Abstract
Purpose:
To examine the effect of kidney recovery on mortality, dialysis and kidney transplantation up to 15 years after AKI.
Materials and Methods:
We studied 29,726 survivors of critical illness and compared these outcomes stratified by AKI and recovery status at hospital discharge. Kidney recovery was defined as a return of serum creatinine to ≤150% of baseline without dialysis prior to hospital discharge.
Results:
Overall AKI occurred in 59.2% in which two thirds developed stage 2–3 AKI. Recovery rate of AKI at hospital discharge was 80.8%. Patients who did not recover experienced the worst 15-year mortality compared to those who recovered and those without AKI (57.8% vs 45.2% vs 30.3%, p<0.001). This pattern was also found in subgroups of patients with suspected sepsis-associated (57.1% vs 47.9% vs 36.5%, p<0.001) and cardiac surgery-associated AKI (60.1% vs 41.8% vs 25.9%, p<0.001). The rates of dialysis and transplantation at 15 years were low and not associated with recovery status.
Conclusions:
Recovery of AKI in critically ill patients at hospital discharge had an effect on long-term mortality for up to 15 years. These results have implications for acute care, follow-up and choice of endpoints for clinical trials.
Keywords: critically ill, outcomes, recovery, renal failure, dialysis, transplantation
INTRODUCTION
Acute kidney injury (AKI) is associated with high morbidity and mortality. Patients who develop AKI carry significantly increased risk for developing chronic kidney disease (CKD), end-stage kidney disease (ESKD), and decreased life expectancy.1 Furthermore, poor long-term outcomes for AKI are strongly associated with AKI severity stage.
During the past decade, many epidemiologic studies found that patients recovering renal function after AKI have dramatically improved short-term survival. For example, patients who had reversal of AKI within 24 hours after documentation of shock experienced a reduced in-hospital mortality (hazard ratio [HR] 0.64).2 An ancillary analysis of the Protocolized Care for Early Septic Shock (ProCESS) trial revealed that, in patients with sepsis-associated AKI who experienced renal recovery by hospital discharge, even if incomplete, had a similar prognosis to those without AKI up to 1 year.3 Srisawat et al. reported the impact of renal recovery at hospital discharge after AKI in 631 patients with community-acquired pneumonia in which those who recovered had significant lower mortality up to 90 days.4 A follow-up study using the same cohort reported long-term outcomes up to 3 years.5 For patients with AKI who recovered renal function by hospital discharge, 3-year survival was comparable to patients without AKI (28% vs 23% mortality), while those who did not recover had much worse prognosis (44% mortality). Recovery status after AKI has been shown to determine the risk of progression to CKD as well.6,7 Over 1 year, CKD developed in 21%, 30%, and 79% of 105 survivors with early reversal, recovery, and nonrecovery following AKI, respectively.7
The capacity of the kidney to increase filtration in the remaining nephrons can restore glomerular filtration rate (GFR) but possibly at the expense of more long-term damage.8,9 Furthermore, any effect of this damage on survival may require many years to manifest. Thus, we examined post-discharge survival, dialysis requirement, and kidney transplantation up to 15 years following an episode of AKI in hospital survivors following critical illness and related these outcomes to renal recovery at hospital discharge. Finally, given that AKI etiology might influence outcomes, we performed subgroup analyses for major causes of AKI in the critically ill (suspected sepsis and cardiac surgery).
MATERIALS AND METHODS
Source population and patients
Approval was obtained from the University of Pittsburgh Institutional Review Board. We analyzed the High-Density Intensive Care (HiDenIC)-08 database including data on a source population of 45,568 adult patients admitted to an intensive care unit (ICU) within a single academic medical center (University of Pittsburgh Medical Center, Pittsburgh, PA) during an 8-year period (July 2000 through October 2008). We excluded patients who did not survive hospitalization, as well as patients with known baseline serum creatinine (SCr) >4 mg/dL, patients with history of dialysis or transplantation prior to hospitalization (determined by querying the U.S. Renal Data System database), and patients with missing SCr data at hospital discharge. In case of multiple ICU admissions, we used only the first admission. Patients were then stratified by maximum AKI stage during hospitalization and recovery status at hospital discharge (Figure 1).
Figure 1.
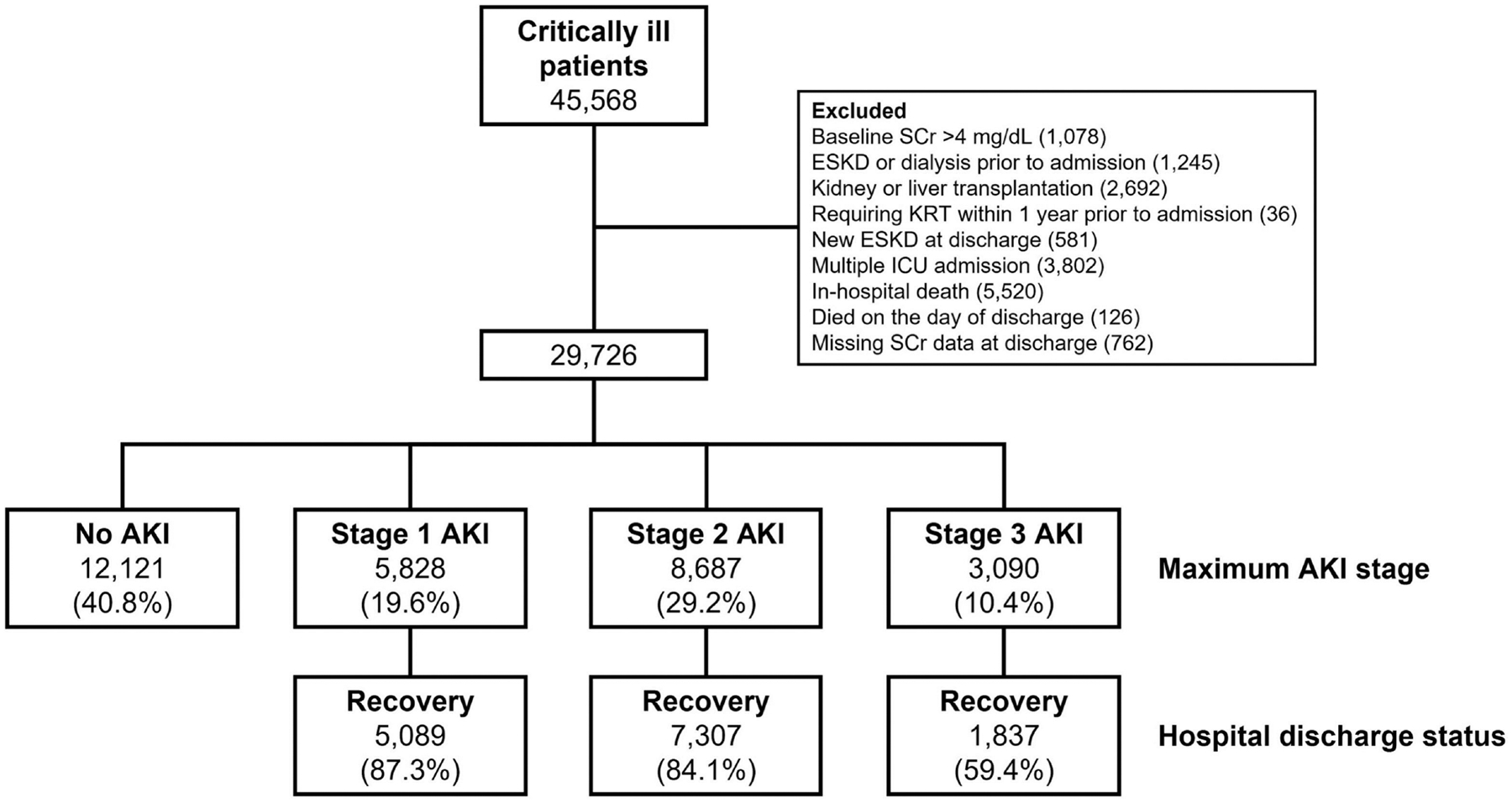
Study Flow.
