Abstract
Mitochondrial complex II is traditionally studied for its participation in two key respiratory processes: the electron transport chain and the Krebs cycle. There is now a rich body of literature explaining how complex II contributes to respiration. However, more recent research shows that not all of the pathologies associated with altered complex II activity clearly correlate with this respiratory role. Complex II activity has now been shown to be necessary for a range of biological processes peripherally related to respiration, including metabolic control, inflammation, and cell fate. Integration of findings from multiple types of studies suggests that complex II both participates in respiration and controls multiple succinate-dependent signal transduction pathways. Thus, the emerging view is that the true biological function of complex II is well beyond respiration. This review uses a semichronological approach to highlight major paradigm shifts that occurred over time. Special emphasis is given to the more recently identified functions of complex II and its subunits because these findings have infused new directions into an established field.
Keywords: complex II, succinate dehydrogenase, succinate signaling, inflammation, hypoxia, cancer metabolism, Krebs cycle, bacterial chemotaxis, respirasome, reverse electron transfer, ischemia-reperfusion, pheochromocytoma, paraganglioma
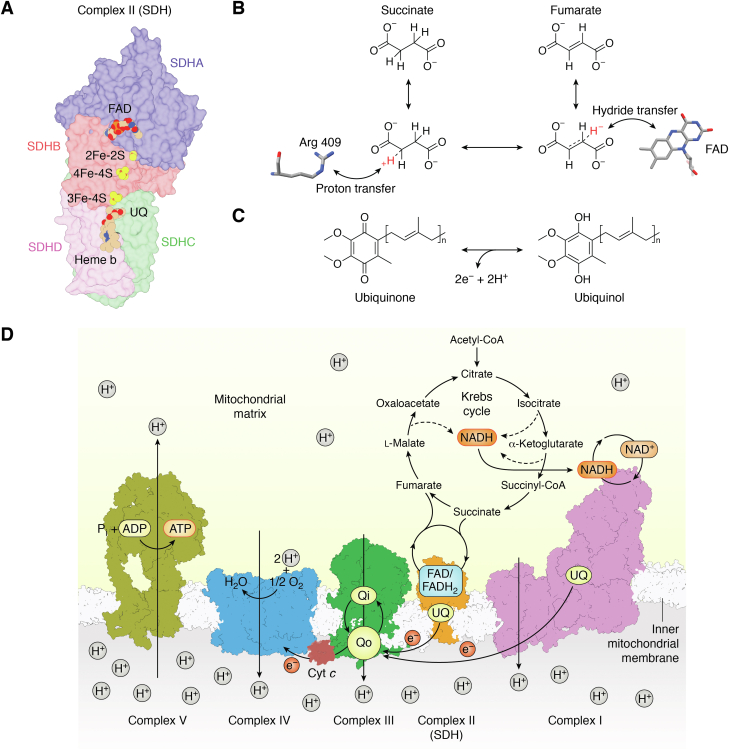
Respiratory complex II (Fig. 1A, succinate dehydrogenase (SDH), canonically SDHA-SDHB-SDHC-SDHD, but with exceptions) is a heterotetrameric membrane-spanning enzyme first described in 1909 (1) and studied in its purified form for around a century. It has been long established that complex II is a key player in multiple respiratory processes (2, 3). The first described role of complex II was as a bioenergetic complex catalyzing two distinct redox reactions in mitochondrial aerobic respiration (Fig. 1, B and C) (2, 3, 4). Here, complex II links oxidative phosphorylation (Fig. 1D) with the Krebs cycle (Fig. 2). Because of the energetics of aerobic respiration, complex II works in a defined catalytic direction under aerobic conditions where the enzyme oxidizes succinate to fumarate and concomitantly reduces the high potential ubiquinone to ubiquinol (Fig. 1C). Under anaerobic conditions with fumarate as the terminal electron acceptor, bacterial complex II homologs can proficiently perform the reverse reaction, i.e., the enzyme reduces fumarate to succinate and concomitantly oxidizes lower potential quinones such as menaquinone or rhodoquinone (5, 6, 7, 8). “Reverse” complex II activity has also been demonstrated in both mammalian mitochondria (9, 10) and bacteria (11). This reverse activity occurs under conditions where the quinone pool is highly reduced and where the fumarate concentration is sufficient to affect the thermodynamic driving force of the reverse reaction (12).
Figure 1.
Mitochondrial complex II and its reactions in mitochondrial aerobic respiration.A, architecture of mitochondrial complex II. Mitochondrial complex II (PDB 1ZOY (75)) contains four subunits: SDHA, SDHB, SDHC, and SDHD. SDHA (blue) is also called the flavoprotein (Fp) subunit; it houses the covalent FAD and the succinate oxidation site. SDHB (red) is also called the iron–sulfur (Ip) subunit; it houses the three Fe-S clusters that support electron transfer between the two active sites. The SDHC (green) and SDHD (purple) subunits are membrane-embedded, contain b-type heme, and house the ubiquinone reduction site. B and C, complex II couples two redox reactions: succinate/fumarate interconversion and quinone/quinol interconversion. The reactions are believed to be dependent as electrons that are coproducts of one reaction are transferred to the second active site to be used as cosubstrates. B, Succinate/fumarate interconversion. The mechanism of succinate/fumarate interconversion is better studied in the fumarate reduction direction. For fumarate reduction, substrate binding involves two active site histidines (His 232 and His 355, from the E. coli QFR) and one active site arginine (Arg 390 of E. coli QFR, which is equivalent to Arg 409 of human SDHA) (58). Catalysis requires an initial transfer of a hydride from FAD to fumarate via a neutral flavin semiquinone (71). This is followed by the transfer of a proton by an active site arginine (Arg 287, E. coli QFR numbering) (72, 207). Supporting the reaction through the transition state is a hydrogen bond between substrate carboxylate and a threonine side chain hydroxyl (Thr 244, E. coli QFR numbering) (73). Because this catalytic threonine is on a different domain than the binding residues, interdomain motion changes the position of this hydrogen-bond donor, which twists the substrate during the reaction. Both a diode effect and the presence of a different flavin intermediate suggest that intermediates of succinate oxidation and fumarate reduction differ (71, 202). C, quinone/quinol interconversion. The second chemical reaction housed by complex II is the 2H+/2e− interconversion of quinone and quinol in the membrane. In mitochondrial aerobic respiration, this involves the electrons harvested from succinate and uses ubiquinone as the quinone. Different types of respiration may use quinones with different potentials, which affects the driving force of the reaction. D, aerobic respiration usually relies upon oxidative phosphorylation to synthesize ATP because this is the most efficient way to convert energy to a biologically useful form. In animals, oxidative phosphorylation involves four membrane-spanning complexes of the electron transfer chain. The figure uses ovine complex I (pink, PDB 7ZD6, (208)), porcine complex II (orange, PDB 1ZOY (75)), bovine complex III (green, PDB 1NTZ (209)), and bovine complex IV (blue, PDB 2OCC (210)). These complexes guide the transfer of electrons down small, energetically favorable electron steps until they reach O2, which is reduced to H2O in complex IV. The strong oxidizing power of oxygen, ∼800 mV, drives the entire process. The overarching theme of all respiration is the coupling of these electron transfer steps with the formation of a transmembrane electrochemical gradient, shown as a proton gradient here. Complex V (mustard yellow, PDB 6ZPO (211)), also called the ATP synthase, converts the energy stored in the electrochemical gradient to a more biologically useful form. To do this, complex V couples the transfer of a proton back along its gradient with conformational changes that promote the formation of a bond between ADP and inorganic phosphate and that release the product, ATP. SDH, succinate dehydrogenase; QFR, quinol:fumarate reductase.
Figure 2.
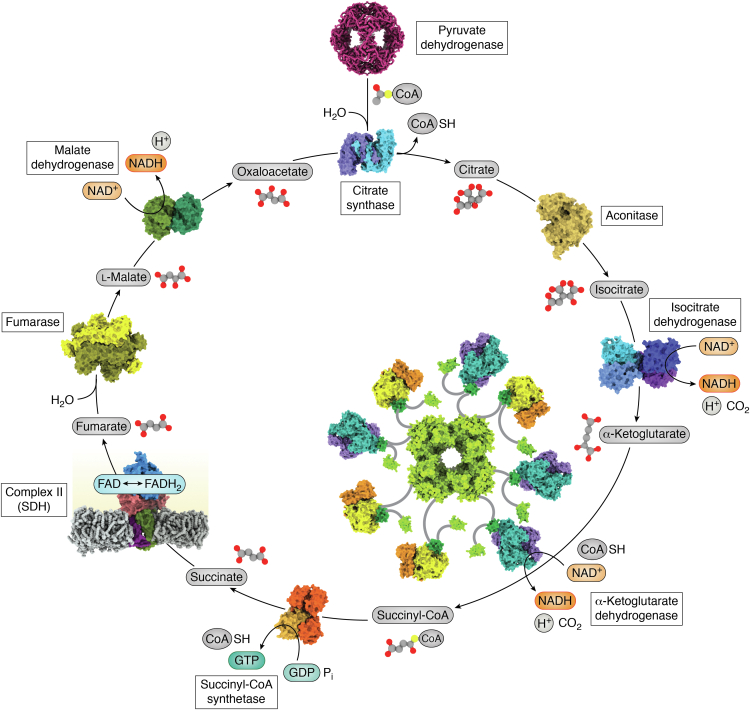
Complex II in the Krebs cycle. The Krebs cycle, also called the tricarboxylic acid cycle or the citric acid cycle, is an energy-harvesting process integral to aerobic respiration. The Krebs cycle uses a series of oxidation-reduction reactions on carboxylate-containing small molecules. The Krebs cycle contributes to oxidative phosphorylation in multiple ways. First, it produces reducing equivalents in the form of NADH, which is used by respiratory complex I. Second, the chemical intermediate succinate is used by complex II to provide another conduit for electrons to enter the respiratory chain. Coordinates used to develop this figure include human pyruvate dehydrogenase (PDB 6H55, (212)), porcine citrate synthase (PDB 1CTS, (213)), porcine aconitase (PDB 7ACN, (214)), human isocitrate dehydrogenase (PDB 7CE3, (215)), porcine succinyl CoA synthase (PDB 2FP4, (216)), porcine succinate dehydrogenase (PDB 1ZOY, (75)), human fumarase (PDB 5UPP, (217)), and porcine malate dehydrogenase (PDB 1MLD, (218)). The α-ketoglutarate dehydrogenase complex was modeled from the E. coli E1 component (PDB 2JGD, (219)), the E. coli E3-binding domain (PDB 1BBL, (220)), the Thermus thermophilus E3 component (PDB 2EQ7, (221)), and the E. coli lipoyl domain (PDB 1PMR, (222)). SDH, succinate dehydrogenase.
In addition to this respiratory role, complex II influences signaling pathways that control metabolism and cell fate (13). There may be multiple ways that complex II controls signaling. First, mature and assembled complex II can form direct complexes with effectors. For example, during bacterial chemotaxis, fumarate can induce a change in the direction of the bacterial flagellar motor, which in turn allows bacteria to reorient and change the direction of swimming. Complex II homologs that preferentially catalyze fumarate reduction directly bind to the flagellar motor and are essential for this chemotaxis with fumarate (14, 15, 16). In animals, complex II likely impacts signaling through the regulation of cellular levels of its substrate, succinate. The importance of succinate as a potent signaling molecule has become apparent over the past few years (13, 17). Complex II is the master controller of succinate levels in eukaryotic cells. As a result, changes in complex II activity and initiation of reverse activity (9, 10) each impact succinate signaling. Succinate signaling in eukaryotic cells proceeds via at least two distinct mechanisms. Succinate inhibits a range of enzymes via product inhibition when succinate is a coproduct of these enzymes (18, 19). Succinate can also stimulate succinate receptor 1 (SUCNR1, formerly GPR91), which is a promiscuous G protein–coupled receptor (GPCR) (20).
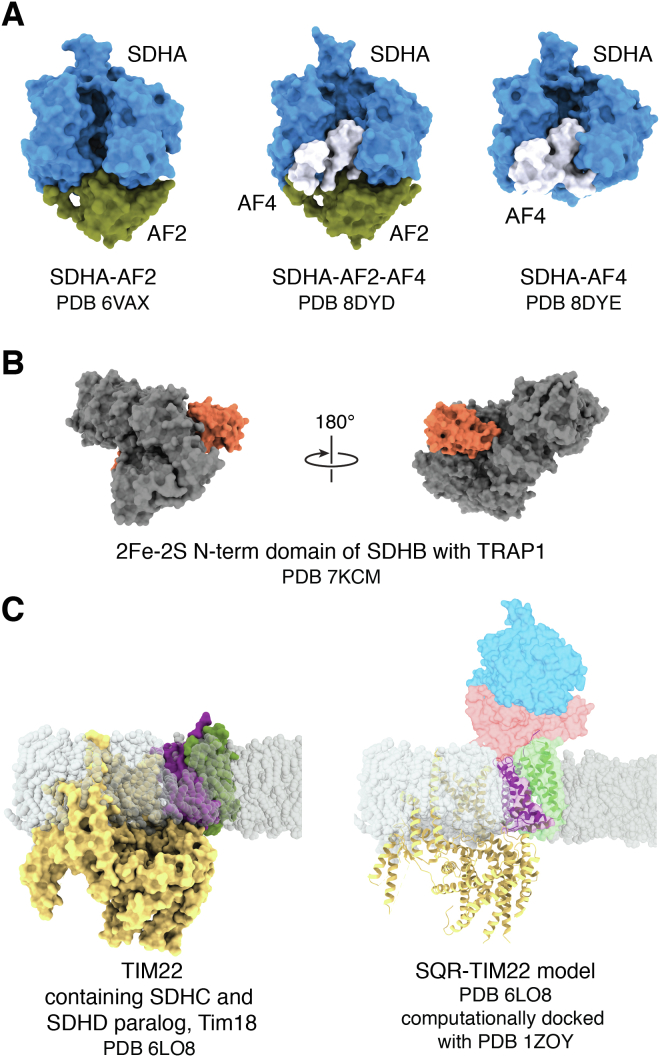
Of note, complex II subunits may affect signaling by participating in alternative assemblies (21, 22, 23, 24) (Fig. 3). SDHA is a stable part of a species that is termed “Complex II-low” (21) because it has a lower molecular weight than complex II. Complex II-low runs as a broad band on native gels, and it is not yet clear whether this is a single, defined species with unusual migration or whether it is a heterogeneous mixture of SDHA-containing species. To date, there are three stable SDHA-containing species that could be parts of complex II-low (Fig. 3A). Biologically, the accumulation of complex II-low correlates with altered signaling (21, 25).
Figure 3.
