Abstract
The COVID-19 pandemic put enormous pressure on the vaccine production chain as billions of vaccines had to be produced in the shortest timeframe possible. Vaccine production chains struggled to keep up with demand, resulting in disruptions and production delays. This study aimed to make an inventory of challenges and opportunities that occurred in the production chain of the COVID-19 vaccine. Insights derived through approximately 80 interviews and roundtable discussions were combined with findings from a scoping literature review. Data were analysed through an inductive process where barriers and opportunities were linked to specific facets of the production chain. Key bottlenecks identified include a lack of manufacturing facilities, a lack of tech-transfer personnel, inefficient arrangement of production stakeholders, critical shortages in raw materials, and restricting protectionist measures. A need for a central governing body to map out shortages and to coordinate allocation of available resource became evident. Other suggested solutions were to repurpose existing facilities and to build in more flexibility in the production process by making materials interchangeable. Also, simplification of the production chain could be achieved through geographical reengagement of processes. Three overarching themes were identified, impacting overall functioning of the vaccine production chain: regulatory and visibility, collaboration and communication, and funding and policy. The results in this study showed a multitude of interdependent processes underlying the vaccine production chain, executed by diverse stakeholders with differing objectives. It characterizes the global complexity of the pharmaceutical production chain and highlights its extreme vulnerability to disruptions. More resilience and robustness must be integrated into the vaccine production chain, and low-middle income countries should be empowered to manufacture vaccines themselves. In conclusion, there’s a need to rethink the production system for vaccines and other essential medicines in order to become better prepared for future health crises.
Keywords: Vaccine, Supply chain, Upscaling production, Barriers
1. Introduction
The COVID-19 pandemic resulted in an enormous pressure on global manufacturing and upscaling processes for vaccines. Mitigating the pandemic was a worldwide priority and vaccines were a critical part of the solution. In high-income countries, this led to the development and mass production of multiple vaccines, allowing for citizens to be vaccinated in multiple-shot vaccination regimens. In low-income countries, however, vaccination rates stayed generally behind due to the lack of sufficient vaccines.
Effective management of vaccine production chains is considered a crucial component in the global response to disease outbreaks. Nevertheless, various challenges inherent to the nature of the industry make it prone to disruptions during health crises. Firstly, the manufacturing of biologicals is technically demanding, lengthy and complex, requiring sophisticated equipment and facilities, and specific know-how. Secondly, due to vulnerability in production, the safety and effectiveness of biologicals can change rapidly, making stringent regulatory guidelines necessary alongside all parts of the production chain. This makes the production chain less adaptable and agile in times of uncertainty, and prolongs the time needed for companies to implement changes [1]. Lastly, due to the complex nature of vaccines and biologicals, manufacturers are ill prepared for novel threats and unable to scale up production of novel vaccines [2]. These properties present unique challenges for rapidly upscaling production of biologicals/vaccines during health emergencies, making management of the vaccine production chain particularly challenging during pandemics.
Despite these challenges, the increase in production capacity experienced during COVID-19 has been remarkable; production scaled up from zero to billions doses of COVID-19 vaccines, representing three to four times the pre-COVID-19 global vaccine demand. Due to this rapid expansion, however, vaccine production chains have been put under exceptionally high strain, facing diverse challenges and supply chain disruptions [3]. To prevent future disruptions to the vaccine production chain, it is important to identify critical bottlenecks and strategies to overcome these in a timely manner. This will enable the world to better cope with situations when rapid production upscaling is required or when pressure is further exacerbated, e.g. due to the rise of new variants or emergence of new pathogens.
Existing literature on vaccine production chain challenges is scattered, and relatively few papers describe actual manufacturing activities; most focus on decision making and distribution processes. In contrast, studies that do focus on manufacturing activities do so in a highly specialized and detailed way, lacking a broader, holistic approach. Moreover, most studies describe challenges in the production chain in non-pandemic situations, which are believed to be different from high demand pandemic situations [4].
The COVID-19 crisis provides a unique opportunity to investigate production chain challenges during a pandemic; a scenario where production chains are required to run at maximum pace under extreme time pressure. To study the pandemic vaccine production chain, we combined insights retrieved through interviews with existing literature on vaccine production chains. The aim was to understand and anticipate where bottlenecks and opportunities may occur in order to find and implement sustainable solutions, not only to challenges in the COVID-19 pandemic, but also for future challenges to come.
2. Methods
The methodology is built on two phases of data collection. First, primary, qualitative data collection was collected during the pandemic itself through 80 interviews and roundtable discussions. At that time, the first vaccine candidates were given (emergency) authorisation, and issues related to upscaling production became apparent. During these interviews and discussions, conversations were held with 120 representatives of diverse stakeholders to learn from their first-hand experience. Among these representatives were researchers, policy makers, regulators, and top managers of the life science industry, including specialists in vaccine manufacturing. A complete list of organizations and companies that participated to the discussions is presented in Table S1 in the Appendix. Second, a scoping literature review was conducted to complement this experiential data with current and ongoing insights in supply chain challenges. Data from both phases were analysed, merged and structured to provide an overview of vaccine production challenges and opportunities. This enables us to triangulate data, and compare and contrast insights gathered during a pandemic.
2.1. Data collection
During a period of five weeks, between February 2021 and March 2021, around 80 virtual discussions with 120 representatives of diverse stakeholders were held. The qualitative data collection was conducted by the Special Envoy Vaccines for the Netherlands, supported by a team of representatives of the Netherlands’ Ministry of Health, Welfare and Sport as well as the Ministry of Economic Affairs and Climate Policy with the aim to identify, inventorize and assess bottlenecks as well as opportunities for upscaling vaccine production. For most interviews or roundtable discussions a summarizing report was written, and primary recommendations were collated in a report for the Dutch Minister of Health on how the Dutch government and relevant industry stakeholders could contribute to the availability of more vaccines [5].
