Abstract
Vitellogenin (Vt) is considered the primary protein precursor of egg yolk, serving as a source of protein- and lipid-rich nutrients for the developing embryo. However, recent research has revealed that the functions of Vt and Vt-derived polypeptides, such as yolkin (Y) and yolk glycopeptide 40 (YGP40), extend beyond their nutritional roles as a source of amino acids. Emerging evidence has demonstrated that both Y and YGP40 possess immunomodulatory properties and can contribute to host immune defenses. Additionally, Y polypeptides have been shown to exhibit neuroprotective activity, participating in the modulation of neurons’ survival and activity, inhibiting neurodegeneration processes, and improving cognitive functions in rats. These non-nutritional functions not only enhance our understanding of the physiological roles of these molecules during embryonic development but also offer a promising basis for the potential application of these proteins in human health.
Key words: yolkin, yolk glycopeptide 40, vitellogenin, immunomodulatory activity, neuroprotective activity
INTRODUCTION
Avian eggs are a remarkable creation of nature, with the primary biological function of giving rise to new life. Consequently, they contain essential elements for life, and numerous egg compounds possess biological activity (Mine, 2007). In addition to being a versatile food product, eggs are a rich source of high-quality nutritive substances, many of which exhibit multifunctional properties such as foaming, gelling, emulsifying, and coloring (Anton, 2013; Li et al., 2021). Hen eggs, in particular, are an excellent source of raw materials for the development of health-promoting functional foods, that is, nutraceuticals, and natural pharmaceuticals, garnering significant attention in recent years (Mine, 2007; Réhault-Godbert and Guyot, 2018; Réhault-Godbert et al., 2019; Kazana et al., 2020, 2022a,b). Although the bioactive compounds in the egg white and yolk have been well-characterized, limited information is available on the proteins present in the egg yolk plasma other than immunoglobulin Y (IgY). Therefore, the aim of this paper was to present the characteristics (including physicochemical description) and significance of relatively recently discovered yolkin (Y) and yolk glycopeptide 40 (YGP40), and their protein precursor, vitellogenin (Vt).
VITELLOGENIN—THE PRECURSOR OF EGG YOLK PROTEINS
Due to its original role as an embryonic chamber, egg yolk contains numerous vital nutrients and preservative substances. Thus, the yolk represents a major source of active principles usable in the medical, pharmaceutical, cosmetic, nutraceutical, and biotechnological industries (Anton, 2007, 2013; Li et al., 2021). Water and dry matter each account for 52% of the mass of egg yolk, fats for 32%, proteins for 16%, and sugars and mineral compounds for approx. 2% (Patil et al., 2022). From the physicochemical point of view, the yolk is a pseudoplastic non-Newtonian liquid and a very complicated system of emulsified protein-lipid complexes. Most of the yolk proteins are bound to lipids and are present in the form of lipoproteins.
When fractionating the yolk using ultracentrifugation, its components separate into 2 fractions, namely granules and plasma (Anton 2013; Li et al., 2021). The granules contain α-lipovitellins and β-lipovitellins, high-density lipoproteins (HDL), phosvitin, and low-density lipoproteins (LDL). The plasma contains low-density lipoproteins and livetins, lipid-free globular proteins (Anton, 2007, 2013; Mine and Kovacs-Nolan, 2016; Li et al., 2021). Recently, 255 unique protein species have been identified in egg yolk plasma by 3 types of combinatorial peptide ligand libraries (Gao et al., 2017).
Plasma proteins include yolk immunoglobulins with high heterogeneity of the variable regions of both heavy and light chains (∼45%), glycoproteins—yolk glycopeptide (YGP) 42 and YGP40 (41%), albumins (14%), and a recently discovered protein YGP30, showing a 75% similarity to YGP40 (Jolivet et al., 2008). Among the livetins, γ-livetin, also referred to as IgY, has so far aroused the greatest interest in researchers. Serum IgY is selectively transferred to the yolk via a receptor on the surface of the oocyte membrane that is specific for IgY translocation (Schade and Chicana, 2007).
The precursor of the main proteins of the granular fraction, such as α- lipovitellins and β-lipovitellins, phosvitin, and HDL lipoproteins, is Vt (Yamamura et al., 1995; Finn, 2007; Réhault-Godbert and Guyot, 2018; Kazana et al., 2020; Zabłocka et al., 2021a). Chicken Vt is synthesized in the hen's liver. This protein is post-translationally glycosylated and phosphorylated in the endoplasmic reticulum and Golgi complex before being tagged for export and is secreted to the circulating plasma in the form of homodimeric lipoprotein complexes (Yamamura et al., 1995; Finn, 2007). During egg formation in the laying hen's reproductive system, Vt is transported from the blood to the oviduct, absorbed by the growing oocyte, and then subjected to proteolytic processing to form proteins of the granular fraction of the yolk, which are N-terminal fragments of the precursor protein ( Elkin et al., 1995; Yamamura et al., 1995; Réhault-Godbert and Guyot, 2018).
Vitellogenin is encoded by numerous genes, and the synthesis of this protein is hormone-dependent, beginning under the influence of a rise in the concentration of estrogens in the bloodstream. Vt contains 3 conserved LPD_N domains, which have also been identified in many other functionally important proteins, including in the D domain of the von Willebrand factor. Vt is a phosphoglycoprotein with a molecular weight of 240 to 600 kDa, belonging to the family of lipid transport proteins that also includes apolipoprotein B and microsomal triglyceride transport protein (MTP) (Avarre et al., 2007). The main function of Vt is to provide a source of essential nutrients (amino acids and peptides) for the developing embryo (Elkin et al., 1995; Yamamura et al., 1995; Avarre et al., 2007; Lindsay et al., 2020).
Vitellogenin isolated from numerous organisms is characterized by high sequence homology and a variety of biological activities (Chen et al. 1997; Lindsay et al., 2020). A homolog of this protein isolated from the crustacean Branchiostoma blecheri has been shown to have antimicrobial activity against Escherichia coli as well as hemagglutination properties (Zhang et al., 2005). Vt in insect Ceanorhabditis elegans shows antioxidant activity as a strong free radical scavenger, and the corresponding fish protein isolated from blood serum can neutralize the IPNV virus (Infectious Pancreatic Necrosis Virus) that induces pancreatic necrosis (Seehuus et al., 2006, Nakamura et al., 1999). It seems that although Vt is a relatively little-known protein, it may become a promising object of research with potential application in many technologies.
