Highlights
-
•
Altered brain connectivity is associated with pain phenotype and severity.
-
•
Neuropathic and nociceptive pain display unique functional connectivity differences.
-
•
Intralimbic and limbostriatal networks may contribute to neuropathic pain severity.
Keywords: Neuropathic pain, Nociceptive pain, Resting state fMRI, Functional connectivity, Spinal cord injury
Abstract
Many individuals with spinal cord injury live with debilitating chronic pain that may be neuropathic, nociceptive, or a combination of both in nature. Identification of brain regions demonstrating altered connectivity associated with the type and severity of pain experience may elucidate underlying mechanisms, as well as treatment targets. Resting state and sensorimotor task-based magnetic resonance imaging data were collected in 37 individuals with chronic spinal cord injury. Seed-based correlations were utilized to identify resting state functional connectivity of regions with established roles in pain processing: the primary motor and somatosensory cortices, cingulate, insula, hippocampus, parahippocampal gyri, thalamus, amygdala, caudate, putamen, and periaqueductal gray matter. Resting state functional connectivity alterations and task-based activation associated with individuals’ pain type and intensity ratings on the International Spinal Cord Injury Basic Pain Dataset (0–10 scale) were evaluated. We found that intralimbic and limbostriatal resting state connectivity alterations are uniquely associated with neuropathic pain severity, whereas thalamocortical and thalamolimbic connectivity alterations are associated specifically with nociceptive pain severity. The joint effect and contrast of both pain types were associated with altered limbocortical connectivity. No significant differences in task-based activation were identified. These findings suggest that the experience of pain in individuals with spinal cord injury may be associated with unique alterations in resting state functional connectivity dependent upon pain type.
1. Introduction
Injury to the spinal cord results in de-afferentation of sensory and motor pathways rostral and caudal to the injury level, with evidence of brain somatomotor atrophy and alterations in network function. Chronic pain affects as many as four out of five individuals with chronic spinal cord injury (SCI) and negatively impacts participation in daily tasks, life satisfaction, and overall health and wellbeing (Felix et al., 2021). The primary chronic pain complaints reported by persons with spinal cord injury (PwSCI) are nociceptive (affects 49%) and neuropathic (affects 56%) in nature (Felix et al., 2021). Nociceptive pain arises from non-neural tissues and often develops due to postural impairments and positioning, compensatory movement patterns, and musculoskeletal overuse injuries during mobility tasks such transfers and wheelchair propulsion in PwSCI (Cardenas and Felix, 2009, Scholz et al., 2019). Nociceptive pain can thus be characterized as a “normal”, albeit disabling consequence of reduced mobility, and is typically managed with modifications to reduce musculoskeletal stresses, physical fitness training, and nonsteroidal anti-inflammatory drugs (NSAIDS) (Cardenas and Felix, 2009). Neuropathic pain stems from poorly understood maladaptive responses to peripheral and central de-afferentation after neural injury and tends to be more severe in intensity and refractory to treatment in most PwSCI (Kosek et al., 2016, Melzack and Loeser, 1978). Current pharmacologic treatment strategies for neuropathic pain consist of gabapentinoids, tricyclic antidepressants, and serotonin norepinephrine reuptake inhibitors, which offer limited efficacy (Bates et al., 2019, Guy et al., 2016). Opioids are not recommended as a first-line therapy for pain after SCI but are still commonly prescribed and often at high dosages as a last resort.
Limitations in pain management support the need for a better understanding of brain mechanisms contributing to nociceptive and neuropathic pain processing for the development of more efficacious treatments. Prior volumetric, diffusion, biochemical, and functional connectivity differences have been demonstrated between individuals with chronic neuropathic pain after SCI and those without pain or healthy controls, with some overlap of regions of interest, but no clear brain phenotype has emerged across studies (Huynh et al., 2021, Huynh et al., 2019, Li et al., 2020b, Solstrand Dahlberg et al., 2018). The majority of existing pain literature in PwSCI has focused on structural differences with reported alterations of gray matter volume of the primary somatosensory and motor cortices (Jutzeler et al., 2016, Mole et al., 2014), anterior cingulate cortex (Jutzeler et al., 2016), thalamus (Jutzeler et al., 2016) and insula (Yoon et al., 2013) in PwSCI with neuropathic pain compared to those without pain or healthy individuals. Alterations in mean diffusivity of the thalamus, amygdala, and insula (Gustin et al., 2010), as well as biochemical differences in the thalamus (Gustin et al., 2014, Pattany et al., 2002, Widerström-Noga et al., 2015), anterior cingulate cortex (Widerström-Noga et al., 2013), and periaqueductal gray matter (Kyathanahally et al., 2021) have also been identified in PwSCI with neuropathic pain compared to those without pain or healthy individuals. Additional regions with established roles in pain processing and reported structural or functional differences in PwSCI compared to healthy individuals include the posterior cingulate cortex (Cermik et al., 2006), basal ganglia (Min et al., 2015), and hippocampus and parahippocampal gyri (Q. Chen et al., 2018). These findings suggest altered neural function of these regions may contribute to the experience of neuropathic pain, yet little is known regarding their functional connectivity in PwSCI.
A limited number of studies reporting alterations in resting state functional connectivity (rsFC) or task-based functional activation associated with neuropathic pain in PwSCI have been performed, with no overlapping results produced between studies (Huynh et al., 2021, Li et al., 2020b), or lack of statistically significant results (Black et al., 2021). Reported alterations in rsFC in PwSCI included limbocerebellar (X. Li et al., 2020), limbocortical (X. Li et al., 2020), and intracortical (Huynh et al., 2021) alterations associated with the presence of neuropathic pain, and intralimbic (X. Li et al., 2020) and thalamocortical (Huynh et al., 2021) rsFC alterations between PwSCI with neuropathic pain compared to healthy individuals. Associations have also been reported between limbocerebellar (X. Li et al., 2020), limbocortical (X. Li et al., 2020), and intralimbic (Huynh et al., 2021) connectivity and neuropathic pain intensity, and limbothalamic connectivity and extent of the spatial distribution of neuropathic pain symptoms (Huynh et al., 2021). In functional activation studies, conflicting results have been reported concerning somatosensory cortex activation and reorganization associated with neuropathic pain presence and intensity (Jutzeler et al., 2015, Wrigley et al., 2009). The lack of overlapping results across rsFC and task-based functional activation studies in this population are likely driven by differences in methodology, the selected seed regions of interest (ROI), and the limited number of studies, along with the diversity of mechanisms, levels, and severity of injuries and symptoms in PwSCI.
Lack of replication of findings and the limited number of rsFC and task-based functional activation studies restrict our understanding of mechanisms contributing to the experience of neuropathic pain and support the need for further investigation of pain-related differences in brain function within the SCI population. Analyses incorporating a wider set of previously reported sensorimotor and limbic seed regions associated with pain processing and SCI could further elucidate pain-related alterations in rsFC and provide improved replicability across studies. Prior studies have typically not accounted for the possible co-occurrence of both neuropathic and nociceptive pain, or for medication use as confounding factors, which may contribute to the discrepancy in results. Cortical reorganization and altered somatosensory cortex activation have been identified in individuals with chronic neuropathic pain, but not chronic nociceptive pain, compared to healthy individuals, which suggests neuropathic and nociceptive pain phenotypes may engage distinct functional connectivity and activation patterns (Widerström-Noga et al., 2015). Similar to experimental activation of a-delta and c-fiber pain, the experience of chronic neuropathic and nociceptive pain may engage both overlapping and segregated neural systems (Bishop et al., 1958, Matre et al., 2010, Veldhuijzen et al., 2009). Moreover, when studying clinical populations, opioids and gabapentin may also limit replicability of findings as these drugs can alter resting state functional connectivity independent of pain relief (Croosu et al., 2021, Gorka et al., 2014, Wanigasekera et al., 2016).
Identification of alterations in rsFC and functional activation associated with specific pain phenotypes, while considering medication effects, could segregate the underlying mechanisms of diverse pain experiences in PwSCI to better inform treatment strategies. The aim of this study is to identify rsFC and functional activation alterations associated uniquely, additively, and non-additively (interactions) with neuropathic and nociceptive pain severity in PwSCI.
2. Materials and methods
2.1. Subjects
This cross-sectional study included PwSCI who were at least three years post injury. Participants were enrolled in a clinical trial assessing changes in bone health (primary outcome) and brain connectivity (secondary outcome) in response to robotic-assisted gait training (ClinicalTrials.gov Identifier: NCT02533713) or in an observational study assessing surgical treatment for severe neuropathic pain (IRB ID 1235452-13). Criteria for enrollment for each study can be found in Table 1. Recruitment strategies included study flyers and clinic and participant referral. Data collection occurred at Craig Hospital and Swedish Medical Center in Englewood, Colorado.
Table 1.
Study criteria.
| Robotic-assisted gait training trial (NCT02533713) |
|---|
| Inclusion criteria |
|
|
|
|
|
|
|
|
| Exclusion criteria |
|
|
|
|
|
|
|
|
|
|
| Surgical intervention study (IRB ID 1235452–13) |
| Inclusion criteria |
|
|
|
| Exclusion criteria: |
|
American Spinal Injury Association Impairment Scale (AIS), spinal cord injury (SCI), Modified Ashworth Scale (MAS), magnetic resonance imaging (MRI).
For all participants, data were derived from baseline testing which occurred between 08/16/2017 and 04/02/2021. All analyses were conducted on de-identified data by a member of the research team not involved in data collection. The study protocols were approved by our Institutional Review Boards and all participants gave their written informed consent to participate. A total of 71 PwSCI were enrolled across both studies. Complete magnetic resonance imaging (MRI) and pain datasets were required for inclusion in this analysis. Out of the full sample, 33 participants were excluded due to unobtainable (n = 31) or poor-quality/motion artifact (n = 2) MRI data resulting in a sample of 37 PwSCI included in this analysis (n = 28 enrolled in NCT02533713, n = 9 enrolled in IRB ID 1235452-13).
2.2. Clinical outcome measures
Neuropathic and nociceptive pain presence and intensity were assessed with the International Spinal Cord Injury Pain Basic Dataset (ISCIPBDS) (Widerström-Noga et al., 2014). The ISCIPBDS collects participant-reported pain outcomes including the description, location, and average weekly intensity of up to their 3 worst pain problems. The ISCIPBDS follows the International Spinal Cord Injury Pain Classification definitions to classify pain as nociceptive or neuropathic, and further classifies neuropathic pain as at-level or below-level SCI pain (Bryce et al., 2012). Example descriptors for classification of neuropathic and nociceptive pain phenotypes can be found in Supplemental Table 1, with full details regarding the ISCIPBDS construct provided in Widerström-Noga et al. (2014). Participants’ worst neuropathic and nociceptive pain intensities were defined as the highest rated average weekly intensity of each pain type on the ISCIPBDS. Pain intensity was rated on a 0–10 scale, with 0 being no pain, and 10 being worst imaginable pain. Individuals who denied neuropathic and/or nociceptive pain were included in analyses with pain intensities of 0. Demographic factors (age, sex), injury characteristics (injury duration, level of injury), and pain medication use were attained from study intake questionnaires. Pain medication use was defined as current use of opioids or gabapentin. American Spinal Injury Association Impairment Scale (AIS) classification was confirmed by physical exam. Clinical outcome measures were collected on the day of (n = 28) or 1–2 days after the scan (n = 9) dependent upon the study protocol.
