Abstract
Objectives
Cancer stem cells (CSCs), a small subpopulation of cells with high tumorigenesis and strong intrinsic drug resistance, exhibit self-renewal and differentiation abilities. CSCs play a crucial role in tumor progression, drug resistance, recurrence and metastasis,and conventional therapy is not enough to eradicate them. Therefore, developing novel therapies targeting CSCs to increase drug sensitivity and preventing relapse is essential. The objective of this review is to present nanotherapies that target and eradicate the tumor “seeds”.
Evidence acquisition
Evidence was collected and sorted from the literature ranging from 2000 to 2022, using appropriate keywords and key phrases as search terms within scientific databases such as Web of Science, PubMed and Google Scholar.
Results
Nanoparticle drug delivery systems have been successfully applied to gain longer circulation time, more precise targeting capability and better stability during cancer treatment. Nanotechnology-based strategies that have been used to target CSCs, include (1) encapsulating small molecular drugs and genes by nanotechnology, (2) targeting CSC signaling pathways, (3) utilizing nanocarriers targeting for specific markers of CSCs, (4) improving photothermal/ photodynamic therapy (PTT/PDT), 5)targeting the metabolism of CSCs and 6) enhancing nanomedicine-aided immunotherapy.
Conclusion
This review summarizes the biological hallmarks and markers of CSCs, and the nanotechnology-based therapies to kill them. Nanoparticle drug delivery systems are appropriate means for delivering drugs to tumors through enhanced permeability and retention (EPR) effect. Furthermore, surface modification with special ligands or antibodies improves the recognition and uptake of tumor cells or CSCs. It is expected that this review can offer insights into features of CSCs and the exploration of targeting nanodrug delivery systems.
Graphical abstract
Keywords: Nanomedicine, Cancer stem cells, Cancer therapy, Drug delivery system
Introduction
A total of 19.3 million new cancer cases and approximately 10.0 million cancer deaths occurred in 2020 [1], suggesting that anticancer treatment requires further improvement. Cancer stem cells (CSCs) are a subset of cancer cells characterized by their ability to self-renew, differentiate and drive tumor initiation, metastasis, drug resistance and relapse. CSCs were first discovered in 1994 in a study of human acute myeloid leukemia (AML), in which a population of CD34+CD38− AML-initiating cells were identified in AML patients by transplanting into severe combined immune-deficient mice.These cells were engrafted in the mice and produced a large number of colony-forming progenitors [2]. In 2003, CD44+CD24− lineage(-) tumorigenic breast cancer cells were isolated. As few as 100 CSCs with this phenotype were able to form tumors in NOD/SCID mice [3]. Additionally, CSCs have been successfully identified in brain tumors [4], prostate cancers [5], ovarian cancers [6] and colon cancers [7]. CSCs can drive tumor initiation, but their origin of is currently unknown. Several hypotheses for this issue have been summarized: (1) genetic or epigenetic changes in stem cells, progenitor cells and differentiated somatic cells; (2) dedifferentiation of normal cancer cells; (3) cell fusion; and (4) the effects of the tumor microenvironment (TME) [8–10].
CSCs are often quiescent and intrinsically drug resistant through the expression of drug efflux transporters, and the activation of antiapoptotic signaling pathways [11, 12]. Thus, conventional chemotherapy and radiotherapy usually fail to kill CSCs, often resulting in an increased CSC fraction in tumors. Thus, effective approaches to eliminate CSCs are quite necessary. With the development of nanotechnology, there are an increasing number of nanomedicines approved by FDA or in clinical or preclinical trials for cancer treatment. In regard to targeting CSCs, nanomedicines demonstrate many advantages. This review summarizes the hallmarks and surface markers of CSCs, and overviews the nanomedicine strategies to kill CSCs.
Hallmarks and surface markers of CSCs
In recent decades, CSCshave been defined as a small and unique subset of cells that exhibitself-renewal, differentiation potential, and intrinsic chemotherapy and radiotherapy resistance [13]. CSCs have acquired chemotherapy and radiotherapy resistance through multiple mechanisms, such as the upregulation of drug-efflux pumps (P-gp, ABCG2, MRP1, MDR1), a superior DNA-repair capacity, several activated survival signaling pathways,and enhanced protection against reactive oxygen species (ROS) [14–16]. In addition the plasticity of CSCs, especially their adoptionof quiescence, has been an important element of their drug resistance [17]. CSCs can release hypoxia-inducible factor 1 to induce the release of proangiogenic factors for tumor angiogenesis [18]. In addition, CSCs demonstrate epigenetic alterations such as histone modifications and DNA methylations, that contribute to tumor heterogeneity [19].
CSC surfaces, such as CD133, CD44, CD166 and EpCAM, play an important roles in the adhesion of the cells to their niche, [20]. ALDH1 is responsible for the oxidation of aldehydes into carboxylic acids and is related to tumorigenesis and metastasis in CSCs [21]. These cellular markers are usually applied to isolate CSCs from heterogeneous tumor cell populations (Table 1). CSCs share many of the same signaling pathways as normal stem cells, including Wnt, Notch and Hedgehog. Signaling pathways, such as TGF β along with PIK3/AKT, STAT or EGFR, are oncogenic cascades in CSCs [22–24]. CSCs are in a quiescent state and can efflux drugs out by highly expressed drug pumps, resulting in resistance to conventional treatment. However, the key CSC markers and signaling pathways demonstrate promise for targeted treatment. Due to in-depth investigations on the self-renewal, drug resistance, abnormal metabolism and embryonic signaling pathways in CSCs, much effort has been put forward for targeting CSCs. Based on the characteristics of CSCs, the possible therapies and strategies that can eliminate CSCs are summarized in Fig. 1.
Table 1.
| Tumor types | CSCs markers |
|---|---|
| Breast cancer | CD44, CD24, ALDH1, CD133, ESA, PROCR, CD29, CD61,CD70, CD90, LGR5, CXCR4, EpCam, ProC-R, Sox-2, Nanog, OCT3/4 |
| Colon cancer | ALDH1, CD44, CD24, CD133, LGR5, EpCam, Letm1, Sall4, Sox-2, Nanog |
| Glioblastoma | CD133, CD15, A2B5, ALDH, Sox-2, BMI1, Nestin, MUSASHI1, Nanog |
| Lung cancer | CD133, ALDH1, CD166, CD90, EpCam, Nanog, OCT4+ |
| Liver cancer | CD24, CD90, CD13, ESA, OV6, CD133, ALDH, IB50-1, EpCam, Sall, AFP, Nanog, OCT3/4, Sox-2 |
| Gastric cancer | CD44, Lgr5,CD24, CD90, CXCR4, LINGO2, ALDH, Letm1, Musashi2, Nanog, OCT3/4 |
| Ovarian cancer | CD44, CD117, CD133, ALDH1, CD24, ROR1 |
| Prostate cancer | CD44, integrin α2β1,CD133+, ALDH1+, FAM65B+ MF12 ,LEF1, Trop2 |
| Leukemia | CD34, CD38, CD117 |
| Melanoma | CD133, CD20,CD271 |
Fig. 1.
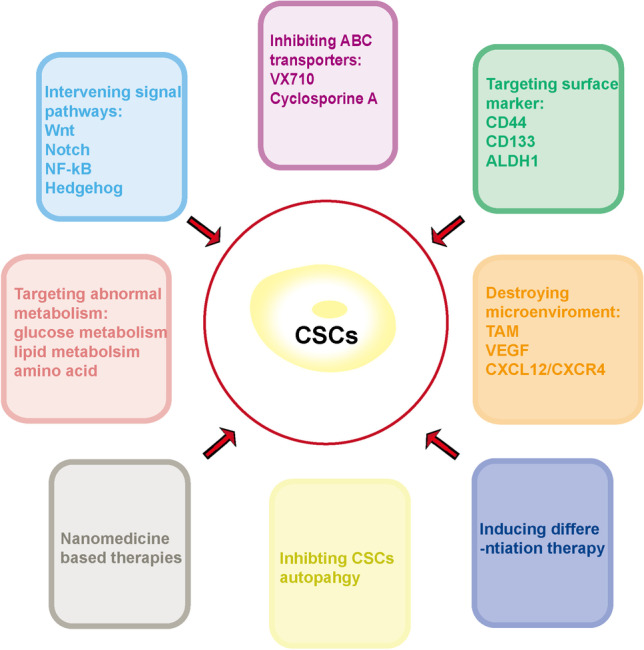
Possible therapies and strategies that can eliminate CSCs
Drugs targeting CSCs
Inhibitors targeting the signaling pathways in CSCs
The pathways affecting CSCs mainly include the Wnt, Hedgehog, Notch, BMP, Bmi, PI3K/Akt, integrin and STAT pathways [31]. Among them, the Wnt, Hedgehog and Notch signaling pathways have been thoroughly studied in CSCs and many inhibitors have entered clinical trials andhave been approved [32]. The Wnt signaling pathway regulates cell proliferation, differentiation, apoptosis and stemness. Many studies have found that tumor cells with high Wnt activity are functionally related to CSCs [33]. For example, as one of the markers of CSCs in cancers, CD44 expression is promoted by Wnt/β-catenin signaling and cytokines secreted from tumor-associated cells [34]. In addition to Wnt signaling, the Notch signaling pathway also plays an important role in CSCs. Wang R. et al. reported that Notch components (Notch1, Hes1 and Hey1) were upregulated in sphere-forming liver CSCs [35]. The Hedgehog pathway has also been shown to regulate the properties of CSCs in glioma, chronic myeloid leukemia (CML) and multiple myeloma, especially through the SMO gene [36]. Therefore, these signaling pathways are potential targets for CSCs. Several inhibitors in clinical trials targeting the Wnt, Hedgehog and Notch signaling pathways are summarized in Fig. 2; Table 2.
