Abstract
Introduction:
Repetitive negative thinking (RNT) is a cognitive process focusing on self-relevant and negative experiences, leading to a poor prognosis of major depressive disorder (MDD). We previously identified that connectivity between the precuneus/posterior cingulate cortex (PCC) and right temporoparietal junction (rTPJ) was positively correlated with levels of RNT.
Objective:
In this double-blind, randomized, sham-controlled, proof-of-concept trial, we employed real-time functional magnetic resonance imaging neurofeedback (rtfMRI-nf) to delineate the neural processes that may be causally linked to RNT and could potentially become treatment targets for MDD.
Methods:
MDD-affected individuals were assigned to either active (n = 20) or sham feedback group (n = 19). RNT was measured by the Ruminative Response Scale-brooding subscale (RRS-B) before and 1 week after the intervention.
Results:
Individuals in the active but not in the sham group showed a significant reduction in the RRS-B; however, a greater reduction in the PCC-rTPJ connectivity was unrelated to a greater reduction in the RRS-B. Exploratory analyses revealed that a greater reduction in the retrosplenial cortex (RSC)-rTPJ connectivity yielded a more pronounced reduction in the RRS-B in the active but not in the sham group.
Conclusions:
RtfMRI-nf was effective in reducing RNT. Considering the underlying mechanism of rtfMIR-nf, the RSC and rTPJ could be part of a network (i.e., default mode network) that might collectively affect the intensity of RNT. Understanding the relationship between the functional organization of targeted neural changes and clinical metrics, such as RNT, has the potential to guide the development of mechanism-based treatment of MDD.
Keywords: fMRI neurofeedback, Functional connectivity, Repetitive negative thinking, Rumination, Depression
Introduction
Major depressive disorder (MDD) is one of the most common psychiatric disorders and a leading cause of disability worldwide [1–3]. Although effective antidepressant treatments are available, almost two-thirds of patients do not respond satisfactorily to first-line treatments [4], and about one-third remain refractory after diverse combinations of antidepressant medications, augmenting strategies, and psychotherapies [5]. MDD is a heterogeneous syndrome with different clinical symptoms [6], and one such symptom is repetitive negative thinking (RNT) [6]. RNT (often referred to as rumination in depression) is a recurrent thought process which is negative in valence and difficult to disengage from [7]. Accumulating evidence supports that RNT is a maladaptive cognitive process, which when modulated, changes the risk for, the severity of, or the recurrence of MDD [8–10]; however, the neurobiological mechanisms underlying RNT are not well understood. The lack of mechanistic understanding of RNT and depression may be a reason why first-line treatments for depression fail for many individuals [11, 12]. Here, we employed real-time functional magnetic resonance imaging neurofeedback (rtfMRI-nf) to delineate the neural processes that may be causally linked to RNT in depression.
Real-time functional magnetic resonance imaging (rtfMRI) provides us blood-oxygen-level-dependent signal processing and displays simultaneously with image acquisition. It has enabled rtfMRI-nf that allows a person to see and regulate the fMRI signal from their brain [13, 14]. It can provide individuals with instantaneous feedback concerning their brain activity and may facilitate control over RNT [15]. Identifying the brain regions/circuits to target with feedback is one of the most important elements in a rtfMRI-nf study [16] (see online suppl. Introduction 1.1; for all online suppl. material, see www.karger.com/doi/10.1159/000528377).
This investigation used a double-blind randomized sham-controlled proof-of-concept trial (RCT) of connectivity-based rtfMRI-nf in MDD-affected participants to examine whether they would be able to decouple the target functional connectivity and reduce RNT. We previously conducted a data-driven resting-state fMRI analysis [17], where we found that the functional connectivity between the precuneus/posterior cingulate cortex (PCC) and right temporoparietal junction (rTPJ) was positively correlated with a higher level of RNT (see online suppl. Introduction 1.2). Thus, we aimed to reduce the PCC-rTPJ connectivity via rtfMRI-nf. In line with recent methodological recommendations [18], this RCT utilized active sham-controlled feedback where participants received artificially generated feedback with similar probabilistic properties to the real feedback. This design allowed participants in both groups to engage in rtfMRI-nf while receiving feedback signals with the same amount of reward experiences or the sense of achievement, regardless of the real or artificially generated feedback signals (see online suppl. Introduction 1.3). The primary neural outcome was the change, relative to the baseline, in RNT-related connectivity between the PCC and rTPJ during a rtfMRI-nf session. The primary clinical outcome was the change, relative to the baseline, in levels of RNT measured by the brooding subscale of the Ruminative Response Scale (RRS-B) [19] at 1-week follow-up. The RRS composes of three subscales; the “depression” subscale (RRS-D) is substantially overlapping with depressive symptom itself, while “brooding” (RRS-B) and “reflection” (RRS-R) are two distinct cognitive processes considered to lead a different trajectory of depression (i.e., brooding leads to worse prognosis but reflection does not) [20]. Thus, we used the RRS-B as a measure of RNT. We hypothesized that compared to the sham group, participants undergoing active rtfMRI-nf would show the following effects: (1) greater reduction in the PCC-rTPJ connectivity during a single session of rtfMRI-nf and (2) greater reduction in the RRS-B at 1-week follow-up. We also explored the association between the change in the primary neural outcome and the change in the primary clinical outcome to examine whether the modulated effects of the target brain regions, rather than placebo effects, account for symptom changes.
Materials and Methods
Study Design
This study was conducted at the Laureate Institute for Brain Research (LIBR) and registered on ClinicalTrials.gov (NCT04941066). Participants were randomized into an active or a sham group with a 1:1 ratio. Participants, assessors, and researchers who were involved in data collection were blind to the group allocation (see online suppl. Methods 2.1). The randomization was conducted independently by a randomization center with the covariate adaptive randomization method [21, 22] using an in-house python script controlling for age, sex, medication status, RRS [19], and Montgomery-Åsberg Depression Rating Scale (MADRS) [23]. Blinding integrity was tested by asking participants to guess group allocation after the intervention. Participants underwent the initial visit including the baseline assessment of clinical symptoms followed by the rtfMRI-nf session (active or sham) and a 1-week follow-up assessment without rtfMRI-nf. Consensus on the reporting and experimental design of clinical and cognitive-behavioral neurofeedback studies (CRED-nf) checklist [24] is available in online supplementary Table S1.
Participants
Between March 2021 and March 2022, 43 individuals with MDD were recruited. Eligible participants were between 18 and 65 years, fluent in English, met the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DMS-5) criteria for uni-polar MDD based on the Mini-International Neuropsychiatric Interview 7.0.2 (MINI) [25], and had current depressive symptoms with MADRS score >6 [23]. Exclusion criteria were as follows: a lifetime history of bipolar disorder, schizophrenia, or any psychotic disorders; met DSM-5 criteria for substance abuse or dependence within 6 months prior to study entry; current severe suicidal ideation or attempt within 12 months prior to study entry; abnormal neuromorphological brain profile; pregnancy; and general contraindications against MRI examinations. Anxiety disorders were acceptable for enrollment if these co-occurring disorders were stable and not the primary disorder. Details of sample size calculation are provided in online supplementary methods 2.2 and online supplementary Figure S1. All participants provided written informed consent and received financial compensation for participation in the study.
Interventions
The supplementary material (online suppl. Methods 2.3–2.5 and online suppl. Fig. S2) provides details on image data acquisition and rtfMRI-nf. The rtfMRI-nf training paradigm was the same as our previous study [15] and is illustrated in online supplementary Figure S3. Briefly, during the rtfMRI-nf session, participants attempted to regulate RNT by applying emotion regulation strategies (i.e., cognitive reappraisal, see online suppl. Methods 2.6) while viewing negative words that describe their personality traits (a list of stimulus words is in online suppl. Table S2). The rtfMRI-nf session started with a baseline run (View1), followed by three rtfMRI-nf training runs (NF1, NF2, and NF3), and ended with a transfer run (View2). No feedback was given during View1 and View2 to assess their ability to regulate the PCC-rTPJ connectivity before and after three rtfMRI-nf training runs. The PCC-rTPJ connectivity during View1 was used as baseline connectivity for each participant.
We used AFNI software (https://afni.nimh.nih.gov/), a set of C programs for processing, analyzing, and displaying fMRI data, to implement rtfMRI-nf and to analyze fMRI data. An in-house program written in Python was used for real-time fMRI data transferring and processing with comprehensive noise reduction and motion correction [26, 27]. Functional connectivity estimates from the PCC and rTPJ were presented graphically to the subjects in real-time. The participants assigned to the sham group received the same feedback presentation as the active group did, except that the feedback was artificially generated unrelated to the target connectivity. We also placed a safeguard process against an accidental correlation between synthesized sham feedback and actual blood-oxygen-level-dependent signal changes from the target connectivity. This ensured that the sham group received a feedback signal with similar probabilistic structures to the active group while irrelevant to their brain activation. Details of the sham feedback are explained in online supplementary methods 2.7 and 2.8.
