Abstract
This systematic review aims to summarize the impact of vaccination against influenza, shingles, and pneumococcus on the incidence on the risk of cardiovascular events in the elderly. This protocol was developed in accordance with PRISMA guidelines. We conducted a literature search and identified all relevant articles published regarding the matter up to September 2022. We retrieved 38 studies (influenza vaccine = 33, pneumococcal vaccine = 5, and zoster vaccine = 2). A total of 28 and 2 studies have shown that influenza and pneumococcal vaccines significantly lower the risk of cardiovascular disease in the elderly. Also, repeated influenza vaccination shows a consistent and dose-dependent protective effect against acute coronary syndromes and stroke. Moreover, dual influenza and pneumococcal vaccination was associated with lower risks of some cardiovascular events (stroke, congestive heart failure, ischemic heart disease, and myocardial infarction). However, the impact of PCV13 on cardiovascular events has not been studied, nor has the currently recommended vaccination schedule (PCV13 + PPV23). As for herpes zoster vaccination, only the protective effect against stroke has been studied with the live attenuated herpes zoster vaccine, but no studies have been conducted with the recombinant subunit herpes zoster vaccine. This review outlines the benefits of the vaccines mentioned above beyond their preventive action on infectious diseases. It is intended for health professionals who wish to inform and advise their elderly patients.
Keywords: Influenza, Herpes zoster, Pneumococci, Vaccine, Cardiovascular event, Elderly person
Introduction
Elderly people are particularly at risk of developing certain infections due to immune senescence, age-related specific organ physiological changes, and other factors (dementia, institutionalization, etc.) frequently met in this population [1–3]. Among these infections, influenza, pneumococcal, and zoster infections are important causes of high morbidity and mortality in the elderly [4–6].
These infections are vaccine-preventable diseases, and the effectiveness of vaccines to reduce them has been largely demonstrated in the elderly [7]. Large cohort studies have shown that influenza vaccination reduces influenza-like diseases, pneumonia, and the risk of death among elderly persons [8]. As for invasive pneumococcal disease (IPD) prevention, many studies and six meta-analyses found significant effectiveness of the 23-valent pneumococcal polysaccharide vaccine (PPV23) [9]. More recently, the 13-valent pneumococcal conjugated vaccine (PCV13) has also demonstrated efficacy against community-acquired pneumonia and IPD episodes in patients over 65 years of age [10]. Regarding the live attenuated varicella zoster virus vaccine (ZVL), it was shown to be 62% effective in preventing herpes zoster and 81% effective in preventing post-herpetic neuralgia [11].
Beyond the burden of infectious diseases, these infections are also associated with several non-infectious complications like cardiovascular events. As a matter of fact, an increased risk of cardiovascular disease during influenza infection has been established by Madjid et al. and Warren-Gash et al. [12, 13]. The risk of acute cardiovascular events and the risk of stroke were shown to increase, respectively, fivefold and threefold in the first 3 days after a respiratory tract infection [14]. Moreover, Chow et al. highlighted that 11.7% of adults with laboratory-confirmed influenza had an acute cardiovascular event during the first years. The most common events (non-mutually exclusive) were acute heart failure (6.2%) and acute ischemic heart disease (IHD) (5.7%) [15]. In recent years, some studies have found that acute infections, especially pneumonia, are associated with a transient increasing risk of acute vascular events [14, 16, 17]. In fact, acute cardiac events are frequent in pneumococcal pneumonia and are associated with increased mortality [18]. Regarding herpes zoster, Seo et al. revealed that severe herpes zoster requiring hospitalization significantly increased the risk of myocardial infarction (MI) (HR, 1.831; 95% CI, 1.354–2.476), ischemic stroke (HR, 1.523; 95% CI, 1.212–1.915), and heart failure (HR, 2.034; 95% CI, 1.615–2.562) [19]. Recent studies suggested that the risk of stroke may increase in the year following an acute episode of herpes zoster, possibly also mediated by varicella-zona virus (VZV) replication in arterial walls, resulting in cerebral vasculopathy [20].
Despite the well-known benefits of vaccines [7], there are significant differences in vaccination programs for adults in Europe [21]. The lack of consensus-based vaccination programs in Europe results in vaccination coverage variations by country. Besides, the coverage of vaccines recommended for the elderly is not optimal. Influenza vaccination coverage almost always falls short of the World Health Organization’s (WHO) target of 75% of older adults [22] with 59.9% in France [23]. Pneumococcal vaccination uptake is around 4.8% in older adults [24], and shingles uptake rates are lower than 10% in France [25]. This low vaccine coverage in the elderly seems to be the result of multiple factors such as vaccine hesitancy [26], a lack of health literacy [27], and a lack of healthcare providers advise [28]. Several updated reviews on infectious diseases among the elderly [3] and associated vaccinations [7] were published and are helping physicians to discuss about the benefits of vaccines in reducing infectious diseases. However, perceived risks of vaccine-preventable diseases may be low in the elderly, and complacency may lead to the absence of vaccination [29]. On the other hand, in a study using data from the 2019 Health Barometer of Santé publique France, 53% of the people aged 18 to 85 years old feared stroke, and 45% of them feared MI [30].
The aim of this systematic review is to identify and measure the impact of influenza, herpes zoster, and pneumococcal vaccinations on the incidence of cardiovascular events in people aged 65 years and older. Providing data highlighting the protective effect of these vaccines against some cardiovascular events can provide new arguments to promote vaccination in this vulnerable population.
Materials and methods
This protocol has been developed following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines, as shown in the PRISMA checklist [31]. The systematic review was prospectively registered with the prospective register of systematic reviews (PROSPERO; registration number: CRD42021261176). No significant changes were made to the protocol.
Literature search strategy
We determined the selection criteria for the data collection and identified all the relevant articles published on the subject up to September 2022. We consulted the Medline database (Pubmed), Embase, and Cochrane Library with our search criteria to retrieve articles published in French and English related to the subject in adults aged over 65 years and individual medical subject heading (MeSH) terms.
The keywords of the searches were for vaccine (1) (“vaccines” [MH] OR “vaccines” [TW] OR “vaccine” [TW] OR “Vaccination” [TW] OR “immunization” [TW] OR “adult immunization” [TW]); for herpes zoster (2) (“herpes zoster” [MH] OR “Zona” [TW] OR “shingles” [TW] OR “post-herpetic neuralgia” [TW]); for pneumococcal (3) (“pneumococcal infections” [MH] OR “Pneumococcal Disease” [TW] OR “streptococcus pneumoniae infection” [TW] OR “pneumococcal” OR “Streptococcus pneumoniae”); for influenza (4) (“influenza, human” [MH] OR “influenza” [TW] OR “flu” [TW] OR “influenza virus” [TW]); and for cardiovascular diseases (5) (“cardiovascular diseases” [MH] OR “cardiovascular accident” [TW] “cardiovascular mortality” OR “cardiovascular morbidity”OR “cardiovascular events”). The search equations in Pubmed, Embase, and Cochrane Library were combinations of the previous equations with a filter setting the age to 65 years and older.
The reference lists of relevant studies and meta-analyses already carried out were examined to identify additional studies. The method used was consistent with the recommendations of Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) [31].
Data management
We downloaded references identified in the searches (electronic database and additional searches) into Zotero® reference management software. Once duplicates are removed, the remaining references were exported into Abstrackr (http://abstrackr.cebm.brown.edu/account/login).
Study selection
Two levels of screening for eligibility were performed. The first level was performed by two independent reviewers (AA, EBN) using titles and abstracts from assess and excluding papers that did not meet eligibility criteria. The screening process was performed using Abstrackr. The second level was carried out using the full-text article, and two authors (AA, EBN) assessed the papers against the inclusion criteria for the review to determine their eligibility for inclusion. In case of disagreement, we called upon a third reviewer (GG). We prioritized randomized controlled trials, case–control studies, prospective and retrospective cohorts, and systematic reviews. We retained studies with subgroup analyses in the over-65 age group.
Types of participants and study
Any study which measures the impact of influenza, herpes zoster, or pneumococcal vaccination on the risk of cardiovascular events compared to non-vaccinated people, conducted in a clinical or real-life population. We excluded studies with participants aged < 65 years of age and who had no unvaccinated control group or self-control group. Studies that included participants without age criteria were retained if subgroup analyses for age were performed, including people 65 and over. Studies were excluded if the different age groups were not well defined or if there was a comparison of different doses or vaccine protocols without an unvaccinated group. The review authors resolved disagreements through a consensus-based decision or, if necessary, discussion with a third reviewer.
Risk of bias for included studies was assessed in duplicate by investigators. The Cochrane risk of bias tool was used to examine the quality of randomized clinical trials [32] and the Newcastle–Ottawa Scale for observational studies [33]. Differences were discussed and settled by the entire study team if unresolved.
Results
Search results
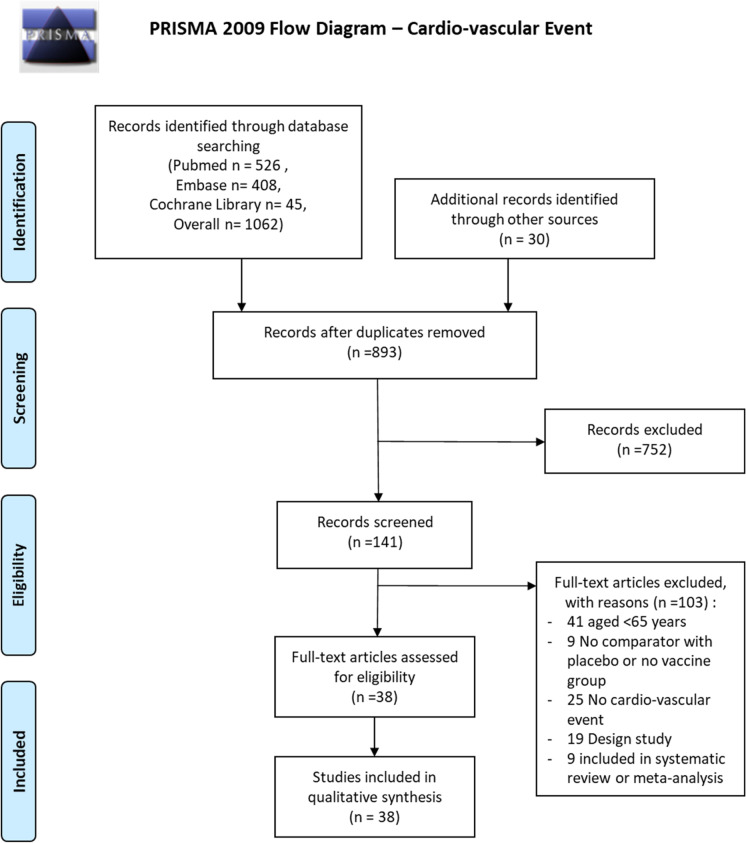
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) study flow diagram of the search for the updated and extended review is shown in Fig. 1. Electronic searches identified 1062 records. On the basis of titles and abstracts, we excluded 752 records and assessed 141 full-text records for eligibility. Of the 141 records examined in full, we considered 38 to be eligible for inclusion, and we found 30 additional studies by searching the reference sections of relevant reviews and by updating our search of ongoing trials on the databases (Fig. 1).
Fig. 1.
Flow diagram–cardiovascular event
Included studies
For details on the characteristics of individual studies retained, see Table 1, 2, and 3.
Table 1.
