Abstract
Over the last few years, intensifying human impact and the deterioration of natural habitats have severely restricted the global distribution of large herbivores. Rucervus duvaucelii, commonly recognized as the swamp deer, is a habitat-specialist endemic large herbivore of the Indian Subcontinent. It is classified as vulnerable by the IUCN and listed in CITES Appendix I due to a steep decline in its population, which is primarily due to anthropogenic causes. In Nepal, the last remaining population of this species is confined to limited pocket areas within the western Terai Arc Landscape. We explored potential habitat for swamp deer across this landscape using species distribution modelling through the MaxEnt algorithm by using 173 field-verified presence points alongside six anthropogenic, four topographic, and four vegetation-related variables. Our study found that out of the total study area (9207 km2), only 6% (590 km2) was suitable for swamp deer. Approximately 45% of suitable habitat was incorporated within protected areas, with Shuklaphanta National Park harboring the largest habitat patch. The suitability of habitat was discovered to be positively associated with low-elevation areas, areas near water sources, and areas far from settlements, implying the need to conserve water sources and minimize the extension of anthropogenic pressure for their long-term conservation. Additionally, we suggest the implications of a swamp deer-centric conservation strategy, with an emphasis on increasing connectivity through the corridors and landscape-level population connectivity through trans-boundary conservation initiatives between Nepal and India. Moreover, considering large herbivores' high vulnerability to extinction, similar researche incorporating anthropogenic factors is of the utmost importance to produce vital information on habitat suitability for conserving other regionally and globally endemic, habitat-specialized herbivores.
Keywords: Swamp deer, MaxEnt, Habitat, TAL, Conservation
1. Introduction
Large herbivores exhibit a significant role in molding the ecosystem through ecological function that benefits other species [1,2]. Despite their critical role in the ecosystem, various anthropogenic pressures such as habitat degradation, resource exhaustion, poaching, and human-wildlife conflicts [[3], [4], [5], [6], [7], [8]] have posed a threat of extinction to 60% of the world's large herbivores [8,9]. Although the South Asia holds preeminent varieties of terrestrial mega herbivores [10,11], 12 out of 15 terrestrial large herbivores are confronting critical conservation hurdles, as they are delegated “threatened” by the IUCN [2,8], owing to severe loss of their habitat and significant population decline.
Swamp deer (Rucervus duvaucelii), commonly referred as Barasingha, are habitat-specialized large grassland herbivores of the order Artiodactyla and the family Cervidae [12]. R. duvaucelii is primarily a grazer [13] but occasionally feeds on aquatic plants [14]. It heavily prefers short grassland [15] and principally feeds on Saccharum spp., Narenga porphyrocoma, Cynodon dactylon, Imperata cylindrica, Oryza rufpogon, and Phragmites karka [13,14,16,17]. R. duvaucelii prefers grasslands with water holes [15]. This preference is due to their requirement for water, as they engage in drinking activities at least twice daily during the winter and monsoon seasons, and three or more times during the summer season [17]. R. duvaucelii is usually known to move 2–3 km a day [18,19], but is acknowledged to avoid thickly forested areas [13]. The IUCN Red List of threatened species designates the species as globally vulnerable [12] and categorized as endangered on the National Red List of mammals at the national level [20,21]. It is also listed in Appendix I by CITES and protected under the National Parks and Wildlife Conservation Act 1973 of Nepal [22]. recognized three subspecies of R. duvaucelii, namely, Rucervus duvaucelii duvaucelii, Rucervus duvaucelii branderi, and Rucervus duvaucelii ranjitsinhi. R. d. duvaucelii (hereafter swamp deer) is the most abundant subspecies, contributing to around 80% of the worldwide population [19,23].
The distribution range of swamp deer encompasses the northern region of the Indo-Gangetic plain, including Nepal and various parts of India along the Ganges [24]. Generally, undisturbed patches of grassland are reported to encompass a substantial number of the swamp deer [12,25]. The swampy grassland within flood plains of Nepal contains the most extensive and suitable habitat for swamp deer [26,27]. In Nepal, the sole surviving population of swamp deer is now restricted to the pocket areas of Bardia National Park (BNP) and Shuklaphanta National Park (SNP), with an estimate of 2325 individuals [21]. With the majority of the population contained within the Terai Arc Landscape (TAL), SNP is thought to have the world's largest swamp deer herd [28,29], making the area critical for conservation [25,30].
In Nepal, swamp deer research has so far focused on a variety of topics, including abundance and density [[31], [32], [33], [34]], the impact of construction [35], activity pattern [17], habitat use [13,15,36], habitat suitability [24]. However, there is a dearth of insights regarding the present distribution status of swamp deer in Nepal. Moreover, the information on distribution of swamp deer in human-dominated landscapes and their response to anthropogenic variables are sparsely known. Since swamp deer is a habitat specialist endemic species [36,37], information regarding its potential distribution, taking anthropogenic aspects into consideration, can be deemed crucial for prioritizing conservation actions.
Escalating anthropogenic activities such as poaching for trophies and food assumption [[38], [39], [40], [41]], deforestation and forest fire [42,43], change in river dynamics, increase in siltation [44], habitat fragmentation [45], infrastructure development [46], disease [29,36,40,41], human-wildlife conflict [47,48], and food deficiency due to agricultural expansion [49] have become a major threat to biodiversity conservation globally. These activities are especially likely to endanger the existence of habitat specialist species like swamp deer, which are already threatened with extinction [50]. Thus, knowledge of human triggered and ecological drivers that influence the habitat use and distribution of threatened wildlife species is crucial for habitat management and conservation [[51], [52], [53], [54], [55], [56]]. These ecological drivers include topography and micro-climate (elevation, slope, aspect, and water distance) and anthropogenic-related factors (distance to human paths, roads, settlements, land use, and population density). These factors could potentially influence the long-term habitat composition, structure, or function, that can be identified with the help of species distribution modeling [52,[57], [58], [59], [60]], as well as potentially weaken species resilience to future disturbances [[61], [62], [63]].
With the evolution of 3S techniques (GIS, GPS, and RS), numerous models have been launched to document the distribution of suitable habitat. These models encompass mechanism models [64,65], regression models [66], and ecological niche models [66,67]. Recognized for its effectiveness in predicting wildlife habitat distribution and risk zone modeling [[68], [69], [70], [71]], the maximum entropy model (MaxEnt) has emerged as an acclaimed ecological niche model. Originally, the MaxEnt model was developed to quantify the presence density of specific species across the landscape [67], but over the years it has been used to predict species distribution pattern and ecological niches, utilizing presence-only data and environmental predictors, thus minimizing bias and elevating accuracy [67,72,73]. Some literature has discussed a few limitations of MaxEnt, including potential biases in the sampling of occurrence points, the complexity of feature selection, and the regularization multiplier [74]. Nevertheless, in terms of performance, this model is generally considered superior to others [75].
This study aimed to investigate the habitat suitability of vulnerable swamp deer using MaxEnt by assessing the contribution of ecological and anthropogenic variables with respect to their present distribution. Since small, isolated populations in patches are far more prone to becoming extinct as a result of demographic and environmental stochasticity, or random events of chance [76,77], it is a dire necessity to identify the potential suitable habitats of endemic large herbivores to augment those favorable characteristics and improve connectivity among habitats for sustainable conservation through the collaborative efforts of government and local people. Therefore, our study aimed to identify the existing distribution as well as viable habitats for new populations of swamp deer using a well-known species distribution model by incorporating anthropogenic, topographic, and vegetation-related variables.
2. Materials and method
2.1. Study area
The study was carried out within the western segment of Nepal's Terai Arc Landscape, including Banke, Bardia, Kailali, and Kanchanpur Districts, with an area of 9207 km2 (Fig. 1). SNP and its buffer zone (BZ), BNP and its BZ, Banke National Park (BaNP) and its BZ, and Krishnasar Conservation Area (KrCA) are the protected areas within the study area [78]. Around 30% (2754 km2) of the study area is encompassed by protected areas [28]. According to the IUCN's Protected Area Categories System, national parks go into category II, whereas conservation areas and buffer zones fall into category VI (www.iucn.org). In national parks, admission without authorization from the park management is prohibited. However, reasonable entry is allowed for locals to generate their subsistence needs in conservation areas and its buffer zone. The lowlands of Nepal are rich in floral and faunal diversity and have noteworthy conservation value [4,79,80]. Sal (Shorea robusta), Asna (Terminalia tomentosa), Botdhamero (Lagestroemia parviflora), and Sindure (Mallatus Philippines) are the dominant floral species [81]. Similarly, Swamp deer (Cervus duvaucelii), Black buck (Antilope cervicapra), hog deer (Axis porcinus), Python (Python molurus), Spotted deer (Axis axis), Sambar deer (Rusa unicolor), Gaur (Bos gaurus), Rhinoceros (Rhinoceros unicornis), Tiger (Panthera tigris tigris), Common leopard (Panthera pardus), Wild Asian elephant (Elephas maximas), and Giant hornbill (Buceros bicornis) are the major faunal species [28,36,48].
