Abstract
Brinjal and okra are being sprayed withdifferent formulations of imidacloprid which leads to significant deposition of residues on fruits. Here in this study, we validated a method that could detect the traces of imidacloprid residues in a short run time using LC-MS/MS. LOD of 0.001 and LOQ of 0.003 μg/g for brinjal and in compliance with the MRL (0.2 mg/kg). The recovery at different spiking levels recorded 89.67 to 115.55 with an RSDr range of 3.02 and 5.22%. For okra, 0.0025 and 0.008 μg/g were observed as LOD and LOQ, respectively. Recovery was obtained between 88.69 and 93.74%. Precision in terms of repeatability (RSDr) and reproducibility (RSDwR) was found satisfactory in both matrices. Two applications of imidacloprid 17.8% SL in both vegetables showed faster dissipation initially and persisted up to 15–25 days. Decontamination with 2% salt solution followed by boiling for 15min. removed 96.43 and 73.66% of imidacloprid residues from brinjal and okra, respectively. Risk assessment was found to be less than one (HI < 1) for brinjal and okra matrix and can be safely consumed.
Keywords: Imidacloprid, LC-MS/MS, Dissipation, Decontamination, Risk assessment
1. Introduction
Brinjal (Solanum melongena L.) is the most consumed vegetable in India. Its entire plant or parts are being used to prepare medicine for treating respiratory diseases in Ayurveda, Siddha, Homeopathy, and Unani [1]. It is being cultivated in 668.72 thousand ha with 12.40 mM T and 35.55 MT ha−1of production and productivity, respectively [2]. Similarly, okra (Abelmoschus esculentus (L.)) is also a familiar vegetable to Indian society, producing about 6.47 million tonnes (3.9%) in 5.28 mha of cultivated area (5.7%) with productivity of 11.63 MTha−1 during 2017–18 [2]. It is valued for its green fruits, rich in proteins, calcium, phosphorus, iron, carotene, and vitamins (A, B, C), and can heal genito-urinary disorders, spermatorrhoea and chronic dysentery problems [3]. About 27 and 72 insect pest species associated with brinjal and okra, from seedling to fruit set and up to maturity of fruits, respectively. Jassid, aphid, whitefly, and fruit and shoot borer are common insect pests infesting the brinjal, and 20–89% of losses are due to fruit and shoot borer in various parts of India, where as leafhopper, whitefly, and shoot and fruit borer collectively cause 36–90% of yield loss in okra [4]. A total of 42 and 30 pesticides/insecticides have been registered for use on brinjal and okra, respectively, in India [5]. For effective management of pest complex in these vegetables, imidacloprid has been recommended in India, either single or combined with synthetic pyrethroids [6].
Imidacloprid is a neonicotinoid insecticide. It is a synthetic nicotine analog and is classified as chloronicotinyl derivative (imidacloprid, thiacloprid, acetamiprid). It acts as an acetylcholine (Ach) binding site of the nicotine acetylcholine receptor as agonist and causes excitation in insects, leading to paralysis and death. It is most effective against insect pests infesting corn, cotton, potato, rice, vegetables, and fruits. This has been formulated and registered as 17.8% SL, 70% WG, 30.5% SC as a foliar spray and 70% WS and 48% FS as a seed treatment in India. Apart from its agriculturaluse, also used for managing the termite's attacking buildings and civil structures, gardens, and turf, controlling fleas on domestic animals and insects boring the trees' trunks and branches [[6], [7], [8], [9]].
Imidacloprid is one of the most important contaminants observed rigorously ininternational trade in various food commodities. The maximum residue limit (MRL) for imidacloprid in brinjal and okra is 0.2 and 2.00 mg/kg, respectively [10]. The analytical method must be sensitive to detect and quantify the traces of imidacloprid at or below the MRLs. Reviews on analytical methods for estimation of imidacloprid in different substrates were reported as; in potato and onion with LOD and LOQ of0.0075 and 0.015 μg/g; 0.006 and 0.012 μg/g, respectively [11]; rice straw with LOQ of 0.015 μg/g [12]; grape leaves, berries and soil with LOD and LOQ of 0.017 and 0.05 μg/g [13]; brinjal and soil with LOD of 0.003 and LOQ of 0.01 μg/g [14]; cotton seed cake with LOQ of 5.0 μg/g [15]; mulberry leaves with LOQ of 0.01 μg/g [16] using HPLC with PDA detector. Further, it was detected through colorimetric using a graphene chemosensor and HPLC-DAD [17,18]. These techniques were inferior to the liquid chromatography-tandem mass spectrometry (LC-MS/MS), whereas imidacloprid in water and soil with LOD of 0.0003 and 0.0005 μg/g and LOQ of 0.001 and 0.0017 μg/g was attended using LC-MS/MS [19]. There is a great need for a reproducible method with a confirmatory technique like mass spectrometry to detect and quantify the traces of imidacloprid in a food matrix, particularly vegetables.
The agricultural use of several neonicotinoids, such as thiacloprid, clothianidin, thiamethoxam, acetamiprid, and imidacloprid, wasrestricted due to their observed negative effects on honeybees and other beneficial animals [20]. Research findings highlighted the toxic effects and unacceptably high risks posed by imidacloprid on honeybees, such as impairment of memory and brain metabolism, poor searching capacity, worker bees unable to provide food for the larvae, and failure of navigational abilities in bees leading to the Colony Collapse Disorder [8]. The low vapour pressure of 1.0 × 10−7 mmHg and the low Henry's law constant of 6.5 × 10−11 atm-m3 per mole indicate its non-volatile nature to retain as potential residues on crop canopy and soil [21]. Brinjal and okra being cross-pollinated crops and honey bee activity is common requirement for pollination and fruit set. Frequent application of this insecticide causes bee toxicity and residue have toxicological implications for consumers. With this scientific background, a method was developed and validated using LC-MS/MS (ESI+) to study the persistence of imidacloprid in brinjal and okra. Further, decontamination of residues from edible fruits was attended.
2. Materials and method
2.1. Field experimentation
Good Agricultural Practices trial (GAP trials) for recording the persistence of imidacloprid insecticide in brinjal and okra was conducted during Kharif 2019 at Main Agricultural Research Station, University of Agricultural Sciences, Raichur, Karnataka, India (Longitude:77.3345° E and Latitude:16.2043°N). To the ecological parameter operated at an experimental location, highly suitable and best-performing cultivars of brinjal and okra viz., Arka Kusumkar and Arka Anamika, respectively, were transplanted for this study for an accurate record of chemical behavior. The experiment was conducted adopting the Randomised Block Design (RBD), having three treatments and eight replications to and minimize the errors in the observation. The treatment plot size was 10 × 16 m2. Two doses of imidacloprid 17.8% SL formulation chemical such as 20 g a. i./ha (recommended dose) and 40 g a. i./ha (double the recommended dose) was applied as a foliar spray, and an untreated control treatment was maintained. Two applications were made during flower initiation and fruit set at 15 days intervals using a high-volume knapsack compression sprayer with 500 L ha−1 of water volume. No rain was recorded during the experimentation. Whereas 18.60 and 33.00 °C of mean maximum and minimum temperature, respectively, and 46 and 58% of relative humidity (RH) were observed.
