Abstract
Introduction
In stage IV NSCLC with solitary or oligometastatic brain metastasis, surgical resection of the primary and definitive management of the brain metastasis is an accepted standard. However, the effect of systemic chemotherapy after surgical resection on overall survival is not well-established.
Methods
We used the National Cancer Database to retrospectively identify individuals with NSCLC as the primary tumor along with synchronous brain metastases who underwent thoracic resection with or without adjuvant chemotherapy. Chi-square and Wilcoxon rank sum tests were performed to compare categorical and continuous variables, respectively, across the treatment groups. Kaplan-Meier and Cox proportional modeling were done to determine the survival benefit.
Results
A total of 310 (71.9%) of the cohort received perioperative chemotherapy, most of whom (79.4%) received it in the adjuvant setting. Patients receiving chemotherapy were likely to be younger (p = 0.002), privately insured (p = 0.01), and receive radiation (p < 0.001). Perioperative chemotherapy was significantly associated with survival on both univariate (hazard ratio = 0.71[0.52 – 0.99]) and multivariable (hazard ratio = 0.66 [0.47 – 0.92]) in addition to age (p = 0.03), Charlson-Deyo score (p = 0.02), pathologic N stage (p = 0.02), and adenocarcinoma histology (p = 0.02). Kaplan-Meier analysis confirmed this result with a significantly better survival with perioperative chemotherapy (p = 0.02). Further subgroup analysis using pathologic N stage revealed similar effect in pN1 (p = 0.001), but not pN0 (p = 0.2) patients.
Conclusions
Perioperative chemotherapy for pN0-1 NSCLC with synchronous brain metastasis is associated with improved OS in this analysis.
Keywords: lung cancer, brain metastasis, adjuvant therapy, chemotherapy
Introduction
Lung cancer is the second most-diagnosed cancer in 2020 with an estimated 116,300 and 112,520 new cases in men and women respectively and is the leading cause of cancer-related deaths in both men and women, with an estimated 5-year survival of 19% (across all stages and races) between 2009-2015. Non-small-cell lung cancer (NSCLC) is the most common type of lung cancer, accounting for 80-85% of lung cancers.1 Patients with advanced NSCLC have poor prognosis with most large phase III trials demonstrating median survival of 8 to 10 months and a 1-year survival rate of 30 to 35% in the preimmunotherapy era.2 Without treatment, brain metastases in NSCLC were typically fatal producing dismal median survival of 1-2 months. Historically, whole-brain radiation therapy (WBRT1) alone was offered as first-line therapy for the management of brain metastases as reduction of neurologic symptoms was exhibited in more than 75% of patients. However, this benefit translated to a median survival of only 3–6 months and came with the risk of significant chronic neurologic morbidities with high-dose WBRT. More recent outcomes regarding metastasectomy of synchronous brain metastases from NSCLC as treatment led to improved median survival.3 A large retrospective series with NSCLC brain metastases previously reported median survival of 12.4 months and the overall 5-year survival rate of 11% with pulmonary resection after resection of the solitary brain metastasis.4 Several retrospective studies reported improved survival times when both the primary lung tumor and brain metastases were resected.3 In a retrospective study conducted in 2006, thoracic stage I patients with solitary brain metastases had a more favorable outcome than expected and was comparable to stage I NSCLC without brain metastases, supporting aggressive treatment in these specific circumstances. However, this was not extended to locally advanced NSCLC with solitary brain metastases.5
Recommendations by the National Comprehensive Cancer Network (NCCN) with regards to stage IV metastatic cancer differ depending on the distribution of metastasis. For locoregional and limited metastases, category 2A recommendations for local intervention of the metastatic site followed by systemic therapy is suggested. In the cases of solitary or limited brain metastases, stereotactic radio surgery (SRS) or surgical resection followed by SRS or WBRT is recommended. This can be followed by treatment of the thoracic disease depending on if definitive therapy is feasible or not. Disseminated metastatic disease therapy depends on the histologic subtype, molecular testing, and the patient.6
The impact that chemotherapy has on survival has previously been shown to be significant. Chemotherapy improved survival and palliated symptoms, improving quality of life in patients with stage IV NSCLC.2 Local consolidative therapy for oligometastatic NSCLC without progression after initial systemic therapy improved progression-free survival compared with maintenance therapy alone, suggesting that aggressive local therapy should be further explored.7 Traditionally, neoadjuvant therapy followed by surgery had been a mainstay of therapy in locally advanced disease, thought to be owing to limitations in an era of open lung surgery in which the delivery of adjuvant therapy after resection by thoracotomy was challenging.8 Now, with more use of video-assisted thoracoscopic surgery (VATS), patients undergoing resection have a higher probability for completing adjuvant therapy.9 Therefore, the alternative option of adjuvant therapy is considered a viable option as well.
