Abstract
Background:
The associations between cognitive domains and odor identification are well-established, but how sociodemographic variables affect these relationships is less clear.
Purpose:
Using the survey-adapted Montreal Cognitive Assessment instrument (MoCA-SA), we assess how age, gender, race, and education shape these relationships.
Methods:
We first used cluster analysis and multidimensional scaling to empirically derive distinct cognitive domains from the MoCA-SA since it is unclear whether the MoCA-SA can be disaggregated into cognitive domains. We then used ordinal logistic regression to test whether these empirically derived cognitive domains were associated with odor identification and how sociodemographic variables modified these relationships.
Study Population:
Nationally representative sample of community-dwelling U.S. older adults.
Results:
We identified five out of the six theoretical cognitive domains, with the language domain unable to be identified. Odor identification was associated with episodic memory, visuospatial ability, and executive function. Stratified analyses by sociodemographic variables reveal that the associations between some of the cognitive domains and odor identification varied by age, gender, or race, but not by education.
Conclusions:
These results suggest that 1) the MoCA-SA can be used to identify cognitive domains in survey research and 2) the performance of smell tests as a screener for cognitive decline may potentially be weaker in certain subpopulations.
Keywords: Cognition, Cognitive Domains, Odor identification
Introduction
The survey-adapted Montreal Cognitive Assessment instrument (MoCA-SA) has successfully measured general cognitive function in a representative sample of U.S. older adults and has much practical applicability in survey research given its shorter administration time and capacity to detect a broad range of cognitive abilities.1 The MoCA-SA used in the National Social Life, Health, and Aging Project (NSHAP) derived 18 questions from the Montreal Cognitive Assessment instrument (MoCA)2 to assess 6 cognitive domains, including “executive function,” “visuospatial skills,” “language,” “attention, concentration, and working memory,” “orientation,” and “short-term memory.”1 While these 6 cognitive domains in the MoCA have been empirically validated,3–5 the cognitive domains in the MoCA-SA, a significantly shorter cognitive test, has not yet been empirically evaluated; thus, it is unclear whether the MoCA-SA is similarly able to assess cognitive domains beyond general cognitive function and whether the MoCA-SA can be used to study how distinct cognitive domains are associated with different types of health outcomes.
Isolating cognitive domains is an important component of understanding how cognition is related to health outcomes, particularly in older adults at risk of cognitive decline; health outcomes in older adults have been associated with different cognitive domains. For instance, Johnson et al found that executive function was a better predictor of functional decline and mortality in elderly women than global cognition.6 Lavery et al similarly found that distinct cognitive domains were associated with mortality, with a decline in processing speed a strong predictor of mortality for the older-old age group.7 In addition, olfactory loss in older adults has been associated with a decline in verbal memory, but not with a decline in executive control or global function.8 Odor identification has also been found to be correlated with language, memory, and general cognitive functioning,9 and Wilson et al found that lower odor identification was associated with a faster rate of decline in perceptual speed and episodic memory, but not with a decline in semantic memory, working memory, or visuospatial ability.10 All these studies lend support to the importance of identifying different cognitive domains to study its associations with health outcomes.
However, many of the studies on cognitive domains have derived these domains from clinical cognitive tests that use a large battery of items that are difficult to incorporate in population-based survey research; this limits our ability to study the consequences of decline in specific cognitive domains at the population level using representative survey data. Therefore, it is important to determine whether distinct cognitive domains can be detected within a large survey dataset that uses a truncated version of a standardized cognitive test. We aim to evaluate the possibility of detecting cognitive domains in the MoCA-SA used by NSHAP, a nationally representative survey of community-dwelling U.S. older adults.
If it is possible to distinguish different cognitive domains in the MoCA-SA, this would enable a more precise study of associations between cognition and a variety of factors measured in NSHAP, including associations with gender, sensory function, sexuality, physical and mental health, and social participation. Additionally, if it is possible to detect cognitive domains in a shortened cognitive test adapted for survey use, this can encourage other large-scale survey studies to use survey-adapted cognitive tests to study cognitive domains and its associations with health outcomes within a population.
Previous studies have validated the MoCA test; however, the survey-adapted version of the MoCA remains unvalidated. Hendershott et al has evaluated the sensitivity and specificity of the MoCA subdomains as well as whether it could identify cognitive impairment within specific domains and found that the MoCA not only was able to screen for global cognitive decline but was able to detect executive function declines as well.4 Freitas et al have confirmed the validity of the MoCA as an appropriate cognitive screening tool that can identify different cognitive domains,3 and Vogel et al have also empirically validated the MoCA domain classifications and supported the MoCA as a cognitive test that can measure similar cognitive constructs as those measured by a more comprehensive battery of tests.5
Although the questions in the MoCA-SA were taken from the MoCA and items were chosen from all 6 cognitive domains in the full MoCA tool, it is not clear whether a shortened form of the MoCA can still effectively distinguish between all 6 cognitive dimensions, and whether any of the isolated cognitive dimensions are associated with health outcomes. To evaluate the possibility of empirically deriving cognitive domains from the MoCA-SA, we used two different methods — cluster analysis and multidimensional scaling — to determine if responses to the MoCA-SA items could detect the classic domains at a population level.