Fig. 2.

Chemical structures of several compounds targeting Wnt and Notch signaling pathways of CSCs
Table 2.
Several inhibitors targeting the Wnt, Hedgehog and Notch signaling pathways in clinical trials
| Compound | Target | Tumor type | Phase state | Efficacy outcomes |
|---|---|---|---|---|
|
LGK974 [37] |
Wnt | Metastatic colorectal cancer |
Phase I |
N/A |
|
ETC-159 [38] |
Wnt | Solid tumor |
Phase I |
Recruiting |
|
PRI-724 [39] |
Wnt | Advanced pancreatic cancer |
Phase I |
Stable disease rate: 40% |
|
MK-0752 [40] |
Notch | T cell acute lymphoblastic leukemia |
Phase I |
Terminated |
|
LY-900,009 [41] |
Notch | Advanced cancer |
Phase I |
Stable disease in 5 patients from dose escalation group |
|
RO4929097 [42] |
Notch | Advanced-stage solid tumors | Phase I | Partial response in 1 of 96 evaluable patients, Stable disease in 28 of 96 patients |
|
BMS-906,024 [43] |
Notch | Advanced cancer |
Phase I |
Terminated |
|
Nirogacestat [44] |
Notch |
1.Metastatic Cancer Pancreas 2.Desmoid tumors |
Phase II |
1.Terminated 2.Confirmed partial response in 5 patients, stable disease in anoth -er patients |
|
Patidegib [45] |
Hedgehog | Basal cell nevus syndrome; skin cancer |
Phase II |
Terminated |
|
BMS-833,923 [46] |
Hedgehog | Leukemia |
Phase I/II |
N/A |
|
Taladegib [47] |
Hedgehog | Advanced cancer |
Phase I/II |
Stable disease rate: 30.9%,Objective respo -nse rate: 26.2% |
Classical drugs
Studies have found that some classical drugs possess anti CSC abilities. Metformin, a conventional drug for the treatment of type 2 diabetes, has been proven to exert potential antitumor effects and also appears to act on CSCs. A landmark study of the effect of metformin on CSCs was conducted by Hirsch et al. In their reserch, CD44+/CD24−/low CSCs were eliminated after metformin treatment, and a synergistic antitumor effect was found with doorubicin [48]. Salinomycin, an antibiotic applied in veterinary medicine, has been isolated from the bacterium Streptomyces albus. It has been shown to eliminate CSCs in different types of tumors, such as human ovarian CSCs and breast CSCs. An increasing number of derivatives of salinomycin have been synthesized to have a higher selectivity for CSCs. The salinomycin C20-propargylamine derivative has shown a strikingly low IC50 of 23 nM against HMLER CD24 low/CD44 high breast CSCs by sequestering iron in lysosomes, providing a highly selective agent to target CSCs [49]. Puromycin is another antibiotic that can suppress CSCs. Puromycin effectively eliminates CSCs through ribosomal protein translation inhibition in tumor spheres and monolayer cultures [50]. Nelfinavir, a human immunodeficiency virus (HIV) protease inhibitor, lowers self-renewal and induces apoptosis in CSCs,ultimately impairing CSC-induced allograft formation in vivo [51].
Targeting the abnormal metabolism in CSCs
CSCs may have highly glycolytic or oxidative phosphorylation phenotypes, depending on their surface markers andTME. CD44 variant isoforms (CD44v), a marker of CSCs, can interact with and stabilize cysteine-glutamate transporter, and promote cysteine uptake [52]. Breast CSCs shift glucose metabolism from mitochondrial oxidative phosphorylation to anaerobic by increasing the pyruvate kinase M2 isoform along with lactate dehydrogenase and glucose 6-phosphate dehydrogenase; additionally, 2-deoxyglucose combined with doxorubicin has been used to inhibit breast CSC proliferation [53]. CD9 promotes the plasma membrane localization of ASCT2, the major glutamine transporter, enhancing glutamine uptake in pancreatic ductal adenocarcinoma cells [54]. Glutamine deprivation significantly inhibits self-renewal, decreases the expression of stemness markers, increases ROS levels in pancreatic cancer stem cells, and finally enhances radiosensitivity [55]. CSCs are also highly dependent on lipid metabolism, especially fatty acid synthesis, lipid desaturation and β-oxidation. Therefore, there are different therapeutic methods for targeting CSCs based on lipid metabolism, mainly by targeting key enzymes including fatty acid synthase (FASN), acetyl‐CoA carboxylase‐1 (ACC), stearoyl CoA desaturase 1 (SCD1), and carnitine palmitoyl-transferase 1 (CPT1). Upregulated FASN has been observed to maintain stemness in CSCs. Once FASN is inhibited by cerulenin, the sphere formation, invasive abilities of CSCs and expression of stemness markers such as SOX2, nestin, CD133, and FABP7 were inhibited [56]. Other therapeutic molecules, such as resveratrol (FASN inhibitor), sorafenib (ACC inhibitor), and etomoxir (CPT1 inhibitor) can also target CSCs.
Problems of conventional drugs targeting CSCs
Although significant progress has been made in drugs targeting CSCs, several problems remain to be resolved. First, the effects of conventional targeting drugs can be hampered by drug properties, such as poor solubility and stability, unfavorable pharmacokinetics, lack of selectivity, and dose-limiting toxicities. Second, CSCs and normal stem cells share many similar properties ; thus, conventional drugs targeting CSCs can also destroy normal stem cells. Third, CSCs are more resistant than other tumor cells, leading to inefficient therapeutic outcomes. Therefore, more precise and efficient CSC targeting strategies need to be developed.
Nanomedicine-based strategies for CSC therapy
Nanocarriers for delivering small molecule drugs and nucleic acid agents
As mentioned above, some drugs have the ability to eliminate CSCs, including salinomycin (Sal), curcumin, disulfiram and chloroquine. However, these drug applications are limited by the physicochemical properties, pharmacokinetics, and stability of the drugs. Applications of Sal are limited by its poor water solubility. Sal-loaded nanomedicines have been developed to improve their pharmacokinetic properties, safety and tolerability. Wang Q et al. designed Sal-loaded gelatinase-responsive nanoparticles for the elimination of cervical cancer stem cells. The CD44-positive HeLa cell level was lower in Sal-loaded nanoparticle group than in the control and Sal solution group. In addition the nanoparticles-treated group significantly suppressed the regenerative ability of HeLa cells [57]. CD24 low/CD44 high breast CSCshave shown high sensitivity to Sal-loaded Gold nanoparticles by ferroptosis-induced cell death [58]. Sal is also used in combination with some classic anticancer drugs to eradicate both bulky tumor cells and CSCs. Sal and docetaxel have been encapsulated in PLGA/TPGS nanoparticles in a synergistic 1:1 ratio of to target both breast cancer cells and stem cells. These coloaded nanoparticles demonstratebetter inhibition of MCF-7 cells in vitro and in vivo. In addtion, the combination is more effective in inhibiting mammosphere formation of MCF-7 CSCs [59]. Kun Y et al. developed curcumin-loaded PLGA nanoparticles modified with sialic acid and anti-ALDH to permeate the blood-brain barrier (BBB) and target brain CSCs (Fig. 3) [60]. Wedelolactone, mainly a liver disease treatment, has been promise as an effective anticancer drug, but its effects on CSCs are unknown. Das S et al. found that wedelolactone-encapsulated PLGA nanoparticles enhanced drug uptake in breast cancer cells and in breast CSCs. The ALDH+ or CD44+/CD24− population decreased after treatment with wedelolactone. In addition, it could increase the chemosensitivity of paclitaxel by downregulating SOX2 and ABCG2 [61]. Doxorubicin and thymoquinone coloaded cocktail shell-derived aragonite CaCO3 nanoparticles significantly reduce ALDH activity, sphere-formation and the percentage of CD44+CD24− in breast cancer cells [62].
