Abstract
Suberoylanilide hydroxamic acid (SAHA) is a histone deacetylase (HDAC) inhibitor with anticancer effects via epigenetic and non-epigenetic mechanisms. The role of SAHA in metabolic rewiring and epigenomic reprogramming to inhibit pro-tumorigenic cascades in lung cancer remains unknown. In this study, we aimed to investigate the regulation of mitochondrial metabolism, DNA methylome reprogramming, and transcriptomic gene expression by SAHA in lipopolysaccharide (LPS)-induced inflammatory model of lung epithelial BEAS-2B cells. Liquid chromatography-mass spectrometry was used for metabolomic analysis, while next-generation sequencing was done to study epigenetic changes. The metabolomic study reveals that SAHA treatment significantly regulated methionine, glutathione, and nicotinamide metabolism with alteration of the metabolite levels of methionine, S-adenosylmethionine, S-adenosylhomocysteine, glutathione, nicotinamide, 1-methylnicotinamide, and nicotinamide adenine dinucleotide in BEAS-2B cells. Epigenomic CpG methyl-seq shows SAHA revoked a list of differentially methylated regions in the promoter region of the genes, such as HDAC11, miR4509–1, and miR3191. Transcriptomic RNA-seq reveals SAHA abrogated LPS-induced differentially expressed genes encoding proinflammatory cytokines, including interleukin 1α (IL-1α), IL-1β, IL-2, IL-6, IL-24, and IL-32. Integrative analysis of DNA methylome-RNA transcriptome displays a list of genes, of which CpG methylation correlated with changes in gene expression. qPCR validation of transcriptomic RNA-seq data shows that SAHA treatment significantly reduced the LPS-induced mRNA levels of IL-1β, IL-6, DNMT1, and DNMT3A in BEAS-2B cells. Altogether, SAHA treatment alters the mitochondrial metabolism, epigenetic CpG methylation, and transcriptomic gene expression to inhibit LPS-induced inflammatory responses in lung epithelial cells, which may provide novel molecular targets to inhibit the inflammation component of lung carcinogenesis.
Keywords: HDAC inhibitor, SAHA, Mitochondrial metabolism, DNA methylation, Gene expression, Lung inflammation
Introduction
Histone deacetylases (HDACs) play an essential role in regulating epigenetic reprogramming by modulating the histone deacetylation process and are involved in the development and progression of cancer (1). Aberrant expression of HDACs has been found in diverse cancer types, including hematological malignancies, lung, breast, colorectal, prostate, ovarian, and gastric cancers (2; 3). Eighteen HDACs have been recognized in humans and categorized as zinc/iron-dependent and -independent deacetylases. Class I (HDACs 1, 2, 3, and 8), class II (HDACs 4, 5, 7, and 9), class IIB (HDACs 6 and 10), and class IV (HDAC11) are zinc/iron-dependent, while class III HDACs (sirtuins 1–7) are zinc/iron-independent that have an absolute requirement for nicotinamide adenine dinucleotide (NAD+) (4). Growing evidence reported that expression of HDACs, particularly HDAC 1, 7, 10, and 11, is associated with the progression and poor prognosis of lung cancer (5–8). Since HDACs contribute to cancer development, targeting HDACs would be logical to inhibit cancer development and progression. To date, four HDAC inhibitors (HDACi), vorinostat (suberanilohydroxamic acid; SAHA will be used hereafter), romidepsin, panobinostat, and belinostat have been approved by the Food and Drug Administration (FDA) to treat cutaneous T cell lymphoma.
Among all cancer types (solid and hematologic), lung cancer is the most common cause of cancer-related death worldwide. The 5-years survival rate of metastatic non-small cell lung cancer is around 7%. Chronic inflammation induced by cigarette smoking, viral and bacterial infection acts as the risk factor in mediating lung cancer initiation, progression, and metastasis (9; 10). Previous studies demonstrate that inflammation increases the number and incidence of lung tumors in carcinogen-driven lung cancer (11; 12). Hence blocking inflammation may reduce the risk/incidence of lung cancer. Lipopolysaccharide (LPS), a component of the outer membrane of gram-negative bacteria, is commonly used as a tumor-promoting agent in combination with the tumor-initiation agent in inflammation-driven lung cancer (11–13). Inflammation profoundly drives chromatin remodeling, including alteration of histone acetylation/deacetylation, alters mitochondrial metabolism, changes epigenetic reprogramming, and causes genetic mutation leading to lung cancer (14). DNA methylation is an essential epigenetic mechanism in inflammatory responses (15). The methylation profile on the cytosine-phosphate−guanine (CpG) regions is involved in inflammation-response-related genes (16). Moreover, numerous literature report that inflammation can alter the regulation of cellular/mitochondrial metabolism, such as the tricarboxylic acid (TCA) cycle, and alter the formation of Acetyl-Coenzyme A (AcCoA) from pyruvate under glycolytic metabolism (17; 18). TCA cycle generates a series of metabolites such as AcCoA, α-ketoglutarate, NAD+/NADH, and S-adenosyl methionine (SAM) which are tightly linked to the basic epigenetic machinery, such as DNA/histone modifications and chromatin remodeling to modulate phenotypic gene expression (19). Hence, mitochondrial metabolites and epigenetics are interconnected either directly or indirectly.
SAHA, one of the first synthetic HDACi, induces cellular differentiation and inhibits HDAC activity (20). SAHA inhibits a broad spectrum of HDACs, including HDAC1, which is overexpressed in human NSCLC (21). A previous study reported that HDAC inhibitors exhibit remarkable antitumor activity in lung cancer cell lines (22). SAHA demonstrated potential cancer interception effects in diverse cancers, including hepatocellular carcinoma and lung cancer (23; 24). However, the role of SAHA in regulating metabolic and epigenetic pathways to inhibit pro-tumorigenic cascades in lung cancer is unknown. In this study, we utilized multi-omic approaches, including next-generation sequencing and liquid chromatography-mass spectrometry (LC-MS) to integrate metabolomic, DNA methylomic, and RNA transcriptomic as a global untargeted approach in profiling the underlying metabolic and epigenetic regulation of SAHA in LPS-induced inflammatory lung model. To our knowledge, this is the first study to examine the regulation of cellular/mitochondrial metabolism, DNA methylome reprogramming, and transcriptomic gene expression by SAHA in an LPS-induced inflammatory lung epithelial cell model relevant to multi-event lung carcinogenesis.
Materials and Methods
Chemicals and reagents
SAHA was procured from Cayman Chemical Company (Ann Arbor, MI, USA). Lipopolysaccharides (LPS) was purchased from Sigma-Aldrich (St. Louis, MO, USA). CellTiter 96® Aqueous One Solution Cell Proliferation Assay System was obtained from Promega (Madison, WI, USA). GeneJET RNA Purification Kit was purchased from Thermo Fisher Scientific (Waltham, MA, USA).
Cell culture and treatment
The BEAS-2B, a normal human epithelial cell line, was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA, RRID: CVCL_0168) and cultured in Bronchial Epithelial Cell Growth Medium (BEGM, Lonza, Walkersville, MD, USA). A549 human lung epithelial carcinoma cells (RRID: CVCL_0023) were obtained from Dr. Tamara Minko’s lab (Rutgers University, NJ) and maintained in Dulbecco’s Modified Eagle Medium (DMEM, Gibco) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. Cell line studies were conducted within 10 passages after thawing. BEAS-2B cell line was authenticated by ATCC using STR profiling. A549 cells were not authenticated. Cells were not tested for Mycoplasma contamination, but the morphology and growth of the cells were monitored regularly. The goal of this study is to investigate the chemopreventive effect of SAHA to reduce the inflammation component of lung cancer. Therefore, we utilized a normal human lung epithelial cell model with LPS treatment to induce inflammation and profiled the impact of 3 or 24 h pre-treatment with a low (0.1 μM; non-antiproliferative) and high (20 μM; antiproliferative) concentration of SAHA on the LPS-induced metabolome (24 h LPS exposure) for “acute” effects of SAHA. We then examined the “chronic” effects (7 days exposure to SAHA at a concentration of 50 nM; non-antiproliferative) on the DNA methylation (methylome) and transcriptome changes (upon 1-day exposure to LPS). The treatment regimen of SAHA and LPS is shown in Fig. 1A and 1B.