2.3. Neuroimaging
Data were obtained on a single 3 T Siemens Trio using a 12-channel head coil. The scanning protocol sequences utilized for resting state functional connectivity and task-based analysis consisted of a high resolution structural T1 image (1x1x1 mm), two sets of 6-min-18-s resting state echo planar imaging (EPI) datasets with a repetition time (TR) of 3 s, and one set each of a 4-min-6-sec finger tap and foot tap task EPI dataset with a 3 s TR. T1 weighted structural images were acquired with a gradient echo sequence with GRAPPA parallel imaging with an acceleration factor of 2, 256 mm field of view (FOV), 1x1x1 mm voxel size, 1 mm slice thickness, sagittal acquisition (interleaved), 20 ms echo time (TE), 4.92 ms TR, flip angle of 25 degrees, and 5:17 scan time. Resting state functional images were acquired with an EPI sequence with a 216 mm FOV, 3x3x3 mm voxel size, 3 mm slice thickness, acquisition interleaved, 30 ms TE, 3000 ms TR, flip angle of 85 degrees, 6:18 scan time (2 repeated scans collected in each subject). Finger and foot tap task-based functional images were each acquired with an EPI sequence with a 200 mm FOV, 3.1x3.1x3.1 mm voxel size, 3.1 mm slice thickness, acquisition interleaved, 30 ms TE, 3000 ms TR, flip angle of 90 degrees, 4:06 scan time (1 finger tap and 1 foot tap scan collected in each subject). The tasks were acquired with a blocked design of active finger or imagined foot tapping (onsets 35, 95, 155, and 215 s; durations 30, 30, 30, 30 s) alternated with rest periods (onsets 0, 65, 125, and 185 s; durations 35, 30, 30, 30 s).
2.4. Resting state connectivity analysis
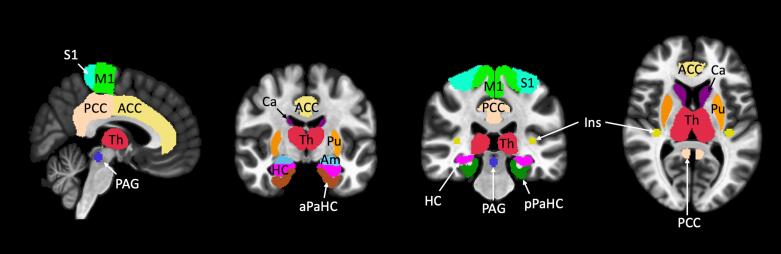
All functional data was preprocessed and statistically analyzed using the CONN Toolbox (version 20b) (Whitfield-Gabrieli and Nieto-Castanon, 2012), a cross-platform software operating under Statistical Parametric Mapping (SPM12) (Friston et al., 1994) and MATLAB (version R2020b, The Mathworks Inc., Natick, MA). Preprocessing was completed using default parameters, including slice timing, motion correction, spatial normalization to the Montreal Neurological Institute (MNI) template, spatial smoothing with an 8-mm Gaussian kernel, and high-pass temporal filtering (cutoff 128 s). The Artifact Detection Toolbox (https://www.nitrc.org/projects/artifact_detect) was used to detect frames with excessive motion (global signal value z > 5, interscan motion > 0.9 mm), which were regressed out of the time-series in CONN’s denoising pipeline in addition to white matter and cerebrospinal fluid signal using aCompCor. Seed-based correlations were utilized to identify brain regions correlated with seed regions of interest. Main analyses consisted of 4 linear regressions conducted to identify alterations in rsFC associated with: 1) worst neuropathic pain intensity (model included 3 regressors controlling for gabapentin use, opioid use, and worst nociceptive pain intensity), 2) worst nociceptive pain intensity (model included 3 regressors controlling for gabapentin use, opioid use, and worst neuropathic pain intensity), 3) the joint effect of each participant’s worst neuropathic and nociceptive pain intensities (model included 2 regressors controlling for gabapentin and opioid use), and 4) the contrast of worst neuropathic and nociceptive pain intensities (model included 2 regressors controlling for gabapentin and opioid use). All analyses controlled for current pain medication use utilizing separate binary regressors for gabapentin and opioids. For the isolated neuropathic and nociceptive analyses (regression models 1 and 2), the intensity of the opposite pain type was included as a covariate of no interest. Supplemental analyses included 1) the effect of gabapentin use (model included 3 regressors controlling for opioid use, and worst neuropathic and nociceptive pain intensities) and 2) the effect of opioid use (model included 3 regressors controlling for gabapentin use, and worst neuropathic and nociceptive pain intensities). 21 seed ROIs were selected based on prior literature supporting their role in pain processing and previously reported neuroimaging differences in PwSCI and consisted of the lower body representation of the primary motor and somatosensory cortices (Bolwerk et al., 2013, Iwabuchi et al., 2023, Jutzeler et al., 2016, Li et al., 2020a, Mole et al., 2014, Zhang et al., 2014, Zhou et al., 2018), anterior (Huynh et al., 2021, Iwabuchi et al., 2023, Jutzeler et al., 2016, Pascoal-Faria et al., 2015, Zhou et al., 2018) and posterior cingulate (Cermik et al., 2006, Freund et al., 2010, Huynh et al., 2021, Keltner et al., 2017), insula (Gustin et al., 2010, Huynh et al., 2021, Iwabuchi et al., 2023, Li et al., 2020b, Zhou et al., 2018), hippocampus (Q. Chen et al., 2018, Iwabuchi et al., 2023, Li et al., 2020b, Ruscheweyh et al., 2018, Vachon-Presseau et al., 2013), parahippocampal gyri (Q. Chen et al., 2018, Ruscheweyh et al., 2018, Vachon-Presseau et al., 2013, Zhang et al., 2014), thalamus (Gustin et al., 2010, Huynh et al., 2021, Iwabuchi et al., 2023, Li et al., 2020a, Zhang et al., 2014, Zhou et al., 2018), amygdala (Gustin et al., 2010, Huynh et al., 2021), caudate (B. Lee et al., 2022, Min et al., 2015, Seixas et al., 2016, Sprenger et al., 2015), putamen (Iwabuchi et al., 2023; B. Lee et al., 2022, Min et al., 2015, Ruscheweyh et al., 2018, Sprenger et al., 2015, Zhang et al., 2014), and the periaqueductal gray matter (Kyathanahally et al., 2021, Li et al., 2020a, Linnman et al., 2012, Sprenger et al., 2015) (Supplemental Fig. 1). Right and left seeds were included for all bilateral structures (note that the medial somatosensory and motor cortex representations of the lower body were analyzed as one midline ROI). Seed ROIs were defined from the Conn Toolbox atlas, except for the insula, somatosensory and motor, and periaqueductal gray matter ROIs. Somatosensory and motor seed ROIs were defined as the intersection of the Conn toolbox defined superior sensorimotor network and the precentral or postcentral gyri respectively. The posterior insula seed was derived from MNI coordinates for the lower leg representation region of the insula as per Bjornsdotter et al. (-36, −25, 8) with a 5 mm spherical radius (Björnsdotter et al., 2009). The periaqueductal gray matter seed ROI was defined from MNI coordinates previously reported by Linnman et al. (1, −29, −12) with a 5 mm spherical radius (Linnman et al., 2012). Significance level was set at p < 0.0024 family-wise error (FWE) corrected, based on Bonferroni correction for multiple comparisons across all seed ROIs (i.e. p < 0.05/21 comparisons). Additionally, we required a minimum of 10 contiguous voxels to consider a cluster significant. The cluster-forming height threshold was p < 0.001, as recommended by Roiser et al. (2016).
2.5. Task-based functional MRI analyses
The finger and foot tap task paradigms were preprocessed in CONN and modeled in SPM12 using a general linear model with boxcar regressors signifying the task onsets and durations (4 sets of 30 s tapping blocks) convolved with the SPM12 canonical hemodynamic response function. The model also included 6 motion regressors and outliers detected by CONN’s Artifact Detection Toolbox. First-level contrast images of finger and foot tapping responses were obtained for each subject and modeled in a second-level analysis to determine the effect of pain phenotype and intensity on task-based functional activation. The same 4 linear regression models applied to the resting-state data were applied to the task data to assess the effects of 1) neuropathic pain intensity, 2) nociceptive pain intensity, 3) the joint effect of neuropathic and nociceptive pain intensities, and 4) the contrast of neuropathic and nociceptive pain intensities, with all models controlling for pain medication use. Significance was set at a voxel-wise cluster height threshold of p < 0.001, and a critical cluster threshold of p < 0.05 FWE-corrected for multiple comparisons.
2.6. Descriptive statistical analyses
Descriptive statistics of demographic variables included percentages, mean, range, and standard deviation. Normality of data distributions was assessed with Shapiro-Wilk goodness-of-fit tests. Associations between pain intensities and continuous variables were determined with Kendall’s Tau correlation and Wilcoxon Rank-Sum and Kruskal-Wallis tests for categorical variables. All analyses were conducted using JMP version 16 (SAS Institute Inc., Cary, NC) at p < 0.05.
3. Results
3.1. Cohort characteristics
Of the 37 PwSCI in our sample, 70.3% (n = 26) reported neuropathic pain, 45.9% (n = 17) reported nociceptive pain, and 16.2% (n = 6) reported no pain. Specific pain presentations included only neuropathic pain 37.8% (n = 14), only nociceptive pain 13.5% (n = 5), and both neuropathic and nociceptive pain 32.4% (n = 12). In those with each pain type, average neuropathic pain intensity (5.92 ± 2.35, range 2–10) was higher than average nociceptive pain intensity (4.71 ± 1.61, range 2–8). Of the 26 participants with neuropathic pain, 8 were classified as at-level and 18 as below-level SCI pain. Additional data regarding pain type, severity, and location and medication use for each participant can be found in Supplemental Table 2. 13 participants (35.1%) utilized opioids and/or gabapentin for pain management at the time of assessment. Additional demographic data are presented in Table 2.
Table 2.
Participant characteristics.
| Variable | Total (n = 37) | Neuropathic Pain Intensity | Nociceptive Pain Intensity |
|---|---|---|---|
| Demographics | |||
| Age (years) | 41.24 ± 13.48 | τ = 0.13, p = 0.29 | τ = -0.03, p = 0.84 |
| Male | 30 (81.1) | W = 117, p = 0.53 | W = 137, p = 0.88 |
| Pain Phenotype | |||
| No pain | 6 (16.2) | – | – |
| Neuropathic pain | 26 (70.3) | – | – |
| Only neuropathic pain | 14 (37.8) | – | – |
| Nociceptive pain | 17 (45.9) | – | – |
| Only nociceptive pain | 5 (13.5) | – | – |
| Neuropathic + Nociceptive Pain | 12 (32.4) | – | – |
| Injury Characteristics | |||
| Injury duration (years) | 10.17 ± 8.05 | τ = 0.06, p = 0.62 | τ = 0.17, p = 0.17 |
| Tetraplegia | 5 (13.5) | W = 74, p = 0.35 | W = 107, p = 0.58 |
AIS classification*
|
26/36 (72.2) 6/36 (16.7) 4/36 (11.1) |
H(2) = 2.82, p = 0.24 | H(2) = 0.65, p = 0.72 |
| Active Medication Use | |||
| Opioid | 10 (27.0) | W = 235, p = 0.12 | W = 209, p = 0.50 |
| Gabapentin | 5 (13.5) | W = 144, p = 0.03 | W = 76, p = 0.37 |
| ISCIPBDS Pain Ratings | |||
| Worst neuropathic | 5.92 ± 2.35 | – | τ = 0.04, p = 0.78 |
| Worst nociceptive | 4.71 ± 1.61 | τ = 0.04, p = 0.78 | – |
Continuous variables are presented as mean ± SD and categorical variables as N (%). *Data provided includes the full sample of participants (n = 37) except for AIS classification (n = 36). American Spinal Injury Association Impairment Scale (AIS), International Spinal Cord Injury Pain Basic Dataset (ISCIPBDS). Associations of participant characteristics with worst neuropathic and nociceptive pain intensities rated on the ISCIPBDS are reported from Kendall’s Tau (τ) for continuous variables and Wilcoxon Rank-Sum (W) or Kruskal-Wallis (H) tests for categorical variables.
