Abstract
Background:
Little is known about patient-specific risk factors for skin neoplasia in individuals with Lynch Syndrome (LS).
Objective:
Identify clinical factors associated with development of skin neoplasms in LS.
Methods:
Clinical data were systematically collected on a cohort of LS carriers (confirmed pathogenic germline variants [PGVs] in MLH1, MSH2, MSH6, PMS2, or EPCAM) age ≥18 undergoing clinical genetics care at Dana-Farber Cancer Institute from 1/2000–3/2020. Multivariable logistic regression was performed to evaluate clinical factors associated with skin neoplasia.
Results:
Of 607 LS carriers, 9.2% had LS-associated skin neoplasia and 15.0% had non-LS-associated skin neoplasia. 58.2% (353/607) had documentation of prior dermatologic evaluation. 29.7% (38/128) with skin neoplasms lacked a history of visceral LS-associated malignancy. LS-associated skin neoplasms were significantly associated with male sex, age, race, MLH1 PGVs, MSH2/EPCAM PGVs, and personal history of non-LS skin neoplasms. Non-LS-associated skin neoplasms was significantly associated with age, number of first- and second-degree relatives with non-LS-associated skin neoplasms, and personal history of LS-associated skin neoplasms.
Limitations:
Single-institution observational study; demographic homogeneity.
Conclusions:
Skin neoplasms are common in individuals with LS. We identified clinical factors associated with LS- and non-LS-associated skin neoplasms. Regular dermatologic surveillance should be considered for all LS carriers.
Keywords: Muir-Torre syndrome, sebaceous, keratoacanthoma, hereditary, HNPCC
INTRODUCTION
Lynch syndrome (LS) is an autosomal dominant disorder that is the most common cause of inherited gastrointestinal cancer.1 LS is caused by pathogenic germline variants in DNA mismatch repair genes (MLH1, MSH2, MSH6, PMS2) or EPCAM, and predisposes individuals to a wide array of visceral malignancies, including colorectal, endometrial, ovarian, and other cancers.2,3 A subset of individuals with LS also develop specific skin neoplasms, including sebaceous carcinomas, sebaceous adenomas, and keratoacanthomas.4–6 LS carriers with cutaneous sebaceous neoplasms are classified as having Muir-Torre Syndrome (MTS).4
Despite skin neoplasms being well-known manifestations of LS, clinical practice guidelines7–13 provide minimal guidance regarding dermatologic management for LS carriers due to a paucity of data about the prevalence and risk factors for such skin neoplasms. Additionlly, recent studies have found microsatellite instability and/or DNA mismatch repair deficiency in squamous cell carcinomas and melanomas in individuals with LS, suggesting that some skin neoplasms not classically thought to be LS-associated might arise through similar biologic pathways as LS-associated skin neoplasms.14–16
Our primary aims were to evaluate the frequency of LS-associated and non-LS associated neoplasms in a large cohort of confirmed LS carriers and evaluate specific clinical factors associated with the development of skin neoplasms in LS carriers. Secondary aims were to characterize the spectrum and anatomic location of skin neoplasms in LS carriers.
METHODS
This was an observational study that retrospectively analyzed individuals who had been prospectively recruited to a cancer genetics registry at the Dana-Farber Cancer Institute (DFCI), Boston, MA. As previously described,17 we analyzed individuals age ≥18 years at the time of germline testing who had confirmed pathogenic/likely pathogenic germline variants (PGVs) in MLH1, MSH2, MSH6, PMS2, or EPCAM who were recruited between 1/2000–3/2020 while receiving clinical LS care at the DFCI cancer genetics clinic. For LS families in whom multiple members were enrolled, we assigned proband status to the individual with the most comprehensive available clinical information. Clinical data were self-reported and reviewed by a genetic counselor and physician before being entered into the Progeny (Progeny Genetics, Delray Beach, FL) software program. Medical chart review was conducted to abstract dermatologic data, including history of skin neoplasia (by formal pathology report and/or mention in a clinical note) and whether the individual had a documented history of having undergone formal dermatologic evaluation. Individuals with a documented skin neoplasm were counted as having seen a dermatologist at least once.
LS-associated skin neoplasms were defined as sebaceous adenomas, sebaceous carcinomas, sebaceous epitheliomas/sebaceomas, and keratoacanthomas. All other primary skin neoplasms were classified as non-LS-associated, including skin neoplasms otherwise not specified. LS-associated visceral malignancies were defined as colorectal, endometrial, ovarian, urinary tract, small bowel, gastric, pancreatic, biliary, and brain cancers. As previously described,17 family history of skin neoplasm was analyzed as a categorical variable (yes/no) and as a continuous variable by examining the number of affected first- and/or second-degree relatives from the same side of the family.
The study cohort was analyzed with descriptive statistics. Bivariate association analyses were conducted to compare individuals with LS-associated skin neoplasm versus those without, and those with non-LS-associated skin neoplasms and those without.
