Key Points
Question
What is the effect of a problem-oriented, culturally adapted, targeted quality improvement intervention on reperfusion therapy, including intravenous thrombolysis or endovascular thrombectomy, for acute ischemic stroke in China?
Findings
In this stepped-wedge cluster randomized clinical trial of 12 132 patients with acute ischemic stroke who were enrolled within 3.5 or 4.5 hours of onset from 16 secondary and 33 tertiary hospitals, the rate of reperfusion therapy for eligible patients was 53.5% in the intervention period and 43.9% in the control period; the improvement was not statistically significant after adjusting for cluster and period, but it was statistically significant when limited to the setting of secondary hospitals.
Meaning
Among patients with acute ischemic stroke in China, a targeted quality improvement intervention did not improve the reperfusion therapy rate compared with usual care but may be effective in secondary hospitals, which may provide a direction for better resource allocation.
This stepped-wedge cluster randomized clinical trial examines the effectiveness of a problem-oriented, culturally adapted, targeted quality improvement intervention to improve reperfusion therapy for acute ischemic stroke among patients in China.
Abstract
Importance
Reperfusion therapy is the most effective treatment for acute ischemic stroke but remains underused in China.
Objective
To evaluate the effect of a problem-oriented, culturally adapted, targeted quality improvement intervention on reperfusion therapy for patients with acute ischemic stroke in China.
Design, Setting, and Participants
In this stepped-wedge cluster randomized clinical trial, patients from 16 secondary and 33 tertiary hospitals in China with acute ischemic stroke within 6 hours of symptom onset were consecutively recruited between July 1, 2018, and June 30, 2020.
Interventions
Hospitals were randomly assigned to 1 of 3 sequences to receive the targeted quality improvement intervention (n = 5689), in which workflow reconstruction was promoted to reduce in-hospital reperfusion treatment delays, or usual care (n = 6443), in which conventional stroke care was left to the discretion of the stroke team.
Main Outcomes and Measures
The primary outcome was the reperfusion therapy rate, a composite outcome of intravenous recombinant tissue plasminogen activator (IV rtPA) or endovascular thrombectomy (EVT) for eligible patients who arrived within 3.5 or 4.5 hours of symptom onset. Secondary outcomes were the IV rtPA administration rate among eligible patients who arrived within 3.5 hours of symptom onset, the EVT rate among eligible participants who arrived within 4.5 hours of symptom onset, the proportion of patients with door-to-needle time within 60 minutes, the proportion of patients with door-to-puncture time within 90 minutes, in-hospital mortality, and 3-month disability as measured by a modified Rankin Scale score greater than 2.
Results
All 12 132 eligible patients (mean [SD] age, 66 [12.1] years; 7759 male [64.0%]) completed the trial. The reperfusion rate was 53.5% (3046 of 5689) for the eligible patients in the intervention period and 43.9% (2830 of 6443) in the control period. No significant improvement in primary outcomes was found for the intervention after adjusting for cluster, period, and imbalanced baseline covariates (adjusted risk difference [ARD], 5.5%; 95% CI, −8.0% to 19.0%; adjusted odds ratio [AOR], 1.26; 95% CI, 0.72-2.21) or for the secondary outcomes. However, significant improvements were found in secondary hospitals for reperfusion therapy (1081 of 1870 patients [57.8%] vs 945 of 2022 patients [42.9%]; ARD, 19.0%; 95% CI, 6.4%-31.6%; AOR, 2.24; 95% CI, 1.29-3.88), IV rtPA administration (1062 of 1826 patients [58.2%] vs 916 of 2170 patients [42.2%]; ARD, 20.3%; 95% CI, 7.4%-33.1%; AOR, 2.37; 95% CI, 1.34-4.19), and EVT (51 of 231 patients [22.1%] vs 37 of 259 patients [14.3%]; ARD, 13.6%; 95% CI, 1.0%-26.3%; AOR, 3.03; 95% CI, 1.11-8.25) in subgroup analyses.
Conclusions and Relevance
In this stepped-wedge cluster randomized clinical trial of patients with acute ischemic stroke in China, the use of a targeted quality improvement intervention compared with usual care did not improve the reperfusion therapy rate. However, the intervention may be effective in secondary hospitals.
Trial Registration
ClinicalTrials.gov Identifier: NCT03578107
Introduction
Stroke remains the leading cause of long-term disability worldwide.1 As the most populous country in the world, China has the highest estimated risk of lifetime stroke, and the disability-adjusted life-years due to stroke increased by 36.7% from 1990 to 2019.2,3 Reperfusion therapy, including intravenous thrombolysis (IVT)4,5,6 with recombinant tissue plasminogen activator (rtPA) and endovascular thrombectomy (EVT),7,8 is the most effective treatment for improving functional outcomes of acute ischemic stroke.9,10,11 However, the rate of intravenous rtPA (IV rtPA) administration within 4.5 hours after stroke onset among eligible patients was only 22.9%, and the rate of EVT was only 0.8% as reported by the Chinese Stroke Center Alliance data from 2015 to 2019,12 which was far below the IVT rate (>73.4%) between 2013 and 2015 and the EVT rate (>3.3%) in 2016 reported by Get With the Guidelines–Stroke in the US.13,14
Previous cluster randomized clinical trials of multifaceted quality improvement interventions to improve adherence to evidence-based performance measures in patients with acute ischemic stroke have shown an inconsistent effect on the rate of IVT, and the EVT rate was not evaluated.15,16 Other trials that specifically aimed to improve thrombolysis by comprehensive intervention have revealed inconsistent conclusions as well.17,18,19,20 The low rate of IVT and EVT was presumably caused by the unequal distribution of high-quality health resources,21,22 the unorganized stroke care in stroke centers that have just begun to be constructed in China,23,24 the eroded patient-physician trust encountered during structural and social transformations,25 and the inadequate medical reimbursement in transitioning health systems in China.26 In addition, the Target: Stroke Initiative, launched by the American Heart Association/American Stroke Association (AHA/ASA), markedly improved the workflow of stroke care and demonstrated the effectiveness of key best practice strategies for reperfusion therapy in stroke centers.27,28,29 However, whether these strategies would be effective in a different health care setting in China remains unknown. Therefore, we developed a problem-oriented, culturally adapted, targeted quality improvement intervention with the support of the AHA/ASA to improve the reperfusion rate for patients with acute ischemic stroke in China.30 To examine whether reperfusion therapy for patients with acute ischemic stroke could be improved with a problem-oriented, culturally adapted, targeted quality improvement intervention, we performed a pragmatic, stepped-wedge cluster randomized clinical trial for sequential implementation across hospitals in China.
Methods
The trial protocol was approved by the central institutional review board at Beijing Tiantan Hospital, Capital Medical University. Written informed consent was obtained from patients or their proxies before enrollment. The study was conducted according to the study protocol (Supplement 1) and analyzed according to the statistical analysis plan (Supplement 1). This stepped-wedge cluster randomized clinical trial was reported according to the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.31
Trial Design and Oversight
The detailed study design has been previously reported.30 Briefly, the Improve Acute Reperfusion Treatment Quality for Stroke in China (IMPROVE Stroke Care in China) trial was a stepped-wedge, pragmatic cluster randomized clinical trial in which 51 hospitals (clusters) in China were randomly assigned to receive a problem-oriented, culturally adapted, and targeted quality improvement intervention for 1 of 3 predefined, 6-month periods during an 18-month period between January 1, 2019, and June 30, 2020, after a 6-month period of usual care.