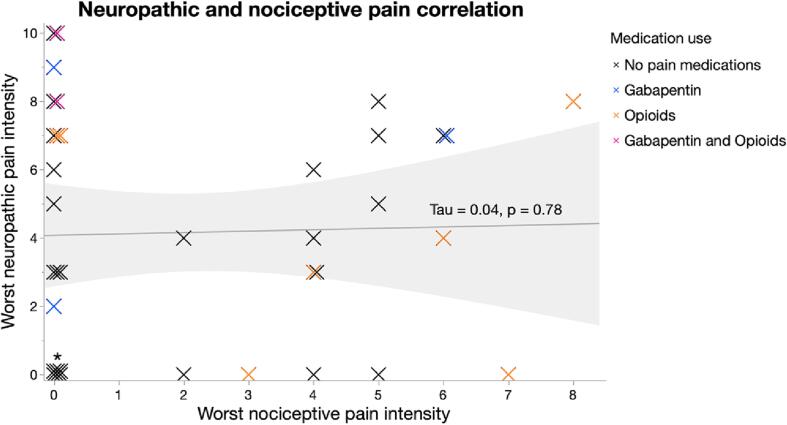
For the analysis attempting to segregate neuropathic and nociceptive pain, no significant correlation between neuropathic and nociceptive pain ratings was identified in the total sample (τ = 0.04, p = 0.78) (Fig. 1). However, in the subgroup of participants with both pain types there was a significant positive correlation between neuropathic and nociceptive pain ratings (τ = 0.54, p = 0.03).
Fig. 1.
Correlation between neuropathic and nociceptive pain intensities. No significant correlation of neuropathic and nociceptive pain severities was identified (τ = 0.04, p = 0.78) in participants with chronic SCI (n = 37). Individual data points reflect participants’ neuropathic pain ratings plotted against their nociceptive pain ratings. Participants are defined as currently taking gabapentin (blue), opioids (orange), both medications (purple), or no medications (black). *Signifies cluster of 6 data points with intensity ratings of 0 for both pain types. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Resting state functional connectivity and task-based functional activation
3.2.1. MRI data quality
All 37 participants were included in rsFC and finger tap functional activation analyses, with 1 participant excluded from the foot tap task analysis due to missing data. No participants required exclusion due to excess motion artifact. An average of 11 ± 18 of a total of 248 resting state frames, 8 ± 13 of a total of 80 finger tap frames, and 10 ± 15 of a total of 80 foot tap frames per participant were regressed out of the model during denoising due to motion that exceeded the framewise displacement cutoff.
3.2.2. Effect of neuropathic pain severity
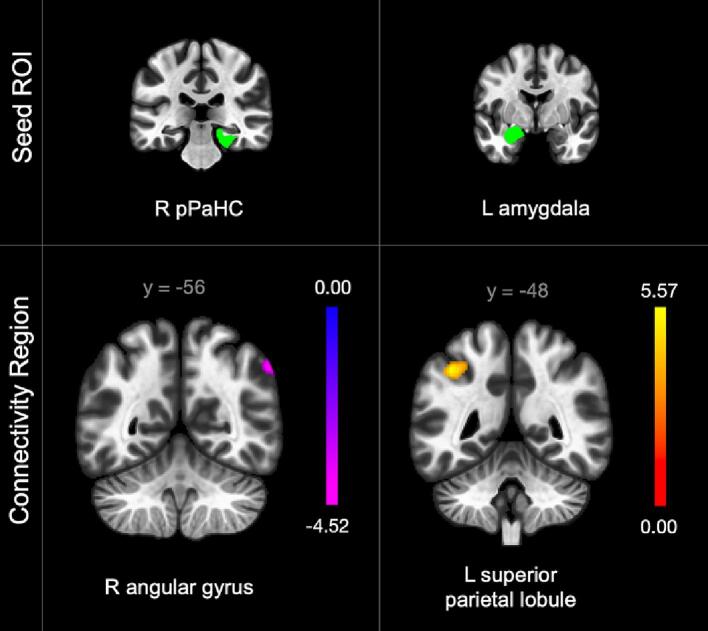
Neuropathic pain severity was associated with intralimbic and limbostriatal connectivity pattern alterations. In the linear regression assessing the effect of worst neuropathic pain intensity on rsFC — while controlling for worst nociceptive pain severity and medication use — higher neuropathic pain severity was significantly associated with lower connectivity between the right posterior parahippocampal gyrus (pPaHC) to the right putamen and amygdala (Fig. 2). Results from all regression models and a summary of connectivity patterns associated with pain phenotypes can be found in Table 3, Table 4 respectively.
Fig. 2.
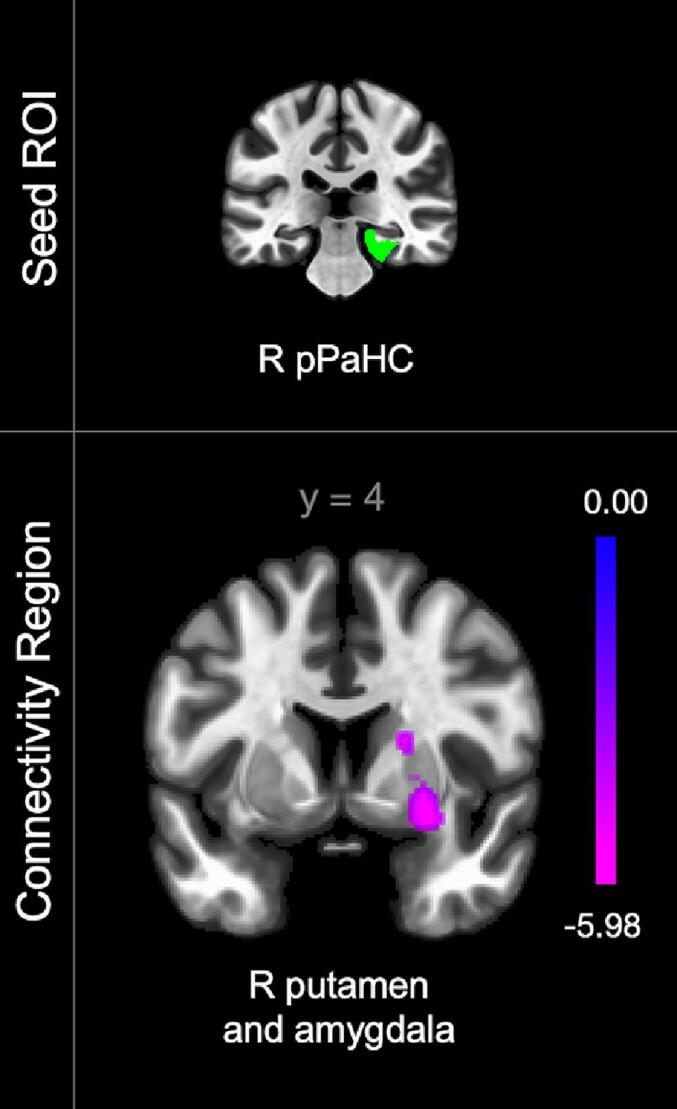
Connectivity alterations associated with neuropathic pain severity in participants with chronic SCI (n = 37). Color bar indicates connectivity strength with purple indicating lowest connectivity value. Posterior parahippocampal gyrus (pPaHC). Laterality indicated by right (R) or left (L). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Connectivity alterations associated with pain severity.
| Seed | Resulting regions | MNI X | MNI Y | MNI Z | Cluster size | T | p-FWE |
|---|---|---|---|---|---|---|---|
| Effect of Neuropathic Pain Severity | |||||||
| Parahippocampal gyrus (posterior division R) | R putamen and amygdala | 26 | 4 | −10 | 366 | −6.41 | 0.0002 |
| Effect of Nociceptive Pain Severity | |||||||
| Thalamus L | R hippocampus, temporal fusiform cortex (anterior and posterior divisions R), inferior temporal gyrus (anterior division R), R amygdala, parahippocampal gyrus (anterior division R) | 38 | −14 | −18 | 379 | −7.29 | 0.0005 |
| Joint Effect of Neuropathic and Nociceptive Pain Severities | |||||||
| Parahippocampal gyrus (posterior division R) | R angular gyrus, lateral occipital cortex (superior division R), supramarginal gyrus (posterior division R) | 56 | −56 | 44 | 276 | −4.94 | 0.0016 |
| Amygdala L | L superior parietal lobule, supramarginal gyrus (posterior and anterior divisions L) | −38 | −50 | 46 | 319 | 6.21 | 0.0007 |
| Contrast of Neuropathic and Nociceptive Pain Severities | |||||||
| Cingulate gyrus (posterior division) | Intracalcarine cortex R and L, lingual gyrus R and L, cuneal cortex R and L, supracalcarine cortex R and L, cerebellum 6 R, occipital pole L | 14 | −74 | 18 | 1422 | −5.39 | <0.000001 |
Positive and negative T-values reflect positive and negative correlation between neuropathic pain severity and connectivity respectively (in the contrast of neuropathic and nociceptive pain severities, negative T-value reflects negative and positive correlation between neuropathic and nociceptive pain severity and connectivity respectively). Laterality is defined as right (R) and left (L). Montreal Neurological Institute (MNI) system coordinates for resulting regions are provided. Significance level was set at p < 0.0024 after family-wise error (FWE) level of p < 0.05 and additional Bonferroni correction for multiple comparisons across all seed ROIs (21 comparisons) and a minimum of 10 contiguous voxels in resulting clusters.
Table 4.
Connectivity patterns associated with pain phenotypes and severity.
| Pain Phenotype and Severity | Connectivity Pattern Alterations |
|---|---|
| ↑ Neuropathic pain intensity | ↓ Intralimbic and limbostriatal |
| ↑ Nociceptive pain intensity | ↓ Thalamocortical and Thalamolimbic |
| ↑ Neuropathic + nociceptive pain intensities | ↑ and ↓ Limbocortical (↓ pPaHC – parietal; ↑ amygdala - parietal) |
| Neuropathic > nociceptive pain intensity | ↓ Limbocortical |
| Neuropathic < nociceptive pain intensity | ↑ Limbocortical |
Summary of connectivity pattern alterations associated with pain phenotypes and severity. Full results and specific regions can be found in Table 3. Posterior parahippocampal gyrus (pPaHC).
3.2.3. Effect of nociceptive pain severity
Severity of nociceptive pain was associated with alterations in thalamocortical and thalamolimbic connectivity patterns. In the linear regression assessing the effect of worst nociceptive pain severity on rsFC — while controlling for worst neuropathic pain severity and medication use —higher nociceptive pain severity was significantly associated with lower connectivity of the left thalamus to the right hippocampus, amygdala, anterior parahippocampal gyrus (aPaHC), temporal fusiform cortex (anterior and posterior divisions), and inferior temporal gyrus (anterior division) (Fig. 3).
Fig. 3.
