Abstract
The COVID-19 pandemic, caused by SARS-CoV-2, has become a global public health crisis. The entry of SARS-CoV-2 into host cells is facilitated by the binding of its spike protein (S1-RBD) to the host receptor hACE2. Small molecule compounds targeting S1-RBD-hACE2 interaction could provide an alternative therapeutic strategy sensitive to viral mutations. In this study, we identified G7a as a hit compound that targets the S1-RBD-hACE2 interaction, using high-throughput screening in the SARS2-S pseudovirus model. To enhance the antiviral activity of G7a, we designed and synthesized a series of novel 7-azaindole derivatives that bind to the S1-RBD-hACE2 interface. Surprisingly, ASM-7 showed excellent antiviral activity and low cytotoxicity, as confirmed by pseudovirus and native virus assays. Molecular docking and molecular dynamics simulations revealed that ASM-7 could stably bind to the binding interface of S1-RBD-hACE2, forming strong non-covalent interactions with key residues. Furthermore, the binding of ASM-7 caused alterations in the structural dynamics of both S1-RBD and hACE2, resulting in a decrease in their binding affinity and ultimately impeding the viral invasion of host cells. Our findings demonstrate that ASM-7 is a promising lead compound for developing novel therapeutics against SARS-CoV-2.
Keywords: SARS2-S pseudovirus model, 7-azaindoles, Molecular dynamics simulation
1. Introduction
As of the end of May 2023, the coronavirus disease 2019 (COVID-19) pandemic has resulted in >766 million confirmed cases and over 6.9 million fatalities worldwide, as reported by the World Health Organization [1]. Extensive research has established that the spike protein receptor-binding domain (S1-RBD) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) interacts with the human angiotensin-converting enzyme 2 (hACE2) receptor on the cellular surface [2]. This interaction is critical in the virus's ability to invade and replicate within host cells [3]. Hence, the development of small molecules capable of inhibiting the binding of S1-RBD and hACE2 has become a critical avenue for the creation of coronavirus treatments [4]. The elucidation of the intricate structure of the SARS-CoV-2 S1-RBD and hACE2 full-length protein complex has uncovered the mechanism behind the virus's infiltration of host cells and furnished an essential foundation for developing targeted new drugs [5]. However, the vast and featureless contact surface shared by the S1-RBD and hACE2 proteins poses a formidable challenge in identifying a suitable small molecule drug capable of stably binding and disrupting this regulatory pathway [6].
Of particular note, the discovery of the Omicron variant in November 2021 has presented a significant challenge, as it features over 14 mutations in the spike protein RBD and has rapidly spread worldwide. In the mutated Omicron variants, several amino acid positions in S1-RBD have been identified as being altered compared to the wild-type strain. These include G339D, S371L, S373P, S375F, N440K, G446S, S477N, T478K, E484A, Q493R, G496S, Q498R, N501Y, and Y505H [7,8]. The S477N mutation is a crucial concern, as it has emerged numerous times in various SARS-CoV-2 lineages and has been demonstrated to enhance the virus's affinity for the host receptor at the S1-RBD-hACE2 interface [9]. Consequently, the effectiveness of current COVID-19 vaccines and antibody therapies may be compromised due to the substantial number of mutations, including S477N [10].
Currently, small molecule drugs targeting the disruption of S1-RBD and hACE2 binding hold promise in mitigating antibody and vaccine resistance resulting from viral mutations. The growing body of research has identified an expanding repertoire of small-molecule drugs capable of inhibiting the interaction between S1-RBD and hACE2. All-trans retinoic acid (ATRA) that binds directly in a deep hydrophobic pocket of the S1-RBD leads to an “all-down” conformations [11], thereby blocking the interaction between RBD and hACE2. The ceftazidime binds to the interface of S1-RBD with ACE2 and blocks the binding of the complex [12]. Compounds such as nilotinib might induce significant conformational changes in the ACE2-RBD complex, intervene with the hydrogen bonds, and destabilize the complex of S1-RBD-hACE2 [13], thus potentially reducing the SARS-CoV-2 infection risk. Nevertheless, the need to conduct live SARS-CoV-2 experiments in biosafety level III laboratories has impeded research and drug development for COVID-19. Conversely, the utilization of SARS-CoV-2 spike protein (SARS2-S) pseudoviruses devoid of specific gene sequences is significantly safer and can be investigated in biosafety level II laboratories, providing a valuable tool for studying SARS-CoV-2 virology [14].
This study employed a combination of virtual and high-throughput screening techniques to identify the hit compound G7a, which can inhibit SARS-CoV-2 entry into host cells by obstructing the interaction between S1-RBD and hACE2. Building on the binding mode of G7a with the S1-RBD-hACE2 protein, we designed and synthesized a collection of 7-azaindole derivatives called ASMs, and conducted preliminary structure-activity relationship (SAR) studies on the compounds using the SARS2-S pseudovirus assay. Subsequently, we tested the most active compound, ASM-7, in vitro through live virus experiments. We employed molecular docking and molecular dynamics (MD) simulation techniques to further elucidate the interaction modes between the compounds ASMs and the S1-RBD-hACE2 protein.
2. Results and discussion
2.1. Discovery of hit compound G7a that inhibits the infection of SARS2-S pseudovirus
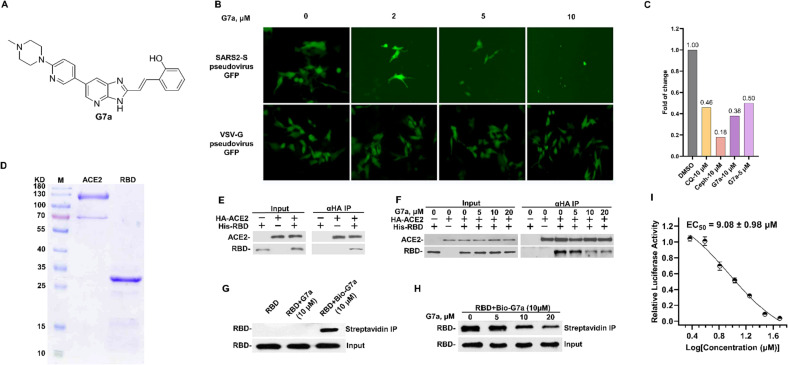
Firstly, we systematically screened our in-house compound library to identify potential hit compounds against SARS-CoV-2. Using a SARS2-S pseudovirus model (Fig. S1), G7a, with a 3H-imidazo[4,5-b]pyridine scaffold, was identified as a potential inhibitor (Fig. 1A). G7a could selectively inhibit the invasion of SARS2-S pseudovirus using GFP as a reporter gene while exhibiting no effect on the invasion of VSV-G pseudovirus (Fig. 1B). Treatment with G7a at 5 μM and 10 μM displayed inhibition of SARS2-S pseudovirus invasion (Fig. 1C).
Fig. 1.
G7a inhibited SARS-CoV-2 entry and blocked the interaction of RBD and hACE2 in pseudovirus model, CQ (Chloroquine) and Ceph (Cepharanthine) were treated as a positive control. (A) Chemical structure of G7a. (B) & (C) Compared with VSV-G pseudovirus, G7a specifically inhibited the entry of SARS2-S pseudovirus into A549-ACE2 cells. (D) Purified hACE2 and RBD protein were detected by Coomassie blue staining. (E) Western Blot detected the interaction of hACE2 and RBD protein by HA-IP in vitro. (F) Western blot was used to detect the interaction changes of hACE2 and RBD proteins after treatment with 0, 5, 10, and 20 μM of G7a. (G) Western blot for detecting RBD protein by streptavidin IP following treatment with DMSO, G7a and Biotin-G7a. (H) RBD protein was pre-incubated with DMSO or G7a (5, 10, 20 μM, 1 h) prior to incubated with the Biotin-G7a (10 μM, 1 h) in PBS. IP products detected by Western blot. (I) G7a inhibited the entry of SARS2-S pseudovirus in dose-dependent manner.
The infectivity of SARS-CoV-2 is reliant on the binding of RBD with the hACE2 receptor. So, we then purified hACE2 and spike RBD proteins using eukaryotic and prokaryotic expression, respectively (Fig. 1D). Meanwhile, the co-immunoprecipitation assay confirmed the interaction between spike RBD and hACE2 (Fig. 1E). G7a was then found to block the interaction between hACE2 and spike RBD proteins in a concentration-dependent manner (Fig. 1F). To evaluate whether G7a directly targeted spike RBD, we synthesized the Biotin-G7a molecular probe. Biotin-G7a retained the ability to bind spike RBD protein (Fig. 1G), while the amount of spike RBD “pulled down” by the beads was competed off by the pretreatment of live cells with G7a in a dose-dependent manner (Fig. 1H). Importantly, G7a potently inhibited SARS2-S pseudovirus infection in host cells with an EC50 of 9.08 μM (Fig. 1I).
2.2. G7a-based molecular design, synthesis, and biological evaluation
2.2.1. Design strategy for compounds ASMs
The binding mode of G7a with S1-RBD-hACE2 protein reveals that the nitrogen-substituted indole scaffold of G7a serves as a hydrogen bond donor and acceptor. It predominantly interacts with GLU37 of hACE2 (chain A) and ARG403 of S1-RBD (chain E) through hydrogen bonds at distances of 2.21 and 2.42 Å, respectively. Additionally, G7a engages in hydrophobic interactions with LYS353 and PRO389 of hACE2 (Fig. 2A). Analysis of the surface map in Fig. 2B indicates that G7a predominantly binds to the surface of hACE2, displaying relatively weak non-covalent interactions with S1-RBD. Although the phenylethylene moiety of G7a contributes to hydrophobic interactions with S1-RBD, its binding affinity is relatively weak. Notably, S1-RBD features a small hydrophobic sub-pocket adjacent to the phenylethylene fragment of G7a. To enhance the interaction between G7a and S1-RBD, aiming to improve its ability to block the binding of S1-RBD to hACE2, we aimed to introduce a hydrophobic fragment capable of binding to this small hydrophobic sub-pocket. As shown in Fig. 2C, we utilized a scaffold hopping approach and introduced a rigid “hinge” carbonyl group at the 3-position of the nitrogen-substituted indole scaffold, which allowed the upcoming aromatic group (—Ar) to face the direction of the sub-pocket formed by S1-RBD and bind to it. After introducing the “hinge” carbonyl group at 7-azaindole's 3-position, the —Ar directly connected to the carbonyl group exhibited strong binding to the sub-pocket of S1-RBD, as shown in Fig. 2D. Based on this finding, we designed and synthesized a series of 7-azaindole derivatives (ASMs) to improve their ability to inhibit the binding of S1-RBD to hACE2.
Fig. 2.
Design scheme of compounds ASMs.
2.2.2. Chemical synthesis of compounds ASMs
The synthetic route of intermediates 3a–3i was presented in Scheme 1 . To commence, the reaction of 2-fluorosubstituted pyridine with various amines was carried out through a substitution reaction in the presence of N,N-diisopropylethylamine (DIPEA). This led to the formation of compounds 2a–2i, which were subsequently coupled with bis(pinacolato)diboron via Suzuki reaction in the presence of Pd(dppf)Cl2 as catalysts and dry KAc. Remarkably, borate intermediates 3a–3i were obtained without the need for further purification.
Scheme 1.
Synthesis of intermediate compounds 3a–3i. Reagents and conditions: (i) DIPEA, DMF, 120 °C, 4 h; (ii) Bis(pinacolato)diboron, KAC, Pd(dppf)Cl2, DMF, 90 °C, 3 h.
Scheme 2 illustrated the synthetic pathway used to synthesize intermediates 6a–6d. The reaction commenced by subjecting 5-bromo-7-azaindole to a Friedel-Crafts reaction with various substituted aryl chlorides at the 3-position in the presence of AlCl3 under a nitrogen atmosphere. It resulted in the formation of intermediates 6a–6d.
Scheme 2.
Synthesis of intermediate compounds 6a–6d. Reagents and conditions: (iii) AlCl3, related acyl chloride, DCM, r.t, 12 h.
The synthetic route of ASMs was depicted in Scheme 3 . First, intermediates 6a–6d were coupled with 3a–3i through a Suzuki reaction catalyzed by Pd(dppf)Cl2 and dry K2CO3 to yield ASMs. Subsequently, the Boc-protected ASMs (ASM-3, ASM-6, ASM-8, ASM-10, ASM-14, ASM-17, ASM-19, and ASM-21) were subjected to trifluoroacetic acid (TFA) deprotection to yield the corresponding products, i.e., ASM-4, ASM-7, ASM-9, ASM-11, ASM-15, ASM-18, ASM-20, and ASM-22, respectively. ASM-21 and ASM-23 were obtained through a different approach. Specifically, ASM-21 or ASM-23 was synthesized via a reaction between ASM-6 and ethyl 3-bromopropanoate or (bromomethyl)cyclopropane in N,N-dimethylformamide (DMF) under the catalysis of NaI. Besides, Biotin-G7a and Biotin-ASM-7 were synthesized via amide condensation, as shown in Scheme 4 .
Scheme 3.
Synthesis of ASMs compound series. Reagents and conditions: (iv) Pd(dppf)Cl2, K2CO3, DME/H2O, 120 °C, 12 h; (v) TFA, DCM, r.t, 3 h; (vi) NaI, DMF, related substituted alkanes, 60 °C, 4 h; (vii) HCl/Dioxane, r.t, 3 h.
Scheme 4.
Synthesis of Biotin probe. Reagents and conditions: (viii) HATU, DIPEA, DMF, r.t, 4 h.
2.2.3. SAR profiling of compounds ASMs
The efficacy of the synthesized compounds in inhibiting SARS-CoV-2 invasion was evaluated using a SARS2-S pseudovirus model at a concentration of 2 μM. Additionally, cytotoxicity assays were performed on A549 and MRC-5 cells at a concentration of 10 μM for all compounds. The results of these experiments provide insight into the potential antiviral properties of the synthesized compounds and their suitability for further development as therapeutic agents.
