Abstract
Osteoid osteoma is a benign bone tumor commonly occurring in the diaphysis and metaphysis of long bones. Only a few cases were reported in the literature about the rare location of epiphyseal osteoid osteoma and all were treated surgically. Herein, we report a rare case of an epiphyseal tibial osteoid osteoma, in a 14-year-old boy, which was initially diagnosed by imaging and confirmed by histopathology. To the best of our knowledge, this is the first case of an epiphyseal osteoid osteoma treated successfully by CT-guided radiofrequency ablation in a pediatric patient with a good outcome and no detrimental effects. The case highlights the rarity of such presentation, the importance of early imaging and diagnosis, and the success of CT-guided radiofrequency ablation in the treatment of epiphyseal osteoid osteoma.
Keywords: Osteoid osteoma, CT-guided radiofrequency ablation, Pediatric radiology, Interventional radiology, Orthopedic surgery
Introduction
Osteoid osteoma is an idiopathic benign tumor-like growth in the bone, usually occurring in the second and third decades of life. They usually present with dull and aching pain that is worse at night and is relieved by salicylates [1]. They commonly occur in the diaphysis and metaphysis of long bones and only rarely in the epiphysis, with only a few cases reported in the literature [2,3]. Intraepiphyseal and intra-articular location of osteoid osteoma can lead to atypical presentation and atypical radiographic findings which further delay the diagnosis and proper treatment [1]. Herein, we report a case of a 14-year-old boy presenting with left knee pain of 3 months duration, who was diagnosed with left tibial epiphyseal osteoid osteoma after computed tomography (CT) scan and magnetic resonance imaging (MRI) of the left knee. He then underwent CT guided Tru-cut bone biopsy confirming the diagnosis by histopathology and subsequently successful treatment and complete remission by CT-guided radiofrequency ablation.
Case presentation
A 14-year-old boy, not known case of any medical illness, presented to the orthopedic department outpatient clinic complaining of left knee pain of 3 months duration. It started suddenly, becoming worse in the evening and is relieved with 200 mg of Ibuprofen. There is no history of trauma or any previous surgery. Initial examination and investigations revealed normal body temperature, white blood cell count, blood hemoglobin levels, platelets, and hematocrit. Physical examination revealed mild tenderness in the medial aspect of the distal left femur and proximal left tibia with minimal limitation of the left knee joint range of motion.
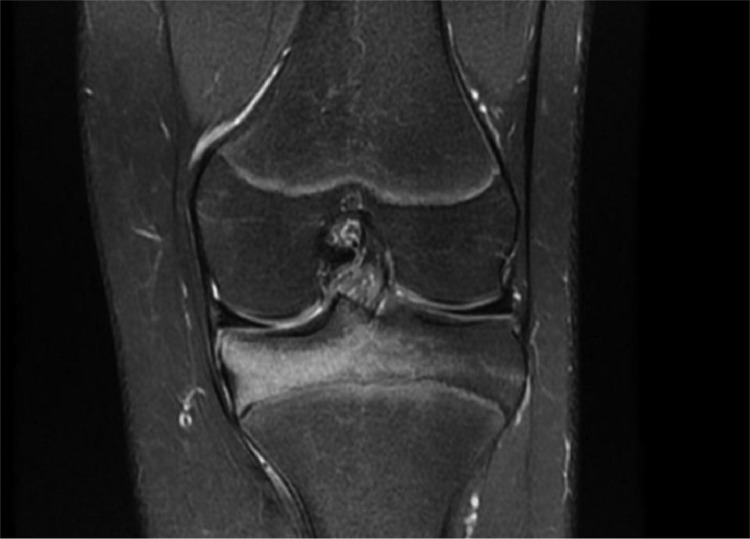
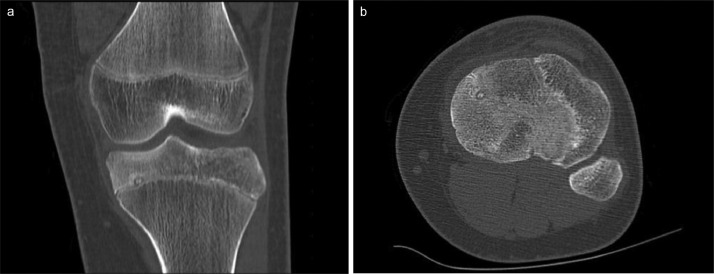
Plain radiography of the left knee, which was done initially, was unremarkable. MRI proton density (PD) weighted fat suppressed (FS) images shows extensive bone marrow hyperintensities indicating bone marrow edema at the medial tibial epiphysis with small round hypointense area abutting the growth plate (Fig. 1). CT scan of the left knee revealed a small focal 5 mm lucent nidus with central area of sclerosis and subtle surrounding sclerosis (Figs. 2A and B). These features are highly suggestive of osteoid osteoma.