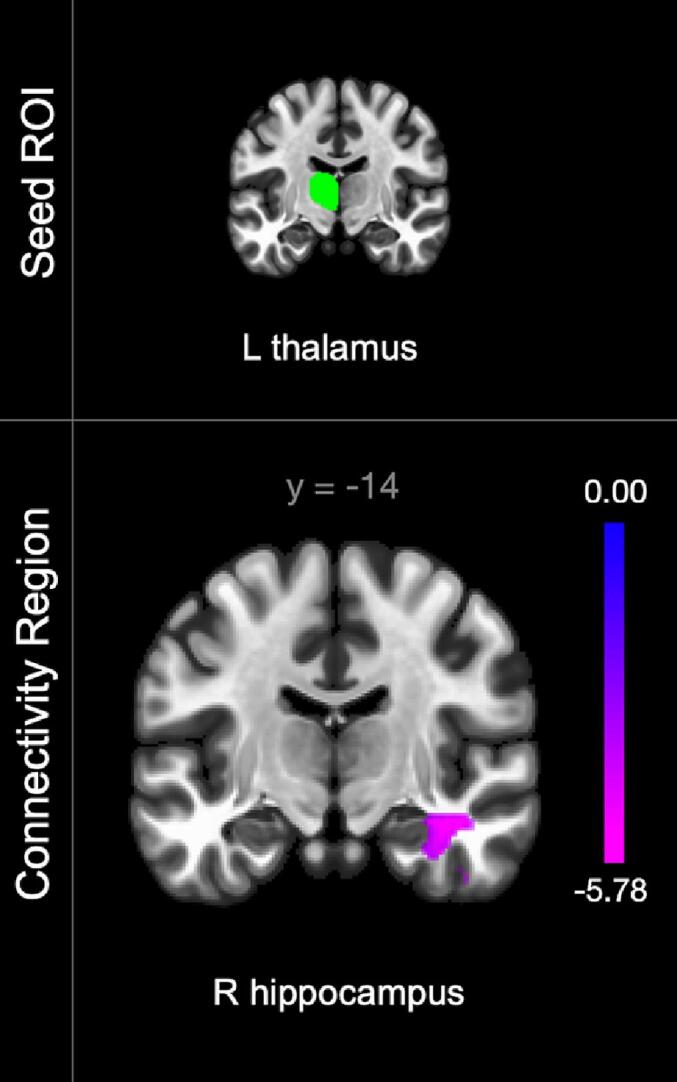
Connectivity alterations associated with nociceptive pain severity in participants with chronic SCI (n = 37). Color bars indicate connectivity strength with purple indicating lowest connectivity values. Laterality indicated by right (R) or left (L). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.4. Joint effect of neuropathic and nociceptive pain severities
The combined effect of neuropathic and nociceptive pain intensities was associated with limbocortical connectivity pattern alterations. In the linear regression assessing the effect of the joint effect of each participant’s neuropathic and nociceptive pain severities on rsFC — while controlling for medication use — significantly altered connectivity was identified between 2 seeds and 2 clusters (Fig. 4). Higher neuropathic and nociceptive pain intensities were significantly associated with 1) lower connectivity between the right pPaHC to right parietal and occipital cortical regions and 2) higher connectivity between the left amygdala to the left superior parietal lobule and supramarginal gyrus.
Fig. 4.
Connectivity alterations associated with additive neuropathic and nociceptive pain severities in participants with chronic SCI (n = 37). Color bars indicate connectivity strength with yellow indicating highest connectivity values and purple indicating lowest connectivity values. Posterior parahippocampal gyrus (pPaHC). Laterality indicated by right (R) or left (L). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.5. Contrast of neuropathic and nociceptive pain severities
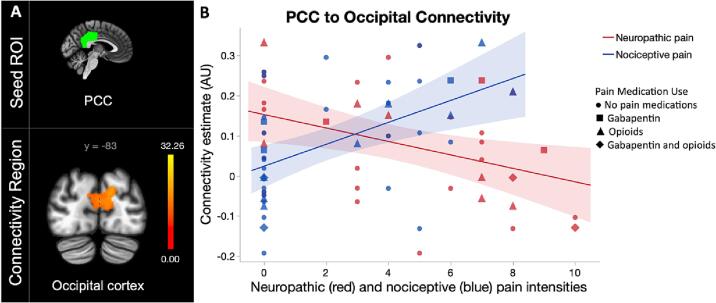
The contrast of neuropathic and nociceptive pain intensities was also associated with limbocortical connectivity pattern alterations. In the linear regression assessing the difference between neuropathic and nociceptive pain severities on rsFC — while controlling for medication use —, connectivity was negatively correlated with neuropathic pain severity and positively correlated with nociceptive pain severity between the posterior cingulate to bilateral occipital regions, bilateral lingual gyri, and left cerebellum (Fig. 5). The cerebellar contribution was 4% (50 voxels) of the total cluster size (1395 voxels) and may be due to a partial volume effect. As such, potential cerebellar involvement should be interpreted with caution.
Fig. 5.
Contrast of neuropathic and nociceptive pain severities in participants with chronic SCI (n = 37). Contrast of both pain intensities identified A) differential connectivity between of the posterior cingulate cortex (PCC) and occipital cortex dependent upon pain type. Color bars indicate connectivity strength and correspond to greater connectivity associated with higher neuropathic pain and lower nociceptive pain intensities, with yellow indicating highest connectivity values. B) Scatter plot representing differences in connectivity alterations associated with neuropathic (red) and nociceptive (blue) pain intensities identified between the PCC to the occipital cortex. The shape of the data points delineates participant medication use defined as no pain medications (circle), gabapentin (square), opioids (triangle), or gabapentin and opioids (diamond). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2.6. Supplemental rsFC analyses assessing effects of pain medications
Supplemental rsFC analyses determining the effects of opioids and gabapentin were performed to assess adequate control for the effect of medication in the primary analyses of pain phenotype and severity. Opioid use was associated with altered connectivity of 4 seeds and 4 clusters, with no overlap identified across findings from the primary pain analyses (Supplemental Table 3). In the supplemental linear regression assessing the effect of opioid use on rsFC — while controlling for gabapentin use and neuropathic and nociceptive pain severity —, opioid use was significantly associated with lower connectivity between the 1) right caudate to the bilateral occipital cortex, 2) right putamen to left occipital cortex, and 3) left amygdala to left occipital cortex, and higher connectivity between the 4) right insula to the right precentral and postcentral gyri. No significant alterations in functional connectivity were identified in the supplemental analysis assessing the effect of gabapentin use (Supplemental Table 4).
3.2.7. Task-based functional activation
While the finger tap and foot tap paradigms generated the expected activations of somatotopically aligned somatosensory and motor cortex regions, analyses assessing the effect of pain phenotype and intensity on sensorimotor task-based functional activation yielded no significant findings. No significant differences in functional activation were identified in the finger or foot tap conditions associated with the effect of neuropathic or nociceptive pain intensity, or the joint-effect or contrast of both pain intensities.
4. Discussion
This study aimed to identify rsFC alterations associated uniquely, additively, and non-additively with neuropathic and nociceptive pain severity in PwSCI. We found specific patterns of rsFC alterations associated with pain phenotype and severity in regions involved in sensory and emotional function, suggestive of pain engaging different pathways which uniquely contribute to neuropathic, nociceptive, and additive pain experiences. To our knowledge, this is the first report of differential rsFC alterations based on neuropathic and nociceptive pain phenotypes in PwSCI, or any other clinical population.
4.1. Neuropathic pain is associated with intralimbic and limbostriatal connectivity alterations
Functional connectivity alterations associated with neuropathic pain severity were isolated to intralimbic and limbostriatal patterns, with lower connectivity identified between the right posterior parahippocampal gyrus and right putamen and amygdala. Prior studies suggest the parahippocampal gyrus and amygdala contribute to psychological and emotional regulation of pain processing (Allen et al., 2021, Meerwijk et al., 2013, Naor et al., 2020) and sensitivity (Grant et al., 2010, Meerwijk et al., 2013, Naor et al., 2020), and have been implicated in other populations with neuralgia (Geha et al., 2007, Tang et al., 2021, Zhang et al., 2018). The putamen, a part of the striatum and basal ganglia, is involved with production of movement, reward, and multisensory integration of noxious and non-noxious stimuli, with previously demonstrated pain-related activation hypothesized to be involved in motor responses for withdrawal from painful stimuli or inhibition of painful movement (Bingel et al., 2002, Borsook et al., 2010, Chudler and Dong, 1995). Alterations in R2* signal, reflective of iron content, in the parahippocampal gyrus and basal ganglia were previously identified in individuals with SCI with neuropathic pain (Kyathanahally et al., 2021), which demonstrates overlap with our identified alterations in the BOLD signal between the parahippocampal gyrus and putamen. A previously identified negative correlation between neuropathic pain severity in individuals with SCI and mean diffusivity of the amygdala suggest microstructural changes could contribute to our findings of altered amygdala connectivity (Gustin et al., 2014). Similar to our findings, increased intralimbic connectivity in PwSCI with neuropathic pain was previously reported by Li et al. (X. Li et al., 2020). Although Li et al. identified altered connectivity between the insula and hippocampus between PwSCI and neuropathic pain and healthy individuals, our complimentary findings offer replicability of intralimbic connectivity pattern differences associated with neuropathic pain in PwSCI.
In contrast, prior studies have identified biochemical differences (Gustin et al., 2014, Pattany et al., 2002, Stanwell et al., 2010, Widerström-Noga et al., 2015) as well as reduced blood flow in the thalamus associated with neuropathic pain after SCI (Gustin et al., 2014). Stronger intracortical (Huynh et al., 2021) and limbocortical (X. Li et al., 2020) rsFC have been reported in PwSCI with neuropathic pain compared to those without pain, as well as stronger thalamocortical connectivity between PwSCI with neuropathic pain and healthy individuals (Huynh et al., 2021). Here, no rsFC alterations were identified in the thalamus or regions classically associated with the spinothalamic tract, suggesting neuropathic pain severity after SCI may be mediated by neuroplastic dysfunction in other pathways, or arise from imbalance in thalamic inhibition by deafferentation. Potential pathways of interest include the spinoreticular, spinomesencephalic, and spinoparabrachial tracts and the sympathetic chain. Afferent tracts such as the spinoreticular, spinomesencephalic, and spinoparabrachial tracts have reported spinal cord projections terminating in limbic, motor, and pain modulatory regions and may contribute to pain processing without directly relaying in the thalamus (Chiang et al., 2020, Willis and Westlund, 1997). Emerging literature suggests the spinoparabrachial pathway may be of particular importance as the parabrachial nucleus located in the brainstem holds a key role in neuropathic pain modulation (Sun et al., 2020). The parabrachial nucleus has been shown to uniquely modulate neuropathic pain, with known projections to the amygdala as well as a role in autonomic function (Chiang et al., 2020).
The spinoparabrachial tract’s involvement in autonomic function could link it to another proposed mechanism of neuropathic pain signaling after SCI via the sympathetic chain, which conveys visceral afferent and efferent information throughout the body (Mansour and Kulesza, 2021). Individuals with complete spinal cord transection with intractable neuropathic pain achieved relief of symptoms after lesioning of hyperactive dorsal root entry zones caudal to the level of cord transection (Falci et al., 2018). Accordingly, it has been suggested that neuropathic pain signaling may be transmitted from below lesion hyperactive dorsal root entry zones bypassing the site of cord injury to cephalad regions through the sympathetic chain and aberrant c-fiber sprouting (Falci et al., 2018).
The spinoreticular and spinomesencephalic tracts could also support mechanisms of neuropathic pain through contributions to limbic and striatal regions without direct thalamic engagement. The spinoreticular tract terminates in the reticular formation, which subsequently projects to limbic structures, and is believed to contribute to emotional and motivational aspects of pain processing (Willis and Westlund, 1997). The spinomesencephalic tract holds a demonstrated role in nociception with known projections to midbrain motor regions, and may plausibly contribute to putamen function in neuropathic pain conditions (Willis and Westlund, 1997). Additional preclinical studies assessing the specific projections of proposed non-thalamic pain pathways and their association with neuropathic pain presence and severity are needed to further elucidate their potential contributions.