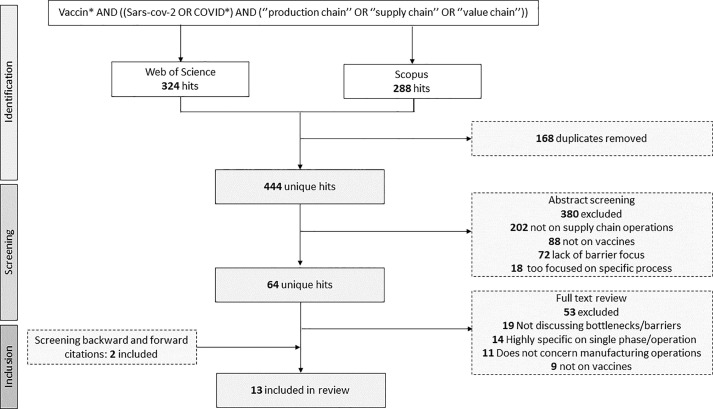
In addition to the interviews, a scoping literature review was conducted with the purpose of exploring the field of vaccine supply chain management, and identifying commonly reported challenges and opportunities. On 6-10-2022, a systematic search was conducted in Web of Science and Embase. Articles had to be published after Nov. 2019 to be included, to ensure they included pandemic considerations. 1831 articles were initially retrieved, out of which 1165 remained eligible for title and abstract screening after deduplication (See Fig. 1 ). Articles were uploaded to Rayyan and screened in a blinded process by two researchers. Conflicting cases were discussed in the presence of a third researcher until consensus was reached. 64 articles were deemed eligible for full-text screening, and a total of 13 studies were ultimately included in the review. A full overview of the search syntax, selection criteria, and included papers can be found Table S2 and S3 in the Appendix.
Fig. 1.
Flowchart of the search and selection process of studies included in the literature review.
2.2. Data analysis
Insights from the qualitative interviews and round table discussions, and studies included in the literature review were analysed through an inductive process. Broad themes and topics describing a particular facet of the vaccine production chain (e.g. capacity building, supply chain operations, regulatory and monitoring) derived naturally from the data. Within these broader themes, a variety of codes and sub-topics were derived and kept in a codebook (e.g. production facilities, human resources, stock management).
The data linking to these codes were grouped together and specific statements were drafted, reflecting their description as bottleneck or opportunity in the data. Statements deriving from the codes were organized among themes into a table. For the literature review the full papers served as input for this analysis; for the qualitative data collection, the insights from the final policy report were the primary basis for analysis. As the qualitative data collection was conducted confidentially, the findings from the report were complemented by one of the co-authors going back to the original summaries to ground them in the data and refine the findings.
To facilitate further analysis and interpretation, the results were structured and divided into three larger segment categories. These categories were formed by splitting the vaccine production chain into three parts, namely: manufacturing capacity, raw input materials, and overarching factors.
-
(1)
Manufacturing capacity: operational and manufacturing activities in the production chain.
-
(2)
Raw input materials: input of materials for functioning of the production chain.
-
(3)
Overarching factors: factors that impact multiple facets of the vaccine production chain.
3. Results
13 articles were deemed eligible and included in this scoping review (Appendix). Included papers were published in 2021 and 2022. All papers discussed the vaccine supply chain in the context of the COVID-19 pandemic. The analysis identified 31 main barriers and 25 opportunities, in 6 different categories (Table 1, Table 2, Table 3, Table 4, Table 5, Table 6). 16 barriers were found both in literature, with primary qualitative data providing more depth to the findings. 13 barriers were only derived from literature, and 2 only from interviews (A6- and E3-, see Table 1 and 5). 13 of the 25 opportunities were found both in literature and in interviews. 9 opportunities were retrieved from literature, and 3 from interviews.
-
1-
Biologicals-derived vaccine manufacturing process
Table 1.
Barriers and opportunities related to the capacity and facilities needed for vaccine manufacturing. IFPMA: international federation of pharmaceutical manufacturers and associations; CDMO = contract development and manufacturing organization, SME: small and medium-sized enterprises. Scaling out: a type of capacity expansion concentrating on the addition of facilities instead of increasing the capacity of already existing facilities. Modular approach: process in which facilities are constructed (partly) off-site in different modules.
|
A. Capacity and facilities | |||
|---|---|---|---|
| A− Barriers | Source(s) | A+ Opportunities | Source(s) |
| A1- Lack of technical facilities for upstream production (e.g., bioreactors) and fill and finish activities | Big pharma representative, 7, 8, 9, 10 |
A1+ “Scaling out’’ of production technologies and partnering with CDMO's to accelerate large commercial-scale production | 6, 7 |
| A2- High financial and time investments to build or repurpose new and existing facilities | IFPMA representative, 7, 9, 10 |
A2+ Exploiting the unutilized fill and finish capacity among small and medium enterprises | Various SME’s |
| A3- Travel restrictions and uncertainties in vaccine composition hinder early construction of new facilities | 8, 10 | A3+ Building upon the willingness of vaccine manufacturers to build a multi-purpose vaccine production facility | CDMO representative/ Big Pharma representative |
| A4- Overreliance upon a limited number of vaccine manufacturers with mass production facilities | 8, 11, 17 | A4+ Incentivizing innovative (e.g., modular) approaches to facility and cold chain design to increase flexibility in manufacturing systems and production capacity | 12,13,14 |
| A5- Specificity and uniqueness of production facilities complicates rapid duplication or repurposing | 6, 10 | A5+ Exploring the possibility of repurposing existing (e.g., veterinary) facilities to facilitate rapid expansion of production capacity | Pharmaceutical company/veterinary pharmaceutical company, 6 |
| A6- Limited global bioreactor capacity due to production of other biologics (e.g., other vaccines, antibodies etc.) | CDMO representative | ||
Table 2.