Fig. 1.
MRI of the left knee PD FS showing extensive bone marrow hyperintensities indicating bone marrow edema at the left medial tibial epiphysis.
Fig. 2.
CT scan of the left knee at the coronal (A) and axial (B) shows 5 mm lucent nidus with central area of sclerosis and subtle surrounding sclerosis.
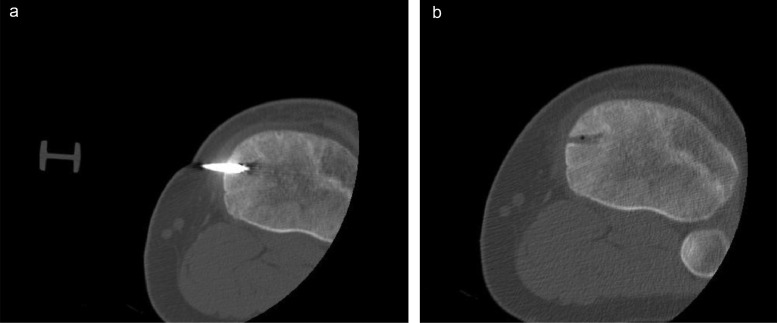
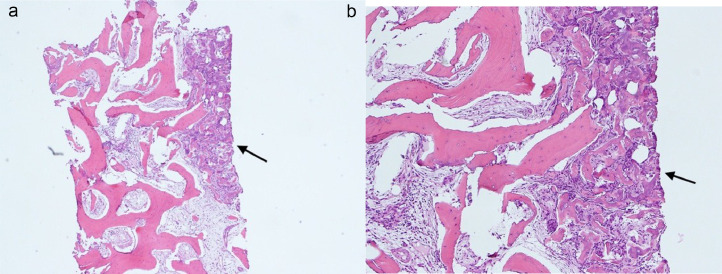
The patient then underwent CT-guided Tru-cut biopsy followed by radiofrequency ablation of the lesion under general anesthesia by the interventional radiology team (Figs. 3A and B). The histopathology report showed a sclerosed nidus of mixed irregular anastomosing bony trabeculae and woven bone with variable mineralization. The bony trabeculae are lined by osteoblasts and in places by multinucleated osteoclasts too. The lesional stroma is formed by a dense, variably vascular fibrous tissue. No atypia is seen (Figs. 4A and B).
Fig. 3.
Knee biopsy and radiofrequency ablation probe inserted in the nidus (A). The images were taken post radiofrequency ablation showing complete elimination of the nidus (B).
Fig. 4.
(A and B) Histopathology slides showing the sclerosed nidus (arrow) of mixed irregular anastomosing bony trabeculae and woven bone with variable mineralization. The bony trabeculae are lined by osteoblasts and in places by multinucleated osteoclasts too. The lesional stroma is formed by a dense, variably vascular fibrous tissue.
Upon follow-up, the patient and his father reported significantly reduced pain scores, with complete resolution of pain after 8 months with no limping. He is resuming normal activities of daily living with normal ambulation and was discharged from our service.
Discussion
Definition, epidemiology, and classification of osteoid osteoma
Osteoid osteoma is a benign osteoblastic skeletal tumor first described as a neoplasm in 1953 by Henry L. Jaffe, differentiating it from other tumors [4]. Histopathologically, osteoid osteoma consists of a center or “nidus” of highly vascularized and innervated immature osteoid, enclosed by a rim of osteoblasts and surrounded by thickened trabecular bone [5]. They typically occur in the second and third decades of life and account for 10%-14% of all benign tumors and 2%-3% of all primary bone tumors [6]. Males are more commonly affected with an approximate male:female ratio of 2:1 [7]. The appendicular skeleton is the commonest location of osteoid osteoma with the lower extremities more frequently affected than the upper extremities. It can occur rarely in the axial skeleton mainly affecting the spine [8]. Osteoid osteomas are classified, according to location of the nidus, as intracortical, subperiosteal, endosteal, and medullary [9]. The commonest type is intracortical lesions (75%), followed by medullary (20%), subperiosteal and endosteal lesions (both accounting for 5%) [10]. They usually occur in the diaphysis and metaphysis of long bones. The occurrence of osteoid osteoma in epiphysis of long bones is rare, as is the case with our patient, with very few reports in the literature [2].
