Abstract
Perivascular spaces are the fluid-filled areas surrounding small blood vessels in the brain, and they may play a role similar to lymphatic vessels in clearing metabolic waste. When their diameters exceed 1 mm, as measured by structural magnetic resonance imaging, they are classified as enlarged perivascular spaces (EPVS). Previously, EPVS were considered to be benign, but increasing evidence suggests that their existence may be associated with various clinical diseases. Here, we review recent clinical studies to understand the potential clinical implications of EPVS. We also review the anatomy and imaging characteristics of EPVS and discuss four causal hypotheses for their formation and associated risk factors. Due to differences in research methods and concerns across studies, unified conclusions are difficult to achieve. Overall, more basic high-quality research is needed to clarify the subject and provide more concrete theoretical support.
Keywords: cerebral small vessels, enlarged perivascular spaces, cognitive decline, cerebral amyloid angiopathy, Parkinson’s disease, aging
Introduction
Perivascular spaces are spaces around perforating cerebral arteries and are also called Virchow–Robin spaces.1,2 The initial research on perivascular spaces mostly focused on anatomy and pathology, however, with the development of magnetic resonance imaging (MRI) technology, they have received an increasing amount of attention, and numerous clinical studies on perivascular spaces have emerged. As a transportation channel from the brain to the cerebrospinal fluid (CSF),3 perivascular spaces connected blood vessel and nerves,4 so it may be of relevance for cerebrovascular, neurodegenerative, and neuroinflammatory diseases.
EPVS is a common occurrence in the aging population,5 and advanced age is a risk factor associated with EPVS.6 Studies have revealed that the presence of EPVS is linked to an increased risk of cognitive decline, dementia, stroke, and cerebral small vessel disease (CSVD), which are considered age-related pathologies.7–9 Although the exact mechanisms underlying the correlation of EPVS in older adults are not fully understood, it is believed that vascular stiffness and aging, brain atrophy, and deposition of metabolic substances may contribute to the development and progression of these conditions. Therefore, in this review, we summarize and discuss the associations between these factors and explore the current knowledge on anatomy, physiology, and major imaging features, as well as the risk factors affecting perivascular spaces and clinical research progress of age-related diseases. Overall, our study provides foundational insights for future clinical studies.
Anatomy and Glial Lymphocytes
Understanding of the anatomical structure of perivascular space is improving. It was initially believed that perivascular space was directly connected to subarachnoid space;10 however, in 1990 it was found that the perivascular spaces of cerebral cortical arteries are surrounded by vascular walls and by a peripheral monolayer of pial meninges and that they are not connected with the subarachnoid space. The PVSs of veins on the other hand do not have this membrane and are directly connected to the subarachnoid space.11 The perivascular spaces of the cortex and basal ganglia are not identical; there is a layer of pial meninges surrounding the vessels of cortical arterioles and venules, and perivascular spaces are located between the vessel wall and the pial meninge. Basilar ganglia arterioles on the other hand are surrounded by two layers of pial meninges, and perivascular spaces are located between these two layers,12 which are connected to subarachnoid space. The perivascular spaces around the veins of the basal ganglia are similar to those of the cortex, but the pial meninges are discontinuous. Now with the development of high-resolution MRI, a better tool to help understand the anatomy of perivascular spaces emerged. The perivascular spaces in the basal ganglia are connected to the basal cisterna, extend significantly from the base of the putamen, and follow the perforating vessels upward with uneven thickness; perivascular spaces in the white matter area are not connected with the subarachnoid space and converge to the ventricle from the subcortex.13
It is generally believed that perivascular spaces play a role in the peripheral circulation of the central nervous system. The main fluid components in the perivascular space are the interstitial fluid, macrophages, and various proteins, including amyloid P, apolipoprotein E, proteoglycan, immunoglobulin G, and albumin.14 The functions of perivascular spaces are to transport CSF and remove metabolic waste from the brain parenchyma. The main circulation process can be outlined as follows: periarterial perivascular spaces are involved in the distribution of CSF and the exchange of CSF with the interstitial fluid, which flows back from perivenous perivascular spaces to meningeal lymphatics and eventually back to systemic circulation for clearance by the kidneys and liver.
EPVS and Age-Related Brain Pathologies
Studies carried in the hospital have shown that PVS in aging population is associated with complex mechanisms, such as arteriolosclerosis,15 Aβ-amyloid16,17 and tau proteins deposition, which are associated with altered fluid hydrodynamics and may be related to neurodegenerative diseases. And in animal experiments, Kress et al found that, compared to young mice, older mice had a 27% loss in vessel pulse ability and a 40% reduction in Aβ-amyloid clearance.18 Zenget al proposed that the microglia inflammatory process may mediate the relationship between glymphatic dysfunction and Alzheimer's Disease, and anti-neuroinflammation therapy may help break the vicious circle between pathology deposition and PVS expansion.19 Besides, A MRI-pathology investigation involving 654 older participants base on a community cohort shows that PVS is related to infarcts independent of other neuro-pathologies regardless of dementia status, which implied that PVS may share similar neurobiological pathways with the infracts.20 In conclusion, current research on PVS and age-related brain pathologies is still in its preliminary stage, and needs further study.
Hypotheses Related to the Cause of EPVSs
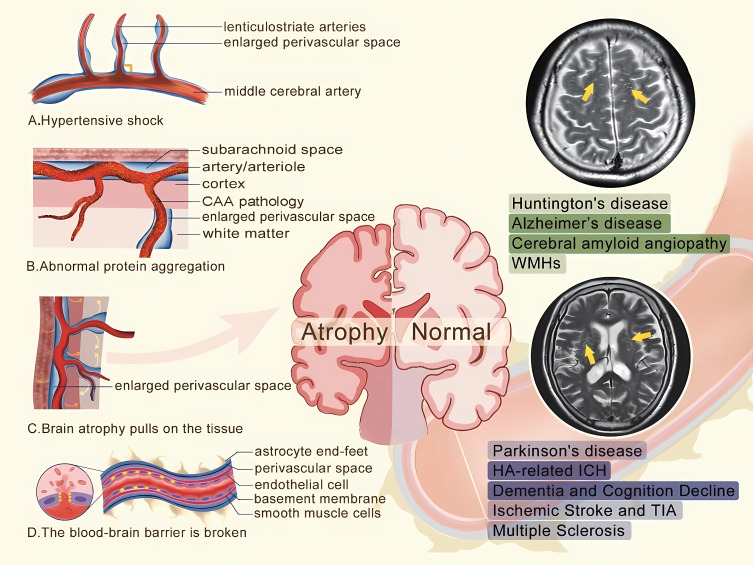
There are several proposed causal mechanisms for perivascular space enlargement: (1) arterial stiffening, (2) protein aggregation (3), brain atrophy (4), and destruction of the blood-brain barrier (BBB) (Figure 1). First, arterial stiffening, a sign of aging, likely contributes to the existence of EPVSs in the basal ganglia.21 It is possible that blood vessel walls are damaged and remodeled22 due to the impact of high pulse waves, and that the perforating arteries in the basal ganglia region are more vulnerable to damage than other blood vessels.23 In addition, the reduced elasticity and thickening of the vascular wall impair the ability of the contractile phenotype of smooth muscle cells to discharge metabolic waste, leading to the widening of the perivascular space.24 Second, the abnormal aggregation of proteins,24 such as β-amyloid, can block the upstream system of cortical arteries, resulting in the drainage of ISF and the widening of perivascular space. White matter has a lower cellular density and is more susceptible to the effects of pressure than gray matter; this is often used to explain the EPVS around the center of the semiovale. Third, brain atrophy with age created a phenomenon known as cavitation by pulling on the tissue around the blood vessels, which may explain the existence of the dilation of PVSs. The fourth proposed mechanism is damage to the Brain Blood Barriers (BBB). High blood pressure, age, or nonspecific inflammation can damage the endothelial cells of the blood vessels and disrupt the tight connections between endothelial cells, resulting in increased BBB permeability. This increases the amount of material leaking from blood vessels, which causes perivascular spaces to widen. These proposed mechanisms are still largely hypothetical and require more concrete evidence.