Summary of relevant observational studies and clinical trials of the efficacy/effectiveness of influenza vaccination on the prevention of cardiovascular events in older adults
| Author | Year of publication | Journal | Study design | Country | Follow-up | Specific-disease | Age (years) | Vaccination (yes) | Outcomes | Protective effect (Y/N) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Chang et al | 2012 | The Journal of Infection | Retrospective cohort study | Taiwan | 12 m | / | 16,284 | ≥ 75 y | 8142 | CHF | Y |
| 2 | Chang et al. (a) | 2016 | Heart Rhythm | Case–control study | Taiwan | / | / | 56,870 | 70.9 ± 13.4 | 11,374 | AF | Y |
| 3 | Chang et al. (b) | 2016 | Scientific Reports | Retrospective cohort study | Taiwan | 1 y | Systemic lupus erythematosus | 10,125 | Subgroup analysis (≥ 65 y) | 1765 | CVD | N |
| 4 | Chen et al | 2013 | Journal of Experimental and Clinical Medicine | Retrospective cohort study | Taiwan | 8 y | COPD | 25,609 | Subgroup analysis (≥ 65 y) | 3345 | CHF | Y |
| 5 | Chen et al | 2022 | Frontiers in Medicine | Retrospective cohort study | Taiwan | / | Chronic obstructive pulmonary disease | 6681 | ≥ 65 y | 3768 | Stroke | Y |
| 6 | Chiang et al | 2017 | American Heart Journal | Case–control study | Taiwan | 13 y | ILI | 160,726 | ≥ 65 y | 62,331 | MACE, MI, stoke | Y |
| 7 | Christiansen et al | 2019 | Intensive Care Medicine | Retrospective cohort study | Denmark | 1 y | / | 89,818 | ≥ 65 y | 34,871 | MI, stroke, CHF | Y |
| 8 | de Abajo et al | 2022 | Heart | Case–control study | Spain | / | / | 144,930 | Subgroup analysis (≥ 65 y) | 45,296 | MI | Y |
| 9 | Fang et al | 2016 | Acta Cardiologica Sinica | Retrospective cohort study | Taiwan | 12 y | Chronic kidney disease | 4406 | ≥ 65 y | 2254 | CHF | Y |
| 10 | Grau et al | 2005 | Stroke | Case–control study | Germany | 18 m | Ischemic or hemorrhagic stroke or TIA | 740 | Subgroup analysis (≥ 65 y) | 187 | Stroke | Y |
| 11 | Heffelfinger et al | 2006 | Human Vaccines | case–control study | USA | / | / | 1735 | ≥ 65 y | 1145 | MI | N |
| 12 | Hsu et al | 2016 | Medicine | Retrospective cohort study | Taiwan | 1 y | / | 202,058 | ≥ 65 y | 93,051 | MI | Y |
| 13 | Huang et al | 2013 | Computer Methods and Programs in Biomedicine | Prospective cohort study | Taiwan | 8 y | COPD | 29,178 | Subgroup analysis (≥ 65 y) | 6713 | IHD | Y |
| 14 | Hung et al | 2010 | Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America | Prospective cohort study | Hong Kong | 64 w | / | 36,636 | ≥ 65 y | 7292 (19.9%) received both PPV and TIV, 2076 (5.7%) received TIV alone, 1875 (5.1%) received PPV alone, and 25,393 (69.3%) were unvaccinated | Stroke, IHD, MI, CHF | Y |
| 15 | Ishigami et al | 2020 | American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation | Retrospective cohort study | USA | 1 y | With and without reduced kidney function | 91,520 | 75, 5 | 54,856 | ACS, CHF | Y |
| 16 | Kao et al | 2017 | Oncotarget | Retrospective cohort study | Taiwan | 8 y | Atrial fibrillation | 5068 | Subgroup analysis (≥ 65 y) | 2233 | Stroke | Y |
| 17 | Lavallée et al | 2002 | Stroke | Case–control study | France | / | / | 270 | Subgroup analysis (≥ 75 y) | 90 | Stroke | Y |
| 18 | Lavallée et al | 2014 | Neurology | Prospective cohort study | Multinational | / | / | 2497 | Subgroup analysis (≥ 75 y) | 1217 | MI, stroke | N |
| 19 | Lin et al | 2014 | International Journal of Environmental Research and Public Health | Case–control study | Taiwan | / | / | 3120 | ≥ 65 y | 2890 | Stroke | Y |
| 20 | Liu et al | 2017 | International Journal of Cardiology | Case–control study | Taiwan | 8 y | Atrial fibrillation | 6570 | 73, 39 | 2547 | Stroke | Y |
| 21 | Liu et al | 2012 | Preventive Medicine | Prospective cohort study | Taiwan | 4 y | Ischemic heart disease | 5048 | Non-vaccinated 75, 7 y vaccinated 74, 8 y | 2760 | CVD | Y |
| 22 | Meyers et al | 2004 | Heart Drug | Case–control study | USA | / | / | 534 | Subgroup analysis (≥ 65 y) | 303 | MI | N |
| 23 | Nichol et al | 2003 | The New England Journal of Medicine | Prospective cohort study | USA | 1 y | / | 146,328 | ≥ 65 y | 87,357 | CVD, IHD, CHF, stroke | Y |
| 24 | Nichol et al | 1994 | The New England Journal of Medicine | Retrospective cohort study | USA | / | / | 78,527 | ≥ 65 y | 41,418 | CHF | Y |
| 25 | Phrommintikul et al | 2011 | European Heart Journal | Prospective randomized open with blinded endpoint (PROBE) study | Thailand | 360 d | ACS | 439 | 66 y | 221 | MACE, ACS, CHF, stroke | Y |
| 26 | Piñol-Ripoll et al | 2008 | Cerebrovascular Diseases (Basel, Switzerland) | Case–control study | Spain | / | Chronic bronchitis and acute infections | 794 |
Cases: 73, 5 y Controls: 73, 2 |
431 | Stroke | N |
| 27 | Seo et al | 2014 | Human Vaccines & Immunotherapeutics | Case–control study | Korea | / | Cardiopulmonary disease | 602 | Subgroup analysis (≥ 65 y) | 430 | IHD, CHF | Y |
| 28 | Siriwardena et al | 2010 | CMAJ: Canadian Medical Association Journal = Journal de l’Association Medicale Canadienne | Case–control study | England and Wales | / | / | 78,706 | Subgroup analysis (≥ 65 y) | 15,575 | MI | Y |
| 29 | Siriwardena et al | 2014 | Vaccine | Case–control study | England and Wales | / | / | 94,022 | Subgroup analysis (≥ 65 y) | 7021 | Stroke | Y |
| 30 | Song et al | 2018 | PloS One | Prospective cohort study | Korea | 1 y | Cardiopulmonary disease | 2119 | 76 | 2119 (vaccined)/ 817(unvaccined) | Acute exacerbation of chronic heart disease | Y |
| 31 | Sung et al | 2014 | Vaccine | Retrospective cohort study | Taiwan | 12 y | COPD | 7722 | Subgroup analysis (≥ 65 y) | 3027 | ACS | Y |
| 32 | Wang et al | 2002 | Vaccine | Prospective cohort study | Taiwan | 12 m | Peritoneal dialysis patients | 2351 | ≥ 65 y | 1326 | CVD | Y |
| 33 | Wu et al | 2019 | PloS One | Retrospective cohort study | Taiwan | 5 y | / | 29,066 | ≥ 65 y | 4554 | CHF | Y |
Table 2.
Summary of relevant observational studies and clinical trials of the efficacy/effectiveness of pneumococcal vaccination on the prevention of cardiovascular events in older adults
| Author | Year of publication | Journal | Study design | Country | Follow-up | Specific-disease | N | Age (years) | Vaccination (yes) | Outcomes | Protective effect (Y/N) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Chang et al | 2012 | The Journal of Infection | Retrospective cohort study | Taiwan | 12 m | / | 16,284 | ≥ 75y | 8142 | CHF | Y |
| 2 | Hedlund et al | 2003 | Vaccine | Prospective cohort study | Sweden | 1 y | / | 259,627 | ≥ 65 y | 100,242 | CHF, MI | N |
| 3 | Hung et al | 2010 | Clinical Infectious Diseases: an official publication of the Infectious Diseases Society of America | Prospective cohort | Hong Kong | 1, 2 y | 36,636 | PPV and TIV: 7292, IV alone: 2076, PPV alone: 1875 (5.1%), and unvaccinated 25,393 | IHD, CHF | N | ||
| 4 | Marra et al | 2020 | International Journal of Infectious Diseases: IJID: official publication of the International Society for Infectious Diseases | Meta-analysis | / | / | / | / | / | / | CVD, MI, IHD, CHF, and stroke | Y |
| 5 | Song et al | 2018 | PloS One | Prospective cohort study | Korea | 1 y | Cardiopulmonary disease | 2 119 | 76 | 2119 | Acute Exacerbation of chronic heart disease | N |
Table 3.
Summary of relevant observational studies and clinical trials of the efficacy/effectiveness of zona vaccination on the prevention of cardiovascular events in older adults
| Author | Year of publication | Journal | Study design | Country | Follow-up | Specific-disease | N | Age (years) | Vaccination (yes) | Outcomes | Protective effect (Y/N) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Klaric et al | 2019 | Military Medicine | Retrospective cohort study | USA | / | / | 265,568 | Subgroup analysis (≥ 65 y) | 63,166 | Strokes | Y |
| Yang et al | 2021 | Stroke | Prospective cohort study | USA | 5.1 y | / | 3,206,812 | ≥ 65 y | 1,603,406 | Stokes | Y |
Design of studies included
The designs of the studies were 1 randomized clinical trial, 13 retrospective cohort studies, 10 prospective cohort studies, 13 case–control studies, and 1 meta-analysis. Follow-up was provided from 64 weeks to 13 years after vaccination.
Outcome measures
We included thirty articles dealing with influenza vaccination compared with a placebo or no vaccination, as reported in Fig. 1. Five articles reported the effect of pneumococcal vaccination on cardiovascular events in vaccinated people compared to a placebo or no vaccination, and two articles discussed the effect of zona vaccination (see Fig. 1). Among these articles, n = 7 reported the vaccination impact on cardiovascular diseases (CVD), n = 2 reported its impact on major adverse cardiovascular events (MACE), n = 11 reported its impact on MI, n = 3 reported its impact on IHD, n = 2 reported its impact on acute coronary syndrome (ACS), n = 1 reported its impact on atrial fibrillation (AF), n = 12 reported its impact on congestive heart failure (CHF), and n = 14 reported its impact on stroke.
Risk of bias in included studies
Based on the Newcastle–Ottawa evaluative scale, the overall quality assessments of the included case–control and cohort studies ranged from fair to good, see Table 4 and Table 5.
Table 4.
Quality assessment for case–control studies
| Selection | Comparability | Exposure | Number of star | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccine | Study | Year of publication | Is the case definition adequate? | Representativeness of the cases | Selection of controls | Definition of controls | Comparability of cases and controls on the basis of the design or analysis | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non-response rate | |
| Influenza | Chang et al | 2016 | a)* | a)* | b) | a)* | * | a)* | a)* | a)* | 7 |
| Influenza | Chiang et al | 2017 | a)* | a)* | a)* | a)* | * | a)* | a)* | c) | 7 |
| Influenza | Grau et al | 2005 | a)* | a)* | a)* | a)* | * | b)* | a)* | c) | 7 |
| Influenza | Heffelfinger et al | 2006 | a)* | a)* | b) | a)* | * | d) | a)* | c) | 5 |
| Influenza | Lavallée et al | 2002 | a)* | a)* | b) | a)* | ** | d) | b) | c) | 5 |
| Influenza | Liu et al | 2017 | a)* | a)* | a)* | a)* | ** | a)* | a)* | a)* | 9 |
| Influenza | Lin et al | 2014 | a)* | a)* | b) | a)* | ** | a)* | a)* | a)* | 8 |
| Influenza | Seo et al | 2014 | c) | a)* | b) | b) | ** | d) | b) | c) | 3 |
| Influenza | Siriwardena et al | 2010 | a)* | a)* | b) | b) | * | a)* | a)* | a)* | 6 |
| Influenza | Piñol-Ripoll et al | 2008 | b) | a)* | b) | a)* | ** | a)* | a)* | a)* | 7 |
| Influenza | Meyers et al | 2004 | a)* | a)* | b) | a)* | ** | d) | a)* | c) | 6 |
| Influenza | Abajo et al | 2021 | a)* | a)* | b) | a)* | ** | a)* | a)* | a)* | 8 |
*a star attests to the quality of the study for each criterion
Table 5.
