Abstract
Introduction
Many countries have begun immunization programs and established protocols to combat pandemics caused by the SARS-CoV-2 virus. Six months after vaccination, the antibody titers produced by the immunization begin to decline, and individuals whose first immunization (either one or two doses) did not provide adequate protection may require a booster dose.
Methods
A quantitative cross-sectional survey of 18-year-olds and older was undertaken in the West Bank from June 15 to June 27, 2022. Each participant had 5 mL of blood drawn to be tested for IgG-S, IgG-N, and blood group.
Results
All participants had positive IgG-S results; IgG-S values ranged between 77 and 40,000 AU/ml, with a mean value of 1254 AU/ml. The value of IgG-N ranged from 0 to 139.3 U/ml for all participants, with a mean value of 22.4 U/ml. 64 (37.2%) of the participants demonstrated positive IgG-N screening results, with mean values of 51.2 U/ml. Female participants' mean IgG concentration was higher than male participants. Furthermore, the results revealed that smokers had lower levels of vaccine-induced antibodies than nonsmokers. High significance was found in the time from the last vaccine till the blood sample test (T = 3.848, P < .001), and the group between 6 and 9 months was found to have higher mean values than the 9-months group (M = 15952).
Conclusions
Participants vaccinated with a higher number of vaccines tend to have higher IgG-S. To elevate total antibodies, booster doses are essential. Additional researchers are needed to examine the positive correlation between IgG-S and IgG-N.
Keywords: SARS-CoV-2, Booster vaccines, COVID-19, IgG-S, IgG-N
1. Introduction
Several countries have started immunization programs and established protocols to counteract the pandemics caused by the COVID-19-causing SARS-CoV-2 virus. A worldwide vaccination program has been successfully launched.1 Herd immunity and reducing the risk of complications from the SARS-CoV-2 virus were the primary priorities of immunization. Vaccines' primary mode of action is stimulating the body to produce antibodies to recognize the SARS-CoV-2 spike (S) protein located on the virus's surface.2
The antibody titers produced by the immunization start to decline six months post-vaccinations, leading to an increased risk of SARS-CoV-2 infections.3, 4, 5, 6 Therefore, most vaccines (Pfizer-BioNTech's, Moderna, AstraZeneca, Sinovac, Sputnik V, and Sputnik Light) provide immunity for approximately six months after completing the total recommended vaccine dosage.7 For the above reasons, the administration of a COVID-19 immunization booster dose (third dose) was approved by the United States Food and Drug Administration (FDA) for individuals 65 years of age and older, after the completion of 6 months from the second dose. Moreover, health workers with a high risk of exposure to SARS-CoV-2 and adults with a greater probability of developing COVID-19 infection were also eligible for the booster dose.8 Additionally, certain individuals whose first immunization (either one or two doses, depending on the product) did not adequately protect them might need a booster dose.
According to studies, those 60 years of age and older who received a booster dose (third dose) of the BNT162b2 vaccine significantly reduced the incidence of serious illness and confirmed COVID-19.9, 10, 11 Recent studies show a significant drop in IgG and total antibodies six months after receiving the BNT162b2b vaccination.12 , 13
The SARS-CoV-2 IgG protein S test utilizes a quantitative method to detect IgG antibodies that are targeted against the region of the SARS-CoV-2 protein that binds to receptors, allowing for the evaluation of the immune response.14 While antibodies to the S protein indicate a previous infection or vaccination, the presence of antibodies to the N protein indicates prior COVID-19.15 , 16 Detection of the S protein but not the N protein in a sample indicated a vaccination in a person who has never been infected, or it might indicate a prior infection in a person whose N protein antibodies have dropped. In those whose infection histories are unclear, measuring S-protein IgG antibody levels right before the second dose of the vaccine may help figure out whether a second dose is necessary.17, 18, 19
In Palestine, vaccination programs with various vaccines started more than two years ago, and as of today, almost 2 million individuals have received vaccinations20 . IgG-N protein levels after infection and IgG-S protein responses to COVID-19 boosters have not been extensively studied. Particularly for individuals who passed away 6 months after receiving the second dose, the effectiveness of vaccines is a widely debated subject. Studies to assess if additional doses of the COVID-19 vaccine are necessary or how long these vaccines will provide immunity to people are still being conducted globally. This study aimed to evaluate the antibody titers rate of the COVID-19 vaccine by evaluating the antibody IgG-S protein and N protein in uninfected SARS-CoV-2 individuals and post-vaccination for at least 6 months after vaccination, in the West Bank, Palestine.