2.2. Sample collection and preparation
Brinjal fruit (1 kg) samples representing the whole treatment plot was picked separately in clean and inert plastic containers at regular interval on 0 (2 h after application), 1, 3, 5, 7, 10, and 15 days after the application of imidacloprid. Okra fruits were drawn at 0 (2 h after application), 1, 3, 5, 7, 10, 15, 20, and 25 days after application. The samples were placed in a container having dry ice and shifted to the analytical laboratory. The whole laboratory sample (1 kg) was grounded thoroughly using a high-volume homogenizer (Robo Coup), stored in the −20 °C and subsequently extracted and analyzed.
2.3. Chemicals and reagents
Imidacloprid 17.8% SL (Confidor®, Bayer India Pvt. Ltd) was purchased from the local authorized dealerfrom Raichur, Karnataka. Imidacloprid (99.60% purity) of Dr. Ehrenstorfer (Augsburg, Germany) was procured through Chromatopack, India. Acetonitrile and methanol (LC-MS grade, purity ≥99.9%) were purchased from J. T. Baker (NJ, USA), and ethyl acetate (≥99.8% purity) from Merck, Mumbai (India). Ammonium formate and formic acid (90% purity) were procured from Sigma Aldrich (India). Anhydrous magnesium sulphate, sodium acetate and sodium sulphate (>99% purity), Sodium chloride (≥99% purity), and primary secondary amine (Solid Phase Extraction sorbent; 40 μm) werepurchased from Agilent Technologies India Pvt. Ltd., Bangalore, India. From the Milli-Q water system, deionized water was collected.
2.4. Extraction of imidacloprid from brinjal and okra fruits
QuEChERS extraction approach and its modification [22], were followed for the extraction and cleanup of brinjal and okra fruits for imidacloprid residues. The extraction process is as follows; weighed 10 g of ground brinjal and okra samples and transferred into 50 mL centrifuge tubes. To this, 20 mL of acetonitrile (for better resolution of analyte peaks) was added, and keep it for 30 min. After time-lapse, homogenized the sample mixture was using a low-volume homogenizer at 10,000–13000 rpm; then 3 g of NaCl was added and vortexed immediately for 2 min. Further, this was centrifuged at 5000 rpm for 5 min (10 °C). After centrifugation, 15 mL of supernatant was collected in a test tube containing 9 g of sodium sulphate. Further, the 8–10 mL of extract was transferred from the test tube into a 15 mL centrifuge tube containing 0.4 g of PSA (primary secondary amine) and 1.05 g anhydrous magnesium sulphate and then vortexed the sample mixture for 1 min and contents were centrifuged at 12,000 rpm for 5 min. Then, 1 mL of supernatant was filtered using 0.22 μm PTFE nylon filter into LC vials.
2.5. Instrumentation (LC-MS/MS)
UHPLC (NEXARA 1200 series) along with thermo-stated column oven and LCMS 8040 (Shimadzu®) with triple quadrupole detector (TQD) systems equipped in LC-MS/MS. Control of whole instrument assembly, data acquisition, and processing were made with LabSolution® (Version 1.5) system software. C-18 column with 150 × 2 mm i. d. (Shimpack XR-ODS) was connected in the oven at 40 °C column oven temperature for separation of the Imidacloprid. A solvent system, such as mobile phases A and B, consisted of 0.0314 g ammonium formate (5 mM) +2 mL MeOH +10 μL formic acid (0.01%) with 100 mL of HPLC water and 0.0314 g ammonium formate (5 mM) +10 μL formic acid (0.01%) with 100 mL of 100% MeOH, respectively was used at 0.4 mL/min flow rate. The gradient programisas follows: at the beginning, 40% B and 60% A for 13 min followed by 100% B up to 1 min and then 40% B for 1 min.
A full scan mass spectrum of imidacloprid with electro-spray ionization positive mode (ESI+) was documented to choose the most intense m/z value. Further, the parent ion (M + H)+ was identified and selected as the precursor ion. The transitions of multiple reaction monitoring (MRM) along with acquisition parameters were optimized for the high abundance of selected ions with ESI+. The MS source parameters used were as follows; interface voltage of 4.5 kV, desolvation temperature of 250 °C, heat block temperature of 400 °C, desolvation gas (N2) of 2.9 L/min, and drying gas at 2.9 L/min. Then collision with argon gas (230 kpa), ascan speed was 6000 u/sec was employed, and different collision energies were optimized for obtaining the highest sensitivity in the developed method.
2.6. Method validation
The different parameters such as linearity, matrix effect, limit of detection (LOD), the limit of quantification (LOQ), specificity, trueness (bias), precision in terms of repeatability (RSDr-intraday), and reproducibility (RSDwR-interday) were validated for imidacloprid in brinjal and okra fruits according to the SANTE/12682/2019 guidelines [[23], [24], [25]]. The blank control matrix of brinjal and okra was extracted and used in the validation process. To detect the presence of possible interferences, six individual non-fortified blank samples were injected. Solvent and matrix-matched linearity were assessed by evaluating the deviation of each linear concentration injected from actual linear concentration (0.002, 0.005, 0.01, 0.02, 0.04, 0.06, 0.08, 0.10 μg/mLfor brinjal and 0.001, 0.002, 0.005, 0.01, 0.02, 0.04, 0.06, 0.08, 0.10 μg/mLfor okra)based on alinear regression equation. The matrix effect was performed by comparing the angular coefficients obtained by the curves in the solvent and matrix according to the following equation:
| Matrix effect (%) = (bm-bs)/bs x 100 |
Where bmand bsare the angular coefficients of the curve in the matrix and in the solvent, respectively.
