Abstract
Background
Incisional herniae (IH) are reported in 5–>20 % of patients undergoing open celiotomy, and can be linked to closure technique. The STITCH randomized trial favors a small bite technique for midline celiotomy closure with a 1-year IH rate of 13 % over larger bites (23 %).
Methods
A continuous musculofascial mass closure with absorbable looped #1 PDS suture with 2-cm bite size was used for all open celiotomies. IH frequency and associated clinicopathologic factors were retrospectively analyzed from prospective data in 336 consecutive patients undergoing visceral resections by a single surgeon.
Results
The study population included 192 men and 144 women, 81 % of whom had a cancer diagnosis, who underwent hepatobiliary, pancreatic, gastroesophageal, and colorectal resections, or a combination. The majority of patients (84 %) had subcostal incisions, and 10 % received a midline incision. At a median follow-up of 19.5 months, the overall IH rate was 3.3 %. Hernia rates were 2.5 % for subcostal margin, 2.9 % for midline, and 5.5 % for other incisions (p = 0.006). Median time to hernia detection was 492 days. Factors associated with IH were increased weight, abdominal depth/girth, male sex, spleen size, visceral fat, and body height (p ≤ 0.04 for all), but not type of resection, prior operations, underlying diagnosis, weight loss, adjuvant chemotherapy or radiation, incision length or suture to incision ratio.
Conclusions
The described technique leads to a low IH rate of <3 % in subcostal or midline incisions, and can be recommended for routine use. The observed results appear superior to those of the STITCH trial, even for the smaller midline incision cohort.
Keywords: Incisional hernia, Abdominal closure, Large bite, STITCH trial, Suture technique, Prevention
Introduction
Incisional herniae (IH) are a common complication after incisions of the abdominal wall, with an prevalence between 5 and 20 % reported in the literature (1). IH have the potential to significantly impact quality of life, and are also associated with a significant cost burden ([2], [3], [4]). Multiple factors play a role in the development of IH, including patient-related risk factors, incision type, closure technique, suture material, and wound infection (4,5). While patient-related risk factors such as obesity, diabetes, malnutrition, and smoking ([5], [6], [7]) are well-accepted associations with the development of IH, the highest quality technique for celiotomy closure has been heavily debated. Factors related to surgical technique include continuous vs. interrupted suture, suture length to wound length ratio, bite size, needle size and type of suture, en masse vs. fascial-only closure, prophylactic mesh vs. primary closure, and presence of tension (5,8).
While randomized controlled trials (RCTs) are generally accepted as the reference standard of clinical trials, a literature review on surgical techniques to prevent IH illustrates the difficulty in using RCTs to establish the optimal operative technique for celiotomy closure. For example, a 2009 multicenter RCT comparing interrupted vs. continuous closure with various materials found a higher than expected IH rate in all three groups, ranging from 0 to 25.5 % among participating centers (9), despite all surgeons being trained to use the same suture materials and techniques. The 2015 European Hernia Society guidelines, which were based on the findings of ten systematic reviews, noted that the data and conclusions from the different reviews were often contradictory, and that the quality of most of the systematic reviews was low (10).
In 2015, the STITCH multicenter randomized trial compared the technique of small tissue bites (5 mm every 5 mm) against the traditional technique of large tissue bites (1 cm every 1 cm) in 560 patients undergoing midline celiotomy (3). The authors found that at the one-year mark post-operatively, the small bite technique was associated with a 13 % IH rate, compared to 23 % for the large bite technique, without significant increase in closure time, pain, or adverse events. However, a 2019 JAMA Surgery editorial noted anecdotally that most surgeons and trainees at their institution continued to default to the 0-looped PDS suture used with the larger bite technique, despite the findings of the STITCH trial (11). The gap between the results of an RCT for surgical technique and its implementation may be due to ingrained surgeon habit or good empiric outcomes with other techniques, or because the technique studied may not be directly applicable to the surgeon's practice. In this practice experience in which the majority of incisions are in subcostal followed by midline location, a tension-free large bite technique for celiotomy closure had been utilized as routine and standard for >25 years. The aim of our study was to qualitatively assess IH rate and potentially contributing risk factors resulting from this standard technique.
