Summary
Background
The 2016 World Health Assembly endorsed the elimination of hepatitis B virus (HBV) infections by 2030. However, the HBV prevalence in Western countries, where the historical prevalence is low and highly impacted by immigration trends, remains uncertain making planning difficult. We aimed to develop a more accurate estimate of HBV prevalence and identify key immigrant populations that need to be screened, vaccinated, and treated to achieve the elimination targets.
Methods
US immigration data from 1900 forward and country-specific modeled prevalence by age and sex were used to estimate immigrated HBV infections entering the US, new infections in the US, mortality (all-cause and liver-related), and disease burden through 2030.
Findings
Using a dynamic Markov model, we estimated 1.8 million (95% uncertainty interval: 1.3–2.6 million) HBV infections in 2020 in all ages, higher than the NHANES national serosurvey. Infections between ages 30–74 accounted for 82% of all cases. Furthermore, HBV infections were concentrated among immigrants. New decompensated cirrhosis, hepatocellular carcinoma, and liver related deaths are expected to increase by 20%, 31% and 25% respectively from 2019 to 2030 at current diagnosis and treatment rate.
Interpretation
National serosurveys can underestimate total infections due to under-sampling in immigrant populations. To meet the WHO elimination targets, culturally appropriate screening and linkage to care programs in the immigrant populations are needed in the US. In their absence, there will be significant increases in the burden of HBV and the US will fail to meet the elimination targets by 2030.
Funding
This analysis was funded by a research grant from Gilead Sciences (IN-US-988-5786) and made possible by grants from John C Martin Foundation (2019-G024), ZeShan Foundation (2021-0101-1-CDA-HEP-10), and EndHep2030 who supported country analyses.
Keywords: Hepatitis B virus, HBV, Immigration, Western countries, United States, Modelling, Prevalence, Mortality, Liver cancer, Cirrhosis, Diagnosis
Research in context.
Evidence before this study
Previous reports of HBsAg prevalence in the United States follow traditional systematic review and meta-analysis procedures while including studies among blood donors among the country of birth and apply this to estimates of immigrant communities by country of birth. Most of these studies exclude the impact of vaccination by utilizing studies that focus on adults. Previous studies have attempted to quantify the annual number or HBsAg positive immigrants entering the country annually for a short period of time but utilize similar methodologies as aforementioned.
Added value of this study
We leveraged our previous work on HBV, that combines meta-analysis and national expert interviews to produce 166 country specific models. These dynamic transmission and disease burden models consider the changing prevalence by age and sex over time as well as the impact of all prophylaxes and treatment. We collected all available annual immigration data by country of birth, age, and sex. The annual age and sex distribution of the infected and non-susceptible individuals by country of birth was combined the age and sex distribution of the annual immigrants by country of birth. This method allowed us to estimate the number of infections by country of birth in 2019, the annual number of infections overall, as well as the infections that occurred in country as a result of the increased prevalence. We then projected the future impact of immigration the morbidity and mortality of HBV in the US through 2030.
Implications of all the available evidence
The prevalence of HBV in the US was found to be significantly higher than the national serosurvey estimates. Over 76% of the estimated number of infections were among immigrants, and the morbidity and mortality associated with HBV will continue to increase through 2030 in the absence of additional interventions. Targeted screening, vaccination, and linkage to care of immigrant communities is integral to working towards the elimination of HBV in the US. Similar studies would be useful to quantify and plan for HBV elimination in other Western European countries that have a low prevalence and a large immigrant community.
Introduction
The 69th World Health Assembly endorsed the Global Health Sector Strategy including a goal to eliminate viral hepatitis infection as a public health threat by 2030,1 and the World Health Organization (WHO) introduced the global targets for the care and management of hepatitis.2 An accurate and updated estimate of hepatitis B virus (HBV) infection prevalence in Western countries is needed to support the hepatitis B elimination efforts along with the populations that need to be targeted for screening. In addition, the disease burden from HBV infection in these countries needs to be updated for the public health planning and appropriate allocation of healthcare and financial resources. However, to track progress against the WHO targets, a realistic estimate of HBV infection disease burden is required. This is particularly difficult in Western countries where prevalence of HBV is low making it difficult to get an accurate estimate of the infected population using national serosurveys.3 In many Western countries immigrants have been identified as a key community impacted by HBV, significantly contributing to the national burden.4, 5, 6, 7, 8, 9 Unfortunately, national serosurveys under-sample in the high-risk population, including immigrants, thus underestimating the total infections present in the country.3
The prevalence of hepatitis B virus (HBV) infection in the United States (US) has been the subject of a long-running debate. The results of the National Health and Nutrition Examination Survey (NHANES) have shown a relatively stable prevalence over time, with the most recent publication estimating a national HBV prevalence of 0.32% (95% confidence interval (CI), 0.24–0.41%), representing 817,000 (95% CI, 613,000–1,100,000) infected individuals, ≥15 years, in the period of 2013–2018 while an older study reported a prevalence of 0.28% (0.22–0.35%) among those ≥6 year-olds representing 862,000 (668,000–105,600) infections in 2011–2016.10,11 Other estimates reported a higher prevalence of HBV infections in the US (1.04–2.49 million).12, 13, 14 Immigrants to the US contribute to a significant portion of the HBV infected population and the under-sampling of the NHANES study in these populations would explain a significantly lower prevalence.10,15 Between 1988 and 2012 there were, on average, 3000 samples per year in NHANES.16 The study design of NHANES, surveys or door knocks, make responding challenging for foreign-born communities, with limited English proficiency, to participate. Furthermore, there can be hesitation to acknowledge foreign-born status and country of birth. While there is the category for place of birth, US or Foreign Born, there is great diversity in immigrants’ country of birth as well as the HBsAg prevalence and vaccination policies in their home country. Thus, it is very difficult to estimate an adjusted HBV prevalence for immigrants using NHANES data.
In the US, previous studies attempted to quantify the impact of immigration, but these studies were limited by utilizing immigrant HBV prevalence estimates that were non-representative of the general population at the time of the study and incorporated studies that reported HBV prevalence in different age groups across countries (most often adults only) applied to the countries’ population.12, 13, 14, 15 Furthermore, they did not consider HBV prevalence by age, nor did they consider the age of the immigrant population, which is important since HBV prevalence in the younger age groups has been declining as a result of the global vaccination effort. Most of the previous studies used a single HBV prevalence estimate over time, while the most recent study did not explicitly take age and sex specific distribution of immigrants or prevalence into account.14 In addition, the previous studies did not attempt to quantify the future HBV prevalence, nor the disease associated morbidity and mortality.
We designed the current methodology to overcome these shortcomings by utilizing modelling to estimate the impact of immigration on the HBV prevalence in Western countries. The United States was selected as a case study given the detailed level of immigration data that is available. The model took several key factors into consideration, which included the changing composition of immigrants by country of birth, age and sex of immigrants, prevalence by age and sex among immigrants, and respective vaccination schedules in the countries of birth. Further, the background and liver-related mortality of the immigrant populations were accounted for as well as their impact on transmission within the US. The primary aim of this study was to quantify the current HBV prevalence in the US. The secondary aim was to quantify which countries of birth have the largest HBV prevalence in the US in 2020. The tertiary aim was to estimate the current and future prevalence of HBV and its impact on the disease burden and mortality in the US through 2030.
Methods
Data selections and modelling methods
The Polaris Observatory maintains 166 country-specific HBV disease burden models—the PRoGReSs model—that are updated annually. PRoGReSs is a dynamic Markov model that considers vertical and horizontal transmission, the impact of HBV prophylaxis programs (timely birth dose, three or more doses of vaccination among one-year-olds, hepatitis B immunoglobulin (HBIG), anti-viral treatment of pregnant women, and catch-up campaigns), disease progression, all-cause mortality, liver-related deaths, and the impact of HBV treatment on disease progression and transmission. It is a compartmental, deterministic, dynamic Markov disease progression model developed in Microsoft Excel and Microsoft Visual Basic (Microsoft Corporation, Redmond, WA, United States) to quantify the annual HBV-infected population by disease stage, sex, and age in a country. Excel was selected due to its transparency, flexibility, and widespread availability.
