 60]. SRC plays a major role for YAP nuclear localization and phosphorylation [61], which results in the transcription of YAP-target genes involved in cell proliferation and survival through Hippo-dependent [62, 63] and Hippo-independent pathways [64].
FAK signaling
Aberrant FAK activation facilitates cancer cell migration, survival, adhesion, and invasion, and is associated with a worse prognosis in PDAC [65–67]. Following integrin engagement or ligand stimulation, FAK undergoes auto-phosphorylation and forms a complex with SRC [68, 69], which induces a conformational change in SRC and its activation. In turn, activated SRC phosphorylates FAK, which serves as a docking site for proteins that stimulate the activation of downstream signaling pathways including RAS, STAT3, and PI3K [70]. The FAK and SRC complex also phosphorylates cytoskeletal adapter proteins (e.g., Paxillin and CAS), which recruit and activate additional signaling molecules involved in cell motility and invasion [69].
Crosstalk with receptor tyrosine kinases (e.g., EGFR and PDGFR)
Aberrant activation of SRC and EGFR is observed in most human malignancies, suggesting functional cooperativity to promote tumor development [71]. In addition to direct tyrosine phosphorylation [72–74], SRC can indirectly activate EGFR signaling by stimulating matrix metalloproteinases (MMPs), which cleave EGFR ligands to promote receptor activation [75]. Another mechanism by which SRC amplifies EGFR signaling is by promoting destruction of the ubiquitin-protein ligase c-CBL, which mediates proteasomal degradation of activated EGFR [76, 77].
In support of the oncogenic synergy between SRC and EGFR in PDAC, stable complexes between SRC and EGFR contribute to more aggressive tumor phenotypes by enhancing DNA synthesis and mitosis [71, 78]. Accordingly, dasatinib (SRC inhibitor) and erlotinib (EGFR inhibitor) treatment reduces pancreatic cancer cell migration and invasion, overcomes STAT3-mediated chemoresistance, and attenuates the growth of PDAC xenografts [79].
SRC also plays versatile roles in mediating cell responses regulated by PDGFR signaling, including cell survival, migration, and actin cytoskeleton rearrangement [80, 81]. Ligand-induced activation of PDGFRβ results in dephosphorylation of SRC on Tyr530 and auto-phosphorylation on Tyr419. In turn, SRC phosphorylates PDGFR and renders it active [82–84]. Constitutive SRC activation due to autocrine PDGF/PDGFR stimulation is observed in genetically-engineered mice that spontaneously develop PDAC, and accelerates tumor development and metastasis [85]. Co-expression of PDGF and SRC is also associated with increased pro-tumorigenic WNT/β-catenin signaling, elevated serum PDGF, and a poorer survival in human PDAC patients [85]. In another study, treatment of mice with the dual PDGFR and SRC tyrosine kinase inhibitor GN963 significantly reduced the growth of orthotopic L3.6pl tumors, and synergized with gemcitabine to abrogate liver metastasis [86].
Cancer cell stemness
Increased SRC activity is observed in pancreatic cancer stem cells [87], and is linked to ligand/receptor signaling pathways commonly implicated in tumor progression [88–90]. Accordingly, therapeutic inhibition of SRC reduces pancreatic cancer stem cell abundance, as well as their colony forming and self-renewing properties in vivo [87].
Hypoxia and metabolic reprograming
In response to hypoxia, pancreatic cancer cells adopt an invasive and treatment-resistant phenotype that enhances tumor growth and metastasis [91–94]. Hypoxia is also accompanied by an accumulation of immunosuppressive myeloid cells and cancer-associated fibroblasts, which promote the exhaustion and exclusion of cytotoxic effector cells [95–97].
SRC activity is increased in hypoxic regions of PDAC tumor-bearing mice, and can be therapeutically targeted using the small molecule SRC inhibitor AZD0530 [98]. Hypoxia-induced activation of SRC also stimulates downstream activation of FAK, NFκB, HIF1α, and STAT3 in cancer cells, which enhances survival, invasion, metastasis, and chemoresistance [99–102].
Tumor cells alter their metabolic needs in response to hypoxia to maintain their survival and proliferation [103]. This process, termed the Warburg effect, describes a process by which cancer cells preferentially use glycolysis for energy production [104]. The Warburg effect is advantageous as it provides rapidly proliferating tumor cells with metabolic intermediates to synthesize cellular components, improves the metastatic potential of cancer cells, and limits oxidative stress [105–108]. SRC has been shown to drive the Warburg effect and therapy resistance by inactivating pyruvate dehydrogenase (PDH), which regulates the metabolic fine-tuning between glycolysis and oxidative phosphorylation [109]. Conversely, treatment of cancer cells with the small molecule SRC inhibitors SU6656 and saracatinib increases PDH phosphorylation and the generation of reactive oxygen species [109]. Together, these findings demonstrate a key involvement of SRC via induction of molecular pathways involved in hypoxia and metabolic reprograming.
Cancer cell invasion and metastasis
SRC expression is most prominent at the invasive border of PDAC, and correlates with enhanced epithelial to mesenchymal transition (EMT) and metastasis [110]. Loss of E-cadherin is a hallmark of EMT [111], and is associated with increased tumor cell invasion and spread [112, 113]. SRC is inversely correlated with E-cadherin levels in human PDAC, while its inhibition restores E-cadherin expression and decreases cellular invasion [114, 115]. The SRC/FAK signaling axis also promotes TGFβ-induced delocalization of E-cadherin [116], while concurrent inhibition of SRC and FAK prevents E-cadherin endocytosis and strengthens E-cadherin-mediated cell adhesions [117].
Detachment of normal cells from the extracellular matrix leads to reduced proliferation and cell death [118]. However, PDAC cells can evade this process by activating signaling pathways that enable adhesion-independent survival. Increased SRC auto-phosphorylation is observed following detachment of pancreatic cancer cells from the extracellular substratum, and results in the activation of downstream signaling proteins (e.g., AKT, JNK) important for cell survival and proliferation [119]. These findings reveal an additional mechanism by which aberrant activation of SRC facilitates tumor invasion and metastasis by preventing the death of migrating cancer cells.
Another way in which aberrant SRC activation contributes to invasion and metastasis is by facilitating the formation of invadopodia. Invadopodia are cell protrusions that mediate actin contractility, membrane trafficking, and focal degradation, thereby enhancing cancer cell extravasation during metastasis [120]. SRC and FAK signaling correlates with the matrix-remodeling ability of invadopodia [121, 122] by regulating the production of MMPs [123–125].
Therapy resistance and modulating treatment response
Increased SRC expression positively correlates with chemoresistance in human pancreatic cancer cell lines [126], while oncogenic KRAS mutations induce a SRC-dependent amplification loop that promotes metastasis and therapy resistance in human PDAC tumors [127]. Conversely, therapeutic or siRNA-mediated SRC inhibition restores sensitivity to gemcitabine [126], 5-flourouracil [128], paclitaxel [129], and cetuximab [130] in preclinical PDAC models. Although the mechanisms underpinning a role for SRC in PDAC chemo-resistance are still unclear, these findings support the use of SRC inhibitors as a complementary strategy to improve response of pancreatic tumors to chemotherapy.
Effects of tumor cell-extrinsic SRC activity in pancreatic cancer
Aberrant activation of SRC in cells of the immediate tumor environment can also promote PDAC development and progression via several mechanisms, including inflammation, immune modulation, desmoplasia, angiogenesis, and lymph-angiogenesis (Fig. 3).
Inflammation and immune modulation
Chronic inflammation is a key component of PDAC, and is linked to tumor progression, metastasis, and chemoresistance [131]. In response to chronic inflammation, pancreatic acinar cells de-differentiate into a ductal-like phenotype in a process known as acinar-to-ductal metaplasia (ADM) [132]. ADM lesions may further develop into pancreatic intraepithelial neoplasia (PanIN) [132], which represent the dominant precursor to PDAC [133, 134].
Increased SRC expression is observed during the progression of chronic pancreatitis to PanIN and PDAC [135], while treatment of mice with the SRC kinase inhibitor PP2 significantly reduces the severity of caerulein-induced pancreatitis in mice and is associated with impaired activation of inflammatory signaling pathways (e.g., STAT3, ERK, NFκB) [136]. Mechanistically, aberrant activation of SRC is observed in circulating monocytes and tissue macrophages during chronic pancreatitis, as well as in tumor-associated macrophages and acinar cells [28, 137]. Accordingly, SRC regulates the production of IL6 by inflammatory macrophages [138], which is required for ADM and progression to PDAC [139]. SRC is also downstream of the SDF1/CXCR4 signaling axis [140], which facilitates mobilization of inflammatory leukocytes and bone marrow-derived mesenchymal cells during pancreatitis and tumor development [141, 142], and enables pancreatic cancer cell invasion and EMT [143, 144].
In addition to inflamed tissues, macrophages are also a major component of PDAC tumors and are associated with poor patient survival [96, 145]. SRC expression and activation is increased in PDAC-associated macrophages compared with resident macrophages in the normal pancreas, and correlates with tumor growth [137]. Activation of SRC in tumor-associated macrophages is induced by tumor-derived cytokines and chemokines (e.g., TNF, MIP, SDF-1), which amplify the production of inflammatory cytokines (e.g., TNF, IL1β, IL6) to reciprocally activate SRC in a feed-forward loop and promote PDAC progression [28, 146].
Surprisingly, the immune modulatory roles of SRC in PDAC remains understudied compared to other cancer types. In preclinical models of B16.OVA melanoma, 1956 sarcoma, MC38 colon, and 4T1 breast cancer, therapeutic inhibition of SRC was shown to enhance antitumor immunity by increasing the infiltration of T- and NK cells, and by reducing the abundance of regulatory T-cells (Tregs) [147]. Similar findings were observed in a mouse model of head and neck squamous cell carcinoma, where dasatinib dramatically reduced tumor growth by inhibiting the recruitment of myeloid-derived suppressor cells [148].
Desmoplasia
Cancer-associated fibroblasts play a major role in the desmoplastic reaction of PDAC via extracellular matrix deposition and remodeling, production of growth factors, as well as reciprocal signaling interactions with cancer and immune cells to promote an immune-suppressive tumor microenvironment [149]. Conversion of normal fibroblasts into cancer-associated fibroblasts is modulated by YAP1-mediated activation of SRC, which stimulates cytoskeletal protein activation and actomyosin contractility [150].
Crosstalk between cancer-associated fibroblasts and the extracellular matrix reinforces the stiffness of the tumor stroma [151]. For example, binding of membrane-bound integrin receptors to extracellular matrix proteins triggers downstream activation of FAK and SRC to induce cytoskeletal remodeling and reinforce cellular stiffness [152]. Reciprocally, increased extracellular matrix stiffness activates the SRC/YAP/MYL9/MYL2 axis in cancer-associated fibroblasts to maintain their tumorigenic phenotype [153]. In contrast, concurrent inhibition of SRCand EGFR increases microvessel density and prevents fibrosis in orthotopic and genetically-engineered PDAC mouse models [154].
Angiogenesis and lymph-angiogenesis
Angiogenesis plays a critical role in PDAC by providing oxygen and nutrients to cancer cells, facilitating tumor cell migration, and promoting the secretion of cytokines by endothelial cells to stimulate tumor growth [155]. VEGF is a key angiogenic molecule that is most frequently upregulated in tumor and immune cells [156], and several studies have demonstrated a requirement of SRC in VEGF-mediated angiogenesis by preventing endothelial cell apoptosis and influencing the stability of sprouting blood vessels [157–160].
Upregulated expression of IL8 is observed in PDAC, and enables tumor growth and metastasis by enhancing angiogenesis via paracrine interactions with endothelial cells [161, 162]. Of note, SRC activity correlates with IL8 production in human L3.6pl and PANC-1 pancreatic cancer cell lines, while pharmacologic inhibition of endogenous SRC or siRNA-mediated knock-down of SRC significantly reduces IL8 production and angiogenesis [56].
SRC also contributes to lymph-angiogenesis, which promotes metastasis to regional lymph nodes [163, 164]. The VEGF-C/VEGFR-3 signaling axis directly activates lymphatic endothelial cells and enhances the secretion of cytokines and growth factors that promote lymph vessel formation [165–168]. SRC promotes IL6-induced VEGF-C expression in lymphatic endothelial cells [169], and VEGF-C stimulation of lymphatic endothelial cells upregulates SRC activity [170]. This functional cooperation suggests paracrine interactions between SRC and VEGF-C signaling in the tumor microenvironment, since therapeutic inhibition of SRC suppresses VEGF-C expression in pancreatic cancer cells, and impairs the proliferation and sprouting of lymphatic endothelial cells [170].