Quality assessment for cohort studies
| Selection | Comparability | Outcome | Number of star | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccine | Study | Year of publication | Representativeness of the exposed cohort | Selection of the non-exposed cohort | Ascertainment of exposure | Demonstration that outcome of interest was not present at start of study | Comparability of cohorts on the basis of the design or analysis | Assessment of outcome | Same method of ascertainment for cases and controls was follow-up long enough for outcomes to occur | Adequacy of follow up of cohorts | |
| Influenza | Christiansen et al | 2019 | a)* | a)* | a)* | a)* | ** | a)* | a)* | d) pas de déclaration | 8 |
| Influenza | Hsu et al | 2016 | a)* | a)* | a)* | b) | * | d) | a)* | d) pas de déclaration | 5 |
| Influenza | Huang et al | 2013 | a)* | a)* | a)* | a)* | 0 | d) | a)* | d) pas de déclaration | 5 |
| Influenza | Hung et al | 2010 | b)* | a)* | a)* | b) | * | a)* | a)* | d) pas de déclaration | 6 |
| Influenza | Lavallée et al | 2014 | b)* | a)* | a)* | a)* | ** | d) | a)* | d) pas de déclaration | 7 |
| Influenza | Liu et al | 2012 | a)* | a)* | a)* | a)* | ** | d) | a)* | a)* | 7 |
| Influenza | Nichol et al | 1994 | a)* | a)* | a)* | a)* | 0 | d) | a)* | a)* | 6 |
| Influenza | Nichol et al | 2003 | a)* | a)* | a)* | a)* | ** | d) | a)* | a)* | 8 |
| Influenza | Song et al | 2018 | a)* | a)* | a)* | a)* | ** | a)* | a)* | a)* | 9 |
| Influenza | Sung et al | 2014 | a)* | a)* | a)* | a)* | ** | d) | a)* | a)* | 8 |
| Influenza | Wang et al | 2002 | b)* | a)* | b)* | b) | 0 | d) | a)* | d) pas de déclaration | 4 |
| Influenza | Wu et al | 2019 | a)* | a)* | a)* | a)* | 0 | d) | a)* | d) pas de déclaration | 5 |
| Influenza | Chen et al | 2013 | a)* | a)* | a)* | a)* | * | d) | a)* | d) pas de déclaration | 6 |
| Influenza | Fang et al | 2016 | a)* | a)* | a)* | a)* | * | d) | a)* | d) pas de déclaration | 6 |
| Influenza | Kao et al | 2017 | a)* | a)* | a)* | a)* | * | d) | a)* | d) pas de déclaration | 6 |
| Influenza | Chang et al | 2016 | a)* | a)* | a)* | a)* | * | d) | a)* | d) pas de déclaration | 6 |
| Influenza | Chen et al | 2022 | a)* | a)* | a)* | a)* | ** | d) | a)* | d) pas de déclaration | 7 |
| Influenza | Ishigami et al | 2020 | a)* | a)* | a)* | a)* | ** | d) | a)* | d) pas de déclaration | 7 |
| Pneumococcal | Chang et al | 2012 | a)* | a)* | a)* | a)* | ** | d) | a)* | a)* | 8 |
| Pneumococcal | Hedlund et al | 2003 | b)* | a)* | a)* | b) | 0 | d) | a)* | a)* | 5 |
| Pneumococcal | Song et al | 2018 | a)* | a)* | a)* | b) | * | d) | a)* | a)* | 6 |
| Pneumococcal | Hung et al | 2010 | a)* | a)* | a)* | a)* | * | d) | a)* | d) pas de déclaration | 6 |
| Herpes zoster | Klaric et al | 2019 | c) | a)* | c) | b) | 0 | c) | a)* | a)* | 3 |
| Herpes zoster | Yang et al | 2021 | b)* | a)* | a)* | a)* | ** | a)* | a)* | a)* | 10 |
*a star attests to the quality of the study for each criterion
Risk of bias for the clinical trial is shown in Table 6, based on the Cochrane scale.
Table 6.
Quality assessment for RCT
| Vaccine | Author | Year of publication | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Losses to follow-up/non-completers | Intention to treat analyses | Selective reporting | Similarity at baseline | Other bias |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Influenza | Phrommintikul et al | 2011 | + | ? | − | + | + | + | + | + | ? | + |
Impact of vaccines on the occurrence of cardiovascular diseases
Some studies have gathered the cardiovascular diseases without going through details on the type of these diseases.
Impact of influenza vaccine
The impact of the influenza vaccine on the occurrence of CVD was assessed in 1 study; 3 other studies reported its impact on hospitalization for CVD. Song et al. [34] evaluated the effectiveness of influenza and pneumococcal vaccines against pneumonia and acute exacerbations of cardiopulmonary diseases among the elderly aged ≥ 65 years with influenza-like illness (ILI). Overall, seasonal influenza vaccination lowered the risk of acute exacerbation of cardiovascular disease (51%) in the elderly aged ≥ 65 years.
For CVD hospitalization, Wang et al. [35] elucidated the cost-effectiveness of a community vaccination program for all the elderly and validated further the consistent effect of influenza vaccination worldwide. The risk difference of hospitalization in cardiovascular diseases among the vaccinated and unvaccinated subjects in the year after the immunization program was risk difference (RD) − 1.6%, (95% CI:0 − 3.0; − 0.1) and the risk ratio (RR) was 0.50, (95% confidence interval (CI): 0.27–0.91). The preventable fractions by vaccination were then 50%. Additionally, Nichol et al. [36] assessed the influence of influenza vaccination on hospital presentations for heart disease. When the odds of hospitalization for IHD and CHF were analyzed separately, the reductions were also significant in every case except that of IHD during the 1999–2000 season (reduction, 10%; p-value = 0.12). In contrast, the study by Chang et al. [37] did not show a significant effect of vaccination on heart disease.
For older patients with heart failure, Liu et al. [38] evaluated the effects of influenza vaccination on all-cause mortality and hospitalization for cardiovascular diseases and IHD in elderly patients. In the Cox proportional-hazards analysis, after adjustments for various factors, influenza vaccination was found to be associated with a reduced risk of hospitalization due to CVD (hazard ratio (HR), 0.84; 95% CI, 0.76–0.93) during the influenza season. During the non-influenza season, influenza vaccination was not statistically associated with a reduced risk (HR, 1.04; 95% CI, 0.91–1.18).
Impact of pneumococcal vaccines
The impact of pneumococcal vaccine on CVD was evaluated in one meta-analysis and one study.
Marra et al. [39] realized a meta-analysis summarizing available evidence on the impact of PPV23 on CVD across all ages. A total of 15 unique studies looked at PPV23 and its effects on any CVD event. When analyzing individuals by age, PPV23 significantly decreased the risk of CVD events (RR: 0.94; 95% CI: 0.89–0.99; I2 = 26.2%; -value = 0.19) for individuals aged ≥ 65 years.
In the study of Song et al. [34], the PPV23 did not lower the risk of acute exacerbation of CVD in the elderly aged ≥ 65 years. During the A/H3N2-dominant season with poor influenza vaccine effectiveness (2014–2015 season), PPV23 showed significant effectiveness against acute exacerbation of cardiopulmonary disease. During seasons with good influenza vaccine effectiveness (2015–2016 and 2016–2017), the PPV23 was effective at preventing acute exacerbation of chronic heart disease (71.0%) during the A/H1N1-dominant 2015–2016 season.
Impact of herpes zoster vaccine
We did not retrieve studies about the herpes zoster vaccine effect on the occurrence of CVD.
Impact of vaccines on occurrence of major adverse cardiovascular events (MACE)
MACE were defined as death, hospitalization for ACS, hospitalization for heart failure, and hospitalization for stroke [40].
Impact of influenza vaccine
Two studies evaluated the impact of the influenza vaccine on the occurrence of MACE. Both found that the influenza vaccine reduced the risk of MACE among elderly patients. Phrommintikul et al. [41] evaluated the effects of the influenza vaccine on cardiovascular outcomes in patients with ACS. The effects of vaccination on MACE were adjusted with age, sex, serum creatinine, ACE-I treatment, and coronary revascularization. Patients in the vaccine group had a significantly lower incidence of MACE than patients in the control group after adjusting for the above parameters (adjusted RR (aRR): 0.69; 95% CI: 0.54–0.90) in the year following vaccination. Chiang et al. [40] conducted a population-based case–control study to determine the protective effect of the influenza vaccine against primary MACE in elderly patients. The adjusted OR (aOR) for MACE in those with influenza vaccination and/or ILI after adjustment, influenza vaccination was associated with reduced odds of MACE (aOR: 0.80; 95% CI: 0.78–0.82; p-value < 0.001).
Impact of pneumococcal and herpes zoster vaccines
Pneumococcal and herpes zoster vaccines effect on the occurrence of MACE was not evaluated in the literature.
Impact of vaccines on the occurrence of myocardial infarction (MI)
Impact of influenza vaccine
Regarding MI, 7 case–control studies, 2 prospective cohort studies, and one retrospective cohort study were included. Meyers et al. [42], Heffelfinger et al. [43], and Lavallée et al. [44] did not reveal an MI risk reduction due to prior influenza vaccination for people over 65 years or 75 years old. But these studies have relatively small numbers (335 and 339). Hsu et al. [45] studied two groups of individuals, those exposed to mismatched vaccine strains during the 2007 influenza period and those exposed to matched strains during the 2008 influenza period. The MI HR in the men and women exposed to mismatched strains in 2007 was 0.990 (95% CI: 0.745–1.316) and 1.102 (95% CI: 0.803–1.513), respectively. The MI HRs were significant in men exposed to the matched strains in 2008 (HR: 0.681; 95% CI: 0.509–0.912) and were barely significant in their female counterparts (HR: 0.737; 95% CI: 0.527–1.029). But the diagnosis period of MI was not consecutive to vaccination for all patients; it ran from January to September 2008 or 2009 of the year following the influenza vaccination campaign (October to December 2007 or 2008). On the other hand, Siriwardena et al. [46] investigated in a case–control study design on a possible association between influenza or pneumococcal vaccination and acute myocardial infarction. Findings were that influenza vaccination was associated with a significantly reduced rate of MI (aOR: 0.79; 95% CI: 0.75–0.83). de Abajo et al. [47] assessed how influenza vaccination was related to a lower risk of a first acute myocardial infarction (AMI) through different epidemic periods. The overall risk reduction of AMI among vaccinated persons was aOR 0.84 (95% CI: 0.81–0.88) and was observed in subgroups of 65 years and older. Moreover, Hung et al. [48] conducted a prospective cohort study by recruiting outpatients aged ≥ 65 years with chronic illness to participate in a pneumococcal polysaccharide vaccine (PPV) and trivalent influenza vaccine (TIV) vaccination program. The TIV-alone had no significant reduction in MI (HR: 0.87; 95% CI: 0.59–1.33; p-value = 0.51). However, dual-vaccines (PPV + TIV) had a 48% reduction in MI (HR: 0.52; 95% CI: 0.38–0.71; p < 0.001) compared to the unvaccinated group. Recently, Christiansen et al. [49] examined the impact of influenza vaccination on the risk of hospitalization for MI among patients aged 65 years or older surviving an intensive care unit admission. The 1-year risk of hospitalization for MI was 1.9% for vaccinated patients and 1.8% for unvaccinated patients, with a corresponding adjusted HR of 0.93 (95% CI: 0.83–1.03). Chiang et al. [40] also determined the protective effect of the influenza vaccine against MI in elderly patients, especially those with ILI. Vaccination had significant protective effects in the MI subgroups (aOR: 0.80; 95% CI: 0.76–0.84; p-value < 0.001). ILI significantly increased the risks of MI (aOR: 1.46; 95% CI: 1.34–1.59; p-value < 0.001). Vaccination neutralized the increased risk of MI due to ILI (aOR: 1.05; 95% CI: 0.92–1.21; p-value = 0.440).
Impact of pneumococcal vaccines
Pneumococcal vaccine effect on the occurrence of MI was evaluated in one meta-analysis.
Marra et al. [39] showed a modest risk reduction in MI following pneumococcal vaccination in people aged 65 years and older (RR: 0.93; 95% CI: 0.88– 0.99; I2 = 31.0%; p-value = 0.20).
Impact of herpes zoster vaccine
Zona vaccine effect on the occurrence of MI was not reported in the literature.
Impact of vaccines on the occurrence of ischemic heart disease
Impact of influenza vaccine
Influenza vaccine effect on the occurrence of IHD was evaluated in 3 prospective cohorts.
Huang et al. [50] investigated the influenza vaccine effect on IHD occurrence secondary to chronic obstructive pulmonary disease (COPD). In the chi-square test analysis, influenza vaccination was associated with a reduced risk of IHD (OR: 0.746; 95% CI: 0.595–0.937) in elderly COPD patients in the age group of 71–80 years old. For the age groups of 61–70 and 81–90 years old, the reduction was not statistically significant (OR: 0.86; 95% CI: 0.71–1.05 and OR: 0.71; 95% CI: 0.41–1.21). When the odds of hospitalization for IHD were analyzed by Nichol et al., the reduction was significant during the 1998–1999 season (OR: 0.80; 95% CI: 0.70–0.91) and not significant during the 1999–2000 season (OR: 0.90; 95% CI: 0.78–1.03) [36]. Hung et al. [48] showed that the dual-vaccine effect (PPV + TIV) was a 35% reduction in IHD (HR: 0.65; 95% CI: 0.54–0.78; p-value < 0.001, compared with the unvaccinated group. In contrast, the TIV alone had no significant reduction in IHD (HR: 0.87; 95% CI: 0.66–1.13; p-value = 0.29).