Though the mechanisms underlying our results are yet unknown, negative associations between neuropathic pain severity and intralimbic and limbostriatal connectivity are suggestive of pain modulatory function, with lower connectivity resulting in higher pain states. Taken together, these findings suggest dysregulation of intralimbic and limbostriatal functional connectivity could be key components in the modulation of neuropathic pain severity after SCI. Further imaging studies, such as positron emission tomography or magnetic resonance spectroscopy, could identify biochemical alterations which may contribute to altered functional connectivity of these regions.
4.2. Nociceptive pain is associated with thalamocortical and thalamolimbic rsFC alterations
Functional connectivity alterations uniquely associated with nociceptive pain severity were isolated to thalamocortical and thalamolimbic patterns. The rsFC presentation of nociceptive pain supports the more traditionally understood transduction of pain signaling via the spinothalamic tract. The spinothalamic tract afferently relays noxious stimuli from the spinal cord to thalamus, a sensory relay center which subsequently projects to cortical regions involved in sensory processing and limbic regions involved in affective aspects of pain perception (De Ridder et al., 2022, Willis and Westlund, 1997). As such, nociceptive signaling via the spinothalamic tract likely contributes to our findings of altered rsFC associated with nociceptive pain severity in thalamic, cortical, and limbic regions.
Prior work has robustly demonstrated nociceptive pain-related thalamic and limbic activation (Apkarian et al., 2005). Importantly, differences in pain processing have been reported in acute versus chronic pain states, with decreased activation of the thalamus and increased activation of limbic regions compared to acute pain conditions (Apkarian et al., 2005, Hashmi et al., 2013). The rsFC alterations we identified in PwSCI were consistent with prior findings in other chronic pain conditions (Apkarian et al., 2005, Hashmi et al., 2013, Vachon-Presseau et al., 2016). Negative associations were identified between nociceptive pain intensity and thalamic connectivity to limbic and cortical regions. Decreased connectivity of the thalamus associated with higher nociceptive pain severity may reflect lower thalamic activation in chronic pain states, consistent with prior literature (Apkarian et al., 2005, Hashmi et al., 2013).
Our results of thalamic involvement being associated exclusively with nociceptive, not neuropathic, pain contradict previously identified literature reporting thalamic differences in PwSCI with neuropathic pain including greater mean diffusivity (Gustin et al., 2014), lower gray matter volume (Jutzeler et al., 2016), biochemical (Gustin et al., 2014, Pattany et al., 2002, Stanwell et al., 2010, Widerström-Noga et al., 2015) and blood flow differences (Gustin et al., 2014), and stronger thalamocortical connectivity (Huynh et al., 2021). In contrast to our findings of diminished thalamocortical functional connectivity associated with nociceptive pain severity in PwSCI, Huynh et al. reported stronger thalamocortical connectivity between PwSCI with neuropathic pain and healthy individuals (Huynh et al., 2021). This discrepancy between our findings may be due to prior studies reporting comparison to healthy controls and additional neuroplastic alterations associated with SCI not specific to pain processing, as well as prior studies’ lack of control for potentially co-occurring nociceptive pain in PwSCI.
In sum, findings from the nociceptive analysis demonstrate patterns of lower thalamocortical and thalamolimbic connectivity contribute to greater nociceptive pain severity in individuals with SCI. Alteration of connectivity in components of traditional pain processing pathways associated with nociceptive pain suggest concurrent nociceptive pain may influence analyses assessing the effect of neuropathic pain in PwSCI and should be considered as a potential confounding variable. Studies assessing rsFC alterations between acute and chronic pain states, or the effect of pain duration, in PwSCI could additionally elucidate alterations in thalamic connectivity associated with pain chronicity.
4.3. The joint effect of neuropathic and nociceptive pain is associated with limbocortical rsFC alterations
Findings specific to the additive analysis assessing the joint effect of both neuropathic and nociceptive pain suggest that overall higher pain burden is associated with limbocortical connectivity alterations. Pain intensity and functional connectivity were negatively correlated in the right pPaHC gyrus to the right angular and supramarginal gyri and lateral occipital cortex, and positively correlated in the left amygdala to the left supramarginal gyrus and superior parietal lobule. Prior studies suggest the parahippocampal gyrus and amygdala contribute to psychological and emotional regulation of pain processing (Allen et al., 2021, Meerwijk et al., 2013, Naor et al., 2020) and sensitivity (Grant et al., 2010, Meerwijk et al., 2013, Naor et al., 2020), and have been implicated in other populations with neuralgia (Geha et al., 2007, Tang et al., 2021, Zhang et al., 2018). The angular and supramarginal gyri hold roles in multisensory integration (Seghier, 2013) and regulation of emotional and empathetic responses to pain (Naor et al., 2020, Zhao et al., 2021). Prior studies also implicated the parietal lobe in pain experience after SCI, with greater pain intensity positively associated with mean diffusivity of the parietal cortex (Gustin et al., 2014) and rsFC of the superior parietal lobule to the angular gyrus (Huynh et al., 2021).
Previously reported altered diffusivity of the amygdala associated with neuropathic pain in PwSCI supports the role of the amygdala in pain processing (Gustin et al., 2014). Likewise, lower gray matter volume of the parahippocampal gyrus has been reported in PwSCI compared to healthy individuals suggestive of potential structural changes in this region after SCI (Q. Chen et al., 2018). Pain-related alterations in rsFC between the parahippocampal gyrus and amygdala to cortical regions have also been previously identified, with the direction of association reported dependent on the cortical region and population (X. F. Chen et al., 2022, Desmarteaux et al., 2021, Mao et al., 2022, Verriotis et al., 2022). In addition to their roles in pain processing, the parahippocampal gyrus and amygdala are involved in processes of emotion, memory, attention, and conditional learning through association of stimuli with positive or negative outcomes (Aminoff et al., 2013, Gallagher and Chiba, 1996). As such, integration of pain intensity with additional roles in behavior in the parahippocampal gyrus and amygdala could influence their interactions with a range of cortical regions involved in these processes. Our findings of pain intensity being associated with alterations in limbocortical functional connectivity may reflect increased activation of the parahippocampal gyrus and amygdala in higher pain states, and subsequent engagement of functions recruiting cortical regions involved in multimodal pain-related behavior modification.
Given the association of the parahippocampal gyrus and amygdala with psychoemotional aspects of pain processing, further analysis of the relationship of these regions, and greater additive pain burden, with other psychological factors such as depression, anxiety, pain catastrophizing, and kinesiophobia is warranted to determine additional factors potentially contributing to limbocortical connectivity alterations in PwSCI (Cherif et al., 2020, de la Rosa-Díaz et al., 2022).
4.4. Posterior cingulate connectivity differs based on pain type
When contrasting the effect of the severity of neuropathic and nociceptive pain severity on rsFC patterns, opposing connectivity patterns were identified from a seed in the posterior cingulate to the occipital cortex (see Fig. 5b). These pain phenotype dependent limbocortical alterations further support differential processing of neuropathic and nociceptive pain. Though the underlying mechanisms contributing to pain phenotype dependent alterations in these regions is unclear, the contributions of these regions to non-noxious sensory function may be influential. The posterior cingulate is a component of both the limbic system and the default mode network and holds a well-established role in pain experience (Benarroch, 2020). Prior evidence suggests the posterior cingulate holds a role in the processing of sensory information not exclusive to pain perception (Oertel et al., 2012). As such, pain phenotype dependent differences in posterior cingulate functional connectivity may reflect alterations in the processing of non-noxious stimuli.
Allodynia, the interpretation of non-noxious stimuli as noxious due to central sensitization is common in individuals with neuropathic pain after SCI (S. Lee et al., 2013). Allodynia has been previously associated with differences in activation of the posterior cingulate suggesting our findings in these regions may be driven in part by the presence or absence of allodynia in those with and without neuropathic pain respectively (Freund et al., 2010, Maleki et al., 2021, Russo et al., 2020). However, activation of the posterior cingulate has also been previously reported with higher levels of noxious stimulation (Schneider et al., 2001). Our findings may also reflect severity related differences in pain processing as neuropathic pain intensities are typically rated higher in severity than nociceptive pain, a trend that is reflected in our sample. Experimental pain studies investigating the relationship between posterior cingulate function, allodynia, and pain intensities within neuropathic and nociceptive pain phenotypes could elucidate underlying mechanisms contributing to our findings of differential connectivity based on pain type.
4.5. Functional activation was not associated with pain phenotype and intensity
We did not identify any significant associations between finger and foot tap induced functional activation and pain phenotype and intensity for neuropathic or nociceptive pain, or the joint effect or contrast of both pain intensities. Our results are consistent with the lack of association between neuropathic pain intensity and evoked responses to sensory stimuli (Wrigley et al., 2009) or extent of cortical reorganization in PwSCI (Jutzeler et al., 2015), as well as the lack of difference in evoked sensorimotor functional activation between PwSCI with and without neuropathic pain and healthy individuals (Gustin et al., 2010, Jutzeler et al., 2015, Solstrand Dahlberg et al., 2018). However, in a prior study on individuals with trigeminal neuropathy compared to those with temporomandibular joint disorder and healthy individuals, only neuropathic pain influenced cortical organization, suggesting a unique influence of neuropathic as opposed to nociceptive pain (Gustin et al., 2012). In PwSCI, contrasting findings regarding the association of cortical organization and neuropathic pain have been reported, with both greater and lesser extent of reorganization identified in PwSCI with neuropathic pain compared to those without pain or healthy controls (Jutzeler et al., 2015, Wrigley et al., 2009). Additional studies assessing cortical reorganization associated with neuropathic pain in PwSCI controlling for the potential confound of nociceptive pain may clarify whether similar patterns reported in other populations may be seen in PwSCI.
4.6. Summary
Our findings suggest that intralimbic and limbostriatal pathway connectivity alterations may be uniquely associated with neuropathic pain severity, whereas thalamocortical and thalamolimbic connectivity alterations may be associated specifically with nociceptive pain severity. Greater overall pain burden considering the joint effect of both pain types resulted in limbocortical connectivity alterations involving multisensory processing regions. Opposing connectivity relationships dependent upon pain type suggest the posterior cingulate differentially modulates the experience of both neuropathic and nociceptive pain. Taken together, these findings suggest altered neural activity in these regions may be driven by different pathways which uniquely contribute to neuropathic and nociceptive pain experiences.
4.7. Limitations and future considerations
Our sample size was modest and as such, we were limited in the number of variables of interest included in our statistical models. Binary classification of pain as either neuropathic or nociceptive is not representative of the clinical reality, where the term “mixed pain” may be a better descriptor (Freynhagen et al., 2019). This was the case for approximately a third of our participants who expressed both types of pain. Our findings are limited by the lack of segregation of our sample into distinct groups (neuropathic only, nociceptive only, both pain types, or no pain), which was not attempted due to the limited number of participants with only nociceptive (n = 5) or no pain (n = 6). While our statistical approach in principle should account for these limitations, future clinical studies with clearer segregation, as well as experimental induction of different pain types are needed to confirm the specificity of the current results. An approach incorporating separate groups for PwSCI with only neuropathic pain, only nociceptive pain, both pain types, and no pain, as well as healthy individuals would enable more optimal investigation of pain phenotypes and associated rsFC and functional activation differences. Clinical outcome measure data collection did not occur at the time of scanning for all participants, though this limitation is mitigated by all data collection occurring either the day of or 1–2 days after the scan, thus capturing pain experienced on the day of the scan in the weekly average reported pain intensities on the ISCIPBDS.