Hospitals and Study Participants
We planned to recruit 51 hospitals in mainland China from the Chinese Stroke Center Alliance hospitals located in 29 provinces, autonomous regions, and municipalities. To ensure the diversity and representativeness of the included hospitals, hospitals were enrolled considering their economic-geographic regions (Eastern, Central, and Western) and hospital level (secondary or tertiary). To be eligible, hospitals were required to be secondary or tertiary public hospitals with emergency department and neurologic wards, have a 24/7 on-call stroke team, have the capacity for IVT or EVT, have administered IVT or EVT to at least 10 patients during the whole year before enrollment, and have at least 5 patients admitted within 6 hours after stroke onset per month. All hospitals had to want to improve the workflow of treating patients with acute ischemic stroke and have good collaboration among the multidisciplinary team. Hospitals that participated in other research projects of reperfusion quality improvement in patients with acute ischemic stroke were all excluded after a questionnaire survey.
At participating hospitals, patients were consecutively enrolled if they were older than 18 years; presented with symptoms of acute ischemic stroke, which was confirmed by computed tomography (CT) or magnetic resonance imaging (MRI); and arrived at the hospital within 6 hours of symptom onset.30 Patients diagnosed with other cerebrovascular diseases, such as transient ischemic attack (TIA), hemorrhagic stroke, cerebral venous sinus thrombosis, or noncerebrovascular diseases, were excluded. All patients were scheduled to be followed up for 90 days to obtain information on the modified Rankin Scale (mRS) score (range, 0 [no symptoms] to 6 [death]) or death through telephone interviews by a blinded centralized follow-up team who was trained by the research team.
Randomization
The cluster randomization was performed at the hospital level centrally using a computer-generated random number sequence. The other members of the study team and the selected sites were informed that they would cross over to the intervention period 1 month before each of the predefined steps to maintain allocation concealment while aiding in training logistics. A total of 17 hospitals were randomly assigned to 1 of the 3 sequences (cohorts) considering hospital location and level to minimize potential imbalance between the intervention and control periods as much as possible. Of the 51 participating hospitals (17 secondary and 34 tertiary hospitals), 2 (1 secondary hospital and 1 tertiary hospital) withdrew from this trial because they were unable to recruit eligible patients, and 1 hospital discontinued the study before the intervention and was replaced by a newly enrolled hospital with the same hospital level.
Intervention and Training
A problem-oriented, culturally adapted, targeted quality improvement intervention, STEP (strategies, toolkit, exploration, and paradigm), was developed based on the theories of implementation science and the consolidated framework of implementation research32 to promote workflow reconstruction in stroke centers and to shorten in-hospital delays of reperfusion treatment for patients with acute ischemic stroke (eFigure 1 in Supplement 2). More detailed descriptions of the STEP intervention are reported in the eMethods in Supplement 2 and the protocol (Supplement 1).30
Outcome Measures
The primary outcome was reperfusion therapy rate, defined as a composite outcome of IVT for eligible patients who arrived within 3.5 hours of symptom onset or EVT for eligible patients who arrived within 4.5 hours of symptom onset. The secondary outcomes included the rate of IV rtPA administration among eligible patients who arrived within 3.5 hours of symptom onset; the rate of EVT among eligible patients who arrived within 4.5 hours of symptom onset; the proportion of patients with a door-to-needle time (DNT) within 60 minutes among participants who received IV rtPA; the proportion of patients with a door-to-puncture time (DPT) within 90 minutes among participants who received EVT; in-hospital mortality; and 3-month disability as measured by an mRS score greater than 2.
Sample Size
Assuming 31 patients per period (6 months) per hospital, 51 hospitals, a 0.03 intraclass correlation coefficient, and a 19% reperfusion treatment rate at baseline, we expected a total of 6324 patients would provide 90% statistical power for detecting a 30% improvement in the primary outcomes (from 19% to 25%) at a 2-sided α level of 0.05. The 19% rate was based on the Chinese Stroke Center Alliance data up to March 2018.12 However, because of the increasing enrollment volumes of participating hospitals in clinical practice and the continuous recruitment design, the final enrollment was much larger than the initial targeted sample size, which may provide abundant power for this analysis.
Statistical Analysis
All results were reported using an intention-to-treat analysis. Baseline characteristics are summarized as the mean (SD) or median (IQR) for continuous variables and as numbers (percentages) for categorical variables. Baseline characteristics between the intervention and control participants were compared using crude differences and 95% CIs.
We first performed the analyses for the primary analysis using mixed-effects logistic regression models with a random effect for the cluster (hospital) and a fixed-time effect for every 6-month period. However, the models did not converge. Therefore, we modeled the outcomes using generalized estimating equation models to account for the clustering effect within hospitals, with a fixed-time effect for the period. To check the robustness of our primary analysis, we also modeled the outcomes using generalized estimating equation models with additional adjustment for baseline imbalanced covariates (covariate-adjusted analysis), including medical history of stroke or TIA, medication history of antiplatelet agent and statin, and hospital location. In all these analyses, we reported the absolute effect measure risk difference (RD) and 95% CI estimated from a binomial regression model with the identity link function, in addition to the relative effect measure odds ratio (OR) and 95% CI.33
A total of 1423 of 11 821 participants discharged alive (12.0%) had missing data for mRS scores at 90 days; therefore, we first reported the results based on the complete data and then used multiple imputations by fully conditional specification to generate 5 complete data sets. The imputation models included all baseline covariates listed in Table 1. The analyses were then performed for each data set separately, and the results were pooled according to the Rubin rules.34
Table 1. Baseline Patient and Hospital Characteristics of the Improve Acute Reperfusion Treatment Quality for Stroke in China (IMPROVE Stroke Care in China) Triala.