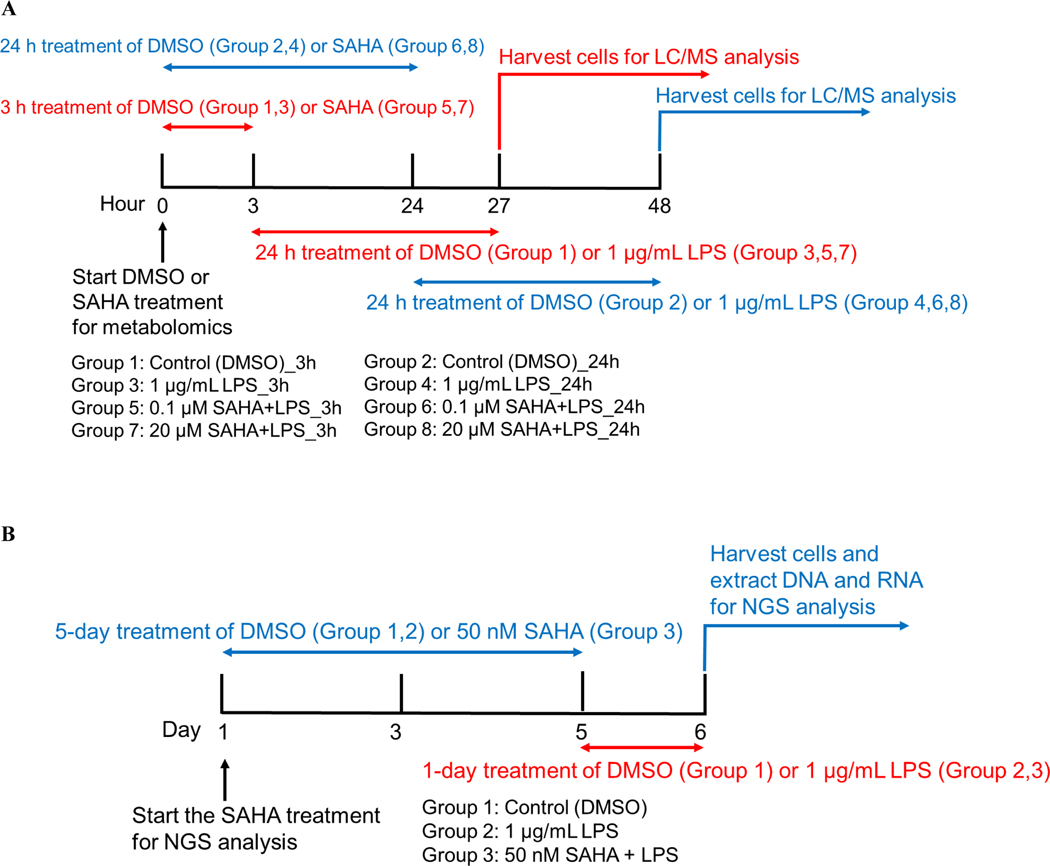
Figure 1. The treatment regimen of suberoylanilide hydroxamic acid (SAHA) and lipopolysaccharide (LPS) for metabolomics and next-generation sequencing (NGS) analysis.
(A) The treatment regimen for metabolomic analysis by LC-MS; the red lines indicated the group of 3 h treatment of SAHA followed by 24 h treatment of LPS. The blue line means the group of 24 h treatment of SAHA followed by 24 h treatment of LPS. After the treatment of LPS, all two groups underwent the harvesting of cells and LC-MS analysis. (B) Treatment regimen for NGS analysis; the blue line means the group of 5-day treatment of SAHA followed by 1-day treatment of LPS. After LPS treatment, all two groups underwent the harvest of cells and the analysis of NGS.
Cell viability assay
MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium] assay was used to detect the cell viability following the method of our previously published paper (25). After seeding 5×103 BEAS-2B cells in a 96-well plate overnight, the cells were treated with different concentrations of SAHA and 0.1% dimethyl sulfoxide (DMSO) as the vehicle control for 24 h, followed by using the cell proliferation assay kit from Promega. For the inflammation study, the cells were treated with 1 μg/mL LPS for another 24 h after DMSO or SAHA treatment.
Metabolite extraction and LC-MS analysis
LC-MS metabolic profiling was performed at Metabolomic Shared Resources of Rutgers Cancer Institute of New Jersey using the method of our recently published papers (26; 27). The event of metabolomic changes after drug treatment is rapid; hence we used 3 and 24 h treatments to investigate the acute effect of SAHA at low and high concentrations, followed by 24 h LPS treatment for metabolomic analysis based on the published papers (28; 29). In brief, 1.0×106 BEAS-2B cells were seeded in a 100 mm × 15 mm cell culture dish (Celltreat Scientific Products) overnight, followed by treatment with 1 μg/mL LPS vs. 1 μg/mL LPS + 0.1 or 20 μM SAHA for 3 and 24 h. LPS was added after 3 or 24 h treatment with 0.1% DMSO and SAHA (Fig. 1A). Total 24 metabolite samples were extracted from 8 groups consisting of control, LPS, LPS + 0.1 or 20 μM SAHA at 3 and 24 h time points (n = 3 per group) and were subjected to metabolomic analysis. The metabolites were extracted using 1 mL ice-cold lysis buffer consisting of methanol:acetonitrile:water (40:40:20) with 0.5% formic acid, followed by incubation on ice for 5 min. Then 50 μL 15% NH4HCO3 was added to neutralize the acetic acid. The cell suspension was collected into an Eppendorf tube by scraping and centrifuged at 15000 × g for 10 min at 4 °C. The clear supernatant solution was collected into new tubes and stored at −80 °C until further analysis by LC-MS. MAVEN software was used to obtain the metabolite data, while statistical and pathway analysis was performed by the web-based tool MetaboAnalyst (V5.0, RRID:SCR_015539) (https://www.metaboanalyst.ca/).
13C Isotope labeling for metabolomic analysis
To validate the metabolic activity of SAHA, we used a methionine-free DMEM medium supplemented with 2 mM 13C-methionine as described previously (26). Here, 1.0×106 BEAS-2B cells were seeded in a 100 mm × 15 mm cell culture dish overnight, followed by treatment with 0.1% DMSO and 0.1 or 20 μM SAHA for 3 and 24 h. Then, metabolites were extracted and analyzed by LC-MS using a similar protocol described for metabolomic profiling. Total 18 metabolite samples were extracted from 6 groups consisting of control, 0.1 or 20 μM SAHA at 3 and 24 h time points (n = 3 per group) and were subjected to metabolomic analysis.
Isolation of nucleic acids and next-generation sequencing (NGS)
BEAS-2B cells were seeded in 10-cm cell culture dishes at a density of 2 × 105 cells/dish overnight and then treated with 0.1% DMSO, 1 μg/mL LPS, or 1 μg/mL LPS + 50 nM SAHA for five days. Our study mainly focuses on prevention strategy rather than treatment, and five days treatment plan with a lower concentration is ideal in the drug-mediated epigenetic study (30; 31). Therefore, we chose a five-day treatment plan with a low concentration of SAHA (50 nM), followed by LPS treatment for 24 h (Fig. 1B). The cell culture medium containing the compound was changed every two days, and cells were harvested at day 5. Then, we extracted the DNA and RNA samples using AllPrep DNA/RNA Mini Kit (Qiagen, Valencia, CA, USA). DNA and RNA concentrations were measured by utilizing the NanoDrop spectrophotometer. 3 DNA samples (n = 1) and 3 RNA samples (n = 1) were sequenced and analyzed. Each DNA and RNA sample was pooled from three replicates. Our previous published paper reported that pooled DNA and RNA isolated from three plates of cells and combined into one sample for DNA-seq and RNA-seq analysis do not differ from individual replicates (31; 32). 3 μg RNA and 3 μg DNA of each sample was used to perform RNA-seq and SureSelect Methyl-seq, respectively. The library preparation and sequencing were accomplished by GENEWIZ, NJ, USA. In brief, the Illumina TruSeq RNA preparation kit (Illumina, San Diego, CA, USA) was used to build the RNA library sequenced on an Illumina NextSeq 500 instrument with 75 bp single-end reads, giving 30–40 million reads/sample. On the other hand, Agilent Mouse SureSelect Methyl-seq Target Enrichment System (Agilent Technologies Inc., Santa Clara, CA, USA) was used to further process the DNA samples. Next, we used EZ DNA Methylation-Gold Kit (Zymo Research, USA) to perform the bisulfite conversion. Similarly, DNA sequencing was performed on the Illumina NextSeq 500 instrument with 76-bp single-end reads, producing 30–40 million reads/sample. The method of RNA-seq and DNA methyl-seq analysis is described in our previously published articles (26; 33).
