Abstract
Since the identification and characterization of gasdermin (GSDM) D as the main effector of inflammatory regulated cell death (or pyroptosis), literature on the GSDM family of pore-forming proteins is rapidly expanding, revealing novel mechanisms regulating their expression and functions that go beyond pyroptosis. Indeed, a growing body of evidence corroborates the importance of GSDMs within the gastrointestinal system, underscoring their critical contributions to the pathophysiology of gastrointestinal cancers, enteric infections and gut mucosal inflammation, such as inflammatory bowel disease. However, with this increase in knowledge, several important and controversial issues have arisen regarding basic GSDM biology and its role(s) during health and disease states. These include critical questions centred around GSDM-dependent lytic versus non-lytic functions, the biological activities of cleaved versus full-length proteins, the differential roles of GSDM-expressing mucosal immune versus epithelial cells, and whether GSDMs promote pathogenic or protective effects during specific disease settings. This Review provides a comprehensive summary and interpretation of the current literature on GSDM biology, specifically focusing on the gastrointestinal tract, highlighting the main controversial issues and their clinical implications, and addressing future areas of research to unravel the specific role(s) of this intriguing, yet enigmatic, family of proteins.
Introduction
Identification of the first gasdermin (GSDM) gene in humans dates back to 2000 (ref.1), with five additional paralogue genes identified thereafter2. GSDMs were initially associated with a plethora of diseases, including hearing loss3, asthma4,5, alopecia6,7 and cancer8,9; however, their specific biological functions remained elusive for over a decade. In 2015, three studies independently described the pore-forming ability of GSDMD, reporting its functional role as the primary effector of pyroptosis10-12. The term ‘pyroptosis’ was first introduced in 2001 to describe a form of regulated cell death, dependent on non-apoptotic caspase-1, that promoted an inflammatory response13. The definition of pyroptosis has changed over the last several years, with the assembly of GSDM pores within the cell plasma membrane now considered its hallmark feature14. Although this definition has restricted the role of GSDMs as indispensable for pyroptosis, reports demonstrate that they can facilitate other forms of regulated cell death14 and several non-lytic processes.
The number of studies focusing on the contribution of GSDMs in gastrointestinal pathology is rapidly increasing and GSDMs have been implicated in diseases affecting the gastrointestinal tract, including gastrointestinal-related malignancies, enteric infections and gut mucosal immune-mediated disorders, such as inflammatory bowel disease (IBD). In this Review, we: (1) summarize the current literature about GSDM biology in the gastroenterology field, (2) provide a comprehensive interpretation of these studies, specifically regarding neoplastic, infectious and immune-mediated diseases of the gastrointestinal tract, (3) address the main controversies concerning the role(s) and function(s) of GSDMs in gastrointestinal pathophysiology, and (4) highlight the potential value of GSDMs in the clinical setting as biomarkers and/or therapeutic targets of gastrointestinal-related disorders.
General concepts in GSDM biology
Six paralogue genes are present in humans: GSDMA, GSDMB, GSDMC, GSDMD, deafness autosomal dominant (DFNA) 5 (also known as GSDME) and deafness autosomal recessive (DFNB) 59 (also known as pejvakin (PJVK)), which share 45% homology in two highly conserved amino-terminal (NT) and carboxy-terminal domains15. Beyond the first observations of GSDMD mediating caspase-1-dependent pyroptosis in immune cells, subsequent studies revealed a number of shared and unique features that characterize GSDMs, including their cellular source and/or localization, activation pathways and biological function(s) (summarized in Fig. 1). The general concepts regarding GSDM structure and activation pathways are outlined in Boxes 1, 2, respectively.
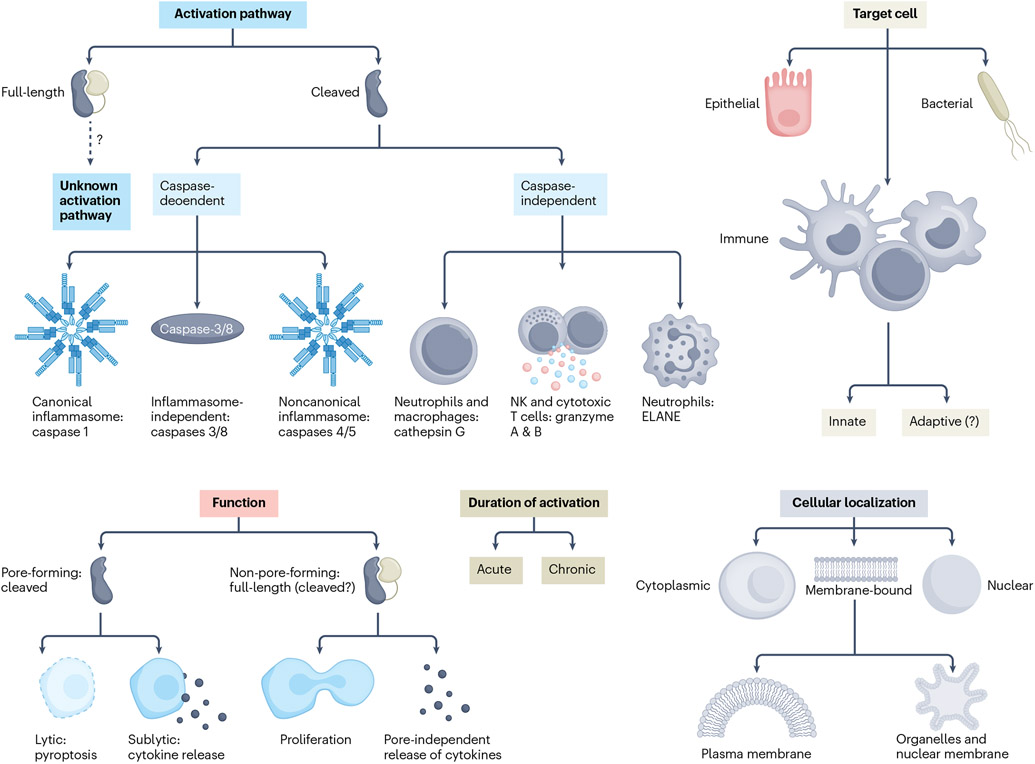
Fig. 1 ∣. Important features of gasdermins.
Schematic representation of the most important features of gasdermins (GSDMs). The activation pathways of full-length GSDMs are not fully understood. Two major pathways have been identified for cleaved GSDMs: caspase-dependent (with or without the involvement of the inflammasome) and caspase-independent (requiring different proteases). GSDMs can target either human cells (immune and non-immune) and/or bacterial cells. GSDM functions can be schematically classified as pore-forming (that is, pyroptosis and cytokine release) and non-pore-forming (including cell proliferation and pore-independent cytokine release). GSDM activation can occur acutely (resulting in either pyroptosis or definitive membrane repair) or chronically (with continuous pore formation counterbalanced by repair mechanisms). Within eukaryotic cells, GSDMs can be found in different locations: intracytoplasmic or nuclear, or bound to lipid membranes (that is, plasma or organelle membranes). ELANE, neutrophil elastase; NK, natural killer.
Box 1. Structure of gasdermins.
Each gasdermin (GSDM) presents with highly conserved amino-terminal (NT) and carboxy-terminal domains16, connected through a linker region unique to each GSDM195, except for pejvakin (PJVK), whose NT domain is directly connected to a shorter C-terminal domain16. The C-terminal domain is almost exclusively composed of α-helices to assume a globular conformation, and it is thought to be responsible for the inhibited state of full-length GSDMs by completely masking the NT hydrophobic pocket that binds lipids16. Three interface regions have been identified in GSDMA3, involving the α1 and α4 helices (both crucial in lipid binding) and the β1–β2 loop on the NT domain187. Once inhibition of the C-terminal domain is released, the NT domain binds to the negatively charged lipids of the plasma membrane and undergoes extensive conformational changes, forms homo-oligomers and inserts within the membrane188. The release of inhibitory states is usually achieved via proteolytic cleavage in the linker region to allow dissociation of the C-terminal domain. However, some mutations affecting the sequence of the C-terminal domain can abolish its inhibitory function and allow full-length GSDM to form pores16. Notably, in the absence of lipid membranes, the C-terminal and NT domains remain associated even when the linker region is cleaved16. The β3–β4–β5 and β7–α4–β8 regions of GSDMA3-NT form transmembrane β-hairpins. Each GSDMA3-NT subunit contributes four β-strands to the formation of the pore; 27 NT fragments comprise the entire pore, characterized by a β-barrel transmembrane region and a globular cytosolic rim, and whose inner diameter measures 18 nm187. For GSDMD, the β1–β2 loop mediates lipid binding, and a dominant 33-fold symmetry has been reported with an inner diameter of 21.5nm189. Evidence suggests that GSDMD pore structure is dynamic, alternating between open and closed states and regulating pore size51.
Box 2. The inflammasome and pyroptosis.
Inflammasomes are multimeric cytosolic complexes expressed in immune and non-immune cells; they assemble in response to pathogen-associated and damage-associated molecular patterns, which are recognized by intracellular sensors (reviewed elsewhere196), and orchestrate several cell responses. These sensors interact (either directly or via an adaptor protein) with pro-caspase 1 (ref.196). In the canonical pathway, pro-caspase 1 undergoes dimerization and auto-proteolysis, generating active caspase 1 (ref.197), which serves as a protease for several cytosolic molecules, including full-length GSDMs12,198, pro-IL-1β and pro-IL-18 (ref.199). GSDMD–amino-terminal (GSDMD-NT) assembles into membrane-inserted pores enabling the secretion of inflammatory mediators28, and it can eventually determine an alteration of the osmotic gradient across the plasma membrane, ultimately leading to membrane disruption and pyroptotic cell lysis. Lactate dehydrogenase, being a higher molecular weight protein, cannot pass through GSDM pores, so its presence in the extracellular space is considered a marker of complete membrane rupture and cell death. In the non-canonical pathway, caspases 4 and 5 (caspase 11 in mice) are directly activated by lipopolysaccharides. They form dimers that process the maturation of GSDMD-NT, after which GSDMD pores enable the influx of K+ ions, which activates the NLRP3 inflammasome10,11,200. The currently accepted definition of pyroptosis is: “a form of regulated cell death that critically depends on the formation of plasma membrane pores by members of the gasdermin protein family, often (but not always) as a consequence of inflammatory caspase activation”14.
The morphological features of pyroptosis in vitro are represented by: (1) chromatin condensation with an intact nucleus, (2) mild cell swelling, (3) membrane blebbing and formation of pyroptotic bodies, and (4) osmotic lysis disrupting membrane integrity201. Research has shown that GSDM pores can also participate in other forms of regulated cell death (such as secondary necrosis), highlighting that active crosstalk exists between different cell death pathways202.
Regulated cell death via GSDM pore formation (that is, pyroptosis (Box 2)) is the best-characterized function of GSDMs. The in vitro ability to form pores within liposomal membranes has been demonstrated for the NT domains of GSDMA-E16, whereas PJVK has lost its pore-forming capabilities, despite retaining a role in inflammation and response to infection17. Besides pyroptosis, GSDM pores contribute to other forms of regulated cell death. Caspase-3-mediated GSDME cleavage causes secondary necrosis in apoptotic cells18. In neutrophils, cleaved GSDMD participates in inflammatory responses and/or regulated cell death. Canonical, caspase-1-mediated activation of GSDMD induces neutrophil pyroptosis19, whereas ELANE-cleaved GSDMD induces lytic neutrophil death during infection20, thereby exerting a regulatory effect on inflammatory responses or conversely, participating in NETosis21, possibly by forming nuclear membrane pores and allowing extrusion of DNA fragments that can form neutrophil extracellular traps (NETs)22. Furthermore, cathepsin G-cleaved GSDMD promotes inflammatory functions in neutrophils without mediating their death23. Proteolytic activation of GSDMs can modulate mitochondrial oxidative stress24,25, suggesting that they participate in mitophagy. GSDM-dependent mitochondrial permeabilization also promotes regulated cell death: GSDMD-NT induces the release of mitochondrial reactive oxygen species (ROS), which stimulates either pyroptosis (via NLRP3 activation and subsequent GSDMD-mediated pyroptosis)25 or necroptosis (via mixed lineage kinase domain-like pseudokinase (MLKL) pores)26. Finally, in human cells lines, GSDMD-NT and GSDME-NT promote apoptosis by releasing mitochondria-derived caspase 3 (ref.27).
GSDMs also regulate the release of intracytoplasmic molecules, which can occur concomitantly with pyroptosis but can also happen in the absence of pyroptosis. GSDM pores are responsible for the unconventional release of IL-1 cytokine family members (for example, IL-1β, IL-18 and IL-33)2,28-34 and other inflammatory mediators, including ATP and HMGB1 (refs. 35,36). GSDMD-NT also contributes to IL-1β release from neutrophils by localizing to azurophilic granules and autophagosomes, possibly through vesicle trafficking37. Finally, biological functions have been described for some full-length GSDMs (GSDM-FL). In intestinal epithelial cells (IECs), GSDMB-FL translocates to the plasma membrane and regulates proliferation, migration and cellular adhesion in vitro38, whereas GSDMD-FL participates in the release of IL-1β-containing small extracellular vesicles (sEVs) from IECs ex vivo during dextran sulfate sodium (DSS)-induced colitis, as confirmed in vitro in mouse IECs39.
Importantly, pyroptosis does not represent an ‘all-or-nothing’ process, and the outcome(s) of GSDM-dependent pore formation can be finely tuned at different levels. Promoter methylation regulates cell-specific GSDM expression8,40-43, and multiple upstream signalling pathways control GSDM gene expression (Table 1). Proteolysis critically regulates GSDM activity: caspase 3 cleaves GSDMB and GSDMD to produce inactive fragments44,45, whereas caspase 7 produces inactive GSDMD-NT44. Post-translational modification, including phosphorylation27, oxidation46, succination47, palmitoylation48 and conjugation to itaconate49, also modulates GSDM activity.
Table 1 ∣.
General biology of the gasdermin family
| GSDMs (aliases) and location on chromosomes (human and mouse) |
Structure | Lipid binding capacity |
Cellular localization |
Proteolytic modulator(s) |
Signalling pathways | Biological functions |
Refs. |
|---|---|---|---|---|---|---|---|
| GSDMA (GSDM1, FKSG9) chr17q21.1 Gsdma1–3 chr11D | NT and CT domains connected through a linker region | Cardiolipin, phosphatidic acid, phosphatidylserine, phosphoinositide | Plasma membrane; cytoplasm; mitochondria | SpeB; unknown human activator? | Upregulated by TGFβ1-LMO1, TNF Interactions with Hsp90–Hsp70–Hop, Trap1–mtHsp75 complexes (GSDMA3)a | Pyroptosis Mitochondrial homeostasis Autophagy | 8,9,16,24,62,64-66,187,188 |
| GSDMB (GSDML) chr17q21.1 | NT and CT domains connected through a linker region | For both NT and FL: phosphatidic acid, phosphatidylglycerol sulfatide, phosphoinositide | Plasma membrane; cytoplasm; nucleus | GZMA; caspase 1 (activation, uncertain); caspases 1, 3, 6, 7 (inactivation, uncertain); caspases 4, 9 (unknown effect) | Upregulated by IFNγ (FL, cytoplasmic); upregulated by methotrexate (FL localized to plasma membrane) Upregulates transcription of TGFβ1, 5-LOX and MMP-9 (isoform 1, nuclear) FAK phosphorylation (downstream effector of GSDMB-FL) Interacts with caspase 4 (FL) | Pyroptosis (NT domain) Adjuvant in GSDMD pyroptosis (FL) Cell proliferation, migration, adhesion (FL) Bactericidal (NT domain) Promotes tissue remodelling | 5,9,16,38,45,68-70,74,189 |
| GSDMC (MLZE) chr8q24.21 Gsdmc1–4 chr15D1 | NT and CT domains connected through a linker region | Uncharacterized | Plasma membrane | Caspase 8 | Downregulated by TGFβa Upregulated by STAT3-PDL1 Upregulated by STAT6 (Gsdmc2–4), IL-4, IL-13 (GSDMC2–4)a ERK and JNK pathways (downstream effectors) | Pyroptosis Control of cell cycle? | 9,16,32,75,76,190 |
| GSDMD (GSDMDC1, FNA5L, FKSG10) chr8q24.3 Gsdmd chr15D3 | NT and CT domains connected through linker region | Cardiolipin, phosphatidic acid, phosphoinositide | Plasma membrane; cytoplasm; nuclear membrane; mitochondria; neutrophilic granules; autophagosome | Canonical inflammasome (caspase 1); non-canonical inflammasome (caspases 4 and 5); RIPK1–FADD–caspase 8; caspase 3; ELANE; cathepsin G | IRF1 and IRF2 (transcription regulation)a Forms complex with Hsp90–Cdc73–NEDD4–caspase 8a | Pyroptosis NETosis; mitophagy Sublytic release of inflammatory mediators Release of inflammatory mediators in microvesicles (FL)a Bactericidal effect? | 9-12,16,21-23,25,28,33,3,39,51,84,86,91-93,99,186,191-194 |
| DFNA5 or GSDME (ICERE-1) chr7p15.3 Dfna5 or Gsdme chr6B2.3 | NT and CT domains connected through a linker region | Cardiolipin, phosphatidylserine, phosphoinositide | Plasma membrane; cytoplasm; mitochondria | Caspase 3 (and caspase 8); GZMB | IRF1 (transcription regulation)a TNF stimulates caspase 3-mediated pyroptosisa | Pyroptosis Sublytic release of inflammatory mediators Autophagy | 36,94-96,98,99,106,116,131 |
| DFNB59 or PJVK (GSDMF?) chr2q31.2 Pjvk or Dfbn59 chr2.3 | NT, shorter CT, no linker region | Uncharacterized | Peroxisomes | Unknown, not required? | Unknown | Pexophagy | 15,16,109,110,195 |
All data refer to human GSDMs, unless otherwise specified. Cdc, cell division cycle; CT, carboxy-terminal; DFNA5, deafness autosomal dominant 5; FADD, FAS-associated death domain protein; ELANE, neutrophil elastase; ERK, extracellular signal-regulated kinase; FAK, focal adhesion kinase; FL, full-length; GSDM, gasdermin; GZMA, granzyme A; GZMB, granzyme B; Hop, Hsp70/Hsp90 organizing protein; Hsp, heat-shock protein; IFNγ, interferon-γ; IRF, interferon regulatory factor; JNK, c-Jun amino-terminal kinase; LMO1, LIM domain only 1; MMP-9, matrix metallopeptidase 9; mtHsp75, mitochondrial Hsp 75; NEDD, neural precursor cell-expressed developmentally downregulated gene; NT, amino-terminal; PJVK, pejvakin; RIPK1, receptor-interacting serine–threonine protein kinase 1; SpeB, streptococcal pyrogenic exotoxin B; STAT, signal transducer and activator of transcription; TGFβ1, transforming growth factor-β1; TNF, tumour necrosis factor; Trap1, TNF receptor associated protein 1; 5-LOX, 5-lipoxygenase.