Because odor identification tests can help screen for dementia,11–13 and given the extensive research on cognitive domains and odor identification,8–10 we asked whether any of our empirically-derived cognitive domains are associated with the ability to identify odors to evaluate whether these cognitive domains can be effectively used in survey research.
Additionally, because age, gender, race, and education have been found to be associated with both odor identification14 and cognition,15 we further examined whether sociodemographic variables modified the relationship between each cognitive domain and odor identification, as well as whether these sociodemographic variables are independently associated with the cognitive domains.
Data
Study Population
NSHAP is a nationally representative longitudinal study of the health and social relationships of community-dwelling U.S. older adults. The NSHAP sample is based on a multistage area probability design that can be generalized to U.S. adults born between 1920–1947.16
In 2010–2011, interviewers administered in-home questionnaires that collected demographic, social, psychological, cognitive, and biological measures from respondents aged 62–91 at the time of the interview. 3,377 interviews were completed during this wave of data collection and the overall unconditional response rate was 74%.17
Cognitive Measures
Cognition was assessed using the MoCA-SA which highly correlates with the complete instrument and includes 18 cognitive items that measure a range of cognitive functions including the modified trail making test-B, clock drawing test, naming, digit span, serial 7 subtractions, sentence repetition, verbal fluency, abstraction, delayed recall, and temporal orientation.1
Each successfully completed cognitive task was scored as 1, while unsuccessfully completed tasks were scored as 0. In our analyses, the serial 7 subtraction item was dichotomized as 0–1 points. If respondents gave 0–2 correct answers to this item, they were scored as unsuccessfully completed; if they gave 3–5 correct answers to this item, they were scored as successfully completed. With this scoring, the global cognition score using the MoCA-SA scale ranges from 0–18.
We excluded respondents who had any missing scores on the cognitive measures. We included only older adults who were cognitively intact in our analyses since cognitive impairment could affect our results. Therefore, we excluded respondents who reported a doctor’s diagnosis of dementia, including Alzheimer’s, dementia, or Parkinson’s. It is possible that there are respondents in the sample who have undiagnosed cognitive impairment. As a conservative measure, we excluded from analyses respondents with extremely low cognition scores (a score of 2 or less on the MoCA-SA); respondents who were unable to successfully complete any cognitive tasks or only able to complete one or two tasks on a battery of 18 items likely has undiagnosed cognitive impairment. 3,046 respondents had data for an analysis of cognitive domains.
Olfactory Measures
Olfactory function was tested in a randomly selected subset of NSHAP respondents (N=2,304; 2,212 participated) using felt-tip Sniffin’ Sticks pens.18 To test odor identification, respondents were asked to choose the correct odor among four word/picture choices; scores were coded as correct or incorrect (don’t know and refused responses were coded as incorrect), resulting in scores ranging from 0–5.
Demographic and Health Variables
We included gender, age, race, education, and health status variables in our models as covariates (Table 1). We used the modified Charlson comorbidity index19 to measure physical health by totaling the number of chronic conditions respondents reported having (range 0–11). Depressive symptoms were measured using the NSHAP’s modified Center for Epidemiologic Studies Depression Scale.20 The frequency of 11 depressive symptoms (never/rarely, some of the time, and occasionally/most of the time) were totaled and standardized by following the recommendation of Payne et al,20 resulting in a possible range of 0–22.
Table 1.
Descriptive Statistics of Demographic and Health Variables in US Older Adults
| Demographics | Mean/% | N | |
|---|---|---|---|
| Age | 72.3 | 3,046 | |
| Female | 52.3 | 3,046 | |
| Race/ethnicity | 3,034a | ||
| White | 81.6 | ||
| Black | 9.1 | ||
| Hispanic, Non-black | 6.6 | ||
| Other | 2.7 | ||
| Education | 3,046 | ||
| <HS | 16.0 | ||
| HS/Equivalent | 25.3 | ||
| Some college/Associate’s | 32.0 | ||
| Bachelors or more | 26.6 | ||
| Health Variables | Mean (SE) | Range | N |
| Charlson comorbidity index | 1.4 (0.04) | (0–11) | 3,044a |
| Depressive symptoms | 4.5 (0.11) | (0–20) | 3,013a |
| Odor identification (# correct) | 4.1 (0.04) | (0–5) | 1,993b |
| Global cognition (Total MoCA-SA) | 12.5 (0.12) | (3–18) | 3,046 |
| Cognitive Subdomains | 3,046 | ||
| Orientation | 1.9 (0.01) | (0–2) | |
| Episodic memory | 2.8 (0.05) | (0–5) | |
| Working memory | 2.3 (0.03) | (0–3) | |
| Visuospatial | 2.2 (0.02) | (0–3) | |
| Executive function | 3.2 (0.05) | (0–5) | |
Note.