Bioinformatics analyses
RNA-seq and computational analysis
The BEAS-2B cells were treated with LPS or LPS + SAHA for five days, and total RNA was isolated from cells using the Allprep DNA/RNA Mini Kit. The library preparation and sequencing were accomplished by GENEWIZ, NJ, USA. We used Cutadapt to eliminate the Illumina universal adapter sequence from the sequencing reads. Cutadapt is a command-line program produced by second-generation sequencers. To align the RNA-seq reads to the genome and remove PCR duplicates, we utilized a splice aligner tool, HISAT2 (hierarchical indexing for spliced alignment of transcripts 2). Next, the featureCounts software program (version 1.5.1) was used to count genomic features with overlapping reads. Data were then scrutinized for differential expression with differentially expressed genes (DEG)-seq (version 1.36.0) in the R package.
DNA-seq and computational analysis
The BEAS-2B cells were treated with LPS or LPS + SAHA for five days, and total DNA was isolated from cells using the Allprep DNA/RNA Mini Kit. Bismark (version 0.15.0) program was used to align bisulfite converted sequence reads and to find cytosine methylation status. At the same time, the computational tool DMRfinder was applied to identify differentially methylated regions (DMRs) from Methyl-seq data. A cut-off value with ≥ 10% methylation differences and P < 0.05 were considered significant. ChIPseeker (version 1.10.3) in R (version 3.4.0) was utilized to perform genomic annotation.
Ingenuity pathway analysis (IPA)
The DEGs with false discovery rates (FDR) adjusted P value (q value) < 0.01 and log2 fold changes ≥ 2 or ≤ −2 were analyzed by IPA 4.0, Ingenuity Systems (www.Ingenuity.com, RRID:SCR_008653) to identify the biological functions, networks, and pathways mediated by LPS +/− SAHA.
Correlation between DNA methylation and gene expression
The genes were sorted from RNA-seq data based on their expression level with a cut-off threshold log2-fold change of gene expression difference ≥ 1 or ≤ −1 to establish the relationship between DNA methylation and gene expression (26; 27). The sorted genes were further organized with a cut-off DNA methylation difference ≥ 10%. Next, we identified the genes of interest in the promoter DNA hypermethylation/RNA downregulation or promoter DNA hypomethylation/RNA upregulation. RStudio software was used to plot the data.
mRNA extraction and qPCR analysis
BEAS-2B cells were seeded in a 6-well plate at a density of 3 × 105 cells per well overnight and then pre-treated with 0.1 and 20 μM of SAHA for 24 h, followed by 1 μg/mL of LPS treatment for 24 h. mRNA was extracted using the mRNA extraction kit from Thermo Fisher Scientific. Then, 0.5 μg of mRNA sample was used to synthesize cDNA by adding SuperScript III First-Strand cDNA Synthesis System purchased from Invitrogen, NY, USA. The cDNA was used as the template for real-time PCR using the SYBR Green PCR master mix (Applied Biosystem, CA, USA) to measure the RNA expression of various genes. The mRNA expression was calculated as the fold change with normalization to the expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) using the 2−ΔΔCT method, while GAPDH was used as an internal loading control. Primers were designed using Primer-Blast software and purchased from Integrated DNA Technologies (IDT, Coralville, Iowa, USA). Primer sequences are listed in Supplementary Table S1. Three independent experiments confirmed the level of mRNA expression.
Statistical analysis
Unless otherwise stated, the results are presented as the mean ± SD (n = 3). P value ≤ 0.05 was considered statistically significant. The false discovery rate (FDR) for differential expression analysis was controlled using Benjamini-Hochberg (BH) method. We used a cut-off of BH adjust p-value (padj) < 0.01 for RNA-seq analysis. For Methyl-seq analysis, methylation differences ≥ 10% and P < 0.05 were considered statistically significant. GraphPad Prism software (GraphPad Software, San Diego, CA, USA, RRID:SCR_002798) was used to conduct the statistical analysis using one-way ANOVA followed by Tukey’s multiple comparison test among various groups and two-way ANOVA followed by Tukey’s multiple comparison test among different groups and time points.
Data availability
The data supporting this study’s findings are available in the methods, results, and/or supplementary material of this article.
Results
SAHA regulates metabolic rewiring in LPS-treated BEAS-2B cells
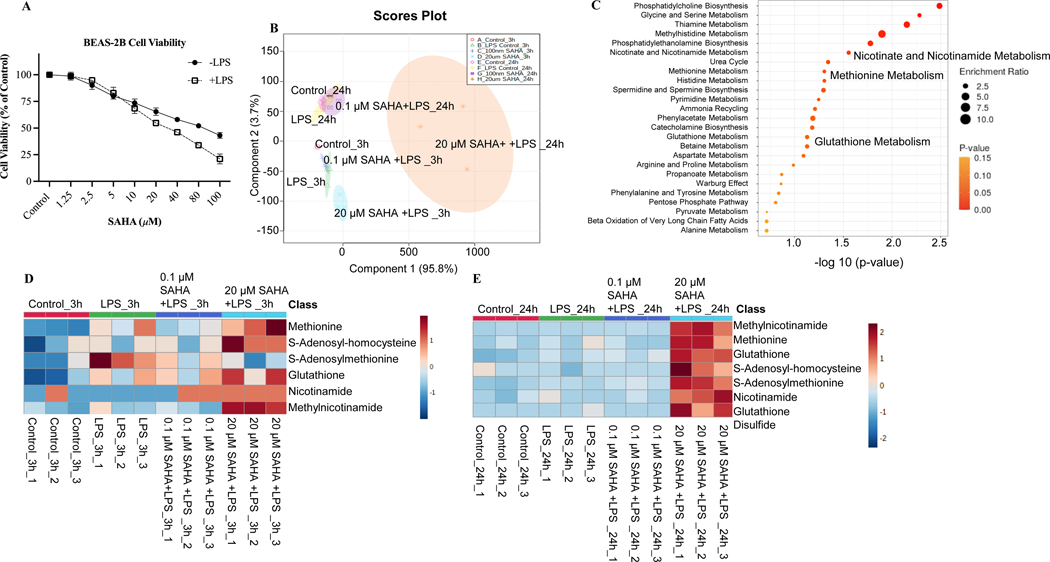
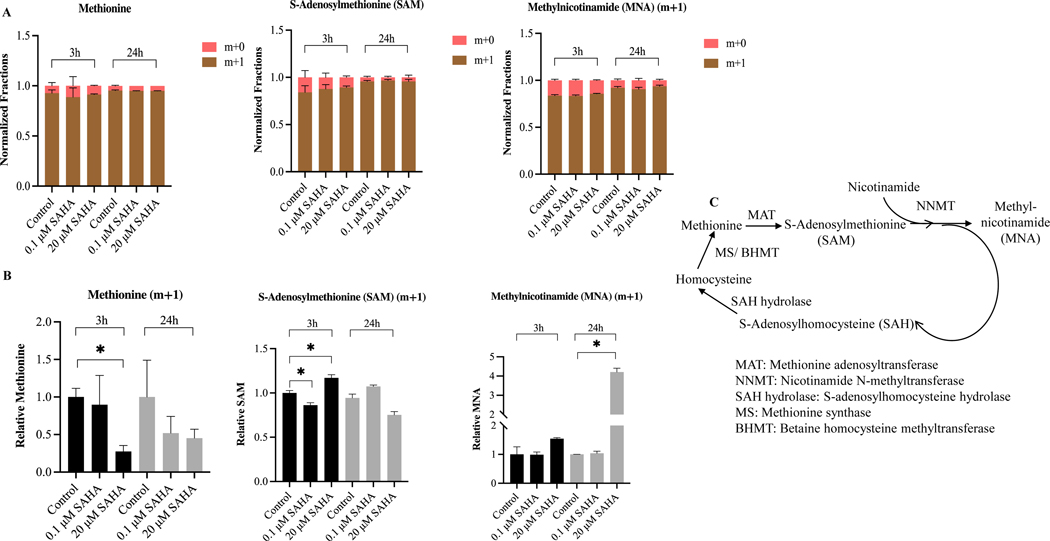