Materials and methods
Patients and data selection
Incisional hernia frequency and associated clinicopathologic factors were analyzed from prospective data in consecutive patients undergoing primary incision closure after visceral resections within a single-surgeon practice between 2014 and 2021. Patients undergoing simultaneous hernia repair or mesh placement were excluded. Demographic and clinical variables collected included age, sex, weight, height, BMI, history of weight loss, smoking history, past medical history (including history of diabetes), and past surgical history (including prior abdominal operations). Diagnostic parameters included underlying diagnosis, cancer stage where applicable, diagnostic laboratory parameters (complete blood count including platelet) and leukocyte counts and comprehensive metabolic panel (including serum creatinine and albumin levels). Morphometric parameters such as abdominal depth, girth, visceral fat and psoas muscle thickness, and spleen volume had been measured using preoperative cross-sectional imaging, with the first four parameters measured at the level of the L3 vertebral body, while the spleen volume was calculated using the depth, length, and height of the spleen on imaging. Operative therapy was assessed through data on type and length of incision, resection type, suture:incision (S:I) ratio at closure, use of abdominal drains, estimated blood loss, and duration of the operation. Postoperative outcomes were charted for occurrence of postoperative complications, postoperative length of stay, and adjuvant systemic and/or radiation therapy. All patients underwent structured follow-up that involved once yearly clinical visits after celiotomies for benign conditions, or more frequent visits with cross-sectional surveillance imaging for cancer patients. Incisional herniae were diagnosed clinically and/or identified or verified on follow-up cross-sectional imaging through computed tomography (CT) or magnetic resonance imaging (MRI), where applicable. Time to hernia formation was calculated.
Technical aspects
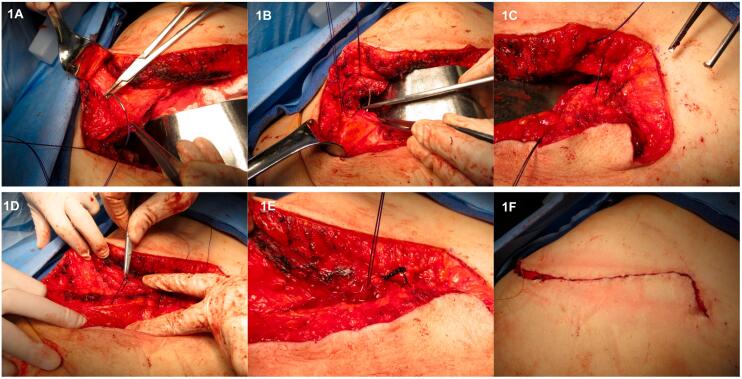
A continuous, single-layer, musculofascial mass closure with absorbable monofilament looped #1 PDS suture with >2 cm bite and a stitch separation of at least 1.5 cm has been used for all open celiotomies including hand port sites (Fig. 1). All 12 mm umbilical port sites were closed with 2 interrupted #0 PDS figure-of-eight sutures, while 5 mm port sites through the muscular abdominal wall remained unclosed in the musculofascial layers. Information of incision length and the length of closing suture used was documented at the time of operative closure. All incisions were then closed with intracutaneous (subcuticular) monofilament Monocryl material, in 2–0 size for major incisions, and 4–0 size for port sites, followed by Dermabond or Steri-Strip application and a sterile gauze dressing.
Fig. 1.
Continuous, single-layer, low-tension mass closure of celiotomy incision. 1A: Start of #1 looped PDS suture with mass bites at incision edge followed by loop interlock. 1B: Continuation of suture with large bites at approximately 1.5 cm distance intervals, achieving apposition of muscular incision edges without tension. 1C: Similar technique of suture at the opposite incision edge. 1D: Stepwise tightening of suture loops after complete apposition of incision edges, without holding tension. 1E: Final knot closure of both suture ends, with subsequent inversion of the knot in muscular layer. 1F: Intracutaneous suture without subcutaneous stitching completing the skin closure.