Fig. 1.
Map depicting study area with elevation gradient and type of protected areas within four districts of western TAL (Terai Arc Landscape).
3. Data collection
3.1. Swamp deer presence points
The data on presence points of swamp deer were collected during the period from 3rd February to 7th April 2021. We held discussions with local people, officials of division forest offices, and officials of protected areas to identify potential habitats for swamp deer and then visited those areas to record the evidence of the presence of swamp deer. Direct observation of individuals, pellet droppings, hoofmarks, and the presence of carcasses were the major methods of determining the presence of the species [82]. Swamp deer were identified with the help of their distinct antlers. Unlike other species, in the top part of the antler beam of swamp deer, the branches are arranged in a dichotomous way. Similarly, their hoof prints have a characteristic splayed pattern, showcasing their adaptive trait for thriving in marshy habitats. While swamp deer pellets are larger compared to those of other coexisting cervids such as hog deer or domestic animals like goats or sheep in the area, they were not considered definitive evidence on their own. Swamp deer presence was inferred in a particular area when at least two out of three indirect indicators (antlers, hoof prints, or pellets) were identified. A total of 173 presence points of swamp deer were documented from the study area through this process.
3.2. Environmental variables
A total of 14 variables, which included four topographic, four vegetation-related, and six anthropogenic variables, were extracted through various sources for the study (Table 1).
Table 1.
Fourteen environmental variables including four topographic, four vegetation related and six anthropogenic variables used for Variance inflation factor (VIF) test in the study.
| Source | Category | Variable | Unit |
|---|---|---|---|
| USGS | Topographic | Elevation | m |
| Aspect | Degree | ||
| Slope | Degree | ||
| GEOFABRIK | Distance to water | m | |
| Landsat | Vegetation-related | Mean EVI, Minimum EVI, Maximum EVI (Enhanced Vegetation Index) | Dimensionless |
| GFC | Forest | Dimensionless | |
| Department of Survey, Nepal | Anthropogenic | Distance to settlement | m |
| GEOFABRIK | Distance to the motor road | m | |
| Distance to path | m | ||
| Distance to building | m | ||
| HUMDATA | Population density | Dimensionless | |
| ICIMOD | Land use/land cover | m |
3.3. Topographic variables
Topographic variables such as elevation, slope, aspect, and proximity to water are known to govern the distribution of large herbivores [24,83]. The digital elevation model data with a resolution of 30 m was obtained from the USGS website (https://earthexplorer.usgs.gov/). Slope and aspect were generated from the digital elevation model using ArcGIS [84]. Shapefiles with information on the sources of water were downloaded from Geobabrik (https://www.geofabrik.de/data/shapefiles.html) and converted by ArcGIS into a distance raster file. Due to the inaccessibility of high-resolution climatic variables, elevation was used as a proxy for temperature.
3.4. Vegetation-related variables
Vegetation-related variables are one of the most important factors influencing the distribution of herbivores like swamp deer [85,86]. Forest cover, the minimum Enhanced Vegetation Index (EVI), the mean EVI, and the maximum EVI were the four variables collected for the study. Forest cover was obtained from Ref. [87] (https://earthenginepartners.appspot.com/science-2013-global-forest).The EVI time-series data used in the study was obtained from MODIS (https://earthexplorer.usgs.gov/). A Savitzky-Golay filter was used to smooth the data in the TIMESAT program [11]. The process reduced the amount of cloud cover in the environment, which helped in visualizing the images, and then the average values were calculated for overall indices to eventually obtain the final index of EVI.
3.5. Anthropogenic variables
Human activities have been identified as one of the primary threats to swamp deer survival and distribution [24,82]. Anthropogenic variables such as distance to a human path, distance to roads, distance to settlements, distance to buildings, population density, and land use data were obtained as variables in this study. Data from Geofabrik's website (https://www.geofabrik.de/data/shapefiles.html) was used to extract data on the location of the path, road, and buildings. Settlements data was extracted from the Department of the Survey, Nepal, and the distance raster file was created using ArcGIS. The ICIMOD website (http://www.icimod.org/) was used to download the data on land use and land cover change. Human population density data was extracted from HUMDATA (https://data.humdata.org/).
3.6. Spatial thinning and reducing multicollinearity among variables
As accounting for unequal sampling bias improves model prediction efficiency [88], spatial thinning in the oversampled zone is considered effective [89]. Spatial thinning can be done by removing spatially autocorrelated points and constructing the data on location to be more calibrated and fit for evaluation [90]. We used the “spThin” package [91] in R for preprocessing the presence data to reduce the spatial autocorrelation and minimize the sampling bias. Since the final spatial resolution of our variables was 30 m, we filtered the localities and retained 158 points out of 173 presence points by removing auto-correlated points and keeping at least a 100-m distance between two presence points to reduce bias and ensure that each location is unique [92]. A variance inflation factor (VIF) test was conducted to measure the multicollinearity among variables due to its ability to explicitly measure the degree of multicollinearity between predictor variables and its sensitivity and reliability in identifying highly correlated variables [93,94]. Those variables with a high VIF (more than 10) were removed to reduce multicollinearity [95,96], hence retaining 12 out of the 14 pre-defined variables to be applied in MaxEnt modeling.
3.7. Prediction of the distribution of swamp deer
MaxEnt, one of the most widely used tools for modeling the distribution of species [88], was utilized to assess the potential distribution of swamp deer in this study based on the collected presence points. The sample data collected in the real-world scenario might contain bias, influencing the performance of the model and causing over-fitting. Therefore, spatial filtering was done to avoid this bias [97,98]. To make modeling robust, MaxEnt was set up to utilize 10,000 background points with a 10-fold cross-validation method to construct binary maps, with the maximum training sensitivity plus specificity as the threshold in order to determine the best model.
We used the ENMeval package in R to optimize the MaxEnt model. For this, a total of 48 possible models with different combinations of the five feature classes (FC) L, Q, H, P, and T (where L is linear, Q is quadratic, H is hinge, P is product, and T is threshold), and eight regularization multiplier (RM) values (0.5–4, 0.5) were evaluated. The ENMeval package [99] was used to test 48 parameter combinations. We used Akaike information criterion (AIC) and 5% training omission rate (OR5) alongside the difference between AUC values to identify the best-fit model [100,101]. After model optimization, the model chosen with the characteristic class (FC) = LH, RM = 0.5 and delta AIC = 0 was established as best fit model. After fixing these parameter settings, the maximum number of iterations was set to 1000, and 70% of the presence points were used to train the model, whereas the remaining data was utilized to test the model [102]. Model evaluation was done with two methods, which included threshold dependent and threshold independent methods. The value of accuracy was directly obtained from the area under the curve (AUC) in the threshold independent model. AUC values of 0.7 denote poor model performance, values greater than 0.7 denote moderate model performance, and values greater than 0.9 denote excellent model performance [103]. Whereas for the threshold-dependent model, we performed true skill statistics (TSS) in R [104]. The TSS equals sensitivity plus specificity and ranges from −1 to 1. A TSS value of less than 0 represents random performance, whereas a value close to 1 represents a perfect fit [105]. The AUC and TSS were calculated for all 10 models, and then those values were averaged to obtain the final value [83,106]. The threshold used to maximize the TSS was used to convert the continuous probability map into binary (suitable/unsuitable) map [107]. The map was classified using reclassify function of ArcGIS, and the binary classified map was intersected with the shapefiles of protected areas and district to obtain the suitable area for swamp deer.
4. Results
4.1. Distribution of swamp deer
Of the total study area, 590 km2 (6% of the total area) was found to be suitable for the swamp deer (Fig. 2). Among the four districts, Kanchanpur had the highest suitable habitat, followed by Kailali, Banke, and Bardia (Table 2).
Fig. 2.
Suitable area for swamp deer (represented in red color) across four districts and three protected areas of Western Terai Arc Landscape (TAL). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 2.
