Abstract
RT Cardiac Systems (RTCS, Raleigh, NC) is developing an intravascular percutaneous mechanical circulatory support (pMCS) device drive system for use during high-risk percutaneous coronary intervention (PCI) and emergent cardiogenic shock (CS). The proprietary pMCS device (US patent 10,780,206) consists of a miniaturized axial flow pump with an integrated motor connected via a short flexible drive system. This novel flexible drive system creates a flexible pump that is advantageous for percutaneous placement and conforming to anatomy. This design also has the benefit of not requiring a continuous external lubrication source. In this article we present engineering development and feasibility testing of the prototype pMCS system. Computational fluid dynamics (CFD) modeling was performed to evaluate candidate blade set designs (impeller leading and trailing edges, diffuser) and predict hydrodynamic performance and hemolysis risk. Bench testing of candidate lip seal designs (radial interference, durometer, seal angle) were evaluated for leak rate. Two 16Fr prototype devices were then fabricated and tested in a static mock flow loop. Experimental testing demonstrated 3 L/min flow against 110mmHg and 4 L/min flow against 80mmHg, which matched the CFD-predicted hydrodynamic performance. These results demonstrate feasibility of the engineering design and performance of the prototype devices.
Keywords: Mechanical circulatory support, percutaneous, cardiogenic shock, percutaneous coronary intervention
INTRODUCTION
Percutaneous mechanical circulatory support (pMCS) devices provide hemodynamic and metabolic support of cardiogenic shock (CS) patients and high-risk patients undergoing percutaneous coronary intervention (PCI).1,2 Their use, outcomes, and associated complications have been investigated through retrospective studies,3-6 prospective trials (ISAR-SHOCK trial,7 IMPRESS in Severe Shock trial,8 PROTECT II trial9), observational studies,10 and meta-analyses.11 CS patient mortality tends to be determined in the acute time period, with cause of death attributed to neurologic damage and refractory shock,8 and is the most common cause of in-hospital mortality after acute myocardial infarction10; thus, early and rapid use of pMCS, even prior to initiation of PCI and pharmacologic use, is critical to improving outcomes.2,12,13
RT Cardiac Systems (RTCS, Raleigh, NC) is developing innovative pMCS system technology featuring an implanted pump and motor assembly connected via a short, flexible drive shaft that does not require lubrication, and a new blade design. These novel features are not available in current clinically-available catheter-based pumps and are designed to increase hemodynamic support, lower the risk of blood trauma, and provide a simple, easy to operate system for improved patient management and outcomes. In this article, the engineering design, computational fluid dynamics (CFD) analysis, prototype feasibility testing in static mock flow loops, and anatomic fit in an acute animal cadaver model are presented.
METHODS
Device Concept.
RTCS is developing a pMCS system comprised of a catheter-based axial flow device and controller (Figure 1). The pump is designed for intraventricular placement via peripheral arterial access and positioned across the aortic valve. The device has a flexible connection between the pump and motor housings which facilitates percutaneous delivery, ensures reliable, continuous flexing, conforms to the patient’s anatomy, and provides peak pump performance by maintaining inflow alignment within the left ventricle (a limitation of clinically-approved MCS devices that use a non-flexing cannula). The pump utilizes a novel inflow cannula that may minimize occlusion risk by allowing the device to utilize its high flow capacity. A unique blade design includes a diffuser to efficiently convert kinetic energy coming off the impeller into the pressure head, which may reduce the risk of hemolysis, and encloses dual journal bearings supporting the drive shaft. The bearings keep the rotating shaft running smoothly to ensure the impeller never contacts the housing (minimizing vibration or cavitation), which may yield more efficient pumping and less blood stress. The bearings work without external lubrication, simplifying the system and reducing resources required to maintain an external lubrication source (required by all other clinically-approved intravascular devices). The diffuser has a lip seal on the fore section resisting blood from entering the diffuser. This location is a high surface washing region that should reduce the risk of thrombus formation. Collectively, the goal is for these novel features to translate into significant clinical benefits for patients.
Figure 1.
A, illustration of RTCS pMCS device placement with left ventricular inflow and aortic outflow. B, the device features a novel non-lubricating flexible drive system advantageous for percutaneous placement and conforming to anatomy.