Statistical analysis
Data were analyzed through descriptive statistics, contingency analysis via chi-square testing, and t-test or Mann-Whitney analysis based on data distribution. Hernia-free survival was calculated via Kaplan-Meier analysis with log-rank comparison, with time of hernia diagnosis or patient death defined as uncensored events. For all comparative statistics, significance of differences was accepted at p < 0.05.
Results
Over the study period, a total of 336 patients were included, of which 192 (57 %) were men and 144 (43 %) were women (Table 1). Median age of the participants was 65 years (range: 17–88 y) and 81 % of patients had a cancer diagnosis. There were 162 hepatobiliary (48 %), 111 pancreatic (33 %), 20 gastroesophageal (6 %), 7 colorectal (2 %) and 36 other procedures (11 %), including multivisceral resections in 14 %. The average weight was 79.5 kg (+/− 18.4), at a body mass index (BMI) of 28.4 (+/− 6.3). Weight loss of >5 lbs. had been experienced by 34 % of patients, at an average of 24.8 lbs. (+/− 18.3). A history of tobacco use was identified in 52 % (32 % active smokers), with a mean of 43 pack-years (+/− 27), while never-smokers comprised 44 % and no information was available in 4 %. In addition, 21 % of patients were diabetic, and 45 % had a prior abdominal operation. Mean laboratory parameter values were for albumin 3.8 g/dL (+/− 0.65), creatinine 0.84 mg/dL (+/− 0.28), leukocyte count 7.4 × 109 cells/L (+/− 2.6), and platelet count 242 × 103/μL (+/− 98).
Table 1.
Demographic and clinical characteristics of patients.
| Patient characteristics | n = 336 |
|---|---|
| Median age (yrs, range) | 65 (17–88) |
| Sex, n (%) | |
| Male | 192 (57 %) |
| Female | 144 (43 %) |
| Cancer diagnosis, n (%) | 273 (81 %) |
| Type of resection, n (%) | |
| Hepatobiliary | 162 (48 %) |
| Pancreatic | 111 (33 %) |
| Gastroesophageal | 20 (6 %) |
| Colorectal | 7 (2 %) |
| Other | 36 (11 %) |
| Multivisceral | 47 (14 %) |
| Incision type, n (%) | |
| Subcostal | 283 (84 %) |
| Midline | 34 (10 %) |
| Other | 19 (6 %) |
The main incision was at the subcostal margin (n = 283, 84 %), midline (n = 34, 10 %) or other (n = 19, 6 %). Incisions categorized as “other” included transverse or hockey stick incisions and hand port sites. An umbilical port site for concurrent laparoscopy access had been utilized in 72 patients. The mean length of the main incision was 28 cm (range: 4–42). The mean S:I ratio was 4.53:1 (SD 0.68, range 2.9–6.8). The mean operation time was 316 min (+/− 117), the median estimated blood loss 150 mL (IQR 233). Postoperative length of stay was 8 days (median, range 1–43), Grade 3+ complications were encountered in 18.4 %, and superficial wound infections seen in 3.7 %. There was one dehiscence requiring reoperation that did not result in an IH afterwards. In 57 % of all patients, adjuvant therapy had been administered. All of these received cytotoxic chemotherapy, while adjuvant radiotherapy was utilized in 16 %. The IH development rate in this adjuvant cohort was 4.8 % (p = NS). In addition, antiangiogenic biologic therapy had been given to 8 patients (2.4 %), of whom one developed an IH (p = NS). None of these patients had received therapy within 8 weeks before or after the operation.
