Abstract
Different parts of Corchorus olitorius and C. capsularis possess different antioxidant compounds. This study investigated the phytochemical components and antioxidant capacities of ultrasound-assisted extraction of different plant parts of both species using spectrophotometry at various phenological stages. Results also indicate that leaves, stems and roots of C. olitorius at various growth stages showed higher phytochemical components and antioxidant potential compared to C. capsularis. The phytochemical components from roots to leaves in C. olitorius including total polyphenol 0.97–11.11 mg GAE/g DW, total flavonoid 0.99–7.78 mg QE/g DW and total tannin 4.02–26.89 TA E/g DW, whereas C. capsularis total polyphenol 1.04–7.93 mg GAE/g DW, total flavonoid 0.77–5.5.92 mg QE/g DW and total tannin content 3.17–22.73 TA E/g DW. C. olitorius produced overall 22.23%, 13.61%, 12.24% higher total polyphenol, total flavonoid and total tannin, respectively compare to C. capsularis. Different parts extract also significantly affected antioxidant capacities including DPPH, ABTS, and FRAP activity with values of 22.03–79.46% inhibition, 10.84–104.10 μmol TE/g DW, and 10.84–104.10 μmol Fe2+/g DW respectively for C. olitorius, while C. capsularis demonstrated 14.03–70.97% of DPPH inhibition, 9.16–95.60 μmol TE/g DW of ABTS and 5.31–71.82 μmol Fe2+/g DW of FRAP activity. Moreover, leaves of the flowering stage, young stems and aged roots of both species displayed a higher content of phytochemical and antioxidant activities than other growth stages. A positive correlation between the phytochemical and antioxidant potential indicated that phenolic constituents solely affected antioxidant activity. Thus, this study established that the plant's parts and phenological growth stages significantly influence the concentration of phytoconstituents and antioxidant activities, and determine the harvesting stages of the different organs of C. olitorius and C. capsularis for considerable medicinal importance as folk and industry.
Keywords: Growth stages, Bioactive compounds, Variation, Antioxidant potential, C. olitorius, C. capsularis
1. Introduction
Corchorus olitorius and C. capsularis are annual herbaceous plant belonging to the Tiliaceae family that grows up to 4–5 m tall with short and branched stems, leaves 6–10 cm long, 3.5–5 cm broad, elliptic-lanceolate which are found in all tropical, sub-tropical regions and warm temperate regions of the world. Both are widely cultivated in India, Bangladesh, Myanmar, Nepal, China, Taiwan, Thailand, Vietnam, Cambodia, Brazil and many other countries [1]. These are mainly farmed for the production of biodegradable natural fibers from stem bark. Moreover, in jute-producing countries tender shoots with young leaves are utilized as a vegetable, and different parts have several nutraceuticals, pharmaceutical, and commercial importance [2]. Vitamin C, carotenoids, α-tocopherol and phenolic content in both of species are significantly greater than in many other vegetables and grains, making it an excellent source of these nutrients [3]. It has been reviewed that C. capsularis leaves are used in ayurvedic medication to treat fever, liver diseases, ascites, algesia, cystitis, dysentery, dysuria, piles, gonorrhea, tumors, and female infertility in various parts of the world [4]. According to reports, different portions of C. olitorius demonstrate a variety of biological effects, including antibacterial, antidiabetic, cardioprotective, hepatoprotective, nephroprotective, anticonvulsant, antiestrogenic, and antimicrobial activity [5]. Additionally, leaves are utilized as a blood purifier, leaf twigs are often used to treat cardiac issues, and leaf infusion is used as a tonic and appetite stimulant. Root scrapings are employed to relieve toothache, and root decoction is used as a tonic to enhance strength. Purgatives and febrifuges are derived from seeds.
It has been reported that plants contain chemical substances that are formed during the plant's normal metabolic process. These biologically active phytochemicals are found in medicinal and edible plants that have been extensively investigated for their natural antioxidant potential. It also has been noted that various plant parts, like leaf, stem, bark, seed, and root, contain huge amounts of bioactive compounds, including phenolics, flavonoids, tannins, etc., with the ability to inhibit excessively synthesized free radicals, and thus can function as antioxidants [6,7]. Epidemiological and clinical studies provide evidence that most of these phytochemicals exhibit protective and disease-preventing functions through their antioxidant activities that inactivate lipid free radicals or inhibit hydroperoxide breakdown into free radicals [8,9]. The phytochemical compositions of different plants are influenced by their phonological growth stages [10]. For example, phenolic compounds and antioxidant activities of the flowering stage of Teucrium polium L. have been reported to be more than those at the seedling and vegetative stage [11]. The concentration of chemical compounds in the same stages of development varies depending on the organs of the plant. For the write up instance, leaves of Myrtus communis L. exhibited higher phytochemical content and antioxidant activity compared to flowers and fruits [12]. Furthermore, the highest antioxidant activity was observed at the full-flowering stage of Satureja rechingeri Jamzad plants [13].
To date, different phytochemicals have been characterized from different parts of Corchorus ssp Yakoub et al. [14]. However, it is undisclosed how variations in phenolic content and antioxidant activity occur in the leaves of C. olitorius and C. capsularis during different developmental stages. To the best knowledge, there is no research has yet been presented on the variations in phytochemical compounds and antioxidant properties of the various parts of C. olitorius and C. capsularis at different phenological stages. The main objective of this study was to evaluate the phytochemical composition and the antioxidant properties in different parts (like leaves, stems, and roots) at four different phenological stages: seedlings, vegetative, flowering, and after flowering as well as find out the best growth stage at which the species possesses the highest phytochemical components and most potent antioxidant capacity.
