Abstract
Background:
Without treatment, approximately half of HIV-infected infants die by age 2 years, and 80% die before age 5 years. Early identification of HIV-infected and HIV-exposed infants provides opportunities for life-saving interventions. We evaluated integration of HIV-related services with routine infant immunization in Tanzania.
Methods:
During April 2009 to March 2010, at 4 urban and 4 rural sites, mothers’ HIV status was determined at first-month immunization using antenatal cards. HIV-exposed infants were offered HIV testing and follow-up care. Impact of integrated service delivery was assessed by comparing average monthly vaccine doses administered during the study period and a 2-year baseline period; acceptance was assessed by interviewing mothers and service providers.
Findings:
During 7569 visits, 308 HIV-exposed infants were identified and registered; of these, 290 (94%) were tested, 15 (5%) were HIV infected. At urban sites, first-month vaccine doses remained stable (+2% for pentavalent vaccine and −4% for polio vaccine), and vaccine doses given later in life (pentavalent, polio, and measles) increased 12%, 8%, and 11%, respectively. At rural sites, first-month vaccine doses decreased 33% and 35% and vaccine doses given later in life decreased 23%, 28%, and 28%. Mothers and service providers generally favored integrated services; however, HIV-related stigma and inadequate confidentiality controls of HIV testing were identified, particularly at rural sites.
Interpretation:
Integration of HIV-related services at immunization visits identified HIV-exposed infants, HIV-infected infants, and HIV-infected mothers; however, decreases in vaccine doses administered at rural sites were concerning. HIV-related service integration with immunization visits needs careful monitoring to ensure optimum vaccine delivery.
Keywords: HIV, early infant diagnosis, routine immunizations, vaccination, integration
INTRODUCTION
HIV transmission from a mother to her fetus or infant can be prevented by detection of maternal HIV infection and administration of antiretroviral therapy (ART) to the mother during pregnancy, labor, and postpartum period and to infants during the postpartum period.1 However, despite the availability of effective interventions for preventing mother to child transmission (PMTCT) of HIV, services remain underutilized; in 2009, an estimated 42,000 to 60,000 pregnant women died because of HIV and 370,000 children became newly infected.1,2 Although half of untreated HIV-infected infants die by age 2 years, and 80% die before age 5 years,3,4 early implementation of ART for HIV-infected infants and of cotrimoxazole (CTX) prophylaxis for all HIV-exposed infants to prevent opportunistic infections can substantially reduce childhood morbidity and mortality.5,6
Postpartum follow-up of HIV-positive mother–infant pairs remains a challenge, highlighting the need to find alternative strategies for early identification and treatment of HIV-exposed infants.7 HIV prevalence among pregnant women is highest in sub-Saharan African countries, reaching 20%–40% in some countries.5 In 2004, a National HIV care and treatment program was launched in Tanzania with support from the Global Fund to Fight AIDS, Tuberculosis, and Malaria and the US President’s Emergency Plan for AIDS Relief.8 In 2010, 1,414,051 (85%) of the estimated 1,665,300 pregnant women in Tanzania were tested for HIV and 87,343 (6%) were found to be HIV infected.9,10 However, of an estimated 84,000 infants born to HIV-infected women in Tanzania, only 10% were started on CTX; and of an estimated 75,000 HIV-infected children 0–14 years of age who were in need of ART in Tanzania, 17% were started on ART.5
Because of their success in achieving high population coverage,11,12 routine immunization services have been used as a platform to deliver other health interventions, including vitamin A supplementation, deworming treatments, and insecticide-treated bed nets.13,14 Integrating other primary health care services with routine immunization visits is recommended in the World Health Organization and United Nations Children’s Fund’s Global Immunization Vision and Strategy, 2006–2015.15 In addition, the World Health Organization PMTCT Strategic Vision, 2010–2015, recommends integration of PMTCT services with maternal, newborn, and child health programs.16
In 2007, the Tanzania Ministry of Health and Social Welfare reported that coverage with the first dose of diphtheria toxoid, tetanus toxoid, and pertusis was 89% and recommended integration of HIV-related services with routine immunization visits, including transferring the HIV status for each mother from her antenatal care (ANC) card to her infant’s routine immunization record. During 2008, HIV-related services were integrated with routine infant immunization visits at a limited number of pilot sites.17 From April 2009 to March 2010, we assessed implementation of integrated HIV-related services at 4 urban heath centers and 4 rural health centers.