Figure 1.
The hypotheses of the cause mechanisms of perivascular space enlargement.
Notes: (A) The reduced elasticity and thickening of the vascular wall caused by high pulse waves impairs the ability of the contractile phenotype of smooth muscle cells to discharge metabolic waste; (B) The abnormal aggregation of proteins can block the upstream system of cortical arteries; (C) Brain atrophy pulls on the tissue around the blood vessels, creating a phenomenon known as cavitation; (D) The tight connections between endothelial cells was disrupted, resulting in increased blood-brain barrier permeability. The yellow arrow in the figure above refers to enlarged perivascular spaces in centrum semiovale, and in the figure below refers to enlarged perivascular spaces in the basal ganglia.
Perivascular spaces present similar to CSF on all MRI sequences, showing a low signal on T1WI and FLAIR and a high signal on T2WI, with a uniform signal, no mass effect, and no contrast enhancement effect. According to the Standards for Reporting Vascular Changes on Euro imaging (STRIVE), perivascular spaces with a diameter >2 mm are considered EPVSs; the normal diameters of perivascular spaces are <2 mm and they rarely extend to 10–20 mm.25 Perivascular spaces are mainly distributed in the basal ganglia, subcortical white matter, and midbrain but can also be seen in the thalamus, cerebellum, and insula. They are constituted by space around the blood vessels, which extends in the direction of the perforator vessel. Therefore, according to the associated perforating arteries, perivascular spaces can roughly be divided into three types: the basal ganglia type around the deep passage branch of the pin-stria artery; the cerebral hemisphere type around the medullary arteries (hemi-oval center); and the midbrain type around the perforating artery of the posterior cerebral artery.1 The commonly used Potter score26 grades perivascular spaces from 0 to 4 according to the occurring numbers; grade 0: the absence of perivascular spaces in the brain plane determined in the basal ganglia and central hemiovale; grade 1: 1 to 10 perivascular spaces; grade 2: 11 to 20 perivascular spaces; grade 3: 21 to 40 perivascular spaces; and grade 4: >40 perivascular spaces. The midbrain is rated as 0 or 1 depending on whether any PVS can be seen. Perivascular spaces can be classified according to the location of blood vessels, as well as according to morphology. The two most common types are the single follicle type that is an isolated and round-like cystic structure and the local cluster type constituted by multiple strips and lines.
Because EPVS are filled with interstitial fluid, their signals and those of CSF on all sequences are often difficult to identify because of overlaps in intensity and distribution with the lacunae.27 Combining pathology and imaging, they can be distinguished as shown in Table 1.
Table 1.
The Differences Between Perivascular Spaces and Lacunes
| Perivascular Space | Lacune | |
|---|---|---|
| Location | Common in lower one thirds of basal ganglia | Common in upper two thirds of basal ganglia |
| Shape | Round, oval, linear, etc. mostly smooth edge | Mostly wedge-shaped irregular edges |
| Size | Usually < 2 mm in diameter | The diameter is 3 ~ 15mm |
| Symmetry | General symmetry | Asymmetry |
| FLAIR | No high density signal ring around it. | Acute phase, high signal; In the non-acute stage, the central signal is low and the peripheral signal is thin. |
There have also been reports of a rare large perivascular space called the giant tumefactive PVS (GTPVS). It is commonly found in the mesencephalothalamic region, paramedial artery, and white matter,5 and it is associated with headaches in approximately 50% of patients.28 In addition, cognitive decline, vertigo, ataxia, vision changes, and seizures have also been reported. The cause of large perivascular spaces is unclear. Regarding treatment, a waiting strategy may be best for asymptomatic patients, and surgical intervention may be best for those with cerebral edema to obtain pathological evidence and improve symptoms, but the selection and comparison of surgical methods are limited by the small number of cases.29
Risk Factors
The risk factors associated with EPVSs include demographic characteristics, genetics, hypertension, and sleep duration and associated inflammation. Advancing age is significantly correlated with perivascular spaces and positively correlated with the severity of EPVSs, which may be an independent risk factor.5 Age is more strongly correlated with EPVSs in the basal ganglia region than in the centrum semiovale.30 Studies have shown that in healthy people and patients with Alzheimer’s disease (AD), EPVSs are more common in men than in women,31 especially in the white matter;32 however, there is no hypothesis for this sex difference. The prevalence of EPVSs is much lower in Chinese patients than in white patients with TIA/ischemic stroke.33 In addition, there may be differences in the classic distribution pattern of perivascular spaces in cerebral amyloid angiopathy (CAA).34 Under the premise of uniform research methods, ethnic differences need to be further explored.
Vascular risk factors are key to understanding the association between EPVS and clinical diseases and hypertension plays an important role in it.35 In the priming phase, chronic stress exposure initiates hypoxia-sensitive gene expression and molecular cascade. The release of inflammatory factors, including cytokines, inflammatory matrix metalloproteinases and cyclooxygenase-2, in turn, acts on the BBB inducing the expression of adhesion molecules in endothelial cells, thereby promoting leukocyte and platelet adhesion and microvascular obstruction.36–38 It becomes an important hypothesis for the formation of EPVS. Yao et al39 have proposed that hypertension may promote the development of EPVS in the whole brain, including BG, WM and hippocampus (HP). Further studies have shown that systolic blood pressure level is independently correlated with EPVS in BG, but not in WM.40,41 The control of blood pressure may play an important role in EPVS.
Perivascular spaces play an important role in the removal of metabolic waste, and studies have shown that deep sleep promotes the metabolism of substances such as beta-amyloid protein. Poor sleep efficiency is independently correlated with the increase of BG-PVSs,42 and the EPVS burden of individuals with a short sleep duration was significantly higher,43 suggesting that sleep may be a risk factor for perivascular space enlargement.