Abbreviation: ESKD; end-stage kidney disease; KRT, kidney replacement therapy; SCr, serum creatinine.
Definitions
AKI diagnosis and staging was determined using either SCr or urine output according to Kidney Disease Improving Global Outcomes (KDIGO) criteria.10 We classified patients if they developed AKI and determined the maximum AKI stage during hospitalization. We determined baseline SCr within 12 months prior to hospital admission taking a median value if multiple measurements were available. Reference SCr was determined by the lower of baseline and admission SCr as previously described.11–13 For patients with missing baseline SCr (without history of CKD), we estimated baseline SCr by solving the Modification of Diet in Renal Disease equation as recommended in the KDIGO AKI guideline assuming a GFR of 75 mL/min/1.73 m2.14
Recovery from AKI was assessed based on SCr at hospital discharge, which was defined as a return of SCr to ≤150% of baseline without need for dialysis.15 Non-recovery AKI was defined as need for dialysis within 48 hours prior to discharge or no return of SCr to ≤150% of baseline. Suspected sepsis was defined as obtaining blood cultures (irrespective of results) and treating with antibiotics.
Outcome
Our primary outcome was survival to 15 years after the index hospitalization stratified by recovery status at hospital discharge up to 30 days. Vital status was determined from the National Center for Health Statistics National Death Index database or the Social Security Administration’s Death Master File. Secondary outcomes included the rates of dialysis and transplantation at 15 years after AKI by recovery status. Long-term outcomes were also examined in subgroup analyses of the two most common etiologies of AKI in ICU—sepsis and cardiac surgery.
Statistical Analysis
Statistical comparisons for categorical and continuous data were performed using Chi-square/Fisher Exact test and Mann-Whitney U test/Kruskal-Wallis test. Data is reported as counts (percentages) for categorical and median with interquartile range for continuous data. No imputation was performed on missing data. Unadjusted survival estimates are estimated using Kaplan-Meier estimates stratified by recovery status. Cox proportional-hazard model was used to adjust long term survival by AKI status for age, sex, race, and comorbidities (CKD, heart disease, diabetes, hypertension, and vascular disease). We also ran the model without covariates to determine the unadjusted hazard. Age-adjusted parametric (Weibull) survival model was used to compute average life expectancy. All-cause mortality was analyzed. All statistical analyses were performed using Stata version 15.1 (STATA Corp, TX). P value of <0.05 was considered significant.
RESULTS
Of 29,726 critically ill patients, 17,605 (59.2%) experienced AKI. Of these, 5,828 (33.1%) had stage 1 AKI, while 8,687 (49.3%) and 3,090 (17.6%) had stage 2 and 3, respectively. Rates of renal recovery at hospital discharge were 80.8% overall, and 87.3%, 84.1%, and 59.4% for patients with stage 1, 2, and 3 AKI, respectively (Figure 1). Patient characteristics stratified by AKI recovery status at hospital discharge are shown in Table 1. Median age was 60 years and 57.4% of the entire cohort were male. Compared to patients who recovered following AKI, those who did not recover from AKI were significantly older (67 vs. 62 years) and had more comorbidities including diabetes, hypertension, heart disease, vascular disease, and preexisting CKD (Table 1). Patients recovering from AKI had higher baseline estimated GFR (83.3 vs. 75.0 mL/min/1.73 m2) and fewer patients in this group experienced anemia, or vasopressor use and had lower Acute Physiology And Chronic Health Evaluation (APACHE) III scores. Surgical admission was associated with higher rates of AKI recovery.
Table 1.
Patient Characteristics Stratified by AKI Recovery Status at Hospital Discharge
| Variable | Total (N=29,726) | Recovery AKI (N=14,233) | Non-Recovery AKI (N=3,372) | No AKI (N=12,121) | p-value* |
|---|---|---|---|---|---|
| Age, year | 60.0 (47.0–73.0) | 62.0 (50.0–74.0) | 67.0 (53.0–77.0) | 55.0 (42.0–69.0) | <0.001 |
| Male | 17,070 (57.4%) | 8,456 (59.4%) | 1,624 (48.2%) | 6,990 (57.7%) | <0.001 |
| Comorbidities | |||||
| - Chronic kidney disease | 739 (2.5%) | 409 (2.9%) | 148 (4.4%) | 182 (1.5%) | <0.001 |
| - Heart disease | 3,675 (12.4%) | 2,013 (14.1%) | 577 (17.1%) | 1,085 (9.0%) | <0.001 |
| - Diabetes mellitus | 3,823 (12.9%) | 2,134 (15.0%) | 602 (17.9%) | 1,087 (9.0%) | <0.001 |
| - Hypertension | 7,921 (26.6%) | 4,218 (29.6%) | 1,066 (31.6%) | 2,637 (21.8%) | <0.001 |
| - Vascular disease | 2,025 (6.8%) | 1,079 (7.6%) | 295 (8.7%) | 651 (5.4%) | <0.001 |
| Severe anemia | 726 (2.4%) | 369 (2.6%) | 122 (3.6%) | 235 (1.9%) | <0.001 |
| Moderate anemia | 7,307 (24.6%) | 3,850 (27.0%) | 1,096 (32.5%) | 2,361 (19.5%) | <0.001 |
| Suspected sepsis | 6,818 (22.9%) | 4,318 (30.3%) | 1,329 (39.4%) | 1,171 (9.7%) | <0.001 |
| Vasopressors | 5,123 (17.2%) | 3,121 (21.9%) | 1,024 (30.4%) | 978 (8.1%) | <0.001 |
| Hypotensive index | 0.0 (0.0–0.7) | 0.0 (0.0–2.1) | 0.0 (0.0–4.7) | 0.0 (0.0–0.0) | <0.001 |
| APACHE III | 49.0 (34.0–68.0) | 56.0 (41.0–75.0) | 65.0 (49.0–87.0) | 38.0 (27.0–51.0) | <0.001 |
| Mechanical ventilation | 14,155 (47.6%) | 8,570 (60.2%) | 1,643 (48.7%) | 3,942 (32.5%) | <0.001 |
| Surgical admission | 17,965 (60.4%) | 9,593 (67.4%) | 1,705 (50.6%) | 6,667 (55.0%) | <0.001 |
| Cardiac surgery | 3,632 (12.2%) | 2,164 (15.2%) | 388 (11.5%) | 1,080 (8.9%) | <0.001 |
| Max AKI stage | |||||
| - Stage 1 | 5828 (19.6%) | 5089 (35.8%) | 739 (21.9%) | NA | <0.001 |
| - Stage 2 | 8687 (29.2%) | 7307 (51.3%) | 1380 (40.9%) | NA | <0.001 |
| - Stage 3 | 3090 (10.4%) | 1837 (12.9%) | 1253 (37.2%) | NA | <0.001 |
| Reference SCr, mg/dL | 0.8 (0.7–1.0) | 0.9 (0.7–1.0) | 0.9 (0.8–1.1) | 0.8 (0.7–1.0) | <0.001 |
| Reference eGFR, mL/min/1.73 m2 | 85.3 (75.0–107.2) | 83.3 (75.0–105.5) | 75.0 (75.0–75.0) | 92.9 (75.6113.1) | <0.001 |
| SCr at hospital discharge, mg/dL | 0.9 (0.7–1.2) | 0.9 (0.7–1.1) | 1.8 (1.5–2.6) | 0.8 (0.7–1.0) | <0.001 |
| SCr at 3 mo after discharge, mg/dL | 0.9 (0.7–1.2) | 0.9 (0.7–1.2) | 1.3 (0.9–1.9) | 0.8 (0.7–1.0) | <0.001 |
| SCr at 12 mo after discharge, mg/dL | 0.9 (0.7–1.2) | 0.9 (0.7–1.3) | 1.3 (0.9–1.9) | 0.8 (0.7–1.0) | <0.001 |
| SCr at 18 mo after discharge, mg/dL | 0.9 (0.7–1.2) | 1.0 (0.7–1.3) | 1.3 (0.9–1.9) | 0.8 (0.7–1.0) | <0.001 |
| LOS in ICU | 3.0 (2.0–6.0) | 4.0 (3.0–9.0) | 4.0 (3.0–9.0) | 2.0 (2.0–3.0) | <0.001 |
| LOS in hospital | 10.0 (6.0–19.0) | 14.0 (8.0–24.0) | 14.0 (8.0–25.0) | 7.0 (4.0–11.0) | <0.001 |
| Mortality after hospital discharge | |||||
| - 30 days | 539 (1.8%) | 265 (1.9%) | 110 (3.3%) | 164 (1.4%) | <0.001 |
| - 90 days | 1,857 (6.2%) | 997 (7.0%) | 410 (12.2%) | 450 (3.7%) | <0.001 |
| - 1 year | 4,349 (14.6%) | 2,330 (16.4%) | 859 (25.5%) | 1,160 (9.6%) | <0.001 |
| - 5 years | 9,329 (31.4%) | 4,961 (34.9%) | 1,585 (47.0%) | 2,783 (23.0%) | <0.001 |
| - 10 years | 11,868 (39.9%) | 6,333 (44.5%) | 1,935 (57.4%) | 3,600 (29.7%) | <0.001 |
| - 15 years | 12,060 (40.6%) | 6,435 (45.2%) | 1,949 (57.8%) | 3,676 (30.3%) | <0.001 |
Data shown as n (%) or median (interquartile range).