Suitable area (km2) for swamp deer within each district of Western TAL.
| District | Suitable Area (km2) | % out of total suitable habitat |
|---|---|---|
| Banke | 62.23933 | 11 |
| Bardia | 56.01832 | 9 |
| Kailali | 216.0011 | 37 |
| Kanchanpur | 256.2041 | 43 |
| Total | 590.4629 | 100 |
Swamp deer distribution was identified in three national parks (NPs). Shuklaphanta NP had the largest suitable habitat for the species, followed by Bardia NP and the Banke buffer zone (BZ) (Table 3). A total of 265.23 km2 of swamp deer habitat was inside the protected areas. Approximately 65% of the total swamp deer habitat in the study area was located outside the protected areas.
Table 3.
Suitable area (km2) for swamp deer within each protected area of Western TAL.
| Protected area | Area deer (km2) | % out of total suitable habitat |
|---|---|---|
| Banke Buffer Zone | 24.48376 | 9 |
| Banke NP | 9.184139 | 3 |
| Bardia Buffer Zone | 3.173636 | 1 |
| Bardia NP | 36.55117 | 14 |
| Shuklaphanta Buffer Zone | 17.44644 | 7 |
| Shuklaphanta NP | 174.3932 | 66 |
| Total | 265.2323 | 100 |
4.2. Importance of variables to build the model
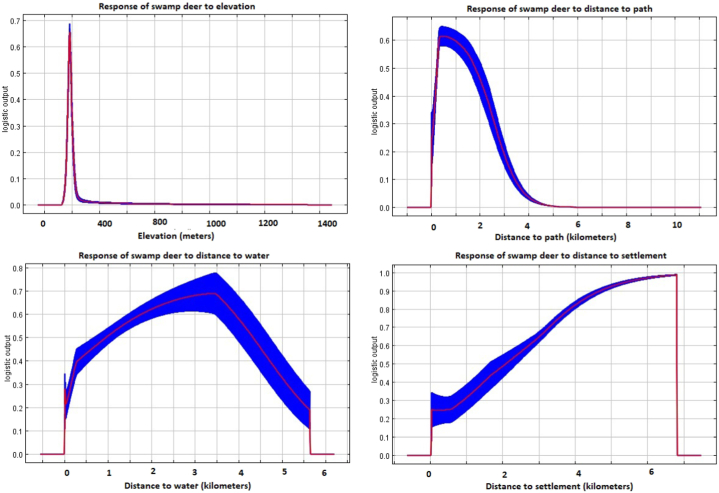
Among the 12 variables used to predict the suitable habitat for swamp deer, the variables with the highest contribution to building the model were elevation, followed by distance to the path, distance to water, and distance to settlement (Fig. 3). According to the response curves, swamp deer were most likely to be found in areas with low elevation (around 200 m), near water sources (<3.5 km), far from settlements (>6 km), but close to paths (<1 km) (Fig. 4).
Fig. 3.
The jackknife outcomes depict the gain from regularized training for a set of 12 variables, along with their respective contribution, both without and with only a particular variable. The lighter colors are indicative of variables that exhibit a significant decrease in gain in their absence, while medium colors correspond to environmental variables that yield the highest gain when utilized in isolation. The darkest color, on the other hand, represents regularized training gain when all variables are used. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
Response curves indicating the influence of four of the important variables (elevation, distance to path, distance to water, and distance to settlement) on habitat suitability of swamp deer. The X axis represents the value for four response variables, and the Y axis represent the logistic output probability. The figure represents that habitat suitability peaks at low elevation, nearby path, near water sources and far from the settlements.
4.3. Model accuracy
The accuracy of the models generated was excellent, with an average AUC value of (0.95 ± 0.08). The TSS value generated by the models also represented a fine fit, with a value of (0.72 ± 0.06). An average threshold of 0.185 was obtained to maximize the sum of sensitivity and specificity (Table 4).
Table 4.
The accuracy generated for the swamp deer distribution model through threshold dependent (TSS) and threshold independent (AUC) methods.
| Accuracy assessment | |||
|---|---|---|---|
| model | AUC | Threshold | TSS |
| 1 | 0.9599 | 0.1853 | 0.7164 |
| 2 | 0.9685 | 0.176 | 0.802 |
| 3 | 0.9589 | 0.2074 | 0.6639 |
| 4 | 0.9569 | 0.1558 | 0.7539 |
| 5 | 0.9699 | 0.2295 | 0.8348 |
| 6 | 0.9661 | 0.1871 | 0.7997 |
| 7 | 0.9443 | 0.1744 | 0.6794 |
| 8 | 0.9447 | 0.1603 | 0.6121 |
| 9 | 0.9643 | 0.2142 | 0.7276 |
| 10 | 0.951 | 0.1596 | 0.6994 |
| Average | 0.9585 | 0.185 | 0.72892 |
| St. dev. | 0.0088 | 0.023749 | 0.065986 |
5. Discussion
Our study sheds light on important component of endemic species conservation by assessing the habitat suitability and attributes associated with the distribution of a habitat specialist mega herbivore, the swamp deer, in an important conservation landscape of Nepal. In the recent scenarios of a drastic decline in the global population of large herbivores, owing to their relatively low population densities, unique habitat requirements, and slow life history characteristics [108,109], large-scale conservation initiatives are deemed imminent for their sustainable conservation. This implies that precise information on distribution, and various habitat parameters are necessary to ensure their future survival. It was especially important to produce detailed data on the potential distribution and identify the effect of anthropogenic variables on the habitat suitability of habitat-specialist large herbivore species like swamp deer, which live in a mosaic of protected and unprotected areas close to human habitations [[110], [111], [112]]. Therefore, this study is distinct in identifying the primary distribution locations (both inside and outside protected areas), investigating the role of variables influencing the distribution, and examining the extent of protected area coverage within highly suitable areas. Thus, our findings give insight into a crucial aspect of swamp deer-centric conservation, emphasizing landscape-level population connectivity through trans-boundary collaboration. Besides, as there is very limited information on the distribution of the habitat specialist large herbivores across their distribution range, the findings of our study are expected to pave a path and broaden our understanding regarding the prerequisites of conserving these endemic species.
As it has been observed that using SDM approaches to construct distribution maps for habitat-specialist species such as swamp deer yields better predictions than habitat-generalist species [37,113], our study included numerous field-based presence locations and important spatial variables on a fine scale (30 m resolution), allowing us to make more accurate predictions than previous studies [114]. We did not use species absence data during our analysis because it was difficult to determine the true absence of swam deer from these mosaic environments. However, since the number of pseudo-absences has the biggest impact on model accuracy for classification and machine learning techniques, we averaged numerous runs with fewer pseudo-absences for generating the most predictive models [102]. Even though ensemble methods of multiple algorithms are known to predict with higher accuracy, given our limited resources and computational power, we utilized single algorithm modeling techniques such as MaxEnt because it is known to generate a distributional map with an accuracy comparable to ensemble techniques [115]. Nevertheless, our model predicted fairly well (AUC of 0.95 and TSS of 0.72), which is to be anticipated quite well for a habitat-specialist species [37,116]. Moreover, studies like [37], have also previously assessed the distribution and identified priority conservation areas for swamp deer through modeling and field surveys in India. However, our study stands out as the first to assess swamp deer distribution in Nepal, employing finer scale variables at 30 m resolution and focusing on the effect of anthropogenic variables on habitat suitability.
5.1. Influence of variables on habitat suitability
Our study's habitat suitability model identified elevation as the most important variable governing swamp deer distribution, with habitat suitability peaking around 200 m elevation. The result coincides with a previous study reporting 100–300 m as the suitable altitude range for swamp deer [12]. Elevation has been widely regarded as one of the most important predictors for species distribution, especially among mammals, all around the world [[117], [118], [119]].
We hypothesized that swamp deer would prefer habitats close to water sources and avoid areas with high anthropogenic pressure. In this context, one of the important predictors governing the swamp deer distribution in our study was the distance from water sources. Our study indicated that habitat suitability decreased as the distance from water sources increased. Swamp deer, mostly feeding on grass species and occasionally on aquatic plants [14], are generally reported to show a high preference for grassland plots with water holes [15]. The species is known to drink water at least twice a day in the winter and even more often in the summer season [17]. Even though swamp deer move 2–3 km a day [18], they are mostly found to stay within the range of water sources. Previous studies, conducted in Nepal [24] as well as India [37], have also indicated the presence of suitable areas within a few kilometers of water sources.
