Highlights
-
•
Herpesviruses modulate carbohydrate-related metabolism within cells during infection.
-
•
Carbohydrate metabolism impacts the replication cycles of herpesviruses.
-
•
Carbohydrate metabolism-related pathways could be novel targets to treat herpesvirus infections.
Keywords: Herpesviruses, Herpes simplex virus type 1 (HSV-1), Herpes simplex virus type 2 (HSV-2), Varicella-zoster virus (VZV), Epstein-Barr virus (EBV), Cytomegalovirus (CMV), Human herpesvirus 6 (HHV-6), Human herpesvirus 7 (HHV-7), Kaposi's sarcoma-associated herpesvirus (KSHV), Carbohydrate metabolism, Glycolysis, Gluconeogenesis, Pentose phosphate pathway
Abstract
Human herpesviruses are enveloped viruses with double-stranded linear DNA genomes highly prevalent in the human population. These viruses are subdivided into three subfamilies, namely alphaherpesvirinae (herpes simplex virus type 1, HSV-1; herpes simplex virus type 2, HSV-2; and varicella-zoster virus, VZV), betaherpesvirinae (human cytomegalovirus, HCMV; human herpesvirus 6, HHV-6; and human herpesvirus 7, HHV-7) and gammaherpesvirinae (Epstein-Barr virus, EBV; and Kaposi's sarcoma-associated herpesvirus, KSHV). Besides encoding numerous molecular determinants to evade the host antiviral responses, these viruses also modulate cellular metabolic processes to promote their replication. Here, we review and discuss existing studies describing an interplay between carbohydrate metabolism and the replication cycle of herpesviruses, altogether highlighting potentially new molecular targets based on these interactions that could be used to block herpesvirus infections.
Graphical abstract

Introduction
Human herpesviruses are enveloped viruses with linear double-stranded DNA (dsDNA) genomes and are divided into three subfamilies: alphaherpesvirinae, betaherpesvirinae and gammaherpesvirinae (Asha and Sharma-Walia, 2021). Viruses within the alphaherpesvirinae subfamily include herpes simplex virus type 1 (HSV-1), herpes simplex virus type 2 (HSV-2), and varicella-zoster virus (VZV) (Cole, 2020; Retamal-Diaz et al., 2015). Cytomegalovirus (HCMV), human herpesvirus 6 (HHV-6), and human herpesvirus 7 (HHV-7) belong to the betaherpesvirinae subfamily, while Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV), belong to the gammaherpesvirinae subfamily (Asha and Sharma-Walia, 2021).
Alphaherpesviruses are highly frequent in the human population. Regarding HSV-1 and HSV-2, available studies report a prevalence nearing 67% and 13%, respectively, for these viruses (James et al., 2020), while the prevalence for VZV nears 93% in the U.S. in individuals between 6 and 19 years old and 98% for adults aged 20–49 (Kourieh et al., 2019). Diseases elicited by HSV-1 and HSV-2 are diverse, although several are skin-related, while others can affect the eye and the central nervous system (Duarte et al., 2019). VZV causes varicella and zoster, also known as chickenpox and shingles, respectively (Gershon et al., 2015). Generally, infections by alphaherpesviruses involve interactions between viral surface glycoproteins, such as glycoprotein B (gB), with heparan sulfates on the surface of host cells (Ibanez et al., 2018). For HSV-1 and HSV-2, the viral gD protein can interact with the cellular receptors nectin-1 or nectin-2 in most anchored cells or the tumor necrosis factor receptor (TNFR)-related herpesvirus entry mediator (HVEM) in immune cells (Ibanez et al., 2018). On the other hand, VZV uses the insulin-degrading enzyme (IDE) expressed on the surface of host cells as a receptor, which interacts with glycoprotein E (gE), as evidenced in a fibroblast cell line (MRC5 cells) and human melanoma cells (MeWo cells) (Table 1) (Li et al., 2006, 2010).
Table 1.
Effect of carbohydrate metabolism inhibitors over the replication cycle of herpesviruses.
| Virus | Drug | Drug target | Viral outcome | Cellular type | Reference |
|---|---|---|---|---|---|
| Herpes simplex virus type 1 (HSV-1) | 2-deoxy-D-glucose (2DG) | Hexokinase (HK) | Prevents viral replication and reduces virus shedding. | Epithelial cells (BSC1 cell line) and bone marrow-derived macrophage (BMDM). | (Courtney et al., 1973; Varanasi et al., 2017). |
| Human cytomegalovirus (HCMV) | Compound C | AMPK | Severe attenuation of HCMV replication. Reduces early and late gene expression and viral DNA synthesis by inhibiting glycolysis rate . | Human fibroblasts (MRC-5 cells). | (McArdle et al., 2012). |
| XCT790 | Estrogen-related receptor α (ERRα) | Reduces the accumulation of viral RNA, proteins, and DNA and the yield of infectious progeny. | Human foreskin fibroblasts (HF cells) and human fibroblasts (MRC-5 cells). | (Hwang et al., 2014) | |
| STO-609 | Calmodulin-dependent kinase kinase (CaMKK) | Blocks viral DNA replication and the activation of glycolysis mediated by HCMV infection. | Human fibroblasts (MRC-5 cells). | (McArdle et al., 2011) | |
| Indinavir | Glucose transporter isoform GLUT4 | Inhibits HCMV production. | Human foreskin fibroblasts (HF cells). | (Yu et al., 2011) | |
| Human herpes virus 6A (HHV-6A) | Rapamycin | mTORC1 activity | Negatively affects viral DNA replication, viral protein synthesis and virion production. | Human T cell line (HSB-2 cells). | (Wu et al., 2020). |
| Kaposi's sarcoma-associated herpesvirus (KSHV) | 2-deoxy-D-glucose (2DG) | Hexokinase (HK) | Inhibits KSHV replication and virus reactivation from latency. | Human embryonic kidney 293 cells (HEK293 cells) and endothelial cells (iSLK.219, a human Kaposi's sarcoma-derived cell line). | (Leung et al., 2012; Myoung and Ganem, 2011) |
| Oxamate | Lactate dehydrogenase (LDH) | Inhibits viral genome replication. | Neuronal cells (TIME cells) and endothelial cells (iSLK cells). | (Sanchez et al., 2017) |
Betaherpesviruses, such as HCMV, are highly prevalent in children, with nearly 59% of those older than six years old having been infected with this virus (Rico et al., 2021). Mononucleosis is the most common disease related to HCMV infection, although this is mainly a hallmark related to EBV (see below) (Frascaroli et al., 2006). This virus predominantly infects epithelial cells and fibroblasts by interacting with platelet-derived growth factor receptor alpha (PDGFRα) and neuropilin-2 (Nrp2) (Kabanova et al., 2016; Martinez-Martin et al., 2018), whereas myeloid cell infection occurs through viral protein interaction with host-encoded HVEM (Sinzger et al., 2008). The binding of herpesvirus surface glycoproteins with HVEM is somewhat common among alphaherpesvirinae and betaherpesvirinae subfamilies viruses. HCMV uses the UL144 viral glycoprotein, an orthologue of HVEM, which binds to the host protein B and T lymphocyte attenuator (BTLA) by utilizing residues from its N-terminal cysteine-rich domain 1 (CRD1) (Bitra et al., 2019). Coincidently, the BTLA site that binds to HVEM also overlaps with the binding site of gD from HSV-1 (Cheung et al., 2005). Importantly, HCMV UL144 binding to its ligand inhibits T cell proliferation by mimicking the inhibitory effects of the HVEM-BTLA interaction, which overall limits the host's antiviral response and favours virus fitness (Cheung et al., 2005). Another betaherpesvirus of interest is HHV-6, which infects children with a prevalence of approximately 90% within the first three years of life (Eliassen et al., 2018). Symptoms associated with HHV-6 subtype A (HHV-6A) and HHV-6 subtype B (HHV-6B) infection depend on the immunological state of the individual, wherein immunosuppressed patients can develop exanthem subitum, encephalitis, dementia, ataxia, or myocarditis (Eliassen et al., 2018; Gravel et al., 2015; Yao et al., 2009). HHV-6A and HHV-6B infect T cells thanks to the interaction of viral surface glycoproteins, namely the complex gH/gL/gQ1/gQ2 to cell surface CD46 expressed in host cells (Akkapaiboon et al., 2004; Santoro et al., 1999). Additionally, CD134, a member of the TNF receptor superfamily, is a specific receptor of HHV-6B and interacts with the viral protein complex gH/gL/gQ1/gQ2 (Tang et al., 2013). Regarding HHV-7, this virus mainly infects CD4+ T cells (Yang et al., 2021), and cell infection is mediated by gB of HHV-7, which interacts with heparan-like molecules (Secchiero et al., 1997). The prevalence of HHV-7 is higher than 85% in the U.S. population, and symptoms associated with its infection are characterized by exanthem subitum, fever, and seizures which may relate to the development of encephalitis (Ablashi et al., 1995; Parra et al., 2017).
Lastly, gammaherpesviruses include EBV and KSHV. Although the prevalence of EBV nears 90% in the human population (Kuri et al., 2020), approximately 90% of these cases are asymptomatic, with infections mainly occurring during childhood (Kuri et al., 2020). During adulthood, EBV infection mainly causes mononucleosis, and this virus is associated with lymphoproliferative diseases, such as Hodgkin's lymphoma (Massini et al., 2009). The main infection targets of EBV are B cells and epithelial cells (Busse et al., 2010; Xiao et al., 2008). EBV glycoprotein 350 (gp-350) is used for infecting B cells through its interaction with the CD21 receptor expressed on the surface of B cells (Busse et al., 2010), while epithelial cell infection is mediated through its interaction with integrin 1-beta (BMRF-2) (Xiao et al., 2008). Remarkably, infected B cells produce viral particles that are infectious for epithelial cells only, while the opposite occurs upon epithelial cell infection, which produces infectious viral particles for B cells (Busse et al., 2010). This differential tropism is possible because EBV modulates it by modifying its gene and protein expression patterns depending on the infected cell type. However, the precise mechanism related to this phenomenon is unknown, although current evidence indicates that glycoproteins in the EBV virion can regulate this viral tropism (Bu et al., 2022; Neuhierl et al., 2002; Ruiss et al., 2011). As its name suggests, KSHV causes all Kaposi's sarcomas (Chang and Moore, 2014). The prevalence of KSHV is highly variable, with infection nearing 5% in Asia and Europe (Rohner et al., 2014), less than 10% in the general population in America (de Sanjose et al., 2009), and 20–80% in African countries (Etta et al., 2018). Cell targets of KSHV are mainly immune cells, such as dendritic cells (DCs), monocytes, and B cells (Chakraborty et al., 2012). However, infection of oral epithelial cells has also been described (Chakraborty et al., 2012). KSHV mainly enters the cells through interactions involving a viral surface glycoprotein complex consisting of gH/gL and the host receptor CD49 (Veettil et al., 2014).