Evidence only available in mice.
Once GSDM pores are assembled, the imbalanced ionic flow that eventually leads to cell death can be counterbalanced by membrane repair mechanisms through the endosomal sorting complexes required for protein transport (ESCRT) III. The ESCRT machinery, that is activated in response to increases in cytosolic Ca2+, is then recruited to the site of GSDM pores, in which damage is repaired through the budding of pore-containing membranes50. This ‘tug-of-war’ between pore formation and membrane repair determines the ultimate fate of the cell (that is, pyroptosis or cell survival). In line with this concept, GSDMD pores are dynamic structures whose open or closed status occurs intermittently, with pore size varying over time51.
GSDMs in the gastrointestinal tract — call them by their names
GSDMs are implicated in several gastrointestinal disorders, including enteric infections52, mucosal immune-mediated diseases2, carcinogenesis and tumour progression53 (Table 2). Evidence indicates that their contribution can be either dependent on or unrelated to their involvement in inflammation. GSDMs are also involved in two other gastrointestinal-relevant biological processes, coagulation and cell differentiation. In mouse models of sepsis, GSDMD pores in macrophages promoted coagulation via tissue factor release54 and phosphatidylserine exposure55. Additionally, GSDMD-dependent release of neutrophil traps during NETosis can capture platelets and promote clot formation in mice56. Whether the role of GSDMD in coagulation might be relevant during hypercoagulatory states associated with gastrointestinal diseases (for example, increased risk of venous thromboembolism during acute severe ulcerative colitis57) is conceivable, yet currently unknown. A role for GSDMs in intestinal epithelial differentiation is also postulated based on their differential patterns of expression throughout the human gastrointestinal tract58. In the oesophagus and stomach, GSDMA is preferentially expressed in the superficial epithelium, whereas GSDMB is primarily found in the basal region, where stem cells reside. In the oesophagus, GSDMC and GSDMD are predominantly expressed in differentiated and differentiating cells, respectively; conversely, GSDMD is mainly found in differentiated gastric cells9. GSDM-dependent regulation of intestinal epithelial differentiation is currently supported by only descriptive observations, however, and functional studies are required to verify this hypothesis.
Table 2 ∣.
Summary of the functions of gasdermins in gastrointestinal diseases
| GSDM | Cancer | Infectious diseases | Immune-mediated diseases |
Refs. |
|---|---|---|---|---|
| GSDMA | Silencing associated with gastric cancer Promotes TGFβ-associated death of neoplastic cells? | Unknown — role described for Streptococcus pyogenes infection in keratinocytes | SNPs associated with susceptibility to IBD | 1,8,9,63,65,196 |
| GSDMB | GZMA-dependent clearance of neoplastic cells Potential role for GMZA-dependent pyroptosis in boosting antitumour immunity |
Bactericidal effect against intracellular pathogens | SNPs associated with susceptibility to IBD Promotes epithelial restitution (GSDMB-FL) Potential pro-inflammatory role with GMZA-dependent pyroptosis |
1,8,9,38,63,69,70,74,196 |
| GSDMC | Increased expression in CRC Promotes growth of CRC cells when TGFβ control failsa Potential role as oncosuppressor in oesophageal squamous cell carcinoma Can switch apoptosis to pyroptosis in neoplastic cells (caspase 8-dependent cleavage) |
Potential role in helminth infectiona | Potential role in type 2 immune responsesa | 1,8,9,32,75-78 |
| GSDMD | Downregulation promotes cell proliferation in gastric cancer cell lines | Pyroptosis of macrophages and IECs infected by intracellular bacteria (protective or pathogenic, depending on the model)a NET formation (response against intracellular and extracellular pathogens) PIT formation (response against intracellular pathogens) Potential direct bactericidal effect against extracellular pathogens Potential role in shaping gut microbiomea Response against parasites (macrophage hyperactivation)a Pyroptosis of macrophages and IECs infected by virus (protective or pathogenic, depending on the model)a |
Controversial role (promoting or protective) in colitisa Mediates IL-1β and IL-18 release from IECsa Mediates cytokine release and pyroptosis in macrophages |
1,8,9,20,22,30,39,82,85,88,90-93,125,134-136,138,139,150,191 |
| GSDME | Silencing and loss-of-function mutations associated with CRC development Increased expression in oesophageal cancer compared to expression in adjacent tissues Caspase 3 and GZMB-dependent clearance of neoplastic cells GZMB-dependent pyroptosis boosts antitumour immunity Promotes the development of colitis-associated cancer via HMGB1 releasea |
Unknown | Promotes intestinal inflammation via HMGB1 releasea TNF-induced caspase 3-dependent pyroptosis in IECsa |
18,36,59,95,96,100-102,105,106 |
All data refer to human GSDMs, unless otherwise specified. CRC, colorectal cancer; FL, full-length; GSDM, gasdermin; GZMA, granzyme A; GZMB, granzyme B; HMGB1, high-mobility group box 1; IBD, inflammatory bowel disease; IECs, intestinal epithelial cells; NET, neutrophil extracellular trap; PIT, pore-induced intracellular traps; SNPs, single nucleotide polymorphisms; TGFβ, transforming growth factor-β; TNF, tumour necrosis factor.
Evidence only available in mice.
Current research on GSDMs generally focuses on each family member separately, but the possibility exists that multiple GSDMs are simultaneously involved in a biological process within targeted anatomical regions. For example, GSDMA and GSDMB expression is observed in the upper gastrointestinal tract, whereas GSDMB, GSDMC, GSDMD and GSDME are more prevalent in the lower part1,5,8,9,15,32,59-61. Although some functions might overlap among GSDM family members, others seem to be decisively different, with even the same result (for example, pyroptosis) mediated by different cellular mechanisms, as discussed below. As such, two approaches can be taken to understand better the roles of GSDMs in the gastrointestinal tract: to evaluate systematically each GSDM separately or to comprehensively consider the family as a whole in the context of a specific biological process. In the following sections, we discuss the unique aspects of each GSDM separately and then address translational studies to frame their functional contributions during specific pathophysiological events.
GSDMA
Genome-wide association studies revealed that polymorphisms in GSDMA are associated with susceptibility to skin disorders62 and IBD63; however, little is known about the stimuli and proteases involved in GSDMA activation. Initial observations verified the presence of GSDMA in normal gastric and oesophageal human tissues and showed its silencing in gastric and oesophageal cancer cell lines and primary oesophageal and gastric cancer cells, purportedly through promoter methylation1,8,9, suggesting its potential role as an oncosuppressor. In addition, TGFβ can upregulate GSDMA to induce GSDMA-mediated cell death8.
The most compelling evidence regarding the function of GSDMA in the epithelium comes from studies on mouse keratinocytes. GSDMA is linked to mitochondrial stress and macroautophagy24. A gain-of-function mutation in Gsdma3 impaired mitochondrial function, with a subsequent increase in ROS that determines autophagy-dependent cell death, indicated by increased LC3-II; similarly, the NT domains of human GSDMA and GSDMD induced LC3-II activation64. Some of these results were replicated in HEK293T cells, indicating that such processes could occur in other epithelial cells besides skin cells24; however, their relevance in gastrointestinal pathophysiology is unknown. Streptococcus pyogenes-derived exotoxin B (SpeB) selectively cleaved GSDMA at its linker region to induce pyroptosis in keratinocytes in vitro65,66. Gsdma1−/− mice infected with S. pyogenes developed less severe skin lesions than wild-type mice but showed more severe systemic infection, suggesting that GSDMA1-mediated pyroptosis might have a role in the development of skin ulcers, but might also be key to confining infection and preventing bacterial dissemination65. Conversely, LaRock and colleagues showed that Gsdma1–3−/− mice develop larger skin lesions66. These two studies disagree on whether SpeB cleaves GSDMA1 or all three mouse GSDMAs, leaving open questions regarding the net effects of GSDMA-mediated pyroptosis upon cutaneous S. pyogenes infection. It would be interesting to explore whether GSDMA has a similar role in response to specific gastrointestinal infections in ulcer development.
GSDMB
The roles of GSDMB have remained somewhat elusive until the past few years, as GSDMB shows some peculiarities that distinguish it from other family members. For example, both GSDMB-FL and GSDMB-NT fragments can bind lipid membranes with specific phospholipids (phosphoinositide and glycolipid sulfatide, but not cardiolipin) in contrast to other GSDMs45. Another distinctive trait is that GSDMB has six splice variants67; however, the specific expression and role(s) of each isoform are not yet completely understood. Selective nuclear localization has been reported for isoform 1 (ref.5), yet whether GSDMB can function as a transcription factor or has other nuclear functions is not clear. Overexpression of GSDMB isoform 1 in airway epithelium correlates with increased levels of mediators implicated in airway remodelling during chronic asthma5. Whether the same process contributes to the development of fibrosis in chronic intestinal inflammation is currently unknown.
In vitro experimentation has produced inconsistent results regarding whether GSDMB is activated by inflammatory and/or apoptotic caspases10,45,68. Caspase 1 has been found to activate GSDMB-NT, which contributed to the pathogenesis of asthma in a human GSDMB knock-in mouse model69. Conversely, other studies have found that caspases 1, 3, 6 and 7 mediate proteolytic inactivation of GSDMB45,68. Finally, evidence indicates that lymphocyte-derived granzyme A (GZMA) mediates proteolytic activation of GSDMB in human intestinal epithelial cell lines70.
Increased levels of GSDMB have been documented in patients with IBD38, and polymorphisms in GSDMB have been linked to several chronic inflammatory diseases, including IBD45. A role for GSDMB in carcinogenesis has also been proposed for several gastrointestinal malignancies, with either pro-tumour or antitumour functions. In oesophageal tumours, GSDMB was initially suggested to function as an oncogene9, but subsequently, expression was found to be reduced in tumours compared with adjacent, uninvolved areas70. Furthermore, GSDMB in oesophageal tumours can be regulated by promoter methylation71. In gastric tumours, GSDMB is either more72 or less70 expressed than in adjacent, non-neoplastic tissues. Finally, tissues derived from colorectal cancer (CRC) lesions usually express GSDMB at levels comparable to those in tissue from corresponding non-neoplastic areas70,73. Interestingly, a differential GSDMB band pattern was observed upon western blotting, comparing involved and non-involved areas from both CRC and diffuse gastric cancer tissues73. Collectively, these observations suggest that GSDMB might have different and potentially opposing roles during carcinogenesis.
Regarding function(s), GSDMB-FL promotes non-canonical pyroptosis in THP-1 macrophages by physically interacting with caspase 4 to enhance its proteolytic activity on GSDMD in vitro68. Zhou and colleagues described a form of GSDMB-mediated pyroptosis in a colon cancer cell line. Specifically, GZMA, derived from natural killer and/or CD8+ T cells, entered IECs via perforin pores, subsequently generating GSDMB-NT fragments that oligomerized into the plasma membrane and mediated pyroptosis70. A subsequent study, adopting a GSDMB knock-in mouse model, identified membranes of Gram-negative bacteria as the exclusive target of GZMA-cleaved GSDMB-NT pores74, sparing host IECs. Finally, non-lytic functions of GSDMB-FL were found in colonocytes: in vitro translocation of GSDMB-FL to the plasma membrane regulated cell proliferation, adhesion and migration through a mechanism involving PDGFA-dependent phosphorylation of focal adhesion kinase38.
The three-pronged roles of GSDMB in IECs (pyroptotic, bactericidal and pro-restitution) are archetypical of the functional complexity that a single protein can possess within a specific cell. To account for such functional differences, one or a combination of the following explanations can be suggested: (1) GSDMB possesses differential specificity for a precise phospholipid composition within the plasma membrane, enabling it to distinguish between host and bacterial membranes; (2) the functions and membrane-binding capacities of GSDMB vary in an isoform-specific fashion; and (3) partner proteins intervene to facilitate GSDMB docking to membranes, triggering a specific function.
GSDMC
GSDMC has been suggested to have roles as an oncogene in CRC72 and an oncosuppressor in oesophageal squamous cell carcinoma9. GSDMC overexpression was observed in CRC tissues compared with normal adjacent areas75. In Apcflox/flox mice (a CRC mouse model), Gsdmc2 and Gsdmc4 are under negative control of the TGFβ signalling pathway; in these animals, colonic deletion of Tgfbr2 was associated with upregulated expression of these two Gsdmc genes and increased tumour proliferation. GSDMC expression in a CRC cell line correlated with cell proliferation and promoted the growth of cell xenografts implanted in nude mice75.
Hou and colleagues reported a pro-tumorigenic role for GSDMC in switching regulated cell death from apoptosis to pyroptosis in a breast cancer cell line76. Under hypoxic conditions, phosphorylated STAT3 interacted with PDL1 and translocated to the nucleus, where it promoted GSDMC transcription. With high expression levels of GSDMC, macrophage-derived tumour necrosis factor (TNF) promoted caspase 8-mediated cleavage of GSDMC, whose NT fragments facilitated pyroptosis76. It would be interesting to investigate whether such observations can be recapitulated in gastrointestinal cancer cell lines. Furthermore, in a cervical cancer cell line, α-ketoglutarate (αKG) was reported to induce the formation of an intracellular DR6-containing receptosome, which provided a physical platform for the pro-pyroptotic activation of GSDMC, mediated by caspase 8 (ref.77). This αKG-induced pyroptosis was also observed in human gastric and CRC cell lines77.
A role of GSDMC in IEC pyroptosis during a type 2 immune response to helminth infection has also been proposed. HEK293 cells transfected with Gsdmc2 and infected with Nippostrongylus brasiliensis initiated spontaneous cleavage of GSDMC2 and underwent pyroptosis, and mouse intestinal organoids treated with IL-4 or IL-13 showed increased lytic cell death, possibly through a GSDMC-mediated mechanism78. Caution should be applied, however, in interpreting these findings as indicating that GSDMC cleavage occurs in organoids, as causal proof of GSDMC-mediated cell death was not shown in this study78. Additionally, Zhao and colleagues found that, in the context of type 2 immunity, STAT6 upregulated Gsdmc2–4 in mouse IECs and GSDMC mediated IL-33 release from goblet cells within intestinal organoids32.
GSDMD
GSDMD is the primary effector of pyroptosis: its pores cause lytic cell death but also serve as channels for the secretion of inflammatory cytokines28,30 involved in several inflammatory diseases79. ‘Hyperactivation’, described in macrophages80, dendritic cells29 and neutrophils81, is a process in which inflammatory mediators are continuously released concomitantly with GSDMD cleavage, without the cell undergoing lytic death; the stimuli promoting cell hyperactivation seem to differ qualitatively, and not just quantitatively, from those that induce pyroptosis28, but this issue remains unclear. Membrane repair mechanisms that occur simultaneously with GSDM pore assembly (such as ESCRT50) have been suggested to be critical for macrophages to enter the hyperactivation state, but this remains to be proven. Conversely, in neutrophils, GSDMD has been found to be capable of localizing to intracellular organelles selectively and not to the cell’s plasma membrane37, explaining why the cells can tonically release inflammatory mediators without undergoing pyroptosis. Whether this hyperactivated state might be relevant in gastrointestinal diseases, particularly those characterized by chronic inflammation, such as IBD, is certainly an intriguing possibility.