METHODS
Intervention Population
Four urban health centers [Kigamboni and Mbagala Round Table in Temeke District in Dar es Salaam, the capital city of Dar es Salaam Region, Butimba (renamed Nayamagana District Hospital in 2009) and Buzuruga in Nyamagana District in Mwanza, the capital city of Mwanza Region] and 4 rural health centers (Chole and Maneromango in Kisarawe District, Pwani Region, Hedaru and Ndungu in Same District, Kilimanjaro Region) were purposively selected as study sites in consultation with Tanzania Ministry of Health and Social Welfare district medical officers using the following criteria: on-site HIV testing for mothers, access to early infant HIV testing, access to adult and pediatric HIV care and treatment, and routine immunization services with ≥30 infant first-month visits per month (Fig. 1). The target study population included all infants attending routine first-month immunization visits between April 1, 2009 and March 31, 2010 at the selected sites. To minimize potential differences among mothers at study sites and mothers at nonstudy sites, all nonstudy health centers from the same district as study sites were used for comparison.
FIGURE 1.
Map of Tanzania and location of the 8 study sites.
Integration of Service Delivery
The routine childhood immunization schedule in Tanzania includes Bacille Calmette-Guérin vaccine (BCG) at birth; oral polio vaccine at birth (OPV0), 4 weeks (OPV1), 8 weeks (OPV2) and 12 weeks (OPV3); pentavalent diphtheria, tetanus toxoid, whole cell pertussis, hemophilus influenzae type B, and hepatitis B vaccine at 4 weeks (Penta1), 8 weeks (Penta2), and 12 weeks (Penta3); and measles vaccine at 9 months. Service providers at the study sites were trained to follow a protocol at first-month routine immunization visits, which included the following: (1) administering Penta1 and OPV1, (2) determining the mother’s HIV status from her ANC card, (3) offering HIV testing and counseling to mothers whose HIV status was not documented on her ANC card, (4) providing health education and infant feeding recommendations, (5) recording the mother’s HIV status on the infant’s health card, (6) registering infants of HIV-positive mothers as HIV exposed for follow-up services, and (7) among HIV-exposed infants, offering early infant diagnosis (EID) HIV testing using DNA polymerase chain reaction (PCR), initiating CTX, conducting a health assessment, and refering HIV-infected infants for immediate follow-up of HIV care and treatment.
Oral consent for HIV testing for both mother and infant was obtained and documented on the specimen testing form. For mothers, a serial rapid test algorithm for HIV-1 antibody testing on whole blood was performed on-site using SD Bioline HIV-1⁄2 3.0 (Standard Diagnostics Inc., Kyonggi-do, Korea) and Determine HIV-1⁄2 (Abbott Laboratories Abbott Park, IL) rapid tests. If the rapid tests were discordant, a con-firmatory test was done using Uni-Gold Recombigen (Trinity Biotech, Wicklow, Ireland). For EID, capillary blood was collected on dried blood spots using filter paper and transported to a regional laboratory for HIV-1 DNA PCR performed using the Abbott m2000 system Real-Time HIV-1 assay (Abbott Laboratories).18 A positive PCR test confirmed HIV infection by the detection of viral nucleic acid.
Data on infant and mother attendance, maternal HIV status, and services offered and received by mothers and infants were recorded by providers on monthly tally sheets. Infants determined to be HIV exposed were registered for follow-up care including monthly physical examinations, growth monitoring, replenishing CTX supply, and HIV testing again at 6 weeks after the cessation of breastfeeding. Monthly supervisory visits were conducted at each study site by the study coordinator.
Vaccine Doses Administered
The impact of integration of HIV-related services on routine immunization service delivery was evaluated by an analysis of the number of doses of each vaccine administered at the study sites. In Tanzania, service providers routinely use a tally sheet to record the number of doses of each vaccine administered. Doses administered during the study period (April 1, 2009 to March 31, 2010) were compared with doses administered during a baseline period (April 1, 2006 to March 31, 2008) before initiation of integrated services; there were no changes in the catchment area for immunization clinics, or of planned immunization, or other health services that would account for changes in the number of doses administered from year to year. For each participating district, we calculated the average monthly number of doses for each vaccine given at study sites and the aggregate average number of doses administered at all nonstudy health centers and dispensaries in the same district. The absolute change, from the baseline period to the study period, in the average monthly number of doses given at each study site was calculated and compared with the change for all nonstudy health centers in the participating district; a change of ± 5% was defined as “stable”. To account for potential variability in the average number of doses due to stock-outs, we conducted a sensitivity analysis excluding stock-out months, defined as a month in which the number of penta1 doses was less than half of the number of OPV1 doses or vice versa or the number of penta1 doses or OPV1 doses was <25% of the average monthly total.