EPVSs and Clinical Diseases
EPVS is a Potential Marker of Cognitive Decline and Dementia
It has been recognized that cerebral small vessel diseases (CSVD) are associated with cognitive decline. Therefore, the role of EPVS, an emerging CSVD imaging marker, in cognitive impairment is a hot topic of current research (see in Table 2 and Table 3). The evidence has shown that EPVS is a potential marker of cognitive decline and dementia.44 A recent community-based study suggested that the association between EPVS and cognitive decline and dementia was independent of other CSVD markers.44 It has been reported that there is a negative correlation between EPVS counts and MoCA scores, wherein an increase in the number of EPVS is related to a decrease in the MoCA score, regardless of other CSVD markers and vascular factors.45 A study involving 6135 person-years of follow-up demonstrated that participants with the highest degree of EPVS (more than 20 EPVSs in the white matter or a cribriform change in the basal ganglia) is related to incident dementia,46 and EPVS in the basal ganglia region were significantly associated with vascular dementia.47 In addition, studies have shown that severe EPVS at baseline experience is related to worsening of information processing, visuospatial testing and executive function.48 No studies have shown the correlation of EPVS with memory impairment, even for EPVS in hippocampal regions.49,50 A study with a follow-up period of up to 8 years showed no significant association between hippocampal EPVS load and baseline cognitive performance or dementia events.39 Surprisingly, a study in elderly without dementia found that EPVS is associated with good memory performance, but the result should be interpreted with caution.51
Table 2.
Characteristics of Non-Dementic Studies Included in the Review
| Study | Study Design | Geographic Region | Subject Characteristics | Sample Size | EPVS Locations | EPVS Assessment | Rating Scale | High Degree/Threshold of EPVS | Age (mean) | F/M | Type of Scanner, MRI Sequence |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Charidimou et al56 | Prospective cohort | USA | ICH | 452 | CSO and BG | Count | 4 points | >20 | 70.6 | 217/235 | 1.5T, T1, T2, FLAIR |
| Martinez-Ramirez et al17 | Retrospective cohort | USA | CAA | 117 | CSO only | Volume | 4 points | >20 | 73.6 | 38/25 | 1.5T, 3T, T2, T2*-GRE |
| Best et al86 | Prospective cohort | UK | ICH in OACs | 14/1386 | CSO and BG | Count | 4 points | >10 | 75.8 | 575/811 | NA, T1, T2, FLAIR, T2*-GRE |
| Raposo et al59 | Prospective cohort | France | ICH | 38 | CSO and BG | Count | 5 points | >20 | 65.8 | 15/23 | 3T, T2, T2*-GRE, FLAIR |
| Tsai34 | Prospective cohort | China | ICH | 108 | CSO and BG | Count | 4 points | >20 | 66.9 | 37/71 | 3T, T1, T2, FLAIR |
| Park et al75 | Retrospective cohort | Korea | PD | 271 | CSO and BG | Count | 5 points | >20 | 66.6 | / | 3T, T2, FLAIR |
| Fang et al72 | Prospective cohort | China | PD and HCs | 343 | CSO and BG and MC | Count | 4 points /binary | >20 | 60.6 | 122/221 | 1.5T or 3T, T2 |
| McKnight et al87 | Retrospective cohort | USA | PD and ET | 181 | The PVS of the medullary veins | Volume | ALPS-index | / | 63.4 | 63/118 | 3T, T1, T2 |
| Chung et al88 | Retrospective cohort | Korea | PD | 248 | BG only | Count | 4 points | >10 | 68.88 | 130/118 | 3T, T2, T2*-GRE, FLAIR |
| Lau et al89 | Prospective cohort | UK and China | TIA/ischemic stroke | 2002 | CSO and BG | Count | <11/ 11–20/ >20 | >20 | 68.5 | 881/1121 | 1.5T/3T, NA |
| Liang et al71 | Retrospective cohort | China | TIA/ischemic stroke | 648 | CSO and BG | Count | 4 points | / | 65.8 | 263/385 | 1.5T, T1, T2, FLAIR |
| Song et al33 | Retrospective cohort | China | Ischemic stroke | 494 | CSO and BG | Count | 5 points | >20 | 66.4 | 207/287 | 3T, T1, T2, FLAIR |
| Wang et al68 | Prospective cohort | China | General population | 161 | CSO and BG | Quantitative measures | 5 points | / | 60.4 | 89/72 | 3T, T1, T2, FLAIR |
| Potter et al67 | Prospective cohort | UK | Acute stroke | 298 | CSO and BG | Count | 5 points | / | 68 | / | 1.5T T1, T2, FLAIR, T2*-GRE |
| Aribisala et al70 | Retrospective cohort | UK | CSVD | 866 | CSO and BG and HP | NA | NA | / | 73 | NA | 1.5T, T1, T2, FLAIR, T2*-GRE |
| Rouhl et al69 | Prospective cohort | Netherlands | CSVD | 163 | CSO and BG and MC | Count | 3 points | / | 63.9 | 63/100 | 1.5T, T1, T2, FLAIR |
| Conforti et al77 | Retrospective cohort | Italy | MS | 37 | CSO and BG and MC and HP | Volume | / | / | 42.7 | 25/12 | 3T, T1, T2, FLAIR, etc |
| Etemadifar et al78 | A case-control study | Italy | MS | 73/73 | CSO and BG and MC | Shape, size | / | / | 32.3 | 55/18 | 1.5T, T1, T2, FLAIR, |
| Wuerfel et al79 | A case-control study | Germany | MS | 45 | / | Volume | / | / | 39.8 | 23/22 | 1.5T, T1, T2, FLAIR, |
Table 3.
Characteristics of Studies of Cognition Decline and Dementia Included in the Review
| Study | Participation | Study Design | Geographic Region | Sample Size | EPVS Locations | EPVS Assessment | Rating Scale | Age (mean) | F/M | Type of Scanner MRI Sequence | Cognitive Evaluation Tool | Congnitive Domain |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hilal et al90 | General population | Meta analysis | Germany | 3575 | BG and CSO and HP and MC | Count | The Rotterdam-Graz EPVS | 68.3 | 1808/1767 | NA, T1, T2, FLAIR | MMSE and G-factor | Visuomotor speed(BG) / memory domain (Hp) |
| Ding et al51 | General population | Prospective cohort | USA | 2612 | BG and CSO | Count | >3 mm | 74.6 | 1542 | 1.5T, T1, T2, FLAIR | Verbal memory, processing speed, and executive function | Information processing speed |
| Javierre-Petit et al20 | General population | Prospective cohort | USA | 654 | Brain hemi-sphere | Count and shape | 3-point scale | 90.1 | 180/474 | 3T | MMSE | Visuospatial abilities, semantic memory |
| Zhu et al46 | General population | Prospective cohort | France | 1778 | BG and CSO | Count | ---- | 72.4 | 1081/691 | 1.5T, T1, T2, | MMSE and ISTet al DSM-IV criteria | Verbal fluency and psychomotor speed |
| Libecap et al45 | General population | Cross-sectional | USA | 110 | CAO, BG, HP and MC | Counts | Numbers | / | 46/64 | 3T, T1, FLAIR | MoCA | / |
| Hurford et al54 | Patients with ischaemic stroke and TIA | Prospective cohort | UK | 246 | BG and CSO | Count | 4 points | 62 | 110/136 | 1.5T, T1, T2, FLAIR, T2*-GRE | NART and seven cognitive domains | None |
| Yao et al39 | General population | Prospective cohort | France | 1818 | HP | Count | 3 points | 72.46 | 1112/706 | 1.5T, T1, T2, FLAIR | MMSE and IST, et al | None |
| Francesco et al53 | Patients with ischaemic stroke and TIA | Retrospective cohort | Italy | 430 | BG and CSO | Count | 4 points | 64.7 | 154/276 | NA, T1, T2, FLAIR | MMSE | NG |
| Philip et al55 | Patients with lacunar stroke syndrome | Prospective cohort | UK | 120 | BG and CSO and MC | Count and volume | 4 points | 70 | 42/78 | 1.5T, T1, T2, FLAIR | Standardized tests | None |
| Passiak et al48 | General population | Cross-sectionally | USA | 327 | BG | Count | / | 134/193 | 1.5T, T1, T2, FLAIR | The California Verbal Learning Test–Second Edition, the Biber Figure Learning Test, DKEFS Test, Letter-Number Switching Test | Multiple information processing and executive function performances | |
| Joan et al49 | Patients with hypertension | Prospective cohort | Spain | 723 | HP and MC | Count | / | 64 | 355/368 | 1.5T, T1, T2, FLAIR | Dementia Rating Scale-2, | |