Barriers and opportunities related to the optimization of production operations and increasing supply chain efficiencies. CDMO: contract development and manufacturing organization.
|
B. Operations and supply chain efficiencies | |||
|---|---|---|---|
| B− Barriers | Source(s) | B+ Opportunities | Source(s) |
| B1− Difficulties in optimization of production process due to time pressures and complexities of biopharmaceuticals | 7, 8, 12 | B1+ Incorporating supply chain experts with appropriate technical and management skills into all vaccine supply chain decisions | 12, 17 |
| B2− Inefficiencies and organizational challenges between actors in globalized production chains | Logistical expert 10, 11 |
B2+ Reducing the geographical distance between production stakeholders and manufacturing processes to decrease production timelines | Logistical expert, Fill & Finish CDMO |
| B3− Logistical challenges due to (ultra) cold chain management | 7, 9, 11, 12, 13, 15, 16 |
B3+ Stimulating the development of a single-dose vaccine with less rigorous storing conditions to reduce cold chain infrastructure complexities | 6, 13 |
| B4− Outdated infrastructure and technical equipment in low-income and low-middle income countries | 7 | B4+ Organizing matchmaking events to exploit undiscovered production opportunities and linking supply chain actors to each other | Taskforce, 6 |
| B5− Lack of human resources to support operational activities (supporting staff, technology transfer, production specialists) | Big pharma representative; 6, 8, 9, 12, 16, 17, 18 |
B5+ Establishing training facilities and/or educational programs to tackle shortages in (e.g., tech transfer) personnel | Big Pharma representative, 12 |
| B6− Difficulties in technology transfer between partnering manufacturers | Big pharma representative 10, 12 |
||
Table 3.
Barriers and opportunities related to the resources and input materials needed for vaccine manufacturing. CDMO: clinical development and manufacturing organization; SME: small and medium-sized enterprises; IFPMA: international federation of pharmaceutical manufacturers and associations; Task Force: this was a vaccine Task Force set up by the government to provide concrete recommendations regarding vaccine production upscaling. MEB: medicines evaluation board. Bullwhip effect: small fluctuations in demand at the retail level can cause progressively larger fluctuations in demand at the raw material supplier level. Ripple effect: a situation in which one event (e.g. a disruption) causes a series of other events to happen.
|
C. Resources and input materials | |||
|---|---|---|---|
| C− Barriers | Source(s) | C+ Opportunities | Source(s) |
| C1− Lack of critical resources and raw materials (e.g., flacons, syringes, cell cultures, filters, single-use bioreactor bags, adjuvants) which can disrupt the entire production chain through compounded risk | CDMO, SME biotech 6, 7, 8, 9, 10, 15, 16 |
C1+ Coordinating and allocating available resources in a centralized manner, e.g., through governments or global initiatives | Task Force, 7, 16 |
| C2− Improper inventory management and material stock-outs | Logistical expert 12, 17, 18 |
C2+ Building flexibility into the production process by making materials interchangeable and by establishing alternative sources of raw materials | 14, 16 |
| C3− Worsening of existing shortages through safety stocking and a bullwhip effect | Logistical expert, 6, 8 |
C3+ Organizing a marketplace and/or matchmaking efforts that link suppliers of raw materials to production chain manufacturers | Task Force, 6 |
| C4− Unfair allocation of available materials amongst countries and manufacturers | CDMO, SME biotech 6, 7, 8 |
C4+ Stretching scare supplies of materials needed in the vaccine production process (e.g., filters) through recycling | 6 |
| C5− Administrative burden posed by export restrictions upon manufacturers and suppliers | Big pharma representative 9 |
C5+ Increasing volumes in flacons or filling vaccines in large multi-dose bags to reduce shortages in glass vials | Logistical expert, vaccine production expert, 7 |
| C6− Reduced availability of materials and disruptions of global supply chains due to export restrictions and protectionist measures | IFPMA representatives 6, 7, 9, 13 |
C6+ Exploring alternative ways of administration (e.g., intranasal, orally or with bio- or microneedle patches) to reduce shortages of traditional syringes | SME biotech, MEB, logistical expert, vaccine production experts, 13, 15 |
| C7− Difficulties in managing supply chains of other life-saving medicines through shortages and a ripple effect | IFMPA representatives 8, 14 |
||
| C8− Lack of reliable data and reduced visibility into available resources | 6, 7, 8, 9, 15, 16 | ||
Table 4.
Barriers and opportunities related to oversight and regulatory compliance in vaccine manufacturing. CEPI coalition for epidemic preparedness innovation; GAVI: global alliance for vaccines and immunizations; MEB: medicines evaluation board; CDMO: contract development and manufacturing organization.’’’. Rolling review: a regulatory tool to speed up the assessment of data on a continuous basis as they become available.
|
D - Regulatory and oversight | |||
|---|---|---|---|
| D− Barriers | Source(s) | D+ Opportunities | Source(s) |
| D1− Interoperability issues due to high product specificity and a lack of regulatory standardization | CEPI/GAVI representative 6, 7, 8, 14 |
D1+ Providing active regulatory support and building in flexibility for manufacturers by allowing e.g., alternative sourcing of materials, innovative trial designs, and rolling reviews | MEB, Big Pharma, 6, 7, 10, 14, |
| D2− Delays due to lengthy and sophisticated quality control (QC) standards |
Big pharma representative 6, 8, 12, 14, 16 |
D2+ Creating more regulatory alignment and harmonization, e.g., in standardization of printed materials and data between manufacturers and countries | 14, 16 |
| D3− Lack of alignment and coordination in regulatory standards and procedures | Vaccine production expert 6, 16, 17 |
D3+ Establishing international monitoring networks to holistically map the flow of materials and information, and to identify unused production capacity. | Big Pharma, CDMO, 8, 12, 16 |
| D4− Lack of a (centralized) monitoring and coordinating system to identify and manage production capacity | MEB, Big Pharma representatives 7, 8, 10, 11, 12, 14, 17, |
||
Table 5.
