Abstract
Rheumatoidarthritis (RA) is an autoimmune disease characterized by uncontrolled joint inflammation and damage to bone and cartilage. B cells are known to play a crucial role in the pathogenesis and development of arthritis. Previous studies have found that B cells may be a potential target for treating RA. Rituximab, a monoclonal antibody targeting B cells, has induced long-term clinical responses in RA. Collagen-induced arthritis (CIA) mouse model is a widely studied autoimmune model of RA. CIA mouse model was used to investigate the effect of rituximab on the RA severity in the mice. Following induction of CIA, animals were treated with rituximab (250 mg/kg/week) intraperitoneally on the days 28, 35, 42, 49, 56, and 63 after collagen induction. We investigated the effect of rituximab on NF-κB p65, IκBα, GM-CSF, MCP-1, iNOS, TNF-α, and IL-6 cells in splenic CD19+ and CD45R+ B cells using flow cytometry. We also assessed the effect of rituximab on NF-κB p65, GM-CSF, IκBα, MCP-1, iNOS, TNF-α, and IL-6 at mRNA levels using RT-PCR analyses of knee tissues. Rituximab treatment significantly decreased CD19+NF-κB p65+, CD45R+NF-κB p65+, CD19+GM-CSF+, CD45R+GM-CSF+, CD19+MCP-1+, CD45R+MCP-1+, CD19+TNF-α+, CD45R+TNF-α+, CD19+iNOS+, CD45R+iNOS+, CD19+IL-6+, and CD45R+IL-6+, and increased CD45R+IκBα+ in spleen cells of CIA mice. We further observed that rituximab treatment downregulated NF-κB p65, GM-CSF, MCP-1, iNOS, TNF-α, and IL-6, whereas it upregulated IκBα, mRNA level. All these findings suggest that rituximab may be a novel therapeutic target for the treatment of RA.
Keywords: Autoimmune disease, Collagen-induced arthritis, Rituximab, B cells, NF-κB/GM-CSF/iNOS signaling
1. Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune inflammatory disease that causes joint pain, swelling, cartilage, and bone destruction [1]. Pathological changes observed in RA are characterized by synovial hyperplasia and destruction of cartilage and bone due to massive inflammatory cell infiltration [2]. The initiating cause of RA has not been fully understood yet. Still, dysregulation of the immune system has been confirmed to play a significant role in the propagation of the disease [3]. The various inflammatory cytokines secreted by T and B cells in the synovial fluids and tissues contribute to the initiation and progression of RA [4,5]. Targeting the reduction of inflammatory mediators can effectively control and prevent chronic inflammatory diseases [6]. Thus, there is an urgency to explore novel anti-inflammatory therapies for treating RA.
Nuclear factor (NF)-κB signaling is one of the key transcriptional pathways in the progression and development of RA [7]. A previous study showed that the blockade of the NF-κB signaling is considered a vital strategy for controlling inflammatory responses in RA [8]. Accumulating evidence suggested that NF-κB is highly activated in synovial tissues of RA patients [9]. IkB-α is an inhibitor of NF-κB, and decreased IκB-α level is indicative of NF-κB pathway activation [2]. Granulocyte macrophage-colony stimulating factor (GM-CSF) is a key mediator of synovial cell migration and inflammation in RA [10]. A previous study showed that elevated GM-CSF level is evident in RA patients' plasma, synovial fluid, and synoviocytes [11]. Blockade of the GM-CSF pathway induced sustained suppression of lymphocyte activities in RA [12]. Therefore, inhibiting proinflammatory cytokines is an important strategy in treating RA.
Monocyte chemotactic protein-1 (MCP-1) plays a critical role in the development of RA [13]. MCP-1 is a potent proinflammatory mediator and a crucial factor triggering inflammation in RA [13,14]. A significant increase in MCP-1 level was observed in experimental arthritis [15] (Zhou et al., 2011). The activation of inducible nitric oxide synthase (iNOS) is known to intensify the disease severity of RA [16], which promotes inflammation and oxidative damage of the arthritic joints [17]. INOS expression on the CIA model increases and contributes to RA's progression [18].
The upregulation of major proinflammatory cytokines, such as TNF-α, and IL-6, reflect dysregulated innate and autoimmune pathophysiology [19]. A previous study has also reported that proinflammatory cytokines regulate a wide range of inflammatory processes associated with the pathogenesis of RA [20]. In the inflammatory cascade of RA, TNF-α enhances the activation of fibroblasts [21]. It is known that TNF-α and IL-6 levels are higher concentrations in RA and have a significant role in inflammation and cartilage destruction [22]. Thus, the upregulation of pro-inflammatory signaling could be an effective approach for treating RA.
Rituximab is a genetically engineered chimeric anti-CD20 monoclonal antibody initially developed for treating B cell non‐Hodgkin's lymphoma [23]. Rituximab anti-CD20 antibody is an established therapy for RA [24]. Rituximab has proved safe and efficacious in patients with RA [25]. A previous study has reported clinically significant improvements in patients with RA following rituximab therapy [26]. Rituximab is shown to treat moderate to severe in patients with RA [27,28]. Rituximab has shown good therapeutic effects in treating RA, suggesting that developing drugs targeting B cells have important clinical significance [29,30]. Rituximab inhibits the NF-κB signaling pathway in non-Hodgkin's lymphoma B-cell lines [31]. Collagen-induced arthritis (CIA) is a classic mouse model for studying the pathogenesis and new therapeutic drugs for RA, owing to similar pathological features of the disease [32]. Inflammatory mediator signaling is a potential therapeutic target in several inflammatory diseases, including RA. Therefore, in this study, we investigated the effect of rituximab on NF-κB/GM-CSF/iNOS signaling in CD19/CD45R-expressing B cells in CIA mice. Hereafter, to extend our hypothesis, rituximab treatment in CIA mice could possess new insight into the novel regulatory mechanism in RA treatment.
2. Materials and methods
2.1. Animals
Male DBA/1J mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA). The mice were housed at 25 ± 2 °C with a 12 h light/dark cycle in a specific pathogen-free environment, fed standard mice chow, and given food and water ad libitum. All experiments were carried out with the approval of the King Saud University Institute's animal use and care committee (KSU-SE-21-64).
2.2. CIA induction and rituximab administration
Bovine type II collagen (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in 0.05 M acetic acid and emulsified with an equal volume of Freund's complete adjuvant (2 mg/ml). The collagen emulsion was administered via intradermal injection at the base of the tail into DBA/1J mice on day 0. On day 21, another emulsion prepared with type II collagen and Freund's incomplete adjuvant was intradermally administered near the primary injection [[33], [34], [35]]. We used rituximab, a monoclonal antibody targeting B cells, which has been shown to induce long-term clinical responses in RA.
The mice were randomly separated into four groups: 1) The NC group received saline (NC; n = 6), 2) The NC group received rituximab (NC + rituximab; n = 6), 3) The CIA group received saline (CIA; n = 6), and 4) The CIA group received rituximab (CIA + rituximab; n = 6). Mice were intraperitoneally injected with rituximab (250 mg/kg/week) every week, starting from day 28 until day 63, after collagen induction. At the same time, normal mice and mice with CIA were administered an equal volume of normal saline as control. The rituximab dose and route of administration were selected based on previous animal studies [33,36,37]. Mice were sacrificed at the end of the treatment period by deep inhalational anesthesia (isoflurane). Different tissues (knee/spleen) were collected for various molecular analyses, i.e., flow cytometry and RT-PCR.
