Abstract
Human papillomavirus (HPV) plays a significant role in the development of cervical cancers in the setting of co-infection with HIV. Botswana has a high prevalence of HIV and cervical cancer. In this study, we investigated the distribution of HPV subtypes in cervical cancer biopsy samples from patients in Botswana using a highly sensitive pan-pathogen microarray technology, PathoChip, to detect both high- (HR-HPV) and low-risk HPV (LR-HPV) subtypes in women living with HIV (WLWH) and women living without HIV. We analyzed samples from 168 patients, of which 73% (n = 123) were WLWH with a median CD4 count of 479.5 cells/μL. Five HR-HPV subtypes were detected in the cohort: HPV 16, 18, 26, 34, and 53. The most prevalent subtypes were HPV 26 (96%) and HPV 34 (92%); 86% of WLWH (n = 106) had co-infection with four or more HR-HPV subtypes compared to 67% (n = 30) of women without HIV (p < 0.01). We detected 66 LR-HPV subtypes among all cervical cancer patients, with HPV 6b and 48 being most prevalent. Notably, signatures for LR-HPV subtypes 10, 41, 90, and 129 were only detected in WLWH. Signal intensity for HPV 18 was significantly weaker in WLWH with CD4 levels ≤200 cells/μL as compared to patients with >200 cells/μL and HIV-negative patients. Although the majority of cervical cancer specimens in this cohort were determined to have multiple HPV infections, the most prevalent HR-HPV subtypes (HPV 26 and HPV34) found in these cervical cancer samples are not covered in the current HPV vaccines. Though no conclusions can be made on the direct carcinogenicity of these subtypes the results do underlie the need for continued screening for prevention of cervical cancer.
Keywords: HPV subtypes, High risk HPV, HIV, Cervical cancer, Africa
Highlights
-
•
In this investigation of high- and low-risk HPV subtypes and viral burden in cervical cancer samples, we identified differences in HPV-subtypes by HIV-status.
-
•
Utilizes novel PathoChip hybridization to evaluate for low-copy number microorganisms in.
-
•
The most prevalent subtypes among all samples were HPV 26 (96%) and HPV 34 (92%).
-
•
Signal intensity for HPV 18 was significantly weaker in WLWH with CD4 levels ≤200 cells/μL.
-
•
Provides data to support the need for continued HPV screening even with HPV vaccination campaigns.
1. Introduction
Human papillomavirus (HPV) is the most common sexually transmitted infections worldwide and plays a significant role in driving the oncogenic phenotype of numerous human malignancies, including cervical cancer [1]. Among the more than 120 identified HPV genotypes, 14 are deemed high-risk subtypes, two of which—HPV 16 and HPV 18—are implicated in approximately 70% of all cervical cancers [2,3]. Worldwide, there are more than 600,000 new cases and more than 300,000 deaths from cervical cancer annually. The overwhelming majority of morbidity and mortality associated with cervical cancer occurs in low- and middle-income countries [4].
Cervical cancer is also the most frequently detected cancer in women living with HIV (WLWH) and is classified as an acquired immunodeficiency syndrome (AIDS)-defining illness. WLWH have an increased risk for HPV infection and persistence, with a higher risk of developing invasive cervical cancer [2,5,6]. With the advent of antiretroviral therapy, people living with HIV have a longer life expectancy bringing an increased prevalence of women susceptible to developing cervical cancer.
Sub-Saharan Africa bears a high burden of cervical cancer, including in the middle-income country Botswana. Cervical cancer is the leading cause of cancer death in Botswana, with more than two thirds of cervical cancer cases occurring in WLWH [7]. Botswana has the fourth highest prevalence of HIV in the world at 20.3% [8]. Therefore, Botswana provides an opportunity to investigate the contributions of HIV in women with HPV-associated cervical cancer.
A critical piece of Botswana's efforts to combat cervical cancer is widespread vaccination campaign. Unvaccinated women in Botswana have high prevalence of cervical HPV infection (60%) [9,10]. Introduced in 2015, the national vaccination campaign utilized the quadrivalent HPV vaccine as a two-dose schedule (1, 6–12 months), for in-school girls. Vaccinations available include: the bivalent vaccine covers HPV 16–18, the quadrivalent HPV vaccine offers protection against 6 and 11 (low-risk subtypes understood to be non-oncogenic, but a causative agent of genital warts) and 16 and 18. Finally, a more recent nonavalent vaccine is available that covers additional subtypes including HPV 31, 33, 45, 52, and 58 [11].
Adding to the environment is the presence of HIV. There is limited data on the efficacy of HPV vaccination in individuals with HIV. In a recent systematic review, Bergman et al. demonstrated that vaccines are safe and effective, but studies on the duration of protection and impact of declining immunity were needed [12].
In this study, through the use of PathoChip, we are able to investigate the distribution of HPV subtypes in a cohort of women with and without HIV, diagnosed with cervical cancer in Botswana. We used the PathoChip pan-pathogen microarray technology, which has high specificity for the majority of HPV subtypes and is highly sensitive to detection of HPV subtypes present in low copy numbers and rare clinical samples. Results may assist in informing the potential efficacy of widespread vaccination programs in an HIV-infected population.
2. Methods
2.1. Study samples
Formalin-fixed paraffin-embedded (FFPE) biopsy samples were collected in Botswana from patients with cervical cancer diagnosed among two hospitals in Gaborone via biopsy during 2013–2017 (n = 168). Patient and control samples from Botswana were received at the University of Pennsylvania as de-identified FFPE samples in 10-μm sections prepared as rolls. Blank FFPE block sections and buffer-only control samples prepared during each batch of patient sample collection were used as processing controls and for multiple corrections in analyses. These disease-free control samples were provided along with the human disease FFPE samples and were generated in the lab when the samples were cut to have the same samples from the same patient microenvironment. Patient demographic and clinical information was also collected for analysis, including patient age, Federation of Gynecology and Obstetrics (FIGO) 2009 cervical cancer staging, and HIV status.
2.2. PathoChip
PathoChip is a comparative genomic hybridization tool that contains conserved and unique probe sets for parallel DNA and RNA detection of viruses, bacteria, fungi, parasites, and other human pathogenic microorganisms at the species and strain level. PathoChip is patented by the University of Pennsylvania (Patent Number U.S. Pat. No. 10,883,145). Inclusion of conserved probes allows identification of previously uncharacterized microorganisms, while non-specific amplification of patient samples enables detection of low-copy number microorganisms and fragmented genomes. These features set PathoChip apart from traditional techniques of microbiome screening, such as PCR and in situ hybridization, and offers rapid identification of predominant and opportunistic infectious agents in patient tumors that could influence a pro-tumorigenic microenvironment.