Chicken Vt consists of 3 species designed Vt I, II, and III (Yamamura et al., 1995; Groche et al., 2000), found in the 0.33:1.0:0.08 ratio in the plasma of birds (Groche et al., 2000). Vt II, a protein with a molecular mass of 240 kDa, has been reported to be proteolytically cleaved into a smaller number of fragments; with the following identified: the N-terminal region, termed lipovitellin I (120 kDa) corresponding to the N-terminal domain of 1096 residues, and the phosphoryl-rich domain, termed phosvitin corresponding to the serine-rich domain of 1112-1328. The 30 kDa chain of lipovitellin (lipovitellin II) is also recognized as a fragment of Vt II of the N-terminal region (Yamamura et al., 1995) (Figure 1).
Figure 1.
Schematic representation of the precursor-product relationship between vitellogenin II (Vt II) and the yolk proteins. In the process of egg formation, Vtg II (from hen's blood plasma) is proteolytically cleaved to the main proteins of the yolk, which are accumulated in immature egg cells (oocytes) located in the antral ovarian follicles. Abbreviations: LvH, Lipovitellin 1; LvL, Lipovitellin 2; Pv, Phosvitin; Vwfd, D domain of the von Willebrand factor; YGP 40, yolk glycopeptide 40.
The specific proteolysis of Vt has been suggested to constitute a regulatory step in the fusion between the endocytic compartments in oocytes. Cathepsin D, which was isolated from the chicken oocyte, is a proteinase putatively responsible for the intraoocytic processing of Vt II (Elkin et al., 1995; Yamamura et al., 1995; Groche et al., 2000; Finn, 2007). Hydrolysis in mildly acidic conditions (in vitro) of Vt II with the use of purified chicken cathepsin D led to obtaining protein fragments indistinguishable from those found in the yolk (Retzek et al., 1992 ). The evident important role of this aspartic endopeptidase in the proteolytic cleavage of plasma Vt into major granule proteins was confirmed by Elkin et al. (1995), who conducted hydrolysis (in vitro) of Japanese quail and hen Vt II.
YOLKIN (Y) POLYPEPTIDE COMPLEX FROM EGG YOLK
Not much is known about the products of proteolysis which regulate correct embryonal development. Of particular interest is Y, a group of peptides naturally occurring in egg yolk. It is a complex mixture accompanying chicken IgY-γ livetin in egg yolk plasma. Y was discovered more than 10 yr ago when conducting comparative studies on the biological activity of IgG from colostrum and IgY from egg yolk plasma, being observed in the presence of the fraction collected by gel permeation chromatography which was the descending arm of the peak corresponding to IgY (Polanowski et al., 2013).
Molecule Structure
Y is rich in acidic amino acid residues and contains a low amount of methionine (Polanowski et al., 2012, 2013). It is a heterogeneous mixture, usually consisting of several polypeptides of molecular weights ranging from 1 to 35 kDa, with fractions from 16 to 23 kDa being the most abundant (Polanowski et al., 2013). Y complex is present in egg yolk from different bird species; however, an electrophoretic analysis revealed significant differences in Y peptide composition (Zabłocka et al., 2021a ). For example, proteins of about 30 to 35 kDa had a small share in the composition of Y obtained from goose and quail egg yolks. In contrast, Y preparations obtained from pigeon and quail eggs were mainly composed of proteins of 25 kDa, and the Y isolated from goose and duck eggs contained dominant proteins of 20 to 25 kDa. It is well worth noting that the peptide composition of Y from eggs laid by wild species is very similar to those from burrowing birds. Also, almost the same Y compositions were observed for eggs from domestic and water birds (Zabłocka et al., 2021a ).
The protein profile of Y does not depend just on the bird species. The proportion of polypeptides can vary greatly depending on the time when the eggs were collected or even on the pen, and other factors (Polanowski et al., 2013; Zambrowicz et al., 2017; Zabłocka et al., 2021a ). Y obtained by Zabłocka et al. (2021a) was composed mainly of proteins of about 30 to 35 kDa and minor proteins of about 20 to 25 kDa, while fractions from 16 to 23 kDa were most abundant in Y obtained by Polanowski et al. (2013). Y preparation obtained by Zabłocka et al. (2014) using gel permeation chromatography was composed of peptides from 20 to 37 kDa, and predominant peptide fractions of about 23 kDa. The protein composition of Y obtained by Zambrowicz et al. (2017) was slightly different from that obtained by Polanowski et al. (2013), which was characterized by protein fractions at 16 to 35 kDa and smaller protein fractions from a few to 16 kDa.
Polanowski et al. (2013) showed a significant difference in peptide composition even between Y preparations obtained from eggs laid by the same hens housed in controlled conditions, and isolated by the same method, except where the time intervals of egg collection may be associated with the age of the hens or the season. The differences in protein patterns may be directly related to habitat, the degree of exposure to pathogens, or the nutrition of hens (Zabłocka et al., 2021a ). However, regardless of the protein profile, Y possesses comparable biological activity.
Y most likely represents Vt II-derived peptides because N-terminal amino acid sequences of the electrophoretically purified Y fragments are homological to some fragments of the C-terminal domain of Vt II. The polypeptides of about 4 and 12 kDa are free of carbohydrates and start at position 1732 in the Vt amino acid sequence, whereas the other larger protein fractions (16, 19, 23, 29, 32, and 35 kDa) are glycoproteins corresponding to the amino acid sequence of Vt starting at position 1572 (Polanowski et al., 2013) (Figure 1). On this basis, it can be assumed that Y may be considered as a set of peptides generated during proteolytic cleavage of YGP40 at positions 1571S-1572A and 1731R-1732M (Szmyt et al., 2021). Based on this, it can be supposed that the protein profile of Y is most likely influenced by the activity of cathepsin D degrading Vt during egg formation. However, this process has not been sufficiently studied.
Extraction and Purification Methods
Y has already been isolated from hen egg yolks using gel permeation chromatography, ultra-filtration processes, and protein fractionation with the use of perchloric acid connected with salt precipitation, or acetone and ethanol extraction (Polanowski et al., 2012; Polanowski et al., 2013; Zabłocka et al., 2014; Zambrowicz et al., 2017). The yield obtained for the Y preparation from the starting material (complex of IgY and Y) ranges from 3.6% to 4.6% (Zambrowicz et al., 2017).
Size-exclusion chromatography on sephacryl S 100 HR resin provided a Y with main proteins of 35, 32, 29, 23, 19, and 16 kDa, and peptide fractions lower than 16 kDa (Polanowski et al., 2013). Another method based on ion-exchange chromatography on DEAE cellulose led to obtaining another yolk plasma glycoprotein, YPG40, with immunomodulatory properties, identified as a C-terminal cysteine-rich fragment of Vt II (Yamamura et al., 1995). The Y preparations isolated using ultrafiltration in working membranes with cut-offs at 50 and 1 kDa, and with perchloric acid fractionation, were predominantly composed of fractions of about 20 and 37 kDa, and minor fractions of about 23, 24, 25, and 35 kDa (Zabłocka et al., 2014). The simple and rapid precipitation method using acetone or ethanol led to Y preparations consisting predominantly of proteins of about 40, 37, 32, and 24 kDa and smaller proteins of 15 to 18 kDa. The effectiveness of Y separation from IgY depends on the concentrations of the precipitating agents, with the purest Y preparations obtained using 70% solutions of either acetone or ethanol. This method can help in shortening the time of Y isolation in comparison to liquid chromatography.