Outcome Measures
The primary neural outcome was the change, relative to the baseline, in the PCC-rTPJ connectivity during the rtfMRI-nf session. The PCC-rTPJ connectivity was calculated with a generalized psychophysiological interaction analysis (online suppl. Methods 2.9) [28]. All clinical measures were self-reported or assessed by blinded interviewers at baseline and 1-week follow-up, except for the self-rated scale measuring state-dependent moods (Positive and Negative Affect Schedule - Expanded Form: PANAS [29]) and the study original scale (Post-neurofeedback Session Questionnaires) to evaluate blind integrity and to collect emotion regulation strategies used during the rtfMRI-nf. Those measures were collected before and right after the rtfMRI-nf (post-rtfMRI-nf). The primary clinical outcome was the change, relative to the baseline, in the intensity of RNT measured by the RRS-B at 1-week follow-up. To further assess the clinically reliable change in RNT, we used the reliable change measurement [30–32] for the RRS-B. Following recent recommendations [33], deteriorations with respect to the primary behavioral outcome were also explored. The secondary RNT-related clinical outcomes were the change, relative to the baseline, in the RRS-D, RRS-R, and the total score of three subscales of the RRS (RRS-T) 1 week after the rtfMRI-nf. Other exploratory outcomes included the change in, relative to the baseline, depression, anxiety, positive and negative moods, and emotion regulation (online suppl. Methods 2.10) 1 week after the rtfMRI-nf (or post-rtfMRI-nf). The details of the Post-neurofeedback Session Questionnaires (study original) are presented in online supplementary Table S3, and the typical emotion regulation strategies used during rtfMRI-nf are explained and summarized in online supplementary Table S4.
Statistical Analyses
Baseline differences between the groups on demographic and clinical variables were calculated using independent t tests for continuous variables and χ2 statistics or Fisher’s exact tests for categorical variables.
Longitudinal Data Analysis
Longitudinal change in each outcome was evaluated with a series of linear mixed effect model analyses (LME, lme4 package) [34] in R. For the primary neural outcome, the longitudinal change of the PCC-rTPJ connectivity at NF1, NF2, NF3, and View2 relative to View1 was tested by the LME. The LME model included fixed effects of time, group, time-by-group interaction, and a random effect of the subject on intercept. For the clinical outcomes, the longitudinal change in symptoms at 1-week follow-up (or post-rtfMRI-nf) relative to baseline was examined by the LME. The LME model included fixed effects of time, group, time-by-group interaction, age, sex, and a random effect of the subject on intercept. For all post hoc comparisons, we performed False Discovery Rate (FDR) [35] corrections for multiple comparisons, except for exploratory outcomes.
Regression Analysis
To causally relate the modulated effects of the target brain regions to the symptom changes, we explored the associations between the change in the primary neural outcome and the change in the primary clinical outcome. A robust regression analysis with MM-estimation [36] (R MASS package) [37] was conducted to investigate the association between the change in the PCC-rTPJ con nectivity during rtfMRI-nf (change in the average connectivity during rtfMRI-nf runs relative to baseline) and the change in the RRS-B at follow-up from baseline. The regression model included the change in the RRS-B as a dependent variable and the change in the PCC-rTPJ connectivity, group, and interaction of these variables as independent variables.
Whole-Brain Exploratory Analysis
In case any associations between the primary neural outcome and primary clinical outcome could not be determined, we conducted a whole-brain exploratory analysis to identify the functional connectivity associated with the primary clinical outcome. We used AFNI 3dLMEr program [38] to conduct an LME analysis on the whole-brain image data. The model included time, group, change in the RRS-B, three-way interaction (time-by-group-by-change in the RRS-B), age, sex, and average head motion as fixed effects, and a random effect of the subject on intercept. See online supplementary methods 2.12 and 2.13 for more details.
Results
Demographic and Behavioral Measures
Among the 43 subjects recruited, four subjects did not have usable data during rtfMRI-nf due to excessive head motion (more than 25% of all three runs of rtfMRI-nf data were censored), resulting in 20 MDD subjects in the active and 19 MDD subjects in the sham group (Fig. 1). There was no drop out at 1-week follow-up. At baseline, there were no group differences in any variables (Tables 1, 2). Results from the Post-Neurofeedback Session Questionnaires indicated that participants could not differentiate the group assignment (χ2(1) = 0.64, p = 0.43), and there were no significant differences in the sense of achievement during the rtfMRI-nf (e.g., item 8: “How successful do you feel you were in modulating your brain activity during this session?,” t(37) = −1.31, p = 0.20; online suppl. Table S3). No significant side effects nor adverse events were reported (Table 2 and online suppl. Table S3).
Fig. 1.
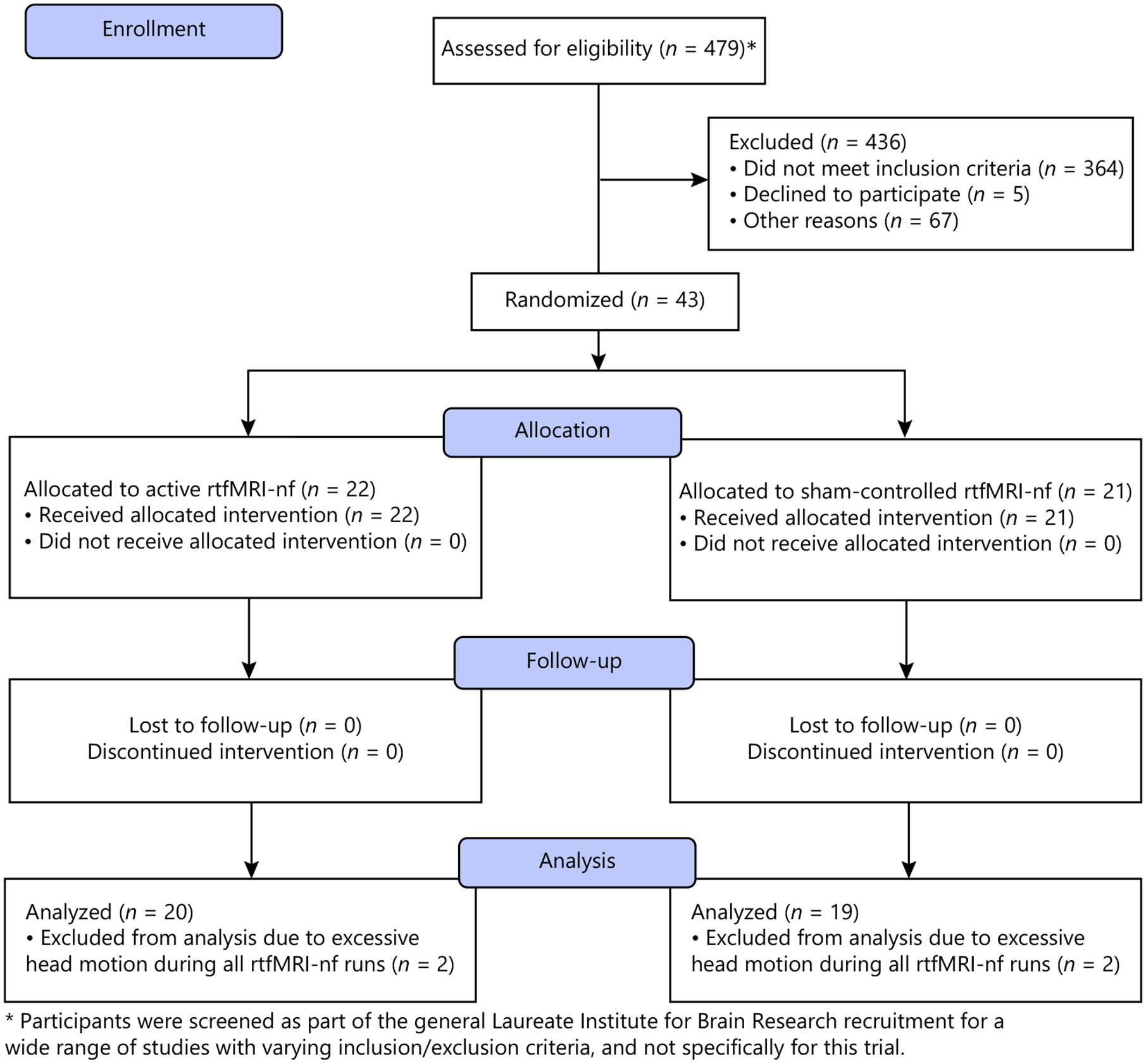
CONSORT flow diagram.