MTS assay that measures cell viability based on the metabolic activity of cells was used to determine the cytotoxic concentrations of SAHA in BEAS-2B cells. Cytotoxicity study showed that SAHA possesses moderate cytotoxicity against BEAS-2B cells with an IC50 69.42 ± 2.39 μM (Fig. 2A). To study the potential metabolic changes after SAHA treatment, BEAS-2B cells were treated with 0.1% DMSO or LPS +/− SAHA for 3 and 24 h. Principal component analysis (PCA) by Metaboanalyst showed a significant differential metabolic profile between LPS versus treatment groups (0.1 and 20 μM SAHA) at 3 and 24 h (Fig. 2B). A total of 163 metabolites were examined among the comparison groups (Supplementary Table S2). We next investigated the specific metabolic pathways by pathway analysis using Metaboanalyst. Treatment with SAHA in LPS-exposed BEAS-2B cells altered the metabolites primarily related to methionine, glutathione, and nicotinamide metabolism (Fig. 2C). The heatmap of the top 25 metabolites after SAHA treatment for 3 and 24 h is presented in Supplementary Fig. S1 A and B. Next, we measured the alteration level of each metabolite after SAHA treatment in BEAS-2B cells. SAHA treatment resulted in significant differential metabolic profiles between LPS and treatment groups (0.1 and 20 μM SAHA). In line with the enrich pathway analysis, the most significantly regulated metabolites were methionine, S-adenosylmethionine (SAM), S-adenosylhomocysteine (SAH), glutathione, nicotinamide adenine dinucleotide (NAD), nicotinamide, and methyl nicotinamide (MNA) after 3 and 24 h treatments (Fig. 2D and E). SAHA elevated the metabolite levels of methionine and SAH while decreasing the SAM level, leading to attenuation in SAM/SAH ratio. Furthermore, the metabolite levels of glutathione, nicotinamide, MNA, and NAD were also increased after SAHA treatment (Supplementary Fig. S2 A-C). In addition, 13C isotope-labeled metabolic data shows an efficient transfer of methyl group from methionine to MNA via SAM after SAHA treatment (Fig. 3A and B) which suggests the involvement of SAM-MNA pathway to provide methyl group to MNA. The pathway describing the transfer of methyl group from methionine to MNA is presented in Fig. 3C. As treatment with SAHA causes significantly altered mitochondrial metabolites in BEAS-2B cells, we hypothesized that regulation of the epigenetic machinery by the above altered TCA metabolites could contribute to the anticancer effect of SAHA by targeting the inflammation component of lung cancer.
Figure 2. Metabolic analysis of suberoylanilide hydroxamic acid (SAHA) treated on lipopolysaccharide (LPS)-challenged BEAS-2B cells.
(A) Cell viability of BEAS-2B cells treated with SAHA and challenged with 1 μg/mL LPS; (B) Principal component analysis (PCA) plot of metabolites after SAHA treatment for 3 and 24 h; (C) Significant enrich pathway analysis; (D) Heatmap of metabolites associated with nicotinate and nicotinamide pathway, methionine metabolism, and glutathione pathway after 3 h treatment; (E) Heatmap of metabolites associated with nicotinate and nicotinamide pathway, methionine metabolism, and glutathione pathway after 24 h treatment. All experiments were conducted in triplicate (n = 3).
Figure 3. The treatment of suberoylanilide hydroxamic acid (SAHA) promotes the transfer of the methyl group from methionine to methyl nicotinamide (MNA) via S-Adenosylmethionine (SAM).
(A) The stacked bar showing the transfer of methyl group from methionine to MNA via SAM. M+1: one 13C carbon is attached; m+0: no 13C is labeled; (B) Relative abundance of intracellular methionine, SAM, and MNA after SAHA treatment for 3 and 24 h; (C) The pathway relating to transferring the methyl group from methionine to methylnicotinamide. *P < 0.05 indicates statistically significant. All experiments were conducted in triplicate (n = 3).
SAHA regulates DNA methylomic reprogramming in LPS-treated BEAS-2B cells
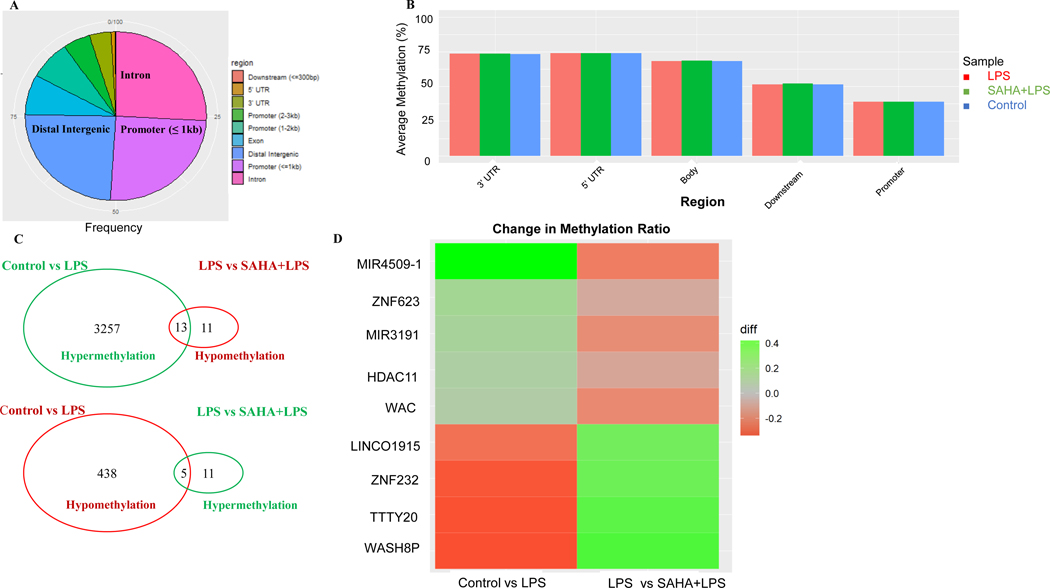
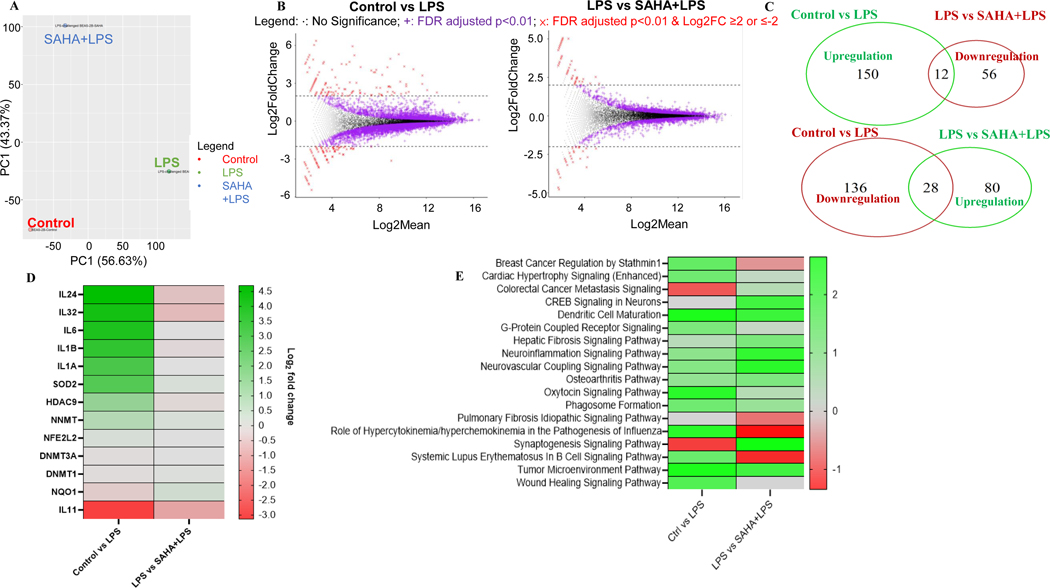
Next, we performed differentially methylated regions (DMRs) profiling from DNA methyl-seq to represent the epigenomic modulations by SAHA in BEAS-2B cells. A cut-off threshold DNA methylation differences ≥ 10% were used to select a subgroup of DMRs (27). The analysis of the annotation region showed that about 75% of DMRs are equally distributed in the distal intergenic, promoter, and intron regions (Fig. 4A and Supplementary Table S3). We then compared the average DNA methylation levels of DMRs based on gene regions for samples in the control, LPS, and LPS + SAHA groups. However, no significant differences in overall methylation levels were observed among the control and treatment groups, but CpG methylation was much lower in the promoters than in other regions for all three groups (Fig. 4B). By examining the CpG methylation alterations, a total 3713 DMRs were identified in control vs. LPS group, of which 3270 DMRs were hypermethylated and 443 DMRs were hypomethylated (Fig. 4C). Among these DMRs, 40 were regulated in the LPS + SAHA group, with 24 hypomethylated and 16 hypermethylated. Comparing both groups together, 18 DMRs were hypo- or hyper-methylated by LPS and reversed by co-treatment with SAHA. The heatmap of the top 9 regulated genes indicates that the most significant modulated genes after SAHA treatment are HDAC11, ZNF-232, TTTY20, miR4509–1, miR3191, and WASH8P (Fig. 4D).