GSDMD overexpression is observed in IBD39, and its contribution to disease pathogenesis revolves around its ability to facilitate the release of IL-1 family members. Specifically, GSDMD-associated pores mediate IL-18 secretion30, whereas chaperoned GSDMD-FL interacts with NEDD4 to promote the release of sEVs containing IL-1β in a caspase 8-dependent, non-lytic manner39. GSDMD pores have also been found to promote IL-33 release from airway epithelial cells33 and hepatic stellate cells34 in vitro. Future studies will elucidate if such a mechanism for IL-33 secretion is also pertinent to the luminal gastrointestinal tract. Furthermore, in mice, GSDMD pores regulate exocytosis in intestinal goblet cells by inducing Ca-dependent conformational changes of the cytoskeleton that enable intracellular granules to fuse with the plasma membrane and mucins to be released into the extracellular space without causing pyroptosis82.
GSDMD has also been suggested to have a role as an oncosuppressor in gastrointestinal malignancies. CRC tissues from 34 patients showed reduced GSDMD levels compared with levels in non-cancerous control tissues, and decreased GSDMD levels correlated with a poor prognosis in patients with CRC (n = 244)83,84. Gastric cancer cell lines also displayed reduced activity of GSDMD, and GSDMD downregulation promoted cell proliferation in vitro85. Also, GSDMD was increased in oesophageal squamous cell carcinoma compared to the levels in normal tissue, and metformin induced GSDMD-mediated pyroptosis in oesophageal cancer cell lines86.
GSDMD can also bind cardiolipin, which is exclusively expressed in the mitochondria of eukaryotic cells and in bacterial walls87. Consistently, a role for GSDMD in mitophagy (discussed above) and bacterial killing was observed in vitro. Indeed, recombinant GSDMD-NT showed bactericidal activity against Listeria monocytogenes, Staphylococcus aureus and Escherichia coli88. Furthermore, the release of NETs (discussed above) is dependent on non-canonical GSDMD activation, which can be initiated by either cytosolic bacteria or extracellular-originating lipopolysaccharide (LPS)22: NETs have a recognized role in sequestering extracellular pathogens89, and they also contribute to restraining the dissemination of intracellular bacteria that are released after NETosis22. Jorgensen and colleagues described a similar mechanism in pyroptotic macrophages, with the formation of ‘pore-induced intracellular traps’ (PITs) to sequester intracellular bacteria in vitro90. Three studies also showed that caspase 8-mediated proteolysis of GSDMD in macrophages can elicit pyroptosis and promote antimicrobial functions91-93.
GSDME
In 2017, two groups reported that caspase 3 mediated proteolytic activation of GSDME to induce lytic cell death in vitro18,94. As caspase 3 is an apoptotic protease, the possibility exists that GSDME determines the fate of cells when exposed to pro-apoptotic stimuli. In the presence of low GSDME levels, cells progress to apoptosis, whereas high levels can skew cells towards pyroptosis in vitro95. Alternatively, Rogers and colleagues showed that GSDME mediated secondary necrosis in vitro; if phagocytes do not promptly remove apoptotic bodies, they can eventually release their intracellular contents via GSDME pores27. Caspase 3-activated GSDME can promote apoptosis by targeting mitochondria to facilitate cytochrome c release and caspase 3 activation in vitro27. Zhang and colleagues found that, in vitro, lymphocyte-derived GZMB cleaved GSDME at the same site as caspase 3 in vitro96. Finally, aside from cell death, GSDME is implicated in the secretion of IL-1α (during caspase-8-dependent pyroptosis)97 and IL-1β (as a complementary and independent mechanism that can occur without lytic cell death)98 from macrophages, IL-1β from neutrophils99, HMGB1 from IECs36, and IL-18 from gastric cancer cell lines31.
Increased intestinal GSDME has been found in patients with Crohn’s disease59. In mice treated with trinitrobenzene sulfonic acid (TNBS), caspase 3-dependent GSDME cleavage in IECs induced pyroptosis and the release of inflammatory mediators (that is, HMGB1, IL-1β, TNF and IL-6), thereby promoting inflammation59. Furthermore, in the same mouse model, TNF-induced shedding of IECs was GSDME-dependent and under the control of interferon regulating factor 1 (IRF1)100.
Regarding carcinogenesis, GSDME silencing, through methylation of its promoter, was found in CRC101, with GSDME generally considered an oncosuppressor in this setting. Conversely, increased GSDME was found in oesophageal squamous cell carcinoma102 and gastric adenocarcinoma103 compared with the levels in non-involved areas, with microRNAs contributing to GSDME regulation in the context of cancer104. The majority of cancer-associated mutations in GSDME seem to impair its pore-forming abilities96. GSDME can switch regulated cell death from apoptosis to pyroptosis in colorectal105 and gastric106 cancer cell lines. Thus, GSDME-mediated cell lysis can reduce tumour burden by killing neoplastic clones18,94 and promote antitumour functions by immunosurveillance via the release of inflammatory mediators96. On the other hand, during experimental colitis, GSDME-dependent release of HMGB1 from IECs promoted carcinogenesis by stimulating cell proliferation36.
Finally, an in vitro study highlighted the potential importance of GSDME during viral infection107. In keratinocytes, the virus-induced shutdown of protein synthesis prompted mitochondrial damage and subsequent caspase 3-dependent GSDME activation, which regulated IL-1α release and pyroptosis107. However, whether a similar mechanism might intervene during viral infections of the gastrointestinal tract is currently unknown.
PJVK
As discussed above, PJVK shows a truncated C-terminal domain with no linker region. Its NT domain does not display pyroptotic capacity in vitro16, and it is currently unknown whether proteolysis is required to activate PJVK. Mutations in DFNB59 are linked to hearing loss in humans108. PJVK localizes to peroxisomes of inner ear hair cells109, and in response to a sound-induced increase in ROS levels, PJVK recruits LC3B to induce pexophagy, which protects against noise-induced damage110. To date, no role for PJVK in gastrointestinal diseases has been described.
The effect of GSDMs on gastrointestinal-related diseases
Cancer
An attempt can be made to distil the role(s) of GSDMs, in the context of gastrointestinal-related cancers, into the following categories: (1) in contributing to tumour-predisposing conditions, tumour development and dissemination; (2) in the GSDM-dependent death of neoplastic cells; (3) in affecting the tumour microenvironment (TME) upon activation; and (4) as effectors of antineoplastic therapies. Figure 2 illustrates the main roles of epithelial-derived GSDMs in the context of gastrointestinal cancers.
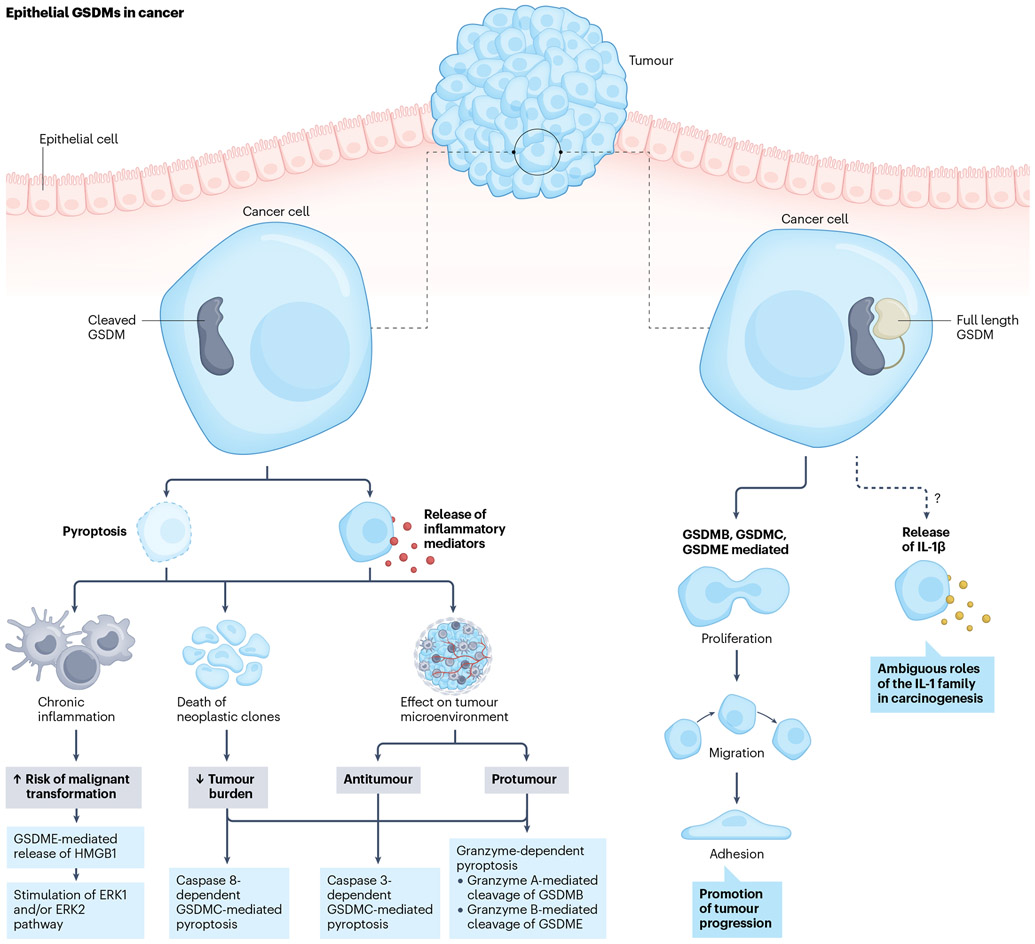
Fig. 2 ∣. Role and contribution of epithelial-derived gasdermins in gastrointestinal cancers.
Cleaved gasdermins (GSDMs) contribute to gastrointestinal carcinogenesis by mediating pyroptotic cell death and/or sublytic release of inflammatory mediators. Chronic intestinal inflammation, secondary to GSDM-dependent release of intracellular mediators, is a pro-carcinogenic stimulus that perpetuates tumorigenesis. GSDM-mediated pyroptosis of cancer cells can exert a direct antitumour effect by killing neoplastic clones, thereby reducing the tumour burden. Finally, the GSDM-dependent release of intracellular mediators from neoplastic cells shapes the tumour microenvironment to promote either antitumour or pro-tumour functions in relation to the specific leukocyte subpopulations that are recruited and activated. Full-length GSDMs can also affect the pathogenesis of cancer. GSDMB, GSDMC and GSDME are involved in the proliferation, migration and adhesion of intestinal epithelial cells, all of which are critical components for the development and growth of malignancies. Lastly, as IL-1β has been reported to play a role in the pathogenesis of gastrointestinal cancer, it is possible that the specific form of IL-1β release (and potentially, of other cytokines belonging to the IL-1 superfamily) mediated by GSDMs might also contribute to carcinogenesis. HMGB1, high-mobility group box protein 1.
GSDMs promote inflammation and influence the biology of neoplastic cells.
As GSDM pores commonly prompt local inflammation, their activation can be considered a general predisposing factor to tumour development as chronic inflammation increases the risk of malignant transformation111-113. Tan et al. described a mouse model of colitis-associated CRC36 in which IEC-derived HMGB1, produced and released in a GSDME-dependent manner, induced tumour proliferation36. A study in a mouse model indicated a more direct role of GSDMs in controlling the proliferation of neoplastic cells. Specifically, in Apcflox/flox mice, GSDMC, once deprived of TGFβ-dependent inhibition, induces tumour cell proliferation in CRC75. Conversely, GSDMD downregulation promoted cell proliferation in gastric cancer cell lines85. Also, the observation that GSDMB-FL mediates IEC proliferation and migration38 in vitro hints at its potential contribution to the development of gastrointestinal cancers, similar to breast cancer, in which GSDMB expression correlates with tumour growth and dissemination114. An analysis of GSDM gene expression in cancer revealed a potential role for the differential regulation of GSDMA, GSDMB, GSDMD and GSDME in CRC; GSDME was suggested to positively regulate cell migration and angiogenesis, as opposed to GSDMA, GSDMB and GSDMD that might downregulate such processes115. Overall, these findings suggest that GSDM-dependent regulation of different cell functions might have an important role in the development and progression of gastrointestinal malignancies.
GSDM-pores lead to the death of neoplastic clones and/or facilitate the release of inflammatory mediators.
Caspase 3 or GZMB can activate GSDME to induce pyroptosis of neoplastic cells. GSDME is frequently silenced in CRC cells, and rescuing its expression can reduce tumour burden, suggesting the role of GSDME as an oncosuppressor105,106. This concept seems to contradict the contribution, as mentioned above, of GSDME pores, to the pathogenesis of colitis-associated cancer36. Chronic GSDME activity in otherwise normal IECs might be a stimulus for carcinogenesis, whereas abrupt pyroptosis of neoplastic cells might exert an overall tumour-suppressive effect. Croes and colleagues demonstrated that Gsdme deletion in both azoxymethane-treated and Apc1638N/+ mice did not affect CRC development116. The possibility exists that, in mouse models of sporadic CRC, for tumours to evade immunosurveillance, neoplastic cells might need GSDME to be silenced. As such, in Gsdme-deficient mice, susceptibility to carcinogenesis would not be increased as, even in wild-type mice, Gsdme is already functionally absent in their tumours. Also, GSDME levels are increased in oesophageal tumour tissues compared with their levels in healthy adjacent tissues, and its gene expression correlates with a better prognosis102, which might depend on caspase 3-mediated GSDME cleavage that induces pyroptosis and subsequent clearance of neoplastic cells.
In line with this concept, Zhou and colleagues proposed that GZMA-dependent GSDMB cleavage promotes pyroptotic clearance of neoplastic cells in CRC70. Conversely, a study using the azoxymethane-DSS mouse model found that Gzma deletion protects from colitis-associated tumorigenesis by reducing the release of pro-inflammatory cytokines from intestinal macrophages117. However, as mice lack a GSDMB orthologue, this observation is difficult to reconcile with the findings of Zhou et al.70. Possibly, GZMA exerts different effects on the inflamed intestinal mucosa as on the neoplastic epithelium. Finally, αKG-dependent GSDMC-mediated pyroptosis was reported in gastric and colon cancer cell lines77, but the translational relevance of these findings has yet to be defined.
GSDM-mediated pyroptosis profoundly affects the tumour microenvironment.
Pyroptosis of neoplastic clones can reduce the tumour burden; however, its antitumorigenic effects also include a profound influence on the TME, which is critical in modulating the biology of cancer cells (Box 3). As opposed to ‘immunologically silent’ apoptosis, pyroptosis is a form of inflammatory cell death52 in which inflammatory mediators, released either through GSDM pores or as a result of membrane rupture, recruit and activate immune cells with antitumour functions. Zhang and colleagues elegantly showed that GZMB, derived from natural killer and/or cytotoxic T cells, induce GSDME-mediated pyroptosis, which in turn, promotes antineoplastic functions within the TME, whereas Gsdme overexpression in cancer cells reduces tumour growth96. In Gsdme-expressing tumours, tumour-associated macrophages (TAMs) and tumour-infiltrating lymphocytes (TILs) exhibited enhanced immunosurveillance functions, and tumour growth in NOD SCID gamma mice (lacking mature lymphocytes) was more rapid than in wild-type mice, regardless of GSDME presence, suggesting that TILs are necessary for the in vivo antitumour effects of GSDME96. Intriguingly, there is also an inherent presence of GSDME-dependent tumour-suppressor function, as xenografted cancer cells devoid of Gsdme grew faster in NOD SCID gamma mice than Gsdme-expressing cells96. Although the researchers in this study reported similar phenotypic observations with a number of other cancer cell lines, their functional and mechanistic studies in vivo utilized a breast cancer model. Hence, caution should be applied in extrapolating this information to gastrointestinal models. In a pan-cancer analysis, increased GSDME in stomach adenocarcinoma correlated with infiltration of tumour-associated fibroblasts103. Finally, PDL1-dependent, GSDMC-mediated pyroptosis occurred in nude mice xenografted with breast cancer cell lines and was under the control of macrophage-derived TNF76. However, this form of GSDMC-mediated pyroptosis in tumour-hypoxic regions supports tumour development and dampens TME antitumour functions76. Collectively, these observations demonstrate that TAMs and TILs critically mediate both pro-tumour and antitumour functions through GSDMC and GSDME cleavage, respectively.
Box 3. The immune tumour microenvironment – overview and insights for the potential role of GSDMs.