Table 1.
Demographic data and head motion
| Active (n = 20), mean (SD) | Sham (n = 19), mean (SD) | Statistics | p | |
|---|---|---|---|---|
| Age | 33.60 (10.60) | 33.42 (11.49) | t(37) = 0.05 | 0.96 |
| Female (%) | 14 (70.00) | 14 (73.00) | χ2(1) < 0.001 | 1.00 |
| Handedness: right (%) | 17 (85.00) | 18 (94.74) | Fisher’s exact test | 0.61 |
| Race/ethnicity: non-white (%) | 2 (10.00) | 6 (31.58) | Fisher’s exact test | 0.12 |
| Black | 0 (0.00) | 1 (5.26) | ||
| Native American | 2 (10.00) | 5 (26.32) | ||
| White | 18 (90.00) | 13 (68.42) | ||
| Diagnosis (%) | ||||
| MDD without comorbidity | 12 (60.00) | 6 (31.57) | χ2(1) = 2.13 | 0.15 |
| MDD and anxiety disorder | 8 (40.00) | 13 (68.42) | χ2(1) = 2.13 | 0.15 |
| Generalized anxiety disorder | 3 (15.00) | 10 (52.00) | ||
| Social anxiety disorder | 5 (10.00) | 5 (26.32) | ||
| Panic disorder | 4 (20.00) | 4 (21.05) | ||
| Depressive episode (%) | ||||
| Single episode | 6 (30.00) | 8 (42.00) | χ2(1) = 0.21 | 0.65 |
| Recurrent | 14 (70.00) | 11 (57.89) | ||
| Medicated (%) | 13 (65.00) | 7 (36.84) | χ2(1) = 2.07 | 0.15 |
| Benzodiazepines | 3 (15.00) | 2 (10.53) | ||
| Anti-psychotics | 0 (0.00) | 0 (0.00) | ||
| Psychotherapy (%) | 5 (10.00) | 4 (21.05) | χ2(1) < 0.001 | 1.00 |
| Average head motion (framewise displacement) | 0.07 (0.03) | 0.07 (0.03) | t(37) = 0.02 | 0.98 |
Table 2.
Estimated means and fixed effect statistics of the clinical outcomes at the pre-, post-real-time fMRI neurofeedback session and follow-up
| Baseline | One-week follow-up | Fixed effects | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| active (n = 20), mean (SD) | sham (n = 19), mean (SD) | active (n = 20), mean (SD) | sham (n = 19), mean (SD) | Time | Group | Time × group | |||||
| F | p | F | p | F | p | ||||||
| Primary cliniail outcome | |||||||||||
| RRS | Brooding | 12.85 (3.13) | 12.37 (3.18) | 9.70 (2.87) | 11.84 (3.66) | 17.08 | <0.001 b | 0.77 | 0.39 | 8.70 | 0.005 e |
| Secondary dirlical outcomes | |||||||||||
| RRS | Depression | 31.30 (7.42) | 29.95 (6.42) | 27.35 (6.28) | 28.16 (6.85) | 16.36 | <0.001 c | 0.05 | 0.83 | 2.32 | 0.14 |
| Reflection | 10.80 (3.78) | 11.16 (3.04) | 10.40 (3.17) | 10.26 (3.81) | 2.47 | 0.12 | 0.00 | 0.95 | 0.36 | 0.55 | |
| Total | 54.95 (12.83) | 53.47 (10.91) | 47.45 (8.83) | 50.26 (13.21) | 20.64 | <0.001 d | 0.02 | 0.89 | 3.31 | 0.08 | |
| Exploratory chinical outcomes | |||||||||||
| MADRS | 18.70 (7.32) | 20.63 (4.95) | 13.79 (6.80)a | 15.16 (7.00) | 25.12 | <0.001 | 0.59 | 0.45 | 0.15 | 0.70 | |
| HAMA | 14.55 (5.50) | 17.37 (5.43) | 12.53 (6.79)a | 14.47 (5.70) | 7.04 | 0.01 | 1.91 | 0.18 | 0.25 | 0.62 | |
| PHQ | 11.30 (5.70) | 10.53 (4.14) | 9.00 (5.32) | 8.21 (3.79) | 17.93 | <0.001 | 0.41 | 0.52 | 0.00 | 0.99 | |
| STAI | Trait | 54.75 (9.31) | 53.58 (9.75) | 51.85 (9.65) | 51.26 (9.02) | 11.17 | 0.002 | 0.21 | 0.65 | 0.14 | 0.71 |
| MCQ | Total | 67.25 (12.47) | 66.21 (13.70) | 62.55 (10.87) | 64.74 (14.39) | 7.84 | 0.01 | 0.01 | 0.92 | 2.14 | 0.15 |
| Lack of cognitive confidence | 13.95 (6.33) | 12.11 (5.10) | 13.45 (6.12) | 12.79 (5.27) | 0.07 | 0.79 | 0.55 | 0.46 | 2.99 | 0.09 | |
| Positive beliefs about worry | 10.15 (3.66) | 10.63 (3.93) | 9.65 (3.10) | 10.84 (5.07) | 0.12 | 0.73 | 0.41 | 0.53 | 0.72 | 0.40 | |
| Cognitive self-consciousness | 15.55 (4.14) | 16.26 (4.94) | 14.35 (4.67) | 15.74 (4.49) | 6.47 | 0.02 | 0.58 | 0.45 | 0.99 | 0.33 | |
| Negative beliefs about uncontrollability and danger | 14.90 (5.59) | 14.79 (5.13) | 13.20 (4.93) | 14.26 (4.68) | 5.21 | 0.03 | 0.07 | 0.79 | 1.45 | 0.24 | |
| Need to control thoughts | 12.70 (3.42) | 12.42 (3.25) | 11.90 (2.75) | 11.11 (2.56) | 11.59 | 0.002 | 0.31 | 0.58 | 0.69 | 0.41 | |
| TCQ | Total | 62.30 (7.21) | 62.74 (7.32) | 62.20 (6.56) | 62.95 (6.71) | 0.01 | 0.92 | 0.05 | 0.82 | 0.08 | 0.78 |
| Distraction | 14.35 (2.92) | 14.32 (2.85) | 15.65 (3.36) | 15.05 (1.87) | 10.25 | 0.003 | 0.18 | 0.67 | 0.78 | 0.38 | |
| Social control | 14.50 (2.37) | 13.79 (1.81) | 14.55 (1.67) | 13.74 (1.82) | 0.00 | 0.10 | 1.89 | 0.18 | 0.03 | 0.86 | |
| Worry | 10.25 (2.29) | 10.58 (3.73) | 9.20 (2.19) | 10.47 (3.85) | 2.69 | 0.11 | 0.71 | 0.40 | 1.80 | 0.19 | |