Barriers and opportunities related to collaboration and communication between supply chain actors. PPP: public–private partnership. Task Force: this was a vaccine Task Force set up by the government to provide concrete recommendations regarding vaccine production upscaling.
|
E. Supply chain collaboration and communication | |||
|---|---|---|---|
| E− Barriers | Source(s) | E+ Opportunities | Source(s) |
| E1− Lack of coordination and communication between production chain actors |
6, 11, 12, 17 |
E1+ Promoting strategic partnerships and dynamic alliances between supply chain actors (e.g., PPP’s, active government engagement) to overcome supply chain challenges | Task Force, 7, 9, 11, 12, 13, 14, |
| E2− Difficulties in demand forecasting and articulation of production needs to manufacturers and suppliers | 6, 8, 11, 12, 15, 17, 18 |
E2+ Improving supply chain visibility and creating better information- and demand forecasting systems by, e.g., use of novel technologies such as blockchain. | 8, 12, 17 |
| E3− Lack of transparency to share supply chain issues and optimize production processes |
8 | E3+ Establishing robust communication and coordination mechanisms between governments, organizations and manufacturers to, e.g., redistribute vaccine production and reduce wastage | Task Force 7, 12, 16, 17 |
| E4− Reduced willingness to communicate production forecasts by manufacturers due to public blaming and shaming | Task Force | ||
Table 6.
Barriers and opportunities related to policy measures and funding. Task Force: this was a vaccine Task Force set up by the government to provide concrete recommendations regarding vaccine production upscaling. Advanced market commitment: a binding contract used to guarantee a viable market for a product once it is successfully developed.
|
F. Policy and funding | |||
|---|---|---|---|
| F− Barriers | Source(s) | F+ Opportunities | Source(s) |
| F1− Lack of public investment/financial support in expanding production capacity of input suppliers | 6 | F1+ Providing direct push funding through e.g., subsidies to develop supply chains and to finance expansion of manufacturing capacity | 6, 7, 10, 11, 16, 17, |
| F2− Lack of financial support mechanisms to support development and manufacturing in low-income and low-middle income countries | 11, 17, 18 |
F2+ Applying financial pull mechanisms (e.g., advanced market commitments) to create market certainty, allowing for alternative risk tolerance approaches by manufacturers (parallel investments, building opportunistic factories) | Task Force 6, 7, 8, 10, 14, |
| F3− Complicated location decisions for vaccine production due to geopolitical tensions |
6, 7 | F3+ Facilitating international policy coordination, addressing export-restricting policies and promoting trade |
Task Force 6, 9, 16 |
The production process for biologicals-derived vaccines is complex and can be divided into three distinct phases. First, upstream manufacturing activities where a mammalian living cell culture is developed, cultivated, and induced to produce the active substance which is then isolated. Second, downstream operations purify the harvested active substance through chromatography, filtration and various other techniques. Lastly, fill and finish processes package the purified active substance in e.g., flacons, and mix it with other ingredients or excipient to enhance the immune response (adjuvants) or reduce the likelihood of (bacterial) contamination (preservatives).
Biologicals-derived vaccine production involves sophisticated facilities and equipment, specialized actors, and many different processes that are unique to the type of vaccine produced. The vulnerability and instability of biologicals through the entire supply chain, makes vaccine manufacturing at times unpredictable. This makes biopharmaceuticals among the most regulated industries. Manufacturing consistency and control, and demonstrating biosimilarity, are paramount to guarantee the quality, efficacy and safety of each vaccine.
-
2-
Bottlenecks and opportunities in capacity building for biologicals manufacturing
4. Production facilities and capacity building
Key respondents indicated there are very limited opportunities to, at a given time, to upscale vaccine production capacity within a period of 2–3 months. However, within 9 to 12 months, there would be possibilities to realize an increase of production capacity through direct expansion of production facilities, production optimization, and by facilitating technology transfer.
With increased vaccine demand, manufacturers ramped up capacity by scaling up facilities and forming new partnerships to facilitate outsourcing of technologies (Table 1 ). Outsourcing of production was explicitly mentioned in literature as facilitator [A1+: [6], [7]] - never before have clinical development and manufacturing organizations (CDMOs) played such a crucial role in a pandemic response. Despite this, a lack of technical facilities for upstream, downstream and fill and finish operations, including bioreactors, remained a key bottleneck, which became evident in interviews with big pharma representatives as well as in literature [A1-: [7], [8], [9], [10]]. Interestingly, discussions with small and medium sized enterprises (SME), revealed an unutilized fill and finish capacity [A2+].
Building new production facilities from scratch was mentioned as option by IFMPA representatives to combat the lack of facilities, although acknowledged to be complicated due to time constraints and increasingly high costs [A2-: [7], [9], [10]]. Moreover, travel restrictions and uncertainties in final vaccine composition were reported as further complicating rapid upscaling and construction of new facilities [A3-: [8], [10]].
4.1. Limited high-capacity manufacturing companies
Another concern mentioned in literature was the overreliance upon a limited number of high-capacity manufacturing companies [A5-: [8], [11], [12]]. Only six manufacturing companies make up for a capacity of 10 billion vaccine doses, which is considered a serious risk for global availability.
Due to the nature of the (biological) vaccine manufacturing process, rapid duplication or sharing of facilities for different types of vaccines is rarely possible and was explicitly described in literature as barrier for upscaling capacity [A6-: [6], [10]]. Facilities, equipment and processes are custom built and designed to produce a specific type of vaccine, essentially making every facility unique. Interestingly big pharma and CDMO’s shared enthusiasm for collaboration and building multi-purpose vaccine production facilities [A3+]. Moreover, innovative (e.g. modular) approaches to facility design were mentioned in literature as opportunity to improve flexibility in manufacturing systems and increase overall production capacity, albeit at higher cost [A4+: [12], [13], [14]].
4.2. Repurposing of existing production facilities
Pharmaceutical companies stated an opportunity for ramping up vaccine production could be to repurpose existing (e.g. veterinary) facilities that make use of large, fixed bioreactors, so that they allow for production of human vaccines [A5+: [6]]. These facilities are largely designed for production of biologicals and would only require several changes to be able to manufacture human vaccines. Some concerns, however, were expressed by CDMO representatives about global bioreactor capacity being required to produce other biologicals such as other vaccines or monoclonal antibodies [A6-].