| Characteristic | Intervention (n = 5689 [46.9%]) | Control (n = 6443 [53.1%]) | Difference (95% CI) |
|---|---|---|---|
| Patient characteristics | |||
| Demographic characteristics | |||
| Age, mean (SD), y | 66.4 (12.1) | 65.9 (12.0) | 0.5 (0.1 to 0.9) |
| Sex | |||
| Male | 3655 (64.2) | 4104 (63.7) | 0.5 (−1.2 to 2.3) |
| Female | 2034 (35.8) | 2339 (36.3) | −0.5 (−2.3 to 1.2) |
| BMI, mean (SD) | 23.6 (3.5) | 23.9 (4.0) | −0.3 (−0.4 to −0.2) |
| NIHSS score at admission, median (IQR) | 5.0 (2.0 to 10.0) | 5.0 (2.0 to 10.0) | 0.0 (0.0 to 0.0) |
| Current smoking | 1570 (27.6) | 1668 (25.9) | 1.7 (0.1 to 3.3) |
| Drinking | 1386 (24.4) | 1607 (24.9) | −0.5 (−2.1 to 1.0) |
| Medical history | |||
| Stroke or TIA | 1358 (23.9) | 2087 (32.4) | −8.5 (−10.1 to −6.9) |
| Hypertension | 3435 (60.4) | 4022 (62.4) | −2.0 (−3.8 to −0.3) |
| Diabetes | 1092 (19.2) | 1309 (20.3) | −1.1 (−2.5 to −0.3) |
| Dyslipidemia | 301 (5.3) | 424 (6.6) | −1.3 (−2.1 to −0.5) |
| Myocardial infarction | 117 (2.1) | 125 (1.9) | 0.2 (−0.4 to 0.6) |
| Atrial fibrillation | 579 (10.2) | 528 (8.2) | 2.0 (1.0 to 3.0) |
| Peripheral vascular disease | 52 (0.9) | 113 (1.8) | −0.9 (−1.2 to −0.4) |
| Medication history | |||
| Antiplatelet agent | 904 (15.9) | 1346 (20.9) | −5.0 (−6.4 to −3.6) |
| Anticoagulant drugs | 182 (3.2) | 370 (5.7) | −2.5 (−3.3 to −1.8) |
| Antihypertensive drugs | 2501 (44.0) | 2876 (44.6) | −0.6 (−2.4 to 1.1) |
| Antidiabetics | 853 (15.0) | 1051 (16.3) | −1.3 (−2.6 to 0.0) |
| Statins | 749 (13.2) | 1212 (18.8) | −5.6 (−6.9 to −4.3) |
| Onset-to-door time, median (IQR), min | 95.0 (55.0 to 144.0) | 94.0 (54.0 to 146.0) | −2.0 (−4.0 to 0.0) |
| Hospital characteristics | |||
| Hospital level | |||
| Tertiary | 3819 (67.1) | 4241 (65.8) | 1.3 (−0.4 to 3.0) |
| Secondary | 1870 (32.9) | 2202 (34.2) | −1.3 (−3.0 to 0.4) |
| Hospital location | |||
| Eastern | 2385 (41.9) | 3189 (49.5) | −7.6 (−9.3 to −5.8) |
| Central | 1857 (32.6) | 1807 (28.0) | 4.6 (3.0 to 6.2) |
| Western | 1447 (25.4) | 1447 (22.5) | 2.9 (1.5 to 4.5) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischemic attack.
Data are presented as number (percentage) unless otherwise indicated.
To check the heterogeneity between subgroups, we performed post hoc subgroup analyses by age (<65 or ≥65 years), sex, and hospital level (secondary or tertiary) in the abovementioned primary and covariable-adjusted analysis. P values were calculated from generalized estimating equation models. A 2-sided P < .05 was considered statistically significant. We conducted all analyses using SAS software, version 9.4 (SAS Institute Inc).
Results
A total of 12 132 participants (5689 [46.9%] in the intervention period and 6443 [53.1%] in the control period) were included in this analysis. The mean (SD) age of the participants was 66.1 (12.1) years; 7759 (64.0% were men and 4373 (36.0%) were women. Details on patient enrollment and identification are shown in Figure 1. Baseline characteristics between the included and excluded participants were largely comparable except that the former group had more patients recruited from hospitals located in Eastern China and fewer patients from Central China (eTable 1 in Supplement 2).
Figure 1. Flow of Hospitals and Participants Through the Improve Acute Reperfusion Treatment Quality for Stroke in China (IMPROVE Stroke Care in China).
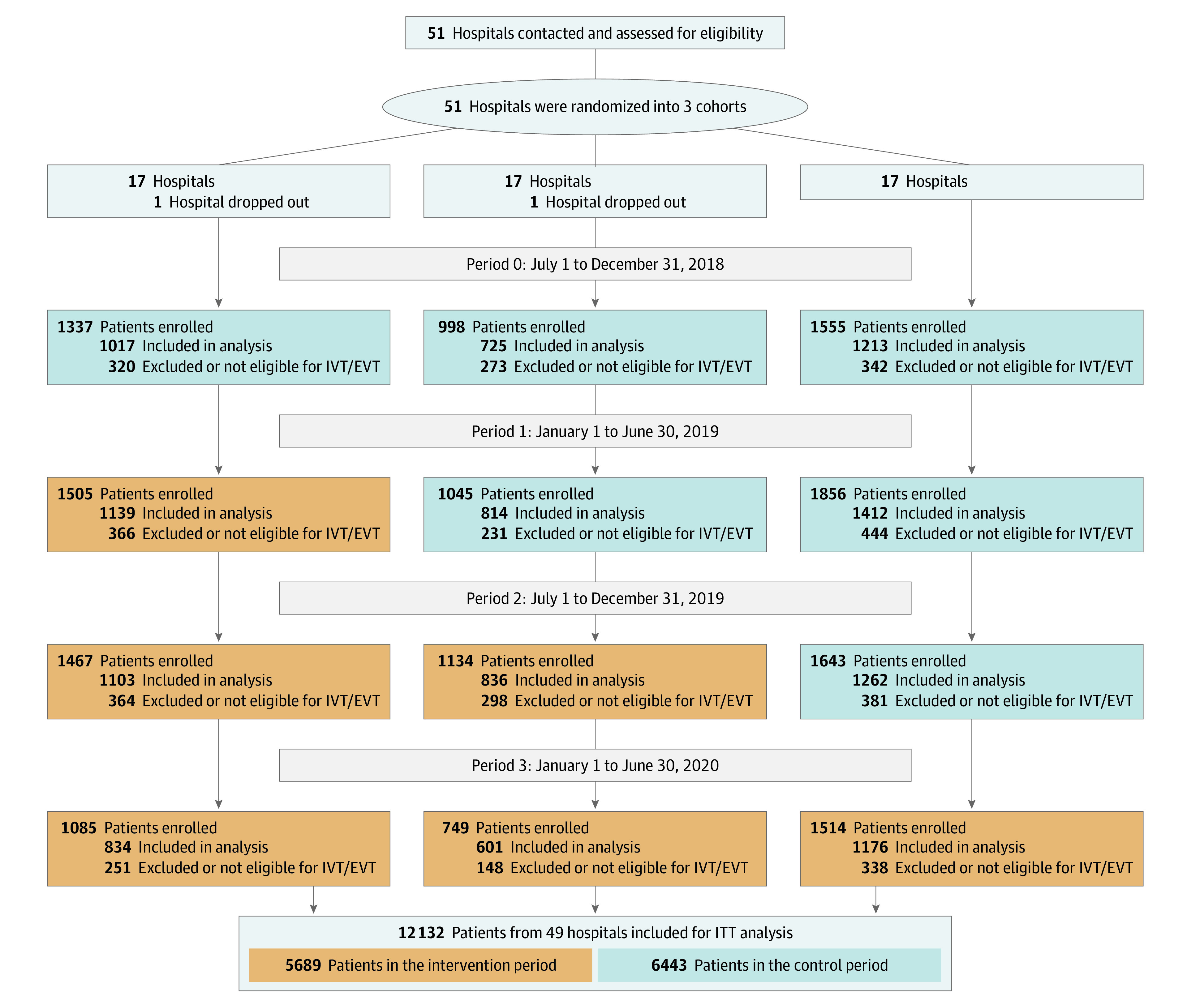
EVT indicates endovascular thrombectomy; ITT, Intention to treat; and IVT, intravenous thrombolysis.