| Punishment | 10.25 (2.92) | 10.16 (2.52) | 9.55 (2.42) | 9.26 (2.18) | 9.36 | 0.004 | 0.10 | 0.76 | 0.14 | 0.71 | |
| Reappraisal | 12.95 (2.70) | 13.89 (2.45) | 13.25 (3.01) | 14.42 (2.59) | 1.70 | 0.20 | 1.70 | 0.20 | 0.13 | 0.72 | |
| ERQ | Cognitive reappraisal | 22.90 (8.06) | 27.05 (7.92) | 26.55 (6.97) | 29.21 (4.50) | 7.33 | 0.01 | 2.88 | 0.10 | 0.48 | 0.49 |
| Expressive suppression | 15.70 (4.60) | 14.79 (4.93) | 16.45 (5.20) | 14.79 (4.89) | 0.28 | 0.60 | 0.75 | 0.39 | 0.28 | 0.60 | |
| Baseline | Post-rtfMRI-nf | Fixed effects | |||||||||
| active (n = 20), mean (SD) | sham (n = 19), mean (SD) | active (n = 20), mean (SD) | sham (n = 19), mean (SD) | Time | Group | Time × group | |||||
| F | p | F | p | F | p | ||||||
| PANAS | Negative affect | 14.60 (4.04) | 15.11 (3.78) | 12.05 (2.09) | 11.47 (2.01) | 34.67 | <0.001 | 0.00 | 0.99 | 1.06 | 0.31 |
| Positive affect | 21.50 (5.51) | 20.21 (3.81) | 24.30 (7.73) | 23.42 (7.39) | 9.44 | 0.004 | 0.31 | 0.58 | 0.04 | 0.83 | |
| Fear | 8.90 (2.99) | 8.74 (2.88) | 7.65 (2.25) | 7.26 (1.91) | 14.33 | <0.001 | 0.13 | 0.72 | 0.10 | 0.75 | |
| Hostility | 8.00 (2.53) | 7.42 (2.27) | 6.75 (1.16) | 6.26 (0.56) | 13.55 | <0.001 | 1.09 | 0.30 | 0.02 | 0.89 | |
| Guilt | 10.15 (4.88) | 10.05 (3.41) | 7.55 (2.61) | 7.00 (1.67) | 24.42 | <0.001 | 0.12 | 0.73 | 0.16 | 0.69 | |
| Sadness | 9.80 (3.91) | 10.11 (4.01) | 6.75 (2.27) | 7.00 (2.60) | 46.34 | <0.001 | 0.10 | 0.76 | 0.00 | 0.95 | |
| Joviality | 13.95 (5.11) | 12.79 (2.80) | 17.30 (8.33) | 17.42 (6.21) | 15.98 | <0.001 | 0.06 | 0.80 | 0.41 | 0.52 | |
| Self-Assurance | 11.30 (4.75) | 10.74 (3.93) | 11.75 (5.38) | 13.11 (5.15) | 7.55 | 0.01 | 0.19 | 0.67 | 3.50 | 0.07 | |
| Attentiveness | 10.80 (2.84) | 10.58 (2.39) | 10.95 (3.07) | 10.21 (2.94) | 0.05 | 0.82 | 0.39 | 0.54 | 0.29 | 0.59 | |
| Shyness | 5.85 (2.52) | 6.21 (1.75) | 4.55 (1.79) | 4.84 (1.30) | 31.84 | <0.001 | 0.49 | 0.49 | 0.02 | 0.89 | |
| Fatigue | 10.85 (5.04) | 10.26 (4.71) | 8.50 (3.91) | 9.89 (4.14) | 3.03 | 0.09 | 0.14 | 0.71 | 1.61 | 0.21 | |
| Serenity | 9.00 (3.26) | 8.53 (2.82) | 9.45 (2.39) | 9.32 (2.21) | 2.41 | 0.13 | 0.11 | 0.74 | 0.18 | 0.67 | |
| Surprise | 3.60 (1.14) | 3.42 (1.02) | 4.50 (1.73) | 5.26 (2.64) | 22.16 | <0.001 | 0.42 | 0.52 | 2.62 | 0.11 | |
Means and standard deviations (SD) of symptom and cognitive outcome measures at the baseline (before rtfMRI-nf), post-rtfMRI-nf, and 1-week follow-up. RRS, Ruminative Response Scale; MADRS, Montgomery-Åsberg Depression Rating Scale; HAMA, Hamilton Anxiety Scale; PHQ, Patient Health Questionnaire-9; STAI, The State-Trait Anxiety Inventor-Trait; PANAS, Positive and Negative Affect Schedule - Expanded Form; MCQ, Metacognition Questionnaire-30; TCQ, Thought Control Questionnaire; ERQ, Emotion Regulation Questionnaire. a n = 19. All p values are presented uncorrected for multiple comparisons. For the primary and secondary clinical outcomes, false discovery rate (FDR) correction for multiple comparisons on the fixed effects yielded pFDR = 0.004b, 0.0002c, 0.001d, and 0.02e. Exploratory clinical outcomes were uncorrected.
Longitudinal Change in the PCC-rTPJ Connectivity
Primary Neural Outcome
There were no significant interactions or main effects on PPI estimates of the PCC-rTPJ connectivity (Fig. 2; time-by-group interaction: F(3, 91) = 0.86, p = 0.46, time: F(3, 91) = 0.30, p = 0.83, group: F(1, 36) = 0.86, p = 0.36). The result of the same LME analysis with age, sex, and average head motion as covariates are presented in online supplementary Results 3.1. The main findings did not change even after controlling for covariates.
Fig. 2.
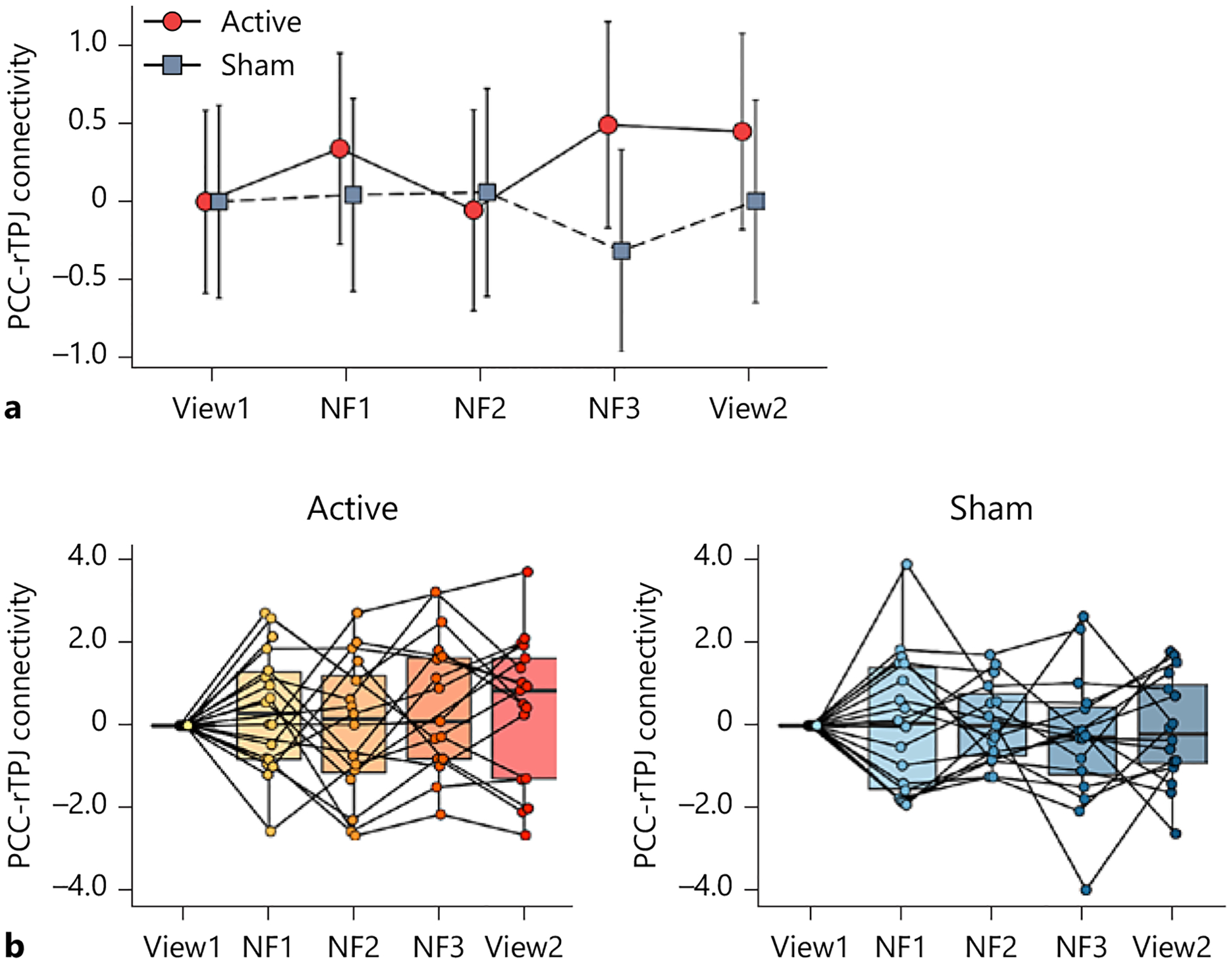
Longitudinal change in the primary neural outcome. a Change in gPPI estimates of the precuneus/posterior cingulate cortex (PCC) and the right temporoparietal junction (rTPJ) functional connectivity between the active and sham groups through the neurofeedback session. View1 and View2: no-neurofeedback run with a self-referential task, NF1, NF2, and NF3: neurofeedback run with a self-referential task. The error bars represent the 95% confidence interval of the mean values. b Boxplots and individual plots of the PCC-rTPJ connectivity. Left: active group, right: sham group.