Based on the design strategy, we synthesized a series of compounds denoted as ASM-1– 13. The biological activity of these compounds was evaluated and summarized in Table 1 . It revealed that most newly designed 7-azaindole derivatives exhibited superior antiviral activity compared to G7a (EC50 = 9.08 μM). The cytotoxicity IC50 values for all newly synthesized compounds to A549 and MRC-5 cell lines were higher than 10 μM. In comparison to G7a, the utilization of only 7-azaindole to substitute the original pyridinimidazole resulted in ASM-1. The luciferase ratio of ASM-1 decreased to approximately 40 %, thereby partially confirming the effectiveness of our design strategy. Subsequently, compounds ASM-2, ASM-4, ASM-7, ASM-9, and ASM-11 exhibited the highest activity among these compounds, with a luciferase ratio lower than 25 %. It was observed that a small methyl group could influence antiviral activity when comparing ASM-2 and ASM-11, which confirmed the importance of the piperazine ring for enhancing activity. Furthermore, it was found that the benzene ring substituted with a fluorine atom displayed greater activity than that substituted with a methoxy group, as observed when comparing ASM-4 and ASM-7. Remarkably, compared to their corresponding -Boc protected counterparts, namely compounds ASM-3, ASM-6, ASM-8, and ASM-10, compounds ASM-4, ASM-7, ASM-9, and ASM-11 exhibited enhanced antiviral activity. On one hand, the substituted -Boc group presented a significant spatial hindrance, resulting in difficulties in accessing the binding pocket. On the other hand, the exposed amino group exhibited greater polarity and was more prone to interact with solvents. However, when R1 was sulfonyl (as in ASM-12 and ASM-13), the activity of the compound almost completely vanished.
Table 1.
SAR exploration of ASM-1– 13.
Considering the previous observations of certain activity exhibited by compounds ASM-1– 13 substituted at the 2,5- position of the pyridine ring, we endeavored to modify the molecular orientation by designing and synthesizing substituted compounds at the 2,4- position, resulting in the formation of ASM-14– 20. Table 2 outlined the bioassay results of ASM-14– 20, indicating that the antiviral activity of the 2,5- substituted pyridine derivatives was more favorable than that of the 2,4- substituted. Additionally, the introduction of chlorine atoms did not appear to provide any noticeable benefits in improving biological activity (ASM-17– 20).
Table 2.
SAR exploration of ASM-14– 20.
Several substituents were introduced as R2 into the NH of 7-azaindole to generate ASM-21– 23 (Table 3 ). The results showed that the antiviral activity of the resulting compounds was lower when a substituent was installed. For example, when a substituted carboxyl (ASM-22) or cycloalkane (ASM-23) was introduced to the 1-position of 7-azaindole, the resulting compound exhibited significantly reduced activity compared to ASM-7.
Table 3.
SAR exploration of ASM-21– 23.
As a result, ASM-7 demonstrated both potent anti-SARS2-S pseudovirus infectivity and low toxicity against the A549 and MRC-5 test cells and was selected for further evaluation of its potential anti-SARS-CoV-2 activity and mechanism of action.
2.2.4. In vitro antiviral activity of ASM-7
As shown in Fig. 3A, ASM-7 inhibited SARS2-S pseudovirus from infecting host cells in a concentration-dependent manner. The EC50 of ASM-7 for blocking virus entry was 0.45 μM (Fig. 3B), representing a 20-fold advantage over G7a (EC50 = 9.08 μM). Besides, the Biotin-ASM-7 probe (Bio-ASM-7) also could pull down the spike RBD protein through streptavidin IP (Fig. 3C). Therefore, we further investigated the cellular antiviral activity of ASM-7 against original SARS-CoV-2 strain. ASM-7 exhibited an excellent inhibition on the infection of SARS-CoV-2 original viral strain in cells, with an EC50 value of 1.001 μM (Fig. S1F).
Fig. 3.
Antiviral activity of ASM-7. (A) & (B) ASM-7 inhibits SARS2-S pseudovirus entry in spike-pseudovirus model. (C) Western blot for detecting RBD protein by streptavidin IP following treatment with DMSO, ASM-7 and Biotin-ASM-7.
2.2.5. Pharmacokinetic profile of G7a and ASM-7
In light of the association between SARS-CoV-2 and pulmonary infection, we investigated the tissue distribution of G7a and ASM-7 in female ICR mice (Fig. 4 ). Our findings indicate a directional target tissue distribution. Following intravenous administration of G7a or ASM-7 in 5 min, we observed that the concentration of G7a in the blood was significantly higher than in the lung. However, compared to plasma drug concentration, the concentration of ASM-7 in the lung was substantially higher, reaching 30 mg/kg (Fig. 4A & B). Furthermore, after oral administration of ASM-7 in 4 h, ASM-7 could still be detected in the lung (Fig. 4B). Subsequently, we assessed the distribution of ASM-7 and observed that it exhibited favorable targetability towards the lung (Fig. 4C). Finally, we evaluated the pharmacokinetic (PK) properties of ASM-7 in mice through oral administration and intravenous injection (Fig. 4D & E). The lung concentration-time curves suggest that ASM-7 may undergo slow metabolism in mice. In conclusion, our results indicate that ASM-7 is suitable for oral administration in lung-related diseases.
Fig. 4.
Distribution of G7a or ASM-7 in female ICR mice (n = 3), compounds were administrated at a dose of 10 mL/kg. (A) Compound concentration in blood at certain time points. (B) Compound concentration in lung at certain time points. (C) Distribution of ASM-7 in different organs (heart, liver, spleen, lung, kidney and brain) after 1 h oral administration (30 mg/kg). (D) & (E) Lung concentration-time curves of the PK study of ASM-7.
2.3. Molecular mechanism of ASMs inhibiting SARS-CoV-2 infection
2.3.1. Molecular docking
In order to validate the identified binding site and elucidate the drug design rationale, we conducted molecular docking studies on the top four active molecules (ASM-2, ASM-4, ASM-7, and ASM-11) from the SARS2-S pseudovirus assay. Employing an induced fit docking approach and referencing the binding site of G7a, we docked these four compounds onto the binding interface of S1-RBD and hACE2. This process led to the identification of optimal conformational binding modes, as depicted in Fig. 5 .
Fig. 5.
Predicted binding patterns for ASM-2 with S1-RBD-hACE2 protein (A–C), ASM-4 with S1-RBD-hACE2 protein (D–F), ASM-7 with S1-RBD-hACE2 protein (G–I), and ASM-11 with S1-RBD-hACE2 protein (J-L). S1-RBD protein was displayed as turquoise cartoon or surface, hACE2 protein was displayed as lightcoral cartoon or surface, key residues were displayed as burlywood sticks, and ASM-2 (aqua), -4 (yellow green), -7 (red), and -11(purple) were displayed as balls and sticks.
ASM-2 bound deeply into the target binding pocket (Fig. 5A) and established multiple non-covalent interactions with surrounding amino acid residues. The N-methylpiperazine group of ASM-2 formed a hydrogen bond with ASP30 of hACE2 at 2.32 Å (Fig. 5B & C), while the 4-fluorophenyl group engaged in a pi-cation interaction with LYS353 of hACE2. Additionally, HIE34 of hACE2 exhibited three pi-pi interactions with the 4-fluorophenyl, pyridine, and benzene rings of ASM-2. Furthermore, ARG403 on S1-RBD formed a pi-cation interaction with the benzene ring at 3.80 Å. Interestingly, ASM-2's binding pattern was similar to that of G7a, with ASM-2 exhibiting a greater degree of interaction with hACE2. The robust pi-pi interactions between HIE34 and the three aromatic rings of ASM-2 resulted in the 4-fluorophenyl moiety facing the hACE2 protein, which led to increased affinity with hACE2 and weaker inhibition of viral infection in the SARS2-S pseudovirus assay.
In contrast to ASM-2, ASM-4 exhibited a distinct binding pattern at the S1-RBD-hACE2 interface. Specifically, the 4-methoxyphenyl group of ASM-4 strategically interacted with a compact hydrophobic sub-pocket of S1-RBD, causing to the hydrophilic pyrimidine component to protrude towards the solvent-exposed region (Fig. 5D). Furthermore, the 7-azaindole moiety of ASM-4 formed a hydrogen bond with ASP30 of hACE2 at a distance of 2.05 Å and engaged in a pi-cation interaction with LYS417 of S1-RBD (Fig. 5E & F). Additionally, the 4-methoxyphenyl moiety interacted through pi-cation interaction with ARG403 of S1-RBD and underwent a pi-pi stacking interaction with HIE34 of hACE2. Moreover, the pyridine-piperazine moiety of ASM-4 was solvent-exposed and established polar interactions with hydrophilic amino acids.
The binding pattern of ASM-7 to the protein resembled that of ASM-4. In ASM-7, the fluorophenyl group bound to the hydrophobic pocket of S1-RBD, while the piperazine hydrophilic tail was exposed to the solvent region (Fig. 5G). Further examination of ASM-7 revealed that its 7-azaindole scaffold formed a hydrogen bond with ASP30 of hACE2 at a distance of 2.30 Å and pi-cation interactions with LYS417 of S1-RBD (Fig. 5H & I). ARG403 of S1-RBD showed both a pi-cation interaction with the fluorophenyl group at a distance of 3.51 Å and a hydrogen bond with the “hinge” carbonyl introduced through our design at a distance of 2.33 Å. Furthermore, the protonated piperazine group, exposed to the solvent region, formed a strong hydrogen bond with SER563 of hACE2 at a distance of 1.99 Å. ASM-7 exhibited firm anchoring to the binding site of the S1-RBD and hACE2 interface through hydrogen bonds and pi-cation interactions.
ASM-11 demonstrated a binding mode to S1-RBD-hACE2 akin to ASM-4 and ASM-7. Specifically, the hydrophobic fluorophenyl group of ASM-11 interacted with the hydrophobic site on S1-RBD, while the hydrophilic piperazine tail remained exposed to the protein solvent (Fig. 5J). Moreover, the interaction between ASM-11 and hACE2 involved the formation of hydrogen bonds with ASP30 and GLN388. Additionally, pi-cation interactions occurred between ASM-11 and ARG403 and LYS417 of S1-RBD, and face-to-face pi-pi interactions were observed with TYR453 of S1-RBD, as illustrated in Fig. 5K & L.
In general, ASM-4, ASM-7, and ASM-11 shared analogous binding orientations, with hydrophobic substituted benzene rings fitting into the sub-pocket of S1-RBD. The 7-azaindole scaffold established robust hydrogen bond and pi-cation interactions with hACE2's ASP30 and S1-RBD's LYS417, while the pyrimidine hydrophilic tail segment was exposed to the solvent. ASM-2, however, did not adopt this binding orientation.
2.3.2. Molecular dynamics simulation
To elucidate the binding stability between the ligand and protein in the docking conformation, we conducted MD simulations that incorporated enhanced sampling techniques and all-atom MD simulations.
2.3.2.1. Binding pose metadynamics simulation
The Binding Pose Metadynamics (BPMD) [15] utilizes enhanced sampling techniques to assess the stability and reliability of protein-ligand binding poses in aqueous environments. A PoseScore <2 indicates a stable ligand binding and reliable binding mode, while a PersScore >0.6 indicates sustained and stable hydrogen bond formation between the ligand and the protein. Results from BPMD simulations of compounds ASM-2, ASM-4, ASM-7, and ASM-11 with the S1-RBD-hACE2 complex were presented in Fig. 6A, D, G, and J, respectively. The PoseScore of all four complexes was <2, indicating reliable docking poses and stable binding. However, their PersScore were < 0.6, suggesting a limited capacity to form sustained hydrogen bonds with surrounding residues of the binding site. The collective variable root-mean-square deviation (CV RMSD) curves of the ASM-2, ASM-4, and ASM-7 complexes reached equilibrium within 10 × 10 ns of simulation time. Especially, the CV RMSD of the ASM-7 complex exhibited a steady convergence after 2 ns and stabilized at around 1.5 Å, indicating the strongest binding stability of ASM-7 to the protein in aqueous solution.
Fig. 6.
BPMD simulations for ASM-2/S1-RBD-hACE2 complex (A), ASM-4/S1-RBD-hACE2 complex (D), ASM-7/S1-RBD-hACE2 complex (G), and ASM-11/S1-RBD-hACE2 complex (J). 100 ns MD simulations for ASM-2/S1-RBD-hACE2 complex (B & C), ASM-4/S1-RBD-hACE2 complex (E & F), ASM-7/S1-RBD-hACE2 complex (H & I), and ASM-11/S1-RBD-hACE2 complex (K & L).
2.3.2.2. 100 ns MD simulation for ASM-2, ASM-4, ASM-7, and ASM-11 with S1-RBD-hACE2 protein
BPMD analysis revealed the stable binding of ASM-2, ASM-4, ASM-7, and ASM-11 to S1-RBD-hACE2. To gain further insight into their interactions with surrounding residues in the binding site, we performed 100 ns all-atom MD simulations for each of the four systems under aqueous conditions.
The RMSD fluctuations during the MD simulations were analyzed for protein backbone and ligand heavy atom in the ASM-2, ASM-4, ASM-7, and ASM-11 systems (Fig. 6B, E, H, and K, respectively). In the ASM-2 complex, the protein backbone RMSD increased from 1.5 Å to 4.6 Å, with equilibration commencing after 80 ns and stabilization at approximately 3.6 Å. The heavy atom RMSD in ASM-2 followed a similar trend, fluctuating within the range of 1–4.2 Å and converging in the final 20 ns. These observations imply that ASM-2 exhibited a certain degree of fluctuation within the binding pocket. In the ASM-4 complex, the protein backbone RMSD remained stable at approximately 2.8 Å for the first 60 ns, rapidly increased to 4.5 Å after 60 ns, and eventually plateaued at 3.3 Å after 80 ns. In contrast, the heavy atoms of ASM-4 exhibited a different pattern, with significant fluctuations ranging from 2 to 6.8 Å initially, followed by stabilizing at approximately 3.0 Å after 60 ns. In the ASM-7 complex system, both protein and ligand RMSD exhibited minimal fluctuations, indicating a high degree of stability in the binding of ASM-7 to S1-RBD-hACE2. The protein backbone RMSD in the ASM-11 system displayed relatively stable fluctuations of 2.0 to 3.8 Å, similarly to the ligand heavy atoms at the binding site with an RMSD fluctuation range of 1–4.2 Å. Overall, except for ASM-4, which induced larger fluctuations within the binding pocket, the other three compounds demonstrated stable binding.