Patient Characteristics
The median (IQR) National Institutes of Health Stroke Scale score (range, 0-42, with a higher score indicating greater stroke severity) at admission was 5 (2-10). A total of 7457 participants (61.5%) had a medical history of hypertension, 3445 (28.4%) had a history of stroke or TIA, and 2401 (19.8%) had diabetes. The baseline characteristics between the intervention and control periods were generally similar except for a lower proportion of patients with a medical history of stroke or TIA (difference, −8.5%; 95% CI, −10.1% to −6.9%), patients with prior use of antiplatelet agents (difference, −5.0%; 95% CI, −6.4% to −3.6%) and statins (difference, −5.6%; 95% CI, −6.9% to −4.3%), and patients recruited from hospitals located in Eastern China (difference, −7.6%; 95% CI, −9.3% to −5.8%) in the former group (Table 1).
Primary and Secondary Outcomes
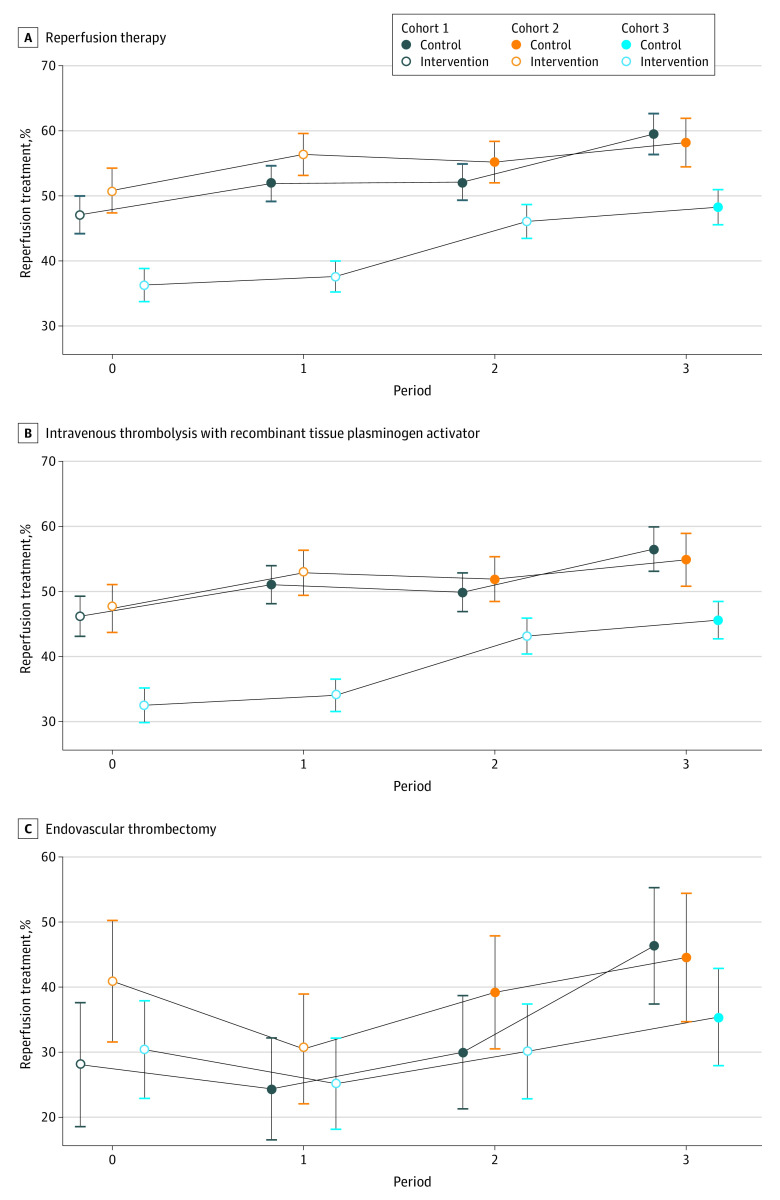
A total of 3046 of 5689 participants (53.5%) in the intervention period and 2830 of 6443 (43.9%) in the control period received reperfusion therapy. The temporal trend for reperfusion therapy is shown in Figure 2A. After adjustment for cluster and period, the implementation of the STEP intervention did not result in a significantly higher rate of reperfusion therapy (adjusted RD [ARD], 7.8%; 95% CI, −7.3% to 23.0%; adjusted OR [AOR], 1.37; 95% CI, 0.74-2.52) (Table 2). The results from the imbalanced baseline covariate-adjusted analysis were consistent with those from the primary analysis (ARD, 5.5%; 95% CI, −8.0% to 19.0%; AOR, 1.26; 95% CI, 0.72-2.21) (Table 2).
Figure 2. Unadjusted Temporal Trends in Reperfusion Therapy.
A, Reperfusion therapy among eligible patients who arrived within 3.5 or 4.5 hours of symptom onset. B, Intravenous thrombolysis among eligible patients who arrived within 3.5 hours of symptom onset. C, Endovascular thrombectomy among eligible patients who arrived within 4.5 hours of symptom onset. Whiskers indicate 95% CIs.
Table 2. Primary and Secondary Trial Outcomes.
| Outcomes | ICC | No. of cases/total No. (%) | Primary analysisa | Covariate-adjusted analysisb | |||
|---|---|---|---|---|---|---|---|
| Intervention | Control | ARD (95% CI) | AOR (95% CI) | ARD (95% CI) | AOR (95% CI) | ||
| Primary outcome | |||||||
| Reperfusion therapy among patients who arrived within 3.5 or 4.5 h of symptom onset | 0.28 | 3046/5689 (53.5) | 2830/6443 (43.9) | 7.8 (−7.3 to 23.0) | 1.37 (0.74 to 2.52) | 5.5 (−8.0 to 19.0) | 1.26 (0.72 to 2.21) |
| Secondary outcomes | |||||||
| IV rtPA among patients who arrived within 3.5 h of symptom onset | 0.28 | 2830/5540 (51.1) | 2612/6316 (41.4) | 8.9 (−6.4 to 24.2) | 1.43 (0.77 to 2.66) | 6.7 (−7.0 to 20.5) | 1.33 (0.75 to 2.36) |
| EVT among patients who arrived within 4.5 h of symptom onset | 0.36 | 270/739 (36.5) | 236/772 (30.6) | 0.8 (−11.7 to 13.4) | 1.05 (0.57 to 1.95) | 0.4 (−10.1 to 11.0) | 0.99 (0.57 to 1.71) |
| DNT within 60 min | 0.22 | 2018/2830 (71.3) | 1701/2612 (65.1) | 7.5 (−6.0 to 21.0) | 1.44 (0.76 to 2.75) | 7.1 (−5.3 to 19.4) | 1.39 (0.77 to 2.54) |
| DPT within 90 min | 0.23 | 62/270 (23.0) | 86/236 (36.4) | −25.6 (−40.6 to −10.7) | 0.26 (0.12 to 0.57) | −18.4 (−29.4 to −7.3) | 0.27 (0.14 to 0.52) |
| In-hospital death | 0.14 | 146/5689 (2.6) | 165/6443 (2.6) | −0.2 (−1.3 to 0.9) | 0.92 (0.58 to 1.45) | −0.3 (−1.2 to 0.6) | 0.93 (0.61 to 1.41) |
| Disability at 90 dc | 0.02 | 1215/4687 (25.9) | 1439/5711 (25.2) | −1.3 (−5.4 to 2.9) | 0.94 (0.75 to 1.16) | −0.9 (−5.1 to 3.2) | 0.96 (0.77 to 1.20) |
Abbreviations: AOR, adjusted odds ratio; ARD, adjusted risk difference; DNT, door-to-needle time; DPT, door-to-puncture time; EVT, endovascular thrombectomy; ICC, intracluster correlation; IV rtPA, intravenous recombinant tissue plasminogen activator.