Change in RNT and Other Clinical Outcomes
Primary Clinical Outcome
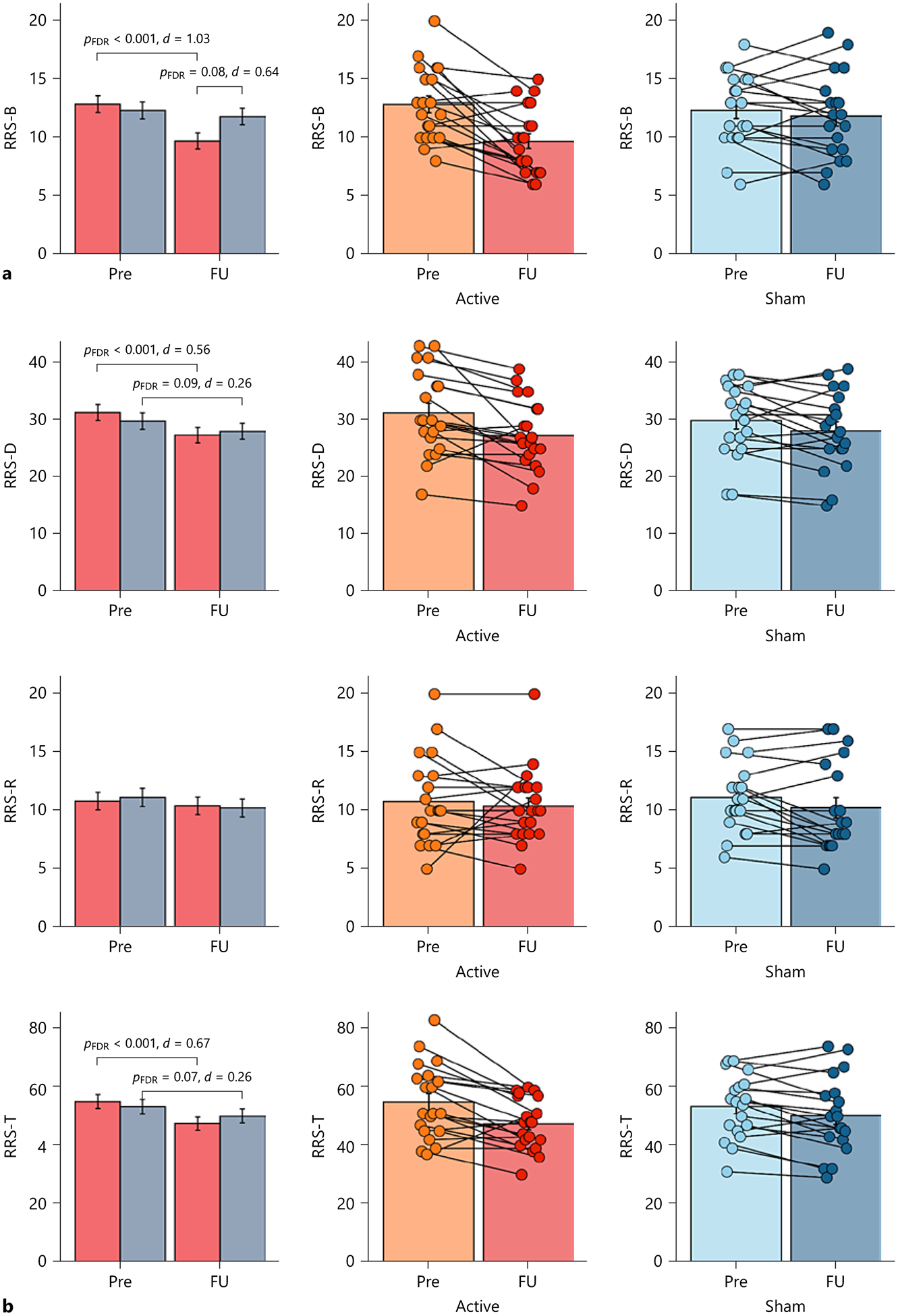
There was a main effect of time and interaction effect on the RRS-B (Fig. 3. A.; time-by-group interaction: F(1, 37) = 8.70, p = 0.005, time: F(1, 37) = 17.08, p < 0.001, group: F(1, 35) = 0.77, p = 0.39). The active group showed a reduced RRS-B score from baseline to follow-up (t(37) = 5.07, pFDR < 0.001, d = 1.03), while the sham group did not (t(37) = 0.83, pFDR = 0.41, d = 0.15). The active group showed a lower RRS-B score at follow-up compared to the sham group (t(51) = −2.10, p = 0.04), although it did not survive the FDR correction (pFDR = 0.08, d = 0.64). The estimated reliable change of the RRS-B was 4.15 of the absolute score change (online suppl. Methods 2.11). Six out of 20 subjects in the active group showed a reliable improvement in RNT (30%), whereas none of the 19 subjects in the sham group did (0%), indicating that the number of subjects who improved was significantly higher in the active compared to the sham group (Fisher’s exact test, p = 0.02). There were no reliable deteriorations in either group.
Fig. 3.
Longitudinal change in repetitive negative thinking. a Primary clinical outcome (brooding subscale of the Ruminative Response Scale, RRS-B). The left panels show the mean values of the RRS-B at baseline (Pre) and 1-week follow-up (FU) in both groups. The error bars represent the standard errors of the mean values. The middle panels show the mean values and individual plots of the RRS-B at Pre and FU in the active group. The right panel shows the mean values and individual plots of the RRS-B at Pre and FU in the sham group. b Secondary clinical outcomes (depression subscale of the RRS, RRS-D; reflection subscale of the RRS, RRS-R; and the RRS total score, RRS-T). The left panels show the mean values of each RRS subscale or the total score at Pre and FU in both groups. The error bars represent the standard errors of the mean values. The middle panels show the mean values of each RRS subscale or the total score at Pre and FU in the active group. The right panel shows the mean values of each RRS subscale or the total score at Pre and FU in the sham group. FDR: false discovery rate correction.
Secondary and Other Clinical Outcomes
The statistical tests for the secondary and other clinical outcomes are summarized in Table 2. In short, there were no significant time-by-group interactions on any variables, although there was a trending time-by-group interaction on the RRS-T (Fig. 3B..; time-by-group interaction: F(1, 37) = 3.31, p = 0.08, time: F(1, 37) = 20.64, p < 0.001, group: F(1, 35) = 0.02, p = 0.88). Only a significant time effect was observed on the secondary clinical out comes including the RRS-T and RRS-D, and other exploratory outcomes such as depression, anxiety, positive and negative moods, and emotion regulation (see online suppl. Results 3.2. for more details).
Association between Change in the PCC-rTPJ Connectivity and Change in RNT
There were no significant group differences in the association between change in the PCC-rTPJ connectivity and change in the RRS-B, and no associations between those variables in either group (online suppl. Table S5).