Further analysis investigated ligand-protein interactions. Fig. 6C demonstrated that during 99 % of the simulation time, the7-azaindole hydrogen atom of ASM-2 formed a hydrogen bond with TYR505 backbone atoms in S1-RBD. Moreover, the pyridine ring of the 7-azaindole engaged in a pi-pi interaction with TYR505. Additionally, p-fluorophenyl exhibited a pi-pi interaction with TYR449 in S1-RBD over 51 % of the simulation. The carbonyl group of ASM-2 formed a hydrogen bond with LYS353 in hACE2 during 76 % of the simulation. Interestingly, HIS34 in hACE2 engaged in a pi-pi interaction with ASM-2's benzene ring in the solvent region for 92 % of the simulation. Furthermore, the side chain of ASP30 in hACE2 formed a hydrogen bond with protonated N-methylpiperazine with 51 % occupancy. Following MD simulation, ASM-2 exhibited significant conformational alteration and developed new non-bonding interactions: pi-pi stacking with TYR449, hydrogen bonds and pi-pi interaction with TYR505, as compared to the docking conformation (Fig. 5C). In Fig. 6F, ASM-4's 7-azaindole formed hydrogen bonds with ASP30 in hACE2 during 79 % of the simulation, and with LYS26 for 30 % of the simulation. Additionally, the p-methoxyphenyl group of ASM-4 formed pi-cation interactions with ARG403 in S1-RBD for 64 % of the simulation. ASM-4 also interacted with binding site residues via water bridges. Loss of pi-cation interactions between ASM-4's 7-azaindole and LYS417 in S1-RBD observed in the docked conformation (Fig. 5F), caused significant fluctuations in the binding site. Fig. 6I illustrated ASM-7's interaction with the protein during a 100 ns MD simulation. Remarkably, for 96 % of the simulation, the 7-azaindole hydrogen on ASM-7 formed a hydrogen bond with ASP30 backbone atom in hACE2, enhancing binding affinity. All non-covalent interactions, except those involving the carbonyl and piperazine, were consistently observed during the MD simulation, validating ASM-7's docked conformation (Fig. 5I) and binding site stability. Fig. 6L presented interaction details between ASM-11 and protein residues during MD simulation. Similar to ASM-7, the 7-azaindole scaffold of ASM-11 established hydrogen bonds with hACE2's ASP30 for 98 % of the simulation. ASM-11 also formed persistent hydrogen bonds (41 %) with hACE2's ASN33 side chain and a water bridge with S1-RBD's GLU406 through a water molecule. Compared to ASM-7, ASM-11 exhibited weaker pi-cation interaction with S1-RBD's LYS417 and lacked a pi-pi interaction with TYR453. These results indicate inferior binding stability of ASM-11 compared to ASM-7. In brief, after a 100 ns all-atom MD simulation, the binding stability of ASM-7 to the S1-RBD-hACE2 protein interface surpassed that of the other three complex systems.
2.3.2.3. 300 ns MD simulation for ASM-7 with S1-RBD-hACE2 protein
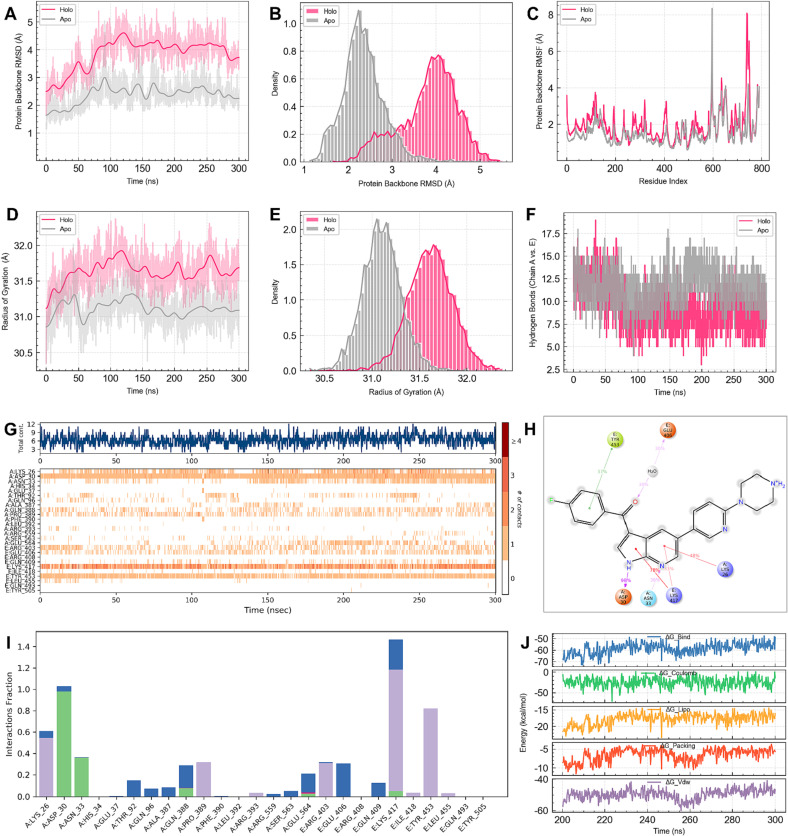
Based on the results of the SARS2-S pseudovirus assay, native SARS-CoV-2 antiviral activity, cytotoxicity test, and MD simulations, we selected the ASM-7 complex for longer time-scale MD simulations (Figs. 7 & S2). Our objective was to systematically investigate the impact of ASM-7 on the binding of S1-RBD to hACE2 in the complex. Fig. 7A presented the protein backbone RMSD over time for the ligand-bound ASM-7 system (Holo) and the ligand-free system (Apo). Both systems reached equilibrium after 100 ns, but the Holo system exhibited higher protein fluctuations (~4.0 Å) compared to the Apo system (~2.5 Å), indicating that ASM-7 could affect the stability of the S1-RBD and hACE2 complex. Fig. 7B showed the protein backbone RMSD distribution histogram, clearly demonstrating smaller protein fluctuations in the Apo system compared to the Holo system. Additionally, Fig. 7C displayed the root mean square fluctuation (RMSF) of the protein backbone in the ASM-7 bound and unbound complex systems. Our analysis showed reduced protein atomic fluctuations in the Apo system, except for residue 600, suggesting that ASM-7 significantly affected the stability of the S1-RBD-hACE2 complex. The radius of gyration (Rg), an important metric for characterizing protein compactness and stability, was examined. A smaller Rg indicates a more stable and compactly folded protein structure. In Fig. 7D, the line graph displayed the Rg of protein backbone atoms over time. The Rg of the Apo system stabilized at approximately 31.0 Å, while the Rg of the Holo system stabilized at around 31.6 Å. These results indicated that the protein structure of the Holo system with ASM-7 was less stable than the ligand-free system. Moreover, Fig. 7E, the Rg distribution histogram, clearly demonstrated that the Apo system exhibited smaller Rg values, indicating greater stability. To explore the impact of ASM-7 binding on non-bonded interactions between S1-RBD and hACE2, we evaluated the number of hydrogen bonds formed between hACE2 (chain A) and S1-RBD (chain E) in the Apo and Holo systems throughout the simulation (Fig. 7F). Our findings indicate that as the simulation reached equilibrium, the Apo system formed a greater number of hydrogen bonds (~12) compared to the Holo system (~8.5), suggesting that ASM-7 may influence the formation of certain hydrogen bonds between hACE2 and S1-RBD.
Fig. 7.
300 ns MD simulations for Holo system (ASM-7/S1-RBD-hACE2 complex) and Apo system (S1-RBD-hACE2 complex). (A) Timeseries analysis of protein backbone RMSD. (B) RMSD distribution histogram of protein backbone atoms. (C) Timeseries analysis of protein backbone RMSF. (D) Timeseries analysis of protein backbone Rg. (E) Rg distribution histogram of protein backbone atoms. (F) Time series of the number of hydrogen bonds formed between hACE2 (chain A) and S1-RBD (chain E). (G) Protein-ligand contacts of ASM7 and S1-RBD-hACE2. (H) Ligand-protein contacts of ASM7 and S1-RBD-hACE2. (I) Protein-ligand interactions of ASM7 and S1-RBD-hACE2. (J) Timeseries analysis of binding energies between ASM-7 and S1-RBD-hACE2.
We analyzed the interaction between ASM-7 and the S1-RBD-hACE2 protein binding interface. Protein-ligand contacts during the 300 ns MD simulation were depicted in Fig. 7G. A total of approximately 8 specific contacts were observed between the protein and ASM-7 throughout the simulation. Notably, residue ASP30 of hACE2 maintained contact with ASM-7, while residues LYS26, ASN33, GLN388, and PRO389 also exhibited varying degrees of sustained contact. Interestingly, ASM-7 consistently interacted with residues LYS417 and TYR453 of S1-RBD throughout the entire MD simulation. Fig. 7H depicted the interaction between ASM-7 and the surrounding binding site residues. The hydrogen on the 7-azaindole group of ASM-7 formed a stable hydrogen bond with the backbone atom of residue ASP30 of hACE2 during 98 % of the simulation time, indicating the stability of this interaction. Furthermore, ASM-7 established stable hydrogen bonds and pi-cation interactions with residues ASN33 and LYS26 of hACE2. Additionally, ASM-7 formed sustained and stable pi-cation, pi-pi, and water bridge interactions with S1-RBD residues, including LYS417, TYR453, and GLU406. Fig. 7I provided a detailed representation of the specific types of protein-ligand interactions observed in Fig. 7G. Specifically, residues ASP30 and ASN33 of hACE2 primarily formed hydrogen bonds (green bars) with ASM-7, while LYS26 and PRO389 mainly participated in hydrophobic interactions (violet bars). On the other hand, residues ARG403, LYS417, and TYR453 of S1-RBD predominantly interacted hydrophobically, and GLU406 primarily formed a water-bridged interaction (blue bars) with ASM-7. Remarkably, the crucial non-covalent bonds between ASM-7 and the S1-RBD region are not prone to mutation in the Omicron variants. This suggests that ASM-7 can stably bind to the S1-RBD-hACE2 interface, even in the presence of Omicron mutations, without developing resistance.
To assess the binding affinity of ASM-7 with the S1-RBD-hACE2 complex, we employed the MMGBSA method to calculate the binding free energy from the final 100 ns MD trajectory. The findings, depicted in Fig. 7J, illustrate the temporal changes in both the binding free energy and the significant energy terms contributing to the overall energy. The calculated binding free energy between ASM-7 and S1-RBD-hACE2 was determined to be −58.65 ± 4.78 kcal/mol. Notably, the van der Waals (Vdw) energy term emerged as the primary contributor to the total energy, with a magnitude of −49.56 ± 3.20 kcal/mol, followed by the Coulomb energy term at −24.58 ± 11.46 kcal/mol. The solvation energy term exhibited a slightly smaller contribution than the Coulomb energy term, amounting to −17.81 ± 1.67 kcal/mol. Taken together, these outcomes suggest that ASM-7 possesses the capacity to disrupt the interaction between hACE2 and S1-RBD and stably bind to the binding interface of S1-RBD-hACE2.
The Dynamical Cross-Correlation Matrix (DCCM) [16] facilitates the analysis of motional patterns and interactions among specific atoms, such as Cα atoms, in amino acids within a protein. DCCM values range from 1 to −1, with 1 representing a strong positive correlation, implying the same direction of movement; −1 representing a negative correlation, implying the opposite direction of movement; and 0 indicating no correlation. Through DCCM mapping, correlated or anti-correlated motions between Cα atoms in the S1-RBD-hACE2 protein can be identified, both with and without ASM-7 (Fig. 8A & D). The Holo system demonstrated darker colors in regions of positively or negatively correlated amino acids, compared to the Apo system, indicating more pronounced movements in the same or opposite direction among residues in the Holo system bound with ASM-7. Notably, the R1 and R2 regions of the S1-RBD and hACE2 binding interface displayed a higher number and stronger negative correlations (red) between Cα atoms in the Holo system than in the Apo system, suggesting that ASM-7 induced greater opposite movements between amino acids in the binding interface of S1-RBD and hACE2.
Fig. 8.
DCCM map for the Holo (A) and Apo (D) complexes showing positive and negative correlative motions between the S1-RBD and hACE2. Blue denotes positive correlations, whereas red denotes negative correlations. The S1-RBD-hACE binding interface regions were labeled as R1 and R2. FEL of Holo (B) and Apo (E) systems. A. Motion of the proteins for Holo (C) and Apo (F) complexes. RINs analysis of the Holo (G) and Apo (H) complexes.
This study utilized protein Cα atoms for principal component analysis (PCA) to capture significant conformational changes in protein. The results revealed that the first ten eigenvectors contribute to approximately 80 % of the total motion in MD simulations of both Holo and Apo systems (Fig. S3B). Specifically, the first two principal components (PCs) explain 58.36 % and 55.52 % of the covariance in the Holo and Apo systems, respectively (Fig. S3A). Hence, PC1 and PC2 describe the most significant protein motions in both MD simulations. To assess the relative free energy of different protein conformations in the simulation system, the free energy landscape (FEL) method was employed. FELs based on PC1 and PC2 were calculated and plotted for the Holo (Fig. 8B) and Apo (Fig. 8E) systems using the Boltzmann relation. The energy-minimum basins are depicted in a color gradient from red to blue, where the deepest blue region represents the most stable conformation with the lowest energy. The Apo system exhibited a greater number of blue and deep blue regions compared to the Holo system, as evident in Fig. 8B & E. Analyzing the distribution of orange-yellow and red regions led to the inference that the Apo system displayed greater stability than the Holo system. These observation suggest that the binding of ASM-7 may influence the stability of the S1-RBD-hACE2 protein.
We analyzed the Holo and Apo systems by extracting their first and last frames from the MD simulations to investigate the protein's motion direction. Fig. 8C displayed the motion direction of the Holo system during the 300 ns MD simulation. The initial state of the system was represented by a white cartoon (protein) and magenta spheres (ASM-7). The arrow in the figure indicates the protein's motion direction, while its length represents the extent of protein displacement. In the Holo system, S1-RBD exhibited a greater displacement opposite to hACE2, and the amino acids at the S1-RBD binding site to ASM-7 moved away from hACE2. Although some hACE2 amino acids moved in the opposite direction of S1-RBD, the overall motion was not significant. In contrast, the Apo system (Fig. 8F), exhibited weaker movement of S1-RBD and hACE2 compared to the Holo system, and there were no significant fluctuations observed at the S1-RBD-hACE2 protein interface. The RMSF values of protein backbone atoms in Fig. 7C were color-mapped onto the simulation proteins, where red, white, and blue represented large, small, and minimal fluctuations, respectively. Fig. S3C & D, clearly indicate that the ASM-7 binding site residues in the Holo system were predominantly colored red, while those in the Apo system were mostly white to light blue. This indicates that ASM-7 significantly impacted the stability of its binding site residues. These findings provide further evidence of ASM-7's potential to influence the binding of S1-RBD to hACE2.