A 16Fr prototype pump was developed to work out the early technical challenges, and these are presented in this article. It is anticipated the 16Fr prototype device design will form the basis for a 14Fr device providing 3.5 L/min flow at 80mmHg tailored for high-risk PCI patients and a 19Fr device providing 6+ L/min flow tailored for CS therapy.
CFD-Aided Hydraulic Design.
Candidate hydraulic designs were numerically simulated and analyzed using ANSYS version 2021 R2 (Canonsburg, PA). ANSYS Design Modeler, CFX Mesh, and CFX solver modules were used for preparing the geometry, meshing, and solution. Hydraulic design performance parameters included pressure generation (i.e. head), hemolysis index (HI), and areas of low wall shear stress. The numerical iterations were carried out by modifying the impeller and diffuser leading and trailing edge angles while keeping the hub designs unchanged.
Atmospheric pressure inlet and mass flow outlet boundary conditions were applied. The model assumed a Newtonian fluid with blood properties of 2.7cP viscosity and 1045 kg/m3 density. Design iterations were performed against a targeted 80mmHg pressure head at 3.5 L/min flow and HI of 0.002 [−]. A wall shear stress threshold of less than 1 Pa was used for flow stasis-related thrombus potential.14 The HI model is based on Eulerian application of power-law expression which relates the hemolytic damage to shear stress and exposure time (eq. 1):15
| (1) |
where t is exposure time and τ is shear stress. The constants C, α, and β were empirical (known as Heuser and Opitz constants). The shear stress was calculated based on the strain rate and the viscosity. Time derivative of the of the right-hand side of eq. 1 was used as a source term in the scalar transport equation to solve HI as a field parameter.15 Steady-state simulations were performed while monitoring the residuals, head, and hemolysis. To ensure convergence, the simulations were continued until the residuals reached below 10−4 and head and hemolysis predictions were stable.
Candidate designs consisted of one pair of impeller and diffuser with uniform thickness blades, which were placed perpendicularly to the hub. Design criteria included a 16Fr diameter and tapered impeller blade to generate a varied clearance of 0.003-0.0015 inch from the leading to trailing edge to provide radial space to enable potential for less stiff impeller operation. Those with high pressure head and low HI were considered for further analysis. An iterative design framework was manually applied to modify the impeller and diffuser blades sequentially. Once the impeller design was selected, candidate diffuser designs were simulated and evaluated, and the final design was fabricated and tested experimentally over a wide range of test conditions.
Engineering Design, Prototype, and Bench Testing.
The pMCS device is a miniaturized axial flow pump with an integrated motor connected via a short flexible drive system and consists of three major subsystems: (1) hydraulic, (2) motor, and (3) flexible drive (Figure 2). The full prototype system with the motor controller is shown in Figure 3. The controller with monitor interface is used to start and stop the device and adjust pump speed (rpm). This development effort concentrated on reducing technical risks for the hydraulic system and the novel drive system. The motor and electronics are important but utilized more mature technology and thus had less focus in this early feasibility study.
Figure 2.
Illustration of the RTCS pMCS device comprised of three subsystems (hydraulic, motor, flexible drive).
Figure 3.
A, photograph of the assembled prototype pMCS system (pump, controller, monitor) used in feasibility testing. B, closeup of prototype pump in mock loop tubing with its corresponding computer aided design (CAD) drawing below.
The 16Fr pump (2-bladed impeller, 3-bladed diffuser) is designed to operate over a range of rotational speeds (20,000 rpm start-up, 35,000-45,000 rpm) to provide up to 2.0 L/min/m2 flow against an 80mmHg load. The hydraulic system is comprised of the blood contacting surfaces that propel blood from the left ventricle via a flexible occlusion-resistant inflow cannula to the ascending aorta. The motor system (Faulhaber MicroMo, Clearwater, FL) provides rotational energy required by the hydraulic system. The drive system connects the rotating motor shaft to the hydraulic impeller. An electrical driveline connects the motor to an external control monitor that controls device operation and displays and stores real-time information. The control and monitoring system operate the pump at start-up (20,000 rpm), over the range of pump speeds (20,000-45,000 rpm), and is also expected to provide feedback parameters enabling clinicians to monitor patient physiology, system performance, and system alarms. The custom controller (Xaplos, Fort Lauderdale, FL) contains motor drive circuitry and has been tuned for the prototype motor.