A solid tumour consists of two main components: neoplastic cells and the tumour microenvironment (TME), which exerts a critical role in carcinogenesis. The TME comprises immune cells, stromal cells, blood vessels, extracellular matrix and extracellular vesicles. Both innate and adaptive immunity have paramount roles in the TME, as they can either promote or suppress tumour growth, depending on the specific cell population residing in the TME and their activation pathways203. The TME usually polarizes monocytes towards an M2 phenotype, which mediates immunosuppressive functions, thereby promoting carcinogenesis204, whereas M1 macrophages have antitumour functions205. Dendritic cells typically present neoplastic antigens to trigger antitumour responses, but they can also be co-opted to promote immunosuppression206. Among tumour-infiltrating lymphocytes, regulatory T (Treg) cells dampen immune responses and support tumour growth207, whereas CD4+ T helper (TH) cells208, CD8+ cytotoxic T (CTL) cells209 and natural killer cells210 are responsible for antitumour functions. Specifically, CTL cells serve as sentinels that detect tumour antigens and kill neoplastic cells. Accessory signals are also required for CD8+ T cells to attack neoplastic clones; conversely, other signals — such as those involving the immune checkpoints PD1 and CTLA-4 — can induce the ‘exhaustion’ of CD8+ T cells211. Gasdermin B (GSDMB) and GSDME have been identified as effectors of CTL-induced cell death in neoplastic epithelial cells70,96. Overall, bidirectional crosstalk exists between neoplastic cells and the TME. Tumour cells attempt to promote a ‘permissive’ environment with immunosuppressive functions, whereas the TME can either support carcinogenesis or be activated to exert immunosurveillance functions that restrict tumour growth. The balance between the two determines whether a malignancy develops and progresses, and the modulation of these interactions surely represents an intriguing target for antineoplastic therapies.
Pyroptosis can also occur in tumour-infiltrating immune cells, usually through inflammasome-dependent GSDM activation, as opposed to the observations in neoplastic cells. The contribution of immune cell-derived GSDMs to gastrointestinal malignancies is controversial and has been extensively discussed elsewhere52,53. For example, IL-1β and IL-18 (two of the main GSDM-dependent cytokines secreted by macrophages) possess both antitumorigenic and pro-tumorigenic functions, as shown in mouse models of CRC52, suggesting that their effects are probably time-dependent and cell-specific. As a general rule, the net effect of GSDM activation in infiltrating immune cells of the TME depends on whether it ultimately leads to the prevalence of cells primed towards either immunosurveillance or tolerogenic functions.
GSDMs as effectors of antineoplastic drugs that can modulate their efficacy.
GSDMs mediate some of the effects of anticancer therapies and might also represent potential therapeutic targets. Indeed, several known chemotherapeutic agents induce pyroptosis of neoplastic cells, primarily via GSDME. For example, cisplatin induces GSDME-dependent pyroptosis in oesophageal squamous cell cancer in vitro, with concomitant STAT3β expression enhancing this effect by disrupting the mitochondrial respiratory chain and enhancing ROS levels118. Similarly, lobaplatin promoted GSDME-mediated lytic cell death in a CRC cell line105, and 5-FU triggered caspase 3-dependent GSDME cleavage to mediate pyroptosis in gastric cancer cell lines106. Photodynamic therapy also induces caspase 3-dependent and caspase 8-dependent GSDME-mediated pyroptosis in oesophageal squamous cell carcinoma119. GSDME was shown to regulate gut radiosensitivity, as radiation can drive caspase 3-dependent GSDME-mediated pyroptosis in IECs119. In a homograft mouse model, Gsdme−/− CRC cells were less sensitive to radiotherapy, whereas Gsdme expression enhanced the number of tumour-infiltrating natural killer cells120. Furthermore, radiotherapy was reported to induce GSDME-mediated pyroptosis in a CRC cell line120. It could be speculated that such GSDM-dependent mechanisms of action might also be responsible for some of the adverse events associated with antineoplastic treatment, primarily through the induction of pyroptosis in high GSDME-expressing normal cells. Proposed strategies to specifically exploit GSDMs in antineoplastic treatment are discussed below.
GSDMs interfere with all phases of cancer, from development to treatment.
Taken together, the contribution of the GSDM family to the pathogenesis of gastrointestinal-related cancers is complex, and their activation can indeed give rise to dichotomous effects. Inflammation sustained by GSDM-dependent processes in IECs can be considered a general tumorigenic stimulus, whereas induction of inflammasome-dependent pathways in immune cells, culminating in GSDM activation, can result in more ambiguous effects. On the other hand, GSDME-mediated pyroptosis of neoplastic clones seemingly has a net tumour-suppressive effect via direct reduction of tumour burden and promotion of TME-dependent antineoplastic functions, whereas the effects of GSDMC-mediated pyroptosis on the TME promote tumour growth. Stimulation of pyroptotic cell death is one of the mechanisms by which antineoplastic treatments exert their functions; however, low GSDM expression might potentially mediate resistance to specific mechanisms of action of antineoplastic agents. Finally, non-pyroptotic GSDM-dependent functions, mostly related to control of the cell cycle, might also contribute to tumour growth and dissemination.
Enteric infections
Evidence shows that GSDMs are activated in response to food-borne pathogens, including Salmonella, Shigella and Yersinia bacteria. Figure 3a shows the functions performed by gut mucosa-derived GSDMs in response to local infection. In this regard, two main issues need to be considered: (1) activation of GSDMs in intestinal haematopoietic versus epithelial cells, and (2) their response to extracellular versus intracellular pathogens.
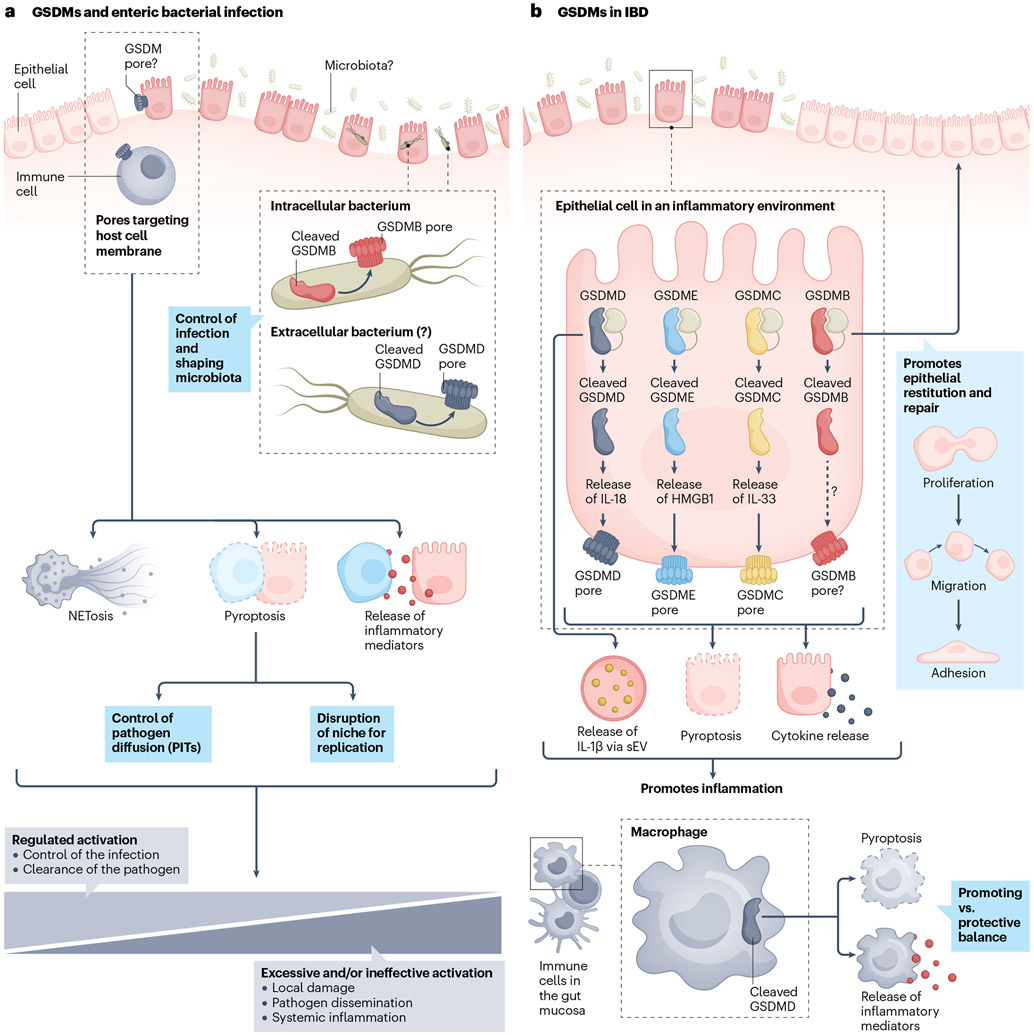
Fig. 3 ∣. Role and contribution of gasdermins in gastrointestinal inflammation.
a, Gasdermins (GSDMs) facilitate host–bacteria interactions within the gut. GSDMD-amino-terminal (GSDMD-NT) mediates NETosis, through which neutrophils extrude neutrophil extracellular traps to confine extracellular bacteria. Pyroptosis of cells infected with intracellular bacteria disrupts the niche for their replication. Furthermore, when undergoing GSDMD-mediated pyroptosis, infected macrophages can form pore-induced intracellular traps (PITs), which further help to restrain intracellular pathogens. Finally, the GSDM-dependent release of mediators is involved in eliciting and regulating local and potentially systemic inflammatory responses associated with infections. GSDM pore formation is a tightly regulated process during infection. Controlled activation can support battling an infection, whereas excessive activation can have detrimental effects. GSDM pores can also target bacterial walls, as described for GSDMB and GSDMD, with intracellular and extracellular pathogens, respectively. b, During the course of inflammatory bowel disease (IBD), GSDMs expressed in both epithelial and immune cells are pivotal in regulating inflammation. In intestinal epithelial cells (IECs), full-length (FL) GSDMB promotes epithelial repair. Conversely, GSDMD-FL mediates the release of IL-1β that fuels intestinal inflammation. Furthermore, GSDM-dependent pyroptosis of IECs is pathogenic in IBD, causing loss of mucosal integrity (by killing epithelial cells) and mediating the release of inflammatory mediators. Macrophage-derived cleaved GSDMD has an ambiguous role, described as both promoting and protecting from colitis. HMGB1, high-mobility group box protein 1; sEVs, small extracellular vesicles.
GSDMs as key effectors of antibacterial functions in haematopoietic cells.
Inflammasome activation was crucial in inducing GSDMD-mediated pyroptosis and IL-1β release in bone marrow-derived mouse macrophages upon infection with E. coli, Salmonella enterica subsp. enterica serovar Typhimurium and Shigella flexneri11. An in vitro bactericidal effect of GSDMD against several bacteria, including E. coli, was also found16,88, but its biological relevance in vivo has yet to be determined16. It was proposed that GSDMD-dependent formation of PITs contributes to controlling intracellular infections, with cellular debris trapping intracellular pathogens, such as S. Typhimurium, which neutrophils could subsequently kill via efferocytosis90. Thurston and colleagues reported that intracellular growth of S. Typhimurium was restricted by caspase 1 and caspase 11 activity in mouse macrophages, independent of GSDMD activity121. Conversely, Zuo and colleagues reported that GSDMD-mediated pyroptosis of macrophages in the caecum of mice infected with S. Typhimurium protected against bacterial dissemination. The researchers observed that S. Typhimurium-derived Salmonella plasmid virulence C inhibited pyroptosis, which correlated with reduced intestinal inflammation, but increased bacterial dissemination and liver damage122. GSDM-mediated cell lysis might be a redundant mechanism to further disrupt the niche for intracellular pathogens and, at the same time, promote immune cell recruitment via the release of inflammatory mediators to fight against infecting microorganisms. Extracellular pathogens also activate GSDMD-mediated defence responses. In vitro, the Shiga toxin 2-LPS complex from extracellular enterohaemorrhagic E. coli (EHEC) activated GSDMD in macrophages via the non-canonical inflammasome pathway to induce mitochondrial damage, ROS production, and subsequent pyroptosis and release of IL-1β25. However, the in vivo pathophysiological consequences of such a mechanism have not yet been explored. Notably, other studies found that Shiga toxin alone (that is, not in complex with LPS)123 and subtilase cytotoxin124 (another EHEC-derived virulence factor) suppressed caspase 11-dependent inflammasome activation in mouse macrophages.
Studies on yersiniosis have uncovered another relevant GSDMD pathway. Yersinia pseudotuberculosis-derived Yersinia outer protein J (YopJ) blocked TAK1 to trigger receptor-interacting serine–threonine-protein kinase 1 (RIPK1)-dependent, caspase 8-mediated GSDMD and GSDME cleavage in mouse macrophages91,92; however, in this scenario, a pathophysiological role has not been identified for GSDME in macrophages92. Yet, human macrophages are resistant to cell death induced by TAK1 inhibition, suggesting that they might have evolved a mechanism to escape this form of cell death92. Caspase 8-dependent GSDMD cleavage has been proven to provide anti-Yersinia defence in vivo; furthermore, concomitant caspase 3-mediated GSDMD inactivation also occurs, which is protective against Y. paratuberculosis infection in vivo, suggesting that tight control over GSDMD activity is fundamental during Yersinia infection93.
An important role in fighting infections has also been described for neutrophil-derived GSDMs. Caspase 11-dependent, GSDMD-mediated NETosis control Salmonella ΔsifA (a mainly cytosolic strain) infection in mice22; conversely, canonical inflammasome pathways, via caspase 1-mediated cleavage, reportedly control GSDMD-dependent pyroptosis and IL-1β secretion in neutrophils during S. Typhimurium infection in mice, but the in vivo implications of such observations have not yet been explored19. Gsdmd−/− mice are protected against E. coli infection, as they exhibit enhanced bactericidal activity attributed to a reduction in GSDMD-dependent neutrophil death20. It is plausible that GSDMD-dependent neutrophil death dampens the host’s defence against extracellular pathogens by reducing the number of active neutrophils recruited to the infection site, whereas in the presence of bacteria capable of intracellular replication, NETosis has a positive effect. Finally, during Yersinia infection, RIPK1 (besides triggering GSDMD-mediated pyroptosis in macrophages) activates neutrophil-derived GSDME to mediate pyroptosis and IL-1β release, notably without NET exclusion. In this model, IL-1β blockade increased bacterial burden in the spleen and liver of Gsdme−/− mice99.
Epithelial-derived GSDMs grapple between bactericidal and pyroptotic functions.
Hansen and colleagues reported that in IECs, GSDMB-NT cleaved by natural killer cell-derived GZMA selectively formed pores in membranes of Gram-negative bacteria, thereby exhibiting direct bactericidal capabilities, sparing host cells74. The researchers found that the Gram-negative bacterium, S. flexneri, has evolved a mechanism to escape GSDMB-mediated killing through bacteria-derived ubiquitin ligase IpaH7.8 that promoted GSDMB ubiquitination and subsequent proteasomal degradation74. Likewise, S. flexneri-derived IpaH7.8 targeted human GSDMD in IECs to promote its proteasomal degradation. GSDMD mediated IEC pyroptosis with protection against infection, but no direct targeting against the pathogen was detected125. Such observations suggest that different GSDMs might have evolved to exert simultaneous yet separate and complementary functions in IECs during intracellular infections.
Specific functions have also been described for epithelial GSDMD in response to Salmonella infection. Activation of the NAIP–NLRC4 inflammasome prompted contraction, pyroptotic cell death and extrusion of IECs. In mice, GSDMD sublytic pore formation was required to mediate epithelial contractions that allowed packing of IECs in specific foci, but their expulsion did not appear to be GSDMD-dependent, with controversial findings regarding the ability of GSDMD to mediate pyroptosis in this setting126,127. IEC expulsion is a crucial, epithelial-inherent process to restrain S. Typhimurium infection that depends on NAIP–NLRC4 (ref.128) and caspase 11 (ref.129), and coincides with other defence responses130. Evidence suggests that extruding cells display both apoptotic and pyroptotic features131. The observation that GSDMD is proteolytically activated during such processes, which is in line with the concept that inflammasome pathways activate GSDMs, reinforces the concept that different defence mechanisms are contemporaneously active during infection and interact with each other, but also provokes the need to investigate further how these processes are orchestrated by the cell. Enteropathogenic E. coli also induces GSDMD-mediated IEC pyroptosis; however, whether such a process promotes or is protective against E. coli infection has not yet been explored132.
Do GSDMs contribute to shaping the gut microbiota?
Extensive studies have shown a relationship between inflammasome activation and the gut microbiota (reviewed elsewhere133), but the specific effect of GSDMs has only been investigated in one study. Gsdmd−/− mice fed a high-fat diet displayed more severe colonic inflammation, gut dysbiosis and systemic endotoxaemia than Gsdmd-expressing controls fed the same diet, and GSDMD-NT exerted direct bactericidal effects on bacteria of the Proteobacteria phylum134. Furthermore, GSDMD was important for mucus secretion from goblet cells in mice, and Gsdmd deficiency was associated with increased bacterial attachment to the colonic epithelium, rendering mice more susceptible to Citrobacter rodentium infection82.