Once the genomes of herpesviruses enter the nucleus of infected cells, these viruses will hijack and modulate cellular processes to carry out their replication cycles, altogether altering transcription, splicing, translation, and post-translational modifications, among other processes (Iarovaia et al., 2021; Imbert and Langford, 2021; Liao and Garcia-Blanco, 2021; Lucic et al., 2021; Soto et al., 2022). Moreover, herpesvirus infections are also known to control epitranscriptomic and epigenetic processes (Soto et al., 2022), endosome trafficking (Tognarelli et al., 2021b), and remodel the cytoskeleton (Naghavi and Walsh, 2017; Robinson et al., 2018; Stradal and Schelhaas, 2018; Walsh and Naghavi, 2019). Additionally, viral infections also modulate cellular signaling pathways, such as those related to phosphatidylinositol phosphate kinases (PIKs), mitogen-activated protein kinases (MAPK), toll-like receptors (TLRs), and interferon (IFN)-related pathways, to name a few (Beziau et al., 2020; Brezgin et al., 2021; Chander et al., 2021). Other routes, such as those related to hypoxia and heat-shock responses, are also modulated by herpesviruses (Reyes et al., 2021, 2022). The function of cellular organelles and key homeostasis cellular processes may also be altered during viral infections, such as the unfolded protein response (UPR), autophagy, apoptosis, and mitochondrial function (Choi and Song, 2019; Fang and Peng, 2021; Jheng et al., 2014; Li et al., 2021; Quarleri et al., 2021; Tognarelli et al., 2021a; Xiao and Cai, 2020). Importantly, alterations in cellular metabolism also occur during viral infections, with numerous viruses altering glucose, lipids, and amino acid metabolism (Farias et al., 2022; Sumbria et al., 2020).
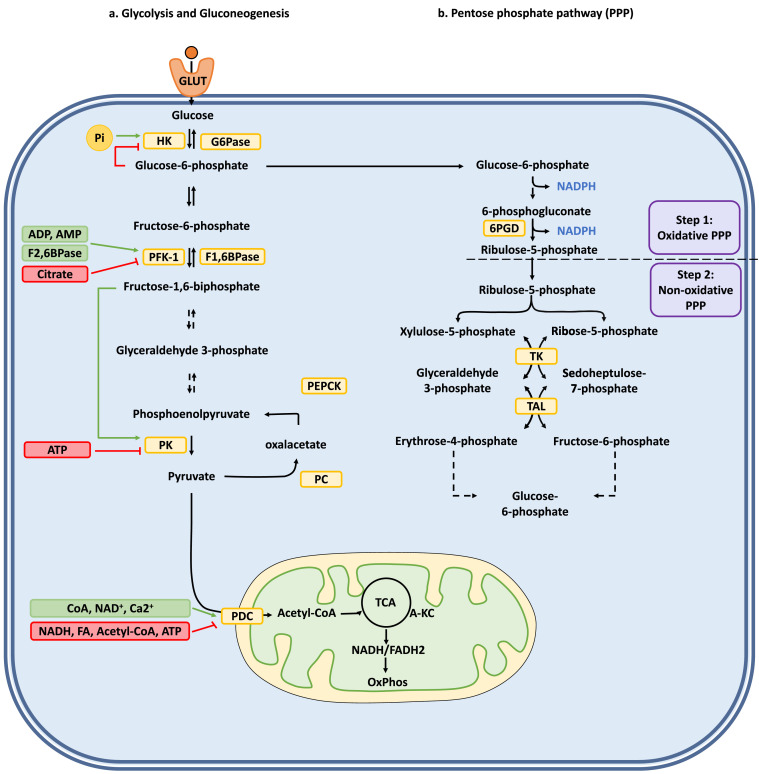
The common understanding of carbohydrate metabolism is mainly focused on glucose degradation (glycolysis), glucose formation (gluconeogenesis), and the phosphate pentose pathway (PPP) (Fig. 1) (Li et al., 2015). Glycolysis provides to the cell energy in the form of adenosine triphosphate (ATP), reducing power in the form of nicotinamide adenine dinucleotide hydrogen (NADH) under aerobic conditions, as well as pyruvate, which is then decarboxylated to acetyl-CoA (Stacpoole and McCall, 2023). Acetyl-CoA, in turn, can enter the tricarboxylic acid cycle (TCA) to generate NADH, flavin adenine dinucleotide (FADH2), and ATP (in the form of guanosine triphosphate, GTP) (Fig. 1a) (Stacpoole and McCall, 2023). Gluconeogenesis, an opposing metabolic process relative to glycolysis, will provide glucose to particular cell types (predominately hepatic and kidney cells) when dietary intake of this molecule is insufficient or absent (Fig. 1a) (Shah and Wondisford, 2020). The PPP, in turn, delivers reducing power to the cell in the form of nicotinamide adenine dinucleotide phosphate hydrogen (NADPH) and ribose-5-phosphate (R5P), a fundamental component needed for nucleic acid synthesis (Fig. 1b) (Ge et al., 2020).
Fig. 1.
Carbohydrate metabolism pathways and their regulation. (a) Glycolysis and gluconeogenesis pathways and their enzymatic regulation in a typical human cell, (b) Pentose phosphate pathway (PPP). The enzymes involved in the different chemical transformations are marked in yellow boxes. Next to the different pathways shown with black arrows, are green and red boxes indicating the type of regulations carried out by different metabolites. Green boxes: Positive regulations; Red boxes: Negative regulations. Green arrows and red lines indicate positive and negative regulations, respectively. Chemical transformations involving multiple reactions are depicted as dashed lines. Abbreviations: 6PGD: 6-phosphogluconate dehydrogenase; ADP: adenosine diphosphate; A-KC: alpha-ketoglutarate; AMP: adenosine monophosphate; ATP: adenosine triphosphate; CoA: coenzyme A; G6Pase: glucose 6-phosphatase; GLUT: glucose transporter; HK: hexokinase; F1,6BPase: fructose 1,6-bisphosphatase; F2,6BPase: fructose 2,6-biphosphatase; FA: Fatty acid; NAD+/NADH: nicotinamide adenine dinucleotide; NADPH: nicotinamide adenine dinucleotide phosphate; PC: pyruvate carboxylase; PDC: pyruvate dehydrogenase complex; PEPCK: phosphoenolpyruvate carboxykinase; PFK-1: phosphofructokinase-1; Pi: Inorganic phosphate; PK: pyruvate kinase; TAL: transaldolase; TCA: tricarboxylic acid cycle; TK: transketolase. FADH2: reduced flavin adenine dinucleotide.
In this review, we revise and discuss current evidence regarding how human herpesviruses modulate carbohydrate metabolism-associated pathways and the processes that could be targeted to hamper herpesvirus replication and herpesvirus-related disease in the host.
Alphaherpesviruses and carbohydrate metabolism
Herpes simplex virus type 1 has been described to affect glucose metabolism mainly by modulating glycolysis and the phosphate pentose pathway (Fig. 2a) (Vastag et al., 2011). Infection of epithelial cells (Vero cells) by HSV-1 has been reported to activate glycolysis by enhancing the activity of phosphofructokinase-1 (PFK-1), a glycolysis key rate-limiting enzyme, namely by increasing both the expression of PFK-1 and its phosphorylation in specific serine residues in this enzyme (Abrantes et al., 2012). Indeed, PFK-1 was also increased at the transcriptional and translational levels (Abrantes et al., 2012). In epithelial cells, PFK-1 synthesis was upregulated with increased glucose uptake, lactate efflux, and ATP content (Abrantes et al., 2012). In this study, the authors also reported that knocking-down PFK-1 reduced viral titters, as determined using plaque-forming unit (PFU) assays, suggesting an essential role for PFK-1 in glycolysis in regulating HSV-1 production (Abrantes et al., 2012). Noteworthy, it was recently reported that transfection of a construct encoding the HSV-1 viral protein UL43 in human umbilical vein endothelial cells (HUVEC cells) moderately enhanced glucose uptake in these cells at early time points during infection (Deng et al., 2022). However, the molecular mechanism mediating this increase in glucose uptake by UL43 has not been elucidated yet. It will be interesting to evaluate the implications of herpesviral proteins in the modulation of carbohydrate metabolism by analyzing the effects of individual viral proteins or by using viruses deleted in specific genes and how these viral factors relate to such metabolic processes. In other viral infections, such as those mediated by adenoviruses, viral gene products such as E4ORF1 have been reported to induce the activation of v-myc avian myelocytomatosis viral oncogene homolog (c-Myc), which promoted glucose metabolism (Mesri and Lampidis, 2021; Thai et al., 2014). Noteworthy, c-Myc was found to act as a transcription factor that influenced glucose metabolism by upregulating the expression of numerous genes encoding glycolytic enzymes, such as glucose transporters (GLUTs), hexokinase-II (HK-II), PFK-1, pyruvate kinase (PK) and lactate dehydrogenase A (LDHA), among others (Dang, 1999; DeBerardinis et al., 2008; Marbaniang and Kma, 2018; Wang et al., 2022). Therefore, it may be interesting to evaluate the role of HSV-1 UL43 over the expression of genes that regulate glucose metabolism, particularly c-Myc.
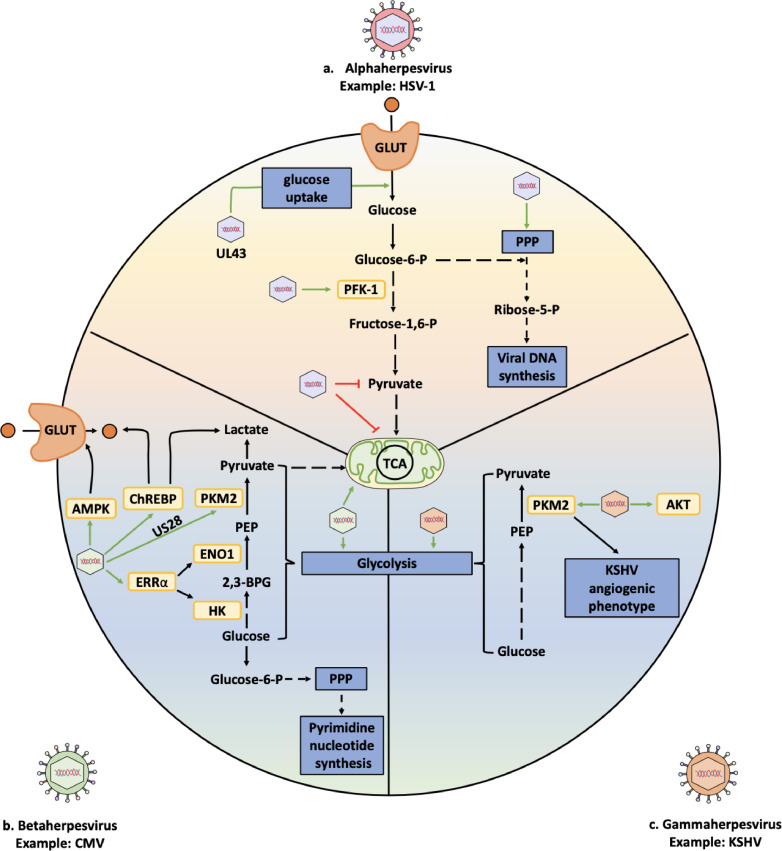
Fig. 2.
