Abstract
Neuroblastoma is the most common extracranial solid tumor in children, often manifests in the retroperitoneal region. We present a case of a 3-year-old boy with no previous medical history, presented for abdominal distension. Physical examination revealed a distinct, mobile, solid mass situated in the left lumbar region. Abdominal magnetic resonance imaging displayed a well delimited, well-encapsulated mass attached to the tail of the pancreas. Urinary catecholamine metabolite levels were negative. Surgical exploration revealed that the tumor was primitively related to the left adrenal gland, and a complete resection was performed. The postoperative recovery was uncomplicated. NMYC oncogene was non-amplified.
Keywords: Neuroblastoma, Pediatrics, Oncology, Adrenal gland neoplasms
1. Introduction
Neuroblastoma is a type of cancer that primarily affects young children with the highest incidence between the ages of two and three.1 Over half of all neuroblastoma cases manifest in the medulla of the adrenal glands.1 Clinical manifestations of neuroblastoma are not specific. This lack of specificity makes the diagnosis and management of neuroblastoma challenging for both clinicians and surgeons. Our objective was to evaluate the difficulties associated with diagnosing and treating neuroblastoma, aiming to provide valuable insights for healthcare professionals involved in its management.
2. Case report
A previously healthy 3-year-old boy was admitted to our department for a one-month history of abdominal distension. Palpation revealed a well-defined, movable, solid mass in the left lumbar region. The abdominal ultrasound examination indicated the presence of a large volume of tissue of retroperitoneal origin, without providing further details regarding its specific location or origin.
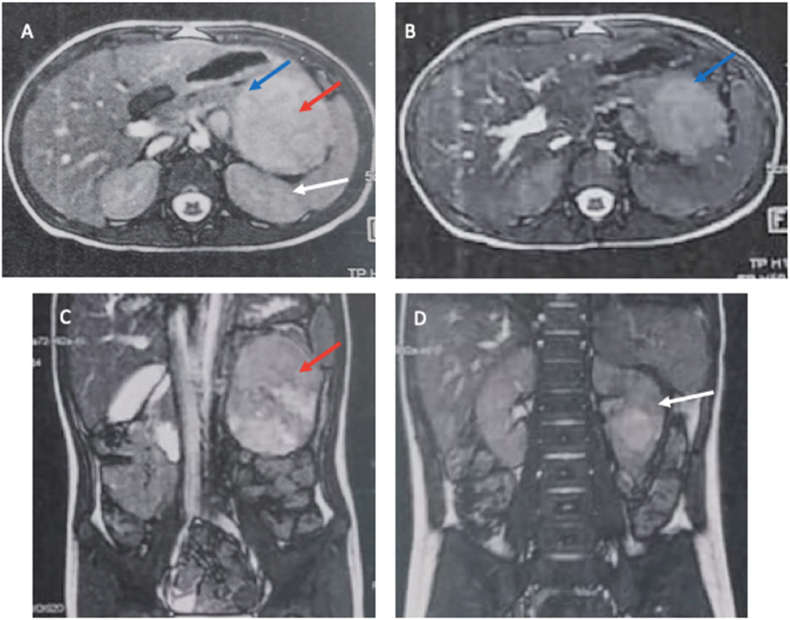
The color Doppler examination did not reveal any evidence of vascular compression or invasion. The abdominal magnetic resonance imaging (MRI) showed a sizable, clearly encapsulated mass located in the splenorenal recess. The mass appeared to be connected to the pancreatic tail, causing the edges of the pancreas to assume a beak-like shape. These findings strongly suggested that the mass originated from the pancreas (Fig. 1). There were no clear indications of vascular invasion. The mass caused displacement of these veins without infiltrating them. Additionally, the left kidney was moderately displaced towards the posterior aspect. Urinary catecholamine metabolite levels were found to be normal.
Fig. 1.
