Abstract
Introduction:
As it may not be feasible to provide cervical cancer screening services to all HIV-infected women in most resource-limited settings, there is a need to identify those who are most at risk. We determined the prevalence, patterns, and associated factors of cervical cytological abnormalities among HIV-infected women in Lagos, Nigeria.
Methods:
This descriptive cross-sectional study was conducted among HIV-infected women at the adult HIV treatment and colposcopy clinics of a university teaching hospital in Lagos, Nigeria between October 2018 to December 2019. A cervical sample was collected from each woman to detect cervical cytological abnormalities.
Results:
Of the 593 enrolled women, cervical cytological abnormalities were present in 40 (6.7%). Most (37.5%) of the women with cytological abnormalities had atypical squamous cells of undetermined significance (ASCUS). Age at coitarche (<20 versus ≥20 years: adjusted odds ratio, 2.42; 95% confidence interval, 1.21–4.83, P=0.01) was the only factor that was independently associated with cervical epithelial abnormalities.
Conclusion:
The prevalence of cervical cytological abnormalities in our study is lower than most previous reports in Africa. Sexual debut at an early age was significantly associated with cytological abnormalities. It is necessary to confirm the findings of this study through a well-designed and adequately powered longitudinal study.
Keywords: ASCUS, Cervical cancer, Cytological abnormalities, HIV, Nigeria, Pap smear
Introduction
Human papillomavirus (HPV) infection is a well-established cause of cervical cancer [1]. Women who are infected with HIV, even while on antiretroviral therapy [2], are more likely to have persistent high-risk HPV infections [3] that lead to precancerous squamous intraepithelial lesions (SIL) of the cervix [4], [5] which if left untreated may progress to invasive cervical cancer [5]. Sub-Saharan Africa accounts for about 80% of the global burden of cervical cancer [6] and is also the region most affected by the global HIV epidemic with an estimated 25 million cases [7]. Nigerian women currently bear the highest burden of cervical cancer in Africa, with an annual incidence of 14,089 new cases and 8,240 deaths [8].
To deliver and scale-up access to antiretroviral therapy (ART) many countries across the sub-Saharan Africa (SSA) region have successfully established dedicated HIV programmes in the past two to three decades to extend the lifespan of people living with HIV (PLVIV) [9], [10]. With a longer lifespan, HIV-infected women are now at increased risk of developing cervical precancer and cancer [3]. Integration of cervical cancer screening and treatment services into HIV treatment programmes is currently used in most settings in SSA including Nigeria to effectively control the unnecessarily high burden of cervical cancer in women living with HIV (WLHIV) [11], [12]. However, due to limited resources in most countries in the region, it may not be feasible to provide these screening services to all HIV-infected women and thus there is a need to identify those who are most at risk of developing cancer. There is currently limited access to diagnostic colposcopy compared to cervical cytology in most resource-limited settings due to a dearth of facilities and skilled manpower [1]. Furthermore, available data, suggest that Pap smear cervical cytology is a more sensitive and specific screening and diagnostic tool for cervical pre(cancer) than colposcopy in HIV-infected women [13]. We, therefore, aimed in this study to determine the prevalence, patterns and factors associated with cervical epithelial abnormalities using Pap smear cytology among HIV-infected women attending the adult HIV treatment clinic of a university teaching in Lagos, Nigeria.
Patients and Methods
Study design and setting
This cross-sectional study involved HIV-infected women at the adult HIV treatment clinic of a university teaching hospital in Lagos, Nigeria who were enrolled in the “Epigenetic Biomarkers of Cervical Cancer in Nigerian Women Study” [14] between October 2018 to December 2019. The HIV treatment clinic provides comprehensive care for people living with HIV in Lagos and its surrounding states in Southwest Nigeria. The clinic also offers integrated reproductive health services to sexually active WLHIV such as routine cervical cancer screening with either a Pap smear, HPV DNA testing or visual inspection with acetic acid (VIA) depending on availability and affordability.
Study population and eligibility criteria
The study participants were consecutively enrolled HIV-infected women who attend care at the adult HIV clinic and consented to undergo cervical cancer screening using the Papanicolaou (Pap) test smear at the Colposcopy clinic of the hospital as part of the “Epigenetic Biomarkers of Cervical Cancer in Nigerian Women Study” [14]. Inclusion criteria at enrolment included sexually active HIV-infected women aged 25–65 years [15] while exclusion criteria were virgo intacta, suspicious cervical lesions, current pregnancy or childbirth within the past 6 weeks, previous hysterectomy, history of HPV DNA testing or Pap smear within the past 3-years, and history of cervical cancer or therapy for benign or malignant cervical lesions. Women who were confirmed as having invasive cervical cancer at any time during the study were also excluded and referred for treatment.
Sample size calculation
The primary study endpoint was the proportion of screened HIV-infected women with cervical cytological abnormalities. We calculated a sample size of 184 WLHIV by imputing an estimated prevalence of 12.2% for cervical cytological abnormalities [16], type I error and precision rates of 5% respectively, and a projected non-response or data recording error rate of 10%. We, however, included all the 593 women enrolled for Pap smear screening during the study period in the final data analyses.