Whole-Brain Exploratory Analysis: Functional Connectivity Associated with a Reduction in RNT
A whole-brain analysis revealed a three-way interaction effect (time-by-group-by-change in the RRS-B) in the left retrosplenial cortex (RSC) (MNI: −7, −53, 11; cluster size k = 79; online suppl. Fig. S4). This RSC cluster included the ventral part of the PCC. The spatial relationship between this RSC cluster and the original PCC target is illustrated in online supplementary Figure S5. See online supplementary material for more details (suppl. Results 3.3 and 3.4, online suppl. Table S6; online suppl. Fig. S6). Subsequently, we investigated the effect of rtfMRI-nf on the connectivity between the RSC and our original rtfMRI-nf targets, i.e., rTPJ or PCC. The results of the RSC-rTPJ connectivity analysis are as follows:
Longitudinal Change in the RSC-rTPJ Connectivity
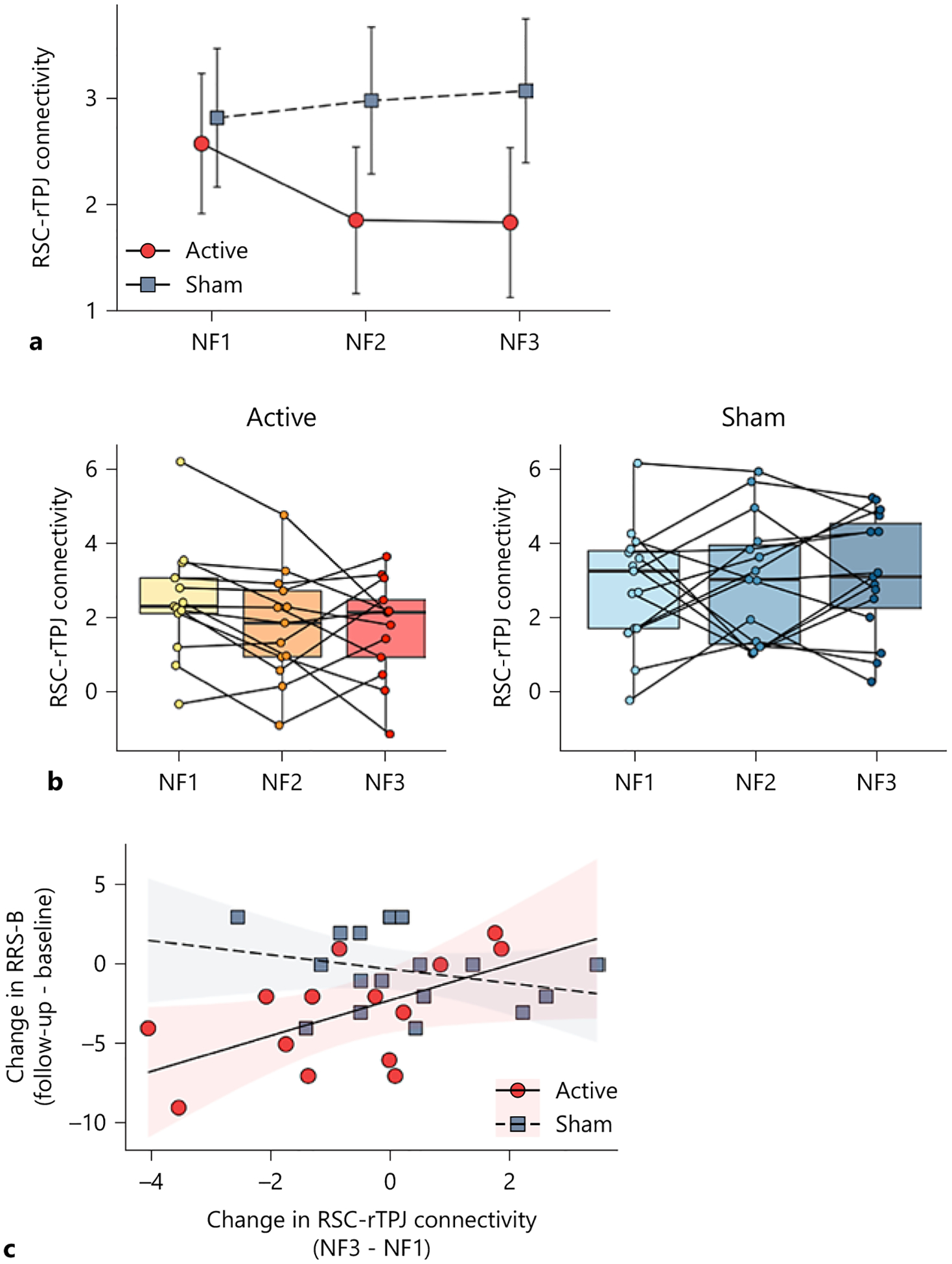
A significant main effect on group (active and sham) was observed in the RSC-rTPJ connectivity (time-by-group interaction: F(2, 64) = 2.37, p = 0.10, time: F(2, 64) = 0.73, p = 0.48, group: F(1, 36) = 4.99, p = 0.03), with significantly lower RSC-rTPJ connectivity at NF2 (t(71) = −2.26, p = 0.03, d = 0.77) and NF3 (t(71) = −2.48, p = 0.02, d = 0.87) in the active compared to the sham group (Fig. 4a, b).
Fig. 4.
Longitudinal change in the functional connectivity between the retrosplenial cortex (RSC) and right temporoparietal junction (rTPJ), and an association between the change in the RSC-rTPJ connectivity and the change in the brooding subscale of the Ruminative Response Scale (RRS-B). a Longitudinal change in the RSC-rTPJ connectivity between two groups through the rtfMRI-nf runs. NF1, NF2, and NF3: neurofeedback run with a self-referential task. b Boxplots and individual plots of the RSC-rTPJ connectivity. Left: active group, Right: sham group. c An association between the change in the RSC-rTPJ connectivity (NF3 from NF1) and the change in the RRS-B (follow-up from baseline). The error bars represent the 95% confidence interval of the mean values. The shaded areas represent the 95% confidence interval of regression slopes.
Association Between Change in the RSC-rTPJ Connectivity and Change in RNT
A decreased RSC-rTPJ connectivity from NF1 to NF3 was associated with a decreased RRS-B score from baseline to follow-up within the active group (adjusted R2 = 0.36, β = 1.14, p = 0.04; Fig. 4. C and online suppl. Table S7). There were no such associations within the sham group (adjusted R2 = 0.28, β = −0.44, p = 0.31). There were no significant findings in the RSC-PCC connectivity (online suppl. Results 3.5, online suppl. Table S8 and online suppl. Fig. S7).
Discussion
This double-blind, sham-controlled proof-of-concept study investigated the feasibility of a novel rtfMRI-nf protocol targeting an RNT-related brain circuit in individuals with MDD. We observed three main findings. First, the active rtfMRI-nf intervention did not differ from the sham intervention in the degree of connectivity between the PCC and rTPJ. Second, despite the lack of the main effect, participants in the active group showed a significant reduction in the RRS-B scores. However, the RRS-B reduction was not associated with the change in the PCC-rTPJ connectivity. Third, we found that the reduced RSC-rTPJ connectivity was associated with the reduced RRS-B score in the active group, but not in the sham. Taken together, these results provide preliminary evidence that rtfMRI-nf could reduce RNT in MDD; however, the mechanism underlying rtfMRI-nf effects may not be due to the engagement of a priori-defined target connectivity.
Unlike in the previous study recruiting healthy participants [15], there was no progressive linear decrease of the target connectivity across rtfMRI-nf runs, nor did the change in the target connectivity correlate with an RNT improvement. Several factors could have mitigated effects. Our target connectivity was selected based on data-driven analyses of the resting-state fMRI data (see online suppl. Introduction 1.2). Neural features of disengagement from RNT during the task (e.g., rtfMRI-nf) may not be simply measured as a reverse finding of the resting-state fMRI data correlating with levels of the RRS scores [17]. Moreover, in this study, we searched for brain regions where a reinforcement learning might have occurred to reduce RNT. The RSC-rTPJ connectivity was significantly decreased during NF2 and NF3 in the active compared to the sham group. This reduced connectivity was correlated with a reduction of RNT in the active group but not in the sham group.
Although the functional differences between each subdivision of the PCC remain unclear [39], one study showed progressive shifts in the PCC function from anterior to posterior and from dorsal to ventral, such that the posterior and ventral portion of PCC, especially the RSC, plays a pivotal role within the DMN in a self-referential processing including an episodic memory retrieval [40]. The RSC has been implicated in conditioned fear memories [41, 42], spatial navigations, and episodic, or autobiographical, memories [39, 40, 43], and has been frequently observed to be activated during recalling of episodic memories along with the PCC [39, 44]. The TPJ, especially in the right hemisphere, has been established as a key region of the theory of mind and is involved in building models of other people’s perspective-taking [45]. Individuals with higher RNT are often preoccupied with self-critical thoughts and past instances of failure, rather than attempting to improve present circumstances by incorporating others’ perspectives [20, 46–49]. Thus, it is possible that individuals in the active group might have learned to regulate the RSC activity together with the function of the rTPJ by applying emotion regulation strategies (i.e., retrieving an emotional episodic memory and reconsolidating it from a third person’s perspective), which might have contributed to a reduction in RNT, although we do not emphasize this finding due to the exploratory nature of the analyses. The possible reason that we observed training effects on the RSC-rTPJ connectivity to reduce RNT in the active group but not in the sham group may be: the feedback signal in the active group was more consistent (reflecting their own brain’s functional connectivity), and it might have been correlated with their experiences of applying emotion regulation strategies (as explained above) compared to the feedback signal presented for the sham group. We do not have detailed information on how they adjusted their emotion regulation strategies based on the feedback. Future studies need to explore how individuals adjusted emotion regulation strategies as described in a recent review paper [50].
Although other clinical measurements, including the MADRS, showed significant improvement in both groups (Table 2), only the RRS-B showed a time-by-group interaction, with a significant reduction in the active group. This finding needs to be interpreted with caution since only 30% in the active group showed reliable improvement in RNT. One small clinical study conducting a six-session of group metacognitive therapy (i.e., one form of cognitive behavioral therapy to modify metacognition of RNT) in nine adolescents with internalizing disorders reported a reliable improvement of RNT in 67% (6/9) of their sample [51]. A relatively modest rate of the reliable change in RNT in our data may be due to the difference in session numbers or time spent for the intervention. Emerging research suggests that improvement in behaviors or symptoms can continue for weeks to months after the final rtfMRI-nf session [52, 53]. The optimal time point to evaluate clinical outcomes after the rtfMRI-nf remains an open discussion for a further investigation.