Stable complexes that contain complex II subunits. Complex II subunits have been shown to functionally integrate into alternative species. A, complex II-low is a heterogeneous mixture of species that contain SDHA, SDHAF2, and SDHAF4 that runs as a broad band on a native gel (21). The predominant species is likely the stable SDHA-AF2 complex (PDB 6VAX, (140, 142)). However, it cannot be excluded that complex II-low contains SDHA-AF2-AF4 (PDB 8DYD (140)) or SDHA-AF4 (PDB 8DYE (140)). B, the SDHB subunit forms complexes with TRAP1, which may have implications for regulating cancer metabolism (PDB 7KCM (133)). C, the SDHC subunit and an SDHD paralog called Tim18 (36% identical, 57% similar) form part of the mitochondrial inner membrane protein importer, TIM22 (22). The left panel shows the experimental structure, while the right panel shows a model with this docked to SDHA and SDHB subunits. Although sterics suggest that this complex could theoretically form, functional studies suggest that the Tim18 cannot support succinate-dependent respiration and SDHD cannot support TIM22 transport activity (22, 26). SDH, succinate dehydrogenase; SDHAF, succinate dehydrogenase assembly factor.
SDHA is not the only subunit found in alternative complexes. SDHC forms a membrane-spanning region of the yeast TIM22 protein importer that is required for function; here, SDHC interacts with an SDHD paralog called Tim18 (22, 24) (Fig. 3B). As a curiosity, yeast contains multiple paralogs of both SDHC and SDHD that are expressed under different respiratory conditions and have partial functional overlap (26). SDHD and the Tim18 paralog share 36% identity and 57% similarity and can both bind to SDHC, but they cannot functionally replace one another (26). Moreover, the SDHC-Tim18 heterodimer does not coordinate heme and therefore differs in cofactor status as compared to SDHC-SDHD. Finally, SDHB can be found in a complex with an HSP90 paralog called tumor necrosis factor receptor–associated protein 1 (TRAP1) (Fig. 3C); the stability of this complex and its functional significance is less clear (27, 28, 29, 30). One possibility is that some or all of these alternative complexes have distinct cellular roles. However, cellular succinate oxidation activity may be regulated by assembly and disassembly of the complex II heterotetramer (23, 31), and some of these complexes could also be storage forms for unassembled complex II subunits that are poised for rapid complex II assembly. As a result, these species may simply correlate with altered signaling.
Taken together, although complex II was among the first enzymes studied, its full biological role is still emerging. A semichronological consideration of findings in the field helps to highlight how the field pivoted in thinking about the roles for complex II in biology.
Biochemistry, spectroscopy, and enzymology of mitochondrial enzymes
From the 1950s through the 1980s, most studies on complex II used mitochondrial enzyme purified from slaughterhouse material, with some studies using the yeast enzyme (32, 33, 34). These early studies not only investigated the details of complex II function but also used complex II as a model to understand the basic principles of enzymology and the role of cofactors in catalysis. Complex II has a number of features conducive to its use as a model system. These features include high-level expression, absence of cellular toxicity, and reasonable stability. Moreover, complex II has a hallmark visible absorbance spectrum imparted by its many cofactors: FAD, iron–sulfur clusters (2Fe-2S, 4Fe-4S, 3Fe-4S), and heme b. This spectrum translates to a reddish-brown color that can be followed by eye. Detailed early investigations assigned redox potentials to each of these species. Among the more important basic findings from this era was the discovery that the FAD cofactor could be covalently attached to protein (35, 36). In complex II, the covalent attachment to the protein raises the redox potential of the FAD cofactor by ∼100 mV (37). This increase in potential is required for the succinate oxidation reaction.
A switch to bacterial model systems
Beginning in the 1980s, the complex II field shifted toward the use of bacterial model systems. One advantage of the bacterial enzymes is that, at that time, these more easily allowed the use of molecular biological techniques. However, because of differences in sequence conservation in different subunits of complex II enzymes, there are limitations on whether some findings can be extrapolated to the entire family. As an example, bacterial complex II enzymes all have detectable sequence similarity with mitochondrial complex II in the soluble SDHA and SDHB subunits (30%–50% identical, 50%–75% similar). However, many complex II homologs lack detectable similarity in the membrane-spanning subunits (38). These membrane-spanning subunits contain the quinone/quinol binding site and sometimes contain heme b. Even though work on bacterial complex II showed fundamental features of quinol chemistry (39, 40, 41) and how heme b could contribute to function (42), many investigations of bacterial complex II focused on the succinate oxidation reaction in the soluble domains.
The first mutagenesis studies on bacterial homologs were, in fact, instrumental in building a deep understanding of the enzymology of complex II (Fig. 1, B and C) (37, 43, 44, 45, 46, 47, 48, 49, 50). An additional early application of site-directed mutagenesis included the recapitulation of a patient mutation in the Escherichia coli complex II homolog (51, 52). A surprising discovery was that the substitution of active site residues resulted in the full elimination of succinate oxidation in the purified enzyme, with the clinical phenotype of optic atrophy, ataxia, and myopathy being milder than might be expected for the loss of a central metabolic enzyme (51, 52). Here, the patient was heterozygous, so had ∼50% of the normal level of complex II activity (51, 52). In fact, given that SDHD knockout in mice is embryonic lethal (53), heterozygous loss of complex II catalytic function via mutation often produces unexpectedly mild clinical symptoms with late onset, usually when the individual is in their 40’s (18, 54, 55, 56). Homozygous loss of complex II activity or activity below 50% of normal levels has been associated with earlier symptom onset and more severe symptoms (57).
Membrane protein crystallography and structure-function approaches
In the late 1990s, bacterial complex II was among the first membrane proteins with structures determined by X-ray crystallography (Fig. 4) (58, 59). Here, the interrogation of both soluble (60, 61, 62) and membrane-associated (58, 59) complex II homologs involved in bacterial anaerobic respiration led the field. Termed fumarate reductases or quinol:fumarate reductases, these complex II enzymes preferentially reduce fumarate to succinate (63, 64, 65, 66). This reaction is the reverse of that catalyzed during oxidative phosphorylation and the Krebs cycle. Once conditions for ready structure determination were identified, the application of a careful structure-function approach on mutant fumarate reductases allowed for a deep understanding of the mechanism of fumarate reduction (Fig. 1B) (67, 68, 69, 70, 71, 72, 73).
Figure 4.
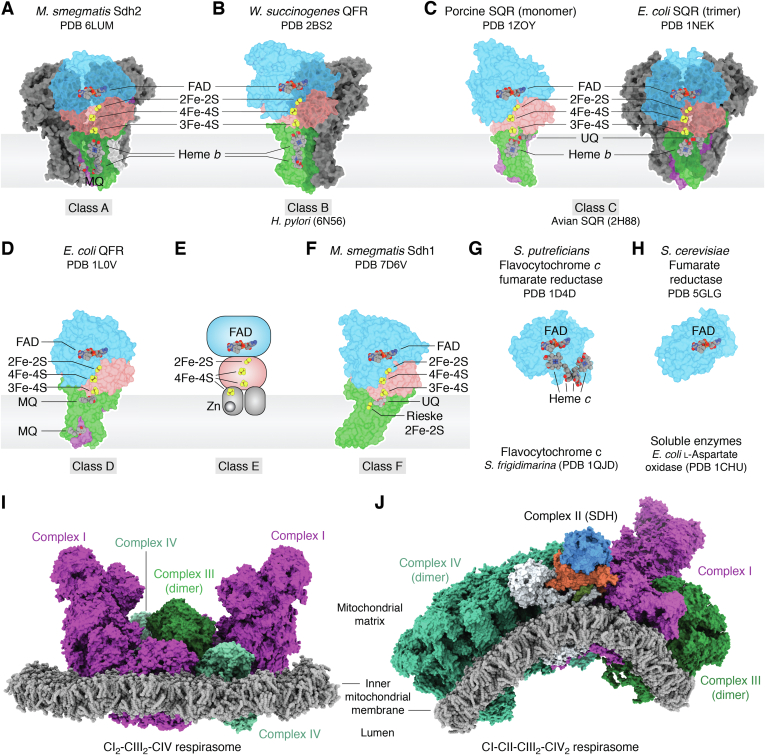
Diversity in structure and megastructure across the greater complex II family. Complex II enzymes are currently categorized into six recognized classes based on their membrane-spanning regions (83). Most members of the complex II superfamily contain a soluble domain with two subunits and between one and three membrane-spanning subunits, although Class B members are believed to peripheral-membrane enzymes and there are fully soluble homologs. While all complex II homologs share significant sequence identity in the soluble domains, the membrane-spanning domains may have evolved independently, possibly more than one time. In addition, some complex II family members contain supernumerary subunits. Depending on whether they were discovered for a physiological role in aerobic or anaerobic respiration, the corresponding complex II genes are termed sdhABCD (aerobic form) or frdABCD (anaerobic form). A, Class A complex II enzymes are predominantly from archaea. These can contain three membrane-spanning subunits, two of which have three membrane-spanning helices, with the additional single-pass membrane-spanning subunit termed SdhF. Class A homologs house two integral-membrane b-type hemes. Shown is the cryoEM structure of Mycobacterium smegmatis Sdh2, which is a trimer (PDB 6LUM (77)). B, Class B complex II contains a single membrane-spanning subunit with five membrane-spanning helices and two b-type hemes. Class B complex II enzymes are fumarate reductases and obligate dimers. Shown is the X-ray crystal W. succinogenes QFR (PDB 2BS2 (59)). C, Class C complex II enzymes contain two membrane-spanning helices and one b-type heme. Members of Class C complex II are of intense interest because they include mitochondrial homologs. Some Class C enzymes are (left) monomers (porcine mitochondrial complex II, PDB 1ZOY (75)) while others are (right) trimers (E coli SQR, PDB 1NEK (42)). D, Class D complex II enzymes are monomers that contain two membrane-spanning helices but no integral membrane heme. A putative cofactor is located at a similar position as heme in other homologs, but the identity of this has not been assigned (223). Shown is the E. coli QFR (PDB 1L0V (58)). E, Class E complex II enzymes contain membrane anchors with an amphipathic nature that suggests they could be peripherally associated with the membrane (224). Class E complex II enzymes coordinate a Zn2+ and 4Fe-4S cluster in the amphipathic helices. A structure representing this class has not yet been reported. F, Class F complex II enzymes contain a single membrane-spanning subunit with six helices. Members of Class F coordinate an integral-membrane Rieske Fe-S cluster. Shown is the structure of Mycobacterium smegmatis Sdh1 (PDB 7D6V (79)). G and H, soluble homologs of the SDHA subunit have distinct biological roles. G, some soluble SDHA homologs contain a fused tetra-heme domain and biologically act as fumarate reductases in anaerobic respiration (60, 61, 62). Shown is the Shewanella oneidensis (formerly putrefaciens) flavocytochrome c fumarate reductase (PDB 1D4D (62)). H, some soluble SDHA homologs are found without additional fused polypeptide and can catalyze redox reactions related to dicarboxylate oxidoreduction. The most extensively studied soluble SDHA homolog is perhaps L-Aspartate oxidase. Shown is the yeast Osm1 fumarate reductase (PDB 5GLG (94)). I and J, numerous structures of respiratory supercomplexes have been reported, with varying stoichiometries. These all contain complex III (CIII) at the core, which is an obligate dimer. Complex I (CI) and complex IV (CIV) can each interact with CIII independently and have one or two copies associated with each CIII2. I, reported respirasomes lacking complex II include: CICIII2, CIII2CIV, CICIII2CIV, and CI2CIII2CIV2. Shown is the human CI2CIII2CIV2 respirasome (PDB 5XTI, (110)) with CI in magenta, CIII in green, and CIV in teal. J, the only reported structure of a respirasome that contains complex II (CII) is from the ciliate Tetrahymena thermophila and has a stoichiometry of CICIICIII2CIV2 (80). Note that Tetrahymena respiratory complexes contain supernumerary subunits as compared to their counterparts in animals (111). This is particularly notable in CIV and in CII, with the latter containing 11 supernumerary subunits (SDHTT1 – SDTT11). For complex II, SDHA is in blue, SDHB is in red, SDHC and SDHD are in green and purple, and the 11 supernumerary subunits are in white. SDH, succinate dehydrogenase; QFR, quinol:fumarate reductase.
Structures of porcine and avian mitochondrial complex II from slaughterhouse sources were determined in the mid-2000s (74, 75). These additional structures were particularly important for revealing the details of the membrane-spanning regions of the mitochondrial enzymes. Structural studies with inhibitors helped to explain how quinone chemistry is supported in the mitochondria (74, 75, 76).
Recent advances in cryoEM have also been applied to members of the complex II family, allowing for structure determination of additional complex II enzymes without crystallization (77, 78, 79, 80). With this combination of crystallography and cryoEM, structures of complex II homologs have now been reported from a range of model organisms and pathogens. There are at least 14 unique complex II enzymes in the protein database. These enzymes reveal broad architectural diversity as they vary in oligomerization, the presence of supernumerary subunits, membrane protein composition, integral-membrane cofactors, and preferred quinone substrates (42, 58, 59, 74, 75, 77, 78, 80, 81, 82). These distinct complex II structures represent five of the six recognized evolutionary subclasses of complex II (Fig. 4, A–F) (83).
The broad structural variability across the membrane subunits allows for the development of class-selective inhibitors that target the quinone binding site. Notably, the ability of complex II to connect the Krebs cycle with respiration is critical for antimicrobial resistance, virulence, biofilm formation, and growth of many pathogens, including bacteria, fungi, and parasites (84, 85, 86, 87, 88, 89, 90). Complex II inhibitors have long been used as antifungal agents. Indeed, complex II inhibitors are currently among the fastest growing classes of agricultural antifungals (91). In addition, complex II inhibitors are being developed to target human pathogens. In this respect, the high-resolution structures of complex II, particularly those from Ascaris suum (81) and Mycobacterium tuberculosis (77, 79), have opened the possibilities for structure-based development of therapeutics for these difficult to treat pathogens (92, 93).
There are also structures available for soluble homologs of complex II; these enzymes do not catalyze the quinone/quinol half-reaction that is supported by the integral-membrane complex II enzymes. Soluble complex II homologs include flavocytochrome c fumarate reductases from Shewanella that contain a cytochrome domain (60, 61, 62) (Fig. 4G), soluble fumarate reductase from yeast that does not contain a cytochrome domain (94) (Fig. 4H), and L-Aspartate oxidase from E. coli (95, 96).
Complex II as a part of megacomplexes
The increasing capacity to interrogate very large protein complexes now allow structures to be determined for respiratory megacomplexes (80, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111). Sometimes called respirasomes, these megacomplexes contain more than one respiratory complex. The close association between individual complexes may improve stability, allow for local coordination of respiration, or optimize the kinetics and efficiency of respiration by forming conduits to pass respiratory intermediates. Complex II is conspicuously absent from all (97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109, 110, 111) but one (80) of these reported respirasome structures. When it is present, complex II induces curvature in the respirasome due to its asymmetric shape (Fig. 4, I and J) (80). This gives rise to a hypothesis that complex II incorporation into the respirasome could stabilize the folds that hallmark the cristae. But this also suggests that most respirasomes do not contain complex II. The lower affinity of complex II for respirasomes may stem from how it interacts with the other proteins. Complex II has the smallest buried protein–protein interface of any of the species within the respirasome. Moreover, some of the complex II surface on the interior of the respirasome is prevented from making direct protein–protein interactions with the remaining complexes by a defined lipid pocket. This suggests that complex II is truly less strongly associated with respirasomes and implies that much of the complex II population is found outside these megacomplexes.