The LOD was fixed based on the lowest concentration of the analyte to produce area response, i.e., three times baseline noise. LOQ was selected as the concentration of imidacloprid that gives an S/N ratio of 10 and recovery of the lowest spike level within the limit of 70–120% with an RSD of ≤20%.Trueness or bias test was conducted through spike and recovery at three fortification levels by spiking the brinjal at 0.005, 0.025, and 0.05 μg/gand okra fruits at 0.008, 0.04, and 0.08 μg/g using imidacloprid analytical standard solution. Each level spiked brinjal and okra sample of six replications was then kept at room temperature of 25 °C for 30min. to reach sample stability. The fortified samples were extracted and injected into LC-MS/MS. The area response in spiked concentration after extraction and matrix-matched standards as compared to calculate the percent recovery. The method precision was determined with regard to the repeatability relative to standard deviation. The repeatability (RSDr) was assessed on the same day, while within-laboratory reproducibility (RSDwR) was measured on two consecutive days by two different analysts. The precision values were calculated as relative standard deviation (RSD) of replicate measurements and percent recovery.
2.7. Statistical analysis
The persistence kinetics, half-life, and the theoretical dissipation time to reach the level of 0.01 μg/gwere computed using the equations of first-order kinetics [26].
| Ct = C0e–kt |
| t1/2 = ln(2)/k |
| t0.01 = In (0.001/C0)/(-k) |
Where Ct (μg/g) is the residual levels of imidacloprid at time t (days), C0 (μg/g) is the initial deposits, and k is the rate constant (day−1).
2.8. Risk assessment
The Estimated daily intake, EDI (mg/kg/day), is a realistic toxicological criterion for pesticide exposure. The per capita consumption of 357 g/body (recommended daily intake of 357 g/day of vegetables for a balanced diet of an average man in the Indian context as per Indian Council of Medical Research) was considered for estimation of human health risk with imidacloprid residues in brinjal, and okra was carried by calculating the EDI, using hazard index (HI) model. The hazard indices were calculated by dividing the EDI (mg/kg/day) by their corresponding Acceptable Daily Intake (ADI) values. If the hazard index (HI) is more than 1, the food is considered as not safe for human consumption [27].
3. Results and discussion
3.1. Optimization of LC-MS/MS parameters
Method for determination and quantification of imidacloprid in brinjal and okra, different acquisition parameters of mass spectrometer were optimized. Initially, the full scan mass spectrum of the imidacloprid was recorded in an array to choose the most abundantm/z value. For imidacloprid, the parent ion (M + H)+ 256.05 was identified and selected as a precursor ion. Based on the known molecular ion, MRM transformation with different collision energies (CE) viz. −17, −9, −19 for imidacloprid. The daughter ions of 209.10, 221.15, and 175.05 for imidacloprid were selected for further quantification and confirmation in brinjal and okra samples with ESI-positive mode. After the determination of the MRM transitions, the chromatographic conditions for better determination of imidacloprid were found. It was emphasized that the total ion chromatogram (TIC) had good separation resolution. The present developed MRM positive mode is accustomed to more sensitive and accurate detection of imidacloprid in brinjal and okra matrix at low concentrations. In this method, imidacloprid was eluted at 1.262 ± 0.1 min of retention time. The developed method can analyze the imidacloprid within a short period of time program of3.50min. (Fig. 1a&b).
Fig. 1.
(a) Product ion mass spectra (m/z: (M + H)+ 256.05; 209.10, 221.15, 175.05); (b) total ion chromatogram for imidacloprid.
3.2. Method validation in brinjal
The sensitivity or linearity was studied in brinjal matrices as well as in methanol solvent in the range of 0.002–0.1 μg/g. Good linearity and strong correlation between the concentration of peak area in terms of residuals obtained at ±20% with the coefficient of determination (R2) higher than 0.999. The LOD and LOQ of 0.001 and 0.003 μg/gfor imidacloprid were defined as the minimum spiked concentration with compliance recovery of 70–120% for imidacloprid in brinjal matrix was identified, and below MRL of 0.5 mg/kg for imidacloprid. The specificity test with respect to retention time and response for imidacloprid in brinjal did not record any interference. The matrix effect of less than 18.26% was observed in the brinjal matrix, and less than 20% which compliance with method validation criteria (Table 1). The trueness was evaluated at different spiking levels of 0.003, 0.015, and 0.030 μg g−1was found to be 89.67, 107.06 and 115.55%, respectively. The precision of imidacloprid in brinjal in terms of repeatability (RSDr) and reproducibility (RSDwR) was estimated, and %RSD and recoveries were 14.23, 15.41, 5.33 and 99.82, 94.80, 111.05% at 0.003, 0.015 and 0.030 μg/g, respectively. The interday day compression for %RSD in terms of RSDwR at the spiking level of 0.003, 0.015, and 0.03 μg/g were16.87, 16.37 and 8.28 respectively, for imidacloprid (Table 2).
Table 1.
Analytical curve, coefficient of determination (R2), LOD, LOQ of imidacloprid in solvent and matrix.
| Matrix | Parameter | Solvent standard |
Brinjal matrix standard |
||||||
|---|---|---|---|---|---|---|---|---|---|
| tcal | tcrit | T test | tcal | tcrit | T test | ||||
| Brinjal | Analytical curve | Y = 4500.8x-22339 | 2.515 | 2.306 | S | Y = 5322.8x-20619 | 2.421 | 2.306 | S |
| R2 | 0.998 | 0.999 | |||||||
| LOD | 0.001 μg mL−1 | 0.001 μg/g | |||||||
| LOQ | 0.003 μg mL−1 | 0.003 μg/g | |||||||
| Matrix effect | 18.26 | ||||||||
| Okra | Analytical curve | Y = 774.9x-812.53 | 3.087 | 2.262 | S* | Y = 841.1x-404.28 | 2.841 | 2.262 | S* |
| R2* | 0.998 | 0.999 | |||||||
| LOD* | 0.001 μg mL−1 | 0.0025 μg/g | |||||||
| LOQ* | 0.002 μg mL−1 | 0.008 μg/g | |||||||
| Matrix effect | 8.54 | ||||||||
R2- Coefficient of determination, LOD-Limit of detection, LOQ-Limit of quantification S-significant tcal-t student calculated, tcrit-t student critical.
Table 2.