2. Methods
2.1. Study design and sampling
A cross-sectional study was carried out at Birzeit University in Palestine between June 15 and June 27, 2022. The blood was collected from participants aged 18 years and older of both sexes. Participants in this study must have no prior history of COVID-19 infection or have not received the COVID vaccination within six months of their enrollment in the study. Furthermore, the study included people who had received the booster dose during the six months before the participation date. Exclusion criteria for the study were those who have not received any vaccinations as well as those who had a positive Polymerase chain reaction (PCR) test within the previous six months of the study to be able to evaluate the positive results of IgG not related to recent infection and vaccination which remain IgG positive for at least 6 months.21, 22, 23, 24
Through advertisements on the university's website and Facebook pages, the public was enrolled in the study. Daily sampling operations were carried out throughout the study which were done by qualified staff nurses and laboratory technicians from research staff. Using the equation with a 90% level of confidence and a 5% error margin, consisting of 271 West Bank residents based on the total West Bank residents whom vaccinated (two million), study had 180 participants (66.4%), but 8 surveys were excluded from the data analysis due to hemolysis of the blood sample, so the actual sample size was 172 participants. The sample size was calculated by the Raosoft sample size calculator website.25
2.2. Data collection procedure and ethical consideration
Each participant's demographic information, such as age, sex, marital status, location of residence, smoking, vaccination kinds and dates of use, chronic conditions, number of medicines, and allergies, was collected before a blood sample was obtained. Additionally, before extracting blood, each participant checked his blood pressure and pulse. All participants signed a consent form outlining the background and goals of the study, its commitment to confidentiality, and the rights to participate, decline, or withdraw from the study. All participants also received their blood group and Immunoglobin G (S and N protein) results. The Institutional Review Board (IRB) committee of Birzeit University with reference number BZU-PNH-2131.
2.3. Instruments
Each participant had two blood tubes drawn at the same time, one EDTA tube (for Blood grouping) and one plain tube were used for the measurement of Immunoglobulin levels. Plain tubes were centrifuged at 5000 rpm for 10 min at room temperature, then the serum from each tube was put in 12 × 75 mm plastic tubes with caps and stored in the refrigerator. Participants's coded plasma was sent to the Medicare Ramallah Central Lab in the West Bank, in Palestine, where Immunoglobulin G (S and N protein) levels were checked. Levels of CoV-19 Ab-IgG protein S were determined by using the SARS-CoV-2 IgG kit (Abbot Company, Cat No. 6560G, USA). The instrument machine is an Architect CI 4100 (Abbott Company, USA). For measurement of COVID-19 N- Protein Human IgG levels, SARS-CoV-2 NP IgG kit (Aeskulisa The company, Cat No. 6122, Germany) was used, while the instrument used was Stat Fax 303 plus Strip Reader (GMI Trusted Laboratory Solutions Company, USA). Antibodies against COVID-19 IgG were quantified as arbitrary units (AU) per milliliter (positive test at or above 50 AU/ml; the maximum allowed value is 40 000 AU/ml). The COVID-19 N-Protein Human IgG is expressed as Units/mL (U/ml), with negative findings below 8 U/ml, the borderline between 8 and 12U/ml, and positive values exceeding 12 U/ml.
2.4. Statistical analysis
To ascertain and assess the participants' demographic and other distinctive features, the data were analyzed using SPSS Version 25, employing frequencies, percentages, means, and standard deviations (SD). Additionally, a one-way ANOVA test and an independent T-test were used to determine whether there are any statistically significant differences between IgG-S or IgG –N and the sociodemographic characteristics of the participant. Furthermore, Pearson's correlation coefficient test was used to assess the statistical association between IgG-S and IgG-N and a Bivariate correlation between IgG –S and vaccine variables.
3. Results
3.1. Sociodemographic characteristics of participants
Blood samples were collected from 180 participants. However, due to hemolysis of the blood sample, 8 survey were not included in the data analysis. The total number of participants in this study was 172. According to Table 1 , 99 respondents (57.6%) were married, and 104 (60.5%) of the participants lived in rural areas. Participants' average age was 39.7 years, and 94 (54.7%) of them were males. Between 6 and 9 months, before the blood draws for this study, 90 (52.3%) of the individuals had received the vaccination.
Table 1.
Sociodemographic characteristics of the participant (N = 172).
| Sociodemographic characteristics | N (%) | |
|---|---|---|
| Gender | Male | 94 (54.7) |
| Female | 78 (45.3) | |
| Age (years) | 18–30 | 63 (36.6) |
| 31–50 | 61(35.5) | |
| More than 50 | 48(27.9) | |
| Marital status | Married | 99(57.6) |
| Single | 66(38.4) | |
| Divorced | 3(1.7) | |
| Widowed | 4(2.3) | |
| Place of residence | Urban | 68(39.5) |
| Rural | 104(60.5) | |
| Time from the last vaccine till the blood sample test (months) | 6–9 | 90(52.3) |
| More than 9 | 82(47.7) | |
3.2. Data of participants
Table 2 summarizes the medical history of the participants. The first vaccination dose was provided to all participants 172 (100%) and 51 (29.7%) participants received the third dose. There were 68 (39.5%) smokers overall among the participants, according to the lifestyle questions. Moreover, the majority of participants were not experiencing any chronic illnesses.