Apart from isolation from a natural source of hen egg yolk, Y can theoretically be obtained by chemical synthesis or by expression of recombinant protein in a microbial cell. Therefore, it is so significant to determine the role of glycosylation of the Y molecules. Zambrowicz et al. (2018) have proved that the presence of carbohydrates in Y polypeptides is not necessary to preserve immunomodulatory activity. The glycosylated and nonglycosylated Y fractions demonstrate only slightly different levels of ability to induce secretion of IL-6, IL-10, and TNF-α. Removal of carbohydrates from Y polypeptides by enzyme PNGase F may significantly improve NO generation in BMDM cells. These authors also have proved that purification of the Y complex to individual peptides is not desirable as the Y fractions and the unfractionated Y exert a similar potency to induce cytokines. It was even found that the isolated low molecular mass fractions (polypeptides from 15 to 25 kDa) showed a weaker ability to stimulate nitric oxide release by BMDM cells than unseparated Y, suggesting that Y constituents act synergistically, and the strongest immunostimulating activity possessed by the whole Y complex (Zambrowicz et al., 2018). Thus, the formulation of a potential therapeutic agent based on the whole Y complex may be more attractive than the expensive deep purification of particular peptides.
Biochemical and Biomedical Properties
Y isolated from hen egg yolk can be considered as a potent therapeutic agent and nutraceutical. The recently obtained results demonstrate that Y possesses immunoregulatory and neuroprotective activity. The mechanisms of action of Y are still under investigation.
Immunoregulatory Activity of Yolkin
Innate immunity is the first line of defense against invading pathogens. It is an antigen-independent defense mechanism that has no immunologic memory and this is unable to recognize or “memorize” a pathogen. The crucial function of innate immunity is the rapid recruitment of immune cells to the site of infection and producing inflammatory factors such as cytokines, chemokines, and also nitric oxide (Marshall et al., 2018).
A preliminary study published in 2012 by Polanowski et al. showed that an unpurified complex of IgY and Y had significantly higher cytokine-inducing activity than purified IgY. This complex at 100 µg/mL possessed the same or even higher cytokine-inducing activity than the lipopolysaccharide (LPS) used as a positive control. Subsequent studies have shown that purified Y polypeptide complex possesses immunomodulatory activity, which was confirmed in the human whole blood (as ex vivo model), mouse bone marrow-derived macrophages of the BMDM cell line, and murine macrophage-like cell line J774.2 (as in vitro models) (Polanowski et al., 2012, 2013; Zambrowicz et al., 2017; Kazana et al., 2020, 2022a).
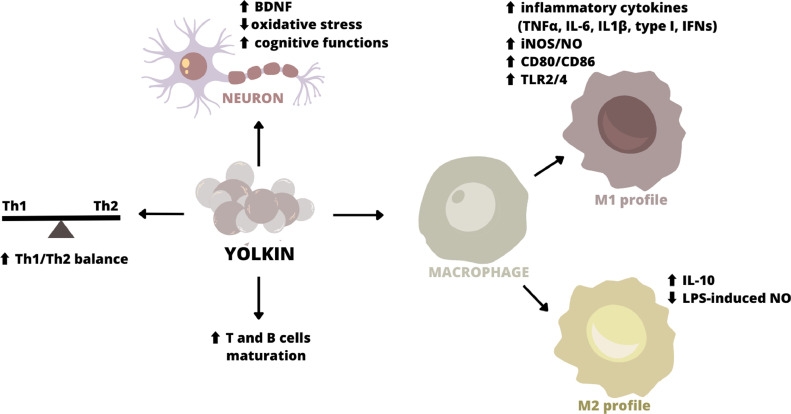
The impact of Y on the secretory activity of the human whole blood ex vivo has been studied most extensively because the use of unseparated whole blood cultures mimicked the natural microenvironment in which the different leukocyte populations (such as TH1 and TH2) and macrophages can cooperate. Y complex purified from IgY was found to efficiently induce pro-inflammatory factors such as interferons α/β (IFNs α/β), interleukin 1 beta (IL-1β), interleukin 6 (IL-6), chemotactic interleukin 8 (IL-8) and tumor necrosis factor-alpha (TNF-α) controlling the cellular immune response, and anti-inflammatory cytokine interleukin 10 (IL-10) (Polanowski et al., 2012, 2013; Zabłocka et al., 2014, 2021a; Kazana et al., 2020, 2022a).
Recently, Obmińska-Mrukowicz et al. (2020) provided some insight regarding the mode of immunomodulatory activity of Y. The results of their study in mice showed that Y influences the development of the immune response and the phenotype of cells in lymphoid organs. It was proved that Y may play an important role in the induction of maturation and stimulatory signals in immature T and B cells. Treating mice with Y diminished the percentage of double-positive cells and caused the growth of the content of single-positive CD4+ and CD8+ cells in the thymus and the level of CD19+B cells in the spleen and mesenteric lymph nodes. T cells were affected by Y as evidenced by a significant increase of mature thymocyte subset, stimulation of mitogen-induced proliferation of thymocytes, and activation of MAP kinases in Jurkat cells (Obmińska-Mrukowicz et al., 2020 ).
The modulatory effect of polypeptide complex Y on the immune system is also due to its ability to activate nitric oxide (NO) production (Zabłocka et al., 2014; Kazana et al., 2020, 2022a). NO is a molecule that exerts beneficial results in the body as an antibacterial, antiparasitic, antitumor, and antiviral agent (Tripathi et al., 2007). It was observed that Y significantly increases iNOS (inducible Nitric Oxide Synthase) expression and upregulates NO production in BMDM cells (Kazana et al., 2020). It was also shown that Y can modulate the effect of LPS on NO production in mouse macrophage-like cells of the J774.2 line. When Y was applied to the cells together with LPS, the concentration of NO in the supernatants was significantly lower than that in cells treated with LPS alone (Zabłocka et al., 2014).
To explain the molecular mechanism of Y immune activity, Kazana et al. (2020, 2022a) examined its impact on the maturation and function of macrophages in bone marrow-derived macrophages of the BMDM cell line in a mouse model.