The habitat suitability for swamp deer increased with increasing distance from settlement, whereas the suitability decreased with increased distance from the path. Areas with high human pressure are reported to negatively influence the space use of most ungulates [120]. Since settlements are highly modified by humans to accommodate their needs, swamp deer will most likely avoid those areas. Both wild ungulates and domestic cattle are forced to share the same area for foraging in human-dominated landscapes within non-protected regions [36,121]. Since paths are usually used by humans to graze their livestock to optimum foraging grounds, it is highly likely that swamp deer will use the same paths to forage.
Similarly, a prominent importance of such anthropogenic variables (distance to settlement, distance to path, distance to water sources, etc.) in governing distribution of habitat specialist herbivore have been presented throughout several studies. These factors include effect of water availability on large herbivores [122,123], and impact of anthropogenic structure in habitat preferences [83,124], indicating the essence of managing water sources and minimizing anthropogenic encroachment for conserving these habitat specialist large herbivores all around the globe.
5.2. Suitable habitat for swamp deer
We hypothesized that a significant portion of the potentially suitable habitat for this species is located outside the protected areas. In this context, approximately 45% of the suitable habitat of swamp deer was found to be incorporated into three protected areas within the study site. Our study revealed that the largest suitable habitat for swamp deer existed in SNP (174.93 km2). At present, SNP constitutes the largest herd of swamp deer in Asia [21,29]. However, according to the SNP's records, the number of swamp deer in 2019 was 2246, down from 2301 in 2014. This decline in population could be attributed to escalating anthropogenic pressure around the national park [16], thereby limiting its population in the area. Changes in land use patterns, ineffective grassland management techniques, the spread of invasive species, and the invasion of woody perennial species within the SNP grasslands [16] are some of the anthropogenic-induced limiting factors for the expansion of the swamp deer population. The proportion of site uses by swamp deer in SNP was reported to be 0.23, which was described as being affected by natural and anthropogenic correlates [125], suggesting species-specific planning for conservation and management of swamp deer, largely focusing on grassland management. BNP, which supports 106 individuals, also harbors a significant suitable habitat for swamp deer, but the population is reported to face a continuous shortage of food, diseases, and anthropogenic stress [29,126]. As per our study, the largest continuous patch of suitable habitat lies in SNP, which connects a conservation priority area (critical corridor) identified by the study of [37]. This critical corridor connects the swamp deer population of Nepal with the Indian population within the Sharda habitat block of India, which is mostly unprotected. This critical corridor should be protected and made functional through the coordination and cooperation of Nepalese and Indian authorities. This transboundary conservation approach will help to generate and conserve the meta-population of swamp deer, which is the largest global intermixing population. Also, the Nepalese population may become genetically separated from the Indian population if this corridor is lost because of accelerating human disturbances. Besides SNP, the suitable areas of swamp deer in other protected areas (Bardia NP and Banke NP) and outside protected areas (mainly Kailali District) have fragmented patches. The largest patch of suitable habitat in the non-protected region lies within the Kailali District, which needs immediate attention for conservation because it has the potential to harbor a swamp deer population.
Since over 65% of the suitable area lies outside protected areas, this presents opportunities as well as challenges in conserving this habitat specialist species. Opportunities in the sense that the suitable area lying outside protected areas could be further expanded as suitable habitat with efficient translocations, planned conservation efforts, and grassland management endeavors. Likewise, small, isolated, and fragmented patches outside protected areas are more susceptible to human encroachment and conversion of land use, which poses great challenges to maintaining those areas intact and favorable for swamp deer. Therefore, identification of viable habitat outside protected areas and establishment of new populations through translocation could be critical for the long-term survival of species [127,128]. Thus, future conservation strategies should focus on either establishing new adjacent protected areas or extending the existing ones by including the suitable habitat patches lying outside protected areas, particularly in the far western region of Nepal, where there is an absence of connectivity and no proper networks of protected areas. This will enable the inclusion of more suitable habitats for swamp deer within the potential protected area networks. However, if the existing and future suitable habitats in protected area networks do not overlap, just expanding protected areas will aid species conservation [129,130]. This shows that appropriate corridor policies should be taken into account in the future when formulating the management plans for swamp deer within concerned protected areas, which will consequently help to strengthen the protected area networks and ultimately promote swamp deer mobility.
Alternatively, if the development or expansion of the current protected area network is not viable due to economic, political, or societal mores, then we suggest collaborative swamp deer habitat conservation initiatives with the assistance of local inhabitants within the fragmented swamp deer habitats. Also, this can be feasible in developing countries like Nepal, which is globally recognized for its successful conservation endeavors through the participatory support of the locals, for example through a community forestry program [131]. Thus, we also suggest promoting community-based conservation interventions in such fragmented habitats. This will not only benefit endemic swamp deer and other highly threatened habitat-specialist species that occur in the region but also greatly contribute to the coexistence of humans and wildlife within the landscape. Therefore, the implementation of socio-ecological arrangements for landscape-level planning in the potential suitable habitats of swamp deer and conservation activities that bind people, their socio-cultural systems, and ecological systems [[132], [133], [134], [135]] could be the appropriate course of action in this scenario.
One limitation of the study is the use of single-algorithm modeling techniques due to limited resources and computational power. Ensemble methods of multiple algorithms are known to predict with higher accuracy, and future studies could potentially utilize these methods to further improve the accuracy of predictions. Additionally, while the study focused on the effect of anthropogenic variables on habitat suitability, there may be other factors that contribute to swamp deer distribution that were not considered. Future studies could explore these additional factors, as well as incorporate data on species absences, to further improve predictive models. Furthermore, while the study focused on the distribution of swamp deer in Nepal, it did not assess the species' population size or demographic trends, which are important considerations for conservation planning. Future studies could address these gaps in knowledge to inform more effective conservation strategies.
6. Implications and conservation recommendations
The results of our study have important implications for the conservation of swamp deer and other habitat specialist large herbivores in the Western Terai Arc Landscape of Nepal. We identified a significant area of suitable habitat for swamp deer, but only a small portion of it is encompassed within the existing protected area system. Therefore, our study recommends expanding the protected area system to include the largest patches of suitable habitat within the non-protected regions. Additionally, connectivity between fragmented patches of suitable habitat should be increased through trans-boundary conservation initiatives.
To ensure sustainable conservation of swamp deer and other sympatric species, it is important to conserve water sources, manage wetlands, reduce encroachment on grasslands, and control anthropogenic expansion in the potential habitat of these species. We recommend using swamp deer as an umbrella species for habitat management, which would also benefit other habitat-specialized herbivores of grasslands and wetlands. Our study emphasizes the need for continued efforts to protect these species and their habitats through integrated conservation initiatives.
7. Conclusion
In conclusion, our study provides critical information on the potential suitable habitat of swamp deer and the effects of anthropogenic variables on their distribution in the Western Terai Arc Landscape of Nepal. We identified a significant area of suitable habitat for swamp deer, and our study recommends expanding the existing protected area system and increasing connectivity between fragmented patches of suitable habitat.
Our study highlights the importance of effective conservation mechanisms in mitigating the risk of extinction for habitat specialist large herbivores. We recommend using swamp deer as an umbrella species for habitat management, which would also benefit other sympatric species of grasslands and wetlands. Overall, our study emphasizes the critical role of continued efforts to protect these species and their habitats for sustainable conservation.
Author contribution statement
Bijaya Dhami: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Binaya Adhikari: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Saroj Panthi; Bijaya Neupane: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Data availability statement
Data will be made available on request.
Additional information
No additional information is available for this paper.
Declaration of competing interest
We declare that there are not any financial or personal relationships that have influenced this work.
Acknowledgements
We express our gratitude to the Department of National Park and Wildlife Conservation for granting permission to conduct this study. We would also like to acknowledge the officials of Suklaphanta NP, Bardiya NP, Banke NP, Blackbuck CA and division forest offices of western TAL region. We would like to express our gratitude to the Institute of Forestry, Pokhara, Tribhuvan University, for their continuous technical support and assistance during the research project.