If the function of complex II is in the chemical reactions that contribute to respiration and signaling, why isn’t it more stably associated with the respirasome? And does it do something unique when it is not associated with the respirasome? One possibility is that complex II participates in other macromolecular supercomplexes. Inspiration for such a role is found in bacterial chemotaxis. Here, the anaerobic complex II homolog, fumarate reductase, binds directly to the flagellar switch complex (14, 15, 16). This interaction supports the assembly of the bacterial flagellum and is required for chemotaxis in response to fumarate (14, 15, 16). In mitochondria, initial reports suggest the existence of a Krebs metabolon (112, 113), which could contain complex II. A Krebs metabolon could increase the efficiency of the Krebs cycle by providing defined conduits for passing small molecule intermediates from one enzyme to the next (112, 113).
Bioinformatics, disease-associated mutations, and altered cell fate
As the field matured in the early 2000s, an increasing number of noncanonical functions for complex II began to be reported. Consistent with this, a long-standing mystery in the field is that complex II deficiency is not associated with a defined set of patient symptoms. This also became clear in the early 2000s, when rapid advances in genome sequencing resulted in an explosion in the identification of disease-associated mutations within the complex II genes, which have been compiled into publicly accessible mutation databases (114).
A small number of mutations, particularly those associated with the SDHA subunit, are associated with different types of neurodegenerative disease. This disease phenotype mirrors what is observed with chemical inhibition of the SDHA subunit by the covalent inhibitor 3-nitropropionate (115). 3-nitropropionate induces specific loss of striatal neurons and has been used in animals to induce Huntington’s disease-like symptoms (115). Mutation of mitochondrial complexes commonly results in neurodegenerative phenotypes, with a theory that neurons have a disproportionately large requirement for oxidative phosphorylation. Indeed, chemical inhibition of complex I is used as a model for Parkinson’s disease as it results in the loss of motor neurons (116). Although it is not clear why the reduced function of electron transfer complexes disproportionately affects different populations of neurons, this could suggest that some mutations of complex II alter mitochondrial bioenergetics (Fig. 1D).
The overwhelming majority of disease-associated complex II mutations, however, are found in the SDHB, SDHC, and SDHD subunits (114). Individuals with complex II mutations in the SDHB, SDHC, or SDHD strongly correlate with many types of genetically linked endocrine neoplasia, in particular pheochromocytomas (38, 114, 117) and paragangliomas (118, 119, 120); more recent studies suggest that there must be additional mutations in one or more proteins outside of complex II in order for tumorigenesis to occur (121). Downregulation of SDHB, SDHC, or SDHD is also a common feature of clear renal cell carcinoma, with the loss of cellular SDH activity correlating with invasiveness (122, 123).
Complex II activity has long been known as a tumor suppressor (124) but the mechanism underlying this phenomenon remains less clear. Confounding the understanding of this process is that mutations in different subunits may result in cells with different propensities to promote metastasis (125, 126). Cancer cells disfavor oxidative phosphorylation and favor glycolysis, which is termed the Warburg effect, and complex II attenuates aerobic glycolysis. Although the role of complex II appears to be intimately linked with metabolism, one possibility is that the shift to neoplastic growth is triggered via a nonrespiratory function of complex II or by its substrate, succinate.
Much more recently, advances in genome editing have allowed for conditional knockout of complex II subunits in specific cell types of model animals. The range of observed phenotypes suggests that there may be functions of complex II that are not yet understood and can even sometimes show opposing phenotypes. For example, attempts to use a conditional knockout to create a mouse model for pheochromocytoma instead resulted in obesity (127). In two recently reported studies, mouse genetic models that affected complex II levels had opposing effects on lifespan (128, 129).
Complex II assembly and the identification of new molecular players
Complex II activity may be at least partially regulated at the level of assembly. Understanding the processes of folding and assembly have been at the forefront of the field in recent years (130). It is now clear that complex II assembly requires both general and dedicated chaperones. The HSP60 chaperone has long been known to promote the folding of apo-SDHA within the mitochondrion (131). A second chaperone, TRAP1, has long been implicated in regulating mitochondrial metabolism (132) but has more recently been suggested as a chaperone for the SDHB subunit of complex II (27, 28), as supported by cryoEM snapshots of this process (133) (Fig. 3C). Notably, there are conflicting reports of how TRAP1 affects complex II activity. Some studies suggest that TRAP1 inhibits complex II and promotes neoplastic growth, and other studies show that TRAP1 is required for full complex II activity and promotes homeostasis (27, 28, 29, 30).
Dedicated assembly factors for the soluble SDHA and SDHB subunits of complex II were identified via genome sequencing of patients with complex II deficiency but no mutations in any of the complex II subunits (134). This led to the eventual discovery of four succinate dehydrogenase assembly factors (SDHAFs), termed SDHAF1–SDHAF4 (134, 135, 136, 137, 138, 139). Of these, SDHAF2 has homologs in all kingdoms of life, with bacterial homologs termed SdhE and yeast homologs termed Sdh5. In contrast, SDHAF4 is pan-eukaryotic with a limited number of homologs in bacterial α- and γ-proteobacteria that are not commonly used as model systems (140). As a result of the low number of bacterial models for SDHAF1, SDHAF3, and SDHAF4, studies of these complex II assembly factors combined work other models, particularly yeast, due to the ease of genetics. In yeast, these assembly factors are termed Sdh5–Sdh8 (134, 136, 139).
Roles for these assembly factors are still being explored. SDHAF2 and SDHAF4 act as assembly chaperones for the SDHA subunit. Of these, the better understood role is for the SdhE/Sdh5/SDHAF2 assembly factor, which works with dicarboxylate to enhance the attachment of the covalent FAD (134, 141, 142, 143, 144, 145). While the bacterial FrdA-SdhE and SdhA-SdhE complexes have little buried surface area and can readily disassociate (146, 147), the human SDHA–SDHAF2 complex (Fig. 3A) is highly stable, long-lived, and appears to accumulate in cancer cell lines (21). The role of SDHAF4 (yeast Sdh8) is more cryptic, but it releases SDHAF2 from the SDHA–SDHAF2 complex (140), which is a requirement for the assembly of functional complex II (136, 137, 148). SDHAF1 and SDHAF3 are proposed to act as chaperones for Fe-S assembly within the SDHB subunit (138, 139). How these assembly factors act and whether there is any interplay with SDHAF4 or TRAP1 remains to be reported.
Finally, there are currently no reports of assembly mechanisms or dedicated chaperones for the integral-membrane SDHC and SDHD subunits. Heme b insertion into the membrane-spanning domain would be required for the full function of complex II. As mechanisms of assembly is a rapidly advancing area of research, more may be known in the future.
Succinate signaling, regulation of transcription, and cell fate
As recently as 2010, advances in cell biology were leveraged to show that the activity of complex II controls cell fate in a way that does not strictly result from a primary metabolic function. For example, the inducible loss of complex II activity is linked to a number of ROS- or succinate-dependent (149) biological activities, including DNA methylation, inflammation, cell fate in adipocytes, and cancer metabolism (18, 54, 55, 56). It is now appreciated that some types of cellular signaling are linked to complex II activity.
To understand the molecular mechanisms underlying how complex II signals, the synthesis of key relevant results that were reported before a signaling role was considered in the field is important. Among the earlier curiosities was the discovery that hypoxia or cellular acidification in response to anticancer drugs could cause the specific disassembly of complex II and the accumulation of a ∼100 kDa soluble species containing the SDHA subunit (23). These soluble species were interpreted as an SDHA–SDHB complex (23), but more recent work in macrophages showed that species observed upon complex II disassembly do not contain SDHB (31). Although the species was not assigned in either work, one possibility is that complex II disassembly species might be a complex between SDHA and an assembly factor. The SDHA-AF2 species (142) has recently been suggested as the dominant molecular component of this species, with this complex being highly stable and migrating at an appropriate molecular weight (150). Despite the significant accumulation of this ∼100 kDa species in cells under these conditions, no independent biological activity of the purified SDHA-AF2 complex has been detected (150). One possibility is that this ∼100 kDa species may be a way to store SDHA subunits in an inactive state following complex II disassembly while poising the cell for rapid reassembly when metabolic conditions change. No matter the nature of the accumulated species, the loss of assembled complex II and the appearance of the ∼100 kD SDHA-containing species correlate with reactive oxygen species generation, increased apoptosis, and the accumulation of the complex II substrate, succinate, in cells (23, 31). One interpretation of this is that dynamic assembly and disassembly of complex II regulates succinate signaling.
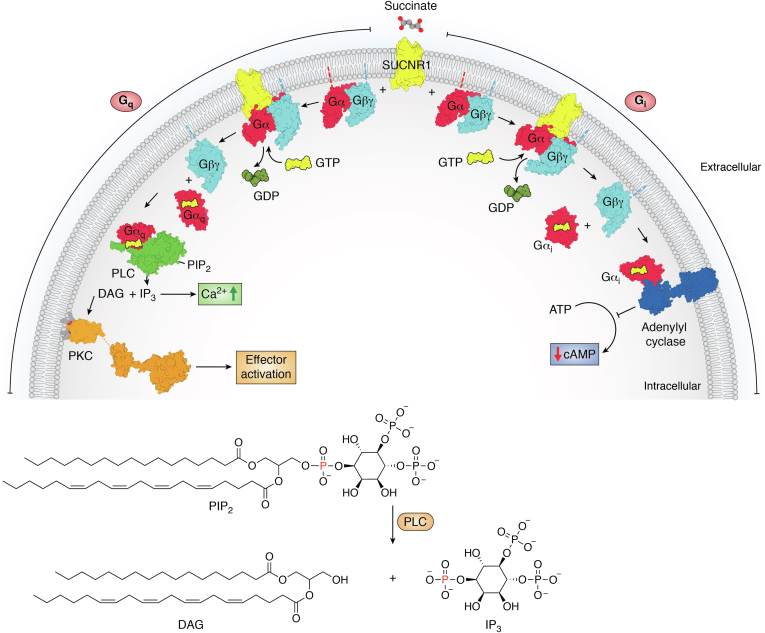
Indeed, within the last 15 years, metabolites including succinate and other Krebs cycle intermediates began to be recognized as signaling molecules (13, 17, 149, 151, 152). Succinate signaling occurs via more than one distinct molecular pathway. One route of signaling by succinate and other Krebs cycles intermediates is receptor-mediated. Here, accumulated cellular succinate stimulates the GPCR, SUCNR1, which is differentially expressed in both immune and adipose cells (153), including in the renin-producing cells of the nephron. This effect is currently believed to be local rather than systemic. SUCNR1 can couple to both Gi and Go (154, 155, 156) and to activate a range of downstream effectors via canonical G protein–dependent pathways (Fig. 5); SUCNR1 does not strongly couple arrestins and has not been demonstrated to initiate arrestin-dependent signaling. Many of the effectors downstream of Gi/Gq, like mitogen-activated protein kinases, Src-family kinases, and phosphoinositol kinases promote cellular survival, growth, proliferation, and metastasis (157, 158, 159, 160, 161). Potentially due to the activation of these pathways, SUCNR1 stimulation by succinate has been linked to several types of cancers. SUCNR1 may be involved in a number of succinate-associated health effects including ischemia-reperfusion injury, hypertension, inflammation, rheumatoid arthritis, nonalcoholic fatty liver disease, angiogenesis, and exercise-induced muscle remodeling (162, 163, 164, 165, 166, 167, 168, 169). These SUCNR1-dependent processes could explain how complex II, which controls cellular levels of the agonist, succinate, is the only respiratory enzyme that regulates metabolism or acts as a tumor suppressor (170, 171). Notably, GPCRs tend to be excellent pharmacological targets. Given the broad number of biological effects and diseases that depend upon succinate signaling through SUCNR1, this receptor has been aggressively pursued as a potential therapeutic target (172, 173, 174, 175) since its deorphanization (20).
Figure 5.
Succinate-stimulated SUCNR1 signaling. SUCNR1 is a GPCR, or 7-TM receptor, that responds to stimulation by succinate (20). Like other GPCRs, SUCNR1 and the proteins in its downstream signaling pathways transmit information through conformational changes that can create or remove binding sites and/or change the catalytic activity of effectors. Although the pharmacology and signaling pathways are still in the process of being mapped, SUCNR1 (PDB 6RNK (225)) has been shown to couple to either Gq or Gi. In the figure, the SUCNR1-G protein complex is modeled from 6RNK (225) and either 1GOT (226) or 3SN6 (227), using 3SN6 as a guide to place the Gα and Gβγ subunits. Both Gq (modeled from 2BCJ (228) and 1GOT (226)) and Gi (modeled from 1GIA (229) and 1GOT (226)) are heterotrimeric G proteins containing Gαβγ subunits. SUCNR1 initiates G protein-dependent signaling by catalyzing GDP release from the Gα subunit. The binding of GTP, which is at a higher concentration in the cell, disassociates the GPCR–Gαβγ complex, releasing GTP-bound Gα (2BCJ (228) or 1GIA (229)) and Gβγ (from 1GOT (226) or 1TBG (230)). Classical Gαq (from 2BCJ (228)) signaling involves the activation of phospholipase C (PLC, 7SQ2 (231)), which catalyzes the cleavage of the membrane-associated PIP2 into two potent signaling molecules: IP3, which is soluble, and diacylglycerol (DAG), which remains membrane-attached. IP3 mobilizes intracellular Ca2+ while DAG activates protein kinase C (PKC, modeled from PDB 3PFQ (232) and PDB 7L92 (233)) via a C1 domain. The kinase activity of PKC leads to the activation of numerous signaling effectors. Among the most potent and best characterized effectors are the Ras-Raf-MEK-ERK signaling cascade, but other mitogen activated protein kinases (MAP kinases), and Src family kinases are downstream of PKC. Signaling via Gαi (PDB 1GIA (229)) inhibits adenylyl cyclase (AC, modeled from PDB 1CJK (234)), which normally converts ATP to cyclic AMP (cAMP). The inhibition of adenyl cyclase, therefore, decreases cAMP, which has a range of effects on cAMP-dependent processes. GPCR, G protein-coupled receptor; SUCNR1, succinate receptor 1.
Cellular succinate, and other Krebs cycle intermediates with a similar chemical structure, can inhibit enzymes where succinate is a product. This has long been known to regulate the Krebs cycle via feedback inhibition. Succinate is also a coproduct of a very large number of α-ketoglutarate-dependent enzymes ((176); also called 2-oxoglutarate-dependent enzymes) and can inhibit these enzymes. Among enzymes affected by Krebs cycle intermediates are members of the nonheme iron α-ketoglutarate-dependent superfamily. Although other Krebs cycle intermediates, such as fumarate, have a higher affinity for the nonheme-iron α-ketoglutarate-dependent enzymes than does succinate (176, 177), succinate is currently believed to be a physiological regulator due to its cellular concentrations.