Variables that were significant in the bivariate association analysis of LS-associated skin neoplasms were considered for inclusion in multivariable analyses of both LS- and non-LS-associated skin neoplasms. Multivariable logistic regressions were performed to examine the association between personal history of LS-associated and non-LS-associated skin neoplasms with the aforementioned significant clinical factors. LS carriers with MSH2 and EPCAM PGVs were analyzed in aggregate as were MSH6 and PMS2 PGVs due to sample size and the genes’ comparable penetrance. Odds ratios (OR) summarized the degree of association, and 95% confidence intervals (CI) were reported. We considered associations to be statistically significant at alpha <0.05 (two-sided). Analyses were conducted in R version 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
Missing age at germline testing data were imputed with the following priority: 1) latest age at diagnosis of any LS cancers (maximum age at diagnosis), 2) current age or age at death, and 3) estimated current age. Missing race data were classified as “unknown” in the multivariable analysis.
The study was approved by the Dana-Farber/Harvard Cancer Center Institutional Review Board (Boston, MA).
RESULTS
607 individuals with LS PGVs were included in the study cohort, 413 (68.0%) of whom were female and 501 (82.5%) of whom were white. Median age at germline testing was 50.0 years (IQR 39.5–61.0). 59.8% (363/607) of individuals had a personal history of a LS-associated visceral malignancy, the most common of which was colorectal cancer (34.8%, 211/607). 353/607 (58.2%) LS carriers had documentation in the medical record of having had formal evaluation by a dermatologist.
128/607 (21.1%) LS carriers had any skin neoplasm, including 56 (9.2%) with LS-associated skin neoplasms, 91 (15.0%) with non-LS-associated skin neoplasms, and 19 (3.1%) with both- LS and non-LS-associated skin neoplasms (Table 1). Median age at first skin neoplasm was 54.0 years (range 25–79). 38/128 (29.7%) LS carriers with skin neoplasms had no history of LS-associated visceral malignancy. Age at diagnosis data were available for 39/43 LS carriers with a personal history of both LS-associated skin neoplasm and LS-associated visceral malignancy, of whom 8/39 (20.5%) had their first skin neoplasm concurrent (n=2) or prior to (n=6) their first visceral malignancy.
Table 1:
Clinical characteristics of a cohort of individuals with Lynch syndrome
| Entire cohort (n=607) |
Participants with Lynch-associated skin neoplasm1 (n=56) |
Participants without Lynch-associated skin neoplasm1 (n=551) |
Participants with non-Lynch-associated skin neoplasm (n=91) |
Participants without non-Lynch-associated skin neoplasm (n=516) |
|||
|---|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | p-value | N (%) | N (%) | p-value | |
|
| |||||||
| Female | 413 (68.0) | 29 (51.8) | 384 (69.7) | 0.010 | 63 (69.2) | 350 (67.8) | 0.903 |
| Male | 194 (32.0) | 27 (48.2) | 167 (30.3) | 28 (30.8) | 166 (32.2) | ||
| Median age at germline testing, years (IQR) | 50.0 (39.5–61.0) | 55.5 (48.0–66.2) | 50.0 (39.0–60.0) | 0.001 | 60.0 (51.0–67.0) | 49.0 (38.0–59.0) | <0.001 |
| Race | |||||||
| White | 501 (82.5) | 53 (94.6) | 448 (81.3) | 0.132 | 81 (89.0) | 420 (81.4) | 0.177 |
| Black | 11 (1.8) | 0 | 11 (2.0) | 0 | 11 (2.1) | ||
| Asian/Pacific Islander | 15 (2.5) | 1 (1.8) | 14 (2.5) | 1 (1.1) | 14 (2.7) | ||
| Other | 19 (3.1) | 1 (1.8) | 18 (3.3) | 0 | 19 (3.7) | ||
| Missing | 61 (10.0) | 1 (1.8) | 60 (10.9) | 9 (9.9) | 52 (10.1) | ||
| Lynch syndrome gene with pathogenic variant | |||||||
| MLH1 | 137 (22.6) | 13 (23.2) | 124 (22.5) | <0.001 | 22 (24.2) | 115 (22.3) | 0.927 |
| MSH2 | 180 (29.7) | 36 (64.3) | 144 (26.1) | 28 (30.8) | 152 (29.5) | ||
| MSH6 | 145 (23.9) | 7 (12.5) | 138 (25.0) | 20 (22.0) | 125 (24.2) | ||
| PMS2 | 138 (22.7) | 0 | 138 (25.0) | 21 (23.1) | 117 (22.7) | ||
| EPCAM | 7 (1.2) | 0 | 7 (1.3) | 0 | 7 (1.4) | ||
| Personal history of any Lynch-associated visceral malignancy | 363 (59.8) | 43 (76.8) | 320 (58.1) | 0.006 | 62 (68.1) | 301 (58.3) | 0.083 |