The drive system (driveshaft, bearings, lip seal) was internally designed to radially and axially support the rotating shaft with attached impeller (Figure 2). Initial drive system testing showed a flexible shaft could not adequately support the rotating impeller, resulting in the impeller contacting the housing; thus, a hybrid solid-flexible shaft with the solid shaft (bore in aft section to insert flexible shaft) extending from the impeller to the thrust bearing was developed to reduce impeller radial motion. The drive shaft, thrust bearing, and motor couple are laser welded (Spectralytics, Dassel, MN) into the drive assembly. Dual journal bearings are placed in the fore and aft sections of the diffuser, which eliminate impeller-to-housing contact.
A lip seal was designed to reduce potential leakage rate between the blood and the motor. A leak tester (Figure 4) was developed to assess the relationship between radial interference of the rotating shaft and seal (0.001”, 0.003”), seal durometer (25, 45 Shore D), and seal geometry fluid/air angles (70°/35°, 40°/20°). Fluid leakage rate across the lip seal was observed (microscope) and quantified for the eight candidate design configurations at 18mmHg (25cm water column) and the pump operating at 20,000 rpm. Lip seal wear was evaluated by measuring any change in seal diameter during the test period. One sample lip seal was fabricated and tested for each of the eight design configurations.
Figure 4.
Illustrations of (A) lip seal placement on the RTCS pMCS device shaft to prevent fluid leak, and (B) test apparatus developed to investigate the relationship of seal leakage rate and the radial interference, material durometer, and seal geometry. Photographs of (C) test mount and lip seal sample, and (D) lip seal test instrument developed and used to quantify leak rate of eight candidate lip seal designs.
Accelerated drive system testing indicated a weakness in the laser welds to the flexible portion of the drive shaft. The motor coupler and the solid shaft were redesigned with dual, opposing laser slots to increase weld area and strength. The thrust bearing was strategically located over the weld slots on the solid shaft. Results from the third weld study indicated that this laser weld slot method provides the required robustness.
Static Mock Flow Loop Model.
Static mock loop experiments (Figure 5) were performed to quantify in vitro pump hydraulic performance (head pressure (H) to flow (Q), H-Q curves) of the pMCS system. Two prototype systems (pump 1 and pump 2; identical pump design and assembled components) were each tested in mock loops with 3 different viscosities. Pressure gradient and flow data were collected over a range of pump speeds by varying outflow resistance.
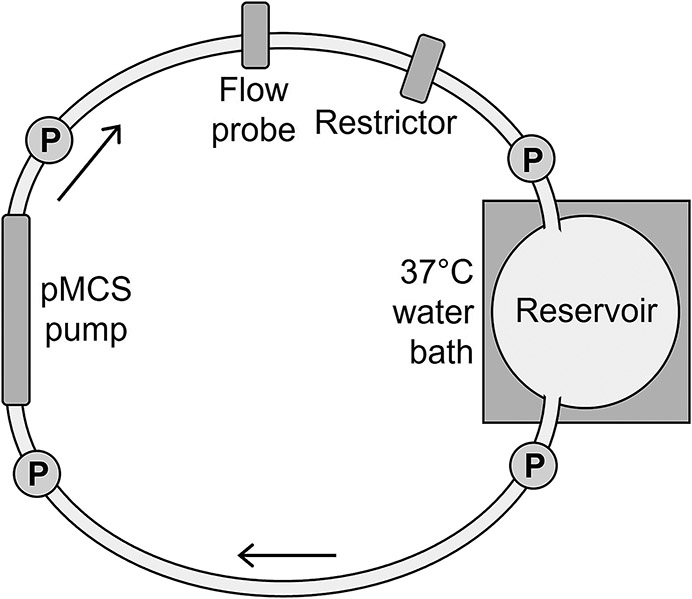
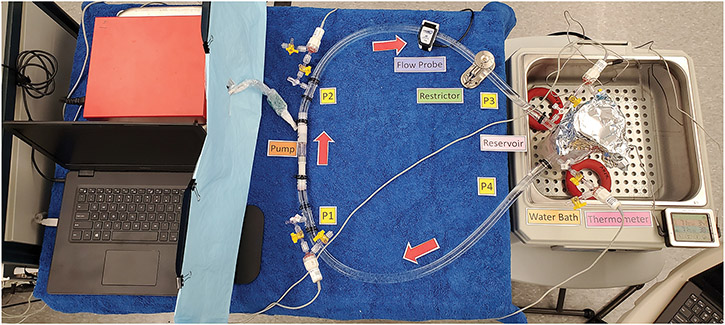
Figure 5.