2. Materials and methods
2.1. Plant material and preparation of extract
T8 variety of C. olitorius and Y1 variety of C. capsularis were sowing in an innovation experimental farm of Institute of Bast Fiber Crop, Changsha, China on May 15, 2020 under the natural condition. The land was fertilized as a recommendation. Irrigation and other cultural activities were performed when necessary. Leaves, stems, and roots of T8 variety of C. olitorius and Y1 variety of C. capsularis were harvested randomly at four different growth stages including seedling (1st stage), vegetative growth (2nd stage), flowering (3rd stage), and post-flowering (4th stage) stage of plants. Each sample contained a minimum of three individual plants of similar variety that were mixed to form one sample. Three biological replicates at the same heights of each species were performed at every harvest for further analysis. The dirt was removed from the root by first using tap water and then double-distilled water. The obtained samples were then allowed for seven days at 25 °C temperature under shade until dried and ground into powdered. After sieving the powder keep it in an airtight polythene bag. Then the obtained powder was employed for extraction in order to prepare the sample for assessing the phenolic profiles and antioxidant activity. Extraction was done ultrasound-assisted extraction (UAE) by the following procedures: concentration of ethanol 60%, ultrasonic temperature 70 °C, ultrasonic time 50 min, and liquid-solid ratio 40 mL/g. After extraction, the mixture was then centrifuged at 6000×g for 10 min and the upper layer was gathered by filtration. The crude extract was preserved at -20°C until further analysis.
2.2. Chemicals
Ethanol and distilled water were used as the extraction solvent. Whereas, gallic acid, tannic acid, quercetin and were purchased (Sigma Chemical, USA) and used to make a calibration standard for total polyphenols and total flavonoids. Aluminum chloride (Sigma-Aldrich), Folin-Ciocalteu, sodium carbonate and sodium hydroxide were received from Aladdin Chemistry Co., Ltd. (Shanghai, China). Unless otherwise specified, all chemical and reagent was of analytical grade.
2.3. Total polyphenol contents determination
Total polyphenol concentration was assessed by utilizing Folin-Ciocalteu (FC) colorimetric technique presented by Biswas et al. [15]. Briefly, Folin–Ciocalteu reagent and 10% Na2CO3 were used to generate a blue colour solution. Then, 1.0 mL extract was combined to 0.75 mL Folin-Ciocalteu reagent, 0.25 mL 10% (w/w) Na2CO3, and 1.0 mL distilled water. The combinations were then digested in the dark for 60 min at room temperature to develop intense blue colour, and absorbance of the intense blue colour solutions was taken with a spectrophotometer at 765 nm. Using a standard curve of gallic acid, the content of total phenolic compounds was estimated and expressed as mg GAE/g DW.
2.4. Total flavonoid content determination
The total flavonoid concentration in sample was measured by utilizing colorimetric method outlined by Ordon et al. [16]. In an Eppendorf tube, 1 mL of sample extract was combined with 1 mL NaNO2 (5%, w/v), 2 mL AlCl3 solution (10%), and 1 mL NaOH solution (1 mol/L). The final volume was set to 5 mL by adding deionized water. After 10 min of incubation, a spectrophotometer (UV 2700, Shimadzu, Japan) was used to read the absorbance at 510 nm. The response was given as mg of quercetin equivalent (mg QE/g DW) per gram of dry weight.
2.5. Total tannin content determination
The total tannin content in the extracts was evaluated utilizing the procedure with little modification [17]. In short, 1 mL of extract was combined with 49 mL of distilled water in a 100 mL volumetric flask, and after vigorously shaking the mixture, adding (HPO3)3 0.1 mL, 1.7 mL of 75% ethanol, FC 2.5 mL and 10 mL of Na2CO3. After 15 min of incubation, the absorbance at 680 nm was read with a spectrophotometer (UV 2700, Shimadzu, Japan). The results were denoted as mg TAE/g DW, where tannic acid served as the standard.
2.6. Determination of antioxidant activities at different growth stages of various anatomical parts
2.6.1. Inhibition of 1,1diphenyl 2-picryl hydrazine (DPPH)
The DPPH scavenging effect of extracted samples was accomplished based on the technique mentioned in the previous studies with minor modifications [15]. In short, 2.0 mL of various concentrations of sample extract was added to 2.0 mL solution of DPPH (0.2 mM in anhydrous ethanol). The well-shaken mixture proceeded in dark for 30 min, and the reduced DPPH radical absorbance was read at 517 nm against the blank. The scavenging rate was estimated using the following formula.
| Scavenge percentage % = [(Abscontrol – Abssample)/Abscontrol] × 100 |
Where, Abscontrol was defined as control absorbance, whereas Abssample was defined as sample absorbance (the extracts).
2.6.2. ABTS+• radical scavenging activity
The ABTS+• radical inhibitor assay was implemented following the methodology stated by researcher with minor alterations [18]. ABTS was first solubilized in phosphate-buffered saline (PBS, 0.01 M, pH 7.4) with a final concentration 7 mM. To make ABTS+•, the ABTS solution was then coupled with an equivalent volume of 2.45 mM K2S2O8 solution and allowed to stand for 16 h in dark. Before using the solution, it was mixed thoroughly with distilled water until the absorbance at 734 nm was 0.70 ± 0.002. Then, 0.5 mL of various concentrations of extract was combined with 2 mL of the prepared ABTS+• solution and incubated for 10 min. The absorbance at 734 nm was read utilizing a Spectra Max microplate reader, and trolox was utilized as a reference. Then, ABTS activity was expressed as trolox equivalents (μmol TE/g DW).