This study has several limitations. First, our sample size was relatively small. We estimated our sample size based on the pilot data [15] to detect a significant difference in the interaction term (time-by-group). However, our estimation could be overly optimistic since we simulated the power and sample size based on the interaction term (see online suppl. Methods 2.2 and online suppl. Fig. S1). Thus, we acknowledge that our sample size might have been underpowered to detect a significant group difference in the final post hoc comparison with an independent t test. Moreover, the results of the secondary outcome, the change in the RRS-B score, need replication in larger sample. Second, we recruited MDD-affected individuals with relatively mild to moderate depression (Table 2), and we did not set inclusion criteria based on the baseline score of the RRS-B. Thus, we are cautious about the generalizability of our findings, especially for severe or treatment-resistant MDD with high levels of RNT. Third, although we determined our target connectivity based on comprehensive data-driven analyses of the resting-state fMRI data [17], an adjustment of the anatomical location of the target using a task-based fMRI paradigm (i.e., a functional localizer) would be beneficial. The challenge is that, unlike motor, affective, or executive functional regions, e.g., [54–62], RNT is a higher order cognitive process, and we are still uncertain what kind of tasks would have the maximum power to identify an RNT-related circuit. Fourth, although we observed a more prominent reduction in RNT for the active group compared to the sham, both groups showed a reduction in general depression. The current design allowed us to collect the follow-up data 1 week after the rtfMRI-nf, and we do not know the longitudinal effect of rtfMRI-nf on RNT and depression. Future investigation will need to clarify whether current results could be maintained in the longer term and how modulating RNT-related circuits could contribute to antidepressant effects.
Conclusion
The goal of this RCT was to determine whether rtfMRI-nf would reduce the PCC-rTPJ connectivity and RNT in individuals with MDD. Although rtfMRI-nf reduced RNT, it failed to show the engagement of original targets, defined based on the resting-state fMRI data [17]. This study revealed a challenge in the connectivity-based rtfMRI-nf to precisely target the specific circuit as intended. It is important to note that it would be overly ambitious to argue that any particular brain activities or connectivity are the primary factors causing RNT. Instead, it would be acceptable to assume that each brain region functions as a separate node in a network to collectively generate or inhibit RNT [63, 64]. To determine the causal involvement in neural alterations and symptom changes, a whole-brain investigation to explore such nodes would be valuable. In line with this notion, our preliminary data indicate that the RSC and rTPJ could be part of a network that might collectively affect the intensity of RNT, and may become treatment targets and treatment monitoring markers for future antidepressant interventions. We suggest that future studies should (1) evaluate how the feedback signals influence a person’s brain beyond the target regions to facilitate the understanding of mechanisms underlying rtfMRI-nf and (2) explore whole-brain regions potentially account for the causal effects on symptom changes.
Supplementary Material
Acknowledgments
We would like to express our appreciation to CoBRE Neuro-Map Investigators at LIBR, and thank all the research participants. We acknowledge the contributions of Sahib S. Khalsa, M.D., Ph.D., Tim Collins, Dara Crittenden, Amy Peterson, Megan Cole, Lisa Kinyon, Lindsey Bailey, Courtney Boone, Natosha Markham, Lisa Rillo, Angela Yakshin, and the LIBR Assessment Team for diagnostic assessments and data collection, and Julie Arterbury, Leslie Walker, Amy Ginn, Bill Alden, Julie DiCarlo, and Greg Hammond for helping with MRI scanning. The authors acknowledge Jerzy Bodurka, Ph.D. (1964-2021) for his intellectual and scientific contributions to the establishment of the EEG, structural and functional MRI, and neurofeedback processes that provided the foundation for the data collection, analysis, and interpretation of findings for the present work.
Funding Sources
This work has been supported in part by The William K. Warren Foundation, and the National Institute of General Medical Sciences Center Grant Award Number (1P20GM121312). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest Statement
Martin P. Paulus, M.D., is an advisor to Spring Care, Inc., a behavioral health start-up; he has received royalties for an article about methamphetamine in up-to-date. The other authors report no financial relationships with commercial interests related to the present study.
Statement of Ethics
The study protocol was reviewed and approved by the WCG IRB (https://www.wcgirb.com) (IRB Tracking Number 20210286). All participants provided written informed consent.
Data Availability Statement
The data that support the findings of this study are not publicly available due to ethics restrictions but available from the corresponding author upon reasonable request.
References
- 1.Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nat Med. 1998;4(11): 1241–3. [DOI] [PubMed] [Google Scholar]
- 2.Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34(1):13–25. [DOI] [PubMed] [Google Scholar]
- 3.Pincus HA, Pettit AR. The societal costs of chronic major depression. J Clin Psychiatry. 2001;62(Suppl 6):5–9. [PubMed] [Google Scholar]
- 4.Cain RA. Navigating the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study: practical outcomes and implications for depression treatment in primary care. Prim Care. 2007;34(3):505–19, vi, [DOI] [PubMed] [Google Scholar]
- 5.Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR D report. Am J Psychiatry. 2006;163(11):1905–17. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg D The heterogeneity of “major de-pression”. World Psychiatry. 2011;10(3):226–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehring T, Watkins ER. Repetitive negative thinking as a transdiagnostic process. Int J Cogn Ther. 2008;1(3):192–205. [Google Scholar]
- 8.Bessette KL, Jacobs RH, Heleniak C, Peters AT, Welsh RC, Watkins ER, et al. Malleability of rumination: an exploratory model of CBT-based plasticity and long-term reduced risk for depressive relapse among youth from a pilot randomized clinical trial. PLoS One. 2020; 15(6):e0233539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watkins ER. Depressive rumination and comorbidity: evidence for brooding as a transdiagnostic process. J Ration Emot Cogn Behav Ther. 2009;27(3):160–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watkins ER. Depressive rumination: investigating mechanisms to improve cognitive behavioural treatments. Cogn Behav Ther. 2009;38 Suppl 1(S1):8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones NP, Siegle GJ, Thase ME. Effects of rumination and initial severity on remission to cognitive therapy for depression. Cognit Ther Res. 2008;32(4):591–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmaling KB, Dimidjian S, Katon W, Sullivan M. Response styles among patients with minor depression and dysthymia in primary care. J Abnorm Psychol. 2002;111(2):350–6. [DOI] [PubMed] [Google Scholar]
- 13.Cox RW, Jesmanowicz A, Hyde JS. Real-time functional magnetic resonance imaging. Magn Reson Med. 1995;33(2):230–6. [DOI] [PubMed] [Google Scholar]
- 14.deCharms RC. Applications of real-time fMRI. Nat Rev Neurosci. 2008;9(9):720–9. [DOI] [PubMed] [Google Scholar]
- 15.Tsuchiyagaito A, Misaki M, Zoubi OA;, Tulsa 1000 Investigators, Paulus M, Bodurka J. Prevent breaking bad: a proof of concept study of rebalancing the brain’s rumination circuit with real-time fMRI functional connectivity neurofeedback. Hum Brain Mapp. 2021; 42(4):922–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fede SJ, Dean SF, Manuweera T, Momenan R. A guide to literature informed decisions in the design of real time fMRI neurofeedback studies: a systematic review. Front Hum Neurosci. 2020;14:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Misaki M, Tsuchiyagaito A, Al Zoubi O, Paulus M, Bodurka J; Tulsa 1000 Investigators 1000 Connectome-wide search for functional connectivity locus associated with pathological rumination as a target for real-time fMRI neurofeedback intervention. Neuroimage Clin. 2020;26:102244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guidi J, Brakemeier EL, Bockting CLH, Cosci F, Cuijpers P, Jarrett RB, et al. Methodological recommendations for trials of psychological interventions. Psychother Psychosom. 2018; 87(5):276–84. [DOI] [PubMed] [Google Scholar]
- 19.Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: the 1989 Loma Prieta Earthquake. J Pers Soc Psychol. 1991;61(1):115–21. [DOI] [PubMed] [Google Scholar]