A, illustration of the static mock flow loop model for testing hydrodynamic and hemodynamic performance of the prototype pumps. P, pressure transducers. B, photo of the static mock flow loop setup.
The pMCS pump was integrated into the mock loop circuit (1/2” ND-100-65 Tygon PVC). Glycerol-water solutions of 2.2cP, 2.7cP, and 3.2cP at 37°C were prepared to mimic the viscosity of blood. A Hoffman clamp was used to adjust the outflow resistance. The mock loop reservoir was maintained at 37°C. Four fluid-filled transducers (ADInstruments, Colorado Springs, CO) were used to acquire pressure data at pump inlet and outlet and at reservoir inlet and outlet. A 11mm flow probe (Transonic Systems, Ithaca, NY) was placed on the pump outflow tubing. Hemodynamic data were collected using a PowerLab 16/35 acquisition system (ADInstruments) with bridge amps (ADInstruments). Data were recorded using LabChart v.8.1.16 (ADInstruments).
Once the static mock loop was primed, the pump was set to 35,000 rpm and no resistance. Flow was decreased in 0.25 L/min increments by increasing the outflow resistance and data were collected at each increment. Pump speed was then increased by 2,500 rpm increments to 45,000 rpm, and stepwise data were reacquired at each rpm increment. Delta pressure (ΔP) was limited to 0-140mmHg. Motor temperature was documented from the controller software. The experimental design was repeated for both pumps tested at each of the 3 viscosities. Mean values for each parameter at each test condition were calculated and H-Q curves were plotted using Prism v.9.3.1 (471) (GraphPad, San Diego, CA).
Anatomical Fit Model.
A device fit study was performed in a healthy calf carcass to assess proof-of-concept and surgical fit/placement of the prototype pump. The animal was housed in a facility in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). The testing protocol16 was approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Louisville.
A Jersey calf (67kg, male, Oak Hill Genetics, Ewing, IL) was sedated (xylazine 0.02 mg/kg IM) and a catheter was placed in an ear vein for administration of ketamine 4 mg/kg IV and midazolam 0.4 mg/kg IV. The animal was intubated and placed under general isoflurane anesthesia. Heparin (300/kg IV) was administered, and the animal was euthanized under general anesthesia (Beauthanasia-D, 0.22mg/kg IV). A left thoracotomy was performed, the left ribs were removed, and the heart, aorta, and head vessels were exposed in situ to facilitate future pump placement strategies and current prototype fit.
RESULTS
CFD-Aided Hydraulic Design.
The predicted hydrodynamic and hemolytic performances for candidate impeller and diffuser designs are shown in Figure 6. From three different leading edge impeller designs (G - reference, P – less inlet angle, K – greater inlet angle), design G was selected for evaluation (Figure 6A). From three different trailing edge impeller designs (G - reference, R – straighter exit, and S – more angled exit), design S was selected for evaluation (Figure 6B). The HI of design R was not considered, as design G and S were the competing candidates. Once the impeller design was finalized, a similar iterative process was applied to the diffuser design. From three different candidate diffuser designs with a combination of leading and trailing edge modifications (3 – steep inlet and straight exit, 6 – less angled inlet and similar outlet to 3, and 7 – similar inlet to 3 with slightly more angled outlet), diffuser design 7 was selected for evaluation (Figure 6C). The predicted H-Q profiles for the final modeled pump design are shown in Figure 6D.
Figure 6.
A-C, pressure development (Head, mmHg) and hemolysis index (HI) calculation from CFD blade set simulations showing three configurations each for simulated performance of impeller leading edge profiles (A), impeller trailing edge profiles (B), and diffuser blade profiles (C). D, predicted head pressure (H) to flow (Q; H-Q) profiles for the final modeled pump design.