2.3. Flowcytometric analysis
For flow cytometry analysis, we used conjugated antibodies to the following: CD19, CD45R, NF-κB p65, IκBα, GM-CSF, MCP-1, iNOS, TNF-α, and IL-6. For the staining of spleen cells, the following conjugated antibodies were used: anti-CD19 FITC, anti-CD19 PE/Dazzle, anti-CD19 APC/Cyanine7, anti-CD45R PE, anti-CD45R PE/Dazzle, anti-CD45R APC, anti–NF–κB p65 Alexa Fluor® 488, anti-IκBα PE, anti-GM-CSF FITC, anti-MCP-1 APC, anti-iNOS PE, anti-TNF-α PE, and anti-IL-6 APC. Antibodies were obtained from BioLegend, San Diego, CA, USA. Splenocytes were incubated with PMA/ionomycin (Sigma-Aldrich) and Golgi-plug (BD Biosciences) for 4 h before staining, as previously described (Ahmad et al., 2015; 2017). Cells were washed, and surface staining of CD19 and CD45R was performed. After fixation and permeabilization, cells were stained with anti–NF–κB p65, anti-IκBα, anti-GM-CSF, anti-MCP-1, anti-iNOS, anti-TNF-α, and anti-IL-6 fluorescent antibodies. The proportions of CD19+NF-κB p65+, CD45R+NF-κB p65+, CD19+GM-CSF+, CD45R+GM-CSF+, CD45R+IκBα+, CD19+MCP-1+, CD45R+MCP-1+, CD19+TNF-α+, CD45R+TNF-α+, CD19+iNOS+, CD45R+iNOS+, CD19+IL-6+, and CD45R+IL-6+ cells were determined in the lymphocyte gate. After washing two to three times, flow cytometry measured fluorescence intensity. Flow cytometry data acquisition and analysis were conducted on FC500 using CXP software (Beckman Coulter, Indianapolis, IN, USA). Each experiment was performed in triplicate.
2.4. RT-PCR analysis
Total RNA was extracted from knee tissues using TRIzol (Invitrogen Carlsbad, CA, USA) according to the manufacturer's instructions. The purity and concentration of RNA were measured using a NanoDrop 2000 (Thermo Scientific). cDNA synthesis was performed by the cDNA reverse transcription kit (Applied Biosystems, Foster City, USA). qRT-PCR was performed using a commercial SYBRGreen fluorescent dye (Applied Biosystems) [38,39]. Primer sequences for NF-κB p65, IκBα, GM-CSF, MCP-1, TNF-α, IL-6, and GAPDH were used for RT-PCR are listed in Table 1. Each experiment was performed in triplicate. The relative mRNA level was calculated by the 2−ΔΔCt method [40].
Table 1.
Primers sequence.
| Targeted gene | Direction and Sequence | |
|---|---|---|
| NF-κB p65 | F: 5′-CTGCCGAGTAAACCGGAACT-3′ | R: 5′-CCCTGTGACATCACCTGCTT-3′ |
| IκBα | F: 5′-AAGGCTACTCCCCCTACCAG-3′ | R: 5′-CAAGAAGGCGACACAGACCT -3′ |
| GM-CSF | F: 5′-AGCTTTACGAGAGCTCTTTTGC-3′ | R: 5′-CACATCCTCCTCAGGACCTT-3′ |
| iNOS | F: 5′-TCAGCCAAGCACTCCAATGT-3′ | R: 5′-AGTGATGGAGGTGCCCTAGT-3′ |
| MCP-1 | F: 5′-CAAAGCCAGGGGCCTTTTTC-3′ | R: 5′-TACCAGGAGCCAGGCATAGT-3′ |
| TNF-α | F: 5′-GGACTAGCCAGGAGGGAGAA-3′ | R: 5′-CGCGGATCATGCTTTCTGTG-3′ |
| IL-6 | F: 5′-GCCTTCTTGGGACTGATGCT-3′ | R: 5′-GACAGGTCTGTTGGGAGTGG-3′ |
| GAPDH | F: 5′-GTCAAGGCCGAGAATGGGAA-3′ | R: 5′-CTCGTGGTTCACACCCATCA-3′ |
NF-κB p65, Nuclear factor kappa B; IκBα, Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha; GM-CSF, Granulocyte-macrophage colony-stimulating factor; iNOS, Inducible nitric oxide synthase; MCP-1, Monocyte Chemoattractant Protein-1; TNFα, Tumour necrosis factor α; IL-6, Interleukin 6; GAPDH, Glyceraldehyde 3-phosphate dehydrogenase.
2.5. Statistical analysis
All data are presented as mean ± standard deviation (SD); six animals comprise each group. Statistical Analysis was performed using the GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA, USA). The data were analyzed by one-way ANOVA followed by Bonferroni's post-hoc comparison test. The level of statistical significance was set at p < 0.05.
3. Results
3.1. Rituximab administration suppresses NF-κB p65 expression in CIA mice
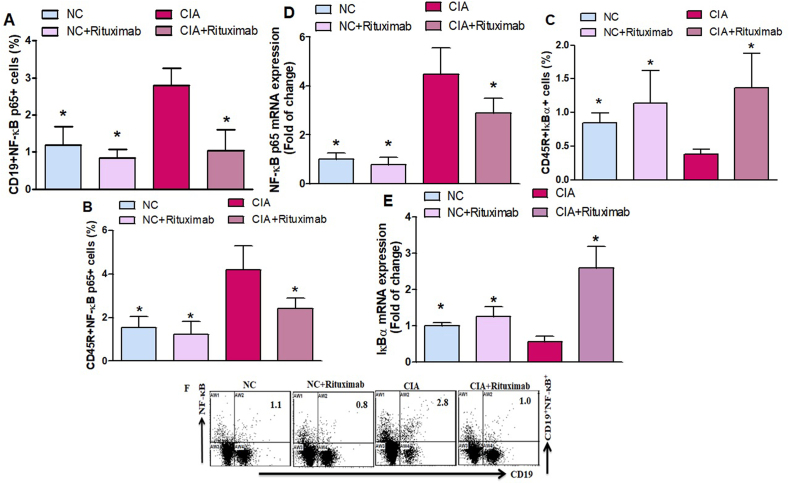
Flow cytometric analysis was performed to evaluate the effect of rituximab on NF-κB p65 and IκBα transcription factors in CIA mice. We found that the number of NF-κB p65-expressing CD19+ and CD45R+ B cells increased in the spleen of untreated CIA mice; however, rituximab-treated CIA mice showed a significant decrease in NF-κB p65-expressing CD19+ and CD45R+ cells (Fig. 1A and B). As shown in Fig. 1C, rituximab-treated CIA mice substantially increased IκBα-expressing CD45R+ B cells compared to untreated CIA mice in the spleen. mRNA level was examined to elucidate the effects of rituximab on NF-κB p65 and IκBα expression in knee tissues. The mRNA expression levels of NF-κB p65 were significantly decreased, and IκBα increased in the rituximab-treated CIA mice compared to untreated CIA mice (Fig. 1D and E). Our results indicated that rituximab exerted anti-inflammatory effects by inhibiting NF-κB p65 and increasing IκBα could attenuate RA progression.
Fig. 1.
A, B, and C. Therapeutic effect of rituximab on NF-κB- and IκBα-expressing CD19+ and CD45R+ B cells were analyzed through flow cytometry in the spleen. D and E The expression levels of NF-κB and IκBα mRNA were analyzed by RT-PCR in the knee tissues. F Representative flow cytometry dot plots of one mouse from each group. The cells were gated on FSC-SSC, and then the lymphocytes were gated for analyzing the percentage of CD19+NF-κB+, CD45R+NF-κB+, CD19+IκBα+, and CD45R+IκBα+ B cells. Normal control (NC) mice received saline and rituximab (250 mg/kg/week) intraperitoneally (ip). CIA mice were treated with rituximab (250 mg/kg/week) ip starting from day 28 until day 63 after collagen induction. The significance level was set at *p < 0.05 compared with the CIA untreated mice. The data present the mean ± SD (n = 6).