Summary of interactions occurring between selected herpesviruses and carbohydrate metabolism processes. (a) Interactions between herpes simplex virus type 1 (HSV-1), an alphaherpesvirus, and glucose metabolism-related processes (glycolysis and the pentose phosphate pathway). (b) Interactions between cytomegalovirus (CMV), a betaherpesvirus, and enzymes related to glycolysis and the pentose phosphate pathway. (c) Interactions between the catabolic glucose pathway and the Kaposi's sarcoma-associated herpesvirus (KSHV), a gammaherpesvirus. The enzymes involved in the different chemical transformations are indicated in yellow boxes. Green arrows and red lines indicate positive and negative regulations mediated by herpesviruses. Chemical transformations involving multiple reactions are depicted as dashed lines. Abbreviations: 2,3-BPG: 2,3 biphosphoglycerate; AKT: Protein kinase B; AMPK: AMP-activated protein kinase; ChREBP: carbohydrate-responsive element-binding protein; ENO1: enolase 1; ERRα: Estrogen-related receptor alpha; GLUT: glucose transporter; HK: hexokinase; PEP: phosphoenolpyruvate; PFK-1: phosphofructokinase-1; PKM2: Pyruvate kinase M2; PPP: Pentose phosphate pathway; TCA: Tricarboxylic acid cycle.
Increases in glucose uptake mediated by HSV-1 infection are consistent with increases in glucose-6-phosphate (G6P) and hexose-6-phosphate (H6P) metabolites in epithelial cells (Vero cells), as well in human embryonic lung fibroblast cells (MRC5 cells), as reported by Vastag et al. using liquid chromatography-tandem mass spectrometry (LC-MS) (Vastag et al., 2011). However, at late time points during HSV-1 infection, it was found that there was an accumulation of fructose-1,6-biphosphate (FBP), indicating that this metabolite and glycolysis are required for HSV-1 replication, as the life-cycle of this virus was affected when glycolysis was blocked by inhibiting PFK-1 (Vastag et al., 2011). Moreover, HSV-1-infected fibroblasts displayed an accumulation of dihydroxyacetone phosphate (DHAP) and phosphoenolpyruvate (PEP), besides FBP, which are metabolites that are upstream of pyruvate synthesis, thus indicating that HSV-1 infection produces a holdup in glycolytic efflux at the level of the conversion of PEP into pyruvate (Vastag et al., 2011). The accumulation of these metabolites was followed by an increase in the levels of PPP intermediates, suggesting that HSV-1 guides carbohydrate metabolism towards the production of ribose-phosphate, likely for the synthesis of nucleotides that may be required for viral DNA synthesis (Abrantes et al., 2012; Vastag et al., 2011). Additionally, HSV-1 infection was found to reduce pyruvate production, which is used to form acetyl-CoA for the TCA cycle and malate, which is related to this cycle, thus suggesting that HSV-1 also inhibits the TCA cycle in epithelial cells (Vero cells) and human embryonic lung fibroblast cells (MRC5 cells) (Vastag et al., 2011). However, more studies are needed to elucidate the mechanism used by HSV-1 to impair the TCA cycle and the impact of this virus on carbohydrate metabolism in different cell types targeted by this virus (e.g., epithelial, neuronal, and immune cells).
Importantly, 2-deoxy-D-glucose (2DG), a glucose analogue that inhibits glycolysis by acting over HK, has been reported to reduce HSV-1 PFU formation in the epithelial cell line BSC1 (Courtney et al., 1973). 2DG treatment also reduced virus shedding during HSV-1 genital infections in humans and decreased the severity of ocular inflammatory lesions in mice mediated by HSV-1 infection (Varanasi et al., 2017). Nevertheless, treatment with 2DG after ocular infection with HSV-1 may be detrimental for the host, eventually resulting in herpes encephalitis, as reported in C57BL/6 adult mice (Berber et al., 2021). While the dose of 2DG used during human genital infections with herpes was 0.19% in topical or intravaginal formulations that were applied four times a day for three weeks (Blough and Giuntoli, 1979), the posology of 2DG used in the HSV-1-infected mice was 250 mg/kg via intraperitoneal injection twice a day for two weeks (Berber et al., 2021; Wang et al., 2016). Thus, it will be important to consider adequate drug-targeting doses and delivery strategies for different tissues if pursuing such an approach for blocking HSV-1 replication at particular sites in the body.
Currently, most of the available studies reporting a role for carbohydrate metabolism during human alphaherpesvirus infection relate to HSV-1. Thus, much remains to be studied regarding the modulation of carbohydrate metabolism by other alphaherpesvirus infections. Indeed, overall more studies focused on HSV-2 and VZV are needed to assess how these viruses may modulate cellular carbohydrate metabolism in their primary target cells and other cell types, such as immune cells, or whether these viruses share similar features as those described so far for HSV-1. Given the relatively few reports on the effects of HSV-2 or VZV over carbohydrate metabolism, we foresee that new studies will emerge in the near future regarding how these viruses may affect this metabolic process, as well as the impact of carbohydrate metabolism over viral replication and the diseases produced by these viruses.
Betaherpesviruses and carbohydrate-related metabolism
Regarding the human cytomegalovirus (HCMV), liquid chromatography-tandem mass spectrometry and microarray analyses have reported significant increases in metabolites and enzymes related to glycolysis, the TCA cycle, and pyrimidine nucleotide biosynthesis upon HCMV infection of human fibroblasts (Fig. 2b) (Munger et al., 2006). Studies in HCMV seropositive patients have found that these individuals have higher glycated hemoglobin (HbA1c) levels than HCMV seronegative individuals (Rector et al., 2015). Importantly, HbA1c is an indicator of glycemic control and average levels of plasma glucose during the previous four months, wherein high levels of HbA1c indicate diabetes mellitus (Higgins, 2012), thus suggesting that HCMV infection is associated with this disease. Mechanistically, the increase in HbA1c observed in HCMV-infected patients was associated with an accumulation of differentiated cytotoxic T cells, such as circulating effector memory T cells (EM: CD27−CD45RA−) and CD45RA re-expressing effector memory T cells (EMRA: CD27−CD45RA+), being this latter T cell phenotype associated with an accelerated immunosenescence phenotype driven by HCMV infection (Rector et al., 2015). Therefore, it will be relevant to know whether glucose accumulation plays a role in the modulation of the immune response elicited against this pathogen and determine if drugs used to treat diabetes mellitus (e.g. metformin, an FDA-approved drug) may have a positive impact on immune cells, considering that human foreskin fibroblasts (HFF cells) infected with HCMV and treated with metformin evidenced a delay in HCMV replication kinetics and reduced viral titers (Combs et al., 2021).
Considering that glycolysis is positively regulated by AMP-activated protein kinase (AMPK) activity, which modulates glucose transporters GLUT1 and GLUT4, HK, and PFK-2, another study focused on the role that these host proteins may have during HCMV infection (Weisova et al., 2009). Interestingly, HCMV infection was reported to induce AMPK activity in human fibroblasts (MRC-5 cells), eliciting the phosphorylation and increase in abundance of AMPK targets, such as acetyl-CoA carboxylase-1 (ACC1), GLUT4 and the negative regulator of mammalian target of rapamycin complex (mTOR) signaling tuberous sclerosis protein (TSC1) (McArdle et al., 2012). Noteworthy, AMPK inhibition using compound C, or AMPK-specific RNAi in HCMV-infected cells, blocked the activation of glycolysis induced by HCMV infection without significantly impacting uninfected cells (McArdle et al., 2012). Further, AMPK inhibition severely attenuated HCMV replication, the expression of early and late viral proteins (i.e. UL44 and pp28 proteins), as well as viral DNA replication without impacting immediate-early protein expression (e.g. IE1 protein) (McArdle et al., 2012). The inhibition of calmodulin-dependent kinase kinase (CaMKK), using the specific inhibitor STO-609 but not calmodulin-dependent kinase II (CaMKII) or protein kinase A (PKA), was found to block the activation of glycolysis mediated by HCMV infection in human fibroblasts (MRC-5 cell line) and attenuated the yield of HCMV particles through a reduction in the expression of the early viral protein UL26, and also impaired viral genome replication decreasing viral PFUs (McArdle et al., 2011).
HCMV infection has also been reported to promote a post-transcriptional induction of estrogen-related receptor alpha (ERRα), an orphan nuclear receptor associated with regulating mitochondrial biogenesis, oxidative metabolism, and gluconeogenesis (Hwang et al., 2014; Villena and Kralli, 2008). Genetic and pharmacological inhibition of ERRα (with ERRα shRNA, or the ERRα inverse agonist XCT790) reduced viral RNA levels, as well as the expression of viral immediate-early proteins IE1 and IE2 and late viral proteins pUL26 and pUL44 (Hwang et al., 2014). It also decreased the accumulation of viral DNA compared to control cells, affecting the yield of infectious progeny (Hwang et al., 2014). Importantly, this inhibition also reduced the expression of enolase-1 (ENO1), triosephosphate isomerase-1 (TPI1), and HK-II glycolysis-associated enzymes relating the observed effects to glucose metabolism (Hwang et al., 2014).
Another study found that HCMV induced the expression of carbohydrate-response element binding proteins alpha and beta (ChREBP-α and ChREBP-β), transcription factors that respond to glucose signaling, and their consequent translocation to the nucleus in human fibroblasts (Yu et al., 2014). Interestingly, the induction of ChREBP was required for effective HCMV infection because the depletion of ChREBP by shRNA (shChREBP1 and shChREBP2) in HCMV-infected cells resulted in reduced titters, but not in the expression of the viral immediate-early protein IE86, early protein pp52, or late proteins pp65 and pp28. Additionally, HCMV infection increased glucose uptake and lactate excretion levels, processes that are regulated by ChREBP expression (Yu et al., 2014). Indeed, the inhibition of ChREBP in infected cells decreased glucose uptake by 39% and lactate production by 35% (Yu et al., 2014). Moreover, GLUT4 is a direct gene target of ChREBP (Yu et al., 2014). This study also reported increased mRNA levels of GLUT2 and GLUT4 in infected cells. In contrast, the inhibition of ChREBP in HCMV-infected cells inhibited GLUT expression (Yu et al., 2014). Therefore, these findings suggest that ChREBP plays an essential role in glucose uptake and glycolysis activity during HCMV infection, particularly through the regulation of glucose transporter expression.
Regarding HCMV viral proteins participating in carbohydrate metabolism-related processes, the HCMV-encoded chemokine receptor US28 has been reported to stimulate hypoxia-inducible factor-1 (HIF-1) and pyruvate kinase M2 (PKM2), promoting a feedforward loop that increases the expression of genes associated with glucose metabolism, namely GLUT1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in fibroblasts (NIH/3T3 cell line) (de Wit et al., 2016). Further, in human fibroblasts (MRC5 cells), the HCMV UL38 protein was found to be needed for eliciting glycolytic activation and inducing leucine/isoleucine and arginine catabolic processes (Rodriguez-Sanchez et al., 2019). In addition, UL38 was found to interact with tuberous sclerosis complex 2 (TSC2), a tumor suppressor protein that inhibits the mTOR signaling pathway (Rodriguez-Sanchez et al., 2019). This positive modulation of glycolysis by a viral protein suggests that these viruses have evolved to modulate carbohydrate metabolism to promote their replication. Therefore, the development of drugs that can interfere with particular glycolysis steps in infected cells, with no collateral inhibition in other cells that highly rely on this metabolic pathway for their antiviral functions, such as immune effector cells (e.g. DCs and T cells), could be used as novel approaches for specifically dampening viral replication and pathology without hampering an effective antiviral response.