Future studies with larger sample sizes should consider including additional factors such as age and duration of injury (Finnerup et al., 2014), sex (Girard-Tremblay et al., 2014, Robertson et al., 2022), pain duration (Hashmi et al., 2013, Vachon-Presseau et al., 2016, Youssef et al., 2019), and injury classification (tetraplegia vs paraplegia) (Kennedy et al., 1997, Siddall et al., 2003), mood disorders, physical activity levels, socioeconomic status, and personal traits,(Mills et al., 2019) which may contribute to pain outcomes after SCI to further elucidate underlying pain mechanisms in this heterogeneous population. We did not control for the number of painful body sites as previous work by Huynh et al. reported no association between pain intensity and pain extent in PwSCI, and no overlap in rsFC differences associated with pain intensity and extent (Huynh et al., 2021). However, additional determination of the effects of specific neuropathic pain presentations after SCI, such as the spatial extent of pain (Huynh et al., 2021), if the pain is at level or below level, and presence of allodynia and/or hyperalgesia in non-hypesthesia regions, is needed (Widerström-Noga, 2017). As NSAIDs were not previously found to alter rsFC (Wanigasekera et al., 2016) and only 2 individuals in our sample currently utilized them, we did not control for NSAID use in our sample. However, as only the short-term effects of NSAID use on connectivity have been assessed after administration in an experimental pain condition (Wanigasekera et al., 2016), future studies investigating the effects of long-term use of NSAIDs as well as other medications typically utilized in individuals with SCI and chronic pain may be warranted. Hypotheses regarding specific tracts and pathways and their association with these findings are speculative and require further preclinical and clinical work to define their projections and association with function.
Of note, in the supplemental analysis assessing the effects of opioid use, opioids were associated with altered limbocortical connectivity in regions traditionally associated with pain processing including the amygdala, insula, and primary somatosensory and motor cortices. Though we controlled for the effects of opioids in our analysis and did not identify any potential confounding results with our primary analyses associated with opioid use, opioids are not consistently included as a potential confounding variable in neuropathic pain rsFC or functional activation studies. Careful consideration of potential confounding variables such as co-occurrence of nociceptive pain and opioid use is recommended for future studies of neuropathic pain in PwSCI as these factors may influence functional connectivity results. Wide discrepancy exists in resting state functional connectivity outcomes in neuropathic pain studies in PwSCI, likely driven by differences in methodology, and small, heterogeneous samples (Huynh et al., 2021, Li et al., 2020b). Reporting overall connectivity patterns in addition to specification of distinct regions may enable greater replicability and identification of connectivity pattern alterations associated with neuropathic pain across studies.
5. Conclusions
Alterations in rsFC between regions involved in sensory and emotional aspects of pain processing after SCI were found to be associated with both pain severity and phenotype. Unique connectivity patterns dependent upon pain phenotype suggest differential relationships between brain regions may exist for neuropathic and nociceptive pain and could guide further study and future interventions.
Funding and Disclosures
This work was supported by the Office of the Secretary of Defense for Health Affairs through the Spinal Cord Injury Clinical Trial Program under Award Number W81XWH-15-2-0078. Opinions, interpretations, conclusions, and recommendations are those of the author and are not necessarily endorsed by the Department of Defense. This work was also supported by the National Institutes of Health’s National Center for Advancing Translational Sciences [TL1R002493, UL1TR002494] and Eunice Kennedy Shriver National Institute of Child Health and Human Development (5R01HD097407); Wings for Life [WFL-US-014/14]; and a Florence P. Kendall Doctoral Scholarship and Promotion of Doctoral Studies I Scholarship from the Foundation for Physical Therapy Research. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health’s National Center for Advancing Translational Sciences. The authors have no conflicts of interest to report.
CRediT authorship contribution statement
Jesse L. Kowalski: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization, Funding acquisition. Leslie R. Morse: Conceptualization, Investigation, Resources, Writing – review & editing, Supervision, Project administration, Funding acquisition. Karen Troy: Investigation, Writing – review & editing. Nguyen Nguyen: Investigation, Data curation, Writing – review & editing. Ricardo A. Battaglino: Investigation, Writing – review & editing. Scott P. Falci: Investigation, Resources, Writing – review & editing. Clas Linnman: Conceptualization, Methodology, Investigation, Data curation, Writing – review & editing, Supervision, Project administration, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2023.103414.
Contributor Information
Jesse L. Kowalski, Email: kowal225@umn.edu.
Leslie R. Morse, Email: morsel@umn.edu.
Karen Troy, Email: ktroy@wpi.edu.
Nguyen Nguyen, Email: nguy4139@umn.edu.
Ricardo A. Battaglino, Email: rbattagl@umn.edu.
Scott P. Falci, Email: spfalci@comcast.net.
Clas Linnman, Email: clinnman@partners.org.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary figure 1.
References
- Allen H.N., Bobnar H.J., Kolber B.J. Left and right hemispheric lateralization of the amygdala in pain. Prog. Neurobiol. 2021;196 doi: 10.1016/j.pneurobio.2020.101891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff E.M., Kveraga K., Bar M. The role of the parahippocampal cortex in cognition. Trends Cogn. Sci. 2013;17(8):379–390. doi: 10.1016/j.tics.2013.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian A.V., Bushnell M.C., Treede R.D., Zubieta J.K. Human brain mechanisms of pain perception and regulation in health and disease. Eur. J. Pain. 2005;9(4):463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- Bates D., Schultheis B.C., Hanes M.C., Jolly S.M., Chakravarthy K.V., Deer T.R., Levy R.M., Hunter C.W. A Comprehensive Algorithm for Management of Neuropathic Pain. Pain Med. 2019;20(Supplement_1):S2–S12. doi: 10.1093/pm/pnz075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch E.E. What is the role of the cingulate cortex in pain? Neurology. 2020;95(16):729–732. doi: 10.1212/wnl.0000000000010712. [DOI] [PubMed] [Google Scholar]
- Bingel U., Quante M., Knab R., Bromm B., Weiller C., Büchel C. Subcortical structures involved in pain processing: evidence from single-trial fMRI. Pain. 2002;99(1–2):313–321. doi: 10.1016/s0304-3959(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Bishop G.H., Landau W.M., Jones M.H. Evidence for a double peripheral pathway for pain. Science. 1958;128(3326):712–714. doi: 10.1126/science.128.3326.712. [DOI] [PubMed] [Google Scholar]
- Björnsdotter M., Löken L., Olausson H., Vallbo A., Wessberg J. Somatotopic organization of gentle touch processing in the posterior insular cortex. J. Neurosci. 2009;29(29):9314–9320. doi: 10.1523/jneurosci.0400-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black S.R., King J.B., Mahan M.A., Anderson J., Butson C.R. Functional Hyperconnectivity and Task-Based Activity Changes Associated With Neuropathic Pain After Spinal Cord Injury: A Pilot Study. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.613630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolwerk A., Seifert F., Maihöfner C. Altered resting-state functional connectivity in complex regional pain syndrome. J. Pain. 2013;14(10):1107–1115.e1108. doi: 10.1016/j.jpain.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Borsook D., Upadhyay J., Chudler E.H., Becerra L. A key role of the basal ganglia in pain and analgesia–insights gained through human functional imaging. Mol. Pain. 2010;6:27. doi: 10.1186/1744-8069-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce T.N., Biering-Sørensen F., Finnerup N.B., Cardenas D.D., Defrin R., Lundeberg T., Norrbrink C., Richards J.S., Siddall P., Stripling T., Treede R.-D., Waxman S.G., Widerström-Noga E., Yezierski R.P., Dijkers M. International Spinal Cord Injury Pain Classification: part I. Background and description. Spinal Cord. 2012;50(6):413–417. doi: 10.1038/sc.2011.156. [DOI] [PubMed] [Google Scholar]
- Cardenas D.D., Felix E.R. Pain after spinal cord injury: a review of classification, treatment approaches, and treatment assessment. PM R. 2009;1(12):1077–1090. doi: 10.1016/j.pmrj.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Cermik T.F., Tuna H., Kaya M., Tuna F., Gültekin A., Yiğitbaşi O.N., Alavi A. Assessment of regional blood flow in cerebral motor and sensory areas in patients with spinal cord injury. Brain Res. 2006;1109(1):54–59. doi: 10.1016/j.brainres.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Chen X.-F., He P., Xu K.-H., Jin Y.-H., Chen Y., Wang B., Hu X.u., Qi L.e., Wang M.-W., Li J. Disrupted Spontaneous Neural Activity and Its Interaction With Pain and Emotion in Temporomandibular Disorders. Front. Neurosci. 2022;16 doi: 10.3389/fnins.2022.941244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Zheng W., Chen X., Li X., Wang L., Qin W., Li K., Chen N. Whether Visual-related Structural and Functional Changes Occur in Brain of Patients with Acute Incomplete Cervical Cord Injury: A Multimodal Based MRI Study. Neuroscience. 2018;393:284–294. doi: 10.1016/j.neuroscience.2018.10.014. [DOI] [PubMed] [Google Scholar]
- Cherif F., Zouari H.G., Cherif W., Hadded M., Cheour M., Damak R. Depression Prevalence in Neuropathic Pain and Its Impact on the Quality of Life. Pain Res. Manag. 2020;2020:7408508. doi: 10.1155/2020/7408508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang M.C., Nguyen E.K., Canto-Bustos M., Papale A.E., Oswald A.M., Ross S.E. Divergent Neural Pathways Emanating from the Lateral Parabrachial Nucleus Mediate Distinct Components of the Pain Response. Neuron. 2020;106(6):927–939.e925. doi: 10.1016/j.neuron.2020.03.014. [DOI] [PubMed] [Google Scholar]
- Chudler E.H., Dong W.K. The role of the basal ganglia in nociception and pain. Pain. 1995;60(1):3–38. doi: 10.1016/0304-3959(94)00172-B. [DOI] [PubMed] [Google Scholar]
- Croosu S.S., Frøkjaer J.B., Drewes A.M., Hansen T.M. Tapentadol and oxycodone affect resting-state functional brain connectivity: A randomized, placebo-controlled trial. J. Neuroimaging. 2021;31(5):956–961. doi: 10.1111/jon.12902. [DOI] [PubMed] [Google Scholar]