The disease stages considered in the PRoGReSs model were chronic hepatitis B, compensated cirrhosis, decompensated cirrhosis, hepatocellular carcinoma, and liver transplant. Populations with decompensated cirrhosis and hepatocellular carcinoma were considered liver-transplant-eligible.
Details on the search strategy, study selection criteria, data collection and processing, and modelling were previously published.3 The appendix outlines details of the updates since the original publication. The country-level models in the Polaris Observatory represent 99% of the estimated global HBV infections. Of the 166 country-specific models, 164 were compatible with US immigration data—the exceptions being Palestine and the United States (see Appendix). Each model estimates the annual number of infected individuals by disease stage, serologic status, HBV viremia (high-viral load, low-viral load), treatment responders, age, and sex from 1900 onward. Models were available and utilized for 99.4% of all immigrants to the US since 1900. For countries in which immigration data existed but models were not available, a regional average, using the Global Burden of Disease regions (Appendix), was used.
Immigrants and data analyses
The annual number of immigrants receiving lawful permanent resident status in the US was collected from 1900 onward by country of birth, age, and sex (Appendix). The study focused on lawful permanent residents as these individuals are most likely to remain in the country permanently, and they are the only group in which reliable data are consistently available. For the purposes of this study, lawful permanent residents will be referred to as immigrants. The term immigrant in this study only refers to first generation migrants. All other individuals, including individuals with migrant heritage are considered separately. The modelling study excludes undocumented immigrants as well as those in the US on temporary visas although sub-analyses estimated the possible impact of both groups.
The US model was then seeded in the year 1900 with the cumulative number of immigrants from 1820 to 1899 and the HBV prevalence in their respective countries of birth. Thereafter, country-level models were used to provide two sets of inputs annually: (1) HBV-positive immigrants entering the US—added to the US prevalent population, and (2) immigrants susceptible to HBV infection entering the US. For the first input, in each year the annual HBV prevalence by age and sex in the country of birth was applied to the number of incoming immigrants by age and sex, then distributed by the stage of liver disease and serologic status. By design, this process considered the impact of HBV prophylaxis programs in the countries of birth when those programs started. For the second set of inputs, the annual proportions of either infected and recovered cases or those previously immunized in the countries of birth were applied to the incoming immigrants by age and sex. This estimate was subtracted from the total chronic HBV cases in a given year and country to estimate the percent of all immigrants to the US susceptible to HBV infection. Once in the US, the entire population was subject to the US background mortality and disease progression of HBV infection that may lead to HBV-related deaths, as well as the impact of vaccination, screening, and treatment schedules within the US. Given the dynamic model of HBV transmission, these added cases impacted the perinatal and horizontal transmission within the US at the population level. The most recent recommendations state that adults and adolescents from countries with a prevalence over two-percent should be screened for HBV.17 Although screening of immigrants from high prevalence countries has been recommended since 2008, it has not been applied consistently.15 Since 2009, immigrants are required to receive the Advisory Committee on Immunization Practices recommended vaccines.18 Based on these guidelines, all uninfected immigrants under the age ≤18 entering the US in 2009 and beyond were assumed to be vaccinated in the analysis.
The model was also populated with US-specific inputs. This included the vaccination schedules of the general population, both childhood and adolescents.19,20 The screening rates of pregnant women and the coverage of HBIG and timely birth dose of the HBV vaccine of infants born to HBV-positive mothers was assumed to be flat from 1984 to 1993 (no annual data available), and then annual published numbers were utilized from 1994–2017.21, 22, 23 The antiviral treatment of pregnant women, to prevent mother-to-child transmission, was assumed to increase from 39% in 2007 to 44.8% by 2020, utilizing annual screening rates to adjust these estimates.22,24,25 Based on recent data, it was estimated that there are 330,400 individuals diagnosed with chronic hepatitis B in the US, 13,900 chronic cases diagnosed annually, and 130,200 individuals on antiviral treatment in 2019.3,26, 27, 28
To estimate the impact of the increased HBV prevalence on the perinatal and horizontal incidence in the US because of immigration, an additional “no-immigration” US model was developed where no immigrants were assumed to have entered the US from 1900 onward. The difference between the total HBV-positive immigrants alive in 2020 in the no-immigration model and the above model was used to estimate the additional impact of immigration on HBV transmission and prevalence, whether they came into the US infected or were infected while residing in the US.
HBV disease burden analyses
In order to estimate the future burden of HBV in the US, the number of immigrants by country of birth and the age distribution of those immigrants were assumed to remain constant from 2019 to 2030. Screening and treatment were also assumed to remain constant. While the aforementioned inputs were assumed to remain constant, the country-level modeled projections for the prevalence and disease burden of the annual immigrants by age and sex remained dynamic. Additionally, the model continued to dynamically estimate the progression and transmission of the disease as a result of the continued constant immigration through 2030.
Uncertainty analysis
An uncertainty analysis was conducted to measure the impact of key uncertainties in this analysis. For each country-of-birth model, the 95% uncertainty intervals in prevalence were considered (Appendix). Low prevalence was defined as the scenario that considered the low prevalence in the country of birth, low transmission probabilities, and high progression rates in the country model. Conversely, the high-prevalence scenario used the high prevalence estimate, high transmission probabilities, and low progression rates. Separately, the impact of HBV infections among temporary and undocumented immigrants on the overall HBV prevalence was estimated.
Role of funding
This analysis was funded by a research grant from Gilead Sciences (IN-US-988-5786) and made possible by grants from John C Martin Foundation (2019-G024), ZeShan Foundation (2021-0101-1-CDA-HEP-10) and EndHep2030 who supported country analyses. The funders had no role in study design, data collection, data analysis, data interpretation, or preparation of the manuscript.
Results
HBV infection among US immigrants
The analysis estimates that in 2020 alone, there were an estimated 35,000 chronic HBV-positive individuals that newly gained lawful permanent resident status. In addition to the “new cases” due to immigration, there were over 8000 acute HBV infections and over 1400 chronic HBV infections occurring within the US in 2020. The analysis took into consideration the age specific acute to chronic transition as previously described.3
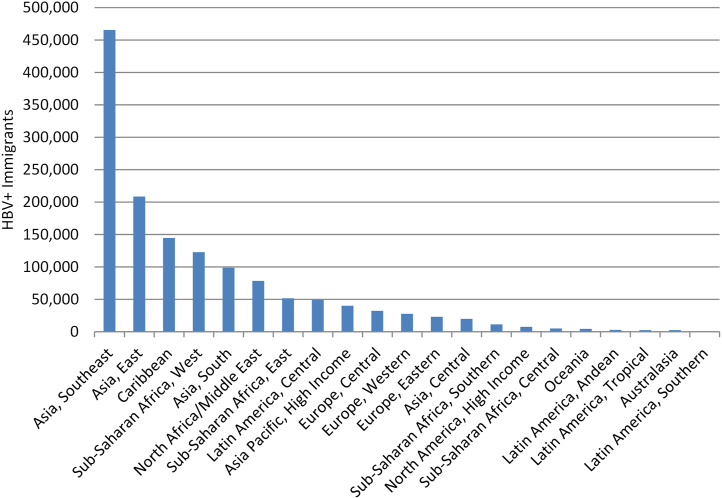
In 2020, 76% (1.4 immigrants out of 1.8 million total infections) of all living chronic HBV-positive immigrants were born in 20 countries, with almost 40% (554,700 HBV + immigrants from these three countries out of 1.4 million total infections among immigrants) coming from the Philippines, China, and Vietnam (Table 1). When Global Burden of Disease regions are examined, 74% (1,039,900 from these regions out of 1.4 million total infected immigrants) of all cases among immigrants are from 5 regions: Asia Southeast, Asia East, Caribbean, Sub-Saharan Africa West, and Asia South (Fig. 1, Table 1).
Table 1.