Table 2.
Medical data of the participants (N = 172).
| Statement | Yes (%) | No (%) |
| Are you currently a smoker? | 68(39.5) | 104(60.5) |
| Have you had any positive PCR test during the beginning of the year 2022? | 0 | 172(100) |
| Have you had any vaccination since the beginning of the year 2022? | 0 | 172(100) |
| Did you receive the first dose of the vaccination? | 172(100) | 0 |
| Did you receive the second dose of the vaccination? | 158(91.9) | 14(8.1) |
| Did you receive the third dose (Booster) dose of the vaccination? | 51(29.7) | 121(70.3) |
| Do you have diabetes mellitus disease? | 15(8.7) | 157(91.3) |
| Do you have cardiac disease? | 16(9.3) | 156(90.7) |
| Do you have hypertension? | 20(11.6) | 152(88.4) |
| Do you have chronic obstructive pulmonary disease? | 2(1.2) | 170(98.8) |
| Do you have a malignant tumor? | 2(1.2) | 170(98.8) |
| Do you have a gastrointestinal disease? | 3(1.7) | 169(98.3) |
| Do you have thyroid disease? | 4(2.3) | 168(97.7) |
| Do you have an autoimmune disease? | 1(0.6) | 171(99.4) |
| Do you have a rheumatoid or joint disease? | 5(2.9) | 167(97.1) |
| Do you have an allergy to medication? | 12(7) | 160(93) |
| Do you have a neuro or mental disease? | 1(0.6) | 171(99.4) |
| Do you take any medication regularly? | 58(33.7) | 114(66.3) |
| Do you take one type of medication regularly? | 30(17.4) | 142(82.6) |
| Do you take two types of medications regularly? | 11(6.4) | 161(93.6) |
| Do you take more than two medications regularly? | 17(9.9) | 155(90.1) |
3.3. Vaccination types administered to participants
Once the various forms of vaccination were considered, six types were identified: Pfizer-BioNTech, Moderna, AstraZeneca, Sinovac, Sputnik V, and Sputnik Light (Table 3 ). In addition, three different dosages were made accessible to the general population. The first dosage of vaccination had been administered to all participants, and 158 (91.8%) received the second dose. Regarding the type of vaccine, the findings revealed that Pfizer-BioNTech was the most widely used vaccine for the first and second doses, followed by Sputnik V, and AstraZeneca was the least popular.
Table 3.
Vaccinations provided to participants (N = 172).
| First dose | N(%) | Second dose | N(%) | Third dose (Booster) | N(%) |
|---|---|---|---|---|---|
| Pfizer-BioNTech's | 91(52.9) | Pfizer-BioNTech's | 91(52.9) | Pfizer-BioNTech's | 51(29.7) |
| Sputnik V | 50(29.1) | Sputnik V | 50(29.1) | ||
| Sinovac | 10(5.8) | Sinovac | 10(5.8) | ||
| Sputnik light | 14(8.2) | ||||
| Moderna | 4(2.3) | Moderna | 4(2.3) | ||
| AstraZeneca | 3(1.7) | AstraZeneca | 3(1.7) | ||
| Total | 172(100) | 158(91.8) | 51(29.7) |
3.4. Participants’ blood group and rhesus (Rh) factor
Depending on their ABO blood group and rhesus factor, the participant's blood group was divided into 8 different blood groups, including A+, A, B+, B-, AB+, AB-, O+, and O-. Fig. 1 demonstrates that participants have a high frequency of blood group O+, followed by A+ and B+, while none of the participants have blood group AB-.
Fig. 1.
Distribution of ABO and Rh blood groups between participants.