3.2. Rituximab downregulates GM-CSF transcription factor signaling
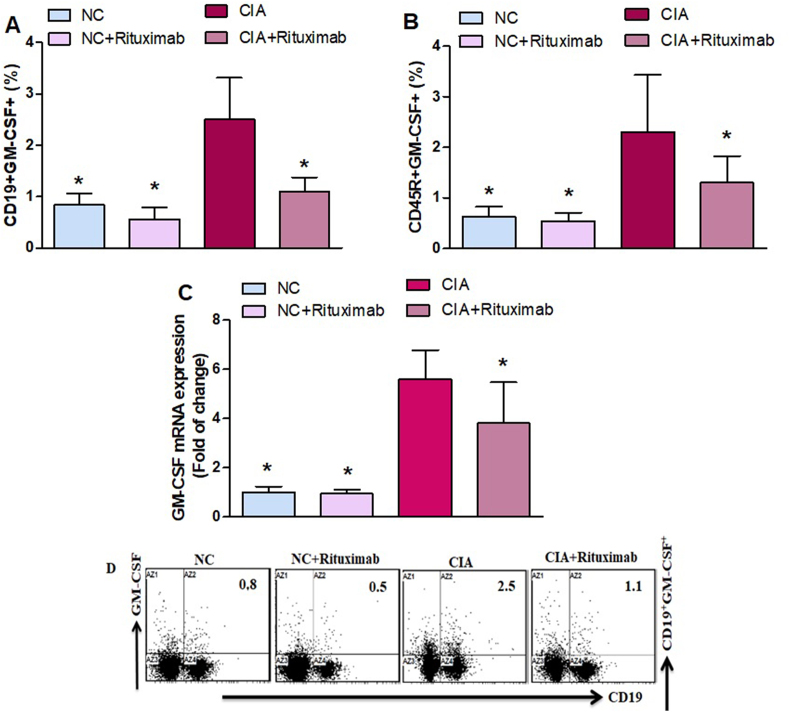
Then, we further investigated the effect of rituximab on B cells. We found that the number of GM–CSF–expressing CD19+ and CD45R+ cells increased in the spleen of untreated CIA mice compared to that of NC mice (Fig. 2A and B). Rituximab-treated CIA mice showed a significant decrease in GM–CSF–expressing CD19+ and CD45R+ B cells compared with untreated CIA mice (Fig. 2A and B). To further clarify the mechanism of rituximab, we used RT-PCR to examine changes in the mRNA level of GM-CSF in knee tissues. The level of GM-CSF mRNA in untreated CIA mice were significantly higher than those in NC mice (Fig. 2C). Rituximab administration in CIA mice decreased GM-CSF mRNA level compared with untreated CIA mice (Fig. 2C). These results demonstrate that rituximab administration decreases GM-CSF levels, which could represent a new target for the RA therapies.
Fig. 2.
A and B. Therapeutic effect of rituximab on GM–CSF–expressing CD19+ and CD45R+ B cells was analyzed through flow cytometry in the spleen. C The expression level of GM-CSF mRNA was analyzed by RT-PCR in the knee tissues. D Representative flow cytometry dot plots of one mouse from each group. The cells were gated on FSC-SSC, and then the lymphocytes were gated to analyze the percentage of CD19+GM-CSF+ and CD45R+GM-CSF+ B cells. Normal control (NC) mice received saline and rituximab (250 mg/kg/week) intraperitoneally (ip). CIA mice were treated with rituximab (250 mg/kg/week) ip starting from day 28 until day 63 after collagen induction. The significance level was set at *p < 0.05 compared with the CIA untreated mice. The data present the mean ± SD (n = 6).
3.3. Rituximab inhibits MCP-1-mediated inflammatory responses in CIA mice
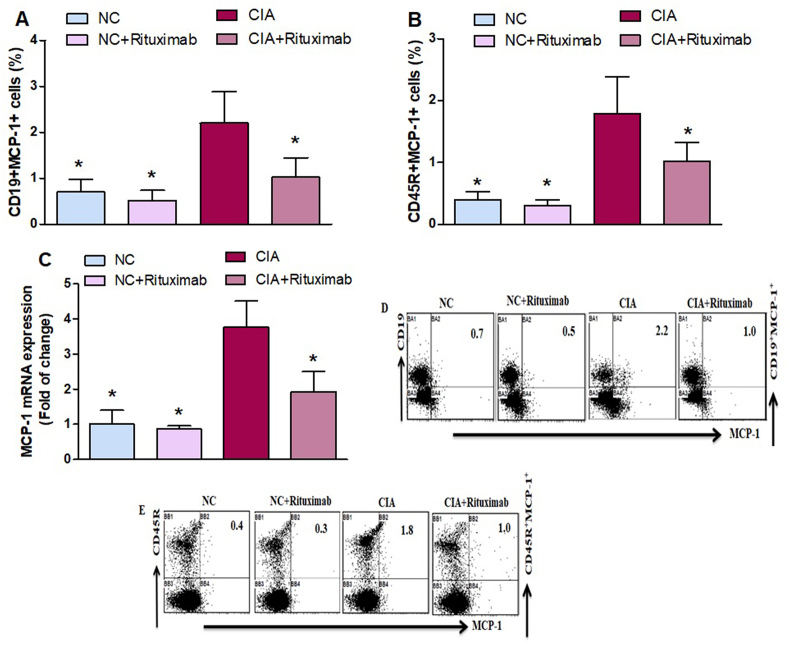
To further investigate the effect of rituximab, the percentage of MCP-1-expressing CD19+ and CD45R+ B cells in the spleen. The lower percentage of MCP-1-expressing CD19+ and CD45R+ cells was observed in rituximab-treated CIA mice compared with that of the untreated CIA mice (Fig. 3A and B), and MCP-1 mRNA level was significantly decreased in the knee tissues of CIA mice treated with rituximab compared with that of the untreated CIA mice (Fig. 3C). Taken together, these results suggest that rituximab has the potential to inhibit the expansion of inflammatory cytokines.
Fig. 3.
A and B. Therapeutic effect of rituximab on MCP-1-expressing CD19+ and CD45R+ B cells was analyzed through flow cytometry in the spleen. C The expression level of MCP-1 mRNA was analyzed by RT-PCR in the knee tissues. D and E Representative flow cytometry dot plots of one mouse from each group. The cells were gated on FSC-SSC, and then the lymphocytes were gated to analyze the percentage of CD19+MCP-1+ and CD45R+MCP-1+ B cells. Normal control (NC) mice received saline and rituximab (250 mg/kg/week) intraperitoneally (ip). CIA mice were treated with rituximab (250 mg/kg/week) ip starting from day 28 until day 63 after collagen induction. The significance level was set at *p < 0.05 compared with the CIA untreated mice. The data present the mean ± SD (n = 6).