HBV infection among US immigrants.
| Country/Region of birth | HBV + Immigrants (UI), 2020 | HBV prevalence among immigrants (UI), 2020 |
|---|---|---|
| Philippines | 298,000 (242,900–354,900) | 14.2% (11.6%–17.0%) |
| China | 136,000 (117,500–151,500) | 7.4% (6.4%–8.2%) |
| Vietnam | 120,700 (108,700–195,600) | 10.0% (9.0%–16.2%) |
| India | 58,000 (46,600–65,600) | 3.2% (2.6%–3.7%) |
| Taiwan | 45,100 (39,900–79,600) | 12.0% (10.6%–21.1%) |
| Nigeria | 43,500 (37,300–55,800) | 13.8% (11.9%–17.8%) |
| Jamaica | 39,700 (4500–52,900) | 4.7% (0.5%–6.3%) |
| Haiti | 38,600 (12,600–53,500) | 5.2% (1.7%–7.2%) |
| Dominican Republic | 37,700 (20,100–49,800) | 2.6% (1.4%–3.4%) |
| South Korea | 36,300 (30,100–46,600) | 3.8% (3.1%–4.9%) |
| Ethiopia | 30,600 (25,000–32,700) | 11.3% (9.3%–12.1%) |
| Hong Kong | 27,500 (22,900–55,900) | 8.6% (7.1%–17.4%) |
| Ghana | 23,400 (11,000–28,600) | 13.1% (6.1%–16.0%) |
| Bangladesh | 20,200 (10,900–29,800) | 6.6% (3.6%–9.8%) |
| Pakistan | 15,700 (4900–58,900) | 3.4% (1.1%–12.9%) |
| Iraq | 13,800 (4500–18,000) | 5.6% (1.8%–7.3%) |
| El Salvador | 13,800 (1100–27,600) | 1.7% (0.1%–3.4%) |
| Iran | 13,700 (12,100–15,300) | 3.0% (2.7%–3.4%) |
| Mexico | 12,400 (9400–31,900) | 0.2% (0.1%–0.4%) |
| Liberia | 12,400 (10,000–14,800) | 13.0% (10.5%–15.5%) |
| Myanmar | 11,400 (3700–20,700) | 6.9% (2.2%–12.5%) |
| Cuba | 11,200 (3000–30,400) | 0.7% (0.2%–2.0%) |
| Uzbekistan | 10,700 (3100–17,600) | 10.0% (2.9%–16.4%) |
| Albania | 10,600 (6600–18,000) | 8.9% (5.5%–15.1%) |
| Thailand | 10,400 (8200–16,100) | 4.3% (3.4%–6.6%) |
| Somalia | 10,200 (8100–12,200) | 8.1% (6.5%–9.7%) |
| Jordan | 9300 (1800–11,900) | 5.8% (1.1%–7.4%) |
| Guyana | 9100 (6500–12,200) | 3.0% (2.2%–4.0%) |
| Russia | 8600 (4400–10,600) | 0.7% (0.3%–0.8%) |
| South Africa | 7900 (7000–9300) | 7.8% (6.9%–9.2%) |
| Cambodia | 7900 (6500–9600) | 4.7% (3.9%–5.7%) |
| Syria | 7900 (1900–10,400) | 6.7% (1.6%–8.8%) |
| Canada | 7700 (3700–15,700) | 0.4% (0.2%–0.8%) |
| Colombia | 7600 (800–43,600) | 1.1% (0.1%–6.1%) |
| Cameroon | 7500 (6500–8400) | 11.5% (10.0%–12.8%) |
| Sierra Leone | 7100 (5800–8500) | 14.1% (11.3%–16.7%) |
| Romania | 6900 (6000–7700) | 3.0% (2.6%–3.4%) |
| Trinidad and Tobago | 6600 (4700–8700) | 2.8% (2.0%–3.7%) |
| Indonesia | 6500 (5500–8900) | 8.2% (7.0%–11.3%) |
| Israel | 6000 (3200–9300) | 3.1% (1.6%–4.8%) |
| Laos | 6000 (3600–8100) | 3.8% (2.3%–5.1%) |
| United Kingdom | 5900 (3300–9500) | 0.4% (0.2%–0.7%) |
| Ukraine | 5600 (3800–8100) | 1.3% (0.9%–1.9%) |
| Cabo Verde | 5600 (4400–6700) | 12.7% (10.1%–15.4%) |
| Guatemala | 5400 (1200–14,800) | 1.2% (0.3%–3.4%) |
| Poland | 5400 (3300–7200) | 1.0% (0.6%–1.3%) |
| Afghanistan | 5000 (3100–7200) | 3.5% (2.2%–5.0%) |
| Moldova | 4800 (3400–6500) | 7.5% (5.3%–10.2%) |
| Turkey | 4800 (3000–7800) | 1.9% (1.2%–3.1%) |
| Yemen | 4800 (1200–12,600) | 5.6% (1.4%–14.9%) |
| Sudan | 4700 (3800–6100) | 6.9% (5.6%–9.0%) |
| Côte d’Ivoire | 4600 (1300–5400) | 16.5% (4.8%–19.3%) |
| Bosnia and Herzegovina | 4400 (2200–6300) | 2.6% (1.3%–3.8%) |
| Venezuela | 4300 (3600–9600) | 1.7% (1.5%–3.9%) |
| Egypt | 4200 (3100–5300) | 1.7% (1.3%–2.2%) |
| Togo | 4100 (3300–4900) | 14.6% (11.7%–17.6%) |
| Belarus | 4000 (2100–5800) | 5.0% (2.5%–7.1%) |
| Greece | 3900 (3100–4800) | 1.1% (0.9%–1.3%) |
| Lebanon | 3800 (3300–4300) | 2.6% (2.3%–2.9%) |
| Senegal | 3700 (2900–4700) | 14.0% (10.8%–17.7%) |
| Nepal | 3500 (3100–4100) | 2.6% (2.2%–3.0%) |
| Guinea | 3500 (2800–4200) | 16.5% (13.1%–19.9%) |
| Zimbabwe | 3500 (2700–4100) | 17.7% (13.9%–21.1%) |
| Nicaragua | 3300 (300–8600) | 1.5% (0.1%–3.9%) |
| Portugal | 3100 (1900–4400) | 1.2% (0.7%–1.6%) |
| Malaysia | 3100 (2400–3800) | 4.5% (3.6%–5.5%) |
| Italy | 2900 (1200–4100) | 0.2% (0.1%–0.2%) |
| Bulgaria | 2900 (1800–7500) | 2.6% (1.7%–7.0%) |
| Japan | 2800 (2500–3100) | 0.7% (0.6%–0.8%) |
| Eritrea | 2800 (2200–3300) | 8.2% (6.6%–9.9%) |
| Kenya | 2700 (1800–4100) | 2.0% (1.3%–3.2%) |
| Democratic Republic of the Congo | 2700 (2200–3000) | 4.3% (3.7%–4.9%) |
| Brazil | 2600 (800–3900) | 0.8% (0.2%–1.2%) |
| Tonga | 2400 (1900–2900) | 11.7% (9.5%–14.4%) |
| Germany | 2200 (1300–5200) | 0.2% (0.1%–0.4%) |
| Morocco | 1800 (900–2300) | 1.8% (0.9%–2.3%) |
| Mali | 1700 (1300–2600) | 18.8% (14.9%–28.4%) |
| Armenia | 1600 (1000–2500) | 1.8% (1.1%–2.7%) |
| Uganda | 1500 (1200–1900) | 4.8% (4.0%–6.1%) |
| Honduras | 1500 (700–3800) | 0.5% (0.3%–1.4%) |
| Kazakhstan | 1400 (800–2200) | 3.5% (1.9%–5.3%) |
| Australia | 1400 (1100–1800) | 1.2% (0.9%–1.5%) |
| Belize | 1400 (300–1900) | 2.9% (0.6%–3.8%) |
| Kuwait | 1400 (600–1800) | 4.3% (1.7%–5.4%) |
| Tanzania | 1400 (700–1600) | 4.5% (2.2%–5.2%) |
| Kyrgyzstan | 1300 (600–2000) | 8.5% (3.9%–12.9%) |
| Sri Lanka | 1300 (1100–1700) | 2.8% (2.2%–3.5%) |
| Bhutan | 1300 (600–2800) | 2.3% (1.1%–4.8%) |
| Peru | 1300 (1000–2200) | 0.3% (0.3%–0.5%) |
| Mauritania | 1300 (1000–1800) | 18.6% (14.8%–27.3%) |