Survival benefit with local intervention of brain metastasis in oligometastatic stage IV lung cancer have been previously demonstrated. Whereas intuitively, it may be extrapolated that patients with oligometastatic disease who underwent definitive local therapies for known/exhibited sites of involvement will benefit from systemic therapy administered with adjuvant intent, the evidence supporting this approach is not well reported. Thus, in this study, the large and well-annotated database, National Cancer Database (NCDB) was utilized to retrospectively analyze better understand the role of chemotherapy administered with adjuvant intent in the treatment of resectable NSCLC (N0-N1) with brain-only oligometastatic involvement.
Material and Methods
Ethics Approval and Informed Consent Statement
The study was conducted following the Declaration of Helsinki. Institutional ethics approval from Institutional Review Board was not required as de-identified data from publicly available databases was used.
Data Source
The NCDB is a joint project of the American Cancer Society and American College of Surgeons Commission on Cancer.10 The NCDB, established in 1989, is a nationwide, facility-based, comprehensive clinical surveillance resource oncology data set that currently captures 69% of all newly diagnosed malignancies in the US annually. It includes data collected from more than 1500 cancer registries in the United States. Ethical review and approval were obtained from NCDB before release of de-identified data for analysis. The data used in this study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Study Population
All patients with classification of NSCLC from 2004-2014 were identified. The International Classification of Diseases for Oncology, third edition (ICD-O-3), codes for histologic types of NSCLC were grouped into squamous cell (8070-8072), adenocarcinoma (8050, 8140-8147, 8230, 8244, 8250, 8251, 8255, 8260, 8310, 8323, 8480, 8481, 8490, 8550, 8574) and other NSCLCs (8022, 8030-8033, 8320, 8571, 572, 8570, 8074, 8570, 8572, 8575, 8012, 8013, 8014, 8020, 8021; 8560, 8075 ). To limit the potential confounding effect of immunotherapy between the two groups, 2014 was chosen as the cutoff year as immune checkpoint inhibitors were initially approved in 2015. Patients who received immunotherapy subsequently on progression were excluded from the analysis. To further avoid confounding the attribution of CNS metastases, patients with more than one primary site of malignancy at diagnosis were excluded. In addition, patients who were not diagnosed or treated at the reporting facility were also excluded. Of the 1,284,846 records contained in the data set provided by the NCDB, 942,374 were identified as the first and only cancer for that patient. 46,120 of the patients identified had synchronous brain metastases (defined as present at time of diagnosis, code available only for cases starting from 2010); of these, 807 cases were identified using Surgical Procedure of the Primary Site codes 30, 33, 45, 55, and 56. 310 patients alive at least 90 days after surgery and with N0-N1 pathologic stage without missing data were included in the final cohort for analysis (Figure 1). This was chosen as a clinically relevant time point as adjuvant chemotherapy is typically administered between 60-90 days after surgery and to minimize immortal-time biases against the no-chemotherapy group. Patients wherein chemotherapy was not administered or recommended owing to contraindications were excluded.
Figure 1.
Cohort selection schema.
Statistical Analysis
The patients were separated into two treatment groups, chemotherapy, and no chemotherapy. Associations between treatment groups and demographic variables and disease status were analyzed using the Pearson Chi-square test for categorical variables and Wilcoxon rank sum test for continuous variables. Overall survival (OS), the primary end point, was defined as the time (in months) from diagnosis to death from any cause. Patients alive at the date of last follow-up were censored. Multivariable Cox proportional hazards modeling was performed using all significant variables on univariate analysis and results were used to assess the independent effect of chemotherapy treatment and other variables on OS. Relative prognosis was summarized using estimates and 95% confidence limits for the hazard ratio (HR). In addition, we performed subgroup Kaplan-Meier survival analysis on the basis of pathologic N stage to potentially account for confounding caused by understaging. All associations were considered statistically significant at an alpha error <0.05 (P value <0.05). All analyses were performed using SAS version 9.4.