- 20.Treynor W, Gonzalez R, Nolen-Hoeksema S. Rumination reconsidered: a psychometric analysis. Cognit Ther Res. 2003;27:247–59. [Google Scholar]
- 21.Kalish LA, Begg CB. Treatment allocation methods in clinical trials: a review. Stat Med. 1985;4(2):129–44. [DOI] [PubMed] [Google Scholar]
- 22.Suresh K An overview of randomization techniques: an unbiased assessment of outcome in clinical research. J Hum Reprod Sci. 2011;4(1):8–11. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–9. [DOI] [PubMed] [Google Scholar]
- 24.Ros T, Enriquez-Geppert S, Zotev V, Young KD, Wood G, Whitfield-Gabrieli S, et al. Consensus on the reporting and experimental design of clinical and cognitive-behavioural neurofeedback studies (CRED-nf checklist). Brain. 2020;143(6):1674–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33; quiz 34–57. [PubMed] [Google Scholar]
- 26.Misaki M, Barzigar N, Zotev V, Phillips R, Cheng S, Bodurka J. Real-time fMRI processing with physiological noise correction: comparison with off-line analysis. J Neurosci Methods. 2015;256:117–21. [DOI] [PubMed] [Google Scholar]
- 27.Misaki M, Bodurka J. The impact of real-time fMRI denoising on online evaluation of brain activity and functional connectivity. J Neural Eng. 2021;18(4):046092. [DOI] [PubMed] [Google Scholar]
- 28.McLaren DG, Ries ML, Xu G, Johnson SC. A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. Neuroimage. 2012;61(4):1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watson D, Clark LA. The PANAS-X: manual for the positive and negative affect schedule: expanded form. Iowa City, IA: The University of Iowa; 1994. [Google Scholar]
- 30.Evans C, Margison F, Barkham M. The contribution of reliable and clinically significant change methods to evidence-based mental health. Evid Based Ment Health. 1998;1(3): 70–2. [Google Scholar]
- 31.Guhn M, Forer B, Zumbo BD. Reliable change index. In: Michalos AC, editor. Encyclopedia of quality of life and well-being research. Dordrecht: Springer; 2014. [Google Scholar]
- 32.Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59(1):12–9. [DOI] [PubMed] [Google Scholar]
- 33.Fava GA, Guidi J, Rafanelli C, Rickels K. The clinical inadequacy of the placebo model and the development of an alternative conceptual framework. Psychother Psychosom. 2017; 86(6):332–40. [DOI] [PubMed] [Google Scholar]
- 34.Bates D, Mächler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67(1):1–48. [Google Scholar]
- 35.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B. 1995;57(1):289–300. [Google Scholar]
- 36.Huber PJ. Robust regression: asymptotics, conjectures and Monte Carlo. Ann Statist. 1973;1(5):799–821. [Google Scholar]
- 37.Venables WN, Ripley BD. Modern applied statistics with S. 4th ed. New York: Springer; 2002. [Google Scholar]
- 38.Chen G, Saad ZS, Britton JC, Pine DS, Cox RW. Linear mixed-effects modeling approach to FMRI group analysis. Neuroimage. 2013; 73:176–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129(Pt 3):564–83. [DOI] [PubMed] [Google Scholar]
- 40.Cauda F, Geminiani G, D’Agata F, Sacco K, Duca S, Bagshaw AP, et al. Functional connectivity of the posteromedial cortex. PLoS One. 2010;5(9):e13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cowansage KK, Shuman T, Dillingham BC, Chang A, Golshani P, Mayford M. Direct reactivation of a coherent neocortical memory of context. Neuron. 2014;84(2):432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang G, Xie H, Wang L, Luo W, Wang Y, Jiang J, et al. Switching from fear to No fear by different neural ensembles in mouse retrosplenial cortex. Cereb Cortex. 2019;29(12): 5085–97. [DOI] [PubMed] [Google Scholar]
- 43.Vann SD, Aggleton JP, Maguire EA. What does the retrosplenial cortex do? Nat Rev Neurosci. 2009;10(11):792–802. [DOI] [PubMed] [Google Scholar]
- 44.Fletcher PC, Frith CD, Grasby PM, Shallice T, Frackowiak RS, Dolan RJ. Brain systems for encoding and retrieval of auditory-verbal memory. An in vivo study in humans. Brain. 1995;118 (Pt 2)(Pt 2):401–16. [DOI] [PubMed] [Google Scholar]
- 45.Bio BJ, Guterstam A, Pinsk M, Wilterson AI, Graziano MSA. Right temporoparietal junction encodes inferred visual knowledge of others. Neuropsychologia. 2022;171:108243. [DOI] [PubMed] [Google Scholar]
- 46.McEvoy PM, Mahoney AEJ, Moulds ML. Are worry, rumination, and post-event processing one and the same? Development of the repetitive thinking questionnaire. J Anxiety Disord. 2010;24(5):509–19. [DOI] [PubMed] [Google Scholar]
- 47.Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspect Psychol Sci. 2008;3(5):400–24. [DOI] [PubMed] [Google Scholar]
- 48.Watkins E, Moulds M. Distinct modes of ruminative self-focus: impact of abstract versus concrete rumination on problem solving in depression. Emotion. 2005;5(3):319–28. [DOI] [PubMed] [Google Scholar]
- 49.Watkins ER, Nolen-Hoeksema S. A habit-goal framework of depressive rumination. J Abnorm Psychol. 2014;123(1):24–34. [DOI] [PubMed] [Google Scholar]
- 50.Lubianiker N, Paret C, Dayan P, Hendler T. Neurofeedback through the lens of reinforcement learning. Trends Neurosci. 2022;45(8): 579–93. [DOI] [PubMed] [Google Scholar]
- 51.Thorslund J, McEvoy PM, Anderson RA. Group metacognitive therapy for adolescents with anxiety and depressive disorders: a pilot study. J Clin Psychol. 2020;76(4):625–45. [DOI] [PubMed] [Google Scholar]
- 52.Mehler DMA, Sokunbi MO, Habes I, Barawi K, Subramanian L, Range M, et al. Targeting the affective brain-a randomized controlled trial of real-time fMRI neurofeedback in patients with depression. Neuropsychopharmacology. 2018;43(13):2578–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rance M, Walsh C, Sukhodolsky DG, Pittman B, Qiu M, Kichuk SA, et al. Time course of clinical change following neurofeedback. Neuroimage. 2018;181:807–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bruhl AB, Scherpiet S, Sulzer J, Stampfli P, Seifritz E, Herwig U. Real-time neurofeedback using functional MRI could improve down-regulation of amygdala activity during emotional stimulation: a proof-of-concept study. Brain Topogr. 2014;27(1):138–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hampson M, Stoica T, Saksa J, Scheinost D, Qiu M, Bhawnani J, et al. Real-time fMRI biofeedback targeting the orbitofrontal cortex for contamination anxiety. J Vis Exp. 2012;59: 3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sampaio-Baptista C, Neyedli HF, Sanders ZB, Diosi K, Havard D, Huang Y, et al. fMRI neurofeedback in the motor system elicits bidirectional changes in activity and in white matter structure in the adult human brain. Cell Rep. 2021;37(4):109890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scheinost D, Stoica T, Saksa J, Papademetris X, Constable RT, Pittenger C, et al. Orbito-frontal cortex neurofeedback produces lasting changes in contamination anxiety and resting-state connectivity. Transl Psychiatry. 2013;3(4):e250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Subramanian L, Hindle JV, Johnston S, Roberts MV, Husain M, Goebel R, et al. Real-time functional magnetic resonance imaging neurofeedback for treatment of Parkinson’s disease. J Neurosci. 2011;31(45):16309–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sukhodolsky DG, Walsh C, Koller WN, Eilbott J, Rance M, Fulbright RK, et al. Randomized, sham-controlled trial of real-time functional magnetic resonance imaging neurofeedback for tics in adolescents with Tourette syndrome. Biol Psychiatry. 2020;87(12): 1063–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takamura M, Okamoto Y, Shibasaki C, Yoshino A, Okada G, Ichikawa N, et al. Antidepressive effect of left dorsolateral prefrontal cortex neurofeedback in patients with major depressive disorder: a preliminary report. J Affect Disord. 2020;271:224–7. [DOI] [PubMed] [Google Scholar]
- 61.Zilverstand A, Sorger B, Sarkheil P, Goebel R. fMRI neurofeedback facilitates anxiety regulation in females with spider phobia. Front Behav Neurosci. 2015;9:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zweerings J, Sarkheil P, Keller M, Dyck M, Klasen M, Becker B, et al. Rt-fMRI neurofeedback-guided cognitive reappraisal training modulates amygdala responsivity in posttraumatic stress disorder. Neuroimage Clin. 2020; 28:102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kvamme TL, Ros T, Overgaard M. Can neurofeedback provide evidence of direct brain-behavior causality? Neuroimage. 2022;258: 119400. [DOI] [PubMed] [Google Scholar]
- 64.Thibault RT, Lifshitz M, Birbaumer N, Raz A. Neurofeedback, self-regulation, and brain imaging: clinical science and fad in the service of mental disorders. Psychother Psychosom. 2015;84(4):193–207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not publicly available due to ethics restrictions but available from the corresponding author upon reasonable request.


