Abstract
Corneal disease threatens vision globally. Among corneal diseases, calcific band keratopathy has severe effects on vision owing to its unique location. Currently, ethylene diamine tetraacetic acid (EDTA) chelation remains the most important treatment. However, only the safety of low-dose topical EDTA eye drops is well established in humans. Therefore, the purpose of this study was to determine the safe dose range of EDTA for calcific band keratopathy surgery and its toxic effects on rabbit eyes. Rabbits were administered different doses of EDTA solutions (0.50, 0.20, 0.10, 0.05, and 0.01 M) for twenty minutes. In day seven, the rabbits were euthanized and pathological examination was performed for cornea. We found severe corneal edema in 0.50 M group, while milder edema in lower-concentration treated groups. Followed by corneal thickness measurement, the measured values increase to the peak in post-operative three day (0.20 M group) or one day (lower-concentration groups), then decreased. Groups comparison shown significant difference between BSS control group and higher concentration groups (0.20 M and 0.10 M) (P < 0.001) in observation period, but no significance was observed between low concentration and control group in the day seven after surgery (P > 0.05). Confocal microscopy examination suggested, the number of corneal endothelial cells significantly decreased from 3428.6 ± 180.3 cells/mm2 to 2808 ± 80.6 cells/mm2 in the 0.50 M group, while the lower-concentration groups showed lesser toxic effects on corneal endothelial cells. Finally, our histological examination demonstrated inflammation in each experimental group and dose-dependent, compared with control group. Our study found 0.05 M and 0.01 M EDTA solutions had no obvious toxic effect on the corneal endothelium compared with higher concentration. However, further study of EDTA side effect by clinical trials, and therapeutic effect observation with different concentration are necessary.
Keywords: Corneal endothelial cells (CECs), Calcific band keratopathy (CBK), Ethylene diamine tetraacetic acid (EDTA), Solution concentration, Rabbits
1. Introduction
Dixon [1] first described calcific band keratopathy (CBK) in 1848 as a degenerative corneal disease characterized by the deposition of grayish to whitish opacities consisting of calcium hydroxyapatite on the corneal surface, most commonly involving the Bowman layer [[1], [2], [3]]. In the early stages, the opacities appear in the nasal or temporal margins of the cornea, leaving a clear space between the corneal limbus and the opacity. As the disease progresses, the opacity gradually extends to the central part of the cornea and becomes band-shaped [4]. Early corneal opacity is slight and not visible to the naked eye. Calcium deposits gradually become dense, leading to thickening and elevation of the upper part of the epithelium. Moreover, roughness, uneven surface, and epithelial erosions cause photophobia, tearing, pain in the eyes, and other symptoms [5]. In the late stages, vision may decrease significantly. Visual loss is not only related to the extent and location of keratopathy but also complicated by uveitis and cataracts. CBK is caused by chronic eye disease, such as chronic uveitis, corneal ulcers, and chemical burns or systematic diseases, such as hypercalcemia due to chronic renal failure [6], hyperparathyroidism [7] and sarcoidosis [8].
The primary mineral deposited in the cornea is hydroxyapatite, a complex crystal composed mainly of calcium and phosphorus, which usually occur in the blood and interstitial tissues in a supersaturated state. Although the exact mechanism of precipitation has not been determined, pH changes, increased tear evaporation, and elevated calcium concentrations are thought to be important causes of calcium precipitation [9].
Various modalities have been used to treat CBK, including superficial keratectomy [10], Nd:YAG laser [11], phototherapeutic keratectomy [2,12], lamellar keratoplasty [13] and chelation therapy using ethylene diamine tetraacetic acid (EDTA) [14]. EDTA is the most widely used method for treating CBK [1,2,11,[14], [15], [16], [17]]. The goal of treatment is to remove the calcium opacities and restore a smooth ocular surface. In most cases, vision can be improved; however, in eyes with poor visual potential, the procedure is mainly performed to improve ocular comfort.
EDTA is a chelating agent, which was originally used mainly for dialysis. One mole of EDTA chelates one mol of metallic ions [18]. EDTA for ophthalmic use was initially derived from Na2EDTA, but that formulation has lost the Food and Drug Administration approval and can now only be obtained at specially equipped compounding pharmacies [19].
In clinical settings, ophthalmologists usually remove the corneal epithelium from the area of the opacity using a golf-club spud and apply a fragment of EDTA-dipped surgical sponge to the opacity for 5 min. The sponge fragment is then removed, and the eye is checked for any remaining opacity using a light guide. If the opacity is not completely removed, another sponge fragment is applied for 5 min, and the procedure is repeated as required [4]. EDTA solutions are usually diluted to different concentrations according to the ophthalmologist's expertise. However, some studies have reported that certain concentrations of EDTA have toxic effects on the cornea and conjunctiva [20]. Moreover, EDTA can penetrate the corneal epithelium to affect the corneal endothelium [21].
To date, no study has compared the toxicity of different concentrations of EDTA solution on the cornea, particularly on non-regeneration corneal endothelial cells. Therefore, rabbit eyes in which the corneal epithelium was removed were used in this study to stimulate CBK surgery and evaluate the degree of corneal endothelial-cell damage caused by different concentrations of EDTA solution.
2. Methods and materials
2.1. Experimental animals
Thirty male 6-month-old New Zealand rabbits (3–3.5 kg) were purchased from Chengdu Dashuo Laboratory Animal Co. Ltd (No. 51203500027176). Each cage (815 × 500 × 340 mm) contained one rabbit. Rabbits were housed in a specific room with an air filtration rate of 10–20 air changes/h and a temperature of 20–26 °C. The humidity was 40–70%, and a fluorescent light was used to ensure a 12-h light (08:00–20:00) and dark cycle. The rabbits were housed in an environment where they could eat and drink freely and were adapted to the environment for at least two weeks before surgery. All animal experiments were approved by the Animal Ethics Committee of the Sichuan Provincial People's Hospital (No. 2022–308).