Results
Patient Characteristics
A total of 310 stage IV NSCLC patients with solitary or oligometastatic disease to the brain were identified who were alive for more than 90 days after surgery. The mean age of the cohort was 58.3 years (SD, 9.7). Most the cohort was male (53.9%), white (87.4%), and resided in a metro area (78.4%). The most common insurance type was private (49.7%) followed by government insurance (42.6%). Most patients received treatment at either an academic research program (44.2%) or a comprehensive community cancer program (35.5%). Upper lobe (66.5%) of the lung was the most common site of the primary tumor followed by the lower lobe (24.2%) and adenocarcinoma was the most common primary tumor histology (68.7%). Pathologic N0 (74.5%), and T2 (51.5%) were the most common primary tumor stages, and 183 (59%) patients were diagnosed with a Grade II/IV tumor. A lobectomy (94.8%) was the typically performed surgery, whereas thoracotomy (56.1%) was the preferred surgical approach. 67.4% of the population had a Charlson Deyo score of 0, and 32.6% had a score of either 1 or 2. 223 (71.9%) patients received chemotherapy of whom 42 (13.5%) received neoadjuvant, 177 (57%) received adjuvant chemotherapy, and chemotherapy sequence was not known for 4 (0.01%) patients. In addition, 261(86.7%) patients received radiation and 49 (13.3%) did not.
Descriptive characteristics of patients receiving (71.9%) and not receiving (28.1%) chemotherapy are compared and summarized in Table 1. Patients who received chemotherapy were more likely to be younger (chemotherapy vs. no chemotherapy; mean [SD], 57 [8.7] vs. 61.4 [11.3]; Wilcoxon rank sum test p = 0.002), insured by a private organization (52% vs. 43.7%; Chi-square test p = 0.01), and received radiation in addition to chemotherapy (93% vs. 71.3%; p < 0.001). There was no significant difference between the two treatment groups in sex, race, Charlson Deyo score, median income, education, residence, treatment facility, primary tumor site, histology, pathologic N and T stage, grade, and surgery type and approach.
Table 1.
Descriptive statistics of the cohort
| Variables | Total (N = 310)1 | Chemotherapy (N = 223) | No Chemotherapy (n = 87) | P value2 |
|---|---|---|---|---|
| Age | 58.3 (9.7) | 57.0 (8.7) | 61.4 (11.3) | 0.002 |
| Sex | ||||
| Male | 167 (53.9%) | 117 (52.5%) | 50 (57.5%) | 0.4 |
| Female | 143 (46.9%) | 106 (47.5%) | 37 (42.35%) | |
| Race | ||||
| White | 271 (87.4%) | 197 (88.3%) | 74 (85.1%) | 0.2 |
| Black | 29 (9.4%) | 21 (9.4%) | 8 (9.2%) | |
| Other | 10 (3.2%) | 5 (2.2%) | 5 (5.7%) | |
| Primary payer | ||||
| Private Insurance | 154 (49.7%) | 116 (52.0%) | 38 (43.7%) | 0.01 |
| Government Insurance | 132 (42.6%) | 85 (38.1%) | 47 (54.0%) | |
| No Insurance or Unknown | 24 (7.7%) | 22 (9.9%) | 2 (2.3%) | |
| Median Income Quartiles | ||||
| <$38,000 | 57 (18.6%) | 36 (16.4%) | 21 (24.1%) | 0.4 |