5. Operations and supply chain inefficiencies
Some respondents further stated that production capacity could be further increased by improving optimization (Table 2 ). The vulnerable and unstable nature of biologicals, however, makes scale-up optimization complicated, requiring significant resource and time investments [B1-: [7], [8], [12]]. Given the necessity of highly-advanced facilities and personnel, vaccine manufacturing networks are global, consisting of collaborations between many different specialized stakeholders. Inherent to such complex global networks are production inefficiencies and organizational challenges, which were identified as a key barrier by logistical experts during interviews, as well as in literature [B2-: [10], [11]]. In addition to that, logistical challenges related to (ultra) cold chain management [B3-: [7], [9], [11], [12], [13], [15], [16]] and outdated technical equipment and infrastructure in low-income countries [B4-: [7]] were mentioned in literature as problematic bottlenecks for supply chain operations.
Insights from interviewees and literature indicated several opportunities that could enhance the efficiency between supply chain actors and mitigate some operational challenges. Firstly, production efficiency could be improved by incorporating supply chain experts into all supply chain decisions [B1+: [12], [17]]. Secondly, logistical experts and CDMO representatives argued that simplification of the production chain could be realized by reducing the geographical distance between production stakeholders [B2 + ]. Aligning processes like active substance production and fill-and-finish operations to take place closer together would reduce logistical complexity, and could, at the same time, utilize the identified capacity of SMEs. Thirdly, manufacturing of a vaccine that does not require extreme storage conditions was emphasised as important in literature as it would substantially reduce cold chain infrastructure complexities [B3+: [6], [16]]. Lastly, European matchmaking events can facilitate cooperation by mobilising and connecting supply chain actors, [B4+: [6]].
5.1. Technology transfer
A common concern among interviewees, and an overarching problem for almost all upscaling scenario’s, was the lack of qualified personnel, which appeared to exist even before COVID-19. Enquiry with multiple vaccine manufacturers and analysis of literature revealed a worrying shortage of supporting staff such as technicians and logistics personnel, as well as technology transfer experts [B5-: [6], [8], [9], [12], [16], [17], [18]].
Big pharma representatives stated that the scale at which their companies collaborate needs to be of sufficient critical mass to allow the technology transfer process to be successful and of added value. The process of technology transfer itself was found challenging both by pharmaceutical companies and in literature [B6-: [10], [12]]. Even for highly experienced manufacturers, successful technology transfer is still very complex and often takes many months up to several years. Interviewees commented that herein lie opportunities for countries to establish training facilities or higher education tracks that can provide specific programs for the field of technology transfer [B5+: [12]].
-
3-
Bottlenecks and opportunities in supply inputs to the biologicals production chain
6. Resource scarcity
The demand for input supplies such as raw materials and specialized equipment increased significantly due to rapid capacity expansion. Interviewees indicated substantial pressure was put on the raw material supply chain, giving rise to challenges across all vaccine manufacturing steps (Table 3 ). Critical shortages of various materials were frequently expressed by CDMOs and in literature, including but not limited to flacons, lipids, syringes, cell cultures, filters, and single-use bioreactors bags. Some of these shortages pose significant compounded risk and could potentially disturb the entire vaccine production chain [C1-: [6], [7], [8], [9], [10], [15], [16]]. To prevent hold-ups or disruptions, interviewees emphasized the importance of maintaining a stable supply and on-time delivery of the hundreds of specific components, derived from multiple countries on a global scale, needed in the manufacturing process.
Improper inventory management and stockpiling of essential materials and equipment have been observed and were identified as a problematic barrier to efficient upscaling of production capacity [C2–: [12], [17], [18]]. Moreover, interviewees expressed that stockpiling behaviour has caused a bullwhip effect and worsened global shortages and stock-outs [C3–: [6], [8]].
6.1. Resource allocation
In the battle for resources, a multi-dimensional dichotomy became evident from different interviewees, characterized by unfair allocation of available materials and resources [C4-: [6], [7], [8]]. This dichotomy existed between large and small companies, between the US and the EU, and between COVID-19 production processes and non-COVID-19 related biopharmaceuticals. Smaller companies expressed rapidly increasing and alarming shortages of components necessary for the preparation of vaccines but also for other biologicals making use of the same components as vaccines. This bottleneck seemed less of an obstacle for large manufacturing firms, presumably due to the long-standing relationship large firms have with global suppliers.
This battle for resources was also taking place on a country and continental level, between the UK and the EU on the shipping of AstraZeneca’s vaccine, but also between the U.S. and the E.U., with regulations such as the U.S. Defence Production Act of 1950 and E.U. export bans. These protectionist measures were not only expressed by pharmaceutical companies to be an administrative burden [C5-: [9]] but they were also claimed to restrict the availability to input material, obstructing the global supply chain networks which specifically require open trade channels to function efficiently [C6-: [6], [7], [9], [13]]. Interviewees warned for a ripple effect, where shortages and protectionist regulations jeopardize not only the input materials for vaccines, but also those of other medicines, inducing a knock-on effect that impacts the global supply chain of other medicines [C7-: [8], [14]].
7. Resource coordination and alternatives
Proper inventory tracking and allocation of raw materials is deemed crucial for an optimal functioning of the vaccine production chain. However, there appeared to be an absence of reliable data along the supply chain to forecast supply and manufacturing needs, which was explicitly mentioned as barrier to effective inventory management, potentially further exacerbating stockpiling behavior [C8-: [6], [7], [8], [9], [15], [16]].
Numerous recommendations were made to ensure more adequate distribution of materials and to prevent disruptions in the production chain due to shortages. Firstly, a central coordinative body was recommended to be set up, to map out and deal with existing and expected shortages, and allocate available resources [C1+:[7], [16]]. In addition, several studies stressed the importance of making raw materials more interchangeable, thus circumventing the scarcity of highly specific raw materials [C2+: [14], [16]]. Lastly, organizing a marketplace and/or facilitating matchmaking efforts between companies could prove effective in linking suppliers of raw materials to vaccine manufacturers [C3+: [6]].