2.2. Animal grouping
The rabbits were randomly assigned to the treatment and control groups using the BioBook system (IDBS). Animals were evenly divided into six groups: in the control group, a surgical sponge fragment soaked in balanced salt solution (BSS) (MQA, Inami, Tokyo, Japan) was applied; in the experimental groups, surgical sponge fragments soaked in 0.01, 0.05, 0.10, 0.20 or 0.50 M EDTA (pH = 8.0, Sigma-Aldrich Trading Co., Ltd.) were applied.
2.3. Surgical methods and EDTA treatment
All animals were anesthetized using pentobarbital sodium (30 mg/kg intravenously). After anesthesia, the rabbit was placed on the operating table in the lateral position, draped with a towel, and the eye was routinely sterilized. The eyelid was opened using an eyelid opener, the conjunctival sac was rinsed with povidone-iodine + saline, and topical anesthesia was achieved using oxybuprocaine. A 1 cm × 1 cm surgical sponge was dipped in different concentrations of EDTA solution or BSS. The corneal epithelium was carefully removed using a golf-club spud, and a surgical sponge was placed in the center of the cornea for 20 min. Finally, the sponge was removed, the eye surface and conjunctival sac were rinsed with approximately 50 mL saline, and antibiotic ointment was applied.
2.4. Animal health observation
The rabbit's weight and food residue were recorded once a day.
2.5. Slit-lamp microscope examination
Slit-lamp microscopic examination of the anterior segment was performed zero, one, three, and seven days after the operation. An S350S slit-lamp microscope (MediWorks Co., Ltd., Shanghai, China) was used for the examination, which required two people. One person observed and collected photos; the other person assisted in placing the eyelid opener in the rabbit's eye and fixing the rabbit so that the corneal plane was parallel to the longitudinal plane of the acquisition lens. Eyes were examined at 20× magnification with diffusion light irradiation. Anterior-segment examination included observations of the conjunctiva, eyelid, cornea, sclera, and iris. We mainly recorded the degree of corneal edema, classified into grades 0–4 as follows: Grade 0, completely transparent cornea, no edema; Grade 1, mild corneal edema, iris, and lens details can be observed; Grade 2, moderate corneal edema, iris, and lens can be observed; Grade 3, severe corneal edema with no visible lens; and Grade 4, diffuse corneal edema, no visible iris.
2.6. Ultrasonic pachymetry
Ultrasonic pachymetry is the most widely used method of central corneal thickness (CCT) measurement in clinical practice [22], and is recognized as the “gold standard” for corneal thickness measurement [23]. CCT was measured at zero, one, three, and seven days postoperatively using an A-mode ultrasonic pachymeter (Tomey Corporation, Japan), and the mean of five measurements was recorded as the CCT value.
2.7. Intraocular pressure measurement
A portable tonometer (TonoVet Tonometer, Finland) was used to measure and record the intraocular pressure (IOP) at zero, one, three, and seven days postoperatively.
2.8. In vivo confocal microscopy
In vivo confocal microscopy allows real-time visualization of the cornea at the cellular level from front to back with high resolution, and is advantageous for observing corneal edema [24]. Images of the corneal endothelium and stroma were collected using the Heidelberg Retinal Tomography (HRT3)/Rostock Cornea Module (RCM) (Heidelberg Engineering Inc., Germany) in vivo as described previously [25].
Confocal microscopy was performed at zero, one, three, and seven days postoperatively. After the rabbits were anesthetized, a drop of carbomer gel was applied to the lens cap as the coupling medium. The central cornea was maintained in contact with the cap. By manually controlling the depth of the z-direction at 2 μm increments, representative images from the corneal stroma to the corneal endothelium were recorded. Corneal endothelial density in cells/mm2 was analyzed using the procedure associated with HRT3/RCM.
2.9. Pathological examination
The rabbits were euthanized seven days postoperatively by injecting an overdose of the anesthetic. The right eyeball of each rabbit was removed, and the cornea was cut into a ring 2 mm outside the limbus for histopathological examination. Corneal tissue was fixed in 10% formaldehyde solution and tissue sections were prepared, dehydrated in gradient ethanol, and embedded in wax. Continuous sections were obtained from the central cornea and routine hematoxylin and eosin staining was performed. The morphology of the corneal endothelium was observed under a light microscope and the types and numbers of corneal inflammatory cells were counted.
2.10. Statistical analysis
Data were analyzed using the Shapiro-–Wilk test to verify their normal distribution. All data were statistically analyzed using SPSS software (version 26.0; SPSS, Inc., Chicago, IL, United States). All experimental data are expressed as the mean ± standard deviation. Comparisons between groups were performed using double-factor variance analysis for statistical analysis, and LSD t-test was used for further pairwise comparison. Preoperative values of these variables and values one, three, and seven days after exposure to EDTA were compared using a paired-samples t-test. Statistical significance was set at P < 0.05.
3. Results
3.1. Grade of corneal edema in rabbits
Preoperatively, the corneas were transparent in all groups. In the BSS and 0.01 M EDTA groups, the rabbits' corneas remained transparent throughout the experimental observation, and the degree of edema was 0–1. In the 0.05 M and 0.10 M EDTA groups, corneal edema was grade 1–2, and it was the most obvious on the first day after surgery; thereafter, corneal edema gradually reduced and returned to normal on the third day after surgery. In the 0.20 M EDTA group, grade 2–3 corneal edema, conjunctival mixed hyperemia, and increased conjunctival sac secretion were observed on the first day after operation. However, the degree of reaction was less than that in the 0.50 M EDTA group, and the corneal edema resolved seven days postoperatively. In the 0.50 M EDTA group, the corneal edema was diffuse, and significant thickening of the cornea was observed postoperatively. The intraocular structure was unclear, with grade 3–4 mixed conjunctival congestion, increased conjunctival-sac secretion, and other signs of irritation, which did not improve significantly during the entire observation period (Fig. 1).
Fig. 1.