Study procedure and data collection
Potentially eligible participants were enrolled by members of the study team using the consecutive sampling technique. Study intent and procedures were introduced, and informed consent was obtained before any study procedure. A data collection form created on REDCap software was used to collect information on socio-demographic variables, duration of HIV diagnosis and use of combination antiretroviral therapy (cART), and other relevant information aimed at evaluating the risk factors for cervical cancer [17]. Information on participants’ immune status (the most recent values CD4+ cell counts, and HIV RNA viral load documented within ±8 weeks from the enrolment date) was extracted from the electronic medical records at the HIV clinic. All the participants then had a pelvic examination and cervical smear sample collection by the study nurse at the Colposcopy clinic. The smear samples were sent to the hospital’s Anatomic and Molecular Pathology laboratory for cytological examination using the conventional Pap smear. The presence or absence of cervical cytological abnormalities was determined and interpreted according to the revised 2014 Bethesda classification system [18]. Internal and external quality assurances were ensured by labelling the slides with the participant’s unique identification code after which a unique laboratory identifier is assigned before being viewed by a third-year histopathology resident doctor. These are then reviewed by either of the two experienced pathologists in the study. Cases with cervical epithelial abnormalities were randomly selected and jointly reviewed by the two pathologists to have an agreement. In addition, the reviewed slides were scanned using a slide scanner and sent to a pathologist at Northwestern University in Chicago, Illinois after which virtual meetings were held to discuss discordant cases.
Statistical analyses
Statistical analyses were carried out using SPSS version 27.0 for Windows. We computed descriptive statistics for the women’s sociodemographic, clinical and laboratory characteristics. Continuous variables were presented as mean and standard deviation if normally distributed or median and interquartile range if skewed while categorical variables were presented as frequencies and percentages. The associations between cervical cytological abnormalities and the women’s characteristics were tested using the χ2 test for categorical variables and the independent sample t-test, one-way analysis of variance or Mann-Whitney U test where applicable for continuous variables. Odds ratios (OR) and 95% confidence interval (CI) of cervical cytological abnormalities were estimated using multivariate binary logistic analyses for all the women’s characteristics. The mean age at enrolment and coitarche as well as the median CD4+ cell count as reported in this study were used as the stratifying cut-off values in our models. Variables with P<0.20 such as participant’s age, the CD4+ cells count, age at coitarche, and tobacco smoking were included in the adjusted multivariate model. We considered P<0.05 as statistically significant.
Results
The characteristics of women enrolled in the study are shown in Table 1. The participants’ mean age at enrolment, BMI, duration of use of cART and age of sexual debut were 43.3 ± 8.0 years, 27.1 ± 5.2 kg/m2, 111.4 ± 49.1 months, and 20.0 ± 3.4 years respectively. The median time since HIV diagnosis was 11.0 (8.0–14.0) years, CD4+ cells count was 407.0 (265.0–583.0) cells/μL, and HIV viral load was 20.0 (0.0–52.0) copies/mL. The women were predominantly multiparous (n=510, 86.0%), married (n=372, 62.7%), and had at least a secondary level of education (n=497, 83.8%). Most of the participants had never previously used any form of hormonal contraceptive (n=407, 68.6%), had no previous STI (n=469, 79.1%), and had never smoked tobacco (n=571, 96.3%) nor consumed any alcoholic beverages (n=464, 79.2%). About four in five of the women have had at least two-lifetime sexual partners (n=487, 82.1%). Cervical cytological abnormalities were present in 40 (6.7%) of the 593 enrolled women.
Table 1:
Characteristics of study participants (n=593)a
| Characteristics | Frequency (%) |
|---|---|
| Mean age at enrolment (± SD) in years | 43.3 ± 8.0 |
| Mean BMI (± SD) in kg/m 2 | 27.1 ± 5.2 |
| Median overall time since HIV diagnosis (IQR) in years | 11.0 (8.0–14.0) |
| Mean duration of cART use (± SD) in months | 111.4 ± 49.1 |
| Median CD4+ cells count (IQR) in cells/μL | 407.0 (265.0–583.0) |
| Median HIV viral load (IQR) in copies/mL | 20.0 (0.0–52.0) |
| Mean age at coitarche (± SD) in years | 20.0 ± 3.4 |
| Parity | |
| Nulliparity | 83 (14.0) |
| Multiparity | 510 (86.0) |
| Marital status | |
| Married | 372 (62.7) |
| Unmarried | 221 (37.3) |
| Educational level | |
| Uneducated | 19 (3.2) |
| At least primary education | 77 (13.0) |
| At least secondary education | 293 (49.4) |
| At least tertiary education | 204 (34.4) |
| Use of hormonal contraceptive | |
| Yes | 186 (31.4) |
| No | 407 (68.6) |
| Number of lifetime sexual partners | |
| One | 106 (17.9) |
| More than one | 487 (82.1) |
| Previous STI | |
| Yes | 124 (20.9) |
| No | 469 (79.1) |
| Tobacco smoking | |
| Yes | 22 (3.7) |
| No | 571 (96.3) |
| Consumption of alcoholic beverages | |
| Yes | 129 (21.8) |
| No | 464 (79.2) |
| Cervical cytological abnormalities | |
| Yes | 40 (6.7) |
| No | 553 (93.3) |
Abbreviations: cART, combination antiretroviral therapy; BMI, body mass index; IQR, interquartile range; SD, standard deviation; STI, sexually transmitted infections
Values are given as mean ± SD, median (interquartile range), or frequency (percentage) unless indicated otherwise.