P-values relate to a 3-way comparison between recovery, non-recovery, and no AKI except where indicated. APACHE III, Acute Physiology And Chronic Health Evaluation III; ICU, intensive care unit; LOS, length of stay; eGFR, estimated glomerular filtration rate; SCr, serum creatinine.
Overall mortality rates at 30 and 90 days were greater for patients who did not recover following AKI compared to patients who did (3.3% vs. 1.9% and 12.2% vs. 7%). By comparison, patients who never experienced AKI had 30- and 90-day mortality rates of 1.4% and 3.7%, respectively. Compared to patients who recovered after AKI, mortality rates were still higher in those with AKI and not recovering at 1 year (25.5% vs 16.4%, p<0.001), 5 years (47% vs 34.9%, p<0.001), and 15 years (57.8% vs 45.2%, p<0.001). Corresponding mortality rates for patients without AKI were 23.0%, 29.7% and 30.3% (Table 1, Figures 2 and 3). In a Cox model analyzing survival over 15 years, using no AKI as the reference, hazard ratios and corresponding 95% confidence intervals for AKI with recovery were 1.37 (1.31 – 1.43) adjusted and 1.67 (1.60 – 1.73) unadjusted. For AKI without recovery hazard ratios and corresponding 95% confidence intervals were 1.84 (1.73 – 1.94) adjusted 2.45 (2.32 – 2.59) unadjusted. The age-adjusted average life expectancy after hospital discharge was highest in those without AKI, while it was lowest in those who did not recover (Figure 4). This phenomenon was more prominent in younger patients.
Figure 2.
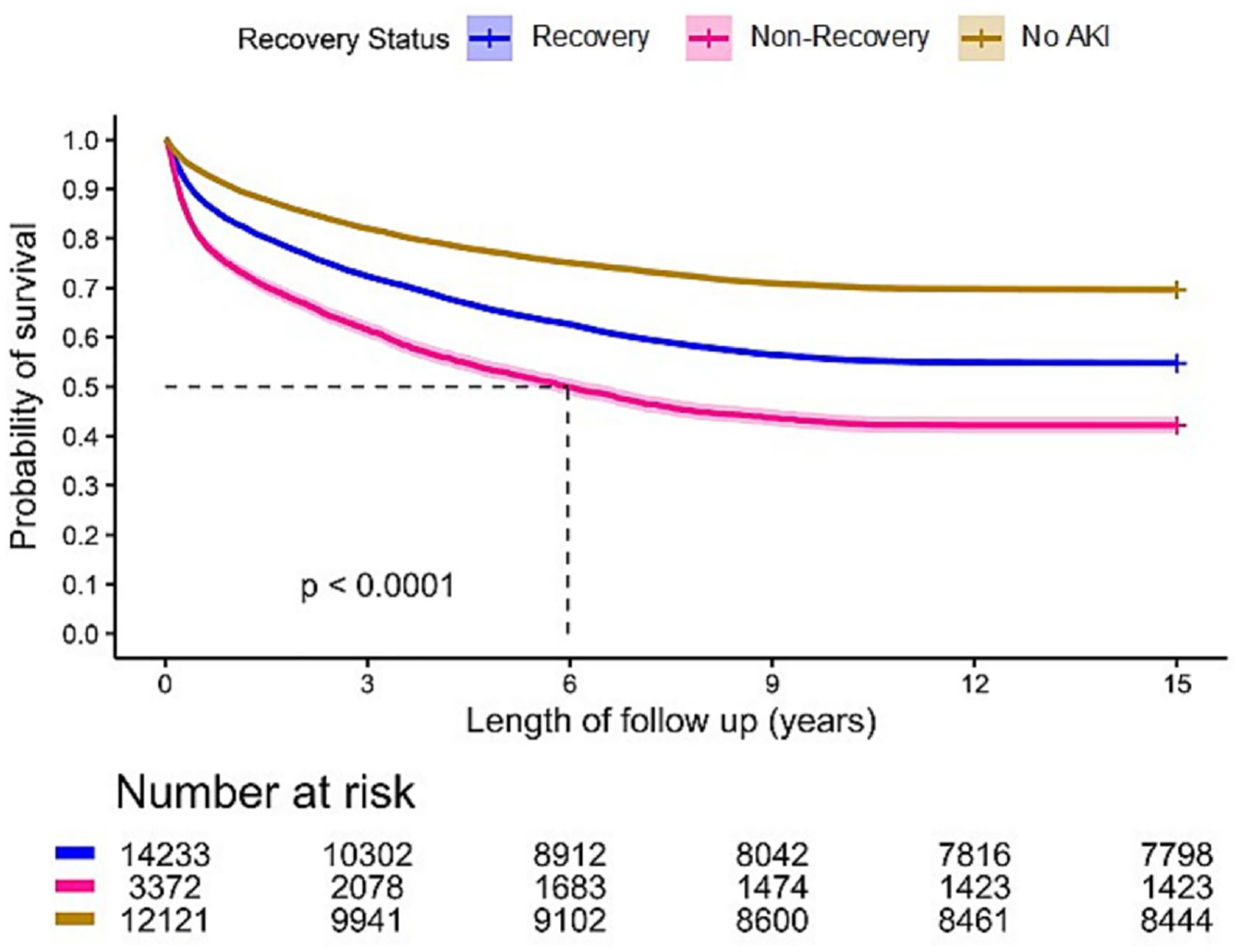
Long-Term Survival by AKI Recovery Status.
Figure 3.