Clinical presentation of osteoid osteoma
The typical clinical presentation of osteoid osteomas is dull, aching pain at the site of involvement, typically worsened at night. The pain is relieved by nonsteroidal anti-inflammatory agents and salicylates which is attributed to the inhibition of the release of prostaglandin E2 and prostacyclins by the tumor. Intra-articular osteoid osteoma might also present with decreased range of motion as well as gait or growth disturbances. If the lesion involves a bone in the subcutaneous region, then the patient usually presents with swelling, erythema, and tenderness. Furthermore, lesions within the joint can also present with synovitis, joint pain, flexion contracture, decreased range of motion, and antalgic gait or limping. In pediatric cases, if the lesion involves the open physis, then limb length discrepancy may be the presentation with coronal and sagittal malalignment [1,5,11,12].
Imaging modalities of osteoid osteoma
The most important imaging finding is the nidus. It is crucial to identify the nidus and assess the nidus mineralization, size, location, and surrounding sclerosis. A nidus size of less than 15 mm is highly suggestive of osteoid osteoma. A nidus size of more than 15 mm should prompt the radiologist to consider other differential diagnosis. Plain radiograph may show an oval, lytic lesion within dense cortical bone surrounded by bone thickening and sclerosis. If the nidus is present at the subperiosteal region, then it is a rounded sclerotic focus elevating the periosteum with limited sclerotic reactions. If located intramedullary, then the tumor is well-circumscribed with complete or partially calcified nidus and minimal or absent surrounding sclerosis [12].
Nonenhanced CT scan is the best imaging modality for diagnosis, further assessment of the location of the nidus and estimation of the surrounding sclerosis. It shows the well-defined nidus, which is round or oval with low attenuation, with varying surrounding reactive sclerosis [12]. On the other hand, MRI is more sensitive to detect reactive changes in soft tissue. On MRI, the nidus shows T1-weighted slightly hyperintense to isointense signal and T2-weighted variable intensity compared to the adjacent normal muscle. The nidus can be hypointense in all sequences depending on its vascularity and mineralization. The surrounding sclerosis appears as low signal on both T1- and T2-weighted sequences. Moreover, T2-weighted short Tau inversion recovery sequence is useful in estimating the surrounding perifocal bone edema. Contrast-enhanced CT and MRI are not necessary but increase the diagnostic accuracy in indeterminate cases as they show rapid early arterial enhancement of the nidus [5,12]. Technetium (Tc) 99 bone scintigraphy findings show high central uptake with surrounding lesser uptake, commonly termed as “double density” sign. Tc 99 scintigraphy is very highly sensitive for the detection of osteoid osteomas and negative results warrant MRI evaluation to look for other causes. In cases where the nidus is not visible or in the presence of equivocal findings, a biopsy should be performed for histopathologic confirmation [5,13].
Differential diagnosis of osteoid osteoma
The main differential diagnosis of epiphyseal osteoid osteoma is bone infarction, chronic osteomyelitis (Brodie abscess), enchondroma and chondroblastoma [12,14]. The main differentials to be excluded are other primary bone tumors that may mimic osteoid osteoma. Chondroblastoma is an uncommon osseous neoplasm, accounting for less than 1% of bone tumors. Radiographically, chondroblastoma can be distinguished from other bony lesions as it presents with an eccentrically or centrally located lesion with smooth or lobulated margins and thin sclerotic rim involving the epiphysis or other secondary ossification centers [15]. Enchondromas can highly mimic osteoid osteoma presenting with osteolytic lesions with well demarcated border and specks of calcification. They may show single or multiple niduses [16]. The differentials are excluded based on radiological findings. As aforementioned, equivocal cases warrant additional imaging techniques such as MRI and radionuclide bone images. A biopsy should be performed in these cases to confirm the diagnosis.
Treatment of osteoid osteoma
The treatment of osteoid osteoma can be classified into medical, percutaneous image-guided treatment and surgical resection. A multidisciplinary management team comprising of interventional radiologist, orthopedic surgeon, anesthesiologist specialized in pain medicine, specialized radiology technologist and nursing staff should be involved to provide the best quality of care to osteoid osteoma patients. The main medical management is the use of nonsteroidal anti-inflammatory drugs for pain relief. However, the prolonged use of nonsteroidal anti-inflammatory drugs can lead to inadequate clinical response, renal, and gastro-intestinal side effects and eventually drug tolerance. To achieve complete symptomatic relief, surgical excision by en bloc resection should involve complete resection of the nidus, which is usually difficult, and the surgery invariably involves the resection of surrounding sclerotic bone for complete excision. This results in bone weakening, especially in the pediatric population, resulting in the necessity of bone grafts, further surgeries, and increased patient morbidity. The gold standard of treatment has become percutaneous image-guided procedures such as CT-guided radiofrequency ablation that we opted for in our patient. The procedures are either done under CT or MRI guidance allowing for accurate localization of the nidus for clinical relief of symptoms, without affecting the bone strength. CT guidance is superior to other imaging modalities due to excellent and rapid visualization of the bones. The eradication of the nidus is done by either physical destruction with trephine needle or bone drill, ethanol ablation, or thermal ablation. Moreover, thermal ablation is done either by radiofrequency ablation, microwave ablation, or laser ablation [5,12,17].