| Colorectal cancer | 211 (34.8) | 27 (48.2) | 184 (33.4) | 0.038 | 36 (39.6) | 175 (33.9) | 0.340 |
| Endometrial cancer* | 129/413 (31.2)* | 15/29 (51.7)* | 114/384 (29.7)* | 0.021 | 27/63 (42.9)* | 102/350 (29.1)* | 0.038 |
| Ovarian cancer* | 30/413 (7.3)* | 2/29 (6.9)* | 28/384 (7.3)* | 1.000 | 10/63 (15.9)* | 20/350 (5.7)* | 0.014 |
| Urinary tract cancer | 27 (4.4) | 8 (14.3) | 19 (3.4) | 0.002 | 8 (8.8) | 19 (3.7) | 0.047 |
| Small bowel cancer | 10 (1.6) | 1 (1.8) | 9 (1.6) | 1.000 | 2 (2.2) | 8 (1.6) | 0.651 |
| Gastric cancer | 9 (1.5) | 3 (5.4) | 6 (1.1) | 0.042 | 0 | 9 (1.7) | 0.369 |
| Pancreatic cancer | 10 (1.6) | 0 | 10 (1.8) | 0.610 | 2 (2.2) | 8 (1.6) | 0.651 |
| Hepatobiliary cancer | 5 (0.8) | 2 (3.6) | 3 (0.5) | 0.070 | 1 (1.1) | 4 (0.8) | 0.557 |
| Brain cancer | 4 (0.7) | 0 | 4 (0.7) | 1.000 | 0 | 4 (0.8) | 1.000 |
| Family history of Lynch-associated neoplasm** | |||||||
| Any Lynch-associated skin neoplasm | 33 (5.4) | 9 (16.1) | 24 (4.4) | 0.002 | 9 (9.9) | 24 (4.7) | 0.073 |
| Colorectal cancer | 405 (66.7) | 46 (82.1) | 359 (65.2) | 0.011 | 66 (72.5) | 339 (65.7) | 0.228 |
| Endometrial cancer | 219 (36.1) | 24 (42.9) | 195 (35.4) | 0.307 | 31 (34.1) | 188 (36.4) | 0.723 |
| Ovarian cancer | 72 (11.9) | 5 (8.9) | 67 (12.2) | 0.664 | 11 (12.1) | 61 (11.8) | 1.000 |
| Urinary tract cancer | 126 (20.8) | 18 (32.1) | 108 (19.6) | 0.037 | 27 (29.7) | 99 (19.2) | 0.035 |
| Small bowel cancer | 21 (3.5) | 5 (8.9) | 16 (2.9) | 0.036 | 6 (6.6) | 15 (2.9) | 0.110 |
| Gastric cancer | 69 (11.4) | 8 (14.3) | 61 (11.1) | 0.505 | 11 (12.1) | 58 (11.2) | 0.858 |
| Pancreatic cancer | 64 (10.5) | 5 (8.9) | 59 (10.7) | 0.822 | 8 (8.8) | 56 (10.9) | 0.711 |
| Hepatobiliary cancer | 31 (5.1) | 2 (3.6) | 29 (5.3) | 1.000 | 5 (5.5) | 26 (5.0) | 0.798 |
| Brain cancer | 15 (2.5) | 0 | 15 (2.7) | 0.383 | 1 (1.1) | 14 (2.7) | 0.711 |
Denominator includes female participants only
Family history in any first- and/or second-degree relative
Lynch-associated skin neoplasms are defined as sebaceous neoplasms and keratoacanthomas
IQR = interquartile range
Of the 128 LS carriers with any skin neoplasm, 36 (28.1%) had multiple histologic types of skin neoplasms. The most common type of skin neoplasm was basal cell carcinoma (43.0%, 55/128), followed by sebaceous adenoma (34.4%, 44/128) and squamous cell carcinoma (27.3%, 35/128) (Table 2). Other skin neoplasms included melanoma (13.3%, 17/128), sebaceous carcinoma (10.9%, 14/128), sebaceous epithelioma/sebaceomas (5.5%, 7/128), keratoacanthoma (3.1%, 4/128), and Merkel cell carcinoma (0.8%, 1/128). One patient with melanoma and one with Merkel cell carcinoma had regional lymph node metastases, but no others had documented metastatic disease.
Table 2:
Number of Lynch syndrome carriers (N) with specific skin neoplasms
| Type of skin neoplasm | N=607 (%) |
|---|---|
| No skin neoplasm | 479 (78.9) |
| Any Lynch-associated skin neoplasm1 | 56 (9.2) |
| Sebaceous adenoma | 44 (7.2) |
| Sebaceous carcinoma | 14 (2.3) |
| Sebaceous epithelioma | 7 (1.2) |
| Keratoacanthoma | 4 (0.7) |
| Any Non-Lynch-associated skin neoplasm | 91 (15.0) |
| Basal cell carcinoma | 55 (9.1) |
| Squamous cell carcinoma | 35 (5.8) |
| Melanoma | 17 (2.8) |
| Merkel cell carcinoma | 1 (0.2) |
| Unspecified skin neoplasm | 3 (0.5) |
Lynch-associated skin neoplasms are defined as sebaceous neoplasms and keratoacanthomas
The 128 LS carriers with skin neoplasia had a total of 268 skin neoplasms, 97 of which were LS-associated and 171 of which were non-LS-associated. Anatomic location was documented for 169/268 (63.1%) skin neoplasms. For sebaceous adenomas, sebaceous epitheliomas/sebaceomas, and keratoacanthomas, the most common anatomic location was the face, while for sebaceous carcinomas, the most common location was the trunk (Figure 1). For non-LS-associated skin neoplasms, the most common anatomic location for basal and squamous cell carcinomas was the face, and for melanoma was the trunk.