Fig. 3.
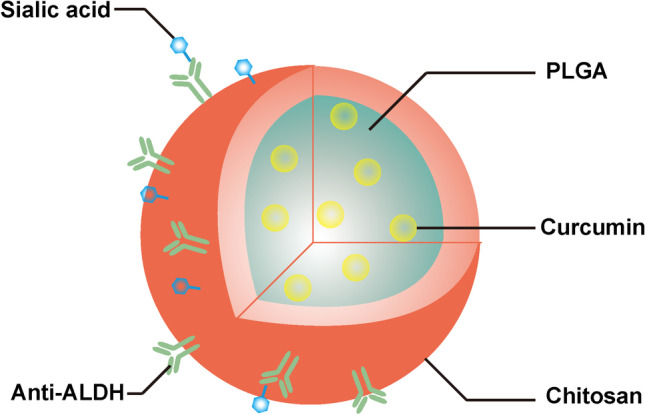
Anti-ALDH and Sialic acid co-modified PLGA nanoparticles for targeting brain CSCs. Adapted from [60]
In contrast to the combination of chemical drugs, the dual delivery of a hepatocyte nuclear factor 4 alpha (HNF4α)-encoding plasmid and cisplatin by mesoporous silica nanoparticles also exhibits excellent inhibition of pluripotency and tumorigenicity in CD133+ hepatoma cells [63] (Fig. 4).HNF4α has been proven to be impaired in hepatocellular carcinoma and is associated with poor differentiation, high metastatic potential, and poor prognosis. RNA nanotechnology has been widely applied to overcome the hurdles of gene delivery in vivo, such as rapid degradation, nonspecific distribution, and gene regulation. In the research of Yin H et al., the pRNA-3WJ motif was utilized as a core scaffold to deliver anti-miR21 and CD133 aptamers for targeting breast CSCs. The 3WJ/CD133apt/anti-miR21 nanoparticles achieved a high accumulation in MDA-MB-231 and CSCs, and significantly increased PTEN and PDCD4 expression [64]. Carbamate-mannose modified PEI has been designed as a nonviral gene vector for NF-κB shRNA delivery. These nanocomplexes are able to decrease mammosphere formation, lower the ratio of ALDH+ breast CSCs, and inhibit migration and invasion. In addition, the above nanocomplexes sensitized doxorubicin-loaded micellar nanoparticles to treatment [65].
Fig. 4.
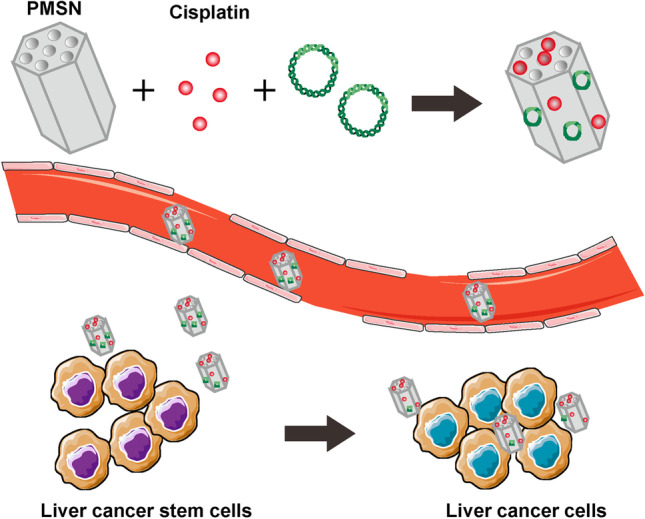
Mesoporous silica nanoparticles co-loaded cisplatin and HNF4α DNA plasmid to reduce the portion of liver CSCs. Adapted from [63]
Nanomedicinebased photothermal/ photodynamic therapy (PTT/PDT)
Photothermal or photodynamic therapy (PTT and PDT, respecitively) is a focus in cancer treatment. This treatment demonstrates higher selectivity, lower toxicity and better repeatability than traditional methods. PTT or PDT is also used to eradicate CSCs. Different types of photothermal agents are listed in Fig. 5. CD271 antibody-functionalized hollow gold nanospheres achieve specific targeting toward CD271+ osteosarcoma stem cells and induce the death of CSCs by apoptosis pathways and DNA injuries upon near-infrared laser irradiation [66]. Because CSCs preferentially reside in hypoxic regions and are centrally located in tumors, small nanoparticles more easily pass through the extracellular matrix for targeting CSCs. The Li YP group applied ferritin to form 26 nm nanocages for deep tumor penetration and binding to the transferrin receptor 1of CSCs. The DIR and epirubicin-loaded nanocages were preferentially accessible to CSCs in a 4T1 breast cancer model [67]. Fernandes S et al. revealed that monotherapy with iron oxide nanocubes could not eliminate quiescent CSCs, and could even restart division. A combination with doxorubicin-loaded thermally responsive nanoparticles caused a stop in quiescent-CSC activities [68]. In addition to chemotherapy combinations, immunotherapy, radiotherapy and PDT are also been reported (Fig. 6). According to Atkinson R, CSCs are more resistant to 6 grays of ionizing radiation, leading to an increased relative proportion after 48–72 h of ionizing radiation. However, a larger reduction in breast tumor size without an increase in the percentage of CSCs was achieved by local hyperthermia for 20 min at 42 °C after exposure to ionizing radiation using optically activated gold nanoshells [69]. Notably, PTT sensitized breast cancer stem cells to radiation therapy.
Fig. 5.

Different types of photothermal agents used for PTT
Fig. 6.
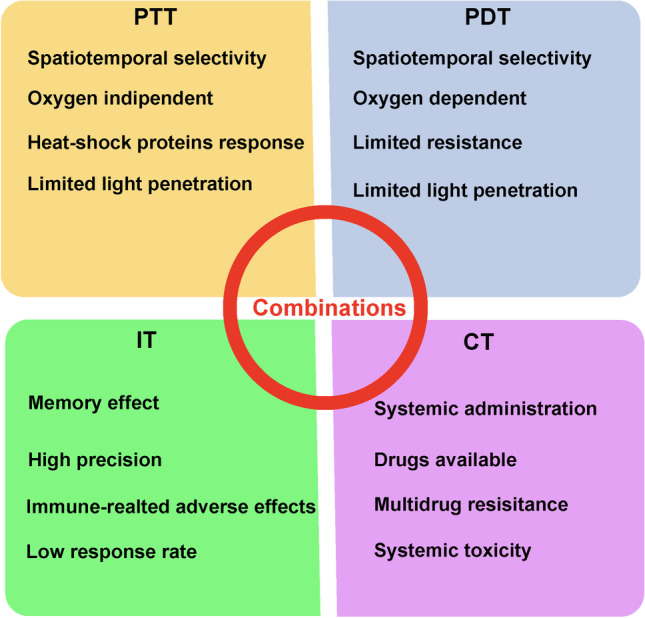
Combination of PTT with other antitumor therapies. Photodynamic therapy (PDT), Chemotherapy (CT), Immunotherapy (IT)
PDT kills tumor cells by generating ROS. PDT has been applied in the clinic for the treatment of various cancers over the past 40 years [70]. The effects of PDT during CSC treatment are under research. AlPcS4Cl-loaded anti-CD133 modified gold nanoparticles show a specific targeting toward lung CSCs. Adequate and continuous ROS exposure can eliminate CSCs [71]. Platinum metallacage-loaded nanoparticles have achieved chemo and photodynamic combination therapy. This platform can penetrate deeply into 3D tumor spheroids, and is able to eradicate liver CSCs, and decrease migration and spheroid formation [72]. In another report, salinomycin, photosensitizer chlorin e6 (Ce6) and vitamin E acetate were coencapsulated into a novel keratin-based nanoformulation to limit the self-renewal capacity and decrease the stemness of breast CSCs. Zebrafish embryo experiments in vivo have shown that the nanoformulations can interfere with the Wnt/β-catenin signaling pathway, which is an important pathway in CSCs [73]. Ginsenoside Rg3, a principal ginseng component, has presented significant anticancer activities and anti CSC stemness. Nanocarriers synthesized by graphene oxide (GO) linked with photosensitizer (PS) indocyanine green (ICG), folic acid, and polyethylene glycol (PEG) have been applied to encapsulate Rg3 to inhibit the progression and stemness of osteosarcoma [74].