3.4. Rituximab reduced spleen and tissue iNOS expression in CIA mice
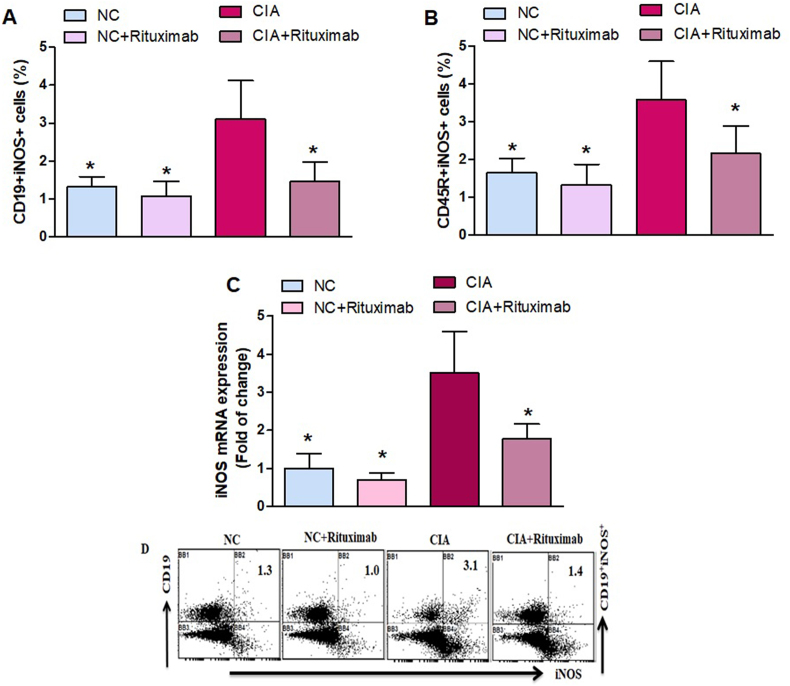
We investigated whether rituximab treatment affected iNOS-expressing CD19+ and CD45R+ B cells in the spleen. Our data show that these cells were significantly increased in the spleen of untreated CIA mice compared with saline-treated mice (Fig. 4A and B). Interestingly, rituximab treatment of CIA mice significantly decreased the number of CD19+iNOS+ and CD45R+iNOS+ B cells (Fig. 4A and B). We further examined the effect of rituximab treatment on iNOS mRNA levels in the knee tissues. We found that reduced in the knee tissues of rituximab-treated CIA mice compared with those of untreated CIA mice (Fig. 4C). These results suggest that rituximab treatment exerts its therapeutic effect through downregulation of iNOS expression during joint inflammation in the CIA model.
Fig. 4.
A and B. Therapeutic effect of rituximab on iNOS-expressing CD19+ and CD45R+ B cells was analyzed through flow cytometry in the spleen. C The expression level of iNOS mRNA was analyzed by RT-PCR in the knee tissues. D Representative flow cytometry dot plots of one mouse from each group. The cells were gated on FSC-SSC, and then the lymphocytes were gated to analyze the percentage of CD19+iNOS+ and CD45R+iNOS+ B cells. Normal control (NC) mice received saline and rituximab (250 mg/kg/week) intraperitoneally (ip). CIA mice were treated with rituximab (250 mg/kg/week) ip starting from day 28 until day 63 after collagen induction. The significance level was set at *p < 0.05 compared with the CIA untreated mice. The data present the mean ± SD (n = 6).
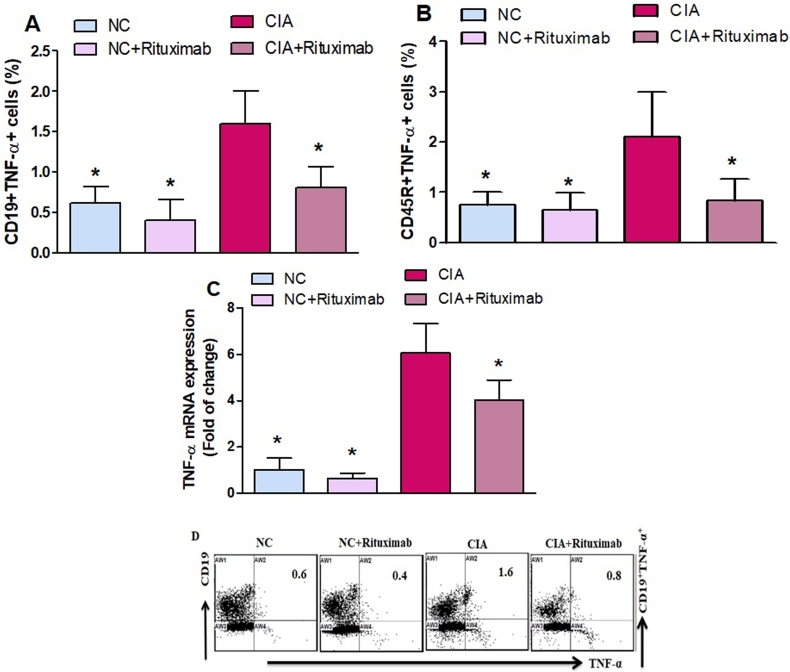
3.5. Rituximab treatment suppresses TNF-α expression in CIA mice
We further evaluated the effect of rituximab administration on TNF-α-expressing CD19+ and CD45RR+ cells in the spleen. In CIA mice treated with rituximab, TNF-expressing CD19+ and CD45R+ B cells significantly decreased compared with untreated CIA mice (Fig. 5A and B). RT-PCR analysis also demonstrated decreased TNF-α mRNA in the knee tissues of CIA mice treated with rituximab compared with untreated CIA mice (Fig. 5C). Our results indicate that rituximab could be an anti-inflammatory agent in RA treatment.
Fig. 5.
A and B. Therapeutic effect of rituximab on TNF-α-expressing CD19+ and CD45R+ B cells was analyzed through flow cytometry in the spleen. C The expression level of TNF-α mRNA was analyzed by RT-PCR in the knee tissues. D Representative flow cytometry dot plots of one mouse from each group. The cells were gated on FSC-SSC, and then the lymphocytes were gated to analyze the percentage of CD19+TNF-α+ and CD45R+TNF-α+ B cells. Normal control (NC) mice received saline and rituximab (250 mg/kg/week) intraperitoneally (ip). CIA mice were treated with rituximab (250 mg/kg/week) ip starting from day 28 until day 63 after collagen induction. The significance level was set at *p < 0.05 compared with the CIA untreated mice. The data present the mean ± SD (n = 6).
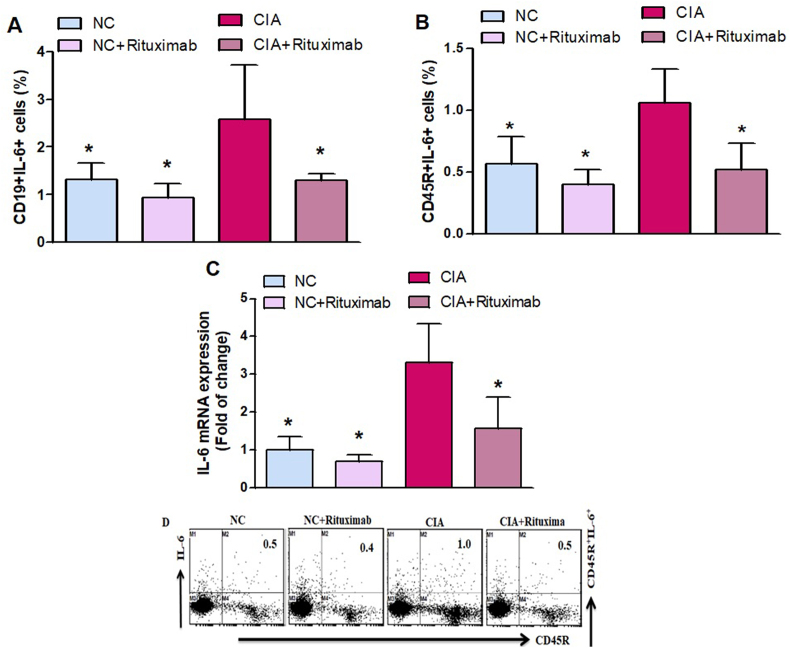
3.6. Rituximab inhibits IL-6 expression in CIA mice
As shown in Fig. 6A and B, rituximab administration in CIA mice significantly decreased the number of IL-6-expressing CD19+ and CD45R+ B cells compared with the untreated CIA mice. To further examine the decreasing effect of rituximab on IL-6 expression, mRNA expression was investigated in knee tissues (Fig. 6C). A significant decrease in the mRNA level of IL-6 was observed in the CIA mice treated with rituximab as compared with the untreated CIA mice (Fig. 6C). Taken together, these results revealed that rituximab decreased IL-6 inflammatory cytokine, which could retard RA development.