The PathoChip array design was previously described [13,14]. SurePrint glass slide microarrays (Agilent Technologies Inc.) contain eight replicate arrays per slide. Each array comprises 60,000 probe sets representing unique and conserved sequences of all known viruses and human pathogenic bacteria, fungi, and parasites in GenBank. Each probe is a 60-nucleotide DNA oligomer, and for each accession there are multiple probes that target various genomic regions of viruses, prokaryotic, and eukaryotic microorganisms. Accession annotations are available in Gene Expression Omnibus (http://www.ncbi.nlm.nih. gov/geo/) [15]. Overall, PathoChip probes can detect a total of 4054 microorganism species. This includes a total of forty HPV subtypes, detecting high-risk subtypes 16, 18, 26, 34, and 53, as well as low-risk subtypes 1, 2, 4, 5, 6b, 7, 9, 10, 32, 41, 48, 49, 50, 54, 60, 61, 63, 88, 90, 92, 96, 101, 103, 108, 109, 112, 116, 121, 126, 128, 129, 131, 132, 134, 148 [32].
Sample preparation and microarray processing
The PathoChip screening procedure has been previously described [10,11,[16], [17], [18]]. DNA and RNA were simultaneously extracted from cancer patients and control FFPE tissue samples using the AllPrep DNA/RNA FFPE Kit (Qiagen, Hilden, Germany), and absorbance was measured at 260/280 nm to assess quality. Patient sample RNA (100 ng) and DNA (50 ng) were used for whole genome and transcriptome amplification (WTA) using the TransPlex Complete Whole Transcriptome Amplification Kit (Sigma-Aldrich, St. Louis, MO). Human DNA and RNA were extracted from the human B cell line BJAB (obtained from ATCC) to serve as a reference for cross-hybridization of probes to the amplified patient genomes and to determine background normalization. Purified WTA product (PCR purification kit, Qiagen, Germantown, MD, USA) quality was determined using A260/280 measurements, and 1 μg was labeled with either Cy3 (amplified patient sample) or Cy5 (amplified human reference) (SureTag labeling kit, Agilent Technologies, Santa Clara, CA). All labeled specimens were purified, and a Cy3-labeled and Cy5-labeled reference were hybridized together on each PathoChIP array in constant rotation at 65 °C for 40 h, as previously described [10,11]. Array slides were washed and then scanned for visualization using an Agilent SureScan G4900DA array scanner.
2.3. Microarray data extraction and statistical analysis
Microarray data extraction and analyses were described previously [10,11,13,14]. Briefly, raw data were extracted from microarray images using Agilent Feature Extraction software, and the R program was used for data normalization and analysis. Human probes on the chip were used as a control for data normalization. Specifically, a scale factor for sample i was calculated as the sum of green and sum of red signal ratios of human probes (H):
Normalized signals for non-human probes were obtained as:
For normalized signals, the background signal from FFPE controls was further subtracted for correction. All human control probes in a cohort were pooled as baseline. A t-test was applied to select probes that were significantly present in the cohort (HIV-positive or HIV-negative), namely those with significantly higher values than baseline. We adjusted p-values using the Benjamini-Hochberg procedure for multiple-testing correction. A probe was regarded as a signature probe if its log2 fold-change was >1 and adjusted p-value was <0.05. Mean value of the baseline (human probes in a cohort) was used as a cutoff to decide if a probe in an individual sample had a positive signal in a cohort. Baseline mean was 0.2792321 for the HIV-positive cohort and 0.2544218 for the HIV-negative cohort. Prevalence of a probe in a cohort was calculated as the percentage of samples with positive signals.
2.4. Clinical data analysis
Array data were grouped by age range, cervical cancer stage, and HIV status, and hybridization signal intensity (HSI) and prevalence were determined for each HPV subtype. Prevalence was calculated by counting the number of samples with signals greater than the average signal of the human probes (cutoff) and represented as a percentage of the total number of samples. Distribution (prevalence and HSI) of low and high-risk HPV subtypes were compared by HIV status, age groups and CD4 counts using two-sided test. All analysis was conducted using R version 3.6.3.
2.5. Ethics
This study was approved by the University of Pennsylvania (IRB # 820,159), the Human Research Development Council at the Ministry of Health and Wellness in Botswana, and the University of Botswana institutional review boards.
3. Results
3.1. Demographic information for cervical cancer patients in Botswana
A total of 168 patients from Botswana diagnosed with cervical cancer were included in this study, and demographic characteristics are presented in Table 1. Median age of the cohort was 49 years [interquartile range (IQR): 43–61]. Most patients presented with stage III (32%) or II (25%) cervical cancer, and most were WLWH (73%, n = 123), 91.3% of whom were reported to be on anti-retroviral medicationMedian CD4 count of WLWH was 479.5 cells/μL (IQR: 324.2–649.2), and most patients (n = 91) had an undetectable viral load at the time of cancer diagnosis. Cervical cancer was diagnosed at a significantly younger age in WLWH (median age: 47 years) than HIV-negative patients (median age: 66 years) (p > 0.001).
Table 1.
Demographic and treatment characteristics of cervical cancer patients by HIV status.
| HIV-positive |
HIV-negative |
Total |
||||
|---|---|---|---|---|---|---|
| n | Percentage (%) | N | Percentage (%) | n | Percentage (%) | |
| Total | 123 | 100.00 | 45 | 100.00 | 168 | 100.00 |
| Age (years) | ||||||
| Median | 47 | 66 | 49 | |||
| IQR | 42–53 | 57–72 | 43–61 | |||
| 20–39 | 21 | 17.07 | 4 | 8.89 | 25 | 14.88 |
| 40–59 | 78 | 63.41 | 9 | 20.00 | 87 | 51.79 |
| ≥60 | 15 | 12.20 | 31 | 68.89 | 46 | 27.38 |
| No records | 9 | 7.32 | 1 | 2.22 | 10 | 5.95 |
| FIGO cervical stage | ||||||
| Stage I | 13 | 10.57 | 5 | 11.11 | 18 | 10.71 |
| Stage II | 30 | 24.39 | 12 | 26.67 | 42 | 25.00 |
| Stage III | 34 | 27.64 | 19 | 42.22 | 53 | 31.55 |
| Stage IV | 22 | 17.89 | 3 | 6.67 | 25 | 14.88 |
| Unknown/no record | 24 | 13.16 | 6 | 13.33 | 30 | 17.86 |
| CD4 count (cells/μL) | ||||||
| Median | 479.5 | n/a | ||||
| IQR | 324.2–649.2 | n/a | ||||
| HIV viral load | ||||||
| Detected | 17 | 13.82 | n/a | n/a | ||
| Not detected | 91 | 73.98 | n/a | n/a | ||
3.2. Distribution of high- and low-risk HPV signatures by HIV status
3.2.1. High-risk HPV subtypes
With the use of PathoChip, we looked at cervical cancer to identify and characterize the presence of HPV subtypes. All forty HPV subtypes were present in the patient cohort (n = 40 in WLWH, n = 37 in HIV-negative patients), where five high-risk HPV subtypes were identified: HPV 16, 18, 26, 34, and 53 (Table 2). The most prevalent high-risk HPV subtypes were HPV 26 (96%) and HPV 34 (92%). All high-risk HPV subtypes were consistently more prevalent among WLWH. Specifically, HPV 26 and HPV 34 were more prevalent in WLWH (both 98%) compared to HIV-negative patients [89% (p < 0.01) and 78% (p < 0.001), respectively]. High-risk HPV 16 was more prevalent in WLWH (83%) than HIV-negative patients (67%, p = 0.02). Co-infection with four or more high-risk subtypes was found in 86% of WLWH (n = 106) vs. 67% of HIV-negative patients (n = 30) (p < 0.01). Approximately 10% of patients in both groups did not have HPV 16 or 18 high-risk subtypes. In this small group of patients, HPV 34 was more prevalent in WLWH (100%) vs. in HIV-negative patients (40%) (p < 0.01). Of patients that were negative for HPV 16 and 18 high-risk subtypes, 91.67% of WLWH were positive for two or more other high-risk HPV subtypes (26, 34, and 53), compared to 60% of HIV-negative patients.