The reduced sample size in these variables stem from missing responses.
This item was only administered to a randomly selected subset of respondents.
Methods
Cluster Analysis & Multidimensional Scaling
We use two different methods to evaluate whether the MoCA-SA can distinguish distinct cognitive domains. While the MoCA-SA was developed using the 6 cognitive domains in the MoCA, the MoCA-SA includes far fewer cognitive items, so it is unclear whether the same discriminant ability exists for the MoCA-SA as it does for the MoCA. Therefore, rather than a priori assigning the MoCA-SA’s 18 cognitive items into cognitive domains, we use cluster analysis and multidimensional scaling to empirically visualize how these cognitive items cluster together, and from these distinct clusters, inductively identify the type of cognitive ability represented. This approach allows us to see how each cognitive item functions in relation to each other without incorporating theoretical preconceptions of how the cognitive domains should be formed since our goal is to test whether it is possible to empirically derive distinct cognitive domains from a shortened version of the MoCA to evaluate its applicability to survey research.
To empirically derive the cognitive domains, we first created a dissimilarity matrix of the 18 cognitive items in the MoCA-SA and then performed a cluster analysis on this dissimilarity matrix. To ensure robustness of our cluster analysis, we also performed multidimensional scaling (MDS) that locates each of the 18 cognitive items in a 3-dimensional space. MDS is a non-parametric technique that uses the relative distance between each variable to locate these items in a space.21 The closer the cognitive items are located to each other in a 3-dimensional space, the more similar they are in how they function.
Regression Analysis
We used ordinal logistic regression to test whether our empirically derived cognitive domains (dependent variables) were associated with odor identification (number correct) to confirm known associations. Because of the known associations between gender, race, age, and education with odor identification14 and cognitive domains,15 we tested whether these variables modified the relationship between cognitive domains and odor identification. We stratified the analyses on cognitive domains and odor identification by gender (male vs female), race (White vs non-White), age group (62–69; 70–79; 80–91), and education (high school or less vs some college or more).