Role of GSDMs during infections with non-bacterial pathogens.
Studies investigating the contribution of GSDMs to non-bacterial gastrointestinal infections are sparse. In macrophages in vitro, Entamoeba histolytica independently induced activation of both caspase 1, which promoted pro-IL-1β maturation, and caspase 4, and mediated proteolytic GSDMD activation, resulting in macrophage hyperactivation135,136. Currently, a role of GSDMC in worm infection has been proposed. In a mouse model of Nippostrongylus brasiliensis infection, hyperactivated tuft cells recruited type 2 innate lymphoid cells, which upregulated Gsdmc2 in IECs, leading to their lytic death78. Similarly, Zhao and colleagues confirmed the importance of GSDMC in helminth infection, showing that Gsdmc1–4ΔIEC mice did not develop hyperplasia of goblet and tuft cells and displayed reduced responses against Heligmosomoides polygyrus owing to the lack of GSDMC-dependent IL-33 secretion32.
Evidence is scarce regarding the role of GSDMs in various viral infections, wherein pyroptosis is suggested to exert either detrimental or favourable functions137. In mouse IECs, rotavirus infection elicited NLRP9B-dependent GSDMD activation and subsequent IL-18 release upon pyroptosis, with purportedly protective effects138. Conversely, a pathogenic role for GSDMD was proposed in norovirus gastrointestinal infection in mice by inhibiting NLRP3-dependent, GSDMD-mediated pyroptosis of macrophages139. GSDMs were also implicated in the response against enterovirus 71 (EV71). Caspase 3-mediated activation of GSDME occurred in epithelial cells infected with EV71 in vitro, and Gsdme−/− mice showed less severe disease symptoms than controls140. Conversely, GSDMD was cleaved in vitro by the viral protease 3C to form an inactive NT fragment141. NLRP3 inflammasome142 and caspase 1 (ref.143) contribute to the defence against EV71; it is plausible that they serve to produce mature IL-1β and IL-18, whose secretion is augmented during EV71 infection143, but they cannot generate active GSDMD-NT owing to viral counteractive mechanisms in play. GSDM activation also occurs concomitantly with fungal infections, particularly Candida albicans and Aspergillus fumigatus infections144. However, whether this is relevant in the gastrointestinal system has not yet been explored.
GSDM-dependent pore formation is paramount in controlling infections.
Overall, evidence suggests that GSDM pores have important roles in controlling bacterial infections of the gastrointestinal tract via different mechanisms. This evidence is in line with evolution studies showing that, in the subkingdom Metazoa, GSDMs evolved under the selective pressure of responding to infections17,145,146. First, GSDMs exert a direct bactericidal effect (that is, pore formation within bacterial membranes) against both intracellular and extracellular microorganisms in vitro; however, the in vivo relevance of this direct killing was established only for the intracellular pathogen, S. flexneri74. Second, pyroptosis of infected cells disrupts the niche for intracellular pathogen replication. Third, GSDM pores are implicated in other mechanisms that restrict pathogen replication and dissemination, such as the formation of NETs and PITs. Finally, lytic cell death mediates local inflammatory damage but might also be critical for immune cell recruitment to contain the infection and prevent systemic dissemination. Evidence supports the involvement of GSDMs in non-bacterial infections, but their precise functions and roles have yet to be elucidated in the gastrointestinal system.
Inflammatory bowel disease
GSDMs are gaining increasing attention in the IBD field as key mediators of host allostatic responses that sustain chronic intestinal inflammation, as summarized in Fig. 3b. Several single-nucleotide polymorphisms (SNPs), mostly in GSDMB38,45,147, but also in GSDMA63,148, GSDMD147 and GSDME147, are associated with the genetic susceptibility to IBD. Furthermore, GSDMs participate in innate responses in immune and epithelial cells and have reciprocal influences on the host gut microbiota.
IEC-derived GSDMs at the crossroads of repair and pyroptosis during intestinal inflammation.
Rana and colleagues reported that GSDMB was increased in IBD, particularly in ulcerative colitis, and made the compelling observation that GSDMB-FL translocation to the plasma membrane promoted epithelial restitution in vitro without causing pyroptosis38, supporting an overall role for GSDMB-dependent wound repair. Notably, dichotomous roles have been ascribed to GSDMB in IECs, particularly GZMA-dependent pyroptosis and epithelial proliferation. It could be speculated that GSDMB is crucial to determining IEC fate and that its function depends on the specific pathway that is activated. In addition, it has yet to be established if GSDMB cleavage contributes to IBD pathogenesis by enhancing intestinal inflammation and if the full-length protein encoded by GSDMB variants preferentially facilitates, or deters, cleavage. Preliminary data suggest that GSDMB-mediated IEC pyroptosis might promote intestinal inflammation in Crohn’s disease and that disease-associated GSDMB SNPs might facilitate or prevent cleavage, with pathogenic or protective effects, respectively149. These competing functions might underlie the different pathogeneses associated with ulcerative colitis and Crohn’s disease and/or the different anatomical regions affected within the gastrointestinal tract, as Crohn’s disease is most commonly found in the terminal ileum and ulcerative colitis is restricted to the colonic mucosa. Future studies should clarify these issues.
GSDMC has been implicated in the promotion of colonic inflammation; however, DSS-induced colitis in GsdmcΔIEC mice did not show differences in the severity of inflammation compared with that in wild-type controls, possibly due to low baseline expression of GSDMC in controls. Conversely, when wild-type and Gsdmc-deficient mice were pre-infected with H. polygyrus (which normally upregulates GSDMC expression in IECs), GsdmcΔIEC mice displayed enhanced recovery. These results support the concept that epithelial GSDMC might promote the pathogenesis of IBD, but only if additional environmental factors intervene to upregulate its expression, therefore suggesting that intestinal pathogens might affect whether and how specific GSDMs contribute to IBD pathophysiology. Furthermore, Il10−/− colitic mice displayed increased expression and cleavage of GSDMC and Gsdmc deficiency in this model significantly (P < 0.001 for weight loss, P < 0.01 for colon length) mitigated DSS-induced colitis32, providing further support for a pathogenic role of GSDMC in IBD.
GSDME has also been suggested to have a role in the pathogenesis of Crohn’s disease. Tan and colleagues observed that GsdmeΔIEC mice were protected from TNBS-induced colitis59. Mechanistically, caspase 3-mediated GSDME cleavage determined pore formation and pyroptotic cell death associated with HMGB1 release, which actively contributed to intestinal inflammation, as the administration of anti-HMGB1 antibodies alleviated colitis in wild-type mice. HMGB1 blockade was not sufficient to completely recapitulate the effects of Gsdme deficiency on histological evaluation, suggesting that other GSDME-dependent functions might also contribute to colitis in this model59. In the same mouse model, the researchers also found that TNF stimulation induced GSDME-mediated pyroptosis in IECs by caspase 3-mediated cleavage under the control of IRF1 (ref.100).
The controversial role(s) of GSDMD in the pathogenesis of IBD.
Although several studies have detected increased levels of GSDMD in patients with IBD, animal studies have found conflicting results concerning its contribution to intestinal inflammation. Specifically, Gsdmd−/− mice were either protected from DSS-induced colitis39 or displayed a more severe phenotype than wild-type control mice150, with controversy also surrounding whether epithelial or immune-derived GSDMD exerts a prominent role during intestinal inflammation. Using a model of acute DSS-induced colitis, Bulek and colleagues showed non-pyroptotic release of sEVs containing IL-1β (via chaperoned GSDMD-FL)39 from IECs ex vivo, whereas another group observed pyroptotic release of IL-18, which promoted colitis by driving goblet cell loss in vivo30. A specific role of GSDMD was also found in a mouse model of ileitis151, in which caspase 8 promoted ileal inflammation by inducing GSDMD-dependent IEC death, independently from MLKL-mediated necroptosis (which also contributed to the development of intestinal inflammation in this model)151. This study suggested a role for GSDMD-mediated pyroptosis in ileitis and underscored the importance of crosstalk between different programmes of regulated cell death during intestinal inflammation. Collectively, these data strongly support the concept that GSDMD activation in IECs mediates intestinal inflammation.
Aside from IECs, GSDM-expressing myeloid cells also contribute to the pathogenesis of IBD. Ma and colleagues showed that selective deletion of Gsdmd in myeloid cells exacerbated DSS-induced colitis in mice, independent of gut microbiota150. The researchers did not detect a difference in colitis phenotype between Gsdmd−/− and GsdmdΔIEC mice150, which suggests that the main contribution to colitis comes from non-epithelial GSDMD. Conversely, two groups found that selective deletion of upstream regulators of GSDMD in macrophages, namely NEK152 and TRIM2 (ref.153), conferred protection against experimental colitis in mice by restricting GSDMD-mediated pyroptosis.
The global effects of GSDMD activation during the pathogenesis of IBD seem to be multifaceted and probably depend on the specific pathway(s) and timing of activation. Although some of the results described above are difficult to reconcile, three main conclusions can be drawn: (1) GSDMD has an active role in colitis and ileitis, (2) IEC-derived GSDMD seems to promote intestinal inflammation via both cell death and secretion of inflammatory mediators, and (3) GSDMD activation in macrophages might have a subtle and context-dependent role in intestinal inflammation.
GSDMs orchestrate inflammation in the gastrointestinal tract.
Overall, it is plausible that GSDMs exert dichotomous roles during IBD pathogenesis. In IECs, GSDM-mediated pyroptosis and/or release of inflammatory mediators promote intestinal inflammation30,32,36,59,151. Furthermore, the crosstalk between pyroptosis and other programmes of regulated cell death involving GSDMs is highly probable. So far, a purported protective role for IECs is described only for GSDMB38. It would be interesting to explore if other GSDMs share similar functions, or if this phenomenon is unique to GSDMB-FL, perhaps through its ability to bind lipid membranes. Regarding GSDMD in myeloid cells, the ambiguous results for its contribution to intestinal inflammation150,152,153 suggest that fine-tuning and regulatory mechanisms are in play and are probably more relevant than GSDMD activation. In this regard, one interesting question pertains to the potential role of cell hyperactivation in chronic intestinal inflammation. As pyroptotic cells release inflammatory mediators but eventually die, therefore ceasing their secretory activity, it is conceivable that hyperactivated cells contribute to the self-sustaining, non-resolving inflammation observed in conditions such as IBD. Future research should focus on the various physiological and pathophysiological stimuli that determine which cells express a specific GSDM, what particular function is specific for each GSDM, and, importantly, what is the net outcome of their activation in both IECs and myeloid cells.
Clinical insights into GSDMs in the gastrointestinal tract
The body of knowledge on GSDMs in gastrointestinal health and disease is rapidly expanding; as such, the logical extension of these findings is to leverage such discoveries into developing clinically useful tools for the management of patient care. Two main areas of such application can be identified: (1) using GSDMs as biomarkers for patient monitoring and/or precision medicine, and (2) exploiting GSDM biology to design effective therapeutics.
GSDMs as biomarkers for disease states
Several studies have shown a correlation between the expression of one or more GSDM and clinical outcome(s) in patients with cancer. Two main approaches are proposed: evaluating the expression of specific GSDMs and attempting to build multi-parametric scores including pyroptosis-related genes154. In rectal adenocarcinoma, high GSDMD expression correlated with a better prognosis and enhanced infiltration and activation of immune cells in the TME155. Differences in GSDMD immunolocalization patterns in epithelial cells of 178 patients with CRC correlated with both prognosis (cytoplasmic GSDMD associated with favourable outcomes and nuclear GSDMD with deeper infiltration depth) and TME composition (membranous GSDMD correlated with enrichment of CD68+ macrophages and CD8+ T cells and nuclear GSDMD with CD3+ cells)156. A specific methylation pattern in GSDME (involving two CpG islands) accurately discriminated CRC from normal colon, suggesting its potential use as a biomarker for CRC detection through blood-based liquid biopsy screening157. In signet ring cell gastric carcinoma, expression of intracellular MUC20 variant 2 correlated with a reduction of apoptosis and GSDME-mediated pyroptosis in cancer cells and with resistance to cisplatin and paclitaxel158. In HER2-positive breast cancer, GSDMB expression correlated with a poor prognosis and reduced response to anti-HER2 therapy159. As HER2 can also be overexpressed in gastric tumours and trastuzumab (anti-HER2 monoclonal antibody) is used for the treatment of HER2-positive gastric and gastroesophageal cancers160, it is conceivable that such associations might also be present in gastrointestinal malignancies. Zeng and colleagues identified a signature of five pyroptosis-related genes that predicted the prognosis in 65 patients with oesophageal adenocarcinoma. Specifically, regarding GSDMs, high GSDMB correlated with improved survival71.
Besides cancer, little is known regarding the potential application(s) of GSDMs as biomarkers in other diseases, namely, infections and immune-mediated diseases. For instance, elevated levels of GSDMD-NT in circulating plasma microparticles were observed in 26 patients who were critically ill with sepsis, but no significant correlations were found with clinical and clinically relevant biochemical parameters161. Regarding gastrointestinal pathologies, the first issue to address would be whether GSDM levels should be investigated explicitly within the gut mucosa or if systemic serum levels might also serve the same purpose. The potential applications span from predicting disease course to precision medicine to tailor specific treatment modalities. Given that most GSDMs require proteolysis to be activated, the levels of NT fragments are likely to be more relevant as biomarkers. An alternative argument could be made for GSDMB, as both full-length and cleaved NT forms are biologically active and exert opposing functions in IECs. In this context, it might be more beneficial to measure the ratio between NT and full-length forms to assess which process prevails (that is, pore-forming or wound healing function(s)). Furthermore, increasing evidence indicates that multiple GSDMs are probably simultaneously expressed in the same tissues and perhaps even within the same cells. Thus, the relative expression of each GSDM compared to other family members might also correlate with clinically meaningful outcomes.
Therapeutic potential of GSDMs in cancer
Anticipation is mounting regarding the potential to manipulate GSDM-mediated pyroptosis for the treatment of specific malignancies. In general, pyroptosis could be exploited in two ways: inducing proteolytic activation of endogenous GSDMs or selectively introducing exogenous GSDMs into cancer cells.
The possibility of combining different therapies to enhance the antitumour effects of GSDM-mediated pyroptosis is intriguing, with preliminary evidence supporting this potential concept. Decitabine restored GSDME expression in CRC cells via demethylation of its promoter94, thereby enhancing the efficacy of chemotherapeutics that act via a GSDME-dependent mechanism. The combination of BIX 01294 (a methyltransferase inhibitor) and cisplatin induced autophagy-dependent GSDME-mediated pyroptosis in gastric cancer cell lines transferred into nude mice162. Furthermore, the PLK1 inhibitor, BI2536, synergized with cisplatin to induce GSDME-mediated pyroptosis in mice xenografted with human oesophageal squamous cell carcinoma cells102. LPS enhanced GSDMD expression in HT-29 cells, making them more sensitive to oxaliplatin-induced pyroptosis84. Tumour grafts of mouse colon carcinoma cells that expressed human GSDMB showed an increased response to anti-PD1 therapy70, suggesting a link between pyroptosis and immune checkpoints that might be exploited for future therapies against colon cancer. Other pharmacological modulators not routinely used for oncology treatments might also be employed to enhance GSDM-mediated pyroptosis in cancer. For instance, famotidine, an antihistamine H2 receptor, induced GSDME pore assembly in gastric cancer cells, with subsequent IL-18 release and pyroptotic cell death31.
It should also be noted that chemotherapy-induced GSDM-dependent pyroptosis might be responsible for some of the adverse events associated with antineoplastic treatment. For example, doxorubicin and cisplatin, which are used for the treatment of gastric cancer and CRC, respectively, induced caspase 3-dependent GSDME-mediated pyroptosis of kidney tubular epithelial cells, both in human cell cultures and in mice, which partially accounts for the nephrotoxicity associated with such therapies163; similarly, cisplatin induced GSDME-dependent weight loss and tissue damage in mice94. Experiments in mice suggest that the vitamin D–vitamin D receptor pathway might mitigate such pyroptosis-related adverse effects164,165. Furthermore, excessive pyroptosis of neoplastic cells resulted in the development of detrimental systemic inflammatory syndromes, such as that described for chimeric antigen receptor T cell therapy that activated GSDME-mediated pyroptosis of tumour cells, but also GSDMD-mediated pyroptosis of macrophages leading to cytokine release syndrome in a mouse model166.