Macrophages are key immune cells participating in the immune response to pathogens and tumors, maintaining tissue homeostasis, promoting the repair processes, and controlling immune response in lifestyle-associated diseases and neurodegenerative disorders (Shapouri-Moghaddam et al., 2018). Macrophages play a pivotal role in the elimination of pathogens via the production of reactive oxygen and nitrogen species, by phagocytosis, and also by releasing a broad spectrum of inflammatory factors, including cytokines and chemokines regulating the induction and duration of an inflammatory process (Oishi et al., 2018). Besides, the ability of macrophages to induce antigen presentation and T and B cell activation makes them strategic cells in the initiation and propagation of the adaptive immune response (Wynn and Vannella, 2016). Macrophages exist in 2 distinct subsets, both closely related to inflammatory response. Classically activated macrophages (M1) produce mainly pro-inflammatory interferons (IFNs), tumor necrosis factor-alpha (TNF-α), and nitric oxide (NO) (Yunna et al., 2020). Alternatively activated macrophages (M2) produce anti-inflammatory cytokines such as interleukin 10 (IL-10) and transforming growth factor beta (TGF-β). M2 cells participate in Th2 response and tissue repair and have immunoregulatory functions (Roszer, 2015). The latest studies show that Y can regulate both M1 and M2 macrophage profiles (Zabłocka et al., 2014; Kazana et al., 2020, 2022a). It was observed that Y peptides significantly down-regulated BMDM cell proliferation and simultaneously increased the cell surface marker expression of CD80/CD86, which indicated macrophage polarization towards the M1 phenotype. Additionally, using HEK-BlueTM hTLR2/hTLR4/NOD2 and hNull1 cellular models, it was shown that Y is strongly recognized and activated by TLR2 and TLR4 receptors but not by NOD2 (Kazana et al., 2022a). TLR2/4-dependent BMDM activation resulted in the up-regulation of mRNA expression and production of pro-inflammatory cytokines, such as type I Interferons, TNF-α, IL-6, iNOS expression, and also nitric oxide synthesis. Besides, Y initiated anti-inflammatory M2-dependent IL-10 expression/production, opposing the switch to the metabolic program induced by inflammatory stimuli in M1 macrophages (Kazana et al., 2020, Kazana et al., 2022a). Finally, the observed yolkin-dependent inhibition of LPS induced nitric oxide production in mouse macrophage-like cells of the J774.2 line which indicates switching into M2-response (Zabłocka et al., 2014).
There is a lot of data indicating that the activation of MAPK and PI3K/Akt kinase pathways are critical in both pro-inflammatory and anti-inflammatory responses in TLR-stimulated macrophages and can be a crucial regulator of TLRs and NF-κB signaling in macrophages (Arthur et al., 2013; Duan et al., 2022). This data and the previously observed effect of Y on cytokines and NO production prompted researchers to explain the molecular basis of these phenomena. It was found that Y can upregulate the phosphorylation of extracellular signal-regulated kinase 1/2(ERK1/2), c-Jun N-terminal kinase (JNK), and PI3K/Akt kinases, which provided an increased mRNA expression and production of cytokines: type I Interferons, TNF-α, IL-6 and IL-10, in BMDM cells. Furthermore, it was shown that the concentrations of NO, TNF-α, and type I IFNs (α/β) produced by BMDM cells in response to Y are sufficient to trigger antiviral activity in VSV-infected BMDM cells (Kazana et al., 2020).
These properties reveal that Y may play an important role in the development of an embryo's innate immune system (Figure 2). It seems that yoking, as in the case of a bird embryo, could have a beneficial effect on human health as an effective natural drug or as a supplement and nutraceutical in the conventional therapy of immunodeficiency.
Figure 2.
Mechanisms of immunomodulatory and neuroprotective activity of yolkin (Y). Y polypeptide complex isolated from hen egg yolk keeps the immune response balance of human whole blood cells by induction of IFN and TNF-alpha (Th1 cytokines), and IL-6 and IL-10 (Th2 cytokines). It may also play an important role in the induction of maturation and stimulatory signals in immature T and B cells. Y regulates both M1 and M2 macrophage profiles. Significantly down-regulates macrophage proliferation and simultaneously increases the cell surface marker expression of CD80/CD86 characterized M1 macrophage phenotype. Y is recognized by, and activated TLR2 and TLR4 receptors, and up-regulates mRNA expression and production of proinflammatory factors (type one IFNs, TNF-α, IL-6, iNOS/NO). Besides, Y initiates anti-inflammatory M2-dependent IL-10 production and inhibits LPS–induced nitric oxide production in macrophages. The neuroprotective activity of Y is connected to its impact on BDNF expression/secretion, and reduction of ROS generation in neurons, resulting in improvement of the cognitive functions.
Neuroprotective Activity of Yolkin
Aging is a fundamental process in the degeneration of neurons and the development of dementia (Nelson and Tabet, 2015; Wyss-Coray, 2016). For unknown reasons, after 20 yr of age, neurons in the brain begin to die. In contrast to other cells in our body, neurons do not grow back, and over time that damage accumulates (Elobeid et al., 2016). In older people, the prevailing causes of death are cardiovascular diseases, cancer, but also neurodegenerative diseases and dementias, which constitute a growing public health problem in developed countries (Gómez-Gómez and Zapico, 2019). Neurodegenerative diseases develop very slowly and for many years do not give any symptoms (Hardy and Selkoe, 2022). They are variable, with symptoms ranging from progressive dysfunction of motor control to mood disorders and cognitive deficits, finally leading to dementia. Therefore, the primary scientific goal is to understand the causes and the mechanism of neuronal degeneration and to find an effective way to both support the function of neurons and prevent their apoptosis. One way is to find potential nutraceuticals, preferably isolated from natural raw materials that demonstrate neuroprotective activity in long-term supplementation. Systematic use of neuroprotective agents may be an important therapeutic aspect, improving our mental performance and significantly slowing down or even reducing the risk of neurodegenerative disease development.
In 2016, Lemieszewska et al. proved the neuroprotective properties of Y in an animal model study, testing episodic and spatial memory, motor functions, and exploratory behavior of rats after oral and intraperitoneal administration of Y. The experiment was carried out in 6- and 12-mo-old rats, along with a series of behavioral tests, including an Open Field Test (OFT), a Novel Object Recognition Test (NORT), and a Morris Water Maze (MWM). It was observed that Y can improve the behavior and cognitive functions in both tested groups of young and aged rats. Particular age-related cognitive deficits observed within the control groups of older rats seemed to be restored during the Y treatment. These included an increased recognition of a novel object in the testing area or higher effectiveness in localizing the target zone in the MWM. The behavioral rat model of age-related cognitive dysfunction has shown the ability of Y to improve spatial and episodic memory. That study showed that Y may find an application for the prevention and treatment of aging-related neurodegenerative disorders. The molecular mechanism of the neuroprotective activity of Y is still under investigation.