Corneal edema of rabbits in each group. Before operation, corneas in each group were transparent. In 0.50 M EDTA group, the corneal edema was diffuse and the iris could not be observed. In 0.20 M EDTA group, corneal edema was obvious and the intraocular structure was not clear. Corneal edema was observed in 0.10 M EDTA group and 0.05 M EDTA group, but iris could be observed. The corneal edema was mild in the 0.01 M EDTA group and the balanced salt solution control group, and the intraocular structure could be seen.
3.2. CCT evaluation
Corneal thickness is closely related to the barrier and pump functions of the endothelium [26]. An ultrasonic pachymeter was employed to evaluate CCT and validate the findings. Preoperatively, CCT in the groups was 351.6 ± 4.7, 348.4 ± 11.1, 356.4 ± 2.9, 357.8 ± 9.1, 348.8 ± 10.8, 360.0 ± 6.2 μm, respectively (Table 1), and the difference was not statistically significant (F = 1.829, P > 0.05) (Fig. 2A). In one and three days after the operation, CCT in experimental groups was significantly higher than in the BSS control group (Fig. 2B, C, P < 0.001), but no significant difference between 0.05 and 0.01 M EDTA and BSS-treated groups was observed in day seven (Fig. 2D).
Table 1.
CCT measured at different time in each group.
| 0.50 M EDTA | 0.20 M EDTA | 0.10 M EDTA | 0.05 M EDTA | 0.01 M EDTA | BSS | |
|---|---|---|---|---|---|---|
| CCT (μm) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Preoperative | 351.6 ± 4.7 | 348.4 ± 11.1 | 356.4 ± 2.9 | 357.8 ± 9.1 | 348.8 ± 10.8 | 360.0 ± 6.2 |
| Postop 1 d | – | 955.8 ± 22.3**** | 877.6 ± 22.7**** | 777.2 ± 32.3**** | 642.2 ± 76.2* | 416.6 ± 27.7* |
| Postop 3 d | – | 1303.6 ± 68.6**** | 740.8 ± 5.2**** | 675.0 ± 29.0**** | 532.0 ± 90.0* | 371.6 ± 5.5* |
| Postop 7 d | – | 961.0 ± 62.0**** | 505.0 ± 14.2**** | 411.4 ± 20.9* | 389.8 ± 6.9**** | 369.4 ± 7.8* |
Note. ****P < 0.001; ***P < 0.005; **P < 0.01; *P < 0.05. All P values were calculated by comparing with the preoperative values in each group.
Fig. 2.
Comparison of the CCT on the different days among 0.50, 0.20, 0.10, 0.05, 0.01 M EDTA solution groups and the BSS group: A, B, C and D represent the comparison of pre-operation, post-operation one, three and seven days. ****P < 0.001; ***P < 0.005; **P < 0.01; *P < 0.05.
Moreover, the mean CCT in the 0.20, 0.10, 0.05, and 0.01 M EDTA groups at one, three, and seven days were as follows, which were significantly different from the preoperative CCT (Table 1). In the BSS group, postoperative CCT was also significantly different from the preoperative CCT (P < 0.05). We believe that the difference between the preoperative and postoperative CCT in the BSS group was due to corneal edema caused by the trauma of the operation itself, because the values in the experimental groups were higher than those in the control group. In the 0.50 M EDTA group, the corneal stroma showed prominent edema and increased thickness, which exceeded the upper limit of automatic thickness measurement (1500 μm) (Table 1).
3.3. Results of intraocular pressure (IOP) measurement
We examined IOP before and after surgery to rule out corneal endothelial damage caused by a possible increase in IOP caused by surgery. However, in each group, IOP decreased postoperatively which was significantly different from the preoperative IOP (Table 2). We speculated that the decrease in IOP may be related to scraping of the corneal epithelium.
Table 2.
IOP measured at different time in each group.
| 0.50 M EDTA | 0.20 M EDTA | 0.10 M EDTA | 0.05 M EDTA | 0.01 M EDTA | BSS | |
|---|---|---|---|---|---|---|
| IOP (mmHg) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Preoperative | 12.0 ± 1.0 | 12.0 ± 1.5 | 12.4 ± 1.3 | 12.0 ± 1.5 | 12.0 ± 1.0 | 11.6 ± 1.5 |
| Postop 1 d | 7.2 ± 1.4**** | 8.8 ± 2.5* | 8.8 ± 1.6* | 8.6 ± 1.9**** | 8.4 ± 0.8* | 8.6 ± 1.1* |
| Postop 3 d | 6.8 ± 0.8**** | 8.6 ± 2.0* | 8.8 ± 1.4**** | 9.4 ± 2.4* | 9.0 ± 1.0* | 8.6 ± 1.1* |
| Postop 7 d | 7.8 ± 1.4* | 10.0 ± 2.5* | 10.4 ± 1.1* | 10.0 ± 1.5* | 10.2 ± 1.3* | 10.4 ± 0.8 |
Note. ****P < 0.001; ***P < 0.005; **P < 0.01; *P < 0.05. All P values were calculated by comparing with the preoperative values in each group.
3.4. In vivo confocal microscopy
In vivo confocal microscopy is a non-invasive technique that evaluates the appearance and structure of endothelial cells in situ in a narrow region of the cornea. Preoperative confocal microscopy showed that the nuclei of the corneal stromal-layer cells were spindle-shaped or oblong, with a clear contour and high reflection. Moreover, the cytoplasm and cell margins were generally not visible. The stromal fibers exhibited good light transmission (Fig. 3A). The morphology changes were not significant at one, three, and seven days postoperatively in BSS group, which was similar to that before surgery (Fig. 3B). In the 0.01 M and 0.05 M solution groups at seven days postoperatively, stromal cells were in a quiescent state; the cytoplasm did not show light change; nuclei were visible under a dark background; and the cytoplasm, cell boundary, and collagen lamina were not visible (Fig. 3C). In the 0.1 M and 0.2 M solution groups, the morphology changes were not significant at one, three, and seven days postoperatively. The stromal layer was activated, suggesting an inflammatory response in the corneal tissue, manifested as stromal cell swelling, enhanced reflection, and cross-linking into a network, and the nuclei lost their normal spindle or oblong shape and were irregularly shaped with blurred contour (indicated by red arrow) (Fig. 3D and E). In the 0.50 M solution group, due to excessive corneal edema, it was difficult to visualize the full-thickness corneal structure one day postoperatively. Three days postoperatively, the corneal stroma appeared as black, low-reflective bands of different widths, which formed different shades of wrinkles together with the translucent stromal fibers. Simultaneously, the outlines of the stromal-cell nuclei were not clear and the nuclear size was reduced. Seven days postoperatively, the stromal-cell nuclei were significantly reduced, the black reflective band of the corneal stroma widened, and wrinkles between the black and normal stromal fibers were obvious (indicated by red arrow) (Fig. 3F).