Nanomedicines targeting the signaling pathways in CSCs
Nanomedicine-based CSC signaling pathway modulations include delivering small molecules to regulate signaling pathways, RNA interference nanotechnology and antibody-nanoparticle conjugates. For abnormal activation of the Wnt signaling pathway in various CSCs, Shamsian A et al. demonstrated that vorinostat (histone-deacetylase inhibitor) and PKF118-310 (Wnt-β-catenin pathway inhibitor) coloaded protein corona-capped gold nanoparticles reduced the stem cell population and Snail marker [75]. Cuprous oxide nanoparticles can also inhibit the stemness of prostate cancer cell lines and the Wnt signaling pathway [76]. Yallapu et al. developed curcumin-loaded PLGA nanoparticles to increase the chemo/radio sensitivity of cisplatinresistant ovarian cancer cells. These nanoparticles significantly reduce the β-catenin and c-Myc protein levels, which are important in CSCs [77]. The Notch signaling pathway is also highly activated in CSCs. In the research, α-mangostin-encapsulated PLGA nanoparticles have been used to inhibit the self-renewal ability, stem cell markers and pluripotency maintaining factors (Sox-2, KLF-4, c-Myc, Nanog) of CSCs by Notch signaling suppression [78]. SHP-1 siRNA-loaded ZnAs@SiO2 nanoparticles have been applied to eliminate hepatocellular carcinoma cells and impede recurrence by the SHP-1/JAK2/STAT3 signaling pathways. These nanoparticles inhibit tumor growth and induce changes in stemness markers (CD133, Sox-2 and OCT-4) and EMT markers (E-cadherin, Vimentin and Slug) [79]. Kaushik NK and colleagues found that cotreatment with low doses of PEG coated gold nanoparticles and cold plasma not only inhibited the proliferation of tumor cells but also inhibited EMT behavior, decreasing sphere formation and the self-renewal capacity of glioma stem cells by abolishing the PI3K/AKT signaling pathway [80].
Nanocarriers targeting the specific markers of CSCs
Specific surface markers are not only separation markers for CSCs, but also therapeutic targets. CD44, CD13, CD36, CD24, CD133, CD20 and CD90 are commonly reported as nanomedicines-targeting biomarkers [81]. Cho JH et al. designed CBP4, a novel small peptide, to target CD133. CBP4 conjugated gold nanoparticles exhibited “signal on-off” properties by quenching depending on the presence of CD133+ glioma CSCs, which improved imaging effects when CSCs were present [82]. Salinomycin-loaded PEG-PLGA nanoparticles conjugated with CD133 aptamers have been applied to target CD133+ osteosarcoma CSCs. Results showed that CD133 aptamer-decorated nanoparticles are 4.92 or 2.33-times more effective than undecorated nanoparticles or salinomycin in CD133+ Saos-2 cells, respectively [83]. CD44 was firstly described in hematopoietic stem cells and then in cancer and leukemia initiating cells [84]. Hyaluronic acid (HA) is a common CD44 specific ligand and has been utilized for active targeting in tumor therapy. Su Z et al. constructed anti-CD44 antibody-modified superparamagnetic iron oxide nanoparticles to kill human oral squamous cell carcinoma stem cells. Results demonstrated that CD44 nanoparticles induced CD44 -overexpressed CSCs to undergo programmed death [85]. Albumin nanoparticles functionalized with HA exhibit high affinity to CD44-enriched B16F10 cells. Moreover, HA-eNPs/ATRA treatment significantly decrease the side population of B16F10 cells in vitro. Finally, tumor growth has been significantly inhibited by HA-eNPs/ATRA in lung metastasis tumors in mice [86]. It is recognized that CSCs also have different phenotypes; thus, it is more efficient to target two surface markers instead of one. CD44 and CD133 are both gastric CSC markers. Chen H and colleagues developed CD44 and CD133 antibody-conjugated all-trans retinoic acid-loaded PLA-lecithin-PEG nanoparticles for targeting gastric cancer stem cells. These dual antibody decorated nanoparticles showed better inhibitory effects than the single targeting and nontargeting ones [87]. Sal-loaded lipid-polymer nanoparticles decorated with anti-CD20 aptamers have shown selective cytotoxicity to CD20+ melanoma CSCs, as proven by suppressing the formation of tumor spheres and decreasing the proportion of CD20+ melanoma cells [88]. To reduce off-target effects and improve safety, several smart strategies to hide the target site during circulation and expose the tumor site for CSC targeting should be further investigated.
Exosome-based nanomedicines for CSCs
Exosomes are small extracellular vesicles secreted by mammalian cells with a diameter of approximately 100 nm. Exosomes deliver various bioactive molecules, such as nucleic acids, proteins and lipids to the parent or distant cells [89]. Compared to conventional synthetic nanoparticles and liposomes, exosome-based delivery systems have high biocompatibility and stability, long circulation time, easy modification, low immunogenicity, and low toxicity [90–92]. Exosomes can be fabricated to target CSCs. In one study, luminescent porous silicon nanoparticles (PSiNPs) loaded with doxorubicin (DOX@PSiNPs) were used. Human hepatocarcinoma Bel7402 cells were incubated with PSiNPs for 6 h and then incubated in nanoparticle-free medium. The results showed that PsiNPs were exocytosed from Bel7402 cells in a time-dependent manner to induce autophagy and could be obtained through centrifugation. Exosome-sheathed DOX-loaded PSiNPs (DOX@E-PSiNPs) were also obtained in a similar way. DOX@E-PSiNPs demonstrated excellent antitumor and anti-CSC efficacy [93] (Fig. 7).
Fig. 7.
Schematic illustration of exosome-sheathed porous silicon nanoparticles (PSiNPs) as nano-carriers for CSCs targeting delivery. Permission by Creative Commons CC BY [93]
Nano-immunotherapy for CSCs
Immune cells that infiltrate in tumors are expected to eradicate cancer cells. However, CSCs alone or with other noncancerous cells can lead to an immunosuppressive TME [94]. CSCs or stromal cells interfere with DC recruitment to the tumor site, impair maturation and improve the formation of immunoregulatory DCs (DCregs) [95]. The crosstalk between regulatory T cells and CSCs also provides an immunosuppressive TME [96]. Therefore, immunotherapy targeting CSCs is a key route to improve antitumor efficacy.
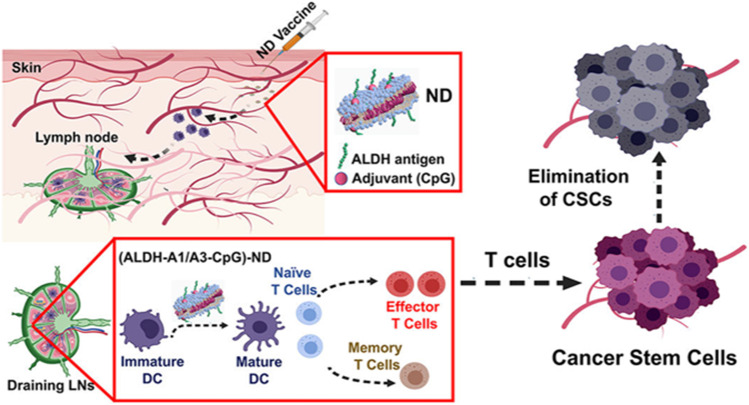
In recent years, extensive preclinical studies and initial clinical data have demonstrated that nanotechnology can overcome some of the challenges that currently limit cancer immunotherapy [97]. ALDH is a surface marker for isolating and identifying CSCs. Najafabadi A et al. developed a synthetic high-density lipoprotein nanodisk vaccination to generate ALDH-specific T-cell responses against ALDH+ CSCs based on ALDH1-A1 and ALDH1-A3 epitopes. The nanodisk vaccination exhibited potent antitumor efficacy in combination with anti-PD-L1 therapy in vivo (Fig. 8) [98]. In another study, sialic acid-modified doxorubicin-loaded liposomes were able to modulate the immunosuppressive TME in multiple ways, including inhibiting tumor-associated macrophage (TAM)-mediated immunosuppression and promoting the infiltration of CD8+ T cells. The combination of liposomes with anti-PD-1 therapy almost completely eliminated B16F10 tumors and effectively inhibited 4T1 tumors. To eradicate breast CSCs, metformin was incorporated into the above combination treatment, and the synergistic effects were beneficial in 4T1 tumors [99]. A cocktail nanodevice composed of paclitaxel, thioridazine (anti-CSC agent) and HY19991 (PD-1/PD-L1 inhibitor) coloaded enzyme/pH dual-sensitive micelles has been designed to reduce the proportion of CSCs and enhance T-cell infiltration [100]. As reported, nanoimmunotherapy can target CSCs and further improve the effects of immunotherapy.
Fig. 8.
Schematic illustration of nanodiscs against ALDHhigh CSCs. Adapted from [98]
Nanomedicines targeting the abnormal metabolism in CSCs
The abnormal metabolism of CSCs provides an opportunity for CSC targeting. Shen Y et al. constructed a novel paclitaxel loaded liposome (Nano-Taxol) to investigate its effects on stemness and metabolic reprogramming in paclitaxel-resistant ovarian cancer. The results showed that the intraperitoneal delivery of Nano-Taxol effectively suppressed CSCs and redirected metabolism from glycolysis to oxidative phosphorylation [101]. Nano-realgar has been used for anticancer treatment. As reported, Nano-realgar inhibits lung cancer stem cell viability by inducing glucose metabolism, and downregulating the expression of the HIF-1α and PI3K/Akt/mTOR pathways [102].