| Matthew et al44 | General population | Prospective cohort | Australia | 414 | BG and CSO | Count | / | 79.8 | 218/196 | 3T, T1, T2, FLAIR | MMSE | Verbal reasoning (HP) |
| Sim J et al50 | General population | Prospective cohort | Italy | 109 | HP | Count | 5-point | 65.2 | 52/57 | 3T, T1, T2, FLAIR, T2*-GRE | MMSE, ADAS-Cog, Montreal Cognitive Assessment | None |
| Ciampa et al63 | Patients with AD cognitively unimpaired | Retrospective cohort | Switzerland | 680 | BG and CSO | Count | 4 points | 59.95 | 220/460 | 3T, T1-TFE, T2-FLAIR | / | / |
| Kim at al60 | Patients with AD–related cognitive impairment | Retrospective cohort | Korea | 144 | BG and CSO | Count | 4 points | 73.35 | 88/56 | 3T, T1, T2, FLAIR | Clinical Dementia Rating Scale–Sum of Boxes score, MMSE, et al | / |
| Vilor-Tejedor et al62 | Patients with AD cognitively unimpaired | Retrospective cohort | Spain | 322 | BG and CSO | Count | 4 points | 60.7 | 204/118 | 3T, T1, T2, FLAIR | / | / |
| Banerjee et al61 | Patients with cognitive impairment | Prospective cohort | UK | 226 | BG and CSO | Count | 4 points | 72.1 | 128/98 | 3T, T1, T2, T2*GRE, FLAIR | NINCDS-ADRDA | / |
Notes: Seven cognitive domains: current intellectual functioning, verbal and visual memory, nominal, perceptual, frontal executive and speed and attention functions.
Abbreviations: CSO, centrum semiovale; BG, basal ganglia; HP, hippocampus; MC, mesencephalon; CAA, cerebral amyloid angiopathy; HA, hypertensive arteriopathy; ICH, Spontaneous intracerebral hemorrhage; MMSE, Mini-Mental State Examination; G-factor, general fluid cognitive ability factor; IST, the Isaacs Set; NART, National Adult Reading Test; ADAS-Cog, The Alzheimer’s Disease Assessment Scale-Cognitive Subscale; PD, Parkinson disease; ET, essential tremor; TIA, transient ischemic attack; CSVD, cerebral small vessel disease.
There are also findings that point away from the association between EPVS and cognitive decline. A recent study in a large memory clinic population suggest that EPVS may not be used as a specific CSVD marker for cognitive impairment.52 Besides, the relationship between EPVS and cognitive decline in patients with ischemic cerebral events has not been established.53,54 Several articles support the theory that EPVS are associated with dementia, but it is not clear whether this relationship is causal or concomitant.24
Due to the widespread use of the 4-point scale method, the location and number of perivascular spaces are the main focus of current studies, but other traits, such as size and volume,55 are also informative. For example, study that reported that the presence of LPVSs (>3 mm in diameter) was significantly associated with impaired information processing performance and an increased risk of vascular dementia has received attention.51
EPVSs May Distinguish Cerebral Amyloid Angiopathy from Spontaneous Intracerebral Hemorrhage
CAA is one of the common causes of spontaneous intracerebral hemorrhage (ICH) which is characterized by strict cerebral hemorrhage and hypertensive arteriopathy (HA)-related ICH is more common in basal ganglia(BG) regions. Studies have shown that different MRI-visible EPVS patterns may be of significance in distinguishing the dominant cause of ICH. The prevalence of high-degree BG-EPVS may be associated with HA-ICH and EPVS in the central semiovale region (CSO) is related to CAA-related ICH.56 CAA is characterized by extensive β-amyloid deposition that erodes cerebral arterioles and replaces smooth muscle, leading to extracellular degradation57 and, ultimately, hemorrhage. The possible cause of the vascular accumulation of β-amyloid is the clearing of mechanical obstacles that affect the CSF outflow pathway and increase interstitial pressure, resulting in dilated perivascular space. A study further validated the associations between CSO-EPVS and pathologically confirmed sporadic CAA and Dutch-Type CAA; the relative volume of CSO-PVs in both groups was significantly higher than that in the non-CAA group and was not associated with bleeding.17 18F-florbetapir PET,58 an emerging imaging test to detect the β-amyloid burden in the human brain, also provides supporting evidence.34,59,60 The role of amyloid deposition in perivascular space enlargement needs to be further elucidated.
Failure to clear β-amyloid may also lead to increased β-amyloid accumulation in the parenchyma, and the association between perivascular space widening in the center of the semiovale and AD is also of concern. One study reported that a high degree of MRI-visible CSO-EPVSs independently predicted β-amyloid positivity in patients with AD-related cognitive impairment.60 Patients with more than 20 EPVSs in the white matter area had a significantly higher risk of AD and related dementias than those with 11–20 EPVSs.61 In addition to the clearance mechanism of brain metabolic waste, some scholars have also elaborated on the relationship between EPVSs and AD from the perspective of tau pathophysiology, neurodegeneration, and synaptic dysfunction62 or AD gene susceptibility.63 Some studies have indicated that β-amyloidosis and EPVSs in patients with AD are not associated with PET findings.60
The Influence of Other Markers on the Assessment of EPVSs Should Not Be Ignored
The interaction between EPVSs and other CSVD markers is complex, and their role in cognitive impairment is widely debated. It is believed that EPVSs, white matter lesions, and lacunae can be explained by arteriolosclerosis-related outcomes, such as increased cortical arterial pulsatility or hypoperfusion.64 But that does not mean that their clinical effects are the same, for example, Philip et al highlighted that the relationship between EPVS and cognitive decline, possibly due to the presence of lacunae, is overestimated.55
EPVS is close related to markers of CSVDs, such as WMHs, lacunae, and cerebral microbleeds. It is generally believed that WMH is a manifestation of white matter degradation and metabolic waste deposition, as well as an active state of the colloid lymphatic clearance mechanism, which is widely associated with other markers.65 Lacunae are small infarcts with diameters of 2–20 mm, and BG-PVSs have been reported to be closely related to lacunae.66,67 Cerebral microbleeds, typically 1–10 mm in diameter, are characterized by hemosiderin deposition. EPVSs are non-bleeding markers with diameters of less than 3 mm. They run along blood vessels anatomically, and it is not unexpected that they are affected by hypertensive damage, especially in the basal ganglions. A study on computational measures, including volume, count, size, length, width, and linearity, demonstrated that BG-EPVS features were related to most CSVD imaging markers.68 Similar differences have been found in previous literature in patients with ischemic stroke.31
The inflammatory mechanism is an important hypothesis for understanding the formation of CSVD, and there is also a close association between EPVS and inflammatory factors reported in existing studies. A study by Rouhl et al69 found that a higher degree of BG-EPVS was independently related to neopterin concentrations, which is a marker of activated monocytes/macrophages. Aribisala et al70 demonstrated that MRI-visible PVSs are weakly associated with circulating inflammatory markers, such as C-reactive protein (CRP) and interleukin-6 (IL-6)). These results are consistent with the hypothesis that inflammation affects CSVD by affecting small perforated arterioles. Longitudinal studies for determining whether inflammatory modulators can prevent small vessel disease are needed.