Fig. 3.
Evaluation of the morphology of corneal stromal layer by IVCM. Representative images demonstrate the morphology of corneal stromal layer preoperative (A); postop 7 days in the BSS group (B); postop 7 days in the 0.05 M group (C); postop 7 days in the 0.10 M group(D); postop 7 days in the 0.20 M group(E); postop 7 days in the 0.50 M group(F).
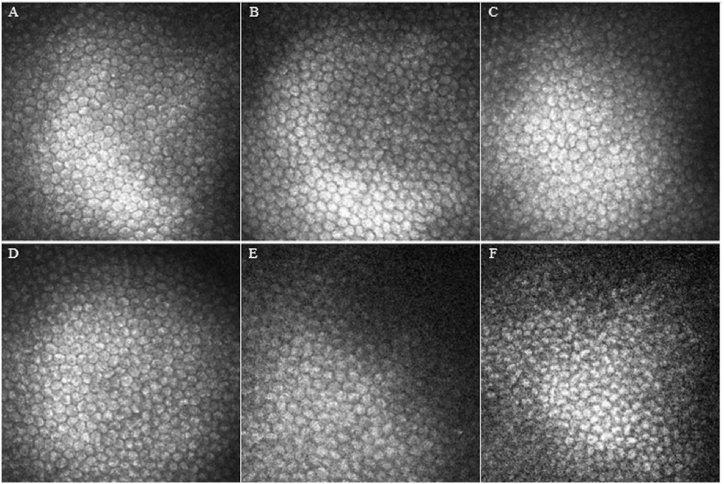
Preoperative confocal microscopy showed that the endothelial cells were arranged in a regular layer of flat honeycomb hexagonal cells, with clear boundaries, highly reflective cell bodies, close cell arrangement, uniform size, and low-reflective cell boundaries and intercellular spaces (not shown). The corneal endothelial cells in the BSS group showed no significant changes seven days postoperatively. The endothelial cells were strongly honeycombed hexagonal cells with tight connections between cells and clear boundaries which was similar with preoperative morphology (Fig. 4A). Postoperatively, no significant difference was observed between the 0.01 M and 0.05 M solution groups. The corneal endothelial cells were regular polygons with clear boundaries, high reflection, and uniform size, and the nucleus was generally not visible (Fig. 4B and C). In 0.10 M and 0.20 M solution groups, corneal endothelial cells showed increased atypia, uneven size, and dark areas between the cells (Fig. 4D and E). In the 0.50 M solution group, corneal endothelial cells showed uneven size, and increased cell volume. Moreover, cells were deformed and fused at postoperative three days. Seven days postoperatively, pleomorphic corneal endothelial cells significantly increased. The morphology of pleomorphic cells was irregular, the cell boundary was unclear, and the cell area of pleomorphic cells was significantly larger than that of normal corneal endothelial cells (Fig. 4F).
Fig. 4.
Evaluation of the morphology of corneal endothelial cells by IVCM. Representative images demonstrate the morphology of corneal endothelial cells BSS group (A); 0.01 M group (B); 0.05 M group (C); 0.10 M group (D); 0.20 M group (E); 0.50 M group(F), these images are from seven days after surgery.
3.5. Endothelial cell density
Corneal endothelial cell density (ECD) varied according to the time of evaluation (F = 102.567, P < 0.001) and between groups (F = 3.229, P < 0.05) before and after surgery, and the change trends in different groups at different time points were different (F = 141.703, P < 0.001). Firstly, the comparison of ECD between the experimental and control groups was analyzed. There is no significant difference between each preoperative group and one day after the operation (Fig. 5A and B), except for a significant difference between 0.50 and 0.05 M EDTA treated groups (P < 0.05) (Fig. 5A), which may result from individual differences among subjects in the 0.05 M EDTA treated group. During the follow-up observation on day three and day seven, compared BSS treated group, only 0.05 M EDTA-treated group has no statistical difference (Fig. 5C and D).
Fig. 5.
Comparison of the ECD on the different days among 0.50, 0.20, 0.10, 0.05, 0.01 M EDTA solution groups and the BSS group: A, B, C and D represent the comparison of pre-operation, post-operation one, three and seven days. ****P < 0.001; ***P < 0.005; **P < 0.01; *P < 0.05.
A paired-sample t-test was performed for ECD changes over time in each treatment group. In BSS group, a significant difference was observed between the ECD at one day after surgery and that before surgery (P < 0.05); however, no significant change was observed three and seven days postoperatively (both P > 0.05). In 0.01 M group, no significant change was observed at one day postoperatively (P > 0.05); however, significant changes were observed three and seven days postoperatively (both P < 0.05). In 0.05 M group, significant changes were observed at one, three, and seven days postoperatively (all P < 0.05). In 0.10 M group significant changes were observed at one, three, and seven days postoperatively, respectively, with significant changes (all P < 0.05). In 0.20 M group, significant changes were observed at one, three, and seven days postoperatively (all P < 0.05). In the 0.50 M EDTA group, ECD could not be measured because of corneal hyperedema and hyperemia. ECD decreased from 3428.6 ± 180.3 to 2892.0 ± 75.0 cells/mm2 (P < 0.05) and 2808.0 ± 80.6 cells/mm2 (P < 0.05) three and seven days postoperatively, respectively (Table 3).