Conclusion and perspectives
.CSCs are often quiescent and intrinsically drug-resistant through the expression of drug efflux transporters, and the activation of antiapoptotic signaling pathways. Conventional therapy often fails to eradicate them. Nanoparticle drug delivery systems (NDDSs) are very attractive carriers for the delivery of drugs to tumors. In our review, nanotechnology-based strategies for CSC targeting are proposed, include (1) encapsulating small molecular drugs and genes by nanotechnology, (2) targeting CSC signaling pathways, (3) utilizing nanocarriers targeting for specific markers of CSCs, (4) improving PTT/PDT, (5) targeting the metabolism of CSCs and (6) enhancing nanomedicine-aided immunotherapy.
Although nanomedicines have successfully been approved by the FDA for cancer treatment and more nanomedicines are in human clinical trials, the application of nanomedicines in anti-CSC therapies is at a relatively early stage. In designing rational nanomedicines for CSC targeting, the following issues could also be addressed: (1) CSCs and normal stem cells share many signaling pathways; therefore, the utilization of inhibitors or siRNA of signaling pathways can also cause the destruction of normal stem cells. Although loading by nanotechnology can reduce toxicity, it is more efficient to choose the abnormal activation of signaling pathways for targeted therapy of different CSCs. (2) A median of 0.7% of a nanoparticle injection dose arrives at solid tumors, and this low value contributed to the poor clinical translation of nanomedicines [103]. Ouyang B et al. found a dose threshold for effective nanoparticle delivery. There are a small number of CSCs in tumors. Thus, for CSC recognition and effective drug delivery, there may be a minimum dose threshold that is worth exploring. (3) CSCs are “cold” seeds, namely in a resting state with low proliferation ability and inherent therapy resistance. Remolding these “cold” CSCs to “hot” tumor epithelial cells with high proliferation ability and active telomerase activity before or during nanomedicine treatment may be a good strategy to enhance the killing effects.
Acknowledgements
This work was supported by the National Nature Science Foundation of China (No.81803029) and the Science and Technology Foundation of Yuzhong District, Chongqing (No.20210179).
Author contributions
Lin Li, Rui Ni and Lin Chen contributed to the idea for this review article. The literature search and analysis were performed by Dan Zheng. Further evidences collection, analysis and arrangement were accomplished by Lin Li and Rui Ni. The first draft of the manuscript was written by Lin Li and Rui Ni. Then, the manuscript was critically revised by Lin Chen (Corresponding Author). All authors read and approved the final manuscript.
Declarations
Conflict of interest
The authors confirm that this article content has no conflicts of interest.
Footnotes
Lin Li and Rui Ni contributed equally to this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 3.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, Dirks PB. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821. [PubMed] [Google Scholar]
- 5.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65:10946. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 6.Szotek PP, Pieretti-Vanmarcke R, Masiakos PT, Dinulescu DM, Connolly D, Foster R, Dombkowski D, Preffer F, MacLaughlin DT, Donahoe PK. Ovarian cancer side population defines cells with stem cell-like characteristics and mullerian inhibiting substance responsiveness. Proc Natl Acad Sci U S A. 2006;103:11154. doi: 10.1073/pnas.0603672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2006;445:106. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- 8.Zhu P, Fan Z. Cancer stem cells and tumorigenesis. Biophys Rep. 2018;4:178–88. doi: 10.1007/s41048-018-0062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Najafi M, Farhood B, Mortezaee K. Cancer stem cells (CSCs) in cancer progression and therapy. J Cell Physiol. 2019;234:8381–95. doi: 10.1002/jcp.27740. [DOI] [PubMed] [Google Scholar]
- 10.Islam F, Qiao B, Smith RA, Gopalan V, Lam AKY. Cancer stem cell: fundamental experimental pathological concepts and updates. Exp Mol Pathol. 2015;98:184–91. doi: 10.1016/j.yexmp.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Paul R, Dorsey JF, Fan Y. Cell plasticity, senescence, and quiescence in cancer stem cells: Biological and therapeutic implications. Pharmacol Ther. 2022;231:107985. doi: 10.1016/j.pharmthera.2021.107985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang D-Y, Monteiro MJ, Liu J-P. Mechanisms of cancer stem cell senescence: current understanding and future perspectives. Clin Exp Pharmacol Physiol. 2021;48:1185–202. doi: 10.1111/1440-1681.13528. [DOI] [PubMed] [Google Scholar]
- 13.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–11. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 14.Nathansen J, Meyer F, Müller L, Schmitz M, Borgmann K, Dubrovska A. Beyond the double-strand breaks: the role of DNA repair proteins in cancer stem-cell regulation. Cancers. 2021;13:4818. doi: 10.3390/cancers13194818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dzobo K, Senthebane DA, Ganz C, Thomford NE, Wonkam A, Dandara C. Advances in therapeutic targeting of cancer stem cells within the tumor microenvironment: an updated review. Cells. 2020;9:1896. doi: 10.3390/cells9081896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toh TB, Lim JJ, Chow EK-H. Epigenetics in cancer stem cells. Mol Cancer. 2017;16:29. doi: 10.1186/s12943-017-0596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23:1124–34. doi: 10.1038/nm.4409. [DOI] [PubMed] [Google Scholar]
- 18.Emami Nejad A, Najafgholian S, Rostami A, Sistani A, Shojaeifar S, Esparvarinha M, Nedaeinia R, Haghjooy Javanmard S, Taherian M, Ahmadlou M, Salehi R, Sadeghi B, Manian M. The role of hypoxia in the tumor microenvironment and development of cancer stem cell: a novel approach to developing treatment. Cancer Cell Int. 2021;21:62. doi: 10.1186/s12935-020-01719-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wainwright EN, Scaffidi P. Epigenetics and Cancer Stem cells: unleashing, hijacking, and restricting Cellular plasticity. Trends Cancer. 2017;3:372–86. doi: 10.1016/j.trecan.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Robertis M, Poeta ML, Signori E, Fazio VM. Current understanding and clinical utility of miRNAs regulation of colon cancer stem cells. Semin Cancer Biol. 2018;53:232–47. doi: 10.1016/j.semcancer.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 21.Bai X, Ni J, Beretov J, Graham P, Li Y. Cancer stem cell in breast cancer therapeutic resistance. Cancer Treat Rev. 2018;69:152–63. doi: 10.1016/j.ctrv.2018.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Nazio F, Po A, Abballe L, Ballabio C, Diomedi Camassei F, Bordi M, Camera A, Caruso S, Caruana I, Pezzullo M, Ferraina C, Milletti G, Gianesello M, Reddel S, De Luca CD, Ceglie D, Marinelli S, Campello S, Papaleo E, Miele E, Cacchione A, Carai A, Vinci M, Velardi E, De Angelis B, Tiberi L, Quintarelli C, Mastronuzzi A, Ferretti E, Locatelli F, Cecconi F. Targeting cancer stem cells in medulloblastoma by inhibiting AMBRA1 dual function in autophagy and STAT3 signalling. Acta Neuropathol. 2021;142:537–64. doi: 10.1007/s00401-021-02347-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Y, Jiang X, Duan L, Xiong Q, Yuan Y, Liu P, Jiang L, Shen Q, Zhao S, Yang C, Chen Y. LncRNA PKMYT1AR promotes cancer stem cell maintenance in non-small cell lung cancer via activating wnt signaling pathway. Mol Cancer. 2021;20:156. doi: 10.1186/s12943-021-01469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marquardt S, Solanki M, Spitschak A, Vera J, Pützer BM. Emerging functional markers for cancer stem cell-based therapies: understanding signaling networks for targeting metastasis. Semin Cancer Biol. 2018;53:90–109. doi: 10.1016/j.semcancer.2018.