3.5. IgG-S and IgG-N protein results
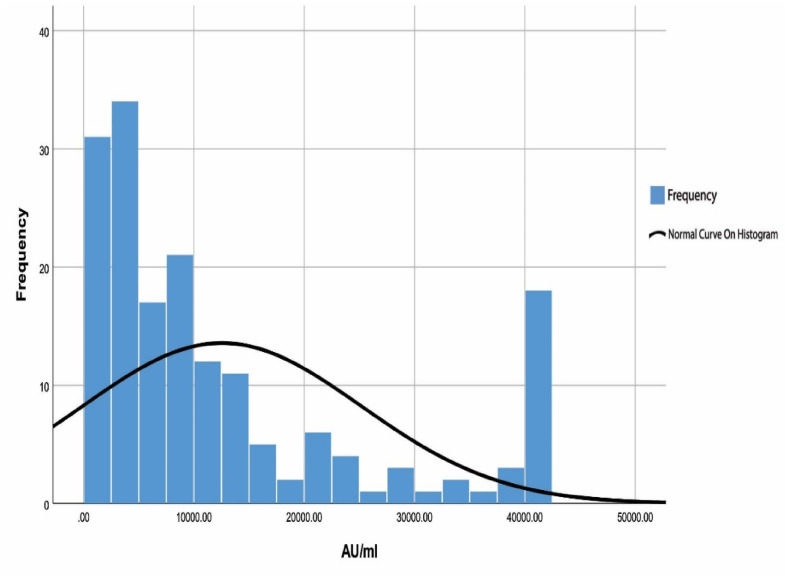
To assess each participant's response to the COVID-19 infection and vaccinations, two blood tests were conducted. The results of the laboratory testing indicated that all participants had positive IgG-S results (Fig. 2 ). IgG-S values ranged between 77 and 40,000 AU/ml, with a mean value of 12547AU/m. Regarding IgG-N, the findings showed that the value ranged from 0 to 139.3 U/ml for all participants, with a mean value of 22.4 U/ml (Fig. 3 ). Moreover, 64 (37.2%) of the participants demonstrated positive IgG-N screening results, with mean values of 51.2 U/ml and ranging between 12 and 139.3 U/ml.
Fig. 2.
Participants IgG Spike protein, N = 172. Note: Positive test at or above 50 AU/ml.
Fig. 3.
Participants IgG Nucleocapsid Protein Spike protein, N = 172
Note: Negative results below 8 U/ml, the borderline results between 8 and 12U/ml, and positive results exceeding 12 U/ml.
3.6. Sociodemographic characteristics of participants and IgG spike protein results
IgG concentrations varied significantly among male and female participants, as shown in Table 4 , where female participants' mean IgG concentration was higher than male participants' (T = −1.278, P = .005). The antibody concentration was higher and significantly different in participants above the age of 50 compared to those under this age. As anticipated, participants who received their vaccinations between the periods of 6 and 9 months had significantly higher IgG-S concentrations than those who had their vaccinations after 9 months (T = 3.848, P < .001). The results showed that the IgG-S concentrations were greater in participants who had received three vaccination doses, followed by two doses, and the lowest concentrations were for those who had received just one dose (F = 14.906, P < .001).
Table 4.
Comparison between the Sociodemographic Characteristics of participants and IgG Spike protein results and IgG Nucleocapsid Protein.
| Variable | Values | Mean AU/ml (SD) IgG-S |
F/T value IgG-S |
p-value IgG-S |
Mean U/ml (SD) IgG-N |
F/T value IgG –N |
p-value IgG –N |
|---|---|---|---|---|---|---|---|
| Gender | Male | 11427 (11583) | T = −1.278 | P = .005 | 24.5(36.9) | T = 0.913 | P = .052 |
| Female | 13897(13767) | 19.8(28.8) | |||||
| Age (years) | 18–30 | 12066(11623) | F = 3.327 | P = .038 | 15.3(24.12) | F = 2.426 | P = .091 |
| 31–50 | 10195(10810) | 25.3(32.9) | |||||
| More than 50 | 16385(15388) | 28.3(42.9) | |||||
| Marital status | Married | 11620(12126) | F = 1.556 | P = .202 | 25.3(36) | F = 0.957 | P = .415 |
| Single | 13133(12540) | 18.3(29.3) | |||||
| Divorced | 26723(22996) | 35.2(46.3) | |||||
| Widowed | 15200(17355) | 8.6(11.9) | |||||
| Place of residence | Urban | 13883(13940) | T = 1.122 | P = .019 | 23.8(33.9) | T = 0.439 | P = .580 |
| Rural | 11673(11704) | 21.5(33.2) | |||||
| Time from the last vaccine till the blood sample test (months) | 6–9 | 15952(13749) | T = 3.848 | P < .001 | 24.25(36.5) | T = 0.775 | P = .176 |
| More than 9 | 8809(10133) | 20.29(29.8) | |||||
| Are you currently a smoker? | Yes | 11219(12094) | T = −1.115 | P = .238 | 24.2(35.5) | T = 0.327 | P = .327 |
| No | 13415(12972) | 21.2(32.2) | |||||
| Participants blood group | O | 11266(12878) | F = 0.628 | P = .598 | 22.7(33.6) | F = 1.107 | P = .348 |
| A | 13130(11830) | 21.9(33.8) | |||||
| B | 15115(14124) | 28.7(36.4) | |||||
| AB | 13054(13051) | 3.8(3.4) | |||||
| Number of vaccination doses | One dose | 5319(4075) | F = 14.906 | P < .00 | 20.48(32.97) | F = 1.809 | P = .167 |
| Two doses | 10031(10570) | 19.07(28.12) | |||||
| Three doses | 19808(14948) | 29.76(42.16) |
Note: one-way ANOVA test and an independent T-test, SD: Standard Deviation; the P value ≤ .05 indicates significant, IgG-S IgG Spike protein, IgG-N: IgG Nucleocapsid Protein.