| Fiji | 1200 (1000–1500) | 2.6% (2.1%–3.1%) |
| Gambia | 1200 (1000–1400) | 7.1% (5.7%–8.1%) |
| Ecuador | 1200 (600–2100) | 0.3% (0.2%–0.6%) |
| Tajikistan | 1200 (900–1800) | 10.2% (7.4%–15.0%) |
| Congo | 1200 (900–1400) | 7.1% (5.6%–8.5%) |
| New Zealand | 1200 (600–2300) | 3.1% (1.6%–6.3%) |
| Singapore | 1100 (800–1300) | 4.2% (3.2%–5.1%) |
| Saudi Arabia | 1000 (400–1300) | 2.8% (1.1%–3.6%) |
| Georgia | 980 (790–1240) | 2.8% (2.2%–3.5%) |
| Mongolia | 980 (830–1100) | 9.9% (8.3%–11.0%) |
| Benin | 970 (810–1160) | 13.8% (11.5%–16.4%) |
| Azerbaijan | 970 (640–1500) | 2.7% (1.8%–4.1%) |
| Spain | 900 (560–1480) | 0.5% (0.3%–0.8%) |
| Niger | 870 (700–1030) | 15.5% (12.5%–18.3%) |
| Slovakia | 770 (10–990) | 1.1% (0.0%–1.4%) |
| Tunisia | 770 (670–860) | 6.9% (6.1%–7.8%) |
| Algeria | 760 (470–1200) | 2.3% (1.4%–3.7%) |
| Zambia | 750 (600–880) | 6.0% (4.8%–7.1%) |
| Turkmenistan | 720 (440–1040) | 13.8% (8.5%–19.8%) |
| Angola | 720 (480–870) | 15.0% (10.0%–18.1%) |
| Kosovo | 710 (510–940) | 2.1% (1.5%–2.7%) |
| Burkina Faso | 660 (530–780) | 9.7% (7.7%–11.4%) |
| Samoa | 640 (510–800) | 6.0% (4.8%–7.5%) |
| Rwanda | 630 (310–740) | 5.0% (2.5%–5.9%) |
| France | 580 (240–1370) | 0.2% (0.1%–0.5%) |
| Panama | 570 (330–1160) | 0.5% (0.3%–1.0%) |
| Argentina | 480 (190–820) | 0.2% (0.1%–0.4%) |
| Austria | 440 (230–610) | 0.1% (0.0%–0.1%) |
| Burundi | 410 (330–510) | 4.7% (3.9%–5.9%) |
| Switzerland | 360 (120–770) | 0.4% (0.1%–0.8%) |
| United Arab Emirates | 350 (40–460) | 1.8% (0.2%–2.3%) |
| Central African Republic | 330 (260–400) | 14.6% (11.3%–17.5%) |
| Chad | 330 (250–390) | 15.1% (11.6%–17.9%) |
| Netherlands | 320 (100–520) | 0.2% (0.1%–0.3%) |
| Hungary | 310 (250–360) | 0.1% (0.0%–0.1%) |
| Guinea-Bissau | 290 (230–340) | 17.9% (14.5%–21.1%) |
| Gabon | 280 (90–330) | 12.3% (4.0%–14.4%) |
| Libya | 270 (210–320) | 2.6% (2.0%–3.1%) |
| Croatia | 270 (220–660) | 0.6% (0.5%–1.5%) |
| Costa Rica | 260 (150–450) | 0.3% (0.2%–0.5%) |
| Belgium | 260 (190–330) | 0.3% (0.2%–0.4%) |
| Malawi | 250 (160–300) | 6.8% (4.5%–8.2%) |
| Mozambique | 240 (190–270) | 10.2% (7.9%–11.3%) |
| Bolivia | 240 (210–340) | 0.3% (0.3%–0.5%) |
| Ireland | 230 (160–350) | 0.1% (0.0%–0.1%) |
| Suriname | 210 (160–290) | 2.7% (1.9%–3.6%) |
| Qatar | 200 (150–250) | 5.0% (3.6%–6.0%) |
| Djibouti | 170 (130–210) | 7.6% (6.0%–9.6%) |
| Chile | 160 (10–300) | 0.2% (0.0%–0.3%) |
| Sweden | 150 (40–200) | 0.1% (0.0%–0.1%) |
| Paraguay | 140 (70–240) | 0.8% (0.4%–1.4%) |
| Czechia | 130 (10–180) | 0.3% (0.0%–0.4%) |
| Madagascar | 120 (100–160) | 7.0% (5.5%–9.0%) |
| Norway | 100 (50–140) | 0.1% (0.0%–0.1%) |
| Papua New Guinea | 90 (70–120) | 11.9% (9.3%–14.8%) |
| Oman | 90 (60–90) | 4.4% (2.9%–4.5%) |
| Eswatini | 80 (70–100) | 15.6% (12.3%–18.6%) |
| South Sudan | 80 (60–100) | 8.5% (6.7%–10.4%) |
| Bahrain | 80 (40–100) | 2.4% (1.4%–2.9%) |
| Lesotho | 80 (60–90) | 16.7% (13.2%–19.9%) |
| Denmark | 70 (70–110) | 0.1% (0.1%–0.1%) |
| Estonia | 50 (40–60) | 0.7% (0.5%–0.9%) |
| Finland | 50 (30–50) | 0.2% (0.1%–0.2%) |
| Democratic People’s Republic of Korea | 40 (20–50) | 6.5% (3.9%–9.4%) |
| Federated States of Micronesia | 30 (20–40) | 11.2% (9.1%–13.9%) |
| São Tomé and Príncipe | 30 (20–30) | 15.7% (12.6%–18.8%) |
| Slovenia | 30 (20–30) | 0.4% (0.4%–0.6%) |
| Kiribati | 30 (10–30) | 14.2% (6.0%–16.4%) |
| Solomon Islands | 20 (20–30) | 17.7% (14.9%–21.2%) |
| Marshall Islands | 20 (10–30) | 2.7% (2.0%–3.8%) |
| Comoros | 10 (10–10) | 7.9% (6.3%–9.5%) |
| Vanuatu | <10 | 10.4% (8.6%–12.6%) |
| Tuvalu | <10 | 7.3% (5.9%–9.0%) |
| Asia Pacific, High Income | 40,100 (33,400–50,900) | 2.9% (2.4%–3.7%) |
| Asia, Central | 20,000 (9000–31,000) | 5.6% (2.5%–8.7%) |
| Asia, East | 208,600 (180,300–287,000) | 8.2% (7.1%–11.3%) |
| Asia, South | 98,700 (66,100–161,300) | 3.6% (2.4%–5.8%) |
| Asia, Southeast | 465,300 (382,700–619,300) | 11.0% (9.1%–14.7%) |
| Australasia | 2600 (1700–4100) | 1.6% (1.1%–2.6%) |
| Caribbean | 144,500 (51,800–209,500) | 2.8% (1.0%–4.0%) |
| Europe, Central | 32,300 (20,900–49,900) | 1.6% (1.0%–2.4%) |
| Europe, Eastern | 23,100 (13,700–31,100) | 1.2% (0.7%–1.6%) |
| Europe, Western | 27,400 (15,700–43,300) | 0.4% (0.2%–0.6%) |
| Latin America, Andean | 2800 (1900–4600) | 0.3% (0.2%–0.6%) |
| Latin America, Central | 49,100 (17,600–141,500) | 0.5% (0.2%–1.4%) |
| Latin America, Southern | 600 (200–1100) | 0.2% (0.1%–0.3%) |
| Latin America, Tropical | 2700 (800–4200) | 0.8% (0.2%–1.2%) |
| North Africa/Middle East | 78,600 (41,300–107,500) | 3.5% (1.8%–4.8%) |
| North America, High Income | 7700 (3700–15,700) | 0.4% (0.2%–0.8%) |
| Oceania | 4500 (3600–5500) | 5.5% (4.4%–6.7%) |
| Sub-Saharan Africa, Central | 5200 (4000–6000) | 5.9% (4.6%–6.9%) |
| Sub-Saharan Africa, East | 51,700 (40,900–59,000) | 7.7% (6.1%–8.9%) |
| Sub-Saharan Africa, Southern | 11,600 (9900–13,600) | 9.5% (8.1%–11.1%) |
| Sub-Saharan Africa, West | 122,800 (91,200–151,600) | 13.5% (10.0%–16.7%) |
Fig. 1.