Table 2.
Prevalence of high- and low-risk subtypes by HIV status.
| HIV-positive |
HIV-negative |
Total |
p | |||||
|---|---|---|---|---|---|---|---|---|
| n | Percentage (%) | n | Percentage (%) | n | Percentage (%) | |||
| Total | 123 | 73.21 | 45 | 26.78 | 168 | 100.00 | ||
| High-risk | HPV 16 | 102 | 82.93 | 30 | 66.67 | 132 | 78.57 | 0.02293 |
| HPV 18 | 105 | 85.37 | 34 | 75.56 | 139 | 82.74 | 0.1362 | |
| HPV 26 | 121 | 98.37 | 40 | 88.89 | 161 | 95.83 | 0.006439 | |
| HPV 34 | 120 | 97.56 | 35 | 77.78 | 155 | 92.26 | 0.00002139 | |
| HPV 53 | 107 | 86.99 | 30 | 66.67 | 137 | 81.55 | 0.002634 | |
| HPV 16 +HPV 18 | 96 | 78.05 | 24 | 53.33 | 120 | 71.43 | 0.001688 | |
| HPV 16 or HPV 18 | 111 | 90.24 | 40 | 88.89 | 151 | 89.88 | 0.7965 | |
| No HPV 16 | 12 | 9.76 | 5 | 11.11 | 17 | 10.12 | 0.7965 | |
| No HPV 18 | ||||||||
| Low-risk | HPV 1 | 115 | 93.50 | 40 | 88.89 | 155 | 92.26 | 0.3223 |
| HPV 2 | 118 | 95.93 | 39 | 86.67 | 157 | 93.45 | 0.0315 | |
| HPV 4 | 113 | 91.87 | 35 | 77.78 | 148 | 88.10 | 0.0125 | |
| HPV 5 | 110 | 89.43 | 43 | 95.56 | 153 | 91.07 | 0.2176 | |
| HPV 6b | 122 | 99.19 | 43 | 95.56 | 165 | 98.21 | 0.1155 | |
| HPV 7 | 120 | 97.56 | 41 | 91.11 | 161 | 95.83 | 0.06393 | |
| HPV 9 | 121 | 98.37 | 41 | 91.11 | 162 | 96.43 | 0.02468 | |
| HPV 10 | 99 | 80.49 | NA | NA | 99 | 58.93 | NA | |
| HPV 32 | 116 | 94.31 | 27 | 60.00 | 143 | 85.12 | 3.15E-08 | |
| HPV 41 | 84 | 68.29 | NA | NA | 84 | 50.00 | NA | |
| HPV 48 | 121 | 98.37 | 43 | 95.56 | 164 | 97.62 | 0.2886 | |
| HPV 49 | 107 | 86.99 | 38 | 84.44 | 145 | 86.31 | 0.6706 | |
| HPV 50 | 120 | 97.56 | 40 | 88.89 | 160 | 95.24 | 0.01942 | |
| HPV 54 | 99 | 80.49 | 29 | 64.44 | 128 | 76.19 | 0.03061 | |
| HPV 60 | 118 | 95.93 | 42 | 93.33 | 160 | 95.24 | 0.4832 | |
| HPV 61 | 119 | 96.75 | 40 | 88.89 | 159 | 94.64 | 0.04514 | |
| HPV 63 | 117 | 95.12 | 32 | 71.11 | 149 | 88.69 | 0.00001351 | |
| HPV 88 | 119 | 96.75 | 44 | 97.78 | 163 | 97.02 | 0.728 | |
| HPV 90 | 93 | 75.61 | NA | NA | 93 | 55.36 | NA | |
| HPV 92 | 119 | 96.75 | 40 | 88.89 | 159 | 94.64 | 0.04514 | |
| HPV 96 | 116 | 94.31 | 36 | 80.00 | 152 | 90.48 | 0.005143 | |
| HPV 101 | 121 | 98.37 | 43 | 95.56 | 164 | 97.62 | 0.2886 | |
| HPV 103 | 121 | 98.37 | 42 | 93.33 | 163 | 97.02 | 0.08864 | |
| HPV 108 | 118 | 95.93 | 36 | 80.00 | 154 | 91.67 | 0.0009352 | |
| HPV 109 | 114 | 92.68 | 39 | 86.67 | 153 | 91.07 | 0.2259 | |
| HPV 112 | 117 | 95.12 | 41 | 91.11 | 158 | 94.05 | 0.3305 | |
| HPV 116 | 112 | 91.06 | 28 | 62.22 | 140 | 83.33 | 0.000008952 | |
| HPV 121 | 116 | 94.31 | 42 | 93.33 | 158 | 94.05 | 0.8129 | |
| HPV 126 | 120 | 97.56 | 35 | 77.78 | 155 | 92.26 | 0.00002139 | |
| HPV 128 | 118 | 95.93 | 40 | 88.89 | 158 | 94.05 | 0.08739 | |
| HPV 129 | 104 | 84.55 | NA | NA | 104 | 61.90 | NA | |
| HPV 131 | 120 | 97.56 | 40 | 88.89 | 160 | 95.24 | 0.01942 | |
| HPV 132 | 122 | 99.19 | 41 | 91.11 | 163 | 97.02 | 0.006374 | |
| HPV 134 | 115 | 93.50 | 41 | 91.11 | 156 | 92.86 | 0.5951 | |
| HPV 148 | 118 | 95.93 | 41 | 91.11 | 159 | 94.64 | 0.2188 | |
Of high-risk subtypes in the samples, HPV 18 had the highest HSI. There was no statistical difference in HSI in regards to any of the high-risk subtypes (HPV 16, 18, 26, 34, and 53). While both HPV 16 and HPV 18 were more prevalent among WLWH, average HSI was greater for HPV 16 and HPV 18 in HIV-negative patients, although not statistically significant (Table 3).