3.7. Sociodemographic characteristics of participants and IgG nucleocapsid protein
The comparison among participants socioeconomic characteristics and IgG-N concentrations is shown in Table 4. In the current study, there were no statistically significant differences between participant socioeconomic characteristics and IgG-N concentration. It is interesting to observe that the mean IgG-N values were higher for males, participants over 50, and participants who received their vaccinations between 6 and 9 months earlier than they were for other comparable groups.
3.8. Correlation between IgG spike protein and IgG nucleocapsid protein
The correlation between IgG-S and IgG-N was found to be significant (less than 0.01) and positive. As a result, IgG-S will increase in response to an increase in IgG-N (Table 5 ).
Table 5.
Pearson's correlation between IgG –S and IgG –N (N = 172).
| Test | Mean | SD | Pearson's Correlation Sig. (2-tailed) (r) |
p-value |
|---|---|---|---|---|
| IgG Spike protein | 12547 | 12642 | 0.224** | .003 |
| IgG Nucleocapsid Protein | 22.4 | 33.4 | 0.224** | .003 |
Note: ** Correlation is significant at the 0.01 level (2-tailed).
3.9. Correlation between IgG spike protein and vaccines variables
A Bivariate Pearson correlation indicated a positive moderate correlation between IgG-S results and a total number of vaccines (r = .378, p < .01). Which means that participants with a higher number of vaccines tend to have higher IgG-S. Moreover, the results indicated a moderate to high positive correlation between IgG-S results and the total number of Pfizer-BioNTech's vaccines (r = .445, p < .001). This means participants vaccinated with a higher number of vaccines tend to have higher IgG-S. However, there is a negative moderate association between IgG –S and time from the last vaccination (r −0.260, p < .01), which means that IgG-S results tend to decrease as the time from the last vaccination increases, as shown in (Table 6 ).
Table 6.
Correlation between IgG spike protein and vaccines variables.
| No. of vaccines (N = 172) | No. of Pfizer-BioNTech's vaccines (N = 91) | Time from last vaccination (N = 172) | |
|---|---|---|---|
| IgG spike protein(r) | .378 | .445 | −.260 |
| p | <.001 | <.001 | .001 |
4. Discussion
Following vaccination, there is a decreased probability that a patient might experience severe disease symptoms, need oxygen therapy, or be hospitalized in the critical care unit; nevertheless, COVID-19 boosters are required to raise total antibodies. However, the results of the current study revealed a correlation between the antibody concentration and the number of doses received as well as the period since vaccination. All subjects who received their vaccines between the period of 6 and 9 months had significantly higher IgG-S concentrations than those who received the vaccination after 9 months. These results are in line with previous research.10 , 13 , 26 The IgG-S levels, meanwhile, were highest in participants who had received three vaccination doses, followed by two doses, and were lowest in participants who had only received one dose.27 , 28
In the current study, it was found that females had higher IgG-S concentrations than males. In the meanwhile, since the results of previous studies are contradictory, more investigation is necessary to establish the relationship between gender and protein concentration. Upon reviewing earlier research, some of these researches showed results that were similar to those of the current study,19 , 29 while other studies indicated that the mean value for all types of vaccines showed no significant differences in IgG-S for vaccinated males and females.30 Inversely, IgG-S concentration was observed to be significantly more in males than in females with Pfizer and AstraZeneca.31
People living in urban areas had significantly higher levels of IgG-S than those living in rural areas, which is consistent with previous studies.32, 33, 34, 35 It might be a result of the accessibility of immunization facilities in urban Palestine. Furthermore, the results revealed that smokers had lower levels of vaccine-induced antibodies than nonsmokers as well as an increase in IgG-N levels in smoker individuals due to the elevated susceptibility to infection, which is consistent with earlier research.36, 37, 38, 39 More research is needed into the mechanisms through which cigarette smoke decreases immunological responses to COVID-19 vaccines.
Moreover, this research revealed that participants with blood group B had significantly higher levels of IgG-S and IgG-N. The data suggest that blood type B poses a significant risk of COVID-19 infection, which is in line with earlier studies.40 Other research, on the other hand, found no influence of blood type on COVID-19 infection.41, 42, 43 During infections, the N protein is extensively produced and has strong immunogenic activity. As a result, both the N and S proteins might be suitable targets for COVID-19 antibody detection. This study showed a high mean of protein N between males, smokers, and over-50-years-old participants (Table 6). In general, antibody testing will aid in determining the degree of infection and transmission in the population.44 , 45 However, further study is needed to determine how long the antibodies will stay and whether they are protective against re-infection.