Total number of HBV-positive immigrants by Global Burden of Disease region, 2020. HBV = hepatitis B virus.
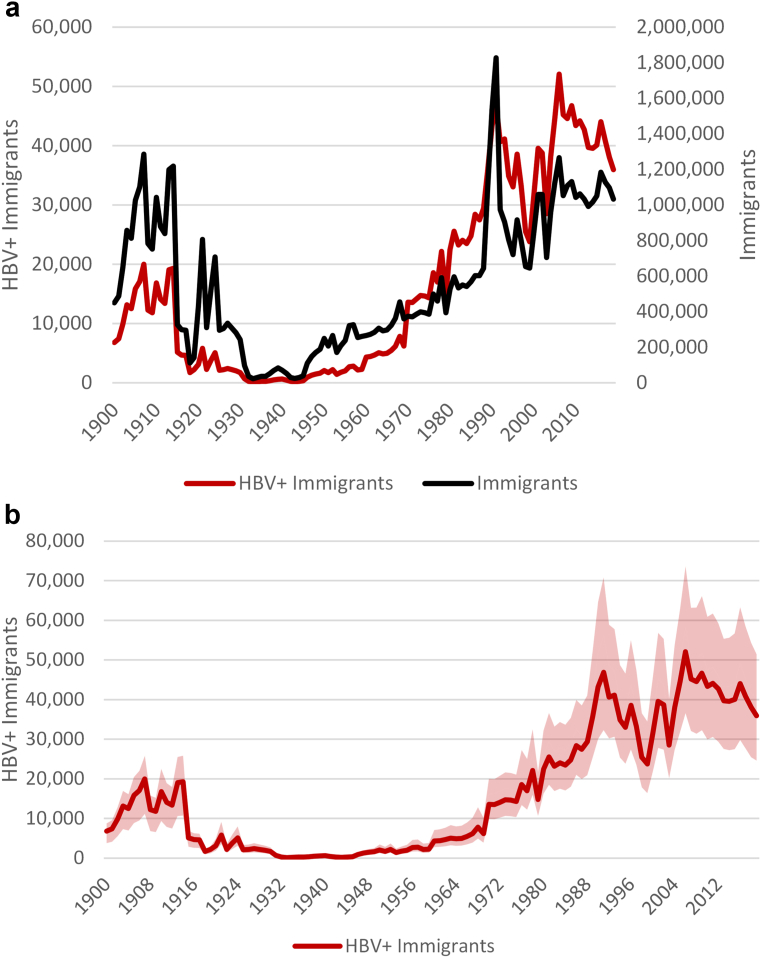
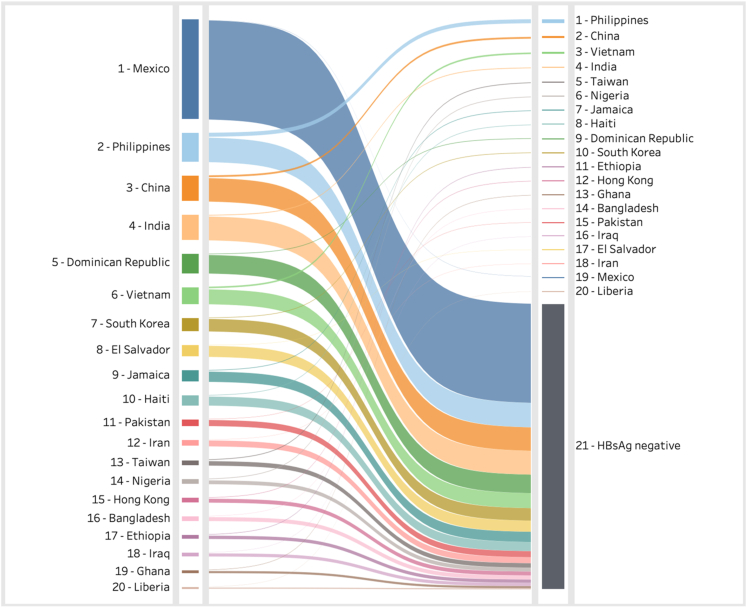
Over the 1900–2019 period, over 67 million individuals received lawful permanent resident status in the US. The annual number of HBV-positive immigrants varied significantly due to the shifting compositions and the change in HBV prevalence over time in the countries of birth (Fig. 2a). This is most noticeable after 1965 when the Immigration and Nationality Act ended national origin quotas, thus decreasing the share of immigrants that were from Europe where HBV prevalence is generally low. An estimated 80% (1.12 million out of 1.4 million)of HBV infections immigrated since 1975. As the number of countries that immigrants were coming from increased, so did the uncertainty around the annual number of HBV-positive cases (Fig. 2b). Fig. 3 shows the top 20 source countries of all imported HBV infections (on the right) and the total number of immigrants to the US who were still alive in 2020 (on the left). The analyses showed that (1) the majority of immigrants to the US are HBV negative, and (2) HBV infection is not proportional to immigration levels.
Fig. 2.
a) Annual number of immigrants and HBV-positive immigrants entering the US, 1900–2019. b) Annual number of HBV-positive immigrants entering the US, 1900–2019, with low and high uncertainty bands. HBV = hepatitis B virus.
Fig. 3.
Immigrant populations and HBV-positive infections in the US by country of birth 2020. HBV = hepatitis B virus.
HBV prevalence in the US
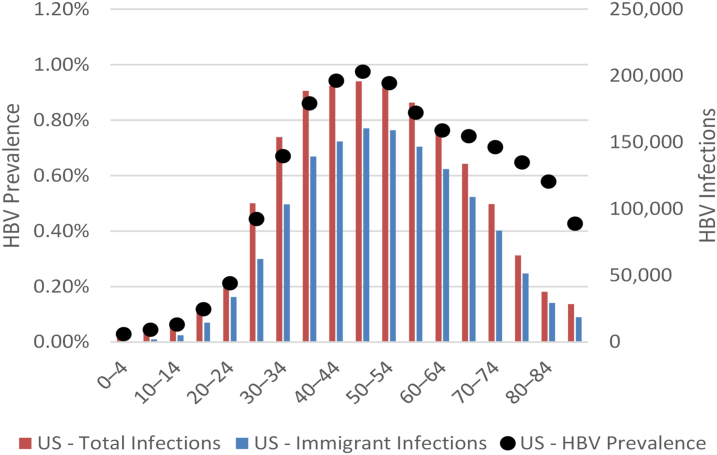
In 2020, it is estimated that there were a total of 1.8 million (95% uncertainty interval: 1.3–2.6 million) HBV-positive individuals in the US, corresponding to a prevalence of 0.55% (UI: 0.41–0.77%) in all ages. The analysis estimated that almost 1.4 million of these infections, or 76% (1.4 million out of 1.8 million total infections) of all estimated infections, were among immigrants. An estimated 243,000 (13%, 243,000 out of 1.8 million total) infections occurred in the absence of any immigration, with the balance an estimated 191,000 (10%, 191,000 out of 1.8 million total infections) being the result of domestic incidence due to immigrants living in the US. The age distribution of the HBV infection in the US is shown in Fig. 4. The analysis took into account the age of immigration from the country of birth and aging of the total population in the US. Of all HBV infections, 82% (1.15 million out of 1.4 million) fell within the ages 30–74.