Table 3.
Hybridization signal intensity of high- and low-risk HPV subtypes by HIV status.
| Subtype | HIV-positive | HIV-negative | Total | p | |
|---|---|---|---|---|---|
| High-risk | HPV 16 | 1.5455 | 1.7658 | 1.6045 | 0.9036 |
| HPV 18 | 1.5960 | 1.9867 | 1.7006 | 0.8365 | |
| HPV 26 | 1.7208 | 1.5558 | 1.6766 | 0.9273 | |
| HPV 34 | 1.5548 | 1.3093 | 1.4891 | 0.8847 | |
| HPV 53 | 1.4770 | 1.3729 | 1.4491 | 0.9508 | |
| Low-risk | HPV 1 | 1.8066 | 1.3021 | 1.6715 | 0.7748 |
| HPV 2 | 1.5683 | 1.4648 | 1.5406 | 0.9526 | |
| HPV 4 | 1.5653 | 1.6121 | 1.5778 | 0.9790 | |
| HPV 5 | 1.9884 | 1.3932 | 1.8290 | 0.7462 | |
| HPV 6b | 1.5609 | 1.4742 | 1.5376 | 0.9603 | |
| HPV 7 | 1.8130 | 1.6027 | 1.7567 | 0.9094 | |
| HPV 9 | 1.5687 | 1.3981 | 1.5230 | 0.9211 | |
| HPV 10 | 1.3500 | NA | 1.3500 | NA | |
| HPV 32 | 1.6993 | 1.6562 | 1.6877 | 0.9812 | |
| HPV 41 | 1.5302 | NA | 1.5302 | NA | |
| HPV 48 | 1.7597 | 1.9968 | 1.8232 | 0.9027 | |
| HPV 49 | 1.8767 | 1.5519 | 1.7897 | 0.8607 | |
| HPV 50 | 1.7594 | 1.5226 | 1.6959 | 0.8960 | |
| HPV 54 | 2.1813 | 1.5938 | 2.0240 | 0.7624 | |
| HPV 60 | 1.9252 | 1.9543 | 1.9330 | 0.9882 | |
| HPV 61 | 1.9120 | 1.8130 | 1.8855 | 0.9591 | |
| HPV 63 | 1.5479 | 1.3200 | 1.4868 | 0.8929 | |
| HPV 88 | 1.6310 | 1.4364 | 1.5789 | 0.9115 | |
| HPV 90 | 1.3605 | NA | 1.3605 | NA | |
| HPV 92 | 1.7411 | 1.6547 | 1.7180 | 0.9626 | |
| HPV 96 | 1.7334 | 1.3480 | 1.6302 | 0.8262 | |
| HPV 101 | 1.5796 | 1.7578 | 1.6273 | 0.9223 | |
| HPV 103 | 1.8379 | 1.9365 | 1.8643 | 0.9595 | |
| HPV 108 | 1.7826 | 2.1112 | 1.8707 | 0.8678 | |
| HPV 109 | 1.8923 | 1.7586 | 1.8565 | 0.9442 | |
| HPV 112 | 1.6614 | 1.6012 | 1.6453 | 0.9734 | |
| HPV 116 | 1.4588 | 1.3075 | 1.4183 | 0.9275 | |
| HPV 121 | 2.1310 | 1.8130 | 2.0458 | 0.8728 | |
| HPV 126 | 1.7106 | 2.1373 | 1.8249 | 0.8278 | |
| HPV 128 | 1.6700 | 1.4686 | 1.6161 | 0.9095 | |
| HPV 129 | 1.6741 | NA | 1.6741 | NA | |
| HPV 131 | 1.7786 | 1.6591 | 1.7466 | 0.9486 | |
| HPV 132 | 1.7237 | 1.6268 | 1.6978 | 0.9578 | |
| HPV 134 | 1.7923 | 1.5866 | 1.7372 | 0.9109 | |
| HPV 148 | 1.9958 | 1.6167 | 1.8942 | 0.8419 |
3.2.2. Low-risk HPV subtypes
Signatures for 66 low-risk HPV subtypes were detected among all cervical cancer patients in our cohort (Table 2). The most prevalent low-risk HPV subtypes were HPV 6b, 48 and 101 (all 98%). Several low-risk HPV subtypes were significantly more prevalent among WLWH: HPV 2, 4, 9, 32, 50, 54, 61, 63, 92, 96, 108, 116, 126, 131, and 132. Notably, signatures for low-risk HPV subtypes 10 (80%), 41 (68%), 90 (76%), and 129 (85%) were only detected in WLWH (Table 2).
Of the low-risk HPV subtypes in the samples, HPV 121 had the highest HSI. There was no significant difference observed in the HSI between low-risk subtypes by HIV status (Table 3).
3.3. Distribution of high- and low-risk HPV signatures by HIV status and age group
3.3.1. High-risk HPV subtypes
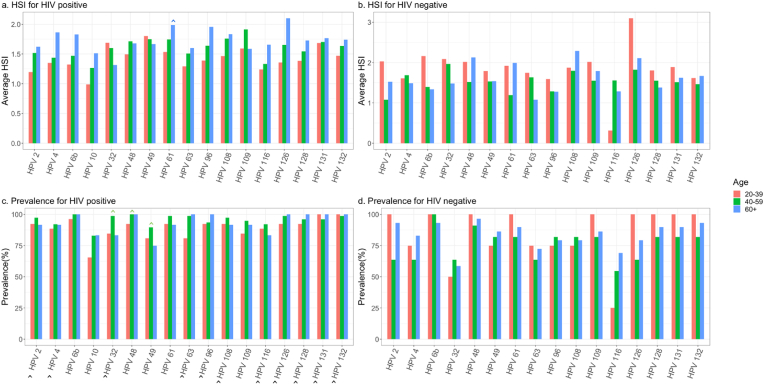
Among WLWH, high-risk HPV 26 and HPV 34 were highly prevalent among all cervical cancer patients regardless of age (≥96% for both) (Fig. 1A). Further, high-risk HPV 34 was significantly more prevalent in WLWH of all age groups compared to HIV-negative patients (p = 0.001). Average HSI was significantly higher for HPV 18 in patients aged ≥60 years, regardless of HIV status, as compared to other age groups (p = 0.03) (Fig. 1A).
Fig. 1A.
High-risk HPV subtype prevalence and hybridization signal intensity (HSI) by HIV status and age group.