2.6.3. Ferric reducing antioxidant activity assay
Benzie and Strain. Method was carried out to assess the ferric-reducing power of the extract based on the inhibitor potentials observed during the reduction of Fe3+-2,4,-tripyridyl-s-trazine (TPTZ) for the presence of antioxidants [19]. The FRAP test was performed by following the instructions in the business kit. By maintaining the ratio 10:1:1 of CH3COONa buffer, TPTZ and FeCl3·6H2O solution FRAP working solution was obtained. After mixing 5 μL of the samples with 180 μL of the FRAP solution was allowed for 10 min at room temperature, the absorbance at 593 nm was observed utilizing a microplate reader against the blank. As a standard, a working solution of FeSO4·7H2O that had just been made was used and the results were stated as μmol Fe2+/g DW.
2.7. Statistical analysis
Analysis of variance (one-way ANOVA) was conducted by applying SPSS version 16 to test significant differences in phytochemical content and antioxidant activities. The finding of this study was outlined in the form of a mean value with standard error (SE) based on triplicate measurements. The least significant difference (LSD) test was utilized to compare the averages by applying Statistix 8.1 software. In addition, using Pearson's correlation coefficient, the interactions between of phytochemical and antioxidant activity were evaluated.
3. Results
3.1. Total polyphenol content in different plant parts
There was a significant difference in total polyphenol content at various stages of growth and anatomical parts were illustrated in Fig. 1. The total phenolic content in C. olitorius ranged from 6.57 to 11.11 mg GAE/g DW for leaves, 0.97–5.09 mg GAE/g DW for stems, and 2.41–5.95 mg GAE/g DW for roots. In the case of C. capsularis varied from 5.89 to 7.93 mg GAE/g DW for leaves, 1.28–3.17 mg GAE/g DW for stems, and 1.04–4.12 mg GAE/g DW for roots. The results indicated that TPC levels in leaves were substantially higher than in stems and roots. TPC increased with an increasing trend and reached its highest at the 3rd stage, then decreased. On the other hand, TPC in stems and roots showed a decreasing and increasing trend with the increasing growth stages, respectively. However, in regards to various growth phases and plant parts, T8 displayed the highest concentration of phenolic compounds, whereas varieties Y1 showed consistently lower content. The highest total polyphenol content was found in leaves at the 3rd stage, whereas stems and roots displayed the highest total polyphenol content at the 1st and 4th stages of growth, respectively. For polyphenol acid, leaves produced the highest content at stage 3rd and about 6 and 3 times higher measured at the same time compared to the stems and roots, respectively, for two varieties of both species.
Fig. 1.
Total polyphenol content in different anatomical parts extracts of C. olitorius (T8) and C. capsularis (Y1) at various growth stages. Values are means ± SE. Different small letters displayed the differences at different growth stages and plant parts of particular varieties.
3.2. Total flavonoid content in different plant parts
Like total polyphenol content, the results demonstrated that the total flavonoid content of each species differed significantly across various growth stages and anatomical parts (Fig. 2). The flavonoids content of T8 of C. olitorius was 5.14–7.78 mg QE/g DW for leaves, 0.99–3.29 mg QE/g DW for stems, and 1.78–3.22 mg QE/g DW for roots. In the case of C. capsularis 3.93–5.93 mg QE/g DW for leaves, 0.74–2.01 mg QE/g DW for the stems, 1.26–2.64 mg QE/g DW for roots. The results showed that TFC increased from 1st (vegetative growth) to the 3rd stage (flowering) of all varieties. However, the TFC content was highest at the 3rd stage (flowering), both of the varieties T8 and Y1, respectively. At the 3rd stage, the highest flavonoid contents (7.78 mg QE/g DW) of leaves was recorded in T8, while the highest level of total flavonoid (5.93 mg QE/g DW) was recorded in Y1, which were approximately 2–3 times higher the value recorded in the stems and roots. Significant differences in flavonoid concentration were observed between stem and root, and accumulation tendencies shifted in the opposite direction as growth stages increased. In the case of the stem of both species displayed a decreasing trend of total flavonoids where the highest content was recorded 1st stage in all varieties. Whereas, root extracts of C. olitorius recorded comparatively higher flavonoid content than C. capsularis and were highest in 4th stage of growth.
Fig. 2.
Total flavonoid content in different anatomical parts extracts of C. olitorius (T8) and C. capsularis (Y1) at various growth stages. Values are means ± SE. Different small letters displayed the differences at different growth stages and plant parts of particular varieties.
3.3. Total tannin content in the different plant parts
Results of total tannin content in the leaves, stems, and roots of both of varieties were presented in Fig. 3. It was observed that there was significant variation for total tannin contents in various growth stages and plant parts of C. olitorius and C. capsularis and total tannin contents were richest in leaves, followed by stems and roots. Total tannin contents of C. olitorius were 16.51–26.89 mg TAE/g DW for leaves, 7.65–14.72 mg TAE/g DW for the stems, and 4.02–13.98 mg TAE/g DW for roots. While total tannin contents in C. capsularis were 13.87–22.73 mg TAE/g DW, 6.07–12.56 mg TAE/g DW and 3.17–11.98 mg TAE/g DW for leaves, stems, and roots, respectively. Like TFC and TPC, the total tannin contents increased from the seedling stage to the flowering stage of both species. However, the total tannin content were highest (26.89 mg TAE/g DW) and (22.73 mg TAE/g DW) in leaves at the 3rd stage (flowering) of T8 and Y1, respectively.
Fig. 3.
Total tannin content in different anatomical parts extracts of C. olitorius (T8) and C. capsularis (Y1) at various growth stages. Values are means ± SE. Different small letters displayed the differences at different growth stages and plant parts of particular varieties.