7.1. Innovative alternatives
A common view amongst interviewees was the necessity for replacements of scarce input materials and solutions that prevent the need for certain materials altogether, in the event of critical supply chain disruptions. However, this was expressed to be complicated due to high product specificity, lack of standardization and stringent regulatory requirements that lower the flexibility of finding alternatives [D1-: [6], [7], [8], [14]]. Nevertheless, numerous solutions to raw material scarcity were discussed in interviews and literature. One study reported that various manufacturers stretched the scarce supplies needed in the production process (e.g. filters) through recycling [C4+: [6]]. Moreover, to the existing shortages of vials, logistical and vaccine production experts suggested increasing the volumes in flacons or filling the vaccine doses in larger multi-dose bags [C5+: [7]]. Exploring alternative ways of administration (e.g. intranasally, orally or by using bio- or microneedle patches) was mentioned by regulatory and vaccine production experts as another promising solution to shortages in the traditional vial and needle syringe system [C6+: [13], [15]]. While there’s various promising advantages to using microneedle patches (i.e. improved immunogenicity, dose-sparing effects, reduction of vaccine wastage), these patches were not available during the COVID pandemic due to a lack of existing manufacturing and regulatory infrastructure needed for rapid development. Continued investments will therefore be needed to make this a reliable alternative and to ensure the infrastructure is in place for future pandemics.
-
4-
Overarching factors that influence the entire production chain
Three overarching factors emerged that influence the production chain on a more aggregate level. When managed correctly, they can improve the flexibility and adaptability of the vaccine production chain and its processes during rapid expansion of capacity.
8. Regulatory alignment and supply chain visibility
All interviewees agreed that the willingness of authorities throughout the world to remove regulatory obstacles and speed up approval procedures have made the rapid development and production of COVID-19 vaccines possible (Table 4 ). Regulatory agencies have shown capability and willingness to be flexible in authorisation procedures, by means of rolling reviews and fast track or conditional approval [D1+: [6], [7], [10], [14]]. However, regulatory flexibility is also deemed important after approval and throughout the entire supply chain. The high degree of material specificity and lack of standardisation as expressed in interviews and literature [D1-: [6], [7], [8], [14]], requires regulatory agencies to remain agile in the approval of alternatives and product modifications, and during inspection of new or repurposed manufacturing facilities.
In interviews with pharmaceutical companies and in literature, the lengthy and sophisticated quality control systems were mentioned as particularly challenging to upscaling production, in some cases leading to significant production delays and vaccine wastage [D2-: [8], [12], [14], [16]]. According to a vaccine production expert, this was made even more challenging by a lack of alignment and coordination in regulatory standards and procedures between countries [D3-: [6], [16], [17]]. Further regulatory harmonization between countries could reduce compliance uncertainties for supply chain actors and streamline global supply chains. Several studies highlighted standardization of printed materials (e.g., labels on vials) as an example and a rather easy solution to prevent costly changeover delays between manufacturers and countries [D2+: [14], [16]].
Another important issue mentioned by regulatory agencies, big pharma representatives and in literature, was the absence of (centralized) monitoring and coordinating system to identify and manage production capacity [D4-: [7], [8], [10], [11], [12], [14], [17]]. This was believed to have hampered overall production capacity due to suboptimal decision making, misallocation of resources and unnecessary wastage. Therefore, it was recommended by various interviewees to establish an international monitoring network between governments to map the flow of materials and information, and to identify unused production capacity [D3+: [8], [12], [16]].
9. Supply chain collaboration and communication
The second factor concerns collaboration and communication (Table 5 ). There was consensus amongst interviewees that the degree of interdisciplinary cooperation between universities, companies and government authorities during COVID-19 has been unique and inspiring. Many new alliances have emerged, even between competing parties where collaboration would have seemed very unlikely before the pandemic. The role of the government in promoting strategic partnerships and dynamic alliances (e.g., through public–private partnerships) was explicitly mentioned as important for overcoming supply chain challenges [E1+: [7], [9], [11], [12], [13], [14]]. Even so, various studies reported a lack of communication between production chain actors as a bottleneck in the manufacturing process [E1: [6], [11], [12], [17]]. Moreover, one study reported a lack of transparency and willingness to share supply chain issues and to optimize production processes [E2-: [8]], suggesting that better communication between actors could improve the overall efficiency of the production chain.
The lack in communication made the long-term forecasting and timely articulation of production needs more complicated. This was frequently mentioned in literature as a barrier [E2-: [6], [8], [11], [12], [15], [17], [18]], and is considered crucial for proper planning and scheduling of all stages of the supply chain. Creating better demand forecasting systems and improving supply chain visibility by e.g., use of novel technologies such as blockchain was specifically mentioned as a promising solution for further improvement of the vaccine supply chain [E2+: [8], [12], [17]].
Additionally, several interviewees commented on the importance of expectation management. Not only by suppliers of materials and equipment, but also by vaccine manufacturers to be able to efficiently manage vaccine roll-out and shelf-life logistics. Unfortunately, it appeared from interviews that big pharma companies have become extremely cautious in communicating forecasts and production expectations due to the risk of negative publicity as happened with AstraZeneca, after they could not live up to the contractually agreed deliveries with the EU [E4-].
Lastly, to increase the predictability of vaccine demand and to ensure efficient redistribution of surplus, proper coordination between governments, organizations and manufacturers is necessary. [E3+: [7], [12], [16], [17]]. Multiple interviewees indicated the presence of a limited and temporary vaccine surplus in certain countries due to particular vaccine preferences of citizens. Communication about these surpluses between countries and manufacturers are important for optimal stock management, and possibly, redistribution to other countries.
10. Funding and policy
The last factor concerns economic and policy decisions (Table 6 ). It was uniformly agreed by interviewees that the rapid public investments made by governments, such as the over $12 billion in Operation Warp Speed, facilitated rapid vaccine development and incentivized many actors to accelerate manufacturing by taking unusual risks to meet global demands. Direct push investments into new or repurposed production facilities have created new capacities and increased overall vaccine production and were pointed to as key facilitators in upscaling production [F1+: [6], [7], [10], [11], [16], [17]]. Additionally, pull incentives such as advanced market commitments (AMC’s) were considered pivotal instruments as they de-risked expansion of manufacturing capacity by removing long term demand uncertainties and by ensuring a profitable market [F2+: [6], [7], [8], [10], [14]]. However, some challenges related to financing were identified in literature. Among these were a lack of public investment into expanding capacity of key input suppliers [F1-:[6]], and a lack of financial support mechanisms to support manufacturing of vaccine in low-middle income countries [F2-: [11], [17], [18]].