3.3.2. Low-risk HPV subtypes
A majority of low-risk HPV subtypes had higher prevalence among WLWH of all age groups compared to HIV-negative patients (Fig. 1B). Specifically, HPV 2, 4, 6b, 32, 63, 96, 108, 116, 126, 128, 131, and 132 were significantly more prevalent in WLWH compared to HIV-negative patients. HPV 32, 48, and 49 were significantly more prevalent for all patients regardless of HIV status at ages of 40–60 years old compared to patients of other age groups (p = 0.01, 0.05, 0.03, respectively). Notably, average HSI was significantly higher among patients aged ≥60 years regardless of HIV status for HPV 61 as compared to other age groups (p = 0.03) (Fig. 1B).
Fig. 1B.
Low-risk HPV prevalence and hybridization signal intensity (HSI) by HIV status and age group.
3.4. Distribution of high- and low-risk HPV signatures by CD4 count and HIV status
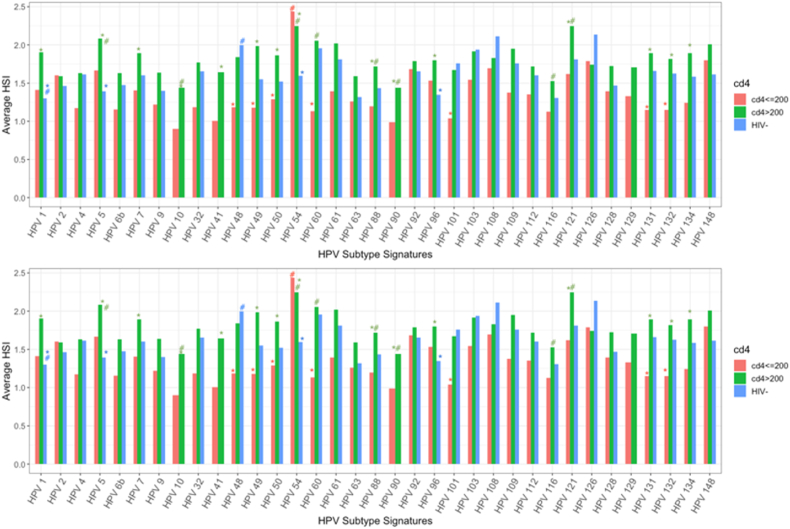
Similar prevalence of high- and low-risk HPV subtypes were noted across various CD4 groups and HIV-negative groups. HSI for HPV 18 was significantly lower in WLWH with CD4 levels ≤200 cells/μL, as compared to WLWH with CD4 >200 cells/μL and HIV-negative patients (p < 0.01) (Fig. 2A). Average HSI was significantly higher for WLWH with CD4 levels >200 cells/μL for HPV 34 and HPV 53 (p = 0.03, 0.04, respectively).
Fig. 2A.
High-risk HPV subtype hybridization signal intensity (HSI) by CD4 cell count and HIV status. *Comparison of HPV subtype across CD4 ranges; #Comparison of HPV subtypes within the same CD4 category.
WLWH with CD4 levels ≤200 cells/μL had significantly lower average HSI for low-risk subtypes HPV 48, 49, 50, 60, 101, 131, and 132, as compared to WLWH with CD4 >200 cells/μL or HIV-negative patients (Fig. 2B). However, average HSI for HPV 1, 5, 7, 41, 32, 49, 50, 54, 88, 90, 96, and 121 was significantly higher among WLWH with CD4 levels >200 cells/μL compared to WLWH with CD4 >200 cells/μL or HIV-negative patients.
Fig. 2B.
Low-risk HPV hybridization signal intensity (HSI) by CD4 cell count and HIV status. *Comparison of HPV subtype across CD4 ranges; #Compare of HPV subtypes with the same CD4 category.
3.5. Distribution of HPV subtype by cancer stage
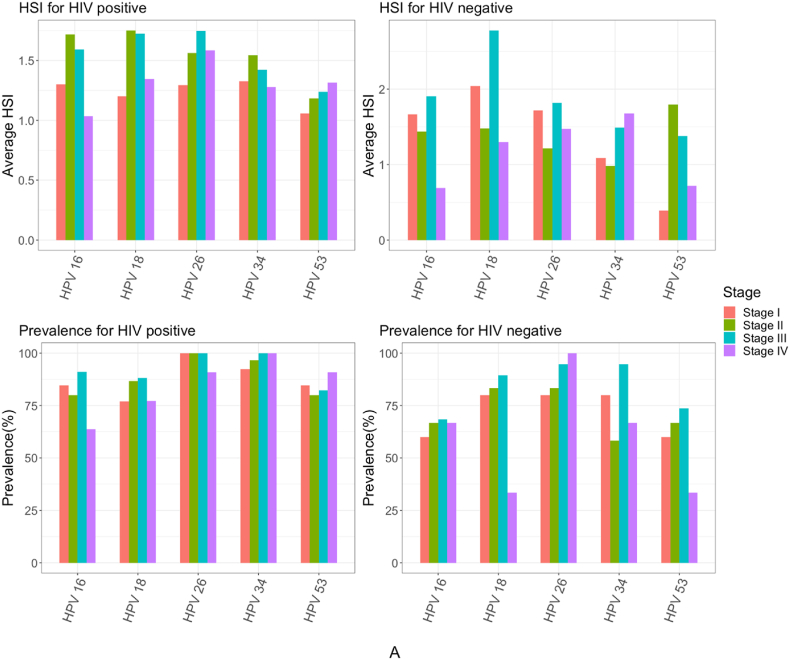
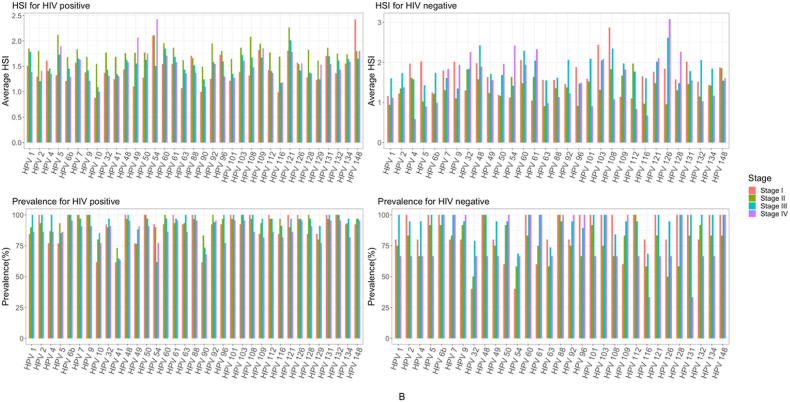
Generally, the prevalence of high-risk HPV subtypes was overall high and similar across FIGO cervical stage groups among WLWH (Figure A). However, the prevalence of high-risk HPV 16 was found significantly higher among WLWH in stage III of cervical cancer, compared to other FIGO stage groups (p = 0.03) (Fig. 3A).
Fig. 3A.