Accuracy, repeatability (RSDr) and Reproducibility (RSDwR) of the proposed method for imidacloprid.
| Replication | Brinjal |
Okra |
||||
|---|---|---|---|---|---|---|
| 0.003 μgg−1 | 0.015 μgg−1 | 0.030 μgg−1 | 0.008 (μgg−1) | 0.04 (μgg−1) | 0.08 (μgg−1) | |
| Accuracy | ||||||
| Mean | 89.67 | 107.06 | 115.55 | 88.69 | 97.27 | 93.74 |
| SD | 15.47 | 8.02 | 3.31 | 7.622 | 6.27 | 7.12 |
| % RSD | 17.25 | 7.49 | 2.87 | 8.60 | 6.44 | 7.59 |
| Repeatability (RSDr) | ||||||
| Mean | 99.82 | 94.80 | 111.05 | 90.28 | 98.96 | 91.84 |
| SD | 14.20 | 14.60 | 5.92 | 15.29 | 6.92 | 7.43 |
| % RSD | 14.23 | 15.41 | 5.33 | 16.93 | 6.99 | 8.09 |
| Reproducibility (RSDwR) | ||||||
| Mean∗ | 96.05 | 89.97 | 105.79 | 85.18 | 77.02 | 77.17 |
| SDa | 16.20 | 14.73 | 8.77 | 8.40 | 4.695 | 4.70 |
| % RSDb | 16.87 | 16.37 | 8.28 | 9.86 | 6.10 | 6.09 |
Mean of between days.
Inter day standard deviation.
Inter day relative standard deviation.
3.3. Method validation in okra
Linearity for imidacloprid in okra matrix, as well as in methanol solvent in the calibration range of 0.001–0.1 μg/g found with good linearity and strong correlation between concentrations and peak area with residuals observed less than ±20% with R2 higher than 0.998. The LOD of 0.0025 μg/gand LOQ of 0.008 μg/g was defined as the minimum spiked concentration with compliance recovery of 70–120% for imidacloprid in the okra matrix was identified, which is well below the MRL of 0.5 μg/kg imidacloprid in okra [10](Table 1). The specificity test for imidacloprid with a standardized method observed not much interference of the matrices during analysis and did not find other peaks at the determined RT and area. The matrix effect calculated was 8.54% in okra for imidacloprid. The trueness was found to be 88.69, 97.27, and 93.74% of recovery at spiking levels of 0.008, 0.04, and 0.08 μgg−1. The precision of imidacloprid in okra in terms of repeatability (RSDr) and reproducibility (RSDwR) was found in the RSDr, and recoveries of 16.93, 6.99, and 8.09 was 90.28, 98.96, and 91.84%, at 0.008, 0.04 and 0.08 μg/gspiking level, respectively. The interday day comparison of per cent RSD in terms of RSDwR at spiking levels of 0.008, 0.04, and 0.08 μg/g were 9.86, 6.09, and 6.09 for imidacloprid, respectively, fulfilled the requirement of SANTE/12682/2019 (Table 2).
3.4. Dissipation of imidacloprid in brinjal
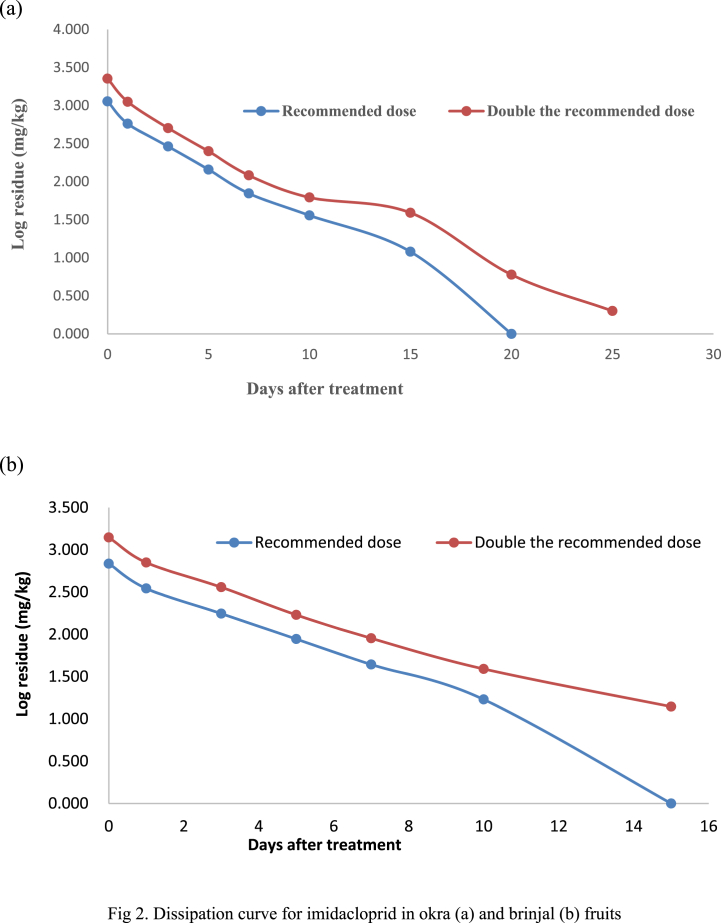
The initial residues of imidacloprid in brinjal fruit were 0.685 and 1.399mg/kgat 20 and 40 g a. i./ha, respectively (Table 3). The double dose (40 g a. i./ha) recorded higher residues due to concentration, chemical nature, formulation, as well as the insecticide applied and uptake on the plant surface. Similarly, imidacloprid on tomato was observed with 0.08 and 0.23 mg/kginitial residues at an applied dose of 35 and 70 g a. i./ha [28]and 1.95 at 20 g a. i./ha [29]. The application of 30 and 60 g a. iha−1 in cucumber recorded 1.93 and 3.65 mg/kg, respectively, which further declined to 0.69 mg/kg at 14 days after application [30]. The imidacloprid residues of 0.349 and 0.707 mg/kg in the brinjal samples contributed to a total loss of 49.05 and 49.46%, and residues gradually dissipated to 0.001 and 0.004 mg/kgon the 15th day accounting to the loss of 99.85 and 98.99% at 20 and 40 g a. i. Ha−1, respectively (Fig. 2b). The initial deposit of imidacloprid on brinjal was 0.652 mg/kg and dissipated to 93.17% on the 10th day and reached BDL after the 10th day [31]. The dissipation of imidacloprid in cucumber was found 94.48 and 99.18% followed first-order dissipation kinetics at single and double dosages, respectively and residue reached below detectable level in 3 days after spray [30].
Table 3.