There were several limitations to the current study that should be acknowledged. To begin, obtaining two or more readings for laboratory tests for IgG-S and IgG-N is preferable to ensure more accurate results. Second, the sample size, cross-sectional design, and nonprobability sampling may distort study results due to unequal chances of being selected to participate in the study, limiting the study's generality. Third, different types of vaccines given to participants affect the immune system response, which alters IgG results.
5. Conclusions
In conclusion, participants vaccinated with a higher number of vaccines tend to have higher IgG-S. In addition, to raise antibodies, booster shots are necessary, though the timing deserves further research. Immunoglobin G (Spike and Nucleocapsid protein) results were positive even though COVID-19 infection and vaccination occurred during the previous six months. Greater antibody levels have been observed in females and urban residents. Smokers have a suboptimal immune response to vaccines, and there is a positive correlation between IgG-S and IgG-N. In order to prevent the spread of the SARS-CoV-2 virus, it is recommended that further studies be done on those who have already been exposed to the virus to determine if they require booster doses or not.
Ethical consideration.
The study was approved by the IRB (Institutional Review Board) committee at Birzeit University's Faculty of Pharmacy, Nursing, and Health Professions (reference number BZU-PNH-2131). Before taking part in the study, all participants gave their permission.
Funding
This work was supported by the Birzeit University Scientific Research Committee in Palestine project number 281164. The study's design, data collection, analysis, and interpretation, as well as the manuscript's composition, were all independent of the funding source.
Declaration of interests statement
There are no conflicts of interest reported by any of the researchers in this work.
Acknowledgments
The authors would like to thank all of the participants in the study, as well as the Birzeit University Scientific Research Committee in Palestine, for funding this research. Finally, we'd like to thank the Medicare Ramallah Central Lab in the West Bank, Palestine, for the antibody results.
References
- 1.Jeyanathan M., Afkhami S., Smaill F., Miller M.S., Lichty B.D., Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020;20(10):615–632. doi: 10.1038/s41577-020-00434-6. 2020. 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronavirus disease (COVID-19) https://www.who.int/emergencies/diseases/novel-coronavirus-2019?gclid=Cj0KCQiAhMOMBhDhARIsAPVml-EHadYEXLEmOs-2t6_gJDyXXOJoy8uLDqtb542lWVCs5nY_1XYHBXgaAtZUEALw_wcB n.d.
- 3.Brown C.M., Vostok J., Johnson H., et al. Outbreak of SARS-CoV-2 infections, including COVID-19 vaccine breakthrough infections, associated with large public gatherings — barnstable county, Massachusetts. MMWR (Morb Mortal Wkly Rep) July 2021;70(2021):1059. doi: 10.15585/MMWR.MM7031E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rode O.Ð., Bodulić K., Zember S., et al. Decline of anti-SARS-CoV-2 IgG antibody levels 6 Months after complete BNT162b2 vaccination in healthcare workers to levels observed following the first vaccine dose. Vaccines. 2022;10(2022):153. doi: 10.3390/VACCINES10020153. 10. 153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salvagno G.L., Henry B.M., Pighi L., De Nitto S., Gianfilippi G., Lippi G. The pronounced decline of anti-SARS-CoV-2 spike trimeric IgG and RBD IgG in baseline seronegative individuals six months after BNT162b2 vaccination is consistent with the need for vaccine boosters. Clin Chem Lab Med. 2022;60:E29–E31. doi: 10.1515/CCLM-2021-1184/MACHINEREADABLECITATION/RIS. [DOI] [PubMed] [Google Scholar]
- 6.Chahla R.E., Tomas-Grau R.H., Cazorla S.I., et al. Long-term analysis of antibodies elicited by SPUTNIK V: a prospective cohort study in Tucumán, Argentina. Lancet. Reg.Heal. Americas. 2022;6 doi: 10.1016/J.LANA.2021.100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lancet. 2021;397:1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FDA Authorizes Booster Dose of Pfizer-BioNTech COVID-19 Vaccine for Certain Populations | FDA. https://www.fda.gov/news-events/press-announcements/fda-authorizes-booster-dose-pfizer-biontech-covid-19-vaccine-certain-populations
- 9.Bar-On Y.M., Goldberg Y., Mandel M., et al. Protection across age groups of BNT162b2 vaccine booster against covid-19. medRxiv. 2021:2021. doi: 10.1101/2021.10.07.21264626. 10.07.21264626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naaber P., Tserel L., Kangro K., et al. Dynamics of antibody response to BNT162b2 vaccine after six months: a longitudinal prospective study. Lancet Reg Health Europe. 2021;10 doi: 10.1016/J.LANEPE.2021.100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vargas-Herrera N., Fernández-Navarro M., Cabezudo N.E., et al. Immunogenicity and reactogenicity of a third dose of BNT162b2 vaccine for COVID-19 after a primary regimen with BBIBP-CorV or BNT162b2 vaccines in Lima, Peru. PLoS One. 2022;17 doi: 10.1371/JOURNAL.PONE.0268419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Letizia A.G., Ge Y., Goforth C.W., et al. SARS-CoV-2 seropositivity among US marine recruits attending basic training, United States, spring–fall 2020. Emerg Infect Dis. 2021;27:1188. doi: 10.3201/EID2704.204732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayart J.L., Douxfils J., Gillot C., et al. Total and neutralizing antibodies 6 Months post-vaccination with BNT162b2 in healthcare workers. Vaccines. 2021;9:1092. doi: 10.3390/VACCINES9101092. 9 (2021) 1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Narasimhan M., Mahimainathan L., Araj E., et al. Clinical evaluation of the abbott alinity sars-cov-2 spike-specific quantitative igg and igm assays among infected, recovered, and vaccinated groups. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.00388-21/SUPPL_FILE/JCM.00388-21-S0001.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coronavirus Disease 2019 (COVID-19) | CDC, (n.d.). https://www.cdc.gov/coronavirus/2019-ncov/index.html (accessed December 19, 2022).