Fig. 4.
Total HBV prevalence and infections by age in 2020. HBV = hepatitis B virus.
Total HBV prevalence including non-immigrants & undocumented
Although immigrants are those individuals that are the likeliest to stay in the US, eventually gain citizenship and access the healthcare system, they are not inclusive of all foreign-born populations. In 2016, it was estimated that there were 2.3 million non-immigrants, which include temporary workers, students, exchange visitors, and diplomats.29 These individuals were not included in the main analysis. When the global prevalence is applied to this population, it would result in another 89,000 additional cases. In 2015, it was estimated that there were 11.97 million undocumented immigrants in the US.30 As this report provided the number of undocumented immigrants by country or region, it was possible to apply the overall prevalence in these countries in 2015 to undocumented individuals as a secondary analysis (Supplementary Table S4). This results in an additional 184,000 infections, although these individuals are less likely to access healthcare. When these populations are added to the aforementioned total, the result is approximately 2.1 (1.6–2.8) million HBV infections in the US corresponding to a prevalence of 0.63% (0.48–0.85%).
Projected HBV infections in the next 10 years
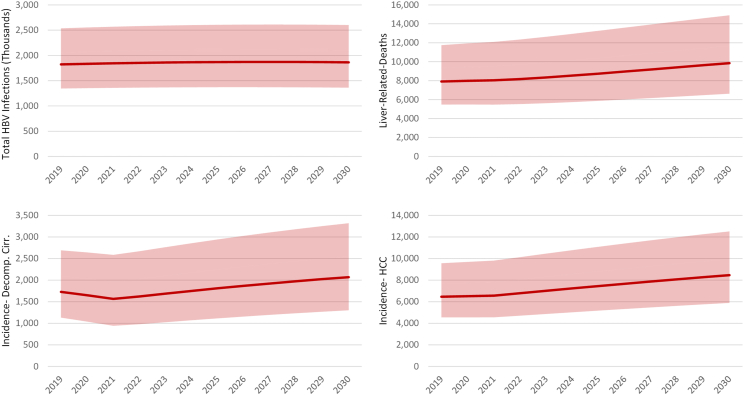
If immigration trends remain constant through 2030, it is estimated that the total HBV infection in the United States would remain relatively constant (Fig. 5). This is in part due to the vaccination policies in the countries of birth resulting in fewer infections entering the US annually (Supplementary Fig. S1). Under these assumptions, it is estimated that the annual number of HBV-positive immigrants will fall to 26,500 (UI: 18,000–38,800) in 2030, from a peak of 52,000 (UI: 36,600–73,600) in 2006. This fall is a direct result of the impact of vaccination in the countries of birth. By 2030, the prevalence in the US is estimated to be 0.52% (UI: 0.38–0.73%) which is statistically flat. The total number of HBV infections is projected to be 1.9 million (UI: 1.4–2.6 million) in 2030.
Fig. 5.
Projected infections, HBV-related morbidity and mortality, 2019–2030 with low and high uncertainty intervals. HBV = hepatitis B virus, Decomp. Cirr. = decompensated cirrhosis, HCC = hepatocellular carcinoma.
Impact of HBV infection on disease burden and mortality in the US
Although the total number of HBV infected is expected to remain relatively constant, HBV-related morbidity and mortality are expected to increase through 2030 (Fig. 5). This is a direct result of the aging infected population, combined with the decrease in infections and prevalence among the younger age cohorts. The annual incidence of decompensated cirrhosis is projected to increase by 20% from 2019 to 2030 from 1700 (UI: 1100–2700) to 2100 (UI: 1300–3300) cases, hepatocellular carcinoma (HCC) annual incidence is forecasted to increase by 31% from 6500 (4500–9600) 8500 (UI: 5900–12,500) cases, and liver related deaths are expected to increase by 25% from 7900 (UI: 5500–11,600) to 9900 (UI: 6600–14,900) deaths annually.
Validation of the model outputs
The model was also run excluding immigration from 1900 forward (the no-immigration model). The adult prevalence in 2016 was projected to be 0.08%, as compared to the most recent estimate of 0.12% (CI: 0.07–0.20%) among those that are US Born.10 The projection of the no-immigration model was within the confidence interval of the reported NHANES prevalence (among US born) even though the latter included a portion of the US Born HBV-positive cases among the descendants of immigrants. In addition, in 2017, the national Perinatal Hepatitis B Prevention Program estimated that there were 20,832 HBV-positive pregnancies in the US.22 The results of our immigration model estimated that in the same year there were 18,900 (UI: 14,100–26,500) HBV-positive pregnancies.
Discussion
This study suggests that after explicitly accounting for the immigrant populations, a projected HBV prevalence of 0.55% (0.41–0.77%), 1.8 million (1.3–2.6 million) infections, in the United States, for all ages, is significantly higher than the nationally reported estimate of 0.32% (0.24–0.41%) representing 817,000 (613,000–1,100,000) in ages ≥15 years or 0.28% (0.22–0.35%) representing 862,000 (668,000–105,600) infections among those ≥6 years-old.10,11 If non-immigrants, including temporary workers, and undocumented immigrants are included, the overall prevalence increases to 0.63% (UI: 0.48–0.85%) or 2.1 (1.6–2.8) million HBV infections. Over 76% (1.4 million out of 1.8 million total infections) of the prevalent population in the US were found to be immigrants, and 35,000, 96% (35,000 imported out of 36,400 new chronic cases), of new chronic infections were imported chronic cases. Our findings suggested HBV prevalence in the US has been underestimated in national serosurveys. This is likely to be the case in other Western countries as well where immigration accounts for the majority of HBV infections.
The World Health Organization’s (WHO) targets require all countries to diagnose 90% of all HBV infections and treat 80% of the eligible population. In 2016, a total of 307,900 individuals were estimated to have been diagnosed with hepatitis B in the US with another 13,900 newly diagnosed annually.26 By 2020, we estimate that approximately 334,800 chronic HBV infections were diagnosed in the US, corresponding to a diagnosis coverage of 18% (334,800 diagnosed out of 1.8 million infected) with just under 17% (90,500 treated out of 534,100 eligible) of all eligible individuals on treatment. US is simply not diagnosing and treating sufficient number of HBV cases to achieve the WHO elimination targets. Thus, significant efforts are needed to screen, diagnose, and treat the HBV population in the US. According to this study, it is imperative that the US healthcare system be optimized to reach immigrant populations to meet the WHO target.
This study highlights that HBV infection in the US disproportionally impacts immigrant communities (76% (1.4 million out of 1.8 million total infections) of all estimated HBV infections), which highlighted the needs for allocating additional resources to these communities. For example, Mexico accounts for the largest group of immigrants, but it is ranked number 19 in terms of immigrants living with HBV in the US, whereas Chinese Americans account for a significantly lower number but are ranked number 2 in terms of immigrants living with HBV in the US (Fig. 3). As shown in Supplementary Fig. S1, the number of HBV infected immigrants to the US will drop as many individuals entering the US will have been vaccinated at birth or in early childhood. The US HBV prevalence among the younger cohorts (less than 24 years old) is low (Fig. 4) because of US and global HBV vaccination programs. Increased screening and linkage to care are needed in the key immigrant populations with a focus on those aged >24 years old.
In 2020, it was estimated that 96% (35,000 imported out of 36,400 new chronic cases) of all chronic HBV incident cases (new chronic infections) were among immigrants entering the country, imported chronic cases, with the rest caused by ongoing transmission occurring within the US. Although the annual number of infected immigrants is expected to decrease through 2030, cumulatively, they add up to 340,000 new chronic HBV infections. This analysis highlights an opportunity to increase diagnosis in the United States. If all new incoming immigrants were screened for HBV starting in 2022, then the estimated total number of diagnosed in 2030 would increase from 374,300, 20% (374,300 diagnosed out of 1.86 million cases) of the infected population, to only 645,100 or 35% (645,100 diagnosed out of 1.86 million cases). In comparison, universal screening of all immigrants would identify >70% of all HBV infections in the country helping the US get close to the WHO diagnosis target of 80% by 2030. Although the screening of immigrants from high prevalence countries has been recommended since 2008, it has not been applied consistently.15 The most recent recommendations state that adults and adolescents from countries with a prevalence over two-percent should be screened for HBV.17 Based on the estimates provided in the appendix, this would exclude over 18% (253,000 HBV + immigrants from these countries out of 1.4 million immigrant cases) of cases among immigrants, representing over 253,000 infections.