Fig. 6.
A and B. Therapeutic effect of rituximab on IL-6-expressing CD19+ and CD45R+ B cells was analyzed through flow cytometry in the spleen. C The expression level of IL-6 mRNA was analyzed by RT-PCR in the knee tissues. D Representative flow cytometry dot plots of one mouse from each group. The cells were gated on FSC-SSC, and then the lymphocytes were gated to analyze the percentage of CD19+IL-6+ and CD45R+IL-6+ B cells. Normal control (NC) mice received saline and rituximab (250 mg/kg/week) intraperitoneally (ip). CIA mice were treated with rituximab (250 mg/kg/week) ip starting from day 28 until day 63 after collagen induction. The significance level was set at *p < 0.05 compared with the CIA untreated mice. The data present the mean ± SD (n = 6).
4. Discussion
This study addressed the hypothesis that rituximab reduces inflammatory mediators in experimental arthritis. NF-κB is involved in cell survival, proliferation, and chronic inflammation characteristic of RA. Its activation plays an important role in the pathogenesis of RA [41]. Recently, there has been increasing interest in developing biological agents that target specific signal transduction pathways in RA. Our results showed that the level of NF-κB-expressing CD19+ and CD45R+ B cells was significantly decreased in the spleen cells of rituximab-treated CIA mice. Our results demonstrated that rituximab increased IκBα-producing CD45R+ B cells in the spleen. Our results also showed that rituximab treatment significantly suppressed the NF-κB mRNA expression but increased that of IκBα, which provides strong evidence that rituximab regulates the NF-κB/IκBα pathway in RA. Therefore, it can be concluded that the anti‐arthritic effects of rituximab are caused by the inhibitory action on NF-κB and by upregulating IκBα expression in the CIA mouse model. These findings indicated that rituximab has a therapeutic impact on CIA by regulating the activation of NF-κB/IκBα signaling pathways which could attenuate RA progression.
A previous study has demonstrated that the GM-CSF level increased in the bone marrow, serum, and peripheral blood of patients with RA [42]. GM-CSF leads to the destruction of the joint structure in mice [43]. Previous data showed that neutralizing GM-CSF is beneficial in RA [44]. Inhibition of GM-CSF reduced the RA disease activity score in arthritis patients in clinical trials [45]. Previous results indicated that increased GM-CSF expression worsened disease symptoms in a mouse model with RA [46]. Clinical trials of agents targeting the GM-CSF pathway in RA have been reported [47]. These data suggest that GM-CSF is a key player in RA and that blocking this pathway may provide benefits. This study found that rituximab treatment reduced the number of GM–CSF–expressing CD19+ and CD45R+ B cells in the spleen of CIA mice. Our study also confirmed the anti-arthritic through the effect of rituximab on the mRNA level. Similarly, the mRNA level of GM-CSF was diminished by rituximab treatment in knee tissues. Our data suggest that rituximab-mediated inhibition of GM-CSF activation could contribute to its efficacy in the CIA mouse model. Although the mechanism underlying the suppression of GM-CSF by rituximab warrants further elucidation, rituximab administration could be used to treat RA.
Inflammatory cytokines and chemokines are involved in the development of RA [5,48]. Accumulating evidence demonstrated that MCP-1 is highly expressed in the joints of patients suffering from RA [49]. It was previously reported that MCP-1 is involved in developing arthritis [50,51]. A previous study reported that MCP-1 is an important indicator for evaluating RA disease activity [49]. A previous study showed that MCP-1 was higher among RA patients [52]. A previous study found that MCP-1 promotes fibroblast-like synoviocyte proliferation, migration, and differentiation potential [53]. A previous study suggested that MCP-1 can destroy joints in CIA mice [54]. Therefore, these results support our hypothesis that MCP-1 inhibition could suppress arthritis development in CIA mice. Our results demonstrated that rituximab-treated CIA mice had decreased MCP-1-expressing CD19+ and CD45R+ levels in the spleen. Our results also revealed that rituximab-treated CIA mice had reduced mRNA levels MCP-1 in knee tissues. These results suggest that rituximab downregulates inflammatory mediators in CIA mice, which may account for the beneficial effect of rituximab on RA. Our results further indicate that rituximab administration may be a potential anti-arthritic agent with novel mechanisms of action.
iNOS is one of the main transcriptional targets of an inflammatory mediator. Increased expression of iNOS has been observed in the synovium and cartilage of RA patients [55]. Previous studies have reported that iNOS expression was induced in CIA mice [17,56]. It has also been observed that iNOS expression significantly increased in the spleen tissue of arthritic animals [57]. Our results demonstrated that rituximab decreased iNOS-expressing the spleen's CD19+ and CD45R+ B cells. We also found that the mRNA expression level of iNOS was reduced in the knee tissues of rituximab-treated CIA mice. Thus, the observed anti-inflammatory effects of rituximab could be due to their ability to decrease the expression level of iNOS. This suggests that the anti-CD20 monoclonal antibody rituximab has marked advantages as a new therapeutic agent for RA.
TNF-α is the main inflammatory mediator in RA development, and various anti-TNF-α therapies have begun to improve clinical outcomes [58]. A previous study reported that TNF-α-blocking agents had improved the management of RA patients [59]. Additionally, it was shown that overproduction of TNF-α induces joint inflammation and pannus formation, leading to cartilage erosion and bone destruction [60]. Furthermore, an increased TNF-α level was confirmed to aggravate inflammation by activating NF-κB and producing cytokines and other inflammatory mediators in RA [61]. It was also reported that TNF-α levels elevated in synovial tissues of CIA mice [62]. This study found that rituximab treatment significantly decreased CD19+TNF-α+ and CD45R+TNF-α+ in CIA mice. Furthermore, in CIA mice treated with rituximab, the mRNA expression level of TNF-α was lowered compared with the CIA untreated mice. Importantly, rituximab treatment provided a significant anti-inflammatory effect, as evidenced by the reduction in TNF-α expression in the spleen and knee tissues of CIA mice. Therefore, these results suggest that rituximab inhibited CIA by downregulating TNF-α.
B cells aggregates in the synovium of inflamed joints and mediate the pathogenesis of RA and proinflammatory cytokine IL-6, which is critically involved [63]. It has been reported that IL-6 plays a significant role in the pathogenesis of RA [64]. It was further reported that IL-6 contributes to the pathogenesis of CIA and has been used as a biomarker for early diagnosis and therapy [65]. Recent studies have shown that IL-6 contributes to the pathophysiology of RA [66,67]. In this study, we found that IL-6 production was significantly elevated in CIA untreated mice; however, production of IL-6 was decreased by treating CIA mice with rituximab. Our results also revealed that rituximab-treated CIA mice had reduced mRNA levels IL-6 in knee tissues. Our data suggest that IL-6 mediates the anti-inflammatory effects of rituximab; however, the mechanism underlying its antiarthritic activities warrants further investigation. Our data suggest that rituximab has great potential as an innovative therapy for suppressing arthritis progression. There are also some limitations in this study. The lack of immunohistochemistry and the absence of Western blotting experiments were limitations of the present study, which may be considered in future relevant studies.
Our study shows that the anti-arthritic effect of rituximab occurs via the downregulation of NF-κB transcription factor and proinflammatory mediators in splenic B cells and knee tissues. Rituximab treatment is expected to ameliorate RA disease progression by suppressing proinflammatory mediators in CIA mice. Therefore, our results suggest that the downregulation of NF-κB signaling and proinflammatory mediators by the anti-CD20 monoclonal antibody rituximab could be helpful to as a potential treatment for RA.