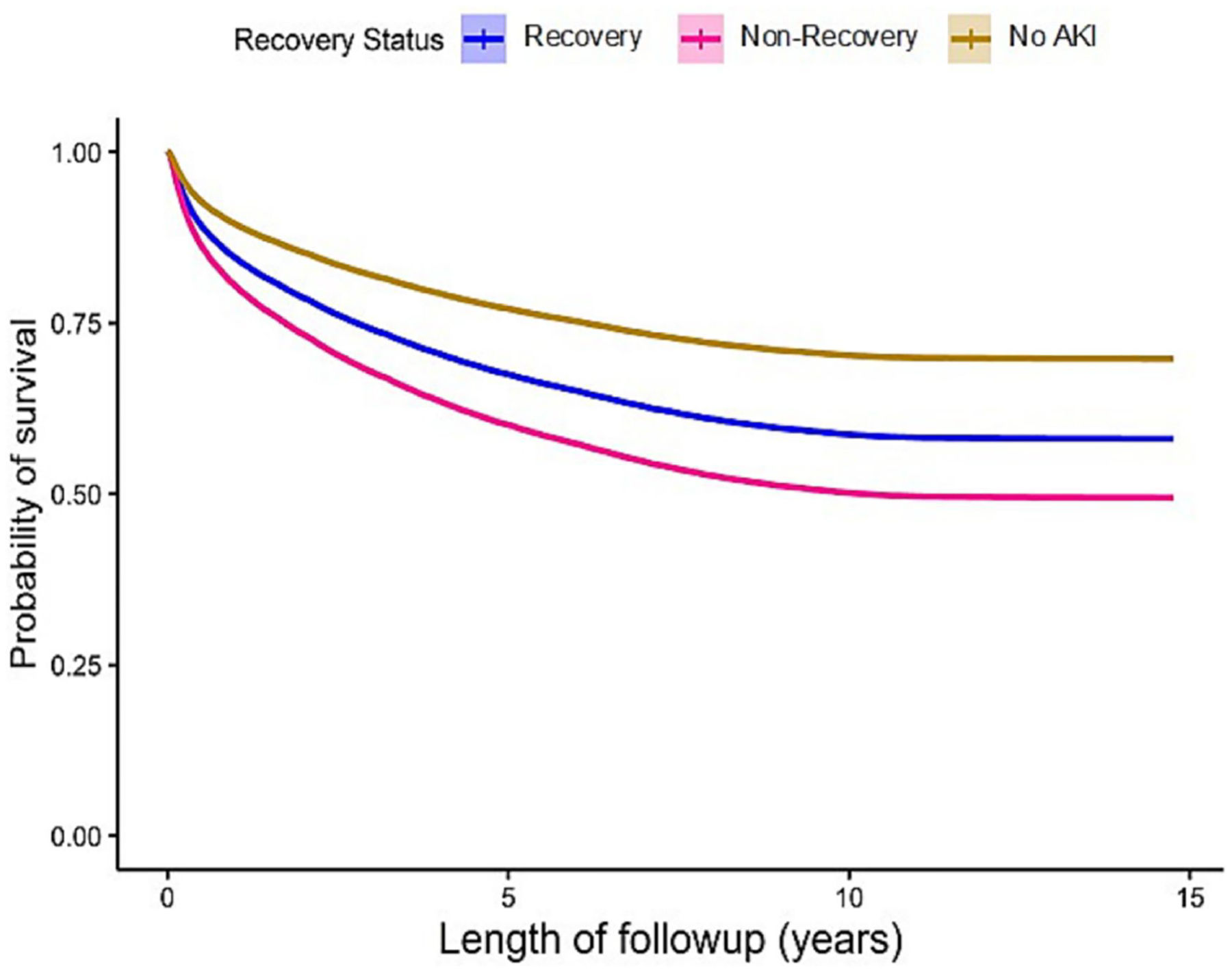
Adjusted Long-Term Survival by AKI Recovery Status. Adjusted by age, race, sex, multiple comorbidities.
Figure 4.
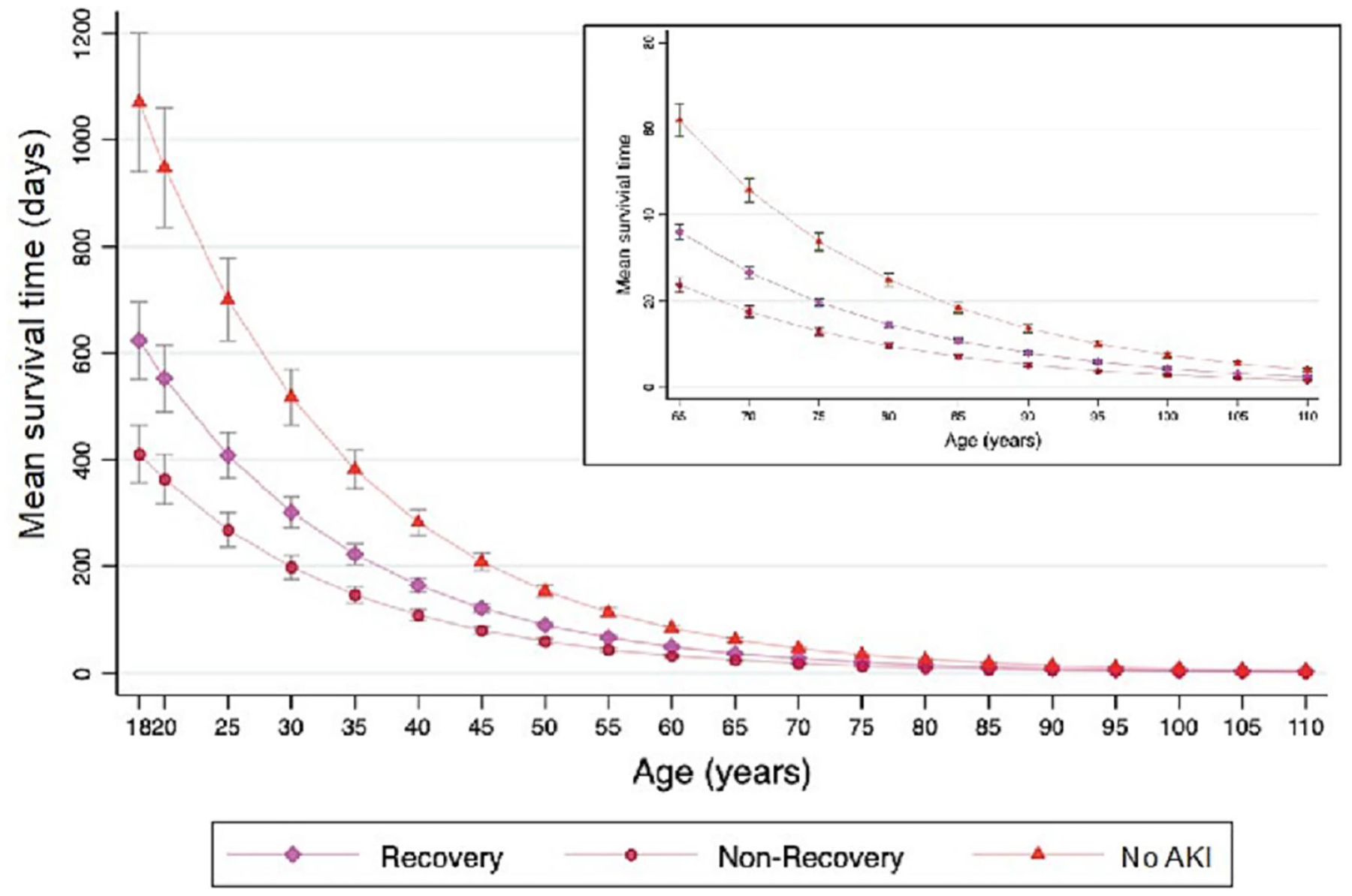
Age-Adjusted Average Life Expectancy by AKI Recovery Status at Hospital Discharge.
Sepsis-associated AKI (S-AKI)
The overall rate of AKI among 6,818 patients with suspected sepsis was extremely high (5,647 patients, 82.8%). Among patients with S-AKI, 79.3% developed moderate to severe (stage 2 or 3) AKI (Table 1S in supplementary data). However, 4,318 (76.5%) patients recovered from AKI by hospital discharge. Overall, mean duration of follow-up was 8.9 (± 6.6) years. Mortality rates at 1 year after hospital discharge were significantly higher for patients not recovering compared to those who did recover (30.5% vs 22.1%) and the lowest mortality was seen for no AKI (16.1%, p<0.001). The same was true at 5 years (48.5% vs 39.1% vs 30.1%, p<0.001) and 15 years (57.1% vs 47.9% vs 36.5%, p<0.001) (Table 2 and Figure 5).
Table 2.