References
- 1.Danell K., Bergström R., Duncan P., Pastor J. Cambridge University Press; 2006. Large Herbivore Ecology, Ecosystem Dynamics and Conservation. [Google Scholar]
- 2.Ripple W.J., Estes J.A., Beschta R.L., Wilmers C.C., Ritchie E.G., Hebblewhite M., Berger J., Elmhagen B., Letnic M., Nelson M.P. Status and ecological effects of the world's largest carnivores. Science (80- 2014;343 doi: 10.1126/science.1241484. [DOI] [PubMed] [Google Scholar]
- 3.Adhikari B., Baral K., Bhandari S., Kunwar R.M., Subedi S.C. Prevalence of mortality in mammals: a retrospective study from wildlife rescue center of Nepal. Conserv. Sci. Pract. 2022;4 [Google Scholar]
- 4.Kunwar R.M., Baral B., Luintel S., Uprety Y., Poudel R.C., Adhikari B., Adhikari Y.P., Subedi S.C., Subedi C.K., Poudel P. Ethnomedicinal landscape: distribution of used medicinal plant species in Nepal. J. Ethnobiol. Ethnomed. 2022;18:34. doi: 10.1186/s13002-022-00531-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhami B., Bhusal A., Adhikari B., Miya M.S., Maharjan S.K., Neupane D., Adhikari H. Habitat suitability and conflict zone mapping for the blue bull (Boselaphus tragocamelus) across Nepal. Animals. 2023;13:937. doi: 10.3390/ani13050937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceballos G., Ehrlich P.R., Dirzo R. Biological annihilation via the ongoing sixth mass extinction signaled by vertebrate population losses and declines. Proc. Natl. Acad. Sci. USA. 2017;114:E6089–E6096. doi: 10.1073/pnas.1704949114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindsey P.A., Chapron G., Petracca L.S., Burnham D., Hayward M.W., Henschel P., Hinks A.E., Garnett S.T., Macdonald D.W., Macdonald E.A. Relative efforts of countries to conserve world's megafauna. Glob. Ecol. Conserv. 2017;10:243–252. [Google Scholar]
- 8.Ripple W.J., Newsome T.M., Wolf C., Dirzo R., Everatt K.T., Galetti M., Hayward M.W., Kerley G.I.H., Levi T., Lindsey P.A. Collapse of the world's largest herbivores. Sci. Adv. 2015;1 doi: 10.1126/sciadv.1400103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trouwborst A. Global large herbivore conservation and international law. Biodivers. Conserv. 2019;28:3891–3914. [Google Scholar]
- 10.Ahrestani F.S., Heitkönig I.M.A., van Langevelde F., Vaidyanathan S., Madhusudan M.D., Prins H.H.T. Moisture and nutrients determine the distribution and richness of India's large herbivore species assemblage. Basic Appl. Ecol. 2011;12:634–642. [Google Scholar]
- 11.Jönsson P., Eklundh L. TIMESAT—a program for analyzing time-series of satellite sensor data. Comput. Geosci. 2004;30:833–845. [Google Scholar]
- 12.Duckworth J.W., Kumar N.S., Pokharel C.P., Sagar B.H., Timmins R. e. T4257A22167675. 2015. (Rucervus Duvaucelii. The IUCN Red List of Threatened Species 2015). [Google Scholar]
- 13.Pokharel C.P. Royal Bardia National Park; Nepal: 1996. Food Habit and Habitat Utilization of Swamp Deer (Cervus duvauceli Duvauceli) [Google Scholar]
- 14.Moe S.R. The importance of aquatic vegetation for the management of the barasingha Cervus duvauceli in Nepal. Biol. Conserv. 1994;70:33–37. [Google Scholar]
- 15.Bhattarai B. Dep. For. Tribhuvan Univ. Kathmandu; Nepal: 2015. Habitat Preference and Evaluaton of Swamp Deer (Cervus duvauceli Duvauceli) in Bardia National Park, BSc Thesis; p. 82pp. [Google Scholar]
- 16.Bhattarai P. Threats on grassland ecosystem services: a case from Shuklaphanta Wildlife Reserve, Nepal. J. Sci. Technol. 2012;13:159–166. [Google Scholar]
- 17.Bhatta B. 2008. Daily Activities, Distribution, and Food Preference of Swamp Deer (Cervus duvauceli Duvauceli) [Google Scholar]
- 18.Martin C. 1977. Status and Ecology of the Barasingha (Cerous Duvauceli Branderi)in Kanha National Park (India) [Google Scholar]
- 19.Qureshi Q., Sawarkar V.B., Mathur P.K. Proj. Report. Wildl. Inst. India, Dehradun; 1995. Ecology and Management of Swamp Deer (Cervus duvauceli) in Dudhwa Tiger Reserve, UP (India) [Google Scholar]
- 20.Jnawali S.R., Baral H., Lee S., Acharya K., Upadhyay G., Pandey M., Griffiths J. The status of Nepal mammals: the national red list series, department of national Parks and wildlife conservation kathmandu, Nepal, Pref. by Simon M. Stuart Chair IUCN Species Surviv. Comm. Status Nepal's Mamm. Natl. Red List Ser. 2011;4 [Google Scholar]
- 21.DNPWC . Department of National Park and Wildlife Conservation; 2020. Cervus duvauceli.http://Dnpwc.Gov.Np/En/Species-Detail/71/ [Google Scholar]
- 22.Groves C.P. Geographic variation in the Barasingha or swamp deer (Cervus duvauceli) J. Bombay Nat. Hist. Soc. 1982;79:620–629. [Google Scholar]
- 23.Sankar K., Goyal S.P. ENVIS Bull. Wildl.; 2004. Ungulates of India. [Google Scholar]
- 24.Ghimire S.K., Dhamala M.K., Lamichhane B.R., Ranabhat R., KC K.B., Poudel S. Identification of suitable habitat for swamp deer Rucervus duvaucelii duvaucelii (mammalia: Artiodactyla: Cervidae) in chitwan national park, Nepal. J. Threat. Taxa. 2019;11:13644–13653. [Google Scholar]
- 25.Qureshi Q., Sawarkar V.B., Rahmani A.R., Mathur P.K. Swamp deer or barasingha (Cervus duvauceli Cuvier, 1823) Envis Bull. 2004;7:181–192. [Google Scholar]
- 26.Mishra H.R. Muntiacus muntjak); 1982. The Ecology and Behaviour of Chital (Axis axis) in the Royal Chitwan National Park, Nepal: with Comparative Studies of Hog Deer (Axis Porcinus), Sambar (Cervus unicolor) and Barking Deer. [Google Scholar]
- 27.Gurung K.K. A. Deutsch; Nepal: 1983. Heart of the Jungle: the Wildlife of Chitwan. [Google Scholar]
- 28.DNPWC . Department of National Parks and Wildlife Conservation.; Nepal: 2019. Annual Report (July 2019–June 2020) [Google Scholar]
- 29.Poudel B.S. Thirty years of managing Suklaphanta, the swamp deer and the tiger: issues and strategies. Initiat. 2007;1:72–76. doi: 10.3126/init.v1i0.2684. [DOI] [Google Scholar]
- 30.Singh A. Threat. Deer. Proc. A Work. Meet. Deer Spec. Gr. Surviv. Serv. Comm. IUCN Threat. Deer Program. a Doss. Plan. Restor. Program. Threat. Mamm. with Spec. Ref. T; 1978. The status of the swamp deer Cervus duvauceli duvauceli in the dudhwa national park; pp. 132–142. [Google Scholar]
- 31.Wegge P., Storaas T. Sampling tiger ungulate prey by the distance method: lessons learned in Bardia National Park, Nepal. Anim. Conserv. 2009;12:78–84. [Google Scholar]
- 32.Adhikari P., Thapa T.B. Estimating abundance of large mammalian prey in Suklaphanta wildlife reserve, Nepal. J. Instr. Sci. Technol. 2013;18:84–89. [Google Scholar]
- 33.Gautam D.N. Assessment of wild ungulates in the KARNALI flood plain of bardia national park, Nepal. Int. J. Conserv. Sci. 2013;4 [Google Scholar]
- 34.Baniya R.K., Baniya C.B., Mou P., Ge J. Reserv. Nepal; 2017. Prey Selection by Tiger (Panthera Tigris Tigris) in Shuklaphanta Wildlife Reserve Nepal, Prey Sel. By Tiger (Panthera Tigris Tigris) Shuklaphanta Wildl. [Google Scholar]
- 35.Henshaw J. The barasingha, or swamp deer, in Suklaphanta Wildlife Reserve, Nepal. Oryx. 1994;28:199–206. [Google Scholar]
- 36.Regmi S., Neupane B., Dhami B., Gautam D., Panthi S., Poudel M. Niche breadth and overlap of spotted deer and domestic cattle with swamp deer in tropical region of Nepal. Ecol. Process. 2022;11:1–10. [Google Scholar]
- 37.Paul S., Sarkar D., Patil A., Ghosh T., Talukdar G., Kumar M., Habib B., Nigam P., Mohan D., Pandav B. Assessment of endemic northern swamp deer (Rucervus duvaucelii duvaucelii) distribution and identification of priority conservation areas through modeling and field surveys across north India. Glob. Ecol. Conserv. 2020 [Google Scholar]