Because the cognitive domains contain different numbers of cognitive items, to enable comparability across the clusters, we converted the scores for each cognitive domain into z-scores. We subtracted the mean for each of these domains from each respondent’s score and divided the difference by the standard deviation. We included age, gender, race, education, depressive symptoms and the Charlson comorbidity index as covariates. We estimated all models in Stata 15 (StataCorp LLC, College Station, TX) using the survey weights provided with the dataset.
Results
Cognitive Domains
Although the MoCA-SA included 18 items from the MoCA to theoretically capture 6 cognitive domains,1 our empirical analyses revealed that the MoCA-SA was only able to effectively distinguish 5 different cognitive domains, including “temporal orientation,” “episodic memory,” “visuospatial ability,” “working memory,” and “executive function.” The “language” domain was not distinguishable (Figure 1).
Figure 1.
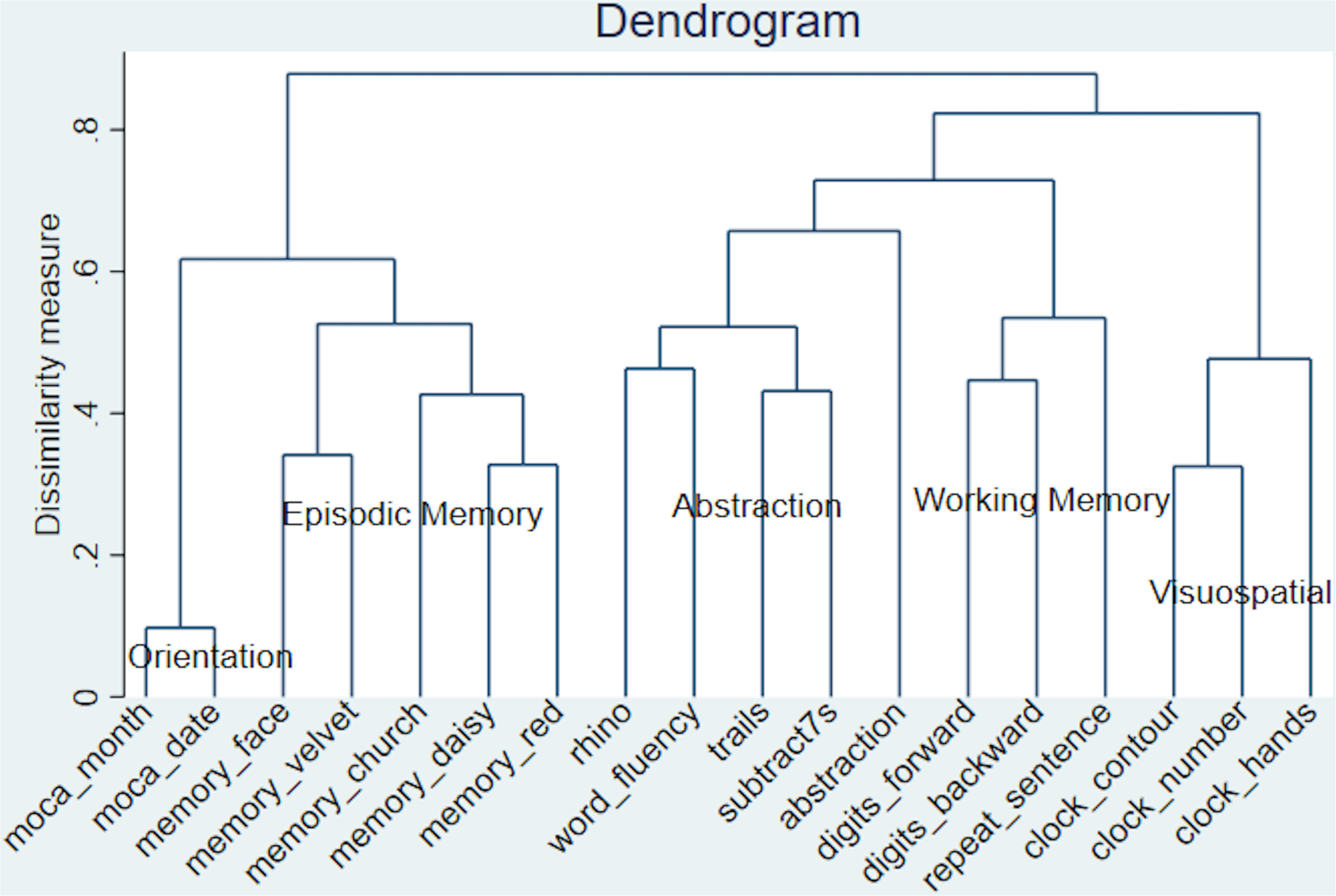
Cluster Analysis of 18 Cognitive Items, N=3,046
In the MDS graph (Figure 2), we are also only able to identify 5 different cognitive domains. The five delayed recall items that measure “episodic memory” are all located to the far right on dimension 1 and in the middle of dimension 2. The “temporal orientation” measures (moca month and date) are located close to the “episodic memory” items, but while the “episodic memory” items are low on dimension 3, the “temporal orientation” measures are high on dimension 3. The three items meant to measure “visuospatial skills” (clock contour, hands, number) are all located towards the far left of dimension 1 and towards the back of dimension 2. Although sentence repetition was theoretically meant to measure “language,” in Figure 2, we see that it is located on the far left of dimension 1 and in the front of dimension 2 near the digit span items; therefore, we included sentence repetition as part of the “working memory” cognitive domain. The items abstraction, trails, serial subtract 7s, word fluency, and naming the rhino are all located in the center-left of the graph, and from these items, we derived the “executive function” domain.