Targeting SRC in pancreatic cancer
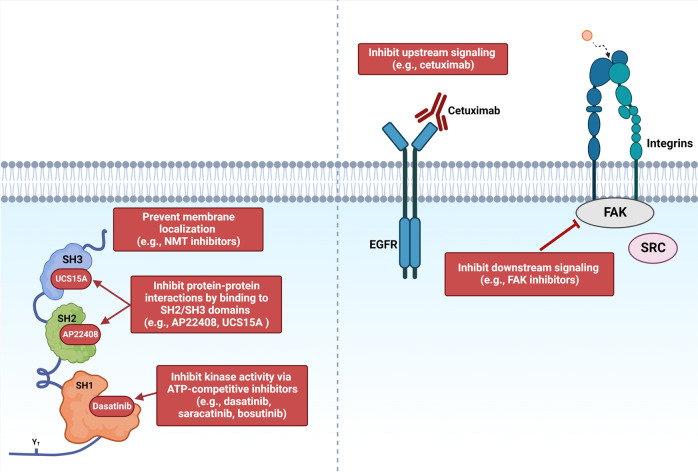
Strategies to inhibit SRC include suppressing its catalytic activity, inhibiting protein stability, interfering with signaling components of the SRC signaling pathway, or by reducing its protein-protein interactions (Fig. 4).
Fig. 4. Therapeutic strategies to inhibit SRC signaling.
Therapeutic approaches to inhibit SRC include (1) interfering with protein stability/membrane localization, (2) reducing protein-protein interactions, (3) inhibiting catalytic kinase activity, or (4) by interfering with upstream/downstream signaling components. Figure created in Biorender.
Inhibiting SRC catalytic activity
Small molecule ATP-competitive SRC inhibitors that target the catalytic activity of SRC include dasatinib (Sprycel, BMS354825, Bristol-Myers Squibb), bosutinib (SKI-606, Wyeth), and saracatinib (AZD0530, AstraZeneca) [171]. The antitumorigenic activity of dasatinib has been extensively studied in preclinical models; however, its efficacy in Phase I/II clinical trials has been disappointing (Table 1). One major issue is the lack of biomarkers to identify patients who are most likely to respond to therapy. This is crucial, since the therapeutic efficacy of SRC inhibitors is influenced by the level of SRC activity in tumors [135]. In one trial, dasatinib failed to improve the overall survival of patients with metastatic pancreatic cancer; however, a durable response was observed in a few patients following brief exposure to therapy (NCT00474812) [172]. These findings provide compelling rationale for studying the biology of “exceptional responders” to identify biomarkers or genetic vulnerabilities that could be exploited to improve treatment response [173]. Currently, small molecule SRC inhibitors are not FDA-approved for the treatment of solid tumors.
Table 1.
Summary of selected clinical trials using SRC inhibitors in pancreatic cancer.
| Drug | Selection criteria | Phase | Combination partners | Side effects | Status | Outcome | References |
|---|---|---|---|---|---|---|---|
| Dasatinib | Locally-advanced pancreatic cancer | II | Gemcitabine | Neutropenia, fatigue, thrombocytopenia, anemia, abdominal pain | Complete | No significant clinical activity | [226] |
| Advanced pancreatic cancer | I | Erlotinib + gemcitabine | Anemia, fatigue, nausea, lymphopenia, leukopenia, neutropenia, thrombocytopenia | Active, not recruiting |
Well tolerated. Stable disease as best response observed in 69% of patients |
[201] |
|
| Metastatic pancreatic cancer | II | Monotherapy | Terminated due to toxicity | Terminated due to toxicity | N/A | NCT00544908 | |
| II | Monotherapy | Fatigue, nausea, edema, pleural effusions | Completed, awaiting results | No significant clinical activity |
[172] |
||
| II | 5-FU and oxaliplatin | Nausea, fatigue, neutropenia, upper gastrointestinal hemorrhage, depression | Complete | Well tolerated. No improvement in efficacy over chemotherapy alone |
[227] |
||
| II | mFOLFOX6 | Results pending | Completed | Results pending | NCT01652976 | ||
| Resected pancreatic cancer (adjuvant) | II | Gemcitabine | Results pending | Completed | Results pending | NCT01234935 | |
| Bosutinib | Advanced solid cancers (including pancreatic) | I | Monotherapy | Nausea, diarrhea, vomiting, fatigue, anorexia | Completed | Well tolerated. Complete response and stable disease observed |
[228] |
| Resected pancreatic cancer | I | Gemcitabine | N/A | Terminated due to slow accrual | N/A | NCT01025570 | |
| Locally advanced/metastatic solid cancers (incl pancreatic) | I/II | Capecitabine | Diarrhea, nausea, vomiting | Terminated (reason unknown) | Limited efficacy |
[229] |
|
| Saracatinib | Advanced pancreatic cancer | I/II | Gemcitabine | Anorexia, diarrhea, anemia, fatigue, | Completed | Well tolerated. No improvement in efficacy over chemotherapy alone |
[230] |
| Recurrent metastatic pancreatic cancer | II | Monotherapy | Results pending | Completed | Results pending | NCT00735917 |
Interfering with protein stability
Another approach to reduce SRC activation includes triggering protein instability or preventing maturation of the protein. The molecular chaperone Heat shock protein 90 (Hsp90) promotes the stability and function of oncoproteins including SRC [174]. Hsp90 inhibitors are divided into several classes based on their mechanism of action, including (1) inhibiting ATP binding, (2) disruption of co-chaperone/Hsp90 interactions, (3) preventing post-translational modifications of Hsp90, and (4) interfering with client/Hsp90 associations [174]. The latter class of Hsp90 inhibitors promote the degradation of client proteins [175], and have shown promising results in Phase I clinical trials [174–176]. Given that Hsp90 is required for the maturation of SRC [177], Hsp90 inhibitors may represent an effective strategy for the treatment of PDAC. Indeed, Hsp90 inhibitors ICPD47 and ICPD62 have been shown to synergize with chemotherapy and reduce the growth of pancreatic cancer cell lines in vitro [178]. Furthermore, treatment of mice with the Hsp90 inhibitor XL888 in combination with anti-PD1 impaired the growth of subcutaneous Panc02 and orthotopic KPC tumors [179]. Further analysis revealed that tumors of mice treated with both XL888 and anti-PD1 showed increased T-cell infiltration and an enrichment of genes associated with immune activation [179]. In separate studies, inhibition of Hsp90 also sensitized treatment-refractory PDAC xenografts to chemotherapy and radiotherapy [180]. A Phase I/II trial of XL888 in combination with anti-PD1 is currently undergoing clinical evaluation in patients with advanced pancreatic cancer (NCT3095781).
N-myristoyltransferases (NMTs) are enzymes that regulate the function of oncogenic proteins by catalyzing myristoylation [181]. Protein N-myristoylation of SRC anchors it to the cell membrane, and helps maintain its structure and kinase activity [181, 182]. Interestingly, NMT levels correlate with SRC activation in human tumors, and is associated with a poor prognosis [183]. Meanwhile, inhibition of NMT1 suppresses SRC-induced oncogenic signaling and significantly reduces the growth of tumor xenografts with limited toxicity in vivo [183]. Loss of myristoylation also suppresses downstream SRC signaling pathways, including FAK and MAPK [184].
Interfering with upstream and/or downstream molecules of the SRC signaling pathway
Given the complexity of the SRC signaling network, therapeutic agents aimed at interfering with upstream and/or downstream SRC signaling components (e.g., integrins, EGFR, FAK, PI3K) represent another promising approach for the treatment of PDAC (Table 2).
Table 2.
Summary of selected clinical trials targeting upstream and/or downstream activators of SRC in pancreatic cancer.
| Target | Drug | Selection criteria | Phase | Combination partners | Side effects | Status | Outcome | References |
|---|---|---|---|---|---|---|---|---|
| Integrins | Cilengitide | Advanced pancreatic cancer | II | Gemcitabine | Nausea, dyspepsia, dyspnea, chills, fever | Completed | Well tolerated; no significant improvement in survival | EMD 121974 [190] |
| IMGN388 | Advanced solid cancers (including pancreatic cancer) | I | Monotherapy | Results pending | Completed | Results pending | NCT00721669 [231] | |
| Volociximab | Metastatic pancreatic cancer | II | Gemcitabine | Results pending | Completed | Results pending | NCT00401570 [232] | |
| EGFR | Erlotinib | Locally advanced pancreatic cancer | III | Gemcitabine | Anemia, neutropenia, diarrhea, rash | Completed | No significant improvement in overall survival | NCT00634725 [193] |
| Advanced pancreatic cancer | II | Gemcitabine | Neutropenia, lymphopenia, fatigue | Completed | Well tolerated; no significant improvement in survival | NCT00810719 [233] | ||
| Advanced pancreatic cancer | III | Gemcitabine | Nausea, vomiting, fatigue, anorexia | Completed | Increased overall and progression free survival in combination group | NCT00026338 [192] | ||
| Cetuximab | Advanced pancreatic cancer | III | Gemcitabine | Diarrhea, rash, fatigue, nausea, vomiting | Completed | No significant improvement in overall survival | NCT00075686 [234] | |
| Nimotuzumab | Advanced pancreatic cancer | II | Gemcitabine | Fatigue, rash | Completed | Well tolerated. Significantly improved progression free and overall survival | NCT00561990 [235] | |
| FAK | PF‐00562271 | Advanced solid cancers (including pancreatic cancer) | I | Monotherapy | Headache, nausea/vomiting, dehydration, edema | Completed | MTD determined | NCT00666926 [236] |
| VS‐4718 | Advanced pancreatic cancer | I | Gemcitabine/ Nab‐paclitaxel | N/A | Terminated | N/A | NCT02651727 | |
| Defactinib | Advanced solid cancers (including pancreatic cancer) | II | Pembrolizumab (anti‐PD1) | N/A | Recruiting | N/A | NCT02758587 | |
| Advanced solid cancers (including pancreatic cancer) | II | Pembrolizumab and Gemcitabine | Fatigue, nausea, myalgia, nausea/vomiting, anorexia, fever | Completed | Well tolerated. No partial or complete responses observed | NCT02546531 | ||
| GSK2256098 | Recurrent pancreatic cancer | II | Trametinib (MEK1/2 inhibitor) | N/A | Active, not recruiting | N/A | NCT02428270 | |
| PI3K/AKT | Oleandrin (PBI‐05204) | Metastatic pancreatic cancer | I | Monotherapy | N/A | Active, not recruiting | N/A | NCT02329717 |
| AZD5363 | Advanced/recurrent solid cancers (including pancreatic cancer) | II | Monotherapy | N/A | Recruiting | N/A | NCT02465060 | |
| Perifosine | Advanced pancreatic cancer | II | Monotherapy | Nausea, vomiting, fatigue | Completed | No objective response observed | NCT00059982 [237] | |
| Buparlisib | Advanced solid cancers (including pancreatic cancer) | I | mFOLFOX6 | Neutropenia, fatigue, leukopenia, hyperglycemia, thrombocytopenia | Completed | MTD determined | NCT01571024 [238] | |
| Advanced solid cancers (including pancreatic cancer) | I | Trametinib (MEK1/2 inhibitor) | Stomatitis, diarrhea, dysphagia, rash | Completed | Minimal activity observed in pancreatic cancer patients | NCT01155453 | ||
| MK2206 | Advanced pancreatic cancer | I/Ib | Dinaciclib (CDK inhibitor) | Results pending | Completed | Results pending | NCT01783171 | |
| Recurrent metastatic pancreatic cancer | II | Selumetinib (MEK1/2 inhibitor) | Nausea, vomiting, fatigue, anorexia | Completed | No improvement in overall survival. | NCT01658943 [239] | ||
| Afuresertib (GSK2110183) | Advanced solid cancers (including pancreatic cancer) | I/II | Trametinib (MEK1/2 inhibitor) | Diarrhea, dermatitis, rash, fatigue, nausea, vomiting | Completed | Poor tolerability: no patients enrolled in Phase II | NCT01476137 [240] | |
| Uprosertib (GSK2141795) | Advanced solid cancers (including pancreatic cancer) | I | Trametinib (MEK1/2 inhibitor) | Diarrhea, rash | Completed | Poor tolerability and minimal clinical activity | NCT01138085 [241] |
MTD Maximum tolerated dose.
Integrins
Integrins are transmembrane receptors that bind with proteins (e.g., vinculin, filamin) to regulate cytoskeleton stability, and phosphorylate kinases (e.g., SRC, FAK) to activate downstream signaling pathways. Activation of SRC by β1 integrin enhances the invasive capacity of pancreatic cancer cells [185], while activation of SRC by β3 integrin promotes anchorage-independent PDAC tumor growth and lymph node metastasis [186]. Integrins are also involved in stellate cell activation [187], and the production of tumor-promoting cytokines [188]. In orthotopic and genetically-engineered models of PDAC, coadministration of cilengitide (avβ3 and avβ5 integrin antagonist) and verapamil (calcium channel blocker) increased chemo-sensitivity to gemcitabine, and significantly reduced tumor growth compared with monotherapy-treated groups [189]. However, a Phase II trial combining cilengitide with gemcitabine in patients with advanced pancreatic cancer did not achieve clinical benefit (EMD 121974) [190] (Table 2).