Figure 1. Anatomic location of skin neoplasms (N=268, where N is number of skin neoplasms) in 128 individuals with Lynch syndrome and skin neoplasms.
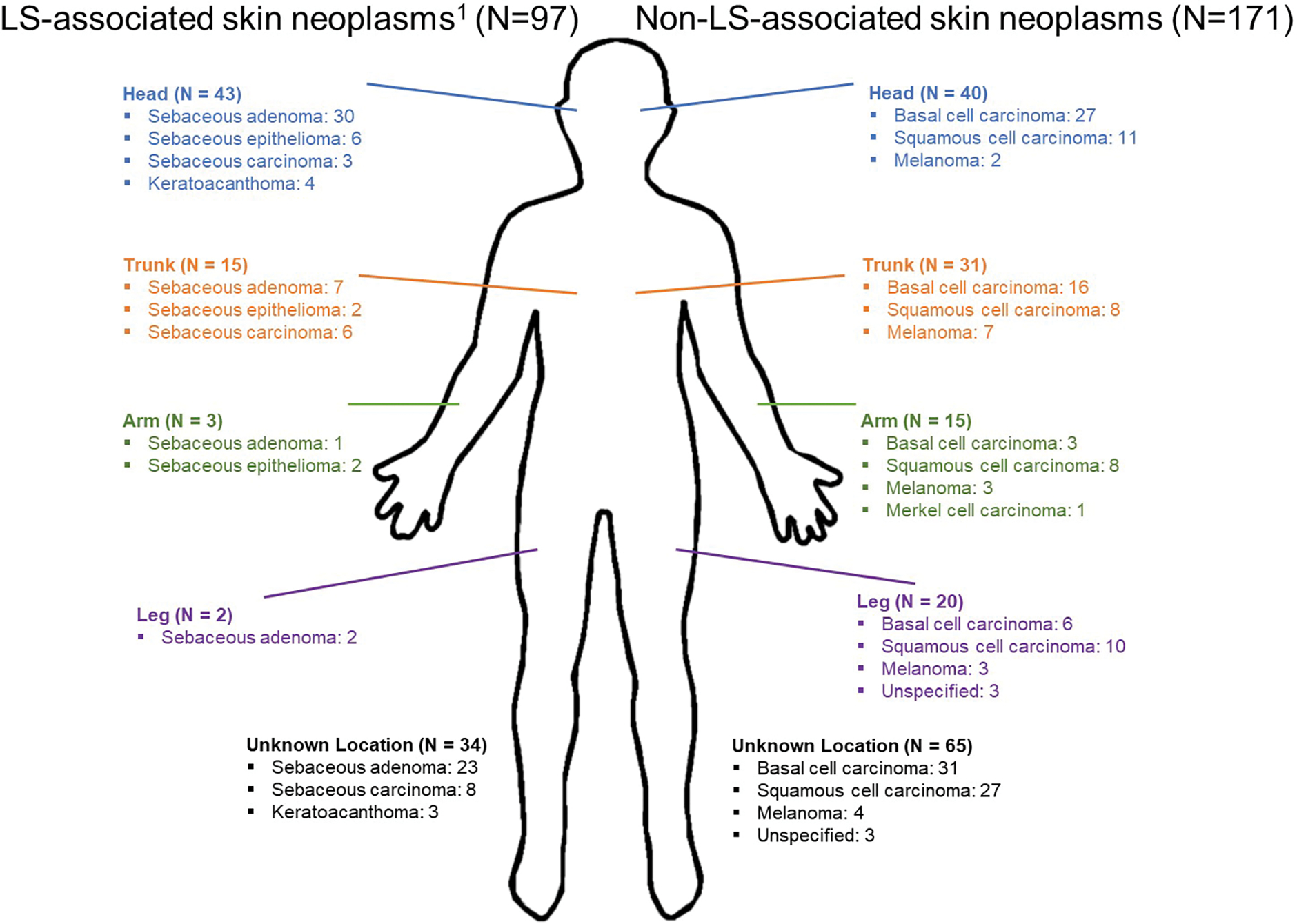
1Lynch-associated skin neoplasms are defined as sebaceous neoplasms and keratoacanthomas
On bivariate association analysis, individuals with LS-associated skin neoplasms were significantly more likely to be male, older at germline testing, and to have MSH2/EPCAM PGVs, compared to those without LS-associated skin neoplasms (Table 1). They were also significantly more likely to have a personal and/or family history of various LS-associated malignancies, including LS-associated skin neoplasms (Table 1). On bivariate association analysis, patients with non-LS skin neoplasms were significantly more likely than those without non-LS skin neoplasms to be older at time of germline testing and to have a personal and/or family history of certain LS-associated malignancies.
On multivariable analysis, personal history of LS-associated skin neoplasm was significantly associated with male sex (OR 2.44, 95% CI 1.30–4.57), increasing age (OR 1.77 per 10 years, 95% CI 1.36–2.32), race (OR 0.09 for unknown race, 95% CI 0.01–0.72; reference: white race), MLH1 PGVs (OR 6.67, 95% CI 2.33–19.13; reference: MSH6/PMS2 PGVs), MSH2/EPCAM PGVs (OR 15.33, 95% CI 5.95, 39.45; reference: MSH6/PMS2 PGVs), and personal history of non-LS skin neoplasms (OR 2.69, 95% CI 1.33–5.46) (Table 3). There were no significant associations between personal history of LS-associated skin neoplasm and either personal history of LS-associated visceral cancer or number of first-/second-degree relatives with LS-associated skin neoplasms.