Qualitative Assessment
During August 1–17, 2010, qualitative data were collected through indepth interviews with service providers who delivered the integrated services and mothers of children at each study site. At each site, 4–5 HIV-positive mothers, 4–5 HIV-negative mothers, and 2 service providers were interviewed by trained qualitative researchers from the Tanzania National Institute for Medical Research. Interview topics included the following: (1) awareness of the delivery method for integrating HIV services with immunization visits, (2) general perception of the integrated service and potential scale-up, (3) awareness of other mothers’ feelings toward integrated delivery method, (4) benefits of integrated delivery method, (5) challenges of integrated delivery method, and (6) recommendations for improving integrated delivery method.
Data Analysis
Quantitative data were entered into an electronic data base using Epi Info for Windows version 3.3.2 [US Centers for Disease Control and Prevention (CDC), Atlanta, GA], and analyzed using SAS version 9.3 (SAS Institute, Cary, NC). The number of vaccine doses administered during each month was entered and analyzed using a Microsoft Excel 2010 database for the baseline period and the study period for study sites and the aggregate for nonstudy health centers in each participating district where the study sites were located. Audio recordings of qualitative interviews were transcribed and translated from Swahili into English. Qualitative data were coded and analyzed using EZ-text version 3.06C (CDC, Atlanta, GA) to identify common themes.
Role of Funding Source
This research has been supported by US President’s Emergency Plan for AIDS Relief through the CDC. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC. The study protocol was approved by the CDC Institutional Review Board and the Tanzania National Institute for Medical Research.
RESULTS
Integrated Service Delivery
Table 1 provides a summary of the integrated service delivery data. Overall, 7423 (98%) of 7569 infants attending first-month vaccination services had mothers with known HIV status by ANC card. At urban sites, 278 (5%) of 6074 mothers with known HIV status were HIV positive; of the 128 mothers with unknown HIV status who accepted HIV testing, 15 (12%) were found to be HIV positive. At urban sites, of the 254 (93%) infants who were identified as HIV exposed and received HIV testing by PCR, 13 (5%) were HIV infected. At rural sites, 40 (3%) of the 1349 mothers with known HIV status were HIV positive; of the 13 mothers with unknown HIV status, 9 (69%) accepted HIV testing and 0 (0%) were found to be HIV positive. At rural sites, of the 36 (100%) infants who were identified as HIV exposed and received HIV testing by PCR, 2 (6%) were HIV infected.
TABLE 1.
Integrated HIV-Related Services Delivered at the First-Month Routine Immunization Visit Among Infants Attending First-Month Immunization Visits at 4 Urban and 4 Rural Study Sites, April 2009 to March 2010, Tanzania
| Urban, n = 6207 | Rural, n = 1362 | Rural, N = 7569 | ||||
|---|---|---|---|---|---|---|
| Variable | n | % | n | % | n | % |
| All mother–infant pairs | ||||||
| Mother’s HIV status by ANC card | ||||||
| Positive | 278 | 4.5 | 40 | 2.9 | 318 | 4.2 |
| Negative | 5796 | 93.4 | 1309 | 96.1 | 7105 | 93.9 |
| Unknown | 133 | 2.1 | 13 | 1.0 | 146 | 1.9 |
| n = 133 | n = 13 | N = 146 | ||||
| Mother–infant pairs with unknown maternal HIV status by ANC card | ||||||
| HIV counseling and testing of mothers | ||||||
| Yes | 128 | 96.2 | 9 | 69.2 | 137 | 93.8 |
| No | 5 | 3.8 | 4 | 30.8 | 9 | 6.2 |
| HIV test results of mothers* | ||||||
| Positive | 15 | 11.7 | 0 | 0.0 | 15 | 10.9 |
| Negative | 113 | 88.3 | 9 | 100.0 | 122 | 89.1 |
| Unknown | 0 | 0.0 | 0 | 0.0 | 0.0 | 0.0 |
| n = 293 | n = 40 | N = 333 | ||||
| Infants identified as HIV exposed | ||||||
| Registered for follow-up care† | ||||||
| Yes | 272 | 92.8 | 36 | 90.0 | 308 | 92.5 |
| No | 21 | 7.2 | 4 | 10.0 | 25 | 7.5 |
| n = 272 | n = 36 | N = 308 | ||||
| Registered infants identified as HIV exposed | ||||||
| Specimen collected and sent to laboratory for HIV testing by PCR | ||||||
| Yes | 254 | 93.4 | 36 | 100.0 | 290 | 94.2 |
| No | 18 | 6.6 | 0 | 0.0 | 18 | 5.8 |
| HIV PCR test results‡ | ||||||
| Positive | 13 | 5.1 | 2 | 5.6 | 15 | 5.2 |
| Negative | 238 | 93.7 | 34 | 94.4 | 272 | 93.8 |
| Unknown | 3 | 1.2 | 0 | 0.0 | 3 | 1.0 |
Five mothers at urban sites and 4 mothers at rural sites did not receive counseling and testing; therefore did not have results.