High-risk HPV subtype prevalence and hybridization signal intensity (HSI) by HIV status and stage group.
In contrast, the prevalence of low-risk HPV subtypes varied across stage groups (Figure B). Between FICO cervical stage groups of WLWH, five (HPV 2, 61, 108, 112, and 121) low-risk HPV subtypes were significantly higher in stage I (p < 0.04), four (HPV 54, 60, 92, 128) in stage II (p < 0.04), and six (HPV 1, 2, 4, 63, 96, and 108) in stage III (p < 0.04) (Figure B). Whereas low-risk HPV subtypes significantly lower in prevalence among WLWH included subtypes 54 in stage III (p = 0.01) and 96 in stage IV (p = 0.04), compared to other stage groups (Fig. 3B).
Fig. 3B.
Low-risk HPV subtype prevalence and hybridization signal intensity (HSI) by HIV status and stage group.
While no high-risk HPV subtypes had significantly higher HSI in any particular stage group, HPV 16 was significantly lower in HSI among WLWH with stage IV cervical cancer (p = 0.03). Of low-risk HPV subtypes, seven (HPV 2, 10, 92, 108, 112, 116, and 128) were significantly higher in HIS among WLWH in stage II, compared to patients of other stage groups (p < 0.03). Whereas HSI of HPV subtypes 2 (p = 0.04) and 54 (p = 0.03) were significantly higher among stage III WLWH.
3.6. Average HSI of HPV 16 and HPV 18 organized by viral gene region
Specifically for HPV 16 probes organized by gene region, WLWH had higher HSI for E2, E5, and L1 regions compared to HIV-negative patients. Probes for HPV 18 viral gene regions E6, E7, and L2 had significantly higher HSI among WLWH compared to HIV-negative patients (Fig. 1A, Fig. 2BB).
4. Discussion
To our knowledge, our study is one of the first to look at the breadth of high- and low-risk HPV subtypes and their respective viral burden within invasive cervical cancer specimens by HIV status from a sub-Saharan Africa setting. Previously published worldwide reviews of HPV subtypes detected in invasive cervical cancer have primarily focused on prevalence of HPV high-risk subtypes, showing that HPV 16 and 18 are present in 48% and 23% of cervical cancers in Africa, which is low compared to major regions of the world [19]. Ermel et al. [20] showed that 42.7% of all cervical cancer samples were positive for HPV 16, compared to 87% in invasive cervical cancers in the US and 80.8% in Kenya. Additionally, HPV 18 was detected in 23.5% of cervical cancer samples from Botswana, 19.2% from Kenya, and 8.7% from the US. Subtypes other than HPV 16 and HPV 18 were detected in 65 of 136 (47.8%) of cervical cancer samples from Botswana, 25 of 146 (1.1%) samples from Kenya, and 5 of 46 (10.9%) samples from the US. A 2015 meta-analysis noted that HPV 16, 18, and 45 are the three most common subtypes among all regions across Africa, followed by HPV 33 as the fourth most common in every region except Western Africa, where HPV 35 is the fourth most common [21]. In our population, ranked by prevalence, high risk HPV subtypes included 26, 34, 18, and 53. HPV16 was less common amongst our total population.
Co-infection of HPV and HIV can accelerate progression from cervical intra-epithelial neoplasia to cervical cancer. HIV-infected individuals are also more likely to have co-infection with multiple HPV subtypes, including non-carcinogenic HPV subtypes or HPV subtypes with unknown carcinogenicity [22]. Maranga et al. [23] examined HPV subtypes in cervical cancer smears from WLWH and HIV-negative women in Kenya and found higher prevalence of high-risk HPV subtypes (52, 58, 68, potential high-risk 53/70; as well as low-risk subtypes 44/55) in WLWH compared to HIV-negative women. The authors determined that coinfection with HIV may alter the composition of HPV subtypes found in women with cervical cancer. Further, previous data from Botswana showed that the most common high-risk HPV subtypes in invasive cervical cancer, regardless of HIV status, were HPV 16 (75.4%) and HPV 18 (28.6%), while other high-risk HPV subtypes were only found in 16.7% of samples [24]. One third of these invasive cervical cancer patients had multiple high-risk HPV subtypes, with higher rates of multiple subtypes found in WLWH. Compared to extant literature, 25 our cohort had a higher prevalence of high-risk subtypes HPV 16, 26, 34, and 53 in WLWH (e.g., 46.1% HPV 16 and 22.6% HPV 18 reported by De Vuyst et al. vs. 79% HPV 16 and 82% HPV 18 in our current study). Similarly, our study population had higher prevalence of most low-risk subtypes detected in WLWH of all age groups compared to HIV-negative patients. Further, WLWH had a higher number of HPV subtypes in any given sample.
A 2016 meta-analysis of 21 studies from Africa included a total of 770 WLWH with cervical cancer, testing HPV DNA from both cervical cells and/or tumor biopsies. While detection of any HPV subtype was similar among WLWH and HIV-negative women (91.2% vs 89.6%, respectively), HIV-positive cervical cancers were more likely to be infected with multiple HPV subtypes (27.8%) than HIV-negative cervical cancers (15.9%). Additionally, HPV 16 was less prevalent in HIV-positive cervical cancers than in HIV-negative cervical cancers (RR 0.88), while all other high-risk subtypes were more frequently detected in HIV-positive cervical cancers [26]. This contrasts with our data showing that HPV 16 was more prevalent in WLWH (83%) than HIV-negative women (67%, p = 0.02), confirmed in a prior study from Botswana as well [25]. As Strickler et al. describes, HPV 16 plays a unique role in WLWH and may be independent from immune status and avoid the impacts of immune surveillance better than other HPV types [27].While other high-risk HPV subtypes may proliferate and persist more readily in WLWH with reduced cell counts and an immunodeficient environment, HPV16 may maintain a baseline infectious rate [23,29]. A potential theory for the discrepancy between HPV16 rates in WLWH in Botswana compared to other countries in Africa, could be attributed to the overall higher average CD4 cell count in our study population and thus other HRHPV subtypes were not able to proliferate [median: 479.5 cells/μL (IQR: 324.2–649.2); most patients (n = 91) had an undetectable viral load at the time of cancer diagnosis].
In patients with detected HPV 18, HSI for HPV 18 was significantly lower in WLWH with CD4 ≤200 cells/μL compared to HIV-negative women and WLWH with CD4 >200 cells/μL. Thus, it can be hypothesized that there is less virus needed to establish HPV 18 infection in WLWH with lower CD4 cell counts. The corollary, that HSI was higher for WLWH with CD4 >200 cells/μL for HPV 34 and HPV 53, may suggest that higher viral load is needed to establish infection in those with higher CD4 cell counts due to a more intact immune system. Historically, HPV 16 is the only subtype for which there is evidence that viral load may predict viral persistence and progression to precancerous lesions [[28], [29], [30]]. These data may further indicate that HPV 18 has a similar role in pathogenicity of invasive cervical cancer. Similarly, Wentzensen et al. showed that viral load measured by signal intensity could predict differentiation between infection and cervical intra-epithelial neoplasia level ≥2 for HPV 18 [31]. The role of HSI for various HPV subtypes in determining clinical course of disease and outcomes needs to be further evaluated and will be an area of future investigation. Of note, while many of the patients were on antiretroviral therapy, we were unable to run an interaction analysis between duration of antiretroviral exposure and HPV subtype detection as this was self reported data that was not always available and unverified by medical accounts.