One possible explanation is the ability of Y to modulate nitric oxide production (Zabłocka et al., 2014). It is known that NO released in physiological concentration plays an important role as a neurotransmitter, while an overproduction may provide inflammation and autoimmune diseases. So, the modulation of NO production seems to be an important therapeutic point in the treatment of neurodegenerative disorders and can participate in the improvement of memory (Tewari et al., 2021). The second possibility can be connected with the impact of Y on IL-10 production (Zabłocka et al., 2021b ), where that level is significantly decreased in neurodegenerative processes (Porro et al., 2020; Weston et al., 2021). IL-10 inhibits pro-inflammatory response by down-regulating the expression of cytokines such as IFN-γ or TNF-α (Silva et al., 2016).
The brain, because of its high oxygen consumption and lipid-rich content, is extremely susceptible to oxidative stress. Latest studies have demonstrated that oxidative stress plays a central role in the pathophysiology of neurodegenerative diseases such as Alzheimer's disease (AD) and Parkinson's disease (PD), and antioxidant therapy seems to be the most proper for the prevention and treatment of such neurodegenerative diseases (Kim et al., 2015). In our studies using DPPH and FRAP methods, we have shown that Y did not possess antioxidant activity. When Y was applied to the PC12 cells alone, no induction of reactive oxygen species (ROS) was observed. However, when Y was applied to the PC12 cells simultaneously with H2O2, a significant reduction in intracellular ROS generation was observed (Zabłocka et al., 2018). Additionally, the Y complex significantly affected lipid peroxidation in the PBMC cells stimulated previously by LPS (Zabłocka et al., 2014).
The level of neurotrophic factors could be associated with the pathogenesis of neurodegenerative disorders. In humans, brain and peripheral levels of neurotrophins may be significantly reduced in patients with AD compared to healthy subjects (Kerschensteiner et al., 1999; Scalzo et al., 2010; Sopova et al., 2014). Neurotrophins play a relevant role in neuron cell physiology. One of them is a brain-derived neurotrophic factor (BDNF) which is involved in the neuron's development, survival, and repair after injury (Scaper, 2008; Bartkowska et al., 2010). In the central nervous system, BDNF contributes to the correct function of the neocortex and hippocampus. The last one is part of the limbic system mainly responsible for memory. Data from numerous studies show that BDNF may have a protective role on both cholinergic and dopaminergic neurons, and also may participate in the regulation of synaptic connectivity (Waterhouse and Xu, 2009; Cunha et al., 2010; Lu et al., 2014). This proves very clearly that BDNF is essential for both the survival and activity of dopaminergic and cholinergic neurons in a particular brain region. It has also been proven that the decrease in the level of BDNF in the brain led to memory and cognitive disorders (Cunha et al., 2010; Allen et al., 2011, 2013). In a previous study, we showed that Y at a dose of 100 μg/mL can stimulate whole blood cells to release significant amounts of BDNF (Zambrowicz et al., 2017). Cytokine and BDNF production by whole blood immune cells provides a novel example of the bidirectional interaction between the nervous and immune systems (Bryson and Lynch, 2016). Furthermore, Y upregulates cAMP production and PKA activation in both rat pheochromocytoma PC12 cells and rat hippocampal precursor cells H19-7, providing increased CREB factor phosphorylation and activation of BDNF expression (Kazana et al., 2022b, 2023). Upregulation of CREB phosphorylation by yolkin is dependent on cyclic adenosine monophosphate/protein kinase A (cAMP/PKA) and phosphoinositide 3-kinases/protein kinase B (PI3K/Akt)-signaling pathway activation. Additionally, yolkin can upregulate the expression of carboxypeptidase E/neurotrophic factor α1 (CPE/NF-α1) (Kazana et al., 2023).
A response by the body to the deposition of amyloid beta 42 is the synchronic reduction of the level of neurotrophins and overexpression of toxic inflammatory mediators, in particular free radicals, which would lead to neuron cell dysfunction and consequently to death (Skaper, 2007; Lyman et al., 2014). Therapy based on the regulation of the secretion of neurotrophins, modulation of the immunological system, and support mechanisms of cell protection against ROS seems to be particularly promising in improving health status in neurodegenerative disease patients. Yolkin, due to the above-mentioned biological properties, may be a potential neuroprotective agent.
EGG YOLK GLYCOPEPTIDE 40
The most known are the products of proteolysis of Vt located in the yolk granule fraction. Relatively recently, the presence of Vt fragments has also been demonstrated in yolk plasma. Yamamura et al. (1995) isolated 2 proteins from the β-livetin fraction of yolk plasma. The first is a yolk glycoprotein of 40 kDa (YGP40) with an asparagine-linked carbohydrate chain, identified as a C-terminal cysteine-rich fragment of Vt II, the cysteine-rich domain homologous to the D2 region of von Willebrand factor. YGP40 is composed of 284 amino acid residues located between Ala-1567 and Thr-1850 of the Vt II amino acid sequence (Yamamura et al., 1995). The 27-amino acid N-terminal sequence of the 40-kDa protein is 56% similar to the last third of the lipovitellin 1-coding region of the Vt II gene, suggesting it may come from an analogous region of the Vt I gene (Groche et al., 2000). The second protein fragment is the yolk plasma glycoprotein of 42 kDa which was probably the proteolytic product of Vt I (Yamamura et al., 1995).
Both glycoproteins were identified in growing oocytes but absent in laying hen serum. A further in vitro study showed that YGP40 dissociated from the lipovitellin–phosvitin complex after the limited cleavage of Vt II using cathepsin D. Probably, after incorporation from the serum into the oocyte, Vt II is cleaved into HDL and LDL lipovitellins, phosvitin, YGP42, and YGP40, which are likely released into the yolk plasma before or during compartmentation of the lipovitellin–phosvitin complex into the yolk granule (Yamamura et al., 1995, Groche et al., 2000).
Limited information is available about the biological properties of YGP 40. Recently, Szmyt et al. (2021) produced YGP40 using a recombinant DNA technology involving genetically modified E. coli BL21 (DE3) expression cells. It has been shown that recombinant YGP40 possesses immunomodulatory activity and can stimulate human whole blood cells to produce TNF-α and IL-10 and induce nitric oxide production. Pro-inflammatory TNF-alpha, secreted by Th1 cells, controls cellular immunity, while anti-inflammatory cytokine IL-10 secreted by Th2 cells is responsible for the control of the humoral immune response. rYGP40 also causes the up-regulation of iNOS expression in murine bone marrow-derived macrophages (BMDM) and has no cytotoxic effects on BMDM (Szmyt et al., 2021).