As shown in Figure 1, of the 40 women with cervical cytological abnormalities, 15 (37.5%) had atypical squamous cells of undetermined significance (ASCUS), 10 (25.0%) had low-grade squamous intraepithelial lesions (LSIL), 4 (10.0%) had atypical squamous cells cannot exclude high-grade squamous intraepithelial lesions (ASC-H), and 11 (27.5%) had high-grade squamous intraepithelial lesions (HSIL). In Figure 2, an increasing but no significant difference in the mean ages of enrolled participants was recorded based on the types of cervical precancerous lesions (P=0.33) from LSIL to HSIL. The women were stratified into two groups based on the presence of cervical precancerous lesions in their cervical cytology samples and the associated factors were compared in Table 2.
Figure 1:
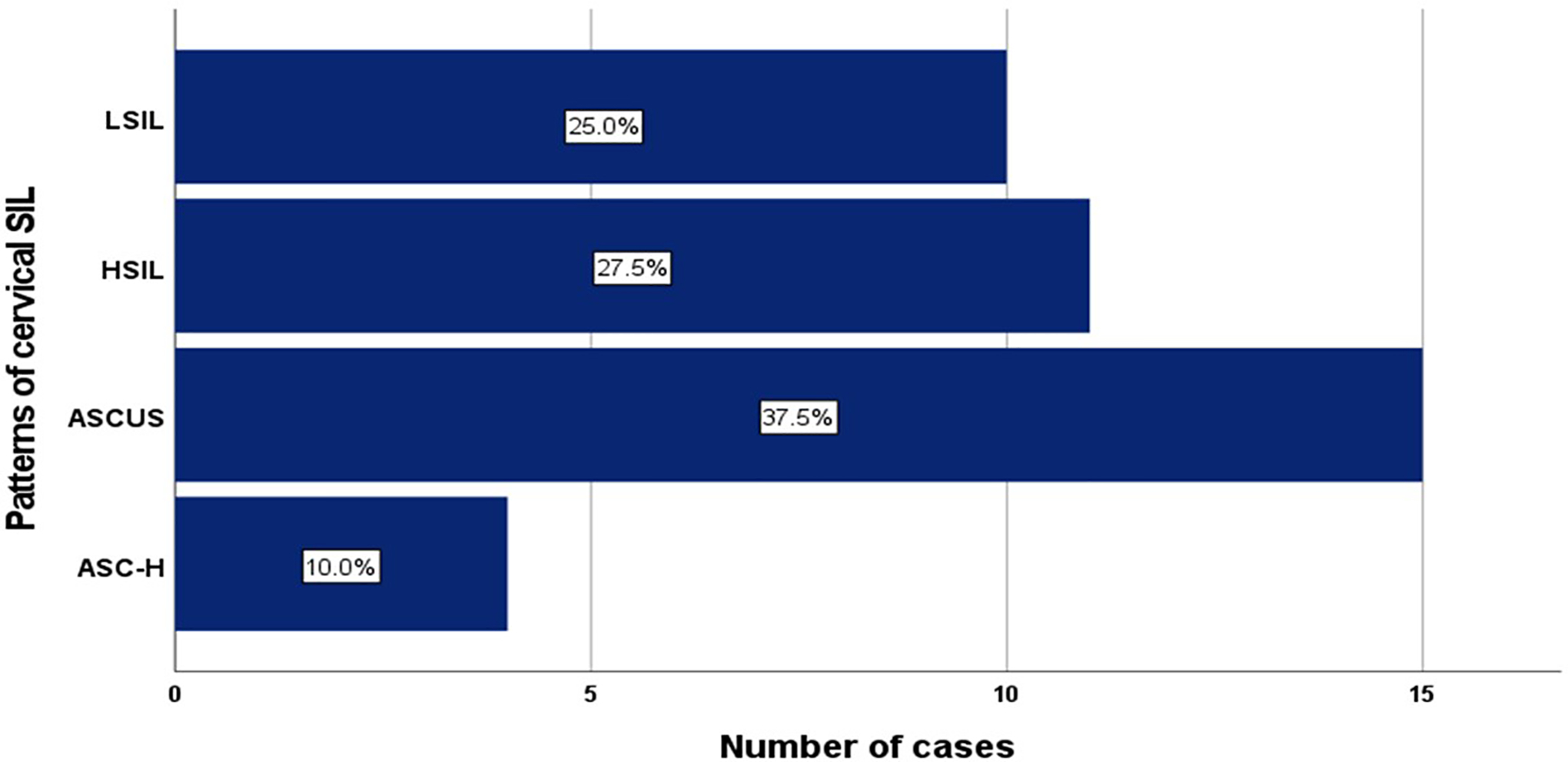
Patterns of cytological abnormalities among HIV-infected women with cervical squamous intraepithelial lesions (SIL).