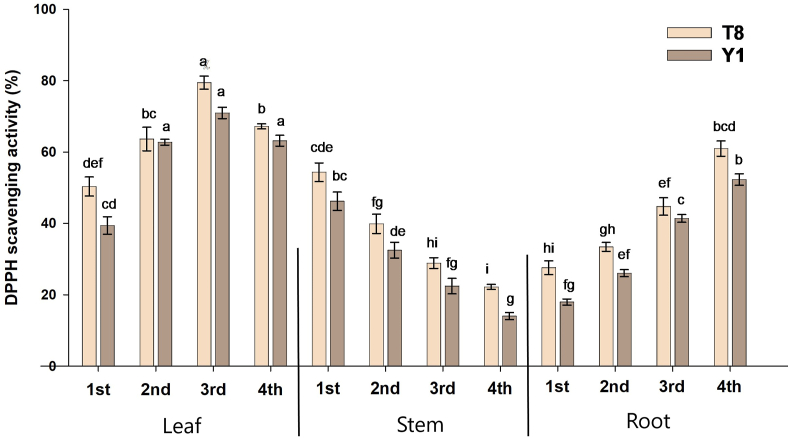
3.4. Free radical scavenging (DPPH) activity
Antioxidants that have the ability to donate electron or hydrogen atoms to neutralize free radicals or convert DPPH• radical or a more stable DPPH• colourless product in the reduced form [20]. Results revealed that leaves extract displayed the highest scavenging activity compared to stems and leaves of both species (Fig. 4). The DPPH• value in different growth stages ranged from 50.33 to 79.46% for leaves, 22.20–54.37% for stems, and 27.58–60.97% for roots of C. olitorius. Whereas the scavenging rate of DPPH• of C. capsularis ranged from 39.41 to 7.97% for leaves, 14.03–46.21% for stems and 17.97–52.30% for roots. The result indicated that variety of C. olitorius demonstrated a higher antioxidant capacity than C. capsularis. In leaves of both varieties, DPPH scavenging activities increased 1st (seedlings) stage to 3rd (flowering) and then decreased. On the other hand, the stems displayed decreasing trend from 1st stage to 4th stage, and the lowest was recorded in 4th stage of both of the species, whereas T8 of C. olitorius scavenged the highest DPPH (46.21%). However, roots from both species were inhibited just as opposed to stems. It showed that all varieties increased the DPPH scavenged percentage with increasing phonological stage, and most aged roots showed a higher percentage of inhibition.
Fig. 4.
Antioxidant activity in different anatomical parts of C. olitorius (T8) and C. capsularis (Y1) extracts using 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity. Values are means ± SE. Various small letters displayed the differences at different growth stages and plant parts of particular varieties.
3.5. ABTS+• radical scavenging activity
The performance of ABTS+• radical scavenging behaviour in different plant parts in different growth stages of C. olitorius and C. capsularis extract was illustrated in Fig. 5. Results revealed that the ABTS+• radical scavenging activity had a significant variation in respect of the growth stage and various anatomical parts. The value of the scavenging effect ranged from 79.67 to 104.10 μmol TE/g DW for leaves, 10.84–69.48 μmol TE/g DW for stems and 13.21–74.26 mmol TE/g DW for root concerning C. olitorius. In the other word, C. capsularis reported the ABTS radical scavenging activity 63.63–95.60 μmol TE/g DW, 9.16–59.21 μmol TE/g DW and 10.64–63.79 μmol TE/g DW respectively for leaves, stems and roots. The flowering stage exhibited the highest activity in both of the varieties; 104.10 μmol TE/g DW for C. olitorius and 95.60 μmol TE/g DW for C. capsularis. This result indicated that C. olitorius extract demonstrated a higher ABTS activity than C. capsularis in the same state of growth. It could be concluded from these reports that the C. olitorius extract was a more potent scavenger of ABTS+• radical.
Fig. 5.
Antioxidant activity in different parts of C. olitorius (T8) and C. capsularis (Y1) extracts using ABTS+• radical scavenging activity. Values are means ± SE. Various small letters displayed the differences at different growth stages and plant parts of particular varieties.
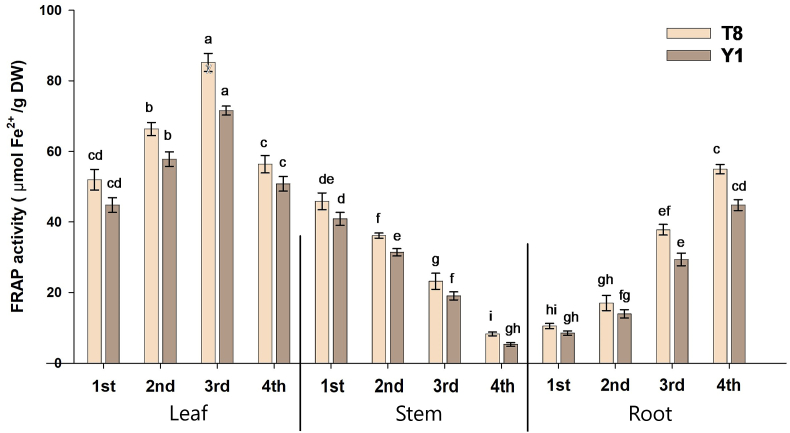
3.6. Ferrous reducing antioxidative power (FRAP)
Like DPPH, the activity of FRAP also showed a similar trend with increasing the growth stages in different organs of both species (Fig. 6). The ferric-reducing antioxidant capacity of C. olitorius ranged from 52.01 to 85.21 μmol Fe2+/g DW leaves, 8.27–45.85 μmol Fe2+/g DW for stems, and 10.53–54.98 μmol Fe2+/g DW for roots. While the C. capsularis ranged from 44.78 to 71.57 μmol Fe2+/g DW for leaves, 5.31–40.87 μmol Fe2+/g DW for stems, 8.51–44.78 μmol Fe2+/g DW for roots. It was observed that, among the different parts, leaves showed the highest FRAP activities, where the activity increased at a certain level and then decreased. The highest scavenging activity was recorded in the 3rd stage. The result of the experiment reflected that of FRAP activities of C. olitorius demonstrated the more elevated than the C. capsularis. The 3rd (flowering) stage of both species displayed higher FRAP activity than the other growth stages when comparing the activities within growth phases. But, both species' roots showed the FRAP activities just as opposed to the leaf.