| $38,000-$47,999 | 79 (25.7%) | 58 (26.4%) | 21 (24.1%) | |
| $48,000-$62,999 | 86 (28.0%) | 65 (29.5%) | 21 (24.1%) | |
| $63,000 + | 85 (27.7%) | 61 (27.7%) | 24 (27.6%) | |
| % No High School Degree | ||||
| >=21% | 58 (18.9%) | 37 (16.8%) | 21 (24.1%) | 0.4 |
| 13-20% | 75 (24.4%) | 53 (24.1%) | 22 (25.3%) | |
| 7.0-12.9% | 103 (33.6%) | 77 (35.0%) | 26 (29.9%) | |
| <7% | 71 (23.1%) | 53 (24.1%) | 18 (20.7%) | |
| Residence | ||||
| Metro | 243 (78.4%) | 180 (80.7%) | 63 (72.4%) | 0.1 |
| Urban | 49 (15.8%) | 30 (13.5%) | 19 (21.8%) | |
| Rural | 6 (1.9%) | 3 (1.3%) | 3 (3.4%) | |
| Unknown | 12 (3.9%) | 10 (4.5%) | 2 (2.3%) | |
| Facility Type | ||||
| Community Cancer Program | 24 (8.0%) | 17 (7.9%) | 7 (8.2%) | 0.8 |
| Comprehensive Community Cancer Program | 107 (35.5%) | 79 (36.6%) | 28 (32.9%) | |
| Academic/Research Program | 133 (44.2%) | 92 (42.6%) | 41 (48.2%) | |
| Integrated Network Cancer Program | 37 (12.3%) | 28 (13.0%) | 9 (10.6%) | |
| Site | ||||
| Main bronchus | 2 (0.6%) | 2 (0.9%) | 0.7 | |
| Upper lobe | 206 (66.5%) | 147 (65.9%) | 59 (67.8%) | |
| Middle lobe | 11 (3.5%) | 7 (3.1%) | 4 (4.6%) | |
| Lower lobe | 75 (24.2%) | 54 (24.2%) | 21 (24.1%) | |
| Overlapping lesion of lung | 9 (2.9%) | 8 (3.6%) | 1 (1.1%) | |
| Lung, NOS | 7 (2.3%) | 5 (2.2%) | 2 (2.3%) | |
| Histology | ||||
| Squamous | 36 (11.6%) | 26 (11.7%) | 10 (11.5%) | 0.9 |
| Adenocarcinoma | 213 (68.7%) | 152 (68.2%) | 61 (70.1%) | |
| Others | 61 (19.7%) | 45 (20.2%) | 16 (18.4%) | |
| Pathologic N Stage | ||||
| N0 | 231 (74.5%) | 164 (73.5%) | 67 (77.0%) | 0.5 |
| N1 | 79 (25.5%) | 59 (26.5%) | 20 (23.0%) | |
| Pathologic T stage | ||||
| T0 | 2 (0.6%) | 2 (0.9%) | 0.1 | |
| T1 | 72 (23.3%) | 44 (19.8%) | 28 (32.2%) | |
| T2 | 159 (51.5%) | 115 (51.8%) | 44 (50.6%) | |
| T3 | 64 (20.7%) | 52 (23.4%) | 12 (13.8%) | |
| T4 | 12 (3.9%) | 9 (4.1%) | 3 (3.4%) | |
| Grade | ||||
| I/II | 81 (26.1%) | 56 (25.1%) | 25 (28.7%) | 0.5 |
| III/IV | 183 (59.0%) | 131 (58.7%) | 52 (59.8%) | |
| Unknown | 46 (14.8%) | 36 (16.1%) | 10 (11.5%) | |
| Surgery | ||||
| Lobectomy | 294 (94.8%) | 210 (94.2%) | 84 (96.6%) | 0.3 |
| Pneumonectomy | 16 (5.2%) | 13 (5.8%) | 3 (3.4%) | |
| Surgical Approach | ||||
| VATS | 67 (21.6%) | 45 (20.2%) | 22 (25.3%) | 0.6 |
| Thoracotomy | 174 (56.1%) | 128 (57.4%) | 46 (52.9%) | |
| Unknown | 69 (22.3%) | 50 (22.4%) | 19 (21.8%) | |
| Charlson Deyo Score | ||||
| 0 | 209 (67.4%) | 150 (67.3%) | 59 (67.8%) | 0.9 |
| 1 or 2 | 101 (32.6%) | 73 (32.7%) | 28 (32.2%) | |
| Radiation | ||||
| Yes | 261 (86.7%) | 199 (93.0%) | 62 (71.3%) | < 0.001 |
| No | 49 (13.3%) | 4 (7%) | 25 (28.7%) | |
Sociodemographic, and clinicopathological variables were compared across the two treatment groups using Pearson’s Chi-square test and Wilcoxon rank sum test for categorical and continuous variables, respectively. All significantly different variable (p < 0.05) p values are highlighted in bold.