Dissipation of imidacloprid 17.8% SL at recommended and double dose in brinjal and okra.
| Days after treatment | Brinjal |
Okra |
||||||
|---|---|---|---|---|---|---|---|---|
| Residues (mgkg−1) Mean ± SD | Dissipation (%) | Residues (mgkg−1) Mean ± SD | Dissipation (%) | Residues (mgkg−1) Mean ± SD | Dissipation (%) | Residues (mgkg−1) Mean ± SD | Dissipation (%) | |
| 20 g a.iha−1 | 40 g a.iha−1 | 20 g a.iha−1 | 40 g a.iha−1 | |||||
| 0 | 0.685 ± 0.066 | 0.00 | 1.399 ± 0.108 | 0.00 | 1.134 ± 0.106 | – | 2.258 ± 0.041 | – |
| 1 | 0.349 ± 0.043 | 49.05 | 0.707 ± 0.086 | 49.46 | 0.577 ± 0.054 | 49.08 | 1.121 ± 0.222 | 50.37 |
| 3 | 0.176 ± 0.013 | 74.30 | 0.361 ± 0.022 | 74.19 | 0.290 ± 0.013 | 74.47 | 0.505 ± 0.046 | 77.64 |
| 5 | 0.088 ± 0.006 | 87.15 | 0.170 ± 0.046 | 87.84 | 0.144 ± 0.010 | 87.31 | 0.251 ± 0.031 | 88.88 |
| 7 | 0.044 ± 0.007 | 93.57 | 0.090 ± 0.015 | 93.56 | 0.070 ± 0.004 | 93.83 | 0.121 ± 0.049 | 94.65 |
| 10 | 0.017 ± 0.005 | 97.51 | 0.039 ± 0.008 | 97.21 | 0.036 ± 0.006 | 96.84 | 0.062 ± 0.015 | 97.27 |
| 15 | 0.001 ± 0.001 | 99.85 | 0.014 ± 0.004 | 98.99 | 0.012 ± 0.03 | 98.94 | 0.039 ± 0.003 | 98.27 |
| 20 | – | – | 0.001 ± 0.001 | 99.91 | 0.006 ± 0.002 | 99.73 | ||
| 25 | – | – | – | – | – | – | 0.002 ± 0.002 | 99.91 |
| Correlation coefficient = 0.993 | Correlation coefficient = 0.989 | Correlation coefficient = 0.993 | Correlation coefficient = 0.961 | |||||
| Regression equation = 0.820–0.178× | Regression equation = 0.985–0.131× | Regression equation y = 0.778–0.126× | Regression equation y = 0.089–0.089× | |||||
| t1/2 = 1.69 days | t1/2 = 2.30 days | t ½ = 2.38 days | t ½ = 3.36 days | |||||
| K = 0.178 day−1 | K = 0.131 day−1 | K = 0.126 day−1 | K = 0.089 day−1 | |||||
t1/2- half-life, K-coefficient of determination.
Fig. 2.
Dissipation curve for imidacloprid in okra (a) and brinjal (b) fruits.
The imidacloprid residues were dissipated to half of its concentration in 1.69 and 2.30 days from brinjal, respectively (Table 3). Few reports on half-life (t1/2) of imidacloprid were 8.9 and 7.5 days for 35 g. a.i./ha and 75 g a. i./hare spectively and [28]; 2.5 days for 20 g a. i/ha on tomato fruits [29]; 3.40 and 2.70 days at single and double dose, respectively in cucumber [30] indicating similar degradation pattern. Further, the degradation rate constant (k) and correlation coefficient (r) values were found to be 0.115/dayand 0.993 for recommended dose and 0.097/dayand 0.979 for double the recommended dose, respectively. The safe waiting period of imidacloprid on brinjal was 0.87 and 1.31 days at single and double doses, respectively. The recorded SWP was closer to the duration observed in (3 days) cucumber [30] and 4.7 days was observed in brinjal [31] (Fig. 2b).
3.5. Dissipation in okra
The initial concentration of 1.134 mg/kgat 20 g a. i. Ha−1 and 2.258 mg/kgin the dose of 40 g a. i./hawere recorded in okra fruits (Table 3). Different factors such as plant form (erect or prostrate), the shape of plant parts (broad, narrow, or linear), and the growth of plant parts (slow or fast) influence the retention and deposition of pesticides on the plant surface [32]. The response of the imidacloprid in both doses in the samples drawn at zero-day is depicted in Fig. 2a. The initial deposit for a chemical change from crop to crop, their deposition site (leaves, fruits,etc.) and dosage applied. The residues gradually dissipated to 0.003 and 0.005 mg/kgonthe21st and 25th day after application, accounting forthe loss of 99.73 and 99.91% in single and double the recommended dose, respectively. The rate of dissipation of residue from okra fruits was slower in the present investigation. Dissipation of 96.20% residues from tomato fruits was noticed on the 10th day after application and dissipated below its detectable limit on the 14th day after application [33]. These reviews indicated that the persistence period of imidacloprid in different crop canopies was in the range of 3–14 days which was greatly influenced by several other factors such as insecticide type and formulation and crop growth dilution and weather parameter operated during the experimentation [24] (Fig. 3).
Fig. 3.
Imidacloprid response in okra fruit at (a) recommended and (b) double the recommended dose at zero-day samples.
The time period of 2.38 and 3.36 days noticed for a loss of 50% of concentration recorded initially in the applied doses of 20 and 40 g a. i./ha in okra fruits, respectively. A similar observation was recorded for imidacloprid with a half-life period of 2.5 days in tomatoes [33]. The half values for imidacloprid were calculated as 2.64 to 3.30 in brinjal, 2.38 to 2.58 in okra, and 1.90 to 2.23 in tomato fruits [34]. The rate of insecticide degradation (fast or slow) affects the safe waiting period of the chemical. The waiting period for imidacloprid on okra fruits was 8.53 and 13.34 days at 20 and 40 g a. i./ha, respectively.
3.6. Decontamination of imidacloprid in brinjal
The decontamination results of imidacloprid in brinjal fruits by different household treatments are presented in Table 4. The reduction of imidacloprid residues from the brinjal fruits collected from the plot applied the dose 20 g a. i/ha to different treatments ranging from 61.03 to 99.43%. Salt solution (2%) followed by boiling for 5 min resulted in a significant reduction (99.43%) and was superior to the other treatments. Dipping in 2% salt solution for 15 min resulted in an 89.11% reduction. In contrast, less reduction of imidacloprid (61.03%) was reported in dipping fruits in 0.1% sodium bicarbonate solution for 15 min. In a similar experiment, imidacloprid and thiabendazole were effectively removed (30.59–65.24%) from cherry tomatoes by soaking them initially in water and then rinsing them with running tap water [35].
Table 4.