- 16.Where Proteins and Innovation Advance Biomedicine, (n.d.). https://www.acrobiosystems.com/?gclid=EAIaIQobChMImJGdzb-G_AIVUOZ3Ch1YWQPgEAAYASAAEgKbKvD_BwE (accessed December 19, 2022).
- 17.Blain H., Gamon L., Tuaillon E., et al. Atypical symptoms, SARS-CoV-2 test results and immunisation rates in 456 residents from eight nursing homes facing a COVID-19 outbreak. Age Ageing. 2021;50:641–648. doi: 10.1093/AGEING/AFAB050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blain H., Tuaillon E., Gamon L., et al. Spike antibody levels of nursing home residents with or without prior COVID-19 3 Weeks after a single BNT162b2 vaccine dose. JAMA. 2021;325:1898–1899. doi: 10.1001/JAMA.2021.6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prakash O., Solanki B., Sheth J., et al. Population-based seropositivity for IgG antibodies against SARS-CoV-2 in Ahmedabad city. J Fam Med Prim Care. 2021;10:2363. doi: 10.4103/JFMPC.JFMPC_2062_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The Palestinian Health Information Center (PHIC) generalizes its documentation experience, (n.d.). https://www.moh.gov.ps/portal/the-palestinian-health-information-center-phic-generalizes-its-documentation-experience/(accessed March 9, 2022).
- 21.Collier A.Y., Yu J., McMahan K., et al. Differential kinetics of immune responses elicited by covid-19 vaccines. N Engl J Med. 2021;385:2010–2012. doi: 10.1056/NEJMC2115596/SUPPL_FILE/NEJMC2115596_DISCLOSURES.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krause P.R., Fleming T.R., Peto R., et al. Considerations in boosting COVID-19 vaccine immune responses. Lancet. 2021;398:1377–1380. doi: 10.1016/S0140-6736(21)02046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Z., Zhang Y., Wang M., et al. Humoral and cellular immune responses of COVID-19 vaccines against SARS-cov-2 omicron variant: a systemic review. Int J Biol Sci. 2022;18:4629. doi: 10.7150/IJBS.73583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brockman M.A., Mwimanzi F., Lapointe H.R., et al. Reduced magnitude and durability of humoral immune responses to COVID-19 mRNA vaccines among older adults. J Infect Dis. 2022;225:1129–1140. doi: 10.1093/INFDIS/JIAB592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sample Size Calculator - Confidence Level, Confidence Interval, Sample Size, Population Size, Relevant Population - Creative Research Systems, (n.d.).