Long-Term Outcomes in Patients with Suspected Sepsis by AKI Recovery Status at Hospital Discharge
| Total (N=6,818) | Recovery AKI (N=4,318) | Non-Recovery AKI (N=1,329) | No AKI (N=1,171) | p-value* | |
|---|---|---|---|---|---|
| Death, n (%) | |||||
| At 1 year | 1,549 (22.7) | 954 (22.1) | 406 (30.5) | 189 (16.1) | <0.001 |
| At 5 years | 2,687 (39.4) | 1,690 (39.1) | 645 (48.5) | 352 (30.1) | <0.001 |
| At 10 years | 3,225 (47.3) | 2,046 (47.4) | 757 (57.0) | 422 (36.0) | <0.001 |
| At 15 years | 3,254 (47.7) | 2,067 (47.9) | 759 (57.1) | 428 (36.5) | <0.001 |
| Dialysis Dependence, n (%) | |||||
| At 1 year | 21 (0.3) | 16 (0.4) | 4 (0.3) | 1 (0.1) | 0.29 |
| At 5 years | 84 (1.2) | 65 (1.5) | 13 (1.0) | 6 (0.5) | 0.015 |
| At 10 years | 127 (1.9) | 87 (2.0) | 25 (1.9) | 15 (1.3) | 0.26 |
| At 15 years | 133 (2.0) | 90 (2.1) | 27 (2.0) | 16 (1.4) | 0.28 |
| Kidney transplantation, n (%) | |||||
| At 1 year | 2 (0.0) | 0 | 1 (0.1) | 1 (0.1) | 0.18 |
| At 5 years | 11 (0.2) | 4 (0.1) | 3 (0.2) | 4 (0.3) | 0.14 |
| At 10 years | 18 (0.3) | 9 (0.2) | 5 (0.4) | 4 (0.3) | 0.49 |
| At 15 years | 20 (0.3) | 10 (0.2) | 5 (0.4) | 5 (0.4) | 0.45 |
P-values relate to a 3-way comparison between recovery, non-recovery, and no AKI.
Figure 5.
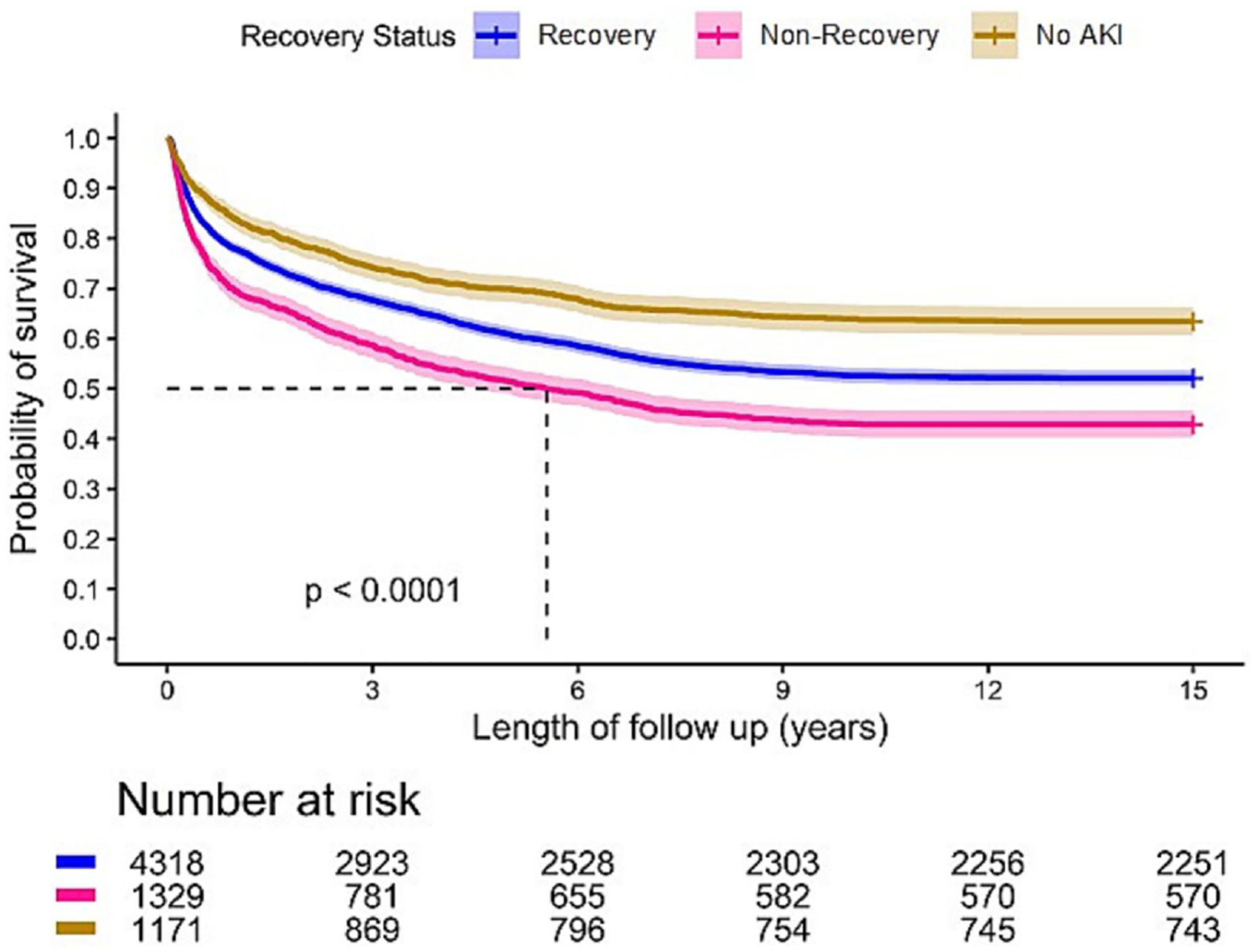
Long-Term Survival in Patients with suspected Sepsis by AKI Recovery Status.
Cardiac surgery-associated AKI (CS-AKI)
Of the 3,632 patients who underwent cardiac surgery, 2,552 (70.3%) developed AKI. Recovery from AKI for these patients was 84.8%. Not surprisingly, patients who did not recover following AKI had the highest mortality at 1 year compared to those who recovered or never developed AKI (19.6% vs 8.8% vs 2.3%, p<0.001). At 15 years, the mortality rate in patients who did not recover from AKI was 60.1% compared to 41.8% for those who recovered, and 25.9% for those without AKI, p<0.001) (Table 3 and Figure 6).
Table 3.
Long-Term Outcomes in Patients Underwent Cardiac Surgery by AKI Recovery Status at Hospital Discharge
| Total (N=3,632) | Recovery AKI (N=2,164) | Non-Recovery AKI (N=388) | No AKI (N=1,080) | p-value* | |
|---|---|---|---|---|---|
| Death, n (%) | |||||
| At 1 year | 291 (8.0) | 190 (8.8) | 76 (19.6) | 25 (2.3) | <0.001 |
| At 5 years | 794 (21.9) | 524 (24.2) | 151 (38.9) | 119 (11.0) | <0.001 |
| At 10 years | 1,371 (37.7) | 877 (40.5) | 228 (58.8) | 266 (24.6) | <0.001 |
| At 15 years | 1,418 (39.0) | 905 (41.8) | 233 (60.1) | 280 (25.9) | <0.001 |
| Dialysis, n (%) | |||||
| At 1 year | 19 (0.5) | 12 (0.6) | 2 (0.5) | 5 (0.5) | 0.94 |
| At 5 years | 70 (1.9) | 45 (2.1) | 5 (1.3) | 20 (1.9) | 0.57 |
| At 10 years | 98 (2.7) | 60 (2.8) | 6 (1.5) | 32 (3.0) | 0.32 |
| At 15 years | 106 (2.9) | 64 (3.0) | 6 (1.5) | 36 (3.3) | 0.20 |
| Kidney transplantation, n (%) | |||||
| At 1 year | 0 | 0 | 0 | 0 | |
| At 5 years | 15 (0.4) | 11 (0.5) | 0 | 4 (0.4) | 0.34 |
| At 10 years | 24 (0.7) | 14 (0.6) | 1 (0.3) | 9 (0.8) | 0.48 |
| At 15 years | 28 (0.8) | 17 (0.8) | 1 (0.3) | 10 (0.9) | 0.43 |
P-values relate to a 3-way comparison between recovery, non-recovery, and no AKI.
Figure 6.