Table 3:
Multivariable analysis of clinical factors associated with personal history of Lynch syndrome-associated skin neoplasms
| Odds Ratio | 95% CI | p-value | |
|---|---|---|---|
|
| |||
| Male sex (ref: female) | 2.44 | (1.30, 4.57) | 0.005 |
| Age | 1.77 (per decade) | (1.36, 2.32) | <0.001 |
| Race (ref: white) | |||
| Non-white | 0.42 | (0.09, 1.93) | 0.266 |
| Unknown | 0.09 | (0.01, 0.72) | 0.023 |
| MMR gene with pathogenic germline variant (ref: MSH6/PMS2) | |||
| MLH1 | 6.67 | (2.33, 19.13) | <0.001 |
| MSH2/EPCAM | 15.33 | (5.95, 39.45) | <0.001 |
| Personal history of LS-associated visceral malignancy | 1.57 | (0.77, 3.23) | 0.217 |
| Number of 1st/2nd degree relatives with LS skin neoplasms | 1.65 (per relative) | (0.79, 3.47) | 0.186 |
| Personal history of non-LS skin neoplasm | 2.69 | (1.33, 5.46) | 0.006 |
On multivariable analysis, personal history of non-LS-associated skin neoplasms was significantly associated with age (OR 1.79 per 10 years, 95% CI 1.45–2.20), number of first- and second-degree relatives with non-LS skin neoplasms (OR 1.60 per first-/second-degree relative, 95% CI 1.19–2.16), and personal history of LS skin neoplasms (OR 2.47, 95% CI 1.23–4.97) (Table 4). Personal history of non-LS-associated skin neoplasms was not significantly associated with race, sex, MLH1 or MSH2/EPCAM PGVs, or personal history of LS-associated visceral cancers.
Table 4:
Multivariable analysis of clinical factors associated with personal history of non-Lynch syndrome-associated skin neoplasms
| Odds Ratio | 95% CI | p-value | |
|---|---|---|---|
|
| |||
| Male sex (ref: female) | 0.82 | (0.49, 1.39) | 0.459 |
| Age | 1.79 (per decade) | (1.45, 2.20) | <0.001 |
| Race (ref: white) | |||
| Non-white | 0.19 | (0.03, 1.44) | 0.108 |
| Unknown | 0.85 | (0.38, 1.90) | 0.686 |
| MMR gene with pathogenic germline variant (ref: MSH6/PMS2) | |||
| MLH1 | 1.91 | (1.00, 3.66) | 0.050 |
| MSH2/EPCAM | 1.25 | (0.67, 2.32) | 0.479 |
| Personal history of LS-associated visceral malignancy | 1.06 | (0.63, 1.80) | 0.819 |
| Number of 1st/2nd degree relatives with non-LS skin neoplasms | 1.60 (per relative) | (1.19, 2.16) | 0.002 |
| Personal history of LS-associated skin neoplasm | 2.47 | (1.23, 4.97) | 0.011 |
Lynch-associated skin neoplasms are defined as sebaceous neoplasms and keratoacanthomas
DISCUSSION
The Muir-Torre Syndrome was first described in 1967 separately by Muir and Torre when they observed patients with multiple sebaceous carcinomas and bowel cancer.18,19 In 1981, Lynch and colleagues identified MTS to be a phenotypic variant of what is now known as LS.20 Since then, skin neoplasms have become a well-recognized manifestation of LS, yet little is known about patient-specific factors that predict for their development. In this study of more than 600 LS carriers with extensive clinical/family history data supported by formal medical record review, we found that LS-associated skin neoplasms (sebaceous neoplasms and keratoacanthomas) were significantly and independently associated with male sex, older age, white race, MLH1 PGVs, MSH2 PGVs, and personal history of non-LS skin neoplasms. We also examined the prevalence and clinical associations of skin neoplasms not classically linked to LS/MTS, which has not been extensively studied in the literature to date.
We found that 21% of individuals in our cohort had a history of skin neoplasia, thereby making skin neoplasms the third most common form of malignancy/neoplasm in these LS carriers, behind colorectal and endometrial cancers. Of LS carriers with both cutaneous and visceral malignancies for whom we had available age data, >20% had a LS-associated skin neoplasm (sebaceous neoplasms and/or keratoacanthomas) precede or coincide with their first LS-associated visceral malignancy. Additionally, nearly 30% of LS carriers with a skin neoplasm lacked any LS-associated visceral malignancy. This highlights that skin neoplasms are common, important, and sometimes early or only manifestations of LS. Our findings also highlight gaps in dermatologic care for LS carriers, with 42% having had no documented care with a dermatologist in their medical chart. As such, the 21% of LS carriers with skin neoplasia may actually underestimate of the true prevalence, since those without prior dermatologic care may have undetected skin neoplasms.