Impact of pneumococcal vaccines
Pneumococcal vaccine effect on the occurrence of IHD was evaluated in one prospective study. Subjects who received PPV alone had no significant reduction of IHD (0.92; 95% CI: 0.69–1.22) compared with the unvaccinated group [48].
Impact of herpes zoster vaccines
Zona vaccine effect on the occurrence of IHD was not reported in the literature.
Impact of vaccines on the occurrence of acute coronary syndrome
Impact of influenza vaccine
The impact of influenza vaccination on the occurrence of acute coronary syndrome (ACS) was reported in two studies.
Sung et al. [51] elucidated the potential protective benefit of influenza vaccination on hospitalization for ACS in a group of elderly Taiwanese patients with COPD. They conducted a population-based cohort study using reimbursement claims from Taiwan’s National Health Insurance Research Database. The rate of hospitalizations for ACS after adjusted for potential confounders was significantly lower, during all seasons, in both sexes and all elderly-age groups (65 − 74 years aHR: 0.48; 95% CI: 0.37–0.61, and 75 years and older aHR: 0.41, 95% CI: 0.29–0.57) in the influenza vaccination group than in the unvaccinated group. For patients who did not experience pneumonic episodes, the vaccine exerted a protective effect, thus preventing from ACS hospitalizations in both groups. Influenza vaccination significantly reduced the number of hospitalizations for ACS regardless of influenza seasonality. When the patients were stratified according to the total number of vaccinations, the aHR for ACS hospitalizations were 0.48 (95% CI: 0.38–0.62) and 0.20 (95% CI: 0.14–0.28) for patients who received 2 − 3 and ≥ 4 vaccinations during the follow-up period (all p-value < 0.001), respectively. More specifically, Ishigami et al. [52] assessed the effectiveness of influenza vaccination among older adults with and without reduced kidney function. Overall, influenza vaccination was associated with lower odds of coronary heart disease (OR: 0.93; 95% CI: 0.88–0.97). When assessing by estimated glomerular filtration rate (eGFR) categories, the association was consistent in eGFR ≥ 30, but not significant in < 30 mL/min/1.73 m2 (OR: 1.03, 95% CI: 0.87–1.23 for ACS).
Impact of pneumococcal and herpes zoster vaccines
Pneumococcal and herpes zoster vaccine effect on the occurrence of IHD was not retrieved in the literature.
Impact of vaccines on the occurrence of atrial fibrillation
Impact of influenza vaccine
The role of influenza vaccination on AF was reported in one study. Chang et al. [53] investigated whether influenza infection was a risk factor for AF and studied whether influenza vaccination could decrease the risk of AF. The subgroup analysis for the patients over 65 years old showed that influenza vaccination (patients receiving vaccination with (OR: 0.881; 95% CI: 0.836–0.928; p-value = 0.0001) or without (OR: 1.136; 95% CI: 0.929–1.389; p-value = 0.214) influenza infection) was consistently associated with a lower risk of AF compared to patients without influenza infection and without vaccination (OR: 1.182, 95% CI: 1.014–1.378; p-value = 0.032).
Impact of pneumococcal and herpes zoster vaccines
Pneumococcal and herpes zoster vaccine effect on the occurrence of AF was not reported in the literature.
Impact of vaccines on the occurrence of congestive heart failure
Impact of influenza vaccine
Influenza vaccine effect on the occurrence of hospitalizations for CHF was evaluated in ten studies.
First, Christiansen et al. did not reveal that the risk of CHF was reduced by prior influenza vaccination for people over 65 years old [49]. Seo et al. [54] estimated the effectiveness of influenza vaccination on preventing hospitalizations in persons with cardiopulmonary disease and established an evidence basis for recommendations on influenza vaccination in this population. The vaccine effectiveness among patients on hospital presentations or IHD and CHF was 56.0% in patients’ aged ≥ 65 y (95% CI: 32.1–71.4%, p-value < 0.001). In 1994, Nichol et al. [55] have already wondered about the efficacy and cost-effectiveness of influenza vaccine administered to older persons living in the community. During the influenza season, vaccination against influenza was associated with significantly fewer hospitalizations for CHF during the epidemic 1991–1992 season (a 37% reduction; p-value = 0.04). For the epidemic 1990–1991 and 1992–1993 seasons, OR for CHF hospitalizations were 0.73 and 1.05, not statistically significant. Besides, in 2019, Wu et al. [56] assessed the association between influenza vaccination and the secondary prevention of CVD among elderly persons. Compared with an unvaccinated cohort, the vaccinated cohort had significantly lower rates of hospitalizations for cardiac failure (HR 0.83, 95% CI: 0.74–0.92). The Kaplan–Meier curves of cumulative incidence showed that the curves of the two groups separated gradually during the first 3 months of the follow-up period and differed significantly at the end of follow-up. When Nichol et al. [36] analyzed the odds of hospitalizations for CHF, the reductions were also significant (OR 0.81, 95% CI: 0.70–0.92). Similar protective results have been reported in elderly patients with chronic kidney disease [57], with reduced kidney function [52] and with chronic obstructive pulmonary disease [58]. More specifically, Fang et al. found a dose effect [57]. When patients were stratified by the total number of vaccinations, hospitalization-adjusted HRs were 0.60 (0.47–0.77), 0.30 (0.23–0.41), and 0.10 (0.06–0.16) for patients receiving 1, 2 to 3, and 4 vaccinations during follow-up, respectively (all p-values < 0.001). More for cardiovascular and cerebrovascular diagnoses, Hung et al. [48] showed that dual-vaccines (PPV + TIV) resulted in a 19% reduction in heart failure (HR, 0.81; 95% CI, 0.70–0.94; p = 0.006), compared to the unvaccinated group. In contrast, the TIV alone had no significant reduction in heart failure (HR 0.92; 95% CI: 0.73–1.16; p = 0.50). Similarly with Chang et al. [59], the cumulative effect of pneumococcal and influenza vaccines on the risk of death, hospitalization, and hospitalization costs in Taiwanese people aged 75 years and older was also assessed. Regarding the risk of hospitalizations, it was significantly lower (24%) in the group receiving both vaccines compared to the unvaccinated group. Getting both vaccines resulted in a significant 29% reduction in CHF risk compared to the flu vaccine alone.
Impact of pneumococcal vaccines
The impact of pneumococcal vaccination in preventing from hospitalization for CHF was evaluated in 3 studies.
Chang et al. [59] assessed the additive effect of PPV and influenza vaccines on the risk of mortality, hospitalization, and hospitalizations expenses/costs in the elderly aged 75 years or older in Taiwan. In terms of hospitalization risks for CHF, compared to the unvaccinated group, significant risk reductions (24%) were observed in the group that received both vaccines. However, those who only received the influenza vaccine had no significant risk decrease (RR 1.07; 95% CI, 0.87–1.37) when compared to the unvaccinated group. Receiving both vaccines was associated with a significant reduction of 29% in the risk of hospitalization for CHF when compared to receiving the influenza vaccine alone. Hedlund et al. [60] investigated prospectively the health effects of a large-scale program of PPV23 vaccination in individuals aged 65 years and older. The incidence of hospital admissions was not lower in the vaccinated cohort compared to the unvaccinated cohort for cardiac failure (RR = 0.95; 95% CI: 0.87–1.05). During the time period when the influenza virus was circulating in the community, December 1998 through May 1999, the incidence of hospital treatment was lower in the vaccinated as in the unvaccinated cohort for cardiac failure (RR = 0.90; 95% CI: 0.80–1.01). Also during the time period without influenza, June through November 1999, the incidence of hospital treatment for cardiac failure was not significantly lower in the vaccinated cohort (1.02; 95% CI: 0.93–1.11)). Futhermore, in Hung et al. [48], subjects who received PPV alone had no significant heart failure reduction (0.99; 95% CI: 0.78–1.27) compared to the unvaccinated group.
Impact of herpes zoster vaccine
Zona vaccine effect on the occurrence of CHF was not evaluated in the literature.
Impact of vaccines on the occurrence of stroke
Impact of influenza vaccine
The effect of influenza vaccination on stroke was retrieved in 11 studies.
Piñol-Ripoll et al. [61] evaluated the importance of acute infections and chronic bronchitis (CB; as a chronic inflammatory state) in several subtypes of ischemic stroke and investigated whether the influenza vaccination was independently associated with a reduced probability of stroke. Influenza vaccination during the last campaign (OR = 1.02, 95% CI = 0.77–1.36) or during every campaign within the last 5 years was not associated with a lower risk of ischemic stroke in any subgroup of age or subtype of stroke. Additionally, Lavallée et al. [44] did not show that the risk of stroke was reduced by prior influenza vaccination for people over 75 years old. Also Chiang et al. [40] have determined the protective effect of influenza vaccine against ischemic stroke. When comparing the vaccinated and unvaccinated groups, vaccination had significant protective effects in ischemic stroke subgroups (aOR 0.80, 95% CI: 0.77–0.82, p < 0.001). When comparing no ILI or vaccination, ILI without vaccination and ILI with vaccination, ILI significantly increased the risks of ischemic stroke (aOR 1.16, 95% CI: 1.10–1.22, p < 0.001). Vaccination neutralized the increased risk of ischemic stroke due to ILI (aOR 0.96, 95% CI: 0.89–1.05, p = 0.398). After adjustment for the traditional risk factors for stroke and other potential confounding factors, Lavallée et al. [62] reported the inverse association between brain infarction and influenza vaccination that was statistically significant during the preceding vaccination campaign (OR 0.45; 95% CI, 0.24 to 0.84; p = 0.012) and every year in the last 5 years (OR 0.37; 95% CI, 0.19 to 0.70; p = 0.002). The strength of the association was similar in men and women. When the analyses were restricted to patients and controls without any cerebrovascular or cardiovascular history, an even stronger negative association between brain infarction and influenza vaccination was observed. Grau et al. [63] investigated whether influenza vaccination was associated independently with reduced odds of stroke and whether effects were confined to stroke subgroups and winter seasons and were shared by other vaccinations. Recent influenza vaccination was associated with a reduced risk of stroke and transient ischemic attack (TIA) in older subjects by 36%. Moreover, Siriwardena et al. [64] investigated whether influenza or pneumococcal vaccination were associated with reduced risk of stroke or transient ischemic attack (TIA). Influenza vaccination given within the same season (as the index date) was associated with a significant 26% reduction in the risk of stroke for those aged 65 years old and over (adjusted OR 0.74, 95% CI: 0.70–0.78) after adjusting for confounding variables. Stroke risk was significantly lower with early influenza vaccination (September to mid-November: 0.73, 95% CI: 0.69–0.77) but less important with late vaccination (mid-November onwards: 0.89, 95% CI: 0.80–0.98). Influenza vaccination within-season was not associated with a reduction in the risk of TIA. Lin et al. [65] determined whether influenza vaccination was associated with a reduced risk of hospitalization for stroke among the elderly. Already vaccinated individuals in the current vaccination season were associated with a reduced risk of stroke admissions (primary intracerebral hemorrhagic stroke (PIH) + ischemic stroke (IS)) OR (OR = 0.80, 95% CI = 0.64–0.98). When they analyzed the data separating PIH and IS, a significant inverse association was observed only for IS (OR = 0.76, 95% CI = 0.60–0.97). When exposure was categorized by cumulative number of influenza vaccinations within the previous 5 years, the adjusted ORs were 0.93 (95% CI = 0.72–1.22) for the group with one or two vaccinations, 0.70 (95% CI = 0.53–0.92) for the group with 3 or 4 vaccinations, and 0.59 (95% CI = 0.42–0.83) for the group with five vaccinations compared with never-vaccinated individuals. There was a significant trend of decreasing risk of hospitalization for stroke with increasing number of vaccinations (chi-square for linear trend = 12.61; p for trend < 0.001). The estimated ORs for IS by cumulative number of influenza vaccinations within the previous 5 years were 0.92 (95% CI = 0.68–1.23), 0.73 (95% CI = 0.54–1.00), and 0.56 (95 CI = 0.38–0.83), respectively (chi-square for linear trend = 9.97; p for trend = 0.001). Again, the association for PIH was not significant. Liu et al. [66] investigated the association of influenza vaccination with the risk of hemorrhagic stroke to develop an efficient strategy for reducing this risk in patients with AF. The adjusted HRs (aHRs) of hemorrhagic stroke significantly decreased in the vaccinated patients during the non-influenza season (influenza season aHR = 0.97, 95% CI: 0.59–1.60, non-influenza season 0.51, 95% CI: 0.30–0.87 and all seasons 0.72, 95% CI 0.50–1.03). Furthermore, the stratified analysis revealed that aHRs decreased significantly in the vaccinated patients during the non-influenza season and all seasons (aHRs = 0.44 and 0.61; p < 0.05 and < 0.05, respectively), particularly in patients aged ≥ 75 years, irrespective of sex. Frequent vaccination significantly reduced the risk of hemorrhagic stroke. During the non-influenza season and all seasons, the aHRs indicated that vaccination significantly reduced the risk of hemorrhagic stroke in a dose-dependent way, in patients aged ≥ 75 years old (non-influenza season ≥ 4 vaccinations: aHR = 0.29, 95% CI: 0.09–0.99; and all seasons aHR = 0.45, 95% CI: 0.22–0.94) and male patients (non-influenza season ≥ 4 vaccinations: aHR = 0.24, 95% CI: 0.07–0.83; and all seasons aHR = 0.24, 95% CI: 0.09–0.62). Christiansen et al. [49] reported that the 1-year risk of hospitalization for stroke was 2.9% in the vaccinated patients and 3.3% in the unvaccinated patients, with a corresponding adjusted HR of 0.84 (95% CI: 0.78–0.92). Nichol et al. [36] showed that influenza vaccination was associated with reductions in the odds of hospitalization for cerebrovascular diseases (reduction of 16% during the 1998–1999 season and 23% during the 1999–2000 season). Similar protective results have been reported in elderly patients with AF [67], and with elderly women with COPD [68]. Finally, by Hung et al. [48], dual-vaccines (PPV + TIV) had a 33% reduction in ischemic stroke compared to the unvaccinated group. In contrast, the TIV alone had no significant reduction in ischemic stroke (HR 0.85; 95% CI: 0.61–1.17; p = 0.31).