Abdominal MRI, (A–B) Axial T2 weighted images and (C–D) coronal T2 weighted images showing a large retro-peritoneal mass (red arrow): The mass is well encapsulated with heterogeneous high signal intensity. It deforms the edge of the tail of pancreas, in beak shape (blue arrow), suggestive that the mass originates from the pancreas. The left kidney (white arrowhead) is moderately displaced posteriorly. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
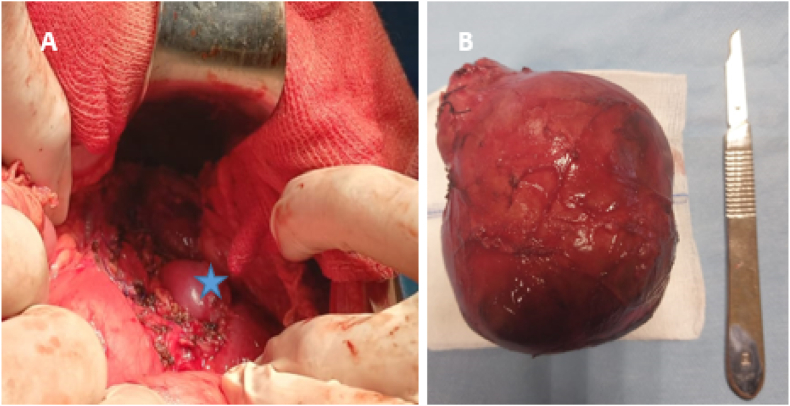
The suspected diagnosis of pancreatoblastoma was strongly considered based on the clinical findings. However, during the surgical exploration, it was discovered that the caudal part of the pancreas was normal and completely separate from the tumor. The tumor was primarily associated with the left adrenal gland and demonstrated adherence to the aorta and the left renal pedicle vessel. However, it did not extend into the left renal capsule. A meticulous process of tumor mobilization was undertaken, resulting in the en-bloc resection of the left adrenal gland (Fig. 2). Upon examination, the specimen exhibited a complete filling of a hemorrhagic encapsulated tumor. The remaining portion of the specimen appeared solid with a brownish color and the presence of cystic formations. Microscopic analysis revealed that the tumor consisted of small round cells arranged in rosettes. The stroma surrounding the cells was lacking Schwannian features. The nuclei appeared hyperchromatic, and atypical mitoses were observed, confirming the diagnosis of differentiating neuroblastoma (Fig. 3).
Fig. 2.
(A) Tumoral bed after en-bloc resection with the left adrenal, leaving the left kidney in place (asterix). (B) Specimen.
Fig. 3.
(A) Macroscopic features of adrenal differentiating neuroblastic tumors. On gross section, the specimen is totally occupied by a largely hemorrhagic encapsulated tumor formation. The rest of the specimen is solid over 4 cm with a brownish color and some cystic formations. (B) Neuroblastoma (Schwannian stroma-poor), differentiating subtype (original, × 400). Microscopy revealed a tumor composed of small round blue cells forming rosettes with scant cytoplasm, Schwannian stroma-poor (arrow), hyperchromatic nuclei, and atypical mitoses. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
The patient's recovery after surgery without any complications. MIBG scintigraphy ruled out the presence of metastasis. Additionally, analysis of the NMYC oncogene indicated a non-amplified status. Our patient did not require any adjuvant treatment, and specifically, chemotherapy was not indicated.
3. Discussion
Neuroblastoma is the most common abdominal tumor in childhood.2 It may be asymptomatic, reach a large size, and be discovered by palpation of a mass, as in our case. The retroperitoneal location allows silent progression to neighboring organs, making the diagnosis challenging.
Ultrasonography is the first-line treatment modality. Cross-sectional imaging is of primary interest for better study of the loco-regional extension of the disease and for preoperative planning. Computed tomography (CT) allows a better detection of calcifications and a more detailed study of vascular relationships. It also highlights the involvement of the spinal canal and the detection of secondary lesions, notably pulmonary and bone metastases. MRI is preferred if accessible; because of its better contrast resolution, absence of radiation, better study of the relationship with adjacent structures without injection of contrast, better detection of bone marrow lesions and secondary hepatic lesions, and better study of spinal canal extension. Metaiodobenzylguanidine (MIBG) imaging, in mapping the disease and detecting neuroblastoma with metastases to the bone and bone marrow offers better sensitivity and specificity of 82% and 91%, respectively.2 Increased levels of catecholamines or catecholamine metabolites are present in greater than 90% of patients with neuroblastoma.1 In our case the patient had a non-secretory neuroblastoma.