Fig. 6.
Antioxidant activity in different parts of C. olitorius (T8) and C. capsularis (Y1) extracts using Ferrous reducing antioxidative power (FRAP) radical scavenging activity. Values are means ± SE. Various small letters displayed the differences at different growth stages and plant parts of particular varieties.
3.7. Pearson correlation coefficient
It has been stated that plant extract's phenolic concentration has been linked or correlated to its antioxidant potential [15,21]. A Pearson's correlation coefficient was utilized to investigate correlations between the extract's phytochemical content of extract and antioxidant characteristics assessed in both C. olitorius and C. capsularis (Table 1). Results demonstrated that the extract phytochemicals exhibited an acceptable and significant association with DPPH, ABTS, and FRAP values by reducing the levels of free radicals. Analytical results revealed that the strongest correlative value was obtained from ABTS and FRAP (R2 = 0.98) followed by TPC and TFC. In addition, all other bioactive compounds had significant positive correlations with rest of the antioxidant method. These correlations in the phytochemical study suggest that both species contained secondary antioxidant metabolites that scavenge the free radicals. Thus a finding of the study justifies the usage of C. olitorius and C. capsularis in traditional medicine practices.
Table 1.
Pearson correlation coefficient for considered variables TPC, TFC, TTC, DPPH, ABTS and FRAP.
| TPC | TFC | TTC | DPPH | ABTS | FRAP | |
|---|---|---|---|---|---|---|
| TPC | 1 | |||||
| TFC | 0.97** | 1 | ||||
| TTC | 0.94** | 0.94** | 1 | |||
| DPPH | 0.87** | 0.88** | 0.92** | 1 | ||
| ABTS | 0.92** | 0.91** | 0.92** | 0.93** | 1 | |
| FRAP | 0.93** | 0.92** | 0.96** | 0.95** | 0.98** | 1 |
∗∗Correlation is significant at 1% level.
4. Discussion
Plants synthesize and accumulate a variety of secondary metabolites with low and high molecular weights that have been demonstrated to have antioxidant activities [22]. These biologically active compounds that belong to various chemical classes like polyphenols and flavonoids have been reported to be reducing agents, singlet oxygen quenchers, and hydrogen donors. Concentrations of these compounds vary with respect to plant parts, planting seasons, solvents used for extraction, and growth stages [11,23]. The variation in phenolic compound concentrations can be related to a variety of factors, including internal characteristics such as plant species, organs, and age, as well as extrinsic factors such as biotic and abiotic influences. In this study, polyphenolic content in various parts extracts of both varieties of C. olitorius and C. capsularis were significantly different at the different phenological stages. The series of complicated reactions that occur during a plant's growth phase ultimately results in changes in its photochemistry; for instance, the phenolic content of plants may increase or decrease steadily toward the conclusion of maturation [24]. Thus, the present study suggests that biosynthesis and accumulation of phenol and flavonoids occur independently in each plant organ, so great variation in their synthesis is evident at different phenological stages.
According to the results of this research finding, both of the varieties had higher levels of phytochemical contents in the leaves than in the stems or roots. Similar results have been found for Portulaca oleracea L. (purslane) leaves, which contain more than stems at various harvest stages [25]. In addition, the flavonoid content at the flowering stage in leaves of both varieties was higher than at any other stage. In agreement with our findings, several researchers reported an increase in flavonoid concentration in different plants such as Teucrium polium L. [11], and Ziziphora clinopodioides Lam [26]. during their flowering stage. In the pre-flowering and flowering stages, the high concentration of flavonoids may be linked to the significant function of fruit coloration, ultraviolet protection, pigmentation, and flower production's aroma, along with their involvement in defense mechanism against pests that might attack the blooms [27]. It also reported flavonoids mainly accumulate in young plants, and their concentration is reduced after the flowering stage when the plant is actively differentiating rather than synthesizing metabolite [28].
Total polyphenol content recorded in leaves was also highest at the flowering stage and then concentration is reduced after the flowering stage both of C. capsularis and C. olitorius. The authors noticed a rise in the leaves during the flowering phase of Portulaca oleracea L. compared to the vegetative phase [29]. Similar results displayed the highest content of polyphenols supported by the findings of Smilax campestris Griseb. Leaves [30], Boerhavia Diffusa L. and Sida cordifolia L [31]. possessed the maximum quantity of total phenolics in the flowering stages. The higher phenolic content detected in the pre-flowering and all flowering stages could result from soil nutrient loss as the plant approaches maturity. It has been reported that the phenolic content increased in the root as the plant advanced in age [32], which was consistent with our observations in the roots of C. capsularis and C. olitorius. Tannins are a distinct class of polyphenolic secondary metabolites that strongly influence the phytoconstituents and phytotherapeutic properties of traditional medicinal plants. Diabetics may benefit by consuming tannins from plant sources due to their ability to lower blood glucose levels, scavenge for free radicals, and increase antioxidant enzymes. The current experiment findings of total tannins in Corchorus capsularis and Corchorus olitorius were consistent to previous study [15] and much higher than previously reported in the leaves of Paederia Foetida L [33]. This comparison indicates that both C. olitorius and C. capsularis are excellent sources of total tannins and are utilized in a variety of therapeutic applications.