In this study of >600 genetically confirmed LS carriers, 9.2% had sebaceous neoplasia and/or keratoacanthomas, thereby classifying them as having the MTS subtype of LS. This is on the high end of prevalence estimates (5–9%) from prior smaller studies, possibly due to the systematic medical record review performed in this study, including review of primary pathology reports.5,6 Additionally, prior data have suggested that specific clinical factors may predict for sebaceous neoplasms in LS carriers, including MSH2 PGVs, and personal histories of colorectal, endometrial, ovarian, and pancreatic cancers (all of which themselves are increased in individuals with MSH2 PGVs).4,5,21 The large study population, including PGV carriers of all 5 LS genes, was a key strength that allowed us to control for numerous clinical variables, including LS gene, sex, age, race, personal and family history, so as to better understand the relative contribution of each factor to an individual’s likelihood of cutaneous neoplasm. While we found that carriers with LS-associated skin neoplasms were more likely to have colorectal, endometrial, gastric, and urinary tract cancers on bivariate analysis, this likely reflects the association of MSH2 PGVs with certain visceral cancers as well as sebaceous cancers, rather than a direct link between sebaceous cancers and certain visceral malignancies.
In the general population, sporadic sebaceous neoplasms are most commonly identified in the head, face, and neck region.22,23 Consistent with this, 75% of the sebaceous adenomas in this cohort were on the face. However, two-thirds of the sebaceous carcinomas in this cohort were located on the trunk, consistent with previous data suggesting that LS-associated sebaceous neoplasms are more likely to be in anatomic locations below the head/neck.21,24 Additionally, more than 60% of the non-LS-associated skin neoplasms in this cohort were also located below the head/neck, all of which emphasize the importance of a full body dermatologic evaluation for all individuals with LS.
Another strength is that we examined clinical factors that may predict for non-sebaceous neoplasms in patients with LS, not just LS-associated skin neoplasms. Although such non-LS-associated skin neoplasms were common in this large population of LS carriers, we found only weak associations with various clinical factors on multivariable analyses. The increased risk of having a LS-associated skin neoplasm with a personal history of a non-LS-associated skin neoplasm, and vice versa, may reflect increased dermatologic screening in these patients, increased risk (e.g., UV exposure) that predisposes to both LS- and non-LS-associated skin neoplasms,25 or LS playing an etiologic role in the pathogenesis of each type of skin neoplasm. While there are reports of MSI-high/MMR-deficient squamous cell carcinomas and other skin malignancies in individuals with LS,15,16,26,27 we did not have such molecular data and were unable to assess whether these are more common in LS carriers versus non-carriers. In general, the percentage of participants in our cohort with melanoma (17/607, 2.8%) and squamous or basal cell cancers (77/607, 12.7%) are within the range of population-level data, where the cumulative risk of developing melanoma by age 75 is 2.05%, squamous cell cancer is 4–14%, and basal cell cancer is 23–39%.28–30 In the future, somatic sequencing of cutaneous neoplasms may help further elucidate the role of LS in predisposing to other cutaneous malignancies.
There is currently no accepted standard-of-care regarding dermatologic surveillance for LS carriers. The National Comprehensive Cancer Network recommends that LS carriers “Consider skin exam every 1–2 years with a health care provider skilled in identifying Lynch syndrome-associated skin manifestations.7 Age to start surveillance is uncertain and can be individualized.” The American Society of Clinical Oncology, on the other hand, does not specifically comment on dermatologic management, but instead states “surveillance is not recommended” for LS-associated cancers other than colorectal, endometrial, ovarian, and gastric cancer, though “may be considered in the context of family history.”12 Other professional society LS clinical practice guidelines, including those from the European Society for Medical Oncology,11 the American College of Gastroenterology,13 and others8–10 lack any mention of dermatologic surveillance.
Limitations of this study include its single-center design, lack of Fitzpatrick skin-type and UV exposure data, and limited racial diversity. Since lighter skin phototypes are associated with squamous and basal cell carcinomas, these may be overrepresented in this cohort, although multivariable analysis found no significant association between race and non-LS-associated skin neoplasms. This analysis lacked a validation cohort so the associations identified should be considered hypothesis-generating rather than proven risk factors. We acknowledge possible ascertainment bias, in that individuals undergoing dermatologic evaluation are more likely to be diagnosed with skin neoplasms. Finally, some patients received dermatologic care outside of our institution, and data on location and age of skin neoplasms may have been incomplete.
These data from >600 LS carriers demonstrate that certain carriers may be at particular risk for developing LS-associated skin neoplasms (e.g., males, MLH1 and EPCAM/MSH2 PGV carriers, personal history of non-LS-associated skin neoplasms) and thus likely to benefit from regular dermatologic surveillance. Skin neoplasms were seen in LS carriers as young as age 25, suggesting that surveillance should begin early in adulthood, especially given the need to counsel for behaviors that can reduce the risk of future UV-induced skin neoplasms. Given the high overall prevalence of skin neoplasms, our data support the notion that all LS carriers should consider regular full-body skin examination as part of routine preventative LS care.
Capsule Summary.
While Lynch syndrome is known to increase risks of cutaneous neoplasia, the data from this analysis demonstrate that cutaneous neoplasms are the third most common malignancy identified in Lynch syndrome, after colorectal and endometrial cancers.