EGFR
Reciprocal signaling between SRC and EGFR contributes to a more cancer-aggressive phenotype by enhancing tumor cell proliferation, invasion, and metastasis [71, 78]. Two main approaches used to target EGFR include monoclonal antibodies (e.g., cetuximab, nimotuzumab) directed against the extracellular domain, as well as small molecule inhibitors (e.g., erlotinib) that compete for the ATP binding site in the tyrosine kinase domain [191]. Erlotinib is the furthest in development for the treatment of PDAC, but has demonstrated mixed results in the clinic (Table 2). In a Phase III trial, erlotinib in combination with gemcitabine significantly improved progression-free and overall survival (NCT00026338) [192], but failed to produce clinical activity in other studies (NCT00634725 [193], NCT00026338 [192]).
FAK
The SRC/FAK signaling axis is implicated in PDAC by increasing tumor cell proliferation, EMT, and metastasis [68, 69, 116, 117]. The FAK inhibitor SK2256098 attenuates the proliferation, motility, and survival of pancreatic cancer cells in vitro [194], while VS-4718 doubled the survival of tumor-bearing mice by restoring sensitivity to chemotherapy and immunotherapy [30, 195]. These changes were associated with reduced tumor fibrosis and decreased numbers of immunosuppressive cells [30]. Based on these encouraging findings, several trials combining FAK inhibitors with chemotherapy (e.g., gemcitabine, nab‐paclitaxel) or immunotherapy (e.g., anti-PD1) are currently underway (Table 2).
PI3K
SRC-mediated activation of PI3K [45, 46] results in downstream phosphorylation of AKT and enhances the growth and survival of pancreatic cancer cells [47]. Accordingly, treatment of mice with the pan-PI3K inhibitor LY294002 inhibited the growth of orthotopic PDAC tumors and decreased peritoneal and liver metastasis [196]. Several pan-PI3K inhibitors have been evaluated in Phase I/II clinical trials in patients with advanced PDAC (Table 2), but have shown poor tolerability and negligible clinical benefit.
Reducing protein-protein interactions
Small molecule non-peptide inhibitors (e.g., AP22408, AP22161, UCS15A) have shown efficacy at reducing SRC protein-protein interactions; however, their effectiveness in cancer remains to be evaluated. AP22408 and AP22161 selectively bind to the SH2 domain of SRC [197, 198], while UCS15A prevents SH3 domain protein-protein interactions [199, 200].
Rationale for combination therapy
Despite encouraging results in preclinical studies, SRC inhibitors have not produced significant clinical benefit in PDAC (Table 1). The development of innovative and rational drug combinations that incorporate SRC inhibition as an adjuvant therapy therefore represents a potential approach to improve patient outcomes with manageable side effects. However, additional studies are required to identify which combination partners are likely to produce the most clinical benefit.
Combining SRC inhibitors with chemotherapy
Aberrant SRC activation plays a role in mediating chemoresistance in PDAC, while therapeutic inhibition of SRC restores chemo-sensitivity of human pancreatic cancer cells [128–130]. Likewise, concomitant inhibition of SRC and EGFR in combination with gemcitabine overcomes STAT3-mediated chemoresistance and attenuates the growth of PDAC xenografts [79]. Encouraging clinical activity was observed in a Phase I trial of patients with metastatic or locally-advanced PDAC, where dual SRC and EGFR inhibition (dasatinib plus erlotinib) in combination with gemcitabine resulted in stable disease in 69% of patients (NCT01660971) [201]. However, other Phase I/II trials combining dasatinib, bosutinib, or saracatinib with gemcitabine have failed to produce significant clinical activity (Table 1).
Combining SRC inhibitors with targeted therapies
Given the synergism between multiple signaling pathways in PDAC, combining SRC inhibitors with additional targeted therapies represents another promising approach to induce robust antitumor responses. For example, inhibition of the SRC/EGFR axis in combination with gemcitabine dramatically reduced cancer cell proliferation, survival, and the growth of orthotopic tumors [79, 154, 201]. This triple combination was also shown to overcome STAT3-mediated chemoresistance and attenuate the growth of PDAC xenografts [79]. Likewise, SKLB261 (multikinase inhibitor of EGFR, SRC, and VEGFR2) potently suppressed the proliferation and invasion of human PDAC cells, restored chemo-sensitivity, and extended the survival of tumor-bearing mice [202]. Dual targeting of SRC and SHP2 (required for full activation of the RAS/ERK pathway) potently inhibited the growth of orthotopic PDAC tumors [203], while concurrent inhibition of STAT3, SRC, and EGFR increased gemcitabine chemo-sensitivity and significantly reduced the growth of PDAC xenografts [204]. Thus, multitargeted therapies have the potential to be more effective at inducing robust antitumor effects in PDAC than blockade of individual pathways alone.
Combining SRC inhibitors with immunotherapy
The poor response of PDAC tumors to immunotherapy is largely attributed to a desmoplastic tumor microenvironment that is densely populated by cancer-associated fibroblasts and immunosuppressive myeloid cells, which promote the exhaustion and exclusion of cytotoxic effector cells [8]. Conversely, therapeutic inhibition of SRC reduces the growth of various solid tumors and hematological malignancies by enhancing the activation, proliferation, and recruitment of cytotoxic CD8 T cells and NK cells, suppressing the recruitment of myeloid-derived suppressor cells and Tregs, and by inhibiting the tumorigenic phenotype of cancer-associated fibroblasts [147, 148, 150, 151]. Thus, SRC inhibition may represent a promising adjunct to immunotherapy; however, the therapeutic benefit of combining SRC inhibitors with immune checkpoint blockade has not been extensively studied in the context of PDAC.
Clinical challenges and therapeutic perspectives
Contributing factors that underpin the poor response to SRC inhibitors in the clinic include the highly aggressive nature of PDAC, rapid development of drug resistance, and lack of patient stratification to identify those who are most likely to benefit from treatment. To maximize the therapeutic benefit from incorporating SRC inhibition into existing cancer treatments, several challenges need to be addressed. One major issue is the lack of effective biomarkers to identify patients who are most likely to benefit from SRC inhibitors. This is crucial, since the therapeutic efficacy of SRC inhibitors is influenced by the extent of SRC activity in tumors [135, 205–207], as well as mutations in other signaling proteins (e.g., c-MET and STAT3) [135].
Maximizing clinical translation
To date, all completed clinical trials of SRC inhibitors in pancreatic cancer have been performed in unselected patients that failed standard-of-care therapies. Genomic analyses have identified the existence of genetically-distinct PDAC subtypes, including: (1) squamous, (2) pancreatic progenitor, (3) immunogenic, and (4) aberrantly differentiated endocrine exocrine [42]. In one study, synergism between SRC inhibitors (e.g., dasatinib, PP2) and a MEK1/2 inhibitor (pimasertinib) enhanced sensitivity to gemcitabine in the squamous subtype of pancreatic cancer cells (e.g., SW1990 and BxP3) and not in PDAC progenitor cells (e.g., AsPC1) [208]. Likewise, clinical stage correlates with increased expression of phosphorylated SRC, and higher baseline levels of phosphorylated SRC is associated with improved progression-free survival following dasatinib therapy [135, 205–207]. Thus, assessment of SRC activation based on phosphorylation status or gene expression analysis may serve as a biomarker to stratify and identify patients who are most likely to benefit from therapy.
Although SRC inhibitors have failed to produce clinical benefit in most PDAC patients, durable and sustained responses are observed in a small subset of patients following brief exposure to therapy (NCT00474812) [172]. Further investigations into the biology of these exceptional responders are warranted to identify biomarkers and/or molecular characteristics that could be exploited to maximize therapeutic response. Specifically, patient-derived tumor organoids or xenografts may help reveal key mechanistic insights to guide the design of clinical trials [173].
Given the unequivocal role of SRC in promoting tumor cell invasion and migration, there is also a need to evaluate the efficacy of SRC inhibitors in early-stage disease or in the adjuvant setting after tumor resection. However, since most PDAC patients are diagnosed with metastasis, recruitment of patients at early stages of the disease remains a significant challenge.
Since the redundancy of cellular pathways may limit the efficacy of inhibiting SRC alone, multitargeted therapies that also block EGFR or STAT3 represent an effective strategy to boost antitumor responses. In line with these findings, encouraging clinical activity was observed in a Phase I trial of patients with metastatic or locally-advanced PDAC, where dual SRC and EGFR inhibition (dasatinib plus erlotinib) in combination with gemcitabine resulted in stable disease in 69% of patients (NCT01660971) [201]. In another study, STAT3 activation correlated with dasatinib resistance in pancreatic cancer cells [135], while dual inhibition of STAT3 and SRC resulted in significantly smaller PDAC tumors in mice compared to monotherapy-treated groups [204]. Likewise, MET amplification mediates resistance to SRC inhibitors in various solid malignancies, while concurrent inhibition of SRC and MET produces a synergistic cytotoxic effect on tumor growth [209–211]. Collectively, these findings provide compelling rationale for the design of innovative and rational combinatorial strategies to improve the clinical activity of SRC inhibitors.
Clinical considerations for direct vs. indirect inhibition of SRC
Although second-generation SRC inhibitors (e.g., Bosutinib, Dasatinib, Saracatinib) are designed to be more selective and potent compared to first-generation inhibitors (e.g., PP1, SU6656), these multikinase inhibitors still confer off-target effects and toxicity in normal cells and tissues. For example, dasatinib inhibits the proliferation and activation of primary human T cells [212], impairs humoral immunity by promoting B-cell apoptosis [213], and reduces the pro-inflammatory capacity of human neutrophils [214].
Inhibition of SRC may also negatively impact bone homeostasis, since SRC plays an essential role in regulating osteoclastic bone resorption and osteoblastic bone formation. Accordingly, Src deficiency results in osteopetrosis in mice [215–218], while dasatinib treatment increases bone mass by reducing bone resorption and stimulating bone anabolism [219]. Thus, the SRC SH2 inhibitors AP22161 and AP22408, which preferentially accumulate on the surface of bones exert potent inhibition of osteoclast-mediated bone resorption [197].
Other studies suggest that SRC inhibition may perturb platelet activation and aggregation, since Src-deficient platelets demonstrate reduced spreading on fibrinogen, and dasatinib treatment increases the tail bleeding time of mice in a dose-dependent manner [220–222]. Likewise, the SRC inhibitors PP2, SU6656, and dasatinib potently inhibit the coagulation-promoting and clot-retracting activities of human platelets [221, 223]. These findings raise important clinical implications, since gastrointestinal, genitourinary, soft tissue hematoma, and central nervous system bleeding is observed in up to 40% of patients during dasatinib therapy [224]. This issue is further complicated by the observation that most patients with bleeding episodes in response to treatment with these drugs also exhibit low platelet counts and advanced-stage cancers that require higher doses of dasatinib for clinical benefit [225].
As an alternative to broad-spectrum SRC inhibitors, indirect strategies that interfere with upstream and/or downstream molecules within the SRC signaling cascade may represent safer and more selective approaches, since these molecules are often less ubiquitously expressed. Likewise, targeting downstream effectors may overcome resistance mechanisms that arise from compensatory activation of other SCR family kinases. Thus, additional studies comparing the advantages between indirect and direct SRC inhibition are warranted.
Concluding remarks
Given the diverse roles of SRC in PDAC and association with a poor prognosis, SRC represents a promising therapeutic target for pancreatic cancer. However, since direct inhibition of SRC still suffers from sufficient target specificity and may have deleterious consequences on cellular processes, alternative approaches aimed at interfering with upstream and/or downstream molecules of the SRC signaling pathway may represent a safer option. Despite encouraging results in preclinical studies, SRC inhibitors have also failed to produce clinical benefit in most PDAC patients. Contributing factors that underpin the poor response to therapy include the highly aggressive nature of PDAC, complexity of the SRC signaling network, rapid development of drug resistance, and lack of patient stratification to identify those who are most likely to benefit from treatment. Thus, additional studies are needed to better understand the diverse roles of SRC in PDAC biology, and to identify prognostic and predictive factors to help stratify patients and maximize therapeutic response.
Author contributions
ARP and ME wrote the manuscript and designed the figures. All authors have read and agreed to the published version of the manuscript.
Funding
ARP is a National Health and Medical Research Council of Australia (NHMRC) Peter Doherty Early Career Fellow (1166447), and is supported by an Avner Collaboration Grant from PanKind, The Australian Pancreatic Cancer Foundation in collaboration with Tour de Cure and Woolworths Limited through Woolies on Wheels and Walks. ME is supported by an Investigator grant from the NHMRC (1173814).
Data availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ashleigh R. Poh, Email: ashleigh.poh@onjcri.org.au
Matthias Ernst, Email: matthias.ernst@onjcri.org.au.