NOS, Not otherwise specified; VATS, video-assisted thoracoscopic surgery
mean (SD); n (%)
Pearson's Chi-square test; Wilcoxon rank sum test
Univariate and Multivariable Analysis of OS
On univariate cox proportional modeling, patient age (HR [95% CI] = 1.03 [1.02 – 1.05]; Wald p < 0.001), 1 or 2 Charlson Deyo score (1.45 [1.06 – 1.99]; p = 0.02), government insurance (1.40 [ 1.02-1.91]; p=0.037), N1 pathologic stage (1.55 [1.12 – 2.16]; p = 0.009) were associated with poorer survival, whereas female sex (0.7 [0.51 – 0.95]; p = 0.02), adenocarcinoma histology (0.61 [0.39 – 0.96]; p = 0.03), and chemotherapy (0.71 [0.52 – 0.99]; p = 0.04) improved survival. Multivariable analysis with patient age, sex, Charlson Deyo score, insurance type, pathologic N stage, histology, and chemotherapy as covariates revealed similar results. Age (1.02 [1.00 – 1.04]; p = 0.03), 1 or 2 Charlson Deyo score (1.45 [1.05 – 2.02]; p = 0.02), and pathologic N1 stage (1.48 [1.05 – 2.08]; p = 0.02) were associated with significantly poorer survival whereas adenocarcinoma histology (0.59 [0.38 – 0.92]; p = 0.02), and perioperative chemotherapy (0.66 [0.47 – 0.92]; p = 0.01) were significantly associated with improved survival (Table 2).
Table 2.
Univariate and Multivariable Cox Proportional Model for Overall Survival
| Univariate HR (95% CI) | p-value1 | Multivariable HR (95% CI) | p value | |
|---|---|---|---|---|
| Age | 1.03 | <0.001 | 1.02 | 0.03 |
| (1.02 - 1.05) | (1.00 - 1.04) | |||
| Sex | ||||
| Female vs. Male | 0.7 | 0.02 | 0.75 | 0.07 |
| (0.51 - 0.95) | (0.55 - 1.03) | |||
| Charlson Deyo Score | ||||
| 1 or 2 vs. 0 | 1.45 | 0.02 | 1.45 | 0.02 |
| (1.06 - 1.99) | (1.05 - 2.02) | |||
| Insurance | ||||
| Government vs. Private Insurance | 1.4 | 0.03 | ||
| (1.02 - 1.91) | ||||
| No insurance/unknown vs. Private Insurance | 1.01 | 0.9 | ||
| (0.54 - 1.91) | ||||
| Pathologic Stage - N | ||||
| N1 vs. N0 | 1.55 | 0.009 | 1.48 | 0.02 |
| (1.12 - 2.16) | (1.05 - 2.08) | |||
| Histology | ||||
| Adenocarcinoma vs. Squamous Cell Carcinoma | 0.57 | 0.01 | 0.59 | 0.02 |
| (0.37 - 0.88) | (0.38 - 0.92) | |||
| Others vs. Squamous Cell Carcinoma | 0.65 | 0.09 | 0.65 | 0.1 |
| (0.39 - 1.09) | (0.39 - 1.10) | |||
| Surgery | ||||
| Pneumonectomy vs. Lobectomy | 1.52 | 0.1 | ||
| (0.82 - 2.8) | ||||
| Surgical Approach | ||||
| Thoracotomy vs.VATS | 1.2 | 0.3 | ||
| (0.78 - 1.85) | ||||
| Radiation | ||||
| Yes vs. No | 0.89 | 0.6 | ||
| (0.58 - 1.37) | ||||
| Chemotherapy | ||||
| Yes vs. No | 0.71 | 0.04 | 0.66 | 0.01 |
| (0.52 - 0.99) | (0.47 - 0.92) |
Multivariable cox modeling was performed using patient age, sex, Charlson Deyo score, insurance type, tumor histology and pathologic N stage, surgery type, radiation therapy status, and adjuvant chemotherapy as covariates. All statistically significant variables (P < 0.05) are highlighted in bold.
Wald p
1-year and 5-year Survival Rate
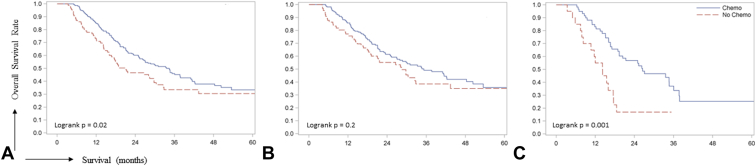
The median follow-up of the entire cohort was 38.1 months. The 1-year and 5-year survival of the entire cohort was 81% (95% CI = 77% - 85%) and 32% (95% CI 26% - 39%), respectively with a median survival of 29.7 months. Patients receiving chemotherapy exhibited significant 1-year (chemotherapy vs. no chemotherapy, 85% [95% CI = 80% - 89%] vs. 72% [61% - 80%]) and 5-year (33% [25% - 42%] vs. 30% [20% - 42%]) survival benefit over patients not receiving any chemotherapy (logrank p = 0.02) (Figure 2). To account for the potential confounding effect of understaging, we performed a subgroup survival analysis in pathologic N0 (N = 231 [74.5%]) and N1 (N = 79 [25.5%]) stage patients. We found that the survival benefit of perioperative chemotherapy was significant in patients with pathologic N1 stage tumors (logrank p = 0.001), but not N0 stage tumors (logrank p = 0.2) suggesting that maybe patients with a higher N stage with brain metastasis would benefit from additional systemic therapy postthoracic resection. Kaplan-Meier survival analysis between patients receiving neoadjuvant chemotherapy (N = 42 [13.5%]) and adjuvant chemotherapy (N = 177 [57%]) revealed no significant survival difference between the two treatment approaches (logrank p = 0.9, Figure S1)
Figure 2.