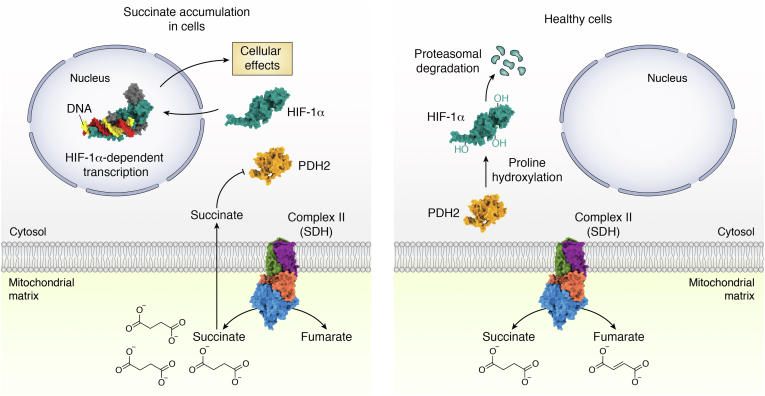
Examples of nonheme iron α-ketoglutarate-dependent dependent enzymes that have been demonstrated to be impacted by complex II activity are prolyl hydroxylases; these enzymes function to hydroxylate proteins to target them for degradation (Fig. 6). One substrate of prolyl hydroxylase-2 (PHD2) is hypoxia-inducible factor 1α (HIF1α) (178, 179, 180, 181), which is a master regulator of metabolism and is implicated in cancer metabolism. Thus, the accumulation of cellular succinate and increase of the cellular succinate:α-ketoglutarate ratio in cells following the reduction, loss, or reversal of complex II activity can inhibit PHD2, stabilize HIF1α, shift metabolism toward aerobic glycolysis (18), and induce cytokines and inflammation (182). This has broad implications for human health. As just one example, recent studies provide indirect evidence that succinate-dependent cytokine response may be a central feature of the severe- and long-COVID that hallmarks a subset of SARS-CoV2 infections. In fact, there is some overlap between the symptoms of complex II deficiency and some of the unusual symptoms of COVID (183, 184, 185). Cell-permeable diethyl succinate boosts viral load (186), while metabolomics identified that succinate accumulation profoundly increases with disease severity (187).
Figure 6.
Control of HIF1α activity through substrate-level inhibition of its degradation. Healthy cells under normoxia use oxidative phosphorylation to synthesize the majority of ATP. During succinate accumulation, cells can redirect metabolism to generate lactate via glycolysis, which is less efficient in producing ATP. One metabolic switch involves changes in complex II (1ZOY (75)) activity, resulting in the accumulation of cellular succinate. Some of the biological situations where succinate might accumulate are hypoxia, during complex II inhibition, in patients with reduced functional complex II, during reverse electron transfer, or during cancer. One theory is that succinate accumulation controls the quantity of the transcription factor HIF1α (modified from 4ZPR (235)). In healthy cells under aerobic conditions, PHD2 (2CGN (177)) covalently attaches hydroxides to proline residues in HIF1α. The end effect is targeting of the HIF1α transcription factor for degradation by the proteasome. Cellular dicarboxylates, including succinate, inhibit prolyl hydroxylase-2. When HIF1α accumulates, it binds to DNA (4ZPR (235)) and induces the transcription of a range of proteins used in the hypoxic response. This shunts respiration away from oxidative phosphorylation and toward glycolysis and the biosynthesis of nucleotides and lipids. When this occurs under aerobic conditions in cancer cells, it is also termed the Warburg effect. HIF1α, hypoxia-inducible factor 1α; SDH, succinate dehydrogenase; PHD2, prolyl hydroxylase-2.
There are also other α-ketoglutarate-dependent enzymes in the cell affected by complex II activity. Notable among these are histone and DNA demethylases. Succinate-induced inhibition of these enzymes attenuates demethylation activity. This correlates with DNA hypermethylation (54) and epigenetic gene silencing, as confirmed by methylome and transcriptome analyses (188). The implications are far reaching because demethylases impact the transcription of genes involved in disparate processes and have epigenetic control over cellular pluripotency, cancer metabolism, cellular transformation, and macrophage activation (189, 190, 191, 192, 193, 194, 195, 196, 197). It should be noted that α-ketoglutarate itself, which could out-compete succinate, is reported to have opposing effects on cellular pluripotency versus differentiation (198, 199). The similarity of the processes triggered via inhibition of histone and DNA methylases and those triggered by SUCNR1 and HIF1α inhibition suggests synergy and functional overlap in the different succinate-dependent signaling cascades.
Finally, covalent succinylation (200, 201) is a posttranslational modification that can modulate the activity of biological macromolecules. Protein succinylation involves destination lysine residues that are also targets of acetylation (200, 201) and requires a succinyl-CoA donor rather than succinate itself (201). While this is therefore distinct from true succinate signaling, changes in complex II activity affect cellular succinate-CoA levels and may also affect succinylation.
These signaling functions have renewed interest in whether the fundamental biological role of complex II is fully understood. One recent area of intense focus has been complex II reversibility in the mitochondria (9, 10). Although all enzymes theoretically can perform bidirectional catalysis, in vitro work shows that both bacterial and mitochondrial complex II perform catalysis via different intermediates in the forward versus reverse directions (71, 202). It was therefore surprising that two groups independently reported that mitochondrial complex II could biologically reverse its direction and reduce fumarate to succinate (9, 10). This allows fumarate to replace O2 as the terminal electron acceptor in the electron transport chain and is an extremely inefficient way to support bioenergetics. Reverse complex II activity may also be part of anaerobic mitochondrial production of hydrogen (203).
Some aspects of reverse electron transfer seem to satisfyingly connect the enzymatic and signaling functions of complex II. However, other aspects bring new questions to the forefront. For example, the ability to use reverse electron transfer in mammals appears to be tissue-dependent, with kidney, liver, and brain more strongly supporting reverse electron transfer (10). Of these, the liver is the most likely to experience anaerobic conditions that might require a shift in metabolism.
Integration over time: an evolving view
Synthesis of the findings on complex II reveals a shifting view over time. At present, it is now appreciated that there may be major roles for complex II in both respiration and in metabolic signaling. A particularly compelling theory is that complex II, or its individual subunits, contributes to multiple biological processes. This could potentially occur via direct protein–protein interactions in noncanonical complexes. Complex II may also move into and out of the megacomplexes during a response to the metabolic state.
Why would complex II have such diverse functions and such plasticity in its structural assemblies? Complex II is an evolutionarily ancient enzyme with ancestors that likely supported respiration before oxygen became abundant on the planet (83). Phylogeny hints that major changes in complex II rapidly occurred around the time of the great oxidation event (2, 204, 205). Perhaps the most important adaptation was the development of a covalent attachment between the FAD cofactor and the protein (35). The SDHAF2 assembly factor that enhances FAD attachment also likely appeared around the time of the great oxidation event (206). SDHAF2 moonlights as a component of the SDHA-AF2 species (142) of complex II-low, which is associated with altered metabolic signaling in cancer cell lines (21). Curiously, while SDHAF2 is found in all kingdoms and in most organisms that have a complex II homolog, the other characterized assembly factors are less broadly distributed.
While speculative, one could consider whether ancient cellular organisms might have benefitted from proteins supporting more than one biological function. It could further be considered whether metabolic signaling was among the earliest signaling systems. The present evidence that complex II connects metabolism and signaling could have assisted an ancient organism in its primordial need to swim toward food. The stable complex II scaffold and its subunits still serve these functions, amongst others. This change in conceptualization brings us one step closer to explaining the range of observations associated with complex II activity. It also directs the field to continually reassess whether we truly know the full role of complex II, or other proteins, in biology.
Conflict of interest
The authors declare no conflicts of interest with contents of this article.
Acknowledgments
We thank Dr Alexy Amunts for providing prepublication coordinates of the Tetrahymena thermophila CICIICIII2CIV2 respirasome used in Figure 4J.
Author contributions
T. M. I. conceptualization; T. M. I. writing–original draft; T. M. I., G. C., and P. K. S. writing-review and editing; T. M. I. and G. C. funding acquisition; P. K. S. visualization.
Funding and additional information
This work was supported by grants from the National Institutes of Health including GM61606 to G. C./T. M. I. and R44DA047146 to T. M. I. G. C. is the recipient of a VA Research Career Scientist award (1K6BX004215). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Reviewed by members of the JBC Editorial Board. Edited by Mike Shipston
Contributor Information
T.M. Iverson, Email: tina.iverson@vanderbilt.edu.
Gary Cecchini, Email: Gary.Cecchini@ucsf.edu.
References
- 1.Thunberg T. Studien über die beeinflussung des gasaustausches des überlebenden froschmuskels durch verschiedene stoffe. Skand. Archiv. Pysiol. 1909;22:430–436. [Google Scholar]
- 2.Cecchini G. Function and structure of complex II of the respiratory chain. Annu. Rev. Biochem. 2003;72:77–109. doi: 10.1146/annurev.biochem.72.121801.161700. [DOI] [PubMed] [Google Scholar]
- 3.Cecchini G. Respiratory complex II: role in cellular physiology and disease. Biochim. Biophys. Acta. 2013;1827:541–542. doi: 10.1016/j.bbabio.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Iverson T.M. Catalytic mechanisms of complex II enzymes: a structural perspective. Biochim. Biophys. Acta. 2013;1827:648–657. doi: 10.1016/j.bbabio.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Hellemond J.J., Klockiewicz M., Gaasenbeek C.P., Roos M.H., Tielens A.G. Rhodoquinone and complex II of the electron transport chain in anaerobically functioning eukaryotes. J. Biol. Chem. 1995;270:31065–31070. doi: 10.1074/jbc.270.52.31065. [DOI] [PubMed] [Google Scholar]
- 6.Van Hellemond J.J., Van Remoortere A., Tielens A.G. Schistosoma mansoni sporocysts contain rhodoquinone and produce succinate by fumarate reduction. Parasitology. 1997;115:177–182. doi: 10.1017/s003118209700125x. [DOI] [PubMed] [Google Scholar]
- 7.Tielens A.G., Van Hellemond J.J. The electron transport chain in anaerobically functioning eukaryotes. Biochim. Biophys. Acta. 1998;1365:71–78. doi: 10.1016/s0005-2728(98)00045-0. [DOI] [PubMed] [Google Scholar]
- 8.Hatchikian E.C. On the role of menaquinone-6 in the electron transport of hydrogen: fumarate reductase system in the strict anaerobe Desulfovibrio gigas. J. Gen. Microbiol. 1974;81:261–266. doi: 10.1099/00221287-81-1-261. [DOI] [PubMed] [Google Scholar]
- 9.Kumar R., Landry A.P., Guha A., Vitvitsky V., Lee H.J., Seike K., et al. A redox cycle with complex II prioritizes sulfide quinone oxidoreductase-dependent H2S oxidation. J. Biol. Chem. 2022;298 doi: 10.1016/j.jbc.2021.101435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spinelli J.B., Rosen P.C., Sprenger H.G., Puszynska A.M., Mann J.L., Roessler J.M., et al. Fumarate is a terminal electron acceptor in the mammalian electron transport chain. Science. 2021;374:1227–1237. doi: 10.1126/science.abi7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maklashina E., Berthold D.A., Cecchini G. Anaerobic expression of Escherichia coli succinate dehydrogenase: functional replacement of fumarate reductase in the respiratory chain during anaerobic growth. J. Bacteriol. 1998;180:5989–5996. doi: 10.1128/jb.180.22.5989-5996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cecchini G. Complexities of complex II: sulfide metabolism in vivo. J. Biol. Chem. 2022;298 doi: 10.1016/j.jbc.2022.101661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy M.P., Chouchani E.T. Why succinate? physiological regulation by a mitochondrial coenzyme Q sentinel. Nat. Chem. Biol. 2022;18:461–469. doi: 10.1038/s41589-022-01004-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koganitsky A., Tworowski D., Dadosh T., Cecchini G., Eisenbach M. A mechanism of modulating the direction of flagellar rotation in bacteria by fumarate and fumarate reductase. J. Mol. Biol. 2019;431:3662–3676. doi: 10.1016/j.jmb.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zarbiv G., Li H., Wolf A., Cecchini G., Caplan S.R., Sourjik V., et al. Energy complexes are apparently associated with the switch-motor complex of bacterial flagella. J. Mol. Biol. 2012;416:192–207. doi: 10.1016/j.jmb.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen-Ben-Lulu G.N., Francis N.R., Shimoni E., Noy D., Davidov Y., Prasad K., et al. The bacterial flagellar switch complex is getting more complex. EMBO J. 2008;27:1134–1144. doi: 10.1038/emboj.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker S.A., Rutter J. Metabolites as signalling molecules. Nat. Rev. Mol. Cell Biol. 2023 doi: 10.1038/s41580-022-00572-w. [DOI] [PubMed] [Google Scholar]
- 18.Selak M.A., Armour S.M., MacKenzie E.D., Boulahbel H., Watson D.G., Mansfield K.D., et al. succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Rose N.R., McDonough M.A., King O.N., Kawamura A., Schofield C.J. Inhibition of 2-oxoglutarate dependent oxygenases. Chem. Soc. Rev. 2011;40:4364–4397. doi: 10.1039/c0cs00203h. [DOI] [PubMed] [Google Scholar]
- 20.He W., Miao F.J., Lin D.C., Schwandner R.T., Wang Z., Gao J., et al. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature. 2004;429:188–193. doi: 10.1038/nature02488. [DOI] [PubMed] [Google Scholar]