References
- 1.Kleeff J, Korc M, Apte M, La Vecchia C, Johnson CD, Biankin AV, et al. Pancreatic cancer. Nat Rev Dis Prim. 2016;2:16022. doi: 10.1038/nrdp.2016.22. [DOI] [PubMed] [Google Scholar]
- 2.Hidalgo M, Cascinu S, Kleeff J, Labianca R, Löhr JM, Neoptolemos J, et al. Addressing the challenges of pancreatic cancer: Future directions for improving outcomes. Pancreatology. 2015;15:8–18. doi: 10.1016/j.pan.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Werner J, Combs SE, Springfeld C, Hartwig W, Hackert T, Büchler MW. Advanced-stage pancreatic cancer: therapy options. Nat Rev Clin Oncol. 2013;10:323–33. doi: 10.1038/nrclinonc.2013.66. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl J Med. 2011;364:1817–25. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, et al. Increased Survival in Pancreatic Cancer with nab-Paclitaxel plus Gemcitabine. N. Engl J Med. 2013;369:1691–703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diamond MS, Lin JH, Vonderheide RH. Site-dependent immune escape due to impaired dendritic cell cross-priming. Cancer Immunol Res. 2021;9:877–90. doi: 10.1158/2326-6066.CIR-20-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218. doi: 10.1038/s41573-018-0007-y. [DOI] [PubMed] [Google Scholar]
- 8.Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer — clinical challenges and opportunities. Nat Rev Clin Oncol. 2020;17:527–40. doi: 10.1038/s41571-020-0363-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rous P. A sarcoma of the fowl transmissible by an agent separable from the tumor cells. J Exp Med. 1911;13:397–411. doi: 10.1084/jem.13.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stehelin D, Fujita DJ, Padgett T, Varmus HE, Bishop JM. Detection and enumeration of transformation-defective strains of avian sarcoma virus with molecular hybridization. Virology. 1977;76:675–84. doi: 10.1016/0042-6822(77)90250-1. [DOI] [PubMed] [Google Scholar]
- 11.Stehelin D, Varmus HE, Bishop JM, Vogt PK. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976;260:170–3. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- 12.Oppermann H, Levinson AD, Varmus HE, Levintow L, Bishop JM. Uninfected vertebrate cells contain a protein that is closely related to the product of the avian sarcoma virus transforming gene (src) Proc Natl Acad Sci USA. 1979;76:1804–8. doi: 10.1073/pnas.76.4.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sen B, Johnson FM. Regulation of SRC family kinases in human cancers. J Signal Transduct. 2011;2011:865819. doi: 10.1155/2011/865819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roskoski R., Jr Src protein-tyrosine kinase structure and regulation. Biochem Biophys Res Commun. 2004;324:1155–64. doi: 10.1016/j.bbrc.2004.09.171. [DOI] [PubMed] [Google Scholar]
- 15.Kurochkina N, Guha U. SH3 domains: modules of protein-protein interactions. Biophys Rev. 2013;5:29–39. doi: 10.1007/s12551-012-0081-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheeler DL, Iida M, Dunn EF. The role of Src in solid tumors. Oncologist. 2009;14:667–78. doi: 10.1634/theoncologist.2009-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cohen GB, Ren R, Baltimore D. Modular binding domains in signal transduction proteins. Cell. 1995;80:237–48. doi: 10.1016/0092-8674(95)90406-9. [DOI] [PubMed] [Google Scholar]
- 18.Jaber Chehayeb R, Boggon TJ. SH2 domain binding: diverse FLVRs of partnership. Front Endocrinol (Lausanne) 2020;11:575220. doi: 10.3389/fendo.2020.575220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ortiz MA, Mikhailova T, Li X, Porter BA, Bah A, Kotula L. Src family kinases, adaptor proteins and the actin cytoskeleton in epithelial-to-mesenchymal transition. Cell Commun Signal. 2021;19:67. doi: 10.1186/s12964-021-00750-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roskoski R., Jr Src protein-tyrosine kinase structure, mechanism, and small molecule inhibitors. Pharm Res. 2015;94:9–25. doi: 10.1016/j.phrs.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Piwnica-Worms H, Saunders KB, Roberts TM, Smith AE, Cheng SH. Tyrosine phosphorylation regulates the biochemical and biological properties of pp60c-src. Cell. 1987;49:75–82. doi: 10.1016/0092-8674(87)90757-4. [DOI] [PubMed] [Google Scholar]
- 22.Yeatman TJ. A renaissance for SRC. Nat Rev Cancer. 2004;4:470–80. doi: 10.1038/nrc1366. [DOI] [PubMed] [Google Scholar]
- 23.Parkin A, Man J, Timpson P, Pajic M. Targeting the complexity of Src signalling in the tumour microenvironment of pancreatic cancer: from mechanism to therapy. Febs J. 2019;286:3510–39. doi: 10.1111/febs.15011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Disco. 2012;2:401–4. doi: 10.1158/2159-8290.CD-12-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lutz MP, Esser IB, Flossmann-Kast BB, Vogelmann R, Lührs H, Friess H, et al. Overexpression and activation of the tyrosine kinase Src in human pancreatic carcinoma. Biochem Biophys Res Commun. 1998;243:503–8. doi: 10.1006/bbrc.1997.8043. [DOI] [PubMed] [Google Scholar]
- 27.Morton JP, Karim SA, Graham K, Timpson P, Jamieson N, Athineos D, et al. Dasatinib inhibits the development of metastases in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology. 2010;139:292–303. doi: 10.1053/j.gastro.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 28.Coppola D. Molecular prognostic markers in pancreatic cancer. Cancer Control. 2000;7:421–7. doi: 10.1177/107327480000700504. [DOI] [PubMed] [Google Scholar]
- 29.Ishizawar R, Parsons SJ. c-Src and cooperating partners in human cancer. Cancer Cell. 2004;6:209–14. doi: 10.1016/j.ccr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Jiang H, Hegde S, Knolhoff BL, Zhu Y, Herndon JM, Meyer MA, et al. Targeting focal adhesion kinase renders pancreatic cancers responsive to checkpoint immunotherapy. Nat Med. 2016;22:851–60. doi: 10.1038/nm.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer. 2014;14:598–610. doi: 10.1038/nrc3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsia DA, Mitra SK, Hauck CR, Streblow DN, Nelson JA, Ilic D, et al. Differential regulation of cell motility and invasion by FAK. J Cell Biol. 2003;160:753–67. doi: 10.1083/jcb.200212114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serrels A, Lund T, Serrels B, Byron A, McPherson RC, von Kriegsheim A, et al. Nuclear FAK controls chemokine transcription, Tregs, and evasion of anti-tumor immunity. Cell. 2015;163:160–73. doi: 10.1016/j.cell.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–6. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walter AO, Peng Z-Y, Cartwright CA. The Shp-2 tyrosine phosphatase activates the Src tyrosine kinase by a non-enzymatic mechanism. Oncogene. 1999;18:1911–20. doi: 10.1038/sj.onc.1202513. [DOI] [PubMed] [Google Scholar]
- 36.Schneeberger VE, Luetteke N, Ren Y, Berns H, Chen L, Foroutan P, et al. SHP2E76K mutant promotes lung tumorigenesis in transgenic mice. Carcinogenesis. 2014;35:1717–25. doi: 10.1093/carcin/bgu025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang J, Zhang F, Niu R. Functions of Shp2 in cancer. J Cell Mol Med. 2015;19:2075–83. doi: 10.1111/jcmm.12618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Masaki T, Okada M, Tokuda M, Shiratori Y, Hatase O, Shirai M, et al. Reduced C-terminal Src kinase (Csk) activities in hepatocellular carcinoma. Hepatology. 1999;29:379–84. doi: 10.1002/hep.510290239. [DOI] [PubMed] [Google Scholar]
- 39.Kim M, Tezuka T, Tanaka K, Yamamoto T. Cbl-c suppresses v-Src-induced transformation through ubiquitin-dependent protein degradation. Oncogene. 2004;23:1645–55. doi: 10.1038/sj.onc.1207298. [DOI] [PubMed] [Google Scholar]
- 40.Yokouchi M, Kondo T, Sanjay A, Houghton A, Yoshimura A, Komiya S, et al. Src-catalyzed phosphorylation of c-Cbl leads to the interdependent ubiquitination of both proteins. J Biol Chem. 2001;276:35185–93. doi: 10.1074/jbc.M102219200. [DOI] [PubMed] [Google Scholar]
- 41.Waddell N, Pajic M, Patch AM, Chang DK, Kassahn KS, Bailey P, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bailey P, Chang DK, Nones K, Johns AL, Patch AM, Gingras MC, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016;531:47–52. doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 43.Bunda S, Heir P, Srikumar T, Cook JD, Burrell K, Kano Y, et al. Src promotes GTPase activity of Ras via tyrosine 32 phosphorylation. Proc Natl Acad Sci. 2014;111:E3785–E3794.. doi: 10.1073/pnas.1406559111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shields DJ, Murphy EA, Desgrosellier JS, Mielgo A, Lau SK, Barnes LA, et al. Oncogenic Ras/Src cooperativity in pancreatic neoplasia. Oncogene. 2011;30:2123–34. doi: 10.1038/onc.2010.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cuevas BD, Lu Y, Mao M, Zhang J, LaPushin R, Siminovitch K, et al. Tyrosine phosphorylation of p85 relieves its inhibitory activity on phosphatidylinositol 3-kinase. J Biol Chem. 2001;276:27455–61. doi: 10.1074/jbc.M100556200. [DOI] [PubMed] [Google Scholar]
- 46.Lu Y, Yu Q, Liu JH, Zhang J, Wang H, Koul D, et al. Src family protein-tyrosine kinases alter the function of PTEN to regulate phosphatidylinositol 3-kinase/AKT cascades. J Biol Chem. 2003;278:40057–66. doi: 10.1074/jbc.M303621200. [DOI] [PubMed] [Google Scholar]
- 47.Murthy D, Attri KS, Singh PK. Phosphoinositide 3-kinase signaling pathway in pancreatic Ductal adenocarcinoma progression, pathogenesis, and therapeutics. Front Physiol (Rev) 2018;9:335. doi: 10.3389/fphys.2018.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcia R, Bowman TL, Niu G, Yu H, Minton S, Muro-Cacho CA, et al. Constitutive activation of Stat3 by the Src and JAK tyrosine kinases participates in growth regulation of human breast carcinoma cells. Oncogene. 2001;20:2499–513. doi: 10.1038/sj.onc.1204349. [DOI] [PubMed] [Google Scholar]
- 49.Byers LA, Sen B, Saigal B, Diao L, Wang J, Nanjundan M, et al. Reciprocal regulation of c-Src and STAT3 in non-small cell lung cancer. Clin Cancer Res. 2009;15:6852–61. doi: 10.1158/1078-0432.CCR-09-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trevino JG, Gray MJ, Nawrocki ST, Summy JM, Lesslie DP, Evans DB, et al. Src activation of Stat3 is an independent requirement from NF-kappaB activation for constitutive IL-8 expression in human pancreatic adenocarcinoma cells. Angiogenesis. 2006;9:101–10. doi: 10.1007/s10456-006-9038-9. [DOI] [PubMed] [Google Scholar]
- 51.Yu CL, Meyer DJ, Campbell GS, Larner AC, Carter-Su C, Schwartz J, et al. Enhanced DNA-binding activity of a Stat3-related protein in cells transformed by the Src oncoprotein. Science. 1995;269:81–83. doi: 10.1126/science.7541555. [DOI] [PubMed] [Google Scholar]
- 52.Cao X, Tay A, Guy GR, Tan YH. Activation and association of Stat3 with Src in v-Src-transformed cell lines. Mol Cell Biol. 1996;16:1595–603. doi: 10.1128/MCB.16.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bromberg J, Darnell JE., Jr The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468–73. doi: 10.1038/sj.onc.1203476. [DOI] [PubMed] [Google Scholar]
- 54.Turkson J, Bowman T, Garcia R, Caldenhoven E, De Groot RP, Jove R. Stat3 activation by Src induces specific gene regulation and is required for cell transformation. Mol Cell Biol. 1998;18:2545–52. doi: 10.1128/MCB.18.5.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Odajima J, Matsumura I, Sonoyama J, Daino H, Kawasaki A, Tanaka H, et al. Full oncogenic activities of v-Src are mediated by multiple signaling pathways. Ras as an essential mediator for cell survival. J Biol Chem. 2000;275:24096–105. doi: 10.1074/jbc.M001606200. [DOI] [PubMed] [Google Scholar]
- 56.Trevino JG, Summy JM, Gray MJ, Nilsson MB, Lesslie DP, Baker CH, et al. Expression and activity of SRC regulate interleukin-8 expression in pancreatic adenocarcinoma cells: implications for angiogenesis. Cancer Res. 2005;65:7214–22. doi: 10.1158/0008-5472.CAN-04-3858. [DOI] [PubMed] [Google Scholar]