Regarding glucose metabolism inhibitors and their impact on HCMV infection, studies with mouse and human natural killer (NK) cells using the glucose metabolism inhibitor 2DG found that this drug impaired NK cytotoxicity activity following priming in vitro (Mah et al., 2017). Moreover, murine cytomegalovirus (MCMV)-infected mice treated with 2DG displayed impaired clearance of NK-specific targets in vivo, associated with higher viral burden and increased susceptibility to infection in the C57BL/6 mouse background (Mah et al., 2017). HCMV-infected human fibroblasts have shown significantly increased glucose consumption compared to uninfected cells, suggesting possible alterations in glucose transport during infection (Yu et al., 2011). Noteworthy, the inhibition of GLUTs using cytochalasin B indicated that infected cells used GLUT4, while uninfected cells used GLUT1 and that the reduction of GLUT1 protein expression in infected cells involved the viral immediate-early protein IE72 (Yu et al., 2011). Further, indinavir, a drug that inhibits GLUT4-mediated glucose uptake, reduced viral production in infected cells (Yu et al., 2011).
Despite HHV-6 and HHV-7 being less studied than HMCV, some reports indicate that these betaherpesviruses also control glucose metabolism to modulate cellular infection. Regarding HHV-6A infection, a study reported that HHV-6A infection promoted glucose metabolism in infected human T cells (HSB-2 cell line), resulting in elevated glycolytic activity with increased glucose uptake, glucose consumption, and augmented lactate secretion (Wu et al., 2020). Furthermore, viral infection increased the expression of mRNAs and proteins related to glucose transporters GLUT1 and GLUT3, as well as the glycolytic enzymes HK2, PFK-1, and LDHA (Wu et al., 2020). In addition, HHV-6A infection was found to activate protein kinase B (AKT)-mTORC1 signaling in infected T cells, wherein the inhibition of mTORC1 blocked HHV-6A-mediated glycolytic activation. The inhibition of glycolysis by 2DG, or the inhibition of mTORC1 activity with the drug rapamycin in HHV-6A-infected T cells, negatively affected HHV-6 DNA replication, the expression of viral proteins (i.e. IE1 and gB protein), and virion production. These results suggest a mechanism by which HHV-6 infection of T cells affects carbohydrate metabolism in these cells, highlighting the importance of glycolysis during the replication cycle of HHV-6. These findings also open new perspectives for HHV-6 treatments considering the participation of carbohydrate metabolism during infection (Wu et al., 2020).
Even though several studies report that carbohydrate metabolism has a significant role in HCMV infection, additional studies are needed for this and other betaherpesviruses, such as HHV-6 and HHV-7. The available studies regarding these viruses have shown that glucose metabolism and carbohydrate-related pathways significantly affect their replication cycles. Therefore, better knowing the role of carbohydrate metabolism during betaherpesvirus infections could allow identifying new therapeutic strategies to treat the diseases that these viruses produce.
Gammaherpesviruses and carbohydrate-related metabolism
The gammaherpesvirus subfamily encompasses EBV and HHV-8 (also known as KSHV), which produce mononucleosis and Kaposi's sarcoma, respectively (Kori and Arga, 2022). Importantly, numerous studies report findings regarding carbohydrate metabolism and its impact on pathologies associated with gammaherpesviruses.
In EBV-transformed lymphoblastoid cells, the aerobic glycolytic pathway has been reported to be activated (Darekar et al., 2012). Indeed, lymphoblastoid cells produced higher lactate, LDHA, and pyruvate levels than mitogen-activated B cells (Darekar et al., 2012). Interestingly, EBV-encoded nuclear antigens 5 (EBNA-5) and EBNA-3 were found to bind to prolyl hydroxylases-1 and −2, respectively, thus inhibiting HIF-1α hydroxylation and its degradation (Darekar et al., 2012). This phenomenon stabilized HIF-1α, which was then able to translocate to the nucleus, form a heterodimer with aryl hydrocarbon receptor nuclear translocator (ARNT), and transactivate genes involved in aerobic glycolysis, such as GLUT1, LDHA, PDK1 (pyruvate dehydrogenase kinase), PGK1 (phosphoglycerate kinase), PFK, SLC16A3 (MCT4, monocarboxylate transporter), HK and PK (Darekar et al., 2012). Furthermore, in EBV-transformed B cells, the inhibition of nuclear factor-kappa B (NF-κB) repressed glucose uptake and induced caspase-independent cell death associated with autophagy (Sommermann et al., 2011). Considering that basal NF-κB activity restrains aerobic glycolysis (Mauro et al., 2011), studying the role of this nuclear factor in herpesvirus infections could open new perspectives regarding its potential impact on carbohydrate metabolism regulation.
A metabolomic approach carried out almost a decade ago found that EBV-encoded latent membrane protein 1 (LMP1) significantly increased glycolysis in nasopharyngeal carcinoma (NPC) biopsies (Xiao et al., 2014). Here, LMP1 promoted the deregulation of HKII, wherein its upregulation inhibited apoptosis and promoted cellular proliferation. Moreover, studies in NPC biopsies correlated positively with the expression levels of HK2 and LMP1, and higher levels of HKII were associated overall with poor survival of NPC patients following radiation therapy (Xiao et al., 2014). Here, c-Myc was shown to be required for LMP1-induced upregulation of HK2 and LMP1-mediated attenuation of the phosphoinositide 3-kinase (PI3K)/AKT-glycogen synthase kinase 3 beta (GSK3beta)-F-box and WD repeat domain-containing 7 (FBW7) through a signaling axis found to stabilize c-Myc (Xiao et al., 2014). Another study indicated that the C-terminal activating region 2 (CTAR2) of LMP1 activates mTORC1, and this latter factor modulates the activation of NF-κB signaling to mediate aerobic glycolysis. Indeed, NF-κB activation was involved in LMP1-induced up-regulation of the transcription of GLUT1 and the growth of nasopharyngeal carcinoma (NPC) cells (Zhang et al., 2017). Blocking mTORC1 and NF-κB altered GLUT1 transcription, and the depletion of GLUT1 led to the suppression of aerobic glycolysis, inhibition of cell proliferation and colony formation, and attenuation of tumorigenic growth properties in LMP1-expressing nasopharyngeal epithelial (NPE) cells (Zhang et al., 2017). LMP1 also promoted the expansion of myeloid-derived suppressor cells (MDSCs) in the tumor microenvironment by promoting glycolysis in malignant cells. Here, LMP1 induced the expression of GLUT1 and glycolytic genes related to HK2, glucose phosphate isomerase (GPI), 6-phosphofructo-2-kinase/fructose 2 and 3 (PFKFB2 and PFKFB3) in MDSCs cells. Interestingly, metabolic reprogramming in these cells resulted in an increase in the expression of the Nod-like receptor family protein 3 (NLRP3) inflammasome, cyclooxygenase 2 (COX-2), and P-p65, and consequently elicited increased production of IL-1β, IL-6 and granulocyte-macrophage colony-stimulating factor (GM-CSF) (Cai et al., 2017). Further, LMP1 increased glucose and glutamine cellular uptake, enhanced LDHA activity and lactate production, and reduced pyruvate kinase activity and pyruvate concentrations. Additionally, LMP1 increased the phosphorylation of PKM2, LDHA, and fibroblast growth factor receptor 1 (FGFR1), as well as the expression of pyruvate dehydrogenase kinase 1 (PDHK1), FGFR1, c-Myc, and HIF-1α, regardless of oxygen availability (Cai et al., 2017). Altogether, these findings evidence a significant role for the EBV protein LMP1 in the modulation of carbohydrate-related metabolism in infected cells, suggesting that this viral factor may be a relevant viral target for future antiviral approaches, given its multiple effects and overall impact on glycolysis (Cai et al., 2017).
Furthermore, LMP1 has also been reported to upregulate fibroblast growth factor 2 (FGF2), leading to constitutive activation of the FGFR1 signaling pathway (Lo et al., 2015). The use of FGFR1 inhibitors (PD161570 and SU5402 drugs) reduced aerobic glycolysis mediated by LMP1, cellular transformation, cell migration, and invasion in non-keratinizing nasopharyngeal carcinoma (NPC) cells (Lo et al., 2015). Moreover, LMP1 was found to regulate carbohydrate metabolic reprogramming by activating insulin-like growth factor-1 (IGF1)-mTORC2 signaling and nuclear acetylation of the Snail promoter by pyruvate dehydrogenase component α subunit (PDHE1α), an enzyme involved in glucose metabolism. Finally, EBV-LMP1 was found to increase the cellular secretion of IGF1, promoting the phosphorylation of insulin-like growth factor 1 receptor (IGF1R) to activate mTORC2/AKT signaling and linking glucose metabolism to cell motility. In addition, LMP1 expression facilitated the translocation of mitochondrial PDHE1α into the nucleus in a phosphorylation-dependent manner at the Ser293 residue. In turn, nuclear PDHE1α promoted histone H3 lysine 9 (H3K9) acetylation of the Snail promoter region to enhance cell motility driving cancer metastasis (Zhang et al., 2019).
Analysis of NPC biopsies by microarray assay showed that uridine diphosphate (UDP)-glucose dehydrogenase (UGDH) gene expression, which is a host factor that participates in glycosaminoglycan synthesis, displayed a high correlation with the expression levels of the EBV latent membrane protein 2A (LMP2A) (Pan et al., 2008). Similar results with UGDH expression were observed in human embryonic kidney 293 cells (HEK293 cells) overexpressing LMP2A (Pan et al., 2008). Regarding the mechanism related to this phenomenon, using signaling pathway-specific inhibitors, it was found that PI3K/Akt and extracellular signal-regulated kinase (ERK), not c-Jun N-terminal kinase (JNK) and p38, participate in LMP2A-induced UGDH expression (Pan et al., 2008).
Besides EBV proteins, EBV microRNAs (i.e. miRNA9 and miR-Bart1–5P) significantly promote glycolysis and angiogenesis in NPC cells in vitro and in vivo (Lyu et al., 2018). EBV-miR-Bart1–5P was found to target the α1 catalytic subunit of AMPK and then regulate the AMPK/mTOR/HIF1 pathway (Lyu et al., 2018). Using siRNA for BART1–5P attenuated protein expression of mTOR, p-mTOR, and the mTOR substrate S6 kinase (S6K1), compared with controls, attenuated the phenotype of EBV-positive NPC cells, by reducing the rate of glycolysis and angiogenesis (Lyu et al., 2018).
Regarding KSHV infection (Fig. 2c) and its impact on glucose metabolism in the cell and its role in this virus' replication cycle, studies in primary endothelial cells (HUVEC) found that KSHV infection induces a substantial enhancement in insulin and glucose uptake (Ingianni et al., 2013). The increase in insulin uptake was most evident during the lytic phase of viral infection and reached a maximum of up to 71% during the latent phase (Ingianni et al., 2013). Glucose uptake was slightly depressed during lytic infection but significantly enhanced compared to controls during the latent phase of viral infection, with an average increase nearing 37% 25 days after cell infection (Ingianni et al., 2013). Latent KSHV infection of endothelial cells induced glycolysis and lactic acid production while decreasing oxygen consumption, thereby eliciting a Warburg effect, a common metabolic alteration found in most tumor cells that is characterized by an increase in aerobic glycolysis, together with a decrease in oxidative phosphorylation as a source of energy (DeBerardinis and Chandel, 2020; Delgado et al., 2010). Interestingly, glycolysis inhibitors such as 2DG selectively induced apoptosis in KSHV-infected endothelial cells but not their uninfected counterparts (Delgado et al., 2010). Therefore, the induction of the Warburg effect was required to maintain KSHV latency in infected cells, such as cancer cells (Delgado et al., 2010).