- 21.Bezawork-Geleta A., Wen H., Dong L., Yan B., Vider J., Boukalova S., et al. Alternative assembly of respiratory complex II connects energy stress to metabolic checkpoints. Nat. Commun. 2018;9:2221. doi: 10.1038/s41467-018-04603-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gebert N., Gebert M., Oeljeklaus S., von der Malsburg K., Stroud D.A., Kulawiak B., et al. Dual function of Sdh3 in the respiratory chain and TIM22 protein translocase of the mitochondrial inner membrane. Mol. Cell. 2011;44:811–818. doi: 10.1016/j.molcel.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 23.Lemarie A., Huc L., Pazarentzos E., Mahul-Mellier A.L., Grimm S. Specific disintegration of complex II succinate:ubiquinone oxidoreductase links pH changes to oxidative stress for apoptosis induction. Cell Death Differ. 2011;18:338–349. doi: 10.1038/cdd.2010.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y., Ou X., Wang X., Sun D., Zhou X., Wu X., et al. Structure of the mitochondrial TIM22 complex from yeast. Cell Res. 2021;31:366–368. doi: 10.1038/s41422-020-00399-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta P., Strange K., Telange R., Guo A., Hatch H., Sobh A., et al. Genetic impairment of succinate metabolism disrupts bioenergetic sensing in adrenal neuroendocrine cancer. Cell Rep. 2022;40 doi: 10.1016/j.celrep.2022.111218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szeto S.S., Reinke S.N., Oyedotun K.S., Sykes B.D., Lemire B.D. Expression of Saccharomyces cerevisiae Sdh3p and Sdh4p paralogs results in catalytically active succinate dehydrogenase isoenzymes. J. Biol. Chem. 2012;287:22509–22520. doi: 10.1074/jbc.M112.344275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sciacovelli M., Guzzo G., Morello V., Frezza C., Zheng L., Nannini N., et al. The mitochondrial chaperone TRAP1 promotes neoplastic growth by inhibiting succinate dehydrogenase. Cell Metab. 2013;17:988–999. doi: 10.1016/j.cmet.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guzzo G., Sciacovelli M., Bernardi P., Rasola A. Inhibition of succinate dehydrogenase by the mitochondrial chaperone TRAP1 has anti-oxidant and anti-apoptotic effects on tumor cells. Oncotarget. 2014;5:11897–11908. doi: 10.18632/oncotarget.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao X., Sun K., Lan Z., Song W., Cheng L., Chi W., et al. Tenofovir and adefovir down-regulate mitochondrial chaperone TRAP1 and succinate dehydrogenase subunit B to metabolically reprogram glucose metabolism and induce nephrotoxicity. Sci. Rep. 2017;7 doi: 10.1038/srep46344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joshi A., Dai L., Liu Y., Lee J., Ghahhari N.M., Segala G., et al. The mitochondrial HSP90 paralog TRAP1 forms an OXPHOS-regulated tetramer and is involved in mitochondrial metabolic homeostasis. BMC Biol. 2020;18:10. doi: 10.1186/s12915-020-0740-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reynolds M.B., Hong H.S., Michmerhuizen B.C., Lawrence A.E., Zhang L., Knight J.S., et al. Cardiolipin coordinates inflammatory metabolic reprogramming through regulation of complex II disassembly and degradation. Sci. Adv. 2023;9 doi: 10.1126/sciadv.ade8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernath P., Kearney E.B., Singer T.P. Studies on succinic dehydrogenase. II. isolation and properties of the dehydrogenase from beef heart. J. Biol. Chem. 1956;223:599–613. [PubMed] [Google Scholar]
- 33.Kearney E.B., Singer T.P. Studies on succinic dehydrogenase. I. preparation and assay of the soluble dehydrogenase. J. Biol. Chem. 1956;219:963–975. [PubMed] [Google Scholar]
- 34.Singer T.P., Thimot N.Z., Massey V., Kearney E.B. Purification and properties of succinic dehydrogenase from yeast. Arch. Biochem. Biophys. 1956;62:497–499. doi: 10.1016/0003-9861(56)90148-5. [DOI] [PubMed] [Google Scholar]
- 35.Kearney E.B., Singer T.P. On the prosthetic group of succinic dehydrogenase. Biochim. Biophys. Acta. 1955;17:596–597. doi: 10.1016/0006-3002(55)90432-7. [DOI] [PubMed] [Google Scholar]
- 36.Singer T.P., Kearney E.B., Massey V. Observations on the flavin moiety of succinic dehydrogenase. Arch. Biochem. Biophys. 1956;60:255–257. doi: 10.1016/0003-9861(56)90415-5. [DOI] [PubMed] [Google Scholar]
- 37.Blaut M., Whittaker K., Valdovinos A., Ackrell B.A., Gunsalus R.P., Cecchini G. Fumarate reductase mutants of Escherichia coli that lack covalently bound flavin. J. Biol. Chem. 1989;264:13599–13604. [PubMed] [Google Scholar]
- 38.Iverson T.M., Maklashina E., Cecchini G. Structural basis for malfunction in complex II. J. Biol. Chem. 2012;287:35430–35438. doi: 10.1074/jbc.R112.408419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horsefield R., Yankovskaya V., Sexton G., Whittingham W., Shiomi K., Omura S., et al. Structural and computational analysis of the quinone-binding site of complex II (succinate-ubiquinone oxidoreductase): a mechanism of electron transfer and proton conduction during ubiquinone reduction. J. Biol. Chem. 2006;281:7309–7316. doi: 10.1074/jbc.M508173200. [DOI] [PubMed] [Google Scholar]
- 40.Ruprecht J., Yankovskaya V., Maklashina E., Iwata S., Cecchini G. Structure of Escherichia coli succinate:quinone oxidoreductase with an occupied and empty quinone-binding site. J. Biol. Chem. 2009;284:29836–29846. doi: 10.1074/jbc.M109.010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruprecht J., Iwata S., Rothery R.A., Weiner J.H., Maklashina E., Cecchini G. Perturbation of the quinone-binding site of complex II alters the electronic properties of the proximal [3Fe-4S] iron-sulfur cluster. J. Biol. Chem. 2011;286:12756–12765. doi: 10.1074/jbc.M110.209874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yankovskaya V., Horsefield R., Tornroth S., Luna-Chavez C., Miyoshi H., Leger C., et al. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science. 2003;299:700–704. doi: 10.1126/science.1079605. [DOI] [PubMed] [Google Scholar]
- 43.Hederstedt L. Succinate dehydrogenase mutants of Bacillus subtilis lacking covalently bound flavin in the flavoprotein subunit. Eur. J. Biochem. 1983;132:589–593. doi: 10.1111/j.1432-1033.1983.tb07404.x. [DOI] [PubMed] [Google Scholar]
- 44.Maguire J.J., Magnusson K., Hederstedt L. Bacillus subtilis mutant succinate dehydrogenase lacking covalently bound flavin: Identification of the primary defect and studies on the iron-sulfur clusters in mutated and wild-type enzyme. Biochemistry. 1986;25:5202–5208. doi: 10.1021/bi00366a033. [DOI] [PubMed] [Google Scholar]
- 45.A A.E., Hederstedt L. Ligands to the 2Fe iron-sulfur center in succinate dehydrogenase. FEBS Lett. 1988;232:298–302. doi: 10.1016/0014-5793(88)80757-9. [DOI] [PubMed] [Google Scholar]
- 46.Werth M.T., Cecchini G., Manodori A., Ackrell B.A., Schroder I., Gunsalus R.P., et al. Site-directed mutagenesis of conserved cysteine residues in Escherichia coli fumarate reductase: modification of the spectroscopic and electrochemical properties of the [2Fe-2S] cluster. Proc. Natl. Acad. Sci. U. S. A. 1990;87:8965–8969. doi: 10.1073/pnas.87.22.8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westenberg D.J., Gunsalus R.P., Ackrell B.A., Cecchini G. Electron transfer from menaquinol to fumarate. Fumarate reductase anchor polypeptide mutants of Escherichia coli. J. Biol. Chem. 1990;265:19560–19567. [PubMed] [Google Scholar]
- 48.Schroder I., Gunsalus R.P., Ackrell B.A., Cochran B., Cecchini G. Identification of active site residues of Escherichia coli fumarate reductase by site-directed mutagenesis. J. Biol. Chem. 1991;266:13572–13579. [PubMed] [Google Scholar]
- 49.Manodori A., Cecchini G., Schroder I., Gunsalus R.P., Werth M.T., Johnson M.K. [3Fe-4S] to [4Fe-4S] cluster conversion in Escherichia coli fumarate reductase by site-directed mutagenesis. Biochemistry. 1992;31:2703–2712. doi: 10.1021/bi00125a010. [DOI] [PubMed] [Google Scholar]
- 50.Kowal A.T., Werth M.T., Manodori A., Cecchini G., Schroder I., Gunsalus R.P., et al. Effect of cysteine to serine mutations on the properties of the [4Fe-4S] center in Escherichia coli fumarate reductase. Biochemistry. 1995;34:12284–12293. doi: 10.1021/bi00038a024. [DOI] [PubMed] [Google Scholar]
- 51.Birch-Machin M.A., Taylor R.W., Cochran B., Ackrell B.A., Turnbull D.M. Late-onset optic atrophy, ataxia, and myopathy associated with a mutation of a complex II gene. Ann. Neurol. 2000;48:330–335. [PubMed] [Google Scholar]
- 52.Taylor R.W., Birch-Machin M.A., Schaefer J., Taylor L., Shakir R., Ackrell B.A., et al. Deficiency of complex II of the mitochondrial respiratory chain in late-onset optic atrophy and ataxia. Ann. Neurol. 1996;39:224–232. doi: 10.1002/ana.410390212. [DOI] [PubMed] [Google Scholar]
- 53.Piruat J.I., Pintado C.O., Ortega-Saenz P., Roche M., Lopez-Barneo J. The mitochondrial SDHD gene is required for early embryogenesis, and its partial deficiency results in persistent carotid body glomus cell activation with full responsiveness to hypoxia. Mol. Cell. Biol. 2004;24:10933–10940. doi: 10.1128/MCB.24.24.10933-10940.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cervera A.M., Bayley J.P., Devilee P., McCreath K.J. Inhibition of succinate dehydrogenase dysregulates histone modification in mammalian cells. Mol. Cancer. 2009;8:89. doi: 10.1186/1476-4598-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mills E.L., Kelly B., Logan A., Costa A.S.H., Varma M., Bryant C.E., et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell. 2016;167:457–470.e13. doi: 10.1016/j.cell.2016.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu K., Lin L., Li Q., Xue Y., Zheng F., Wang G., et al. Scd1 controls de novo beige fat biogenesis through succinate-dependent regulation of mitochondrial complex II. Proc. Natl. Acad. Sci. U. S. A. 2020;117:2462–2472. doi: 10.1073/pnas.1914553117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fullerton M., McFarland R., Taylor R.W., Alston C.L. The genetic basis of isolated mitochondrial complex II deficiency. Mol. Genet. Metab. 2020;131:53–65. doi: 10.1016/j.ymgme.2020.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iverson T.M., Luna-Chavez C., Cecchini G., Rees D.C. Structure of the Escherichia coli fumarate reductase respiratory complex. Science. 1999;284:1961–1966. doi: 10.1126/science.284.5422.1961. [DOI] [PubMed] [Google Scholar]
- 59.Lancaster C.R., Kroger A., Auer M., Michel H. Structure of fumarate reductase from Wolinella succinogenes at 2.2 A resolution. Nature. 1999;402:377–385. doi: 10.1038/46483. [DOI] [PubMed] [Google Scholar]
- 60.Bamford V., Dobbin P.S., Richardson D.J., Hemmings A.M. Open conformation of a flavocytochrome c3 fumarate reductase. Nat. Struct. Biol. 1999;6:1104–1107. doi: 10.1038/70039. [DOI] [PubMed] [Google Scholar]
- 61.Taylor P., Pealing S.L., Reid G.A., Chapman S.K., Walkinshaw M.D. Structural and mechanistic mapping of a unique fumarate reductase. Nat. Struct. Biol. 1999;6:1108–1112. doi: 10.1038/70045. [DOI] [PubMed] [Google Scholar]
- 62.Leys D., Tsapin A.S., Nealson K.H., Meyer T.E., Cusanovich M.A., Van Beeumen J.J. Structure and mechanism of the flavocytochrome c fumarate reductase of Shewanella putrefaciens MR-1. Nat. Struct. Biol. 1999;6:1113–1117. doi: 10.1038/70051. [DOI] [PubMed] [Google Scholar]
- 63.Warringa M.G., Smith O.H., Giuditta A., Singer T.P. Studies on succinic dehydrogenase. VIII. isolation of a succinic dehydrogenase-fumaric reductase from an obligate anaerobe. J. Biol. Chem. 1958;230:97–109. [PubMed] [Google Scholar]
- 64.Deibel R.H., Kvetkas M.J. Fumarate reduction and its role in the diversion of glucose fermentation by Streptococcus faecalis. J. Bacteriol. 1964;88:858–864. doi: 10.1128/jb.88.4.858-864.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pichinoty F., Leminor L., Mollaret H.H. [Study of fumarate reductase in facultative anaerobic bacteria] Can. J. Microbiol. 1965;11:287–290. [PubMed] [Google Scholar]
- 66.Gray C.T., Wimpenny J.W., Hughes D.E., Mossman M.R. Regulation of metabolism in facultative bacteria. I. structural and functional changes in Escherichia coli associated with shifts between the aerobic and anaerobic states. Biochim. Biophys. Acta. 1966;117:22–32. doi: 10.1016/0304-4165(66)90148-6. [DOI] [PubMed] [Google Scholar]
- 67.Doherty M.K., Pealing S.L., Miles C.S., Moysey R., Taylor P., Walkinshaw M.D., et al. Identification of the active site acid/base catalyst in a bacterial fumarate reductase: a kinetic and crystallographic study. Biochemistry. 2000;39:10695–10701. doi: 10.1021/bi000871l. [DOI] [PubMed] [Google Scholar]
- 68.Reid G.A., Miles C.S., Moysey R.K., Pankhurst K.L., Chapman S.K. Catalysis in fumarate reductase. Biochim. Biophys. Acta. 2000;1459:310–315. doi: 10.1016/s0005-2728(00)00166-3. [DOI] [PubMed] [Google Scholar]
- 69.Mowat C.G., Pankhurst K.L., Miles C.S., Leys D., Walkinshaw M.D., Reid G.A., et al. Engineering water to act as an active site acid catalyst in a soluble fumarate reductase. Biochemistry. 2002;41:11990–11996. doi: 10.1021/bi0203177. [DOI] [PubMed] [Google Scholar]
- 70.Pankhurst K.L., Mowat C.G., Miles C.S., Leys D., Walkinshaw M.D., Reid G.A., et al. Role of His505 in the soluble fumarate reductase from Shewanella frigidimarina. Biochemistry. 2002;41:8551–8556. doi: 10.1021/bi020155e. [DOI] [PubMed] [Google Scholar]
- 71.Maklashina E., Iverson T.M., Sher Y., Kotlyar V., Andrell J., Mirza O., et al. Fumarate reductase and succinate oxidase activity of Escherichia coli complex II homologs are perturbed differently by mutation of the flavin binding domain. J. Biol. Chem. 2006;281:11357–11365. doi: 10.1074/jbc.M512544200. [DOI] [PubMed] [Google Scholar]
- 72.Pankhurst K.L., Mowat C.G., Rothery E.L., Hudson J.M., Jones A.K., Miles C.S., et al. A proton delivery pathway in the soluble fumarate reductase from Shewanella frigidimarina. J. Biol. Chem. 2006;281:20589–20597. doi: 10.1074/jbc.M603077200. [DOI] [PubMed] [Google Scholar]