This study employed Residue Interaction Networks (RINs) as a visualization method for protein interactions. RINs represent residues as nodes and non-covalent interactions as edges. These networks were utilized to investigate the impact of ASM-7 on the interactions between S1-RBD and hACE2. Specifically, the last 100 ns of equilibrated Holo and Apo system trajectories were extracted. Non-covalent interactions between residues within the protein structures were then calculated. Fig. 8G & H illustrated the resulting RINs map of S1-RBD (turquoise nodes) and hACE2 (lightcoral nodes). Hydrogen bonds were represented by blue lines, ionic bonds by yellow dashed lines, and van der Waals interactions by dark green dotted lines. In the Holo system (Fig. 8G), 32 nodes and 47 edges were retained, with edges having a non-bonded interaction frequency >0.1. The highest frequency interaction was a hydrogen bond (1.44), and the residue with the highest degree was TYR41 of hACE2 (degree = 9.88). Similarly, in the Apo system (Fig. 8H), 35 nodes and 69 edges were retained, with edges having a non-bonded interaction frequency >0.1. The highest frequency interaction was also a hydrogen bond (1.70), and the residue with the highest degree was TYR41 of hACE2 (degree = 11.01). Our RINs analyses of the MD simulation trajectories revealed that ASM-7 has the potential to weaken the non-covalent interactions between S1-RBD and hACE2 residues.
The MMGBSA method was also employed to determine the binding free energy of the S1-RBD and hACE2 complex during the final 100 ns trajectories of the Holo and Apo systems (Fig. S3E & F). The Holo system exhibited a binding free energy of −82.97 ± 15.10 kcal/mol, with van der Waals (Vdw) energy contributing significantly at −108.93 ± 11.85 kcal/mol, while hydrogen bonds had the lowest contribution at −4.92 ± 2.21 kcal/mol. Conversely, the Apo system displayed a binding free energy of −93.05 ± 9.80 kcal/mol, where Vdw energy played a major role at −97.17 ± 7.95 kcal/mol, and hydrogen bonds had the lowest contribution at −11.42 ± 1.58 kcal/mol. These results align with the hydrogen bond analysis and RINs analysis, demonstrating that the presence of ASM-7 weakened the interaction between S1-RBD and hACE2. Consequently, the energy contribution of hydrogen bonds decreased in the Holo system, ultimately leading to reduced stability of the complex. In general, ASM-7 negatively impacted the binding affinity of S1-RBD and hACE2, resulting in decrease complex stability.
3. Methods and materials
3.1. Chemistry
All compounds were purified by silica gel chromatography. Unless otherwise noted, materials were obtained from commercial suppliers (Bide Pharmatech Ltd., Shanghai, China) and were used without further purification. All reactions were performed under a positive pressure of nitrogen at an ambient temperature (unless otherwise stated). Analytical thin-layer chromatography (TLC) visualized by UV was performed on glass-backed silica gel 60 F254 plates (Qingdao Haiyang Chemical, Qingdao, China) and eluted with the appropriate solvent ratios (v/v). The reactions were assayed by TLC and terminated as judged by the consumption of starting material. High-resolution mass spectra (HRMS) data were acquired by a quadrupole-orbitrap (Q-Exactive) mass spectrometer (Thermo Scientific, Hemel Hempstead, UK) equipped with a heated electrospray ionization source (HESI-II). 1H NMR and 13C NMR spectra were recorded on a Bruker AV2 600 Ultra shield spectrometer (Bruker, Switzerland) at 600 and 151 MHz respectively. Proton resonances were reported in parts per million (ppm) downfield from tetramethylsilane (TMS). Spin multiplicities were described as (s, singlet; d, doublet; t, triplet; q, quartet; quint, quintuplet; sept, septuplet; dd, doublet of doublets; dt, doublet of triplets; bs, broad singlet). Spectra were obtained in DMSO‑d 6.
3.1.1. tert-Butyl 4-(5-bromopyridin-2-yl)piperazine-1-carboxylate (2a)
To a 100 mL dry round bottom flask was added 1a (1.76 g, 10.0 mmol), DIPEA (3.87 g, 30.0 mmol), tert-butyl piperazine-1-carboxylate (1.96 g, 10.5 mmol) and DMF (20 mL), which was stirred for 4 h at 120 °C, the resulting mixture was evacuated and refilled with N2 three times. After the reaction was completed, the mixture was cooled to room temperature and then poured into ice water (100 mL). The resulting precipitate was filtered and washed with water (20 mL) to give 2a (2.49 g) as white solid, yield 73 %, LRMS (ESI) m/z: 343.2 [M + H]+.
3.1.2. 1-(5-Bromopyridin-2-yl)-4-methylpiperazine (2b)
To a 100 mL dry round bottom flask was added 1a (1.76 g, 10.0 mmol), DIPEA (3.87 g, 30.0 mmol), 1-methylpiperazine (1.05 g, 10.5 mmol) and DMF (20 mL), which was stirred for 4 h at 120 °C, the resulting mixture was evacuated and refilled with N2 three times. After the reaction was completed, the mixture was cooled to room temperature and then poured into ice water (100 mL). The resulting precipitate was filtered and washed with water (20 mL) to give 2b (2.04 g) as white solid, yield 80 % LRMS (ESI) m/z: 257.2 [M + H]+.
3.1.3. 1-(5-Bromopyridin-2-yl)-4-(methylsulfonyl)piperazine (2c)
To a 100 mL dry round bottom flask was added 1a (1.76 g, 10.0 mmol), DIPEA (3.87 g, 30.0 mmol), 1-methylpiperazine (1.72 g, 10.5 mmol) and DMF (20 mL), which was stirred for 4 h at 120 °C, the resulting mixture was evacuated and refilled with N2 three times. After the reaction was completed, the mixture was cooled to room temperature and then poured into ice water (100 mL). The resulting precipitate was filtered and washed with water (20 mL) to give 2c (2.23 g) as brown solid, yield 70 %, LRMS (ESI) m/z: 321.2 [M + H]+.
3.1.4. 4-(5-Bromopyridin-2-yl)morpholine (2d)
To a 100 mL dry round bottom flask was added 1a (1.76 g, 10.0 mmol), DIPEA (3.87 g, 30.0 mmol), morpholine (0.92 g, 10.5 mmol) and DMF (20 mL), which was stirred for 4 h at 120 °C, the resulting mixture was evacuated and refilled with N2 three times. After the reaction was completed, the mixture was cooled to room temperature and then poured into ice water (100 mL). The resulting precipitate was filtered and washed with water (20 mL) to give 2d (1.58 g) as white solid, yield 65 %, LRMS (ESI) m/z: 244.2 [M + H]+.
3.1.5. tert-Butyl 4-(4-bromopyridin-2-yl)piperazine-1-carboxylate (2g)
To a 100 mL dry round bottom flask was added 1b (1.76 g, 10.0 mmol), DIPEA (3.87 g, 30.0 mmol), tert-butyl piperazine-1-carboxylate (1.96 g, 10.5 mmol) and DMF (20 mL), which was stirred for 4 h at 120 °C, the resulting mixture was evacuated and refilled with N2 three times. After the reaction was completed, the mixture was cooled to room temperature and then poured into ice water (100 mL). The resulting precipitate was filtered and washed with water (20 mL) to give 2 g (2.35 g) as white solid, yield 69 %, LRMS (ESI) m/z: 343.2 [M + H]+.
3.1.6. 4-(4-Bromopyridin-2-yl)morpholine (2h)
To a 100 mL dry round bottom flask was added 1b (1.76 g, 10.0 mmol), DIPEA (3.87 g, 30.0 mmol), morpholine (0.92 g, 10.5 mmol) and DMF (20 mL), which was stirred for 4 h at 120 °C, the resulting mixture was evacuated and refilled with N2 three times. After the reaction was completed, the mixture was cooled to room temperature and then poured into ice water (100 mL). The resulting precipitate was filtered and washed with water (20 mL) to give 2h (1.45 g) as white solid, yield 60 %, LRMS (ESI) m/z: 244.2 [M + H]+.
3.1.7. tert-Butyl ((1r,4r)-4-((4-bromo-5-chloropyridin-2-yl)amino)cyclohexyl)carbamate (2i)
To a 100 mL dry round bottom flask was added 1c (2.10 g, 10.0 mmol), DIPEA (3.87 g, 30.0 mmol), tert-butyl ((1r, 4r)-4-aminocyclohexyl)carbamate (2.25 g, 10.5 mmol) and DMF (20 mL), which was stirred for 4 h at 120 °C, the resulting mixture was evacuated and refilled with N2 three times. After the reaction was completed, the mixture was cooled to room temperature and then poured into ice water (100 mL). The resulting precipitate was filtered and washed with water (20 mL) to give 2i (2.50 g) as white solid, yield 62 %, LRMS (ESI) m/z: 405.5 [M + H]+.
3.1.8. (5-Bromo-1H-pyrrolo[2,3-b]pyridin-3-yl)(2-hydroxyphenyl)methanone(6a)
To a suspension of AlCl3 (11.98 g, 90.0 mmol) in dichloromethane (200 mL) was added 5-bromo-1H-pyrrolo[2,3-b]pyridine (5.88 g, 30.0 mmol). After stirring for 45 min, 2-hydroxybenzoyl chloride (7.02 g, 45.0 mmol) was added and the reaction mixture was stirred for 12 h, before quenching with MeOH at 0 °C, the mixture was concentrated in vacuum, the pH was changed to 4 by addition of 1 N aqueous NaOH, and the aqueous layer was extracted with EA. The organic layer was dried over MgSO4 and concentrated, the residue was purified by flash column chromatography (EA/PE = 1:2) to give the product 6a (5.02 g) as white solid, yield 53 %, LRMS (ESI) m/z: 318.2 [M + H]+.
3.1.9. (5-Bromo-1H-pyrrolo[2,3-b]pyridin-3-yl)(4-fluorophenyl)methanone (6b)
To a suspension of AlCl3 (11.98 g, 90.0 mmol) in dichloromethane (200 mL) was added 5-bromo-1H-pyrrolo[2,3-b]pyridine (5.88 g, 30.0 mmol). After stirring for 45 min, 4-fluorobenzoyl chloride (7.11 g, 45.0 mmol) was added and the reaction mixture was stirred for 12 h, before quenching with MeOH at 0 °C, the mixture was concentrated in vacuum, the pH was changed to 4 by addition of 1 N aqueous NaOH, and the aqueous layer was extracted with EA. The organic layer was dried over MgSO4 and concentrated, the residue was purified by flash column chromatography (EA/PE = 1:5) to give the product 6b (5.71 g) as white solid, yield 60 %, LRMS (ESI) m/z: 320.2 [M + H]+.
3.1.10. (5-Bromo-1H-pyrrolo[2,3-b]pyridin-3-yl)(4-methoxyphenyl)methanone (6c)
To a suspension of AlCl3 (11.98 g, 90.0 mmol) in dichloromethane (200 mL) was added 5-bromo-1H-pyrrolo[2,3-b]pyridine (5.88 g, 30.0 mmol). After stirring for 45 min, 4-methoxybenzoyl chloride (7.65 g, 45.0 mmol) was added and the reaction mixture was stirred for 12 h, before quenching with MeOH at 0 °C, the mixture was concentrated in vacuum, the pH was changed to 4 by addition of 1 N aqueous NaOH, and the aqueous layer was extracted with EA. The organic layer was dried over MgSO4 and concentrated, the residue was purified by flash column chromatography (EA/PE = 1:5) to give the product 6c (5.05 g) as white solid, yield 51 %, LRMS (ESI) m/z: 332.2 [M + H]+.
3.1.11. (5-Bromo-1H-pyrrolo[2,3-b]pyridin-3-yl)(3-fluorophenyl)methanone (6d)
To a suspension of AlCl3 (11.98 g, 90.0 mmol) in dichloromethane (200 mL) was added 5-bromo-1H-pyrrolo[2,3-b]pyridine (5.88 g, 30.0 mmol). After stirring for 45 min, 2-hydroxybenzoyl chloride (7.02 g, 45.0 mmol) was added and the reaction mixture was stirred for 12 h, before quenching with MeOH at 0 °C, the mixture was concentrated in vacuum, the pH was changed to 4 by addition of 1 N aqueous NaOH, and the aqueous layer was extracted with EA. The organic layer was dried over MgSO4 and concentrated, the residue was purified by flash column chromatography (EA/PE = 1:2) to give the product 6d (5.26 g) as white solid, yield 55 %, LRMS (ESI) m/z: 318.2 [M + H]+.
3.1.12. General procedures for the synthesis of ASMs and biotin probes
6a–6d (1 eq) were coupled with 3a-3i (1.5 eq) under the catalysis of Pd(dppf)Cl2 (0.1 eq) and dry K2CO3 (3 eq) at 120 °C for 12 h via Suzuki reaction to obtain ASMs, in which Boc deprotection of ASM-3, ASM-6, ASM-8, ASM-10, ASM-14, ASM-17, ASM-19 and ASM-21 under TFA condition gave the products ASM-4, ASM-7, ASM-9, ASM-11, ASM-15, ASM-18, ASM-20 and ASM-22, respectively. For ASM-21 and ASM-23, it was prepared through ASM-6 (1 eq) and ethyl 3-bromopropanoate (1.5 eq) or (bromomethyl)cyclopropane (1.5 eq), respectively, under the action of NaI (3 eq) in DMF. As for the Suzuki coupling reaction, the solvent was DME:H2O = 4:1(v/v), when TLC detected reaction completion, the mixture extracted by ethyl acetate (EA) and H2O, the organic layer was concentrated and purified by flash chromatography.
N-Biotinyl-6-amihexaic acid (7, 1.1 eq) and corresponding original molecules (1 eq) were mixed in the presence of HATU (1.1 eq), DIPEA (3 eq) in DMF at room temperature. When TLC detected reaction completion, the mixture was poured into ice water and extracted by EA for three times, the organic layers were collected and purified by flash chromatography to obtain Biotin-G7a and Biotin-ASM-7.