Perivascular Space May Be a Prognostic Indicator of Poor Ischemic Cerebrovascular Disease
EPVSs may serve as early prognostic markers of poor health-related quality of life in patients with mild to moderate acute ischemic stroke.71 A study involving two independent prospective cohorts from UK and China, predominantly comprising patients with transient ischemic attack (TIA)/ischemic stroke, indicated that BG-EPVS but not CSO-EPVS is a prognostic marker of stroke and death, independent of other neuroimaging markers of SVD.71 Multivariate analysis showed that patients with >20 BG-EPVSs showed a 1.8-fold increase in the risk of recurrent ischemic stroke. It should be noted that significant ethnic differences in the prevalence of EPVSs exist, and more prospective multicenter studies are needed to confirm them.
EPVSs are Associated with the Severity of Parkinson’s Disease
Parkinson’s disease (PD), a neurodegenerative disorder, is also of concern in relation to dilated perivascular space. EPVSs may be valuable markers of PD progression.72 Increased BBB permeability in the striatum of the brain73 and the deposition of abnormal proteins such as α- synuclein74 may lead to the formation of enlarged perivascular spaces in patients with PD. Yi et al found that a high degree of BG-EPVSs was associated with a higher movement disorder score, while a low degree of BG-EPVSs was more common during the early stage of the disease.72 In addition, EPVSs may play an important role in predicting cognitive decline in patients with PD75 and may be associated with specific higher neurological symptoms, such as the speed of olfactory and general cognitive processing.72 However, the type and location of abnormal protein deposition in PD and its role in disease progression remain to be clarified.
The Role of Perivascular Space Enlargement in Multiple Sclerosis is Unclear
PVSs clear abnormal metabolites and are considered to be the intersection of nerves, blood vessels, and immunity.4 According to a meta-analysis published in 2020 on EPVSs and multiple sclerosis (MS),76 there is no conclusive evidence that EPVSs have diagnostic and prognostic value for MS. However, several studies have reported a significant increase in the number77–79 and volume79 of PVSs in MS patients relative to the healthy population. This may indicate impaired central nervous system fluid drainage and/or excessive fluid leakage from the vasculature.80 Since most of the included studies were retrospective, it is difficult to determine whether EPVS is an accompanying feature or a risk factor. Compared with the number of EPVSs that may reflect cognitive decline, the diameter may better reflect the increase of local immune cells.
EPVSs are Associated with a Variety of Diseases
In addition to the diseases mentioned above, some diseases have been less studied but are relevant. A study81 of Huntington’s disease and the perivascular space reflected the burden of perivascular spaces by measuring the volume of EPVS and showed that a greater number of PVSs was associated with higher severity of the disease. More importantly, because impaired BBB and the glial lymphatic system may affect the efficacy of intrathecal injections, EPVS, which are closely related to these factors, are expected to be important indicators for assessing treatment eligibility. A study on epilepsy82 focused on conventional traits such as location or number but also on the asymmetrical distribution of EPVSs and found that the largest asymmetrical region of perivascular spaces may help to locate or confirm the onset of seizures. The relationship between EPVS and systemic lupus erythematosus83 validated the inflammatory sensitivity of CSO-EPVS relative to the basal ganglia region. Studies on aneurysmal subarachnoid hemorrhage84 and glioma infiltration85 also provide valuable insights that improve our understanding of the pathophysiological process of EPVS.
Discussion
EPVS is closely related to a variety of clinical diseases and is a promising potential indicator of cognitive impairment, disease course, and prognosis. However, it is worth noting that there is great heterogeneity among research methods. Therefore, researchers should aim for unification and standardization of research methods in the future. Additionally, some previous studies did not investigate the role of other CSVD in their evaluation of EPVS; thus, more systematic work is needed to further confirm this conclusion.
Conclusions
In this review, we have summarized the clinical consequences, anatomy, risk factors, and imaging characteristics of EPVS. We identified several studies showing that EPVS have strong clinical significance. However, most studies on EPVS rely on artificial vision scales with limitations such as dependence on vision, low accuracy, limited scope, and difficulty responding to changes during long-term follow-up. Automated and reproducible methods relying on computerized systems are expected to be used more often in the future. Given the possible clinical consequences of EPVSs, more studies are needed to explore this underexplored area.
Disclosure
The authors report no conflicts of interest in this work. Funded by Qingdao University of Science and Technology (number, WST 2021020) and the Affiliated Hospital of Qingdao University (number, X2021010).
References
- 1.Kwee RM, Kwee TC. Virchow-Robin spaces at MR imaging. Radiographics. 2007;27:1071–1086. [DOI] [PubMed] [Google Scholar]
- 2.Groeschel S, Chong WK, Surtees R, Hanefeld F. Virchow-Robin spaces on magnetic resonance images: normative data, their dilatation, and a review of the literature. Neuroradiology. 2006;48:745–754. doi: 10.1007/s00234-006-0112-1 [DOI] [PubMed] [Google Scholar]
- 3.Carare RO, Bernardes-Silva M, Newman TA, et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34:131–144. doi: 10.1111/j.1365-2990.2007.00926.x [DOI] [PubMed] [Google Scholar]
- 4.Troili F, Cipollini V, Moci M, et al. Perivascular unit: this must be the place. The anatomical crossroad between the immune, vascular and nervous system. Front Neuroanatomy. 2020;14:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heier LA, Bauer CJ, Schwartz L, Zimmerman RD, Morgello S, Deck MD. Large Virchow-Robin spaces: MR-clinical correlation. AJNR Am J Neuroradiol. 1989;10:929–936. [PMC free article] [PubMed] [Google Scholar]
- 6.Patankar TF, Mitra D, Varma A, Snowden J, Neary D, Jackson A. Dilatation of the Virchow-Robin space is a sensitive indicator of cerebral microvascular disease: study in elderly patients with dementia. AJNR Am J Neuroradiol. 2005;26:1512–1520. [PMC free article] [PubMed] [Google Scholar]
- 7.Rouhl RP, van Oostenbrugge RJ, Knottnerus IL, Staals JE, Lodder J. Virchow-Robin spaces relate to cerebral small vessel disease severity. Journal of Neurology. 2008;255:692–696. doi: 10.1007/s00415-008-0777-y [DOI] [PubMed] [Google Scholar]