Kaplan-Meier curves comparing overall survival between the chemotherapy and no-chemotherapy groups in the entire cohort (A), pathologic N0 stage (B), and pathologic N1 stage (C) patients.
Discussion
Fundamental assumptions regarding the disease pathogenesis in NSCLC continue to be refined over the years. Surgical or nonsurgical locally ablative therapies such as radiotherapy alone can be presumed to be curative for localized disease if cancer spread progresses at an orderly fashion. However, the actual clinical experience of metastatic relapse occurring after localized disease is treated with surgery alone demonstrates that histopathologic features aside, TNM staging can be prognostic for unfavorable outcomes in some patients who likely had subclinical distant disease despite what appears to be locoregionally restricted disease with available staging technologies.11,12 Moreover, the scope of locally directed therapies in patients with disseminated cancers has increased in recent years on greater recognition of the oligometastasis hypothesis that posit the improvement in OS of patients when focal therapies are applied to evident sites of residual disease if metastases are truly limited in extent.13 Indeed, with better diagnostic technologies and prognostic tools in recent years, it is becoming more relevant to define the optimal treatment approach in patients with oligometastatic disease, such as the recent demonstration in a small, randomized trial of OS improvement with consolidation radiation compared with maintenance chemotherapy alone in patients with oligometastatic NSCLC.14
Although administering systemic therapy after achieving locoregional control for oligometastatic NSCLC is intuitively presumed to lead to better OS, there is variability in outcomes reported in the literature. Whereas some studies favor systemic therapy, other studies were unable to exhibit benefit of chemotherapy administered with adjuvant intent, likely because of considerable heterogeneity among cases and inherent biases in such retrospective analyses,15, 16, 17 including lack of accounting for the impact of social determinants of health outcomes known to impact survival.18 Our study attempts to address this gap in the literature utilizing data collected through NCDB which captures more than two-thirds of newly diagnosed cancers nationwide,10 obtaining not only pertinent tumor- and treatment-related clinical information but also providing patient-level socioeconomic information on factors such as household income, education, insurance coverage which have previously been shown to be independently associated with risk for short-term mortality after lung cancer surgery.19 We also utilized a 90-day landmark for patient selection to further reduce the negative bias against the no-chemotherapy group which may purely arise from surgically related issues.20
In addition, our data has particular relevance to NSCLC patients with otherwise T1-2 N0 pathologic staging status wherein chemotherapy is typically not recommended in the adjuvant setting but the value in the oligometastatic setting is uncertain. Notably, more than half of the patients who underwent surgery after definitive treatment of brain metastases had either more advanced nodal involvement documented after surgery and/or died within 90 days postprocedure, attesting to the poor prognosis of these patients in general. Nevertheless, even after adjusting for confounding variables known to influence survival, such as sex, age, insurance status, chemotherapy administration remains an independent variable associated with superior OS, with most patients in this series receiving this in the adjuvant setting. The effect of chemotherapy is perhaps not surprising if one views that NSCLC is preponderantly a systemically driven disease which is relatively sensitive to chemotherapy and that the truly oligometastatic state is an exception rather than the rule for this disease.