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Lathia JD, Mack SC, Mulkearns-Hubert EE, Valentim CLL, Rich JN. Cancer stem cells in glioblastoma. Genes Dev. 2015;29:1203–17. doi: 10.1101/gad.261982.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tu S-M. Prostate cancer stem cells. Clin Genitourin Cancer. 2012;10:69–76. doi: 10.1016/j.clgc.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keyvani V, Farshchian M, Esmaeili S-A, Yari H, Moghbeli M, Nezhad S-RK, Abbaszadegan MR. Ovarian cancer stem cells and targeted therapy. J Ovarian Res. 2019;12:120. doi: 10.1186/s13048-019-0588-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walcher L, Kistenmacher A-K, Suo H, Kitte R, Dluczek S, Strauß A, Blaudszun A-R, Yevsa T, Fricke S, Kossatz-Boehlert U. Cancer Stem Cells-Origins and biomarkers: perspectives for targeted personalized therapies. Front Immunol. 2020;11:1280. doi: 10.3389/fimmu.2020.01280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Read T-A, Fogarty MP, Markant SL, McLendon RE, Wei Z, Ellison DW, Febbo PG, Wechsler-Reya RJ. Identification of CD15 as a marker for tumor-propagating cells in a mouse model of medulloblastoma. Cancer Cell. 2009;15:135–47. doi: 10.1016/j.ccr.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tchoghandjian A, Baeza N, Colin C, Cayre M, Metellus P, Beclin C, Ouafik LH, Figarella-Branger D. A2B5 cells from human glioblastoma have Cancer Stem Cell Properties. Brain Pathol. 2010;20:211–21. doi: 10.1111/j.1750-3639.2009.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.An SM, Ding Q, Zhang J, Xie J, Li L. Targeting stem cell signaling pathways for drug discovery: advances in the notch and wnt pathways. Sci China Life Sci. 2014;57:575–80. doi: 10.1007/s11427-014-4665-7. [DOI] [PubMed] [Google Scholar]
- 32.Takebe N, Miele L, Harris PJ, Jeong W, Bando H, Kahn M, Yang SX, Ivy SP. Targeting notch, hedgehog, and wnt pathways in cancer stem cells: clinical update. Nat Rev Clin Oncol. 2015;12:445–64. doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Sousa E, Melo F, Vermeulen L. Wnt Signal cancer stem cell biology. Cancers (Basel) 2016;8:60. doi: 10.3390/cancers8070060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Todaro M, Gaggianesi M, Catalano V, Benfante A, Iovino F, Biffoni M, Apuzzo T, Sperduti I, Volpe S, Cocorullo G, Gulotta G, Dieli F, De Maria R, Stassi G. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell. 2014;14:342–56. doi: 10.1016/j.stem.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Wang R, Sun Q, Wang P, Liu M, Xiong S, Luo J, Huang H, Du Q, Geller DA, Cheng B. Notch and Wnt/β-catenin signaling pathway play important roles in activating liver cancer stem cells. Oncotarget. 2016;7:5754–68. doi: 10.18632/oncotarget.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsui WH. Cancer stem cell signaling pathways. Med (Baltim) 2016;95:8–S19. doi: 10.1097/MD.0000000000004765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu J, Pan S, Hsieh MH, Ng N, Sun F, Wang T, Kasibhatla S, Schuller AG, Li AG, Cheng D, Li J, Tompkins C, Pferdekamper A, Steffy A, Cheng J, Kowal C, Phung V, Guo G, Wang Y, Graham MP, Flynn S, Brenner JC, Li C, Villarroel MC, Schultz PG, Wu X, McNamara P, Sellers WR, Petruzzelli L, Boral AL, Seidel HM, McLaughlin ME, Che J, Carey TE, Vanasse G, Harris JL. Targeting wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc Natl Acad Sci U S A. 2013;110:20224–9. doi: 10.1073/pnas.1314239110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Madan B, Ke Z, Harmston N, Ho SY, Frois AO, Alam J, Jeyaraj DA, Pendharkar V, Ghosh K, Virshup IH, Manoharan V, Ong EHQ, Sangthongpitag K, Hill J, Petretto E, Keller TH, Lee MA, Matter A, Virshup DM. Wnt addiction of genetically defined cancers reversed by PORCN inhibition. Oncogene. 2016;35:2197–207. doi: 10.1038/onc.2015.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ko AH, Chiorean EG, Kwak EL, Lenz H-J, Nadler PI, Wood DL, Fujimori M, Inada T, Kouji H, McWilliams RR. Final results of a phase ib dose-escalation study of PRI-724, a CBP/beta-catenin modulator, plus gemcitabine (GEM) in patients with advanced pancreatic adenocarcinoma (APC) as second-line therapy after FOLFIRINOX or FOLFOX. J Clin Oncol. 2016;34:e15721. [Google Scholar]
- 40.Krop I, Demuth T, Guthrie T, Wen PY, Mason WP, Chinnaiyan P, Butowski N, Groves MD, Kesari S, Freedman SJ, Blackman S, Watters J, Loboda A, Podtelezhnikov A, Lunceford J, Chen C, Giannotti M, Hing J, Beckman R, LoRusso P. Phase I pharmacologic and pharmacodynamic study of the Gamma secretase (notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J Clin Oncol. 2012;30:2307–13. doi: 10.1200/JCO.2011.39.1540. [DOI] [PubMed] [Google Scholar]
- 41.Pant S, Jones SF, Kurkjian CD, Infante JR, Moore KN, Burris HA, McMeekin DS, Benhadji KA, Patel BKR, Frenzel MJ, Kursar JD, Zamek-Gliszczynski MJ, Yuen ESM, Chan EM, Bendell JC. A first-in-human phase I study of the oral notch inhibitor, LY900009, in patients with advanced cancer. Eur J Cancer. 2016;56:1–9. doi: 10.1016/j.ejca.2015.11.021. [DOI] [PubMed] [Google Scholar]
- 42.Tolcher AW, Messersmith WA, Mikulski SM, Papadopoulos KP, Kwak EL, Gibbon DG, Patnaik A, Falchook GS, Dasari A, Shapiro GI, Boylan JF, Xu ZX, Wang K, Koehler A, Song J, Middleton SA, Deutsch J, DeMario M, Kurzrock R, Wheler JJ. Phase I study of RO4929097, a Gamma secretase inhibitor of Notch Signaling, in patients with refractory metastatic or locally advanced solid tumors. J Clin Oncol. 2012;30:2348–53. doi: 10.1200/JCO.2011.36.8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morgan KM, Fischer BS, Lee FY, Shah JJ, Bertino JR, Rosenfeld J, Singh A, Khiabanian H, Pine SR. Gamma Secretase Inhibition by BMS-906024 enhances efficacy of paclitaxel in lung adenocarcinoma. Mol Cancer Ther. 2017;16:2759–69. doi: 10.1158/1535-7163.MCT-17-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kummar S, O’Sullivan Coyne G, Do KT, Turkbey B, Meltzer PS, Polley E, Choyke PL, Meehan R, Vilimas R, Horneffer Y, Juwara L, Lih A, Choudhary A, Mitchell SA, Helman LJ, Doroshow JH, Chen AP. Clinical activity of the γ-Secretase inhibitor PF-03084014 in adults with desmoid tumors (aggressive fibromatosis) J Clin Oncol. 2017;35:1561–9. doi: 10.1200/JCO.2016.71.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gutzmer R, Solomon JA. Hedgehog pathway inhibition for the treatment of basal cell carcinoma. Target Oncol. 2019;14:253–67. doi: 10.1007/s11523-019-00648-2. [DOI] [PubMed] [Google Scholar]
- 46.AlMuraikhi N, Almasoud N, Binhamdan S, Younis G, Ali D, Manikandan M, Vishnubalaji R, Atteya M, Siyal A, Alfayez M, Aldahmash A, Kassem M. Alajez NM. Hedgehog signaling inhibition by smoothened antagonist BMS-833923 reduces osteoblast differentiation and ectopic bone formation of human skeletal (Mesenchymal) stem cells. Stem Cells Int. 2019; 2019: 3435901. [DOI] [PMC free article] [PubMed]
- 47.Bendell J, Andre V, Ho A, Kudchadkar R, Migden M, Infante J, Tiu RV, Pitou C, Tucker T, Brail L, Von Hoff D. Phase I study of LY2940680, a smo antagonist, in patients with advanced cancer including treatment-naïve and previously treated basal cell carcinoma. Clin Cancer Res. 2018;24:2082–91. doi: 10.1158/1078-0432.CCR-17-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–11. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Versini A, Colombeau L, Hienzsch A, Gaillet C, Retailleau P, Debieu S, Müller S, Cañeque T, Rodriguez R. Salinomycin derivatives kill breast cancer stem cells by lysosomal Iron targeting. Chemistry. 2020;26:7416–24. doi: 10.1002/chem.202000335. [DOI] [PubMed] [Google Scholar]