- 57.Liu X, Guo X, Li H, Chen J, Qi X. Src/STAT3 signaling pathways are involved in KAI1-induced downregulation of VEGF-C expression in pancreatic cancer. Mol Med Rep. 2016;13:4774–8. doi: 10.3892/mmr.2016.5093. [DOI] [PubMed] [Google Scholar]
- 58.Wei D, Le X, Zheng L, Wang L, Frey JA, Gao AC, et al. Stat3 activation regulates the expression of vascular endothelial growth factor and human pancreatic cancer angiogenesis and metastasis. Oncogene. 2003;22:319–29. doi: 10.1038/sj.onc.1206122. [DOI] [PubMed] [Google Scholar]
- 59.Hayashi H, Uemura N, Zhao L, Matsumura K, Sato H, Shiraishi Y, et al. Biological significance of YAP/TAZ in pancreatic ductal adenocarcinoma. Front Oncol. 2021;11:700315. doi: 10.3389/fonc.2021.700315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murakami S, Shahbazian D, Surana R, Zhang W, Chen H, Graham GT, et al. Yes-associated protein mediates immune reprogramming in pancreatic ductal adenocarcinoma. Oncogene. 2017;36:1232–44. doi: 10.1038/onc.2016.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sinnet-Smith J, Anwar T, Reed EF, Teper Y, Eibl G, Rozengurt E. Opposite effects of Src family kinases on YAP and ERK activation in pancreatic cancer cells: implications for targeted therapy. Mol Cancer Ther. 2022;21:1652–62. doi: 10.1158/1535-7163.MCT-21-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Si Y, Ji X, Cao X, Dai X, Xu L, Zhao H, et al. Src inhibits the hippo tumor suppressor pathway through tyrosine phosphorylation of Lats1. Cancer Res. 2017;77:4868–80. doi: 10.1158/0008-5472.CAN-17-0391. [DOI] [PubMed] [Google Scholar]
- 63.Lamar JM, Xiao Y, Norton E, Jiang ZG, Gerhard GM, Kooner S, et al. SRC tyrosine kinase activates the YAP/TAZ axis and thereby drives tumor growth and metastasis. J Biol Chem. 2019;294:2302–17. doi: 10.1074/jbc.RA118.004364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosenbluh J, Nijhawan D, Cox AG, Li X, Neal JT, Schafer EJ, et al. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis. Cell. 2012;151:1457–73. doi: 10.1016/j.cell.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaghdoudi S, Decaup E, Belhabib I, Samain R, Cassant-Sourdy S, Rochotte J, et al. FAK activity in cancer-associated fibroblasts is a prognostic marker and a druggable key metastatic player in pancreatic cancer. EMBO Mol Med. 2020;12:e12010. doi: 10.15252/emmm.202012010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee BY, Timpson P, Horvath LG, Daly RJ. FAK signaling in human cancer as a target for therapeutics. Pharm Ther. 2015;146:132–49. doi: 10.1016/j.pharmthera.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 67.Murphy KJ, Zhu J, Trpceski M, Pereira BA, Timpson P, Herrmann D. Focal adhesion kinase priming in pancreatic cancer, altering biomechanics to improve chemotherapy. Biochem Soc Trans. 2022;50:1129–41. doi: 10.1042/BST20220162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parsons JT. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116:1409–16. doi: 10.1242/jcs.00373. [DOI] [PubMed] [Google Scholar]
- 69.Schlaepfer DD, Mitra SK. Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev. 2004;14:92–101. doi: 10.1016/j.gde.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 70.Schlaepfer DD, Broome MA, Hunter T. Fibronectin-stimulated signaling from a focal adhesion kinase-c-Src complex: involvement of the Grb2, p130cas, and Nck adaptor proteins. Mol Cell Biol. 1997;17:1702–13. doi: 10.1128/MCB.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Maa MC, Leu TH, McCarley DJ, Schatzman RC, Parsons SJ. Potentiation of epidermal growth factor receptor-mediated oncogenesis by c-Src: implications for the etiology of multiple human cancers. Proc Natl Acad Sci USA. 1995;92:6981–5. doi: 10.1073/pnas.92.15.6981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem. 1999;274:8335–43. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- 73.Stover DR, Becker M, Liebetanz J, Lydon NB. Src phosphorylation of the epidermal growth factor receptor at novel sites mediates receptor interaction with Src and P85 alpha. J Biol Chem. 1995;270:15591–7. doi: 10.1074/jbc.270.26.15591. [DOI] [PubMed] [Google Scholar]
- 74.Tice DA, Biscardi JS, Nickles AL, Parsons SJ. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc Natl Acad Sci USA. 1999;96:1415–20. doi: 10.1073/pnas.96.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guerrero J, Santibañez JF, González A, Martínez J. EGF receptor transactivation by urokinase receptor stimulus through a mechanism involving Src and matrix metalloproteinases. Exp Cell Res. 2004;292:201–8. doi: 10.1016/j.yexcr.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 76.Thien CBF, Langdon WY. Cbl: many adaptations to regulate protein tyrosine kinases. Nat Rev Mol Cell Biol. 2001;2:294–307. doi: 10.1038/35067100. [DOI] [PubMed] [Google Scholar]
- 77.Bao J, Gur G, Yarden Y. Src promotes destruction of c-Cbl: Implications for oncogenic synergy between Src and growth factor receptors. Proc Natl Acad Sci. 2003;100:2438–43. doi: 10.1073/pnas.0437945100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Biscardi JS, Belsches AP, Parsons SJ. Characterization of human epidermal growth factor receptor and c-Src interactions in human breast tumor cells. Mol Carcinog. 1998;21:261–72. doi: 10.1002/(SICI)1098-2744(199804)21:4<261::AID-MC5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 79.Nagaraj NS, Washington MK, Merchant NB. Combined blockade of Src kinase and epidermal growth factor receptor with gemcitabine overcomes STAT3-mediated resistance of inhibition of pancreatic tumor growth. Clin Cancer Res: Off J Am Assoc Cancer Res. 2011;17:483–93. doi: 10.1158/1078-0432.CCR-10-1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.DeMali KA, Godwin SL, Soltoff SP, Kazlauskas A. Multiple Roles for Src in a PDGF-Stimulated Cell. Exp Cell Res. 1999;253:271–9. doi: 10.1006/excr.1999.4669. [DOI] [PubMed] [Google Scholar]
- 81.Thomas SM, Brugge JS. Cellular functions regulated by SRC family kinases. Annu Rev Cell Developmental Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 82.Kmiecik TE, Johnson PJ, Shalloway D. Regulation by the autophosphorylation site in overexpressed pp60c-src. Mol Cell Biol. 1988;8:4541–6. doi: 10.1128/mcb.8.10.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Roskoski R., Jr Src kinase regulation by phosphorylation and dephosphorylation. Biochem Biophys Res Commun. 2005;331:1–14. doi: 10.1016/j.bbrc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 84.Hansen K, Johnell M, Siegbahn A, Rorsman C, Engström U, Wernstedt C, et al. Mutation of a Src phosphorylation site in the PDGF beta-receptor leads to increased PDGF-stimulated chemotaxis but decreased mitogenesis. Embo J. 1996;15:5299–313. doi: 10.1002/j.1460-2075.1996.tb00915.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kuo TL, Cheng KH, Shan YS, Chen LT, Hung WC. β-catenin-activated autocrine PDGF/Src signaling is a therapeutic target in pancreatic cancer. Theranostics. 2019;9:324–36. doi: 10.7150/thno.28201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baker CH, Trevino JG, Summy JM, Zhang F, Caron A, Nesbit M, et al. Inhibition of PDGFR phosphorylation and Src and Akt activity by GN963 leads to therapy of human pancreatic cancer growing orthotopically in nude mice. Int J Oncol. 2006;29:125–38. [PubMed] [Google Scholar]
- 87.Alcalá S, Mayoral-Varo V, Ruiz-Cañas L, López-Gil JC, Heeschen C, Martín-Pérez J, et al. Targeting SRC kinase signaling in pancreatic cancer stem cells. Int J Mol Sci. 2020;21:7437. doi: 10.3390/ijms21207437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jin W. Regulation of Src family kinases during colorectal cancer development and its clinical implications. Cancers (Basel) 2020;12:1339. doi: 10.3390/cancers12051339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang S, Yu D. Targeting Src family kinases in anti-cancer therapies: turning promise into triumph. Trends Pharm Sci. 2012;33:122–8. doi: 10.1016/j.tips.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rönnstrand L. Signal transduction via the stem cell factor receptor/c-Kit. Cell Mol Life Sci. 2004;61:2535–48. doi: 10.1007/s00018-004-4189-6. [DOI] [PubMed] [Google Scholar]
- 91.Erkan M, Kurtoglu M, Kleeff J. The role of hypoxia in pancreatic cancer: a potential therapeutic target? Expert Rev Gastroenterol Hepatol. 2016;10:301–16. doi: 10.1586/17474124.2016.1117386. [DOI] [PubMed] [Google Scholar]
- 92.Hapke RY, Haake SM. Hypoxia-induced epithelial to mesenchymal transition in cancer. Cancer Lett. 2020;487:10–20. doi: 10.1016/j.canlet.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chang Q, Jurisica I, Do T, Hedley DW. Hypoxia predicts aggressive growth and spontaneous metastasis formation from orthotopically grown primary xenografts of human pancreatic cancer. Cancer Res. 2011;71:3110–20. doi: 10.1158/0008-5472.CAN-10-4049. [DOI] [PubMed] [Google Scholar]
- 94.Büchler P, Reber HA, Lavey RS, Tomlinson J, Büchler MW, Friess H, et al. Tumor hypoxia correlates with metastatic tumor growth of pancreatic cancer in an orthotopic murine model. J Surg Res. 2004;120:295–303. doi: 10.1016/j.jss.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 95.Mello AM, Ngodup T, Lee Y, Donahue KL, Li J, Rao A, et al. Hypoxia promotes an inflammatory phenotype of fibroblasts in pancreatic cancer. Oncogenesis. 2022;11:56. doi: 10.1038/s41389-022-00434-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Poh AR, Ernst M. Tumor-associated macrophages in pancreatic ductal adenocarcinoma: therapeutic opportunities and clinical challenges. Cancers (Basel) 2021;13:2860. doi: 10.3390/cancers13122860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li N, Li Y, Li Z, Huang C, Yang Y, Lang M, et al. Hypoxia inducible factor 1 (HIF-1) recruits macrophage to activate pancreatic stellate cells in pancreatic ductal adenocarcinoma. Int J Mol Sci. 2016;17:799. doi: 10.3390/ijms17060799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pham NA, Magalhaes JM, Do T, Schwock J, Dhani N, Cao PJ, et al. Activation of Src and Src-associated signaling pathways in relation to hypoxia in human cancer xenograft models. Int J Cancer. 2009;124:280–6. doi: 10.1002/ijc.23912. [DOI] [PubMed] [Google Scholar]
- 99.Dai Y, Siemann D. c-Src is required for hypoxia-induced metastasis-associated functions in prostate cancer cells. Onco Targets Ther. 2019;12:3519–29. doi: 10.2147/OTT.S201320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lluis JM, Buricchi F, Chiarugi P, Morales A, Fernandez-Checa JC. Dual role of mitochondrial reactive oxygen species in hypoxia signaling: activation of nuclear factor-{kappa}B via c-SRC and oxidant-dependent cell death. Cancer Res. 2007;67:7368–77. doi: 10.1158/0008-5472.CAN-07-0515. [DOI] [PubMed] [Google Scholar]
- 101.Xu Q, Briggs J, Park S, Niu G, Kortylewski M, Zhang S, et al. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24:5552–60. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- 102.Guo Q, Lu L, Liao Y, Wang X, Zhang Y, Liu Y, et al. Influence of c-Src on hypoxic resistance to paclitaxel in human ovarian cancer cells and reversal of FV-429. Cell Death Dis. 2018;8:e3178–e3178. doi: 10.1038/cddis.2017.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hao X, Ren Y, Feng M, Wang Q, Wang Y. Metabolic reprogramming due to hypoxia in pancreatic cancer: Implications for tumor formation, immunity, and more. Biomedicine Pharmacother. 2021;141:111798. doi: 10.1016/j.biopha.2021.111798. [DOI] [PubMed] [Google Scholar]
- 104.Courtnay R, Ngo DC, Malik N, Ververis K, Tortorella SM, Karagiannis TC. Cancer metabolism and the Warburg effect: the role of HIF-1 and PI3K. Mol Biol Rep. 2015;42:841–51. doi: 10.1007/s11033-015-3858-x. [DOI] [PubMed] [Google Scholar]