Our findings are limited by the inherent nature of this type of investigation and by the data available for analysis. That patients who received chemotherapy had better OS compared with patients who did not receive chemotherapy, ceteris paribus, may merely reflect the confounding effect of the collective clinical acumen of medical oncologists and/or patient self-awareness resulting in a selection bias toward patients with better prognosis overall and ability to better withstand the toxicity of chemotherapy. Another major limitation is the assumption that patients had only a solitary or oligometastatic spread to the brain. Newer noninvasive technologies for diagnosis and prognosis, such as blood-based assays for monitoring and tracking biomarkers, such as circulating tumor DNA, neoantigen-specific T cells, etc. will be necessary for better characterization of the oligometastatic state in stratifying patient groups when comparing outcomes arising from different interventions. Other imbalances that cannot be accounted for with certainty include, lack of data on driver gene mutations, chemotherapy regimen, and subsequent exposure to therapeutic agents with known survival benefit, such as targeted therapies or immunotherapies. Future efforts will require more longitudinal follow-up and incorporation of genomic and immune profiling data to better characterize patients with oligometastatic disease for whom additional systemic chemotherapy is unnecessary, particularly in the pN0 patient population wherein the magnitude of benefit from perioperative chemotherapy appears to be less pronounced. This clinical question remains relevant, even in the era of immune checkpoint inhibitor therapy, as clinical trials to date of first-line immune checkpoint inhibitor therapy alone in NSCLC patients with brain metastases reveal less robust evidence of survival benefit compared with trials incorporating chemotherapy to the immunotherapy regimen.21, 22, 23, 24, 25
In conclusion, perioperative chemotherapy primarily consisting of treatment administered with adjuvant intent, demonstrates OS benefit in NSCLC patients with synchronous brain metastasis at diagnosis and pN0-1 stage primary lung cancer after invasive mediastinal staging who undergo surgical resection of their primary tumor. This has a greater impact in patients with pN1 compared with pN0 tumors. Our study provides an updated benchmark for future studies in this population, demonstrating a median survival of 35.3 months and 26.5 months, with 5-year survival rates of 36% and 25% in pN0 and pN1 patients respectively, who received chemotherapy with adjuvant intent.
Acknowledgments
This work was supported by National Cancer Institute (NCI) grant numbers R01CA25551 (to Sai Yendamuri) and P30CA016056 (involving the use of Roswell Park Cancer Institute’s Biostatistics Shared Resources). All authors declare no conflict of interest regarding this study. The funders had no role in the design of the study, collection, analysis, or interpretation of data, writing of the manuscript, or the decision the publish the study.
CRediT Authorship Contribution Statement
Yeshwanth R. Vedire: Data curation, Visualization, Writing – review and editing.
Sarah Shin: Data curation, Formal analysis, Software, Investigation, Writing – original draft.
Adrienne Groman: Data curation, Software, Validation, Investigation, Visualization.
Mark Hennon: Methodology, Supervision, Writing – review and editing.
Sai Yendamuri: Funding acquisition, Methodology, Project administration, Resources, Supervision, Writing – review and editing.
Grace K. Dy: Methodology, Resources, Supervision, Writing – review and editing.
Footnotes
Drs. Vedire and Shin contributed equally to this work.
Drs. Dy and Yendamuri contributed equally to this work.
Disclosure: The authors declare no conflict of interest.
ICD-O-3. 3rd ed of International Classification of Disease for Oncology; NCCN, National Comprehensive Cancer Network; NCDB, National cancer Database; SRS, Stereotactic Radio Surgery; VAT, Video-assisted Thoracoscopic Surgery; WBRT, Whole Body Radiation Therapy.
Note: To access the supplementary material accompanying this article, visit the online version of the JTO Clinical and Research Reports at www.jtocrr.org and at https://doi.org/10.1016/j.jtocrr.2023.100522.