Figure 2:
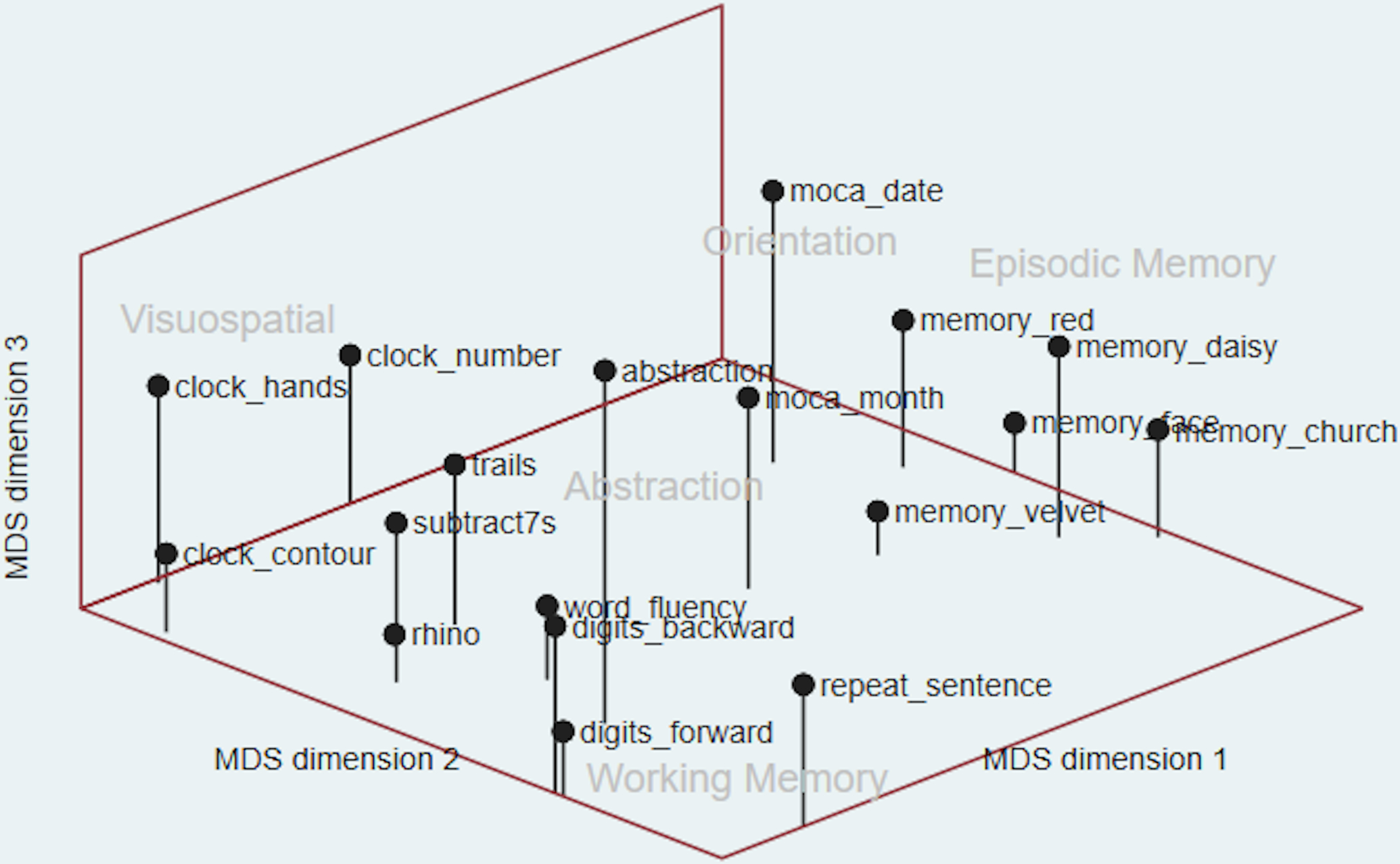
Multidimensional Scaling of 18 Cognitive Items, N=3,046
Olfaction
It is well-established that odor identification is associated with episodic memory.8–10 Table 2 shows that, using our empirically derived cognitive domains, for the overall sample, we similarly found that odor identification was associated with episodic memory (OR = 1.20, 95% CI = [1.09, 1.31]). In addition, we found that odor identification was associated with visuospatial ability (OR = 1.23, 95% CI = [1.14, 1.33]) and executive function (OR = 1.24, 95% CI = [1.16, 1.33]).
Table 2.
Associations Between Odor Identification and Cognitive Domains, Stratified by Gender, Race/Ethnicity, Education, and Age Group, Odds Ratio (95% CI)a
| Orientation, OR (95% CI) | Episodic Memory, OR (95% CI) | Working Memory, OR (95% CI) | Visuospatial, OR (95% CI) | Executive Function, OR (95% CI) | |
|---|---|---|---|---|---|
| Overall, N=1,963 | 1.11 (0.97–1.27) | 1.20 (1.09–1.31)*** | 1.02 (0.91–1.15) | 1.23 (1.14–1.33)*** | 1.24 (1.16–1.33)*** |
| Gender | |||||
| Men, N=929 | 1.00 (0.82–1.22) | 1.09 (0.95–1.26) | 1.01 (0.85–1.19) | 1.27 (1.15–1.40)*** | 1.19 (1.09–1.30)*** |
| Women, N=1,034 | 1.25 (1.02–1.52)* | 1.33 (1.18–1.50)*** | 1.05 (0.92–1.20) | 1.17 (1.04–1.32)** | 1.32 (1.17–1.48)*** |
| Race/Ethnicity | |||||
| White, N=1,420 | 1.06 (0.90–1.26) | 1.23 (1.10–1.38)*** | 1.00 (0.86–1.16) | 1.22 (1.11–1.34)*** | 1.23 (1.13–1.33)*** |
| Non-White, N=543 | 1.29 (1.04–1.60)* | 1.05 (0.89–1.23) | 1.11 (0.91–1.36) | 1.24 (1.10–1.40)*** | 1.35 (1.10–1.65)** |
| Education | |||||
| <HS/=HS, N=873 | 1.07 (0.92–1.25) | 1.17 (1.01–1.35)* | 0.99 (0.86–1.13) | 1.27 (1.10–1.46)** | 1.32 (1.18–1.47)*** |
| Some College or More, N=1,090 | 1.10 (0.91–1.34) | 1.21 (1.08–1.37)** | 1.05 (0.89–1.23) | 1.18 (1.06–1.31)** | 1.17 (1.06–1.30)** |
| Age Group | |||||
| 62–69, N=744 | 1.21 (0.84–1.73) | 0.99 (0.82–1.20) | 0.98 (0.77–1.26) | 1.15 (0.98–1.35) | 1.29 (1.08–1.53)** |
| 70–79, N=770 | 1.24 (1.03–1.51)* | 1.21 (1.05–1.39)** | 0.98 (0.83–1.16) | 1.18 (1.06–1.32)** | 1.20 (1.05–1.37)** |
| 80–91, N=449 | 1.02 (0.83–1.26) | 1.44 (1.23–1.69)*** | 1.12 (1.00–1.26) | 1.38 (1.19–1.59)*** | 1.28 (1.14–1.44)*** |
p<.001;
p<.01;
p<.05
Note.