Lastly, geopolitical tensions were mentioned as having formed a barrier to rapid worldwide upscaling of production. Such tensions have not only been reported to hinder the international trade and flow of goods, but also to have delayed location decision-making for vaccine production facilities [F3-: [6], [7]]. As such, facilitation of international policy coordination and facilitating trade were identified in literature as potential facilitators to overcome the challenges imposed by protectionist measures and export restrictions [F3+: [6], [9], [16]].
11. Discussion
This study shows a gamut of interdependent activities and processes along the vaccine production chain, highlighting its extreme vulnerability to disruptions. The study also revealed a variety of production chain challenges caused by competing interests and objectives between stakeholders (e.g., hoarding, vaccine nationalism, expectation management). These challenges lead to increased supply chain inefficiencies in times of crises. The interdependence of supply chain components (facilities, operations and resources) with funding, collaboration and oversight between stakeholders is depicted in Fig. 2 . Nevertheless, the world has shown an unprecedented collaborative effort to successfully produce billions of vaccines in record time. We must think ahead and use the knowledge gained during this pandemic to help prepare for the next global health crises, regardless of how close or far it may be in the future.
Fig. 2.
The interdependence of supply chain components. Overarching themes of “policy & funding”, “communication & collaboration”, and “regulatory & oversight” impact the entire supply chain. The theme of “policy & funding” mainly concerns the role of governments in supporting, facilitating and stimulating optimal conditions for the supply chains, including funding programs or advanced market conditions, or lack thereof. The theme of “communication & collaboration” concerns the importance or lack of optimal collaboration and communication between stakeholders in the value chain, including strategic partnerships and dynamic alliances, and sharing of information and resources. The theme of “regulatory & oversight” pertains to the role of governments and regulatory agencies to achieve regulatory standardization, support, alignment and harmonization.
Extreme vulnerability typifies the pharmaceutical supply chain – it is driven to be efficient but remains rooted in brittle/fragile system designs [6]. Disruptions are therefore likely to occur, even more so due to the technical and interconnected nature of the vaccine production chain. The vaccine production chain appears to lack robustness and built-in resilience, which would provide the reliability needed in today’s volatile, uncertain, complex and ambiguous (VUCA) world [19]. Instead, current practices favour traditional risk mitigation and management strategies that optimize for efficiency and reduce vulnerabilities [20]. The findings in this study revealed disruptions in supply chain nodes being tackled mostly individually and reactively. However, risk management strategies should be more pro-active, implementing robustness and resilience in the entire supply chain in advance. Supply chains cannot be effectively managed or protected through a siloed approach but require a holistic understanding of how stakeholders and networks interact [21]. This will be critical to implementing resilience and robustness into the system and maintaining normal operations during (inevitable) disruptions in a health crisis.
Challenges due to competing interests are not unique for complex production chains involving many stakeholders – aligning supply chain decisions of separate entities with differing interests is considered one of the key difficulties in health supply chain management [22], [23]. This is even more true during times of high uncertainty and time pressure encountered during pandemics. It might not be in a stakeholder’s interests to commit to full transparency of information as it can undermine their competitive advantage [24]. The key challenge here is to rightly balance stakeholders’ self-interests with the common good. This is particularly challenging as there is not a single coordinating body that takes all decision along the production chain. While governments do set the health objectives that need to be tackled, the production chain is controlled by stakeholders with different incentives. Rethinking the governance of critical public health supply chains, especially during grand challenges, could be a way forward [25]. In the end, a production chain’s efficiency depends highly on how well the interests and objectives of its stakeholders can be aligned, and to what extent stakeholders can create agreement towards a common goal.
Another issue that emerged from the findings in this study is that the current organization of the vaccine production chain (e.g. few manufacturers, globalised production chains, mass production in high-income countries) comes with certain risks and challenges for achieving global vaccine equity. In the current system, global vaccine production is controlled by just a few high-capacity manufacturers mass producing several types of vaccines [26]. While this is cost-efficient, this centralized production also makes potential disruptions among these manufacturers very impactful. There is a need to transition towards a decentralized system where production takes places more locally in different regions [27]. Such a transition would mitigate some of the current risks whilst empowering low- and middle income countries to manufacture vaccines themselves. This would make them less dependent on donations and enable a focus on vaccines that fit local infrastructure and health needs based on local epidemiology [26]. To achieve global vaccine equity, it is important to empower regions/continents to mass-produce their own (type of) vaccine that best fits their local needs, and to spread the manufacturing risks across different production chains. At the same time, due to more spread-out, multiple smaller manufacturing sites, the risk of hoarding, and as a result a potentially increased scarcity of components, becomes higher. Thus, global vaccine equity is not just about a widespread infrastructure for vaccine manufacturing, but also about the availability of critical components and human resources. Moreover, as this study shows, in times of crises, oversight, guidance and coordination are needed to alleviate supply chain inefficiencies that are exacerbated due to peak demand and scarcity.
12. Limitations
Several limitations need to be noted regarding this study. The most important limitation lies in the fact that the literature search was conducted after the interviews. Therefore, the findings of the review could not be used to serve as input for the interviews, nor could the barriers and opportunities described in literature be validated among interviewees. Another arguable weakness is that the interviews conducted with over 100 representatives, did not serve the primary purpose of scientific research, but were aimed at identifying practical solutions to increase the availability of vaccines when they were needed most.