(2-Hydroxyphenyl)(5-(2-(4-methylpiperazin-1-yl)pyridin-4-yl)-1H-pyrrolo[2,3-b]pyridin-3-yl)methanone (ASM-1): white solid. Yield: 32 %, 1H NMR (600 MHz, DMSO‑d 6) δ 12.92 (br s, 1H), 9.60 (br s, 2H), 8.73 (d, J = 2.02 Hz, 1H), 8.70 (d, J = 2.02 Hz, 1H), 8.51 (d, J = 2.02 Hz, 1H), 8.24 (br dd, J = 2.12, 9.09 Hz, 1H), 8.24 (s, 1H), 7.72 (d, J = 7.70 Hz, 1H), 7.60–7.68 (m, 2H), 7.53 (dt, J = 2.20, 8.53 Hz, 1H), 7.32 (d, J = 9.16 Hz, 1H), 3.93–4.03 (m, 4H), 3.27 (br s, 4H), 2.33 (s, 3H); HRMS (ESI) calcd for C24H24N5O2 + [M + H]+ 414.1925, found 414.1926.
(4-Fluorophenyl)(5-(4-(4-methylpiperazin-1-yl)phenyl)-1H-pyrrolo[2,3-b]pyridin-3-yl)methanone (ASM-2): yellow solid. Yield: 51 %, 1H NMR (600 MHz, DMSO‑d 6) δ 8.66 (d, J = 2.20 Hz, 1H), 8.62 (d, J = 2.20 Hz, 1H), 8.14 (s, 1H), 7.93 (dd, J = 5.59, 8.53 Hz, 2H), 7.59 (d, J = 8.62 Hz, 2H), 7.39 (t, J = 8.80 Hz, 2H), 7.07 (d, J = 8.62 Hz, 2H), 3.19–3.22 (m, 4H), 2.44–2.49 (m, 4H), 2.24 (s, 3H); 13C NMR (151 MHz, DMSO‑d 6) δ 188.9, 165.3 (d, J = 246.5 Hz, 1C), 163.7, 150.8, 148.6, 143.6, 136.9, 136.7, 131.7 (d, J = 8.8 Hz, 1C), 131.4, 128.9, 128.0 (2C), 127.0, 119.3, 116.2(d, J = 22.0 Hz, 2C), 116.1, 115.9, 114.0, 55.0 (2C), 48.3 (2C), 46.2; HRMS (ESI) calcd for C25H24FN4O+ [M + H]+ 415.1929, found 415.1928.
tert-Butyl-4-(5-(3-(4-methoxybenzoyl)-1H-pyrrolo[2,3-b]pyridin-5-yl)pyridin-2-yl)piperazine-1-carboxylate (ASM-3): white solid. Yield: 39 %, 1H NMR (600 MHz, DMSO‑d 6) δ 12.56–12.76 (m, 1H), 8.63–8.65 (m, 1H), 8.60–8.63 (m, 1H), 8.50 (d, J = 2.38 Hz, 1H), 8.13 (d, J = 2.75 Hz, 1H), 7.94 (dd, J = 2.57, 8.80 Hz, 1H), 7.85–7.89 (m, 2H), 7.10 (m, J = 8.80 Hz, 2H), 6.99 (d, J = 8.80 Hz, 1H), 3.86–3.89 (s, 3H), 3.55–3.59 (m, 4H), 3.44–3.49 (m, 4H), 1.44 (s, 9H); 13C NMR (151 MHz, DMSO‑d 6) δ 189.0, 177.0, 162.5, 155.0, 146.1, 136.8, 131.3 (2C), 127.1, 114.3 (2C), 107.9 (2C), 103.7, 103.3, 102.6, 90.8, 89.6, 89.4, 79.5, 66.0, 63.8, 55.9 (2C), 44.9 (2C), 28.6 (3C); HRMS (ESI) calcd for C29H31N5O4 + [M + H]+ 514.2449, found 514.2449.
(4-Methoxyphenyl)(5-(6-(piperazin-1-yl)pyridin-3-yl)-1H-pyrrolo[2,3-b]pyridin-3-yl)methanone (ASM-4): white solid. Yield: 46 %, 1H NMR (600 MHz, DMSO‑d 6) δ 12.82 (br d, J = 1.83 Hz, 1H), 9.50 (br s, 2H), 8.75 (d, J = 2.20 Hz, 1H), 8.73 (d, J = 2.20 Hz, 1H), 8.55 (d, J = 2.20 Hz, 1H), 8.27 (br d, J = 8.44 Hz, 1H), 8.23 (d, J = 2.94 Hz, 1H), 7.93 (d, J = 8.62 Hz, 2H), 7.34 (br d, J = 8.80 Hz, 1H), 7.17 (d, J = 8.80 Hz, 2H), 3.98 (br d, J = 4.77 Hz, 4H), 3.93 (s, 3H), 3.31 (br s, 4H); 13C NMR (151 MHz, DMSO‑d 6) δ 188.9, 162.6, 155.0, 148.9, 143.2, 140.9, 136.4, 132.4, 131.3 (2C), 127.8, 126.6, 124.9, 119.5, 114.3(2C), 114.2, 111.8, 56.1, 55.9, 43.4 (2C), 42.4 (2C); HRMS (ESI) calcd for C24H24N5O2 + [M + H]+ 414.1925, found 414.1922.
(4-Fluorophenyl)(5-(6-morpholinopyridin-3-yl)-1H-pyrrolo[2,3-b]pyridin-3-yl)methanone (ASM-5): white solid. Yield: 32 %, 1H NMR (600 MHz, DMSO‑d 6) δ 8.62–8.66 (m, 2H), 8.51 (d, J = 2.38 Hz, 1H), 8.16 (s, 1H), 7.94–7.97 (m, 1H), 7.92 (dd, J = 5.59, 8.53 Hz, 2H), 7.39 (t, J = 8.80 Hz, 2H), 6.99 (d, J = 8.80 Hz, 1H), 3.72–3.79 (m, 4H), 3.50–3.58 (m, 4H); 13C NMR (151 MHz, DMSO‑d 6) δ 188.7, 165.3 (d, J = 246.5 Hz, 1C), 158.9, 149.2, 146.3, 144.6, 143.3, 136.7, 135.5, 131.7 (d, J = 8.8 Hz, 2C), 128.8, 127.0, 124.3, 119.5, 116.0 (d, J = 22.0 Hz, 2C), 114.0, 107.8, 66.4 (2C), 45.6 (2C); HRMS (ESI) calcd for C23H20FN4O2 + [M + H]+ 403.1565, found 403.1563.
tert-Butyl-4-(5-(3-(4-fluorobenzoyl)-1H-pyrrolo[2,3-b]pyridin-5-yl)pyridin-2-yl)piperazine-1-carboxylate (ASM-6): white solid. Yield: 31 %, 1H NMR (600 MHz, DMSO‑d 6) δ 8.69–8.71 (m, 1H), 8.68–8.69 (m, 1H), 8.56 (d, J = 2.20 Hz, 1H), 8.21 (s, 1H), 8.00 (m, 3H), 7.45 (t, J = 8.80 Hz, 2H), 7.05 (d, J = 8.80 Hz, 1H), 3.60–3.65 (m, 4H), 3.49–3.53 (m, 4H), 1.50 (s, 9H); 13C NMR (151 MHz, DMSO‑d 6) δ 188.8, 165.3 (d, J = 246.5 Hz, 1C), 163.7, 158.5, 154.4, 149.0, 146.1, 143.3, 137.3 (br s, 1C), 136.9–136.5 (m, 1C), 131.7 (br d, J = 8.8 Hz, 1C), 128.8, 127.0, 124.1, 119.4, 116.0 (br d, J = 22.0 Hz, 2C), 114.0, 107.9 (2C), 79.5 (2C), 44.9 (2C), 28.5(3C); HRMS (ESI) calcd for C28H29FN5O3 + [M + H]+ 502.2249, found 502.2249.
(4-Fluorophenyl)(5-(6-(piperazin-1-yl)pyridin-3-yl)-1H-pyrrolo[2,3-b]pyridin-3-yl)methanone (ASM-7): white solid. Yield: 42 %, 1H NMR (600 MHz, DMSO‑d 6) δ 8.61–8.69 (m, 2H), 8.52 (d, J = 2.38 Hz, 1H), 8.17 (s, 1H), 7.97 (dd, J = 2.48, 8.89 Hz, 1H), 7.91–7.95 (m, 2H), 7.39 (t, J = 8.80 Hz, 2H), 7.03 (d, J = 8.80 Hz, 1H), 3.63–3.71 (m, 5H), 3.00–3.11 (m, 4H); 13C NMR (151 MHz, DMSO‑d 6) δ 188.8, 165.4 (d, J = 246.5 Hz, 1C), 163.7, 158.5, 148.8, 146.1 (2C), 143.4, 136.6 (d, J = 2.2 Hz, 1C), 136.5, 131.7 (d, J = 8.8 Hz, 1C), 128.9, 127.1, 124.1, 119.3, 116.0 (d, J = 22.0 Hz, 2C), 114.1, 107.8, 48.6 (2C), 44.6 (2C); HRMS (ESI) calcd for C23H21FN5O+ [M + H]+ 402.1725, found 402.1724.
tert-Butyl-4-(5-(3-(3-fluorobenzoyl)-1H-pyrrolo[2,3-b]pyridin-5-yl)pyridin-2-yl)piperazine-1-carboxylate (ASM-8): white solid. Yield: 42 %, 1H NMR (600 MHz, DMSO‑d 6) δ 12.79 (br s, 1H), 8.62–8.70 (m, 2H), 8.51 (d, J = 2.57 Hz, 1H), 8.21 (s, 1H), 7.95 (dd, J = 2.48, 8.90 Hz, 1H), 7.68 (d, J = 7.70 Hz, 1H), 7.59–7.65 (m, 2H), 7.49 (dt, J = 2.02, 8.53 Hz, 1H), 7.00 (d, J = 8.80 Hz, 1H), 3.54–3.60 (m, 4H), 3.46 (br s, 4H), 1.44 (s, 9H); 13C NMR (151 MHz, DMSO‑d 6) δ 189.0, 165.3 (d, J = 246.5 Hz, 1C), 163.6, 158.7, 154.4, 149.0, 146.0 (2C), 143.3, 137.5 (br s, 1C), 136.8–136.4 (m, 1C), 131.6 (br d, J = 8.8 Hz, 1C), 128.9, 127.1, 124.2, 119.6, 116.3, 114.0, 107.6, 107.4, 79.4, 52.9 (2C), 45.0 (2C), 28.6 (3C); HRMS (ESI) calcd for C28H29FN5O3 + [M + H]+ 502.2249, found 502.2248.
(3-Fluorophenyl)(5-(6-(piperazin-1-yl)pyridin-3-yl)-1H-pyrrolo[2,3-b]pyridin-3-yl)methanone (ASM-9): yellow solid. Yield: 40 %, 1H NMR (600 MHz, DMSO‑d 6) δ 12.92 (br s, 1H), 9.60 (br s, 2H), 8.71 (d, J = 2.02 Hz, 1H), 8.69 (d, J = 2.02 Hz, 1H), 8.48 (d, J = 2.02 Hz, 1H), 8.24 (br dd, J = 2.11, 9.08 Hz, 1H), 8.23 (s, 1H), 7.69 (d, J = 7.70 Hz, 1H), 7.58–7.66 (m, 2H), 7.49 (dt, J = 2.20, 8.53 Hz, 1H), 7.31 (d, J = 9.17 Hz, 1H), 3.90–4.01 (m, 4H), 3.25 (br s, 4H); 13C NMR (151 MHz, DMSO‑d 6) δ 188.8, 175.5 (d, J = 246.5 Hz, 1C), 163.3 (d, J = 5.5 Hz, 1C), 161.6, 150.8, 143.6, 137.7, 131.3, 127.4, 125.2, 124.8, 119.0 (br s, 1C), 119.2, 115.7, 115.5, 113.9, 92.7, 90.3, 57.1 (2C), 42.7 (2C); HRMS (ESI) calcd for C23H21FN5O+ [M + H]+ 402.1725, found 402.1728.
tert-Butyl-4-(4-(3-(4-fluorobenzoyl)-1H-pyrrolo[2,3-b]pyridin-5-yl)phenyl)piperazine-1-carboxylate (ASM-10): white solid. Yield: 49 %, 1H NMR (600 MHz, DMSO‑d 6) δ 12.71 (br s, 1H), 8.65 (dd, J = 2.3, 15.1 Hz, 2H), 8.15 (s, 1H), 7.98–7.88 (m, 2H), 7.62 (d, J = 8.6 Hz, 2H), 7.39 (t, J = 8.8 Hz, 2H), 7.09–7.08 (m, 1H), 7.11 (d, J = 8.8 Hz, 1H), 3.50 (br s, 4H), 3.23–3.12 (m, 4H), 1.44 (s, 8H), 1.48 (s, 1H); 13C NMR (151 MHz, DMSO‑d 6) δ 188.9, 165.3 (d, J = 246.5 Hz, 1C), 163.7, 154.3, 150.7, 148.6, 143.6, 137.2 (2C), 131.7 (d, J = 8.8 Hz, 2C), 131.3, 129.5, 128.0, 127.1 (2C), 119.3, 116.8 (2C), 116.0 (d, J = 20.9 Hz, 2C), 114.0, 79.5, 48.6 (2C), 28.5 (3C); HRMS (ESI) calcd for C29H30FN4O3 + [M + H]+ 501.2296, found 501.2300.
(4-Fluorophenyl)(5-(4-(piperazin-1-yl)phenyl)-1H-pyrrolo[2,3-b]pyridin-3-yl)methanone (ASM-11): yellow solid. Yield: 36 %, 1H NMR (600 MHz, DMSO‑d 6) δ 12.74 (br s, 1H), 8.66 (dd, J = 2.29, 15.86 Hz, 2H), 8.16 (s, 1H), 7.89–7.97 (m, 2H), 7.66 (d, J = 8.80 Hz, 2H), 7.40 (t, J = 8.80 Hz, 2H), 7.15 (d, J = 8.80 Hz, 2H), 3.41–3.47 (m, 4H), 3.25–3.30 (m, 4H); 13C NMR (151 MHz, DMSO‑d 6) δ 188.9, 165.3 (d, J = 246.5 Hz, 1C), 163.7, 149.8, 148.7, 143.7, 137.0, 136.6 (d, J = 3.3 Hz, 1C), 131.7 (d, J = 8.8 Hz, 1C), 131.1, 130.3, 128.2 (2C), 127.2, 119.3, 117.0 (2C), 116.0 (d, J = 22.0 Hz, 1C), 114.0 (2C), 45.9 (2C), 43.1 (2C); HRMS (ESI) calcd for C24H22FN4O+ [M + H]+ 401.1772, found 401.1771.