A relationship has been found between PKB (also known as AKT) hyperactivation induced by KSHV infection and GLUT1 expression in the plasma membrane (Gonnella et al., 2013). Increased GLUT1 expression in the membrane in KSHV-infected monocyte cells (THP-1 cells) increased the sensitivity of cells to death induced by 2DG (Gonnella et al., 2013). Moreover, the glycolysis inhibitor 2DG stimulated endoplasmic reticulum (ER) stress, shutting down the activity of eukaryotic translation initiation factor 2A (eIF2A), through the phosphorylation of this factor, consequently inhibiting KSHV and murine herpesvirus 68 replication, as well as KSHV reactivation from latency (Leung et al., 2012). The expression of immediate-early viral genes delayed early and late viral lytic genes involved in the reactivation of this virus, including the master trans activator (RTA) gene, glycoprotein B, glycoprotein K8.1, and angiogenesis-regulating genes, which were markedly decreased upon 2DG treatment (Leung et al., 2012). Overall, these results suggest that the activation of UPR by 2DG elicits an early antiviral response via eIF2A inactivation, which impairs protein synthesis required to drive viral replication and oncogenesis (Leung et al., 2012). Further, oxamate, an inhibitor of LDH, inhibited viral genome replication and was required for early gene transcription (Sanchez et al., 2017).
Noteworthy, PKM2 has been reported to be upregulated upon KSHV infection in endothelial cells and is needed to maintain aerobic glycolysis in infected cells (Ma et al., 2015). PKM2 regulates the angiogenic phenotype of Kaposi's sarcoma by acting as a coactivator of HIF-1α and increasing the levels of HIF-1 angiogenic factors, including vascular endothelial growth factor (VEGF), angiopoietin 2 (ANGPT2) and angiopoietin-like protein 4 (ANGPTL4) (Ma et al., 2015). Consistent with this, the inhibition of PKM2 expression with siRNA blocked endothelial cell migration and their differentiation and the angiogenic potential of KSHV-infected cells (Ma et al., 2015).
Studies intended to measure the potential relationship between KSHV infection and diabetes mellitus type 2 (DM2) in patients show that the seroprevalence of KSHV is 49.1% (95% confidence interval (CI) 43.6–54.5%) in diabetic patients and 23.7% (95% CI 19.4– 28.0%) in control groups. Importantly, when adjusting data based on variables such as ethnicity, sex, body mass index, occupation, educational level, marital status, age, smoking, and alcohol consumption habits, the association between DM2 and KSHV infection still existed (odds ratio (OR) 2.94, 95% CI 2.05–4.22), and the risk of KSHV infection increased with glucose concentration (OR 1.35, 95% CI 1.21–1.51) (Cui et al., 2019). Furthermore, KSHV was more likely to express both latent and lytic antigens in diabetic patients (latent: OR 3.27, 95% CI 2.25–4.75; lytic: OR 3.99, 95% CI 2.68–5.93), and antibody titters against KSHV and viral loads increased in patients with higher blood glucose levels. Considering these results, patients with DM2 have an elevated risk of KSHV infection (Cui et al., 2019). In this line, another study assessed whether KSHV is associated with insulin resistance ketosis and prone type 2 diabetes (KPD) in approximately ten patients. This study did not report differences in non-esterified fatty acid (NEFA) release, endogenous glucose production, or insulin sensitivity considering the amount of infused glucose divided by body weight and time (M value), and overall found that asymptomatic KSHV infection does not appear to be associated with decreased insulin sensitivity in diabetic patients (Nguewa et al., 2017). Moreover, KSHV-infected and non-infected sub-Saharan African patients having DM2 and KPD had blood samples with higher levels of low-density lipoprotein (LDL) and total cholesterol than non-infected patients (Lontchi-Yimagou et al., 2018). However, C-peptide and homeostatic model assessment of β-cell function (HOMA-β) were significantly lower in DNA-KSHV+ than DNA-KSHV– participants, indicating low insulin secretion in this sub-Saharan African diabetes population (Lontchi-Yimagou et al., 2018). A study focused on describing the contribution of KSHV infection over the production of reactive oxygen species (ROS) and their relationship with pathogenesis and development of DM2 in patients found that the plasma levels of the antioxidant α-tocopherol (α-toc) significantly decreased in both DM2- and KSHV-positive subjects (Incani et al., 2020). In contrast, the levels of products related to oxidative stress, such as malondialdehyde (MDA), FA hydroperoxides (HP), and 7-ketocholesterol (7-keto), were much higher in plasma samples of KSHV-positive and DM2 subjects than KSHV-negative and non-DM2 subjects (Incani et al., 2020). Additionally, KSHV-infected body cavity-based lymphoma cell line (BCBL-1) cultured in high-glucose medium (RPMI 1640 medium containing 100, 300, and 600 mg/dl of D-glucose) displayed elevated levels of viral lytic gene expression (i.e. RTA and ORF65) as compared to infected cells grown without D-glucose supplementation (Ye et al., 2016). Similar results were observed in infected hyperglycemic mice, wherein higher levels of lytic genes (i.e. RTA and ORF65) were expressed compared to mice with normal glycemia levels (Ye et al., 2016). Regarding the mechanism of this observation, high glucose levels in BCBL1 cells were found to induce the production of hydrogen peroxide (H2O2), which mediated the downregulation of class III histone deacetylase (HDAC) silent information regulator 1 (SIRT1), resulting in the transactivation of the RTA gene through the acetylation of H3K9 and H4K12 in the RTA promoter region (Ye et al., 2016).
Studies with primary dermal microvascular lymphatic endothelial cells (LEC) have identified that KSHV microRNAs clusters cooperate to decrease mitochondria biogenesis and induce aerobic glycolysis in infected cells, promoting the proliferation of latently infected cells and the maintenance of latency (Yogev et al., 2014). This study also showed that KSHV alters the host's cell energy metabolism through microRNA-mediated downregulation of Egl-9 family hypoxia-inducible factor 2 (EGLN2) and heat shock protein family A (Hsp70) member 9 (HSPA9) (Yogev et al., 2014).
Overall, numerous studies focus on the role of glucose metabolism in the replication cycle of gammaherpesviruses, mainly during cancer progression. Although much has been learned in this regard, additional studies are needed for a more comprehensive understanding of how carbohydrate metabolism is modulated upon infection with these viruses. Hopefully, clinical studies that may help better understand the role of carbohydrate-related processes in the context of the disease elicited by these viruses will be carried out soon, as well as studies that assess the role of these viruses in the context of metabolic disorders, such as DM2. Furthermore, studying the effects of carbohydrate-related metabolism inhibitors could help identify the role of host factors participating in the replication cycle of gammaherpesviruses and their potential therapeutic use, as long as such drugs do not significantly affect tissue homeostasis.
Concluding remarks
Carbohydrate metabolism is a highly regulated process in the cell through numerous metabolic steps and metabolites. Human herpesviruses modulate these steps and the production of carbohydrate metabolic products to favor their replication cycles. Therefore, inhibiting carbohydrate-related metabolic pathways or its regulated steps, such as the activity of hexokinases in glycolysis, may have beneficial effects for the host, being this approach an effective way for reducing herpesvirus infection in vitro. Noteworthy, the observation that carbohydrate metabolism modulation by some herpesviruses relates to metabolic diseases opens important questions regarding the possibility that related herpesviruses may also contribute to such types of pathologies, either directly or indirectly, and that drugs aimed at resolving carbohydrate metabolism-related diseases may eventually have a favorable effect in limiting herpesvirus infections in vivo. However, the potential use of such drugs, repurposed for controlling herpesvirus replication, requires further studies. Nevertheless, the overall effects of such drugs on herpesviral infection may vary significantly depending on the cell type infected. Additionally, these drugs could have detrimental consequences for the host depending on whether inhibiting such cellular processes is toxic for the affected tissue or the cell type infected. Furthermore, some in vivo studies have shown that inhibiting particular glucose metabolism processes may enhance viral infection, as seen in mice infected with HSV-1 and treated with 2DG (Berber et al., 2021; Courtney et al., 1973).
Currently, most studies assessing the role of carbohydrate-related metabolism during viral infections are focused on epithelial cells. Nonetheless, given the key role of immune cells in controlling viral infections and their susceptibility to herpesvirus infections, research focused on virus-mediated modulation of carbohydrate metabolism should also extend to these cells. Also, it will be important to determine the effects of herpesvirus modulation of carbohydrate metabolism during viral persistence and reactivation. However, at present, there are somewhat few studies focused on this area. Similarly, there is relatively poor evidence regarding the identification of herpesviral proteins that directly participate in the modulation of carbohydrate-related metabolism in infected cells, suggesting a need for more-in-detail studies dedicated to elucidating the molecular determinants and mechanisms used by these viruses to modulate carbohydrate-related metabolism in the cell that results in viral replication or that support the replication cycles of these viruses.
Overall, better knowledge of the role of carbohydrate-related metabolism during herpesvirus infections should help identify potential new therapeutic strategies to treat the diseases produced by these viruses. Furthermore, based on the increased number of reports combining multiple omics approaches with functional assays, we foresee that novel studies assessing the role of carbohydrate-related metabolism during herpesvirus infections will contribute in the near future to expanding our understanding of the role played by carbohydrate metabolism over viral infections.
Funding
This work was funded by ANID - Millennium Science Initiative Program - ICN2021_045: Millennium Institute on Immunology and Immunotherapy (former ICN09_016 and P09/016-F) and FONDECYT grant #1190864 from the Agencia Nacional de Investigación y Desarrollo (ANID). The Regional Government of Antofagasta also supported this work through the Innovation Fund for Competitiveness FIC-R 2017 (BIP Code: 30488811-0). MAF is ANID fellow #21191390.
CRediT authorship contribution statement
Farías MA: Conceptualization, Writing – original draft, Writing – review & editing. Cancino FA: Writing – original draft. Navarro AJ: Writing – original draft. Soto AA: Writing – original draft. Pastén-Ferrada IA: . Carreño LJ: Writing – review & editing. González PA: Conceptualization, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Pablo Gonzalez reports financial support was provided by National Agency for Research and Development Chile (ANID).
Acknowledgments
This work was funded by ANID - Millennium Science Initiative Program - ICN2021_045: Millennium Institute on Immunology and Immunotherapy (former ICN09_016 and P09/016-F) and FONDECYT grant #1190864 from the Agencia Nacional de Investigación y Desarrollo (ANID). The Regional Government of Antofagasta also supported this work through the Innovation Fund for Competitiveness FIC-R 2017 (BIP Code: 30488811–0). MAF is ANID fellow #21191390.
Data Availability
Data sharing does not apply to this article as no new data were created or analyzed in this review.