Author contribution statement
Sheikh F. Ahmad, Mushtaq A. Ansari, Ahmed Nadeem, Sabry M. Attia, Saleh A. Bakheet, Abdullah F. Alasmari: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools, or data; Wrote the paper.
Hatun A. Alomar, Haneen A. Al-Mazroua, Abdullah S. Alhamed, Mudassar Shahid, Mohammed Alqinyah, Mohammed A. Assiri, Mohammed A. Al-Hamamah, Yasseen A. Alassmrry: Performed the experiments; Analyzed and interpreted the data; materials, analysis tools or data; Wrote the paper.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors extend their appreciation to the Deputyship for Research & Innovation, Ministry of Education in Saudi Arabia for funding this research work through the project no. (IFKSUOR3-065-2).
References
- 1.Park J.-Y., Kwon Y.-W., Kim S.-A., Park S.-D., Kim C.-H., Kim J.-H., Lee J.-H. Polyherbal formula SC-E3 inhibits rheumatoid arthritis activity in a mouse model of type-II collagen-induced arthritis. J. Integ. Med. 2021;19:265–273. doi: 10.1016/j.joim.2020.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Li Q., Verma I.M. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 3.McInnes I.B., Schett G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 4.Asif Amin M., Fox D.A., Ruth J.H. Synovial cellular and molecular markers in rheumatoid arthritis. Semin. Immunopathol. 2017;39:385–393. doi: 10.1007/s00281-017-0631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choy E.H.S., Panayi G.S. Cytokine pathways and joint inflammation in rheumatoid arthritis. N. Engl. J. Med. 2001;344:907–916. doi: 10.1056/NEJM200103223441207. [DOI] [PubMed] [Google Scholar]
- 6.Furst D.E., Emery P. Rheumatoid arthritis pathophysiology: update on emerging cytokine and cytokine-associated cell targets. Rheumatology. 2014;53:1560–1569. doi: 10.1093/rheumatology/ket414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L., Luo J., Wen H., Zhang T., Zuo X., Li X. MDM2 promotes rheumatoid arthritis via activation of MAPK and NF-κB. Int. Immunopharm. 2016;30:69–73. doi: 10.1016/j.intimp.2015.11.030. [DOI] [PubMed] [Google Scholar]
- 8.Min S.-Y., Yan M., Du Y., Wu T., Khobahy E., Kwon S.-R., Taneja V., Bashmakov A., Nukala S., Ye Y., Orme J., Sajitharan D., Kim H.-Y., Mohan C. Intra-articular nuclear factor-κB blockade ameliorates collagen-induced arthritis in mice by eliciting regulatory T cells and macrophages. Clin. Exp. Immunol. 2013;172:217–227. doi: 10.1111/cei.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tak P.P., Gerlag D.M., Aupperle K.R., van de Geest D.A., Overbeek M., Bennett B.L., Boyle D.L., Manning A.M., Firestein G.S. Inhibitor of nuclear factor kappaB kinase beta is a key regulator of synovial inflammation. Arthritis Rheum. 2001;44:1897–1907. doi: 10.1002/1529-0131. (200108)44:8<1897::AID-ART328>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Cook A.D., Braine E.L., Campbell I.K., Rich M.J., Hamilton J.A. Blockade of collagen-induced arthritis post-onset by antibody to granulocyte-macrophage colony-stimulating factor (GM-CSF): requirement for GM-CSF in the effector phase of disease. Arthritis Res. 2001;3:293. doi: 10.1186/ar318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright H.L., Bucknall R.C., Moots R.J., Edwards S.W. Analysis of SF and plasma cytokines provides insights into the mechanisms of inflammatory arthritis and may predict response to therapy. Rheumatology. 2012;51:451–459. doi: 10.1093/rheumatology/ker338. [DOI] [PubMed] [Google Scholar]
- 12.Guo X., Higgs B.W., Bay-Jensen A.-C., Wu Y., Karsdal M.A., Kuziora M., Godwood A., Close D., Ryan P.C., Roskos L.K., White W.I. Blockade of GM-CSF pathway induced sustained suppression of myeloid and T cell activities in rheumatoid arthritis. Rheumatology. 2018;57:175–184. doi: 10.1093/rheumatology/kex383. [DOI] [PubMed] [Google Scholar]
- 13.Pavkova Goldbergova M., Lipkova J., Pavek N., Gatterova J., Vasku A., Soucek M., Nemec P. RANTES, MCP-1 chemokines and factors describing rheumatoid arthritis. Mol. Immunol. 2012;52:273–278. doi: 10.1016/j.molimm.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Zhou H., Hu B., Zhaopeng Z., Liu J., Zhong Q., Fan Y., Li L. Elevated circulating T cell subsets and cytokines expression in patients with rheumatoid arthritis. Clin. Rheumatol. 2019;38:1831–1839. doi: 10.1007/s10067-019-04465-w. [DOI] [PubMed] [Google Scholar]
- 15.Zhou B., Yuan J., Zhou Y., Ghawji M., Deng Y.-P., Lee A.J., Lee A.J., Nair U., Kang A.H., Brand D.D., Yoo T.J. Administering human adipose-derived mesenchymal stem cells to prevent and treat experimental arthritis. Clin. Immunol. 2011;141:328–337. doi: 10.1016/j.clim.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 16.Zheng H., Yu X., Collin-Osdoby P., Osdoby P. RANKL Stimulates inducible nitric-oxide synthase expression and nitric oxide production in developing Osteoclasts. J. Biol. Chem. 2006;281:15809–15820. doi: 10.1074/jbc.M513225200. [DOI] [PubMed] [Google Scholar]
- 17.Hemshekhar M., Anaparti V., Hitchon C., Mookherjee N. Buprenorphine Alters inflammatory and oxidative stress molecular markers in arthritis. Mediat. Inflamm. 2017;2017:1–10. doi: 10.1155/2017/2515408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jing R., Ban Y., Xu W., Nian H., Guo Y., Geng Y., Zang Y., Zheng C. Therapeutic effects of the total lignans from Vitex negundo seeds on collagen-induced arthritis in rats. Phytomedicine. 2019;58 doi: 10.1016/j.phymed.2019.152825. [DOI] [PubMed] [Google Scholar]
- 19.Firestein G.S., McInnes I.B. Immunopathogenesis of rheumatoid arthritis. Immunity. 2017;46:183–196. doi: 10.1016/j.immuni.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alghasham A., Rasheed Z. Therapeutic targets for rheumatoid arthritis: Progress and promises. Autoimmunity. 2014;47:77–94. doi: 10.3109/08916934.2013.873413. [DOI] [PubMed] [Google Scholar]
- 21.McInnes I.B., Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 22.Umar S., Hedaya O., Singh A.K., Ahmed S. Thymoquinone inhibits TNF-α-induced inflammation and cell adhesion in rheumatoid arthritis synovial fibroblasts by ASK1 regulation. Toxicol. Appl. Pharmacol. 2015;287:299–305. doi: 10.1016/j.taap.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coiffier B., Haioun C., Ketterer N., Engert A., Tilly H., Ma D., Johnson P., Lister A., Feuring-Buske M., Radford J.A., Capdeville R., Diehl V., Reyes F. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998;92:1927–1932. [PubMed] [Google Scholar]
- 24.Cohen S.B., Emery P., Greenwald M.W., Dougados M., Furie R.A., Genovese M.C., Keystone E.C., Loveless J.E., Burmester G.-R., Cravets M.W., Hessey E.W., Shaw T., Totoritis M.C. REFLEX Trial Group, Rituximab for rheumatoid arthritis refractory to anti–tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54:2793–2806. doi: 10.1002/art.22025. [DOI] [PubMed] [Google Scholar]