The recent Center for Disease Control and Prevention (CDC) draft recommendations for the universal screening of adults for HBV should be lauded. However, it should be ensured that there are adequate resources for outreach, education, screening, linkage to care and vaccination for immigrant communities. As they face different cultural and linguistic barriers than the general population and they have a higher burden, they should be prioritized for funding focus. As there is the potential that the continued targeting of immigrant communities could result in additional stigma and marginalization, these programs must work directly with the communities being most impacted to attempt to mitigate these undue burdens.
Further analysis is required for state and local planning. Immigration patterns and numbers are heterogenous throughout the country and the largest immigrant communities as listed in Table 1 will vary greatly depending on the locality. Thus, similar analyses will be needed at the state and local level to develop appropriate strategies to access the HBV infected populations. The work of screening, linking to care, and vaccination should be facilitated by community and patient advocacy groups that are able to properly address the needs of their community in their own language. A recent study showed the promise of utilizing these organizations for screening and linkage to care.31
While this analysis adds to the body of evidence that the vast majority of HBV infections in the US are among immigrants, it must be noted that there are still 10–20% of infections, an estimated 200,000–400,000 Americans, that would not be diagnosed and linked to care via a program focused solely on immigrants. While universal programs with strong support and funding will have the largest impact; in the absence of systemic support, targeted programs will produce stronger results. An important distinction needs to be made between the prevention and diagnosis of acute infections and the diagnosis of chronic infections. The most recent CDC data on acute cases of HBV finds that the largest risk factors are people who inject drugs, multiple sexual partners, and men who have sex with men respectively.26 Unfortunately, the CDC does not collect risk data for chronic cases, but by comparing the relative rates in the 2019 acute and chronic cases by demographic characteristic, one can clearly see that these are two separate groups that require different strategies. For acute cases in 2019, the highest risk in each category was among 40–49 year olds, males, non-Hispanic whites, and rural residents, whereas for chronic cases the highest risk was among 30–39 year olds, males, Asian/Pacific Islanders, and urban residents.26 It is among this latter group, chronic infections in need of diagnosis, in which immigrants represent the majority and for which our analysis provides additional support.
Similar to the HCV programs targeting baby boomers, immigrants are the starting point for an HBV elimination program in the US. There needs to be national guidelines and appropriate funding that can aid local health departments and community organizations in developing their specific elimination plans and identifying the communities most in need. Once a program like this proves successful, it can be broadened to ensure that other positive cases are captured and that the morbidity and mortality associated with hepatitis B is reduced.
To reduce HBV incidence in the US, all immigrants found to be negative, with no history of vaccination should be vaccinated to prevent in-country transmission. This is of particular importance if a household member is found to be positive, to reduce to the risk of household transmission. In addition, to address 1400 new chronic HBV infections each year within the US, there needs to be universal screening of all pregnant women, antiviral treatment of those eligible, and birth dose vaccination within the first 24 h of life, along with hepatitis B immunoglobulin screening of infants of all HBV-positive mothers. Another strategy to reduce future new cases of HBV is to strengthen the vaccination programs in the countries of birth from which many HBV-positive immigrants come. By helping countries, particularly those with low or no timely birth dose coverage, work towards the elimination of HBV in source countries could also avert future cases among immigrants.
There were several limitations to this analysis. It assumes that the year of lawful permanent resident status is the year of entry. Over 40% of immigrants gain their status in the year of entry and the majority of those “adjusted” gain it within five years of entry.32,33 While the analysis does not explicitly take into consideration refugees, 97% of refugees admitted between 2000 and 2018 received lawful permanent resident status by 2020.34 Thus, these are individuals that do not receive their status in the year of entry. The birth cohort is the same regardless of when an individual gains their status and thus the impact of this assumption on the forecasts is minor.
The analysis also assumes the prevalence and the disease stage distribution of immigrants by age and sex is the same as in the general population in the country of birth. The prevalence in the immigrant population may be lower than the general population of the birth country since they may represent higher-income individuals from urban areas that would have access to hospital births and HBV vaccination. Thus, the true HBV prevalence among immigrants may be lower than forecasted here. On the other hand, the HBV prevalence may be higher among immigrants if they entered as refugees.
The analysis assumed that immigrants in the US had the same non-hepatic mortality rate as the rest of the US population. In fact, a more limited access to healthcare may result in higher all-cause mortality rates leading to a lower total HBV prevalence among immigrants. Similarly, the US HBV vaccination programs were applied uniformly to everyone living in the US. The immigrant population living in the US may have more limited access to the HBV prophylaxis programs leading to a higher HBV prevalence in this population.
The forward projection assumes a constant level of immigration both numerically as well as by country of birth. Although the country of birth and number of immigrants has historically changed, it is difficult to predict future changes. While the current assumption is unlikely, especially due to the reduced number of immigrants as a result of COVID-19; in the absence of better data, it was deemed the most defensible assumption. The forward projections also assume a constant number of individuals being treated through 2030. Although it is possible that new treatments may become available in this time period, they were not considered.
Finally, this analysis does not consider emigration—immigrants leaving the country. This would result in an overestimate of the number of HBV infections among immigrants in the US. However, this combined with the above limitations are expected to have been captured within the uncertainty ranges reported here. Despite these limitations, we believe that our estimates are far more accurate than the previous publication because the current modelling has included most of the important factors associated with the HBV prevalence in the US, namely age at entry and vaccination status.
This study provides a new approach to estimating HBV prevalence in countries with a low HBV prevalence in the native population. The current approach provides the estimated HBV prevalence and associated disease burden at the national population level for all ages. Much of Western countries have a low prevalence but large immigrant populations. To develop robust strategies to reach the WHO viral hepatitis elimination targets, these countries need a strong understanding of the burden among immigrant communities. The data from said analyses would provide valuable insight that could inform programs and resource allocation.
Contributors
Devin Razavi-Shearer and Ivane Gamkrelidze conducted the immigration analysis, Devin Razavi-Shearer, Ivane Gamkrelidze and Homie Razavi prepared the first draft and Calvin Pan provided feedback to finalize the manuscript. Kathryn Razavi-Shearer extracted immigration data. Devin Razavi-Shearer, Kathryn Razavi-Shearer, Sarah Blach, Chris Estes, and Ellen Mooneyhan populated and calibrated the underlying HBV models. Devin Razavi-Shearer, Homie Razavi, and Ivane Gamkrelidze verified all underlying data. All authors had full access to all the data and accept responsibility for the publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Data sharing statement
The sources of all underlying data and modelling assumptions can be found in the Supplementary Materials. These data can also be found at The Polaris Observatory, https://cdafound.org/dashboard/polaris/. For additional inquiries please contact the authors within 1 year of publication.
Declaration of interests
Calvin Q. Pan: Consultant – Gilead, AbbVie; Advisory Board – Gilead, BMS, AbbVie; Research – Gilead, Merck. Homie Razavi: Employee – CDA Foundation, Advisory Board – Gilead, AbbVie, VBI Vaccine, Merck, Jansson, Roche; Research – Gilead, AbbVie, Assembly Biosciences, Intercept, Pfizer. Devin Razavi-Shearer, Ivane Gamkrelidze, Kathryn Razavi-Shearer, Sarah Blach, Chris Estes, Ellen Mooneyhan: Employee – CDA Foundation.
Acknowledgments
The authors thank the Polaris Observatory Collaborators who provided inputs for individual country models used in this analysis. Without their contributions and review of country model outputs, this work would not have been possible.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2023.100516.