- 105.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 106.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–33. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kamarajugadda S, Stemboroski L, Cai Q, Simpson NE, Nayak S, Tan M, et al. Glucose oxidation modulates anoikis and tumor metastasis. Mol Cell Biol. 2012;32:1893–907. doi: 10.1128/MCB.06248-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lu J, Tan M, Cai Q. The Warburg effect in tumor progression: Mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015;356:156–64. doi: 10.1016/j.canlet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jin Y, Cai Q, Shenoy AK, Lim S, Zhang Y, Charles S, et al. Src drives the Warburg effect and therapy resistance by inactivating pyruvate dehydrogenase through tyrosine-289 phosphorylation. Oncotarget. 2016;7:25113–24. doi: 10.18632/oncotarget.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nobis M, McGhee EJ, Morton JP, Schwarz JP, Karim SA, Quinn J, et al. Intravital FLIM-FRET imaging reveals dasatinib-induced spatial control of src in pancreatic cancer. Cancer Res. 2013;73:4674–86. doi: 10.1158/0008-5472.CAN-12-4545. [DOI] [PubMed] [Google Scholar]
- 111.Kang Y, Massagué J. Epithelial-mesenchymal transitions: twist in development and metastasis. Cell. 2004;118:277–9. doi: 10.1016/j.cell.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 112.Joo YE, Rew JS, Park CS, Kim SJ. Expression of E-cadherin, alpha- and beta-catenins in patients with pancreatic adenocarcinoma. Pancreatology. 2002;2:129–37. doi: 10.1159/000055903. [DOI] [PubMed] [Google Scholar]
- 113.Pignatelli M, Ansari TW, Gunter P, Liu D, Hirano S, Takeichi M, et al. Loss of membranous E-cadherin expression in pancreatic cancer: correlation with lymph node metastasis, high grade, and advanced stage. J Pathol. 1994;174:243–8. doi: 10.1002/path.1711740403. [DOI] [PubMed] [Google Scholar]
- 114.Dosch AR, Dai X, Gaidarski Iii AA, Shi C, Castellanos JA, VanSaun MN, et al. Src kinase inhibition restores E-cadherin expression in dasatinib-sensitive pancreatic cancer cells. Oncotarget. 2019;10:1056–69. doi: 10.18632/oncotarget.26621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nagathihalli NS, Merchant NB. Src-mediated regulation of E-cadherin and EMT in pancreatic cancer. Front Biosci (Landmark Ed) 2012;17:2059–69. doi: 10.2741/4037. [DOI] [PubMed] [Google Scholar]
- 116.Cicchini C, Laudadio I, Citarella F, Corazzari M, Steindler C, Conigliaro A, et al. TGFbeta-induced EMT requires focal adhesion kinase (FAK) signaling. Exp Cell Res. 2008;314:143–52. doi: 10.1016/j.yexcr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 117.Canel M, Serrels A, Miller D, Timpson P, Serrels B, Frame MC, et al. Quantitative in vivo imaging of the effects of inhibiting integrin signaling via Src and FAK on cancer cell movement: effects on E-cadherin dynamics. Cancer Res. 2010;70:9413–22. doi: 10.1158/0008-5472.CAN-10-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–26. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Connelly SF, Isley BA, Baker CH, Gallick GE, Summy JM. Loss of tyrosine phosphatase-dependent inhibition promotes activation of tyrosine kinase c-Src in detached pancreatic cells. Mol Carcinog. 2010;49:1007–21. doi: 10.1002/mc.20684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Clark ES, Whigham AS, Yarbrough WG, Weaver AM. Cortactin is an essential regulator of matrix metalloproteinase secretion and extracellular matrix degradation in invadopodia. Cancer Res. 2007;67:4227–35. doi: 10.1158/0008-5472.CAN-06-3928. [DOI] [PubMed] [Google Scholar]
- 121.Bowden ET, Onikoyi E, Slack R, Myoui A, Yoneda T, Yamada KM, et al. Co-localization of cortactin and phosphotyrosine identifies active invadopodia in human breast cancer cells. Exp Cell Res. 2006;312:1240–53. doi: 10.1016/j.yexcr.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 122.Spinardi L, Rietdorf J, Nitsch L, Bono M, Tacchetti C, Way M, et al. A dynamic podosome-like structure of epithelial cells. Exp Cell Res. 2004;295:360–74. doi: 10.1016/j.yexcr.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 123.Artym VV, Zhang Y, Seillier-Moiseiwitsch F, Yamada KM, Mueller SC. Dynamic interactions of cortactin and membrane type 1 matrix metalloproteinase at invadopodia: defining the stages of invadopodia formation and function. Cancer Res. 2006;66:3034–43. doi: 10.1158/0008-5472.CAN-05-2177. [DOI] [PubMed] [Google Scholar]
- 124.Nakahara H, Howard L, Thompson EW, Sato H, Seiki M, Yeh Y, et al. Transmembrane/cytoplasmic domain-mediated membrane type 1-matrix metalloprotease docking to invadopodia is required for cell invasion. Proc Natl Acad Sci USA. 1997;94:7959–64. doi: 10.1073/pnas.94.15.7959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Monsky WL, Kelly T, Lin CY, Yeh Y, Stetler-Stevenson WG, Mueller SC, et al. Binding and localization of M(r) 72,000 matrix metalloproteinase at cell surface invadopodia. Cancer Res. 1993;53:3159–64. [PubMed] [Google Scholar]
- 126.Duxbury MS, Ito H, Zinner MJ, Ashley SW, Whang EE. Inhibition of SRC tyrosine kinase impairs inherent and acquired gemcitabine resistance in human pancreatic adenocarcinoma cells. Clin Cancer Res. 2004;10:2307–18. doi: 10.1158/1078-0432.CCR-1183-3. [DOI] [PubMed] [Google Scholar]
- 127.Kelber JA, Reno T, Kaushal S, Metildi C, Wright T, Stoletov K, et al. KRas induces a Src/PEAK1/ErbB2 kinase amplification loop that drives metastatic growth and therapy resistance in pancreatic cancer. Cancer Res. 2012;72:2554–64. doi: 10.1158/0008-5472.CAN-11-3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ischenko I, Camaj P, Seeliger H, Kleespies A, Guba M, De Toni EN, et al. Inhibition of Src tyrosine kinase reverts chemoresistance toward 5-fluorouracil in human pancreatic carcinoma cells: an involvement of epidermal growth factor receptor signaling. Oncogene. 2008;27:7212–22. doi: 10.1038/onc.2008.326. [DOI] [PubMed] [Google Scholar]
- 129.Ma L, Wei J, Su GH, Lin J. Dasatinib can enhance paclitaxel and gemcitabine inhibitory activity in human pancreatic cancer cells. Cancer Biol Ther. 2019;20:855–65. doi: 10.1080/15384047.2019.1579956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kim YJ, Jung K, Baek DS, Hong SS, Kim YS. Co-targeting of EGF receptor and neuropilin-1 overcomes cetuximab resistance in pancreatic ductal adenocarcinoma with integrin β1-driven Src-Akt bypass signaling. Oncogene. 2017;36:2543–52. doi: 10.1038/onc.2016.407. [DOI] [PubMed] [Google Scholar]
- 131.Shadhu K, Xi C. Inflammation and pancreatic cancer: An updated review. Saudi J Gastroenterol. 2019;25:3–13. doi: 10.4103/sjg.SJG_390_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang L, Xie D, Wei D. Pancreatic acinar-to-ductal metaplasia and pancreatic cancer. Methods Mol Biol. 2019;1882:299–308. doi: 10.1007/978-1-4939-8879-2_26. [DOI] [PubMed] [Google Scholar]
- 133.Liou GY, Döppler H, Necela B, Krishna M, Crawford HC, Raimondo M, et al. Macrophage-secreted cytokines drive pancreatic acinar-to-ductal metaplasia through NF-κB and MMPs. J Cell Biol. 2013;202:563–77. doi: 10.1083/jcb.201301001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–83. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 135.Nagaraj NS, Smith JJ, Revetta F, Washington MK, Merchant NB. Targeted inhibition of SRC kinase signaling attenuates pancreatic tumorigenesis. Mol Cancer Ther. 2010;9:2322–32. doi: 10.1158/1535-7163.MCT-09-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ramnath RD, Sun J, Bhatia M. Involvement of SRC family kinases in substance P-induced chemokine production in mouse pancreatic acinar cells and its significance in acute pancreatitis. J Pharm Exp Ther. 2009;329:418–28. doi: 10.1124/jpet.108.148684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Yokoi K, Hawke D, Oborn CJ, Jang JY, Nishioka Y, Fan D, et al. Identification and validation of SRC and phospho-SRC family proteins in circulating mononuclear cells as novel biomarkers for pancreatic cancer. Transl Oncol. 2011;4:83–91. doi: 10.1593/tlo.10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Liu ST, Pham H, Pandol SJ, Ptasznik A. Src as the link between inflammation and cancer. Front Physiol. 2013;4:416. doi: 10.3389/fphys.2013.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang Y, Yan W, Collins MA, Bednar F, Rakshit S, Zetter BR, et al. Interleukin-6 is required for pancreatic cancer progression by promoting MAPK signaling activation and oxidative stress resistance. Cancer Res. 2013;73:6359–74. doi: 10.1158/0008-5472.CAN-13-1558-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cheng M, Huang K, Zhou J, Yan D, Tang YL, Zhao TC, et al. A critical role of Src family kinase in SDF-1/CXCR4-mediated bone-marrow progenitor cell recruitment to the ischemic heart. J Mol Cell Cardiol. 2015;81:49–53. doi: 10.1016/j.yjmcc.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Gelmini S, Mangoni M, Serio M, Romagnani P, Lazzeri E. The critical role of SDF-1/CXCR4 axis in cancer and cancer stem cells metastasis. J Endocrinol Invest. 2008;31:809–19. doi: 10.1007/BF03349262. [DOI] [PubMed] [Google Scholar]
- 142.Gong J, Meng H-B, Hua J, Song Z-S, He ZG, Zhou B, et al. The SDF-1/CXCR4 axis regulates migration of transplanted bone marrow mesenchymal stem cells towards the pancreas in rats with acute pancreatitis. Mol Med Rep. 2014;9:1575–82. doi: 10.3892/mmr.2014.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Koshiba T, Hosotani R, Miyamoto Y, Ida J, Tsuji S, Nakajima S, et al. Expression of stromal cell-derived factor 1 and CXCR4 ligand receptor system in pancreatic cancer: a possible role for tumor progression. Clin Cancer Res. 2000;6:3530–5. [PubMed] [Google Scholar]
- 144.Gao Z, Wang X, Wu K, Zhao Y, Hu G. Pancreatic stellate cells increase the invasion of human pancreatic cancer cells through the stromal cell-derived factor-1/CXCR4 axis. Pancreatology. 2010;10:186–93. doi: 10.1159/000236012. [DOI] [PubMed] [Google Scholar]
- 145.Poh AR, Ernst M. Targeting macrophages in cancer: from bench to bedside. Front Oncol. 2018;8:49. doi: 10.3389/fonc.2018.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim HS, Han HD, Armaiz-Pena GN, Stone RL, Nam EJ, Lee JW, et al. Functional roles of Src and Fgr in ovarian carcinoma. Clin Cancer Res. 2011;17:1713–21. doi: 10.1158/1078-0432.CCR-10-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hekim C, Ilander M, Yan J, Michaud E, Smykla R, Vähä-Koskela M, et al. Dasatinib changes immune cell profiles concomitant with reduced tumor growth in several murine solid tumor models. Cancer Immunol Res. 2017;5:157–69. doi: 10.1158/2326-6066.CIR-16-0061-T. [DOI] [PubMed] [Google Scholar]
- 148.Mao L, Deng WW, Yu GT, Bu LL, Liu JF, Ma SR, et al. Inhibition of SRC family kinases reduces myeloid-derived suppressor cells in head and neck cancer. Int J Cancer. 2017;140:1173–85. doi: 10.1002/ijc.30493. [DOI] [PubMed] [Google Scholar]
- 149.Sahai E, Astsaturov I, Cukierman E, DeNardo DG, Egeblad M, Evans RM, et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020;20:174–86. doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Shen T, Li Y, Zhu S, Yu J, Zhang B, Chen X, et al. YAP1 plays a key role of the conversion of normal fibroblasts into cancer-associated fibroblasts that contribute to prostate cancer progression. J Exp Clin Cancer Res. 2020;39:36. doi: 10.1186/s13046-020-1542-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu T, Zhou L, Li D, Andl T, Zhang Y. Cancer-associated fibroblasts build and secure the Tumor microenvironment. Front Cell Dev Biol. 2019;7:60. doi: 10.3389/fcell.2019.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, et al. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol. 2013;15:637–46. doi: 10.1038/ncb2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dosch AR, Dai X, Reyzer ML, Mehra S, Srinivasan S, Willobee BA, et al. Combined Src/EGFR inhibition targets STAT3 signaling and induces stromal remodeling to improve survival in pancreatic cancer. Mol Cancer Res. 2020;18:623–31. doi: 10.1158/1541-7786.MCR-19-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77:1745–70. doi: 10.1007/s00018-019-03351-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Eriksson U, Alitalo K. Structure, expression and receptor-binding properties of novel vascular endothelial growth factors. Curr Top Microbiol Immunol. 1999;237:41–57. doi: 10.1007/978-3-642-59953-8_3. [DOI] [PubMed] [Google Scholar]