In people with a food allergy to egg proteins, YGP40 may have an adverse effect on the immune system, causing IgE-dependent allergic reactions. It has been identified as allergen Gad 6 (Gavrovic-Jankulovic et al., 2014).
CONCLUSIONS
The mechanism and timing of Y formation during egg development are not known. It seems very interesting that these polypeptides do not accompany the main proteins of the yolk of the granular fraction, which is the same protein precursor, but occur in the form of a complex with IgY located in the plasma fraction of the yolk. While IgY is secreted by plasma cells—B lymphocytes in the course of a humoral immune response, and then, through receptors located in the oocyte membrane, it is transported from the laying hen blood to the fallopian tubes and is accumulated in the maturing reproductive cell—the egg.
Probably, the presence of Y in the complex with IgY is related to their synergistic biological activity. Therefore, Y, similar to IgY, may be a key protein for the protection of the developing embryo in the egg, whose main role is to induce a passive immune response and maintain this response until the hatched chick acquires the ability to produce antibodies.
The results of research on Y carried out in recent years in research centers in Wrocław (Poland) prove that it has strong immunomodulatory and neuroprotective properties (Figure 2). Due to these abilities, Y can be of great importance in the development of new drugs and nutraceuticals. Until recently, Vt has been recognized as a protein providing a number of nutrients for developing embryos and as an immunocompetent factor capable of protecting embryos against pathogens and protecting their cells from damage by free radicals. This property results from the antimicrobial and antioxidant activity of the well-known egg yolk protein—phosvitin, which is a product of Vt proteolytic degradation. Vt, in addition to being a precursor for Y and YGP40, plays a new role via functioning as an immune-modulating and neuroprotective molecule.
ACKNOWLEDGMENTS
This work was supported by the Wrocław University of Environmental and Life Sciences (Poland) as part of the Ph.D. research program “MISTRZ”, No N090/0016/22. The APC/BPC is co-financed by Wrocław University of Environmental and Life Sciences.
DISCLOSURES
The authors declare no conflict of interest toward the development, submission, and publication of this manuscript.
REFERENCES
- Allen S.J., Watson J.J., Dawbarn D. The neurotrophins and their role in Alzheimer's disease. Curr. Neuropharmacol. 2011;9:559–573. doi: 10.2174/157015911798376190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen S.J., Watson J.J., Shoemark D.K., Barua N.U., Patel N.K. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 2013;138:155–175. doi: 10.1016/j.pharmthera.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Anton M. In: Pages 1–6 in Bioactive egg Compounds. Huopalahti R., López-Fandiño R., Anton M., Schade R., editors. Springer; Berlin, Germany: 2007. Composition and structure of hen egg yolk. [Google Scholar]
- Anton M. Egg yolk: structures, functionalities and processes. J. Sci. Food Agric. 2013;93:2871–2880. doi: 10.1002/jsfa.6247. [DOI] [PubMed] [Google Scholar]
- Arthur J.S.C., Ley S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013;13:679–692. doi: 10.1038/nri3495. [DOI] [PubMed] [Google Scholar]
- Avarre J.C., Lubzens E., Babin P.J. Apolipocrustacein, formerly vitellogenin, is the major egg yolk precursor protein in decapod crustaceans and is homologous to insect apolipophorin II/I and vertebrate apolipoprotein B. BMC Evol. Biol. 2007;7:3. doi: 10.1186/1471-2148-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkowska K., Turlejski K., Djavadian R.L. Neurotrophins and their receptors in early development of the mammalian nervous system. Acta Neurobiol. Exp. (Wars) 2010;70:454–467. doi: 10.55782/ane-2010-1816. [DOI] [PubMed] [Google Scholar]
- Bryson K.J., Lynch A. Linking T cells to Alzheimer's disease: from neurodegeneration to neurorepair. Curr. Opin. Pharmacol. 2016;26:67–73. doi: 10.1016/j.coph.2015.10.003. [DOI] [PubMed] [Google Scholar]
- Chen J.S., Sappington T.W., Raikhel A.S. Extensive sequence conservation among insect, nematode and vertebrate vitellogenins reveals ancient common ancestry. J. Mol. Evol. 1997;44:440–451. doi: 10.1007/pl00006164. [DOI] [PubMed] [Google Scholar]
- Cunha C., Brambilla R., Thomas K.L. A simple role of BDNF in learning and memory? Front. Mol. Neurosci. 2010;3:1. doi: 10.3389/neuro.02.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan T., Du Y., Xing C., Wang H.Y., Wang R-F. Toll-like receptor signaling and its role in cell-mediated immunity. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.812774. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkin R.G., Freed M.B., Danetz S.A.H., Bidwell C. Proteolysis of Japanese quail and chicken plasma apolipoprotein B and vitellogenin by cathepsin D: Similarity of resulting protein fragments with egg yolk polypeptides. Comp. Biochem. Physiol. 1995;112:191–196. doi: 10.1016/0305-0491(95)00062-3. [DOI] [PubMed] [Google Scholar]
- Elobeid A., Libard S., Leino M., Popova S.N., Alafuzoff I. Altered proteins in the aging brain. J Neuropathol. Exp. Neurol. 2016;75:316–325. doi: 10.1093/jnen/nlw002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn R.N. Vertebrate yolk complexes and the functional implications of phosvitins and other subdomains in vitellogenins1. Biol. Reprod. 2007;76:926–935. doi: 10.1095/biolreprod.106.059766. [DOI] [PubMed] [Google Scholar]
- Gao D., Qiu N., Liu Y., Ma M. Comparative proteome analysis of egg yolk plasma proteins during storage. J. Sci. Food Agric. 2017;97:2392–2400. doi: 10.1002/jsfa.8052. [DOI] [PubMed] [Google Scholar]
- Gavrovic-Jankulovic M., Cirkovic Velickovic T. Food Allergens- Biochemistry and Molecular Nutrition. Food Microbiology and Food Safety, Springer; New York, NY: 2014. Accessed March 2023. [DOI] [Google Scholar]
- Gómez-Gómez E.M., Zapico S.C. Frailty, cognitive decline, neurodegenerative diseases and nutrition interventions. Int. J Mol. Sci. 2019;20:2842. doi: 10.3390/ijms20112842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groche D., Rashkovetsky L.G., Falchuk K.H., Auld D.S. Subunit composition of the zinc proteins alpha- and beta-lipovitellin from chicken. J. Protein. Chem. 2000;5:379–387. doi: 10.1023/a:1026487414167. [DOI] [PubMed] [Google Scholar]
- Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2022;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Jolivet P., Boulard C., Chardot T., Anton M. New insights into the structure of apolipoprotein B from low-density lipoproteins and identification of a novel YGP-like protein in hen egg yolk. Agric. Food Chem. 2008;56:5871–5879. doi: 10.1021/jf800321m. [DOI] [PubMed] [Google Scholar]
- Kazana W., Jakubczyk D., Pacyga-Prus K., Leszczyńska K., Górska S., Siednienko J., Macała J., Piechowiak G., Zabłocka A. A novel mechanism of macrophage activation by the natural yolkin polypeptide complex from egg yolk. Int. J. Mol. Sci. 2022;23:3125. doi: 10.3390/ijms23063125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazana W., Jakubczyk D., Siednienko J., Zambrowicz A., Macała J., Zabłocka A. Mechanism of molecular activity of yolkin—a polypeptide complex derived from hen egg yolk—in PC12 cells and immortalized hippocampal precursor cells H19-7. Mol. Neurobiol. 2023 doi: 10.1007/s12035-023-03246-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazana W., Mitkiewicz M., Ochnik M., Sochocka M., Zambrowicz A., Piechowiak G., Macała J., Miernikiewicz P., Zabłocka A. Yolkin isolated from hen egg yolk as a natural immunoregulator, activating innate immune response in BMDM macrophages. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/5731021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazana W., Zambrowicz A., Macała J., Zabłocka A. Yolkin—a polypeptide complex isolated from chicken egg yolk upregulates the expression of brain-derived neurotrophic factor (BDNF) in PC12 and H19-7 cells. Folia Neuropathol. 2022;60(Special Issue):15. [Google Scholar]
- Kerschensteiner M., Gallmeier E., Behrens L., Leal V.V., Misgeld T., Klinkert W.E., Kolbeck R., Hoppe E., Oropeza-Wekerle R.L., Bartke I., Stadelmann C., Lassmann H., Wekerle H., Hohlfeld R. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J. Exp. Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim G.H., Kim J.E., Rhie S.J., Yoon S. The role of oxidative stress in neurodegenerative diseases. Exp. Neurobiol. 2015;24:325–340. doi: 10.5607/en.2015.24.4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemieszewska M., Jakubik-Witkowska M., Stańczykiewicz B., Zambrowicz A., Zabłocka A., Polanowski A., Trziszka T., Rymaszewska J. Pro-cognitive properties of the immunomodulatory polypeptide complex. Yolkin, from chicken egg yolk and colostrum-derived substances: analyses based on animal model of age-related cognitive deficits. Arch. Immun. Ther. Exp. 2016;64:425–434. doi: 10.1007/s00005-016-0392-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Zhai J., Gu L., Su Y., Gong L., Yang Y., Chang C. Hen egg yolk in food industry: a review of emerging functional modifications and applications. Trends Food Sci. Technol. 2021;115:12–21. [Google Scholar]
- Lindsay W.R., Friesen C.R., Sihlbom C., Bergström J., Berger E., Wilson M.R., Olsson M. Vitellogenin offsets oxidative costs of reproduction in female painted dragon lizards. J. Exp. Biol. 2020;223 doi: 10.1242/jeb.221630. [DOI] [PubMed] [Google Scholar]
- Lu B., Nagappan G., Lu Y. BDNF and synaptic plasticity, cognitive function and dysfunction. Handb. Exp. Pharmacol. 2014;220:223–250. doi: 10.1007/978-3-642-45106-5_9. [DOI] [PubMed] [Google Scholar]
- Lyman M., Lloyd D.G., Ji X., Vizcaychipi M.P., Ma D. Neuroinflammation: the role and consequences. Neurosci. Res. 2014;79:1–12. doi: 10.1016/j.neures.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Marshall J.S., Warrington R., Watson W., Kim H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018;14:49. doi: 10.1186/s13223-018-0278-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mine Y. Egg proteins and peptides in human health- chemistry, bioactivity and production. Curr. Pharm. Des. 2007;13:875–884. doi: 10.2174/138161207780414278. [DOI] [PubMed] [Google Scholar]
- Mine Y., Kovacs-Nolan J. New insights in biologically active proteins and peptides derived from hen egg. Poult. Sci. J. 2016;62:87–95. [Google Scholar]
- Nakamura A., Yasuda K., Adachi H., Sakurai Y., Ishii N., Goto S. Vitellogenin-6 is a major carbonylated protein in aged nematode, Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 1999;264:580–583. doi: 10.1006/bbrc.1999.1549. [DOI] [PubMed] [Google Scholar]
- Nelson L., Tabet Y. Slowing the progression of Alzheimer's disease; what works? Ageing Res. Rev. 2015;23:193–209. doi: 10.1016/j.arr.2015.07.002. [DOI] [PubMed] [Google Scholar]
- Obmińska-Mrukowicz B., Szczypka M., Lis M., Pawlak A., Suszko-Pawłowska A., Sysak A., Zambrowicz A., Burster T., Kocięba M., Artym J., Zaczyńska E., Kochanowska I., Zimecki M. Effects of yolkin on the immune response of mice and its plausible mechanism of action. Immunol. Lett. 2020;220:21–31. doi: 10.1016/j.imlet.2020.01.003. [DOI] [PubMed] [Google Scholar]
- Oishi Y., Manabe I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018;30:511–528. doi: 10.1093/intimm/dxy054. [DOI] [PubMed] [Google Scholar]
- Patil S., Bhakti R., Matondkar M., Bhushette P., Sonawane S.K. A review on understanding of egg yolk as functional ingredients. JMBFS. 2022;11:e4627. doi: 10.55251/jmbfs.4627. [DOI] [Google Scholar]
- Polanowski A., Sosnowska A., Zabłocka A., Janusz M., Trziszka T. Immunologically active peptides that accompany hen egg yolk immunoglobulin Y: separation and identification. Biol. Chem. 2013;394:879–887. doi: 10.1515/hsz-2012-0337. [DOI] [PubMed] [Google Scholar]
- Polanowski A., Zabłocka A., Sosnowska A., Janusz M., Trziszka T. Immunomodulatory activity accompanying chicken egg yolk immunoglobulin Y. Poult. Sci. 2012;91:3091–3096. doi: 10.3382/ps.2012-02546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porro C., Cianciulli A., Panaro M.A. The regulatory role of Il-10 in neurodegenerative diseases. Biomolecules. 2020;10:1017. doi: 10.3390/biom10071017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Réhault-Godbert S., Guyot N. Encyclopedia of Reproduction, Volume 6: Comparative Reproduction. 2 ed. Elsevier, Academic Press; 2018. Vitellogenesis and yolk proteins, birds. [DOI] [Google Scholar]