- 73.Tomasiak T.M., Maklashina E., Cecchini G., Iverson T.M. A threonine on the active site loop controls transition state formation in Escherichia coli respiratory complex II. J. Biol. Chem. 2008;283:15460–15468. doi: 10.1074/jbc.M801372200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang L.S., Sun G., Cobessi D., Wang A.C., Shen J.T., Tung E.Y., et al. 3-nitropropionic acid is a suicide inhibitor of mitochondrial respiration that, upon oxidation by complex II, forms a covalent adduct with a catalytic base arginine in the active site of the enzyme. J. Biol. Chem. 2006;281:5965–5972. doi: 10.1074/jbc.M511270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun F., Huo X., Zhai Y., Wang A., Xu J., Su D., et al. Crystal structure of mitochondrial respiratory membrane protein complex II. Cell. 2005;121:1043–1057. doi: 10.1016/j.cell.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 76.Huang L.S., Lummen P., Berry E.A. Crystallographic investigation of the ubiquinone binding site of respiratory Complex II and its inhibitors. Biochim. Biophys. Acta Proteins Proteom. 2021;1869 doi: 10.1016/j.bbapap.2021.140679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gong H., Gao Y., Zhou X., Xiao Y., Wang W., Tang Y., et al. Cryo-EM structure of trimeric Mycobacterium smegmatis succinate dehydrogenase with a membrane-anchor SdhF. Nat. Commun. 2020;11:4245. doi: 10.1038/s41467-020-18011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Su C.C., Lyu M., Morgan C.E., Bolla J.R., Robinson C.V., Yu E.W. A 'Build and Retrieve' methodology to simultaneously solve cryo-EM structures of membrane proteins. Nat. Methods. 2021;18:69–75. doi: 10.1038/s41592-020-01021-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou X., Gao Y., Wang W., Yang X., Yang X., Liu F., et al. Architecture of the mycobacterial succinate dehydrogenase with a membrane-embedded Rieske FeS cluster. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2022308118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mühleip A., Flygaard R.K., Haapanen O., Baradaran R., Gruhl T., Tobiasson V., et al. Structural basis of mitochondrial membrane bending by I-II-III2-IV2 supercomplex. Nature. 2023;615:934–938. doi: 10.1038/s41586-023-05817-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shimizu H., Osanai A., Sakamoto K., Inaoka D.K., Shiba T., Harada S., et al. Crystal structure of mitochondrial quinol-fumarate reductase from the parasitic nematode Ascaris suum. J. Biochem. 2012;151:589–592. doi: 10.1093/jb/mvs051. [DOI] [PubMed] [Google Scholar]
- 82.Tang Y., Mu A., Zhang Y., Zhou S., Wang W., Lai Y., et al. Cryo-EM structure of Mycobacterium smegmatis DyP-loaded encapsulin. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2025658118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Karavaeva V., Sousa F.L. Modular structure of complex II: an evolutionary perspective. Biochim. Biophys. Acta Bioenerg. 2023;1864 doi: 10.1016/j.bbabio.2022.148916. [DOI] [PubMed] [Google Scholar]
- 84.Spiga L., Winter M.G., Furtado de Carvalho T., Zhu W., Hughes E.R., Gillis C.C., et al. An oxidative central metabolism enables Salmonella to utilize microbiota-derived succinate. Cell Host Microbe. 2017;22:291–301.e6. doi: 10.1016/j.chom.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kuo C.J., Wang S.T., Lin C.M., Chiu H.C., Huang C.R., Lee D.Y., et al. A multi-omic analysis reveals the role of fumarate in regulating the virulence of enterohemorrhagic Escherichia coli. Cell Death Dis. 2018;9:381. doi: 10.1038/s41419-018-0423-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hartman T., Weinrick B., Vilcheze C., Berney M., Tufariello J., Cook G.M., et al. Succinate dehydrogenase is the regulator of respiration in Mycobacterium tuberculosis. PLoS Pathog. 2014;10 doi: 10.1371/journal.ppat.1004510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pecsi I., Hards K., Ekanayaka N., Berney M., Hartman T., Jacobs W.R., Jr., et al. Essentiality of succinate dehydrogenase in Mycobacterium smegmatis and its role in the generation of the membrane potential under hypoxia. mBio. 2014;5 doi: 10.1128/mBio.01093-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gaupp R., Schlag S., Liebeke M., Lalk M., Gotz F. Advantage of upregulation of succinate dehydrogenase in Staphylococcus aureus biofilms. J. Bacteriol. 2010;192:2385–2394. doi: 10.1128/JB.01472-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mercado-Lubo R., Gauger E.J., Leatham M.P., Conway T., Cohen P.S. A Salmonella enterica serovar typhimurium succinate dehydrogenase/fumarate reductase double mutant is avirulent and immunogenic in BALB/c mice. Infect. Immun. 2008;76:1128–1134. doi: 10.1128/IAI.01226-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Eoh H., Rhee K.Y. Multifunctional essentiality of succinate metabolism in adaptation to hypoxia in Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. U. S. A. 2013;110:6554–6559. doi: 10.1073/pnas.1219375110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li S., Li X., Zhang H., Wang Z., Xu H. The research progress in and perspective of potential fungicides: succinate dehydrogenase inhibitors. Bioorg. Med. Chem. 2021;50 doi: 10.1016/j.bmc.2021.116476. [DOI] [PubMed] [Google Scholar]
- 92.Inaoka D.K., Shiba T., Sato D., Balogun E.O., Sasaki T., Nagahama M., et al. Structural insights into the molecular design of flutolanil derivatives targeted for fumarate respiration of parasite mitochondria. Int. J. Mol. Sci. 2015;16:15287–15308. doi: 10.3390/ijms160715287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hards K., Adolph C., Harold L.K., McNeil M.B., Cheung C.Y., Jinich A., et al. Two for the price of one: attacking the energetic-metabolic hub of mycobacteria to produce new chemotherapeutic agents. Prog. Biophys. Mol. Biol. 2020;152:35–44. doi: 10.1016/j.pbiomolbio.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 94.Kim S., Kim C.M., Son Y.J., Choi J.Y., Siegenthaler R.K., Lee Y., et al. Molecular basis of maintaining an oxidizing environment under anaerobiosis by soluble fumarate reductase. Nat. Commun. 2018;9:4867. doi: 10.1038/s41467-018-07285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mattevi A., Tedeschi G., Bacchella L., Coda A., Negri A., Ronchi S. Structure of L-aspartate oxidase: implications for the succinate dehydrogenase/fumarate reductase oxidoreductase family. Structure. 1999;7:745–756. doi: 10.1016/s0969-2126(99)80099-9. [DOI] [PubMed] [Google Scholar]
- 96.Bossi R.T., Negri A., Tedeschi G., Mattevi A. Structure of FAD-bound L-aspartate oxidase: insight into substrate specificity and catalysis. Biochemistry. 2002;41:3018–3024. doi: 10.1021/bi015939r. [DOI] [PubMed] [Google Scholar]
- 97.Letts J.A., Fiedorczuk K., Sazanov L.A. The architecture of respiratory supercomplexes. Nature. 2016;537:644–648. doi: 10.1038/nature19774. [DOI] [PubMed] [Google Scholar]
- 98.Gu J., Wu M., Guo R., Yan K., Lei J., Gao N., et al. The architecture of the mammalian respirasome. Nature. 2016;537:639–643. doi: 10.1038/nature19359. [DOI] [PubMed] [Google Scholar]
- 99.Vercellino I., Sazanov L.A. Structure and assembly of the mammalian mitochondrial supercomplex CIII(2)CIV. Nature. 2021;598:364–367. doi: 10.1038/s41586-021-03927-z. [DOI] [PubMed] [Google Scholar]
- 100.Steimle S., van Eeuwen T., Ozturk Y., Kim H.J., Braitbard M., Selamoglu N., et al. Cryo-EM structures of engineered active bc(1)-cbb(3) type CIII(2)CIV super-complexes and electronic communication between the complexes. Nat. Commun. 2021;12:929. doi: 10.1038/s41467-021-21051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berndtsson J., Aufschnaiter A., Rathore S., Marin-Buera L., Dawitz H., Diessl J., et al. Respiratory supercomplexes enhance electron transport by decreasing cytochrome c diffusion distance. EMBO Rep. 2020;21 doi: 10.15252/embr.202051015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gong H., Li J., Xu A., Tang Y., Ji W., Gao R., et al. An electron transfer path connects subunits of a mycobacterial respiratory supercomplex. Science. 2018;362 doi: 10.1126/science.aat8923. [DOI] [PubMed] [Google Scholar]
- 103.Hartley A.M., Lukoyanova N., Zhang Y., Cabrera-Orefice A., Arnold S., Meunier B., et al. Structure of yeast cytochrome c oxidase in a supercomplex with cytochrome bc(1) Nat. Struct. Mol. Biol. 2019;26:78–83. doi: 10.1038/s41594-018-0172-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hartley A.M., Meunier B., Pinotsis N., Marechal A. Rcf2 revealed in cryo-EM structures of hypoxic isoforms of mature mitochondrial III-IV supercomplexes. Proc. Natl. Acad. Sci. U. S. A. 2020;117:9329–9337. doi: 10.1073/pnas.1920612117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wiseman B., Nitharwal R.G., Fedotovskaya O., Schafer J., Guo H., Kuang Q., et al. Structure of a functional obligate complex III(2)IV(2) respiratory supercomplex from Mycobacterium smegmatis. Nat. Struct. Mol. Biol. 2018;25:1128–1136. doi: 10.1038/s41594-018-0160-3. [DOI] [PubMed] [Google Scholar]
- 106.Kao W.C., Ortmann de Percin Northumberland C., Cheng T.C., Ortiz J., Durand A., von Loeffelholz O., et al. Structural basis for safe and efficient energy conversion in a respiratory supercomplex. Nat. Commun. 2022;13:545. doi: 10.1038/s41467-022-28179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jeon T.J., Lee S.G., Yoo S.H., Kim M., Song D., Ryu J., et al. A dynamic substrate pool revealed by cryo-EM of a lipid-preserved respiratory supercomplex. Antioxid. Redox Signal. 2022 doi: 10.1089/ars.2021.0114. [DOI] [PubMed] [Google Scholar]
- 108.Wu M., Gu J., Guo R., Huang Y., Yang M. Structure of mammalian respiratory supercomplex I(1)III(2)IV(1) Cell. 2016;167:1598–1609.e10. doi: 10.1016/j.cell.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 109.Klusch N., Dreimann M., Senkler J., Rugen N., Kuhlbrandt W., Braun H.P. Cryo-EM structure of the respiratory I + III(2) supercomplex from Arabidopsis thaliana at 2 A resolution. Nat. Plants. 2023;9:142–156. doi: 10.1038/s41477-022-01308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guo R., Zong S., Wu M., Gu J., Yang M. Architecture of human mitochondrial respiratory megacomplex I(2)III(2)IV(2) Cell. 2017;170:1247–1257.e12. doi: 10.1016/j.cell.2017.07.050. [DOI] [PubMed] [Google Scholar]
- 111.Zhou L., Maldonado M., Padavannil A., Guo F., Letts J.A. Structures of Tetrahymena's respiratory chain reveal the diversity of eukaryotic core metabolism. Science. 2022;376:831–839. doi: 10.1126/science.abn7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wu F., Minteer S. Krebs cycle metabolon: structural evidence of substrate channeling revealed by cross-linking and mass spectrometry. Angew. Chem. Int. Ed. Engl. 2015;54:1851–1854. doi: 10.1002/anie.201409336. [DOI] [PubMed] [Google Scholar]
- 113.Wu F., Pelster L.N., Minteer S.D. Krebs cycle metabolon formation: metabolite concentration gradient enhanced compartmentation of sequential enzymes. Chem. Commun. (Camb.) 2015;51:1244–1247. doi: 10.1039/c4cc08702j. [DOI] [PubMed] [Google Scholar]
- 114.Bayley J.P., Devilee P., Taschner P.E. The SDH mutation database: an online resource for succinate dehydrogenase sequence variants involved in pheochromocytoma, paraganglioma and mitochondrial complex II deficiency. BMC Med. Genet. 2005;6:39. doi: 10.1186/1471-2350-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Upadhayay S., Yedke N.G., Rahi V., Singh S., Kumar S., Arora A., et al. An overview of the pathophysiological mechanisms of 3-nitropropionic acid (3-NPA) as a neurotoxin in a huntington's disease model and its relevance to drug discovery and development. Neurochem. Res. 2023;48:1631–1647. doi: 10.1007/s11064-023-03868-1. [DOI] [PubMed] [Google Scholar]
- 116.Ibarra-Gutierrez M.T., Serrano-Garcia N., Orozco-Ibarra M. Rotenone-induced model of Parkinson's disease: beyond mitochondrial complex I inhibition. Mol. Neurobiol. 2023;60:1929–1948. doi: 10.1007/s12035-022-03193-8. [DOI] [PubMed] [Google Scholar]
- 117.Hensen E.F., Bayley J.P. Recent advances in the genetics of SDH-related paraganglioma and pheochromocytoma. Fam. Cancer. 2011;10:355–363. doi: 10.1007/s10689-010-9402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hoekstra A.S., Bayley J.P. The role of complex II in disease. Biochim. Biophys. Acta. 2013;1827:543–551. doi: 10.1016/j.bbabio.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 119.Hoekstra A.S., Devilee P., Bayley J.P. Models of parent-of-origin tumorigenesis in hereditary paraganglioma. Semin. Cell Dev. Biol. 2015;43:117–124. doi: 10.1016/j.semcdb.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 120.Gottlieb E., Tomlinson I.P. Mitochondrial tumour suppressors: a genetic and biochemical update. Nat. Rev. Cancer. 2005;5:857–866. doi: 10.1038/nrc1737. [DOI] [PubMed] [Google Scholar]
- 121.Armstrong N., Storey C.M., Noll S.E., Margulis K., Soe M.H., Xu H., et al. SDHB knockout and succinate accumulation are insufficient for tumorigenesis but dual SDHB/NF1 loss yields SDHx-like pheochromocytomas. Cell Rep. 2022;38 doi: 10.1016/j.celrep.2022.110453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Aggarwal R.K., Luchtel R.A., Machha V., Tischer A., Zou Y., Pradhan K., et al. Functional succinate dehydrogenase deficiency is a common adverse feature of clear cell renal cancer. Proc. Natl. Acad. Sci. U. S. A. 2021;118 doi: 10.1073/pnas.2106947118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fuchs T.L., Maclean F., Turchini J., Vargas A.C., Bhattarai S., Agaimy A., et al. Expanding the clinicopathological spectrum of succinate dehydrogenase-deficient renal cell carcinoma with a focus on variant morphologies: a study of 62 new tumors in 59 patients. Mod. Pathol. 2021;35:836–849. doi: 10.1038/s41379-021-00998-1. [DOI] [PubMed] [Google Scholar]
- 124.Baysal B.E. On the association of succinate dehydrogenase mutations with hereditary paraganglioma. Trends Endocrinol. Metab. 2003;14:453–459. doi: 10.1016/j.tem.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 125.Goncalves J., Moog S., Morin A., Gentric G., Muller S., Morrell A.P., et al. Loss of SDHB promotes dysregulated iron homeostasis, oxidative stress, and sensitivity to ascorbate. Cancer Res. 2021;81:3480–3494. doi: 10.1158/0008-5472.CAN-20-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bayley J.P., Devilee P. Hypothesis: why different types of SDH gene variants cause divergent tumor phenotypes. Genes (Basel) 2022;13:1025. doi: 10.3390/genes13061025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Al Khazal F., Kang S., Nelson Holte M., Choi D.S., Singh R., Ortega-Saenz P., et al. Unexpected obesity, rather than tumorigenesis, in a conditional mouse model of mitochondrial complex II deficiency. FASEB J. 2021;35 doi: 10.1096/fj.202002100R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Baysal B.E., Alahmari A.A., Rodrick T.C., Tabaczynski D., Curtin L., Seshadri M., et al. Succinate dehydrogenase inversely regulates red cell distribution width and healthy life span in chronically hypoxic mice. JCI Insight. 2022;7 doi: 10.1172/jci.insight.158737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Harada K., Yahata T., Onizuka M., Ishii T., Aziz Ibrahim A., Kikkawa E., et al. Mitochondrial electron transport chain complex II dysfunction causes premature aging of hematopoietic stem cells. Stem Cells. 2023;41:39–49. doi: 10.1093/stmcls/sxac072. [DOI] [PubMed] [Google Scholar]