- 157.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4:915–24. doi: 10.1016/S1097-2765(00)80221-X. [DOI] [PubMed] [Google Scholar]
- 158.Wallez Y, Cand F, Cruzalegui F, Wernstedt C, Souchelnytskyi S, Vilgrain I, et al. Src kinase phosphorylates vascular endothelial-cadherin in response to vascular endothelial growth factor: identification of tyrosine 685 as the unique target site. Oncogene. 2007;26:1067–77. doi: 10.1038/sj.onc.1209855. [DOI] [PubMed] [Google Scholar]
- 159.Chou MT, Wang J, Fujita DJ. Src kinase becomes preferentially associated with the VEGFR, KDR/Flk-1, following VEGF stimulation of vascular endothelial cells. BMC Biochem. 2002;3:32. doi: 10.1186/1471-2091-3-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schimmel L, Fukuhara D, Richards M, Jin Y, Essebier P, Frampton E, et al. c-Src controls stability of sprouting blood vessels in the developing retina independently of cell-cell adhesion through focal adhesion assembly. Development. 2020;147:dev185405. doi: 10.1242/dev.185405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Koch AE, Polverini PJ, Kunkel SL, Harlow LA, DiPietro LA, Elner VM, et al. Interleukin-8 as a Macrophage-Derived Mediator of Angiogenesis. Science. 1992;258:1798–801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- 162.Matsuo Y, Sawai H, Funahashi H, Takahashi H, Sakamoto M, Yamamoto M, et al. Enhanced angiogenesis due to inflammatory cytokines from pancreatic cancer cell lines and relation to metastatic potential. Pancreas. 2004;28:344–52. doi: 10.1097/00006676-200404000-00025. [DOI] [PubMed] [Google Scholar]
- 163.Kurahara H, Takao S, Shinchi H, Maemura K, Mataki Y, Sakoda M, et al. Significance of lymphangiogenesis in primary tumor and draining lymph nodes during lymphatic metastasis of pancreatic head cancer. J Surgical Oncol. 2010;102:809–15. doi: 10.1002/jso.21744. [DOI] [PubMed] [Google Scholar]
- 164.Wang Z, Wu J, Li G, Zhang X, Tong M, Wu Z, et al. Lymphangiogenesis and biological behavior in pancreatic carcinoma and other pancreatic tumors. Mol Med Rep. 2012;5:959–63. doi: 10.3892/mmr.2012.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Su JL, Yen CJ, Chen PS, Chuang SE, Hong CC, Kuo IH, et al. The role of the VEGF-C/VEGFR-3 axis in cancer progression. Br J Cancer. 2007;96:541–5. doi: 10.1038/sj.bjc.6603487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schneider M, Büchler P, Giese N, Giese T, Wilting J, Büchler MW, et al. Role of lymphangiogenesis and lymphangiogenic factors during pancreatic cancer progression and lymphatic spread. Int J Oncol. 2006;28:883–90. [PubMed] [Google Scholar]
- 167.Scavelli C, Vacca A, Di Pietro G, Dammacco F, Ribatti D. Crosstalk between angiogenesis and lymphangiogenesis in tumor progression. Leukemia. 2004;18:1054–8. doi: 10.1038/sj.leu.2403355. [DOI] [PubMed] [Google Scholar]
- 168.Gomes FG, Nedel F, Alves AM, Nör JE, Tarquinio SB. Tumor angiogenesis and lymphangiogenesis: tumor/endothelial crosstalk and cellular/microenvironmental signaling mechanisms. Life Sci. 2013;92:101–7. doi: 10.1016/j.lfs.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Huang YH, Yang HY, Hsu YF, Chiu PT, Ou G, Hsu MJ. Src contributes to IL6-induced vascular endothelial growth factor-C expression in lymphatic endothelial cells. Angiogenesis. 2014;17:407–18. doi: 10.1007/s10456-013-9386-1. [DOI] [PubMed] [Google Scholar]
- 170.Ischenko I, Seeliger H, Camaj P, Kleespies A, Guba M, Eichhorn ME, et al. Src tyrosine kinase inhibition suppresses lymphangiogenesis in vitro and in vivo. Curr Cancer Drug Targets. 2010;10:546–53. doi: 10.2174/156800910791517181. [DOI] [PubMed] [Google Scholar]
- 171.Araujo J, Logothetis C. Dasatinib: a potent SRC inhibitor in clinical development for the treatment of solid tumors. Cancer Treat Rev. 2010;36:492–500. doi: 10.1016/j.ctrv.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chee CE, Krishnamurthi S, Nock CJ, Meropol NJ, Gibbons J, Fu P, et al. Phase II study of dasatinib (BMS-354825) in patients with metastatic adenocarcinoma of the pancreas. Oncologist. 2013;18:1091–2. doi: 10.1634/theoncologist.2013-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kaiser J. Biomedicine. Rare cancer successes spawn ‘exceptional’ research efforts. Science. 2013;340:263. doi: 10.1126/science.340.6130.263. [DOI] [PubMed] [Google Scholar]
- 174.Li Y, Zhang T, Schwartz SJ, Sun D. New developments in Hsp90 inhibitors as anti-cancer therapeutics: mechanisms, clinical perspective and more potential. Drug resistance updates: Rev Commentaries Antimicrob Anticancer Chemother. 2009;12:17–27. doi: 10.1016/j.drup.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Neckers L. Hsp90 inhibitors as novel cancer chemotherapeutic agents. Trends Mol Med. 2002;8:S55–S61. doi: 10.1016/S1471-4914(02)02316-X. [DOI] [PubMed] [Google Scholar]
- 176.Mahadevan D, Rensvold DM, Kurtin SE, Cleary JM, Gandhi L, Lyons JF, et al. First-in-human phase I study: Results of a second-generation non-ansamycin heat shock protein 90 (HSP90) inhibitor AT13387 in refractory solid tumors. J Clin Oncol. 2012;30:3028–3028. doi: 10.1200/jco.2012.30.15_suppl.3028. [DOI] [Google Scholar]
- 177.Xu Y, Singer MA, Lindquist S. Maturation of the tyrosine kinase c-src as a kinase and as a substrate depends on the molecular chaperone Hsp90. Proc Natl Acad Sci USA. 1999;96:109–14. doi: 10.1073/pnas.96.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Daunys S, Matulis D, Petrikaitė V. Synergistic activity of Hsp90 inhibitors and anticancer agents in pancreatic cancer cell cultures. Sci Rep. 2019;9:16177. doi: 10.1038/s41598-019-52652-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Zhang Y, Ware MB, Zaidi MY, Ruggieri AN, Olson BM, Komar H, et al. Heat shock protein-90 inhibition alters activation of pancreatic stellate cells and enhances the efficacy of PD-1 blockade in pancreatic cancer. Mol Cancer Ther. 2021;20:150–60. doi: 10.1158/1535-7163.MCT-19-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nagaraju GP, Zakka KM, Landry JC, Shaib WL, Lesinski GB, El-Rayes BF. Inhibition of HSP90 overcomes resistance to chemotherapy and radiotherapy in pancreatic cancer. Int J Cancer. 2019;145:1529–37. doi: 10.1002/ijc.32227. [DOI] [PubMed] [Google Scholar]
- 181.Patwardhan P, Resh MD. Myristoylation and membrane binding regulate c-Src stability and kinase activity. Mol Cell Biol. 2010;30:4094–107. doi: 10.1128/MCB.00246-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Resh MD. Myristylation and palmitylation of Src family members: the fats of the matter. Cell. 1994;76:411–3. doi: 10.1016/0092-8674(94)90104-X. [DOI] [PubMed] [Google Scholar]
- 183.Kim S, Alsaidan OA, Goodwin O, Li Q, Sulejmani E, Han Z, et al. Blocking myristoylation of Src inhibits its kinase activity and suppresses prostate cancer progression. Cancer Res. 2017;77:6950–62. doi: 10.1158/0008-5472.CAN-17-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ducker CE, Upson JJ, French KJ, Smith CD. Two N-myristoyltransferase isozymes play unique roles in protein myristoylation, proliferation, and apoptosis. Mol cancer Res: MCR. 2005;3:463–76. doi: 10.1158/1541-7786.MCR-05-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ashour AA, Gurbuz N, Alpay SN, Abdel-Aziz AA, Mansour AM, Huo L, et al. Elongation factor-2 kinase regulates TG2/β1 integrin/Src/uPAR pathway and epithelial-mesenchymal transition mediating pancreatic cancer cells invasion. J Cell Mol Med. 2014;18:2235–51. doi: 10.1111/jcmm.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Desgrosellier JS, Barnes LA, Shields DJ, Huang M, Lau SK, Prévost N, et al. An integrin alpha(v)beta(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat Med. 2009;15:1163–9. doi: 10.1038/nm.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yan C, Yang Q, Gong Z. Activation of hepatic stellate cells during liver carcinogenesis requires fibrinogen/Integrin αvβ5 in Zebrafish. Neoplasia. 2018;20:533–42. doi: 10.1016/j.neo.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 188.Zhao W, Ajani JA, Sushovan G, Ochi N, Hwang R, Hafley M, et al. Galectin-3 mediates tumor cell-stroma interactions by activating pancreatic stellate cells to produce cytokines via integrin signaling. Gastroenterology. 2018;154:1524–1537.e1526. doi: 10.1053/j.gastro.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 189.Wong PP, Demircioglu F, Ghazaly E, Alrawashdeh W, Stratford MR, Scudamore CL, et al. Dual-action combination therapy enhances angiogenesis while reducing tumor growth and spread. Cancer Cell. 2015;27:123–37. doi: 10.1016/j.ccell.2014.10.015. [DOI] [PubMed] [Google Scholar]
- 190.Friess H, Langrehr JM, Oettle H, Raedle J, Niedergethmann M, Dittrich C, et al. A randomized multi-center phase II trial of the angiogenesis inhibitor Cilengitide (EMD 121974) and gemcitabine compared with gemcitabine alone in advanced unresectable pancreatic cancer. BMC Cancer. 2006;6:285. doi: 10.1186/1471-2407-6-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Starling N, Neoptolemos J, Cunningham D. Role of erlotinib in the management of pancreatic cancer. Ther Clin Risk Manag. 2006;2:435–45. doi: 10.2147/tcrm.2006.2.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–6. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 193.Hammel P, Huguet F, van Laethem J-L, Goldstein D, Glimelius B, Artru P, et al. Effect of chemoradiotherapy vs chemotherapy on survival in patients With locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the LAP07 randomized clinical trial. JAMA. 2016;315:1844–53. doi: 10.1001/jama.2016.4324. [DOI] [PubMed] [Google Scholar]
- 194.Zhang J, He DH, Zajac-Kaye M, Hochwald SN. A small molecule FAK kinase inhibitor, GSK2256098, inhibits growth and survival of pancreatic ductal adenocarcinoma cells. Cell Cycle. 2014;13:3143–9. doi: 10.4161/15384101.2014.949550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Begum A, Ewachiw T, Jung C, Huang A, Norberg KJ, Marchionni L, et al. The extracellular matrix and focal adhesion kinase signaling regulate cancer stem cell function in pancreatic ductal adenocarcinoma. PLoS One. 2017;12:e0180181. doi: 10.1371/journal.pone.0180181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bondar VM, Sweeney-Gotsch B, Andreeff M, Mills GB, McConkey DJ. Inhibition of the phosphatidylinositol 3’-kinase-AKT pathway induces apoptosis in pancreatic carcinoma cells in vitro and in vivo. Mol Cancer Ther. 2002;1:989–97. [PubMed] [Google Scholar]
- 197.Shakespeare W, Yang M, Bohacek R, Cerasoli F, Stebbins K, Sundaramoorthi R, et al. Structure-based design of an osteoclast-selective, nonpeptide src homology 2 inhibitor with in vivo antiresorptive activity. Proc Natl Acad Sci USA. 2000;97:9373–8. doi: 10.1073/pnas.97.17.9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Violette SM, Shakespeare WC, Bartlett C, Guan W, Smith JA, Rickles RJ, et al. A Src SH2 selective binding compound inhibits osteoclast-mediated resorption. Chem Biol. 2000;7:225–35. doi: 10.1016/S1074-5521(00)00090-9. [DOI] [PubMed] [Google Scholar]
- 199.Sharma SV, Oneyama C, Yamashita Y, Nakano H, Sugawara K, Hamada M, et al. UCS15A, a non-kinase inhibitor of Src signal transduction. Oncogene. 2001;20:2068–79. doi: 10.1038/sj.onc.1204296. [DOI] [PubMed] [Google Scholar]
- 200.Oneyama C, Nakano H, Sharma SV. UCS15A, a novel small molecule, SH3 domain-mediated protein–protein interaction blocking drug. Oncogene. 2002;21:2037–50. doi: 10.1038/sj.onc.1205271. [DOI] [PubMed] [Google Scholar]