- 38.Duffy R., John F.A. V St, Büscher B., Brockington D. Toward a new understanding of the links between poverty and illegal wildlife hunting. Conserv. Biol. 2016;30:14–22. doi: 10.1111/cobi.12622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esmail N., Wintle B.C., t Sas-Rolfes M., Athanas A., Beale C.M., Bending Z., Dai R., Fabinyi M., Gluszek S., Haenlein C. Emerging illegal wildlife trade issues: a global horizon scan. Conserv. Lett. 2020;13 [Google Scholar]
- 40.Dhami B., Timilsina S., Adhikari A., Neupane B., Chhetri N.B., Sharma A., Paudel A., Miya M.S., Sharma B., Chhetri A. Research trends, conservation issues and approaches for the endangered Red panda (Ailurus fulgens): a systematic review of literatures across their home-range. J. Anim. Divers. 2021;3:57–68. [Google Scholar]
- 41.Khulal R., Neupane B., Dhami B., Regmi S., Tiwari G.P., Parajuli M. Habitat use and conservation threats to wild water buffalo Bubalus arnee (mammalia: Artiodactyla: bovidae) in Koshi Tappu wildlife reserve, Nepal. J. Threat. Taxa. 2021;13:19714–19724. [Google Scholar]
- 42.Fearnside P.M. Deforestation in Brazilian Amazonia: history, rates, and consequences. Conserv. Biol. 2005;19:680–688. [Google Scholar]
- 43.Sadadev B.M., Silwal T., Dhami B., Thapa N., Neupane B., Rana A., Singh H.B. Do grassland burning practices affect the distribution of the Hispid hare, Caprolagus hispidus (Pearson, 1839)? A study at the Shuklaphanta National Park, Nepal. J. Anim. Divers. 2021;3:0. [Google Scholar]
- 44.Naithani S., Bedi P., Areendran G. Habitat suitability analysis for swamp deer (Rucervus duvaucelii) using Geo-spatial tools in dudhwa national park. Am. J. Rem. Sens. 2018;6:52–58. [Google Scholar]
- 45.Primack R.B. Sinauer Associates Sunderland; 2006. Essentials of Conservation Biology. [Google Scholar]
- 46.Dirzo R., Raven P.H. Global state of biodiversity and loss. Annu. Rev. Environ. Resour. 2003;28:137–167. [Google Scholar]
- 47.Bhandari S., Adhikari B., Baral K., Subedi S.C. Greater one-horned rhino (Rhinoceros unicornis) mortality patterns in Nepal. Glob. Ecol. Conserv. 2022;38 [Google Scholar]
- 48.Koirala P., Neupane B., Silwal T., Dhami B., Regmi S., Dahal D. Distribution of blue bull (Boselaphus tragocamelus) and its conservation threats in Bardia national park, Nepal. J. For. Nat. Resour. Manag. 2020;2:50–60. [Google Scholar]
- 49.Laurance W.F., Sayer J., Cassman K.G. Agricultural expansion and its impacts on tropical nature. Trends Ecol. Evol. 2014;29:107–116. doi: 10.1016/j.tree.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 50.Pereira H.M., Daily G.C., Roughgarden J. A framework for assessing the relative vulnerability of species to land‐use change. Ecol. Appl. 2004;14:730–742. [Google Scholar]
- 51.Baral K., Adhikari B., Bhandari S., Kunwar R.M., Sharma H.P., Aryal A., Ji W. Impact of climate change on distribution of common leopard (Panthera pardus) and its implication on conservation and conflict in Nepal. Heliyon. 2023 doi: 10.1016/j.heliyon.2023.e12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dhami B., Neupane B., Devkota B.P., Maraseni T., Sadadev B.M., Bista S., Adhikari A., Chhetri N.B., Panta M., Stewart A.B. Factors affecting the occupancy of Chinese pangolins (Manis pentadactyla) suggest a highly specialized ecological niche. Ecosphere. 2023;14 [Google Scholar]
- 53.Bhandari S., Adhikari B., Baral K., Panthi S., Kunwar R.M., Thapamagar T., Psaralexi M., Bhusal D.R., Youlatos D. Climate change threatens striped hyena (Hyaena hyaena) distribution in Nepal. Mammal Res. 2022;67:433–443. [Google Scholar]
- 54.Bajaj S., Amali D.G.B. Emerg. Res. Comput. Information, Commun. Appl. Springer; 2019. Species environmental niche distribution modeling for Panthera tigris tigris ‘royal bengal Tiger'Using machine learning; pp. 251–263. [Google Scholar]
- 55.Aarts G., MacKenzie M., McConnell B., Fedak M., Matthiopoulos J. Estimating space‐use and habitat preference from wildlife telemetry data. Ecography. 2008;31:140–160. [Google Scholar]
- 56.Balakrishnan M., Easa P.S. Habitat preferences of the larger mammals in the Parambikulam wildlife sanctuary, Kerala, India. Biol. Conserv. 1986;37:191–200. [Google Scholar]
- 57.Franklin J.F., Spies T.A., Van Pelt R., Carey A.B., Thornburgh D.A., Berg D.R., Lindenmayer D.B., Harmon M.E., Keeton W.S., Shaw D.C. Disturbances and structural development of natural forest ecosystems with silvicultural implications, using Douglas-fir forests as an example. For. Ecol. Manage. 2002;155:399–423. [Google Scholar]
- 58.Palik B.J., Mitchell R.J., Hiers J.K. Modeling silviculture after natural disturbance to sustain biodiversity in the longleaf pine (Pinus palustris) ecosystem: balancing complexity and implementation. For. Ecol. Manage. 2002;155:347–356. [Google Scholar]
- 59.Hessburg P.F., Spies T.A., Perry D.A., Skinner C.N., Taylor A.H., Brown P.M., Stephens S.L., Larson A.J., Churchill D.J., Povak N.A. Tamm review: management of mixed-severity fire regime forests in Oregon, Washington, and Northern California. For. Ecol. Manage. 2016;366:221–250. [Google Scholar]
- 60.Baral K., Bhandari S., Adhikari B., Kunwar R.M., Sharma H.P., Aryal A., Ji W. Anthropogenic mortality of large mammals and trends of conflict over two decades in Nepal. Ecol. Evol. 2022;12 doi: 10.1002/ece3.9381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hessburg P.F., Miller C.L., Parks S.A., Povak N.A., Taylor A.H., Higuera P.E., Prichard S.J., North M.P., Collins B.M., Hurteau M.D. Climate, environment, and disturbance history govern resilience of western North American forests. Front. Ecol. Evol. 2019;7:239. [Google Scholar]
- 62.Leclerc J.-C., Brante A., Viard F. Rapid recovery of native habitat-builders following physical disturbance on pier pilings offsets colonization of cryptogenic and non-indigenous species in a Chilean port. Mar. Environ. Res. 2021;163 doi: 10.1016/j.marenvres.2020.105231. [DOI] [PubMed] [Google Scholar]
- 63.Radeloff V.C., Mladenoff D.J., Boyce M.S. Effects of interacting disturbances on landscape patterns: budworm defoliation and salvage logging. Ecol. Appl. 2000;10:233–247. [Google Scholar]
- 64.Xu W., Ouyang Z., Jiang Z., Zheng H., Liu J. Assessment of giant panda habitat in the daxiangling mountain range, sichuan, China. Biodivers. Sci. 2006;14:223. [Google Scholar]
- 65.Zhiyun O., Jianguo L., Han X., Yingchun T., Hemin Z. An assessment of giant panda habitat in Wolong Nature Reserve. Acta Ecol. Sin. 2001;21:1869–1874. [Google Scholar]
- 66.Schadt S., Revilla E., Wiegand T., Knauer F., Kaczensky P., Breitenmoser U., Bufka L., Červený J., Koubek P., Huber T. Assessing the suitability of central European landscapes for the reintroduction of Eurasian lynx. J. Appl. Ecol. 2002;39:189–203. [Google Scholar]
- 67.Phillips S.J., Anderson R.P., Schapire R.E. Maximum entropy modeling of species geographic distributions. Ecol. Model. 2006;190:231–259. [Google Scholar]
- 68.Clements G.R., Rayan D.M., Aziz S.A., Kawanishi K., Traeholt C., Magintan D., Yazi M.F.A., Tingley R. Predicting the distribution of the Asian tapir in Peninsular Malaysia using maximum entropy modeling. Integr. Zool. 2012;7:400–406. doi: 10.1111/j.1749-4877.2012.00314.x. [DOI] [PubMed] [Google Scholar]