CT-guided radiofrequency ablation as the gold standard treatment of osteoid osteoma
CT-guided radiofrequency ablation, first described in 1989, is a percutaneous, minimally invasive, rapid procedure that uses thermal ablation. It has outcomes comparable to surgery and is more feasible in locations that are difficult to access, like the acetabulum [5,12,17,18]. The mechanism of action is by passing high-frequency alternating current (>50 KHz) from a radiofrequency generator into the patient's body through a noninsulated delivery probe. This results in increased ionic energy in the body tissues and subsequent increased ionic vibrations with loss of energy as heat or thermal energy. The thermal energy generated in the adjacent tissue induces tissue necrosis resulting in tissue ablation. The procedure is performed in the CT room under aseptic conditions. Local anesthesia is sufficient to control the pain; however, the use of general, spinal, or epidural anesthesia should be discussed with the anesthesiologist. CT scan is obtained to precisely locate the lesion for percutaneous approach. A percutaneous cannula with inner stylet is introduced through the skin and soft tissue to the lesion on the bone surface. Once the position is ensured with CT imaging, the Kirshner wire is inserted in place of the stylet, and once within the nidus, it is replaced by electrode that is connected to the radiofrequency ablator. In cases where the lesion is peri-articular, like our patient, a minimum of 10 mm distance between the needle tip and the adjacent cartilage is recommended. Moreover, in cases of osteoid osteomas of subcutaneous bones like the tibia, a minimum distance of 1 cm from the skin and neurovascular bundles should be followed to avoid skin burn and iatrogenic injury. After the procedure, the patients can undergo weightbearing and can be discharged from the hospital on the same day with follow-up. Rare postprocedure complications include skin burn, muscular hematoma, infections, and nerve injury [5,18].
Follow-up and prognosis after treatment
A complete pain relief is indicative of the treatment success. Absence of pain 2 years after the procedure is considered a clinical success. Incomplete ablation should be suspected in patients with incomplete pain relief. There are other radiographic findings suggesting complete ablation including the infilling of the nidus and normalization of the bone density, and these can be seen in 2-27 months after treatment. Further follow-up imaging is usually done by MRI, which is preferred over CT. Follow-up MRI images show resolution of the bone edema, peri-lesional synovial reaction, presence of bone remodeling, and the “ring sign.” The ring sign is the presence of central hypointensity indicating necrosis with surrounding hyperintense rim denoting demarcation zone between the healthy and necrotic tissue. This sign indicates ablation within the tissue and usually is seen up to 6 months [5].
Conclusion
In summary, we reported a rare case of tibial epiphyseal osteoid osteoma treated successfully with CT-guided radiofrequency ablation. The case highlights the rarity of such presentation and emphasizes the importance of proper diagnosis and the success of CT-guided radiofrequency ablation. In our case, CT-guided radiofrequency ablation resulted in complete resolution of the patient's symptoms without side effects like limping or difficulty with ambulation. Our study provides the impetus for further studies to confirm the superiority of CT-guided radiofrequency ablation especially in the case of epiphyseal osteoid osteomas where surgical resection can lead to prolonged side effects like limping and limb length discrepancy.
Patient consent
An informed consent was obtained from the patient's father, consented for publishing patient anonymous case details, imaging data, without personal images or identifiable data. The approval for publication was granted from our institute.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Davis J. Intraepiphyseal Osteoid Osteoma of Proximal Tibial Epiphysis Treated by En bloc Excision under CT Guidance: A Case Report. J Orthop Oncol 7:155. doi: 10.4172/2472-016X.1000155. Available at: https://www.omicsonline.org/open-access/intraepiphyseal-osteoid-osteoma-of-proximal-tibial-epiphysis-treated-by-en-bloc-excision-under-ct-guidance-a-case-report-117882.html [DOI]
- 2.Light J., Retrouvey M., Conran R.M. Vol. 8. 2021. Educational case: osteoid osteoma. (Acad Pathol). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villas C, Garbayo AJ, Martínez Denegri J, Cañadell J. Osteoma osteoide epifisario [Epiphyseal osteoid osteoma] Rev Med Univ Navarra. 1990;34(4):191–193. https://pubmed.ncbi.nlm.nih.gov/2152745/ [PubMed] [Google Scholar]
- 4.Jaffe H.L. Osteoid-osteoma: a benign osteoblastic tumor composed of osteoid and atypical bone. Arch Surg. 1935;31(5):709–728. [Google Scholar]
- 5.Singh D.K., Katyan A., Kumar N., Nigam K., Jaiswal B., Misra R.N., et al. CT-guided radiofrequency ablation of osteoid osteoma: established concepts and new ideas. Br J Radiol. 2020 Oct 1;93(1114) doi: 10.1259/bjr.20200266. [DOI] [PMC free article] [PubMed] [Google Scholar]; Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7548372/#b5.