Supplementary Data
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 2.Socinski M.A., Morris D.E., Masters G.A., Lilenbaum R., American College of Chest P. Chemotherapeutic management of stage IV non-small cell lung cancer. Chest. 2003;123(1)(suppl):226S–243S. doi: 10.1378/chest.123.1_suppl.226s. [DOI] [PubMed] [Google Scholar]
- 3.Pfannschmidt J., Dienemann H. Surgical treatment of oligometastatic non-small cell lung cancer. Lung Cancer (Amsterdam, Netherlands) 2010;69:251–258. doi: 10.1016/j.lungcan.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Bonnette P., Puyo P., Gabriel C., et al. Surgical management of non-small cell lung cancer with synchronous brain metastases. Chest. 2001;119:1469–1475. doi: 10.1378/chest.119.5.1469. [DOI] [PubMed] [Google Scholar]
- 5.Hu C., Chang E.L., Hassenbusch S.J., 3rd, et al. Nonsmall cell lung cancer presenting with synchronous solitary brain metastasis. Cancer. 2006;106:1998–2004. doi: 10.1002/cncr.21818. [DOI] [PubMed] [Google Scholar]
- 6.Ettinger D.S., Wood D.E., Akerley W., et al. NCCN Guidelines Insights: Non-small Cell Lung Cancer. Version 4.2016. J Natl Compr Canc Netw. 2016;Vol. 14:255–264. doi: 10.6004/jnccn.2016.0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez D.R., Blumenschein G.R., Jr., Lee J.J., et al. Local consolidative therapy versus maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer without progression after first-line systemic therapy: a multicentre, randomised, controlled, phase 2 study. Lancet Oncol. 2016;17:1672–1682. doi: 10.1016/S1470-2045(16)30532-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zheng M. Classification and pathology of lung cancer. Surg Oncol Clin N Am. 2016;25:447–468. doi: 10.1016/j.soc.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Shah R.D., D’Amico T.A. Modern impact of video assisted thoracic surgery. J Thorac Dis. 2014;6(suppl 6):S631–S636. doi: 10.3978/j.issn.2072-1439.2014.08.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Cancer Society A.C. o. S. https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/ accessed June 1st, 2020.
- 11.Roth J.A., Fossella F., Komaki R., et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. J Natl Cancer Inst. 1994;86:673–680. doi: 10.1093/jnci/86.9.673. [DOI] [PubMed] [Google Scholar]
- 12.Yoshizawa A., Motoi N., Riely G.J., et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24:653–664. doi: 10.1038/modpathol.2010.232. [DOI] [PubMed] [Google Scholar]
- 13.Hellman S., Weichselbaum R.R. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 14.Gomez D.R., Tang C., Zhang J., et al. Local consolidative therapy vs. maintenance therapy or observation for patients with oligometastatic non-small-cell lung cancer: long-term results of a multi-institutional, Phase II, randomized study. J Clin Oncol. 2019;37:1558–1565. doi: 10.1200/JCO.19.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang S.Y., Kim D.G., Lee S.H., et al. Pulmonary resection in patients with nonsmall-cell lung cancer treated with gamma-knife radiosurgery for synchronous brain metastases. Cancer. 2008;112:1780–1786. doi: 10.1002/cncr.23357. [DOI] [PubMed] [Google Scholar]
- 16.Tanvetyanon T., Robinson L.A., Schell M.J., et al. Outcomes of adrenalectomy for isolated synchronous versus metachronous adrenal metastases in non-small-cell lung cancer: a systematic review and pooled analysis. J Clin Oncol. 2008;26:1142–1147. doi: 10.1200/JCO.2007.14.2091. [DOI] [PubMed] [Google Scholar]
- 17.Flannery T.W., Suntharalingam M., Regine W.F., et al. Long-term survival in patients with synchronous, solitary brain metastasis from non-small-cell lung cancer treated with radiosurgery. Int J Radiat Oncol Biol Phys. 2008;72:19–23. doi: 10.1016/j.ijrobp.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Finke I., Behrens G., Weisser L., Brenner H., Jansen L. Socioeconomic differences and lung cancer survival-systematic review and meta-analysis. Front Oncol. 2018;8:536. doi: 10.3389/fonc.2018.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Melvan J.N., Sancheti M.S., Gillespie T., et al. Nonclinical factors associated with 30-day mortality after lung cancer resection: an analysis of 215,000 patients using the National cancer data base. J Am Coll Surg. 2015;221:550–563. doi: 10.1016/j.jamcollsurg.2015.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMillan R.R., Berger A., Sima C.S., et al. Thirty-day mortality underestimates the risk of early death after major resections for thoracic malignancies. Ann Thorac Surg. 2014;98:1769–1774. doi: 10.1016/j.athoracsur.2014.06.024. discussion 1774–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reck M., Rodriguez-Abreu D., Robinson A.G., et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive non-small-Cell Lung Cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 22.Gandhi L., Rodriguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 23.Hellmann M.D., Paz-Ares L., Bernabe Caro R., et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381:2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 24.Reck M., Ciuleanu T.E., Cobo M., et al. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open. 2021;6 doi: 10.1016/j.esmoop.2021.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goldberg S.B., Schalper K.A., Gettinger S.N., et al. Pembrolizumab for management of patients with NSCLC and brain metastases: long-term results and biomarker analysis from a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2020;21:655–663. doi: 10.1016/S1470-2045(20)30111-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.