Table 3.
ECD measured at different time in each group.
| 0.50 M EDTA | 0.20 M EDTA | 0.10 M EDTA | 0.05 M EDTA | 0.01 M EDTA | BSS | |
|---|---|---|---|---|---|---|
| ECD (cells/mm2) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) |
| Preoperative | 3428.6 ± 180.3 | 3315.2 ± 178.5 | 3259.2 ± 45.5 | 3358.2 ± 182.4 | 3115.2 ± 192.1 | 3352.6 ± 72.2 |
| Postop 1 d | – | 3133.0 ± 146.5* | 3106.2 ± 74.7* | 3259.0 ± 151.1* | 3072.8 ± 210.8 | 3337.2 ± 71.9* |
| Postop 3 d | 2892.0 ± 75.0* | 3008.6 ± 142.2* | 3029.2 ± 127.7* | 3143.4 ± 77.4* | 3036.4 ± 242.0* | 3307.2 ± 69.5 |
| Postop 7 d | 2808.0 ± 80.6* | 2918.6 ± 93.9* | 2990.6 ± 105.1* | 3119.0 ± 96.6* | 3019.4 ± 241.5* | 3297.0 ± 73.2 |
Note. ****P < 0.001; ***P < 0.005; **P < 0.01; *P < 0.05. All P values were calculated by comparing with the preoperative values in each group.
3.6. Pathological examination
No animal died during the experiment, and at the end of the recovery period, all rabbits were euthanized for examination.
In the BSS group, the corneal morphology was regular, with a clear structure of each layer. The corneal epithelium is intact and arranged regularly, the stromal layer contained dense connective tissue, no edema or inflammatory cell infiltration was observed, the posterior elastic layer was uniform (Fig. 6A). In the 0.01 M and 0.05 M solution groups, the cornea was smooth and continuous, with regular morphology, mild tissue edema, and fibrous connective tissue hyperplasia. The corneal epithelium of some rabbit eyes is incomplete, containing 2–3 layers of cells, irregular arrangement. Occasionally, few inflammatory cells infiltrated (mainly lymphocytes) (Fig. 6B and C). In the 0.10 M and 0.20 M solution groups, corneal edema was obvious, the corneal epithelium is incomplete, containing 2–3 layers of cells, arranged disordered, the stromal layer was irregular and contained dense connective tissue, and the arrangement was not orderly. Few cracks and inflammatory-cell infiltration (mainly lymphocytes) were observed (Fig. 6D and E). In the 0.50 M solution group, diffuse corneal edema was observed, the corneal epithelium is incomplete, necrosis and exfoliation can be seen in some areas, with only 1–2 layers of cells and disordered arrangement, the stromal connective tissue was disordered and loose, and significant inflammatory-cell infiltration was observed (granulocytes, lymphocytes, and mononuclear macrophages were all observed) (Fig. 6F).
Fig. 6.
Hematoxylin-eosin staining of rabbit eye specimens in each group, (200 ×).
BSS group (A); 0.01 M group (B); 0.05 M group (C); 0.10 M group (D); 0.20 M group (E); 0.50 M group(F), these images are from seven days after surgery.
In the BSS group, the corneal endothelial cells were monolayer flat epithelium and were continuous and clear, nuclei were arranged neatly, and no inflammatory cell infiltration was observed (Fig. 7A). In the 0.01 M and 0.05 M solution groups, the corneal endothelium was continuous and clear without inflammatory cell infiltration, and endothelial cell nuclei were visible (Fig. 7B and C). In the 0.10 M and 0.20 M solution groups, the endothelium was partially continuous and poorly defined, and the endothelial-cell nuclei were partially exfoliated with occasional cell hyperplasia (Fig. 7D and E). In the 0.50 M solution group, the endothelium was not continuously clear, endothelial-cell nuclei were rare, endothelial cells were obviously swollen, the cytoplasm was lightly stained, the arrangement was disordered, and some endothelial cells were lost (Fig. 7F).
Fig. 7.
Hematoxylin-eosin staining of rabbit eye specimens in each group, (400 ×).
Representative images demonstrate the morphology of corneal endothelial cells BSS group (A); 0.01 M group (B); 0.05 M group (C); 0.10 M group (D); 0.20 M group (E); 0.50 M group(F), these images are from seven days after surgery.
4. Discussion
CBK is a degenerative corneal disease associated with various ocular and systemic pathological conditions. The name is derived from the distinctive appearance of calcium deposits in a band across the central interpalpebral corneal area. This is thought to be a result of the precipitation of calcium salts on the corneal surface under the epithelium. Calcium salts can normally be found in the serum or body fluids, such as tears and aqueous humor. Calcium precipitation can occur with even minor alterations in the corneal microenvironment (tear osmolality, pH), as calcium exists in near-saturation concentrations in the corneal stroma [27,28]. Due to the release of carbon dioxide from the interpalpebral exposure zone and the absence of limbal blood vessels providing a buffer against a considerable pH change, the pH level in the interpalpebral fissure is higher than that on the rest of the ocular surface [28,29].
Previous reports have suggested that dysfunctional endothelial pumps allow the slow diffusion of calcium and phosphate from the aqueous humor into the corneal stroma and Bowman layer, where they tend to precipitate due to the more alkaline pH [30]. Elevated serum calcium or phosphate levels due to systemic conditions can favor precipitation. Similarly, local conditions, such as chronically inflamed eyes, elevate the pH of the eye surface and favor precipitation.
EDTA and its salts are chelating agents. Alkali-metal hydroxides neutralize them to form water-soluble salts or chelates that contain metal cations [[31], [32], [33]]. EDTA forms a tetranegative anion that is strongly attracted to alkaline-earth and transition-metal ions. The chelating action of EDTA occurs at alkaline pH as long as metallic ions are available until all EDTA molecules are utilized [18]. Owing to the strong complexation of metal ions, EDTA is used to treat CBK caused by metal ions deposited in the cornea due to various reasons.