Figure 4. CpG methylation ratio changes regulated by suberoylanilide hydroxamic acid (SAHA) and lipopolysaccharide (LPS) treatment.
(A) Distribution of differentially methylated regions (DMRs) annotated by region; (B) Average methylation ratio of DMRs in diverse gene regions and treatment groups; (C) Venn diagrams showing the numbers of overlapping DMRs between LPS and SAHA+LPS treatment groups; (D) The Heatmap of the significant change in methylation ratio of genes. DNA samples in all groups (n = 1) were pooled from three replicates.
SAHA regulates RNA transcriptome in LPS-treated BEAS-2B cells
To investigate the transcriptomic modulations of SAHA in BEAS-2B cells, we conducted differentially expressed genes (DEGs) analysis. For this purpose, PCA and Euclidean distance clustering were performed to identify the difference in RNA-seq data between control and LPS +/− SAHA groups. As indicated in Fig. 5A, the features of gene expression were clearly separated between treatment and control groups. We further analyzed the global gene expression using a cut-off p-value < 0.01 coupled to log2 fold change ≥ 2.0 or ≤ −2 (26; 27). In control vs. LPS, 15,674 genes were analyzed for significant differential expression. Among these genes, 6974 genes exhibited FDR-adjusted p-value < 0.01. In LPS vs. LPS+SAHA, 15,660 genes were tested for significant differential expression, of which 3079 genes were reported with FDR-adjusted p-values < 0.01 (Supplementary Table S4). A total of 326 genes were differentially expressed, with 162 up- and 164 down-regulated in the LPS-treated group compared to the control. On the other hand, 176 genes were modulated (108 up- and 68 down-regulated) in the LPS+SAHA group in comparison to the control group (Fig. 5B and C). In both comparisons, SAHA treatment reverses the expression of 40 genes with 28 up- and 12 down-regulated (Fig. 5C). We further generated a heatmap presenting the top 31 genes that were most up- and down-regulated based on FDR-adjusted p-value < 0.01 to account for the transcriptional profiles of SAHA-treated BEAS-2B cells (Fig. 5D). Of the most downregulated genes, 5 genes were proinflammatory cytokines such as IL-1α, IL-1β, IL-6, IL-24, and IL-32. IPA analysis shows that several signaling pathways, such as tumor microenvironment, wound healing, breast cancer regulation by stathmin1, and colorectal cancer metastasis signaling, were related to the anticancer effect of SAHA (Fig. 5E).
Figure 5. Transcriptomic changes by suberoylanilide hydroxamic acid (SAHA) in lipopolysaccharide (LPS)-challenged BEAS-2B cells.
(A) PCA plot transcriptomic profiles and Dendrogram of the gene expression profiles clustered by Euclidean distance; (B) MA plots showing DEGs in response to LPS vs. Control group and LPS+SAHA vs. LPS group with a cut-off of p<0.01 and log2(fold change) ≥ 2 or ≤ −2; (C) Venn diagrams comparing the upregulated and downregulated genes in LPS vs. Control group and LPS+SAHA vs. LPS group; (D) Circos plot of top 11 DEGs that appeared in the completely opposite direction in LPS vs. Control group and LPS+SAHA vs. LPS group; (E) Signaling pathway analysis of SAHA by IPA. RNA samples in all groups (n = 1) were pooled from three replicates.
Correlation between DNA methylation and gene expression
To establish the underlying association between DNA methylation and transcriptomic gene expression, we performed a correlation analysis between methyl-seq and RNA-seq data. A cut-off threshold methylation difference ≥ 10% coupled to a log2-fold change of gene expression difference ≥ 1 or ≤ −1 was applied to the selection. The association between RNA-seq gene expression and DNA-seq CpG methylation is presented in the Starbust plot (Supplementary Fig. S3 A and B). Several genes showed an inverse relationship between RNA expression and DMRs in the SAHA-treated group.
Validation of RNA-seq data with qPCR
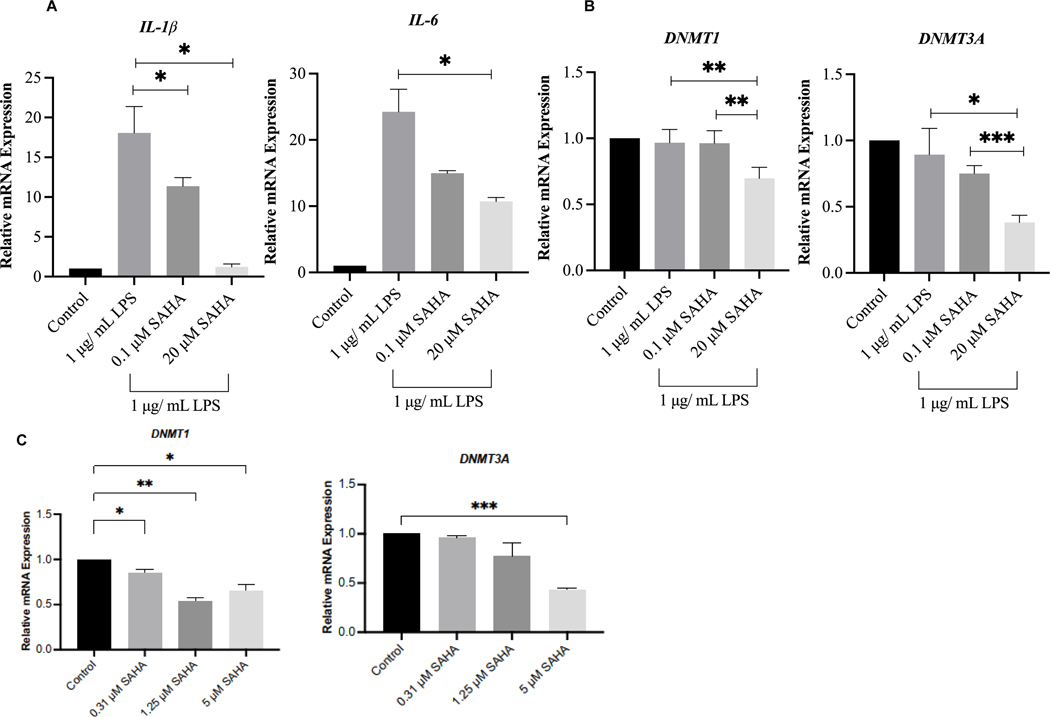
To validate the transcriptomic RNA-seq data obtained from NGS analysis, we performed qPCR analysis of four significantly expressed genes, namely IL-1β, IL-6, DNMT1, and DNMT3A, after SAHA treatment. The relative expression of these genes at the mRNA level is presented in Fig. 6A and B. Among these genes, SAHA treatment significantly reduced the LPS-induced mRNA levels of IL-1β, IL-6, DNMT1, and DNMT3A in BEAS-2B cells. The expression pattern of these genes from the qPCR analysis is consistent with the NGS RNA-seq data, thus validating the DEGs generated from RNA-seq results. We also validated the expression of DNMT1 and DNMT3A in A549 lung cancer cells and found that SAHA significantly inhibited mRNA levels of DNMT1 and DNMT3A up to 5 μM (Fig. 6C). The concentration of SAHA in A549 cells was selected from previously published paper (34).