References
- Ablashi D.V., Berneman Z.N., Kramarsky B., Whitman J., Jr., Asano Y., Pearson G.R. Human herpesvirus-7 (HHV-7): current status. Clin. Diagn. Virol. 1995;4:1–13. doi: 10.1016/0928-0197(95)00005-s. [DOI] [PubMed] [Google Scholar]
- Abrantes J.L., Alves C.M., Costa J., Almeida F.C., Sola-Penna M., Fontes C.F., Souza T.M. Herpes simplex type 1 activates glycolysis through engagement of the enzyme 6-phosphofructo-1-kinase (PFK-1) Biochim. Biophys. Acta. 2012;1822:1198–1206. doi: 10.1016/j.bbadis.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Akkapaiboon P., Mori Y., Sadaoka T., Yonemoto S., Yamanishi K. Intracellular processing of human herpesvirus 6 glycoproteins Q1 and Q2 into tetrameric complexes expressed on the viral envelope. J. Virol. 2004;78:7969–7983. doi: 10.1128/JVI.78.15.7969-7983.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asha K., Sharma-Walia N. Targeting host cellular factors as a strategy of therapeutic intervention for herpesvirus infections. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.603309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berber E., Sumbria D., Newkirk K.M., Rouse B.T. Inhibiting glucose metabolism results in herpes simplex encephalitis. J. Immunol. 2021;207:1824–1835. doi: 10.4049/jimmunol.2100453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beziau A., Brand D., Piver E. The role of phosphatidylinositol phosphate kinases during viral infection. Viruses. 2020;12 doi: 10.3390/v12101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitra A., Nemcovicova I., Picarda G., Doukov T., Wang J., Benedict C.A., Zajonc D.M. Structure of human cytomegalovirus UL144, an HVEM orthologue, bound to the B and T cell lymphocyte attenuator. J. Biol. Chem. 2019;294:10519–10529. doi: 10.1074/jbc.RA119.009199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blough H.A., Giuntoli R.L. Successful treatment of human genital herpes infections with 2-deoxy-D-glucose. JAMA. 1979;241:2798–2801. [PubMed] [Google Scholar]
- Brezgin S., Kostyusheva A., Bayurova E., Volchkova E., Gegechkori V., Gordeychuk I., Glebe D., Kostyushev D., Chulanov V. Immunity and viral infections: modulating antiviral response via CRISPR-Cas systems. Viruses. 2021;13 doi: 10.3390/v13071373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bu G.L., Xie C., Kang Y.F., Zeng M.S., Sun C. How EBV infects: the tropism and underlying molecular mechanism for viral infection. Viruses. 2022;14 doi: 10.3390/v14112372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse C., Feederle R., Schnolzer M., Behrends U., Mautner J., Delecluse H.J. Epstein-Barr viruses that express a CD21 antibody provide evidence that gp350′s functions extend beyond B-cell surface binding. J. Virol. 2010;84:1139–1147. doi: 10.1128/JVI.01953-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai T.T., Ye S.B., Liu Y.N., He J., Chen Q.Y., Mai H.Q., Zhang C.X., Cui J., Zhang X.S., Busson P., et al. LMP1-mediated glycolysis induces myeloid-derived suppressor cell expansion in nasopharyngeal carcinoma. PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S., Veettil M.V., Chandran B. Kaposi's sarcoma associated herpesvirus entry into target cells. Front. Microbiol. 2012;3(6) doi: 10.3389/fmicb.2012.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander Y., Kumar R., Khandelwal N., Singh N., Shringi B.N., Barua S., Kumar N. Role of p38 mitogen-activated protein kinase signalling in virus replication and potential for developing broad spectrum antiviral drugs. Rev. Med. Virol. 2021;31:1–16. doi: 10.1002/rmv.2217. [DOI] [PubMed] [Google Scholar]
- Chang Y., Moore P. Twenty years of KSHV. Viruses. 2014;6:4258–4264. doi: 10.3390/v6114258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung T.C., Humphreys I.R., Potter K.G., Norris P.S., Shumway H.M., Tran B.R., Patterson G., Jean-Jacques R., Yoon M., Spear P.G., et al. Evolutionarily divergent herpesviruses modulate T cell activation by targeting the herpesvirus entry mediator cosignaling pathway. Proc. Natl. Acad. Sci. U. S. A. 2005;102:13218–13223. doi: 10.1073/pnas.0506172102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.A., Song C.H. Insights into the role of endoplasmic reticulum stress in infectious diseases. Front. Immunol. 2019;10:3147. doi: 10.3389/fimmu.2019.03147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S. Herpes simplex virus: epidemiology, diagnosis, and treatment. Nurs. Clin. North Am. 2020;55:337–345. doi: 10.1016/j.cnur.2020.05.004. [DOI] [PubMed] [Google Scholar]
- Combs J.A., Monk C.H., Harrison M.A.A., Norton E.B., Morris C.A., Sullivan D.E., Zwezdaryk K.J. Inhibiting cytomegalovirus replication through targeting the host electron transport chain. Antiviral Res. 2021;194 doi: 10.1016/j.antiviral.2021.105159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney R.J., Steiner S.M., Benyesh-Melnick M. Effects of 2-deoxy-D-glucose on herpes simplex virus replication. Virology. 1973;52:447–455. doi: 10.1016/0042-6822(73)90340-1. [DOI] [PubMed] [Google Scholar]
- Cui M., Fang Q., Zheng J., Shu Z., Chen Y., Fan Y., Zhao J., Wood C., Zhang T., Zeng Y. Kaposi's sarcoma-associated herpesvirus seropositivity is associated with type 2 diabetes mellitus: a case-control study in Xinjiang, China. Int. J. Infect. Dis. 2019;80:73–79. doi: 10.1016/j.ijid.2019.01.003. [DOI] [PubMed] [Google Scholar]
- Dang C.V. c-Myc target genes involved in cell growth, apoptosis, and metabolism. Mol. Cell. Biol. 1999;19:1–11. doi: 10.1128/MCB.19.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darekar S., Georgiou K., Yurchenko M., Yenamandra S.P., Chachami G., Simos G., Klein G., Kashuba E. Epstein-Barr virus immortalization of human B-cells leads to stabilization of hypoxia-induced factor 1 alpha, congruent with the Warburg effect. PLoS ONE. 2012;7:e42072. doi: 10.1371/journal.pone.0042072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sanjose S., Mbisa G., Perez-Alvarez S., Benavente Y., Sukvirach S., Hieu N.T., Shin H.R., Anh P.T., Thomas J., Lazcano E., et al. Geographic variation in the prevalence of Kaposi sarcoma-associated herpesvirus and risk factors for transmission. J. Infect. Dis. 2009;199:1449–1456. doi: 10.1086/598523. [DOI] [PubMed] [Google Scholar]
- de Wit R.H., Mujic-Delic A., van Senten J.R., Fraile-Ramos A., Siderius M., Smit M.J. Human cytomegalovirus encoded chemokine receptor US28 activates the HIF-1alpha/PKM2 axis in glioblastoma cells. Oncotarget. 2016;7:67966–67985. doi: 10.18632/oncotarget.11817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis R.J., Chandel N.S. We need to talk about the Warburg effect. Nat. Metab. 2020;2:127–129. doi: 10.1038/s42255-020-0172-2. [DOI] [PubMed] [Google Scholar]
- DeBerardinis R.J., Lum J.J., Hatzivassiliou G., Thompson C.B. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- Delgado T., Carroll P.A., Punjabi A.S., Margineantu D., Hockenbery D.M., Lagunoff M. Induction of the Warburg effect by Kaposi's sarcoma herpesvirus is required for the maintenance of latently infected endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 2010;107:10696–10701. doi: 10.1073/pnas.1004882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng J., Zhong Z., Geng C., Dai Z., Zheng W., Li Z., Yan Z., Yang J., Deng W., Tan W., et al. Herpes simplex type 1 UL43 multiple membrane-spanning protein increases energy metabolism in host cells through interacting with ARL2. Cells. 2022;11 doi: 10.3390/cells11223594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte L.F., Farias M.A., Alvarez D.M., Bueno S.M., Riedel C.A., Gonzalez P.A. Herpes simplex virus type 1 infection of the central nervous system: insights into proposed interrelationships with neurodegenerative disorders. Front. Cell Neurosci. 2019;13(46) doi: 10.3389/fncel.2019.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliassen E., Lum E., Pritchett J., Ongradi J., Krueger G., Crawford J.R., Phan T.L., Ablashi D., Hudnall S.D. Human herpesvirus 6 and malignancy: a review. Front. Oncol. 2018;8:512. doi: 10.3389/fonc.2018.00512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etta E.M., Alayande D.P., Mavhandu-Ramarumo L.G., Gachara G., Bessong P.O. HHV-8 seroprevalence and genotype distribution in Africa, 1998(-)2017: a systematic review. Viruses. 2018;10 doi: 10.3390/v10090458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Peng K. Regulation of innate immune responses by cell death-associated caspases during virus infection. FEBS J. 2021 doi: 10.1111/febs.16051. [DOI] [PubMed] [Google Scholar]
- Farias M.A., Diethelm-Varela B., Navarro A.J., Kalergis A.M., Gonzalez P.A. Interplay between lipid metabolism, lipid droplets, and DNA virus infections. Cells. 2022;11 doi: 10.3390/cells11142224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frascaroli G., Varani S., Mastroianni A., Britton S., Gibellini D., Rossini G., Landini M.P., Soderberg-Naucler C. Dendritic cell function in cytomegalovirus-infected patients with mononucleosis. J. Leukoc. Biol. 2006;79:932–940. doi: 10.1189/jlb.0905499. [DOI] [PubMed] [Google Scholar]
- Ge T., Yang J., Zhou S., Wang Y., Li Y., Tong X. The role of the pentose phosphate pathway in diabetes and cancer. Front. Endocrinol. (Lausanne) 2020;11:365. doi: 10.3389/fendo.2020.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon A.A., Breuer J., Cohen J.I., Cohrs R.J., Gershon M.D., Gilden D., Grose C., Hambleton S., Kennedy P.G., Oxman M.N., et al. Varicella zoster virus infection. Nat. Rev. Dis. Primers. 2015;1:15016. doi: 10.1038/nrdp.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonnella R., Santarelli R., Farina A., Granato M., D'Orazi G., Faggioni A., Cirone M. Kaposi sarcoma associated herpesvirus (KSHV) induces AKT hyperphosphorylation, bortezomib-resistance and GLUT-1 plasma membrane exposure in THP-1 monocytic cell line. J. Exp. Clin. Cancer Res. 2013;32(79) doi: 10.1186/1756-9966-32-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravel A., Dubuc I., Morissette G., Sedlak R.H., Jerome K.R., Flamand L. Inherited chromosomally integrated human herpesvirus 6 as a predisposing risk factor for the development of angina pectoris. Proc. Natl. Acad. Sci. U. S. A. 2015;112:8058–8063. doi: 10.1073/pnas.1502741112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins T. HbA(1c)–an analyte of increasing importance. Clin. Biochem. 2012;45:1038–1045. doi: 10.1016/j.clinbiochem.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Hwang J., Purdy J.G., Wu K., Rabinowitz J.D., Shenk T. Estrogen-related receptor alpha is required for efficient human cytomegalovirus replication. Proc. Natl. Acad. Sci. U. S. A. 2014;111 doi: 10.1073/pnas.1422361112. E5706-5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iarovaia O.V., Ioudinkova E.S., Velichko A.K., Razin S.V. Manipulation of cellular processes via nucleolus hijaking in the course of viral infection in mammals. Cells. 2021;10 doi: 10.3390/cells10071597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez F.J., Farias M.A., Gonzalez-Troncoso M.P., Corrales N., Duarte L.F., Retamal-Diaz A., Gonzalez P.A. Experimental dissection of the lytic replication cycles of herpes simplex viruses in vitro. Front. Microbiol. 2018;9:2406. doi: 10.3389/fmicb.2018.02406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbert F., Langford D. Viruses, SUMO, and immunity: the interplay between viruses and the host SUMOylation system. J. Neurovirol. 2021;27:531–541. doi: 10.1007/s13365-021-00995-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incani A., Marras L., Serreli G., Ingianni A., Pompei R., Deiana M., Angius F. Human herpesvirus 8 infection may contribute to oxidative stress in diabetes type 2 patients. BMC Res. Notes. 2020;13:75. doi: 10.1186/s13104-020-4935-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingianni A., Piras E., Laconi S., Angius F., Batetta B., Pompei R. Latent herpesvirus 8 infection improves both insulin and glucose uptake in primary endothelial cells. New Microbiol. 2013;36:257–265. [PubMed] [Google Scholar]