Impact of pneumococcal vaccines
According to Marra et al.’s meta-analysis, the impact of PPV23 on cerebrovascular disease (stroke/TIA) was evaluated in five studies, four cohort studies, and one case control study. A significant reduction in the risk of having cerebrovascular disease following pneumococcal vaccination was not observed (RR: 0.96; 95% CI: 0.83–1.10; I2 = 74.3%; p < 0.001) [39].
Impact of herpes zoster vaccines
The effect of herpes zoster vaccination on stroke was retrieved in 2 studies.
Klaric et al. [69] investigated the hypothesis of decreased odds of stroke in persons who have received zoster vaccine. They have carried out several types of analyses in which those without zoster vaccination were significantly at higher risk of stroke compared to those receiving zoster vaccinations. Adjusted odds ratios (aOR) indicated that people in the 65–69 years’ age group who were not vaccinated against shingles were 50% more likely to report a stroke than those who were vaccinated (OR = 1.51, 99% CI: 1.21, 1.88). Also, Yang et al. [70] examined the effect of Zoster vaccine live on risk of stroke among adults aged 66 years or older who enrolled in the Medicare Fee-for-Service Program during 2008 to 2017 in the USA. Adjusted HRs comparing vaccinated to unvaccinated beneficiaries were 0.84 (95% CI; 0.83–0.85), 0.83 (95% CI; 0.82–0.84), and 0.88 (95% CI; 0.85–0.91) for all stroke, AIS, and hemorrhagic stroke, respectively.
Discussion
In this review, we summarized for the first time the impact of vaccines in the elderly against various cardiovascular events. Indeed, a growing body of literature has looked at the impact of each vaccine separately on these types of non-infectious outcomes in the general population. Clar et al. [71] realized a systematic review and a meta-analysis to assess the potential benefits of influenza vaccination for primary and secondary prevention of cardiovascular diseases. Marra et al. [39] realized a meta-analysis summarizing available evidence on the impact of PPV23 on cardiovascular diseases across all ages. More recently, several publications have been issued regarding the cardioprotective impact of vaccinations in the general population [72–76]. In this current work, the prevention of cardiovascular events associated with influenza, pneumococcal, and zoster vaccines among the elderly was reviewed, as this population is particularly concerned about these vaccine-preventable diseases. A search on the impact of COVID-19 vaccination on cardiovascular events was also conducted, but the studies identified referred to thrombotic events. This allows to have an overview for the healthcare provider who wants to advise elderly patients and inform them about the benefits of vaccines beyond their preventive action against infectious diseases, as summarized in Table 7.
Table 7.
Summary of the effects of influenza, pneumococcal, and herpes zoster vaccinations on cardiovascular events in the literature
| Influenza vaccine | ||||
| Protective effect | No evidence of protective effect | |||
| Outcome | Study | Magnitudes of facts % [CI 95%] | Study | Magnitudes of facts % [CI 95%] |
| CVD | Liu et al., 2012 (with IHD) | − 16% [− 24%; − 7%] | Chang et al., 2016 (b) (systemic lupus erythematosus) | − 24% [− 50%; + 13%] |
| Song et al., 2018 (a) (exacerbation CVD) | − 51% [− 73%; − 9%] | |||
| Wang et al., 2002 (hospitalization) | − 50% [− 73%; − 9%] | |||
| Nichol et al., 2003 (hospitalization) | − 19% [− 23%; − 11%] | |||
| MACE | Chiang et al., 2017 (ILI) | − 20% [− 22%; − 18%] | ||
| Phrommintikul et al., 2011 (with ACS) | − 31% [− 46%; − 10%] | |||
| MI | Abajo et al., 2021 | − 16% [− 19%; − 12%] | Christiansen et al., 2019 (hospitalization) | − 7% [− 17%; + 19%] |
| Chiang et al., 2017 (ILI) | − 20% [− 24%; − 16%] | Heffelfinger et al., 2006 | − 3% [− 25%; + 27%] | |
| Hsu et al., 2016 | − 32% [− 50%; − 9%] (men) | Hung et al., 2010 (TIV alone) | − 13% [− 41%; + 33%] | |
| Hung et al., 2010 (double vaccination) | − 48% [− 29%; − 62%] | Lavallée et al., 2014 | − 50% [− 78%; + 22%] | |
| Siriwardena et al., 2010 | − 21% [− 17%; − 25%] | Meyers et al., 2004 | − 44% [− 75%; + 27%] | |
| IHD | Huang et al., 2013 (with COPD) | − 26% [− 7%; − 41%] (71–80 years old) | Hung et al., 2010 (TIV alone) | − 13% [− 34%; + 13%] |
| Hung et al., 2010 (double vaccination) | − 35% [− 22%; − 46%] | |||
| Nichol, 2003 | − 20% [− 9%; − 30%] | |||
| ACS | Ishigami et al., 2020 (reduced kidney function) | − 7% [− 3%; − 12%] | ||
| Sung et al., 2014 (hospitalization) | − 52% [− 39%; − 63%] (65 − 74 years old); − 59% [− 43%; − 71%] (75 years and older) | |||
| AF | Chang et al., 2016 (a) | − 12% [− 8%; − 17%] | ||
| CHF | Chang et al., 2012 (TIV alone + double vaccination) | − 24% [− 1%; − 42%]/ − 29% [− 8%; − 46%] | Hung et al., 2010 (TIV alone) | − 8% [− 27%; + 16%] |
| Chen et al., 2013 (COPD) | − 63% [− 48%; − 74%] (65 − 74 y); − 64% [− 48%; − 75%] (75 years and older) | Christiansen et al., 2019 | − 2% [− 14%; + 12%] | |
| Fang et al., 2016 (CKD) | − 69% [− 61%; − 74%] | |||
| Hung et al., 2010 (double vaccination) | − 19% [− 6%; − 30%] | |||
| Ishigami et al., 2020 (reduced kidney function) | − 8%; [− 1%; − 14%] | |||
| Nichol et al., 1994 | 37% [NA] | |||
| Nichol et al., 2003 (Hospitalization) | − 19% [− 8%; − 30%] | |||
| Seo et al., 2014 (AF + CHF) | − 56% [− 32%; − 71%] | |||
| Wu et al., 2019 (CVD) | − 17% [− 8%; − 26%] | |||
| Stroke | Chen et al., 2022 (women COPD) | − 40% [− 33%; − 46%] | Hung et al., 2010 (TIV alone) | − 15% [− 39%; + 17%] |
| Chiang et al | − 20% [− 18%; − 23%] | Lavallée et al., 2014 | − 8% [− 38%; + 37] | |
| Christiansen et al., 2019 (hospitalization) | − 16% [− 8%; − 22%] | Piñol-Ripoll et al., 2008 | + 2% [− 23%; 36%] | |
| Grau et al. 2005 | − 64% [− 37%; − 80%] | |||
| Hung et al., 2010 (double vaccination) | − 33% [− 17%; − 46%] | |||
| Kao et al., 2017 (AF) | − 52% [− 39%; − 61%] (65–74 y); − 55% [− 47%; − 60%](75 years and older) | |||
| Lavallée et al., 2002 | − 55% [− 16%; − 76%] | |||
| Lin et al., 2014 (hospitalization) | − 20% [− 2%; − 36%] | |||
| Liu et al., 2017 (hemorrhagic) | − 39% [− 1%; − 62%] (75 years and older) | |||
| Nichol et al., 2003 (hospitalization) | − 16% [− 3%; − 28%] | |||
| Siriwardena et al., 2014 | − 26% [− 22%; − 30%] | |||
| Pneumococcal vaccines | ||||
| Protective effect | No evidence of protective effect | |||
| Outcome | Study | Magnitudes of facts % [CI 95%] | Study | Magnitudes of facts % [CI 95%] |
| Cardiovascular disease | Marra et al., 2020 | − 6% [− 1%; − 11%] | Song et al., 2018 (a) | − 6% [− 98%; + 44%] |
| MI | Marra et al., 2020 | − 7% [− 1%; − 12%] | ||
| IHD | Hung et al., 2010 (double vaccination) | − 35% [− 22%; − 46%] | Hung et al., 2010 (PPV alone) | − 8% [− 31%; + 22%] |
| CHF | Chang et al., 2012 (double vaccination) | − 29% [− 8%; − 46%] | Chang et al., 2012(PPV alone) | + 7% [− 16%, + 37%] |
| Hung et al., 2010 (PPV alone) | − 1% [− 22%; 27%] | |||
| Hedlund et al. 2003 (double vaccination) | − 5% [− 13%; + 5%] | |||
| Stroke | Marra et al. 2020 | − 8% [− 19%; + 4%] | ||
| Herpes zoster vaccine | ||||
| Protective effect | No evidence of protective effect | |||
| Outcome | Study | Magnitudes of facts | Study | Magnitudes of facts |
| Stroke | Klaric et al., 2019 | − 49% [− 12%; − 79%] | ||
| Yang et al., 2021 | − 16% [− 17; − 15%] | |||
Influenza and pneumococcal mortality in older adults appear to be well established [36, 38, 77–80]. The impact of these vaccinations on specific cardiovascular mortality is also known [41, 77, 79–85]. Data about the impact of influenza on cardiovascular events in the elderly are numerous and have been reported since the early 1900s. Moreover, influenza vaccine was shown to protect against major non-fatal cardiovascular events, with a significantly lower risk of cardiovascular diseases [34, 38, 86], MACE [40, 41], MI [40, 45–47], IHD occurrence secondary to COPD [50], ASC [51, 52], AF [53], CHF [36, 52–60], and stroke [36, 40, 49, 62, 63, 65–68] in older adults. Observational and clinical studies still show discordant results with regards to the protective association with AMI and MI, although the association with IHD is more consistent (Table 7). Finally, repeated vaccination shows a consistent, dose-dependent, protective association with CHF [57] and stroke [40, 62, 66, 67]. In fact, in a Danish study among adults with HF, influenza vaccination was associated with a reduced risk of both all-cause and cardiovascular death. Frequent vaccination and early vaccination in the year were associated with larger reductions in the risk of death compared to intermittent and late vaccination [87]. Although there are still some gray areas, the studies on repeated vaccination fit with the observational studies and emphasize the need for more robust clinical research to investigate the beneficial effects of influenza vaccine in older adults. Accordingly, the seroprotection provided by influenza vaccination enables a secondary immune response that neutralizes the virus, prevents host cell colonization, and results in the elimination of the pathogen [88]. This widely accepted mechanism is underpinned by a large number of epidemiological, clinical, and experimental studies. It has been suggested that the reduced short-term risk of cardiovascular events offered by the influenza vaccination is due to a reduction in infection, which can be a trigger in the inflammatory cascade that leads to the progression of atherosclerosis [89]. However, these results do not explain the beneficial effects on the prevention of cardiovascular events observed during generally virus-free periods (i.e., the summer months) [38, 40, 41, 81] and the cumulative effect of repeated vaccination [40, 62]. Furthermore, Aidoud et al. reported an emerging mechanism independent of influenza infection [89]. However, few studies of these mechanisms have been performed.