- Réhault-Godbert S., Guyot N., Nys Y. The golden egg: nutritional value, bioactivities, and emerging benefits for human health. Nutrients. 2019;11:684. doi: 10.3390/nu11030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retzek H., Steyrer E., Sanders E.J., Nimpf J., Schneider W.J. Molecular cloning and functional characterization of chicken cathepsin D, a key enzyme for yolk formation. DNA Cell Biol. 1992;11:661–672. doi: 10.1089/dna.1992.11.661. [DOI] [PubMed] [Google Scholar]
- Roszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Med. Inflamm. 2015;2015 doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalzo P., Kümmer A., Bretas T.L., Cardoso F., Teixeira A.L. Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson's disease. J. Neurol. 2010;257:540–545. doi: 10.1007/s00415-009-5357-2. [DOI] [PubMed] [Google Scholar]
- Scaper S.D. The biology of neurotrophins, signaling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol Disord Drug Targets. 2008;7:46–62. doi: 10.2174/187152708783885174. [DOI] [PubMed] [Google Scholar]
- Schade R., Chicana P.A. In: Pages 25–32 in Bioactive Egg Compounds. Huopalahti R., López-Fandiño R., Anton M., Schade R., editors. Springer; Berlin, Germany: 2007. Livetin fractions (Ig Y) [Google Scholar]
- Seehuus S.C., Krekling T., Amdam G.V. Cellular senescence in honey bee brain is largely independent of chronological age. Exp. Gerontol. 2006;41:1117–1125. doi: 10.1016/j.exger.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapouri-Moghaddam A., Mohammadian S., Vazini H., Taghadosi M., Esmaeili S.A., Mardani F., Seifi B., Mohammadi A., Afshari J.T., Sahebkar A. Macrophage plasticity, polarization, and function in health and disease. J. Cell Physiol. 2018;233:6425–6440. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- Silva D.L., Carriche G.M., Castro A.G., Roque S., Saraiva M. Balancing the immune response in the brain: IL-10 and its regulation. J. Neuroinflamm. 2016;13:297. doi: 10.1186/s12974-016-0763-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper S.D. The brain as a target for inflammatory processes and neuroprotective strategies. Ann. N Y Acad. Sci. 2007;1122:23–34. doi: 10.1196/annals.1403.002. [DOI] [PubMed] [Google Scholar]
- Sopova K., Gatsiou K., Stellos K., Laske C. Dysregulation of neurotrophic and haematopoietic growth factors in Alzheimer's disease: from pathophysiology to novel treatment strategies. Curr. Alzheimer Res. 2014;11:27–39. doi: 10.2174/1567205010666131120100743. [DOI] [PubMed] [Google Scholar]
- Szmyt A., Zabłocka A., Macała J., Chrzanowska J., Dąbrowska A. C-terminal fragment of vitellogenin II, a potential yolkin polypeptide complex precursor protein—heterologous expression, purification, and immunoregulatory activity. Int. J. Mol. Sci. 2021;22:7223. doi: 10.3390/ijms22137223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari D., Sah A.N., Bawari S., Nabavi S.F., Dehpour A.R., Shirooie S., Braidy N., Fiebich B.L., Vacca R.A., Nabavi S.M. Role of nitric oxide in neurodegeneration: Function, regulation, and inhibition. Curr. Neuropharmacol. 2021;19:114–126. doi: 10.2174/1570159X18666200429001549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi P., Tripathi P., Kashyap L., Singh V. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 2007;51:443–452. doi: 10.1111/j.1574-695X.2007.00329.x. [DOI] [PubMed] [Google Scholar]
- Waterhouse E., Xu B. New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Mol. Cell Neurosci. 2009;42:81–89. doi: 10.1016/j.mcn.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston L.L., Jiang S., Chisholm D., Jantzie L.L., Bhaskar K. Interleukin-10 deficiency exacerbates inflammation-induced tau pathology. J. Neuroinflamm. 2021;18:161. doi: 10.1186/s12974-021-02211-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn T.A., Vannella K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462. doi: 10.1016/j.immuni.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T. Ageing, neurodegeneration and brain rejuvenation. Nature. 2016;539:180–186. doi: 10.1038/nature20411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura J., Adachi T., Aoki N., Nakjima H., Nakamura R., Matsuda T. Precursor-product relationship between chicken vitellogenin and the yolk proteins: The 40 kDa yolk plasma glycoprotein is derived from C terminal cysteine-rich domain of vitellogenin II. Biochim. Biophys. Acta. 1995;1244:384–394. doi: 10.1016/0304-4165(95)00033-8. [DOI] [PubMed] [Google Scholar]
- Yunna C., Mengru H., Lei W., Chen W. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020;877 doi: 10.1016/j.ejphar.2020.173090. [DOI] [PubMed] [Google Scholar]
- Zabłocka A., Bobak Ł., Macała J., Rymaszewska J., Kazana W., Zambrowicz A. Comparative studies of yolkin preparations isolated from egg yolks of selected bird species. Chem. Biodivers. 2021;18 doi: 10.1002/cbdv.202100178. [DOI] [PubMed] [Google Scholar]
- Zabłocka A., Kazana W., Sochocka M., Stańczykiewicz B., Janusz M., Leszek J., Orzechowska B. Inverse correlation between Alzheimer's disease and cancer: Short Overview. Mol. Neurobiol. 2021;58:6335–6349. doi: 10.1007/s12035-021-02544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabłocka A., Sosnowska A., Urbaniak A., Janusz M., Polanowski A. Peptides accompanying chicken egg yolk IgY: alternative methods of isolation and immunoregulatory activity. Food Funct. 2014;5:724–733. doi: 10.1039/c3fo60391a. [DOI] [PubMed] [Google Scholar]
- Zabłocka A., Zambrowicz A., Macała J., Kazana W., Polanowski A. Yolkin—a polypeptide complex isolated from chicken egg yolk with potential neuroprotective and antioxidative activity. Neuropsychiatry. 2018;8:833–842. [Google Scholar]
- Zambrowicz A., Zabłocka A., Bobak Ł., Macała J., Janusz M., Polanowski A., Trziszka T. A simple and rapid method of isolation of active polypeptide complex, yolkin, from chicken egg yolk. Food. Chem. 2017;230:705–711. doi: 10.1016/j.foodchem.2017.03.101. [DOI] [PubMed] [Google Scholar]
- Zambrowicz A., Zabłocka A., Sudoł M., Bobak Ł., Sosicka P., Trziszka T. The effect of carbohydrate moieties on immunoregulatory activity of yolkin polypeptide naturally occurring in egg yolk. LWT-Food Sci. Technol. 2018;88:165–173. [Google Scholar]
- Zhang S., Sun Y., Pang Q., Shi X. Hemagglutinating and antibacterial activities of vitellogenin. Fish Shellfish Immunol. 2005;19:93–95. doi: 10.1016/j.fsi.2004.10.008. [DOI] [PubMed] [Google Scholar]