Figure 2:
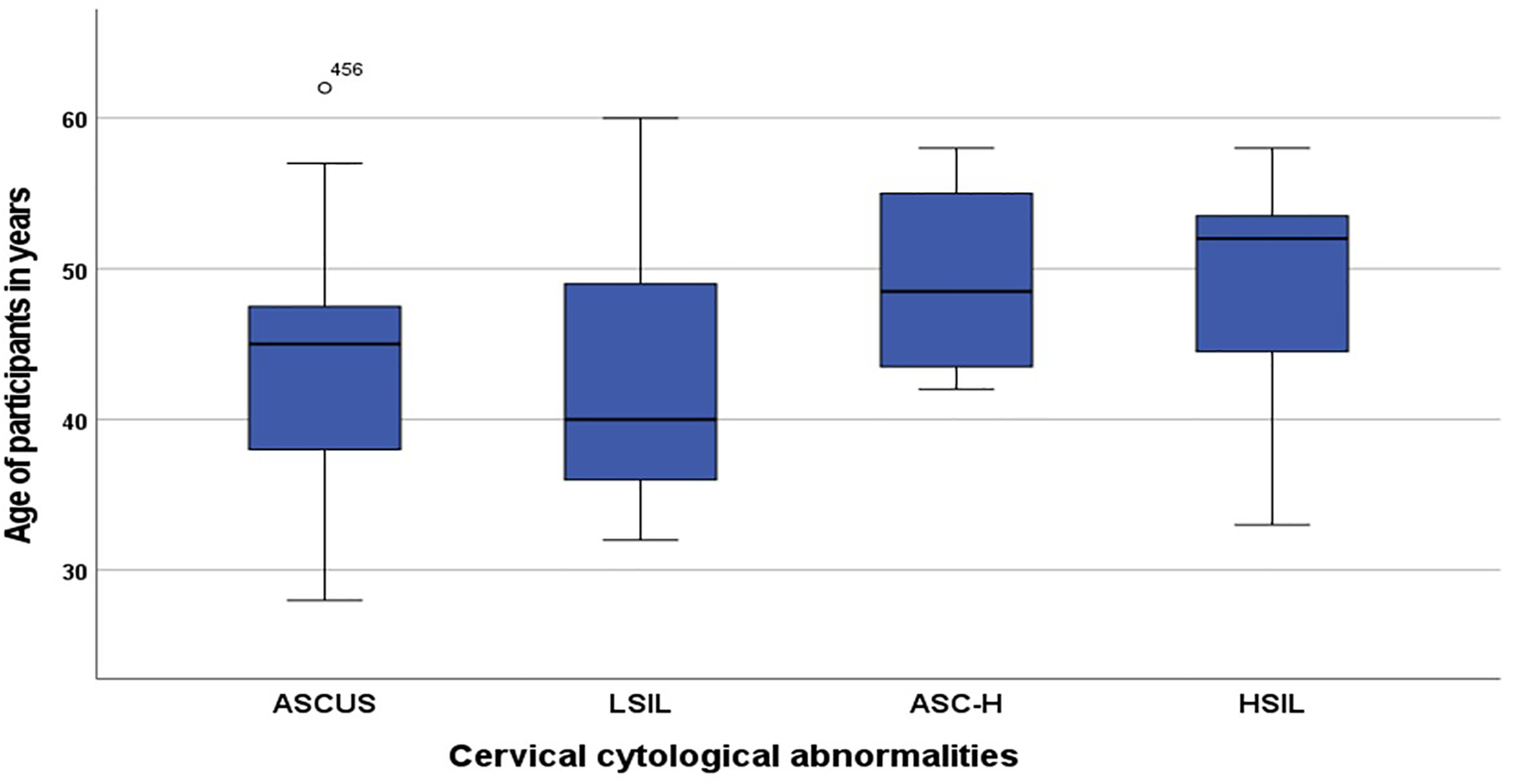
Box plot showing the mean ages of HIV-infected women with ASCUS (43.6±8.8 years), LSIL (43.0±9.6 years), ASC-H (49.3±7.2 years), and HSIL (48.4±7.6 years).
Table 2:
Bivariate analyses of study participants by Pap smear cytology results (n=593).a
| Characteristics | Abnormal Pap smear | P-value | |
|---|---|---|---|
| Yes (n=40) | No (n=553) | ||
| Age at enrolment (± SD) in years | 45.3 ± 8.6 | 43.2 ± 7.9 | 0.09 |
| BMI (± SD) in kg/m 2 | 27.9 ± 4.4 | 27.0 ± 5.3 | 0.31 |
| Overall time since HIV diagnosis (IQR) in years | 11 (8.0 – 15.0) | 11 (8.0 – 13.0) | 0.62 |
| Duration of ART use (± SD) in months | 116.9 ± 55.5 | 111.0 ± 48.6 | 0.46 |
| CD4+ cells count (IQR) in cells/μL | 369.0 (148.0 – 521.0) | 414.0 (266.0 – 591.5) | 0.15 |
| Viral load (IQR) in copies/mL | 20.0 (0.0 – 182.7) | 20.0 (0.0 – 51.0) | 0.35 |
| Age at coitarche (± SD) in years | 18.8 ± 3.1 | 20.1 ± 3.4 | 0.02 |
| Parity | 0.22 | ||
| Nulliparity | 3 (3.6) | 80 (96.4) | |
| Multiparity | 37 (7.3) | 473 (92.7) | |
| Marital status | 0.48 | ||
| Married | 23 (6.2) | 349 (93.8) | |
| Unmarried | 17 (7.7) | 204 (92.3) | |
| Educational level | 0.73 | ||
| Uneducated | 1 (5.3) | 18 (94.7) | |
| At least primary education | 7 (9.1) | 70 (90.9) | |
| At least secondary education | 17 (5.8) | 276 (94.2) | |
| At least tertiary education | 15 (7.4) | 189 (92.6) | |
| Use of hormonal contraceptive | 0.57 | ||
| Yes | 11 (5.9) | 175 (94.1) | |
| No | 29 (7.2) | 376 (92.8) | |
| Number of lifetime sexual partners | 0.62 | ||
| One | 6 (5.7) | 100 (94.3) | |
| More than one | 34 (7.0) | 453 (93.0) | |
| Previous STI | 0.58 | ||
| Yes | 7 (5.6) | 117 (94.4) | |
| No | 33 (7.0) | 436 (93.0) | |
| Tobacco smoking | 0.03 | ||
| Yes | 4 (18.2) | 18 (81.8) | |
| No | 36 (6.3) | 535 (93.7) | |
| Consumption of alcoholic beverages | 0.61 | ||
| Yes | 10 (7.8) | 119 (92.2) | |
| No | 30 (6.5) | 434 (93.5) | |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; IQR, interquartile range; SD, standard deviation; STI, sexually transmitted infections
Values are given as mean ± SD, median (interquartile range), or frequency (percentage) unless indicated otherwise.