Regular dermatologic surveillance should be considered for all individuals with Lynch syndrome.
Funding:
Supported by the National Institutes of Health (National Cancer Institute) R01CA132829 (SS), and The Helen Gurley Brown Presidential Initiative (CU), The Lesswitz Fund for Lynch Syndrome (MBY), The Sweet Family Fund (MBY), and The Terry T. Sweet Fund for Lynch Syndrome (MBY).
Footnotes
Disclosures:
Dr. Uno reports prior consulting/advisory payments from Roche. Dr. Syngal reports prior consulting fees from Myriad Genetic Laboratories, Inc., and inventor/royalties from the PREMM® model. Dr. Yurgelun reports consulting/scientific advisory board fees and research funding from Janssen Pharmaceuticals as well as payments for peer review services from UpToDate.
Prior presentation:
Preliminary data from this manuscript were presented as an abstract at the 2019 Annual Meeting of the Collaborative Group of the Americas on Inherited Gastrointestinal Cancer in Salt Lake City, UT, USA, November 3-5, 2019.
IRB statement/Patient consent:
The study was approved by the Dana-Farber/Harvard Cancer Center Institutional Review Board (Boston, MA), which deemed this to be secondary research, and thus a waiver of the need for informed consent was granted.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Win AK, Jenkins MA, Dowty JG, et al. Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2017;26(3):404–412. doi: 10.1158/1055-9965.EPI-16-0693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lynch HT, Snyder CL, Shaw TG, Heinen CD, Hitchins MP. Milestones of Lynch syndrome: 1895–2015. Nat Rev Cancer. 2015;15(3):181–194. doi: 10.1038/nrc3878 [DOI] [PubMed] [Google Scholar]
- 3.Koornstra JJ, Mourits MJ, Sijmons RH, Leliveld AM, Hollema H, Kleibeuker JH. Management of extracolonic tumours in patients with Lynch syndrome. Lancet Oncol. 2009;10(4):400–408. doi: 10.1016/S1470-2045(09)70041-5 [DOI] [PubMed] [Google Scholar]
- 4.Roberts ME, Riegert-Johnson DL, Thomas BC, et al. A clinical scoring system to identify patients with sebaceous neoplasms at risk for the Muir-Torre variant of Lynch syndrome. Genet Med. 2014;16(9):711–716. doi: 10.1038/gim.2014.19 [DOI] [PubMed] [Google Scholar]
- 5.South CD, Hampel H, Comeras I, Westman JA, Frankel WL, De La Chapelle A. The frequency of Muir-Torre syndrome among Lynch syndrome families. J Natl Cancer Inst. 2008;100(4):277–281. doi: 10.1093/jnci/djm291 [DOI] [PubMed] [Google Scholar]
- 6.Adan F, Crijns MB, Zandstra WSE, et al. Cumulative risk of skin tumours in patients with Lynch syndrome. Br J Dermatol. 2018;179(2):522–523. doi: 10.1111/bjd.16552 [DOI] [PubMed] [Google Scholar]
- 7.National Comprehensive Cancer Network Clinical Practice Guidelines - Genetic/Familial High-Risk Assessment: Colorectal. https://www.nccn.org/professionals/physician_gls/pdf/genetics_colon.pdf. Published 2022. Accessed June 13, 2022.
- 8.Giardiello FM, Allen JI, Axilbund JE, et al. Guidelines on genetic evaluation and management of lynch syndrome: A consensus statement by the US multi-society task force on colorectal cancer. Am J Gastroenterol. 2014;109(8):1159–1179. doi: 10.1038/ajg.2014.186 [DOI] [PubMed] [Google Scholar]
- 9.Vangala DB, Cauchin E, Balmaña J, et al. Screening and surveillance in hereditary gastrointestinal cancers: Recommendations from the European Society of Digestive Oncology (ESDO) expert discussion at the 20th European Society for Medical Oncology (ESMO)/World Congress on Gastrointestinal Cancer, Barcelona, June 2018. Eur J Cancer. 2018;104:91–103. doi: 10.1016/j.ejca.2018.09.004 [DOI] [PubMed] [Google Scholar]
- 10.Seppälä TT, Latchford A, Negoi I, et al. European guidelines from the EHTG and ESCP for Lynch syndrome: an updated third edition of the Mallorca guidelines based on gene and gender. Br J Surg. 2021;108(5):484–498. doi: 10.1002/BJS.11902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stjepanovic N, Moreira L, Carneiro F, et al. Hereditary gastrointestinal cancers: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol Off J Eur Soc Med Oncol. 2019;30(10):1558–1571. doi: 10.1093/ANNONC/MDZ233 [DOI] [PubMed] [Google Scholar]