Effect of different decontamination treatments in the removal of imidacloprid in brinjal and okra.
| Treatment No. | Treatment details | Brinjal |
Reduction (%) | Okra |
Reduction (%) | ||
|---|---|---|---|---|---|---|---|
| Recommended dose (20 g a.i. ha−1) |
Recommended dose (20 g a.i. ha−1) |
||||||
| Initial Residues levels mgkg−1 ± SD | Residue after treatment mgkg1 ± SD | Initial Residue levels mgkg−1± SD | Residue after treatment mgkg−1± SD | ||||
| T1 | Dipping in 2% Tamarind solution for 15 min | 0.349 ± 0.049 | 0.118 ± 0.01 | 66.19e | 0.577 ± 0.054 | 0.219 ± 0.010 | 62.05bc |
| T2 | Dipping in 2% of sodium bicarbonate solution | 0.349 ± 0.049 | 0.136 ± 0.03 | 61.03e | 0.577 ± 0.054 | 0.339 ± 0.002 | 41.25f |
| T3 | Dipping in 1% turmeric solution for 15 min | 0.349 ± 0.049 | 0.082 ± 0.02 | 76.50d | 0.577 ± 0.054 | 0.233 ± 0.022 | 59.62c |
| T4 | 1 lemon in 1 L of water | 0.349 ± 0.049 | 0.077 ± 0.02 | 77.94cd | 0.577 ± 0.054 | 0.201 ± 0.02 | 65.17b |
| T5 | Dipping in 4% of acetic acid solution for 15 min | 0.349 ± 0.049 | 0.060 ± 0.08 | 82.81c | 0.577 ± 0.054 | 0.311 ± 0.012 | 46.10e |
| T6 | Dipping in 2% salt solution + boiling for 5 min | 0.349 ± 0.049 | 0.002 ± 0.05 | 99.43a | 0.577 ± 0.054 | 0.502 ± 0.023 | 12.99h |
| T7 | Dipping in 2% salt solution for 15 min | 0.349 ± 0.049 | 0.038 ± 0.23 | 89.11b | 0.577 ± 0.054 | 0.276 ± 0.003 | 52.16d |
| T8 | Dipping in hot water for 15 min | 0.349 ± 0.049 | 0.125 ± 0.09 | 64.18e | 0.577 ± 0.054 | 0.223 ± 0.012 | 59.219c |
| T9 | Dipping in tap water for 15 min | 0.349 ± 0.049 | 0.064 ± 0.03 | 81.66cd | 0.577 ± 0.054 | 0.490 ± 0.032 | 28.77g |
| T10 | Boiling for 5 min | 0.349 ± 0.049 | 0.076 ± 0.03 | 78.22cd | 0.577 ± 0.054 | 0.152 ± 0.021 | 73.65a |
| SEM± | 1.15 | 0.67 | |||||
| CD (%) | 3.39 | 2.05 | |||||
| CV | 0.03 | 0.03 | |||||
SD: Standard deviation; Significant Differences (p < 0.05, student's paired t-test) are mentioned with different alphabets in the same test item.
3.7. Decontamination of imidacloprid in okra
The decontamination process in removing imidacloprid residues from okra fruits ranged from 12.99 to 73.66% (Table 4). Okra fruits boiled for 15 min were found effective in the removal of imidacloprid (73.66%) and significantly superior to all other treatments. Lemon solution (65.17%) and dipping in 2% tamarind solution (62.05%) were the next best processing approaches for removing imidacloprid from okra. Present observations were supported by the findings which recorded 18.80 and 22.17% of dimethoate and profenofos were removed from tomato fruits with tap water wash, respectively [36]. Further, 2% salt solution found 35.7 and 27.0% removal of dimethoate and profenofos, respectively, from tomato fruits.
3.8. Risk assessment of imidacloprid in brinjal and okra
The application of imidacloprid 17.80% SL has persisted for 21 and 25 days when applied at 20 and 40 g a. i. Ha−1 in okra fruits. The risk assessment with residues had no significant hazard index values at the beginning and end of sampling dates indicating no likelihood of posing a risk upon consumption of okra fruits (Table 5). Similar observations were recorded in brinjal fruits. The imidacloprid persisted for 15 days, and residues obtained in all the doses had no significant hazard Index. Brinjal and okra have MRL of 0.2 and 2 mgkg−1. Residues were more than MRL at 0 and 1 day at an applied dose of 20 g a. i./ha where as, 0, 1, and 3 days at 40 g a. i./hain brinjal fruits and risk assessment shown in significant HI values. The dose applied at 40 g a. i.ha−1only showed residues more than MRL in the case of Okra, and the HI value was less than 1, indicating no potential hazards to the consumers upon consumption of okra fruits a day after application of imidacloprid 17.80% SL formulation product for the management of insect pest under commercial cultivation. The low application rate of insecticides and non-target dietary exposure assessment for the residues recorded in the harvested fruits, pods, and berries are expected to be very low [37].
Table 5.
Risk assessment for residues obtained in imidacloprid at 20 g.a.i/ha and 40 g.a.i/ha in okra and brinjal.
| Sampling days after treatment | Okra |
Brinjal |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean residue (mg/kg) at 20 g.a.i.ha | EDI (mg/kg) | ADI (mg/kg) | HI | Mean residue (mg/kg) at 40 g.a.i.ha | EDI (mg/kg) | HI | Mean residue (mg/kg) at 20 g.a.i.ha | EDI (mg/kg) | HI | Mean residue (mg/kg) at 40 g.a.i.ha | EDI (mg/kg) | HI | |
| 0 | 1.134 | 0.007 | 0.060 | 0.112 | 2.258 | 0.013 | 0.224 | 0.685 | 0.004 | 0.068 | 1.399 | 0.008 | 0.139 |
| 1 | 0.577 | 0.003 | 0.060 | 0.057 | 1.121 | 0.007 | 0.111 | 0.349 | 0.002 | 0.035 | 0.707 | 0.004 | 0.070 |
| 3 | 0.290 | 0.002 | 0.060 | 0.029 | 0.505 | 0.003 | 0.050 | 0.176 | 0.001 | 0.017 | 0.361 | 0.002 | 0.036 |
| 5 | 0.144 | 0.001 | 0.060 | 0.014 | 0.251 | 0.001 | 0.025 | 0.088 | 0.001 | 0.009 | 0.17 | 0.001 | 0.017 |
| 7 | 0.070 | 0.000 | 0.060 | 0.007 | 0.121 | 0.001 | 0.012 | 0.044 | 0.000 | 0.004 | 0.09 | 0.001 | 0.009 |
| 10 | 0.036 | 0.000 | 0.060 | 0.004 | 0.062 | 0.000 | 0.006 | 0.017 | 0.000 | 0.002 | 0.039 | 0.000 | 0.004 |
| 15 | 0.012 | 0.000 | 0.060 | 0.001 | 0.039 | 0.000 | 0.004 | 0.001 | 0.000 | 0.000 | 0.014 | 0.000 | 0.001 |
| 21 | 0.001 | 0.000 | 0.060 | 0.000 | 0.006 | 0.000 | 0.001 | ||||||
| 25 | 0.002 | 0.000 | 0.000 | ||||||||||
EDI-estimated daily intake, ADI-Acceptable daily intake, HI- Hazard index.