Figure 6. qPCR analysis of mRNA expression after suberoylanilide hydroxamic acid (SAHA) treatment in lipopolysaccharide (LPS)-challenged BEAS-2B cells and in A549 cells.
(A) The expression level of IL-1 and IL-6 in BEAS-2B cells; (B) The expression level of DNMT1 and DNMT3A in BEAS-2B cells; C) The expression level of DNMT1 and DNMT3A in A549 cells. *P < 0.05, **P < 0.01, and ***P < 0.001 indicate statistically significant. All experiments were conducted in triplicate (n = 3).
Discussion
Growing evidence suggests that lung carcinogenesis is a multi-step process, which develops through progressive pathologic changes (also known as premalignant lesions) with corresponding genetic, epigenetic, and metabolic changes in the airway epithelium (35). Inflammation plays a pivotal role in lung carcinogenesis. Studies have shown that inflammation resulting from cigarette smoking, environmental tobacco smoke, chronic obstructive pulmonary disease, pathogenic pulmonary infections, and pulmonary fibrosis promotes widespread inflammatory and mutagenic effects in the lungs that promote pro-carcinogenic events (36). Thus, interception of the inflammation component of the multi-event lung carcinogenesis may reduce the public health burden and mortality of this fatal malignancy. HDAC inhibitors displayed remarkable antitumor potential in many human lung cancer cell line models (22). In the current study, for the first time, we examined the biological effects (metabolic rewiring, CpG methylomic reprogramming, and transcriptomic gene expression) of HDAC inhibitor SAHA in LPS-induced inflammatory human lung epithelial BEAS-2B cell model to mimic prevention of inflammation during early stage lung carcinogenesis.
Cellular metabolism and epigenetics play a critical role in tumorigenesis by supporting cell proliferation, metastasis, and pluripotency (37). Previous studies demonstrated that extensive metabolic rewiring occurs within the cells to obtain nutrients, such as amino acids, lipids, glucose, and nucleotides, to support cell proliferation and growth (26). To unravel the impact of SAHA on metabolic rewiring, we examined the changes in the metabolic profile in BEAS-2B cells. We identified the regulation of several metabolites from the SAHA treatment that are critical to the growth and survival of cancer cells. Pathway enrichment analysis of metabolomic data shows the top metabolic pathways involved were methionine, glutathione, and nicotinamide metabolism. One of the altered metabolites is methionine, which acts as a precursor of S-adenosyl methionine (SAM), the principal methyl donor required for all epigenetic methylation reactions, including CpG and histone methylation (19; 38). The addition of adenosine to the sulfur group of methionine causes the activation of methionine’s methyl group, which is converted to S-adenosylhomocysteine (SAH) after methyl group donation. SAH is then converted to homocysteine, cystathionine, cysteine, and finally to glutathione, a physiological antioxidant involved in the epigenetic regulation of redox signaling (39). SAM/SAH ratio is commonly known as the methylation index, whereby a decline in this ratio indicates decreased cellular methylation potential (40). In the current study, SAHA significantly increased the methionine and SAH levels and suppressed the production of SAM, leading to a reduced SAM/SAH ratio, indicating SAHA regulates methionine metabolism to modulate DNA methylation, hence potentially driving epigenetic reprogramming. Apart from methionine and its metabolic pathway, another key epigenetic metabolite is nicotinamide (NAM) which is methylated by the metabolic enzyme nicotinamide N-methyltransferase (NNMT) using the universal methyl donor SAM to generate SAH and 1-methyl nicotinamide (MNA) (41). Previous studies demonstrated the anti-inflammatory effect of NAM and MNA by inhibiting proinflammatory cytokines IL-1, IL-6, IL-8, and TNF-α (42). Moreover, NAM is required to synthesize NAD and NADP, which act as the co-factors in many redox reactions in cellular metabolism (43). Here, we found increased metabolite levels of NAM, MNA, and NAD after treatment with 20 μM SAHA, suggesting the nicotinamide metabolism pathway could be involved in the epigenetic regulation by SAHA. Moreover, our qPCR data indicate a significant reduction of IL-1β and IL-6 mRNA in the SAHA-treated BEAS-2B cells, indicating the SAHA-induced anti-inflammatory regulation for its anticancer effect. 13C metabolic labeling data further validated the ability of SAHA to regulate metabolic rewiring in transferring the methyl group from methionine to MNA via SAM. Methionine transfers a methyl group to MNA via SAM. SAM is one of the basic epigenetic co-factors for CpG and histone methylation, implicating the impact of SAHA on epigenetic reprogramming via CpG/histone methylation.
DNA methylation at 5′ carbon of cytosine nucleotides adjacent to guanines (CpG dinucleotides) is a common DNA modification process that plays an important role in the transcriptional regulation of normal physiological processes (44). Aberrant DNA methylation is closely connected to the event of oncogenesis, including lung adenocarcinoma (45). CpG methylation is mainly catalyzed by three DNA methyltransferases (DNMTs) family members found in higher eukaryotes, namely, DNMT1, DNMT3A, and DNMT3B, by transferring a methyl group from SAM to cytosine. DNMTs transfer methyl group to the C-5 position of cytosine residues. The CpG methylation patterns are tissue-specific, heritable, and associated with gene expression. Subsequently, CpG methylation plays a key role in differentiation and gene expression (46). Overexpression of DNMTs has been implicated in diverse cancers, including breast, prostate, colorectal, and lung cancers (47). We found a series of DMRs linked to SAHA treatment using SureSelect CpG Methyl-seq analysis. Our study further displayed that dysregulated DMRs-related genes are closely associated with regulating HDAC11, miR4509–1, and miR3191. There is a strong association between HDAC inhibition and the anticancer effect of SAHA (48). Herein, our DNA-seq data shows the inhibitory effect of SAHA on HDAC11, the latest but least studied HDAC, that reaffirms a previously published paper’s results (49). Previous studies demonstrated that miR4509–1 and miR3191 regulate cell proliferation, invasion, and migration in cancer (50; 51). Since SAHA treatment changed the microRNA (miR4509–1 and miR3191) methylation ratio in the LPS-induced inflammatory model of lung epithelial BEAS-2B cells, these two microRNA could be potential targets for the antitumor effect of SAHA in lung cancer. Moreover, in our LPS-induced inflammatory lung model, SAHA significantly downregulated the DNMT1 and DNMT3A mRNA, indicating regulation of CpG methylation via DNMTs (46).
Gene expression profiling by RNA sequencing (RNA-seq) offers a more comprehensive understanding of the phenotypic expression underlying the potential mechanisms of different diseases and drugs (52). Several studies have applied this approach to compare transcriptomes in various drug development research (33). A subset of differentially expressed genes, such as IL-1α, IL-1β, IL-6, IL-24, IL-32, NQO1, and HDAC9, was identified between the SAHA and control groups from the RNA-seq data. The regulation of IL-1β and IL-6 genes is consistent with the results obtained from qPCR data. Ingenuity pathway analysis (IPA) of RNA-seq data showed that the signaling pathways, such as breast cancer regulation by stathmin-1, tumor microenvironment, wound healing, and colorectal cancer metastasis signaling pathways were regulated after SAHA treatment. A previous study has reported that SAHA exerts anticancer effects via modulation of the tumor microenvironment and wound healing signaling pathways (53). Moreover, preclinical and clinical trials with SAHA or in combination with other drugs also reported the anticancer effect of SAHA in metastatic colorectal cancer (54). Next, an integrated correlation study between DNA-seq CpG methylation and RNA-seq identified a subgroup of genes associated with SAHA, as shown in Supplementary Fig. S3. Previous experimental sequencing data described the significance of integrating RNA-seq with DNA-seq to detect the expression of mutant alleles, to assess known and unknown gene fusions, and to distinguish splice variants (55).