- de la Rosa-Díaz I., Barrero-Santiago L., Acosta-Ramírez P., Martín-Peces-Barba M., Iglesias-Hernández E., Plisset B., Lutinier N., Belzanne M., La Touche R., Grande-Alonso M. Cross-Sectional Comparative Study on Central Sensitization-Psychosocial Associated Comorbidities and Psychological Characteristics in Breast Cancer Survivors with Nociceptive Pain and Pain with Neuropathic Features and without Pain. Life (Basel) 2022;12(9):1328. doi: 10.3390/life12091328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ridder D., Vanneste S., Smith M., Adhia D. Pain and the Triple Network Model. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.757241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmarteaux C., Streff A., Chen J.I., Houzé B., Piché M., Rainville P. Brain Responses to Hypnotic Verbal Suggestions Predict Pain Modulation. Front Pain Res (Lausanne) 2021;2 doi: 10.3389/fpain.2021.757384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falci S., Indeck C., Barnkow D. Spinal cord injury below-level neuropathic pain relief with dorsal root entry zone microcoagulation performed caudal to level of complete spinal cord transection. J. Neurosurg. Spine. 2018;28(6):612–620. doi: 10.3171/2017.9.SPINE17373. [DOI] [PubMed] [Google Scholar]
- Felix E.R., Cardenas D.D., Bryce T.N., Charlifue S., Lee T.K., MacIntyre B., Mulroy S., Taylor H. Prevalence and Impact of Neuropathic and Nonneuropathic Pain in Chronic Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2021;103(4):729–737. doi: 10.1016/j.apmr.2021.06.022. [DOI] [PubMed] [Google Scholar]
- Finnerup N.B., Norrbrink C., Trok K., Piehl F., Johannesen I.L., Sørensen J.C., Jensen T.S., Werhagen L. Phenotypes and predictors of pain following traumatic spinal cord injury: a prospective study. J. Pain. 2014;15(1):40–48. doi: 10.1016/j.jpain.2013.09.008. [DOI] [PubMed] [Google Scholar]
- Freund W., Wunderlich A.P., Stuber G., Mayer F., Steffen P., Mentzel M., Schmitz B. Different activation of opercular and posterior cingulate cortex (PCC) in patients with complex regional pain syndrome (CRPS I) compared with healthy controls during perception of electrically induced pain: a functional MRI study. Clin. J. Pain. 2010;26(4):339–347. doi: 10.1097/AJP.0b013e3181cb4055. [DOI] [PubMed] [Google Scholar]
- Freynhagen R., Parada H.A., Calderon-Ospina C.A., Chen J., Rakhmawati Emril D., Fernández-Villacorta F.J., Franco H., Ho K.-Y., Lara-Solares A., Li C.-F., Mimenza Alvarado A., Nimmaanrat S., Dolma Santos M., Ciampi de Andrade D. Current understanding of the mixed pain concept: a brief narrative review. Curr. Med. Res. Opin. 2019;35(6):1011–1018. doi: 10.1080/03007995.2018.1552042. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Holmes A.P., Worsley K.J., Poline J.-P., Frith C.D., Frackowiak R.S.J. Statistical parametric maps in functional imaging: A general linear approach. Hum. Brain Mapp. 1994;2(4):189–210. doi: 10.1002/hbm.460020402. [DOI] [Google Scholar]
- Gallagher M., Chiba A.A. The amygdala and emotion. Curr. Opin. Neurobiol. 1996;6(2):221–227. doi: 10.1016/s0959-4388(96)80076-6. [DOI] [PubMed] [Google Scholar]
- Geha P.Y., Baliki M.N., Chialvo D.R., Harden R.N., Paice J.A., Apkarian A.V. Brain activity for spontaneous pain of postherpetic neuralgia and its modulation by lidocaine patch therapy. Pain. 2007;128(1–2):88–100. doi: 10.1016/j.pain.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard-Tremblay L., Auclair V., Daigle K., Léonard G., Whittingstall K., Goffaux P. Sex differences in the neural representation of pain unpleasantness. J. Pain. 2014;15(8):867–877. doi: 10.1016/j.jpain.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Gorka S.M., Fitzgerald D.A., de Wit H., Angstadt M., Phan K.L. Opioid modulation of resting-state anterior cingulate cortex functional connectivity. J. Psychopharmacol. 2014;28(12):1115–1124. doi: 10.1177/0269881114548436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J.A., Courtemanche J., Duerden E.G., Duncan G.H., Rainville P. Cortical thickness and pain sensitivity in zen meditators. Emotion. 2010;10(1):43–53. doi: 10.1037/a0018334. [DOI] [PubMed] [Google Scholar]
- Gustin S.M., Wrigley P.J., Siddall P.J., Henderson L.A. Brain anatomy changes associated with persistent neuropathic pain following spinal cord injury. Cereb. Cortex. 2010;20(6):1409–1419. doi: 10.1093/cercor/bhp205. [DOI] [PubMed] [Google Scholar]
- Gustin S.M., Peck C.C., Cheney L.B., Macey P.M., Murray G.M., Henderson L.A. Pain and plasticity: is chronic pain always associated with somatosensory cortex activity and reorganization? J. Neurosci. 2012;32(43):14874–14884. doi: 10.1523/jneurosci.1733-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustin S.M., Wrigley P.J., Youssef A.M., McIndoe L., Wilcox S.L., Rae C.D., Edden R.A.E., Siddall P.J., Henderson L.A. Thalamic activity and biochemical changes in individuals with neuropathic pain after spinal cord injury. Pain. 2014;155(5):1027–1036. doi: 10.1016/j.pain.2014.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy S.D., Mehta S., Casalino A., Côté I., Kras-Dupuis A., Moulin D.E., Parrent A.G., Potter P., Short C., Teasell R., Bradbury C.L., Bryce T.N., Craven B.C., Finnerup N.B., Harvey D., Hitzig S.L., Lau B., Middleton J.W., O'Connell C., Orenczuk S., Siddall P.J., Townson A., Truchon C., Widerström-Noga E., Wolfe D., Loh E. The CanPain SCI Clinical Practice Guidelines for Rehabilitation Management of Neuropathic Pain after Spinal Cord: Recommendations for treatment. Spinal Cord. 2016;54(S1):S14–S23. doi: 10.1038/sc.2016.90. [DOI] [PubMed] [Google Scholar]
- Hashmi J.A., Baliki M.N., Huang L., Baria A.T., Torbey S., Hermann K.M., Schnitzer T.J., Apkarian A.V. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. 2013;136(9):2751–2768. doi: 10.1093/brain/awt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh V., Rosner J., Curt A., Kollias S., Hubli M., Michels L. Disentangling the Effects of Spinal Cord Injury and Related Neuropathic Pain on Supraspinal Neuroplasticity: A Systematic Review on Neuroimaging. Front. Neurol. 2019;10:1413. doi: 10.3389/fneur.2019.01413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh V., Lütolf R., Rosner J., Luechinger R., Curt A., Kollias S., Hubli M., Michels L. Supraspinal nociceptive networks in neuropathic pain after spinal cord injury. Hum. Brain Mapp. 2021;42(12):3733–3749. doi: 10.1002/hbm.25401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabuchi S.J., Drabek M.M., Cottam W.J., Tadjibaev A., Mohammadi‐Nejad A.-R., Sotiropoulos S., Fernandes G.S., Valdes A.M., Zhang W., Doherty M., Walsh D.A., Auer D.P. Medio-dorsal thalamic dysconnectivity in chronic knee pain: A possible mechanism for negative affect and pain comorbidity. Eur. J. Neurosci. 2023;57(2):373–387. doi: 10.1111/ejn.15880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutzeler C.R., Freund P., Huber E., Curt A., Kramer J.L.K. Neuropathic Pain and Functional Reorganization in the Primary Sensorimotor Cortex After Spinal Cord Injury. J. Pain. 2015;16(12):1256–1267. doi: 10.1016/j.jpain.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Jutzeler C.R., Huber E., Callaghan M.F., Luechinger R., Curt A., Kramer J.L., Freund P. Association of pain and CNS structural changes after spinal cord injury. Sci. Rep. 2016;6:18534. doi: 10.1038/srep18534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keltner, J. R., Connolly, C. G., Vaida, F., Jenkinson, M., Fennema-Notestine, C., Archibald, S., . . . Ellis, R. J. (2017). HIV Distal Neuropathic Pain Is Associated with Smaller Ventral Posterior Cingulate Cortex. Pain Med, 18(3), 428-440. doi:10.1093/pm/pnw180. [DOI] [PMC free article] [PubMed]
- Kennedy P., Frankel H., Gardner B., Nuseibeh I. Factors associated with acute and chronic pain following traumatic spinal cord injuries. Spinal Cord. 1997;35(12):814–817. doi: 10.1038/sj.sc.3100569. [DOI] [PubMed] [Google Scholar]
- Kosek E., Cohen M., Baron R., Gebhart G.F., Mico J.-A., Rice A.S.C., Rief W., Sluka A.K. Do we need a third mechanistic descriptor for chronic pain states? Pain. 2016;157(7):1382–1386. doi: 10.1097/j.pain.0000000000000507. [DOI] [PubMed] [Google Scholar]
- Kyathanahally S.P., Azzarito M., Rosner J., Calhoun V.D., Blaiotta C., Ashburner J., Weiskopf N., Wiech K., Friston K., Ziegler G., Freund P. Microstructural plasticity in nociceptive pathways after spinal cord injury. J. Neurol. Neurosurg. Psychiatry. 2021;92(8):863–871. doi: 10.1136/jnnp-2020-325580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B., Di Pietro F., Henderson L.A., Austin P.J. Altered basal ganglia infraslow oscillation and resting functional connectivity in complex regional pain syndrome. J. Neurosci. Res. 2022;100(7):1487–1505. doi: 10.1002/jnr.25057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Zhao X., Hatch M., Chun S., Chang E. Central Neuropathic Pain in Spinal Cord Injury. Crit Rev Phys Rehabil Med. 2013;25(3–4):159–172. doi: 10.1615/CritRevPhysRehabilMed.2013007944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Li X., Feng Y., Gao F., Kong Y., Hu L. Deficits in ascending and descending pain modulation pathways in patients with postherpetic neuralgia. Neuroimage. 2020;221 doi: 10.1016/j.neuroimage.2020.117186. [DOI] [PubMed] [Google Scholar]
- Li X., Wang L., Chen Q., Hu Y., Du J., Chen X., Zheng W., Lu J., Chen N. The Reorganization of Insular Subregions in Individuals with Below-Level Neuropathic Pain following Incomplete Spinal Cord Injury. Neural Plast. 2020;2020:1–9. doi: 10.1155/2020/2796571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C., Moulton E.A., Barmettler G., Becerra L., Borsook D. Neuroimaging of the periaqueductal gray: state of the field. Neuroimage. 2012;60(1):505–522. doi: 10.1016/j.neuroimage.2011.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maleki N., Szabo E., Becerra L., Moulton E., Scrivani S.J., Burstein R., Borsook D., Antal A. Ictal and interictal brain activation in episodic migraine: Neural basis for extent of allodynia. PLoS One. 2021;16(1):e0244320. doi: 10.1371/journal.pone.0244320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour Y., Kulesza R.J. Premature termination of the sympathetic chain. Folia Morphol (Warsz) 2021;81(4):1054–1057. doi: 10.5603/FM.a2021.0089. [DOI] [PubMed] [Google Scholar]
- Mao C.P., Yang H.J., Yang Q.X., Sun H.H., Zhang G.R., Zhang Q.J. Altered Amygdala-prefrontal Connectivity in Chronic Nonspecific Low Back Pain: Resting-state fMRI and Dynamic Causal Modelling Study. Neuroscience. 2022;482:18–29. doi: 10.1016/j.neuroscience.2021.12.003. [DOI] [PubMed] [Google Scholar]
- Matre D.A., Hernandez-Garcia L., Tran T.D., Casey K.L. “First pain” in humans: convergent and specific forebrain responses. Mol. Pain. 2010;6:81. doi: 10.1186/1744-8069-6-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meerwijk E.L., Ford J.M., Weiss S.J. Brain regions associated with psychological pain: implications for a neural network and its relationship to physical pain. Brain Imaging Behav. 2013;7(1):1–14. doi: 10.1007/s11682-012-9179-y. [DOI] [PubMed] [Google Scholar]
- Melzack R., Loeser J.D. Phantom body pain in paraplegics: evidence for a central “pattern generating mechanism” for pain. Pain. 1978;4(3):195–210. doi: 10.1016/0304-3959(77)90133-6. [DOI] [PubMed] [Google Scholar]