4. Conclusion
Brinjal and okra, sprayed using different formulations of imidacloprid, have significant residues left on fruits. This chemical has well-documented ecotoxicological effects on several non-targets through its trace-level residue. A method that could detect and confirm the traces of imidacloprid within 1.5 min through LC-MS/MS was developed and validated according to the SANTE/11813/2019 guidelines. Precision in terms of repeatability (RSDr) and reproducibility (RSDwR) was found satisfactory in brinjal and okra. The matrix effect was 18.26 and 8.54% in brinjal and okra, respectively. Dissipation of imidacloprid revealed that imidacloprid 17.8% SL persisted up to 15 and 25 days with t1/2 (half-life) 1.69 and 2.30 for brinjal; and 2.38 and 3.36 days for okra at 20 and 40 g a. i./ha, respectively. Decontamination with 2% salt solution followed by boiling for 15 min removed 96.43 and 73.66% imidacloprid residues from brinjal and okra, respectively. The risk of imidacloprid at both doses was negligible depending on the hazard index (HI) value, which was lower than 1 and could be safely consumed.
Author contribution statement
Harischandra Naik R, Pallavi M S,Paramasivam Mariappan: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Ratnamma, Sangamesh Vijaykumar, Saraswati Mahato,Pavankumar:Performed the experiments; Analyzed and interpreted the data.
Saroja Narsing Rao:Performed the experiments; Contributed reagents, materials, analysis tools or data.
Arunkumar Hosamani, Bheemanna M,Prabhuraj Aralimarada: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
Dr. Harischandra Naik R was supported by University of Agricultural Sciences, Raichur [No.COM/UAS/5842/2019-20)].
Data availability statement
The authors do not have permission to share data.
Declaration of interest's statement
The authors declare no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are indebted to the Pesticide Residue and Food Quality Analysis Laboratory (PRFQAL), University of Agricultural Sciences, Raichur, Karnataka, for laboratory research facility support.
References
- 1.Kumar S., Misra A., Verma A.K., Roy R., Tripathi A., Ansari K.M., Das M., Dwivedi P.D. Bt brinjal in India A long way to go. GM Crops. 2011;2:92–98. doi: 10.1016/B978-0-12-802259-7.00004-X. [DOI] [PubMed] [Google Scholar]
- 2.Indian horticulture database, national horticulture board (NHB) J. Hortic. Sci. 2018;36:175–179. [Google Scholar]
- 3.Nadkarni K.M. 1996. Indian Materia Medica: with Ayurvedic, Unani-Tibbi, Siddha, Allopathic, Homeopathic, Naturopathic & Home Remedies, Appendices & indexes,Popular Prakashan1. [Google Scholar]
- 4.Gupta S., Sharma R.K., Gupta R.K., Sinha S.R., Singh R., Gajbhiye V.T. Persistence of new insecticides and their efficacy against insect pests of okra. Bull. Environ. Contam. Toxicol. 2009;82:243–247. doi: 10.1007/s00128-008-9581-8. [DOI] [PubMed] [Google Scholar]
- 5.CIBRC . Directorate of Plant Protection, Quarantine & Storage, Government of India; 2019. Major Use of Insecticides.https://ppqs.gov.in/divisions/cib-rc/major-uses-of-pesticides [Google Scholar]
- 6.Bhargava K.K., Bhatnagar A., Sharma H.C. Bio-efficacy of imidacloprid and beta-cyfluthrin for the management of insect pests of Brinjal. Indian J.Plant Prot. 2003;31:111–113. [Google Scholar]
- 7.Gopal M., Mukherjee I., Chander S. Behaviour of β- cyfluthrin and imidacloprid in mustard crop: alternative insecticide for aphid control. Bull. Environ. Contam. Toxicol. 2002;68:406–411. doi: 10.1007/s001280269. [DOI] [PubMed] [Google Scholar]
- 8.Decourtye A.C., Armengaud M., Renou J., Devillers M., Cluzeau G., Pham-Delegue M.H. Imidacloprid impairs memory and brain metabolism in the honeybee (ApismelliferaL.) Pestic.Biochem. Phys. 2004;78:83–92. doi: 10.1016/j.pestbp.2003.10.001. [DOI] [Google Scholar]
- 9.Overmyer J.P., Mason B.N., Armbrust K.L. Acute toxicity of imidacloprid and fipronil to a non target aquaticinsect Simuliumvittatum Zettersted tcytospecies IS-7. Bull. Environ. Contam. Toxicol. 2005;74:872–879. doi: 10.1007/s00128-005-0662-7. [DOI] [PubMed] [Google Scholar]
- 10.FSSAI . Ministry of Health and Family Welfare; 2018. Notification-pesticide/stds-FSSAI/2017, food safety and standards authority of India.http://www.indiaenvironmentportal.org.in/content/460465/gazette-notification-on-food-safety-and-standards-contaminants-toxins-and-residues-amendment-regulation-related-to-mrl-of-pesticide/ [Google Scholar]
- 11.Mandic A.I., Lazic S.D., Okresz S.N., Gaal F.F. Determination of the insecticide imidacloprid in Potato (SolanumtuberosumL) and Onion (Allium cepa) by high-performance liquid chromatography with diode-array detection. J. Anal. Chem. 2005;60:1134–1138. doi: 10.1007/s10809-005-0256-x. [DOI] [Google Scholar]
- 12.Prasad B.R., Singh V.S., Chander S., Kumar J. Residue evaluation of controlled-release formulations of imidacloprid against rice leaf folder. Bull. Environ. Contam. Toxicol. 2007;78:235–238. doi: 10.1007/s00128-007-9139-1. [DOI] [PubMed] [Google Scholar]
- 13.Arora P.K., Jyot G., Singh B., Battu R.S., Singh B., Aulakh P.S. Persistence of imidacloprid on grape leaves, grape berries and soil. Bull. Environ. Contam. Toxicol. 2009;82:239–242. doi: 10.1007/s00128-008-9554-y. [DOI] [PubMed] [Google Scholar]
- 14.Mandal K., Chahil G.S., Sahoo S.K., Battu R.S., Singh B. Dissipation kinetics of β-cyfluthrin and imidacloprid in brinjal and soil under subtropical conditions of Punjab, India. Bull. Environ. Contam. Toxicol. 2010;84:225–229. doi: 10.1007/s00128-009-9903-5. [DOI] [PubMed] [Google Scholar]