Based on a predefined P<0.20, cervical precancer was found to be associated with the participant’s age (P=0.09), the CD4+ cells count (P=0.35), and age at coitarche (P=0.02) and tobacco smoking (P=0.03). However, after adjustments for other factors in the final multivariate analysis, the age of coitarche (Adjusted OR=2.42, 95%CI: 1.21–4.83, P=0.01) was the only factor that was independently associated with the presence of cervical precancerous lesions in HIV-infected women [Table 3].
Table 3:
Multivariate logistic regression of cervical cytological abnormalities and associated factors
| Factors | Presence of cervical cytological abnormalities | P-value | |
|---|---|---|---|
| Adjusted OR | 95%CI | ||
| Age at enrolment in years | 0.33 | ||
| ≥40 | 1.43 | 0.69 – 2.96 | |
| <40 | 1.00 | Reference | |
| CD4+ cells count in cells/μL | 0.35 | ||
| ≥400 | 0.73 | 0.38 – 1.42 | |
| <400 | 1.00 | Reference | |
| Age at coitarche in years | 0.01 | ||
| <20 | 2.42 | 1.21 – 4.83 | |
| ≥20 | 1.00 | Reference | |
| Tobacco smoking | 0.16 | ||
| Yes | 2.34 | 0.73 – 7.57 | |
| No | 1.00 | Reference | |
Abbreviations: CI, confidence interval; OR, odds ratio
Discussion
This cross-sectional study conducted among HIV-infected women in Lagos, Nigeria determined the prevalence, patterns, and associated factors of cervical epithelial abnormalities using a cytology-based screening method. The prevalence of cervical epithelial abnormalities was 6.7% and this was relatively lower than the reported 8.7% by Liu et al in Dar es Salaam, Tanzania [10], 12.2% by Daniel et al in Jos, Nigeria [16], 17.8% by Getinet et al in East Gojjam, Northwest Ethiopia [19] and 29.4% by Branca et al in Rome, Italy [20] that utilized the same screening method among similar populations of WLHIV. The relatively lower prevalence of cervical epithelial abnormalities in our study may reflect the population of women enrolled as the study site is an HIV treatment centre where almost all clinic attendees are already on antiretroviral therapy. Furthermore, the prevalence of cervical cytological abnormalities reflects closely the burden of HIV reported in the geographical settings of these respective studies [21], [22]. The higher prevalence rates of cervical epithelial abnormalities in this study and others [16], [19], [23] compared to the figures reported in the general women population (3.5–4.1%) [24]–[26] comprising predominantly of HIV seronegative women are not unexpected as HIV-infected women are more likely to have persistent oncogenic HPV infections which predispose them to cervical precancers and cancer [2], [4].
There are variations in the patterns of cervical cytological abnormalities reported in this study and that of previous studies. Most of the cytological abnormalities reported in this study were ASCUS (37.5%) unlike in the studies by Daniel et al [16] and Liu et al [10] that reported LSIL as the commonest abnormalities. The higher prevalence of ASCUS in our study may be attributed mostly to the predominantly higher mean age (43.3±8.0 years) of this study cohort who are prone to having atrophic cervix and vagina that largely mimic atypical epithelial changes as commonly found in postmenopausal women [27]. The recorded patterns of cytological abnormalities are however the same as that of the cross-sectional study conducted by Getinet et al in 2014 among HIV-infected women attending gynaecological examination in a cervical cancer screening centre at the Debre Markos referral hospital in Ethiopia [19].