- 8.Zhu YC, Tzourio C, Soumaré A, Mazoyer B, Dufouil C, Chabriat H. Severity of dilated Virchow-Robin spaces is associated with age, blood pressure, and MRI markers of small vessel disease: a population-based study. Stroke. 2010;41:2483–2490. doi: 10.1161/STROKEAHA.110.591586 [DOI] [PubMed] [Google Scholar]
- 9.Maclullich AM, Wardlaw JM, Ferguson KJ, Starr JM, Seckl JR, Deary IJ. Enlarged perivascular spaces are associated with cognitive function in healthy elderly men. J Neurol Neurosurg Psychiatry. 2004;75:1519–1523. doi: 10.1136/jnnp.2003.030858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mourad FH, Barada KA, Bou Rached NA, Khoury CI, Saadé NE, Nassar CF. Inhibitory effect of experimental colitis on fluid absorption in rat jejunum: role of the enteric nervous system, VIP, and nitric oxide. Am J Physiol Gastrointest Liver Physiol. 2006;290:G262–8. doi: 10.1152/ajpgi.00271.2005 [DOI] [PubMed] [Google Scholar]
- 11.Zhang ET, Inman CB, Weller RO. Interrelationships of the pia mater and the perivascular (Virchow-Robin) spaces in the human cerebrum. J Anat. 1990;170:111–123. [PMC free article] [PubMed] [Google Scholar]
- 12.Pollock H, Hutchings M, Weller RO, Zhang ET. Perivascular spaces in the basal ganglia of the human brain: their relationship to lacunes. J Anat. 1997;191(Pt 3):337–346. doi: 10.1046/j.1469-7580.1997.19130337.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouvy WH, Biessels GJ, Kuijf HJ, Kappelle LJ, Luijten PR, Zwanenburg JJ. Visualization of perivascular spaces and perforating arteries with 7 T magnetic resonance imaging. Investigative Radiology. 2014;49:307–313. doi: 10.1097/RLI.0000000000000027 [DOI] [PubMed] [Google Scholar]
- 14.Esiri MM, Gay D. Immunological and neuropathological significance of the Virchow-Robin space. J Neurol Sci. 1990;100:3–8. doi: 10.1016/0022-510X(90)90004-7 [DOI] [PubMed] [Google Scholar]
- 15.van Swieten JC, van den Hout JH, van Ketel BA, Hijdra A, Wokke JH, van Gijn J. Periventricular lesions in the white matter on magnetic resonance imaging in the elderly. A morphometric correlation with arteriolosclerosis and dilated perivascular spaces. Brain. 1991;114(Pt 2):761–774. doi: 10.1093/brain/114.2.761 [DOI] [PubMed] [Google Scholar]
- 16.van Veluw SJ, Biessels GJ, Bouvy WH, et al. Cerebral amyloid angiopathy severity is linked to dilation of juxtacortical perivascular spaces. J Cereb Blood Flow Metab. 2016;36:576–580. doi: 10.1177/0271678X15620434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinez-Ramirez S, van Rooden S, Charidimou A, et al. Perivascular spaces volume in sporadic and hereditary (Dutch-Type) cerebral amyloid angiopathy. Stroke. 2018;49:1913–1919. doi: 10.1161/STROKEAHA.118.021137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kress BT, Iliff JJ, Xia M, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76:845–861. doi: 10.1002/ana.24271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng Q, Li K, Luo X, et al. The association of enlarged perivascular space with microglia-related inflammation and Alzheimer’s pathology in cognitively normal elderly. Neurobiology of Disease. 2022;170:105755. doi: 10.1016/j.nbd.2022.105755 [DOI] [PubMed] [Google Scholar]
- 20.Javierre-Petit C, Schneider JA, Kapasi A, et al. Neuropathologic and cognitive correlates of enlarged perivascular spaces in a community-based cohort of older adults. Stroke. 2020;51:2825–2833. doi: 10.1161/STROKEAHA.120.029388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riba-Llena I, Jiménez-Balado J, Castañé X, et al. Arterial stiffness is associated with basal ganglia enlarged perivascular spaces and cerebral small vessel disease load. Stroke. 2018;49:1279–1281. doi: 10.1161/STROKEAHA.118.020163 [DOI] [PubMed] [Google Scholar]
- 22.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol. 2008;105:1652–1660. doi: 10.1152/japplphysiol.90549.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivera-Rivera LA, Schubert T, Turski P, et al. Changes in intracranial venous blood flow and pulsatility in Alzheimer’s disease: a 4D flow MRI study. J Cereb Blood Flow Metab. 2017;37:2149–2158. doi: 10.1177/0271678X16661340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bown CW, Carare RO, Schrag MS, Jefferson AL. Physiology and clinical relevance of enlarged perivascular spaces in the aging brain. Neurology. 2021;98:107–117. doi: 10.1212/WNL.0000000000013077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. The Lancet Neurology. 2013;12(8):822–838. doi: 10.1016/S1474-4422(13)70124-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Potter GM, Chappell FM, Morris Z, Wardlaw JM. Cerebral perivascular spaces visible on magnetic resonance imaging: development of a qualitative rating scale and its observer reliability. Cerebrovascular Diseases (Basel, Switzerland). 2015;39:224–231. doi: 10.1159/000375153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dubost F, Yilmaz P, Adams H, et al. Enlarged perivascular spaces in brain MRI: automated quantification in four regions. NeuroImage. 2019;185:534–544. doi: 10.1016/j.neuroimage.2018.10.026 [DOI] [PubMed] [Google Scholar]
- 28.Salzman KL, Osborn AG, House P, et al. Giant tumefactive perivascular spaces. AJNR Am J Neuroradiol. 2005;26:298–305. [PMC free article] [PubMed] [Google Scholar]
- 29.Al Abdulsalam H, Alatar AA, Elwatidy S. Giant tumefactive perivascular spaces: a case report and literature review. World Neurosurgery. 2018;112:201–204. doi: 10.1016/j.wneu.2018.01.144 [DOI] [PubMed] [Google Scholar]
- 30.Charidimou A, Meegahage R, Fox Z, et al. Enlarged perivascular spaces as a marker of underlying arteriopathy in intracerebral haemorrhage: a multicentre MRI cohort study. J Neurol Neurosurg Psychiatry. 2013;84:624–629. doi: 10.1136/jnnp-2012-304434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C, Chen Q, Wang Y, et al. Risk factors of dilated Virchow-Robin spaces are different in various brain regions. PLoS One. 2014;9:e105505. doi: 10.1371/journal.pone.0105505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ramirez J, Berezuk C, McNeely AA, Scott CJ, Gao F, Black SE. Visible Virchow-robin spaces on magnetic resonance imaging of Alzheimer’s disease patients and normal elderly from the Sunnybrook dementia study. J Alzheimer’s Dis. 2015;43:415–424. doi: 10.3233/JAD-132528 [DOI] [PubMed] [Google Scholar]
- 33.Song Q, Cheng Y, Wang Y, Liu J, Wei C, Liu M. Enlarged perivascular spaces and hemorrhagic transformation after acute ischemic stroke. Annals of Translational Medicine. 2021;9:1126. doi: 10.21037/atm-21-1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai HH, Pasi M, Tsai LK, et al. Centrum semiovale perivascular space and amyloid deposition in spontaneous intracerebral hemorrhage. Stroke. 2021;52:2356–2362. doi: 10.1161/STROKEAHA.120.032139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu Y, Dong YH, Lyu PY, Chen WH, Li R. Hypertension-induced cerebral small vessel disease leading to cognitive impairment. Chinese Medical Journal. 2018;131:615–619. doi: 10.4103/0366-6999.226069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenberg GA. Extracellular matrix inflammation in vascular cognitive impairment and dementia. Clinical Science (London, England. 1979;2017(131):425–437. [DOI] [PubMed] [Google Scholar]