Antioxidants facilitate and help the body do normal things like normal cell growth, support the immune system, and the prevention of molecular degeneration, which includes slowing down of the aging process. Previous studies have indicated that the effect of antioxidant activity at the plant's developmental stage [29,34,35]. Incase of antioxidant activities, the high DPPH radical scavenging capacity was noted in the leaf extract of flowering stages, seedling stages of stems, and post-flowering stages of roots of both the species. At the stage of phenological and organ development where the greatest amount of antioxidant activity was achieved, the highest phytochemical contents were obtained at the same stage and from the same organ. The combined effects of the phenolic and flavonoid compounds as well as other components may influence the extract's antioxidant activity by scavenging free radicals and suppressing oxidative damages induced by the hydroxyl radicals or metal chelation [36]. Similar results were also recorded in Achillea Millefolium L. whereas a high level of antioxidant potential at the flowering stage was substantially greater than the fruit set [35].
In the case of ABTS+•, the highest activity was found in leaves of C. olitorius than C. capsularis, even higher compared to stems and roots. The high phenolic content may be representative of the observed antioxidant activity, as phenols and flavonoids are precursors to bioactivity and important contributors to the antioxidative property of plants [37]. In the FRAP is a well-known, reliable approach model where Fe3+/ferricyanide complex is turned into greenish ferrous by reacting with an atom of hydrogen to break the chain of free radicals. Similar to the DPPH, it was observed in this study reported that flowering stages leaves, young seedlings stems, and aged roots extract demonstrated greater scavenging potential against ABTS activity. Previous findings of C. olitorius leaves extract revealed a wide range of FRAP activity [38] that was much higher than the current study. This variation is due to differences in growth stages, drying methods, extraction conditions, analysis method and solvent used for extraction as well the presence of phytochemicals. It was reported that Satureja rechingeri Jamzad extract obtained at the full-flowering stage had the greatest FRAP values and was most effective in neutralizing DPPH radicals [13]. This may be due to high total phenol compounds during the same growth stages, which supported the antioxidant activity of these compounds. Numerous studies have documented a positive correlation between the amount of phenolic compounds and the antioxidant activity of plants [[39], [40], [41]]. By evaluating the correlation coefficients, it is possible to conclude that phytochemicals are notably accountable for antioxidant activities of interest in the selected plant species. Pearson correlation findings also support by the previous study where bioactive compounds of leaves of both species correlated with bioactive compounds and the best correlation was observed between TFC and FRAP as well as between TPC and FRAP followed by TFC and ABTS at the 1% level of significance [15]. It has been conferred the leaves, stems, and roots of both species as potent sources of antioxidants and suggests that secondary metabolites of C. olitorius and C. capsularis make a significant contribution to antioxidant potential [42]. These findings were consistent with our study due to the presence of phenols and flavonoids and their excellent hydrogen-donating antioxidant capacities, which play a protective function against caused by hydroxyl radical-induced oxidative damage. Thus, the current study confirms that relative concentrations of phytochemical compounds that are collected at various phenological stages play an important role in their antioxidative capacities.
5. Conclusion
It is important to note that phenolic chemicals can be extracted from plant parts and growth stages for medicinal applications, as these offer a great deal of potential for pharmaceutical formulations. This study demonstrated that quantitative differences exist in phytochemical content and antioxidant potential in respect to different plant parts and each phenological stage. In addition, study findings also express bioactive compounds and antioxidant activities differ regarding species where C. olitorius demonstrated higher content and antioxidant activities than C. capsularis in different parts of the plant. The flowering stage leaves, stems at seedling and aged roots suggesting for harvesting as a decent source of phytochemical contents with adequate antioxidant activity. Further research is recommended to profiling and therapeutic potential of different parts at the phenological stage. The results suggest that the C. olitorius and C. capsularis leaves should be incorporated into the human diet for therapeutic potentials, while their stems and roots could serve as natural antioxidant sources.
Author contribution statement
Defang Li, Ashok Biswas and Susmita Dey: Conceived and designed the experiments.
Ashok Biswas and Susmita Dey: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Aiping Xiao, Yong Deng and Ziggiju Mesenbet Birhanie: Contributed reagents, materials, analysis tools or data.
Siqi Huang, Liangliang Liu, Defang Li: Analyzed and interpreted the data.
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Ashok Biswas, Email: ashok.ag1sau@gmail.com.
Defang Li, Email: lidefang@caas.cn, chinakenaf@126.com.