In this regard, selective targeting of neoplastic cells could prove to be extremely beneficial to maximize treatment effectiveness and, at the same time, reduce potential deleterious adverse effects. Xiao and colleagues proposed a sophisticated approach based on a designed nanoprodrug that enhanced the cancer-targeted distribution of paclitaxel, which then activated GSDME-mediated pyroptosis via chemophototherapy in mice167. In this study, the researchers showed that the efficacy of this approach was dependent on the ability of GSDME-mediated pyroptosis to evoke an antitumour response in the TME, presenting the advantage of avoiding potential adverse effects related to diffuse GSDME-dependent cell lysis. A different strategy was proposed by Liu and colleagues, who used an adenovirus to deliver apoptin, a protein of the chicken anaemia virus, to target HCT116 cells, a human CRC cell line. This strategy induced GSDME-dependent pyroptosis via the mitochondrial pathway through caspase 3 and caspase 9 activation, and in a tumour xenograft model in mice, significantly reduced tumour growth (P < 0.0001 for both tumour volume and weight)168.
A different argument can be made for those malignancies in which GSDMs seemingly exert tumour-promoting functions. For example, the delivery of a monoclonal antibody targeting GSDMB in HER2-positive breast cancer cells was proposed as a therapeutic strategy. This approach led to reduced GSDMB-dependent cell migration and enhanced sensitivity to trastuzumab (anti-HER2 therapy) in vitro, as well as reduced tumour growth and lung metastases in xenograft mouse models169. Similarly, preliminary findings showed that GSDMB mediated resistance to anti-HER2 therapy, which upregulated GSDMB expression in HER2-expressing breast and gastric cancer cells170. This phenomenon occurred through a mechanism that involved the interaction of GSDMB-NT and LC3B (an autophagy protein), which upregulated protective autophagy-dependent functions170. Interestingly, concomitant blockade of autophagy pathways increased the efficacy of anti-HER2 therapy in a mouse model of xenograft breast cancer170.
Direct delivery of GSDMs has also been proposed for the treatment of malignancies. Wang et al. developed a biorthogonal system that allowed the targeted intracellular release of active GSDMA3 (GSDMA3-NT) in breast cancer cells171. In this study, GSDMA3-mediated pyroptosis in about 15% of 4T1 cells comprising the tumour graft was sufficient to induce clearance of the entire mass, further reinforcing the concept that the anticancer effects of pyroptosis, besides lytic death of cancer cells, is conferred through immune activation to mount specific antineoplastic responses171.
Therapeutic potential of GSDMs in non-malignant diseases
Pharmacological modulation of GSDM activity during intestinal inflammation, either owing to infection or immune-mediated conditions, also represents a tantalizing possibility. Chemical inhibitors and activators of GSDMs are extensively reviewed elsewhere172, and their discussion goes beyond the scope of the present Review. However, briefly, small molecules can target specific residues of GSDMs to modulate their cleavage47 or oligomerization173,174. In this regard, it is worth mentioning that, in a preliminary study utilizing a mouse model of LPS-induced sepsis, disulfiram, a drug approved for the treatment of alcohol addiction that inhibits GSDMD oligomerization, either prevented or delayed lethality when administered before or right after LPS injection, respectively174. Furthermore, a study showed that the administration of disulfiram-loaded lactoferrin nanoparticles in mice had a protective effect against DSS-induced colitis; curiously, however, the researchers did not investigate GSDM cleavage and pore formation175. Caution should be applied when interpreting the results from this study as, although a trend towards increased efficacy was observed with disulfiram-loaded particles, it did not reach statistical significance when compared with treatment with free nanoparticles, therefore leaving some ambiguity as to whether disulfiram would provide a therapeutic benefit in IBD.
Overall, GSDMD inhibition seems to exert its therapeutic effects by alleviating pyroptosis-dependent inflammation. Nonetheless, considering the importance of appropriate inflammatory responses in counteracting local infections and the more than often contradictory results regarding the roles of GSDMs in IBD, caution should also be applied in assuming that the mere inhibition of GSDMs results in the dampening of intestinal inflammation. The bactericidal effects of GSDMs also need to be considered, especially with intracellular pathogens. In this regard, GSDM inhibition might be detrimental, as it might impair the host’s defence against infection with pathogens. As such, it is tempting to speculate that an appropriate strategy would be to either induce activation of endogenous GSDMs skewed towards promoting bacterial killing or introduce exogenous GSDMs engineered to target bacterial wall selectively. An alternative approach could be to induce the repair processes conferred by GSDMB-FL by enhancing its role in epithelial restitution. Indeed, expression of GSDMB and its translocation to the plasma membrane might serve as an effective strategy to promote mucosal healing, particularly in pathologies that damage the gut mucosa, such as IBD. The potential of methotrexate, an immunomodulator approved for the treatment of Crohn’s disease176,177, in achieving such success was discussed in an editorial published in 2022 (ref.178).
Conclusions
In less than a decade, GSDMs have progressively taken centre stage regarding their contribution(s) to innate immune responses, particularly within mucosal surfaces at the interface between the host and external environment, such as in the gastrointestinal tract. However, many key questions remain unanswered, and further research is needed to clarify the role of GSDMs and their contributions to gastrointestinal health and disease (Box 4). Although the present Review is restricted to discussing the role of GSDMs in the luminal gastrointestinal tract, there is strong evidence also showing their contributions to the pathophysiology of hepatic179-182 and, potentially, pancreatic183,184 diseases.
Box 4. Future directions and outstanding questions.
Current evidence regarding the characterization and function(s) of gasdermins (GSDMs) has shed light upon the main roles of this intriguing family of proteins, particularly in the gastrointestinal tract; however, it has also opened the door to several new questions. Conflicting results concerning the contribution and effects of GSDM activity in specific diseases should be framed following these general observations: (1) the formation of GSDM pores does not necessarily result in pyroptotic cell death, (2) GSDM-driven inflammation can promote different effects, depending on its duration and the specific cell population(s) undergoing pyroptosis (that is, immune or non-immune cells), and (3) some GSDMs also possess functions beyond pyroptosis and pore formation, particularly in their full-length forms. These considerations suggest that a more systematic approach should be taken when investigating GSDMs, accounting for several aspects of their inherent biology (Fig. 1). Three main areas of research are warranted for future investigation of GSDMs in the gastrointestinal tract:
- Basic science:
- Relative expression of different GSDMs in the same tissues and/or cells
- Non-lytic and pore-independent functions of GSDMs
- Specificity of activation pathways in terms of which GSDM is upregulated or downregulated and which GSDM-dependent function is controlled
- GSDM interactions/cooperation with other GSDM family members and/or with partner proteins
- Translational studies:
- Functional phenotype(s) depending on pyroptotic and non-pyroptotic functions of GSDMs
- Functional phenotype(s) depending on GSDMs expressed by immune and non-immune cells
- Functional phenotype(s) depending on GSDMs with time-restricted and anatomically restricted activities and on GSDMs with uncontrolled activity
- Clinical research:
- Potential use of GSDMs as diagnostic and prognostic and/or predictive biomarkers: gene expression in cancer, and the consideration of measuring ratios of proteins (amino-terminal and full-length forms; N-terminal to full-length ratio)
- Potential use of GSDMs as therapeutic targets: pharmacological modulation of expression and activity, the introduction of exogenous GSDMs, and synergy with other therapies (for example, checkpoint inhibitors)
The cellular mechanisms that activate and control GSDMs are one of the most prominent issues that should be investigated more thoroughly. A better understanding of the signals that regulate cell fate upon GSDM proteolytic activation (that is, whether they do or do not undergo lytic cell death) is greatly needed. Furthermore, it will be necessary to discover whether such signals differ qualitatively or quantitatively. As functional and mechanistic evidence indicates that GSDMs can also exert roles beyond pore formation, characterizing these processes and elucidating the specific stimuli that control them will also be essential. In this regard, a prominent line of future research should be to investigate the post-translational modifications that regulate GSDM activation and subsequent functions.
Indeed, individual GSDMs can be selectively upregulated, and their functions do not seem to overlap completely with other family members. Different GSDMs are simultaneously expressed by IECs and/or immune cells during active disease phases. In this context, specific GSDM-inducing signals will need to be identified. Additionally, it should be considered that the same stimulus might control different GSDMs in a dose-dependent manner (as described for UVB stimulation of keratinocytes that upregulate GSDMC at lower doses61, whereas increasing irradiation activates GSDMD and GSDME-mediated pyroptosis185,186). Furthermore, it would be interesting to investigate whether the expression of not j ust a single GSDM but also the relative expression among different GSDMs is relevant to the biology and phenotype of GSDM-expressing cells.
Finally, although GSDMs have been traditionally described as inactive in their full-length forms, evidence for biological activities for at least some of the GSDMs (namely, GSDMB-FL and GSDMD-FL in IECs) has been uncovered. Unanswered questions remain as to whether other GSDMs also share these characteristics and which specific stimuli control the activity of their full-length and cleaved forms. Mechanisms that promote cell proliferation can, in parallel, shut down the cellular functions associated with the GSDM pyroptotic machinery, and vice versa, with the contribution of other GSDMs, aside from GSDMB, implicated in regulating the cell cycle. Indeed, the dichotomy of functions reported, specifically for GSDMB-FL versus GSDMB-NT in IECs, is a fascinating conundrum. It is conceivable that diverging pathways for GSDMB activation exist, and alterations in signals emanating from intracellular and extracellular environments are responsible for switching the momentum of epithelial GSDMB from pyroptotic to pro-restitution functions and vice versa. A deeper understanding of such mechanisms and their regulation will be paramount in further dissecting the role of GSDMs, in general, and in leveraging this information to design future therapeutics for GSDM-dependent disease processes.
Key points.
The gasdermin (GSDM) family of lipid-binding proteins is involved in several biological processes, with its five members (GSDMA to GSDME) serving as major factors during gastrointestinal health and disease.
GSDMs are primarily known as mediators of pyroptosis; however, evidence supports other roles, including the non-lytic release of inflammatory cytokines, regulation of vital cell functions and targeted bactericidal effects.
The contribution of each GSDM during gastrointestinal health and disease is unequivocal, albeit ambiguous, with reports of both promotion of and protection from gastrointestinal cancers, infections and immune-mediated disorders.
Preliminary evidence suggests potential applications for GSDMs in clinical gastrointestinal practice, with future investigation warranted to leverage their use as predictive or prognostic biomarkers and/or specific therapeutic targets.
Knowledge gaps and controversies exist in GSDM biology regarding their pyroptotic and non-pyroptotic functions and associated signalling pathways, the biological activities of full-length forms, and their roles in immune and non-immune cells.
Acknowledgements
The authors acknowledge continued support for this work from the NIH/NIDDK and NIAID: DK091222 (P01 Project 3), DK042191, DK125293 and AI141350 (P01 Project 4) (to T.T.P.).
Glossary
- Apoptosis
Form of regulated cell death under the control of executioner caspases that results in the formation of intact vesicles (apoptotic bodies).
- Enterovirus 71
Enterovirus with faecal-oral transmission that is the primary causative agent of ‘hand, foot and mouth disease’.
- Inflammatory bowel disease
Chronic inflammatory condition with a relapsing-remitting course that affects (primarily) the gastrointestinal tract; it arises from dysregulated interactions between the host immune system and its gut microbiome, and its main clinical phenotype is represented by Crohn’s disease and ulcerative colitis.
- Macroautophagy
Catabolic process characterized by the formation of intracellular vesicles (‘autophagosomes’) and aiming at the degradation of cellular components through the lysosomal system.
- Mitophagy
Selective form of autophagy targeting damaged mitochondria.
- Necroptosis
Form of regulated cell death mediated by mixed lineage kinase domain-like pseudokinase pores, whose activation depends on RIPK3 (and RIPK1).
- NETosis
Form of regulated cell death restricted to neutrophils that results in the extrusion of neutrophil extracellular traps (NETs).
- Pexophagy
Removal of damaged peroxisomes via autophagy.
- Pyroptosis
Form of regulated cell death that depends on the formation of GSDM pores on the cell plasma membrane; it is often (but not always) a consequence of inflammatory caspase activation, and is usually associated with the release of inflammatory mediators into the extracellular space.
- Receptosome
Intracellular vesicle that is formed via concentrative, receptor-mediated endocytosis, possibly with the ligand-receptor complex still bound to the membrane.
- Regulated cell death
Form of cell death dependent on the activation of specific signal transducers and terminal effectors; it is susceptible to chemical and/or pharmacological modulations (including both apoptosis and a form of inflammatory cell death).
- Salmonella enterica subsp. enterica serovar Typhimurium
Facultative intracellular pathogen that causes food-borne infectious colitis in mice; it is used as a model of human typhoid fever (caused by S. Typhi).
- Secondary necrosis
Event following end-stage apoptosis in vitro or in vivo when phagocytic clearance of apoptotic bodies fails resulting in plasma membrane rupture and release of cytoplasmic contents.
- Yersiniosis
Food-borne gastrointestinal infection with pathogens localizing to intestinal lymphoid tissues and mesenteric lymph nodes: it is clinically characterized by abdominal pain and diarrhoea and is usually caused by Yersinia enterocolitica; Y. pseudotuberculosis is commonly used in mouse models of yersiniosis but rarely causes yersiniosis in humans.
Footnotes
Competing interests
All authors declare no competing interests.