- 130.Sharma P., Maklashina E., Cecchini G., Iverson T.M. Maturation of the respiratory complex II flavoprotein. Curr. Opin. Struct. Biol. 2019;59:38–46. doi: 10.1016/j.sbi.2019.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Robinson K.M., Lemire B.D. Covalent attachment of FAD to the yeast succinate dehydrogenase flavoprotein requires import into mitochondria, presequence removal, and folding. J. Biol. Chem. 1996;271:4055–4060. doi: 10.1074/jbc.271.8.4055. [DOI] [PubMed] [Google Scholar]
- 132.Yoshida S., Tsutsumi S., Muhlebach G., Sourbier C., Lee M.J., Lee S., et al. Molecular chaperone TRAP1 regulates a metabolic switch between mitochondrial respiration and aerobic glycolysis. Proc. Natl. Acad. Sci. U. S. A. 2013;110:E1604–E1612. doi: 10.1073/pnas.1220659110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Liu Y., Elnatan D., Sun M., Myasniko A.G., Agard D.A. Cryo-EM reveals the dynamic interplay between mitochondrial Hsp90 and SdhB folding intermediates. bioRxiv. 2020 doi: 10.1101/2020.10.06.327627. [preprint] [DOI] [Google Scholar]
- 134.Hao H.X., Khalimonchuk O., Schraders M., Dephoure N., Bayley J.P., Kunst H., et al. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325:1139–1142. doi: 10.1126/science.1175689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huang S., Taylor N.L., Stroher E., Fenske R., Millar A.H. Succinate dehydrogenase assembly factor 2 is needed for assembly and activity of mitochondrial complex II and for normal root elongation in Arabidopsis. Plant J. 2013;73:429–441. doi: 10.1111/tpj.12041. [DOI] [PubMed] [Google Scholar]
- 136.Van Vranken J.G., Bricker D.K., Dephoure N., Gygi S.P., Cox J.E., Thummel C.S., et al. SDHAF4 promotes mitochondrial succinate dehydrogenase activity and prevents neurodegeneration. Cell Metab. 2014;20:241–252. doi: 10.1016/j.cmet.2014.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Belt K., Van Aken O., Murcha M., Millar A.H., Huang S. An assembly factor promotes assembly of flavinated SDH1 into the succinate dehydrogenase complex. Plant Physiol. 2018;177:1439–1452. doi: 10.1104/pp.18.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ghezzi D., Goffrini P., Uziel G., Horvath R., Klopstock T., Lochmuller H., et al. SDHAF1, encoding a LYR complex-II specific assembly factor, is mutated in SDH-defective infantile leukoencephalopathy. Nat. Genet. 2009;41:654–656. doi: 10.1038/ng.378. [DOI] [PubMed] [Google Scholar]
- 139.Na U., Yu W., Cox J., Bricker D.K., Brockmann K., Rutter J., et al. The LYR factors SDHAF1 and SDHAF3 mediate maturation of the iron-sulfur subunit of succinate dehydrogenase. Cell Metab. 2014;20:253–266. doi: 10.1016/j.cmet.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sharma P., Maklashina E., Voehler M., Balintova S., Dvorakova S., Kraus M., et al. Disordered-to-ordered transitions in assembly factors allow the complex II catalytic subunit to switch binding partners. Res. Square. 2023 doi: 10.21203/rs.3.rs-2070392/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Maklashina E., Iverson T.M., Cecchini G. How an assembly factor enhances covalent FAD attachment to the flavoprotein subunit of complex II. J. Biol. Chem. 2022;298 doi: 10.1016/j.jbc.2022.102472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sharma P., Maklashina E., Cecchini G., Iverson T.M. The roles of SDHAF2 and dicarboxylate in covalent flavinylation of SDHA, the human complex II flavoprotein. Proc. Natl. Acad. Sci. U. S. A. 2020;117:23548–23556. doi: 10.1073/pnas.2007391117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.McNeil M.B., Clulow J.S., Wilf N.M., Salmond G.P., Fineran P.C. SdhE is a conserved protein required for flavinylation of succinate dehydrogenase in bacteria. J. Biol. Chem. 2012;287:18418–18428. doi: 10.1074/jbc.M111.293803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.McNeil M.B., Hampton H.G., Hards K.J., Watson B.N., Cook G.M., Fineran P.C. The succinate dehydrogenase assembly factor, SdhE, is required for the flavinylation and activation of fumarate reductase in bacteria. FEBS Lett. 2014;588:414–421. doi: 10.1016/j.febslet.2013.12.019. [DOI] [PubMed] [Google Scholar]
- 145.McNeil M.B., Fineran P.C. The conserved RGxxE motif of the bacterial FAD assembly factor SdhE is required for succinate dehydrogenase flavinylation and activity. Biochemistry. 2013;52:7628–7640. doi: 10.1021/bi401006a. [DOI] [PubMed] [Google Scholar]
- 146.Maher M.J., Herath A.S., Udagedara S.R., Dougan D.A., Truscott K.N. Crystal structure of bacterial succinate:quinone oxidoreductase flavoprotein SdhA in complex with its assembly factor SdhE. Proc. Natl. Acad. Sci. U. S. A. 2018;115:2982–2987. doi: 10.1073/pnas.1800195115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sharma P., Maklashina E., Cecchini G., Iverson T.M. Crystal structure of an assembly intermediate of respiratory Complex II. Nat. Commun. 2018;9:274. doi: 10.1038/s41467-017-02713-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang X., Lv W., Xu J., Zheng A., Zeng M., Cao K., et al. Hepatic suppression of mitochondrial complex II assembly drives systemic metabolic benefits. Adv. Sci. (Weinh.) 2022;9 doi: 10.1002/advs.202105587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Murphy M.P., O'Neill L.A.J. Krebs cycle reimagined: the emerging roles of succinate and itaconate as signal transducers. Cell. 2018;174:780–784. doi: 10.1016/j.cell.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 150.Maklashina E., Rajagukguk S., Iverson T.M., Cecchini G. The unassembled flavoprotein subunits of human and bacterial complex II have impaired catalytic activity and generate only minor amounts of ROS. J. Biol. Chem. 2018;293:7754–7765. doi: 10.1074/jbc.RA118.001977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Thompson C.B. Into thin air: how we sense and respond to hypoxia. Cell. 2016;167:9–11. doi: 10.1016/j.cell.2016.08.036. [DOI] [PubMed] [Google Scholar]
- 152.Xiao M., Yang H., Xu W., Ma S., Lin H., Zhu H., et al. Inhibition of alpha-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012;26:1326–1338. doi: 10.1101/gad.191056.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Regard J.B., Sato I.T., Coughlin S.R. Anatomical profiling of G protein-coupled receptor expression. Cell. 2008;135:561–571. doi: 10.1016/j.cell.2008.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Trauelsen M., Hiron T.K., Lin D., Petersen J.E., Breton B., Husted A.S., et al. Extracellular succinate hyperpolarizes M2 macrophages through SUCNR1/GPR91-mediated Gq signaling. Cell Rep. 2021;35 doi: 10.1016/j.celrep.2021.109246. [DOI] [PubMed] [Google Scholar]
- 155.Robben J.H., Fenton R.A., Vargas S.L., Schweer H., Peti-Peterdi J., Deen P.M., et al. Localization of the succinate receptor in the distal nephron and its signaling in polarized MDCK cells. Kidney Int. 2009;76:1258–1267. doi: 10.1038/ki.2009.360. [DOI] [PubMed] [Google Scholar]
- 156.Mossa A.H., Velasquez Flores M., Cammisotto P.G., Campeau L. Succinate, increased in metabolic syndrome, activates GPR91 receptor signaling in urothelial cells. Cell. Signal. 2017;37:31–39. doi: 10.1016/j.cellsig.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 157.Li T., Hu J., Du S., Chen Y., Wang S., Wu Q. ERK1/2/COX-2/PGE2 signaling pathway mediates GPR91-dependent VEGF release in streptozotocin-induced diabetes. Mol. Vis. 2014;20:1109–1121. [PMC free article] [PubMed] [Google Scholar]
- 158.Aguiar C.J., Rocha-Franco J.A., Sousa P.A., Santos A.K., Ladeira M., Rocha-Resende C., et al. Succinate causes pathological cardiomyocyte hypertrophy through GPR91 activation. Cell Commun. Signal. 2014;12:78. doi: 10.1186/s12964-014-0078-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lu Y.T., Li L.Z., Yang Y.L., Yin X., Liu Q., Zhang L., et al. Succinate induces aberrant mitochondrial fission in cardiomyocytes through GPR91 signaling. Cell Death Dis. 2018;9:672. doi: 10.1038/s41419-018-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hogberg C., Gidlof O., Tan C., Svensson S., Nilsson-Ohman J., Erlinge D., et al. Succinate independently stimulates full platelet activation via cAMP and phosphoinositide 3-kinase-beta signaling. J. Thromb. Haemost. 2011;9:361–372. doi: 10.1111/j.1538-7836.2010.04158.x. [DOI] [PubMed] [Google Scholar]
- 161.Wu J.Y., Huang T.W., Hsieh Y.T., Wang Y.F., Yen C.C., Lee G.L., et al. Cancer-derived succinate promotes macrophage polarization and cancer metastasis via succinate receptor. Mol. Cell. 2020;77:213–227.e5. doi: 10.1016/j.molcel.2019.10.023. [DOI] [PubMed] [Google Scholar]
- 162.Li X., Xie L., Qu X., Zhao B., Fu W., Wu B., et al. GPR91, a critical signaling mechanism in modulating pathophysiologic processes in chronic illnesses. FASEB J. 2020;34:13091–13105. doi: 10.1096/fj.202001037R. [DOI] [PubMed] [Google Scholar]
- 163.Atallah R., Olschewski A., Heinemann A. Succinate at the crossroad of metabolism and angiogenesis: roles of SDH, HIF1alpha and SUCNR1. Biomedicines. 2022;10:3089. doi: 10.3390/biomedicines10123089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Liu H., Zhang H., Zhang X., Chen Q., Xia L. Role of succinic acid in the regulation of sepsis. Int. Immunopharmacol. 2022;110 doi: 10.1016/j.intimp.2022.109065. [DOI] [PubMed] [Google Scholar]
- 165.Liang C., Li J., Tian B., Tian L., Liu Y., Li J., et al. Foresight regarding drug candidates acting on the succinate-GPR91 signalling pathway for non-alcoholic steatohepatitis (NASH) treatment. Biomed. Pharmacother. 2021;144 doi: 10.1016/j.biopha.2021.112298. [DOI] [PubMed] [Google Scholar]
- 166.Reddy A., Bozi L.H.M., Yaghi O.K., Mills E.L., Xiao H., Nicholson H.E., et al. pH-gated succinate secretion regulates muscle remodeling in response to exercise. Cell. 2020;183:62–75.e17. doi: 10.1016/j.cell.2020.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Guo Y., Xu F., Thomas S.C., Zhang Y., Paul B., Sakilam S., et al. Targeting the succinate receptor effectively inhibits periodontitis. Cell Rep. 2022;40 doi: 10.1016/j.celrep.2022.111389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang T., Xu Y.Q., Yuan Y.X., Xu P.W., Zhang C., Li F., et al. Succinate induces skeletal muscle fiber remodeling via SUCNR1 signaling. EMBO Rep. 2021;22 doi: 10.15252/embr.202153027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chouchani E.T., Pell V.R., Gaude E., Aksentijevic D., Sundier S.Y., Robb E.L., et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Matlac D.M., Hadrava Vanova K., Bechmann N., Richter S., Folberth J., Ghayee H.K., et al. Succinate mediates tumorigenic effects via succinate receptor 1: potential for new targeted treatment strategies in succinate dehydrogenase deficient paragangliomas. Front. Endocrinol. (Lausanne) 2021;12 doi: 10.3389/fendo.2021.589451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Keiran N., Ceperuelo-Mallafre V., Calvo E., Hernandez-Alvarez M.I., Ejarque M., Nunez-Roa C., et al. SUCNR1 controls an anti-inflammatory program in macrophages to regulate the metabolic response to obesity. Nat. Immunol. 2019;20:581–592. doi: 10.1038/s41590-019-0372-7. [DOI] [PubMed] [Google Scholar]
- 172.Geubelle P., Gilissen J., Dilly S., Poma L., Dupuis N., Laschet C., et al. Identification and pharmacological characterization of succinate receptor agonists. Br. J. Pharmacol. 2017;174:796–808. doi: 10.1111/bph.13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Trauelsen M., Rexen Ulven E., Hjorth S.A., Brvar M., Monaco C., Frimurer T.M., et al. Receptor structure-based discovery of non-metabolite agonists for the succinate receptor GPR91. Mol. Metab. 2017;6:1585–1596. doi: 10.1016/j.molmet.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Velcicky J., Wilcken R., Cotesta S., Janser P., Schlapbach A., Wagner T., et al. Discovery and optimization of novel SUCNR1 inhibitors: design of zwitterionic derivatives with a salt bridge for the improvement of oral exposure. J. Med. Chem. 2020;63:9856–9875. doi: 10.1021/acs.jmedchem.0c01020. [DOI] [PubMed] [Google Scholar]
- 175.McGarry T., Fearon U. Cell metabolism as a potentially targetable pathway in RA. Nat. Rev. Rheumatol. 2019;15:70–72. doi: 10.1038/s41584-018-0148-8. [DOI] [PubMed] [Google Scholar]
- 176.Hausinger R.P. FeII/alpha-ketoglutarate-dependent hydroxylases and related enzymes. Crit. Rev. Biochem. Mol. Biol. 2004;39:21–68. doi: 10.1080/10409230490440541. [DOI] [PubMed] [Google Scholar]
- 177.Hewitson K.S., Lienard B.M., McDonough M.A., Clifton I.J., Butler D., Soares A.S., et al. Structural and mechanistic studies on the inhibition of the hypoxia-inducible transcription factor hydroxylases by tricarboxylic acid cycle intermediates. J. Biol. Chem. 2007;282:3293–3301. doi: 10.1074/jbc.M608337200. [DOI] [PubMed] [Google Scholar]