Information on the reasons for nonregistration was not collected.
Eighteen infants at urban sites did not have a specimen collected and sent to the laboratory; therefore did not have test results.
Vaccine Doses Administered
Figure 2 shows the changes in the average number of vaccine doses administered per month during study period compared with the baseline period at study sites and non-study sites. Vaccines assessed included those given during the infant’s first-month visit (Penta1, OPV1) and those administered later in life (Penta3, OPV3, measles). At the 8 study sites, overall first-month vaccine doses administered decreased 7% for Penta1 and 12% for OPV1, while vaccine doses administered later in life remained stable (+3%, −1%, and 0%). At the 4 urban study sites, first month vaccine doses remained stable (+2% and −4%) and vaccine doses administered later in life increased 12%, 8%, and 11%. At the 4 rural study sites first-month vaccine doses decreased 33% and 35% and vaccine doses administered later in life decreased 23%, 28%, and 28%. At nonstudy sites, overall first-month vaccine doses administered remained stable (0% and +1%) and vaccine doses administered later in life remained stable (−1%, −1%, and +4%). At urban nonstudy sites, only measles vaccine administered later in life increased 6%, while all others remained stable. At rural nonstudy sites, vaccine doses administered in the first month of life decreased 6% and 4% and vaccine doses administered later in life decreased 11%, 8% and 7%. When stock-out months were excluded, results were similar to results when the stock outs were included, in both urban and rural areas.
FIGURE 2.
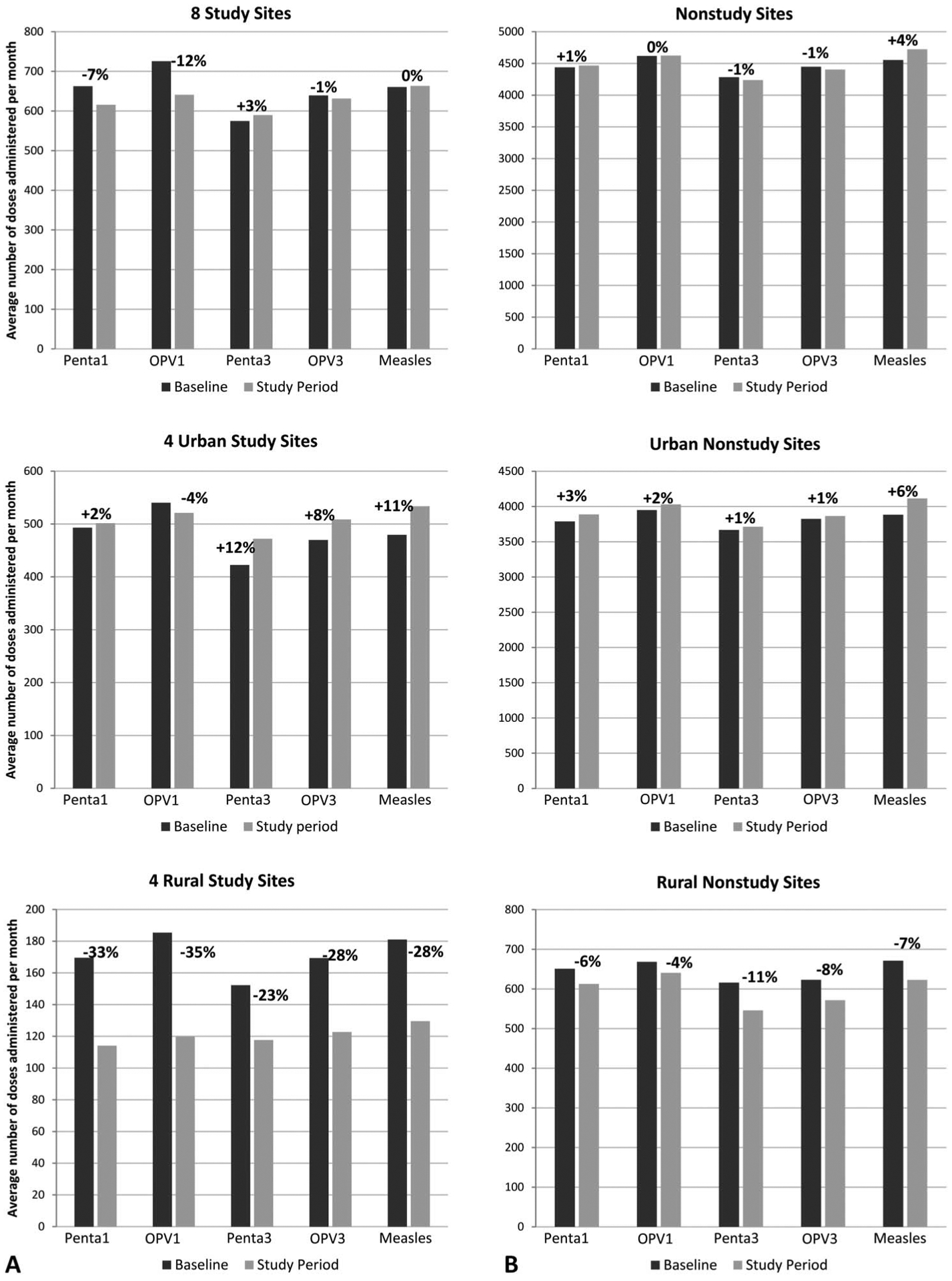
Average number of vaccine* doses administered per month during study period (April 1, 2009 to March 31, 2010) and baseline period (April 1, 2006 to March 31, 2008) at study sites (A) and nonstudy sites** in the same districts (B). Percentages indicate change in vaccine doses administered from baseline period to study period. *Penta1—pentavalent diphtheria, tetanus