The purpose of this table is to examine whether the association between odor identification and each of the cognitive domains is modified by demographic factors. Thus, all the odds ratios in this table refer to the strength of the relationship between each of the cognitive domains (dependent variables) and odor identification.
Table 2 includes the results from each of the stratified analyses evaluating how sociodemographic variables modified the relationship between each of the cognitive domains and odor identification. Our results reveal that the associations between certain cognitive domains and odor identification vary by age, gender, or race, but not by education.
The association between cognitive domains and odor identification tends to be stronger among women. For men, visuospatial ability (OR = 1.27, 95% CI = [1.15, 1.40]) and executive function (OR = 1.19, 95% CI = [1.09, 1.30]) were both significantly associated with odor identification, while for women, all the cognitive domains except for working memory were associated with odor identification. When the analyses were stratified by race, both visuospatial ability and executive function were associated with odor identification among Whites and non-Whites. However, among Whites, episodic memory was also found to be associated with odor identification (OR = 1.23, 95% CI [1.10, 1.38]), while for non-Whites, orientation was weakly associated with odor identification (OR = 1.29, 95% CI [1.04, 1.60]). We also found that the strength of the association between cognitive domains and odor identification generally increases with age. Episodic memory, visuospatial ability, and executive function were all associated with odor identification in the two oldest age groups, while for the youngest age group (62–69), only executive function was significantly associated with odor identification (OR = 1.29, 95% CI [1.08, 1.53]).
Sociodemographic Variables
To better understand how the sociodemographic variables might be shaping the relationship between odor identification and cognitive domains, we also examined the independent associations between the sociodemographic variables and each of the cognitive domains (Table 3). We found that women performed better than men in orientation (OR = 1.88, 95% CI [1.26, 2.80]) and episodic memory (OR = 2.71, 95% CI [2.27–3.23]). Older adults scored worse on all cognitive domains. The Black and Hispanic racial groups performed significantly worse than Whites on episodic memory and visuospatial ability, and all non-White racial groups performed significantly worse on working memory and executive function than Whites. Higher education is associated with better performance on all cognitive domains, while individuals with more depressive symptoms performed worse on episodic memory (OR = 0.96, 95% CI [0.95, 0.98]), visuospatial ability (OR = 0.97, 95% CI [0.95, 0.99]), and executive function (OR = 0.97, 95% CI [0.95, 0.99]). The number of comorbidities was not significantly associated with any of the cognitive domains.
Table 3.