- 201.Cardin DB, Goff LW, Chan E, Whisenant JG, Dan Ayers G, Takebe N, et al. Dual Src and EGFR inhibition in combination with gemcitabine in advanced pancreatic cancer: phase I results. Investigational N. Drugs. 2018;36:442–50. doi: 10.1007/s10637-017-0519-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Pan Y, Zheng M, Zhong L, Yang J, Zhou S, Qin Y, et al. A preclinical evaluation of SKLB261, a multikinase inhibitor of EGFR/Src/VEGFR2, as a therapeutic agent against pancreatic cancer. Mol Cancer Ther. 2015;14:407–18. doi: 10.1158/1535-7163.MCT-14-0485. [DOI] [PubMed] [Google Scholar]
- 203.Gomes EG, Connelly SF, Summy JM. Targeting the yin and the yang: combined inhibition of the tyrosine kinase c-Src and the tyrosine phosphatase SHP-2 disrupts pancreatic cancer signaling and biology in vitro and tumor formation in vivo. Pancreas. 2013;42:795–806. doi: 10.1097/MPA.0b013e3182793fd7. [DOI] [PubMed] [Google Scholar]
- 204.Jaganathan S, Yue P, Turkson J. Enhanced sensitivity of pancreatic cancer cells to concurrent inhibition of aberrant signal transducer and activator of transcription 3 and epidermal growth factor receptor or Src. J Pharm Exp Ther. 2010;333:373–81. doi: 10.1124/jpet.109.162669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tsao AS, Wistuba II, Mehran RJ, Gil J, Nunez M, Lee J, et al. Evaluation of Src Tyr419 as a predictive biomarker in a neoadjuvant trial using dasatinib in resectable malignant pleural mesothelioma. J Clin Oncol. 2010;28:7042–7042. doi: 10.1200/jco.2010.28.15_suppl.7042. [DOI] [Google Scholar]
- 206.Arcaroli JJ, Touban BM, Tan AC, Varella-Garcia M, Powell RW, Eckhardt SG, et al. Gene array and fluorescence in situ hybridization biomarkers of activity of saracatinib (AZD0530), a Src inhibitor, in a preclinical model of colorectal cancer. Clin Cancer Res. 2010;16:4165–77. doi: 10.1158/1078-0432.CCR-10-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Luo F, Barrett YC, Yang Z, Camuso A, McGlinchey K, Wen M-L, et al. Identification and validation of phospho-SRC, a novel and potential pharmacodynamic biomarker for dasatinib (SPRYCEL™), a multi-targeted kinase inhibitor. Cancer Chemother Pharmacol. 2008;62:1065–74. doi: 10.1007/s00280-008-0699-5. [DOI] [PubMed] [Google Scholar]
- 208.Er JL, Goh PN, Lee CY, Tan YJ, Hii LW, Mai CW, et al. Identification of inhibitors synergizing gemcitabine sensitivity in the squamous subtype of pancreatic ductal adenocarcinoma (PDAC) Apoptosis. 2018;23:343–55. doi: 10.1007/s10495-018-1459-6. [DOI] [PubMed] [Google Scholar]
- 209.Bertotti A, Bracco C, Girolami F, Torti D, Gastaldi S, Galimi F, et al. Inhibition of Src impairs the growth of met-addicted gastric tumors. Clin Cancer Res. 2010;16:3933–43. doi: 10.1158/1078-0432.CCR-10-0106. [DOI] [PubMed] [Google Scholar]
- 210.Sen B, Peng S, Saigal B, Williams MD, Johnson FM. Distinct interactions between c-Src and c-Met in mediating resistance to c-Src inhibition in head and neck cancer. Clin Cancer Res. 2011;17:514–24. doi: 10.1158/1078-0432.CCR-10-1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yoshida T, Okamoto I, Okamoto W, Hatashita E, Yamada Y, Kuwata K, et al. Effects of Src inhibitors on cell growth and epidermal growth factor receptor and MET signaling in gefitinib-resistant non-small cell lung cancer cells with acquired MET amplification. Cancer Sci. 2010;101:167–72. doi: 10.1111/j.1349-7006.2009.01368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Schade AE, Schieven GL, Townsend R, Jankowska AM, Susulic V, Zhang R, et al. Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood. 2008;111:1366–77. doi: 10.1182/blood-2007-04-084814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Oksvold MP, Duyvestyn JM, Dagger SA, Taylor SJ, Forfang L, Myklebust JH, et al. The targeting of human and mouse B lymphocytes by dasatinib. Exp Hematol. 2015;43:352–363.e354. doi: 10.1016/j.exphem.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 214.Futosi K, Németh T, Pick R, Vántus T, Walzog B, Mócsai A. Dasatinib inhibits proinflammatory functions of mature human neutrophils. Blood. 2012;119:4981–91. doi: 10.1182/blood-2011-07-369041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Marzia M, Sims NA, Voit S, Migliaccio S, Taranta A, Bernardini S, et al. Decreased c-Src expression enhances osteoblast differentiation and bone formation. J Cell Biol. 2000;151:311–20. doi: 10.1083/jcb.151.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Lowe C, Yoneda T, Boyce BF, Chen H, Mundy GR, Soriano P. Osteopetrosis in Src-deficient mice is due to an autonomous defect of osteoclasts. Proc Natl Acad Sci USA. 1993;90:4485–9. doi: 10.1073/pnas.90.10.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-O. [DOI] [PubMed] [Google Scholar]
- 218.Boyce BF, Yoneda T, Lowe C, Soriano P, Mundy GR. Requirement of pp60c-src expression for osteoclasts to form ruffled borders and resorb bone in mice. J Clin Investig. 1992;90:1622–7. doi: 10.1172/JCI116032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Garcia-Gomez A, Ocio EM, Crusoe E, Santamaria C, Hernández-Campo P, Blanco JF, et al. Dasatinib as a bone-modifying agent: anabolic and anti-resorptive effects. PLoS One. 2012;7:e34914. doi: 10.1371/journal.pone.0034914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Séverin S, Nash CA, Mori J, Zhao Y, Abram C, Lowell CA, et al. Distinct and overlapping functional roles of Src family kinases in mouse platelets. J Thromb Haemost: JTH. 2012;10:1631–45. doi: 10.1111/j.1538-7836.2012.04814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Obergfell A, Eto K, Mocsai A, Buensuceso C, Moores SL, Brugge JS, et al. Coordinate interactions of Csk, Src, and Syk kinases with αIIbβ3 initiate integrin signaling to the cytoskeleton. J Cell Biol. 2002;157:265–75. doi: 10.1083/jcb.200112113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Gratacap MP, Martin V, Valéra MC, Allart S, Garcia C, Sié P, et al. The new tyrosine-kinase inhibitor and anticancer drug dasatinib reversibly affects platelet activation in vitro and in vivo. Blood. 2009;114:1884–92. doi: 10.1182/blood-2009-02-205328. [DOI] [PubMed] [Google Scholar]
- 223.Beke Debreceni I, Mezei G, Batár P, Illés Á, Kappelmayer J Dasatinib Inhibits Procoagulant and Clot Retracting Activities of Human Platelets. International Journal of Molecular Sciences 2019; 10.3390/ijms20215430. [DOI] [PMC free article] [PubMed]
- 224.Kostos L, Burbury K, Srivastava G, Prince HM. Gastrointestinal bleeding in a chronic myeloid leukaemia patient precipitated by dasatinib-induced platelet dysfunction: Case report. Platelets. 2015;26:809–11. doi: 10.3109/09537104.2015.1049138. [DOI] [PubMed] [Google Scholar]
- 225.Ozgur Yurttas N, Eskazan AE. Tyrosine Kinase Inhibitor–Associated Platelet Dysfunction: Does This Need to Have a Significant Clinical Impact? Clin Appl Thromb/Hemost. 2019;25:1076029619866925. doi: 10.1177/1076029619866925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Evans TRJ, Van Cutsem E, Moore MJ, Bazin IS, Rosemurgy A, Bodoky G, et al. Phase 2 placebo-controlled, double-blind trial of dasatinib added to gemcitabine for patients with locally-advanced pancreatic cancer. Ann Oncol. 2017;28:354–61. doi: 10.1093/annonc/mdw607. [DOI] [PubMed] [Google Scholar]
- 227.George TJ, Ali A, Wang Y, Lee JH, Ivey AM, DeRemer D, et al. Phase II study of 5-fluorouracil, oxaliplatin plus dasatinib (FOLFOX-D) in first-line metastatic pancreatic adenocarcinoma. Oncologist. 2021;26:825–e1674. doi: 10.1002/onco.13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Daud AI, Krishnamurthi SS, Saleh MN, Gitlitz BJ, Borad MJ, Gold PJ, et al. Phase I study of bosutinib, a src/abl tyrosine kinase inhibitor, administered to patients with advanced solid tumors. Clin Cancer Res. 2012;18:1092–100. doi: 10.1158/1078-0432.CCR-11-2378. [DOI] [PubMed] [Google Scholar]
- 229.Isakoff SJ, Wang D, Campone M, Calles A, Leip E, Turnbull K, et al. Bosutinib plus capecitabine for selected advanced solid tumours: results of a phase 1 dose-escalation study. Br J Cancer. 2014;111:2058–66. doi: 10.1038/bjc.2014.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Renouf DJ, Moore MJ, Hedley D, Gill S, Jonker D, Chen E, et al. A phase I/II study of the Src inhibitor saracatinib (AZD0530) in combination with gemcitabine in advanced pancreatic cancer. Invest N. Drugs. 2012;30:779–86. doi: 10.1007/s10637-010-9611-3. [DOI] [PubMed] [Google Scholar]
- 231.Thompson DS, Patnaik A, Bendell JC, Papadopoulos K, Infante JR, Mastico RA, et al. A phase I dose-escalation study of IMGN388 in patients with solid tumors. J Clin Oncol. 2010;28:3058–3058. doi: 10.1200/jco.2010.28.15_suppl.3058. [DOI] [Google Scholar]
- 232.Ricart AD, Tolcher AW, Liu G, Holen K, Schwartz G, Albertini M, et al. Volociximab, a chimeric monoclonal antibody that specifically binds alpha5beta1 integrin: a phase I, pharmacokinetic, and biological correlative study. Clin Cancer Res. 2008;14:7924–9. doi: 10.1158/1078-0432.CCR-08-0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Semrad T, Barzi A, Lenz HJ, Hutchins IM, Kim EJ, Gong IY, et al. Pharmacodynamic separation of gemcitabine and erlotinib in locally advanced or metastatic pancreatic cancer: therapeutic and biomarker results. Int J Clin Oncol. 2015;20:518–24. doi: 10.1007/s10147-014-0730-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Philip PA, Benedetti J, Corless CL, Wong R, O’Reilly EM, Flynn PJ, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605–10. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Schultheis B, Reuter D, Ebert MP, Siveke J, Kerkhoff A, Berdel WE, et al. Gemcitabine combined with the monoclonal antibody nimotuzumab is an active first-line regimen in KRAS wildtype patients with locally advanced or metastatic pancreatic cancer: a multicenter, randomized phase IIb study. Ann Oncol. 2017;28:2429–35. doi: 10.1093/annonc/mdx343. [DOI] [PubMed] [Google Scholar]
- 236.Infante JR, Camidge DR, Mileshkin LR, Chen EX, Hicks RJ, Rischin D, et al. Safety, pharmacokinetic, and pharmacodynamic phase I dose-escalation trial of PF-00562271, an inhibitor of focal adhesion kinase, in advanced solid tumors. J Clin Oncol. 2012;30:1527–33. doi: 10.1200/JCO.2011.38.9346. [DOI] [PubMed] [Google Scholar]
- 237.Marsh Rde W, Rocha Lima CM, Levy DE, Mitchell EP, Rowland KM, Jr, Benson AB., 3rd A phase II trial of perifosine in locally advanced, unresectable, or metastatic pancreatic adenocarcinoma. Am J Clin Oncol. 2007;30:26–31. doi: 10.1097/01.coc.0000251235.46149.43. [DOI] [PubMed] [Google Scholar]
- 238.McRee AJ, Sanoff HK, Carlson C, Ivanova A, O’Neil BH. A phase I trial of mFOLFOX6 combined with the oral PI3K inhibitor BKM120 in patients with advanced refractory solid tumors. Invest N. Drugs. 2015;33:1225–31. doi: 10.1007/s10637-015-0298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Chung V, McDonough S, Philip PA, Cardin D, Wang-Gillam A, Hui L, et al. Effect of selumetinib and MK-2206 vs oxaliplatin and fluorouracil in patients with metastatic pancreatic cancer after prior therapy: SWOG S1115 study randomized clinical trial. JAMA Oncol. 2017;3:516–22. doi: 10.1001/jamaoncol.2016.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Tolcher AW, Patnaik A, Papadopoulos KP, Rasco DW, Becerra CR, Allred AJ, et al. Phase I study of the MEK inhibitor trametinib in combination with the AKT inhibitor afuresertib in patients with solid tumors and multiple myeloma. Cancer Chemother Pharm. 2015;75:183–9. doi: 10.1007/s00280-014-2615-5. [DOI] [PubMed] [Google Scholar]
- 241.Tolcher AW, Kurzrock R, Valero V, Gonzalez R, Heist RS, Tan AR, et al. Phase I dose-escalation trial of the oral AKT inhibitor uprosertib in combination with the oral MEK1/MEK2 inhibitor trametinib in patients with solid tumors. Cancer Chemother Pharm. 2020;85:673–83. doi: 10.1007/s00280-020-04038-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.