- 69.Wilting A., Cord A., Hearn A.J., Hesse D., Mohamed A., Traeholdt C., Cheyne S.M., Sunarto S., Jayasilan M.-A., Ross J. Modelling the species distribution of flat-headed cats (Prionailurus planiceps), an endangered South-East Asian small felid. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Adhikari B., Baral K., Bhandari S., Szydlowski M., Kunwar R.M., Panthi S., Neupane B., Koirala R.K. Potential risk zone for anthropogenic mortality of carnivores in Gandaki Province, Nepal. Ecol. Evol. 2022;12 doi: 10.1002/ece3.8491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adhikari B., Bhandari S., Baral K., Lamichhane S., Subedi S.C. Raptors at risk: attributes of mortality within an anthropogenic landscape in the Mid-Hills region of Nepal. Glob. Ecol. Conserv. 2022;38 doi: 10.1016/j.gecco.2022.e02258. [DOI] [Google Scholar]
- 72.Merow C., Smith M.J., Silander J.A., Jr. A practical guide to MaxEnt for modeling species' distributions: what it does, and why inputs and settings matter. Ecography. 2013;36:1058–1069. [Google Scholar]
- 73.Pearson R.G., Raxworthy C.J., Nakamura M., Townsend Peterson A. Predicting species distributions from small numbers of occurrence records: a test case using cryptic geckos in Madagascar. J. Biogeogr. 2007;34:102–117. [Google Scholar]
- 74.Lissovsky A.A., V Dudov S. Species-distribution modeling: advantages and limitations of its application. 2. MaxEnt. Biol. Bull. Rev. 2021;11:265–275. [Google Scholar]
- 75.Wisz M.S., Hijmans R.J., Li J., Peterson A.T., Graham C.H., Guisan A., Group N.P.S.D.W. Effects of sample size on the performance of species distribution models. Divers. Distrib. 2008;14:763–773. [Google Scholar]
- 76.Lande R. Anthropogenic, ecological and genetic factors in extinction and conservation. Popul. Ecol. 1998;40:259–269. [Google Scholar]
- 77.Smith K.G., Almeida R.J. When are extinctions simply bad luck? Rarefaction as a framework for disentangling selective and stochastic extinctions. J. Appl. Ecol. 2020;57:101–110. [Google Scholar]
- 78.DNPWC, Protected areas of Nepal . Department of national parks and wildlife conservation; 2022. Kathmandu, Nepal.http://Dnpwc.Gov.Np/En/ [Google Scholar]
- 79.Kunwar R.M., Thapa-Magar K.B., Subedi S.C., Kutal D.H., Baral B., Joshi N.R., Adhikari B., Upadhyaya K.S., Thapa-Magar S., Ansari A.S. Distribution of important medicinal plant species in Nepal under past, present, and future climatic conditions. Ecol. Indicat. 2023;146 [Google Scholar]
- 80.Kutal D.H., Kunwar R.M., Uprety Y., Adhikari Y.P., Bhattarai S., Adhikari B., Kunwar L.M., Bhatt M.D., Bussmann R.W. Selection of medicinal plants for traditional medicines in Nepal. J. Ethnobiol. Ethnomed. 2021;17:1–11. doi: 10.1186/s13002-021-00486-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.DFRS . For. Resour. Assess; 2015. State of Nepal's Forests. Forest Resource Assessment (FRA) Nepal, Department of Forest Research and Survey (DFRS). Kathmandu, Nepal.DFRS, State of Nepal’s forests.https://jukuri.luke.fi/bitstream/handle/10024/532218/State%20of%20Nepals%20Forests%20(DFRS)_1457244174_1457602126.pdf?sequence=1 [Google Scholar]
- 82.Paul S., Pandav B., Mohan D., Habib B., Nigam P., Mondol S. Current distribution and status of swamp deer Rucervus duvaucelii duvaucelii in the upper Gangetic plains of north India. Oryx. 2018;52:646–653. [Google Scholar]
- 83.Sharma P., Panthi S., Yadav S.K., Bhatta M., Karki A., Duncan T., Poudel M., Acharya K.P. Suitable habitat of wild Asian elephant in western Terai of Nepal. Ecol. Evol. 2020;10:6112–6119. doi: 10.1002/ece3.6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.ESRI . Environ. Syst. Res. Institute; CA: 2017. ArcGIS Desktop: Release 10. [Google Scholar]
- 85.Gandiwa E. Vegetation factors influencing density and distribution of wild large herbivores in a southern A frican savannah. Afr. J. Ecol. 2014;52:274–283. [Google Scholar]
- 86.Perea R., Perea-García-Calvo R., Díaz-Ambrona C.G., San Miguel A. The reintroduction of a flagship ungulate Capra pyrenaica: assessing sustainability by surveying woody vegetation. Biol. Conserv. 2015;181:9–17. [Google Scholar]
- 87.Hansen M.C., V Potapov P., Moore R., Hancher M., Turubanova S.A., Tyukavina A., Thau D., V Stehman S., Goetz S.J., Loveland T.R. High-resolution global maps of 21st-century forest cover change. Science. 2013;342:850–853. doi: 10.1126/science.1244693. 80- [DOI] [PubMed] [Google Scholar]
- 88.Kramer-Schadt S., Niedballa J., Pilgrim J.D., Schröder B., Lindenborn J., Reinfelder V., Stillfried M., Heckmann I., Scharf A.K., Augeri D.M. The importance of correcting for sampling bias in MaxEnt species distribution models. Divers. Distrib. 2013;19:1366–1379. [Google Scholar]
- 89.Phillips S.J., Dudík M., Elith J., Graham C.H., Lehmann A., Leathwick J., Ferrier S. Sample selection bias and presence‐only distribution models: implications for background and pseudo‐absence data. Ecol. Appl. 2009;19:181–197. doi: 10.1890/07-2153.1. [DOI] [PubMed] [Google Scholar]
- 90.Boria R.A., Olson L.E., Goodman S.M., Anderson R.P. Spatial filtering to reduce sampling bias can improve the performance of ecological niche models. Ecol. Model. 2014;275:73–77. [Google Scholar]
- 91.Aiello-Lammens M.E., Boria R.A., Radosavljevic A., Vilela B., Anderson R.P. spThin: an R package for spatial thinning of species occurrence records for use in ecological niche models. Ecography. 2015;38:541–545. [Google Scholar]
- 92.Karki A., Panthi S. Factors affecting livestock depredation by snow leopards (Panthera uncia) in the Himalayan region of Nepal. PeerJ. 2021;9 doi: 10.7717/peerj.11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O’brien R.M. A caution regarding rules of thumb for variance inflation factors. Qual. Quantity. 2007;41:673–690. [Google Scholar]
- 94.Neter J., Kutner M.H., Nachtsheim C.J., Wasserman W. 1996. Applied Linear Statistical Models. [Google Scholar]
- 95.Hair J.F., Black W.C., Babin B.J., Anderson R.E. Pearson College division, Pers; London, UK: 2010. Multivariate Data Analysis. [Google Scholar]
- 96.Kutner M.H., Nachtsheim C.J., Neter J., Li W. McGraw-Hill Irwin; New York): 2005. Applied Linear Statistical Models. [Google Scholar]
- 97.Griffth D.A. 2003. Spatial Autocorrelation and Spatial Filtering. [Google Scholar]
- 98.Segurado P., Araujo M.B., Kunin W.E. Consequences of spatial autocorrelation for niche‐based models. J. Appl. Ecol. 2006;43:433–444. [Google Scholar]
- 99.Kass J.M., Muscarella R., Galante P.J., Bohl C.L., Pinilla-Buitrago G.E., Boria R.A., Soley-Guardia M., Anderson R.P. ENMeval 2.0: redesigned for customizable and reproducible modeling of species' niches and distributions. Methods Ecol. Evol. 2021;12:1602–1608. [Google Scholar]
- 100.Steen B., Cardoso A.C., Tsiamis K., Nieto K., Engel J., Gervasini E. Modelling hot spot areas for the invasive alien plant Elodea nuttallii in the EU. Manag. Biol. Invasions. 2019;10:151. [Google Scholar]
- 101.Mao M., Chen S., Ke Z., Qian Z., Xu Y. Using MaxEnt to predict the potential distribution of the little fire Ant (Wasmannia auropunctata) in China. Insects. 2022;13:1008. doi: 10.3390/insects13111008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barbet-Massin M., Jiguet F., Albert C.H., Thuiller W. Selecting pseudo‐absences for species distribution models: how, where and how many? Methods Ecol. Evol. 2012;3:327–338. [Google Scholar]