Several modalities have been used for the treatment of CBK. The most widely used method is EDTA chelation [14]. As standardized guidelines for EDTA chelation in CBK are not available, the concentration used varies widely from one clinician to another (from 0.05 M to 0.10 M) [1,34,35]. In addition, there is a significant variation in the methods of EDTA dilution in the literature.
Corneal endothelial cells play an essential role in maintaining corneal transparency. The corneal endothelium is comprised of a layer of flat hexagonal cells with a thickness of approximately 5 μm. The gap junctions between the endothelial cells and matrix constitute a mechanical barrier and the ionic pump function of the cells prevents excessive water from entering the corneal stroma and actively expels excess water from the cells. This maintains corneal transparency. The human cornea contains approximately 2800–4000 endothelial cells/mm2, and the number gradually decreases with increasing age, generally at a rate of 0.6% per year. In addition, trauma, glaucoma, diabetes, and a variety of primary corneal diseases, such as Fuchs' corneal endothelial dystrophy, Peters’ abnormality, and keratoconus [36] can cause corneal endothelial-cell injury. Human corneal endothelial cells are non-regenerative and their loss is compensated by expansion and migration of surrounding cells after injury. Goktas et al. [37] found through animal experiments that the danger threshold of corneal endothelial-cell density is 1000 cells/mm2; when the cell density is below the threshold, the incidence of corneal edema increases exponentially due to decompensation of the corneal endothelial cells.
Grant [38] reported that a 1-M EDTA solution applied to the rabbit cornea produced pain and chemosis, and repeated exposure of de-epithelialized eyes for 15 min caused marked swelling of the corneal stroma, intense conjunctival chemosis with small conjunctival hemorrhages, and hemorrhagic iritis. A 0.1-M solution applied continuously for 15 min on the rabbit cornea with the corneal epithelium removed, caused corneal edema, chemosis, and hyperemia of the iris. A 0.01-M solution of EDTA dropped on de-epithelialized rabbit corneas caused mild, pale conjunctival edema and mild edema of the corneal stroma. A 0.01-M solution injected intracorneally produced a pronounced, bluish swelling of the cornea at the injection site. A 0.01-M solution of EDTA injected into the anterior chamber caused diffuse corneal edema and marked hyperemia of the iris and conjunctiva. Sugar and Waltman [39] also reported that one drop of a 20% 0.2-M solution of calcium disodium EDTA applied four times daily for 10 days to rabbit corneas denuded by repeated scraping caused a diffuse superficial ground-glass appearance in the stroma. The haze persisted for two weeks after stopping the treatment. No animal that received BSS showed corneal opacity. However, their study simply examined the symptoms of irritation of the cornea and conjunctiva. To the best of our knowledge, our study is the first to evaluate endothelial-cell damage in de-epithelialized corneas caused by various concentrations of EDTA solution used during CBK surgery.
In this study, we investigated the concentrations of EDTA commonly used in clinical practice and compared the damage to corneal endothelial cells. In clinical practice, EDTA application for 5–45 min of is required, depending on the calcium density. Therefore, we chose an average operation time of 20 min. Unlike human and cat endothelium, rabbit corneal endothelium can regenerate [40,41]; therefore, a rabbit model of corneal endothelium has limitations. Rabbit corneal endothelium is reestablished and the corneal thickness returns to near-normal within 30 days, depending on the extent of the injury [42]. Therefore, we conducted our experiments for seven days after surgery to measure corneal endothelial damage in a rabbit model before endothelial regeneration occurred. The safety of EDTA solutions was evaluated based on ophthalmologists’ clinical observations and pathological examinations for our subjects. Throughout the study, no significant differences in mental state, weight, or food intake were noted among the animals in the different groups. In this study, 0.50 M EDTA produced a very strong response to ocular surface stimulation, including severe conjunctival hyperemia and edema, increased secretion, corneal edema, and corneal endothelial-cell injury. Histopathological results showed that a large number of inflammatory cells infiltrated the corneal stroma and endothelial cells exfoliated in the 0.50 M EDTA group. The 0.20 M and 0.10 M groups also showed obvious ocular surface irritation and corneal stromal edema, but the degree was less than that in the 0.50 M group. Histopathological findings showed a small to moderate amount of inflammatory cell infiltration in the corneal stroma and partial detachment of the corneal endothelial cells. In 0.05 M and 0.01 M groups, mild ocular surface irritation and corneal stromal edema were observed. Histopathological findings revealed occasional inflammatory cell infiltration in the corneal stroma. In the BSS group, no obvious postoperative ocular-surface irritation was observed. However, slight corneal stromal edema was observed, which was believed to be related to the operation itself.
Intriguingly, we found there was a significant difference between 0.50 M and 0.01 M experimental groups before surgery. This may be explained by individual differences of rabbits since ECD of one subject in 0.01 M group was lower than mean value of all other rabbits (2868.0 vs 3319.9 cells/mm2). This clarification may also contribute to significant decrease of ECD in 0.01 M group compared with BSS control group. Moreover, in our study, we only evaluated the damage of different concentrations of EDTA solution for corneal endothelial cells based on animal experiment, which may indicate the side effect of EDTA solution with various concentration. However, more clinical trials should be performed to obtain the data of damage to corneal endothelial cells in human individuals. Meanwhile, no studies have explored whether high concentrations of EDTA require a shorter surgical time and low concentrations require a longer surgical time. And we also did not study therapeutic effect of EDTA. Therefore, further study about treatment evaluation by EDTA with different concentration and the corresponding damage are necessary.
In conclusion, 0.50 M EDTA solution has considerable biological toxicity to the de-epithelialized cornea, and 0.20 M and 0.10 M EDTA solutions also have some toxic effects on the de-epithelialized cornea. Moreover, 0.05 M and 0.01 M EDTA solutions had no obvious toxic effect on the corneal endothelium. However, further study of EDTA side effect by clinical trials, and therapeutic effect observation with different concentration are necessary.
Author contribution statement
Qian Wei: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Chao Qu: Conceived and designed the experiments; Analyzed and interpreted the data.