- 37.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wallin A, Ohrfelt A, Bjerke M. Characteristic clinical presentation and CSF biomarker pattern in cerebral small vessel disease. J Neurol Sci. 2012;322:192–196. doi: 10.1016/j.jns.2012.07.068 [DOI] [PubMed] [Google Scholar]
- 39.Yao M, Zhu YC, Soumaré A, et al. Hippocampal perivascular spaces are related to aging and blood pressure but not to cognition. Neurobiology of Aging. 2014;35:2118–2125. [DOI] [PubMed] [Google Scholar]
- 40.Yang S, Yuan J, Zhang X, et al. Higher ambulatory systolic blood pressure independently associated with enlarged perivascular spaces in basal ganglia. Neurol Res. 2017;39:787–794. doi: 10.1080/01616412.2017.1324552 [DOI] [PubMed] [Google Scholar]
- 41.Yang S, Qin W, Yang L, et al. The relationship between ambulatory blood pressure variability and enlarged perivascular spaces: a cross-sectional study. BMJ open. 2017;7:e015719. doi: 10.1136/bmjopen-2016-015719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Del Brutto OH, Mera RM, Del Brutto VJ, Castillo PR. Enlarged basal ganglia perivascular spaces and sleep parameters. A population-based study. Clinical Neurology and Neurosurgery. 2019;182:53–57. doi: 10.1016/j.clineuro.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 43.Opel RA, Christy A, Boespflug EL, et al. Effects of traumatic brain injury on sleep and enlarged perivascular spaces. J Cereb Blood Flow Metab. 2019;39:2258–2267. doi: 10.1177/0271678X18791632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paradise M, Crawford JD, Lam BCP, et al. Association of dilated perivascular spaces with cognitive decline and incident dementia. Neurology. 2021;96:e1501–e11. doi: 10.1212/WNL.0000000000011537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Libecap TJ, Zachariou V, Bauer CE, et al. Enlarged perivascular spaces are negatively associated with Montreal cognitive assessment scores in older adults. Front Neurol. 2022;13:888511. doi: 10.3389/fneur.2022.888511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhu YC, Dufouil C, Soumaré A, Mazoyer B, Chabriat H, Tzourio C. High degree of dilated Virchow-Robin spaces on MRI is associated with increased risk of dementia. J Alzheimer’s Dis. 2010;22:663–672. doi: 10.3233/JAD-2010-100378 [DOI] [PubMed] [Google Scholar]
- 47.Smeijer D, Ikram MK, Hilal S. Enlarged perivascular spaces and dementia: a systematic review. J Alzheimer’s Dis. 2019;72:247–256. doi: 10.3233/JAD-190527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Passiak BS, Liu D, Kresge HA, et al. Perivascular spaces contribute to cognition beyond other small vessel disease markers. Neurology. 2019;92:e1309–e21. doi: 10.1212/WNL.0000000000007124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiménez-Balado J, Riba-Llena I, Garde E, et al. Prevalence of hippocampal enlarged perivascular spaces in a sample of patients with hypertension and their relation with vascular risk factors and cognitive function. J Neurol Neurosurg Psychiatry. 2018;89:651–656. doi: 10.1136/jnnp-2017-316724 [DOI] [PubMed] [Google Scholar]
- 50.Sim JE, Park MS, Shin HY, et al. Correlation between hippocampal enlarged perivascular spaces and cognition in non-dementic elderly population. Front Neurol. 2020;11:542511. doi: 10.3389/fneur.2020.542511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ding J, Sigurðsson S, Jónsson PV, et al. Large perivascular spaces visible on magnetic resonance imaging, cerebral small vessel disease progression, and risk of dementia: the age, gene/environment susceptibility-reykjavik study. JAMA Neurology. 2017;74:1105–1112. doi: 10.1001/jamaneurol.2017.1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Choe YM, Baek H, Choi HJ, et al. Association between enlarged perivascular spaces and cognition in a memory clinic population. Neurology. 2022;99:e1414–e1421. doi: 10.1212/WNL.0000000000200910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arba F, Quinn TJ, Hankey GJ, et al. Enlarged perivascular spaces and cognitive impairment after stroke and transient ischemic attack. International Journal of Stroke: Official Journal of the International Stroke Society. 2018;13:47–56. doi: 10.1177/1747493016666091 [DOI] [PubMed] [Google Scholar]
- 54.Hurford R, Charidimou A, Fox Z, Cipolotti L, Jager R, Werring DJ. MRI-visible perivascular spaces: relationship to cognition and small vessel disease MRI markers in ischaemic stroke and TIA. J Neurol Neurosurg Psychiatry. 2014;85:522–525. doi: 10.1136/jnnp-2013-305815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Benjamin P, Trippier S, Lawrence AJ, et al. Lacunar infarcts, but not perivascular spaces, are predictors of cognitive decline in cerebral small-vessel disease. Stroke. 2018;49:586–593. doi: 10.1161/STROKEAHA.117.017526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Charidimou A, Boulouis G, Pasi M, et al. MRI-visible perivascular spaces in cerebral amyloid angiopathy and hypertensive arteriopathy. Neurology. 2017;88:1157–1164. doi: 10.1212/WNL.0000000000003746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Damodarasamy M, Vernon RB, Pathan JL, et al. The microvascular extracellular matrix in brains with Alzheimer’s disease neuropathologic change (ADNC) and cerebral amyloid angiopathy (CAA). Fluids Barriers CNS. 2020;17:60. doi: 10.1186/s12987-020-00219-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hutton C, Declerck J, Mintun MA, Pontecorvo MJ, Devous MD, Joshi AD. Quantification of 18F-florbetapir PET: comparison of two analysis methods. Eur J Nucl Med Mol Imaging. 2015;42:725–732. doi: 10.1007/s00259-015-2988-7 [DOI] [PubMed] [Google Scholar]
- 59.Raposo N, Planton M, Payoux P, et al. Enlarged perivascular spaces and florbetapir uptake in patients with intracerebral hemorrhage. Eur J Nucl Med Mol Imaging. 2019;46:2339–2347. doi: 10.1007/s00259-019-04441-1 [DOI] [PubMed] [Google Scholar]
- 60.Kim HJ, Cho H, Park M, et al. MRI-visible perivascular spaces in the centrum semiovale are associated with brain amyloid deposition in patients with Alzheimer disease-related cognitive impairment. AJNR Am J Neuroradiol. 2021;42:1231–1238. doi: 10.3174/ajnr.A7155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Banerjee G, Kim HJ, Fox Z, et al. MRI-visible perivascular space location is associated with Alzheimer’s disease independently of amyloid burden. Brain. 2017;140:1107–1116. doi: 10.1093/brain/awx003 [DOI] [PubMed] [Google Scholar]