- 25.Edwards J.C.W., Szczepański L., Szechiński J., Filipowicz-Sosnowska A., Emery P., Close D.R., Stevens R.M., Shaw T. Efficacy of B-Cell–Targeted therapy with rituximab in patients with rheumatoid arthritis. N. Engl. J. Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 26.Leandro M.J. Clinical outcome in 22 patients with rheumatoid arthritis treated with B lymphocyte depletion. Ann. Rheum. Dis. 2002;61:883–888. doi: 10.1136/ard.61.10.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saag K.G., Teng G.G., Patkar N.M., Anuntiyo J., Finney C., Curtis J.R., Paulus H.E., Mudano A., Pisu M., Elkins-Melton M., Outman R., Allison J.J., Almazor M.S., Bridges S.L., Chatham W.W., Hochberg M., Maclean C., Mikuls T., Moreland L.W., O’dell J., Turkiewicz A.M., Furst D.E. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–784. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 28.Smolen J.S., Keystone E.C., Emery P., Breedveld F.C., Betteridge N., Burmester G.R., Dougados M., Ferraccioli G., Jaeger U., Klareskog L., Kvien T.K., Martin-Mola E., Pavelka K. The Working Group on the Rituximab Consensus Statement, Consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2006;66:143–150. doi: 10.1136/ard.2006.061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurosaki T., Shinohara H., Baba Y. B cell signaling and fate decision. Annu. Rev. Immunol. 2010;28:21–55. doi: 10.1146/annurev.immunol.021908.132541. [DOI] [PubMed] [Google Scholar]
- 30.Reddy V., Cambridge G., Isenberg D.A., Glennie M.J., Cragg M.S., Leandro M. Internalization of rituximab and the Efficiency of B cell depletion in rheumatoid arthritis and systemic Lupus Erythematosus. Arthritis Rheumatol. 2015;67:2046–2055. doi: 10.1002/art.39167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jazirehi A.R., Huerta-Yepez S., Cheng G., Bonavida B. Rituximab (chimeric anti-CD20 monoclonal antibody) inhibits the constitutive nuclear factor-{kappa}B signaling pathway in non-Hodgkin’s lymphoma B-cell lines: role in sensitization to chemotherapeutic drug-induced apoptosis. Cancer Res. 2005;65:264–276. [PubMed] [Google Scholar]
- 32.Alabarse P.V.G., Lora P.S., Silva J.M.S., Santo R.C.E., Freitas E.C., De Oliveira M.S., Almeida A.S., Immig M., Teixeira V.O.N., Filippin L.I., Xavier R.M. Collagen-induced arthritis as an animal model of rheumatoid cachexia: CIA as an animal model of RA. J. Cach. Sarcop. Mus. 2018;9:603–612. doi: 10.1002/jcsm.12280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al-Hamamah M.A., Alotaibi M.R., Ahmad S.F., Nadeem A., Attia M.S.M., Ansari M.A., Bakheet S.A., Alanazi M.M., Attia S.M. Treatment with the anti-CD20 monoclonal antibody rituximab mitigates gonadal disruptions in the collagen-induced arthritis in male DBA/1 J mouse model. Mut. Res. Fund. Mol. Mech. Mutag. 2022;825 doi: 10.1016/j.mrfmmm.2022.111799. [DOI] [PubMed] [Google Scholar]
- 34.Bakheet S.A., Ansari M.A., Nadeem A., Attia S.M., Alhoshani A.R., Gul G., Al-Qahtani Q.H., Albekairi N.A., Ibrahim K.E., Ahmad S.F. CXCR3 antagonist AMG487 suppresses rheumatoid arthritis pathogenesis and progression by shifting the Th17/Treg cell balance. Cell. Signal. 2019;64 doi: 10.1016/j.cellsig.2019.109395. [DOI] [PubMed] [Google Scholar]
- 35.Lo C.K.C., Lam Q.L.K., Sun L., Wang S., Ko K.-H., Xu H., Wu C.-Y., Zheng B.-J., Lu L. Natural killer cell degeneration exacerbates experimental arthritis in mice via enhanced interleukin-17 production. Arthritis Rheum. 2008;58:2700–2711. doi: 10.1002/art.23760. [DOI] [PubMed] [Google Scholar]
- 36.Behrens M., Luckey D., Luthra H., David C., Taneja V. B cells influence sex specificity of arthritis via myeloid suppressors and chemokines in humanized mice. Clin. Immunol. 2017;178:10–19. doi: 10.1016/j.clim.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 37.Yanaba K., Hamaguchi Y., Venturi G.M., Steeber D.A., Clair E.W. St, Tedder T.F. B cell depletion Delays collagen-induced arthritis in mice: arthritis induction Requires Synergy between humoral and cell-mediated immunity. J. Immunol. 2007;179:1369–1380. doi: 10.4049/jimmunol.179.2.1369. [DOI] [PubMed] [Google Scholar]
- 38.Ahmad S.F., Ansari M.A., Nadeem A., Zoheir K.M.A., Bakheet S.A., Al-Shabanah O.A., Al Rikabi A.C., Attia S.M. The tyrosine kinase inhibitor tyrphostin AG126 reduces activation of inflammatory cells and increases Foxp3+ regulatory T cells during pathogenesis of rheumatoid arthritis. Mol. Immunol. 2016;78:65–78. doi: 10.1016/j.molimm.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 39.Bakheet S.A., Alrwashied B.S., Ansari M.A., Nadeem A., Attia S.M., Alanazi M.M., Aldossari A.A., Assiri M.A., Mahmood H.M., Al-Mazroua H.A., Ahmad S.F. CXC chemokine receptor 3 antagonist AMG487 shows potent anti-arthritic effects on collagen-induced arthritis by modifying B cell inflammatory profile. Immunol. Lett. 2020;225:74–81. doi: 10.1016/j.imlet.2020.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using Real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z., Zhuo F., Chu P., Yang X., Zhao G. Germacrone alleviates collagen-induced arthritis via regulating Th1/Th2 balance and NF-κB activation. Biochem. Biophys. Res. Commun. 2019;518:560–564. doi: 10.1016/j.bbrc.2019.08.084. [DOI] [PubMed] [Google Scholar]
- 42.Kuroda T., Tanabe N., Sakatsume M., Nozawa S., Mitsuka T., Ishikawa H., Tohyama C.T., Nakazono K., Murasawa A., Nakano M., Gejyo F. Interleukin-2 levels are elevated in the bone marrow serum of patients with mutilans-type rheumatoid arthritis. Clin. Rheumatol. 2002;21:23–27. doi: 10.1007/s100670200006. [DOI] [PubMed] [Google Scholar]
- 43.Van Nieuwenhuijze A.E.M., Van De Loo F.A., Walgreen B., Bennink M., Helsen M., Van Den Bersselaar L., Wicks I.P., Van Den Berg W.B., Koenders M.I. Complementary action of granulocyte macrophage colony-stimulating factor and interleukin-17A induces interleukin-23, receptor activator of nuclear factor-κB ligand, and matrix metalloproteinases and drives bone and cartilage pathology in experimental arthritis: rationale for combination therapy in rheumatoid arthritis. Arthritis Res. Ther. 2015;17:163. doi: 10.1186/s13075-015-0683-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burmester G.R., Feist E., Sleeman M.A., Wang B., White B., Magrini F. Mavrilimumab, a human monoclonal antibody targeting GM-CSF receptor- , in subjects with rheumatoid arthritis: a randomised, double-blind, placebo-controlled, phase I, first-in-human study. Ann. Rheum. Dis. 2011;70:1542–1549. doi: 10.1136/ard.2010.146225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huizinga T.W.J., Batalov A., Stoilov R., Lloyd E., Wagner T., Saurigny D., Souberbielle B., Esfandiari E. Phase 1b randomized, double-blind study of namilumab, an anti-granulocyte macrophage colony-stimulating factor monoclonal antibody, in mild-to-moderate rheumatoid arthritis. Arthritis Res. Ther. 2017;19:53. doi: 10.1186/s13075-017-1267-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell I.K., Rich M.J., Bischof R.J., Hamilton J.A. The colony-stimulating factors and collagen-induced arthritis: exacerbation of disease by M-CSF and G-CSF and requirement for endogenous M-CSF. J. Leukoc. Biol. 2000;68:144–150. [PubMed] [Google Scholar]