Results were yielded from generalized estimating equation logistic regression models and adjusted for the hospital clustering effect and temporal trend (period).
Results were yielded from generalized estimating equation logistic regression models and adjusted for the hospital clustering effect, temporal trend (period), and imbalanced baseline covariates (medical history of stroke or transient ischemic attack, medication history of antiplatelet agent and statin, and hospital location).
A total of 1423 of 11 821 patients discharged alive (12.0%; 856 of 5543 [15.4%] in the intervention period vs 567 of 6278 [9.0%] in the control period) had missing modified Rankin Scale scores because of loss of follow-up.
We also assessed the components of reperfusion therapy. The rate of IV rtPA administration among eligible patients who arrived within 3.5 hours of symptom onset was 51.1% (2830 of 5540) in the intervention period compared with 41.4% (2612 of 6316) in the control period; the rate of EVT among eligible patients who arrived within 4.5 hours of symptom onset was 36.5% (270 of 739) in the intervention period compared with 30.6% (236 of 772) in the control period. Figure 2B and C shows the temporal trends for IV rtPA administration and EVT, respectively. The difference in the rate of IV rtPA administration (ARD, 8.9%; 95% CI, −6.4% to 24.2%; AOR, 1.43; 95% CI, 0.77-2.66) or EVT (ARD, 0.8%; 95% CI, −11.7% to 13.4%; AOR, 1.05; 95% CI, 0.57-1.95) between the intervention and control periods was not significant after adjusting for the cluster and period. The imbalanced baseline covariate-adjusted analyses show similar results (Table 2). Improvements in other secondary outcomes were not statistically significant, including a DNT within 60 minutes, a DPT within 90 minutes, in-hospital death, and disability at 90 days.
Baseline characteristics between patients with and without mRS scores at 90 days were largely comparable (eTable 2 in Supplement 2). Therefore, we also reported sensitivity analysis from multiple imputed data for mRS scores at 90 days, which revealed comparable results with previous analyses (eTable 3 Supplement 2).
Subgroup Analysis
No significant statistical heterogeneity was found in the intervention effect by age, sex, or hospital level. However, we found that the intervention among secondary hospitals resulted in significantly higher rates of reperfusion therapy (1081 of 1870 patients [57.8%] vs 945 of 2202 patients [42.9%]; ARD, 19.0%; 95% CI, 6.4% to 31.6%; AOR, 2.24; 95% CI, 1.29-3.88), intravenous thrombolysis (1062 of 1826 [58.2%] vs 916 of 2170 patients [42.2%]; ARD, 20.3%; 95% CI, 7.4% to 33.1%; AOR, 2.37; 95% CI, 1.34-4.19), and EVT (51 of 231 patients [22.1%] vs 37 of 259 patients [14.3%]; ARD, 13.6%; 95% CI, 1.0%-26.3%; AOR, 3.03; 95% CI, 1.11-8.25) in the intervention period after adjusting for cluster, period, and imbalanced baseline covariates (Figure 3).
Figure 3. Subgroup Analysis for the Effect of Intervention on Reperfusion Therapy.
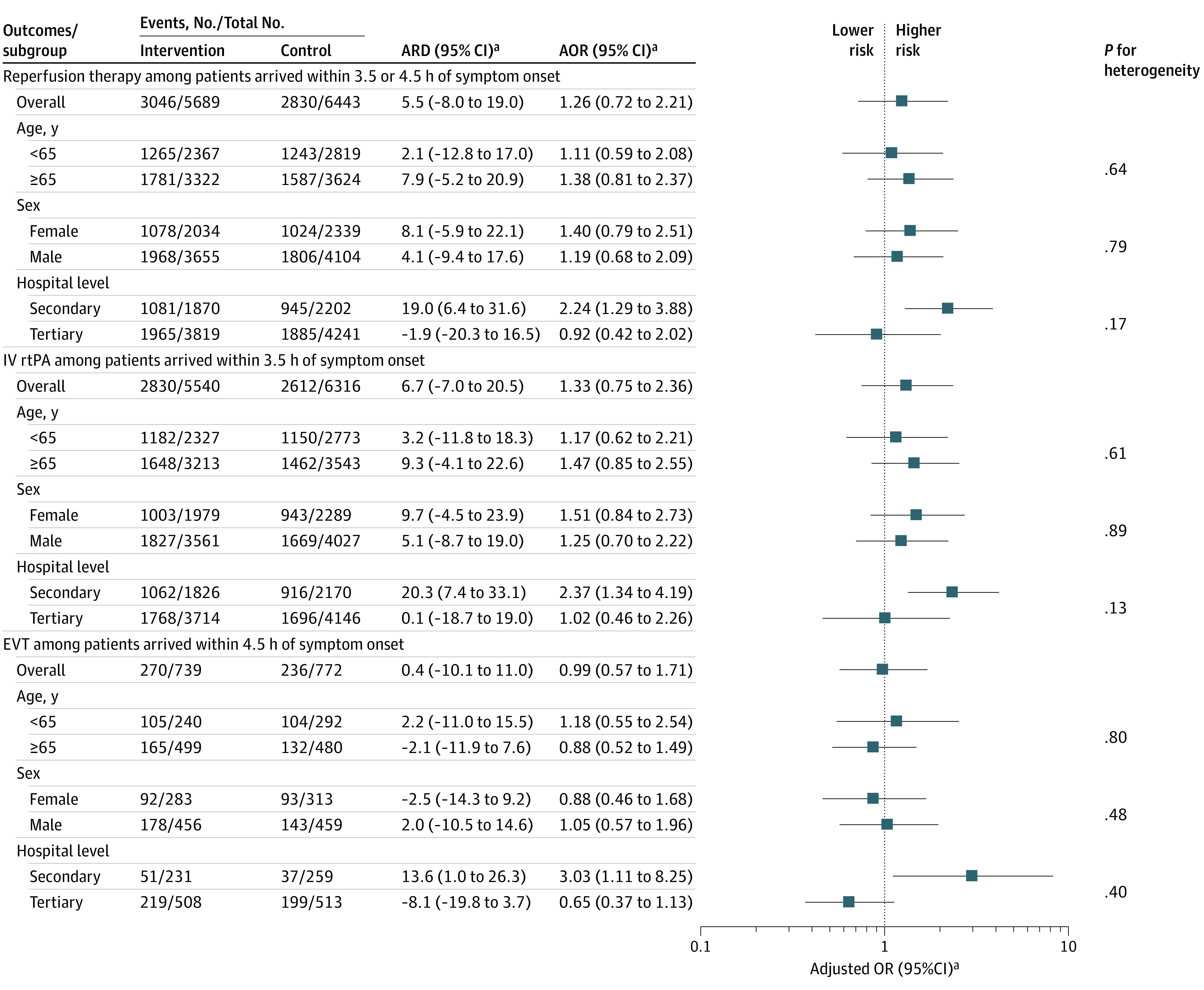
AOR indicates adjusted odds ratio; ARD, adjusted risk difference; EVT, endovascular thrombectomy; IV rtPA, intravenous recombinant tissue plasminogen activator; and OR, odds ratio.
aResults were yielded from generalized estimating equation logistic regression models and adjusting for a medical history of stroke or transient ischemic attack, medication history of antiplatelet agent and statin, and hospital location.