- 15.Mohan C., Kumar Y., Madan J., Saxena N. Multiresidue analysis of neonicotinoids by solid-phase extraction technique using high-performance liquid chromatography. Environ. Monit. Assess. 2010;165:573–576. doi: 10.1007/s10661-009-0968-8. [DOI] [PubMed] [Google Scholar]
- 16.Paramasivam M., Chandrasekaran S., Harischandra Naik R., Karthik P., Thangachamy P., Mahalingam C.A. Determination of imidacloprid residues in mulberry leaves by QuEChERS and Liquid Chromatography with diode array detection. J. Liq. Chromatogr. Relat. Technol. 2014;37:122–129. doi: 10.1080/10826076.2012.738616. [DOI] [Google Scholar]
- 17.Babazadeh S., Moghaddam P.A., Keshipour S., Mollazade K. Analysis of imidacloprid and penconazole residues during their pre-harvest intervals in the greenhouse cucumbers by HPLC–DAD. J. Iran. Chem. Soc. 2020;17:1439–1446. doi: 10.1007/s13738-020-01868-4. [DOI] [Google Scholar]
- 18.Babazadeh S., Moghaddam P.M., Keshipour S., Mollazade K. Colorimetric sensing of imidacloprid in cucumber fruits using a graphene quantum dot/Au (III) chemosensor. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-71349-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thuyet D.Q., Yamazaki K., Phong T.K., Watanabe H., Nhung D.D.T., Takagi K. Determination of imidacloprid in Paddy, water and soil by liquid chromatography electrospray ionization-tandem mass spectrometry. J. Anal. Chem. 2010;65:843–847. doi: 10.1134/S1061934810080149. [DOI] [Google Scholar]
- 20.Carrington D. The guardian; 2012. Pesticides Linked to Honeybee Decline.https://www.theguardian.com/environment/2012/mar/29/crop-pesticides-honeybee-decline [Google Scholar]
- 21.Fossen M. 2006. Environmental Fate of Imidacloprid.http://www.cdpr.ca.gov/docs/emon/fatememo/imidaclprdfate2.pdf [Google Scholar]
- 22.Anastassiades M., Lehotay S.J., Stajnbaher D., Schenck F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and dispersive solid-phase extraction for the determination of pesticide residues in produce. J. AOAC Int. 2003;86:412–431. doi: 10.1093/jaoac/86.2.412. [DOI] [PubMed] [Google Scholar]
- 23.European Commission . 2017. SANTE/12682/2019 of 1st January 2016, Guidance Document on Analytical Quality Control and Method Validation Procedures for Pesticide Residues and Analysis in Food and Feed; pp. 1–34. [Google Scholar]
- 24.Naik H.R., Pallavi M.S., Chawan R., Bheemanna M., Anand N., Paramasivam M. Method development and validation for determination of indoxacarb using LC-ESI-MS/MS and its dissipation kinetics in Pigeonpea. Food Anal. Methods. 2020;13:647–657. doi: 10.1007/s12161-019-01681-7. [DOI] [Google Scholar]
- 25.Harischandra Naik R., Pallavi M.S., Kumar Pavan K., Vanitha B.K., Chandra Sekhara Reddy V., Shwetha A., UdaykumarNidoni R., Bheemanna M. Determination of 72 chemical pesticides and estimation of measurement of uncertainty in rice using LC-MS/MS and GC-MS/MS. Food Anal. Methods. 2021;14:1788–1805. doi: 10.1007/s12161-021-02000-9. [DOI] [Google Scholar]
- 26.Dong B., Hu J. Dissipation and residue determination of flupyram and tebuconazole residues in watermelon and soil by GC-MS. Int. J. Environ. Anal. Chem. 2014;94:493–505. doi: 10.1080/03067319.2013.841152. [DOI] [Google Scholar]
- 27.Darko G., Akoto O. Dietary intake of organophosphorus pesticide residues through vegetables from Kumasi Ghana. Food Chem. Toxicol. 2008;46:3703–3706. doi: 10.1016/j.fct.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 28.Shukla V.R., Patel D.J., Patel A.R., Shah P.G. Dissipation of imidacloprid in tomato (Lycopersicon esculentum mill.) Pestic. Res. J. 2016;28:123–127. [Google Scholar]
- 29.Ahmed A.A., Mekky T.M., Ramadan R.A., Hendawi M.Y. Dissipation of profenofos, imidacloprid and penconazole in tomato fruits and products. Bull. Environ. Contam. Toxicol. 2009;83:812–817. doi: 10.1007/s00128-009-9852-z. [DOI] [PubMed] [Google Scholar]
- 30.Hassanzadeh N., Esmaili S.A., Bahramifar N. Dissipation of imidacloprid in greenhouse cucumbers at single and double dosages spraying. J. Agric. Sci. Technol. 2012;14:557–564. [Google Scholar]
- 31.Gupta S.P., Singh S.P., Paidi S., Nagendra K. Dissipation and decontamination of imidacloprid and lambda-cyhalothrin residues in brinjal. Int.J.Plant. Prot. 2015;8:379–383. doi: 10.15740/HAS/IJPP/8.2/379-383. [DOI] [Google Scholar]
- 32.Ebeling W. Analysis of the basic processes involved in the deposition, degradation, persistence and effectiveness of pesticides. Residue Rev. 1963;3:35–163. doi: 10.1007/978-1-4615-8377-6_3. [DOI] [Google Scholar]
- 33.Romesh A.A., Mekky T.M., Ramadan R.A., Hendawi M.Y. Dissipation of profenofos, imidacloprid and penconazole in tomato fruits and products. Bull. Environ. Contam. Toxicol. 2009;83:812–817. doi: 10.1007/s00128-009-9852-z. [DOI] [PubMed] [Google Scholar]
- 34.Banerjee T., Banerjee D., Roy S., Banerjee H., Pal S. A comparative study on the persistence of imidacloprid and beta-cyfluthrin in vegetables. Bull. Environ. Contam. Toxicol. 2012;89:193–196. doi: 10.1007/s00128-012-0644-5. [DOI] [PubMed] [Google Scholar]
- 35.Zhi Y.M., Yue Y., Xiao J.C. Comparison of removal efficiencies of different household cleaning methods in reducing imidacloprid and thiabendazole residues on cherry and tomatoes. J. Agric. Sci. 2016;8:83–86. [Google Scholar]
- 36.Abou-Arab A.A.K. Behavior of pesticides in tomatoes during commercial and home preparation. Food Chem. 1999;65:509–514. doi: 10.1016/S0308-8146(98)00231-3. [DOI] [Google Scholar]
- 37.Brugger K.E., Dpx-Mp062 Prospective tier I ecological effects assessment for non-target organisms. DuPont Agric. Products Document No. AMR. 1997:4782–4797. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.