Conclusion
In conclusion, using epigenomic and metabolomic approaches, our study indicates that SAHA rescues LPS-mediated changes in metabolic profile, DNA methylation, and gene expression, which may contribute to reducing the severity of the inflammation component of lung cancer. The metabolomic study reveals SAHA reversed LPS-mediated alterations in methionine, glutathione, and nicotinamide metabolism, while transcriptomic analysis shows SAHA blocked or attenuated proinflammatory cytokines and signaling pathways. The analysis of epigenetic CpG methylation identifies several differentially methylated regions in the promoter region of genes, including HDAC11 regulated by LPS, which is rescued by SAHA treatment. The current study’s findings are based on in vitro cell line model, which needs further validation in in vivo animal models and clinical trials.
Supplementary Material
Prevention Relevance:
Inflammation increases the risk of lung cancer and blocking inflammation could reduce the incidence of lung cancer. Herein we demonstrate that HDAC inhibitor suberoylanilide hydroxamic acid regulates metabolic rewiring and epigenetic reprogramming to attenuate lipopolysaccharide-driven inflammation in lung epithelial cells.
Acknowledgments
This study was supported in part by institutional funds and by the National Center for Complementary and Integrative Health (NCCIH) (R01 AT009152 to A.N. Kong), the National Cancer Institute (NCI) (R01 CA200129 to A.N. Kong and P30CA072720–5923 to X. Su), and the National Institute of Environmental Health Sciences (NIEHS) (P30 ES005022 to A.N. Kong). We are grateful to Davit Sargsyan for his help in DNA-seq and RNA-seq analysis. We thank all the members of Professor Ah-Ng Kong’s laboratory for their support and invaluable discussion during the preparation of this manuscript.
Abbreviations
- HDAC
histone deacetylase
- HDACi
HDAC inhibitor
- SAHA
suberoylanilide hydroxamic acid
- DMRs
differentially methylated regions
- DEGs
differentially expressed genes
- LPS
lipopolysaccharide; IL-6, interleukin-6
- DNMT1
DNA methyltransferase 1
- NNMT
nicotinamide N-methyltransferase
- TCA
tricarboxylic acid cycle
- SAM
S-adenosylmethionine
- SAH
S-adenosylhomocysteine
- NAM
nicotinamide
- MNA
methylnicotinamide
- NAD+
nicotinamide adenine dinucleotide
- PCA
principal component analysis
- LC-MS
liquid-chromatography–mass spectrometry
- NGS
next-generation sequencing
- FDA
Food and Drug Administration
- NSCLC
non-small cell lung cancer
- HISAT2
hierarchical indexing for spliced alignment of transcripts 2
Footnotes
Conflicts of Interest:
The authors declare no potential conflicts of interest
References
- 1.Gallinari P, Di Marco S, Jones P, Pallaoro M, Steinkuhler C. 2007. HDACs, histone deacetylation and gene transcription: from molecular biology to cancer therapeutics. Cell Research 17:195–211 [DOI] [PubMed] [Google Scholar]
- 2.Haberland M, Johnson A, Mokalled MH, Montgomery RL, Olson EN. 2009. Genetic dissection of histone deacetylase requirement in tumor cells. P Natl Acad Sci USA 106:7751–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weichert W. 2009. HDAC expression and clinical prognosis in human malignancies. Cancer Lett 280:168–76 [DOI] [PubMed] [Google Scholar]
- 4.Sauve AA, Wolberger C, Schramm VL, Boeke JD. 2006. The Biochemistry of Sirtuins. Annual Review of Biochemistry 75:435–65 [DOI] [PubMed] [Google Scholar]
- 5.Minamiya Y, Ono T, Saito H, Takahashi N, Ito M, et al. 2011. Expression of histone deacetylase 1 correlates with a poor prognosis in patients with adenocarcinoma of the lung. Lung Cancer 74:300–4 [DOI] [PubMed] [Google Scholar]
- 6.Li YX, Zhang XY, Zhu SQ, Dejene EA, Peng WQ, et al. 2020. HDAC10 Regulates Cancer Stem-Like Cell Properties in KRAS-Driven Lung Adenocarcinoma. Cancer Res 80:3265–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lei Y, Liu L, Zhang S, Guo S, Li X, et al. 2017. Hdac7 promotes lung tumorigenesis by inhibiting Stat3 activation. Mol Cancer 16:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bora-Singhal N, Mohankumar D, Saha B, Colin CM, Lee JY, et al. 2020. Novel HDAC11 inhibitors suppress lung adenocarcinoma stem cell self-renewal and overcome drug resistance by suppressing Sox2. Sci Rep 10:4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keohavong P, Kahkonen B, Kinchington E, Yin JL, Jin J, et al. 2010. Pulmonary inflammation and lung tumorigenesis in mice. Cancer Res 70 [Google Scholar]
- 10.Tan Z, Xue H, Sun Y, Zhang C, Song Y, Qi Y. 2021. The Role of Tumor Inflammatory Microenvironment in Lung Cancer. Front Pharmacol 12:688625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu CH, Chen Z, Chen K, Liao FT, Chung CE, et al. 2021. Lipopolysaccharide-Mediated Chronic Inflammation Promotes Tobacco Carcinogen-Induced Lung Cancer and Determines the Efficacy of Immunotherapy. Cancer Research 81:144–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Di ME, Kahkonen B, Liu CH, Di YP. 2021. Lung carcinomas induced by NNK and LPS. Method Cell Biol 163:175–85 [DOI] [PubMed] [Google Scholar]
- 13.Melkamu T, Qian X, Upadhyaya P, O’Sullivan MG, Kassie F. 2013. Lipopolysaccharide Enhances Mouse Lung Tumorigenesis: A Model for Inflammation-Driven Lung Cancer. Vet Pathol 50:895–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karin M, Shalapour S. 2022. Regulation of antitumor immunity by inflammation-induced epigenetic alterations. Cell Mol Immunol 19:59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji J, Xu YB, Zheng MZ, Luo CL, Lei HT, et al. 2019. Methionine Attenuates Lipopolysaccharide-Induced Inflammatory Responses via DNA Methylation in Macrophages. Acs Omega 4:2331–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perez-Novo CA, Bachert C. 2015. DNA methylation, bacteria and airway inflammation: latest insights. Curr Opin Allergy Cl 15:27–32 [DOI] [PubMed] [Google Scholar]
- 17.Palsson-McDermott EM, O’Neill LAJ. 2020. Targeting immunometabolism as an anti-inflammatory strategy. Cell Res 30:300–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kominsky DJ, Campbell EL, Colgan SP. 2010. Metabolic Shifts in Immunity and Inflammation. J Immunol 184:4062–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu R, Li S, Hudlikar R, Wang L, Shannar A, et al. 2022. Redox signaling, mitochondrial metabolism, epigenetics and redox active phytochemicals. Free Radic Biol Med 179:328–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Richon VM, Emiliani S, Verdin E, Webb Y, Breslow R, et al. 1998. A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. Proc Natl Acad Sci U S A 95:3003–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beckers T, Burkhardt C, Wieland H, Gimmnich P, Ciossek T, et al. 2007. Distinct pharmacological properties of second generation HDAC inhibitors with the benzamide or hydroxamate head group. International Journal of Cancer 121:1138–48 [DOI] [PubMed] [Google Scholar]
- 22.Petta V, Gkiozos I, Strimpakos A, Syrigos K. 2013. Histones and lung cancer: are the histone deacetylases a promising therapeutic target? Cancer Chemotherapy and Pharmacology 72:935–52 [DOI] [PubMed] [Google Scholar]
- 23.Hsieh YH, Su IJ, Yen CJ, Tsai TF, Tsai HW, et al. 2013. Histone deacetylase inhibitor suberoylanilide hydroxamic acid suppresses the pro-oncogenic effects induced by hepatitis B virus pre-S-2 mutant oncoprotein and represents a potential chemopreventive agent in high-risk chronic HBV patients. Carcinogenesis 34:475–85 [DOI] [PubMed] [Google Scholar]
- 24.Desai D, Das A, Cohen L, El-Bayoumy K, Amin S. 2003. Chemopreventive efficacy of suberoylanilide hydroxamic acid (SAHA) against 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced lung tumorigenesis in female A/J mice. Anticancer Res 23:499–503 [PubMed] [Google Scholar]