No significant heterogeneity was found in the intervention effect by subgroup for the other secondary outcomes, except that the intervention significantly reduced the rate of in-hospital death (0.8% vs 1.2%; ARD, −0.8%; 95% CI, −1.5% to −0.1%; AOR, 0.39; 95% CI, 0.18-0.83) and may have reduced the rate of disability at 90 days (14.8% vs 16.2%; ARD, −2.8%; 95% CI, −5.7% to 0.2%; AOR, 0.81; 95% CI, 0.63-1.02) among patients younger than 65 years but not among those 65 years or older (eTable 4 in Supplement 2).
Discussion
In this stepped-wedge cluster randomized clinical trial, we found that the STEP targeted quality intervention did not increase the overall rate of reperfusion therapy, the rate of a DNT within 60 minutes, or the rate of a DPT within 90 minutes. However, it increased the rate of reperfusion therapy in secondary hospital settings. The results showed that the problem-oriented, culturally adapted, targeted quality intervention might be more effective in secondary hospital settings, which may provide a direction for better resource allocation. According to the latest statistics, there are more than 10 000 secondary hospitals in China,35 and more than 27 million patients with acute ischemic stroke are admitted to secondary hospitals yearly.36 Therefore, providing more resources to resource-constrained secondary hospitals to efficiently improve reperfusion therapy adoption would benefit most patients with acute ischemic stroke in China and have profound implications for the whole health care system and public health.
There are several potential reasons why the STEP targeted quality improvement intervention was not effective for the improvement of reperfusion therapy overall but may be effective for secondary hospitals. First, because the effect of reperfusion therapy was confirmed in 5 milestone trials in 2015,7 efforts for the adoption of reperfusion therapy have been advocated for by national and local health authorities and medical associations in China, which finally led to increasing the rate of reperfusion therapy. In 2021, increasing the rate of reperfusion therapy became 1 of the 10 National Health Quality and Safety Improvement Goals released by the National Health Commission of the People's Republic of China.37 Although the enrollment of our trial was completed before the start of the goals, under this circumstance, the temporal trend of the rate of reperfusion therapy has been increasing steadily, as data from the Chinese Stroke Center Alliance Nationwide registry38 illustrates (eFigure 2 in Supplement 2), which made it even harder for the STEP targeted quality improvement intervention to show the overall effect. Second, the admission of eligible patients in this study in the last 6 months was reduced because of the COVID-19 pandemic. During this time, most of the medical resources (especially staff) involved in this trial were redirected to the COVID-19 outbreak in China, which seriously influenced the implementation of strategies, the exploration of expert guidance, and the use of the Plan-Do-Study-Act paradigm.39 Therefore, the total effect size of the intervention may have been reduced as well. Third, the baseline reperfusion rate (43.9%) in our trial was much higher than estimated and higher than the rates in other hospitals, which may suggest another reason why the STEP intervention is not effective overall. Fourth, almost 2 times more hospitals and participants were enrolled in tertiary hospitals than in secondary hospitals. Because tertiary hospitals receive more research projects and physicians are more overworked than that in secondary hospitals,40 participating in research programs and obtaining training is not as incentivizing for physicians in tertiary hospitals compared with those in secondary hospitals; therefore, the implementation or adoption of the intervention may be compromised in tertiary hospitals. This issue could be another reason why the intervention was not effective in tertiary hospital settings but was effective in secondary hospital settings.
We noted that fewer patients had a DPT within 90 minutes in the intervention period than in the control period, which may be explained by the higher proportion of patients having a DPT within 90 to 150 minutes in the intervention period (eFigure 3 in Supplement 2). The intervention did not result in a lower rate of disability at 90 days. However, analysis of the effect of reperfusion therapy showed a lower risk of disability at 90 days after adjustment for imbalanced baseline covariates (ARD, −4.0%; 95% CI, −6.1% to −1.9; AOR, 0.80; 95% CI, 0.70-0.91) (eTables 5 and 6 in Supplement 2).
Several studies focusing on the improvement of thrombolysis have been launched. The TIPS (Thrombolysis Implementation in Pediatric Stroke) trial,17 the AVC II (Impact of a Training Program and Organization on the Management of Stroke in the Acute Phase II) trial,19 and the PASTA (Paediatric Arteriopathy Steroid Aspirin Project) trial20 aimed for the adoption of thrombolysis through comprehensive intervention and yielded inconsistent results. The ANGEL-ACT (Endovascular Treatment Key Technique and Emergency Work Flow Improvement of Acute Ischemic Stroke)41 and IMPACT (Improving Patient Access to Stroke Therapy Study) (NCT01870492) studies aim to identify barriers to reperfusion therapy through observation studies, whereas the MISSION (Multicomponent Intervention to Shorten Thrombolytic Door-to-Needle Time in Stroke Patients in China) trial18 and the Target: Stroke trial27 aim to shorten DNT, and the ANGEL-ACT II trial41 aims to shorten DPT through multimodal medical quality improvement by a cluster randomized, parallel controlled design. To the best of our knowledge, the IMPROVE Stroke Care trial is the first and most well-powered clinical trial to evaluate a problem-oriented, culturally adapted, targeted quality improvement intervention for increasing the adoption of reperfusion therapy among eligible patients with acute ischemic stroke in China.
Strengths and Limitations
The IMPROVE Stroke Care trial has several strengths. First, the STEP intervention package was created based on potential causes of China’s low rate of reperfusion therapy, as well as incorporating some successful practices from other countries. Second, it was built on previous observational data and the organization of the National Registry Program Chinese Stroke Center Alliance,12,38 which provided a solid foundation for the design and implementation of the trial. Third, the study used a stepped-wedge, cluster-randomized, pragmatic trial design, allowing all hospitals to eventually receive the intervention, which would maximally benefit clinical practice. Fourth, the causal inference was notably improved with appropriate and strict statistical methods and elaborate sensitivity analysis.