When non-secretory neuroblastoma is suspected, a biopsy of the tumor may be necessary to confirm the diagnosis. This procedure involves obtaining a small tissue sample from the tumor for further examination and analysis by a pathologist. MYCN amplification is used to assess risk and should be systematically sought.3 Currently, fluorescence in situ hybridization (FISH) is the reference technique for the detection of MYCN amplification. It is associated with advanced stages of disease, rapid tumor progression, and poor outcomes; its presence in low-stage and advanced disease is 5%_10% and 40%, respectively.2 In 2009, the International Neuroblastoma Risk Group (INRG) proposed a new staging system (INRGSS) based on preoperative images, allowing the selection of treatment strategies for patients with neuroblastic tumors.4 Operability without vital or functional risk is the cornerstone of management. It is linked to the presence or absence of image-defined risk factors (IDRF).
IDRFs has definitively changed the approach of neuroblastoma.5 Neoadjuvant chemotherapy can be indicated in order to reduce the tumor, making resection easier. Usually, in adrenal neuroblastoma, regional lymph nodes are frequently involved, and the primary tumor and involved lymph nodes often create a confluent mass that encases but does not invade the great vessels of the abdomen.2 In our case, the aorta and left renal vessels were intimately adherent.
The per operative discovery of the adrenal origin of the mass and the requirement of complete resection of the tumor with regionally involved lymph nodes, avoiding the removal of the left kidney was a critical situation that could have been better managed if neuroblastoma was evoked and neoadjuvant therapy was administered.
4. Conclusion
Neuroblastoma is a tumor that affects young children. To enhance the overall survival rate, a multidisciplinary approach is strongly recommended. This approach involves the collaboration of various medical specialists, such as pediatric oncologists, surgeons, radiation oncologists, and other healthcare professionals. By combining their expertise and utilizing a range of treatment modalities, including surgery, chemotherapy, radiation therapy, and immunotherapy, the goal is to improve outcomes and enhance the survival rate for children with neuroblastoma.
Consent
Written informed consent was obtained from the patient's parents for publication of this case report and accompanying images.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare no conflicts of interest regarding the publication of this article.
Contributor Information
Amina Karray, Email: karrayamina04@gmail.com.
Walid Cherifi, Email: walidcherifi89@gmail.com.
Farah Sassi, Email: sassi.farah@outlook.fr.
Abir Boussetta, Email: abirbousetta@gmail.com.
Slim Haouet, Email: haouetfamily@yahoo.fr.
Tahar Gargah, Email: tahar.gargah@rns.tn.
References
- 1.Matthay K., Maris J., Schleiermacher G., et al. Neuroblastoma. Nat Rev Dis Primers. 2016;2(2) doi: 10.1038/nrdp.2016.78. [DOI] [PubMed] [Google Scholar]
- 2.Croteau N., Nuchtern J., LaQuaglia M.P. Management of neuroblastoma in pediatric patients. Surg Oncol Clin. 2021;30(2):291–304. doi: 10.1016/j.soc.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Irwin M.S., Naranjo A., Zhang F.F., et al. Neuroblastoma risk classification system: a report from the children's Oncology Group. J Clin Oncol. 2021;39(29):3229–3241. doi: 10.1200/JCO.21.00278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monclair T., Brodeur G.M., Ambros P.F., et al. The international neuroblastoma risk Group (INRG) staging system: an INRG task force report. J Clin Oncol. 2009;27(2):298–303. doi: 10.1200/JCO.2008.16.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanza C., Galeazzi V., Carboni N., et al. Neuroblastoma image-defined risk factors in adrenal neuroblastoma: role of radiologist. Gland Surg. 2019;8(3):S168–S177. doi: 10.21037/gs.2019.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]