The incidence of cervical precancer and cancer is only marginally affected by the introduction of effective antiretroviral therapy in WLHIV [28] unlike that of other AIDS-defining cancers such as non-Hodgkin’s lymphoma and Kaposi’s sarcoma. This is largely supported by data from HIV/AIDS cohorts in developed countries [28], [29] and thus this reinforced the need to identify women that are mostly at risk of developing cervical precancer and cancer for targeted evidence-based screening in an integrated HIV treatment and reproductive health setting. There are varying results reported in previous studies that examined the association between age and cervical cytological abnormalities among WLHIV. In similarity to our study, findings from the study by Daniel et al in Jos, Nigeria [16] and Kafuruki et al in Mwanza-Tanzania [30] reported no association between age and cervical cytological abnormalities while others conducted by Ononogbu et al in Abuja, Nigeria [31], Kassa et al in Amhara Region, Northwest Ethiopia [32] found that age was a significant predictor for cervical epithelial changes. The variations reported across these various studies including the current study concerning the association between age and cervical epithelial abnormalities could therefore be attributed to the residual effects of other confounding factors which may or may not be adequately controlled for during statistical analyses. CD4+ cell count is a marker of HIV-induced immunosuppression and viral replication and correlates inversely with the risk of acquisition and persistence of high-risk HPV infection, cervical precancer and progression to cancer [23], [33]. This has also been corroborated in several studies conducted among HIV-infected women in sub-Saharan Africa [10], [31], [32]. However, in converse to this proven belief, our current study and the study by Daniel and colleagues [16] conducted in Southwest and North central regions of Nigeria found no association between CD4+ cell counts and cervical precancerous lesions. This, therefore, shows that the interaction effects of HIV immunosuppression and genital high-risk HPV infection on the development of cervical precancer and cancer still deserve further examination, especially in the context of cART.
In similarity to our study, tobacco smoking was not associated with the risk of developing cervical cytological abnormalities in the study conducted by Guarisi et al among a cohort of women in Campinas, Brazil [34]. However, when Guarisi and colleagues restricted their analysis to women with high-grade CIN only, smoking significantly increased the probability of developing the disease [34]. Among behavioural factors weakening the immune system, smoking appeared to strongly increase the risk of cervical precancer and cancer [35]–[37], however, there is an interplay of multiple factors concerning smoking and cervical carcinogenesis, especially by direct local carcinogenic effect and local immunosuppression [32]. It is therefore almost always difficult to isolate the epidemiologic contribution of smoking to cervical carcinogenesis as confirmed in our current study and other recent studies that found no independent correlation between smoking and cervical precancer and/or cancer [16], [32]. Nonetheless, smoking should always be considered in clinical practice and research concerning cervical precancer and cancer.
The mean age at coitarche reported in our study (20.0±3.4 years) is higher than the average age recorded in most industrialized parts of the world [38], [39]. The later age of coitarche recorded in our study may be attributed mostly to the societal premium placed on delaying sexual intercourse before marriage among most ethnic groups in Nigeria. Furthermore, in agreement with the study conducted by Getinet et al in East Gojjam, Northwest Ethiopia that identified earlier initiation of first sexual contact before age 15 years as a significant risk factor for the development of epithelial cervical abnormalities [19], our study also illustrated that women whose age was less than 20 years at their first sexual intercourse were 2.42 times more likely to have precancerous cervical lesions compared to those whose age at first sexual intercourse was at or after 20 years of age. This is probably because women who experience sexual debut very early in life may be at greater risk of exposure to high-risk HPV infection [32]. This finding was, however, at variance with the studies conducted in various parts of Africa by Daniel et al in Jos, Nigeria [16], Ononogbu et al in Abuja, Nigeria [31], Liu et al in Dar es Salaam, Tanzania [10] and Kassa et al in Amhara, Ethiopia [32].
We have a few limitations in this study. First, as this was a cross-sectional study, it was not possible to infer any cause-and-effect relationships. Secondly, there was a chance that some women did not divulge accurate information about their sexual behaviour due to the inherent social desirability bias in society. Thirdly, as this is a cytology-based study, we did not include data on the gold standard diagnosis of cervical precancerous lesions using biopsy and histology that could have confirmed the results of the Pap smear cytological abnormalities. Finally, the study site is an HIV treatment centre where all the enrolled women were on combination ART and thus our findings in the study may not be representative of HIV-infected women who are not on therapy or those who are HIV-negative. However, this study is the first step in generating the hypothesis for a well-designed and adequately powered longitudinal study necessary to confirm the findings of this study.
Conclusions
We found a relatively lower prevalence of cervical precancerous lesions among HIV-infected women in this study compared to results from some other studies conducted within and outside the country. In addition, we found that the initiation of sexual intercourse at an early age was associated with higher odds of cervical precancerous lesions. It is, however, necessary to further confirm the findings of this study through a well-designed and adequately powered longitudinal study using histology as the gold standard diagnostic method for cervical precancerous lesions. Meanwhile, as resources are mostly limited with reduced capacity to provide cervical cancer prevention services to all HIV-infected women in settings such as ours, greater efforts should be made in the interim to identify and scale up screening services in women who initiated sexual intercourse at a relatively younger age.
Acknowledgements
We thank all the participating women and staff of the HIV treatment clinic of the hospital who have contributed to this study. We also appreciate Professor Jian-Jun Wei and Professor Jorge E. Novo of the Department of Pathology, Northwestern University, Chicago, Illinois for their expert support in the cytopathology review.
Funding Sources
The research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number U54CA221205. The protected time for the contribution of Kehinde S. Okunade towards the research reported in this publication was supported by the National Cancer Institute and Fogarty International Center of the National Institutes of Health under Award Numbers K43TW011930, D43TW010934 and D43TW010543. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute, Fogarty International Center, or the National Institutes of Health.