- 50.Cuyàs E, Martin-Castillo B, Corominas-Faja B, Massaguer A, Bosch-Barrera J, Menendez JA. Anti-protozoal and anti-bacterial antibiotics that inhibit protein synthesis kill cancer subtypes enriched for stem cell-like properties. Cell Cycle. 2015;14:3527–32. doi: 10.1080/15384101.2015.1044173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Darini CY, Martin P, Azoulay S, Drici MD, Hofman P, Obba S, Dani C, Ladoux A. Targeting cancer stem cells expressing an embryonic signature with anti-proteases to decrease their tumor potential. Cell Death Dis. 2013;4:e706. doi: 10.1038/cddis.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nagano O, Okazaki S, Saya H. Redox regulation in stem-like cancer cells by CD44 variant isoforms. Oncogene. 2013;32:5191–8. doi: 10.1038/onc.2012.638. [DOI] [PubMed] [Google Scholar]
- 53.Ciavardelli D, Rossi C, Barcaroli D, Volpe S, Consalvo A, Zucchelli M, De Cola A, Scavo E, Carollo R, D’Agostino D, Forlì F, D’Aguanno S, Todaro M, Stassi G, Di Ilio C, De Laurenzi V, Urbani A. Breast cancer stem cells rely on fermentative glycolysis and are sensitive to 2-deoxyglucose treatment. Cell Death Dis. 2014;5:e1336. doi: 10.1038/cddis.2014.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang VMY, Ferreira RMM, Almagro J, Evan T, Legrave N, Zaw Thin M, Frith D, Carvalho J, Barry DJ, Snijders AP, Herbert E, Nye EL, MacRae JI, Behrens A. CD9 identifies pancreatic cancer stem cells and modulates glutamine metabolism to fuel tumour growth. Nat Cell Biol. 2019;21:1425–35. doi: 10.1038/s41556-019-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li D, Fu Z, Chen R, Zhao X, Zhou Y, Zeng B, Yu M, Zhou Q, Lin Q, Gao W, Ye H, Zhou J, Li Z, Liu Y, Chen R. Inhibition of glutamine metabolism counteracts pancreatic cancer stem cell features and sensitizes cells to radiotherapy. Oncotarget. 2015;6:31151–63. doi: 10.18632/oncotarget.5150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yasumoto Y, Miyazaki H, Vaidyan LK, Kagawa Y, Ebrahimi M, Yamamoto Y, Ogata M, Katsuyama Y, Sadahiro H, Suzuki M, Owada Y. Inhibition of fatty acid synthase decreases expression of stemness markers in glioma stem cells. PLoS ONE. 2016;11:e0147717. doi: 10.1371/journal.pone.0147717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Q, Liu F, Wang L, Xie C, Wu P, Du S, Zhou S, Sun Z, Liu Q, Yu L, Liu B, Li R. Enhanced and prolonged antitumor effect of salinomycin-loaded gelatinase-responsive nanoparticles via targeted drug delivery and inhibition of cervical cancer stem cells. Int Nanomedicine. 2020;15:1283–95. doi: 10.2147/IJN.S234679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao Y, Zhao W, Lim YC, Liu T. Salinomycin-loaded gold nanoparticles for treating Cancer Stem cells by Ferroptosis-Induced cell death. Mol Pharm. 2019;16:2532–9. doi: 10.1021/acs.molpharmaceut.9b00132. [DOI] [PubMed] [Google Scholar]
- 59.Gao J, Liu J, Xie F, Lu Y, Yin C, Shen X. Co-delivery of docetaxel and salinomycin to target both breast cancer cells and stem cells by PLGA/TPGS nanoparticles. Int Nanomedicine. 2019;14:9199–216. doi: 10.2147/IJN.S230376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kuo Y-C, Wang L-J. Targeting human brain cancer stem cells by curcumin-loaded nanoparticles grafted with anti-aldehyde dehydrogenase and sialic acid: colocalization of ALDH and CD44. Mater Sci Eng C Mater Biol Appl. 2019;102:362–72. doi: 10.1016/j.msec.2019.04.065. [DOI] [PubMed] [Google Scholar]
- 61.Das S, Mukherjee P, Chatterjee R, Jamal Z, Chatterji U. Enhancing chemosensitivity of breast Cancer stem cells by downregulating SOX2 and ABCG2 using wedelolactone-encapsulated nanoparticles. Mol Cancer Ther. 2019;18:680–92. doi: 10.1158/1535-7163.MCT-18-0409. [DOI] [PubMed] [Google Scholar]
- 62.Ibiyeye KM, Zuki ABZ. Cockle Shell-Derived Aragonite CaCO(3) nanoparticles for co-delivery of doxorubicin and thymoquinone eliminates cancer stem cells. Int J Mol Sci. 2020;21:1900. doi: 10.3390/ijms21051900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsai P-H, Wang M-L, Chang J-H, Yarmishyn AA, Nhi Nguyen PN, Chen W, Chien Y, Huo T-I, Mou C-Y, Chiou S-H. Dual delivery of HNF4α and cisplatin by mesoporous silica nanoparticles inhibits cancer pluripotency and tumorigenicity in hepatoma-derived CD133-expressing stem cells. ACS Appl Mater Interfaces. 2019;11:19808–18. doi: 10.1021/acsami.9b04474. [DOI] [PubMed] [Google Scholar]
- 64.Yin H, Xiong G, Guo S, Xu C, Xu R, Guo P, Shu D. Delivery of anti-miRNA for triple-negative breast cancer therapy using RNA nanoparticles targeting stem cell marker CD133. Mol Ther. 2019;27:1252–61. doi: 10.1016/j.ymthe.2019.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ke X, Yang C, Cheng W, Yang YY. Delivery of NF-κB shRNA using carbamate-mannose modified PEI for eliminating cancer stem cells. Nanomedicine. 2018;14:405–14. doi: 10.1016/j.nano.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 66.Tian J, Gu Y, Li Y, Liu T. CD271 antibody-functionalized HGNs for targeted photothermal therapy of osteosarcoma stem cells. Nanotechnology. 2020;31:305707. doi: 10.1088/1361-6528/ab8593. [DOI] [PubMed] [Google Scholar]
- 67.Tan T, Wang H, Cao H, Zeng L, Wang Y, Wang Z, Wang J, Li J, Wang S, Zhang Z, Li Y. Deep Tumor-Penetrated Nanocages improve accessibility to Cancer Stem cells for photothermal-chemotherapy of breast Cancer metastasis. Adv Sci(Weinh) 2018;5:1801012. doi: 10.1002/advs.201801012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fernandes S, Fernandez T, Metze S, Balakrishnan PB, Mai BT, Conteh J, De Mei C, Turdo A, Di Franco S, Stassi G, Todaro M, Pellegrino T. Magnetic nanoparticle-based hyperthermia mediates drug delivery and impairs the tumorigenic capacity of quiescent colorectal cancer stem cells. ACS Appl Mater Interfaces. 2021;13:15959–72. doi: 10.1021/acsami.0c21349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Atkinson RL, Zhang M, Diagaradjane P, Peddibhotla S, Contreras A, Hilsenbeck SG, Woodward WA, Krishnan S, Chang JC, Rosen JM. Thermal enhancement with optically activated gold nanoshells sensitizes breast cancer stem cells to radiation therapy. Sci Transl Med. 2010;2:55ra79. doi: 10.1126/scitranslmed.3001447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17:657–74. doi: 10.1038/s41571-020-0410-2. [DOI] [PubMed] [Google Scholar]
- 71.Crous A, Abrahamse H. Effective gold nanoparticle-antibody-mediated drug delivery for photodynamic therapy of lung cancer stem cells. Int J Mol Sci. 2020;21:3742. doi: 10.3390/ijms21113742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang B, Liu H, Yang H, Chen W, Wu J, Feng X, Tong R, Yu H, Chen Y, Lv Z, Sun W, He B, Wu J, Yu G, Mao Z, Zheng S. Combinatorial photochemotherapy on liver cancer stem cells with organoplatinum(ii) metallacage-based nanoparticles. J Mater Chem B. 2019;7:6476–87. doi: 10.1039/c9tb01299k. [DOI] [PubMed] [Google Scholar]
- 73.Avancini G, Guerrini A, Ferroni C, Tedesco D, Ballestri M, Columbaro M, Menilli L, Reddi E, Costa R, Leanza L, Varchi G, Moret F. Keratin nanoparticles and photodynamic therapy enhance the anticancer stem cells activity of salinomycin. Mater Sci Eng C Mater Biol Appl. 2021;122:111899. doi: 10.1016/j.msec.2021.111899. [DOI] [PubMed] [Google Scholar]