Appendix A. Supplementary data
References
- 1.World Health Organization . Accountability for the global health sector strategies 2016–2021: actions for impact. World Health Organization; Geneva, Switzerland: 2021. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021. [Google Scholar]
- 2.World Health Organization . World Health Organization; Geneva, Switzerland: 2016. Combating hepatitis B and C to reach elimination by 2030. [Google Scholar]
- 3.Razavi-Shearer D., Gamkrelidze I., Nguyen M.H., et al. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study. Lancet Gastroenterol Hepatol. 2018;3(6):383–403. doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 4.Cuenca-Gomez J.A., Salas-Coronas J., Soriano-Perez M.J., Vazquez-Villegas J., Lozano-Serrano A.B., Cabezas-Fernandez M.T. Viral hepatitis and immigration: a challenge for the healthcare system. Rev Clin Esp (Barc) 2016;216(5):248–252. doi: 10.1016/j.rce.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Picchio C.A., Nomah D.K., Araujo S.G., et al. A novel model of care for simplified testing of HBV in African communities during the COVID-19 pandemic in Spain. Sci Rep. 2021;11(1) doi: 10.1038/s41598-021-96350-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma S., Carballo M., Feld J.J., Janssen H.L.A. Immigration and viral hepatitis. J Hepatol. 2015;63(2):515–522. doi: 10.1016/j.jhep.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 7.Ng E., Quinlan J., Giovinazzo G., et al. Hospitalization related to chronic hepatitis B and C in recent immigrants in Canada: an immigration administrative data-linked, population-based cohort study. Health Rep. 2022;33(6):30–45. doi: 10.25318/82-003-x202200600003-eng. [DOI] [PubMed] [Google Scholar]
- 8.Koc O.M., Kremer C., Bielen R., et al. Prevalence and risk factors of hepatitis B virus infection in Middle-Limburg Belgium, year 2017: importance of migration. J Med Virol. 2019;91(8):1479–1488. doi: 10.1002/jmv.25457. [DOI] [PubMed] [Google Scholar]
- 9.Duberg A.S., Lybeck C., Falt A., Montgomery S., Aleman S. Chronic hepatitis B virus infection and the risk of hepatocellular carcinoma by age and country of origin in people living in Sweden: a national register study. Hepatology communications. 2022;6(9):2418–2430. doi: 10.1002/hep4.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts H., Jiles R., Harris A.M., Gupta N., Teshale E. Incidence and prevalence of sexually transmitted hepatitis B, United States, 2013-2018. Sex Transm Dis. 2021;48(4):305–309. doi: 10.1097/OLQ.0000000000001359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel E.U., Thio C.L., Boon D., Thomas D.L., Tobian A.A.R. Prevalence of hepatitis B and hepatitis D virus infections in the United States, 2011-2016. Clin Infect Dis. 2019;69(4):709–712. doi: 10.1093/cid/ciz001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kowdley K.V., Wang C.C., Welch S., Roberts H., Brosgart C.L. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology. 2012;56(2):422–433. doi: 10.1002/hep.24804. [DOI] [PubMed] [Google Scholar]
- 13.Lim J.K., Nguyen M.H., Kim W.R., Gish R., Perumalswami P., Jacobson I.M. Prevalence of chronic hepatitis B virus infection in the United States. Am J Gastroenterol. 2020;115(9):1429–1438. doi: 10.14309/ajg.0000000000000651. [DOI] [PubMed] [Google Scholar]
- 14.Wong R.J., Brosgart C.L., Welch S., et al. An updated assessment of chronic hepatitis B prevalence among foreign-born persons living in the United States. Hepatology. 2021;74(2):607–626. doi: 10.1002/hep.31782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell T., Armstrong G.L., Hu D.J., Wasley A., Painter J.A. The increasing burden of imported chronic hepatitis B--United States, 1974-2008. PLoS One. 2011;6(12) doi: 10.1371/journal.pone.0027717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roberts H., Kruszon-Moran D., Ly K.N., et al. Prevalence of chronic hepatitis B virus (HBV) infection in U.S. Households: National health and nutrition examination survey (NHANES), 1988-2012. Hepatology. 2016;63(2):388–397. doi: 10.1002/hep.28109. [DOI] [PubMed] [Google Scholar]
- 17.US Preventive Services Task Force Screening for hepatitis C virus infection in adolescents and adults: US preventive Services task force recommendation statement. JAMA. 2020;323(10):970–975. doi: 10.1001/jama.2020.1123. [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention . Centers for Disease Control and Prevention; 2010. Vaccination requirements for U.S. immigration: technical instructions for panel physicians. [Google Scholar]
- 19.Lu P.J., Yankey D., Jeyarajah J., et al. Hepatitis B vaccination among adolescents 13-17 years, United States, 2006-2012. Vaccine. 2015;33(15):1855–1864. doi: 10.1016/j.vaccine.2015.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.WHO/UNICEF . 2020. Reported official target population, number of doses administered and official coverage.http://www.who.int/immunization/monitoring_surveillance/data/en/ [Google Scholar]
- 21.Smith E.A., Jacques-Carroll L., Walker T.Y., Sirotkin B., Murphy T.V. The national perinatal hepatitis B prevention program, 1994-2008. Pediatrics. 2012;129(4):609–616. doi: 10.1542/peds.2011-2866. [DOI] [PubMed] [Google Scholar]
- 22.Koneru A., Fenlon N., Schillie S., Williams C., Weng M.K., Nelson N. National perinatal hepatitis B prevention program: 2009-2017. Pediatrics. 2021;147(3) doi: 10.1542/peds.2020-1823. [DOI] [PubMed] [Google Scholar]
- 23.Centers for Disease Control and Prevention Hepatitis B vaccination--United States, 1982-2002. MMWR Morb Mortal Wkly Rep. 2002;51(25):549–552. [PubMed] [Google Scholar]
- 24.Kubo A., Shlager L., Marks A.R., et al. Prevention of vertical transmission of hepatitis B: an observational study. Ann Intern Med. 2014;160(12):828–835. doi: 10.7326/M13-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris A.M., Isenhour C., Schillie S., Vellozzi C. Hepatitis B virus testing and care among pregnant women using commercial claims data, United States, 2011-2014. Infect Dis Obstet Gynecol. 2018;2018 doi: 10.1155/2018/4107329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention . Division of viral hepatitis, national center for HIV/AIDS, viral hepatitis, STD, and TB prevention. 2021. Viral hepatitis surveillance report 2019. [Google Scholar]
- 27.IQVIA . 2021. IQVIA GPM national audit for HBV/antivirals market, january 2019–december 2020. [Google Scholar]
- 28.Spradling P.R., Rupp L., Moorman A.C., et al. Hepatitis B and C virus infection among 1.2 million persons with access to care: factors associated with testing and infection prevalence. Clin Infect Dis. 2012;55(8):1047–1055. doi: 10.1093/cid/cis616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baker B. Office of immigration statistics PD. U.S. Department of Homeland Security; 2018. Nonimmigrants residing in the United States: fiscal year 2016. [Google Scholar]
- 30.Baker B. U.S. Department of Homeland Security; 2018. Population estimate: illegal alien population residing in the United States: January 2015. [Google Scholar]
- 31.Harris A.M., Link-Gelles R., Kim K., et al. Community-Based Services to Improve Testing and Linkage to Care Among Non-U.S.-Born Persons with Chronic Hepatitis B Virus Infection - Three U.S. Programs, October 2014–September 2017. MMWR Morb Mortal Wkly Rep. 2018;67(19):541–546. doi: 10.15585/mmwr.mm6719a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Office of Immigration Statistics PD, U.S. Department of Homeland Security . U.S. Department of Homeland Security; Washington, DC: 2020. 2019 Yearbook of immigration statistics. [Google Scholar]
- 33.Office of Immigration Statistics PD, U.S. Department of Homeland Security . U.S. Department of Homeland Security; Washington, DC: 2019. 2018 Yearbook of immigration statistics. [Google Scholar]
- 34.Baugh R. Department of Homeland Security; 2022. Refugees and asylees: 2020. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.