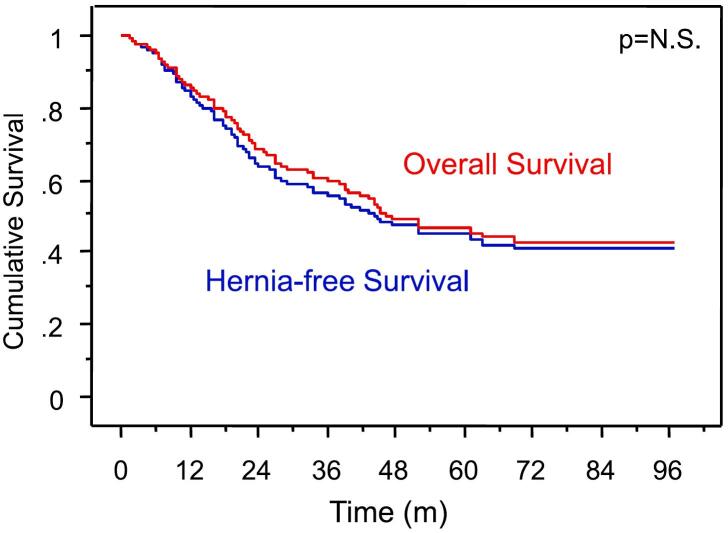
At a median follow-up of 19.5 months (range 1–97, 20.6 for survivors), 11 patients had developed an incisional hernia (3.3 %). Hernia rates were 2.5 % for subcostal margin, 2.9 % for midline, and 5.5 % for other incisions (p = 0.006). The median time to hernia detection was 492 days (40–2059 d). No significant difference between hernia-free survival (HFS) and overall survival (OS) was identified in this patient population, reflecting the low numbers of IH encountered (Fig. 2). A patient subset of 23 % was alive but had the last follow-up within <12 months, and 12.5 % of patients had died within one year. Factors associated with development of IH included increased weight and height, abdominal depth and girth, male sex, spleen size, visceral fat (all at p < 0.05, Table 2). Although the BMI group correlated with IH development (0 % for BMI < 24, 2.8 % for BMI 24–30, and 13.5 % for BMI > 30, p = 0.008), BMI as continuous variable did not (p = 0.07). There was no significant association between IH formation and other parameters such as type of resection, prior operations, underlying diagnosis, weight loss, albumin, estimated blood loss, postoperative complications, adjuvant treatment with cytotoxic, biologic, or radiation therapy, length of incision, S:I ratio at closure or use of abdominal drains.
Fig. 2.
Overall and hernia-free survival in 336 patients undergoing celiotomy.
Table 2.
Factors associated with incisional hernia formation on univariate analysis.
For continuous variables, means ± standard deviations are shown.
| Parameter | Unit/Subset | No incisional hernia (n = 325) | Incisional Hernia (n = 11) | P value |
|---|---|---|---|---|
| Weight | kg | 78.5 (±18.1) | 97.0 (±14.8) | 0.003 |
| Abdominal depth | mm | 114.4 (± 32.2) | 150.4 (±14.5) | 0.015 |
| Abdominal girth | mm2 | 64,457 (±18,269) | 84,663 (±20,399) | 0.019 |
| Sex | Male/female ratio (%) | 56/44 | 91/9 | 0.021 |
| Visceral fat area | mm2 | 5721 (±6340) | 12,503 (±7036) | 0.022 |
| Spleen transectional area | cm2 | 41.5 (±12.6) | 54.4 (6.1) | 0.023 |
| Body height | cm | 168 (±10.6) | 176 (±7.9) | 0.033 |
Discussion
This study found a low IH rate of 3.3 % in patients undergoing abdominal operations primarily for a malignancy. Other studies which were performed in cancer patients have reported hernia formation rates of 6.0–41 % (6,7,[12], [13], [14]). In 2016, Baucom et al. reported that factors independently associated with hernia formation included midline, periumbilical, and subcostal incisions, laparoscopic-assisted procedure, increasing BMI, postoperative surgical site infection (SSI), and a cancer diagnosis other than colorectal or urologic/gynecologic malignancy (7). Similarly in this study, increased weight, increased abdominal depth and girth, and visceral fat were associated with IH development. This may relate to increased mechanical forces at the sutured abdominal wall site but other factors such as impaired healing activity remain possible but uncertain. However, we found no association between the underlying cancer diagnosis and IH formation, nor was there an association between superficial wound infection and IH formation. While prior studies have reported that preoperative chemotherapy is associated with an increased incidence of IH (6,15), postoperative chemotherapy was not found to be linked to IH formation (6,7), which is in line with our findings here. The number of patients on antiangiogenic therapy was low, and no IH problem was encountered. We also found that the highest IH rate in our study population was associated with laparoscopic-associated incisions, including umbilical trocar sites larger than 10 mm and hand port incisions, which has previously been reported by other authors (7), although yet other studies have reported the contrary (16). As a result of this analysis, we have minimized the use of umbilical ports wherever possible, and prefer port sites of <10 mm diameter, too.