Associations Between Sociodemographic Variables and Cognitive Domains, N=2,999
| Orientation, OR (95% CI) | Episodic Memory, OR (95% CI) | Working Memory, OR (95% CI) | Visuospatial, OR (95% CI) | Executive Function, OR (95% CI) | |
|---|---|---|---|---|---|
| Gender (ref. Male) | |||||
| Female | 1.88 (1.26–2.80)** | 2.71 (2.27–3.23)*** | 1.06 (0.87–1.29) | 0.93 (0.79–1.10) | 1.02 (0.86–1.22) |
| Age | 0.95 (0.92–0.99)** | 0.94 (0.93–0.95)*** | 0.96 (0.95–0.97)*** | 0.95 (0.94–0.96)*** | 0.95 (0.94–0.96)*** |
| Race/Ethnicity (ref. White) | |||||
| Black | 0.87 (0.57–1.34) | 0.40 (0.33–0.49)*** | 0.46 (0.33–0.62)*** | 0.41 (0.30–0.55)*** | 0.24 (0.19–0.30)*** |
| Hispanic | 0.74 (0.46–1.20) | 0.48 (0.34–0.68)*** | 0.26 (0.19–0.37)*** | 0.43 (0.31–0.61)*** | 0.34 (0.22–0.52)*** |
| Other | 0.30 (0.08–1.18) | 0.83 (0.42–1.62) | 0.24 (0.14–0.41)*** | 0.63 (0.32–1.25) | 0.39 (0.23–0.67)*** |
| Education | 1.42 (1.15–1.75)** | 1.33 (1.21–1.46)*** | 1.62 (1.50–1.75)*** | 1.47 (1.32–1.63)*** | 2.29 (2.11–2.49)*** |
| Depressive Symptoms | 0.97 (0.93–1.00) | 0.96 (0.95–0.98)*** | 0.98 (0.96–1.01) | 0.97 (0.95–0.99)** | 0.97 (0.95–0.99)** |
| Charlson Comorbidity Index | 1.12 (0.98–1.27) | 1.04 (0.98–1.09) | 1.04 (0.98–1.10) | 0.99 (0.93–1.05) | 0.97 (0.92–1.03) |
p<.001;
p<.01;
p<.05
Discussion
Using two different methods, we inductively derived 5 different cognitive domains (working memory, executive function, visuospatial ability, episodic memory, temporal orientation) in the MoCA-SA. Although the MoCA-SA was developed to capture the 6 distinct cognitive domains in the full MoCA instrument, we were unable to derive the language domain using either of our methods. In our analyses, the cognitive items that were chosen to measure language were instead more closely located to items that measured executive function and working memory − both the animal naming and word fluency tasks were closely located to the “executive function” items, while the sentence repetition task was closely located to the “working memory” items (Figure 2). These findings do not invalidate the use of sentence repetition, animal naming, and word fluency as measures of language, but rather, we suggest that more comprehensive language measures need to be added to the MoCA-SA to effectively derive a language domain and to detect declines in language ability in older adults.
Although we were unable to identify all 6 cognitive domains in the MoCA-SA, we nevertheless showed that it is possible to derive 5 cognitive domains in a shortened, survey-adapted cognitive test and that these cognitive domains were associated with odor identification and sociodemographic variables – associations that have been established in studies with more extensive cognitive measures.
We found that odor identification was associated with episodic memory, visuospatial ability, and executive function, but sociodemographic variables modified some of these associations. The association between cognitive domains and odor identification tends to be stronger among women, with all the cognitive domains except for working memory associated with odor identification, while for men, only visuospatial ability and executive function were significantly associated with odor identification. There were also racial differences in these associations: episodic memory was associated with odor identification among Whites but not among non-Whites. The strength of the association between cognitive domains and odor identification generally increases with age. Episodic memory, visuospatial ability, and executive function were all associated with odor identification in the two oldest age groups, while for the youngest age group, only executive function was associated with odor identification. Independent associations between sociodemographic variables and cognitive domains generally confirm known associations − there were gender, age, education, and racial differences in performance on cognitive domains. Interestingly, while higher education levels were associated with better performance in all the cognitive domains, education did not modify the relationship between odor identification and cognitive domains.
Because sociodemographic variables impact the strength of the associations between odor identification and cognitive domains, this may suggest that there are demographic differences − such as gender22 and age differences23,24 − at the cognitive level of olfactory processing. These results indicate that future research can use fMRI to examine how demographic and health characteristics shape how olfactory information is cognitively processed. Moreover, these variations in how odor identification is associated with cognitive domains highlight the importance of disaggregating general cognitive function into cognitive domains to better understand how different aspects of cognition (and different brain regions) are affected by aging, olfactory dysfunction, and other health issues.
While odor identification tests can be used to screen for dementia,11–13 given our results showing that sociodemographic variables modify the relationship between odor identification and cognitive domains, it may be the case that the performance of smell tests as a screener for cognitive decline is weaker in certain subpopulations. For instance, in the case of younger patients, the weaker association between odor identification and cognitive domains could mean that smell tests are less effective at detecting cognitive decline in these patients. Further research is needed to examine the implications of these results by studying whether there are variations in the ability of odor identification tests to identify cognitive decline in different subgroups.
While studies have empirically validated the MoCA as a cognitive test that can measure cognitive domains,3–5 the MoCA-SA has not yet been empirically validated; our results provide evidence that support the use of the MoCA-SA in survey research. Not only was it possible to inductively derive distinct cognitive domains in the MoCA-SA, but these empirical cognitive domains also functioned in expected ways with health and demographic variables. Based on these results, we argue that the MoCA-SA remains a useful tool in survey research.
Conclusion
It is difficult to administer comprehensive cognitive tests during survey research given time constraints and burden on respondents; although the MoCA-SA may be an abbreviated cognitive screening tool, it is still able to effectively capture broad cognitive abilities given these practical considerations. Future research can use the MoCA-SA as more than a screening test for cognitive impairment – it can also be fruitfully used as a tool to identify cognitive domains and its associations with demographic and health outcomes in population-based survey research.