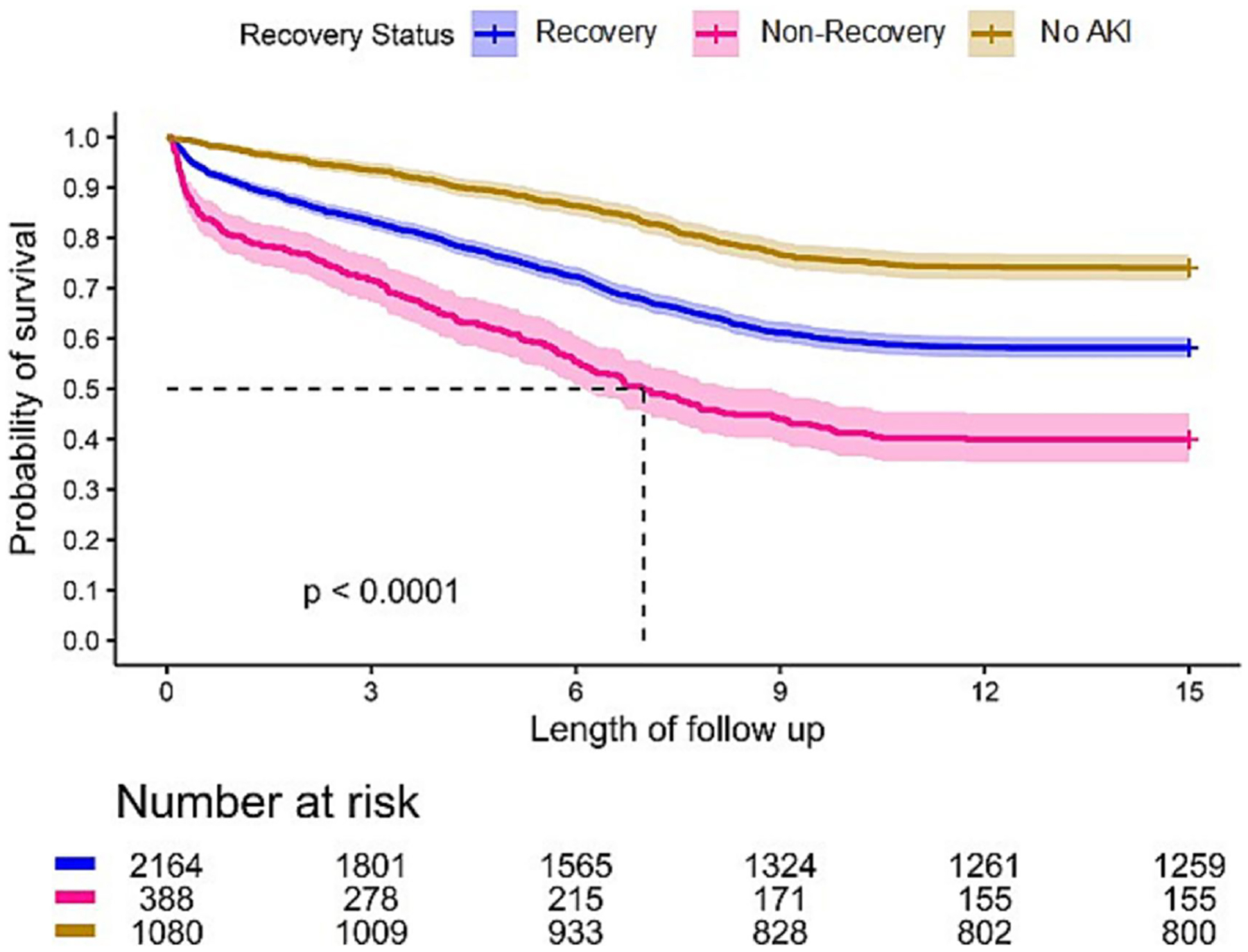
Long-Term Survival in Patients Underwent Cardiac Surgery by AKI Recovery Status.
DISCUSSION
We found that the chance of recovery after AKI in critically ill patients decreased by maximum severity of AKI. Both AKI and recovery status at hospital discharge had an effect on long-term survival and kidney outcomes for up to 15 years. Recovery from AKI in critically ill patients was associated with significantly better outcomes compared to non-recovery of AKI. Recovery status also had an impact on the remaining life expectancy of hospital survivors following AKI. However, patients recovering still faired substantially worse compared to those without AKI. Whereas short-term survival (i.e., <1 year) looked quite favorable for patients recovering after AKI, approaching that of patients without AKI, longer-term survival was clearly reduced such that by 15 years avoiding AKI resulted in 1.93 years longer post-discharge survival (and 3.76 years compared to patients not recovering from AKI) (Table 2S in supplementary data). This long-term effect on mortality was also shown in patients with S-AKI as well as in CS-AKI. Consistent with other reports,16,17 the rates of dialysis and kidney transplant after an episode of AKI during 15 years of follow-up were very low.
Long-term effect of AKI recovery on mortality
Few studies have explored the long-term impact of kidney recovery after AKI on mortality and dialysis dependence. Loef et al.18 included 843 patients who underwent cardiac surgery with median follow-up time of 8.3 years (maximum of 14.3 years) and found that patients with kidney recovery still had a higher risk of long-term mortality (HR 1.66, 95% CI 1.09–2.53) compared to patients without AKI, while those not recovering kidney function following AKI had the highest risk of death (HR 1.72, 95% CI 1.00–2.96). Bihorac et al.19 analyzed a cohort of 10,518 patients hospitalized in a surgical ICU in which 32% had AKI. Compared to no-AKI controls, those with complete and partial kidney recovery at hospital discharge still had higher risk of long-term mortality with maximum follow-up duration of 14 years (adjusted HRs [aHR] 1.20, 95% CI 1.10–1.31; and 1.45, 95% CI 1.32–1.58; respectively). The risk was highest in AKI without recovery (aHR 2.76, 95% CI 2.09–3.43). Another large study by Hobson et al.20 reported a cohort including 2,973 patients in the cardiothoracic ICU. Patients with complete kidney recovery after AKI at hospital discharge still had an increased risk of death (aHR 1.28, 95% CI 1.11–1.48) compared to patients without AKI with maximum follow-up duration of 10 years. Patients with partial kidney recovery and non-recovery AKI had even higher risk of death (aHRs 1.49, 95% CI 1.27–1.74; and 3.79, 95% CI 2.46–5.74; respectively). Another study found that early kidney recovery after CS-AKI, determined by percentage decrease of SCr in 24 hours after its peak, reduced the risk of long-term mortality up to 10 years of follow-up (HR 0.82, 95% CI 0.74–0.90).21 A recent systematic review of nine observational studies including 35,021 patients showed that CS-AKI was associated with a significantly increased risk of long-term mortality (HR 1.68, 95% CI 1.45–1.95).22 The risk was much higher in those not recovering kidney function (HR 2.71, 95% CI 1.26–5.82) compared to those with complete recovery (HR 1.31, 95% CI 1.16–1.47). Our results confirmed long-term effects of kidney recovery after AKI on mortality. This effect continued to impact the outcomes beyond 15 years after an episode of AKI in critically ill patients.
In sepsis, the long-term outcomes of patients with AKI in terms of survival appear to be determined by recovery status at hospital discharge. Secondary analysis in patients with septic shock from ProCESS trial showed that recovery after AKI seem to have similar survival at 1 year to those without AKI,3 while those with S-AKI who did not recover had the worst prognosis. These results were also observed up to 3 years after hospitalization with community-acquired pneumonia.5 However, data on long-term survival after AKI secondary to sepsis is lacking. Our results show that the survival curves for AKI with recovery and no-AKI begin to separate after 1 year (Figures 2 and 3). Thus, even when there is complete recovery of kidney function after AKI, permanently damaged nephrons may be present, and this condition may require many years to clinically manifest.