The most impactful RCT evaluating the influence of celiotomy closure technique on IH rate is the 2015 STITCH trial, which specifically included only midline incisions (3), unlike our cohort. Several prior studies have demonstrated that midline incisions are associated with higher IH formation rates, compared to other incisions such as subcostal, transverse, or paramedian incisions (6,10,17). However, our smaller midline incision cohort of 34 patients also had a lower rate of IH (2.9 %) compared to both the “large bite” and “small bite” groups in the STITCH trial (21 % and 13 % respectively), suggesting that other factors likely played a role. A 2020 systematic review evaluating IH rate in 5427 patients undergoing hepatobiliary surgical procedures via transverse (right subcostal and chevron) and hybrid (transverse with midline extension) incisions reported a 6.0 % pooled incidence of IH in patients with transverse incisions (14). However, there was considerable variation in suture type and method of closure between groups. In studies which specifically evaluated right subcostal incisions, the IH rate ranged from 0 to 7.7 % ([18], [19], [20], [21]). These studies include both patients undergoing operations for complex benign disease (primarily cholecystectomy), as well as transplantation, and therefore do represent a widely heterogenous population. In patients who received a right subcostal incision for benign disease, Sans-López et al. reported that obesity and chronic bronchitis were associated with IH (20), and in patients undergoing liver transplantation via a subcostal incision, Donataccio et al. found that persistent postoperative ascites was the only risk factor for IH occurrence (21). It must be presumed, that a considerable reporting bias exists regarding IH formation rates after major celiotomy incisions, and that the actual frequency of IH formation may be higher than in selcted reported studies.
Another interesting parallel between our data and the STITCH trial is the comparable suture to incision ratio that reflects in this case fewer bites with much greater distance from the edge and incorporation of muscle tissue. Consequently, while the distance between bites and distance from the fascial edge was constant throughout the study, a fairly high variance of the S:I ratio has been observed in this experience due to significant variability of abdominal wall muscular thickness in our patient population, but without identifiable impact on subsequent IH formation.
The strengths of this study include the single-surgeon patient cohort and the presence of the attending surgeon at every celiotomy closure, which minimizes changes in technique and eliminates variation in materials used between patients, and thus allows for assessing other patient-related factors that may or may not contribute to IH formation. The tension applied through traction on a continuous suture is difficult to measure, and if too high may lead to tissue necrosis with decreased healing implications; this has also been purposefully avoided in this practice setting. In addition, a longer follow-up period with a median of 20.6 months for survivors provided more appropriate time to capture the majority of IH, as it has previously been reported that it may take two years for 75 % of herniae to develop (17). The median time to IH development in our study was 492 days, or 1.35 years. Finally, due to the high proportion of patients with cancer or precancerous conditions, clinical follow-up and CT scan surveillance imaging were conducted in a larger proportion of this study population than in series with benign operative indications.
The limitations of this study include its retrospective nature. As both the clinical examination and surveillance CT scans were performed with oncologic intent, rather than with a specific focus on IH detection, it is theoretically possible that some asymptomatic, subclinical herniae remained radiographically undetected during the follow-up period. A retrospective study by Claes et al. did note that CT assessed by a radiologist focused on the occurrence of an IH showed a significantly higher number of IH compared to routine CT assessment or routine clinical examination in an oncologic setting (12). Additionally, some baseline data on patient factors that may have contributed to IH formation were not recorded given the retrospective data analysis. With regard to statistical power, a multivariate logistic regression was not performed due to the low frequency of diagnosed IH in our study. Finally, the low number of patients with midline celiotomy incisions in this study limits the direct comparisons to previously published series that have primarily evaluated outcomes in midline incisions.