Impeller S and diffuser 7 yielded the best results that met pump design criteria. Over the range of flow conditions from 2.5-4.0 L/min at the targeted pressure head, the predicted hemolytic potential was minimally impacted (HI from 0.00100-0.00113 [−]) and flow stasis potential was insignificant (area of critical WSS from 10−6-10−3 mm2). Therefore, the predicted hydrodynamic performance for impeller S and diffuser 7 suggested that design criteria was achieved. The predicted hydraulic performance was found to be comparable to the measurement with a maximum 3% error at 80mmHg and 3.5 L/min conditions.
Lip Seal.
The continuous pump speed (20,000 rpm) test period varied ranging from 0.1 hour (gross leak, terminated early) to 6 hours (0.06 mL/hr leak rate). Lip seal leak rates for all eight candidate designs varied from 0.05-1.23 mL/hr. Anecdotal observations, due to limited sample size (n=1) and variable test period (2-6 hours), suggested that radial interference of 0.003” produced the lowest leak rate (0.34 mL/hr). The lower 25D durometer Pebax material resulted in the least wear (0.0007” decrease in diameter). Subsequently, the design configuration with 0.001” radial interference, 25D Pebax, and geometry with 70° (fluid side) and 35° (air side) angles was selected for the two fabricated prototype devices.
Static Mock Loop Model.
Experiments were performed to quantify in vitro pump hydraulic performance (H-Q curves) of the two prototype pMCS pumps using static mock flow loops with 3 different fluid viscosities. Results (six experiments) demonstrated both pumps performed comparably: against a ΔP of approximately 80mmHg at 43,000-45,000 rpm, both pumps delivered flows of 3.5 L/min at all viscosities (Figure 7 A-F). These H-Q curves acquired experimentally are comparable to those predicted during pump design with the CFD model (Figure 6D).
Figure 7.
Relationship of head pressure (H) to flow (Q) for the two prototype pumps in a static mock flow loop model. H-Q curves for pump 1 (A, C, and E) and pump 2 (B, D, and F) were each tested against viscosities of 2.2cP (A and B), 2.7cP (C and D), and 3.2cP (E and F). Note that at 2.2 cP, pump 2 achieved a maximum 43,000 rpm.
In the 2.2cP loop, pump 1 delivered 4.3 L/min flow against a ΔP of 9mmHg at 45,000 rpm, while pump 2 delivered 4.2 L/min flow against a ΔP of 15mmHg at 43,000 rpm (Figure 7 A and B). In the 2.7cP loop, pump 1 delivered 4.4 L/min flow against a ΔP of 10mmHg at 45,000 rpm, while pump 2 delivered 4.3 L/min flow against a ΔP of 15mmHg at 45,000 rpm (Figure 7, C and D). In the 3.2cP loop, pump 1 delivered 4.3 L/min flow against a ΔP of 13mmHg at 45,000 rpm, while pump 2 delivered 4.2 L/min flow against a ΔP of 17mmHg at 45,000 rpm (Figure 7, E and F).
Motor temperature of both pumps progressively increased from 32°C (pump 1) and 37°C (pump 2) during static mock loop testing. Pump 1 motor temperature reached 53°C and pump 2 motor temperature reached 58°C at their highest point (3.2cP, 45,000 rpm). Pump 2 was unable to reach 45,000 rpm at 2.2cP and no outflow resistance, and it experienced significant vibrations at 43,000 rpm with outflow resistance set to 2.75 L/min.
Animal Model.
The device fit study in the calf carcass (Figure 8) provided useful information for the next iteration of pump design improvements and potential surgical placement options. The primary observational information was inflow cannula length for the bovine model, motor driveline improvements for intravascular use, and the critical need to complete drive shaft development to facilitate the desired flexibility to ease implantation.
Figure 8.
Anatomic fit study in a calf carcass for assessing feasibility of placement for future device iterations. A, left lateral thoracotomy with left ribs removed. The prototype pump is resting on the heart and positioned to approximate the left ventricular inflow and aortic outflow. B, view of the prototype pump in the same carcass with the aorta (foreground) and left ventricle opened and with the pump placed across the opened aortic valve.
DISCUSSION
RTCS is developing an innovative pMCS device drive system for use during high-risk PCI and CS. In this study, we presented engineering development and performance and demonstrated proof-of-concept and feasibility of the early prototype system. Using CFD and static mock flow loops, several features requiring design improvements were identified.