(4-Fluorophenyl)(5-(6-(4-(methylsulfonyl)piperazin-1-yl)pyridin-3-yl)-1H-pyrrolo[2,3-b]pyridin-3-yl)methanone (ASM-12): brown solid. Yield: 34 %, 1H NMR (600 MHz, DMSO‑d 6) δ 12.88 (br s, 1H), 8.75 (d, J = 2.02 Hz, 1H), 8.72–8.74 (m, 1H), 8.54 (d, J = 2.38 Hz, 1H), 8.25 (d, J = 2.93 Hz, 1H), 7.97–8.02 (m, 2H), 7.76–7.80 (m, 1H), 7.72–7.75 (m, 1H), 7.45 (t, J = 8.80 Hz, 2H), 3.83–3.86 (m, 4H), 3.30–3.36 (m, 4H), 3.00 (s, 3H); HRMS (ESI) calcd for C24H23FN5O3S+ [M + H]+ 480.1500, found 480.1505.
(4-Methoxyphenyl)(5-(6-(4-(methylsulfonyl)piperazin-1-yl)pyridin-3-yl)-1H-pyrrolo[2,3-b]pyridin-3-yl)methanone (ASM-13): brown solid. Yield: 34 %, 1H NMR (600 MHz, DMSO‑d 6) δ 9.08 (s, 1H), 7.93–8.00 (m, 2H), 7.76 (d, J = 2.93 Hz, 1H), 7.65–7.73 (m, 1H), 7.45 (t, J = 8.80 Hz, 2H), 7.10 (dd, J = 2.93, 8.99 Hz, 1H), 6.78 (d, J = 8.99 Hz, 1H), 3.38–3.45 (m, 4H), 3.16–3.22 (m, 4H), 2.90 (s, 3H), 2.41 (s, 3H); HRMS (ESI) calcd for C25H26N5O4S+ [M + H]+ 492.1700, found 492.1703.
tert-Butyl-4-(4-(3-(4-fluorobenzoyl)-1H-pyrrolo[2,3-b]pyridin-5-yl)pyridin-2-yl)piperazine-1-carboxylate (ASM-14): white solid. Yield: 44 %, 1H NMR (600 MHz, DMSO‑d 6) δ 12.91 (br d, J = 2.38 Hz, 1H), 8.80–8.86 (m, 2H), 8.29 (d, J = 5.50 Hz, 1H), 8.28 (d, J = 3.12 Hz, 1H), 7.96–8.03 (m, 1H), 7.94–8.05 (m, 1H), 7.46 (t, J = 8.80 Hz, 2H), 7.21 (s, 1H), 7.10 (dd, J = 1.10, 5.14 Hz, 1H), 3.62–3.70 (m, 4H), 3.52 (br s, 4H), 1.48 (s, 9H); 13C NMR (151 MHz, DMSO‑d 6) δ 188.8, 165.4 (d, J = 246.5 Hz, 1C), 163.8, 160.0, 154.5, 149.8 (d, J = 5.5 Hz, 1C), 148.8, 147.9, 144.2, 137.3, 136.5, 131.8 (2C), 129.6, 128.3, 119.1, 116.1 (2C), 114.3, 112.2, 108.3, 105.3, 79.5, 45.0 (2C), 28.6 (3C); HRMS (ESI) calcd for C28H29FN5O3 + [M + H]+ 502.2249, found 502.2247.
(4-Fluorophenyl)(5-(2-(piperazin-1-yl)pyridin-4-yl)-1H-pyrrolo[2,3-b]pyridin-3-yl)methanone (ASM-15): white solid. Yield: 34 %, 1H NMR (600 MHz, DMSO‑d 6) δ 8.79 (dd, J = 2.1, 9.1 Hz, 2H), 8.26 (d, J = 5.1 Hz, 1H), 8.22 (s, 1H), 7.95 (dd, J = 5.6, 8.5 Hz, 2H), 7.40 (br t, J = 8.8 Hz, 2H), 7.23 (s, 1H), 7.10 (br d, J = 5.1 Hz, 1H), 3.88–3.75 (m, 4H), 3.14 (br d, J = 4.8 Hz, 4H); 13C NMR (151 MHz, DMSO‑d 6) δ 188.8, 165.4 (d, J = 246.5 Hz, 1C), 163.8, 159.5, 158.6 (d, J = 30.8 Hz, 1C), 149.8, 148.8, 144.2, 137.4, 136.4, 131.8 (d, J = 8.8 Hz, 2C), 129.4, 128.4, 116.1 (2C), 114.2, 112.7, 105.5, 46.1 (2C), 43.0 (2C); HRMS (ESI) calcd for C23H21FN5O+ [M + H]+ 402.1725, found 402.1727.
(4-Fluorophenyl)(5-(2-morpholinopyridin-4-yl)-1H-pyrrolo[2,3-b]pyridin-3-yl)methanone (ASM-16): white solid. Yield: 44 %, 1H NMR (600 MHz, DMSO‑d 6) δ 12.85 (br s, 1H), 8.78 (q, J = 2.2 Hz, 2H), 8.24 (d, J = 5.3 Hz, 1H), 8.22 (d, J = 2.6 Hz, 1H), 7.97–7.90 (m, 2H), 7.43–7.36 (m, 2H), 7.14 (s, 1H), 7.06 (dd, J = 1.3, 5.1 Hz, 1H), 3.78–3.69 (m, 4H), 3.59–3.54 (m, 4H); 13C NMR (151 MHz, DMSO‑d 6) δ 188.8, 165.4 (d, J = 246.6 Hz, 1C), 160.4, 149.8, 148.8, 147.8, 144.2, 137.3, 136.7, 131.8 (d, J = 9.9 Hz, 2C), 129.6, 128.3, 119.1, 116.1 (2C), 114.3, 112.4, 105.1, 66.5 (2C), 45.7 (2C); HRMS (ESI) calcd for C23H20FN4O2 + [M + H]+ 403.1565, found 403.1563.
tert-Butyl-((1r,4r)-4-((5-chloro-4-(3-(4-fluorobenzoyl)-1H-pyrrolo[2,3-b]pyridin-5-yl)pyridin-2-yl)amino)cyclohexyl)carbamate (ASM-17): white solid. Yield: 45 %, 1H NMR (600 MHz, DMSO‑d 6) δ 12.88 (br s, 1H), 8.66 (d, J = 1.86 Hz, 1H), 8.50 (d, J = 1.84 Hz, 1H), 8.26 (d, J = 2.75 Hz, 1H), 8.18 (s, 1H), 7.89 (dd, J = 5.60, 8.45 Hz, 2H), 7.40 (d, J = 8.80 Hz, 2H), 7.00 (s, 1H), 3.23 (m, 1H), 3.09 (m, 1H), 2.00–2.10 (m, 4H), 1.56–1.62 (m, 2H), 1.31–1.40 (m, 2H); 1.45 (s, 9H); HRMS (ESI) calcd for C30H32ClFN5O3 + [M + H]+ 564.2172, found 564.2168.
(5-(2-(((1r,4r)-4-Aminocyclohexyl)amino)-5-chloropyridin-4-yl)-1H-pyrrolo[2,3-b]pyridin-3-yl)(4-fluorophenyl)methanone (ASM-18): yellow solid. Yield: 41 %, 1H NMR (600 MHz, DMSO‑d 6) δ 13.01 (br s, 1H), 8.63 (d, J = 1.83 Hz, 1H), 8.48 (d, J = 1.83 Hz, 1H), 8.26 (d, J = 2.75 Hz, 1H), 8.16 (s, 1H), 7.94 (dd, J = 5.59, 8.53 Hz, 2H), 7.40 (t, J = 8.80 Hz, 2H), 6.99 (br s, 1H), 3.19 (m, 1H), 3.03 (m, 1H), 1.98–2.10 (m, 4H), 1.52–1.60 (m, 2H), 1.34–1.41 (m, 2H); HRMS (ESI) calcd for C25H24ClFN5O+ [M + H]+ 464.1648, found 464.1649.
tert-Butyl-((1r,4r)-4-((5-chloro-4-(3-(4-methoxybenzoyl)-1H-pyrrolo[2,3-b]pyridin-5-yl)pyridin-2-yl)amino)cyclohexyl)carbamate (ASM-19): white solid. Yield: 40 %, 1H NMR (600 MHz, DMSO‑d 6) δ 12.78 (br s, 1H), 8.56 (d, J = 2.02 Hz, 1H), 8.42 (d, J = 2.02 Hz, 1H), 8.21 (d, J = 2.75 Hz, 1H), 8.11 (s, 1H), 7.87 (d, J = 8.80 Hz, 2H), 7.11 (d, J = 8.80 Hz, 2H), 6.57 (s, 1H), 3.87 (s, 3H), 3.59–3.67 (m, 1H), 3.18–3.23 (m, 1H), 1.97–2.01 (m, 2H), 1.90 (br d, J = 11.92 Hz, 2H), 1.74–1.84 (m, 4H), 1.39 (br s, 9H); HRMS (ESI) calcd for C31H35ClN5O4 + [M + H]+ 576.2372, found 576.2375.
(5-(2-(((1r,4r)-4-Aminocyclohexyl)amino)-5-chloropyridin-4-yl)-1H-pyrrolo[2,3-b]pyridin-3-yl)(4-methoxyphenyl)methanone (ASM-20): yellow solid. Yield: 37 %, 1H NMR (600 MHz, DMSO‑d 6) δ 12.86 (br s, 1H), 8.58 (d, J = 2.02 Hz, 1H), 8.43 (d, J = 2.20 Hz, 1H), 8.21 (d, J = 3.12 Hz, 1H), 8.15 (br s, 2H), 7.87 (d, J = 8.62 Hz, 2H), 7.11 (d, J = 8.80 Hz, 2H), 3.87 (s, 3H), 3.70 (m, 1H), 3.02 (br d, J = 4.77 Hz, 1H), 2.01 (m, 4H), 1.50 (br d, J = 12.47 Hz, 2H), 1.30 (br s, 2H); HRMS (ESI) calcd for C26H27ClN5O2 + [M + H]+ 476.1848, found 476.1849.
tert-Butyl-4-(5-(1-(3-ethoxy-3-oxopropyl)-3-(4-fluorobenzoyl)-1H-pyrrolo[2,3-b]pyridin-5-yl)pyridin-2-yl)piperazine-1-carboxylate (ASM-21): white solid. Yield: 51 %, 1H NMR (600 MHz, DMSO‑d 6) δ 8.68 (d, J = 2.20 Hz, 1H), 8.65 (d, J = 2.20 Hz, 1H), 8.51 (d, J = 2.38 Hz, 1H), 8.31 (s, 1H), 7.95 (dd, J = 2.57, 8.80 Hz, 1H), 7.92 (dd, J = 5.59, 8.71 Hz, 2H), 7.37–7.46 (m, 2H), 7.00 (d, J = 8.80 Hz, 1H), 4.61 (t, J = 6.97 Hz, 2H), 4.03 (q, J = 7.09 Hz, 2H), 3.54–3.60 (m, 4H), 3.46 (br s, 4H), 3.01 (t, J = 6.97 Hz, 2H), 1.44 (s, 9H), 1.10 (s, 3H); 13C NMR (151 MHz, DMSO‑d 6) δ 188.4, 171.2, 163.8 (d, J = 246.5 Hz, 1C), 158.6, 154.4, 147.4, 146.2, 143.2, 139.9, 136.8, 131.7 (d, J = 8.8 Hz, 2C), 129.4, 127.4, 123.8, 119.8, 116.1 (2C), 112.8, 107.9, 79.5, 76.5, 60.7 (2C), 44.9 (2C), 41.3, 40.5, 34.3, 28.6 (3C), 14.4; HRMS (ESI) calcd for C33H37FN5O5 + [M + H]+ 602.2773, found 602.2771.
3-(3-(4-Fluorobenzoyl)-5-(6-(piperazin-1-yl)pyridin-3-yl)-1H-pyrrolo[2,3-b]pyridin-1-yl)propanoic acid (ASM-22): white solid. Yield: 54 %, 1H NMR (600 MHz, DMSO‑d 6) δ 9.66 (br s, 2H), 8.73 (d, J = 2.02 Hz, 1H), 8.72 (d, J = 2.20 Hz, 1H), 8.47 (d, J = 2.38 Hz, 1H), 8.36 (s, 1H), 8.29 (br d, J = 8.62 Hz, 1H), 7.90–7.97 (m, 2H), 7.42 (t, J = 8.80 Hz, 2H), 7.36 (br d, J = 8.99 Hz, 1H), 4.63 (br t, J = 6.88 Hz, 2H), 3.98 (br d, J = 4.77 Hz, 4H), 3.26 (br s, 4H), 3.02 (t, J = 6.97 Hz, 2H); 13C NMR (151 MHz, DMSO‑d 6) δ 188.4, 172.7, 163.8 (d, J = 246.5 Hz, 1C), 147.6, 146.6, 143.4, 140.3 (br s, 1C), 137.0, 136.4, 131.8 (2C), 128.0, 124.7, 119.7, 116.2 (2C), 112.7, 43.1 (2C), 42.5 (2C), 41.5, 40.5 (br s, 1C), 34.3; HRMS (ESI) calcd for C26H25FN5O3 + [M + H]+ 474.1936, found 474.1936.
tert-Butyl-4-(5-(1-(cyclopropylmethyl)-3-(4-fluorobenzoyl)-1H-pyrrolo[2,3-b]pyridin-5-yl)pyridin-2-yl)piperazine-1-carboxylate (ASM-23): white solid. Yield: 47 %, 1H NMR (600 MHz, DMSO‑d 6) δ 8.68 (d, J = 2.02 Hz, 1H), 8.66 (d, J = 2.02 Hz, 1H), 8.51 (d, J = 2.20 Hz, 1H), 8.38 (s, 1H), 7.94–7.97 (m, 2H), 7.90–7.93 (m, 2H), 7.85–7.89 (m, 1H), 7.42 (br t, J = 8.80 Hz, 3H), 7.00 (d, J = 8.80 Hz, 1H), 4.23 (br d, J = 7.15 Hz, 2H), 3.54–3.60 (m, 4H), 3.51 (br dd, J = 3.67, 6.24 Hz, 1H), 3.46 (br s, 4H), 1.44 (s, 9H), 0.47–0.54 (m, 4H); 13C NMR (151 MHz, DMSO‑d 6) δ 188.2, 158.5 (d, J = 246.5 Hz, 1C), 154.4, 147.5, 146.2, 143.2, 139.6, 136.8, 131.7 (d, J = 8.8 Hz, 1C), 129.3, 127.3, 123.9, 119.7, 116.3 (2C), 112.8, 107.9 (2C), 97.8, 79.5 (2C), 49.5, 44.9 (2C), 28.5 (3C), 12.0, 4.2 (2C); HRMS (ESI) calcd for C32H35FN5O3 + [M + H]+ 556.2718, found 556.2715.