- 178.Jaakkola P., Mole D.R., Tian Y.M., Wilson M.I., Gielbert J., Gaskell S.J., et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- 179.Ivan M., Kondo K., Yang H., Kim W., Valiando J., Ohh M., et al. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 180.Masson N., Willam C., Maxwell P.H., Pugh C.W., Ratcliffe P.J. Independent function of two destruction domains in hypoxia-inducible factor-alpha chains activated by prolyl hydroxylation. EMBO J. 2001;20:5197–5206. doi: 10.1093/emboj/20.18.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yu F., White S.B., Zhao Q., Lee F.S. HIF-1alpha binding to VHL is regulated by stimulus-sensitive proline hydroxylation. Proc. Natl. Acad. Sci. U. S. A. 2001;98:9630–9635. doi: 10.1073/pnas.181341498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tannahill G.M., Curtis A.M., Adamik J., Palsson-McDermott E.M., McGettrick A.F., Goel G., et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature. 2013;496:238–242. doi: 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rojbi I., Adel M., Affes M., Hantous S., Jrad M., Ben Nacef I., et al. Pheochromocytoma presenting as fulminant myocarditis mimicking COVID-19 pneumonia. Clin. Case Rep. 2021;9 doi: 10.1002/ccr3.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Naghshineh H., Hasanpour A., Ziaei N., Sadeghi M., Meftah N. Pheochromocytoma triggered by coronavirus disease 2019: a case report. J. Med. Case Rep. 2022;16:233. doi: 10.1186/s13256-022-03378-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Haji N., Jr., Ali S., Wahashi E.A., Khalid M., Ramamurthi K. Johnson and Johnson COVID-19 vaccination triggering pheochromocytoma multisystem crisis. Cureus. 2021;13 doi: 10.7759/cureus.18196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Codo A.C., Davanzo G.G., Monteiro L.B., de Souza G.F., Muraro S.P., Virgilio-da-Silva J.V., et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1alpha/glycolysis-dependent axis. Cell Metab. 2020;32:498–499. doi: 10.1016/j.cmet.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Xiao N., Nie M., Pang H., Wang B., Hu J., Meng X., et al. Integrated cytokine and metabolite analysis reveals immunometabolic reprogramming in COVID-19 patients with therapeutic implications. Nat. Commun. 2021;12:1618. doi: 10.1038/s41467-021-21907-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Letouze E., Martinelli C., Loriot C., Burnichon N., Abermil N., Ottolenghi C., et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell. 2013;23:739–752. doi: 10.1016/j.ccr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 189.Aspuria P.P., Lunt S.Y., Varemo L., Vergnes L., Gozo M., Beach J.A., et al. Succinate dehydrogenase inhibition leads to epithelial-mesenchymal transition and reprogrammed carbon metabolism. Cancer Metab. 2014;2:21. doi: 10.1186/2049-3002-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dominguez-Andres J., Novakovic B., Li Y., Scicluna B.P., Gresnigt M.S., Arts R.J.W., et al. The itaconate pathway is a central regulatory node linking innate immune tolerance and trained immunity. Cell Metab. 2019;29:211–220.e5. doi: 10.1016/j.cmet.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 191.Flavahan W.A., Drier Y., Johnstone S.E., Hemming M.L., Tarjan D.R., Hegazi E., et al. Altered chromosomal topology drives oncogenic programs in SDH-deficient GISTs. Nature. 2019;575:229–233. doi: 10.1038/s41586-019-1668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lampropoulou V., Sergushichev A., Bambouskova M., Nair S., Vincent E.E., Loginicheva E., et al. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodeling and regulation of inflammation. Cell Metab. 2016;24:158–166. doi: 10.1016/j.cmet.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Li S.T., Huang D., Shen S., Cai Y., Xing S., Wu G., et al. Myc-mediated SDHA acetylation triggers epigenetic regulation of gene expression and tumorigenesis. Nat. Metab. 2020;2:256–269. doi: 10.1038/s42255-020-0179-8. [DOI] [PubMed] [Google Scholar]
- 194.Liu P.S., Wang H., Li X., Chao T., Teav T., Christen S., et al. alpha-ketoglutarate orchestrates macrophage activation through metabolic and epigenetic reprogramming. Nat. Immunol. 2017;18:985–994. doi: 10.1038/ni.3796. [DOI] [PubMed] [Google Scholar]
- 195.Narne P., Pandey V., Phanithi P.B. Interplay between mitochondrial metabolism and oxidative stress in ischemic stroke: an epigenetic connection. Mol. Cell. Neurosci. 2017;82:176–194. doi: 10.1016/j.mcn.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 196.Salminen A., Kaarniranta K., Hiltunen M., Kauppinen A. Krebs cycle dysfunction shapes epigenetic landscape of chromatin: novel insights into mitochondrial regulation of aging process. Cell. Signal. 2014;26:1598–1603. doi: 10.1016/j.cellsig.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 197.Sowers M.L., Tang H., Singh V.K., Khan A., Mishra A., Restrepo B.I., et al. Multi-OMICs analysis reveals metabolic and epigenetic changes associated with macrophage polarization. J. Biol. Chem. 2022;298 doi: 10.1016/j.jbc.2022.102418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.TeSlaa T., Chaikovsky A.C., Lipchina I., Escobar S.L., Hochedlinger K., Huang J., et al. Alpha-ketoglutarate accelerates the initial differentiation of primed human pluripotent stem cells. Cell Metab. 2016;24:485–493. doi: 10.1016/j.cmet.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Carey B.W., Finley L.W., Cross J.R., Allis C.D., Thompson C.B. Intracellular alpha-ketoglutarate maintains the pluripotency of embryonic stem cells. Nature. 2015;518:413–416. doi: 10.1038/nature13981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Weinert B.T., Scholz C., Wagner S.A., Iesmantavicius V., Su D., Daniel J.A., et al. Lysine succinylation is a frequently occurring modification in prokaryotes and eukaryotes and extensively overlaps with acetylation. Cell Rep. 2013;4:842–851. doi: 10.1016/j.celrep.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 201.Zhang Z., Tan M., Xie Z., Dai L., Chen Y., Zhao Y. Identification of lysine succinylation as a new post-translational modification. Nat. Chem. Biol. 2011;7:58–63. doi: 10.1038/nchembio.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hudson J.M., Heffron K., Kotlyar V., Sher Y., Maklashina E., Cecchini G., et al. Electron transfer and catalytic control by the iron-sulfur clusters in a respiratory enzyme, E. coli fumarate reductase. J. Am. Chem. Soc. 2005;127:6977–6989. doi: 10.1021/ja043404q. [DOI] [PubMed] [Google Scholar]
- 203.Boxma B., de Graaf R.M., van der Staay G.W., van Alen T.A., Ricard G., Gabaldon T., et al. An anaerobic mitochondrion that produces hydrogen. Nature. 2005;434:74–79. doi: 10.1038/nature03343. [DOI] [PubMed] [Google Scholar]
- 204.Lyons T.W., Reinhard C.T., Planavsky N.J. The rise of oxygen in Earth's early ocean and atmosphere. Nature. 2014;506:307–315. doi: 10.1038/nature13068. [DOI] [PubMed] [Google Scholar]
- 205.Cecchini G., Maklashina E., Yankovskaya V., Iverson T.M., Iwata S. Variation in proton donor/acceptor pathways in succinate:quinone oxidoreductases. FEBS Lett. 2003;545:31–38. doi: 10.1016/s0014-5793(03)00390-9. [DOI] [PubMed] [Google Scholar]
- 206.Lim K., Doseeva V., Demirkan E.S., Pullalarevu S., Krajewski W., Galkin A., et al. Crystal structure of the YgfY from Escherichia coli, a protein that may be involved in transcriptional regulation. Proteins. 2005;58:759–763. doi: 10.1002/prot.20337. [DOI] [PubMed] [Google Scholar]
- 207.Mowat C.G., Moysey R., Miles C.S., Leys D., Doherty M.K., Taylor P., et al. Kinetic and crystallographic analysis of the key active site acid/base arginine in a soluble fumarate reductase. Biochemistry. 2001;40:12292–12298. doi: 10.1021/bi011360h. [DOI] [PubMed] [Google Scholar]
- 208.Kravchuk V., Petrova O., Kampjut D., Wojciechowska-Bason A., Breese Z., Sazanov L. A universal coupling mechanism of respiratory complex I. Nature. 2022;609:808–814. doi: 10.1038/s41586-022-05199-7. [DOI] [PubMed] [Google Scholar]
- 209.Gao X., Wen X., Esser L., Quinn B., Yu L., Yu C.A., et al. Structural basis for the quinone reduction in the bc1 complex: a comparative analysis of crystal structures of mitochondrial cytochrome bc1 with bound substrate and inhibitors at the Qi site. Biochemistry. 2003;42:9067–9080. doi: 10.1021/bi0341814. [DOI] [PubMed] [Google Scholar]
- 210.Yoshikawa S., Shinzawa-Itoh K., Nakashima R., Yaono R., Yamashita E., Inoue N., et al. Redox-coupled crystal structural changes in bovine heart cytochrome c oxidase. Science. 1998;280:1723–1729. doi: 10.1126/science.280.5370.1723. [DOI] [PubMed] [Google Scholar]
- 211.Spikes T.E., Montgomery M.G., Walker J.E. Structure of the dimeric ATP synthase from bovine mitochondria. Proc. Natl. Acad. Sci. U. S. A. 2020;117:23519–23526. doi: 10.1073/pnas.2013998117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Prajapati S., Haselbach D., Wittig S., Patel M.S., Chari A., Schmidt C., et al. Structural and functional analyses of the human PDH complex suggest a “Division-of-Labor” mechanism by local E1 and E3 clusters. Structure. 2019;27:1124–1136.e4. doi: 10.1016/j.str.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 213.Remington S., Wiegand G., Huber R. Crystallographic refinement and atomic models of two different forms of citrate synthase at 2.7 and 1.7 A resolution. J. Mol. Biol. 1982;158:111–152. doi: 10.1016/0022-2836(82)90452-1. [DOI] [PubMed] [Google Scholar]
- 214.Lauble H., Kennedy M.C., Beinert H., Stout C.D. Crystal structures of aconitase with isocitrate and nitroisocitrate bound. Biochemistry. 1992;31:2735–2748. doi: 10.1021/bi00125a014. [DOI] [PubMed] [Google Scholar]
- 215.Sun P., Liu Y., Ma T., Ding J. Structure and allosteric regulation of human NAD-dependent isocitrate dehydrogenase. Cell Discov. 2020;6:94. doi: 10.1038/s41421-020-00220-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fraser M.E., Hayakawa K., Hume M.S., Ryan D.G., Brownie E.R. Interactions of GTP with the ATP-grasp domain of GTP-specific succinyl-CoA synthetase. J. Biol. Chem. 2006;281:11058–11065. doi: 10.1074/jbc.M511785200. [DOI] [PubMed] [Google Scholar]
- 217.Ajalla Aleixo M.A., Rangel V.L., Rustiguel J.K., de Padua R.A.P., Nonato M.C. Structural, biochemical and biophysical characterization of recombinant human fumarate hydratase. FEBS J. 2019;286:1925–1940. doi: 10.1111/febs.14782. [DOI] [PubMed] [Google Scholar]
- 218.Gleason W.B., Fu Z., Birktoft J., Banaszak L. Refined crystal structure of mitochondrial malate dehydrogenase from porcine heart and the consensus structure for dicarboxylic acid oxidoreductases. Biochemistry. 1994;33:2078–2088. doi: 10.1021/bi00174a014. [DOI] [PubMed] [Google Scholar]
- 219.Frank R.A., Price A.J., Northrop F.D., Perham R.N., Luisi B.F. Crystal structure of the E1 component of the Escherichia coli 2-oxoglutarate dehydrogenase multienzyme complex. J. Mol. Biol. 2007;368:639–651. doi: 10.1016/j.jmb.2007.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Robien M.A., Clore G.M., Omichinski J.G., Perham R.N., Appella E., Sakaguchi K., et al. Three-dimensional solution structure of the E3-binding domain of the dihydrolipoamide succinyltransferase core from the 2-oxoglutarate dehydrogenase multienzyme complex of Escherichia coli. Biochemistry. 1992;31:3463–3471. doi: 10.1021/bi00128a021. [DOI] [PubMed] [Google Scholar]
- 221.Nakai T., Kamiya N., RIKEN Structural Genomics/Proteomics Initiative (RSGI) Crystal structure of lipoamide dehydrogenase from thermus thermophilus HB8 with psbdo. RCSB Protein Data Bank. 2007 2EQ7. [Google Scholar]
- 222.Ricaud P.M., Howard M.J., Roberts E.L., Broadhurst R.W., Perham R.N. Three-dimensional structure of the lipoyl domain from the dihydrolipoyl succinyltransferase component of the 2-oxoglutarate dehydrogenase multienzyme complex of Escherichia coli. J. Mol. Biol. 1996;264:179–190. doi: 10.1006/jmbi.1996.0632. [DOI] [PubMed] [Google Scholar]
- 223.Iverson T.M., Luna-Chavez C., Croal L.R., Cecchini G., Rees D.C. Crystallographic studies of the Escherichia coli quinol-fumarate reductase with inhibitors bound to the quinol-binding site. J. Biol. Chem. 2002;277:16124–16130. doi: 10.1074/jbc.M200815200. [DOI] [PubMed] [Google Scholar]
- 224.Juhnke H.D., Hiltscher H., Nasiri H.R., Schwalbe H., Lancaster C.R. Production, characterization and determination of the real catalytic properties of the putative 'succinate dehydrogenase' from Wolinella succinogenes. Mol. Microbiol. 2009;71:1088–1101. doi: 10.1111/j.1365-2958.2008.06581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Haffke M., Fehlmann D., Rummel G., Boivineau J., Duckely M., Gommermann N., et al. Structural basis of species-selective antagonist binding to the succinate receptor. Nature. 2019;574:581–585. doi: 10.1038/s41586-019-1663-8. [DOI] [PubMed] [Google Scholar]
- 226.Lambright D.G., Sondek J., Bohm A., Skiba N.P., Hamm H.E., Sigler P.B. The 2.0 A crystal structure of a heterotrimeric G protein. Nature. 1996;379:311–319. doi: 10.1038/379311a0. [DOI] [PubMed] [Google Scholar]
- 227.Rasmussen S.G., DeVree B.T., Zou Y., Kruse A.C., Chung K.Y., Kobilka T.S., et al. Crystal structure of the beta2 adrenergic receptor-Gs protein complex. Nature. 2011;477:549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Tesmer V.M., Kawano T., Shankaranarayanan A., Kozasa T., Tesmer J.J. Snapshot of activated G proteins at the membrane: the galphaq-GRK2-gbetagamma complex. Science. 2005;310:1686–1690. doi: 10.1126/science.1118890. [DOI] [PubMed] [Google Scholar]
- 229.Coleman D.E., Berghuis A.M., Lee E., Linder M.E., Gilman A.G., Sprang S.R. Structures of active conformations of Gi alpha 1 and the mechanism of GTP hydrolysis. Science. 1994;265:1405–1412. doi: 10.1126/science.8073283. [DOI] [PubMed] [Google Scholar]
- 230.Sondek J., Bohm A., Lambright D.G., Hamm H.E., Sigler P.B. Crystal structure of a G-protein beta gamma dimer at 2.1A resolution. Nature. 1996;379:369–374. doi: 10.1038/379369a0. [DOI] [PubMed] [Google Scholar]
- 231.Waldo G.L., Ricks T.K., Hicks S.N., Cheever M.L., Kawano T., Tsuboi K., et al. Kinetic scaffolding mediated by a phospholipase C-beta and Gq signaling complex. Science. 2010;330:974–980. doi: 10.1126/science.1193438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Leonard T.A., Rozycki B., Saidi L.F., Hummer G., Hurley J.H. Crystal structure and allosteric activation of protein kinase C betaII. Cell. 2011;144:55–66. doi: 10.1016/j.cell.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Katti S.S., Krieger I.V., Ann J., Lee J., Sacchettini J.C., Igumenova T.I. Structural anatomy of protein Kinase C C1 domain interactions with diacylglycerol and other agonists. Nat. Commun. 2022;13:2695. doi: 10.1038/s41467-022-30389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tesmer J.J., Sunahara R.K., Johnson R.A., Gosselin G., Gilman A.G., Sprang S.R. Two-metal-ion catalysis in adenylyl cyclase. Science. 1999;285:756–760. doi: 10.1126/science.285.5428.756. [DOI] [PubMed] [Google Scholar]
- 235.Wu D., Potluri N., Lu J., Kim Y., Rastinejad F. Structural integration in hypoxia-inducible factors. Nature. 2015;524:303–308. doi: 10.1038/nature14883. [DOI] [PubMed] [Google Scholar]