Long-term effect of AKI recovery on kidney outcomes
Many studies have shown comparative findings in terms of long-term kidney outcomes after an episode of AKI. However, follow-up has been much shorter. Wu et al.23 examined 1,363 patients who underwent cardiac surgery and found that those with AKI had a significant higher incidence of CKD stages 3–5 than the non-AKI patients at 3-year follow-up (9.9% vs. 2.3%, respectively). Even a minimal increase in SCr values (<25% increase) after cardiac surgery is associated with the development of these long-term adverse outcomes.24 About 0.2% of patients who never experienced postoperative AKI developed ESKD during the mean follow-up of 4.3 years in a nationwide Swedish study which included 29,330 patients after coronary artery bypass grafting (CABG).25
Several studies have indicated that development of CKD following AKI may still occur even where there is recovery of kidney function acutely. A retrospective study by Jones et al.26 included 719 hospitalized patients with complete recovery of AKI at hospital discharge found that 15% of these developed incident stage 3 CKD during a median follow-up of 2.5 years (HR 3.82, 2.81–5.19). Xu et al.27 showed that 2-year mortality and progressive CKD incidence even after complete recovery of kidney function were significantly increased in 1,295 patients with CS-AKI. Progressive CKD prevalence was significantly higher in patients who had AKI compared to patients who did not (6.8% vs 0.2%) in the 2 years after surgery. Even with complete recovery at hospital discharge, AKI was still a risk factor for accumulated progressive CKD (RR 1.92, 95% CI 1.37–2.69). Xu et al.28 investigated 3,869 patients who underwent cardiac surgery and demonstrated that AKI with complete kidney recovery by Acute Disease Quality Initiative Workgroup definition14 (returned to within 50% of baseline SCr) was a risk factor of 3-year composite endpoint of all-cause mortality, new dialysis and progressive CKD (OR 1.45, 95% CI 1.03–2.04) compared to patients without AKI. A single center retrospective study found about 4% of patients with CS-AKI receiving dialysis continued to receive dialysis at 1 year.16 Another prospective cohort study in 777 patients with CS-AKI showed that 3% of these patients developed ESKD during a mean follow-up of 4.4 years and the risk was increased in a dose-dependent fashion with AKI severity.17
On the other hand, the association between AKI and long-term kidney outcomes has been found to be complex. AKI and CKD share common risk factors such as older age, diabetes, hypertension, and metabolic syndrome,29 hence those who are at higher risk of progression toward CKD or ESKD after an episode of AKI are already at increased risk of CKD progression independent of the AKI event. The impact of AKI on long-term outcomes may be related to the residual kidney function and repair capacity after kidney injury. Our study could not show a difference in the rate of dialysis by recovery status of AKI during 15 years of follow-up in patients experiencing CS-AKI. Surprisingly, at 5 years, we found a significantly higher rate of dialysis in patients with suspected sepsis who recovered from AKI compared to those who did not (1.5% vs 1.0%) (Table 2). However, this result is likely explained by a higher mortality rate in the non-recovery group.
In addition, evidence from a large multicenter randomized controlled trial (CORONARY trial)30 showed that one fourth of patients undergoing CABG lost estimated GFR of at least 10 mL/min/1.73 m2 by 1 year regardless of whether they developed AKI or not. However, Palomba et al.31 reported rates of incident CKD 12 months after cardiac surgery in patients without AKI of 9% compared to 25% for patients with AKI (p<0.001). These findings might be explained by insensitivity of SCr for AKI recognition and for detection of residual kidney damage to quantify recovery. Priyanka et al.32 found that in cardiac surgery patients only manifesting AKI by transient oliguria (i.e., no change in SCr) increased the risk of subsequent de novo or worsening CKD over 6 months by 69% (from 4.5% to 7.6%) compared to patients without AKI.
Implications for research and clinical practice
Little is known about the process of recovery after AKI. Animal models indicate that return of function may belie permanent cellular injury and subsequent fibrosis.29,33–37 AKI often results in patchy injury with some tubules severely affected and others spared.29 Moreover, even after clinical recovery, hyperfiltration can mask structural kidney damage. Recently, reduction in renal functional reserve (RFR) after AKI was found to be an indicator of residual kidney damage and may play a novel role in determining a ‘true’ kidney function recovery and risk of progression to CKD. Husain-Syed et al.38 found that patients with subclinical AKI by positive postoperative cell cycle arrest markers experienced a significant reduction in RFR at 3 months despite having normal estimated GFR. Thus, as we have hypothesized previously,39 some patients may have “apparent recovery” in that their function returns to baseline but there is still loss of nephrons such that with time function declines faster than it would have without the AKI event. Furthermore, short term survival is unlikely to be affected by AKI if function is not severely impaired. Over the long term though, even modest reductions in kidney function (e.g., equivalent to stage 3 CKD) may result decreased survival.
Our findings have implications for improving AKI care. We encourage all AKI survivors, particularly those who experienced moderate to severe or persistent AKI, even if they recover completely, to be followed by a nephrologist within a few weeks or months after hospital discharge as recently recommended by KDIGO.40 Some experts recommend at least one-year duration of close monitoring at post-AKI clinic for ensuing CKD development before referral to general nephrology or family physician.41 Clinicians should also realize that these patients are also at high risk of cardiovascular consequences, thus the primary and secondary prevention could be combined in the goals of care with other specialists and multidisciplinary teams. However, the optimal strategy and duration of post-AKI follow-up is not known and needs further study.
Strengths and limitations
To our knowledge, this is the largest and longest observational study in critically ill patients showing the associations between AKI and kidney recovery after AKI with long-term outcomes including death and ESKD. Our cohort was comprised of critically ill patients from several types of ICUs, including both medical and surgical. Our results have broad implications for the continuum of care for AKI patients including post-AKI care, since AKI recovery status will affect the patient outcomes for more than a decade.
Our study has limitations. We ascertained renal recovery at discharge rather than at a fixed timepoint such as 90 days. While discharge dates may be influenced by non-clinical variables, the date is clinically important since discharge planning may be adapted to subsequent risk. Dialysis use and transplantation rates may have been underestimated as these results relied on positive identification within the USRDS database and were not independently confirmed. Similarly, our use of archived eMR data precluded the clinical diagnosis of sepsis and we could not ensure against any overlap between sepsis and cardiac surgery. Although we investigated a very large number of critically ill patients from several ICUs, epidemiologic analyses cannot exclude every potential confounder. We could only show the association of kidney recovery and long-term effect on adverse outcomes but not causality.
In conclusion, kidney recovery after AKI in critically ill patients at hospital discharge has a long-term relationship with mortality for more than 15 years. While recovery following AKI is associated with significantly improved survival, there remained higher risk of adverse outcomes compared to no AKI. Thus, efforts to both reduce AKI events and to facilitate recovery of kidney function in cases where they still occur, are crucial.
Supplementary Material
Table 1S. Recovery Status at Hospital Discharge by Maximum AKI Stage in Patients with suspected sepsis
Table 2S. Life Expectancy of AKI Survivors Compared to Patients without AKI
Key Learning Points.
What is already known about this subject:
Acute Kidney Injury is associated with severe short and long-term health consequences
When kidney function recovers following acute kidney injury, short-term prognosis is markedly improved, sometimes even similar to patients not experiencing acute kidney injury.
Long-term outcomes for patients recovering from acute kidney injury are poorly understood.
What this study adds:
Patients who experience acute kidney injury during an episode of critically illness have reduced long-term survival even if they recover kidney function acutely.
This effect persists for at least 15 years and occurs in various forms of acute kidney injury such as secondary to infection or cardiac surgery.
What impact this may have on practice or policy:
These results have important implications for acute care, follow-up and choice of endpoints for clinical trials.
Broader assessment of recovery including biomarkers and functional renal reserve may better characterize patients following an AKI event.
ACKNOWLEDGEMENTS
The authors would like to thank all the investigators and data personnel for their contribution to the study, all the supports from Center for Critical Care Nephrology, the CRISMA (Clinical Research, Investigation and Systems Modeling of Acute Illness) Center, and Department of Critical Care Medicine, University of Pittsburgh School of Medicine.
FUNDING
This study was funded in part by NIH/NIDDK (R01 DK083961).
Source of support:
This work was supported in part by grants from the National Institute of Diabetes and Digestive and Kidney Diseases R01DK070910 (JAK) and R01DK106256 (RM).