toxoid, whole cell pertussis, haemophilus influenzae type B, and hepatitis B vaccination at 4 weeks of age; OPV1—oral polio vaccination at 4 weeks of age, Penta3—pentava-lent diphtheria, tetanus toxoid, whole cell pertussis, haemophilus influenzae type B, and hepatitis B vaccination at 12 weeks of age; OPV3—oral polio vaccination at 4 weeks of age 12 weeks of age; Measles—measles vaccination at 9 months of age. **At the start of the baseline period and of the study period, the number of nonstudy sites was 146 and 148, urban nonstudy sites was 93 and 93, and rural non-study sites was 53 and 55.
Qualitative Assessment
Sixty-six mothers (34 at urban health centers, 32 at rural health centers) and 16 providers (2 per health center) were interviewed. Among mothers, 32 (48%) were HIV positive and 34 (52%) were HIV negative. Mothers and service providers were generally in favor of integrating HIV related services with routine immunization visits. Benefits reported by mothers included time savings, having an opportunity to learn their HIV status and the HIV status of their infants, and having an opportunity to receive HIV care and ART. The primary benefit mentioned by service providers was the ability to reach mothers who likely would not attend clinics administering standalone HIV testing and services.
Mothers perceived service providers as trustworthy for maintaining confidentiality. However, mothers, especially at rural sites, expressed fear of HIV-related stigma because of perceived inadequate privacy during HIV testing and the physical layout and patient flow of the sites. Several mothers reported knowing other mothers who did not bring infants to the first vaccination visit because of fear of being identified in the community as being HIV positive and fear of conflict if their spouse or partner discovered they had been tested for HIV. At urban sites, mothers complained of long wait times. Finally, some mothers and service providers reported a perception that HIV testing was required for infants to receive first-month immunizations.