(E)-N-(6-(4-(5-(2-(2-Hydroxystyryl)-3H-imidazo[4,5-b]pyridin-6-yl)pyridin-2-yl)piperazin-1-yl)-6-oxohexyl)-5-(2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanamide (Biotin-G7a): yellow solid. Yield: 36 %, 1H NMR (600 MHz, DMSO‑d 6) δ 10.21 (s, 1H), 8.57 (br s, 1H), 8.54 (d, J = 2.38 Hz, 1H), 8.09 (br s, 1H), 7.95–8.03 (m, 2H), 7.76 (br t, J = 5.59 Hz, 1H), 7.60 (br d, J = 7.52 Hz, 1H), 7.30 (d, J = 16.51 Hz, 1H), 7.18–7.23 (m, 1H), 6.97 (dd, J = 8.53, 15.86 Hz, 2H), 6.88 (t, J = 7.43 Hz, 1H), 6.43 (s, 1H), 6.37 (s, 1H), 4.27–4.34 (m, 1H), 4.09–4.17 (m, 1H), 3.52–3.57 (m, 2H), 3.59 (br d, J = 7.52 Hz, 5H), 3.07–3.13 (m, 1H), 3.03 (q, J = 6.66 Hz, 2H), 2.82 (dd, J = 5.04, 12.38 Hz, 1H), 2.58 (d, J = 12.47 Hz, 1H), 2.36 (br t, J = 7.43 Hz, 2H), 2.05 (t, J = 7.34 Hz, 2H), 1.58–1.66 (m, 1H), 1.48–1.57 (m, 4H), 1.36–1.44 (m, 2H), 1.22–1.35 (m, 4H); HRMS (ESI) calcd for C39H48N9O4S+ [M + H]+ 738.3544, found 738.3545.
N-(6-(4-(5-(3-(4-Fluorobenzoyl)-1H-pyrrolo[2,3-b]pyridin-5-yl)pyridin-2-yl)piperazin-1-yl)-6-oxohexyl)-5-(2-oxohexahydro-1H-thieno[3,4-d]imidazol-4-yl)pentanamide (Biotin-ASM-7): pale yellow solid. Yield: 32 %, 1H NMR (600 MHz, DMSO‑d 6) δ 10.21 (s, 1H), 8.57 (br s, 1H), 8.54 (d, J = 2.38 Hz, 1H), 8.09 (br s, 1H), 7.95–8.03 (m, 2H), 7.76 (br t, J = 5.59 Hz, 1H), 7.60 (br d, J = 7.52 Hz, 1H), 7.30 (d, J = 16.51 Hz, 1H), 7.18–7.23 (m, 1H), 6.97 (dd, J = 8.53, 15.86 Hz, 2H), 6.88 (t, J = 7.43 Hz, 1H), 6.43 (s, 1H), 6.37 (s, 1H), 4.27–4.34 (m, 1H), 4.09–4.17 (m, 1H), 3.52–3.57 (m, 2H), 3.59 (br d, J = 7.52 Hz, 5H), 3.07–3.13 (m, 1H), 3.03 (q, J = 6.66 Hz, 2H), 2.82 (dd, J = 5.04, 12.38 Hz, 1H), 2.58 (d, J = 12.47 Hz, 1H), 2.36 (br t, J = 7.43 Hz, 2H), 2.05 (t, J = 7.34 Hz, 2H), 1.58–1.66 (m, 1H), 1.48–1.57 (m, 4H), 1.36–1.44 (m, 2H), 1.22–1.35 (m, 4H); HRMS (ESI) calcd for C39H46FN8O4S+ [M + H]+ 741.3341, found 741.3339.
3.2. Biological evaluation
3.2.1. SARS2-S pseudovirus assay
The luciferase/GFP-expressing pseudoviruses were produced by lentivirus packaging with 293 T cells. The constructed A549-ACE2 cells (5000) were seeded in 96-well plates at 37 °C overnight. Then luciferase-expressing VSV-G or SARS2-S pseudovirus diluted in complete Dulbecco's Modification of Eagle's Medium (DMEM, Cat. No. MA0212, Meilunbio, China) with 10 % Fetal Bovine Serum (FBS). Compounds were added to above mixture and added to each well. The cells were incubated in a 5 % CO2 environment for 72 h at 37 °C, together with the positive compound control and negative cell control wells. The supernatant was removed and washed by 1 × PBS. Then 30 μL luciferase lysis buffer was added to each well for 1 h at −80 °C. 20 μL lysate was transferred to white 96-well plates and added 20 μL substrate for the detection of luciferase activity. The luciferase assay system was purchased from Promega Biotech (Madison, USA). The PureProteome Streptavidin Magnetic Beads were purchased from Merck Millipore (Cat. No. LSKMAGT02, USA). The luciferase activity was calculated using GraphPad prism8 software (San Diego, CA, USA). For the GFP-expressing SARS2-S pseudovirus, the fluorescence intensity was served as an indicator of the extent of the pseudovirus to enter the cells. Fluorescence microscopy analysis was performed to detect GFP intensity treated with compounds after viral infection for 72 h. Images were captured with fluorescence microscopy (Leica Dmi8, Germany).
3.2.2. SDS-PAGE and Western blot
The purified RBD and ACE2 proteins were analyzed by SDS-PAGE. 5 × SDS loading buffer was added into samples for input and IP. The samples were boiled (100 °C,10 min) and subjected to Western blot analysis. The primary antibodies ACE2 and RBD were purchased form Proteintech (Cat. No. 21115–1-AP, USA) and Sino Biological (Cat. No.40592-T62, Beijing, China), respectively.
3.2.3. Cells and viruses
Vero E6 cells (ATCC, USA) were infected with SARS-CoV-2 original strain and tittered by a plaque assay. Vero E6 cells were treated with different concentration test compounds and exposed to SARS-CoV-2 at a MOI of 0.01. Cells cocultured with virus were maintained in DMEM with 2 % FBS. Virus RNA was quantified by real-time RT-PCR according to the manufacturer's protocol.
3.2.4. MTT assay
A549 and MRC-5 were obtained from the Cell Bank of the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cells were cultured at 5000 cells/well in a 96-well plate in the presence of certain concentration of compounds for 48 h. MTT reagent (Sigma, Cat. No. M2128) was added to each well and cells were cultured for another 4 h. Then 150 μL dimethyl sulfoxide (DMSO, Sigma, Cat. No. D8418) was added for dissolution. After shaking for 15 min, the absorbance was measured by Varioskan Flash (Thermo Fisher Scientific, USA) at 492 nm. The IC50 values were calculated using GraphPad Prism 8 software.
3.2.5. Tissue distribution
All animal experiments were approved by the Institutional Animal Care and Use Committee of Xiamen University (Xiamen, China). Female ICR mice (6–8 weeks, 18–22 g) were used in the pharmacokinetic study. The mice were administered a single dose of compound G7a/ASM-7, suspended in DMSO/Tween 80/D5W (1, 0.2, and 98.8 %) by oral gavage (30 mg/kg) route and by intravenous (3 mg/kg) route. Before the test, the mice were fasted for 12 h. Blood (50 μL) collected from the eye was taken at 5 min, 1 h, 2 h and 4 h in EDTA-K2 nebulization tube. The blood sample was placed on ice and centrifuged to separate the plasma within 30 min and quantified by liquid chromatography-mass spectrometry (LC-MS). Lungs were taken at 0.25 h, 0.5 h, 1 h, 2 h, 3 h, 4 h, 6 h, 8 h, 12 h, 18 h and 24 h. Residual blood was washed by cold PBS. Then, lungs were added certain tissue homogenate (m/v = 1:5) for grinding and centrifugation, the supernatant were collected and quantified by LCMS.
3.3. Molecular modeling
3.3.1. Molecular docking
The crystal structure of the SARS-CoV-2 spike protein RBD in complex with hACE2 was retrieved from the Protein Data Bank (PDB) database (https://www.rcsb.org/, PDB ID: 6M0J). The crystal structure was prepared using the Protein Preparation Wizard panel of Schrödinger's Maestro (version 2021–2) with default settings. This involved removing water and crystal solvent molecules, completing missing amino acid side chains and loops using the Prime module [17,18], and optimizing hydrogen-bonding networks. Subsequently, a 100 ns all-atom MD simulation was performed in an aqueous environment using the method specified below. The last stable conformation from the trajectory was extracted for further analysis. To predict the binding site, the SiteMap tool [19,20] was used with default settings on the stable conformation obtained from the MD simulation. The predicted binding pocket at the S1-RBD-hACE2 protein binding interface (Fig. S4A) had SiteScore and Druggability score (Dscore) values of 1.04 and 0.99, respectively. It is worth noting that literature suggests that Dscores for protein-protein interaction sites vary, and sites with Dscores ranging from 0.75 to 1.0 are considered druggable [21]. Additionally, the CavityPlus web server [22] was utilized to identify potential cavities in the protein, revealing a favorable druggability at the interface of S1-RBD and hACE2 (Fig. S4B). Taking into account the results from both prediction methods, the ligand binding site selected for the present study is the binding site at the S1-RBD-hACE2 protein interface.
The study employed Schrödinger's LigPrep panel (LigPrep, Schrödinger, LLC, New York, NY, 2021) to convert small molecules from 2D to 3D structures. Subsequently, the Glide module of Schrödinger (Glide v 9.1117, Schrödinger, LLC, New York, NY, 2021) [23] was utilized for molecular docking on an in-house compound library based on predicted binding sites. Docking parameters were set to the software's default settings, unless otherwise specified. The receptor grid file was generated using the default options of Receptor Grid Generation tool. The grid box was centered on the centroid of the dummy atoms predicted by Sitemap, and its size was set to “Dock ligands similar in size to the Workspace ligand”. No constraints, rotatable groups, or excluded volumes were applied. Initially, the Ligand Docking tool was employed to screen an in-house compound library using the standard precision (SP) method. Subsequently, the top 40 % compounds from the SP docking results underwent further docking analysis employing the extra precision (XP) protocol. Additionally, the binding affinity between ligands and the protein was evaluated. The Prime MM-GBSA module (Prime MMGBSA v3.000, Schrödinger, LLC, New York, NY, 2021) was used to calculate the binding free energy for the top 30 % XP docking results, utilizing the default settings. Compounds with potential were chosen for subsequent in vitro validation based on their top 10 % ranked docking score and binding free energy. Inspiringly, G7a was identified as the hit compound based on molecular docking and in vitro SARS2-S pseudovirus assay. To optimize the binding mode between G7a and S1-RBD-hACE2 protein, the induced fit docking method was employed. Induced Fit Docking panel (Induced Fit Docking, Schrödinger, LLC, New York, NY, 2021) of Schrödinger was utilized to conduct induced docking activities on G7a and subsequent compounds ASMs, using standard docking protocol and default simulation parameters. The top-ranked conformation of G7a binding to S1-RBD-hACE2 protein served as the template for docking compounds ASMs. This docking process yielded potential binding modes of ASMs with S1-RBD-hACE2 protein. Docking results and MD trajectories were analyzed and visualized using the Maestro tool. Binding mode diagrams of small molecule ligands and proteins were drawn using ChimeraX (Version 1.5) [24], and protein structure motions before and after MD simulation were compared using PyMol (The PyMOL Molecular Graphics System, Version 2.3 Schrödinger, LLC).
3.3.2. Binding pose metadynamics simulation
The stability of ASMs binding to the S1-RBD-hACE2 protein binding interface in aqueous solution was investigated using Schrödinger's Binding Pose Metadynamics panel. The simulation parameters for the complexes were set to the software's default settings. Receptor-ligand complexes were employed as input structure types for the simulations, with each pose subjected to a trial count of 10.
3.3.3. Molecular dynamics simulation
This study utilized Desmond software (Desmond, Schrödinger, LLC, New York, NY, 2021) [25,26] to perform all-atom MD simulations of the target system in aqueous solution under the OPLS4 force field [27]. The target complex was embedded into a cubic simulation box with periodic boundary conditions using the System Builder panel, with a minimum distance of 10 Å between the box boundaries and the complex. The simulation box was then filled with predefined TIP3P water molecules. To maintain a neutral system and achieve a salt concentration of 0.15 M KCl, solvent molecules were replaced with counter ions K+ and Cl−. Next, the system was equilibrated through a series of protocols consisting of restrained minimization and MD simulations to achieve sufficient relaxation [28]. The relaxed system was then subjected to either 100 or 300 ns of unconstrained production run using the NPT (constant number of atoms N, pressure P, and temperature T) ensemble. Trajectories were outputted every 100 ps during the MD sampling process. The particle mesh Ewald (PME) [29,30] method was used to calculate long-range electrostatic interactions, and a 9 Å cutoff was set for van der Waals forces. To maintain a temperature of 310 K and a pressure of 1.01325 bar, Nose-Hoover chain thermostats and Martyna-Tobias-Klein barostats were employed.