Footnotes
Statement of Ethics
This study protocol was reviewed and approved by the Health Research Ethics Committee of the Lagos University Teaching Hospital with approval number ADM/DSCST/HREC/APP/5204. The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. All study participants gave their written informed consent before enrolment and strict adherence to the privacy and confidentiality of participants’ information were ensured during and after the conduct of the study.
Conflicts of Interest
The authors declare that they have no competing interests.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
- 1.Okunade KS. Human papillomavirus and cervical cancer. Journal of Obstetrics and Gynaecology 2020; 40(5): 602–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Branca M, Garbuglia AR, Benedetto A, Cappiello T, Leoncini L, Migliore G, et al. Factors predicting the persistence of genital human papillomavirus infections and PAP smear abnormality in HIV-positive and HIV-negative women during prospective follow-up. Int J STD AIDS. 2003; 14(6): 417–25. [DOI] [PubMed] [Google Scholar]
- 3.Clifford GM, Gonçalves MAG, Franceschi S and HPV and HIV Study Group. Human papillomavirus types among women infected with HIV: a meta-analysis. AIDS 2006: 20(18): 2337–44. [DOI] [PubMed] [Google Scholar]
- 4.Sun XW, Kuhn L, Ellerbrock TV, Chiasson MA, Bush TJ, Wright TC. Human papillomavirus infection in women infected with the human immunodeficiency virus. The New England Journal of Medicine 1997; 337(19): 1343–9. [DOI] [PubMed] [Google Scholar]
- 5.de Araujo Souza PS, Villa LL. Genetic susceptibility to infection with human papillomavirus and development of cervical cancer in women in Brazil. Mutation Research 2003; 544(2–3): 375–83. [DOI] [PubMed] [Google Scholar]
- 6.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians 2021; 71(3): 209–49. [DOI] [PubMed] [Google Scholar]
- 7.Chua KL, Hjerpe A. Persistence of human papillomavirus (HPV) infections preceding cervical carcinoma. Cancer 1996; 77(1): 121–27. [DOI] [PubMed] [Google Scholar]
- 8.Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, et al. The Global Burden of Cancer 2013. JAMA Oncol 2015; 1(4): 505–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghebre RG, Grover S, Xu MJ, Chuang LT, Simonds H. Cervical cancer control in HIV-infected women: Past, present and future. Gynecologic Oncology Reports 2017; 21: 101–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu E, McCree R, Mtisi E, Fawzi WW, Aris E, Lema IA, et al. Prevalence and risk factors of cervical squamous intraepithelial lesions among HIV-infected women in Dar es Salaam, Tanzania. International Journal of STD & AIDS 2016; 27(3): 219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franceschi S, Ronco G. The prevention of cervical cancer in HIV-infected women. AIDS 2010; 24(16): 2579–80. [DOI] [PubMed] [Google Scholar]
- 12.Mwanahamuntu MH, Sahasrabuddhe VV, Kapambwe S, Pfaendler KS, Chibwesha C, Mkumba G, et al. Advancing cervical cancer prevention initiatives in resource-constrained settings: insights from the Cervical Cancer Prevention Program in Zambia. PLoS Medicine 2011; 8(5): e1001032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Branca M, Rossi E, Alderisio M, Migliore G, Morosini PL, Vecchione A, et al. Performance of cytology and colposcopy in diagnosis of cervical intraepithelial neoplasia (CIN) in HIV-positive and HIV-negative women. Cytopathology. 2001; 12(2): 84–93. [DOI] [PubMed] [Google Scholar]
- 14.Musa J, Kim K, Zheng Y, Qu Y, Joyce BT, Wang J, et al. Accelerated Epigenetic Age Among Women with Invasive Cervical Cancer and HIV-Infection in Nigeria. Front Public Health. 2022; 10: 834800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fontham ETH, Wolf AMD, Church TR, Etzioni R, Flowers CR, Herzig A, et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA: A Cancer Journal for Clinicians 2020; 70(5): 321–46. [DOI] [PubMed] [Google Scholar]
- 16.Daniel GO, Musa J, Akindigh TM, Shinku F, Shuaibu SI, Kwaghe B, et al. Prevalence and predictors of precancerous cervical lesions among HIV-positive women in Jos, north-central Nigeria. International Journal of Gynaecology and Obstetrics 2020; 151(2): 253–9. [DOI] [PubMed] [Google Scholar]
- 17.Okunade KS, Nwogu CM, Oluwole AA, Anorlu RI. Prevalence and risk factors for genital high-risk human papillomavirus infection among women attending the outpatient clinics of a university teaching hospital in Lagos, Nigeria. Pan African Medical Journal 2017; 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Getinet M, Gelaw B, Sisay A, Mahmoud EA, Assefa A. Prevalence and predictors of Pap smear cervical epithelial cell abnormality among HIV-positive and negative women attending gynecological examination in cervical cancer screening center at Debre Markos referral hospital, East Gojjam, Northwest Ethiopia. BMC Clin Pathol 2015; 15: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nayar R, Wilbur DC. The Pap test and Bethesda 2014. Cancer Cytopathology 2015; 123(5): 271–81. [DOI] [PubMed] [Google Scholar]