Jun Jiang: Contributed reagents, materials, analysis tools or data.
Guanghong Zhang: Contributed reagents, materials, analysis tools or data; Performed the experiments.
Data availability statement
Data will be made available on request.
Ethics statement
All animal experiments were approved by the Animal Ethics Committee of the Sichuan Provincial People's Hospital (No. 2022–308).
Funding statement
This work was funded by the Natural Science Foundation of China (no. 82171026).
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Contributor Information
Qian Wei, Email: weirenqing1102@163.com.
Chao Qu, Email: lucyjeffersonqu@hotmail.com.
References
- 1.Najjar D.M., Cohen E.J., Rapuano C.J., Laibson P.R. EDTA chelation for calcific band keratopathy: results and long-term follow-up. Am. J. Ophthalmol. 2004;137:1056–1064. doi: 10.1016/j.ajo.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 2.O'Brart D.P., Gartry D.S., Lohmann C.P., Patmore A.L., Kerr Muir M.G., Marshall J. Treatment of band keratopathy by excimer laser phototherapeutic keratectomy: surgical techniques and long term follow up. Br. J. Ophthalmol. 1993;77:702–708. doi: 10.1136/bjo.77.11.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jhanji V., Rapuano C.J., Vajpayee R.B. Corneal calcific band keratopathy. Curr. Opin. Ophthalmol. 2011;22:283–289. doi: 10.1097/ICU.0b013e3283477d36. [DOI] [PubMed] [Google Scholar]
- 4.Kobayashi W., Yokokura S., Hariya T., Nakazawa T. Two percent ethylenediaminetetraacetic acid chelation treatment for band-shaped keratopathy, without blunt scratching after removal of the corneal epithelium. Clin. Ophthalmol. 2015;9:217–223. doi: 10.2147/OPTH.S75938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Hity A., Ramaesh K., Lockington D. EDTA chelation for symptomatic band keratopathy: results and recurrence. Eye. 2018;32:26–31. doi: 10.1038/eye.2017.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Porter R., Crombie A.L. Corneal and conjunctival calcification in chronic renal failure. Br. J. Ophthalmol. 1973;57:339–343. doi: 10.1136/bjo.57.5.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Golan A., Savir H., Bar-Meir S., Oliver I., De Vries A. Band keratopathy due to hyperparathyroidism. Ophthalmologica. Journal international d'ophtalmologie. International Journal of Ophthalmology. Zeitschrift fur Augenheilkunde. 1975;171:119–122. doi: 10.1159/000307477. [DOI] [PubMed] [Google Scholar]
- 8.Johnston R.L., Stanford M.R., Verma S., Green W.T., Graham E.M. Resolution of calcific band keratopathy after lowering elevated serum calcium in a patient with sarcoidosis. Br. J. Ophthalmol. 1995;79:1050. doi: 10.1136/bjo.79.11.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Connor G.R. Calcific band keratopathy. Trans. Am. Ophthalmol. Soc. 1972;70:58–81. [PMC free article] [PubMed] [Google Scholar]
- 10.Wood T.O., Walker G.G. Treatment of band keratopathy. Am. J. Ophthalmol. 1975;80:550. [PubMed] [Google Scholar]
- 11.Baltatzis S., Papaefthimiou J. Treatment of calcific band keratopathy by Nd:YAG laser. Eur. J. Ophthalmol. 1992;2:27–29. doi: 10.1177/112067219200200106. [DOI] [PubMed] [Google Scholar]
- 12.Dighiero P., Boudraa R., Ellies P., Saragoussi J.J., Legeais J.M., Renard G. [Therapeutic photokeratectomy for the treatment of band keratopathy] J. Fr. Ophtalmol. 2000;23:345–349. [PubMed] [Google Scholar]
- 13.Burillon C., Durand L., Berne E., Bouvier R. [Band keratopathy. Symptomatological value based on 23 cases] J. Fr. Ophtalmol. 1992;15:579–586. [PubMed] [Google Scholar]
- 14.Bokosky J.E., Meyer R.F., Sugar A. Surgical treatment of calcific band keratopathy. Ophthalmic Surg. 1985;16:645–647. [PubMed] [Google Scholar]
- 15.Stewart O.G., Morrell A.J. Management of band keratopathy with excimer phototherapeutic keratectomy: visual, refractive, and symptomatic outcome. Eye. 2003;17:233–237. doi: 10.1038/sj.eye.6700327. [DOI] [PubMed] [Google Scholar]
- 16.Maloney R.K., Thompson V., Ghiselli G., Durrie D., Waring G.O., 3rd, O'Connell M. A prospective multicenter trial of excimer laser phototherapeutic keratectomy for corneal vision loss. The Summit Phototherapeutic Keratectomy Study Group. Am. J. Ophthalmol. 1996;122:149–160. doi: 10.1016/s0002-9394(14)72006-9. [DOI] [PubMed] [Google Scholar]
- 17.Dogru M., Katakami C., Miyashita M., Hida E., Uenishi M., Tetsumoto K., Kanno S., Nishida T., Yamanaka A. Ocular surface changes after excimer laser phototherapeutic keratectomy. Ophthalmology. 2000;107:1144–1152. doi: 10.1016/s0161-6420(00)00113-5. [DOI] [PubMed] [Google Scholar]
- 18.Saquy P.C., Maia Campos G., Sousa Neto M.D., Guimarães L.F., Pécora J.D. Evaluation of chelating action of EDTA in association with Dakin's solution. Braz. Dent. J. 1994;5:65–70. [PubMed] [Google Scholar]
- 19.Narvaez J., Chang M., Ing J., De Chance D., Narvaez J.J. Simplified, readily available method for the treatment of band keratopathy with ethylenediaminetetraacetic acid. Cornea. 2021;40:1360–1362. doi: 10.1097/ICO.0000000000002635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanigan R.S., Yamarik T.A. Final report on the safety assessment of EDTA, calcium disodium EDTA, diammonium EDTA, dipotassium EDTA, disodium EDTA, TEA-EDTA, tetrasodium EDTA, tripotassium EDTA, trisodium EDTA, HEDTA, and trisodium HEDTA. Int. J. Toxicol. 2002;21(Suppl 2):95–142. doi: 10.1080/10915810290096522. [DOI] [PubMed] [Google Scholar]