3.3.4. Trajectory analysis
The Desmond module's Simulation Interaction Diagram tool was utilized to perform preliminary analysis of the MD trajectory. This analysis encompassed various aspects, such as time series analysis of RMSD for protein backbone atoms and heavy atoms of small molecules, RMSF analysis for protein backbone atoms, examination of protein-ligand contacts, and evaluation of ligand-protein interactions. The Simulation Event Analysis panel was used for time series analysis of the number of hydrogen bonds formed between S1-RBD and hACE2, Rg time series analysis, and visualization of amino acid fluctuations mapped to the protein structure using different colors (blue-white-red) based on the magnitude of protein backbone atoms' RMSF. To study the relative motion of protein internal atoms during the MD simulation process, the trj_essential_dynamics.py script provided by Schrödinger was used for DCCM and PCA analysis of the equilibrated trajectory. Then, based on PC1 and PC2 of Cα motion in the protein, the gmx sham tool of GROMACS (V. 2022.1) [31,32] was used to draw the FEL with the temperature set to 310 K, which was the same as the temperature used for the MD simulation. The thermal_mmgbsa.py script provided by Schrödinger was used to calculate the binding free energy of ligands with S1-RBD-hACE2 and S1-RBD with hACE2. Finally, the modevectors.py script in PyMol software was used to visualize the direction of motion from the initial to final MD conformations.
The Residue Interaction Network Generator 3.0 (RING 3.0) [33] online server was used with default parameters to generate RINs maps of the last 100 ns MD trajectories of Holo and Apo simulated systems. The resulting network files with the suffix of “.json” were analyzed using Cytoscape 3.9.1 [34]. Only the edges and nodes that interact between the hACE2 (chain A) and S1-RBD (chain E) were retained, and RINs with edge frequency >0.1 were used for visualization.
4. Conclusion
In this study, we identified G7a as a hit through virtual screening and SARS2-S pseudovirus assay. G7a demonstrated the ability to inhibit the interaction between hACE2 and S1-RBD. Utilizing the binding mode of G7a, we have designed and synthesized 23 novel 7-azaindole derivatives to explore more effective binders targeting SARS-CoV-2 S1-RBD-hACE2 interface. The results of the SARS2-S pseudovirus assay and MD simulations indicate that several of these derivatives, known as ASM-2, -4, -7, and -11, exhibit good antiviral activity and stable binding to S1-RBD-hACE2, respectively. Among them, ASM-7 demonstrated activity against the original strain of SARS-CoV-2 with an EC50 of 1.001 μM and exhibited better pharmaceutical properties and a more abundant distribution in the lung when compared to hit compound G7a. Furthermore, molecular docking and MD simulation studies demonstrated that ASM-7 could stably bind to the interface of S1-RBD-hACE2, attenuating the non-covalent interaction between S1-RBD and hACE2. Notably, the key residues of ASM-7's binding site were not mutated in the Omicron mutation strains, indicating that ASM-7 might not lead to drug resistance. Based on these promising results, ASM-7 may be considered a potential candidate for blocking the SARS-CoV-2 S1-RBD-hACE2 interaction, even if the virus mutates in the future.
Abbreviations
- COVID-19
coronavirus disease 2019
- S1-RBD
spike protein receptor-binding domain
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- hACE2
human angiotensin-converting enzyme 2
- ATRA
all-trans retinoic acid
- SARS2-S
SARS-CoV-2 spike protein
- SAR
structure-activity relationship
- MD
molecular dynamics
- CQ
chloroquine
- Ceph
cepharanthine
- DIPEA
N,N-diisopropylethylamine
- DCM
dichloromethane
- EA
ethyl acetate
- DME
1,2-dimethoxyethane
- DMSO
dimethyl sulfoxide
- TFA
trifluoroacetic acid
- DMF
N,N-dimethylformamide
- BPMD
binding pose metadynamics
- CV RMSD
collective variable root-mean-square deviation
- RMSF
root mean square fluctuation
- Rg
radius of gyration
- Vdw
Van der Waals
- DCCM
dynamical cross-correlation matrix
- PCA
principal component analysis
- RINs
residue interaction networks
- TLC
thin-layer chromatography
- HRMS
high-resolution mass spectra
- ppm
parts per million
- TMS
tetramethylsilane
- DMEM
Dulbecco's Modification of Eagle's Medium
- FBS
fetal bovine serum
- LC-MS
liquid chromatography-mass spectrometry
- PDB
Protein Data Bank
- SP
standard precision
- XP
extra precision
- PME
particle mesh Ewald
CRediT authorship contribution statement
Chaojie Wang: Investigation, Conceptualization, Methodology, and Writing-original draft. Fengming He: Methodology, Software, Investigation, and Writing-original draft. Ke Sun: Data curation and Investigation. Kaiqiang Guo: Validation and Data curation. Sheng Lu: Formal analysis. Tong Wu: Data curation. Xiang Gao: Supervision. Meijuan Fang: Project administration and Conceptualization. All authors reviewed the results and approved the manuscript submitted for publication.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (nos. 22274136 and 92256203) and the Fundamental Research Funds for the Central Universities of China (no. 20720180051).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijbiomac.2023.125182.
Appendix A. Supplementary data
Supplementary material
References
- 1.Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (Accessed on May 22, 2023)
- 2.Rabbani G., Ahn S.N. Review: roles of human serum albumin in prediction, diagnoses and treatment of COVID-19. Int. J. Biol. Macromol. 2021;193:948–955. doi: 10.1016/j.ijbiomac.2021.10.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H., Geng Q., Auerbach A., Li F. Structural basis of receptor recognition by SARS-CoV-2. Nature. 2020;581(7807):221–224. doi: 10.1038/s41586-020-2179-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rabbani G., Ahn S.N., Kwon H., Ahmad K., Choi I. Penta-peptide ATN-161 based neutralization mechanism of SARS-CoV-2 spike protein. Biochem. Biophys. Rep. 2021;28 doi: 10.1016/j.bbrep.2021.101170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Q., Zhang Y., Wu L., Niu S., Song C., Zhang Z., Lu G., Qiao C., Hu Y., Yuen K.Y., Wang Q., Zhou H., Yan J., Qi J. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181(4):894–904 e9. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan K.K., Dorosky D., Sharma P., Abbasi S.A., Dye J.M., Kranz D.M., Herbert A.S., Procko E. Engineering human ACE2 to optimize binding to the spike protein of SARS coronavirus 2. Science. 2020;369(6508):1261–1265. doi: 10.1126/science.abc0870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rath S.L., Padhi A.K., Mandal N. Scanning the RBD-ACE2 molecular interactions in omicron variant. Biochem. Biophys. Res. Commun. 2022;592:18–23. doi: 10.1016/j.bbrc.2022.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han P., Li L., Liu S., Wang Q., Zhang D., Xu Z., Han P., Li X., Peng Q., Su C., Huang B., Li D., Zhang R., Tian M., Fu L., Gao Y., Zhao X., Liu K., Qi J., Gao G.F., Wang P. Receptor binding and complex structures of human ACE2 to spike RBD from omicron and delta SARS-CoV-2. Cell. 2022;185(4):630–640 e10. doi: 10.1016/j.cell.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodcroft E.B., Zuber M., Nadeau S., Vaughan T.G., Crawford K.H.D., Althaus C.L., Reichmuth M.L., Bowen J.E., Walls A.C., Corti D., Bloom J.D., Veesler D., Mateo D., Hernando A., Comas I., Candelas F.G., Stadler T., Neher R.A., Consortium S.-S. Spread of a SARS-CoV-2 variant through Europe in the summer of 2020. Nature. 2021;595(7869):707–712. doi: 10.1038/s41586-021-03677-y. [DOI] [PubMed] [Google Scholar]
- 10.Liu L., Iketani S., Guo Y., Chan J.F., Wang M., Liu L., Luo Y., Chu H., Huang Y., Nair M.S., Yu J., Chik K.K., Yuen T.T., Yoon C., To K.K., Chen H., Yin M.T., Sobieszczyk M.E., Huang Y., Wang H.H., Sheng Z., Yuen K.Y., Ho D.D. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602(7898):676–681. doi: 10.1038/s41586-021-04388-0. [DOI] [PubMed] [Google Scholar]
- 11.Tong L., Wang L., Liao S., Xiao X., Qu J., Wu C., Zhu Y., Tai W., Huang Y., Wang P., Li L., Zhang R., Xiang Y., Cheng G. A retinol derivative inhibits SARS-CoV-2 infection by interrupting spike-mediated cellular entry. MBio. 2022;13(4) doi: 10.1128/mbio.01485-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin C., Li Y., Zhang Y., Liu Z., Mu X., Gu C., Liu J., Li Y., Li G., Chen J. Ceftazidime is a potential drug to inhibit SARS-CoV-2 infection in vitro by blocking spike protein-ACE2 interaction. Signal Transduct. Target Ther. 2021;6(1):198. doi: 10.1038/s41392-021-00619-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Razizadeh M., Nikfar M., Liu Y. Small molecule therapeutics to destabilize the ACE2-RBD complex: a molecular dynamics study. Biophys. J. 2021;120(14):2793–2804. doi: 10.1016/j.bpj.2021.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen M., Zhang X.E. Construction and applications of SARS-CoV-2 pseudoviruses: a mini review. Int. J. Biol. Sci. 2021;17(6):1574–1580. doi: 10.7150/ijbs.59184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fusani L., Palmer D.S., Somers D.O., Wall I.D. Exploring ligand stability in protein crystal structures using binding pose metadynamics. J. Chem. Inf. Model. 2020;60(3):1528–1539. doi: 10.1021/acs.jcim.9b00843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amadei A., Linssen A.B., Berendsen H.J. Essential dynamics of proteins. Proteins. 1993;17(4):412–425. doi: 10.1002/prot.340170408. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson M.P., Friesner R.A., Xiang Z., Honig B. On the role of the crystal environment in determining protein side-chain conformations. J. Mol. Biol. 2002;320(3):597–608. doi: 10.1016/s0022-2836(02)00470-9. [DOI] [PubMed] [Google Scholar]
- 18.Jacobson M.P., Pincus D.L., Rapp C.S., Day T.J.F., Honig B., Shaw D.E., Friesner R.A. A hierarchical approach to all-atom protein loop prediction. Proteins. 2004;55(2):351–367. doi: 10.1002/prot.10613. [DOI] [PubMed] [Google Scholar]
- 19.Halgren T. New method for fast and accurate binding-site identification and analysis. Chem. Biol. Drug Des. 2007;69(2):146–148. doi: 10.1111/j.1747-0285.2007.00483.x. [DOI] [PubMed] [Google Scholar]
- 20.Halgren T.A. Identifying and characterizing binding sites and assessing druggability. J. Chem. Inf. Model. 2009;49(2):377–389. doi: 10.1021/ci800324m. [DOI] [PubMed] [Google Scholar]
- 21.Alzyoud L., Bryce R.A., Al Sorkhy M., Atatreh N., Ghattas M.A. Structure-based assessment and druggability classification of protein-protein interaction sites. Sci. Rep. 2022;12(1):7975. doi: 10.1038/s41598-022-12105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y., Wang S., Hu Q., Gao S., Ma X., Zhang W., Shen Y., Chen F., Lai L., Pei J. CavityPlus: a web server for protein cavity detection with pharmacophore modelling, allosteric site identification and covalent ligand binding ability prediction. Nucleic Acids Res. 2018;46(W1):W374–W379. doi: 10.1093/nar/gky380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friesner R.A., Banks J.L., Murphy R.B., Halgren T.A., Klicic J.J., Mainz D.T., Repasky M.P., Knoll E.H., Shelley M., Perry J.K., Shaw D.E., Francis P., Shenkin P.S. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004;47(7):1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 24.Pettersen E.F., Goddard T.D., Huang C.C., Meng E.C., Couch G.S., Croll T.I., Morris J.H., Ferrin T.E., ChimeraX U.C.S.F. Structure visualization for researchers, educators, and developers. Protein Sci. 2021;30(1):70–82. doi: 10.1002/pro.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Desmond . Vol. 2021. Maestro-Desmond Interoperability Tools, Schrödinger; New York, NY: 2021. Desmond Molecular Dynamics System, D. E. Shaw Research, New York, NY, 2021. [Google Scholar]
- 26.Bowers K.J., Chow D.E., Xu H., Dror R.O., Eastwood M.P., Gregersen B.A., et al. 2006. Scalable Algorithms for Molecular Dynamics Simulations on Commodity Clusters, SC ’06: Proceedings of the 2006 ACM/IEEE Conference on Supercomputing; p. 43. [Google Scholar]
- 27.Lu C., Wu C., Ghoreishi D., Chen W., Wang L., Damm W., Ross G.A., Dahlgren M.K., Russell E., Von Bargen C.D., Abel R., Friesner R.A., Harder E.D. OPLS4: improving force field accuracy on challenging regimes of chemical space. J. Chem. Theory Comput. 2021;17(7):4291–4300. doi: 10.1021/acs.jctc.1c00302. [DOI] [PubMed] [Google Scholar]
- 28.He F., Wang X., Wu Q., Liu S., Cao Y., Guo X., Yin S., Yin N., Li B., Fang M. Identification of potential ATP-competitive cyclin-dependent kinase 1 inhibitors: De novo drug generation, molecular docking, and molecular dynamics simulation. Comput. Biol. Med. 2023;155 doi: 10.1016/j.compbiomed.2023.106645. [DOI] [PubMed] [Google Scholar]
- 29.Toukmaji A., Sagui C., Board J.A., Darden T.A. Efficient particle-mesh Ewald based approach to fixed and induced dipolar interactions. J. Chem. Phys. 2000;113:10913–10927. [Google Scholar]
- 30.Darden T., York D., Pedersen L. Particle mesh Ewald: an N·log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993;98(12):10089–10092. [Google Scholar]
- 31.Abraham M.J., Murtola T., Schulz R., Páll S., Smith J.C., Hess B., Lindahl E. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1-2:19–25. [Google Scholar]
- 32.Van Der Spoel D., Lindahl E., Hess B., Groenhof G., Mark A.E., Berendsen H.J.C. GROMACS: fast, flexible, and free. J. Comput. Chem. 2005;26(16):1701–1718. doi: 10.1002/jcc.20291. [DOI] [PubMed] [Google Scholar]
- 33.Clementel D., Del Conte A., Monzon A.M., Camagni G.F., Minervini G., Piovesan D., Tosatto S.C.E. RING 3.0: fast generation of probabilistic residue interaction networks from structural ensembles. Nucleic Acids Res. 2022;50(W1):W651–W656. doi: 10.1093/nar/gkac365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., Amin N., Schwikowski B., Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material