- 47.Avci A.B., Feist E., Burmester G.-R. Targeting GM-CSF in rheumatoid arthritis. Clin. Exp. Rheumatol. 2016;34:39–44. [PubMed] [Google Scholar]
- 48.Koch A.E., Kunkel S.L., Harlow L.A., Mazarakis D.D., Haines G.K., Burdick M.D., Pope R.M., Strieter R.M. Macrophage inflammatory protein-1 alpha. A novel chemotactic cytokine for macrophages in rheumatoid arthritis. J. Clin. Invest. 1994;93:921–928. doi: 10.1172/JCI117097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liou L., Tsai W., Chang C.J., Chao W., Chen M. Blood monocyte chemotactic protein-1 (MCP-1) and Adapted disease activity Score28-MCP-1: favorable indicators for rheumatoid arthritis activity. PLoS One. 2013;8 doi: 10.1371/journal.pone.0055346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kagari T., Doi H., Shimozato T. The importance of IL-1β and TNF-α, and the noninvolvement of IL-6, in the development of monoclonal antibody-induced arthritis. J. Immunol. 2002;169:1459–1466. doi: 10.4049/jimmunol.169.3.1459. [DOI] [PubMed] [Google Scholar]
- 51.Takagi T., Naito Y., Inoue M., Akagiri S., Mizushima K., Handa O., Kokura S., Ichikawa H., Yoshikawa T. Inhalation of carbon monoxide ameliorates collagen-induced arthritis in mice and regulates the articular expression of IL-1β and MCP-1. Inflammation. 2009;32:83–88. doi: 10.1007/s10753-009-9106-6. [DOI] [PubMed] [Google Scholar]
- 52.Rantapaa-Dahlqvist S., Boman K., Tarkowski A., Hallmans G. Up regulation of monocyte chemoattractant protein-1 expression in anti-citrulline antibody and immunoglobulin M rheumatoid factor positive subjects precedes onset of inflammatory response and development of overt rheumatoid arthritis. Ann. Rheum. Dis. 2007;66:121–123. doi: 10.1136/ard.2006.057331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tong X., Zeng H., Gu P., Wang K., Zhang H., Lin X. Monocyte chemoattractant protein-1 promotes the proliferation, migration and differentiation potential of fibroblast-like synoviocytes via the PI3K/P38 cellular signaling pathway. Mol. Med. Rep. 2020;21:1623–1632. doi: 10.3892/mmr.2020.10969. [DOI] [PubMed] [Google Scholar]
- 54.Ogata H., Takeya M., Yoshimura T., Takagi K., Takahashi K. The role of monocyte chemoattractant protein-1 (MCP-1) in the pathogenesis of collagen-induced arthritis in rats. J. Pathol. 1997;182:106–114. doi: 10.1002/(SICI)1096-9896. (199705)182:1<106::AID-PATH816>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 55.Cedergren J., Forslund T., Sundqvist T., Skogh T. Inducible nitric oxide synthase is expressed in synovial fluid granulocytes. Clin. Exp. Immunol. 2002;130:150–155. doi: 10.1046/j.1365-2249.2002.01959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Castejón M.L., Alarcón-de-la-Lastra C., Rosillo M.Á., Montoya T., Fernández-Bolaños J.G., González-Benjumea A., Sánchez-Hidalgo M. A new Peracetylated Oleuropein Derivative ameliorates joint inflammation and destruction in a murine collagen-induced arthritis model via activation of the nrf-2/Ho-1 Antioxidant pathway and suppression of MAPKs and NF-κB activation. Nutrients. 2021;13:311. doi: 10.3390/nu13020311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gul A., Kunwar B., Mazhar M., Faizi S., Ahmed D., Shah M.R., Simjee S.U. Rutin and rutin-conjugated gold nanoparticles ameliorate collagen-induced arthritis in rats through inhibition of NF-κB and iNOS activation. Int. Immunopharm. 2018;59:310–317. doi: 10.1016/j.intimp.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 58.Taylor P.C., Feldmann M. Anti-TNF biologic agents: still the therapy of choice for rheumatoid arthritis. Nat. Rev. Rheumatol. 2009;5:578–582. doi: 10.1038/nrrheum.2009.181. [DOI] [PubMed] [Google Scholar]
- 59.Rubbert-Roth A., Atzeni F., Masala I.F., Caporali R., Montecucco C., Sarzi-Puttini P. TNF inhibitors in rheumatoid arthritis and spondyloarthritis: are they the same? Autoimmun. Rev. 2018;17:24–28. doi: 10.1016/j.autrev.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 60.Tracey D., Klareskog L., Sasso E.H., Salfeld J.G., Tak P.P. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharm. Therap. 2008;117:244–279. doi: 10.1016/j.pharmthera.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 61.Lee Y.R., Hwang J.K., Lee H.S., Cheon Y.J., Ryu J.H., Lee S.I., Kwak H.B., Lee S.M., Kim J.S., Park J.W., Jeon R., Park B.H. SPA0355, a thiourea analogue, inhibits inflammatory responses and joint destruction in fibroblast-like synoviocytes and mice with collagen-induced arthritis. Br. J. Pharmacol. 2011;164:794–806. doi: 10.1111/j.1476-5381.2011.01441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang T., Jia Q., Chen T., Yin H., Tian X., Lin X., Liu Y., Zhao Y., Wang Y., Shi Q., Huang C., Xu H., Liang Q. Alleviation of synovial inflammation of juanbi-tang on collagen-induced arthritis and TNF-tg mice model. Front. Pharmacol. 2020;11:45. doi: 10.3389/fphar.2020.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lai Kwan Lam Q., King Hung Ko O., Zheng B.-J., Lu L. Local BAFF gene silencing suppresses Th17-cell generation and ameliorates autoimmune arthritis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:14993–14998. doi: 10.1073/pnas.0806044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mihara M., Kasutani K., Okazaki M., Nakamura A., Kawai S., Sugimoto M., Matsumoto Y., Ohsugi Y. Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family. Int. Immunopharmacol. 2005;5:1731–1740. doi: 10.1016/j.intimp.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 65.Avci A.B., Feist E., Burmester G.R. Targeting IL-6 or IL-6 receptor in rheumatoid arthritis: what's the difference? BioDrugs. 2018;32:531–546. doi: 10.1007/s40259-018-0320-3. [DOI] [PubMed] [Google Scholar]
- 66.Lally F., Smith E., Filer A., Stone M.A., Shaw J.S., Nash G.B., Buckley C.D., Ed Rainger G. A novel mechanism of neutrophil recruitment in a coculture model of the rheumatoid synovium. Arthritis Rheum. 2005;52:3460–3469. doi: 10.1002/art.21394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yue C., You X., Zhao L., Wang H., Tang F., Zhang F., Zhang X., He W. The effects of adalimumab and methotrexate treatment on peripheral Th17 cells and IL-17/IL-6 secretion in rheumatoid arthritis patients. Rheumatol. Int. 2010;30:1553–1557. doi: 10.1007/s00296-009-1179-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.