- 62.Vilor-Tejedor N, Ciampa I, Operto G, et al. Perivascular spaces are associated with tau pathophysiology and synaptic dysfunction in early Alzheimer’s continuum. Alzheimer Res Therap. 2021;13:135. doi: 10.1186/s13195-021-00878-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ciampa I, Operto G, Falcon C, et al. Genetic predisposition to Alzheimer’s disease is associated with enlargement of perivascular spaces in centrum semiovale region. Genes. 2021;12:825. doi: 10.3390/genes12060825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fazekas F, Kleinert R, Offenbacher H, et al. The morphologic correlate of incidental punctate white matter hyperintensities on MR images. AJNR Am J Neuroradiol. 1991;12:915–921. [PMC free article] [PubMed] [Google Scholar]
- 65.Huang P, Zhang R, Jiaerken Y, et al. Deep white matter hyperintensity is associated with the dilation of perivascular space. J Cereb Blood Flow Metab. 2021;41:2370–2380. doi: 10.1177/0271678X211002279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhu YC, Dufouil C, Mazoyer B, et al. Frequency and location of dilated Virchow-Robin spaces in elderly people: a population-based 3D MR imaging study. AJNR Am J Neuroradiol. 2011;32:709–713. doi: 10.3174/ajnr.A2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Potter GM, Doubal FN, Jackson CA, et al. Enlarged perivascular spaces and cerebral small vessel disease. International Journal of Stroke: Official Journal of the International Stroke Society. 2015;10:376–381. doi: 10.1111/ijs.12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang S, Huang P, Zhang R, et al. Quantity and morphology of perivascular spaces: associations with vascular risk factors and cerebral small vessel disease. Journal of Magnetic Resonance Imaging: JMRI. 2021;54:1326–1336. doi: 10.1002/jmri.27702 [DOI] [PubMed] [Google Scholar]
- 69.Rouhl RP, Damoiseaux JG, Lodder J, et al. Vascular inflammation in cerebral small vessel disease. Neurobiology of Aging. 2012;33:1800–1806. doi: 10.1016/j.neurobiolaging.2011.04.008 [DOI] [PubMed] [Google Scholar]
- 70.Aribisala BS, Wiseman S, Morris Z, et al. Circulating inflammatory markers are associated with magnetic resonance imaging-visible perivascular spaces but not directly with white matter hyperintensities. Stroke. 2014;45:605–607. doi: 10.1161/STROKEAHA.113.004059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liang Y, Deng M, Chen YK, et al. Enlarged perivascular spaces are associated with health-related quality of life in patients with acute ischemic stroke. CNS Neurosci Ther. 2017;23:973–979. doi: 10.1111/cns.12766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fang Y, Gu LY, Tian J, et al. MRI-visible perivascular spaces are associated with cerebrospinal fluid biomarkers in Parkinson’s disease. Aging. 2020;12:25805–25818. doi: 10.18632/aging.104200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gray MT, Woulfe JM. Striatal blood-brain barrier permeability in Parkinson’s disease. J Cereb Blood Flow Metab. 2015;35:747–750. doi: 10.1038/jcbfm.2015.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zou W, Pu T, Feng W, et al. Blocking meningeal lymphatic drainage aggravates Parkinson’s disease-like pathology in mice overexpressing mutated α-synuclein. Translational Neurodegeneration. 2019;8:7. doi: 10.1186/s40035-019-0147-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park YW, Shin NY, Chung SJ, et al. Magnetic resonance imaging-visible perivascular spaces in basal ganglia predict cognitive decline in Parkinson’s disease. Mov Disord. 2019;34:1672–1679. doi: 10.1002/mds.27798 [DOI] [PubMed] [Google Scholar]
- 76.Granberg T, Moridi T, Brand JS, et al. Enlarged perivascular spaces in multiple sclerosis on magnetic resonance imaging: a systematic review and meta-analysis. Journal of Neurology. 2020;267:3199–3212. doi: 10.1007/s00415-020-09971-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Conforti R, Cirillo M, Saturnino PP, et al. Dilated Virchow-Robin spaces and multiple sclerosis: 3 T magnetic resonance study. La Radiologia medica. 2014;119:408–414. doi: 10.1007/s11547-013-0357-9 [DOI] [PubMed] [Google Scholar]
- 78.Etemadifar M, Hekmatnia A, Tayari N, et al. Features of Virchow-Robin spaces in newly diagnosed multiple sclerosis patients. European Journal of Radiology. 2011;80:e104–8. doi: 10.1016/j.ejrad.2010.05.018 [DOI] [PubMed] [Google Scholar]
- 79.Wuerfel J, Haertle M, Waiczies H, et al. Perivascular spaces--MRI marker of inflammatory activity in the brain? Brain. 2008;131:2332–2340. doi: 10.1093/brain/awn171 [DOI] [PubMed] [Google Scholar]
- 80.Wardlaw JM, Doubal F, Armitage P, et al. Lacunar stroke is associated with diffuse blood-brain barrier dysfunction. Ann Neurol. 2009;65:194–202. doi: 10.1002/ana.21549 [DOI] [PubMed] [Google Scholar]
- 81.Chan ST, Mercaldo ND, Ravina B, Hersch SM, Rosas HD. Association of dilated perivascular spaces and disease severity in patients with Huntington disease. Neurology. 2021;96:e890–e4. doi: 10.1212/WNL.0000000000011121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feldman RE, Rutland JW, Fields MC, et al. Quantification of perivascular spaces at 7T: a potential MRI biomarker for epilepsy. Seizure. 2018;54:11–18. doi: 10.1016/j.seizure.2017.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miyata M, Kakeda S, Iwata S, et al. Enlarged perivascular spaces are associated with the disease activity in systemic lupus erythematosus. Sci Rep. 2017;7:12566. doi: 10.1038/s41598-017-12966-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim J, Joo B, Kim JW, et al. Aggravation of enlarged perivascular spaces in the centrum semiovale of patients with aneurysmal subarachnoid hemorrhage. Clinical Neuroradiology. 2021;2021:1–9. [DOI] [PubMed] [Google Scholar]
- 85.Pacioni S, D’Alessandris QG, Buccarelli M, et al. Brain invasion along perivascular spaces by glioma cells: relationship with blood-brain barrier. Cancers. 2019;12:18. doi: 10.3390/cancers12010018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Best JG, Barbato C, Ambler G, et al. Association of enlarged perivascular spaces and anticoagulant-related intracranial hemorrhage. Neurology. 2020;95:e2192–e9. doi: 10.1212/WNL.0000000000010788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.McKnight CD, Trujillo P, Lopez AM, et al. Diffusion along perivascular spaces reveals evidence supportive of glymphatic function impairment in Parkinson disease. Parkinsonism Relat Disord. 2021;89:98–104. doi: 10.1016/j.parkreldis.2021.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chung SJ, Yoo HS, Shin NY, et al. Perivascular spaces in the basal ganglia and long-term motor prognosis in newly diagnosed Parkinson disease. Neurology. 2021;96:e2121–e31. doi: 10.1212/WNL.0000000000011797 [DOI] [PubMed] [Google Scholar]
- 89.Lau KK, Li L, Lovelock CE, et al. Clinical correlates, ethnic differences, and prognostic implications of perivascular spaces in transient ischemic attack and ischemic stroke. Stroke. 2017;48:1470–1477. doi: 10.1161/STROKEAHA.117.016694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hilal S, Tan CS, Adams HHH, et al. Enlarged perivascular spaces and cognition: a meta-analysis of 5 population-based studies. Neurology. 2018;91:e832–e42. doi: 10.1212/WNL.0000000000006079 [DOI] [PMC free article] [PubMed] [Google Scholar]