References
- 1.Islam M.T., Sultana I., Hossain J., Homa Z., Chowdhury M.M.U., de Freitas R.M. A comprehensive review of Corchorus capsularis: a source of nutrition, essential phytoconstituents and biological activities. J. Biomed. Pharmaceut. Res. 2013;2:1–8. [Google Scholar]
- 2.Biswas A., Dey S., Huang S., Deng Y., Birhanie Z.M., Zhang J., Akhter D., Liu L., Li D. A comprehensive review of C. capsularis and C. olitorius: a source of nutrition, essential phytoconstituents and pharmacological activities. Antioxidants. 2022;11 doi: 10.3390/antiox11071358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeghichi S., Kallithraka S., Simopoulos A.P. Nutritional composition of molokhia (Corchorus olitorius) and stamnagathi (Cichorium spinosum) World Rev. Nutr. Diet. 2003;91:1–21. doi: 10.1159/000069924. [DOI] [PubMed] [Google Scholar]
- 4.Islam M.M. Biochemistry, medicinal and food values of jute (Corchorus capsularis L. and C. olitorius L.) leaf: a review. Int. J. Enhanc. Res. Sci. Technol. Eng. 2013;2:35–44. [Google Scholar]
- 5.Ahmed F. Nutraceutical potential of molokhia (Corchorus olitorius L.): a versatile green leafy vegetable. Pharmacogn. Res. 2021;10:24–30. doi: 10.4103/pr.pr. [DOI] [Google Scholar]
- 6.Adebo O.A., Medina-Meza I.G. Impact of fermentation on the phenolic compounds and antioxidant activity of whole cereal grains: a mini review. Molecules. 2020;25:1–19. doi: 10.3390/molecules25040927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atanasov A.G., Waltenberger B., Pferschy-Wenzig E.M., Linder T., Wawrosch C., Uhrin P., Temml V., Wang L., Schwaiger S., Heiss E.H., Rollinger J.M., Schuster D., Breuss J.M., Bochkov V., Mihovilovic M.D., Kopp B., Bauer R., Dirsch V.M., Stuppner H. Discovery and resupply of pharmacologically active plant-derived natural products: a review. Biotechnol. Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maisuthisakul P., Suttajit M., Pongsawatmanit R. Assessment of phenolic content and free radical-scavenging capacity of some Thai indigenous plants. Food Chem. 2007;100:1409–1418. doi: 10.1016/j.foodchem.2005.11.032. [DOI] [Google Scholar]
- 9.Unuofin J.O., Otunola G.A., Afolayan A.J. Polyphenolic content, antioxidant and antimicrobial activities of Vernonia mespilifolia less. Used in Folk Medicine in the Eastern Cape Province, South Africa. J. Evidence-Based Integr. Med. 2018;23:1–9. doi: 10.1177/2515690X18773990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toker Z. Variation of total hypericin, phenolic and flavonoid compounds in Hypericum triquetrifolium during its phenological cycle. Pharm. Biol. 2009;47:285–288. doi: 10.1080/13880200802578983. [DOI] [Google Scholar]
- 11.Sharifi-Rad M., Pohl P., Epifano F., Zengin G., Jaradat N., Messaoudi M. Teucrium polium (L.): phytochemical screening and biological activities at different phenological stages. Molecules. 2022;27 doi: 10.3390/molecules27051561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hazrati S., Hosseini S.J., Ebadi M.T., Nicola S. Evolution of phytochemical variation in myrtle (Myrtus communis L.) organs during different phenological stages. Horticulturae. 2022;8:1–17. doi: 10.3390/horticulturae8090757. [DOI] [Google Scholar]
- 13.Alizadeh A. Essential oil composition, phenolic content, antioxidant, and antimicrobial activity of cultivated Satureja rechingeri Jamzad at different phenological stages. Zeitschrift Fur Naturforsch. - Sect. C J. Biosci. 2015;70:51–58. doi: 10.1515/znc-2014-4121. [DOI] [PubMed] [Google Scholar]
- 14.Ben Yakoub A.R., Abdehedi O., Jridi M., Elfalleh W., Nasri M., Ferchichi A. Flavonoids, phenols, antioxidant, and antimicrobial activities in various extracts from Tossa jute leave (Corchorus olitorus L.) Ind. Crop. Prod. 2018;118:206–213. doi: 10.1016/j.indcrop.2018.03.047. [DOI] [Google Scholar]
- 15.Biswas A., Dey S., Li D., Liu Y., Zhang J., Huang S., Pan G., Deng Y. Comparison of phytochemical profile, mineral content, and in vitro antioxidant activities of Corchorus capsularis and Corchorus olitorius leaf extracts from different populations. J. Food Qual. 2020;2020 doi: 10.1155/2020/2931097. [DOI] [Google Scholar]
- 16.Ordoñez A.A.L.L., Gomez J.D., Vattuone M.A., Isla M.I. Antioxidant activities of Sechium edule (jacq.) swartz extracts. Food Chem. 2006;97:452–458. doi: 10.1016/j.foodchem.2005.05.024. [DOI] [Google Scholar]
- 17.Shad Muhammad Aslam, Shad M.A., Nawaz H., Rehman T., Ahmad H.B. Optimization of extraction efficiency of tannins from Cichorium intybus L.: application of response surface methodology. J. Med. Plants Res. 2012;6:4467–4474. doi: 10.5897/jmpr11.928. [DOI] [Google Scholar]
- 18.Van Den Berg R., G.R.M.M.M.M. Haenen, Van Den Berg H., Bast A. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of antioxidant capacity measurements of mixtures. Food Chem. 1999;66:511–517. doi: 10.1016/S0308-8146(99)00089-8. [DOI] [Google Scholar]
- 19.Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “ antioxidant power”. The FRAP Assay. 1996;76:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 20.Ben Yakoub A.R., Abdehedi O., Jridi M., Elfalleh W., Bkhairia I., Nasri M., Ferchichi A. Bioactive polysaccharides and their soluble fraction from Tossa jute (Corchorus olitorius L.) leaves. Food Biosci. 2020;37 doi: 10.1016/j.fbio.2020.100741. [DOI] [Google Scholar]