The primary challenge was hydraulic performance. The impeller and diffuser blade design were iteratively optimized to maximize device output capacity while maintaining predicted risk of hemolysis. The second primary challenge was ensuring robustness of the flexible drive system. Initially, the flexible drive shaft extended from the impeller to the motor; however, we observed a single component flexible shaft allowed too much impeller radial movement causing impeller contact with the stationary housing; a single journal bearing was insufficient to maintain rotational support, and the laser welding needed to be more robust to allow drive shaft rotation during flexing. These observations led to enhancements of a hybrid solid-flexible drive shaft, selection of dual journal bearings on the solid portion of the drive shaft, and laser weld slots.
A secondary challenge was the motor. We selected a commercial motor for the feasibility testing which was oversized and worked well to confirm feasibility; however, a motor with the required torque-speed characteristics and sized within the requirements will be necessary to advance toward design freeze with continued evaluation in future in vivo studies. The motor also needs to have a smaller diameter driveline appropriate for in vivo studies. The prototype motor driveline had a small, printed circuit board which prohibited placement in the animal cadaver model.
Several tertiary challenges were identified, including completion of the inflow cannula design, lip seal for the drive shaft, drive sheath, and control and monitoring system enhancements for data storage and real-time display. A previously designed inflow cannula tip (patent 9,050,418) will be evaluated next. A rigorous testing protocol of future lip seal design iterations will need to be completed and a selection will be made based on leakage rate and seal material wear.
In conclusion, we completed engineering development and feasibility testing of the prototype 16Fr RTCS pMCS device. Significant design achievements were accomplished, including confirmation of the robust drive system and high-performance hydraulic blade set. These preliminary experiments provided critical insights into the remaining engineering work needed and are planned for the next formal development phase. RTCS envisions a future 14Fr configuration tailored specifically for high-risk PCI and a larger 19Fr configuration tailored for CS therapy with expected capacity of 3.5 L/min and 6+ L/min flow at 80mmHg, respectively. The high-level performance robustness provides confidence that these products will be commercially competitive and facilitate improved outcomes in these patient populations.
ACKNOWLEDGEMENTS
The authors thank Brett M. Cook (Alstonvale, Australia). The authors also thank Jiapeng Huang MD, PhD, Karen S. Powell DVM, PhD, Siddharth Pahwa MD, Todd Adams, and the staff of the Comparative Medicine Research Unit at the University of Louisville for study support.
Conflict of Interest and Sources of Funding Statement:
Conflict of Interest: This study was supported by a National Institutes of Health SBIR grant 1R43HL152774-01 (PI LaRose, Co-Is Slaughter, Koenig, Monreal). Mr. LaRose is the founder and owner of RT Cardiac Systems and a consultant for CDX Medical Technologies. Dr. Taskin is an employee of Medtronic Inc and consultant for RT Cardiac Systems. This presentation is derived from Dr. Taskin's opinions and experiences and does not represent the position of Medtronic. Mr. Shambaugh is a consultant for RT Cardiac Systems and CDX Medical Technologies. Dr. Slaughter is co-founder of Cor Habere and MAST developing medical devices supported by NIH SBIR phase I grants (R43HL142337-01, R43HL142385-01, R43HL152894-01) and is a consultant with Magenta Medical; however, these affiliations and funded projects are unrelated to the subcontract with RT Cardiac Systems and independent of the work presented in this manuscript. Dr. Koenig is co-founder of Cor Habere and MAST developing medical devices supported by NIH SBIR phase I grants (R43HL142337-01, R43HL142385-01, R43HL152894-01); however, these affiliations and funded projects are unrelated to the subcontract with RT Cardiac Systems and independent of the work presented in this manuscript. Dr. Monreal is supported in part by a gift from Robert M. Prizant to the Legacy Foundation of Kentuckiana.
Sources of Funding: This study was supported by a National Institutes of Health SBIR grant 1R43HL152774-01 (PI Larose, Co-Is Slaughter, Koenig, Monreal). The sponsor of this study, RT Cardiac Systems had a role in the study design, data collection and analysis, decision to publish, and preparation of this manuscript.