In conclusion, the large bite technique utilized in this practice experience was associated with an acceptably low IH rate of <3 % in both subcostal and midline incisions, and a HFS that closely parallels OS. Despite the for the most part extensive nature of these operative interventions, only a small proportion of patients appears to be negatively impacted by incisional healing aspects. Our data also highlight the challenges in generalizing the results of randomized controlled trials on surgical technique, as multiple other factors in addition to bite size likely seem to play a role in IH formation. Based on our empirical findings, the described large bite, low tension technique has remained our preferred closure standard, despite the level 1 data availability of the STITCH trial for midline incisions, and our results suggest that the question of best technique for celiotomy closure remains relevant. The described technique can be recommended as a reliable celiotomy closure option and as a technique for future comparative trial evaluation.
Funding sources
None.
Ethics approval
The study analysis was conducted using de-identified data and had been approved by the Institutional Review Board (IRB) with a waiver of consent requirement.
CRediT authorship contribution statement
JS: Drafted manuscript.
MJM: Collected and compiled data.
LYS: Collected and analyzed data.
ANH: Contributed data.
RES: Developed the concept, managed the project, analyzed data, revised the manuscript, and provided final approval of the version to be submitted.
Declaration of competing interest
No conflicts declared.
References
- 1.Patel S.V., Paskar D.D., Nelson R.L., Vedula S.S., Steele S.R. Closure methods for laparotomy incisions for preventing incisional hernias and other wound complications. Cochrane Database Syst Rev. 2017;11(11):CD005661. doi: 10.1002/14651858.CD005661.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alli V.V., Zhang J., Telem D.A. Impact of incisional hernia development following abdominal operations on total healthcare cost. Surg Endosc. 2018;32(5):2381–2386. doi: 10.1007/s00464-017-5936-8. [DOI] [PubMed] [Google Scholar]
- 3.Deerenberg E.B., Harlaar J.J., Steyerberg E.W., Lont H.E., van Doorn H.C., Heisterkamp J., et al. Small bites versus large bites for closure of abdominal midline incisions (STITCH): a double-blind, multicentre, randomised controlled trial. Lancet. 2015;386(10000):1254–1260. doi: 10.1016/S0140-6736(15)60459-7. [DOI] [PubMed] [Google Scholar]
- 4.Diener M.K., Voss S., Jensen K., Buchler M.W., Seiler C.M. Elective midline laparotomy closure: the INLINE systematic review and meta-analysis. Ann Surg. 2010;251(5):843–856. doi: 10.1097/SLA.0b013e3181d973e4. [DOI] [PubMed] [Google Scholar]
- 5.Theodorou A., Banysch M., Gok H., Deerenberg E.B., Kalff J.C., von Websky M.W. Don’t fear the (small) bite: a narrative review of the rationale and misconceptions surrounding closure of abdominal wall incisions. Front Surg. 2022;9:1002558. doi: 10.3389/fsurg.2022.1002558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nilsson J.H., Strandberg Holka P., Sturesson C. Incisional hernia after open resections for colorectal liver metastases - incidence and risk factors. HPB (Oxford) 2016;18(5):436–441. doi: 10.1016/j.hpb.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baucom R.B., Ousley J., Beveridge G.B., Phillips S.E., Pierce R.A., Holzman M.D., et al. Cancer survivorship: defining the incidence of incisional hernia after resection for intra-abdominal malignancy. Ann Surg Oncol. 2016;23(Suppl. 5):764–771. doi: 10.1245/s10434-016-5546-z. [DOI] [PubMed] [Google Scholar]