- 12.Stoffel EM, Mangu PB, Gruber SB, et al. Hereditary colorectal cancer syndromes: American society of clinical oncology clinical practice guideline endorsement of familial risk-colorectal cancer: European Society for medical oncology clinical practice guidelines. J Clin Oncol. 2015;33(2):209–217. doi: 10.1200/JCO.2014.58.1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Syngal S, Brand RE, Church JM, Giardiello FM, Hampel HL, Burt RW. ACG Clinical Guideline: Genetic Testing and Management of Hereditary Gastrointestinal Cancer Syndromes HHS Public Access. Am J Gastroenterol. 2015;110(2):223–263. doi: 10.1038/ajg.2014.435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ponti G, Losi L, Pellacani G, et al. Malignant melanoma in patients with hereditary nonpolyposis colorectal cancer. Br J Dermatol. 2008;159(1):162–168. doi: 10.1111/j.1365-2133.2008.08575.x [DOI] [PubMed] [Google Scholar]
- 15.Adan F, Crijns MB, Dekker E, et al. A squamous cell carcinoma in a young woman with Lynch syndrome. Fam Cancer. 2019;18(2):193–196. doi: 10.1007/s10689-018-00113-5 [DOI] [PubMed] [Google Scholar]
- 16.Ykema BLM, Adan F, Crijns MB, et al. Cutaneous squamous cell carcinoma is associated with Lynch syndrome; widening the spectrum of Lynch syndrome associated tumours. Br J Dermatol. 2021;1:0–3. doi: 10.1111/bjd.20139 [DOI] [PubMed] [Google Scholar]
- 17.Biller LH, Horiguchi M, Uno H, Ukaegbu C, Syngal S, Yurgelun MB. Familial burden and other clinical factors associated with various types of cancer in individuals with Lynch syndrome. Gastroenterology. March 2021. doi: 10.1053/j.gastro.2021.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muir EG, Bell AJY, Barlow KA. Multiple primary carcinomata of the colon, duodenum, and larynx associated with kerato-acanthomata of the face. Br J Surg. 1967;54(3):191–195. doi: 10.1002/bjs.1800540309 [DOI] [PubMed] [Google Scholar]
- 19.Torre D Multiple sebaceous tumors. Arch Dermatol. 1968;98(5):549–551. doi: 10.1001/archderm.98.5.549 [DOI] [PubMed] [Google Scholar]
- 20.Lynch HT, Lynch PM, Pester J, Fusaro RM. The Cancer Family Syndrome Rare Cutaneous Phenotypic Linkage of Torre’s Syndrome. Arch Intern Med. 1981;141(5):607–611. doi: 10.1001/archinte.1981.00340050059016 [DOI] [PubMed] [Google Scholar]
- 21.Dores GM, Curtis RE, Toro JR, Devesa SS, Fraumeni JF. Incidence of cutaneous sebaceous carcinoma and risk of associated neoplasms: insight into Muir-Torre syndrome. Cancer. 2008;113(12):3372–3381. doi: 10.1002/cncr.23963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dasgupta T, Wilson LD, Yu JB. A retrospective review of 1349 cases of sebaceous carcinoma. Cancer. 2009;115(1):158–165. doi: 10.1002/CNCR.23952 [DOI] [PubMed] [Google Scholar]
- 23.Konstantinova AM, Kastnerova L, Michal M, Kolm I, Kazakov DV. Sebaceous Tumors of the Skin: A Study of 145 Lesions From 136 Patients Correlating Pathologic Features and DNA Mismatch Repair Staining Pattern. Am J Dermatopathol. 2021;43(3):174–181. doi: 10.1097/DAD.0000000000001691 [DOI] [PubMed] [Google Scholar]
- 24.Marcoval J, Talavera-Belmonte A, Fornons-Servent R, Bauer-Alonso A, Penín RM, Servitje O. Cutaneous sebaceous tumours and Lynch syndrome: long-term follow-up of 60 patients. Clin Exp Dermatol. 2019;44(5):506–511. doi: 10.1111/ced.13828 [DOI] [PubMed] [Google Scholar]
- 25.Sargen MR, Mai ZM, Engels EA, et al. Ambient Ultraviolet Radiation and Sebaceous Carcinoma Incidence in the United States, 2000–2016. JNCI Cancer Spectr. 2020;4(2). doi: 10.1093/JNCICS/PKAA020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Assam JH, Powell S, Spanos WC. Unresectable Cutaneous Squamous Cell Carcinoma of the Forehead With MLH1 Mutation Showing Dramatic Response to Programmed Cell Death Protein 1 Inhibitor Therapy. Clin Ski Cancer. 2016;1(1):26–29. doi: 10.1016/j.clsc.2016.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khaddour K, Fields RC, Ansstas M, Rosman IS, Ansstas G. Metachronous cutaneous squamous cell carcinoma in a young patient as the only presenting symptom to uncover Lynch syndrome with MLH1 Germline mutation. Hered Cancer Clin Pract. 2020;18(1). doi: 10.1186/s13053-020-00155-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancer Query System: Probability of Developing or Dying of Cancer. https://surveillance.cancer.gov/devcan/canques.html. Accessed June 9, 2022.
- 29.Stern RS. Prevalence of a history of skin cancer in 2007: results of an incidence-based model. Arch Dermatol. 2010;146(3):279–282. doi: 10.1001/ARCHDERMATOL.2010.4 [DOI] [PubMed] [Google Scholar]
- 30.Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: Incidence. J Am Acad Dermatol. 30:774–778. doi: 10.1016/S0190-9622(08)81509-5 [DOI] [PubMed] [Google Scholar]