References
- 1.Saeki N, Kuwahara Y, Sasaki H, Satoh H & Shiroishi T Gasdermin (Gsdm) Localizing to mouse chromosome 11 is predominantly expressed in upper gastrointestinal tract but significantly suppressed in human gastric cancer cells. Mamm. Genome 11, 718–724 (2000). [DOI] [PubMed] [Google Scholar]
- 2.Liu X, Xia S, Zhang Z, Wu H & Lieberman J Channelling inflammation: gasdermins in physiology and disease. Nat. Rev. Drug Discov 20, 384–405 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delmaghani S et al. Mutations in the gene encoding pejvakin, a newly identified protein of the afferent auditory pathway, cause DFNB59 auditory neuropathy. Nat. Genet 38, 770–778 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Yu J et al. Polymorphisms in GSDMA and GSDMB are associated with asthma susceptibility, atopy and BHR. Pediatr. Pulmonol 46, 701–708 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Das S et al. GSDMB induces an asthma phenotype characterized by increased airway responsiveness and remodeling without lung inflammation. Proc. Natl Acad. Sci. USA 113, 13132–13137 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Runkel F et al. The dominant alopecia phenotypes Bareskin, Rex-denuded, and Reduced Coat 2 are caused by mutations in gasdermin 3. Genomics 84, 824–835 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Lunny DP et al. Mutations in gasdermin 3 cause aberrant differentiation of the hair follicle and sebaceous gland. J. Invest. Dermatol 124, 615–621 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Saeki N et al. GASDERMIN, suppressed frequently in gastric cancer, is a target of LMO1 in TGF-β-dependent apoptotic signalling. Oncogene 26, 6488–6498 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Saeki N et al. Distinctive expression and function of four GSDM family genes (GSDMA-D) in normal and malignant upper gastrointestinal epithelium. Genes Chromosomes Cancer 48, 261–271 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Shi J et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Kayagaki N et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature 526, 666–671 (2015). [DOI] [PubMed] [Google Scholar]
- 12.He WT et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 25, 1285–1298 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cookson BT & Brennan MA Pro-inflammatory programmed cell death. Trends Microbiol. 9, 113–114 (2001). [DOI] [PubMed] [Google Scholar]
- 14.Galluzzi L et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 25, 486–541 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamura M et al. Members of a novel gene family, Gsdm, are expressed exclusively in the epithelium of the skin and gastrointestinal tract in a highly tissue-specific manner. Genomics 89, 618–629 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Ding J et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature 535, 111–116 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Angosto-Bazarra D et al. Evolutionary analyses of the gasdermin family suggest conserved roles in infection response despite loss of pore-forming functionality. BMC Biol. 20, 9 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rogers C et al. Cleavage of DFNA5 by caspase-3 during apoptosis mediates progression to secondary necrotic/pyroptotic cell death. Nat. Commun 8, 14128 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chauhan D et al. GSDMD drives canonical inflammasome-induced neutrophil pyroptosis and is dispensable for NETosis. EMBO Rep. 10.15252/EMBR.202154277 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kambara H et al. Gasdermin D exerts anti-inflammatory effects by promoting neutrophil death. Cell Rep. 22, 2924–2936 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sollberger G et al. Gasdermin D plays a vital role in the generation of neutrophil extracellular traps. Sci. Immunol 3, eaar6689 (2018). [DOI] [PubMed] [Google Scholar]
- 22.Chen KW et al. Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Sci. Immunol 3, eaar6676 (2018). [DOI] [PubMed] [Google Scholar]
- 23.Burgener SS et al. Cathepsin G inhibition by Serpinb1 and Serpinb6 prevents programmed necrosis in neutrophils and monocytes and reduces GSDMD-driven inflammation. Cell Rep. 27, 3646–3656.e5 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin PH, Lin HY, Kuo CC & Yang LT N-terminal functional domain of Gasdermin A3 regulates mitochondrial homeostasis via mitochondrial targeting. J. Biomed. Sci 22, 44 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Platnich JM et al. Shiga toxin/lipopolysaccharide activates caspase-4 and Gasdermin D to trigger mitochondrial reactive oxygen species upstream of the NLRP3 inflammasome. Cell Rep. 25, 1525–1536.e7 (2018). [DOI] [PubMed] [Google Scholar]
- 26.Weindel CG et al. Mitochondrial ROS promotes susceptibility to infection via gasdermin D-mediated necroptosis. Cell 185, 3214–3231.e23 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rogers C et al. Gasdermin pores permeabilize mitochondria to augment caspase-3 activation during apoptosis and inflammasome activation. Nat. Commun 10, 1689 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evavold CL et al. The pore-forming protein Gasdermin D regulates interleukin-1 secretion from living macrophages. Immunity 48, 35–44.e6 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zanoni I et al. An endogenous caspase-11 ligand elicits interleukin-1 release from living dendritic cells. Science 352, 1232–1236 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao H et al. Dysregulated microbiota-driven gasdermin D activation promotes colitis development by mediating IL-18 release. Front. Immunol 12, 750841 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang J et al. Famotidine promotes inflammation by triggering cell pyroptosis in gastric cancer cells. BMC Pharmacol. Toxicol 22, 62 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhao M et al. Epithelial STAT6 O-GlcNAcylation drives a concerted anti-helminth alarmin response dependent on tuft cell hyperplasia and Gasdermin C. Immunity 10.1016/J.IMMUNI.2022.03.009 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen W et al. Allergen protease-activated stress granule assembly and gasdermin D fragmentation control interleukin-33 secretion. Nat. Immunol 23, 1021–1030 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamagishi R et al. Gasdermin D-mediated release of IL-33 from senescent hepatic stellate cells promotes obesity-associated hepatocellular carcinoma. Sci. Immunol 7, eabl7209 (2022). [DOI] [PubMed] [Google Scholar]
- 35.De Torre-Minguela C, Barberà-Cremades M, Gómez AI, Martín-Sánchez F & Pelegrín P Macrophage activation and polarization modify P2X7 receptor secretome influencing the inflammatory process. Sci. Rep 6, 22586 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan G, Huang C, Chen J & Zhi F HMGB1 released from GSDME-mediated pyroptotic epithelial cells participates in the tumorigenesis of colitis-associated colorectal cancer through the ERK1/2 pathway. J. Hematol. Oncol 13, 149 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Karmakar M et al. N-GSDMD trafficking to neutrophil organelles facilitates IL-1β release independently of plasma membrane pores and pyroptosis. Nat. Commun 11, 2212 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rana N et al. GSDMB is increased in IBD and regulates epithelial restitution/repair independent of pyroptosis. Cell 10.1016/j.cell.2021.12.024 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bulek K et al. Epithelial-derived gasdermin D mediates nonlytic IL-1β release during experimental colitis. J. Clin. Invest 140, 4218–4234 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Schutter E et al. GSDME and its role in cancer: from behind the scenes to the front of the stage. Int. J. Cancer 148, 2872–2883 (2021). [DOI] [PubMed] [Google Scholar]
- 41.Moussette S et al. Role of DNA methylation in expression control of the IKZF3-GSDMA region in human epithelial cells. PLoS ONE 12, e0172707 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muhammad JS et al. Gasdermin D hypermethylation inhibits pyroptosis and LPS-induced IL-1β release from NK92 cells. Immunotargets Ther. 8, 29–41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stein MM et al. A decade of research on the 17q12-21 asthma locus: piecing together the puzzle. J. Allergy Clin. Immunol 142, 749–764.e3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taabazuing CY, Okondo MC & Bachovchin DA Pyroptosis and apoptosis pathways engage in bidirectional crosstalk in monocytes and macrophages. Cell Chem. Biol 24, 507–514.e4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chao KL, Kulakova L & Herzberg O Gene polymorphism linked to increased asthma and IBD risk alters gasdermin-B structure, a sulfatide and phosphoinositide binding protein. Proc. Natl Acad. Sci. USA 114, E1128–E1137 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Devant P et al. Gasdermin D pore-forming activity is redox-sensitive. Cell Rep. 42, 112008 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Humphries F et al. Succination inactivates gasdermin D and blocks pyroptosis. Science 369, 1633–1637 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu L et al. Chemotherapy-induced pyroptosis is mediated by BAK/BAX-caspase-3-GSDME pathway and inhibited by 2-bromopalmitate. Cell Death Dis. 11, 281 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bambouskova M et al. Itaconate confers tolerance to late NLRP3 inflammasome activation. Cell Rep. 34, 108756 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rühl S et al. ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362, 956–960 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Santa Cruz Garcia AB, Schnur KP, Malik AB & Mo GCH Gasdermin D pores are dynamically regulated by local phosphoinositide circuitry. Nat. Commun 13, 52 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hou J, Hsu J-M & Hung M-C Molecular mechanisms and functions of pyroptosis in inflammation and antitumor immunity. Mol. Cell 10.1016/j.molcel.2021.09.003 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Privitera G, Rana N, Scaldaferri F, Armuzzi A & Pizarro TT Novel insights into the interactions between the gut microbiome, inflammasomes, and gasdermins during colorectal cancer. Front. Cell. Infect. Microbiol 11, 806680 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu C et al. Inflammasome activation triggers blood clotting and host death through pyroptosis. Immunity 50, 1401–1411.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang X et al. Bacterial endotoxin activates the coagulation cascade through Gasdermin D-dependent phosphatidylserine exposure. Immunity 51, 983–996.e6 (2019). [DOI] [PubMed] [Google Scholar]
- 56.McDonald B et al. Platelets and neutrophil extracellular traps collaborate to promote intravascular coagulation during sepsis in mice. Blood 129, 1357–1367 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Solem CA, Loftus EV, Tremaine WJ & Sandborn WJ Venous thromboembolism in inflammatory bowel disease. Am. J. Gastroenterol 99, 97–101 (2004). [DOI] [PubMed] [Google Scholar]
- 58.Zou J et al. The versatile gasdermin family: their function and roles in diseases. Front. Immunol 12, 751533 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tan G, Huang C, Chen J, Chen B & Zhi F Gasdermin-E-mediated pyroptosis participates in the pathogenesis of Crohn’s disease by promoting intestinal inflammation. Cell Rep. 35, 109265 (2021). [DOI] [PubMed] [Google Scholar]
- 60.Katoh M & Katoh M Identification and characterization of human DFNA5L, mouse Dfna5l, and rat Dfna5l genes in silico. Int. J. Oncol 25, 765–770 (2004). [PubMed] [Google Scholar]
- 61.Kusumaningrum N et al. Gasdermin C is induced by ultraviolet light and contributes to MMP-1 expression via activation of ERK and JNK pathways. J. Dermatol. Sci 90, 180–189 (2018). [DOI] [PubMed] [Google Scholar]
- 62.Terao C et al. Transethnic meta-analysis identifies GSDMA and PRDM1 as susceptibility genes to systemic sclerosis. Ann. Rheum. Dis 76, 1150–1158 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Söderman J, Berglind L & Almer S Gene expression-genotype analysis implicates GSDMA, GSDMB, and LRRC3C as contributors to inflammatory bowel disease susceptibility. Biomed. Res. Int 2015, 834805 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shi P et al. Loss of conserved Gsdma3 self-regulation causes autophagy and cell death. Biochem. J 468, 325–336 (2015). [DOI] [PubMed] [Google Scholar]
- 65.Deng W et al. Streptococcal pyrogenic exotoxin B cleaves GSDMA and triggers pyroptosis. Nature 602, 496–502 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.LaRock DL et al. Group A Streptococcus induces GSDMA-dependent pyroptosis in keratinocytes. Nature 605, 527–531 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ruan J Structural insight of Gasdermin family driving pyroptotic cell death. Adv. Exp. Med. Biol 1172, 189–205 (2019). [DOI] [PubMed] [Google Scholar]
- 68.Chen Q et al. GSDMB promotes non-canonical pyroptosis by enhancing caspase-4 activity. J. Mol. Cell Biol 11, 496–508 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Panganiban RA et al. A functional splice variant associated with decreased asthma risk abolishes the ability of gasdermin B to induce epithelial cell pyroptosis. J. Allergy Clin. Immunol 142, 1469–1478.e2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhou Z et al. Granzyme A from cytotoxic lymphocytes cleaves GSDMB to trigger pyroptosis in target cells. Science 368, eaaz7548 (2020). [DOI] [PubMed] [Google Scholar]
- 71.Zeng R et al. Predicting the prognosis of esophageal adenocarcinoma by a pyroptosis-related gene signature. Front. Pharmacol 12, 3142 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Komiyama H et al. Alu-derived cis-element regulates tumorigenesis-dependent gastric expression of GASDERMIN B (GSDMB). Genes Genet. Syst 85, 75–83 (2010). [DOI] [PubMed] [Google Scholar]
- 73.Carl-McGrath S, Schneider-Stock R, Ebert M & Röcken C Differential expression and localisation of gasdermin-like (GSDML), a novel member of the cancer-associated GSDMDC protein family, in neoplastic and non-neoplastic gastric, hepatic, and colon tissues. Pathology 40, 13–24 (2008). [DOI] [PubMed] [Google Scholar]
- 74.Hansen JM et al. Pathogenic ubiquitination of GSDMB inhibits NK cell bactericidal functions. Cell 184, 3178–3191.e18 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Miguchi M et al. Gasdermin C is upregulated by inactivation of transforming growth factor β receptor type II in the presence of mutated Apc, promoting colorectal cancer proliferation. PLoS ONE 11, e0166422 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hou J et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol 22, 1264–1275 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang J-Y et al. The metabolite α-KG induces GSDMC-dependent pyroptosis through death receptor 6-activated caspase-8. Cell Res. 31, 980–997 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xi R et al. Up-regulation of gasdermin C in mouse small intestine is associated with lytic cell death in enterocytes in worm-induced type 2 immunity. Proc. Natl Acad. Sci. USA 118, e2026307118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dinarello CA Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev 281, 8–27 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zanoni I, Tan Y, Di Gioia M, Springstead JR & Kagan JC By capturing inflammatory lipids released from dying cells, the receptor CD14 induces inflammasome-dependent phagocyte hyperactivation. Immunity 47, 697–709.e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen KW et al. The neutrophil NLRC4 inflammasome selectively promotes IL-1β maturation without pyroptosis during acute Salmonella challenge. Cell Rep. 8, 570–582 (2014). [DOI] [PubMed] [Google Scholar]
- 82.Zhang J et al. Epithelial Gasdermin D shapes the host-microbial interface by driving mucus layer formation. Sci. Immunol 7, eabk2092 (2022). [DOI] [PubMed] [Google Scholar]
- 83.Ma Y, Chen Y, Lin C & Hu G Biological functions and clinical significance of the newly identified long non-coding RNA RP1-85F18.6 in colorectal cancer. Oncol. Rep 40, 2648–2658 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu LS et al. LPS enhances the chemosensitivity of oxaliplatin in HT29 cells via GSDMD-mediated pyroptosis. Cancer Manag. Res 12, 10397–10409 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang WJ et al. Downregulation of gasdermin D promotes gastric cancer proliferation by regulating cell cycle-related proteins. J. Dig. Dis 19, 74–83 (2018). [DOI] [PubMed] [Google Scholar]
- 86.Wang L et al. Metformin induces human esophageal carcinoma cell pyroptosis by targeting the miR-497/PELP1 axis. Cancer Lett. 450, 22–31 (2019). [DOI] [PubMed] [Google Scholar]
- 87.Mileykovskaya E & Dowhan W Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim. Biophys. Acta 1788, 2084 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu X et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature 535, 153–158 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Papayannopoulos V Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol 18, 134–147 (2018). [DOI] [PubMed] [Google Scholar]
- 90.Jorgensen I, Zhang Y, Krantz BA & Miao EA Pyroptosis triggers pore-induced intracellular traps (PITs) that capture bacteria and lead to their clearance by efferocytosis. J. Exp. Med 213, 2113–2128 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Orning P et al. Pathogen blockade of TAK1 triggers caspase-8-dependent cleavage of gasdermin D and cell death. Sci 362, 1064–1069 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sarhan J et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl Acad. Sci. USA 115, E10888–E10897 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Demarco B et al. Caspase-8-dependent gasdermin D cleavage promotes antimicrobial defense but confers susceptibility to TNF-induced lethality. Sci. Adv 6, 3465–3483 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Y et al. Chemotherapy drugs induce pyroptosis through caspase-3 cleavage of a gasdermin. Nature 547, 99–103 (2017). [DOI] [PubMed] [Google Scholar]
- 95.Jiang M, Qi L, Li L & Li Y The caspase-3/GSDME signal pathway as a switch between apoptosis and pyroptosis in cancer. Cell Death Discov. 6, 112 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang Z et al. Gasdermin E suppresses tumour growth by activating anti-tumour immunity. Nature 579, 415–420 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Aizawa E et al. GSDME-dependent incomplete pyroptosis permits selective IL-1α release under caspase-1 inhibition. iScience 23, 101070 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhou B & Abbott DW Gasdermin E permits interleukin-1 beta release in distinct sublytic and pyroptotic phases. Cell Rep. 35, 108998 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen KW et al. RIPK1 activates distinct gasdermins in macrophages and neutrophils upon pathogen blockade of innate immune signaling. Proc. Natl Acad. Sci. USA 118, e2101189118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tan G et al. An IRF1-dependent pathway of TNFα-induced shedding in intestinal epithelial cells. J. Crohns Colitis 16, 133–142 (2022). [DOI] [PubMed] [Google Scholar]
- 101.Yokomizo K et al. Methylation of the DFNA5 gene is frequently detected in colorectal cancer. Anticancer. Res 32, 1319–1322 (2012). [PubMed] [Google Scholar]
- 102.Wu M et al. A PLK1 kinase inhibitor enhances the chemosensitivity of cisplatin by inducing pyroptosis in oesophageal squamous cell carcinoma. EBioMedicine 41, 244–255 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang Z et al. Prognostic and immunological role of Gasdermin E in pan-cancer analysis. Front. Oncol 11, 2879 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Su F et al. Long non-coding RNA nuclear paraspeckle assembly transcript 1 regulates ionizing radiation-induced pyroptosis via microRNA-448/gasdermin E in colorectal cancer cells. Int. J. Oncol 59, 79 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yu J et al. Cleavage of GSDME by caspase-3 determines lobaplatin-induced pyroptosis in colon cancer cells. Cell Death Dis. 10, 193 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang Y et al. GSDME mediates caspase-3-dependent pyroptosis in gastric cancer. Biochem. Biophys. Res. Commun 495, 1418–1425 (2018). [DOI] [PubMed] [Google Scholar]