Source of Funding:
The National Social Life, Health and Aging Project is supported by the National Institute on Aging and the National Institutes of Health (R01AG033903, R01AG043538, and R01AG048511). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest The authors report no conflicts of interest.
References
- 1.Kotwal AA, Schumm P, Kern DW, et al. Evaluation of a brief survey instrument for assessing subtle differences in cognitive function among older adults. Alzheimer Dis Asssoc Disord. 2015;29:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 3.Freitas S, Simões MR, Marôco J, et al. Construct validity of the Montreal Cognitive Assessment (MoCA). J Int Neuropsychol Soc. 2012;18:242–250. [DOI] [PubMed] [Google Scholar]
- 4.Hendershott TR, Zhu D, Llanes S, et al. Domain-specific accuracy of the Montreal Cognitive Assessment subsections in Parkinson’s disease. Parkinsonism Relat Disord. 2017;38:31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vogel SJ, Banks SJ, Cummings JL, et al. Concordance of the Montreal Cognitive Assessment with standard neuropsychological measures. Alzheimers Dement (Amst). 2015;1:289–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson JK, Lui L-Y, Yaffe K. Executive function, more than global cognition, predicts functional decline and mortality in elderly women. J Gerontol A Bio Sci Med Sci. 2007;62:1134–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lavery LL, Dodge HH, Snitz B, et al. Cognitive decline and mortality in a community-based cohort: The Monongahela Valley Independent Elders Survey. J Am Geriatr Soc. 2009;57:94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swan GE, Carmelli D. Impaired olfaction predicts cognitive decline in nondemented older Adults. Neuroepidemiology. 2002;21:58–67. [DOI] [PubMed] [Google Scholar]
- 9.Westervelt HJ, Ruffolo JS, Tremont G. Assessing olfaction in the neuropsychological exam: The relationship between odor identification and cognition in older adults. Arch Clin Neuropsychol. 2005;20:761–769. [DOI] [PubMed] [Google Scholar]
- 10.Wilson RS, Arnold SE, Tang Y, et al. Odor identification and decline in different cognitive domains in old age. Neuroepidemiology. 2006;26:61–67. [DOI] [PubMed] [Google Scholar]
- 11.Fullard ME, Morley JF, Duda JE. Olfactory dysfunction as an early biomarker in Parkinson’s disease. Neurosci Bull. 2017;33:515–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lafaille-Magnan M-L, Poirier J, Etienne P, et al. Odor identification as a biomarker of preclinical AD in older adults at risk. Neurology. 2017;89:327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Growdon ME, Schultz AP, Dagley AS, et al. Odor identification and Alzheimer disease biomarkers in clinically normal elderly. Neurology. 2015;84:2153–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pinto JM, Schumm LP, Wroblewsi EK, et al. Racial disparities in olfactory loss among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2014;69A:323–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rexroth DF, Tennstedt SL, Jones RN, et al. Relationship of demographic and health factors to cognition in older adults in the ACTIVE study. J Aging Health. 2013;25;128S–146S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Muircheartaigh C, Eckman S, Smith S. Statistical design and estimation for the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2009;64B:i12–i19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Muircheartaigh C, English N, Pedlow S, et al. Sample design, sample augmentation, and estimation for Wave 2 of the NSHAP. J Gerontol B Psychol Sci Soc Sci. 2014;69:S15–S26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kern DW, Wroblewski KE, Schumm LP, et al. Field survey measures of olfaction: The Olfactory Function Field Exam (OFFE). Field Methods. 2014;26:421–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Katz JN, Chang LC, Sangha O, et al. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34:73–84. [DOI] [PubMed] [Google Scholar]
- 20.Payne C, Hedberg EC, Kozloski M, et al. Using and interpreting mental health measures in the National Social Life, Health, and Aging Project. J Gerontol B Psychol Sci Soc Sci. 2014;69:S99–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borg I, Groenen PJF. Modern multidimensional scaling: Theory and applications. 2nd ed. New York, NY: Springer; 2005. [Google Scholar]
- 22.Bengtsson S, Berglund H, Gulyas B, et al. Brain activation during odor perception in males and females. Neuroreport. 2001;12:2027–2033. [DOI] [PubMed] [Google Scholar]
- 23.Cerf-Ducastel B, Murphy C. FMRI brain activation in response to odors is reduced in primary olfactory areas of elderly subjects. Brain Res. 2003;986:36–53. [DOI] [PubMed] [Google Scholar]
- 24.Reske M, Kellermann TN, Shah NJ, et al. Impact of valence and age on olfactory induced brain activation in healthy women. Behav Neurosci. 2010;124:414–422. [DOI] [PubMed] [Google Scholar]