This study also has several limitations. First, the rate of reperfusion therapy and the intraclass correlation coefficient are higher than those estimated in the sample size estimation stage. However, the final sample size was much larger than the planned sample size because of the increasing enrollment volumes of participating hospitals in clinical practice and the continuous recruitment design, which made the trial well powered to detect the current difference. Second, the COVID-19 pandemic slowed enrollment during the last period of the trial, which increased the imbalance between the intervention and control period and may also have introduced enrollment bias. However, the baseline characteristics were largely comparable. The COVID-19 pandemic led to difficulties in the implementation of the intervention, which may also have impaired the intervention effect. Third, the complex intervention included many components (eg, workflow reconstruction, toolkits, technical trainings, and workshops) that may not have been well received and fully implemented at all sites, especially at tertiary hospitals, or not implemented for a sufficient duration to take effect. On the basis of the survey after the intervention, a single call activation of the stroke team was implemented in only approximately 75% of hospitals (73% in tertiary vs 80% in secondary hospitals), stroke-specific order sets were used in only 77% (72% in tertiary vs 83% in secondary hospitals), and a weekly or biweekly improvement meeting was held in 85.4% (78% in tertiary vs 89% in secondary hospitals). Fourth, the effect of intervention was seen in secondary hospitals. However, this result was drawn from the post hoc subgroup analysis without prespecified sample size calculation or adjustment for multiple subgroup tests, and the interaction P value was greater than .10. Therefore, this result should be interpreted as an exploratory analysis.
Conclusions
Among patients with acute ischemic stroke, the implication of a problem-oriented and culturally adapted, targeted quality improvement intervention compared with usual care at the hospital level did not result in a statistically significant improvement in the rate of reperfusion therapy. However, the intervention may be effective in resource-constrained secondary hospitals. Additional randomized clinical trials are needed to confirm the efficacy of the targeted quality improvement intervention among secondary hospitals, and further analyses are needed to understand the mechanism underlying the lack of efficacy in tertiary hospitals.
Trial Protocol and Statistical Analysis Plan
eMethods.
eTable 1. Baseline Characteristics of the IMPROVE Stroke Care in China Patients and Hospitals Between Included and Excluded Patients
eTable 2. Baseline Characteristics of the IMPROVE Stroke Care China Patients and Hospitals Among Patients With and Without Values of mRS at 90 Days
eTable 3. Results of Disability at 90 Days From Multiple Imputations
eTable 4. Subgroup Analysis for the Effect of Intervention on Reperfusion Treatment for Other Secondary Outcomes
eTable 5. Baseline Characteristics of the IMPROVE Stroke Care in China Patients and Hospitals by Reperfusion
eTable 6. Results of Disability at 90 Days From Raw Data
eFigure 1. The Design of “STEP” Intervention
eFigure 2. Reperfusion Therapy in the Chinese Center Association Alliance Hospitals Not Participating in the IMPROVE Stroke Care in China Trial
eFigure 3. Distribution of Door-to-Puncture Time in the IMPROVE Stroke Care In China Trial
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Vos T, Lim SS, Abbafati C, et al. ; GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204-1222. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin VL, Nguyen G, Cercy K, et al. ; GBD 2016 Lifetime Risk of Stroke Collaborators . Global, regional, and country-specific lifetime risks of stroke, 1990 and 2016. N Engl J Med. 2018;379(25):2429-2437. doi: 10.1056/NEJMoa1804492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ma Q, Li R, Wang L, et al. Temporal trend and attributable risk factors of stroke burden in China, 1990-2019: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2021;6(12):e897-e906. doi: 10.1016/S2468-2667(21)00228-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emberson J, Lees KR, Lyden P, et al. ; Stroke Thrombolysis Trialists’ Collaborative Group . Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet. 2014;384(9958):1929-1935. doi: 10.1016/S0140-6736(14)60584-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012;379(9834):2364-2372. doi: 10.1016/S0140-6736(12)60738-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomalla G, Boutitie F, Ma H, et al. ; Evaluation of unknown Onset Stroke thrombolysis trials (EOS) investigators . Intravenous alteplase for stroke with unknown time of onset guided by advanced imaging: systematic review and meta-analysis of individual patient data. Lancet. 2020;396(10262):1574-1584. doi: 10.1016/S0140-6736(20)32163-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. doi: 10.1016/S0140-6736(16)00163-X [DOI] [PubMed] [Google Scholar]
- 8.Rodrigues FB, Neves JB, Caldeira D, Ferro JM, Ferreira JJ, Costa J. Endovascular treatment versus medical care alone for ischaemic stroke: systematic review and meta-analysis. BMJ. 2016;353:i1754. doi: 10.1136/bmj.i1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211 [DOI] [PubMed] [Google Scholar]
- 10.Liu L, Chen W, Zhou H, et al. ; Chinese Stroke Association Stroke Council Guideline Writing Committee . Chinese Stroke Association guidelines for clinical management of cerebrovascular disorders: executive summary and 2019 update of clinical management of ischaemic cerebrovascular diseases. Stroke Vasc Neurol. 2020;5(2):159-176. doi: 10.1136/svn-2020-000378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berge E, Whiteley W, Audebert H, et al. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur Stroke J. 2021;6(1):I-LXII. doi: 10.1177/2396987321989865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu HQ, Yang X, Wang CJ, et al. Clinical characteristics, management, and in-hospital outcomes in patients with stroke or transient ischemic attack in China. JAMA Netw Open. 2021;4(8):e2120745. doi: 10.1001/jamanetworkopen.2021.20745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Man S, Zhao X, Uchino K, et al. Comparison of acute ischemic stroke care and outcomes between comprehensive stroke centers and primary stroke centers in the United States. Circ Cardiovasc Qual Outcomes. 2018;11(6):e004512. doi: 10.1161/CIRCOUTCOMES.117.004512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith EE, Saver JL, Cox M, et al. Increase in endovascular therapy in Get With The Guidelines-Stroke after the publication of pivotal trials. Circulation. 2017;136(24):2303-2310. doi: 10.1161/CIRCULATIONAHA.117.031097 [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Li Z, Zhao X, et al. ; GOLDEN BRIDGE—AIS Investigators . Effect of a multifaceted quality improvement intervention on hospital personnel adherence to performance measures in patients with acute ischemic stroke in China: a randomized clinical trial. JAMA. 2018;320(3):245-254. doi: 10.1001/jama.2018.8802 [DOI] [PubMed] [Google Scholar]