CONFLICT OF INTEREST STATEMENT
JAK discloses grant support and/or consulting fees paid by Astute Medical, BioMerieux. RM reports receiving grants and personal fees from La Jolla Inc; grants from Bioporto, Inc, and the National Institute of Diabetes and Digestive and Renal Diseases; and personal fees from Beckman Coulter and AM Pharma, Inc, outside the submitted work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.See EJ, Jayasinghe K, Glassford N, et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int. 2019;95(1):160–172. [DOI] [PubMed] [Google Scholar]
- 2.Sood MM, Shafer LA, Ho J, et al. Early reversible acute kidney injury is associated with improved survival in septic shock. J Crit Care. 2014;29(5):711–717. [DOI] [PubMed] [Google Scholar]
- 3.Kellum JA, Chawla LS, Keener C, et al. The Effects of Alternative Resuscitation Strategies on Acute Kidney Injury in Patients with Septic Shock. Am J Respir Crit Care Med. 2016;193(3):281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srisawat N, Murugan R, Lee M, et al. Plasma neutrophil gelatinase-associated lipocalin predicts recovery from acute kidney injury following community-acquired pneumonia. Kidney Int. 2011;80(5):545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiorentino M, Tohme FA, Wang S, Murugan R, Angus DC, Kellum JA. Long-term survival in patients with septic acute kidney injury is strongly influenced by renal recovery. PLoS One. 2018;13(6):e0198269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE. The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int. 2011;79(12):1361–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chua HR, Wong WK, Ong VH, et al. Extended Mortality and Chronic Kidney Disease After Septic Acute Kidney Injury. J Intensive Care Med. 2018:885066618764617. [DOI] [PubMed] [Google Scholar]
- 8.Hostetter TH, Olson JL, Rennke HG, Venkatachalam MA, Brenner BM. Hyperfiltration in remnant nephrons: a potentially adverse response to renal ablation. Am J Physiol. 1981;241(1):F85–93. [DOI] [PubMed] [Google Scholar]
- 9.Fortrie G, de Geus HRH, Betjes MGH. The aftermath of acute kidney injury: a narrative review of long-term mortality and renal function. Crit Care. 2019;23(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Group KDIGOKAKIW. KDIGO clinical practice guideline for acute kidney injury Kidney Int Suppl. 2012;2:1–138. [Google Scholar]
- 11.Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G. Classifying AKI by Urine Output versus Serum Creatinine Level. J Am Soc Nephrol. 2015;26(9):2231–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care. 2006;10(3):R73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zavada J, Hoste E, Cartin-Ceba R, et al. A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol Dial Transplant. 2010;25(12):3911–3918. [DOI] [PubMed] [Google Scholar]
- 14.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P, Acute Dialysis Quality Initiative w. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care. 2004;8(4):R204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017;13(4):241–257. [DOI] [PubMed] [Google Scholar]
- 16.Thongprayoon C, Cheungpasitporn W, Shah IK, et al. Long-term Outcomes and Prognostic Factors for Patients Requiring Renal Replacement Therapy After Cardiac Surgery. Mayo Clin Proc. 2015;90(7):857–864. [DOI] [PubMed] [Google Scholar]
- 17.Chew ST, Ng RR, Liu W, Chow KY, Ti LK. Acute kidney injury increases the risk of end-stage renal disease after cardiac surgery in an Asian population: a prospective cohort study. BMC Nephrol. 2017;18(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loef BG, Epema AH, Smilde TD, et al. Immediate postoperative renal function deterioration in cardiac surgical patients predicts in-hospital mortality and long-term survival. J Am Soc Nephrol. 2005;16(1):195–200. [DOI] [PubMed] [Google Scholar]
- 19.Bihorac A, Yavas S, Subbiah S, et al. Long-term risk of mortality and acute kidney injury during hospitalization after major surgery. Ann Surg. 2009;249(5):851–858. [DOI] [PubMed] [Google Scholar]
- 20.Hobson CE, Yavas S, Segal MS, et al. Acute kidney injury is associated with increased long-term mortality after cardiothoracic surgery. Circulation. 2009;119(18):2444–2453. [DOI] [PubMed] [Google Scholar]
- 21.Swaminathan M, Hudson CC, Phillips-Bute BG, et al. Impact of early renal recovery on survival after cardiac surgery-associated acute kidney injury. Ann Thorac Surg. 2010;89(4):1098–1104. [DOI] [PubMed] [Google Scholar]
- 22.Corredor C, Thomson R, Al-Subaie N. Long-Term Consequences of Acute Kidney Injury After Cardiac Surgery: A Systematic Review and Meta-Analysis. J Cardiothorac Vasc Anesth. 2016;30(1):69–75. [DOI] [PubMed] [Google Scholar]
- 23.Wu B, Ma L, Shao Y, et al. Effect of Cardiac Surgery-Associated Acute Kidney Injury on Long-Term Outcomes of Chinese Patients: A Historical Cohort Study. Blood Purif. 2017;44(3):227–233. [DOI] [PubMed] [Google Scholar]
- 24.Ishani A, Nelson D, Clothier B, et al. The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med. 2011;171(3):226–233. [DOI] [PubMed] [Google Scholar]
- 25.Ryden L, Sartipy U, Evans M, Holzmann MJ. Acute kidney injury after coronary artery bypass grafting and long-term risk of end-stage renal disease. Circulation. 2014;130(23):2005–2011. [DOI] [PubMed] [Google Scholar]
- 26.Jones J, Holmen J, De Graauw J, Jovanovich A, Thornton S, Chonchol M. Association of complete recovery from acute kidney injury with incident CKD stage 3 and all-cause mortality. Am J Kidney Dis. 2012;60(3):402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu JR, Zhu JM, Jiang J, et al. Risk Factors for Long-Term Mortality and Progressive Chronic Kidney Disease Associated With Acute Kidney Injury After Cardiac Surgery. Medicine. 2015;94(45):e2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu J, Xu X, Shen B, et al. Evaluation of five different renal recovery definitions for estimation of long-term outcomes of cardiac surgery associated acute kidney injury. BMC Nephrol. 2019;20(1):427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371(1):58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garg AX, Devereaux PJ, Yusuf S, et al. Kidney function after off-pump or on-pump coronary artery bypass graft surgery: a randomized clinical trial. JAMA. 2014;311(21):2191–2198. [DOI] [PubMed] [Google Scholar]
- 31.Palomba H, Castro I, Yu L, Burdmann EA. The duration of acute kidney injury after cardiac surgery increases the risk of long-term chronic kidney disease. Journal of nephrology. 2017;30(4):567–572. [DOI] [PubMed] [Google Scholar]
- 32.Priyanka P, Zarbock A, Izawa J, Gleason TG, Renfurm RW, Kellum JA. The impact of acute kidney injury by serum creatinine or urine output criteria on major adverse kidney events in cardiac surgery patients. J Thorac Cardiovasc Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 33.Wen X, Li S, Frank A, et al. Time-dependent effects of histone deacetylase inhibition in sepsis-associated acute kidney injury. Intensive Care Med Exp. 2020;8(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonventre JV, Basile D, Liu KD, et al. AKI: a path forward. Clin J Am Soc Nephrol. 2013;8(9):1606–1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16(5):535–543, 531p following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basile DP, Donohoe D, Roethe K, Osborn JL. Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol. 2001;281(5):F887–899. [DOI] [PubMed] [Google Scholar]
- 37.Basile DP, Leonard EC, Beal AG, Schleuter D, Friedrich J. Persistent oxidative stress following renal ischemia-reperfusion injury increases ANG II hemodynamic and fibrotic activity. Am J Physiol Renal Physiol. 2012;302(11):F1494–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Husain-Syed F, Ferrari F, Sharma A, et al. Persistent decrease of renal functional reserve in patients after cardiac surgery-associated acute kidney injury despite clinical recovery. Nephrol Dial Transplant. 2019;34(2):308–317. [DOI] [PubMed] [Google Scholar]
- 39.Kellum JA, Ronco C, Bellomo R. Conceptual advances and evolving terminology in acute kidney disease. Nat Rev Nephrol. 2021;17(7):493–502. [DOI] [PubMed] [Google Scholar]
- 40.Ostermann M, Bellomo R, Burdmann EA, et al. Controversies in acute kidney injury: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int. 2020;98(2):294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silver SA, Goldstein SL, Harel Z, et al. Ambulatory care after acute kidney injury: an opportunity to improve patient outcomes. Can J Kidney Health Dis. 2015;2:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 1S. Recovery Status at Hospital Discharge by Maximum AKI Stage in Patients with suspected sepsis
Table 2S. Life Expectancy of AKI Survivors Compared to Patients without AKI