- James C., Harfouche M., Welton N.J., Turner K.M., Abu-Raddad L.J., Gottlieb S.L., Looker K.J. Herpes simplex virus: global infection prevalence and incidence estimates, 2016. Bull World Health Organ. 2020;98:315–329. doi: 10.2471/BLT.19.237149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jheng J.R., Ho J.Y., Horng J.T. ER stress, autophagy, and RNA viruses. Front. Microbiol. 2014;5:388. doi: 10.3389/fmicb.2014.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabanova A., Marcandalli J., Zhou T., Bianchi S., Baxa U., Tsybovsky Y., Lilleri D., Silacci-Fregni C., Foglierini M., Fernandez-Rodriguez B.M., et al. Platelet-derived growth factor-alpha receptor is the cellular receptor for human cytomegalovirus gHgLgO trimer. Nat. Microbiol. 2016;1:16082. doi: 10.1038/nmicrobiol.2016.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kori M., Arga K.Y. Human oncogenic viruses: an overview of protein biomarkers in viral cancers and their potential use in clinics. Expert Rev. Anticancer Ther. 2022;22:1211–1224. doi: 10.1080/14737140.2022.2139681. [DOI] [PubMed] [Google Scholar]
- Kourieh A., Gheit T., Tommasino M., Dalstein V., Clifford G.M., Lacau St Guily J., Clavel C., Franceschi S., Combes J.D. Prevalence of human herpesviruses infections in nonmalignant tonsils: the SPLIT study. J. Med. Virol. 2019;91:687–697. doi: 10.1002/jmv.25338. [DOI] [PubMed] [Google Scholar]
- Kuri A., Jacobs B.M., Vickaryous N., Pakpoor J., Middeldorp J., Giovannoni G., Dobson R. Epidemiology of Epstein-Barr virus infection and infectious mononucleosis in the United Kingdom. BMC Public Health. 2020;20:912. doi: 10.1186/s12889-020-09049-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung H.J., Duran E.M., Kurtoglu M., Andreansky S., Lampidis T.J., Mesri E.A. Activation of the unfolded protein response by 2-deoxy-D-glucose inhibits Kaposi's sarcoma-associated herpesvirus replication and gene expression. Antimicrob. Agents Chemother. 2012;56:5794–5803. doi: 10.1128/AAC.01126-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Ali M.A., Cohen J.I. Insulin degrading enzyme is a cellular receptor mediating varicella-zoster virus infection and cell-to-cell spread. Cell. 2006;127:305–316. doi: 10.1016/j.cell.2006.08.046. 10.1016/j.cell.2006.08.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Ali M.A., Wang K., Sayre D., Hamel F.G., Fischer E.R., Bennett R.G., Cohen J.I. Insulin degrading enzyme induces a conformational change in varicella-zoster virus gE, and enhances virus infectivity and stability. PLoS ONE. 2010;5:e11327. doi: 10.1371/journal.pone.0011327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Wu K., Zeng S., Zhao F., Fan J., Li Z., Yi L., Ding H., Zhao M., Fan S., Chen J. Viral infection modulates mitochondrial function. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22084260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.B., Gu J.D., Zhou Q.H. Review of aerobic glycolysis and its key enzymes - new targets for lung cancer therapy. Thorac. Cancer. 2015;6:17–24. doi: 10.1111/1759-7714.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao K.C., Garcia-Blanco M.A. Role of alternative splicing in regulating host response to viral infection. Cells. 2021;10 doi: 10.3390/cells10071720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo A.K., Dawson C.W., Young L.S., Ko C.W., Hau P.M., Lo K.W. Activation of the FGFR1 signalling pathway by the Epstein-Barr virus-encoded LMP1 promotes aerobic glycolysis and transformation of human nasopharyngeal epithelial cells. J. Pathol. 2015;237:238–248. doi: 10.1002/path.4575. [DOI] [PubMed] [Google Scholar]
- Lontchi-Yimagou E., Legoff J., Nguewa J.L., Boudou P., Balti E.V., Noubiap J.J., Kamwa V., Atogho-Tiedeu B., Azabji-Kenfack M., Djahmeni E.N., et al. Human herpesvirus 8 infection DNA positivity is associated with low insulin secretion: a case-control study in a sub-Saharan African population with diabetes. J. Diabetes. 2018;10:866–873. doi: 10.1111/1753-0407.12777. [DOI] [PubMed] [Google Scholar]
- Lucic B., de Castro I.J., Lusic M. Viruses in the nucleus. Cold Spring Harb. Perspect. Biol. 2021;13 doi: 10.1101/cshperspect.a039446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu X., Wang J., Guo X., Wu G., Jiao Y., Faleti O.D., Liu P., Liu T., Long Y., Chong T., et al. EBV-miR-BART1-5P activates AMPK/mTOR/HIF1 pathway via a PTEN independent manner to promote glycolysis and angiogenesis in nasopharyngeal carcinoma. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1007484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T., Patel H., Babapoor-Farrokhran S., Franklin R., Semenza G.L., Sodhi A., Montaner S. KSHV induces aerobic glycolysis and angiogenesis through HIF-1-dependent upregulation of pyruvate kinase 2 in Kaposi's sarcoma. Angiogenesis. 2015;18:477–488. doi: 10.1007/s10456-015-9475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah A.Y., Rashidi A., Keppel M.P., Saucier N., Moore E.K., Alinger J.B., Tripathy S.K., Agarwal S.K., Jeng E.K., Wong H.C., et al. Glycolytic requirement for NK cell cytotoxicity and cytomegalovirus control. JCI Insight. 2017;2 doi: 10.1172/jci.insight.95128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbaniang C., Kma L. Dysregulation of glucose metabolism by oncogenes and tumor suppressors in cancer cells. Asian Pac. J. Cancer Prev. 2018;19:2377–2390. doi: 10.22034/APJCP.2018.19.9.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Martin N., Marcandalli J., Huang C.S., Arthur C.P., Perotti M., Foglierini M., Ho H., Dosey A.M., Shriver S., Payandeh J., et al. An unbiased screen for human cytomegalovirus identifies neuropilin-2 as a central viral receptor. Cell. 2018;174:1158–1171. doi: 10.1016/j.cell.2018.06.028. e1119. [DOI] [PubMed] [Google Scholar]
- Massini G., Siemer D., Hohaus S. EBV in Hodgkin lymphoma. Mediterr. J. Hematol. Infect. Dis. 2009;1 doi: 10.4084/MJHID.2009.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauro C., Leow S.C., Anso E., Rocha S., Thotakura A.K., Tornatore L., Moretti M., De Smaele E., Beg A.A., Tergaonkar V., et al. NF-kappaB controls energy homeostasis and metabolic adaptation by upregulating mitochondrial respiration. Nat. Cell Biol. 2011;13:1272–1279. doi: 10.1038/ncb2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle J., Moorman N.J., Munger J. HCMV targets the metabolic stress response through activation of AMPK whose activity is important for viral replication. PLoS Pathog. 2012;8 doi: 10.1371/journal.ppat.1002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McArdle J., Schafer X.L., Munger J. Inhibition of calmodulin-dependent kinase kinase blocks human cytomegalovirus-induced glycolytic activation and severely attenuates production of viral progeny. J. Virol. 2011;85:705–714. doi: 10.1128/JVI.01557-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesri E.A., Lampidis T.J. 2-Deoxy-d-glucose exploits increased glucose metabolism in cancer and viral-infected cells: relevance to its use in India against SARS-CoV-2. IUBMB Life. 2021;73:1198–1204. doi: 10.1002/iub.2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munger J., Bajad S.U., Coller H.A., Shenk T., Rabinowitz J.D. Dynamics of the cellular metabolome during human cytomegalovirus infection. PLoS Pathog. 2006;2:e132. doi: 10.1371/journal.ppat.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myoung J., Ganem D. Generation of a doxycycline-inducible KSHV producer cell line of endothelial origin: maintenance of tight latency with efficient reactivation upon induction. J. Virol. Methods. 2011;174:12–21. doi: 10.1016/j.jviromet.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naghavi M.H., Walsh D. Microtubule regulation and function during virus infection. J. Virol. 2017;91 doi: 10.1128/JVI.00538-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhierl B., Feederle R., Hammerschmidt W., Delecluse H.J. Glycoprotein gp110 of Epstein-Barr virus determines viral tropism and efficiency of infection. Proc. Natl. Acad. Sci. U. S. A. 2002;99:15036–15041. doi: 10.1073/pnas.232381299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguewa J.L., Lontchi-Yimagou E., Agbelika F., AitDjoudi M., Boudou P., Choukem S., Sobngwi E., Gautier J.F. Relationship between HHV8 infection markers and insulin sensitivity in ketosis-prone diabetes. Diabetes Metab. 2017;43:79–82. doi: 10.1016/j.diabet.2016.05.004. [DOI] [PubMed] [Google Scholar]
- Pan Y.R., Vatsyayan J., Chang Y.S., Chang H.Y. Epstein-Barr virus latent membrane protein 2A upregulates UDP-glucose dehydrogenase gene expression via ERK and PI3K/Akt pathway. Cell Microbiol. 2008;10:2447–2460. doi: 10.1111/j.1462-5822.2008.01221.x. [DOI] [PubMed] [Google Scholar]
- Parra M., Alcala A., Amoros C., Baeza A., Galiana A., Tarrago D., Garcia-Quesada M.A., Sanchez-Hellin V. Encephalitis associated with human herpesvirus-7 infection in an immunocompetent adult. Virol. J. 2017;14:97. doi: 10.1186/s12985-017-0764-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quarleri J., Cevallos C., Delpino M.V. Apoptosis in infectious diseases as a mechanism of immune evasion and survival. Adv. Protein Chem. Struct. Biol. 2021;125:1–24. doi: 10.1016/bs.apcsb.2021.01.001. [DOI] [PubMed] [Google Scholar]
- Rector J.L., Thomas G.N., Burns V.E., Dowd J.B., Herr R.M., Moss P.A., Jarczok M.N., Hoffman K., Fischer J.E., Bosch J.A. Elevated HbA(1c) levels and the accumulation of differentiated T cells in CMV(+) individuals. Diabetologia. 2015;58:2596–2605. doi: 10.1007/s00125-015-3731-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retamal-Diaz A.R., Suazo P.A., Garrido I., Kalergis A.M., Gonzalez P.A. [Immune evasion by herpes simplex viruses] Rev. Chilena Infectol. 2015;32:58–70. doi: 10.4067/S0716-10182015000200013. [DOI] [PubMed] [Google Scholar]