DISCUSSION
In 2000, the United Nations General Assembly adopted a series of Millennium Development Goals; the target of goal six focuses on combatting HIV/AIDS, malaria, and other diseases.19 During 2005–2009, the estimated number of infants who received ART globally, increased from 75,000 to 356,400; however, only 28% of children in need received ART.20 In December 2010, the Joint United Nations Program on HIV/AIDS established goals to achieve virtual elimination of mother-to-child HIV transmission and to provide universal ART access to children in need by 2015.1,2 To achieve these goals, innovative strategies to integrate HIV-related services with existing maternal and child health services are being implemented.2,21 However, it is critical to ensure that these activities lead to synergy and strengthening of existing service delivery platforms along with increased HIV-related services.22
We found that 98% of mothers who brought their infants to first-month routine immunization visits had a previously documented HIV test result, and HIV testing was accepted by mothers with unknown status. These findings indicate the success of efforts to scale-up PMTCT services through ANC clinics. Study sites successfully identified and registered 308 HIV-exposed infants, among whom, 5% were confirmed to be HIV infected. These results show that using the routine immunization service delivery platform to identify exposed and infected infants, which is the first step in ensuring optimal care and treatment for these infants, might be feasible. However, nearly all mothers arrived with documented HIV status, suggesting an unusually high uptake of prenatal HIV testing. In other settings with a higher proportion of mothers arriving with an unknown HIV status, the need and workload for HIV testing would be greater; the acceptance of integrated services and impact on immunizations should be carefully evaluated in these settings. A follow-up analysis of dropout rates, administration of ART, and clinical outcomes of registered infants is ongoing at the study sites.
Although the success of integrating HIV-related services with routine immunization service delivery seems to be promising for identification of HIV-infected infants; the implications for the routine immunization program need to be carefully considered. In particular, at the rural clinics, we found substantial decreases in the number of vaccine doses administered during the study period compared with the baseline period and we are unaware of any policy or programmatic changes effecting routine immunization services or other health care services that might have accounted for these changes. Similar to our results, previous studies in Tanzania have reported HIV-related fear and stigma and distrust among rural communities as major challenges to delivering HIV services.23–25 In our study, concern regarding a lack of confidentiality was found to be an important factor that might undermine acceptability of integrating EID and HIV services with routine immunizations. In addition, there was a misperception among mothers that having a documented HIV status or accepting an HIV test was required to receive immunization services. This might motivate mothers with unknown HIV status to seek immunizations at other sites or not seek immunization services at all, partially explaining why there was decreased vaccination at the first visit in the rural sites and why an unexpectedly high percentage (98%) of mothers attending study sites arrived with known HIV status. Addressing these concerns is critical to ensure that all mothers bring their infants to the nearest immunization clinic to benefit from services.
These study findings should be considered in light of limitations. First, site-specific tallies of the numbers of vaccination doses administered may not be representative of vaccination coverage in the population if some parents chose to bring their infants to a nonparticipating site to avoid HIV testing; however, continuity of care is critical to optimal health care provision, and if integrated services were scaled up nationwide, then nonparticipating alternatives may not exist. Second, site-specific data from each nonstudy site were not available; therefore, study sites could not be compared with each individual nonstudy site in the same district to assess the number of vaccinations delivered at nonstudy sites in close proximity to study sites that potentially could suggest nonstudy site preference by mothers. Third, qualitative assessment findings may not be representative of all study participants and did not assess practices of mothers who did not bring infants to study sites for vaccination visits or follow-up care. Finally, the provision of immunizations may be affected by stock-outs of vaccines or other supplies and the difference in the decreases between Penta1 and OPV1, both first-month vaccinations, was likely due to differences in stock availability. A sensitivity analysis for all, urban, and rural study sites accounting for stock-out variability had similar results to those found in our primary analyses.