- 6.Zhang Y, Rosenberg AE. Bone-forming tumors. Surg Pathol Clin. 2017;10(3):513–535. doi: 10.1016/j.path.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Ward WG, Eckardt JJ, Shayestehfar S, Mirra J, Grogan T, Oppenheim W. Osteoid osteoma diagnosis and management with low morbidity. Clin Orthop Relat Res. 1993;291:229–235. [PubMed] [Google Scholar]
- 8.Bednar MS, Weiland AJ, Light TR. Osteoid osteoma of the upper extremity. Hand Clin. 1995;11(2):211–221. [PubMed] [Google Scholar]
- 9.Kayser F, Resnick D, Haghighi P, Pereira Edo R, Greenway G, Schweitzer M, et al. Evidence of the subperiosteal origin of osteoid osteomas in tubular bones: analysis by CT and MR imaging. AJR Am J Roentgenol. 1998;170(3):609–614. doi: 10.2214/ajr.170.3.9490939. [DOI] [PubMed] [Google Scholar]
- 10.Bhure U, Roos JE, Strobel K. Osteoid osteoma: multimodality imaging with focus on hybrid imaging. Eur J Nucl Med Mol Imaging. 2019;46(4):1019–1036. doi: 10.1007/s00259-018-4181-2. [DOI] [PubMed] [Google Scholar]
- 11.Endo RR, Gama NF, Nakagawa SA, Tyng CJ, Chung WT, Pinto FFE. Osteoid osteoma: radiofrequency ablation treatment guided by computed tomography: a case series. Rev Bras Ortop Engl Ed. 2017;52:337–343. doi: 10.1016/j.rboe.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noordin S, Allana S, Hilal K, Nadeem N, Lakdawala R, Sadruddin A, et al. Osteoid osteoma: contemporary management. Orthop Rev. 2018;10:7496. doi: 10.4081/or.2018.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Türkölmez S, Cayir D, Korkmaz M. Osteoid osteoma simulating osteomyelitis: differentiation with Tc-99m hig scintigraphy. Ann Nucl Med. 2006;20:217–220. doi: 10.1007/BF03027433. [DOI] [PubMed] [Google Scholar]
- 14.Becce F., Theumann N., Rochette A., Larousserie F., Campagna R., Cherix S. Osteoid osteoma and osteoid osteoma-mimicking lesions: biopsy findings, distinctive MDCT features and treatment by radiofrequency ablation. Eur Radiol. 2010;20(10):2439–2446. doi: 10.1007/s00330-010-1811-x. [DOI] [PubMed] [Google Scholar]
- 15.Alkadumi M, Duggal N, Kaur S, Dobtsis J. Chondroblastoma of the knee in a teenager. Radiol Case Rep. 2021;16(12):3729–3733. doi: 10.1016/j.radcr.2021.08.065. Erratum in: Radiol Case Rep. 2023 Jan 24;18(4):1645-1646. PMID: 34630808PMCID: PMC8493501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao X, Ren Q, Li X, Tian Y, Wang Z. Epiphyseal enchondroma masking as osteoid osteoma: a case report. Eur J Med Res. 2021;26(1):42. doi: 10.1186/s40001-021-00504-y. Erratum in: Eur J Med Res. 2021 Jun 13;26(1):53. PMID: 33962677PMCID: PMC8106184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Çakar M, Esenyel CZ, Seyran M, Çağrı TA, Adaş M, Bayraktar MK, et al. Osteoid osteoma treated with radiofrequency ablation. Adv Orthop. 2015;2015:1–5. doi: 10.1155/2015/807274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tillotson C.L., Rosenberg A.E., Rosenthal D.I. Controlled thermal injury of bone: report of a percutaneous technique using radiofrequency electrode and generator. Investig Radiol. 1989;24(11):888–892. doi: 10.1097/00004424-198911000-00009. [DOI] [PubMed] [Google Scholar]