Strengths of the present study include the use of PathoChIP, which can characterize HPV subtypes and detect very low viral loads, while previous literature has used only PCR analysis. PathoChIP technology allowed us to capture more accurate data on HPV genotyping. Additionally, we are able to analyze low-risk HPV types, which have not been fully evaluated in previous studies.
However, our study also has limitations. We recognize that we do not have information on temporality or acuity of HIV infection. Enrolled patients were newly diagnosed with cervical cancer, so advanced HIV infection may have been present at enrollment. Therefore, it is difficult to determine whether HIV or HPV infection preceded the other. This limits our ability to predict whether infection with HIV prompted immune dysregulation that led to development of cancer.
5. Conclusions
This is the first study to our knowledge to use the PathoChip technique with high sensitivity to the majority of HPV subtypes to detect the breadth of HPV subtypes in cervical cancer specimens in Botswana. The five most common HR-HPV subtypes in this cohort were 26, 34, 16, 18, and 53. We have demonstrated that co-infection with multiple HPV subtypes was more common in WLWH. This study also demonstrates the heterogeneity and high variability in low risk HPV subtypes present in this population. These results reinforce the need for continued screening in addition to vaccination given the various subtypes detected.
Author contributions
Surbhi Grover: Conceptualization, Resources, Formal Analysis, Manuscript writing, Manuscript Editing and Approval.
Tyler Seckar: Data curation, Formal Analysis, Manuscript writing, manuscript editing and approval.
Le Gao: Data curation, Formal analysis, manuscript writing, manuscript editing and approval.
Xiang Lin: Data curation, manuscript writing, manuscript editing and approval.
Rohini Bhatia: Formal analysis, manuscript writing, manuscript editing and approval.
Nicola Zetola: Conceptualization and resources, data curation, manuscript editing and approval.
Doreen Ramogola-Masire: Conceptualization and resources, data curation, manuscript editing and approval.
Erle Robertson: Conceptualization and resources, manuscript editing and approval.
The work reported in the paper has been performed by the authors, unless clearly specified in the text.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Surbhi Grover is supported by a training grant from the National Cancer Institute (K08CA230170-03S1). This work was supported by Sub-Saharan African Collaborative HIV and Cancer Consortia-U54 (1U54 CA190158-01). We are grateful to the research staff of the Botswana-UPenn Partnership and all participants who made this work possible.
Data availability
Data will be made available on request.
References
- 1.zur Hausen H. Molecular pathogenesis of cancer of the cervix and its causation by specific human papillomavirus types. Curr. Top. Microbiol. Immunol. 1994;186:131–156. doi: 10.1007/978-3-642-78487-3_8. [DOI] [PubMed] [Google Scholar]
- 2.Bosch F.X., Manos M.M., Munoz N., Sherman M., Jansen A.M., Peto J., Schiffman M.H., Moreno V., Kurman R., Shah K.V. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J. Natl. Cancer Inst. 1995;87:796–802. doi: 10.1093/jnci/87.11.796. [DOI] [PubMed] [Google Scholar]
- 3.High Risk human papillomavirus genotype distribution in the Northern region of Portugal: data from regional cervical cancer screening program. Papillomavirus Res. 2019 Dec;8 doi: 10.1016/j.pvr.2019.100179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bray F., Ferlay J., Soerjomataram I., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca - Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 5.Singh D.K., Anastos K., Hoover D.R., Burk R.D., Shi Q., Ngendahayo L., Mutimura E., Cajigas A., Bigirimani V., Cai X., Rwamwejo J., Vuolo M., Cohen M., Castle P.E. Human papillomavirus infection and cervical cytology in HIV-infected and HIV uninfected Rwandan women. J. Infect. Dis. 2009;199:1851–1861. doi: 10.1086/599123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stelzle D., Tanaka L.F., Lee K.K., Ibrahim Khalil A., Iacopo Baussano, Shah A.S.V., Gottlieb S., Klug S.J., Winkler A.S., Bray F., Baggaley R., Clifford G.M., Broutet N., Dalal S. Estimates of the global burden of cervical cancer associated with HIV. Lancet Global Health. 2021;9:e161–e169. doi: 10.1016/S2214-109X(20)30459-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sung H., Ferlay J., Siegel R., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 8.Preliminary results of the fifth Botswana AIDS impact survey. November 23, 2021. https://www.statsbots.org.bw/sites/default/files/publications/BOTSWANA%20AIDS%20IMPACT%20SURVEY%20IV%202013.pdf
- 9.Ramatlho P., Grover S., Mathoma A., Tawe L., Mathagela K., Molebatsi K., Chilisa B., Zetola N.M., Robertson E.S., Ramogola-Masire D. Human papillomavirus prevalence among unvaccinated young female college students in Botswana: a cross-sectional study. S. Afr. Med. J. 2022;112:335–340. [PubMed] [Google Scholar]
- 10.Ramogola-Masire D., McClung N., Mathoma A., Gargano J.W., Nyepetsi N.G., Querec T.D., Onyekwuluje J., Mine M., Morroni C., Luckett R., Markowitz L.E. Human papillomavirus prevalence in male and female university students in Gaborone, Botswana. Epidemiol. Infect. 2022;150 doi: 10.1017/S0950268822000619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raesima M.M., Forhan S.E., Voetsch A.C., Hewitt S., Hariri S., Wang S.A., Pelletier A.R., Letebele M., Pheto T., Ramogola-Masire D., El-Halabi Human papillomavirus vaccination coverage among school girls in a demonstration project –Botswana. MMWR Weekly. 2013;(40):1147–1149. doi: 10.15585/mmwr.mm6440a5. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6440a5.htm October 16, 02015/64. [DOI] [PubMed] [Google Scholar]