- 20.Branca M, Migliore G, Giuliani M, Leoncini L, Ippolito G, Cappiello G, et al. Squamous intraepithelial lesions (SILs) and HPV associated changes in HIV infected women or at risk of HIV. DIANAIDS Cooperative Study Group. Eur J Gynaecol Oncol. 2000; 21(2): 155–9. [PubMed] [Google Scholar]
- 21.USAID 2020. Tanzania HIV/AIDS Fact Sheet [Cited July 29, 2022]. Available from: https://www.usaid.gov/sites/default/files/documents/Tz-HIV-AIDS_Fact_Sheet_Sep_2020.pdf
- 22.O’Brien-Carelli C, Steuben K, Stafford KA, Aliogo R, Alagi M, Johanns CK, et al. Mapping HIV prevalence in Nigeria using small area estimates to develop a targeted HIV intervention strategy. PLOS ONE 2022; 17(6): e0268892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu G, Sharma M, Tan N, Barnabas RV. HIV-positive women have higher risk of human papillomavirus infection, precancerous lesions, and cervical cancer. AIDS 2018; 32(6): 795–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Obure J, Olola O, Swai B, Mlay P, Masenga G, Walmer D. Prevalence and severity of cervical squamous intraepithelial lesion in a tertiary hospital in northern Tanzania. Tanzania Journal of Health Research 2009; 11(4): 163–9. [DOI] [PubMed] [Google Scholar]
- 25.Agaba PA, Thacher TD, Ekwempu CC, Idoko JA. Cervical dysplasia in Nigerian women infected with HIV. International Journal of Gynecology & Obstetrics 2009; 107(2): 99–102. [DOI] [PubMed] [Google Scholar]
- 26.Mbazor JO, Umeora OUJ, Egwuatu VE. Cervical cytology profile of infertility patients in Abakaliki, South-eastern Nigeria. Journal of Obstetrics and Gynaecology 2011; 31(2): 173–7. [DOI] [PubMed] [Google Scholar]
- 27.Bruno MT, Coco A, di Pasqua S, Bonanno G. Management of ASC-US/HPV positive post-menopausal woman. Virology Journal 2019; 16(1): 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mbulaiteye SM, Bhatia K, Adebamowo C, Sasco AJ. HIV and cancer in Africa: mutual collaboration between HIV and cancer programs may provide timely research and public health data. Infectious Agents and Cancer 2011; 6(1): 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel P, Hanson DL, Sullivan PS, Novak RM, Moorman AC, Tong TC, et al. Incidence of Types of Cancer among HIV-Infected Persons Compared with the General Population in the United States, 1992–2003. Annals of Internal Medicine 2008; 148(10): 728. [DOI] [PubMed] [Google Scholar]
- 30.Kafuruki L, Fabian Rambau P, Massinde A, Masalu N. Prevalence and predictors of Cervical Intraepithelial Neoplasia among HIV infected women at Bugando Medical Centre, Mwanza-Tanzania. Infectious Agents and Cancer 2013; 8(1): 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ononogbu U, Almujtaba M, Modibbo F, Lawal I, Offiong R, Olaniyan O. Cervical cancer risk factors among HIV-infected Nigerian women. BMC Public Health 2013; 13(2013): 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kassa LS, Dile WM, Zenebe GK, Berta AM. Precancerous lesions of the cervix among women infected with HIV in Referral Hospitals of Amhara Region, Northwest Ethiopia: a cross-sectional study. Afri Health Sci 2019; 19(1): 1695–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mondatore D, Bai F, Augello M, Giovenzana M, Ceretti AP, Bono V, et al. Persistence of High Percentage of Peripheral Activated CD8+ T Cells Predict Cytologic HPV-Related Dysplasia in cART-Treated, HIV-Positive Subjects. Open Forum Infectious Diseases 2022; 9(4): ofac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guarisi R, Sarian LO, Hammes LS, Longatto-Filho A, Derchain SF, Roteli-Martins C, et al. Smoking worsens the prognosis of mild abnormalities in cervical cytology. Acta Obstet Gynecol Scand. 2009; 88(5): 514–20. [DOI] [PubMed] [Google Scholar]
- 35.Roura E, Castellsagu X, Pawlita M, Waterboer T, Uria Margall N, Bosch FX, et al. Smoking as a major risk factor for cervical cancer and pre-cancer: Results from the EPIC cohort. International Journal of Cancer 2014; 17: 453–66. [DOI] [PubMed] [Google Scholar]
- 36.Fonseca-Moutinho JA. Smoking and cervical cancer. ISRN Obstet Gynecol. 2011; 2011: 847684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dugué P-A, Rebolj M, Garred P, Lynge E. Immunosuppression and risk of cervical cancer. Expert Review of Anticancer Therapy 2013; 13(1): 29–42. [DOI] [PubMed] [Google Scholar]
- 38.Hansen BT, Kjaer SK, Arnheim-Dahlström L, Liaw K-L, Juul KE, Thomsen LT, et al. Age at first intercourse, number of partners and sexually transmitted infection prevalence among Danish, Norwegian and Swedish women: estimates and trends from nationally representative cross-sectional surveys of more than 100 000 women. Acta Obstet Gynecol Scand 2020; 99: 175–85. [DOI] [PubMed] [Google Scholar]
- 39.MacQuarrie KLD, Mallick L, Allen C. Sexual and Reproductive Health in Early and Later Adolescence: DHS Data on Youth Age 10–19. DHS Comparative Reports No. 45. 2017. Rockville, Maryland, USA: ICF. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.