- 26.Bar-On Y.M., Goldberg Y., Mandel M., et al. Protection of BNT162b2 vaccine booster against covid-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMOA2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pérez-Then E., Lucas C., Monteiro V.S., et al. Neutralizing antibodies against the SARS-CoV-2 Delta and Omicron variants following heterologous CoronaVac plus BNT162b2 booster vaccination. Nat Med. 2022;28(3):481–485. doi: 10.1038/s41591-022-01705-6. 28 (2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao Y., Hao X., Wang X., et al. Humoral immunogenicity and reactogenicity of CoronaVac or ZF2001 booster after two doses of inactivated vaccine. Cell Res. 2021;32(1):107–109. doi: 10.1038/s41422-021-00596-5. 32 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zeng F., Dai C., Cai P., et al. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: a possible reason underlying different outcome between sex. J Med Virol. 2020;92:2050–2054. doi: 10.1002/JMV.25989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Al-Asadi A.B., Al-Nuaimi B.N., Abdul-Ghani M.N., Al-Maadhidi J.F. Immune response among different types of SARS-CoV-2 vaccines in Iraq. J Comm Dis. 2022:103–108. doi: 10.24321/0019.5138.202216. E-ISSN: 2581-351X & P-ISSN: 0019-5138). 2022. [DOI] [Google Scholar]
- 31.Hassan R.T., Mohammed S.H. Evaluation of immunoglobulin G level among subjects vaccinated with different types of COVID-19 vaccines in the karbala population, Iraq. Biomed Biotechnol Res J. 2022;6:466. doi: 10.4103/BBRJ.BBRJ_213_22. [DOI] [Google Scholar]
- 32.Abdella S., Riou S., Tessema M., et al. Prevalence of SARS-CoV-2 in urban and rural Ethiopia: randomized household serosurveys reveal level of spread during the first wave of the pandemic. EClinicalMedicine. 2021;35 doi: 10.1016/J.ECLINM.2021.100880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhuiyan T.R., Akhtar M., Akter A., et al. Seroprevalence of SARS-CoV-2 antibodies in Bangladesh related to novel coronavirus infection. IJID Regions. 2022;2:198–203. doi: 10.1016/J.IJREGI.2022.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dayanand D., Irudhayanathan I., Kundu D., et al. Community seroprevalence and risk factors for SARS-CoV-2 infection in different subpopulations in Vellore, India, and their implications for future prevention. Int J Infect Dis. 2022;116:138. doi: 10.1016/J.IJID.2021.12.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoda T., Katsuyama H. Willingness to receive COVID-19 vaccination in Japan. Vaccines. 2021;9(2021):48. doi: 10.3390/VACCINES9010048. Page 48. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bühler K.-M., Echeverry-Alzate V., Calleja-Conde J., et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies: relationship with COVID-19 diagnosis, symptoms, smoking, and method of transmission. IJID Regions. 2022;4:10–16. doi: 10.1016/J.IJREGI.2022.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrara P., Ponticelli D., Agüero F., et al. Does smoking have an impact on the immunological response to COVID-19 vaccines? Evidence from the VASCO study and need for further studies. Publ Health. 2022;203:97–99. doi: 10.1016/J.PUHE.2021.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrara P., Gianfredi V., Tomaselli V., Polosa R. The effect of smoking on humoral response to COVID-19 vaccines: a systematic review of epidemiological studies. Vaccines. 2022;10(2022):303. doi: 10.3390/VACCINES10020303. Page 303. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mitani A., Hamada K., Yoshikawa N., et al. Epidemiological study using IgM and IgG antibody titers against SARS-CoV-2 in the university of tokyo, Japan (UT-CATS) J Infect Chemother. 2021;27:1342–1349. doi: 10.1016/J.JIAC.2021.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yadav S., Saluja D., Bhardwaj P., Yadav S.K., Taneja J. Association of ABO blood group with susceptibility, severity and breakthrough COVID-19 infections in Indian Population. J. Integr. Sci. Technol. 2022;(n.d.):24–28. http://pubs.iscience.in/jist [Google Scholar]
- 41.Ray J.G., Park A.L. SARS-CoV-2 vaccination, ABO blood group and risk of COVID-19: population-based cohort study. BMJ Open. 2022;12 doi: 10.1136/BMJOPEN-2021-059944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allan J.D., McMillan D., Levi M.L. COVID-19 mRNA vaccination, ABO blood type and the severity of self-reported reactogenicity in a large healthcare system: a brief report of a cross-sectional study. Cureus. 2021;13 doi: 10.7759/CUREUS.20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pasko B.E., Abbott D., Bocsi G.T., Draper N.L. ABO blood groups are not associated with COVID-19 disease incidence and severity when correcting for ethnicity differences in blood type. Am J Clin Pathol. 2022 doi: 10.1093/AJCP/AQAC036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suthar M.S., Zimmerman M.G., Kauffman R.C., et al. Rapid generation of neutralizing antibody responses in COVID-19 patients. Cell Reports Medicine. 2020;1 doi: 10.1016/J.XCRM.2020.100040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.N.M.A. Okba, M.A. Müller, W. Li, C. Wang, C.H. Geurtsvankessel, V.M. Corman, M.M. Lamers, R.S. Sikkema, E. De Bruin, F.D. Chandler, Y. Yazdanpanah, Q. Le Hingrat, D. Descamps, N. Houhou-Fidouh, C.B.E.M. Reusken, B.-J. Bosch, C. Drosten, M.P.G. Koopmans, B.L. Haagmans, SARS-CoV-2 specific antibody responses in COVID-19 patients, (n.d.). 10.1101/2020.03.18.20038059. [DOI]