Other studies have shown that pneumococcal vaccination (PPV23) protects against major non-fatal cardiovascular events, with a significantly lower risk of cardiovascular diseases [39] and MI [39] in older adults. There is less literature on the effects of this vaccination against cardiovascular events than there is on influenza. In the literature, the effect of pneumococcal vaccination has not been studied on MACE, ACS, and AF. It would be interesting to explore these outcomes. No protective effect of this vaccination was shown for CHF and stroke (Table 4). Unlike the influenza and pneumococcal double vaccination that was shown to protect against MI [48], IHD [48], CHF [48, 59], and stroke [48], in addition, both vaccinations were compared to administration of the influenza vaccine alone by Hung et al. and Chang et al. on the occurrence of MI [48], IHD [48], CHF [59], and stroke [48]. Both vaccinations appear to be more protective than influenza alone. However, the impact of PCV13 on the cardiovascular event was not studied yet, as well as the currently recommended vaccine regimen with PCV13 followed by PPV23. The link between cardiovascular events and pneumococcal infections seems to be related to the phosphorylcholine lipid antigens of the S. pneumoniae cell wall that induce the production of antibodies that cross-react with oxLDL, a component of atherosclerotic plaques. These antibodies may bind and facilitate the regression of the plaques. In fact, pneumococcal vaccination has been shown to induce anti-oxLDL antibodies [90]. A significant association has also been observed between pneumococcal IgG and anti-oxLDL titers [91]. It is therefore suggested that the PPV, by inducing the production of anti-phosphorylcholine and anti-oxLDL antibodies that block the uptake of LDL by macrophages, may have a protective effect on cardiovascular diseases in humans [75, 90].
For shingles vaccination, only the protective effect against stroke has been studied with the live attenuated herpes zoster vaccine (ZVL), but no studies were performed with the recombinant subunit herpes zoster vaccine (RZV). Associations between VZV-induced vasculopathy and stroke events have been reported since the early 1970’s [92–95]; clinical associations have been extensively reviewed [20]. Several potential multifactorial physiologic mechanisms have been proposed for this interaction between VZV activation and such vascular events that can lead to stroke. Due to VZV’s replication in human cerebral arteries, one hypothesis postulates that the shingles virus directly affects blood vessels as it spreads along nerve fibers. [94] A second hypothesis is that higher levels of inflammation in the body during a severe systemic infection lead to endothelial dysfunction, resulting in the disruption of atheromatous plaques and hypercoagulability [94, 95]. Results from the present review suggest that vaccination against VZV by reducing zoster protects against these possible disruptive events leading to stroke when administered to elderly adults at specific ages.
Despite scientific evidence on the effectiveness, safety, and benefits of the vaccines recommended, doubts about vaccine necessity or efficacy and concerns about possible adverse effects have always followed, including in the elderly [96, 97]. “Vaccine hesitancy” refers to delayed acceptance or refusal of vaccines despite their availability [98]. The reasons for vaccine hesitancy are complex and involve socio-demographic, contextual, physical, and psychosocial factors [96]. These psychosocial factors are amenable to change through intervention [99]. Moreover, vaccination-related knowledge and receiving advice or recommendations, by healthcare providers or relatives, as well as participants’ general vaccination attitudes, also appear important [100, 101], but all of these elements are often not considered in the research investigating older adults’ vaccine hesitancy.
Greater awareness and targeted education around disease risk and vaccine benefits may be required to increase vaccine coverage, particularly regarding influenza, pneumococcal, and shingles vaccines. Informing elderly patients of the substantial risk of cardiovascular events following an infection and its modification by vaccination may constitute a simple, effective, costless, and time-saving method of changing many patients’ negative attitudes towards vaccination. We postulate that this may reduce the complacency component of vaccine hesitancy. Schattner et al. [102] have shown that a short physician-administered verbal instruction on the risk of “heart attack and stroke” following influenza results in a change in behavior and an increase in seasonal influenza vaccination in a population of over 65 years.
This study has a potential limitation; we have not studied the link between cardiovascular events and the COVID-19 vaccine. It has been reported that pre-existing CVD may contribute to adverse early clinical outcomes and that COVID-19 infection may have longer terms of implications for overall cardiovascular health [103]. The American College of Cardiology and the European Society of Cardiology also pointed out that patients with cardiovascular risk factors and established CVD represent a vulnerable population when suffering from COVID-19 [104, 105]. Recent studies have showed that the risk of in-hospital death among patients with severe COVID-19 was significantly associated with elderly, inflammatory response, and cardiovascular comorbidities [106]. Tessitore et al. [107] found that patients with a history of CVD had their COVID-19 hospitalization significantly associated with an increased risk of MACE and death. COVID-19-triggered AMI in individuals with high coronary risks may become a public health issue. Interestingly, during the COVID-19 pandemic, Mahmud et al. [108] analyzed the benefits of influenza vaccination in preventing AMI and estimated that the effectiveness of flu vaccines in AMI prevention was 29%. The merits of vaccination go beyond preventing the original viral infection to its possible prevention of cardiovascular complications. In addition, the respiratory syncytial virus (RSV) is also an infection associated with increased cardiovascular risk in adults and the elderly [109]. We could not be interested in the RSV vaccine and cardiovascular event association because the RSV vaccine has not been launched and we do not yet have real-life data. However, the phase 3 trial seems to be successful against RSV-related lower respiratory tract disease in people over 60 years of age [110]. Moreover, we did not include the diphtheria tetanus poliomyelitis vaccine in our review as it is not a vaccine specific to the elderly; also, it is considered as well followed by the older adults.
There is growing evidence of a link between influenza, pneumococcal, and herpes zoster vaccinations and a reduction in the incidence of cardiovascular events. This is firstly based on the prevention of the cardiovascular complications of infections. We also suggest that vaccination has other infection-independent cardioprotective effects. The scientific interest in vaccination and its cardiovascular protection is growing and will continue to grow. In the literature, the protective effect of the vaccine against influenza, pneumococci, and shingles goes beyond cardiovascular events such as dementia, falls, fractures, and loss of quality of life. This is why we must look for other protective effects of vaccination and communicate these benefits to the aging population and health professionals in order to improve vaccination coverage in this population.
Acknowledgements
We want to express our gratitude to Essalim Soumaya for its reading and correction.
Funding
This study is supported by the ANRT funding under CIFRE, La Conférence des financeurs de la Prévention de la Perte d’Autonomie de la Loire, Solidaire à Fond(s): Le Fonds de dotation de la Caisse d’épargne Loire Drôme Ardèche.
Data Availability
Data used to support the findings of the study are included within the article.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gavazzi G, Krause KH. Infections in the elderly. Praxis (Bern 1994) 2004;93(33):1297–1303. doi: 10.1024/0369-8394.93.33.1297. [DOI] [PubMed] [Google Scholar]
- 2.Pera A, et al. Immunosenescence: implications for response to infection and vaccination in older people. Maturitas. 2015;82(1):50–55. doi: 10.1016/j.maturitas.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 3.El Chakhtoura NG, Bonomo RA, Jump RLP. Influence of aging and environment on presentation of infection in older adults. Infect Dis Clin North Am. 2017;31(4):593–608. doi: 10.1016/j.idc.2017.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonsen L. The global impact of influenza on morbidity and mortality. Vaccine. 1999;17:S3–S10. doi: 10.1016/S0264-410X(99)00099-7. [DOI] [PubMed] [Google Scholar]
- 5.Schmader K. Herpes zoster in the elderly: issues related to geriatrics. Clin Infect Dis. 1999;28(4):736–739. doi: 10.1086/515205. [DOI] [PubMed] [Google Scholar]
- 6.Ruiz M, et al. Etiology of community-acquired pneumonia: impact of age, comorbidity, and severity. Am J Respir Crit Care Med. 1999;160(2):397–405. doi: 10.1164/ajrccm.160.2.9808045. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham AL, McIntyre P, Subbarao K, Booy R, Levin MJ. Vaccines for older adults. BMJ. 2021;372:n188. doi: 10.1136/bmj.n188. [DOI] [PubMed] [Google Scholar]
- 8.Nichol KL, Nordin JD, Nelson DB, Mullooly JP, Hak E. Effectiveness of influenza vaccine in the community-dwelling elderly. N Engl J Med. 2007;357(14):1373–1381. doi: 10.1056/NEJMoa070844. [DOI] [PubMed] [Google Scholar]
- 9.Berild JD, et al. A systematic review of studies published between 2016 and 2019 on the effectiveness and efficacy of pneumococcal vaccination on pneumonia and invasive pneumococcal disease in an elderly population. Pathogens. 2020;9(4):E259. doi: 10.3390/pathogens9040259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webber C, et al. Exploratory efficacy endpoints in the Community-Acquired Pneumonia immunization Trial in Adults (CAPiTA)’. Vaccine. 2017;35(9):1266–1272. doi: 10.1016/j.vaccine.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 11.Walker JL, Andrews NJ, Amirthalingam G, Forbes H, Langan SM, Thomas SL. Effectiveness of herpes zoster vaccination in an older United Kingdom population. Vaccine. 2018;36(17):2371–2377. doi: 10.1016/j.vaccine.2018.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madjid M, et al. Influenza epidemics and acute respiratory disease activity are associated with a surge in autopsy-confirmed coronary heart disease death: results from 8 years of autopsies in 34 892 subjects. Eur Heart J. 2007;28(10):1205–1210. doi: 10.1093/eurheartj/ehm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warren-Gash C, Blackburn R, Whitaker H, McMenamin J, Hayward AC. Laboratory-confirmed respiratory infections as triggers for acute myocardial infarction and stroke: a self-controlled case series analysis of national linked datasets from Scotland. Eur Respir J. 2018;51(3):1701794. doi: 10.1183/13993003.01794-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smeeth L, Thomas SL, Hall AJ, Hubbard R, Farrington P, Vallance P. Risk of myocardial infarction and stroke after acute infection or vaccination. N Engl J Med. 2004;351(25):2611–2618. doi: 10.1056/NEJMoa041747. [DOI] [PubMed] [Google Scholar]
- 15.Chow EJ, et al. Acute cardiovascular events associated with influenza in hospitalized adults: a cross-sectional study. Ann Intern Med. 2020;173(8):605–613. doi: 10.7326/M20-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musher DM, Rueda AM, Kaka AS, Mapara SM. The association between pneumococcal pneumonia and acute cardiac events. Clin Infect Dis. 2007;45(2):158–165. doi: 10.1086/518849. [DOI] [PubMed] [Google Scholar]
- 17.Ramirez J, et al. Acute myocardial infarction in hospitalized patients with community-acquired pneumonia. Clin Infect Dis. 2008;47(2):182–187. doi: 10.1086/589246. [DOI] [PubMed] [Google Scholar]
- 18.Rombauts A, et al. Host- and pathogen-related factors for acute cardiac events in pneumococcal pneumonia. Open Forum Infect Dis. 2020;7(12):ofaa522. doi: 10.1093/ofid/ofaa522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seo H-M, et al. Reciprocal relationship between herpes zoster and cardiovascular diseases: a nationwide population-based case-control study in Korea. J Dermatol. 2018;45(11):1312–1318. doi: 10.1111/1346-8138.14597. [DOI] [PubMed] [Google Scholar]
- 20.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Varicella zoster virus vasculopathies: diverse clinical manifestations, laboratory features, pathogenesis, and treatment. Lancet Neurol. 2009;8(8):731–740. doi: 10.1016/S1474-4422(09)70134-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cassimos DC, Effraimidou E, Medic S, Konstantinidis T, Theodoridou M, Maltezou HC. Vaccination programs for adults in Europe, 2019. Vaccines (Basel) 2020;8(1):34. doi: 10.3390/vaccines8010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.WMA - The world medical association-WMA Declaration of Helsinki – ethical principles for medical research involving human subjects. https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ . Accessed 14 Oct 2021. [PubMed]
- 23.Données de couverture vaccinale grippe par groupe d’âge. https://www.santepubliquefrance.fr/determinants-de-sante/vaccination/donnees-de-couverture-vaccinale-grippe-par-groupe-d-age. Accessed 12 Nov 2021.