- 107.Orzalli MH et al. Virus-mediated inactivation of anti-apoptotic Bcl-2 family members promotes Gasdermin-E-dependent pyroptosis in barrier epithelial cells. Immunity 54, 1447–1462.e5 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mujtaba G, Bukhari I, Fatima A & Naz S A p.C343S missense mutation in PJVK causes progressive hearing loss. Gene 504, 98–101 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Delmaghani S et al. Hypervulnerability to sound exposure through impaired adaptive proliferation of peroxisomes. Cell 163, 894–906 (2015). [DOI] [PubMed] [Google Scholar]
- 110.Defourny J et al. Pejvakin-mediated pexophagy protects auditory hair cells against noise-induced damage. Proc. Natl Acad. Sci. USA 116, 8010–8017 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Spechler SJ Barrett esophagus and risk of esophageal cancer: a clinical review. JAMA 310, 627–636 (2013). [DOI] [PubMed] [Google Scholar]
- 112.Correa P & Houghton JM Carcinogenesis of Helicobacter pylori. Gastroenterology 133, 659–672 (2007). [DOI] [PubMed] [Google Scholar]
- 113.Axelrad JE, Lichtiger S & Yajnik V Inflammatory bowel disease and cancer: the role of inflammation, immunosuppression, and cancer treatment. World J. Gastroenterol 22, 4794 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hergueta-Redondo M et al. Gasdermin-B promotes invasion and metastasis in breast cancer cells. PLoS ONE 9, e90099 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mu M et al. A pan-cancer analysis of molecular characteristics and oncogenic role of gasdermins. Cancer Cell Int. 22, 80 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Croes L et al. Determination of the potential tumor-suppressive effects of Gsdme in a chemically induced and in a genetically modified intestinal cancer mouse model. Cancers 11, 1214 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Santiago L et al. Extracellular granzyme A promotes colorectal cancer development by enhancing gut inflammation. Cell Rep. 32, 107847 (2020). [DOI] [PubMed] [Google Scholar]
- 118.Zheng ZY et al. STAT3β disrupted mitochondrial electron transport chain enhances chemosensitivity by inducing pyroptosis in esophageal squamous cell carcinoma. Cancer Lett. 522, 171–183 (2021). [DOI] [PubMed] [Google Scholar]
- 119.Li L et al. Photodynamic therapy induces human esophageal carcinoma cell pyroptosis by targeting the PKM2/caspase-8/caspase-3/GSDME axis. Cancer Lett. 520, 143–159 (2021). [DOI] [PubMed] [Google Scholar]
- 120.Tan G et al. Radiosensitivity of colorectal cancer and radiation-induced gut damages are regulated by gasdermin E. Cancer Lett. 529, 1–10 (2022). [DOI] [PubMed] [Google Scholar]
- 121.Thurston TLM et al. Growth inhibition of cytosolic Salmonella by caspase-1 and caspase-11 precedes host cell death. Nat. Commun 7, 13292 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zuo L et al. Salmonella spvC gene inhibits pyroptosis and intestinal inflammation to aggravate systemic infection in mice. Front. Microbiol 11, 3135 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Havira MS et al. Shiga toxin suppresses noncanonical inflammasome responses to cytosolic LPS. Sci. Immunol 5, eabc0217 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tsutsuki H et al. Subtilase cytotoxin from Shiga-toxigenic Escherichia coli impairs the inflammasome and exacerbates enteropathogenic bacterial infection. iScience 25, 104050 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Luchetti G et al. Shigella ubiquitin ligase IpaH7.8 targets gasdermin D for degradation to prevent pyroptosis and enable infection. Cell Host Microbe 29, 1521–1530.e10 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rauch I et al. NAIP-NLRC4 inflammasomes coordinate intestinal epithelial cell expulsion with eicosanoid and IL-18 release via activation of caspase-1 and -8. Immunity 46, 649–659 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ventayol PS et al. Bacterial detection by NAIP/NLRC4 elicits prompt contractions of intestinal epithelial cell layers. Proc. Natl Acad. Sci. USA 118, e2013963118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sellin ME et al. Epithelium-intrinsic NAIP/NLRC4 inflammasome drives infected enterocyte expulsion to restrict Salmonella replication in the intestinal mucosa. Cell Host Microbe 16, 237–248 (2014). [DOI] [PubMed] [Google Scholar]
- 129.Knodler LA et al. Noncanonical inflammasome activation of caspase-4/caspase-11 mediates epithelial defenses against enteric bacterial pathogens. Cell Host Microbe 16, 249–256 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fattinger SA, Sellin ME & Hardt WD Epithelial inflammasomes in the defense against Salmonella gut infection. Curr. Opin. Microbiol 59, 86–94 (2021). [DOI] [PubMed] [Google Scholar]
- 131.Fattinger SA et al. Epithelium-autonomous NAIP/NLRC4 prevents TNF-driven inflammatory destruction of the gut epithelial barrier in Salmonella-infected mice. Mucosal Immunol. 14, 615–629 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhong Q et al. Clustering of Tir during enteropathogenic E. coli infection triggers calcium influx–dependent pyroptosis in intestinal epithelial cells. PLoS Biol. 18, e3000986 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Man SM Inflammasomes in the gastrointestinal tract: infection, cancer and gut microbiota homeostasis. Nat. Rev. Gastroenterol. Hepatol 15, 721–737 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shi Y et al. Host Gasdermin D restrains systemic endotoxemia by capturing Proteobacteria in the colon of high-fat diet-feeding mice. Gut Microbes 13, 1946369 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Quach J, Moreau F, Sandall C & Chadee K Entamoeba histolytica-induced IL-1β secretion is dependent on caspase-4 and gasdermin D. Mucosal Immunol. 12, 323–339 (2019). [DOI] [PubMed] [Google Scholar]
- 136.Wang S, Moreau F & Chadee K The colonic pathogen Entamoeba histolytica activates caspase-4/1 that cleaves the pore-forming protein gasdermin D to regulate IL-1β secretion. PLoS Pathog. 18, e1010415 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li J et al. Insight to pyroptosis in viral infectious diseases. Health 13, 574–590 (2021). [Google Scholar]
- 138.Zhu S et al. Nlrp9b inflammasome restricts rotavirus infection in intestinal epithelial cells. Nature 546, 667–670 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Dubois H et al. Nlrp3 inflammasome activation and Gasdermin D-driven pyroptosis are immunopathogenic upon gastrointestinal norovirus infection. PLoS Pathog. 15, e1007709 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dong S et al. Gasdermin E is required for induction of pyroptosis and severe disease during enterovirus 71 infection. J. Biol. Chem 298, 101850 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lei X et al. Enterovirus 71 inhibits pyroptosis through cleavage of Gasdermin D. J. Virol 91, e01069–17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang W et al. EV71 3D protein binds with NLRP3 and enhances the assembly of inflammasome complex. PLoS Pathog. 13, e1006123 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang Y et al. Pyroptosis induced by enterovirus 71 and coxsackievirus B3 infection affects viral replication and host response Sci. Rep 8, 2887 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Briardi B, Malireddi RKS & Kannegantii TD Role of inflammasomes/pyroptosis and PANoptosis during fungal infection. PLoS Pathog. 17, e1009358 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.De Schutter E et al. Punching holes in cellular membranes: biology and evolution of gasdermins. Trends Cell Biol. 31, 500–513 (2021). [DOI] [PubMed] [Google Scholar]
- 146.Jiang S, Zhou Z, Sun Y, Zhang T & Sun L Coral gasdermin triggers pyroptosis. Sci. Immunol 5, eabd2591 (2020). [DOI] [PubMed] [Google Scholar]
- 147.IBD Exomes Portal. Gene: GSDMB. IBD Exomes Browser https://dmz-ibd.broadinstitute.org/gene/ENSG00000073605 (2023). [Google Scholar]
- 148.Jostins L et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 491, 119–124 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Gong W, Liu P & Ren J GSDMB-mediated pyroptosis exacerbates intestinal inflammation by destroying intestinal epithelium in Crohn’s disease [abstract P010]. Presented at the 16th Congress of ECCO. https://www.ecco-ibd.eu/publications/congress-abstracts/item/p010-gsdmb-mediated-pyroptosis-exacerbates-intestinal-inflammation-by-destroying-intestinal-epithelium-in-crohn-s-disease.html (2021). [Google Scholar]
- 150.Ma C et al. Gasdermin D in macrophages restrains colitis by controlling cGAS-mediated inflammation. Sci. Adv 6, eaaz6717 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Schwarzer R, Jiao H, Wachsmuth L, Tresch A & Pasparakis M FADD and caspase-8 regulate gut homeostasis and inflammation by controlling MLKL- and GSDMD-mediated death of intestinal epithelial cells. Immunity 52, 978–993.e6 (2020). [DOI] [PubMed] [Google Scholar]
- 152.Chen X et al. NEK7 interacts with NLRP3 to modulate the pyroptosis in inflammatory bowel disease via NF-κB signaling. Cell Death Dis. 10, 906 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gao W et al. TRIM21 regulates pyroptotic cell death by promoting Gasdermin D oligomerization. Cell Death Differ. 29, 439–450 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Luo B, Lin J, Cai W & Wang M Identification of the pyroptosis-related gene signature and risk score model for colon adenocarcinoma. Front. Genet 12, 2450 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Qiu S, Hu Y & Dong S Pan-cancer analysis reveals the expression, genetic alteration and prognosis of pyroptosis key gene GSDMD. Int. Immunopharmacol 101, 108270 (2021). [DOI] [PubMed] [Google Scholar]
- 156.Wang J et al. Gasdermin D in different subcellular locations predicts diverse progression, immune microenvironment and prognosis in colorectal cancer. J. Inflamm. Res 14, 6223–6235 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ibrahim J et al. Methylation analysis of Gasdermin E shows great promise as a biomarker for colorectal cancer. Cancer Med. 8, 2133–2145 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fu L et al. Intracellular MUC20 variant 2 maintains mitochondrial calcium homeostasis and enhances drug resistance in gastric cancer. Gastric Cancer 25, 542–557 (2022). [DOI] [PubMed] [Google Scholar]
- 159.Hergueta-Redondo M et al. Gasdermin B expression predicts poor clinical outcome in HER2-positive breast cancer. Oncotarget 7, 56295–56308 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gunturu KS, Woo Y, Beaubier N, Remotti HE & Saif MW Gastric cancer and trastuzumab: first biologic therapy in gastric cancer. Ther. Adv. Med. Oncol 5, 143 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Homsy E et al. Circulating Gasdermin-D in critically ill patients. Crit. Care Explor 1, e0039 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Deng BB, Jiao BP, Liu YJ, Li YR & Wang GJ BIX-01294 enhanced chemotherapy effect in gastric cancer by inducing GSDME-mediated pyroptosis. Cell Biol. Int 44, 1890–1899 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Shen X, Wang H, Weng C, Jiang H & Chen J Caspase 3/GSDME-dependent pyroptosis contributes to chemotherapy drug-induced nephrotoxicity. Cell Death Dis. 12, 186 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Huang Z et al. Inhibition of caspase-3-mediated GSDME-derived pyroptosis aids in noncancerous tissue protection of squamous cell carcinoma patients during cisplatin-based chemotherapy. Am. J. Cancer Res 10, 4287 (2020). [PMC free article] [PubMed] [Google Scholar]
- 165.Jiang S et al. Vitamin D/VDR attenuate cisplatin-induced AKI by down-regulating NLRP3/caspase-1/GSDMD pyroptosis pathway. J. Steroid Biochem. Mol. Biol 206, 105789 (2021). [DOI] [PubMed] [Google Scholar]
- 166.Liu Y et al. Gasdermin E-mediated target cell pyroptosis by CAR T cells triggers cytokine release syndrome. Sci. Immunol 5, eaax7969 (2020). [DOI] [PubMed] [Google Scholar]
- 167.Xiao Y et al. Microenvironment-responsive prodrug-induced pyroptosis boosts cancer immunotherapy. Adv. Sci 8, 2101840 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Liu Z et al. Apoptin induces pyroptosis of colorectal cancer cells via the GSDME-dependent pathway. Int. J. Biol. Sci 18, 717 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Molina-Crespo A et al. Intracellular delivery of an antibody targeting gasdermin-B reduces HER2 breast cancer aggressiveness. Clin. Cancer Res 25, 4846–4858 (2019). [DOI] [PubMed] [Google Scholar]
- 170.Gámez-Chiachio M et al. Gasdermin B over-expression modulates HER2-targeted therapy resistance by inducing protective autophagy through Rab7 activation. J. Exp. Clin. Cancer Res 41, 285 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wang Q et al. A bioorthogonal system reveals antitumour immune function of pyroptosis. Nature 579, 421–426 (2020). [DOI] [PubMed] [Google Scholar]
- 172.Ryder CB, Kondolf HC, O’Keefe ME, Zhou B & Abbott DW Chemical modulation of gasdermin-mediated pyroptosis and therapeutic potential. J. Mol. Biol 434, 167183 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rathkey JK et al. Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci. Immunol 3, eaat2738 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hu JJ et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol 21, 736–745 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ou Ate et al. Disulfiram-loaded lactoferrin nanoparticles for treating inflammatory diseases. Acta Pharmacol. Sin 42, 1913–1920 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Feagan BG et al. A comparison of methotrexate with placebo for the maintenance of remission in Crohn’s disease. N. Engl. J. Med 342, 1627–1632 (2000). [DOI] [PubMed] [Google Scholar]
- 177.Feagan BG et al. Methotrexate for the treatment of Crohn’s disease. N. Engl. J. Med 332, 292–297 (1995). [DOI] [PubMed] [Google Scholar]
- 178.Privitera G & Pizarro TT Live or let die: translational insights and clinical perspectives of gasdermin B-dependent intestinal epithelial cell fate. Clin. Transl. Med 12, e787 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Al Mamun A et al. Role of pyroptosis in liver diseases. Int. Immunopharmacol 84, 106489 (2020). [DOI] [PubMed] [Google Scholar]
- 180.Rodríguez-Antonio I, López-Sánchez GN, Uribe M, Chávez-Tapia NC & Nuño-Lámbarri N Role of the inflammasome, gasdermin D, and pyroptosis in non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol 36, 2720–2727 (2021). [DOI] [PubMed] [Google Scholar]
- 181.Gaul S et al. Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J. Hepatol 74, 156–167 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Xu WF et al. Gasdermin E-derived caspase-3 inhibitors effectively protect mice from acute hepatic failure. Acta Pharmacol. Sin 42, 68–76 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lin T et al. Downregulating Gasdermin D reduces severe acute pancreatitis associated with pyroptosis. Med. Sci. Monit 27, e927968 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Wu J et al. Treatment of severe acute pancreatitis and related lung injury by targeting Gasdermin D-mediated pyroptosis. Front. Cell Dev. Biol 9, 780142 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Chen Y et al. Ultraviolet B induces proteolytic cleavage of the pyroptosis inducer gasdermin E in keratinocytes. J. Dermatol. Sci 100, 160–163 (2020). [DOI] [PubMed] [Google Scholar]
- 186.Grossi S et al. Inactivation of the cytoprotective major vault protein by caspase-1 and -9 in epithelial cells during apoptosis. J. Invest. Dermatol 140, 1335–1345.e10 (2020). [DOI] [PubMed] [Google Scholar]
- 187.Ruan J, Xia S, Liu X, Lieberman J & Wu H Cryo-EM structure of the gasdermin A3 membrane pore. Nature 557, 62–67 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Liu Z et al. Crystal structures of the full-length murine and human Gasdermin D reveal mechanisms of autoinhibition, lipid binding, and oligomerization. Immunity 51, 43–49.e4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Xia S et al. Gasdermin D pore structure reveals preferential release of mature interleukin-1. Nature 593, 607–611 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Chen X et al. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 26, 1007–1020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zheng Z et al. The lysosomal Rag-Ragulator complex licenses RIPK1- and caspase-8-mediated pyroptosis by Yersinia. Science 372, eabg0269 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lei M et al. Gsdma3 is a new factor needed for TNF-α-mediated apoptosis signal pathway in mouse skin keratinocytes. Histochem. Cell Biol 138, 385–396 (2012). [DOI] [PubMed] [Google Scholar]
- 193.Kayagaki N et al. IRF2 transcriptionally induces GSDMD expression for pyroptosis. Sci. Signal 12, eaax4917 (2019). [DOI] [PubMed] [Google Scholar]
- 194.Benaoudia S et al. A genome-wide screen identifies IRF2 as a key regulator of caspase-4 in human cells. EMBO Rep. 20, e48235 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Saeki N & Hiroki S in Endothelium and Epithelium: Composition, Functions, and Pathology (eds Carrasco J & Mota M) 193–211 (Nova Biomedical, 2012). [Google Scholar]
- 196.Pandey A, Shen C, Feng S & Man SM Cell biology of inflammasome activation. Trends Cell Biol. 31, 924–939 (2021). [DOI] [PubMed] [Google Scholar]
- 197.Pandey A, Shen C & Man SM Inflammasomes in colitis and colorectal cancer: mechanism of action and therapies. Yale J. Biol. Med 92, 481–498 (2019). [PMC free article] [PubMed] [Google Scholar]
- 198.Liu Z et al. Caspase-1 engages full-length Gasdermin D through two distinct interfaces that mediate caspase recruitment and substrate cleavage. Immunity 53, 106–114.e5 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Dinarello C et al. IL-1 family nomenclature. Nat. Immunol 11, 973 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Rivers-Auty J & Brough D Potassium efflux fires the canon: potassium efflux as a common trigger for canonical and noncanonical NLRP3 pathways. Eur. J. Immunol 45, 2758–2761 (2015). [DOI] [PubMed] [Google Scholar]
- 201.Yu P et al. Pyroptosis: mechanisms and diseases. Signal. Transduct. Target. Ther 6, 128 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Bedoui S, Herold MJ & Strasser A Emerging connectivity of programmed cell death pathways and its physiological implications. Nat. Rev. Mol. Cell Biol 21, 678–695 (2020). [DOI] [PubMed] [Google Scholar]
- 203.Labani-Motlagh A, Ashja-Mahdavi M & Loskog A The tumor microenvironment: a milieu hindering and obstructing antitumor immune responses. Front. Immunol 11, 940 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Colegio OR et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gül N et al. Macrophages eliminate circulating tumor cells after monoclonal antibody therapy. J. Clin. Invest 124, 812–823 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yu J et al. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J. Immunol 190, 3783–3797 (2013). [DOI] [PubMed] [Google Scholar]
- 207.Kobayashi N et al. FOXP3+ regulatory T cells affect the development and progression of hepatocarcinogenesis. Clin. Cancer Res 13, 902–911 (2007). [DOI] [PubMed] [Google Scholar]
- 208.Hoepner S et al. Synergy between CD8 T cells and Th1 or Th2 polarised CD4 T cells for adoptive immunotherapy of brain tumours. PLoS ONE 8, e63933 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Weigelin B et al. Cytotoxic T cells are able to efficiently eliminate cancer cells by additive cytotoxicity. Nat. Commun 12, 5217 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Melaiu O, Lucarini V, Cifaldi L & Fruci D Influence of the tumor microenvironment on NK cell function in solid tumors. Front. Immunol 10, 3038 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jiang W et al. Exhausted CD8+ T cells in the tumor immune microenvironment: new pathways to therapy. Front. Immunol 11, 3739 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]