- 12.Bergman H., Buckley B.S., Villanueva G., Petkovic J., Garritty C., Lutje V., Riveros-Balta A.X., Low N., Henschke N. Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of HPV-related disease in females and males. Cochrane Database Syst. Rev.2019. 2019 doi: 10.1002/14651858.CD013479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banerjee S., Tian T., Wei Z., Shih N., Feldman M., Peck K., DeMichele A., Alwine J., Robertson E.S. Distinct microbiological signatures associated with triple negative breast cancer. Sci. Rep. 2015;5 doi: 10.1038/srep15162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baldwin D.A., Feldman M., Alwine J.C., Robertson E.S. Metagenomic assay for identification of microbial pathogens in tumor tissues. mBio. 2014;5 doi: 10.1128/mBio.01714-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zitvogel L., Ma Y., Raoult D., Kroemer G., Gajewski T.F. The microbiome in cancer immunotherapy: diagnostic tools and therapeutic strategies. Science. 2018;359:1366–1370. doi: 10.1126/science.aar6918. [DOI] [PubMed] [Google Scholar]
- 16.Banerjee S., Tian T., Wei Z., Shih N., Feldman M., Peck K., DeMichele A., Alwine J., Robertson E.S. Distinct microbial signatures associated with different breast cancer types. Front. Microbiol. 2018;9:951. doi: 10.3389/fmicb.2018.00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banerjee S., Tian T., Wei Z., Shih N., Feldman M., Alwine J., Coukos G., Robertson E.S. The ovarian cancer oncobiome. Oncotarget. 2017;8:36225–36245. doi: 10.18632/oncotarget.16717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banerjee S., Tian T., Wei Z., Peck K., Shih N., Chalian A., O'Malley B.W., Weinstein G.S., Feldman M., Alwine J., Robertson E.S. Microbial signatures associated with oropharyngeal and oral squamous cell carcinomas. Sci. Rep. 2017;7:4036. doi: 10.1038/s41598-017-03466-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Sanjose S., Quint W.G., Alemany L., Geraets D.T., Klaustermeier J.E., Lloveras B., Tous S., Felix A., Bravo L.E., Shin H.R., et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 20.Ermel A., Qadadri B., Tong Y., Orang’o O., Macharia B., Ramogola-Masire D., Zetola N.M., Brown D.R. Invasive cervical cancers in the United States, Botswana and Kenya: HPV type distribution and health policy implications. Infect. Agents Cancer. 2016;11:56. doi: 10.1186/s13027-016-0102-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogembo R.K., Gona P.N., Seymour A.J., Park H.S.-M., Bain P.A., Maranda L., Ogembo J.G. Prevalence of human papillomavirus genotypes among african women with normal cervical cytology and neoplasia: a systematic review and meta-analysis. PLoS One. 2015;10(4) doi: 10.1371/journal.pone.0122488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broker T.R., Jin G., Croom-Rivers A., Bragg S.M., Richardson M., Chow L.T., Vermund S.H., Alvarez R.D., Pappas P.G., Squires K.E., Hoesley C.J. Viral latency--the papillomavirus model. Dev. Biol. 2001;106:443–451. ; discussion 452-3, 465-75. PMID: 11761260. [PubMed] [Google Scholar]
- 23.Maranga I.O., Hampson L., Oliver A.W., He X., Gichangi P., Rana F., Opiyo A., Hampson I.N. HIV infection alters the spectrum of HPV subtypes found in cervical smears and carcinomas from Kenyan women. Open Virol. J. 2013;7:19–27. doi: 10.2174/1874357901307010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tawe L., MacDuffie E., Narasimhamurthy M., Wang Q., Gaseitsiwe S., Moyo S., Kasvosve I., Shin S.S., Zetola N.M., Paganotti G., Grover S. Human papillomavirus genotypes in women with invasive cervical cancer with and without human immunodeficiency virus infection in Botswana. Int. J. Cancer. 2020;146:1667–1673. doi: 10.1002/ijc.32581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Vuyst H., Ndirangu G., Moodley M., Tenet V., Estambale B., Meijer C.J.L.M., Snijders P.J.F., Clifford G., Franceschi S. Prevalence of human papillomavirus in women with invasive cervical carcinoma by HIV status in Kenya and South Africa. Int. J. Cancer. 2012;131:949–955. doi: 10.1002/ijc.26470. [DOI] [PubMed] [Google Scholar]
- 26.Clifford G.M., de Vuyst H., Tenet V., Plummer M., Tully S., Franceschi S. Effect of hiv infection on human papillomavirus types causing invasive cervical cancer in Africa. J. Acquir. Immune Defic. Syndr. 2016;73(3):332–339. doi: 10.1097/QAI.0000000000001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strickler H.D., Palefsky J.M., Shah K.V., Anastos K., Klein R.S., Minkoff H., Duerr A., Massad L.S., Celentano D.D., Hall C., Fazzari M., Cu-Uvin S., Bacon M., Schuman P., Levine A.M., Durante A.J., Gange S., Melnick S., Burk R.D. Human papillomavirus type 16 and immune status in human immunodeficiency virus-seropositive women. J. Natl. Cancer Inst. 2003;95:1062–1071. doi: 10.1093/jnci/95.14.1062. [DOI] [PubMed] [Google Scholar]
- 28.Boulet G.A., Benoy I.H., Depuydt C., Horvath C.A.J., Aerts M., Hens N., Vereecken A.J., Bogers J.J. Human papillomavirus 16 load and E2/E6 ratio in HPV16-positive women: biomarkers for cervical intraepithelial neoplasia ≥2 in a liquid-based cytology setting? Cancer Epidemiol. Biomarkers Prev. 2009;18:2992–2999. doi: 10.1158/1055-9965.EPI-09-0025. [DOI] [PubMed] [Google Scholar]
- 29.Gravitt P.E., Kovacic M.B., Herrero R., Schiffman M., Bratti C., Hildesheim A., Morales J., Alfaro M., Sherman M.E., Wacholder S., Rodriguez A.C., Burk R.D. High load for most high risk human papillomavirus genotypes is associated with prevalent cervical cancer precursors but only HPV16 load predicts the development of incident disease. Int. J. Cancer. 2007;121:2787–2793. doi: 10.1002/ijc.23012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xi L.F., Hughes J.P., Castle P.E., Edelstein Z.R., Wang C., Galloway D.A., Koutsky L.A., Kiviat N.B., Schiffman M. Viral load in the natural history of human papillomavirus type 16 infection: a nested case-control study. J. Infect. Dis. 2011;203:1425–1433. doi: 10.1093/infdis/jir049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wentzensen N., Gravitt P.E., Long R., Schiffman M., Dunn S.T., Carreon J.D., Allen R.A., Gunja M., Zuna R.E., Sherman M.E., Gold M.A., Walker J.L., Wang S.S. Human papillomavirus load measured by Linear Array correlates with quantitative PCR in cervical cytology specimens. J. Clin. Microbiol. 2012;50(5):1564–1570. doi: 10.1128/JCM.06240-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouvard V., Baan R., Straif K., Grosse Y., Secretan B., El Ghissassi F., Benbrahim-Tallaa L., Guha N., Freeman C., Galichet L., Cogliano V. WHO international agency for research on cancer monograph working group. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009 Apr;10(4):321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.