- 24.Données de couverture vaccinale pneumocoque par groupe d’âge. https://www.santepubliquefrance.fr/determinants-de-sante/vaccination/articles/donnees-de-couverture-vaccinale-pneumocoque-par-groupe-d-age. Accessed 22 May 2023.
- 25.Vaccination-info-service.fr. https://professionnels.vaccination-info-service.fr/Recommandations-vaccinales-specifiques/Personnes-exposees-a-des-risques-specifiques/Personnes-agees. Accessed 15 Dec 2020.
- 26.Roller-Wirnsberger R, et al. The role of health determinants in the influenza vaccination uptake among older adults (65+): a scope review. Aging Clin Exp Res. 2021;33(8):2123–2132. doi: 10.1007/s40520-021-01793-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lorini C, et al. Health literacy and vaccination: a systematic review. Hum Vaccin Immunother. 2018;14(2):478–488. doi: 10.1080/21645515.2017.1392423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagata JM, Hernández-Ramos I, Kurup AS, Albrecht D, Vivas-Torrealba C, Franco-Paredes C. Social determinants of health and seasonal influenza vaccination in adults ≥65 years: a systematic review of qualitative and quantitative data. BMC Public Health. 2013;13:388. doi: 10.1186/1471-2458-13-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.González-Block MÁ, et al. Influenza vaccination hesitancy in large urban centers in South America. Qualitative analysis of confidence, complacency and convenience across risk groups. PLoS One. 2021;16(8):e0256040. doi: 10.1371/journal.pone.0256040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grave C, et al. Perception des maladies cardiovasculaires et connaissance des facteurs de risque cardiovasculaires en France : Baromètre de Santé publique France 2019. Bull Epidémiologique Hebdomadaire, pp. p475–479. 2020. https://www.santepubliquefrance.fr/import/perception-des-maladies-cardiovasculaires-et-connaissance-des-facteurs-de-risque-cardiovasculaires-en-france-barometre-de-sante-publique-france-2019. Accessed 16 Nov 2021. [Online].
- 31.Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W-65. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 32.RoB 2: A revised Cochrane risk-of-bias tool for randomized trials’. https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials. Accessed 2 Nov 2021.
- 33.Ottawa Hospital Research Institute. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed 2 Nov 2021.
- 34.Song JY, et al. Effectiveness of influenza and pneumococcal polysaccharide vaccines against influenza-related outcomes including pneumonia and acute exacerbation of cardiopulmonary diseases: analysis by dominant viral subtype and vaccine matching. PLoS One. 2018;13(12):e0207918. doi: 10.1371/journal.pone.0207918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C-S, Wang S-T, Chou P. Efficacy and cost-effectiveness of influenza vaccination of the elderly in a densely populated and unvaccinated community. Vaccine. 2002;20(19–20):2494–2499. doi: 10.1016/s0264-410x(02)00181-0. [DOI] [PubMed] [Google Scholar]
- 36.Nichol KL, Nordin J, Mullooly J, Lask R, Fillbrandt K, Iwane M. Influenza vaccination and reduction in hospitalizations for cardiac disease and stroke among the elderly. N Engl J Med. 2003;348(14):1322–1332. doi: 10.1056/NEJMoa025028. [DOI] [PubMed] [Google Scholar]
- 37.Chang C-C, Chang Y-S, Chen W-S, Chen Y-H, Chen J-H. Effects of annual influenza vaccination on morbidity and mortality in patients with systemic lupus erythematosus: a nationwide cohort study. Sci Rep. 2016;6:37817. doi: 10.1038/srep37817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu I-F, et al. Effects of annual influenza vaccination on mortality and hospitalization in elderly patients with ischemic heart disease: a nationwide population-based study. Prev Med. 2012;54(6):431–433. doi: 10.1016/j.ypmed.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 39.Marra F, Zhang A, Gillman E, Bessai K, Parhar K, Vadlamudi NK. The protective effect of pneumococcal vaccination on cardiovascular disease in adults: a systematic review and meta-analysis. Int J Infect Dis. 2020;99:204–213. doi: 10.1016/j.ijid.2020.07.038. [DOI] [PubMed] [Google Scholar]
- 40.Chiang M-H, Wu H-H, Shih C-J, Chen Y-T, Kuo S-C, Chen T-L. Association between influenza vaccination and reduced risks of major adverse cardiovascular events in elderly patients. Am Heart J. 2017;193:1–7. doi: 10.1016/j.ahj.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 41.Phrommintikul A, Kuanprasert S, Wongcharoen W, Kanjanavanit R, Chaiwarith R, Sukonthasarn A. Influenza vaccination reduces cardiovascular events in patients with acute coronary syndrome. Eur Heart J. 2011;32(14):1730–1735. doi: 10.1093/eurheartj/ehr004. [DOI] [PubMed] [Google Scholar]
- 42.Meyers DG, Beahm DD, Jurisich PD, Milford CJ, Edlavich S. Influenza and pneumococcal vaccinations fail to prevent myocardial infarction. Heart Drug. 2004;4(2):96–100. doi: 10.1159/000077705. [DOI] [Google Scholar]
- 43.Heffelfinger JD, et al. Influenza vaccination and risk of incident myocardial infarction. Hum Vaccin. 2006;2(4):161–166. doi: 10.4161/hv.2.4.2943. [DOI] [PubMed] [Google Scholar]
- 44.Lavallée PC, et al. Influenza vaccination and cardiovascular risk in patients with recent TIA and stroke. Neurology. 2014;82(21):1905–1913. doi: 10.1212/WNL.0000000000000456. [DOI] [PubMed] [Google Scholar]
- 45.Hsu S-Y, Chen F-L, Liaw Y-P, Huang J-Y, Nfor ON, Chao D-Y. A matched influenza vaccine strain was effective in reducing the risk of acute myocardial infarction in elderly persons: a population-based study. Medicine (Baltimore) 2016;95(10):e2869. doi: 10.1097/MD.0000000000002869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siriwardena AN, Gwini SM, Coupland CAC. Influenza vaccination, pneumococcal vaccination and risk of acute myocardial infarction: matched case-control study. CMAJ. 2010;182(15):1617–1623. doi: 10.1503/cmaj.091891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Abajo FJ, et al. Influenza vaccine and risk of acute myocardial infarction in a population-based case-control study. Heart. 2022;108(13):1039–1045. doi: 10.1136/heartjnl-2021-319754. [DOI] [PubMed] [Google Scholar]
- 48.Hung IFN, et al. Prevention of acute myocardial infarction and stroke among elderly persons by dual pneumococcal and influenza vaccination: a prospective cohort study. Clin Infect Dis. 2010;51(9):1007–1016. doi: 10.1086/656587. [DOI] [PubMed] [Google Scholar]
- 49.Christiansen CF, Thomsen RW, Schmidt M, Pedersen L, Sørensen HT. Influenza vaccination and 1-year risk of myocardial infarction, stroke, heart failure, pneumonia, and mortality among intensive care unit survivors aged 65 years or older: a nationwide population-based cohort study. Intensive Care Med. 2019;45(7):957–967. doi: 10.1007/s00134-019-05648-4. [DOI] [PubMed] [Google Scholar]
- 50.Huang C-L, Nguyen PA, Kuo P, Iqbal U, Hsu Y-HE, Jian W-S. Influenza vaccination and reduction in risk of ischemic heart disease among chronic obstructive pulmonary elderly. Comput Methods Programs Biomed. 2013;111(2):507–511. doi: 10.1016/j.cmpb.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Sung L-C, et al. Influenza vaccination reduces hospitalization for acute coronary syndrome in elderly patients with chronic obstructive pulmonary disease: a population-based cohort study. Vaccine. 2014;32(30):3843–3849. doi: 10.1016/j.vaccine.2014.04.064. [DOI] [PubMed] [Google Scholar]
- 52.Ishigami J, Sang Y, Grams ME, Coresh J, Chang A, Matsushita K. Effectiveness of influenza vaccination among older adults across kidney function: pooled analysis of 2005–2006 through 2014–2015 influenza seasons. Am J Kidney Dis. 2020;75(6):887–896. doi: 10.1053/j.ajkd.2019.09.008. [DOI] [PubMed] [Google Scholar]
- 53.Chang T-Y, et al. The association between influenza infection, vaccination, and atrial fibrillation: a nationwide case-control study. Heart Rhythm. 2016;13(6):1189–1194. doi: 10.1016/j.hrthm.2016.01.026. [DOI] [PubMed] [Google Scholar]
- 54.Seo YB, et al. Effectiveness of the influenza vaccine at preventing hospitalization due to acute exacerbation of cardiopulmonary disease in Korea from 2011 to 2012. Hum Vaccin Immunother. 2014;10(2):423–427. doi: 10.4161/hv.26858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nichol KL, Margolis KL, Wuorenma J, Von Sternberg T. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N Engl J Med. 1994;331(12):778–784. doi: 10.1056/NEJM199409223311206. [DOI] [PubMed] [Google Scholar]
- 56.Wu H-H, Chang Y-Y, Kuo S-C, Chen Y-T. Influenza vaccination and secondary prevention of cardiovascular disease among Taiwanese elders-a propensity score-matched follow-up study. PLoS One. 2019;14(7):e0219172. doi: 10.1371/journal.pone.0219172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fang Y-A, Chen C-I, Liu J-C, Sung L-C. Influenza vaccination reduces hospitalization for heart failure in elderly patients with chronic kidney disease: A population-based cohort study. Acta Cardiol Sin. 2016;32(3):290–298. doi: 10.6515/ACS20150424L. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen C-I, et al. Influenza vaccination may lead to reduction of hospitalization for heart failure in elderly patients with chronic obstructive pulmonary disease. J Exp Clin Med. 2013;5(2):65–68. doi: 10.1016/j.jecm.2013.02.002. [DOI] [Google Scholar]
- 59.Chang Y-C, Chou Y-J, Liu J-Y, Yeh T-F, Huang N. Additive benefits of pneumococcal and influenza vaccines among elderly persons aged 75 years or older in Taiwan–a representative population-based comparative study. J Infect. 2012;65(3):231–238. doi: 10.1016/j.jinf.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 60.Hedlund J, Christenson B, Lundbergh P, Ortqvist A. Effects of a large-scale intervention with influenza and 23-valent pneumococcal vaccines in elderly people: a 1-year follow-up. Vaccine. 2003;21(25–26):3906–3911. doi: 10.1016/s0264-410x(03)00296-2. [DOI] [PubMed] [Google Scholar]
- 61.Piñol-Ripoll G, de la Puerta I, Santos S, Purroy F, Mostacero E. Chronic bronchitis and acute infections as new risk factors for ischemic stroke and the lack of protection offered by the influenza vaccination. Cerebrovasc Dis. 2008;26(4):339–347. doi: 10.1159/000151636. [DOI] [PubMed] [Google Scholar]
- 62.Lavallée P, Perchaud V, Gautier-Bertrand M, Grabli D, Amarenco P. Association between influenza vaccination and reduced risk of brain infarction. Stroke. 2002;33(2):513–518. doi: 10.1161/hs0202.102328. [DOI] [PubMed] [Google Scholar]
- 63.Grau AJ, Fischer B, Barth C, Ling P, Lichy C, Buggle F. Influenza vaccination is associated with a reduced risk of stroke. Stroke. 2005;36(7):1501–1506. doi: 10.1161/01.STR.0000170674.45136.80. [DOI] [PubMed] [Google Scholar]
- 64.Siriwardena AN, Asghar Z, Coupland CCA. Influenza and pneumococcal vaccination and risk of stroke or transient ischaemic attack-matched case control study. Vaccine. 2014;32(12):1354–1361. doi: 10.1016/j.vaccine.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 65.Lin H-C, Chiu H-F, Ho S-C, Yang C-Y. Association of influenza vaccination and reduced risk of stroke hospitalization among the elderly: a population-based case-control study. Int J Environ Res Public Health. 2014;11(4):3639–3649. doi: 10.3390/ijerph110403639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liu J-C, et al. ‘Influenza vaccination reduces hemorrhagic stroke risk in patients with atrial fibrillation: a population-based cohort study. Int J Cardiol. 2017;232:315–323. doi: 10.1016/j.ijcard.2016.12.074. [DOI] [PubMed] [Google Scholar]