ABBREVIATIONS
- CFD
computational fluid dynamics
- CS
cardiogenic shock
- HI
hemolysis index
- H-Q
head pressure (H) to flow (Q)
- PCI
percutaneous coronary intervention
- pMCS
percutaneous mechanical circulatory support
REFERENCES
- 1.Manian N, Thakker J, Nair A: The use of mechanical circulatory assist devices for ACS patients with cardiogenic shock and high-risk PCI. Curr Cardiol Rep 24: 699–709, 2022. [DOI] [PubMed] [Google Scholar]
- 2.Asleh R, Resar JR: Utilization of percutaneous mechanical circulatory support devices in cardiogenic shock complicating acute myocardial infarction and high-risk percutaneous coronary interventions. J Clin Med 8: 1209, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elbadawi A, Elgendy IY, Omer MA et al. : Hospital volume and in-hospital outcomes with Impella guided percutaneous coronary interventions: insights from a national database. Am J Cardiol 125: 1753–1754, 2020. [DOI] [PubMed] [Google Scholar]
- 4.Schrage B, Ibrahim K, Loehn T, et al. : Impella support for acute myocardial infarction complicated by cardiogenic shock. Circulation 139: 1249–1258, 2019. [DOI] [PubMed] [Google Scholar]
- 5.Ameloot K, Bastos BM, Daemen J, et al. New-generation mechanical circulatory support during high-risk PCI: a cross-sectional analysis. EuroIntervention 15: 427–433, 2019. [DOI] [PubMed] [Google Scholar]
- 6.Dhruva SS, Ross JS, Mortazavi BJ, et al. : Use of mechanical circulatory support devices among patients with acute myocardial infarction complicated by cardiogenic shock. JAMA Netw Open 4: e2037748, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seyfarth M, Sibbing D, Bauer I, et al. : A randomized clinical trial to evaluate the safety and efficacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol 52: 1584–8, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Karami M, Eriksen E, Ouweneel DM, et al. : Long-term 5-year outcome of the randomized IMPRESS in severe shock trial: percutaneous mechanical circulatory support vs. intra-aortic balloon pump in cardiogenic shock after acute myocardial infarction. Eur Heart J Acute Cardiovasc Care 10: 1009–15, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Neill WW, Kleiman NS, Moses J, et al. : A prospective, randomized clinical trial of hemodynamic support with Impella 2.5 versus intra-aortic balloon pump in patients undergoing high-risk percutaneous coronary intervention: the PROTECT II study. Circulation 126: 1717–27, 2012. [DOI] [PubMed] [Google Scholar]
- 10.Tehrani BN, Truesdell AG, Sherwood MW, et al. : Standardized team-based care for cardiogenic shock. J Am Coll Cardiol 73: 1659–1669, 2019. [DOI] [PubMed] [Google Scholar]
- 11.Moustafa A, Khan MS, Saad M, Siddiqui S, Eltahawy E: Impella support versus intra-aortic balloon pump in acute myocardial infarction complicated by cardiogenic shock: a meta-analysis. Cardiovasc Revasc Med 34: 25–31, 2022. [DOI] [PubMed] [Google Scholar]
- 12.Basir MB, Schreiber TL, Grines CL, et al. : Effect of early initiation of mechanical circulatory support on survival in cardiogenic chock. Am J Cardiol 119: 845–851, 2017. [DOI] [PubMed] [Google Scholar]
- 13.Al-Atta A, Zaidan M, Abdalwahab A, et al. : Mechanical circulatory support in acute myocardial infarction complicated by cardiogenic shock. Rev Cardiovasc Med 23: 071, 2022. [DOI] [PubMed] [Google Scholar]
- 14.Hochareon P, Manning KB, Fontaine AA, et al. : Correlation of in vivo clot deposition with the flow characteristics in the 50 cc Penn State artificial heart: a preliminary study. ASAIO J 50: 537–542, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Taskin ME, Fraser KH, Zhang T, et al. : Evaluation of Eulerian and Lagrangian models for hemolysis estimation. ASAIO J 58: 363–72, 2012. [DOI] [PubMed] [Google Scholar]
- 16.National Research Council (US) Committee for the Update of the Guide for the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. 8th ed. Washington (DC): National Academies Press (US); 2011. [PubMed] [Google Scholar]