- 16.Machline-Carrion MJ, Santucci EV, Damiani LP, et al. ; BRIDGE-Stroke Investigators . Effect of a quality improvement intervention on adherence to therapies for patients with acute ischemic stroke and transient ischemic attack: a cluster randomized clinical trial. JAMA Neurol. 2019;76(8):932-941. doi: 10.1001/jamaneurol.2019.1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levi CR, Attia JA, D’Este C, et al. ; TIPS (Thrombolysis Implementation in Stroke) Study Group . Cluster-randomized trial of thrombolysis implementation support in metropolitan and regional Australian stroke centers: lessons for individual and systems behavior change. J Am Heart Assoc. 2020;9(3):e012732. doi: 10.1161/JAHA.119.012732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhong W, Lin L, Gong X, et al. ; MISSION investigators . Evaluation of a Multicomponent Intervention to Shorten Thrombolytic Door-to-Needle Time in Stroke Patients in China (MISSION): a cluster-randomized controlled trial. PLoS Med. 2022;19(7):e1004034. doi: 10.1371/journal.pmed.1004034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haesebaert J, Nighoghossian N, Mercier C, et al. ; AVC II Trial group . Improving access to thrombolysis and inhospital management times in ischemic stroke: a stepped-wedge randomized trial. Stroke. 2018;49(2):405-411. doi: 10.1161/STROKEAHA.117.018335 [DOI] [PubMed] [Google Scholar]
- 20.Price CI, Shaw L, Islam S, et al. Effect of an enhanced paramedic acute stroke treatment assessment on thrombolysis delivery during emergency stroke care. JAMA Neurol. 2020;77(7):840-848. doi: 10.1001/jamaneurol.2020.0611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li D, Zhou Z, Si Y, et al. Unequal distribution of health human resource in mainland China: what are the determinants from a comprehensive perspective? Int J Equity Health. 2018;17(1):29. doi: 10.1186/s12939-018-0742-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong E, Liu S, Chen M, et al. Differences in regional distribution and inequality in health-resource allocation at hospital and primary health centre levels: a longitudinal study in Shanghai, China. BMJ Open. 2020;10(7):e035635. doi: 10.1136/bmjopen-2019-035635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Li Z, Zhao X, et al. Stroke care quality in China: substantial improvement, and a huge challenge and opportunity. Int J Stroke. 2017;12(3):229-235. doi: 10.1177/1747493017694392 [DOI] [PubMed] [Google Scholar]
- 24.Wu S, Wu B, Liu M, et al. ; China Stroke Study Collaboration . Stroke in China: advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 2019;18(4):394-405. doi: 10.1016/S1474-4422(18)30500-3 [DOI] [PubMed] [Google Scholar]
- 25.Tucker JD, Wong B, Nie JB, Kleinman A; Patient-Physician Trust Team . Rebuilding patient-physician trust in China. Lancet. 2016;388(10046):755. doi: 10.1016/S0140-6736(16)31362-9 [DOI] [PubMed] [Google Scholar]
- 26.Yip W, Fu H, Chen AT, et al. 10 years of health-care reform in China: progress and gaps in Universal Health Coverage. Lancet. 2019;394(10204):1192-1204. doi: 10.1016/S0140-6736(19)32136-1 [DOI] [PubMed] [Google Scholar]
- 27.Fonarow GC, Zhao X, Smith EE, et al. Door-to-needle times for tissue plasminogen activator administration and clinical outcomes in acute ischemic stroke before and after a quality improvement initiative. JAMA. 2014;311(16):1632-1640. doi: 10.1001/jama.2014.3203 [DOI] [PubMed] [Google Scholar]
- 28.Fonarow GC, Smith EE, Saver JL, et al. Improving door-to-needle times in acute ischemic stroke: the design and rationale for the American Heart Association/American Stroke Association’s Target: Stroke initiative. Stroke. 2011;42(10):2983-2989. doi: 10.1161/STROKEAHA.111.621342 [DOI] [PubMed] [Google Scholar]
- 29.Man S, Xian Y, Holmes DN, et al. Target: Stroke was associated with faster intravenous thrombolysis and improved one-year outcomes for acute ischemic stroke in Medicare beneficiaries. Circ Cardiovasc Qual Outcomes. 2020;13(12):e007150. doi: 10.1161/CIRCOUTCOMES.120.007150 [DOI] [PubMed] [Google Scholar]
- 30.Li Z, Wang C, Zhang X, et al. Rationale and design of a stepped wedge cluster randomised trial to improve acute reperfusion treatment quality for stroke: IMPROVE stroke care in China. Stroke Vasc Neurol. 2022;7(5):451-456. doi: 10.1136/svn-2021-001461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hemming K, Taljaard M, McKenzie JE, et al. Reporting of stepped wedge cluster randomised trials: extension of the CONSORT 2010 statement with explanation and elaboration. BMJ. 2018;363:k1614. doi: 10.1136/bmj.k1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Damschroder LJ, Aron DC, Keith RE, Kirsh SR, Alexander JA, Lowery JC. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci. 2009;4:50. doi: 10.1186/1748-5908-4-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pedroza C, Truong VT. Performance of models for estimating absolute risk difference in multicenter trials with binary outcome. BMC Med Res Methodol. 2016;16(1):113. doi: 10.1186/s12874-016-0217-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubin DB. Multiple Imputation for Nonresponse in Surveys. Wiley; 1987. [Google Scholar]
- 35.National Health Commission Statistical Information Center . Number of the National Health Institutions by November 2021. Published 2022. Accessed July 11, 2022. http://www.nhc.gov.cn/mohwsbwstjxxzx/s7967/202201/e043142f1df54175a3860d4776891b9e.shtml
- 36.Wang YJ, Li ZX, Gu HQ, et al. ; China Stroke Statistics Writing Committee . China Stroke Statistics: an update on the 2019 report from the National Center for Healthcare Quality Management in Neurological Diseases, China National Clinical Research Center for Neurological Diseases, the Chinese Stroke Association, National Center for Chronic and Non-communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention and Institute for Global Neuroscience and Stroke Collaborations. Stroke Vasc Neurol. 2022;7(5):415-450. doi: 10.1136/svn-2021-001374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.The State Council of the People’s Public of China . Notice of the General Office of the National Health Commission on Printing and Distributing the National Health Quality and Safety Improvement Goals in 2021. Published 2021. Accessed July 3, 2022. http://www.gov.cn/zhengce/zhengceku/2021-02/22/content_5588240.htm
- 38.Wang Y, Li Z, Wang Y, et al. Chinese Stroke Center Alliance: a national effort to improve healthcare quality for acute stroke and transient ischaemic attack: rationale, design and preliminary findings. Stroke Vasc Neurol. 2018;3(4):256-262. doi: 10.1136/svn-2018-000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor MJ, McNicholas C, Nicolay C, Darzi A, Bell D, Reed JE. Systematic review of the application of the plan-do-study-act method to improve quality in healthcare. BMJ Qual Saf. 2014;23(4):290-298. doi: 10.1136/bmjqs-2013-001862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen J, Cheng Y, Hu X, Yuan P, Hao T, Shi Y. Workload, burnout, and medical mistakes among physicians in China: a cross-sectional study. Biosci Trends. 2016;10(1):27-33. doi: 10.5582/bst.2015.01175 [DOI] [PubMed] [Google Scholar]
- 41.Jia B, Ren Z, Mokin M, et al. ; ANGEL-ACT Study Group . Current status of endovascular treatment for acute large vessel occlusion in China: a real-world nationwide registry. Stroke. 2021;52(4):1203-1212. doi: 10.1161/STROKEAHA.120.031869 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eMethods.
eTable 1. Baseline Characteristics of the IMPROVE Stroke Care in China Patients and Hospitals Between Included and Excluded Patients
eTable 2. Baseline Characteristics of the IMPROVE Stroke Care China Patients and Hospitals Among Patients With and Without Values of mRS at 90 Days
eTable 3. Results of Disability at 90 Days From Multiple Imputations
eTable 4. Subgroup Analysis for the Effect of Intervention on Reperfusion Treatment for Other Secondary Outcomes
eTable 5. Baseline Characteristics of the IMPROVE Stroke Care in China Patients and Hospitals by Reperfusion
eTable 6. Results of Disability at 90 Days From Raw Data
eFigure 1. The Design of “STEP” Intervention
eFigure 2. Reperfusion Therapy in the Chinese Center Association Alliance Hospitals Not Participating in the IMPROVE Stroke Care in China Trial
eFigure 3. Distribution of Door-to-Puncture Time in the IMPROVE Stroke Care In China Trial
Nonauthor Collaborators
Data Sharing Statement