- 67.Kao P-F, et al. Influenza vaccination might reduce the risk of ischemic stroke in patients with atrial fibrillation: a population-based cohort study. Oncotarget. 2017;8(68):112697–112711. doi: 10.18632/oncotarget.22352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen C-C, et al. Influenza vaccination and risk of stroke in women with chronic obstructive pulmonary disease: a nationwide, population-based, propensity-matched cohort study. Front Med. 2022;9, no. (Chen C.-C.; Chiu C.-C.; Yang T.Y.; Fang Y.-A.; Hao W.-R., b8501043@tmu.edu.tw; Liu J.-C., liumdcv@tmu.edu.tw) Division of Cardiology, Department of Internal Medicine, Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan. 10.3389/fmed.2022.811021. [DOI] [PMC free article] [PubMed]
- 69.Klaric JS, Beltran TA, McClenathan BM. An Association between herpes zoster vaccination and stroke reduction among elderly individuals. Mil Med. 2019;184(Suppl 1):126–132. doi: 10.1093/milmed/usy343. [DOI] [PubMed] [Google Scholar]
- 70.Yang Q, Chang A, Tong X, Merritt R. Herpes zoster vaccine live and risk of stroke among medicare beneficiaries: a population-based cohort study. Stroke. 2021;52(5):1712–1721. doi: 10.1161/STROKEAHA.120.032788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clar C, Oseni Z, Flowers N, Keshtkar-Jahromi M, Rees K. Influenza vaccines for preventing cardiovascular disease. Cochrane Database Syst Rev. 2015;(5):CD005050. 10.1002/14651858.CD005050.pub3. [DOI] [PMC free article] [PubMed]
- 72.Fröbert O, et al. Influenza vaccination after myocardial infarction: a randomized, double-blind, placebo-controlled, multicenter trial. Circulation. 2021;144(18):1476–1484. doi: 10.1161/CIRCULATIONAHA.121.057042. [DOI] [PubMed] [Google Scholar]
- 73.Jaiswal V, et al. Effect of Pneumococcal vaccine on mortality and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Med. 2022;11(13):3799. doi: 10.3390/jcm11133799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma J, et al. Associations between combined influenza and pneumococcal pneumonia vaccination and cardiovascular outcomes. Cardiology. 2021;146(6):772–780. doi: 10.1159/000519469. [DOI] [PubMed] [Google Scholar]
- 75.Ren S, et al. Generation of cardio-protective antibodies after pneumococcal polysaccharide vaccine: early results from a randomised controlled trial. Atherosclerosis. 2022;346:68–74. doi: 10.1016/j.atherosclerosis.2022.02.011. [DOI] [PubMed] [Google Scholar]
- 76.Yedlapati SH, et al. Effects of influenza vaccine on mortality and cardiovascular outcomes in patients with cardiovascular disease: a systematic review and meta-analysis. J Am Heart Assoc. 2021;10(6):e019636. doi: 10.1161/JAHA.120.019636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Armstrong BG, et al. Effect of influenza vaccination on excess deaths occurring during periods of high circulation of influenza: cohort study in elderly people. BMJ. 2004;329(7467):660. doi: 10.1136/bmj.38198.594109.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Drijkoningen JJC, Rohde GGU. Pneumococcal infection in adults: burden of disease. Clin Microbiol Infect. 2014;20(Suppl 5):45–51. doi: 10.1111/1469-0691.12461. [DOI] [PubMed] [Google Scholar]
- 79.Voordouw ACG, et al. Annual revaccination against influenza and mortality risk in community-dwelling elderly persons. JAMA. 2004;292(17):2089–2095. doi: 10.1001/jama.292.17.2089. [DOI] [PubMed] [Google Scholar]
- 80.Wang C-S, Wang S-T, Lai C-T, Lin L-J, Chou P. Impact of influenza vaccination on major cause-specific mortality. Vaccine. 2007;25(7):1196–1203. doi: 10.1016/j.vaccine.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 81.Ciszewski A, et al. Influenza vaccination in secondary prevention from coronary ischaemic events in coronary artery disease: FLUCAD study. Eur Heart J. 2008;29(11):1350–1358. doi: 10.1093/eurheartj/ehm581. [DOI] [PubMed] [Google Scholar]
- 82.Gurfinkel EP, Leon de la Fuente R, Mendiz O, Mautner B. Flu vaccination in acute coronary syndromes and planned percutaneous coronary interventions (FLUVACS) study: one-year follow-up. Eur Heart J. 2004;25(1):25–31. doi: 10.1016/j.ehj.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 83.Gurfinkel EP, de la Fuente RL, Mendiz O, Mautner B. Influenza vaccine pilot study in acute coronary syndromes and planned percutaneous coronary interventions. Circulation. 2002;105(18):2143–2147. doi: 10.1161/01.CIR.0000016182.85461.F4. [DOI] [PubMed] [Google Scholar]
- 84.Jackson ML, et al. The burden of community-acquired pneumonia in seniors: results of a population-based study. Clin Infect Dis. 2004;39(11):1642–1650. doi: 10.1086/425615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Udell JA, et al. Association between influenza vaccination and cardiovascular outcomes in high-risk patients: a meta-analysis. JAMA. 2013;310(16):1711–1720. doi: 10.1001/jama.2013.279206. [DOI] [PubMed] [Google Scholar]
- 86.Wang YH, et al. Real world study of influenza vaccination intervention among key population of chronic disease management in Shanghai community. Zhonghua Yu Fang Yi Xue Za Zhi. 2020;54(4):425–429. doi: 10.3760/cma.j.cn112150-20191031-00828. [DOI] [PubMed] [Google Scholar]
- 87.Modin D, et al. Influenza vaccine in heart failure. Circulation. 2019;139(5):575–586. doi: 10.1161/CIRCULATIONAHA.118.036788. [DOI] [PubMed] [Google Scholar]
- 88.Cox RJ, Brokstad KA, Ogra P. Influenza virus: immunity and vaccination strategies. Comparison of the immune response to inactivated and live, attenuated influenza vaccines. Scand J Immunol. 2004;59(1):1–15. doi: 10.1111/j.0300-9475.2004.01382.x. [DOI] [PubMed] [Google Scholar]
- 89.Aidoud A, Marlet J, Angoulvant D, Debacq C, Gavazzi G, Fougère B. Influenza vaccination as a novel means of preventing coronary heart disease: effectiveness in older adults. Vaccine. 2020;38(32):4944–4955. doi: 10.1016/j.vaccine.2020.05.070. [DOI] [PubMed] [Google Scholar]
- 90.Binder CJ, et al. Pneumococcal vaccination decreases atherosclerotic lesion formation: molecular mimicry between Streptococcus pneumoniae and oxidized LDL. Nat Med. 2003;9(6):736–743. doi: 10.1038/nm876. [DOI] [PubMed] [Google Scholar]
- 91.Suthers B, Hansbro P, Thambar S, McEvoy M, Peel R, Attia J. Pneumococcal vaccination may induce anti-oxidized low-density lipoprotein antibodies that have potentially protective effects against cardiovascular disease. Vaccine. 2012;30(27):3983–3985. doi: 10.1016/j.vaccine.2012.03.084. [DOI] [PubMed] [Google Scholar]
- 92.Kang J-H, Ho J-D, Chen Y-H, Lin H-C. Increased risk of stroke after a herpes zoster attack: a population-based follow-up study. Stroke. 2009;40(11):3443–3448. doi: 10.1161/STROKEAHA.109.562017. [DOI] [PubMed] [Google Scholar]
- 93.Kleinschmidt-DeMasters BK, Gilden DH. Varicella-zoster virus infections of the nervous system: clinical and pathologic correlates’. Arch Pathol Lab Med. 2001;125(6):770–780. doi: 10.5858/2001-125-0770-VZVIOT. [DOI] [PubMed] [Google Scholar]
- 94.Langan SM, Minassian C, Smeeth L, Thomas SL. Risk of stroke following herpes zoster: a self-controlled case-series study. Clin Infect Dis. 2014;58(11):1497–1503. doi: 10.1093/cid/ciu098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xu J, Lupu F, Esmon CT. Inflammation, innate immunity and blood coagulation. Hamostaseologie. 2010;30(1):5–6, 8–9. [PubMed]
- 96.Nicholls LAB, Gallant AJ, Cogan N, Rasmussen S, Young D, Williams L. Older adults’ vaccine hesitancy: psychosocial factors associated with influenza, pneumococcal, and shingles vaccine uptake. Vaccine. 2021;39(26):3520–3527. doi: 10.1016/j.vaccine.2021.04.062. [DOI] [PubMed] [Google Scholar]
- 97.Schmid P, Rauber D, Betsch C, Lidolt G, Denker M-L. Barriers of influenza vaccination intention and behavior – a systematic review of influenza vaccine hesitancy, 2005–2016. PLOS One. 2017;12(1):e0170550. doi: 10.1371/journal.pone.0170550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.MacDonald NE. Vaccine hesitancy: definition, scope and determinants. Vaccine. 2015;33(34):4161–4164. doi: 10.1016/j.vaccine.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 99.Betsch C, Böhm R, Chapman GB. Using behavioral insights to increase vaccination policy effectiveness. Policy Insights Behav Brain Sci. 2015;2(1):61–73. doi: 10.1177/2372732215600716. [DOI] [Google Scholar]
- 100.Nowalk MP, Middleton DB, Zimmerman RK, Hess MM, Skledar SJ, Jacobs MA. Increasing pneumococcal vaccination rates among hospitalized patients. Infect Control Hosp Epidemiol. 2003;24(7):526–531. doi: 10.1086/502240. [DOI] [PubMed] [Google Scholar]
- 101.Wu S, et al. Factors associated with the uptake of seasonal influenza vaccination in older and younger adults: a large, population-based survey in Beijing, China. BMJ Open. 2017;7(9):e017459. doi: 10.1136/bmjopen-2017-017459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schattner A. Cardiovascular-targeted patient education and uptake of influenza vaccination in elderly patients. Patient Educ Couns. 2020;103(5):1052–1054. doi: 10.1016/j.pec.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 103.Xiong T-Y, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long-term implications. Eur Heart J. 2020;41(19):1798–1800. doi: 10.1093/eurheartj/ehaa231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.ACC clinical bulletin focuses on cardiac implications of coronavirus (COVID-19). American College of Cardiology. https://www.acc.org/latest-in-cardiology/articles/2020/02/13/12/42/http%3a%2f%2fwww.acc.org%2flatest-in-cardiology%2farticles%2f2020%2f02%2f13%2f12%2f42%2facc-clinical-bulletin-focuses-on-cardiac-implications-of-coronavirus-2019-ncov. Accessed 14 Oct 2021.
- 105.ESC guidance for the diagnosis and management of cv disease during the COVID-19 pandemic. https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance, https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance. Accessed 14 Oct 2021.
- 106.Shi S, et al. Characteristics and clinical significance of myocardial injury in patients with severe coronavirus disease 2019. Eur Heart J. 2020;41(22):2070–2079. doi: 10.1093/eurheartj/ehaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tessitore E, et al. Mortality and high risk of major adverse events in patients with COVID-19 and history of cardiovascular disease. Open Heart. 2021;8(1):e001526. doi: 10.1136/openhrt-2020-001526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mahmud E, et al. Management of acute myocardial infarction during the COVID-19 pandemic: a consensus statement from the Society for Cardiovascular Angiography and Interventions (SCAI), the American College of Cardiology (ACC), and the American College of Emergency Physicians (ACEP) Catheter Cardiovasc Interv. 2020;96(2):336–345. doi: 10.1002/ccd.28946. [DOI] [PubMed] [Google Scholar]
- 109.Ivey KS, Edwards KM, Talbot HK. Respiratory syncytial virus and associations with cardiovascular disease in adults. J Am Coll Cardiol. 2018;71(14):1574–1583. doi: 10.1016/j.jacc.2018.02.013. [DOI] [PubMed] [Google Scholar]
- 110.Papi A, et al. Respiratory syncytial virus prefusion F protein vaccine in older adults. N Engl J Med. 2023;388(7):595–608. doi: 10.1056/NEJMoa2209604. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used to support the findings of the study are included within the article.