- 74.Lu S-L, Wang Y-H, Liu G-F, Wang L, Li Y, Guo Z-Y. Graphene Oxide nanoparticle-loaded Ginsenoside Rg3 improves photodynamic therapy in inhibiting malignant progression and stemness of Osteosarcoma. Front Mol Biosci. 2021;8:663089. doi: 10.3389/fmolb.2021.663089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shamsian A, Sepand MR, Javaheri Kachousangi M, Dara T, Ostad SN, Atyabi F, Ghahremani MH. Targeting tumorigenicity of breast cancer stem cells using SAHA/Wnt-b catenin antagonist loaded onto protein corona of gold nanoparticles. Int J Nanomedicine. 2020;15:4063–78. doi: 10.2147/IJN.S234636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang Y, Yang Q-W, Yang Q, Zhou T, Shi M-F, Sun C-X, Gao X-X, Cheng Y-Q, Cui X-G, Sun Y-H. Cuprous oxide nanoparticles inhibit prostate cancer by attenuating the stemness of cancer cells via inhibition of the wnt signaling pathway. Int J Nanomedicine. 2017;12:2569–79. doi: 10.2147/IJN.S130537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yallapu MM, Maher DM, Sundram V, Bell MC, Jaggi M, Chauhan SC. Curcumin induces chemo/radio-sensitization in ovarian cancer cells and curcumin nanoparticles inhibit ovarian cancer cell growth. J Ovarian Res. 2010;3:11. doi: 10.1186/1757-2215-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chandra Boinpelly V, Verma RK, Srivastav S, Srivastava RK. Shankar S. α-Mangostin-encapsulated PLGA nanoparticles inhibit colorectal cancer growth by inhibiting notch pathway. J Cell Mol Med. 2020;24:11343–54. doi: 10.1111/jcmm.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang Y, Zhou B, Luo H, Mao J, Huang Y, Zhang K, Mei C, Yan Y, Jin H, Gao J, Su Z, Pang P, Li D, Shan H. ZnAs@SiO(2) nanoparticles as a potential anti-tumor drug for targeting stemness and epithelial-mesenchymal transition in hepatocellular carcinoma via SHP-1/JAK2/STAT3 signaling. Theranostics. 2019;9:4391–408. doi: 10.7150/thno.32462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaushik NK, Kaushik N, Yoo KC, Uddin N, Kim JS, Lee SJ, Choi EH. Low doses of PEG-coated gold nanoparticles sensitize solid tumors to cold plasma by blocking the PI3K/AKT-driven signaling axis to suppress cellular transformation by inhibiting growth and EMT. Biomaterials. 2016;87:118–30. doi: 10.1016/j.biomaterials.2016.02.014. [DOI] [PubMed] [Google Scholar]
- 81.Mokhtarzadeh A, Hassanpour S, Vahid ZF, Hejazi M, Hashemi M, Ranjbari J, Tabarzad M, Noorolyai S, de la Guardia M. Nano-delivery system targeting to cancer stem cell cluster of differentiation biomarkers. J Control Release. 2017;266:166–86. doi: 10.1016/j.jconrel.2017.09.028. [DOI] [PubMed] [Google Scholar]
- 82.Cho J-H, Kim AR, Kim S-H, Lee S-J, Chung H, Yoon M-Y. Development of a novel imaging agent using peptide-coated gold nanoparticles toward brain glioma stem cell marker CD133. Acta Biomater. 2017;47:182–92. doi: 10.1016/j.actbio.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 83.Ni M, Xiong M, Zhang X, Cai G, Chen H, Zeng Q, Yu Z. Poly(lactic-co-glycolic acid) nanoparticles conjugated with CD133 aptamers for targeted salinomycin delivery to CD133 + osteosarcoma cancer stem cells. Int J Nanomedicine. 2015;10:2537–54. doi: 10.2147/IJN.S78498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zöller M. CD44, Hyaluronan, the hematopoietic stem cell, and leukemia-initiating cells. Front Immunol. 2015;6:235. doi: 10.3389/fimmu.2015.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Su Z, Liu D, Chen L, Zhang J, Ru L, Chen Z, Gao Z, Wang X. CD44-Targeted magnetic nanoparticles kill Head and Neck squamous cell carcinoma stem cells in an alternating magnetic field. Int J Nanomedicine. 2019;14:7549–60. doi: 10.2147/IJN.S215087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Y, Shi S, Ming Y, Wang L, Li C, Luo M, Li Z, Li B, Chen J. Specific cancer stem cell-therapy by albumin nanoparticles functionalized with CD44-mediated targeting. J Nanobiotechnol. 2018;16:99. doi: 10.1186/s12951-018-0424-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen H, Lin J, Shan Y, Zhengmao L. The promotion of nanoparticle delivery to two populations of gastric cancer stem cells by CD133 and CD44 antibodies. Biomed Pharmacother. 2019;115:108857. doi: 10.1016/j.biopha.2019.108857. [DOI] [PubMed] [Google Scholar]
- 88.Zeng Y-b, Yu Z-c, He Y-n, Zhang T, Du L-b, Dong Y-m, Chen H-w, Zhang Y-y, Wang W-q. Erratum: Salinomycin-loaded lipid-polymer nanoparticles with anti-CD20 aptamers selectively suppress human CD20 + melanoma stem cells. Acta Pharmacol Sin. 2018;39:330. doi: 10.1038/aps.2017.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim H, Jang H, Cho H, Choi J, Hwang KY, Choi Y, Kim SH, Yang Y Y. Recent advances in exosome-based drug delivery for cancer therapy. Cancers. 2021;13:4435. doi: 10.3390/cancers13174435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gutiérrez-Vázquez C, Villarroya-Beltri C, Mittelbrunn M. Transfer of extracellular vesicles during immune cell-cell interactions. Immunol Rev. 2013;251:125–42. doi: 10.1111/imr.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Purushothaman A, Bandari SK, Liu J, Mobley JA, Brown EE, Sanderson RD. Fibronectin on the Surface of Myeloma Cell-derived Exosomes mediates exosome-cell interactions. J Biol Chem. 2016;291:1652–63. doi: 10.1074/jbc.M115.686295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mulcahy LA, Pink RC, Carter DRF. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014; 3. [DOI] [PMC free article] [PubMed]
- 93.Yong T, Zhang X, Bie N, Zhang H, Zhang X, Li F, Hakeem A, Hu J, Gan L, Santos HA, Yang X. Tumor exosome-based nanoparticles are efficient drug carriers for chemotherapy. Nat Commun. 2019;10:3838. doi: 10.1038/s41467-019-11718-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dianat-Moghadam H, Mahari A, Salahlou R, Khalili M, Azizi M, Sadeghzadeh H. Immune evader cancer stem cells direct the perspective approaches to cancer immunotherapy. Stem Cell Res Ther. 2022;13:150. doi: 10.1186/s13287-022-02829-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tsuchiya H, Shiota G. Immune evasion by cancer stem cells. Regen Ther. 2021;17:20–33. doi: 10.1016/j.reth.2021.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dianat-Moghadam H, Heidarifard M, Jahanban-Esfahlan R, Panahi Y, Hamishehkar H, Pouremamali F, Rahbarghazi R, Nouri M. Cancer stem cells-emanated therapy resistance: implications for liposomal drug delivery systems. J Control Release. 2018;288:62–83. doi: 10.1016/j.jconrel.2018.08.043. [DOI] [PubMed] [Google Scholar]
- 97.Bockamp E, Rosigkeit S, Siegl D, Schuppan D. Nano-enhanced cancer immunotherapy: immunology encounters nanotechnology. Cells. 2020;9:2102. doi: 10.3390/cells9092102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hassani Najafabadi A, Zhang J, Aikins ME, Najaf Abadi ZI, Liao F, Qin Y, Okeke EB, Scheetz LM, Nam J, Xu Y, Adams D, Lester P, Hetrick T, Schwendeman A, Wicha MS, Chang AE, Li Q, Moon JJ. Cancer Immunotherapy via targeting cancer stem cells using vaccine nanodiscs. Nano lett. 2020;20:7783–92. doi: 10.1021/acs.nanolett.0c03414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li C, Qiu Q, Gao X, Yan X, Fan C, Luo X, Liu X, Wang S, Lai X, Song Y, Deng Y. Sialic acid conjugate-modified liposomal platform modulates immunosuppressive tumor microenvironment in multiple ways for improved immune checkpoint blockade therapy. J Control Release. 2021;337:393–406. doi: 10.1016/j.jconrel.2021.06.027. [DOI] [PubMed] [Google Scholar]
- 100.Lang T, Liu Y, Zheng Z, Ran W, Zhai Y, Yin Q, Zhang P, Li Y. Cocktail Strategy based on Spatio-Temporally controlled Nano device improves therapy of breast Cancer. Adv Mater. 2019;31:1806202. doi: 10.1002/adma.201806202. [DOI] [PubMed] [Google Scholar]
- 101.Shen YA, Li WH, Chen PH, He CL, Chang YH, Chuang CM. Intraperitoneal delivery of a novel liposome-encapsulated paclitaxel redirects metabolic reprogramming and effectively inhibits cancer stem cells in Taxol(®)-resistant ovarian cancer. Am J Transl Res. 2015;7:841–55. [PMC free article] [PubMed] [Google Scholar]
- 102.Yang Fr, Zhao Yf, Hu Xw, Liu Zk, Yu Xd, Li Cy, Li Xr, Li Hj. Nano-realgar suppresses lung cancer stem cell growth by repressing metabolic reprogramming. Gene. 2021;788:145666. doi: 10.1016/j.gene.2021.145666. [DOI] [PubMed] [Google Scholar]
- 103.Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WCW. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1:16014. [Google Scholar]