- 21.Nowicka A., Kucharska A.Z., Sokół-Łętowska A., Fecka I. Comparison of polyphenol content and antioxidant capacity of strawberry fruit from 90 cultivars of Fragaria × ananassa Duch. Food Chem. 2019;270:32–46. doi: 10.1016/j.foodchem.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Kasote D.M., Katyare S.S., Hegde M.V., Bae H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. 2015;11:982–991. doi: 10.7150/ijbs.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lagnika L., Amoussa A.M.O., Adjileye R.A.A., Laleye A., Sanni A. Antimicrobial, antioxidant, toxicity and phytochemical assessment of extracts from Acmella uliginosa, a leafy-vegetable consumed in Bénin, West Africa, BMC Complement. Alternative Med. 2016;16:1–12. doi: 10.1186/s12906-016-1014-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buriro M., Wadhayo Gandahi A., Chand Oad F., Ibrahim Keerio M., Tunio S., Waseem Hassan S.U.L., Mal Oad S. Wheat seed germination under the influence of temperature regimes. Sarhad. 2011;27:539–543. https://www.researchgate.net/publication/266463463 [Google Scholar]
- 25.Petropoulos S.A., Fernandes Â., Dias M.I., Vasilakoglou I.B., Petrotos K., Barros L., Ferreira I.C.F.R. Nutritional value, chemical composition and cytotoxic properties of common purslane in relation to harvesting stage and plant part. Antioxidants. 2019;8:1–15. doi: 10.3390/antiox8080293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding W., Yang T., Liu F., Tian S. Effect of different growth stages of Ziziphora clinopodioides Lam. on its chemical composition. Phcog. Mag. 2014;10:S1–S5. doi: 10.4103/0973-1296.127329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D.S., Mamta Saxena A.G., Saxena Jyoti, Nema Rajeev, Gupta A. Phytochemistry of medicinal plants. Med. Plants Cent. Asia Uzb. Kyrg. 2013;1:13–14. doi: 10.1007/978-1-4614-3912-7_4. [DOI] [Google Scholar]
- 28.Riipi M., Ossipov V., Lempa K., Haukioja E., Koricheva J., Ossipova S., Pihlaja K. Seasonal changes in birch leaf chemistry: are there trade-offs between leaf growth and accumulation of phenolics? Oecologia. 2002;130:380–390. doi: 10.1007/s00442-001-0826-z. [DOI] [PubMed] [Google Scholar]
- 29.Saffaryazdi A., Ganjeali A., Farhoosh R., Cheniany M. Variation in phenolic compounds, α-linolenic acid and linoleic acid contents and antioxidant activity of purslane (Portulaca oleracea L.) during phenological growth stages. Physiol. Mol. Biol. Plants. 2020;26:1519–1529. doi: 10.1007/s12298-020-00836-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rugna A., Ricco R., Gurni A., Wagner M. Variaciones en el contenido de los polifenoles foliares en Smilax campestris Griseb. - smilacaceae - según su grado de desarrollo. Lat. Am. J. Pharm. 2008;27:247–249. [Google Scholar]
- 31.Verma V., Kasera P.K. Short communication variations in secondary metabolites in some arid zone medicinal plants in relation to season and plant growth. Indian J. Plant Physiol. 2007;12:203–206. 203. [Google Scholar]
- 32.Oloyede F.M.A., Oloyede F.M.A., Obuotor E.M. Effect of plant maturity on the antioxidant profile of Amaranthus cruentus L . and celosia argentea L. Bull. Environ. Pharmacol. Life Sci. 2012;2:18–21. [Google Scholar]
- 33.Ojha S., Raj A., Roy A., Roy S. Extraction of total phenolics, flavonoids and tannins from Paederia foetida L. Leaves and their relation with antioxidant activity. Phcog. J. 2018;10:541–547. doi: 10.5530/pj.2018.3.88. [DOI] [Google Scholar]
- 34.Vlaisavljević S., Kaurinović B., Popović M., Vasiljević S. Profile of phenolic compounds in Trifolium pratense L. extracts at different growth stages and their biological activities. Int. J. Food Prop. 2017;20:3090–3101. doi: 10.1080/10942912.2016.1273235. [DOI] [Google Scholar]
- 35.Farhadi N., Babaei K., Farsaraei S., Moghaddam M., Ghasemi Pirbalouti A. Changes in essential oil compositions, total phenol, flavonoids and antioxidant capacity of Achillea millefolium at different growth stages. Ind. Crop. Prod. 2020;152 doi: 10.1016/j.indcrop.2020.112570. [DOI] [Google Scholar]
- 36.Nimse S.B., Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–28006. doi: 10.1039/c4ra13315c. [DOI] [Google Scholar]
- 37.Bendary E., Francis R.R., Ali H.M.G., Sarwat M.I., El Hady S. Antioxidant and structure–activity relationships (SARs) of some phenolic and anilines compounds. Ann. Agric. Sci. 2013;58:173–181. doi: 10.1016/j.aoas.2013.07.002. [DOI] [Google Scholar]
- 38.Sadat A., Hore M., Chakraborty K., Roy S. Phytochemical analysis and antioxidant activity of methanolic extract of leaves of Corchorus olitorius. Int. J. Curr. Pharmaceut. Res. 2017;9:59–63. doi: 10.22159/ijcpr.2017v9i5.22138. [DOI] [Google Scholar]
- 39.Abreu-Naranjo R., Paredes-Moreta J.G., Granda-Albuja G., Iturralde G., González-Paramás A.M., Alvarez-Suarez J.M. Bioactive compounds, phenolic profile, antioxidant capacity and effectiveness against lipid peroxidation of cell membranes of Mauritia flexuosa L. fruit extracts from three biomes in the Ecuadorian Amazon. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e05211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brasileiro B.G., Paulo J., Leite V., Wagner V., Casali D. The influence of planting and harvesting times on the total phenolic content and antioxidant activity of Talinum triangulare (Jacq.) Willd. Acta Sci. 2015:249–255. doi: 10.4025/actasciagron.v37i2.19130. [DOI] [Google Scholar]
- 41.Rodrigues I., Carolyne A., Masrouah C., Pimentel C. Variation of biochemical and antioxidant activity with respect to the phenological stage of Tithonia diversifolia Hemsl . (Asteraceae) populations. Ind. Crop. Prod. 2018;121:241–249. doi: 10.1016/j.indcrop.2018.04.080. [DOI] [Google Scholar]
- 42.Mahomoodally M.F., Sinan K.I., Bene K., Zengin G., Orlando G., Menghini L., Veschi S., Chiavaroli A., Recinella L., Brunetti L., Leone S., Angelini P., Hubka V., Covino S., Venanzoni R., Picot-Allain M.C.N., De Lellis L., Cama A., Cziáky Z., Jekő J., Ferrante C. Bridelia speciosa müll.Arg. stem bark extracts as a potential biomedicine: from tropical western africa to the pharmacy shelf. Antioxidants. 2020;9 doi: 10.3390/antiox9020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.