- 103.Pearce J., Ferrier S. Evaluating the predictive performance of habitat models developed using logistic regression. Ecol. Model. 2000;133:225–245. [Google Scholar]
- 104.R Core Team . 2018. R: A Language and Environment for Statistical Computing. R Found. Stat. Comput. Ing, Vienna, Austria 706. [Google Scholar]
- 105.Allouche O., Tsoar A., Kadmon R. Assessing the accuracy of species distribution models: prevalence, kappa and the true skill statistic (TSS) J. Appl. Ecol. 2006;43:1223–1232. [Google Scholar]
- 106.Bista M., Panthi S., Weiskopf S.R. Habitat overlap between Asiatic black bear Ursus thibetanus and red panda Ailurus fulgens in Himalaya. PLoS One. 2018;13 doi: 10.1371/journal.pone.0203697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liu C., White M., Newell G. Selecting thresholds for the prediction of species occurrence with presence‐only data. J. Biogeogr. 2013;40:778–789. [Google Scholar]
- 108.Wallach A.D., Izhaki I., Toms J.D., Ripple W.J., Shanas U. What is an apex predator? Oikos. 2015;124:1453–1461. [Google Scholar]
- 109.Ripple W.J., Chapron G., López-Bao J.V., Durant S.M., Macdonald D.W., Lindsey P.A., Bennett E.L., Beschta R.L., Bruskotter J.T., Campos-Arceiz A. Saving the world's terrestrial megafauna. Bioscience. 2016;66:807–812. doi: 10.1093/biosci/biw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jathanna D., Karanth K.U., Johnsingh A.J.T. Estimation of large herbivore densities in the tropical forests of southern India using distance sampling. J. Zool. 2003;261:285–290. [Google Scholar]
- 111.Linkie M., Guillera-Arroita G., Smith J., Ario A., Bertagnolio G., Cheong F., Clements G.R., Dinata Y., Duangchantrasiri S., Fredriksson G. Cryptic mammals caught on camera: assessing the utility of range wide camera trap data for conserving the endangered Asian tapir. Biol. Conserv. 2013;162:107–115. [Google Scholar]
- 112.Marshal J.P. Survival estimation of a cryptic antelope via photographic capture–recapture. Afr. J. Ecol. 2017;55:21–29. [Google Scholar]
- 113.Rhoden C.M., Peterman W.E., Taylor C.A. Maxent-directed field surveys identify new populations of narrowly endemic habitat specialists. PeerJ. 2017;5 doi: 10.7717/peerj.3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Aubry K.B., Raley C.M., McKelvey K.S. The importance of data quality for generating reliable distribution models for rare, elusive, and cryptic species. PLoS One. 2017;12 doi: 10.1371/journal.pone.0179152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kaky E., Nolan V., Alatawi A., Gilbert F. A comparison between Ensemble and MaxEnt species distribution modelling approaches for conservation: a case study with Egyptian medicinal plants. Ecol. Inf. 2020;60 [Google Scholar]
- 116.Connor T., Hull V., Viña A., Shortridge A., Tang Y., Zhang J., Wang F., Liu J. Effects of grain size and niche breadth on species distribution modeling. Ecography. 2018;41:1270–1282. [Google Scholar]
- 117.Sathyakumar S., Bashir T., Bhattacharya T., Poudyal K. 2011. Assessing Mammal Distribution and Abundance in Intricate Eastern Himalayan Habitats of Khangchendzonga. Sikkim, India. [Google Scholar]
- 118.Santos M.J., Smith A.B., Thorne J.H., Moritz C. The relative influence of change in habitat and climate on elevation range limits in small mammals in Yosemite National Park, California, USA. Clim. Chang. Responses. 2017;4:1–12. [Google Scholar]
- 119.Musila S., Chen Z.-Z., Li Q., Yego R., Zhang B., Onditi K., Muthoni I., He S.-W., Omondi S., Mathenge J. Diversity and distribution patterns of non-volant small mammals along different elevation gradients on Mt. Kenya, Kenya. Zool. Res. 2019;40:53. doi: 10.24272/j.issn.2095-8137.2019.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lahkar D., Ahmed M.F., Begum R.H., Das S.K., Harihar A. Responses of a wild ungulate assemblage to anthropogenic influences in Manas National Park, India. Biol. Conserv. 2020;243 [Google Scholar]
- 121.Bhandari S., Crego R.D., Stabach J.A. Spatial segregation between wild ungulates and livestock outside protected areas in the lowlands of Nepal. PLoS One. 2022;17 doi: 10.1371/journal.pone.0263122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shannon G., Matthews W.S., Page B.R., Parker G.E., Smith R.J. The affects of artificial water availability on large herbivore ranging patterns in savanna habitats: a new approach based on modelling elephant path distributions. Divers. Distrib. 2009;15:776–783. [Google Scholar]
- 123.Ogutu J.O., Reid R.S., Piepho H.-P., Hobbs N.T., Rainy M.E., Kruska R.L., Worden J.S., Nyabenge M. Large herbivore responses to surface water and land use in an East African savanna: implications for conservation and human-wildlife conflicts. Biodivers. Conserv. 2014;23:573–596. [Google Scholar]
- 124.Leblond M., Dussault C., Ouellet J. Avoidance of roads by large herbivores and its relation to disturbance intensity. J. Zool. 2013;289:32–40. [Google Scholar]
- 125.Lamichhane S., Khanal G., Karki J.B., Aryal C., Acharya S. Natural and anthropogenic correlates of habitat use by wild ungulates in Shuklaphanta National Park, Nepal. Glob. Ecol. Conserv. 2020;24 [Google Scholar]
- 126.IUCN . 2020. The IUCN Red List of Threatened Species.http://Www.Iucnredlist.Org Version 2020-2. [Google Scholar]
- 127.Berger-Tal O., Blumstein D.T., Swaisgood R.R. Conservation translocations: a review of common difficulties and promising directions. Anim. Conserv. 2020;23:121–131. [Google Scholar]
- 128.Langridge J., Sordello R., Reyjol Y. Outcomes of wildlife translocations in protected areas: what is the type and extent of existing evidence? A systematic map protocol. Environ. Evid. 2020;9:1–11. [Google Scholar]
- 129.Araújo M.B., Alagador D., Cabeza M., Nogués‐Bravo D., Thuiller W. Climate change threatens European conservation areas. Ecol. Lett. 2011;14:484–492. doi: 10.1111/j.1461-0248.2011.01610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hannah L., Midgley G., Hughes G., Bomhard B. The view from the Cape: extinction risk, protected areas, and climate change. Bioscience. 2005;55:231–242. [Google Scholar]
- 131.Lamsal P., Kumar L., Aryal A., Atreya K. Future climate and habitat distribution of Himalayan musk deer (moschus chrysogaster) Ecol. Inf. 2018;44:101–108. [Google Scholar]
- 132.Berkes F., Colding J., Folke C. Cambridge university press; 2008. Navigating Social-Ecological Systems: Building Resilience for Complexity and Change. [Google Scholar]
- 133.Virapongse A., Brooks S., Metcalf E.C., Zedalis M., Gosz J., Kliskey A., Alessa L. A social-ecological systems approach for environmental management. J. Environ. Manag. 2016;178:83–91. doi: 10.1016/j.jenvman.2016.02.028. [DOI] [PubMed] [Google Scholar]
- 134.Lamsal P., Kumar L., Atreya K., Pant K.P. Vulnerability and impacts of climate change on forest and freshwater wetland ecosystems in Nepal: a review. Ambio. 2017;46:915–930. doi: 10.1007/s13280-017-0923-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Martín-López B., Palomo I., García-Llorente M., Iniesta-Arandia I., Castro A.J., Del Amo D.G., Gómez-Baggethun E., Montes C. Delineating boundaries of social-ecological systems for landscape planning: a comprehensive spatial approach. Land Use Pol. 2017;66:90–104. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.