- 8.Aiolfi A., Cavalli M., Gambero F., Mini E., Lombardo F., Gordini L., et al. Prophylactic mesh reinforcement for midline incisional hernia prevention: systematic review and updated meta-analysis of randomized controlled trials. Hernia. Apr 2023;27(2):213–224. doi: 10.1007/s10029-022-02660-4. [DOI] [PubMed] [Google Scholar]
- 9.Seiler C.M., Bruckner T., Diener M.K., Papyan A., Golcher H., Seidlmayer C., et al. Interrupted or continuous slowly absorbable sutures for closure of primary elective midline abdominal incisions: a multicenter randomized trial (INSECT: ISRCTN24023541) Ann Surg. 2009;249(4):576–582. doi: 10.1097/SLA.0b013e31819ec6c8. [DOI] [PubMed] [Google Scholar]
- 10.Muysoms F.E., Antoniou S.A., Bury K., Campanelli G., Conze J., Cuccurullo D., et al. European hernia society guidelines on the closure of abdominal wall incisions. Hernia. 2015;19(1):1–24. doi: 10.1007/s10029-014-1342-5. [DOI] [PubMed] [Google Scholar]
- 11.Yheulon C., Davis S.S., Jr. Adopting the STITCH trial: crossing the chasm from publication to practice. JAMA Surg. 2019;154(12):1087–1088. doi: 10.1001/jamasurg.2019.3358. [DOI] [PubMed] [Google Scholar]
- 12.Claes K., Beckers R., Heindryckx E., Kyle-Leinhase I., Pletinckx P., Claeys D., et al. Retrospective observational study on the incidence of incisional hernias after colorectal carcinoma resection with follow-up CT scan. Hernia. 2014;18(6):797–802. doi: 10.1007/s10029-014-1214-z. [DOI] [PubMed] [Google Scholar]
- 13.Collaborative H. Incisional hernia following colorectal cancer surgery according to suture technique: Hughes Abdominal Repair Randomized Trial (HART) Br J Surg. 2022;109(10):943–950. doi: 10.1093/bjs/znac198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davey S., Rajaretnem N., Harji D., Rees J., Messenger D., Smart N.J., et al. Incisional hernia formation in hepatobiliary surgery using transverse and hybrid incisions: a systematic review and meta-analysis. Ann R Coll Surg Engl. 2020;102(9):663–671. doi: 10.1308/rcsann.2020.0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.K. I., Yokoyama Y., Sugawara G., Kubota H., Tojima Y., Kurumiya Y., et al. Incidence of and risk factors for incisional hernia after abdominal surgery. Br J Surg. 2014;101(11) doi: 10.1002/bjs.9600. [DOI] [PubMed] [Google Scholar]
- 16.P. A.L., Klein M., Gögenur I., Rosenberg Incisional hernia after open versus laparoscopic sigmoid resection. Surg Endosc. 2008;22(9) doi: 10.1007/s00464-008-9924-x. [DOI] [PubMed] [Google Scholar]
- 17.Bickenbach K.A., Karanicolas P.J., Ammori J.B., Jayaraman S., Winter J.M., Fields R.C., et al. Up and down or side to side? A systematic review and meta-analysis examining the impact of incision on outcomes after abdominal surgery. Am J Surg. 2013;206(3):400–409. doi: 10.1016/j.amjsurg.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Halm J.A., Lip H., Schmitz P.I., Jeekel J. Incisional hernia after upper abdominal surgery: a randomised controlled trial of midline versus transverse incision. Hernia. 2009;13(3):275–280. doi: 10.1007/s10029-008-0469-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorfman S., Rincon A., Shortt H. Cholecystectomy via Kocher incision without peritoneal closure. Invest Clin. 1997;38(1):3–7. [PubMed] [Google Scholar]
- 20.Sanz-Lopez R., Martinez-Ramos C., Nunez-Pena J.R., Ruiz de Gopegui M., Pastor-Sirera L., Tamames-Escobar S. Incisional hernias after laparoscopic vs open cholecystectomy. Surg Endosc. 1999;13(9):922–924. doi: 10.1007/s004649901135. [DOI] [PubMed] [Google Scholar]
- 21.Donataccio M., Genco B., Donataccio D. Right subcostal incision in liver transplantation: prospective study of feasibility. Transplant Proc. 2006;38(4):1109–1110. doi: 10.1016/j.transproceed.2006.03.044. [DOI] [PubMed] [Google Scholar]