- 25.Yang J, Wu R, Li W, Gao L, Yang Y, et al. 2018. The triterpenoid corosolic acid blocks transformation and epigenetically reactivates Nrf2 in TRAMP-C1 prostate cells. Mol Carcinog 57:512–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li SY, Wu RY, Wang LJ, Kuo HCD, Sargsyan D, et al. 2022. Triterpenoid ursolic acid drives metabolic rewiring and epigenetic reprogramming in treatment/prevention of human prostate cancer. Molecular Carcinogenesis 61:111–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hudlikar RR, Sargsyan D, Cheng D, Kuo HD, Wu R, et al. 2022. Tobacco carcinogen 4-[methyl(nitroso)amino]-1-(3-pyridinyl)-1-butanone (NNK) drives metabolic rewiring and epigenetic reprograming in A/J mice lung cancer model and prevention with diallyl sulphide (DAS). Carcinogenesis 43:140–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han JJ, Wang SY, Yeung K, Yang DW, Gu W, et al. 2020. Proteome-wide effects of naphthalene-derived secondary organic aerosol in BEAS-2B cells are caused by short-lived unsaturated carbonyls. P Natl Acad Sci USA 117:25386–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song YY, Li RJ, Zhang YH, Wei JT, Chen W, et al. 2019. Mass spectrometry-based metabolomics reveals the mechanism of ambient fine particulate matter and its components on energy metabolic reprogramming in BEAS-2B cells. Sci Total Environ 651:3139–50 [DOI] [PubMed] [Google Scholar]
- 30.Hudlikar RR, Sargsyan D, Li WJ, Wu RY, Zheng MNZ, Kong AN. 2021. Epigenomic, Transcriptomic, and Protective Effect of Carotenoid Fucoxanthin in High Glucose-Induced Oxidative Stress in Mes13 Kidney Mesangial Cells. Chem Res Toxicol 34:713–22 [DOI] [PubMed] [Google Scholar]
- 31.Wang C, Wu RY, Sargsyan D, Zheng MNZ, Li SY, et al. 2019. CpG methyl-seq and RNA-seq epigenomic and transcriptomic studies on the preventive effects of Moringa isothiocyanate in mouse epidermal JB6 cells induced by the tumor promoter TPA. Journal of Nutritional Biochemistry 68:69–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang LJ, Shannar AAF, Wu RY, Chou PC, Sarwar MS, et al. 2022. Butyrate Drives Metabolic Rewiring and Epigenetic Reprogramming in Human Colon Cancer Cells. Molecular Nutrition & Food Research 66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu R, Li S, Sargsyan D, Yin R, Kuo HC, et al. 2021. DNA methylome, transcriptome, and prostate cancer prevention by phenethyl isothiocyanate in TRAMP mice. Mol Carcinog 60:391–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee KW, Kim JH, Park JH, Kim HP, Song SH, et al. 2006. Antitumor activity of SK-7041, a novel histone deacetylase inhibitor, in human lung and breast cancer cells. Anticancer Res 26:3429–38 [PubMed] [Google Scholar]
- 35.Kadara H, Scheet P, Wistuba II, Spira AE. 2016. Early Events in the Molecular Pathogenesis of Lung Cancer. Cancer Prev Res 9:518–27 [DOI] [PubMed] [Google Scholar]
- 36.O’Callaghan DS, O’Donnell D, O’Connell F, O’Byrne KJ. 2010. The Role of Inflammation in the Pathogenesis of Non-small Cell Lung Cancer. Journal of Thoracic Oncology 5:2024–36 [DOI] [PubMed] [Google Scholar]
- 37.Kaoutari AE, Fraunhoffer NA, Hoare O, Teyssedou C, Soubeyran P, et al. 2021. Metabolomic profiling of pancreatic adenocarcinoma reveals key features driving clinical outcome and drug resistance. EBioMedicine 66:103332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shyh-Chang N, Locasale JW, Lyssiotis CA, Zheng Y, Teo RY, et al. 2013. Influence of threonine metabolism on S-adenosylmethionine and histone methylation. Science 339:222–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garcia-Gimenez JL, Roma-Mateo C, Perez-Machado G, Peiro-Chova L, Pallardo FV. 2017. Role of glutathione in the regulation of epigenetic mechanisms in disease. Free Radic Biol Med 112:36–48 [DOI] [PubMed] [Google Scholar]
- 40.Caudill MA, Wang JC, Melnyk S, Pogribny IP, Jernigan S, et al. 2001. Intracellular S-adenosylhomocysteine concentrations predict global DNA hypomethylation in tissues of methyl-deficient cystathionine beta-synthase heterozygous mice. J Nutr 131:2811–8 [DOI] [PubMed] [Google Scholar]
- 41.Ulanovskaya OA, Zuhl AM, Cravatt BF. 2013. NNMT promotes epigenetic remodeling in cancer by creating a metabolic methylation sink. Nat Chem Biol 9:300–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Biedron R, Ciszek M, Tokarczyk M, Bobek M, Kurnyta M, et al. 2008. 1-Methylnicotinamide and nicotinamide: two related anti-inflammatory agents that differentially affect the functions of activated macrophages. Arch Immunol Ther Ex 56:127–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Frederick DW, Davis JG, Davila A Jr, Agarwal B, Michan S, et al. 2015. Increasing NAD synthesis in muscle via nicotinamide phosphoribosyltransferase is not sufficient to promote oxidative metabolism. J Biol Chem 290:1546–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uddin MS, Mamun AA, Alghamdi BS, Tewari D, Jeandet P, et al. 2022. Epigenetics of glioblastoma multiforme: From molecular mechanisms to therapeutic approaches. Semin Cancer Biol 83:100–20 [DOI] [PubMed] [Google Scholar]
- 45.Saghafinia S, Mina M, Riggi N, Hanahan D, Ciriello G. 2018. Pan-Cancer Landscape of Aberrant DNA Methylation across Human Tumors. Cell Rep 25:1066–80 e8 [DOI] [PubMed] [Google Scholar]
- 46.Gao L, Emperle M, Guo Y, Grimm SA, Ren W, et al. 2020. Comprehensive structure-function characterization of DNMT3B and DNMT3A reveals distinctive de novo DNA methylation mechanisms. Nat Commun 11:3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheng Y, He C, Wang M, Ma X, Mo F, et al. 2019. Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials. Signal Transduct Target Ther 4:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Munshi A, Tanaka T, Hobbs ML, Tucker SL, Richon VM, Meyn RE. 2006. Vorinostat, a histone deacetylase inhibitor, enhances the response of human tumor cells to ionizing radiation through prolongation of gamma-H2AX foci. Mol Cancer Ther 5:1967–74 [DOI] [PubMed] [Google Scholar]
- 49.Xu WS, Parmigiani RB, Marks PA. 2007. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene 26:5541–52 [DOI] [PubMed] [Google Scholar]
- 50.He HJ, Zhao XJ, Zhu ZQ, Du L, Chen EF, et al. 2019. MicroRNA-3191 promotes migration and invasion by downregulating TGFBR2 in colorectal cancer. J Biochem Mol Toxic 33 [DOI] [PubMed] [Google Scholar]
- 51.AL Zeyadi M, Dimova I, Ranchich V, Rukova B, Nesheva D, et al. 2015. Whole genome microarray analysis in non-small cell lung cancer. Biotechnol Biotec Eq 29:111–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Byron SA, Van Keuren-Jensen KR, Engelthaler DM, Carpten JD, Craig DW. 2016. Translating RNA sequencing into clinical diagnostics: opportunities and challenges. Nat Rev Genet 17:257–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolf IM, Fan Z, Rauh M, Seufert S, Hore N, et al. 2014. Histone deacetylases inhibition by SAHA/Vorinostat normalizes the glioma microenvironment via xCT equilibration. Sci Rep 4:6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arora SP, Tenner LL, Sarantopoulos J, Morris JL, Longoria L, et al. 2019. Modulation of autophagy: A phase II study of vorinostat (VOR) plus hydroxychloroquine (HCQ) vs regorafenib (RGF) in chemo-refractory metastatic colorectal cancer (mCRC). J Clin Oncol 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mody RJ, Wu YM, Lonigro RJ, Cao XH, Roychowdhury S, et al. 2015. Integrative Clinical Sequencing in the Management of Refractory or Relapsed Cancer in Youth. Jama-J Am Med Assoc 314:913–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this study’s findings are available in the methods, results, and/or supplementary material of this article.