- Reyes A., Duarte L.F., Farias M.A., Tognarelli E., Kalergis A.M., Bueno S.M., Gonzalez P.A. Impact of hypoxia over human viral infections and key cellular processes. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22157954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A., Navarro A.J., Diethelm-Varela B., Kalergis A.M., Gonzalez P.A. Is there a role for HSF1 in viral infections? FEBS Open Bio. 2022 doi: 10.1002/2211-5463.13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rico A., Dollard S.C., Valencia D., Corchuelo S., Tong V.T., Laiton-Donato K., Amin M.M., Benavides M., Wong P., Newton S., et al. Epidemiology of cytomegalovirus Infection among mothers and infants in Colombia. J. Med. Virol. 2021;93:6393–6397. doi: 10.1002/jmv.26815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M., Schor S., Barouch-Bentov R., Einav S. Viral journeys on the intracellular highways. Cell Mol. Life Sci. 2018;75:3693–3714. doi: 10.1007/s00018-018-2882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Sanchez I., Schafer X.L., Monaghan M., Munger J. The human cytomegalovirus UL38 protein drives mTOR-independent metabolic flux reprogramming by inhibiting TSC2. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohner E., Wyss N., Trelle S., Mbulaiteye S.M., Egger M., Novak U., Zwahlen M., Bohlius J. HHV-8 seroprevalence: a global view. Syst. Rev. 2014;3(11) doi: 10.1186/2046-4053-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiss R., Jochum S., Mocikat R., Hammerschmidt W., Zeidler R. EBV-gp350 confers B-cell tropism to tailored exosomes and is a neo-antigen in normal and malignant B cells–a new option for the treatment of B-CLL. PLoS ONE. 2011;6:e25294. doi: 10.1371/journal.pone.0025294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez E.L., Pulliam T.H., Dimaio T.A., Thalhofer A.B., Delgado T., Lagunoff M. Glycolysis, glutaminolysis, and fatty acid synthesis are required for distinct stages of Kaposi's sarcoma-associated herpesvirus lytic replication. J Virol. 2017;91 doi: 10.1128/JVI.02237-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro F., Kennedy P.E., Locatelli G., Malnati M.S., Berger E.A., Lusso P. CD46 is a cellular receptor for human herpesvirus 6. Cell. 1999;99:817–827. doi: 10.1016/s0092-8674(00)81678-5. [DOI] [PubMed] [Google Scholar]
- Secchiero P., Sun D., De Vico A.L., Crowley R.W., Reitz M.S., Jr., Zauli G., Lusso P., Gallo R.C. Role of the extracellular domain of human herpesvirus 7 glycoprotein B in virus binding to cell surface heparan sulfate proteoglycans. J. Virol. 1997;71:4571–4580. doi: 10.1128/JVI.71.6.4571-4580.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah A.M., Wondisford F.E. Tracking the carbons supplying gluconeogenesis. J. Biol. Chem. 2020;295:14419–14429. doi: 10.1074/jbc.REV120.012758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinzger C., Digel M., Jahn G. Cytomegalovirus cell tropism. Curr. Top. Microbiol. Immunol. 2008;325:63–83. doi: 10.1007/978-3-540-77349-8_4. [DOI] [PubMed] [Google Scholar]
- Sommermann T.G., O'Neill K., Plas D.R., Cahir-McFarland E. IKKbeta and NF-kappaB transcription govern lymphoma cell survival through AKT-induced plasma membrane trafficking of GLUT1. Cancer Res. 2011;71:7291–7300. doi: 10.1158/0008-5472.CAN-11-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto A.A., Ortiz G., Contreras S., Soto-Rifo R., Gonzalez P.A. Role of epitranscriptomic and epigenetic modifications during the lytic and latent phases of herpesvirus infections. Microorganisms. 2022;10 doi: 10.3390/microorganisms10091754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacpoole P.W., McCall C.E. The pyruvate dehydrogenase complex: life's essential, vulnerable and druggable energy homeostat. Mitochondrion. 2023 doi: 10.1016/j.mito.2023.02.007. [DOI] [PubMed] [Google Scholar]
- Stradal T.E.B., Schelhaas M. Actin dynamics in host-pathogen interaction. FEBS Lett. 2018;592:3658–3669. doi: 10.1002/1873-3468.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumbria D., Berber E., Mathayan M., Rouse B.T. Virus infections and host metabolism-can we manage the interactions? Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.594963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H., Serada S., Kawabata A., Ota M., Hayashi E., Naka T., Yamanishi K., Mori Y. CD134 is a cellular receptor specific for human herpesvirus-6B entry. Proc. Natl. Acad. Sci. U. S. A. 2013;110:9096–9099. doi: 10.1073/pnas.1305187110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai M., Graham N.A., Braas D., Nehil M., Komisopoulou E., Kurdistani S.K., McCormick F., Graeber T.G., Christofk H.R. Adenovirus E4ORF1-induced MYC activation promotes host cell anabolic glucose metabolism and virus replication. Cell Metab. 2014;19:694–701. doi: 10.1016/j.cmet.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognarelli E.I., Retamal-Diaz A., Farias M.A., Duarte L.F., Palomino T.F., Ibanez F.J., Riedel C.A., Kalergis A.M., Bueno S.M., Gonzalez P.A. Pharmacological inhibition of IRE-1 alpha activity in herpes simplex virus type 1 and type 2-infected dendritic cells enhances T cell activation. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.764861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognarelli E.I., Reyes A., Corrales N., Carreno L.J., Bueno S.M., Kalergis A.M., Gonzalez P.A. Modulation of endosome function, vesicle trafficking and autophagy by human herpesviruses. Cells. 2021;10 doi: 10.3390/cells10030542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varanasi S.K., Donohoe D., Jaggi U., Rouse B.T. Manipulating glucose metabolism during different stages of viral pathogenesis can have either detrimental or beneficial effects. J. Immunol. 2017;199:1748–1761. doi: 10.4049/jimmunol.1700472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vastag L., Koyuncu E., Grady S.L., Shenk T.E., Rabinowitz J.D. Divergent effects of human cytomegalovirus and herpes simplex virus-1 on cellular metabolism. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1002124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veettil M.V., Bandyopadhyay C., Dutta D., Chandran B. Interaction of KSHV with host cell surface receptors and cell entry. Viruses. 2014;6:4024–4046. doi: 10.3390/v6104024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villena J.A., Kralli A. ERRalpha: a metabolic function for the oldest orphan. Trends Endocrinol. Metab. 2008;19:269–276. doi: 10.1016/j.tem.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh D., Naghavi M.H. Exploitation of cytoskeletal networks during early viral infection. Trends Microbiol. 2019;27:39–50. doi: 10.1016/j.tim.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A., Huen S.C., Luan H.H., Yu S., Zhang C., Gallezot J.D., Booth C.J., Medzhitov R. Opposing effects of fasting metabolism on tissue tolerance in bacterial and viral inflammation. Cell. 2016;166:1512–1525. doi: 10.1016/j.cell.2016.07.026. e1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Zhang P., Deng K. MYC promotes LDHA expression through MicroRNA-122-5p to potentiate glycolysis in hepatocellular carcinoma. Anal. Cell Pathol. (Amst) 2022;2022 doi: 10.1155/2022/1435173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisova P., Concannon C.G., Devocelle M., Prehn J.H., Ward M.W. Regulation of glucose transporter 3 surface expression by the AMP-activated protein kinase mediates tolerance to glutamate excitation in neurons. J. Neurosci. 2009;29:2997–3008. doi: 10.1523/JNEUROSCI.0354-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Jia J., Xu X., Xu M., Peng G., Ma J., Jiang X., Yao J., Yao K., Li L., Tang H. Human herpesvirus 6A promotes glycolysis in infected T cells by activation of mTOR signaling. PLoS Pathog. 2020;16 doi: 10.1371/journal.ppat.1008568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao J., Palefsky J.M., Herrera R., Berline J., Tugizov S.M. The Epstein-Barr virus BMRF-2 protein facilitates virus attachment to oral epithelial cells. Virology. 2008;370:430–442. doi: 10.1016/j.virol.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L., Hu Z.Y., Dong X., Tan Z., Li W., Tang M., Chen L., Yang L., Tao Y., Jiang Y., et al. Targeting Epstein-Barr virus oncoprotein LMP1-mediated glycolysis sensitizes nasopharyngeal carcinoma to radiation therapy. Oncogene. 2014;33:4568–4578. doi: 10.1038/onc.2014.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Cai W. Autophagy and viral infection. Adv. Exp. Med. Biol. 2020;1207:425–432. doi: 10.1007/978-981-15-4272-5_30. [DOI] [PubMed] [Google Scholar]
- Yang J., Wu P., Liu X., Xia H., Lai Z. Autoimmune encephalitis with multiple auto-antibodies with concomitant human herpesvirus-7 and ovarian teratoma: a case report. Front. Med. (Lausanne) 2021;8 doi: 10.3389/fmed.2021.759559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao K., Honarmand S., Espinosa A., Akhyani N., Glaser C., Jacobson S. Detection of human herpesvirus-6 in cerebrospinal fluid of patients with encephalitis. Ann. Neurol. 2009;65:257–267. doi: 10.1002/ana.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F., Zeng Y., Sha J., Jones T., Kuhne K., Wood C., Gao S.J. High glucose induces reactivation of latent Kaposi's sarcoma-associated herpesvirus. J. Virol. 2016;90:9654–9663. doi: 10.1128/JVI.01049-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev O., Lagos D., Enver T., Boshoff C. Kaposi's sarcoma herpesvirus microRNAs induce metabolic transformation of infected cells. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Maguire T.G., Alwine J.C. Human cytomegalovirus activates glucose transporter 4 expression to increase glucose uptake during infection. J. Virol. 2011;85:1573–1580. doi: 10.1128/JVI.01967-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Maguire T.G., Alwine J.C. ChREBP, a glucose-responsive transcriptional factor, enhances glucose metabolism to support biosynthesis in human cytomegalovirus-infected cells. Proc. Natl. Acad. Sci. U. S. A. 2014;111:1951–1956. doi: 10.1073/pnas.1310779111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Jia L., Lin W., Yip Y.L., Lo K.W., Lau V.M.Y., Zhu D., Tsang C.M., Zhou Y., Deng W., et al. Epstein-barr virus-encoded latent membrane protein 1 upregulates glucose transporter 1 transcription via the mTORC1/NF-kappaB signaling pathways. J. Virol. 2017;91 doi: 10.1128/JVI.02168-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Jia L., Liu T., Yip Y.L., Tang W.C., Lin W., Deng W., Lo K.W., You C., Lung M.L., et al. mTORC2-mediated PDHE1alpha nuclear translocation links EBV-LMP1 reprogrammed glucose metabolism to cancer metastasis in nasopharyngeal carcinoma. Oncogene. 2019;38:4669–4684. doi: 10.1038/s41388-019-0749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing does not apply to this article as no new data were created or analyzed in this review.