- Mills S.E.E., Nicolson K.P., Smith B.H. Chronic pain: a review of its epidemiology and associated factors in population-based studies. Br. J. Anaesth. 2019;123(2):e273–e283. doi: 10.1016/j.bja.2019.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Y.-S., Park J.W., Jin S.U., Jang K.E., Nam H.U., Lee Y.-S., Jung T.-D., Chang Y. Alteration of Resting-State Brain Sensorimotor Connectivity following Spinal Cord Injury: A Resting-State Functional Magnetic Resonance Imaging Study. J. Neurotrauma. 2015;32(18):1422–1427. doi: 10.1089/neu.2014.3661. [DOI] [PubMed] [Google Scholar]
- Mole T.B., MacIver K., Sluming V., Ridgway G.R., Nurmikko T.J. Specific brain morphometric changes in spinal cord injury with and without neuropathic pain. Neuroimage Clin. 2014;5:28–35. doi: 10.1016/j.nicl.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naor N., Rohr C., Schaare L.H., Limbachia C., Shamay-Tsoory S., Okon-Singer H. The neural networks underlying reappraisal of empathy for pain. Soc. Cogn. Affect. Neurosci. 2020;15(7):733–744. doi: 10.1093/scan/nsaa094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel B.G., Preibisch C., Martin T., Walter C., Gamer M., Deichmann R., Lötsch J. Separating brain processing of pain from that of stimulus intensity. Hum. Brain Mapp. 2012;33(4):883–894. doi: 10.1002/hbm.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascoal-Faria P., Yalcin N., Fregni F. Neural markers of neuropathic pain associated with maladaptive plasticity in spinal cord injury. Pain Pract. 2015;15(4):371–377. doi: 10.1111/papr.12237. [DOI] [PubMed] [Google Scholar]
- Pattany P.M., Yezierski R.P., Widerström-Noga E.G., Bowen B.C., Martinez-Arizala A., Garcia B.R., Quencer R.M. Proton magnetic resonance spectroscopy of the thalamus in patients with chronic neuropathic pain after spinal cord injury. AJNR Am. J. Neuroradiol. 2002;23(6):901–905. [PMC free article] [PubMed] [Google Scholar]
- Robertson R.V., Crawford L.S., Meylakh N., Macey P.M., Macefield V.G., Keay K.A., Henderson L.A. Regional hypothalamic, amygdala, and midbrain periaqueductal gray matter recruitment during acute pain in awake humans: A 7-Tesla functional magnetic resonance imaging study. Neuroimage. 2022;259 doi: 10.1016/j.neuroimage.2022.119408. [DOI] [PubMed] [Google Scholar]
- Roiser, J. P., Linden, D. E., Gorno-Tempinin, M. L., Moran, R. J., Dickerson, B. C., & Grafton, S. T. (2016). Minimum statistical standards for submissions to Neuroimage: Clinical. In Neuroimage Clin (Vol. 12, pp. 1045-1047). Netherlands. [DOI] [PMC free article] [PubMed]
- Ruscheweyh R., Wersching H., Kugel H., Sundermann B., Teuber A. Gray matter correlates of pressure pain thresholds and self-rated pain sensitivity: a voxel-based morphometry study. Pain. 2018;159(7):1359–1365. doi: 10.1097/j.pain.0000000000001219. [DOI] [PubMed] [Google Scholar]
- Russo A., Silvestro M., Trojsi F., Bisecco A., De Micco R., Caiazzo G., Di Nardo F., Esposito F., Tessitore A., Tedeschi G. Cognitive Networks Disarrangement in Patients With Migraine Predicts Cutaneous Allodynia. Headache. 2020;60(7):1228–1243. doi: 10.1111/head.13860. [DOI] [PubMed] [Google Scholar]
- Schneider F., Habel U., Holthusen H., Kessler C., Posse S., Müller-Gärtner H.W., Arndt J.O. Subjective ratings of pain correlate with subcortical-limbic blood flow: an fMRI study. Neuropsychobiology. 2001;43(3):175–185. doi: 10.1159/000054887. [DOI] [PubMed] [Google Scholar]
- Scholz J., Finnerup N.B., Attal N., Aziz Q., Baron R., Bennett M.I., Benoliel R., Cohen M., Cruccu G., Davis K.D., Evers S., First M., Giamberardino M.A., Hansson P., Kaasa S., Korwisi B., Kosek E., Lavand'homme P., Nicholas M., Nurmikko T., Perrot S., Raja S.N., Rice A.S.C., Rowbotham M.C., Schug S., Simpson D.M., Smith B.H., Svensson P., Vlaeyen J.W.S., Wang S.-J., Barke A., Rief W., Treede R.-D. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. 2019;160(1):53–59. doi: 10.1097/j.pain.0000000000001365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier M.L. The angular gyrus: multiple functions and multiple subdivisions. Neuroscientist. 2013;19(1):43–61. doi: 10.1177/1073858412440596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seixas D., Palace J., Tracey I. Chronic pain disrupts the reward circuitry in multiple sclerosis. Eur. J. Neurosci. 2016;44(3):1928–1934. doi: 10.1111/ejn.13272. [DOI] [PubMed] [Google Scholar]
- Siddall P.J., McClelland J.M., Rutkowski S.B., Cousins M.J. A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain. 2003;103(3):249–257. doi: 10.1016/s0304-3959(02)00452-9. [DOI] [PubMed] [Google Scholar]
- Solstrand Dahlberg L., Becerra L., Borsook D., Linnman C. Brain changes after spinal cord injury, a quantitative meta-analysis and review. Neurosci. Biobehav. Rev. 2018;90:272–293. doi: 10.1016/j.neubiorev.2018.04.018. [DOI] [PubMed] [Google Scholar]
- Sprenger C., Finsterbusch J., Büchel C. Spinal cord-midbrain functional connectivity is related to perceived pain intensity: a combined spino-cortical FMRI study. J. Neurosci. 2015;35(10):4248–4257. doi: 10.1523/jneurosci.4897-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwell P., Siddall P., Keshava N., Cocuzzo D., Ramadan S., Lin A., Herbert D., Craig A., Tran Y., Middleton J. Neuro magnetic resonance spectroscopy using wavelet decomposition and statistical testing identifies biochemical changes in people with spinal cord injury and pain. Neuroimage. 2010;53(2):544–552. doi: 10.1016/j.neuroimage.2010.06.051. [DOI] [PubMed] [Google Scholar]
- Sun L.i., Liu R., Guo F., Wen M.-Q., Ma X.-L., Li K.-Y., Sun H., Xu C.-L., Li Y.-Y., Wu M.-Y., Zhu Z.-G., Li X.-J., Yu Y.-Q., Chen Z., Li X.-Y., Duan S. Parabrachial nucleus circuit governs neuropathic pain-like behavior. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-19767-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.u., Ren C., Wang M., Dai G., Xiao Y., Wang S., Han F., Chen G. Altered gray matter volume and functional connectivity in patients with herpes zoster and postherpetic neuralgia. Brain Res. 2021;1769:147608. doi: 10.1016/j.brainres.2021.147608. [DOI] [PubMed] [Google Scholar]
- Vachon-Presseau E., Roy M., Martel M.-O., Caron E., Marin M.-F., Chen J., Albouy G., Plante I., Sullivan M.J., Lupien S.J., Rainville P. The stress model of chronic pain: evidence from basal cortisol and hippocampal structure and function in humans. Brain. 2013;136(3):815–827. doi: 10.1093/brain/aws371. [DOI] [PubMed] [Google Scholar]
- Vachon-Presseau E., Tétreault P., Petre B., Huang L., Berger S.E., Torbey S., Baria A.T., Mansour A.R., Hashmi J.A., Griffith J.W., Comasco E., Schnitzer T.J., Baliki M.N., Apkarian A.V. Corticolimbic anatomical characteristics predetermine risk for chronic pain. Brain. 2016;139(7):1958–1970. doi: 10.1093/brain/aww100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldhuijzen D.S., Nemenov M.I., Keaser M., Zhuo J., Gullapalli R.P., Greenspan J.D. Differential brain activation associated with laser-evoked burning and pricking pain: An event-related fMRI study. Pain. 2009;141(1–2):104–113. doi: 10.1016/j.pain.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verriotis M., Sorger C., Peters J., Ayoub L.J., Seunarine K.K., Clark C.A., Moayedi M. Amygdalar Functional Connectivity Differences Associated With Reduced Pain Intensity in Pediatric Peripheral Neuropathic Pain. Front Pain Res (Lausanne) 2022;3 doi: 10.3389/fpain.2022.918766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanigasekera V., Mezue M., Andersson J., Kong Y., Tracey I. Disambiguating Pharmacodynamic Efficacy from Behavior with Neuroimaging: Implications for Analgesic Drug Development. Anesthesiology. 2016;124(1):159–168. doi: 10.1097/aln.0000000000000924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect. 2012;2(3):125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Widerström-Noga E. Neuropathic Pain and Spinal Cord Injury: Phenotypes and Pharmacological Management. Drugs. 2017;77(9):967–984. doi: 10.1007/s40265-017-0747-8. [DOI] [PubMed] [Google Scholar]
- Widerström-Noga E., Pattany P.M., Cruz-Almeida Y., Felix E.R., Perez S., Cardenas D.D., Martinez-Arizala A. Metabolite concentrations in the anterior cingulate cortex predict high neuropathic pain impact after spinal cord injury. Pain. 2013;154(2):204–212. doi: 10.1016/j.pain.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widerström-Noga E., Biering-Sørensen F., Bryce T.N., Cardenas D.D., Finnerup N.B., Jensen M.P., Richards J.S., Siddall P.J. The International Spinal Cord Injury Pain Basic Data Set (version 2.0) Spinal Cord. 2014;52(4):282–286. doi: 10.1038/sc.2014.4. [DOI] [PubMed] [Google Scholar]
- Widerström-Noga E., Cruz-Almeida Y., Felix E.R., Pattany P.M. Somatosensory phenotype is associated with thalamic metabolites and pain intensity after spinal cord injury. Pain. 2015;156(1):166–174. doi: 10.1016/j.pain.0000000000000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis W.D., Westlund K.N. Neuroanatomy of the pain system and of the pathways that modulate pain. J. Clin. Neurophysiol. 1997;14(1):2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley P.J., Press S.R., Gustin S.M., Macefield V.G., Gandevia S.C., Cousins M.J., Middleton J.W., Henderson L.A., Siddall P.J. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain. 2009;141(1):52–59. doi: 10.1016/j.pain.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Yoon E.J., Kim Y.K., Shin H.I., Lee Y., Kim S.E. Cortical and white matter alterations in patients with neuropathic pain after spinal cord injury. Brain Res. 2013;1540:64–73. doi: 10.1016/j.brainres.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Youssef A.M., Azqueta‐Gavaldon M., Silva K.E., Barakat N., Lopez N., Mahmud F., Lebel A., Sethna N.F., Zurakowski D., Simons L.E., Kraft E., Borsook D. Shifting brain circuits in pain chronicity. Hum. Brain Mapp. 2019;40(15):4381–4396. doi: 10.1002/hbm.24709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Liu J., Li L., Du M., Fang W., Wang D., Jiang X., Hu X., Zhang J., Wang X., Fang J. A study on small-world brain functional networks altered by postherpetic neuralgia. Magn. Reson. Imaging. 2014;32(4):359–365. doi: 10.1016/j.mri.2013.12.016. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Mao Z., Pan L., Ling Z., Liu X., Zhang J., Yu X. Dysregulation of Pain- and Emotion-Related Networks in Trigeminal Neuralgia. Front. Hum. Neurosci. 2018;12:107. doi: 10.3389/fnhum.2018.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y., Zhang, L., Rütgen, M., Sladky, R., & Lamm, C. (2021). Neural dynamics between anterior insular cortex and right supramarginal gyrus dissociate genuine affect sharing from perceptual saliency of pretended pain. Elife, 10. doi:10.7554/eLife.69994. [DOI] [PMC free article] [PubMed]
- Zhou F., Gu L., Hong S., Liu J., Jiang J., Huang M., Gong H. Altered low-frequency oscillation amplitude of resting state-fMRI in patients with discogenic low-back and leg pain. J. Pain Res. 2018;11:165–176. doi: 10.2147/jpr.s151562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.