Few published studies have evaluated integration of HIV services with routine immunization services.26,27 In South Africa,27 acceptance by mothers of HIV testing at immunization visits was observed in one high HIV prevalence setting. Another South African study found EID of HIV using dried blood spot testing at the first-month immunization visit to be feasible.26 Evaluations in Rwanda and Haiti suggested no negative impact on service delivery when health care provider training, medical supplies, and revitalization of physical structures were provided at the time integrated services were first implemented.28,29
In general, we found that interviewed mothers and service providers were in favor of the integrated service delivery method due to time savings and the wide reach of the immunization program; however, several key challenges were identified. Based on results from this study, more work is needed to address community stigma and perceived confidentiality issues, particularly in rural settings. Studies are needed to assess the knowledge, attitudes, and practices of mothers who did not bring infants to vaccination visits or follow-up care. Workflow studies should be conducted to improve confidentiality and reduce wait times. Finally, efforts are needed to ensure that providers and mothers understand that opting out of HIV testing does not preclude their infants from receiving routine immunizations. As countries consider integrating additional services into successfully established immunization service delivery platforms, it is critical to monitor implementation and prevent erosion of hard-won public trust, acceptability, and workforce development for successful infant immunization programs built over decades of local efforts and global support.
ACKNOWLEDGMENTS
The authors would like to recognize the great efforts of all maternal and child health staff in Tanzania. the authors thank former Tanzania EPI manager, Mary Kitambi for her support. We are grateful for guidance from Lisa Cairns from the Global Immunization Division at the CDC and Nathan Shaffer at the World Health Organization for their guidance and support. The authors also acknowledge the support from John Vertefeuille, former CDC Tanzania country director. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
The US Centers for Disease Control and Prevention provided funding and participated in study design, data analysis, report writing, and decisions to submit the article for publication.
Footnotes
The authors have no conflicts of interest to disclose.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
The authors do not have a financial or proprietary interest in a product, method, or material or lack thereof.
REFERENCES
- 1.UNAIDS. Getting to zero: 2011–2015 strategy Joint United Nations Programme on HIV/AIDS. 2010. Available at: http://www.unaids.org/en/media/unaids/contentassets/documents/unaidspublication/2010/JC2034_UNAIDS_Strategy_en.pdf. Accessed March 14, 2012.
- 2.UNICEF. Children and AIDS: Fifth Stocktaking Report: UNICEF, UNAIDS, WHO, UNFPA and UNESCO 2010. 2011. Available at: http://www.unicef.org/publications/index_57005.html. Accessed March 14, 2012.
- 3.Newell ML, Brahmbhatt H, Ghys P. Child mortality and HIV infection in Africa: a review. AIDS. 2004;18(suppl 2):S27–S34. [DOI] [PubMed] [Google Scholar]
- 4.Newell M, Coovadia H, Cortina-Borja M, et al. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: a pooled analysis. Lancet. 2004;364:1236–1243. [DOI] [PubMed] [Google Scholar]
- 5.UNAIDS. UNAIDS report on the global AIDS epidemic, 2010 Available at: http://www.unaids.org/globalreport/Global_report.htm. Accessed March 14, 2012.
- 6.World Health Organization. Report of the WHO Technical Reference Group, Paediatric HIV/ART Care Guideline Group Meeting. Geneva, Switzerland: World Health Organization; April 10–11, 2008. Available at: http://www.who.int/hiv/pub/paediatric/WHO_Paediatric_ART_guideline_rev_mreport_2008.pdf. Accessed March 14, 2012. [Google Scholar]
- 7.Kellerman S, Essajee S. HIV testing for children in resource-limited settings: what are we waiting for? PLoS Med. 2010;7:e1000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanzania Commission for AIDS. UNGASS Country Progress Report Tanzania Mainland. Dar es Salaam, Tanzania: UNAIDS; 2008. Available at: http://data.unaids.org/pub/Report/2008/tanzania_2008_country_progress_report_en.pdf. Accessed March 14, 2012. [Google Scholar]
- 9.The United Republic of Tanzania Ministry of Health and Social Welfare. The Tanzania health sector strategic plan, 2009–2015. Available at: http://www.scribd.com/doc/30040268/Tanzania-Health-Sector-Strategic-Plan-III. Accessed March 14, 2012.
- 10.The United Republic of Tanzania Ministry of Health and Social Welfare. 2010 National PMTCT annual report. 2011.