- 21.López Bernal D., Ubels J.L. Quantitative evaluation of the corneal epithelial barrier: effect of artificial tears and preservatives. Curr. Eye Res. 1991;10:645–656. doi: 10.3109/02713689109013856. [DOI] [PubMed] [Google Scholar]
- 22.Barkana Y., Gerber Y., Elbaz U., Schwartz S., Ken-Dror G., Avni I., Zadok D. Central corneal thickness measurement with the Pentacam Scheimpflug system, optical low-coherence reflectometry pachymeter, and ultrasound pachymetry. J. Cataract Refract. Surg. 2005;31:1729–1735. doi: 10.1016/j.jcrs.2005.03.058. [DOI] [PubMed] [Google Scholar]
- 23.Chakrabarti H.S., Craig J.P., Brahma A., Malik T.Y., McGhee C.N. Comparison of corneal thickness measurements using ultrasound and Orbscan slit-scanning topography in normal and post-LASIK eyes. J. Cataract Refract. Surg. 2001;27:1823–1828. doi: 10.1016/s0886-3350(01)01089-6. [DOI] [PubMed] [Google Scholar]
- 24.Zheng T., Le Q., Hong J., Xu J. Comparison of human corneal cell density by age and corneal location: an in vivo confocal microscopy study. BMC Ophthalmol. 2016;16:109. doi: 10.1186/s12886-016-0290-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trinh L., Brignole-Baudouin F., Labbé A., Raphaël M., Bourges J.L., Baudouin C. The corneal endothelium in an endotoxin-induced uveitis model: correlation between in vivo confocal microscopy and immunohistochemistry. Mol. Vis. 2008;14:1149–1156. [PMC free article] [PubMed] [Google Scholar]
- 26.Mergler S., Pleyer U. The human corneal endothelium: new insights into electrophysiology and ion channels. Prog. Retin. Eye Res. 2007;26:359–378. doi: 10.1016/j.preteyeres.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Bellhorn R.W., Korte G.E., Abrutyn D. Spontaneous corneal degeneration in the rat. Lab. Anim. Sci. 1988;38:46–50. [PubMed] [Google Scholar]
- 28.Pucket J.D., Boileau M.J., Sula M.J. Calcific band keratopathy in an alpaca. Vet. Ophthalmol. 2014;17:286–289. doi: 10.1111/vop.12097. [DOI] [PubMed] [Google Scholar]
- 29.Taravella M.J., Stulting R.D., Mader T.H., Weisenthal R.W., Forstot S.L., Underwood L.D. Calcific band keratopathy associated with the use of topical steroid-phosphate preparations. Arch. Ophthalmol. 1994;112:608–613. doi: 10.1001/archopht.1994.01090170052021. [DOI] [PubMed] [Google Scholar]
- 30.Moisseiev E., Gal A., Addadi L., Caspi D., Shemesh G., Michaeli A. Acute calcific band keratopathy: case report and literature review. J. Cataract Refract. Surg. 2013;39:292–294. doi: 10.1016/j.jcrs.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 31.Gennaro A., Hervey A.S., Gennaro A.R.J. Remington's pharmaceutical sciences. 1990;60:993. [Google Scholar]
- 32.S.B. eleventh ed. 1989. The Merck Index: an Encyclopedia of Chemicals, Drugs, and Biologicals. [Google Scholar]
- 33.Lewis R. 1993. Hawley's Condensed Chemical Dictionary, Hawley's Condensed Chemical Dictionary. [Google Scholar]
- 34.Grant W.M. New treatment for calcific corneal opacities. A.M.A. Archives of Ophthalmology. 1952;48:681–685. doi: 10.1001/archopht.1952.00920010693002. [DOI] [PubMed] [Google Scholar]
- 35.Lam H.Y., Wiggs J.L., Jurkunas U.V. Unusual presentation of presumed posterior polymorphous dystrophy associated with iris heterochromia, band keratopathy, and keratoconus. Cornea. 2010;29:1180–1185. doi: 10.1097/ICO.0b013e3181d007e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serrao S., Lombardo M. Corneal endothelial damage after simultaneous PRK and corneal cross-linking in stable keratoconus. Am J Ophthalmol Case Rep. 2019;14:32–34. doi: 10.1016/j.ajoc.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goktas A., Gumus K., Mirza G.E., Crockett C., Karakucuk S., Cavanagh H.D. Corneal endothelial characteristics and central corneal thickness in a population of Turkish cataract patients. Eye Contact Lens. 2012;38:142–145. doi: 10.1097/ICL.0b013e318243e7d2. [DOI] [PubMed] [Google Scholar]
- 38.W.M.J.A.M.A.a.o.o. Grant . vol. 48. 1953. pp. 681–685. (New Treatment for Calcific Corneal Opacities). [Google Scholar]
- 39.Sugar A., Waltman S.R. Corneal toxicity of collagenase inhibitors. Invest. Ophthalmol. 1973;12:779–782. [PubMed] [Google Scholar]
- 40.Van Horn D.L., Sendele D.D., Seideman S., Buco P.J. Regenerative capacity of the corneal endothelium in rabbit and cat. Invest. Ophthalmol. Vis. Sci. 1977;16:597–613. [PubMed] [Google Scholar]
- 41.Ling T.L., Vannas A., Holden B.A. Long-term changes in corneal endothelial morphology following wounding in the cat. Invest. Ophthalmol. Vis. Sci. 1988;29:1407–1412. [PubMed] [Google Scholar]
- 42.Wilson M.R., Yoshizumi M.O., Lee D.A., Martin W., Higginbotham E.J. Use of intraocular gas in flat anterior chamber after filtration surgery. Arch. Ophthalmol. 1988;106:1345. doi: 10.1001/archopht.1988.01060140509004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.