12.1. Implications & conclusion
Given the volatile, uncertain, complex and ambiguous world we live in, and the likelihood of more infectious disease outbreaks in the coming decades, it is expected that strains on production chains will become ever more increased. Many of the supply chain bottlenecks and opportunities identified in this study are not specific to the COVID-19 pandemic – they are also relevant for addressing other health crises that require global collaboration and production upscaling. As such, the findings in this study can form the basis for more in-depth (quantitative) analyses to facilitate establishment of robust supply chains the world can rely upon during future health crises, not only of a pandemic nature, but possibly also for similar events, where supply chains play a critical role in overcoming unanticipated challenges.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was partly financed supported by the Dutch Research Council (NWO), project number VI.Veni.201S.044. [grant number 713732].
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2023.05.027.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1.
Supplementary figure 2.
Supplementary figure 3.
Data availability
Data will be made available on request.
References
- 1.Shire S.J. Formulation and manufacturability of biologics. Curr Opin Biotechnol. 2009;20(6):708–714. doi: 10.1016/j.copbio.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Sell T.K., Gastfriend D., Watson M., Watson C., Richardson L., Cicero A., et al. Building the global vaccine manufacturing capacity needed to respond to pandemics. Vaccine. 2021;39(12):1667–1669. doi: 10.1016/j.vaccine.2021.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golan M, Trump B, Cegan J. The vaccine supply chain: a call for resilience analytics to support COVID-19 vaccine production and distribution; 2020.
- 4.Abbasi B., Fadaki M., Kokshagina O., Saeed N., Chhetri P. Modeling vaccine allocations in the COVID-19 pandemic: a case study in Australia. SSRN Electron J. 2020 [Google Scholar]
- 5.Schikan H. Vaccins - van productie tot preparedness. Den Haag: De Rijksoverheid; 2021 15-03-2021.
- 6.Bown C.P., Bollyky T.J. How COVID-19 vaccine supply chains emerged in the midst of a pandemic. World Econ. 2022;45(2):468–522. doi: 10.1111/twec.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fisher M.K., Ucel C., Yadav P. Building the supply chain for COVID-19 vaccines. Havard Medical School. 2021:34. [Google Scholar]
- 8.Dakin J. Supply chain challenges creating hurdles to COVID-19 vaccine production. Pharmaceut Technol. 2021;45(4) 60-4-4. [Google Scholar]
- 9.Le V.A., Samson L. Are IPRs and patents the real barriers to COVID-19 vaccine supplies? Manchester. J Int Econ Law. 2021;18(2) [Google Scholar]
- 10.Rele S. COVID-19 vaccine development during pandemic: gap analysis, opportunities, and impact on future emerging infectious disease development strategies. Hum Vaccin Immunother. 2021;17(4):1122–1127. doi: 10.1080/21645515.2020.1822136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alam S.T., Ahmed S., Ali S.M., Sarker S., Kabir G., Ul-Islam A. Challenges to COVID-19 vaccine supply chain: implications for sustainable development goals. Int J Prod Econ. 2021;239 doi: 10.1016/j.ijpe.2021.108193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yadav A.K., Kumar D. A fuzzy decision framework of lean-agile-green (LAG) practices for sustainable vaccine supply chain. Int J Product Perform Manag. 2022 [Google Scholar]
- 13.Md Khairi L.N.H., Fahrni M.L., Lazzarino A.I. The race for global equitable access to COVID-19 vaccines. Vaccines. 2022;10(8):1306. doi: 10.3390/vaccines10081306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGovern Ā.T., Salisbury C.M., Nyberg G.B. The pandemic and resilience for the future: AccBio 2021. Biotechnol Prog. 2022;38(1):e3207. doi: 10.1002/btpr.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dai T., Song J.-S. Transforming COVID-19 vaccines into vaccination: Challenges and opportunities for management scientists. Health Care Manag Sci. 2021;24(3):455–459. doi: 10.1007/s10729-021-09563-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finkenstadt D.J., Handfield R.B. Tuning value chains for better signals in the post-COVID era: vaccine supply chain concerns. Int J Oper Prod Manag. 2021;41(8):1302–1317. [Google Scholar]
- 17.Chandra D., Vipin B., Kumar D. A fuzzy multi-criteria framework to identify barriers and enablers of the next-generation vaccine supply chain. Int J Product Perform Manag. 2021;72(3):827–847. [Google Scholar]
- 18.Olutuase V.O., Iwu-Jaja C.J., Akuoko C.P., Adewuyi E.O., Khanal V. Medicines and vaccines supply chains challenges in Nigeria: a scoping review. BMC Public Health. 2022;22:1–15. doi: 10.1186/s12889-021-12361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Btandon-Jones E., Squire B., Autry C., Petersen K. A contingent resource-based perspective of supply chain resilience and robustness. J Supply Chain Manage. 2014;50 [Google Scholar]
- 20.Trump B., Golan M., Keisler J., Cegan J. Vaccine supply chain: resilience-by-design and resilience-by-intervention. Vaccine. 2022;40 doi: 10.1016/j.vaccine.2022.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J., Dou R., Muddada R.R., Zhang W. Management of a holistic supply chain network for proactive resilience: Theory and case study. Comput Ind Eng. 2018;125:668–677. [Google Scholar]
- 22.Kraiselburd S., Yadav P. Supply chains and global health: an imperative for bringing operations management scholarship into action. Prod Oper Manag. 2013;22 [Google Scholar]
- 23.Vosooghidizaji M., Taghipour A., Canel-Depitre B. Supply chain coordination under information asymmetry: a review. Int J Prod Res. 2019;58:1–30. [Google Scholar]
- 24.Ha A.Y., Tong S. Contracting and information sharing under supply chain competition. Manag Sci. 2008;54:701+. [Google Scholar]
- 25.Wang J., Ran B. Sustainable collaborative governance in supply chain. Sustainability [Internet] 2018;10(1) [Google Scholar]
- 26.Bedford H., Attwell K., Danchin M., Marshall H., Corben P., Leask J. Vaccine hesitancy, refusal and access barriers: The need for clarity in terminology. Vaccine. 2018;36(44):6556–6558. doi: 10.1016/j.vaccine.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Kitney R.I., Bell J., Philp J. Build a sustainable vaccines industry with synthetic biology. Trends Biotechnol. 2021;39(9):866–874. doi: 10.1016/j.tibtech.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.