- 11.Victora C, Fenn B, Bryce J, et al. Co-coverage of preventive interventions and implications for child-survival strategies: evidence from national surveys. Lancet. 2005;366:1460–1466. [DOI] [PubMed] [Google Scholar]
- 12.Arevshatian L, Clements C, Lwanga S, et al. An evaluation of infant immunization in Africa: is a transformation in progress? Bull World Health Organ. 2007;85:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuchat A, De Cock KM. The value of science in integration of services. J Infect Dis. 2012;205(suppl 1):S1–S3. [DOI] [PubMed] [Google Scholar]
- 14.Wallace A, Ryman T, Dietz V. Experiences integrating delivery of maternal and child health services with childhood immunization programs: systematic review update. J Infect Dis. 2012;205(suppl 1):S6–S19. [DOI] [PubMed] [Google Scholar]
- 15.World Health Organization, United Nations Children’s Fund. Global immunization vision and strategy. 2005. Available at: http://www.who.int/immunization/givs/en/index.html. Accessed May 3, 2012. [Google Scholar]
- 16.World Health Organization. PMTCT strategic vision 2010–2015: preventing mother-to-child transmission of HIV to reach the UNGASS and Millennium Development Goals. Available at: http://www.who.int/hiv/pub/mtct/strategic_vision.pdf. Accessed May 3, 2012.
- 17.Nuwagaba-Biribonwoha H, Werq-Semo B, Abdallah A, et al. Introducing a multi-site program for early diagnosis of HIV infection among HIV-exposed infants in Tanzania. BMC Pediatr. 2010;10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lofgren S, Morrissey A, Chevallier C, et al. Evaluation of a dried blood spot HIV-1 RNA program for early infant diagnosis and viral load monitoring at rural and remote healthcare facilities. AIDS. 2009;23:2459–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.United Nations. The Millennium Development Goals Report 2009. 2009. Available at: http://mdgs.un.org/unsd/mdg/Resources/Static/Products/Progress2009/MDG_Report_2009_En.pdf. Accessed March 14, 2012.
- 20.World Health Organization. Towards Universal Access—Scaling Up Priority HIV/AIDS Interventions in the Health Sector 2010. Geneva, Switzerland: WHO; 2010. [Google Scholar]
- 21.Ferguson L, Grant AD, Watson-Jones D, et al. Linking women who test HIV-positive in pregnancy-related services to long-term HIV care and treatment services: a systematic review. Trop Med Int Health. 2012;17: 564–580. doi: 10.1111/j.1365-3156.2012.02958.x). [DOI] [PubMed] [Google Scholar]
- 22.De Cock K, El Sadr W, Ghebreyesus T. Game changers: why did the scale-up of HIV treatment work despite weak health systems? J Acquir Immune Defic Syndr. 2011;57(suppl 2):S61–S63. [DOI] [PubMed] [Google Scholar]
- 23.Ostermann J, Reddy E, Shorter M, et al. Who tests, who doesn’t, and why? Uptake of mobile HIV counseling and testing in the Kilimanjaro Region of Tanzania. PLoS One. 2011;6:e16488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Njau B, Watt MH, Ostermann J, et al. Perceived acceptability of home-based couples voluntary HIV counseling and testing in Northern Tanzania. AIDS Care. 2012;24:413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Agnarson AM, Masanja H, Ekström AM, et al. Challenges to ART scale-up in a rural district in Tanzania: stigma and distrust among Tanzanian health care workers, people living with HIV and community members. Trop Med Int Health. 2010;15:1000–1007. [DOI] [PubMed] [Google Scholar]
- 26.Rollins N, Little K, Mzolo S, et al. Surveillance of mother-to-child transmission prevention programmes at immunization clinics: the case for universal screening. AIDS. 2007;21:1341–1347. [DOI] [PubMed] [Google Scholar]
- 27.Rollins N, Mzolo S, Moodley T, et al. Universal HIV testing of infants at immunization clinics: an acceptable and feasible approach for early infant diagnosis in high HIV prevalence settings. AIDS. 2009;23: 1851–1857. [DOI] [PubMed] [Google Scholar]
- 28.Price J, Leslie J, Welsh M, et al. Integrating HIV clinical services into primary health care in Rwanda: a measure of quantitative effects. AIDS Care. 2009;21:608–614. [DOI] [PubMed] [Google Scholar]
- 29.Walton D, Farmer P, Lambert W, et al. Integrated HIV prevention and care strengthens primary health care: lessons from rural Haiti. J Public Health Policy. 2004;25:137–158. [DOI] [PubMed] [Google Scholar]


